Submitted:
04 March 2025
Posted:
05 March 2025
You are already at the latest version
Abstract
A deficient vitamin D (VitD) status has been associated with SARS-CoV-2 infections, severity, and mortality. However, this status related to SARS-CoV-2 reinfections has been little studied. Our aim was to quantify the risk of reinfections considering VitD status before reinfection. Methods: We performed a population-based prospective cohort study in Borriana (Valencia Community, Spain) during 2020-2023 years, measuring 25-hydroxyvitamin D [25(OH)D] levels by electrochemiluminescence. Cox proportional hazards models were employed. Results: Of a total of 644 SARS-CoV-2 cases with confirmed laboratory tests, 378 (58.9%) were included in out study with an average age of 38.8 years, 241 were females (63.8%), and 127 reinfections occurred (33.6%). SARS-CoV-2 reinfection incidence rates per 1000 person-days by VitD status were for a deficient status 0.4983 (< 20 ng/ml), 0.4977 for insufficient (20-29 ng/ml), and 0.3713 for sufficient (≥30ng/ml). Compared with sufficient VitD status, adjusted hazard ratios were 1.79 (95% Confidence Interval [CI] 0.89-3.59) for deficient status and 1.59 (95% CI 1.06-2.38) for insufficient status with a significant inverse dose-response (p=0.02). These results can help improve nutritional actions against SARS-CoV-2 reinfections. Conclusions: These results suggest that a VitD status lower than 30 ng/ml showed a higher risk of SARS-CoV-2 reinfection. Achieving and maintaining a sufficient VitD status is recommended to prevent reinfections.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Description
2.2. Statistical Methods
2.3. Sensitivity Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [CrossRef]
- Singh, S.; Singh, C.M.; Ranjan, A.; Kumar, S.; Singh, D.K. Evidences suggesting a possible role of Vitamin D in COVID 19: The missing link. Indian J Pharmacol 2021, 53, 394-402. [CrossRef]
- Manson, J.E.; Bassuk, S.S. Commentary. Eliminating vitamin D deficiency during the COVID-19 pandemic: A call to action. Metabolism 2020, 112, 154322. [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde AA.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017,356, i6583. [CrossRef]
- Aibana, O.; Huang, C.C.; Aboud, S.; Arnedo-Pena, A.; Becerra, M.C.; Bellido-Blasco, J.B.; Bhosale, R.; Calderon, R.; Chiang, S.; Contreras, C.; et al. Vitamin D status and risk of incident tuberculosis disease: A nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med 2019, 16, e1002907. [CrossRef]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 2020, 31, 3361. [CrossRef]
- Kaya, M.O.; Pamukçu, E.; Yakar, B. The role of vitamin D deficiency on COVID-19: a systematic review and meta-analysis of observational studies. Epidemiol Health 2021, 43, e2021074. [CrossRef]
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low serum 25-hydroxyvitamin D (Vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: A systematic review and meta-analysis. Front Nutr 2021, 8, 660420.
- Teshome, A.; Adane, A.; Girma, B.; Mekonnen, Z.A. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front Public Health 2021, 9, 624559. [CrossRef]
- Hosseini, B.; El Abd, A.; Ducharme, F.M. Effects of vitamin D supplementation on COVID-19 related outcomes: A systematic review and meta-analysis. Nutrients 2022, 14, 2134. [CrossRef]
- Jamilian, A.; Ghalichi, F,; Hamedi Kalajahi, F.; Radkhah, N.; Jourabchi, N.; Musazadeh, V.; Amini-Salehi, E.; Zarezadeh, M.; Ostadrahimi, A. The role of vitamin D in outcomes of critical care in COVID-19 patients: evidence from an umbrella meta-analysis of interventional and observational studies. Public Health Nutr 2024, 27, e127. [CrossRef]
- Sartini, M.; Del Puente, F.; Oliva, M.; Carbone, A.; Bobbio, N.; Schinca, E.; Giribone, L.; Cristina, M.L. Preventive vitamin D supplementation and risk for COVID-19 infection: A systematic review and meta-analysis. Nutrients 2024, 16, 679. [CrossRef]
- Albergamo, A.; Apprato, G.; Silvagno, F. The role of vitamin D in supporting health in the COVID-19 Era. Int J Mol Sci 2022, 23, 3621. [CrossRef]
- Laporte, J.R. Crònica d’una societat intoxicada, 3rd ed; Ediciones Península: Barcelona, Spain, 2024, pp. 384-387.
- Sinopoli, A.; Sciurti, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The efficacy of multivitamin, vitamin A, vitamin B, vitamin C, and vitamin D supplements in the prevention and management of COVID-19 and Long-COVID: An updated systematic review and meta-analysis of randomized clinical trials. Nutrients 2024, 16, 1345. [CrossRef]
- Bilezikian, J.P.; Binkley, N.; De Luca, H.F.; Fassio, A.; Formenti, A.M.; El-Hajj Fuleihan, G.; Heijboer, A.C.; Giustina, A. consensus and controversial aspects of vitamin D and COVID-19. J Clin Endocrinol Metab 2023, 108, 1034-1042. [CrossRef]
- Jenkinson, C.; Desai, R.; McLeod, M.D.; Wolf Mueller, J.; Hewison, M.; Handelsman, D.J. Circulating conjugated and unconjugated vitamin D metabolite measurements by liquid chromatography mass spectrometry. J Clin Endocrinol Metab 2022, 107, 435-449. [CrossRef]
- Abdollahzadeh, R.; Shushizadeh, M.H.; Barazandehrokh, M.; Choopani, S.; Azarnezhad, A.; Paknahad, S.; Pirhoushiaran, M.; Makani, S.Z.; Yeganeh, R.Z.; Al-Kateb, A.; et al. Association of vitamin D receptor gene polymorphisms and clinical/severe outcomes of COVID-19 patients. Infect Genet Evol 2021, 96, 105098. [CrossRef]
- Hamed, E.R.; Abdelhady, S.A.; Al-Touny, S.A.; Kishk, R.M.; Mohamed, M.H.; Rageh, F.; Othman, A.A.A.; Abdelfatah, W.; Azab, H. Correlation between rs7041 and rs4588 polymorphisms in vitamin D binding protein gene and COVID-19-related severity and mortality. BMC Med Genomics 2024, 17, 284. [CrossRef]
- AlGhamdi, S.A.; Ghosh Dastidar, R.; Rybiński, M.; Alsufiani, H.M.; Khoja, S.O.; Enaibsi, N.N.; Saif, S.F.; Carlberg C. Evaluation of the vitamin D response index in a Saudi cohort. Saudi Pharm J. 2024, 32, 102137.
- Smolders, J.; van den Ouweland, J.; Geven, C.; Pickkers, P.; Kox, M. Letter to the Editor: Vitamin D deficiency in COVID-19: mixing up cause and consequence. Metabolism 2021, 115, 154434. [CrossRef]
- COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet 2023, 401, 833-842.
- Deng, J.; Ma, Y.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Severity and outcomes of SARS-CoV-2 reinfection compared with primary infection: A systematic review and meta-analysis. Int J Environ Res Public Health 2023, 20, 3335. [CrossRef]
- Hu, W.H.; Cai, H.L.; Yan, H.C.; Wang, H.; Sun, H.M.; Wei, Y.Y.; Hao, Y.T. Protective effectiveness of previous infection against subsequent SARS-Cov-2 infection: systematic review and meta-analysis. Front Public Health 2024, 12, 1353415. [CrossRef]
- Bøås, H.; Storm, M.L.; Tapia, G.; Kristoffersen, A.B.; Løvlie, A.L.; Størdal, K.; Lyngstad, T.M.; Bragstad, K.; Hungnes, O.; Veneti, L. Frequency and risk of SARS-CoV-2 reinfections in Norway: a nation-wide study, February 2020 to January 2022. BMC Public Health 2024, 24, 181. [CrossRef]
- Gómez-Gonzales, W.; Chihuantito-Abal, L.A.; Gamarra-Bustillos, C.; Morón-Valenzuela, J.; Zavaleta-Oliver, J.; Gomez-Livias, M.; Vargas-Pancorbo, L.; Auqui-Canchari, M.E.; Mejía-Zambrano, H. Risk factors contributing to reinfection by SARS-CoV-2: A systematic review. Adv Respir Med 2023, 91, 560-570. [CrossRef]
- Ismail, N.F.; Rahman, A.E.; Kulkarni, D.; Zhu, F.; Wang, X.; Del Carmen Morales, G.; Srivastava, A.; Allen, K.E.; Spinardi, J.; Kyaw, M.H.; et al. Incidence and outcome of SARS-CoV-2 reinfection in the pre-Omicron era: A global systematic review and meta-analysis. J Glob Health. 2023, 13, 06051. [CrossRef]
- Domènech-Montoliu S, Pac-Sa MR, Vidal-Utrillas P, Latorre-Poveda M, Del Rio-González A, Ferrando-Rubert S, Ferrer-Abad G, Sánchez-Urbano M, Aparisi-Esteve L, Badenes-Marques G, et al. Mass gathering events and COVID-19 transmission in Borriana (Spain): A retrospective cohort study. PLoS One 2021, 16, e0256747. [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Pac-Sa, M.R.; Vidal-Utrillas, P.; Latorre-Poveda, M.; Del Rio-González, A.; Ferrando-Rubert, S.; Ferrer-Abad, G.; Sánchez-Urbano, M.; Aparisi-Esteve, L.; et al. Persistence of anti-SARS-CoV-2 antibodies six months after infection in an outbreak with five hundred COVID-19 cases in Borriana (Spain): A prospective cohort study. COVID 2021, 1, 71-82. [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Guerra-Murcia, O.; Pac-Sa, M.R.; Orrico-Sanchéz, A.; Gómez-Lanas, L.; Sala-Trull, D.; Domènech-León, C.; Del Rio-González, A.; Sánchez-Urbano, M.; et al. ABO blood groups and incidence of COVID-19 in the mass gathering events in borriana (Spain), March 2020: A retrospective cohort study. Epidemiologia (Basel) 2023, 4, 63-73. [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Pac-Sa, M.R.; Orrico-Sanchéz, A.; Gómez-Lanas, L.; Sala-Trull, D.; Domènech-León, C.; Del Rio-González, A.; Sánchez-Urbano, M.; Satorres-Martinez, P.; et al. Cellular immunity of SARS-CoV-2 in the Borriana COVID-19 Cohort: A nested case-control study. Epidemiologia (Basel) 2024, 5, 167-186. [CrossRef]
- Asif, M.; Groboske, S.E.; Leung, E.K.Y.; Yeo, K.J.; van Wijk, X.M.R. Evaluation of a new generation automated assay for 25-Hydroxy vitamin D based on competitive protein binding. J Appl Lab Med 2019, 4, 247-253. [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011, 96, 1911-30. [CrossRef]
- European Centre for Disease Prevention and Control. Reinfection with SARS-CoV-2: implementation of a surveillance case definition within the EU/EEA. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/reinfection-sars-cov-2-implementation-surveillance-case-definition-within-eueea (accessed on 26 June 2024).
- Narasimhan, M.; Mahimainathan, L.; Araj, E.; Clark, A.E.; Markantonis, J.; Green, A.; Xu, J.; SoRelle, J.A.; Alexis, C.; Fankhauser, K.; et al. Clinical evaluation of the Abbott Alinity SARS-CoV-2 sike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J Clin Microbiol 2021, 59, e0038821.
- Egger, M.; Bundschuh, C.; Wiesinger, K.; Gabriel, C.; Clodi, M.; Mueller, T.; Dieplinger, B. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin Chim Acta 2020, 509, 18-21. [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 2016, 45, 1887-1894. [CrossRef]
- Robins, J.M.; Hernán, M.A.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550-60.
- Oskarsson, V.; Eliasson, M.; Salomaa, V.; Reinikainen, J.; Männistö, S.; Palmieri, L.; Donfrancesco, C.; Sans, S.; Costanzo, S.; de Gaetano, G.; et al. Influence of geographical latitude on vitamin D status: cross-sectional results from the BiomarCaRE consortium. Br J Nutr 2022, 128, 2208-2218. [CrossRef]
- Fernández-Vicente, M.; Miján-de-la-Torre, A.; Vella-Ramírez, J.C.; Martí-Bonmatí, E.; Benito-Ibáñez, V.V.; Martínez-de-Arriba, R. Influencing variables on total and free 25(OH)D levels in healthy population. Rev Clin Esp (Barc) 2022, 222, 313-320.
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front Nutr 2023, 10, 1070808. [CrossRef]
- Rivelli, A.; Fitzpatrick, V.; Blair, C.; Copeland, K.; Richards, J. Incidence of COVID-19 reinfection among Midwestern healthcare employees. PLoS One. 2022, 17, e0262164. [CrossRef]
- Yu, W.; Guo, Y.; Hu, T.; Liu, Y.; Fan, Q.; Guo, L.; Zheng, B.; Kong, Y.; Zhu, H.; Yu, J, et al.. Incidence and severity of SARS-CoV-2 reinfection, a multicenter cohort study in Shanghai, China. J Med Virol 2023, 95, e28997.
- Mokhayeri, Y.; Taherpour, N.; Shahbazi, F.; Ghorbani, S.S.; Fallah, S.; Etemad, K.; Izadi, N.; Mehri, A.; Farhadi-Babadi, K.; Rahimi, E.; et al. Estimation of outpatient SARS-CoV-2 reinfection and recurrence rates and associated factors among COVID-19 hospitalized patients over one-year old: a multicenter retrospective cohort study. BMC Infect Dis 2024, 24, 999. [CrossRef]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020-2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg Health Eur. 2022, 20, 100453.
- Zheng, H.; Wu, S.; Chen, W.; Cai, S.; Zhan, M.; Chen, C.; Lin, J.; Xie, Z.; Ou, J.; Ye. W. Meta-analysis of hybrid immunity to mitigate the risk of Omicron variant reinfection. Front Public Health. 2024, 12, 1457266. [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Khera, T.; Gethings, O.; Diamond, I.; Studley, R.; Taylor, N.; Peto, T.E.A.; Walker, A.S.; et al. Risk of SARS-CoV-2 reinfection during multiple Omicron variant waves in the UK general population. Nat Commun 2024, 15, 1008. [CrossRef]
- Hodcroft, E. CoVariants. Overview of variants in countries. Covariants.org. Available online: https://covariants.org/per-country?country=Spain (Accessed on 1 January 2025).
- Chen, J.; Lu, F.; Shen, B.; Xu, H.; Chen, Y.; Hu, Q.; Xu, A.; Tung, T.H.; Hong, D. Associations between pre-infection serum vitamin D concentrations and Omicron COVID-19 incidence, severity and reoccurrence in elderly individuals. Public Health Nutr 2024, 27, e197. [CrossRef]
- Abu Fanne, R.; Moed, M.; Kedem, A.; Lidawi, G.; Maraga, E.; Mohsen, F.; Roguin, A.; Meisel, S.R. SARS-CoV-2 infection-blocking immunity post natural infection: The role of vitamin D. Vaccines (Basel) 2023, 11, 475. [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A., Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J Endocrinol Invest 2022, 45, 167-179. [CrossRef]
- Basińska-Lewandowska, M.; Lewandowski, K.; Horzelski, W.; Lewiński, A.; Skowrońska-Jóźwiak, E. Frequency of COVID-19 infection as a function of vitamin D levels. Nutrients 2023, 15, 1581. [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open 2020, 3, e2019722. [CrossRef]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Golan Cohen, A.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J 2020, 287, 3693-3702. [CrossRef]
- Israel, A.; Cicurel, A.; Feldhamer, I.; Stern, F.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. Vitamin D deficiency is associated with higher risks for SARS-CoV-2 infection and COVID-19 severity: a retrospective case-control study. Intern Emerg Med 2022, 17, 1053-1063. [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One 2020, 15, e0239252. [CrossRef]
- Zeidan, N.M.S.; Lateef, H.M.A.E., Selim, D.M., Razek, S.A., Abd-Elrehim, G.A.B.; Nashat, M.; ElGyar, N.; Waked, N.M.; Soliman, A.A.; Elhewala, A.A.; et al. Vitamin D deficiency and vitamin D receptor FokI polymorphism as risk factors for COVID-19. Pediatr Res 2023, 93, 1383-1390. [CrossRef]
- Seal, K.H.; Bertenthal, D.; Carey, E.; Grunfeld, C.; Bikle, D.D.; Lu, C.M. Association of vitamin D status and COVID-19-related hospitalization and mortality. J Gen Intern Med 2022, 37, 853-861. [CrossRef]
- Dror, A.A., Morozov, N.; Daoud, A.; Namir, Y.; Yakir, O.; Shachar, Y.; Lifshitz, M.; Segal, E.; Fisher, L.; Mizrachi, M.; et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS One 2022, 17, e0263069. [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F., Jude, E.B. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: A cross-sectional multi-centre observational study. Nutrients 2020, 12, 3799.
- Singh, A.; Rastogi, A.; Puri, G.D.; Ganesh, V.; Naik, N.B.; Kajal, K.; Kahlon, S.; Soni, S.L.; Kaloria, N.; Saini, K.; et al. Therapeutic high-dose vitamin D for vitamin D-deficient severe COVID-19 disease: randomized, double-blind, placebo-controlled study (SHADE-S). J Public Health (Oxf) 2024, 46, 256-266. [CrossRef]
- Ma, W.; Nguyen, L.H.; Yue, Y.; Ding, M.; Drew, D.A.; Wang, K.; Merino, J.; Rich-Edwards, J.W.; Sun, Q.; Camargo, C.A.; et al. Associations between predicted vitamin D status, vitamin D intake, and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) severity. Am J Clin Nutr 2022, 115, 1123-1133. [CrossRef]
- Li, Y.; Tong, C.H.; Bare, L.A.; Devlin. J.J. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw Open 2021,4, e2111634. [CrossRef]
- Liu, Y.; Clare, S.; D'Erasmo, G.; Heilbronner, A.; Dash, A.; Krez, A.; Zaworski, C.; Haseltine, K.; Serota, A.; Miller, A.; et al. Vitamin D and SARS-CoV-2 infection: SERVE Study (SARS-CoV-2 exposure and the role of vitamin D among hospital employees). J Nutr 2023, 153, 1420-1426. [CrossRef]
- Ferrari, D.; Locatelli, M. No significant association between vitamin D and COVID-19: A retrospective study from a northern Italian hospital. Int J Vitam Nutr Res 2021, 91, 200-203.
- Crandell, I.; Rockwell, M.; Whitehead, P.; Carter, K.F.; Hanlon, A. Examination of the moderating effect of race on the relationship between vitamin D status and COVID-19 test positivity using propensity score methods. J Am Nutr Assoc 2022, 41, 646-657. [CrossRef]
- Ganmaa, D.; Chinbayar, T.; Khudaykov, P.; Nasantogtoh, E.; Ariunbuyan, S.; Enkhtsetseg, T.; Sarangua, G.; Chan, A.; Tserendagva, D. Latent TB infection, vitamin D status and COVID-19 severity in Mongolian patients. Nutrients 2023, 15, 3979. [CrossRef]
- da Rocha, A.P.; Atallah, A.N.; Aldrighi, J.M.; Pires, A.L.R.; Dos Santos Puga, M.E.; Pinto, A.C.P.N. Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review. Int J Clin Pract 2021, 75, e14649. [CrossRef]
- Butler-Laporte, G.; Nakanishi, T.; Mooser, V.; Morrison, D.R.; Abdullah, T.; Adeleye, O.; Mamlouk, N.; Kimchi, N.; Afrasiabi, Z.; Rezk, N.; et al. Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative: A Mendelian randomization study. PLoS Med 2021, 18, e1003605. [CrossRef]
- Villasis-Keever, M.A.; López-Alarcón, M.G.; Miranda-Novales, G.; Zurita-Cruz, J.N.; Barrada-Vázquez, A.S.; González-Ibarra, J.; Martínez-Reyes, M.; Grajales-Muñiz, C.; Santacruz-Tinoco, C.E.; Martínez-Miguel, B.; et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch Med Res 2022, 53, 423-430. [CrossRef]
- Jolliffe, D.A.; Holt, H.; Greenig, M.; Talaei, M.; Perdek, N.; Pfeffer, P.; Vivaldi, G.; Maltby, S.; Symons, J.; Barlow, N.L.; et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and covid-19: phase 3 randomised controlled trial (CORONAVIT). BMJ 2022, 378, e071230. [CrossRef]
- Brunvoll, S.H.; Nygaard, A.B.; Ellingjord-Dale, M.; Holland, P.; Istre, M.S.; Kalleberg, K.T.; Søraas, C.L.; Holven, K.B.; Ulven, S.M.; Hjartåker, A.; et al. Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial. BMJ 2022, 378, e071245.
- Karonova, T.L.; Chernikova, A.T.; Golovatyuk, K.A.; Bykova, E.S.; Grant, W.B.; Kalinina, O.V.; Grineva, E.N.; Shlyakhto, E.V. Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers. Nutrients 2022, 14, 505. [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol 2018, 177, 266-269. [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grübler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann ,A.; März, W. Critical appraisal of large vitamin D randomized controlled trials. Nutrients 2022, 14, :303. [CrossRef]
- Sobczak, M.; Pawliczak, R. Effect of Vitamin D3 supplementation on severe COVID-19: A meta-analysis of randomized clinical trials. Nutrients 2024, 16, 1402. [CrossRef]
- Subramanian, S.; Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Laird, E.; Quinton, R.; Thickett, D.; Rhodes, J.M. Vitamin D and COVID-19 revisited. J Intern Med 2022, 292, 604-626.
- Martineau, A.R. Vitamin D in the prevention or treatment of COVID-19. Proc Nutr Soc 2023, 82, 200-207. [CrossRef]
- Autier, P.; Doi, G.; Mullie, P.; Vankrunkelsven, P.; D'Ecclesiis, O.; Gandini, S. Vitamin D, acute respiratory infections, and Covid-19: The curse of small-size randomised trials. A critical review with meta-analysis of randomised trials. PLoS One 2025, 20, e0303316. [CrossRef]
- L Bishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-Inflammatory. JBMR Plus 2020, 5, e10405.
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev Endocr Metab Disord 2022, 23, 265-277.
- Peng, M.Y.; Liu, W.C.; Zheng, J.Q.; Lu, C.L.; Hou, Y.C.; Zheng, C.M.; Song, J.Y.; Lu, K.C. Chao, Y.C. Immunological aspects of SARS-CoV-2 infection and the putative beneficial role of vitamin-D. Int J Mol Sci 2021, 22, 5251. [CrossRef]
- Holick, M.F. The one-hundred-year anniversary of the discovery of the sunshine vitamin D3: Historical, personal experience and evidence-based perspectives. Nutrients 2023,15, 593.
- Arora, J.; Patel, D.R.; Nicol, M.J.; Field, C.J.; Restori, K.H.; Wang, J.; Froelich, N.E.; Katkere, B.; Terwilliger, J.A.; Weaver, V.; et al. Vitamin D and the ability to produce 1,25(OH)2D are critical for protection from viral infection of the lungs. Nutrients 2022, 14, 3061.
- Campolina-Silva, G.; Andrade, A.C.D.S.P.; Couto, M.; Bittencourt-Silva, P.G.; Queiroz-Junior, C.M.; Lacerda, L.S.B.; Chaves, I.M.; de Oliveira, L.C.; Marim, F.M.; Oliveira, C.A.; et al. Dietary vitamin D mitigates coronavirus-induced lung inflammation and damage in mice. Viruses 2023, 15, 2434. [CrossRef]
- Jafarpoor, A.; Jazayeri, S.M.; Bokharaei-Salim, F.; Ataei-Pirkooh, A.; Ghaziasadi, A.; Soltani, S.; Sadeghi, A.; Marvi, S.S.; Poortahmasebi, V.; Khorrami, S.M.S.; et al. VDR gene polymorphisms are associated with the increased susceptibility to COVID-19 among iranian population: A case-control study. Int J Immunogenet 2022, 49, 243-253. [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Pac-Sa, M.R.; Vidal-Utrillas, P.; Latorre-Poveda, M.; Del Rio-González, A.; Ferrando-Rubert, S.; Ferrer-Abad, G.; Sánchez-Urbano, M.; Aparisi-Esteve, L.; et al. Complications post-COVID-19 and risk factors among patients after six months of a SARS-CoV-2 infection: A population-based prospective cohort study. Epidemiologia (Basel) 2022, 3, 49-67. [CrossRef]
- Domènech-Montoliu, S.; Puig-Barberà, J.; Badenes-Marques, G.; Gil-Fortuño, M.; Orrico-Sánchez,A.; Pac-Sa, M.R.; Perez-Olaso, O.; Sala-Trull, D.; Sánchez-Urbano, M.; Arnedo-Pena, A. Long COVID prevalence and the impact of the third SARS-CoV-2 vaccine dose: A cross-sectional analysis from the third follow-up of the Borriana Cohort, Valencia, Spain (2020-2022). Vaccines (Basel) 2023, 11, 1590. [CrossRef]
- Monson, R. Occupational Epidemiology; CRC Press: Boca Raton, Florida, USA, 1982; pp. 94–95.
- DiNicolantonio, J.J.; O'Keefe, J.H. Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in Covid-19 patients. Mo Med 2021, 118, 68-73.
- Guerrero-Romero, F.; Micke, O.; Simental-Mendía, L.E.; Rodríguez-Morán, M.; Vormann, J.; Iotti, S.; Banjanin, N.; Rosanoff, A.; Baniasadi, S.; Pourdowlat, G. Importance of magnesium status in COVID-19. Biology (Basel) 2023,12, 735. [CrossRef]
- Hosseini, SJ.; Moradi, B.; Marhemati, M.; Firouzian, A,A.; Ildarabadi, E.; Abedi, A.; Firooz, M. Comparing serum levels of vitamin D and Zinc in novel coronavirus-infected patients and healthy individuals in Northeastern Iran, 2020. Infect Dis Clin Pract (Baltim Md) 2021, 29, e390-e394.
- Rizwan, M.; Cheng, K.; Gang, Y.; Hou, Y.; Wang, C. Immunomodulatory effects of vitamin D and zinc on viral infection. Biol Trace Elem Res 2025, 203:1-17. [CrossRef]
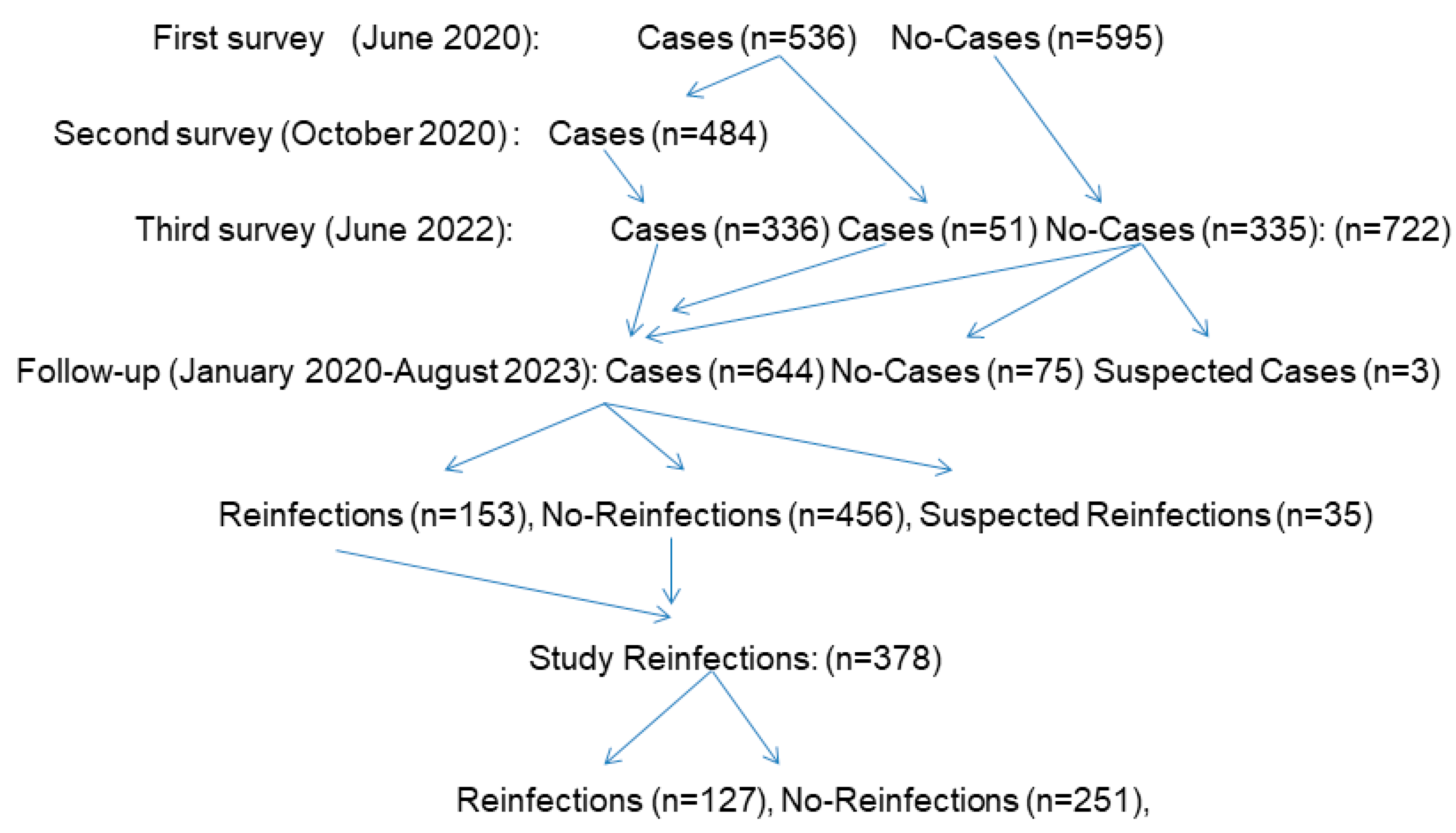
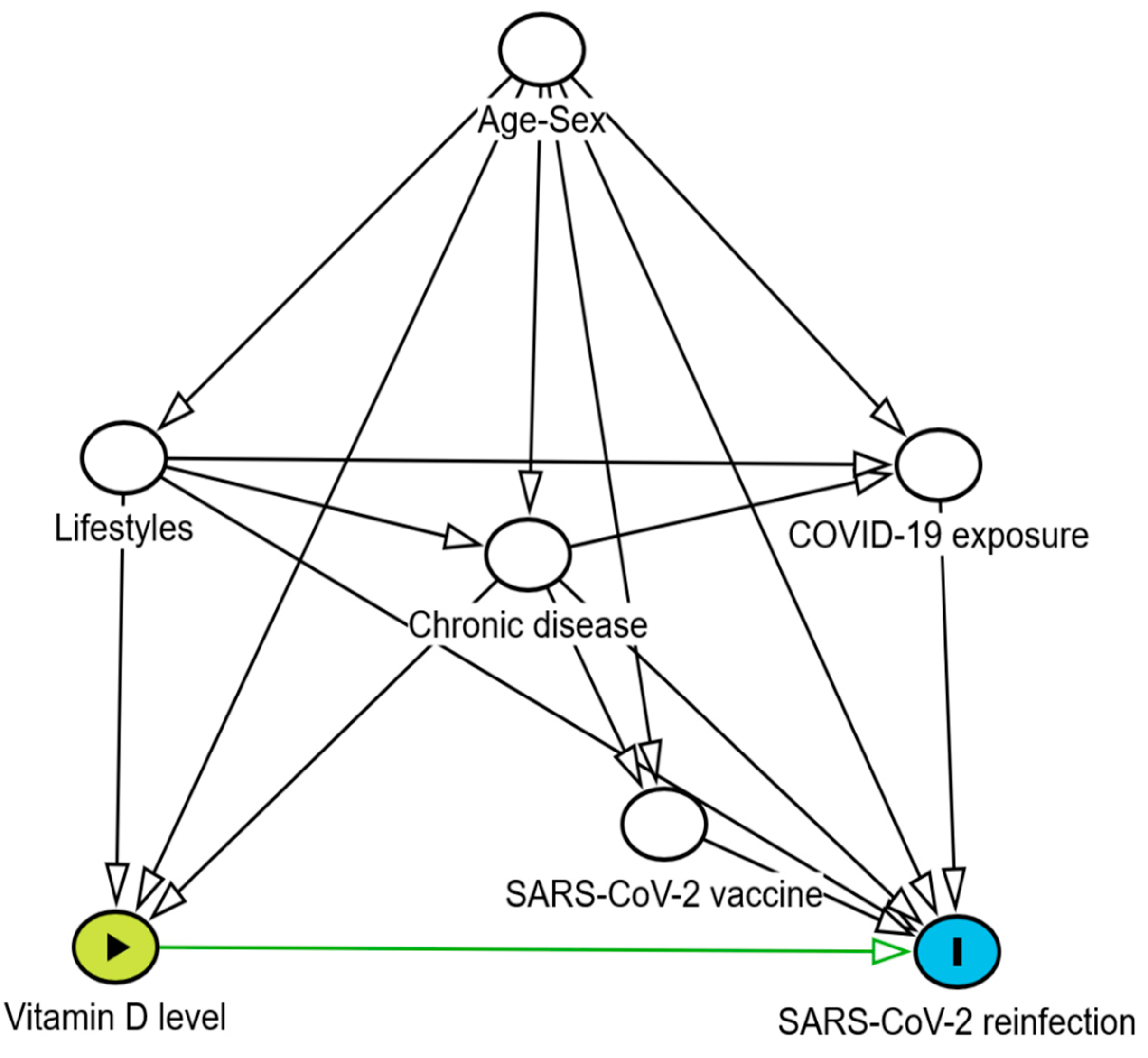
| Variables | Reinfections n=127 N (%) |
No reinfections n=251 N (%) |
p-value |
|---|---|---|---|
| Age (years) + SD1 | 37.9±17.0 | 39.2±16.4 | 0.534 |
| Sex Female | 82(64.6) | 159(63.3) | 0.910 |
| Male | 45(35.4) | 92 (36.7) | |
| Chronic disease2 Yes | 48 (38.4) | 95 (38.2) | 1.000 |
| Chronic disease No | 77 (61.6) | 154(61.8) | |
| Obesity3 BMI4≥30 | 24 (18.9) | 51 (20.7) | 0.785 |
| BMI<30 | 103 (81.1) | 195(79.3) | |
| Alcohol consumption5 Yes | 28 (22.8) | 50 (20.7) | 0.591 |
| Alcohol consumption No | 95 (77.2) | 196 (79.3) | |
| Never smoker6 | 70 (42.6) | 155 (63.3) | 0.306 |
| Current smoker and ex-smoker | 52 (57.4) | 90 (36.7) | |
| Doses SARS-CoV-2 vaccine | 0.095 | ||
| 0 | 6 (4.7) | 5 (2.0) | |
| 1 | 11 (8.7) | 11 (4.4) | |
| 2 | 40 (31.5) | 70 (27.9) | |
| 3-4 | 70 (55.1) | 165 (65.7) | |
| Family CoVID-19 case Yes | 83(65.4) | 151 (60.2) | 0.370 |
| Family CoVID-19 case No | 44 (34.5) | 100 (39.8) | |
| High exposure COVID-19 case7 Yes | 70 (55.6) | 199 (60.3) | 0.436 |
| High exposure COVID-19 case No | 56 (44.4) | 98 (39.7) |
| Vitamin D | Reinfections | No-reinfections | Total | |
|---|---|---|---|---|
| Three levels | N=127 (%) | N=251 (%) | N (%) | p-value |
| 0-19 ng/ml | 11(8.7) | 17 (6.8) | 28 (39.2) | 0.099 |
| 20-29 ng/ml | 69(54.3) | 112 (44.6) | 181 (38.1) | |
| ≥30ng/ml | 47 (37.0) | 122 (48.6) | 169 (27.8) | |
| Two levels | ||||
| 0-29 ng/ml | 80 (63.0) | 129 (51.4) | 209 | 0.037 |
| ≥30ng/ml | 47 (37.0) | 122 (48.6) | 169 | |
| Vitamin D ng/ml+SD1 | 29.0±8.3 | 30.4 ±8.9 | 0.168 |
| Vitamin D levels | SARS-CoV-2 Reinfections |
Person-days | Incidence rate 1000 person-days | 95% CI |
|---|---|---|---|---|
| <20 ng/ml | 11 | 22074 | 0.4983 | 0.2760-0.8998 |
| 20-29 ng/ml | 69 | 138627 | 0.4977 | 0.3931-0.6302 |
| ≥30 ng/ml | 47 | 126578 | 0.3713 | 0.2789-0.4942 |
| <30 ng/ml | 80 | 160701 | 0.4978 | 0.3999-0.6198 |
| ≥30 ng/ml | 47 | 126578 | 0.3713 | 0.2789-0.4942 |
| Total | 127 | 287348 | 0.4421 | 0.3715-0.5261 |
| Vitamin D | Crude hazard ratios | Adjusted hazard ratios1 | ||
|---|---|---|---|---|
| Levels | HR (95% CI) | p-value | HR (95% CI) | p-value |
| <20 ng/ml | 1.25(0.65-2.42) | 0.498 | 1.79 (0.89-3.59) | 0.101 |
| 20-29 ng/ml | 1.26 (0.87-1.83) | 0.219 | 1.59 (1.06-2.38) | 0.024 |
| ≥30 ng/ml | 1.00 | 1.00 | ||
| Trend | 1.17 (0.89-1.55) | 0.256 | 1.42 (1.06-1.92) | 0.020 |
| <30 ng/ml | 1.26 (0.88-1.82) | 0.207 | 1.61 (1.09-2.39) | 0.017 |
| ≥30 ng/ml | 1.00 | 1.00 | ||
| Vitamin D levels | Crude Incidence Rate | 95% CI | Adjusted1 Incidence Rate |
95% CI |
|---|---|---|---|---|
| <20 ng/ml | 0.393 | 0.212-0.573 | 0.323 | 0.168-0.478 |
| 20-29 ng/ml | 0.381 | 0.310-0.452 | 0.396 | 0.325-0.466 |
| ≥30 ng/ml | 0.278 | 0.211-0346 | 0.251 | 0.182-0.313 |
| Trend | Z=2.09 | p-value = 0.036 | Z=2.54 | p-value = 0.011 |
| <30 ng/ml | 0.383 | 0.317-0.449 | 0.399 | 0.332-0.467 |
| ≥30 ng/ml | 0.278 | 0.211-0.346 | 0.254 | 0.183-0.320 |
| Vitamin D | Crude relative risk | Adjusted relative risk1 | ||
|---|---|---|---|---|
| Levels (ng/ml) | RR (95% CI) | p-value | RR (95% CI) | p-value |
| <20 ng/ml | 1.41 (0.84-2.38) | 0.193 | 1.29 (0.74-2.22) | 0.367 |
| 20-29 ng/ml | 1.37 (1.01-1.87) | 0.043 | 1.57 (1.15-2.16) | 0.005 |
| ≥30 ng/ml | 1.00 | 1.00 | ||
| <30 ng/ml | 1.38 (1.02-1.85) | 0.035 | 1.57 (1.15-2.15) | 0.004 |
| ≥30 ng/ml | 1.00 | 1.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





