1. Introduction
Ocular cancers, although relatively rare, often lead to devastating outcomes if not detected and treated early - not only deterioration of vision, but poor survival due to the high percentage of metastatic cases [
1,
2]. The current therapeutic approaches for ocular cancers, such as radiation therapy, and local or systemic chemotherapy, often come with severe side effects and limitations. These include suboptimal targeting of cancer cells, collateral damage to surrounding healthy tissues, and inadequate penetration of ocular structures [
3]. The eye, despite its small size, is one of the most complex organs of the human body, protected by a range of anatomical and physiological barriers that makes drug delivery especially challenging [
4,
5]. Compounding these issues, the tumor microenvironment features very particular conditions, such as acidic pH and hypoxia, further impeding the penetration and efficacy of therapeutic agents. These challenges highlight the urgent need for innovative strategies and improvement of cancer treatments.
Nanomedicine, the application of nanotechnology in healthcare, leverages the unique physicochemical properties of nanoscale materials to enhance drug delivery, improve pharmacokinetics, and enable advanced therapeutic strategies [
6]. Nanoparticles, typically ranging from 1 to 100 nm, offer a high surface area-to-volume ratio and outstandingly customizable surfaces, allowing for large drug loads, controlled drug release, as well as active targeting through ligand-based interactions [
6,
7]. . In oncology, the tumour microenvironment allows for further precision through passive targeting due to the Enhanced Permeability and Retention (EPR) effect [
8,
9] and can even act as a trigger for stimuli responsive formulations [
10,
11], consolidating nanomedicine as a powerful tool for improving treatment efficacy while minimizing systemic toxicity.
With several nanoformulations already commercially and clinically available for ophthalmic applications [
12], and several more in preclnical studies [
13], the integration of nanomedicine into ocular oncology is becoming increasingly feasible. Research efforts have explored innovative strategies to overcome drug penetration barriers, achieve targeted and sustained delivery, and enhance treatment modalities such as imaging-guided therapy, phototherapy, and radiotherapy. Additionally, nanotechnology is paving the way for next-generation approaches, such as gene therapy, offering great promise to revolutionalize ocular cancer treatment [
14,
15].
2. Nanoparticles in Chemotherapy
2.1. Etoposide and Carboplatin
Over a decade ago, Mitra et al. first demonstrated the genetic impact of etoposide-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles, showing an upregulation of apoptotic gene activity in Y-79 cancer cells when compared to native etoposide, achieving about 100 times greater anti-proliferative activity. This finding highlighted the potential of nanoparticulate delivery systems to not only improve drug delivery but also to influence cellular mechanisms at a genetic level, paving the way for innovative approaches in retinoblastoma therapy through toxicogenomics [
16].
Further advancements in this field were made by Godse et al., who formulated PLGA nanoparticles of etoposide specifically for intravitreal injection. They modified the surface of these nanoparticles with chitosan, a mucoadhesive polymer known for its ability to stabilise drug-loaded PLGA complexes and promote sustained drug release at the target site. This modification leads to a uniform intracellular concentration of etoposide over extended periods, enhancing its therapeutic potential [
17]. Chitosan’s free amino groups also allow for the conjugation of targeting ligands, which is particularly beneficial in targeting retinoblastoma cells because they overexpress specific sugar receptors, such as lectins with a preference for galactose and mannose residues. Consequently, galactose was selected as a ligand by Godse et al. for this application. The resulting galactose-chitosan anchored etoposide PLGA NPs (GC-ENP) were characterised by favourable topology, size, polydispersity index, zeta potential, and encapsulation efficiency. In vitro testing demonstrated that these nanoparticles exhibited a sustained drug release pattern and significantly enhanced cellular uptake by Y-79 retinoblastoma cells that overexpressed lectins, compared to non-conjugated PLGA - etoposide NPs. Notably, the cytotoxicity and apoptosis rates in Y-79 cells treated with GC-ENP were greater than those observed with plain etoposide formulations, indicating a marked improvement in therapeutic efficacy [
17].
Other types of NPs that have been used for ocular delivery of etoposide include SLNs. In a study by Ahmad et al., etoposide-loaded SLNs were optimised using a Design of Experiments (DoE) approach to enhance drug bioavailability and retention in ocular tissues. The etoposide-loaded SLNs were characterised for size, drug encapsulation efficiency, and release profile and were able to sustain a therapeutic concentration in the vitreous for 7 days after a signal intravitreal injection , with enhanced deposition in areas like the cornea, conjunctiva, and retina [
18].
Carboplatin, a widely used anticancer drug often co-administered with etoposide, has also been the focus of research aimed at optimising its delivery. Kang et al. crafted PAMAM dendritic nanoparticles which they loaded with carboplatin and administered subconjunctivally in mice. They found that the same dose of carboplatin had better results in terms of tumour shrinkage as a nanoparticulate formulation than the conventional carboplatin in aqueous solution. They were also able to administer higher doses of the drug in its NP form than is possible with plain carboplatin due to its instability. With the higher dose, they had comparable results to low dose NP carboplatin in the treated eye, but they also observed tumour regression in the fellow eye, which suggests a systematic effect [
19].
Mesoporous silica nanoparticles are another type of nanoparticle that has been used for the delivery of carboplatin. Specifically, Qu et al. conjugated MSNP with an Epithelial Cell Adhesion Molecule (EpCAM) - antibody, as EpCAM is a transmembrane glycoprotein that is often overexpressed in rapidly proliferating epithelial tumour cells. The addition of the EpCAM- antibody offered a remarkable advantage to carboplatin-MSNPs compared to MSNPs without it, in terms of targeting retinoblastoma cells and enhancing their cellular uptake, resulting in better efficacy of the drug [
20].
Another strategy to improve targeted delivery is decorating nanoparticles with proteins that belong in the transferrin group, leveraging the overexpression of transferrin receptors in tumour cells. Yet, Ahmad et al. followed a different approach and directly used the proteins apotransferrin and lactoferrin as carriers for carboplatin. The resulting Apo-nano-carbo and Lacto-nano-carbo exhibited high specificity and efficient cellular uptake by Y-79 retinoblastoma cells during in vitro testing. They also demonstrated pH-dependent release of carboplatin, responding to the acidic environment typical of tumour cells, and exhibited prolonged residence time compared to soluble carboplatin [
21].
Taking it a step further, Narayana et al. fabricated lactoferrin protein Nanoparticles which they loaded with both etoposide and carboplatin. When tested in vitro on cancer stem cells in the Y-79 retinoblastoma cell line, the nanoparticles exhibited significantly higher cellular uptake compared to non-cancer cells, demonstrating remarkable specificity, and with the added benefit of dual chemotherapy, notable cytotoxicity [
22].
Following a similar approach, Shinde et al. also developed PLGA nanoparticles that combined carboplatin and etoposide (CE-MIP). There in vitro cytotoxicity studies revealed that CE-MIP outperformed the plain drug mixture against Y-97 cells. Subsequent testing on an orthotopic mouse model via subconjunctival administration showed that CE-MIP elicited double the apoptotic effect compared to control groups. These studies underscore the potential of nanoparticle-based drug delivery systems not only to enhance drug efficacy and synergy but also to provide targeted therapy for retinoblastoma, marking significant progress in ocular oncology research [
23].
2.2. Vincristine
Vincristine is a powerful chemotherapeutic agent that has been used in both retinoblastoma and uveal melanoma, amongst other non-ocular cancers. Notably, Marqibo®, a liposomal formulation of vincristine sulfate, was first approved in 2012 for the treatment of Philadelphia chromosome-negative acute lymphoblastic leukaemia (ALL). Its potential but also toxicity have been explored for metastatic melanoma of uveal, cutaneous or mucosal origin with a good safety profile [
24]. Subsequently a higher dose was tested in a Phase 2 trial for patients with metastatic uveal melanoma [
25]. Liposomal Vincristine was later investigated in two Phase 3 trials led by the Children’s Oncology Group, as part of an adjuvant chemotherapy regimen for paediatric patients with intraocular retinoblastoma [
26,
27].
Further research is being conducted into vincristine delivery through the development of nanoparticle-based systems. For example, Vincristine-loaded Pluronic F127 polymer-coated magnetic nanoparticles were synthesised by Sadri et al. [
28]. This group used two different ligands simultaneously, folic acid and transferrin, in order to increase selectivity and drug uptake. The VCR- loaded nanoparticles demonstrated significantly lower IC50s against Y79 cells compared to healthy ARPE19 cells which suggests higher selectivity and better drug penetration into Y79 cells.
In addition to these advantages, the magnetic nanoparticles exhibited superparamagnetic properties, allowing them to absorb heat effectively and to even generate ROS. Under alternating magnetic field (AMF) irradiation, the nanoparticles heated Y79 cells more efficiently, demonstrating that the combination of hyperthermia and chemotherapy was far more effective than either treatment alone, highlighting the synergistic benefits of this dual approach [
28].
2.3. Doxorubicin
Doxorubicin is a widely used chemotherapy drug with applications for different types of cancer. It has thus been a point of interest in research to be implemented into targeted delivery systems. In fact, Doxil is the first chemotherapeutic drug and one of the first nanoformulations overall to have been made commercially available. This unilamellar PEGylated-liposomal formulation of doxorubicin was first introduced in 1995, and is approved for the treatment of ovarian cancer, AIDS-related Kaposi’s sarcoma and multiple myeloma. Since then, more nanoformulations of doxorubicin have emerged. Lipo Dox® is the second generation of PEG-ylated liposomal doxorubicin with a longer half-life and better stability than its predecessor. A non-PEGylated version of liposomal doxorubicin, Myocet, has also been made available since 2000 for the treatment of metastatic breast cancer. Myocet’s performance has been equal to that of traditional doxorubicin but without the cardiotoxic effects associated with it.
The continuous interest in refining doxorubicin formulations is exemplified by innovations such as PLGA nanocarriers, which were developed by Park et al. These nanocarriers were further enhanced with gold half-shells, forming PLGA-Au NPs loaded with doxorubicin, that could be used in phototherapy. These nanoparticles, upon NIR irradiation, could simultaneously deposit heat and release the drug at the tumour site, drastically improving the cytotoxicity for cancer cells while sparing healthy tissues [
29].
Similar principles have been applied in the treatment of retinoblastoma by different research groups. Boddu et al. investigated the potential of folate-receptor targeted PLGA-PEG-FOL micelles for sustained doxorubicin delivery to retinoblastoma cells. Their study focused on optimising the fabrication process of these micelles, experimenting with different solvents to achieve desirable entrapment efficiency, particle size, and polydispersity. They found that dimethylformamide (DMF) yielded the most favourable characteristics for the micelles. To further control the release of doxorubicin, the researchers incorporated the optimised micelles into a thermosensitive PLGA-PEG-PLGA gel, achieving a sustained drug release profile over two weeks. This sustained release is crucial for maximising drug exposure to the tumour cells while minimising the need for frequent administration. The study also demonstrated the targeted delivery capability of these micelles, revealing a fourfold increase in doxorubicin uptake by Y-79 retinoblastoma cells compared to free doxorubicin. This enhanced uptake was attributed to the presence of folate receptors on the surface of Y-79 cells, which readily bind to the folate conjugated on the micelles [
30].
Parveen et al. also capitalised on the overexpression of folate receptors in Y-79 cells and created folate-decorated chitosan nanoparticles. In vitro studies with Y-79 cells demonstrated the superior cytotoxic effect of the folate-conjugated doxorubicin-loaded chitosan nanoparticles compared to free doxorubicin or unconjugated nanoparticles, as they had an advantage in cellular internalisation via receptor-mediated endocytosis. This targeted approach not only improves drug delivery to the tumour site but also has the potential to reduce the dosage required, thereby minimising off-target effects and improving the overall safety profile of doxorubicin treatment [
31].
Another revolutionary approach presented by Gao et al. are pH-responsive nanoceria, to specifically address the challenge of drug delivery to the tumour microenvironment. As has been discussed previously, nanoceria act as antioxidants in physiological pH environments. However, the acidic tumour environment leads to changes in their electronic structure causing them to act as oxidases and to generate ROS, thus exhibiting cytotoxic activity. [
10] Additionally, the nanoceria were coated with glycol chitosan and conjugated with Doxorubicin and AMD11070, a CXCR4 antagonist. The chemokine receptor CXCR4 has been found to be overexpressed in various cancers, and the group showed that retinoblastoma is amongst those. Interactions between CXCR4 and its ligand, CXCL12, support tumour growth by enhancing angiogenesis, immune evasion, and resistance to therapies, as well as tumour metastasis. AMD11070 not only disrupts the CXCR4 - CXCL12 axis but also facilitates tumour - specific targeting. This triple approach of pH - activated cytotoxic nanoceria, the CXCR4 antagonist and the traditional chemotherapeutic agent, doxorubicin, demonstrated a synergistic effectiveness against retinoblastoma, both in vitro and in vivo [
32].
2.4. Other Chemotherapeutic Agents
Melphalan is one of the novel chemotherapeutic agents that has been applied in the treatment of retinoblastoma, injected either intravitreally or intra-arterially, as mentioned. In the past few years, it has been involved in nanoparticle-mediated delivery studies. Sims et al. created melphalan loaded PLGA nanoparticles which they compared to free melphalan in vitro against retinoblastoma. They also experimented with modifying the surface of the NPs with biotinylated peptides, namely PEG, TET1 and MPG to improve stability and enhance targeted delivery. All modified NPs performed better than unmodified ones, with the latter being the most effective and achieving the highest internalisation by Y-79 cells [
33].
Even more ambitious projects with melphalan have been overtaken. To overcome the risks related to injections altogether, Moheni et al. developed chitosan-alginate melphalan NPs for topical administration. However, since sodium alginate is anionic and hydrophilic, its ability to diffuse through the cornea—which typically favours hydrophobic and cationic or neutral compounds—poses a significant challenge. To address this, the NPs were modified with lauric acid, which not only enhances the formulation's hydrophobicity but also possesses mucoadhesive properties. Ex vivo and in vivo testing in bovine eyes, along with imaging, showed that the modified chitosan-alginate NPs successfully permeated the cornea and delivered melphalan to the vitreous cavity, potentially revolutionising the treatment of retinoblastoma [
34].
Topotecan is another chemotherapeutic drug, approved for various cancers, such as small cell lung carcinoma, ovarian and cervical cancer, and has also been investigated for retinoblastoma [
35]. However, its potential use has suffered due to low bioavailability, side effects and pH-Dependent hydrolysis, which converts it from its active lactone form to a biologically inactive carboxylate species under physiological conditions. Delrish et al. aimed to find a more viable option by incorporating topotecan into chitosan nanoparticles. In vitro tests in human retinoblastoma cells exhibited better rates of cytotoxicity compared to free topotecan and those results were further demonstrated by in vivo testing in a rat model [
36]. The same group further experimented with thiolated and methylated chitosan carboxymethyl dextran nanoparticles (CMD-TCs-NPs and CMD-TMC-NPs) labelled with Cy5, to determine which nanoparticles had the optimal distribution in the virtuous post intravitreal injection in rats with retinoblastoma. They determined that the former had better diffusion capabilities due to lower positive surface charge, and the thiol groups facilitated their interaction with the Y79 cells, potentially improving drug delivery and efficacy [
37].
Following their experiments with carboplatin-loaded MSNPs, Qu et al. also developed topotecan-loaded MSNPs, which they decorated with folic acid. Their study showed remarkable internalisation of the decorated MSNPs through receptor-mediated endocytosis, resulting in significantly higher levels of cytotoxicity in retinoblastoma cells and shrinkage of the tumour in vivo, compared to both free topotecan and unconjugated NPs [
38].
Dasatinib is a small molecule tyrosine kinase inhibitor that has received approval and is used for the treatment of imatinib-resistant chronic myelogenous leukemia (CML) and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). As a dual inhibitor of both Src and ABL kinases, the deregulation of which plays a key role in oncogenetic processes, it has been studied and demonstrated anti-tumor effects in a plethora of human cancers, such as triple-negative breast cancer, gastric, pancreatic and has even progressed to phase I clinical trials against metastatic cutaneous melanoma [
39].
Given its broad spectrum of activity, the potential application of dasatinib in uveal melanoma has also been explored. In preclinical trials, dasatinib was shown to inhibit the growth of primary uveal melanoma cells by disrupting the ERK signaling pathway, a MAPK pathway that is responsible for malignant growth and whose activation depends on the Src kinase. That was further supported by the observation that uveal melanoma cells with higher Src activity and MAPK activation were more susceptible to growth inhibition by dasatinib. [
40,
41] It is worth noting that dasatinib-loaded polymeric micelles have been constructed for intravitreal use and evaluated for safety both in vivo and in vitro [
42]. Although these experiments were focused on proliferative vitreoretinopathy, they lay the groundwork for future applications in treating other ocular diseases, including uveal melanoma.
Other, less conventional agents like curcumin have also been explored in nanomedicine for treating ocular cancers. Curcumin has been shown to sensitise drug resistant tumours by reversing multidrug resistance (MDR) through acting on several MDR pathways, such as the PI3K/Akt/NF-κB, JAK/STAT, MAPK and p53 pathways. [
43,
44,
45] In the Y-79 cell line specifically, it was shown by Thiyagarajan et al. that it modulates the lung-resistance protein (LRP), which contributes to a particularly challenging form of drug resistance in retinoblastoma. These findings, along with the instability and poor bioavailability of curcumin, inspired Das et al. to incorporate it in PLGA NPs, which they decorated with folate. They also added nutlin-3a in the PLGA NPs, an anticancer drug whose efficiency they hypothesised would be enhanced by the presence of curcumin. Indeed, their in vitro experiments against Y-79 cells showed that curcumin inhibits the MPD genes and proteins - MRP-1 and LRP. It also acts synergistically with nutlin-3a to improve cytotoxicity, cell cycle arrest, loss of mitochondrial membrane potential (MMP), and induction of apoptosis. In fact, Folate/nutlin-3a/Curcumin NPs performed superiorly compared to single drug treatments, unconjugated dual drug-loaded NPs, and native drugs in combination [
46].
Curcumin's potential has also been explored in other ocular cancers, such as uveal melanoma. Xie et al. developed an in-situ hydrogel that uses hyaluronic acid and collagen, which are basic components of the vitreous body. Curcumin-loaded nanoparticles were prepared via an emulsion-solvent-evaporation method within this hydrogel, and they were investigated for their rheological properties, payload release, and in vitro anti-UM effects. This delivery system could pass from its liquid form in room temperature to gel at 37 °C within two minutes, it proved to be biocompatible and slow degrading, allowing a sustainable release. Most importantly, the gel that contained curcumin loaded NP resulted in significantly lower cell viability of UM cells compared to the gel containing the same amount of curcumin without the NP carriers. The group suggests this may be due to the instability of curcumin in the aqueous environment and proves the necessity of NP for a more stable, sustainable and effective drug delivery [
47].
Latorre et al. employed albumin-stabilised gold nanoclusters loaded with AZD8055, an mTOR kinase inhibitor that exerts its oncolytic effect via the PI3K/AKT/mTOR pathway for the treatment of UM. While free AZD8055 is impossible to administer intravenously due to its hydrophilicity, the albumin-based NPs allowed its systemic administration in mice and its reach to UM (Mel202) cells. They also demonstrated excellent specificity by sparing healthy keratinocytes as opposed to unconjugated AZD8055 [
48].
Pentacyclic triterpenes have also demonstrated anti-tumour potential and with the help of nanomedicine, they can achieve desirable solubility, stability and bioavailability to be considered in cancer treatment. [
49,
50] Silva et al. developed PLGA NPs loaded with Oleanolic (OA) or its isomer, ursolic acid (UA) which they tested against three different human cancer cell lines, among which the Y-79 cells. For the retinoblastoma cell line in particular, both types of PLGA NPs exhibited significant cytotoxicity, accentuating their potential in retinoblastoma treatment [
51].
Lastly, Methotrexate, which is the main chemotherapeutic drug for intraocular lymphoma, has been successfully conjugated with dendrimers by two different research groups. Both groups used PAMAM-based formulations functionalized with FA, and demonstrated that the slower diffusion rate of the nanosystem compared to the free drug resulted in controlled release with better therapeutic results, as well as reduced toxicity [
52,
53].
3. Nanoparticles in Ocular Imaging
It has already been discussed how specific imaging modalities, like OCT and PAI can be improved with the use of inorganic nanoparticles, especially AuNPs. OCT functions by detecting the backscattering of light from the tissues, typically using NIR light, to create an interference pattern and provide cross-sectional images. PAI is a hybrid imaging technique that depends on the photoacoustic effect and combines light (laser) and ultrasound to visualise tissues. It works by using laser pulses to heat tissues which then, due to thermoelastic expansion, generate pressure waves detectable as ultrasound that ultimately generate the PAI image. PAI also remains largely unaffected by scattered lighting, allowing deeper tissue imaging than OCT. Inorganic nanoparticles, especially AuNPs, have been extensively studied as signal enhancers and their application is also extended in ocular cancer imaging [
54,
55].
Over a decade ago, Prabhulkar et al. used gold nanorods to detect squamous cell carcinoma in conjunctival cells. They functionalized the nanorods with antibodies specific for glucose transporter-1 (GLUT-1), a protein overexpressed in ocular surface squamous neoplasia (OSSN) to visualise neoplastic cells on the OCT. Their study faced a few notable limitations, including the low specificity of GLUT-1 antibodies, which also bound to erythrocytes, and the need for a "threshold" concentration of Au nanorods in the tissue for detection via OCT. This threshold was only reached by more advanced OSSN lesions due to higher GLUT-1 overexpression. Yet, they successfully showed that Au nanorods can be used as molecular markers for OSSN on the OCT [
56].
Raveendran et al. were the first to use gold nanoparticles for ocular PAI. Their nanocages, which can be synthesised remarkably fast, were demonstrated to emit strong PA signals when excited with a nanosecond pulsed laser and produced high contrast PA and US images. In fact, ex vivo experiments in porcine eyes demonstrated approximately 50% stronger PA signals in the presence of nanocages, which the team postulates could be leveraged to more precisely visualise the size, depth, and location of uveal melanoma [
57].
Warther et al. crafted mannose-functionalized fluorescently labelled MSNPs designed to target retinoblastoma (Rb) cells. This strategy was based on their previous work demonstrating that both mannose and galactose can facilitate the uptake of nanoparticles by Rb cells through carbohydrate receptor-mediated endocytosis, a process driven by the overexpression of these receptors on Rb cells. The researchers focused on synthesising MSNPs with a diameter of approximately 25 nm, small enough to potentially bypass the BRB. To address the challenge of aggregation commonly encountered with small MSNPs, PEG chains were incorporated onto the nanoparticle surface. The successful uptake of the fluorescently labelled MSNPs by the target cells was confirmed by confocal microscopy allowing the detection of RB in vitro [
58].
While the aforementioned studies have primarily focused on imaging in ocular cancers, most nanoparticle-mediated imaging is actually conducted in combination with therapeutic interventions. The following sections will explore several multi-modal applications of nanoparticles, highlighting their dual role in both diagnosis and treatment.
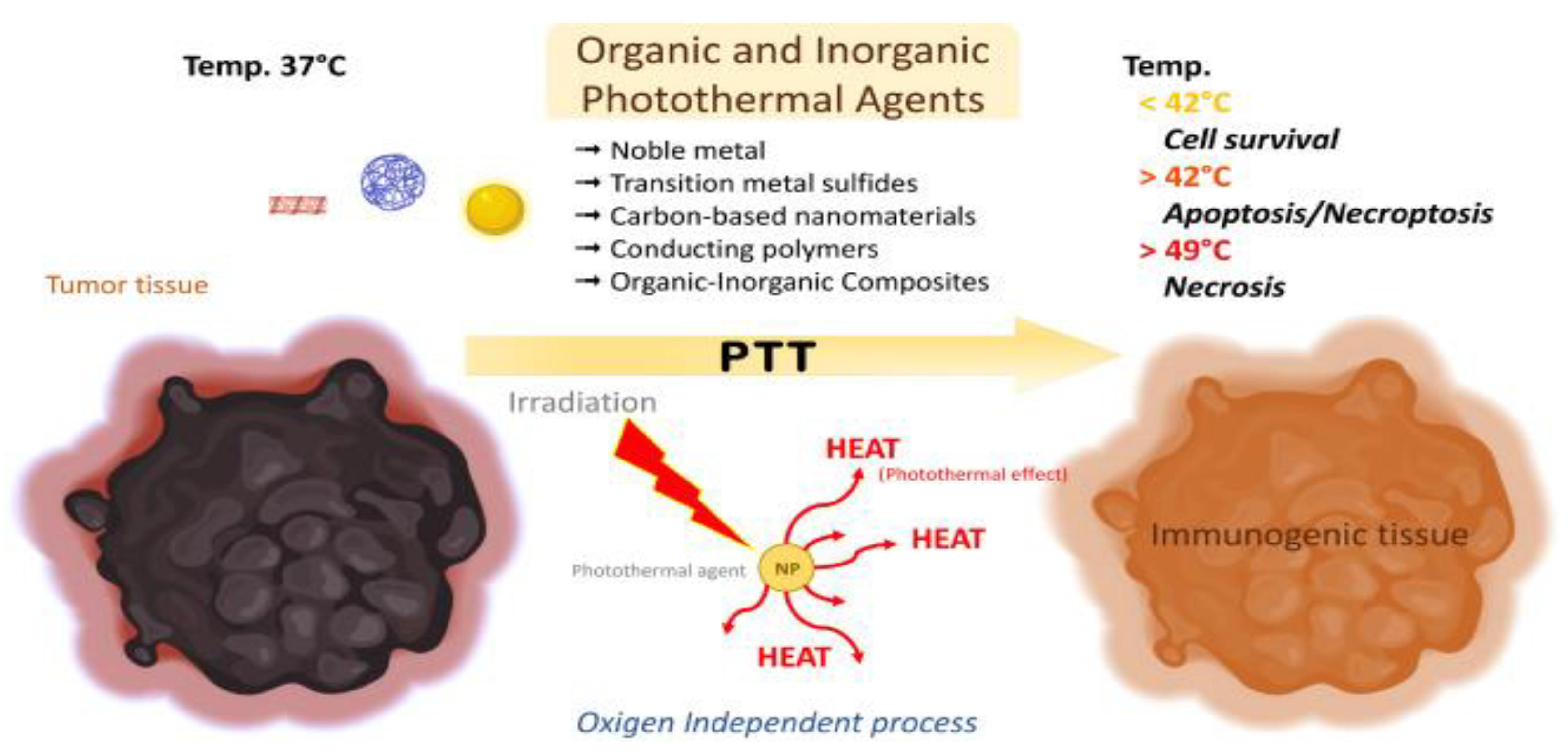
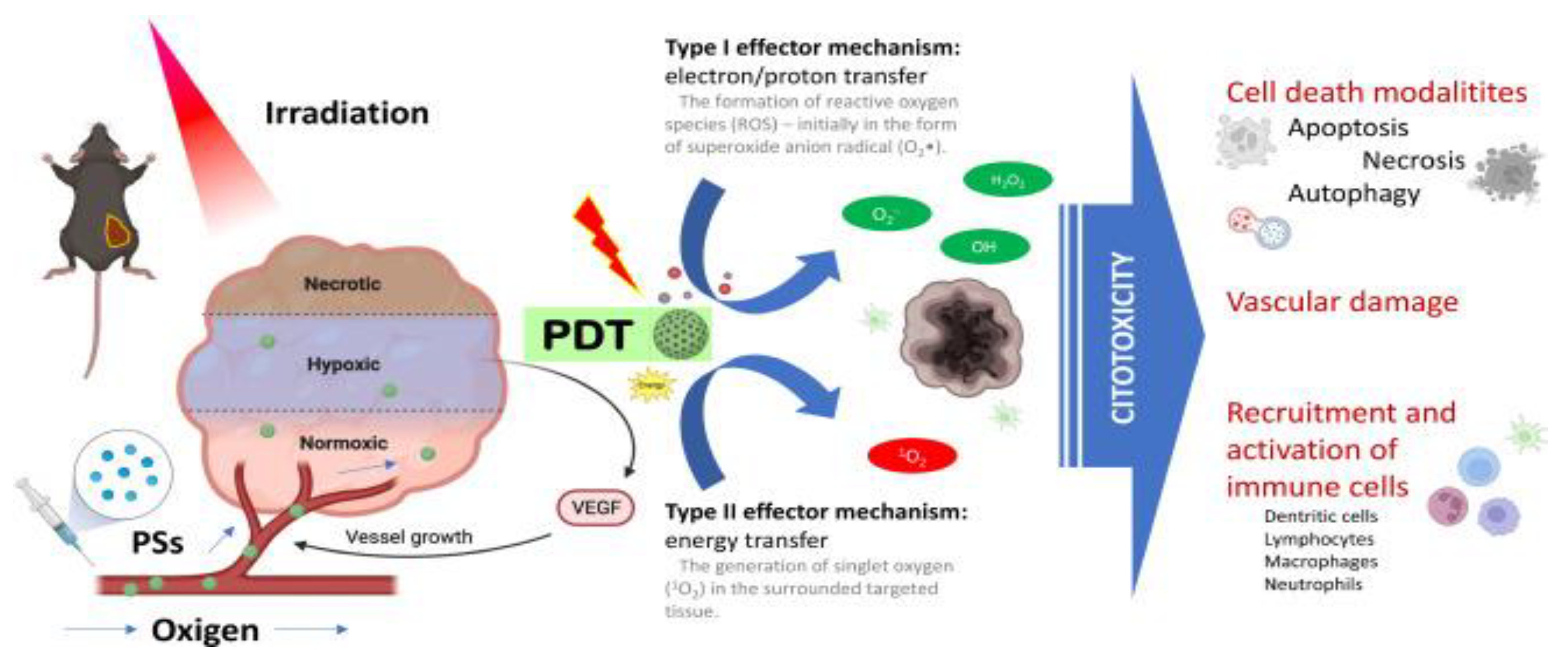
4. Nanoparticles in Phototherapy
Nanoparticles have gained popularity as agents for controlled and targeted heat deposition, thus causing minimal damage to healthy tissue. Their optical properties in combination with their specific binding at tumour cells has given rise to promising types of therapies that rely on hyperthermia or photoactivation. In particular, Photothermal Therapy (PTT) and Photodynamic Therapy (PDT) were initially developed without nanoparticles, relying on direct tissue heating or conventional photosensitizers, respectively, but the introduction of nanotechnology has greatly enhanced them.
PTT uses light sensitive heating nanoparticles which, after being delivered to the tumour site, are activated by a particular wavelength, usually in the near-infrared (NIR) spectrum. Different types of nanoparticles, such as gold nanoparticles (AuNPs), quantum dots and graphene oxide have been employed for their optical properties, but conventional NIR agents such as indocyanine green can also be used in conjugation with other types of nanoparticles. (
Figure 1) Nanomaterial-mediated PTT has been a subject of investigation for several types of tumours such as oral epithelial [
59], colon [
60] and lung carcinoma [
61], and due to it being effective and minimally invasive, it is only logical that it would be studied for ocular cancer treatment as well [
62].
PDT uses photosensitizers that upon light irradiation can transfer absorbed photon energy or excited electrons to surrounding oxygen to generate reactive oxygen species (ROS), thus damaging specific target-cells. Due to their rapid growth, abnormal metabolism and poor blood supply, tumour cells exhibit higher levels of ROS species than normal cells, thus PDT capitalises on their higher redox potential. (
Figure 2) Nanotechnology addresses some important limitations of traditional photosensitizers by offering more stable solutions that do not form aggregates and do not degrade as fast, resulting in more effective and prolonged action.
4.1. Hyperthermia
Tumour cells are known to be more susceptible to heat than healthy cells due to hypoxic and acidic conditions that occur from their poor vasculature, and thus heat-induced apoptosis has been explored as an anti-cancer strategy. [
63] Nevertheless, achieving further precision is crucial to minimise damage to surrounding healthy tissues. The advantage of using nanotechnology for improved selectivity and less collateral damage has been well demonstrated in research.
Demirci et al. crafted dextran-coated iron oxide nanoparticles (DCIONs) to induce magnetic hyperthermia. When an external alternating magnetic field is applied, magnetic nanoparticles are caused to oscillate, generating heat through three mechanisms: Néel relaxation, Brownian relaxation and hysteresis loses. In addition, they can produce ROS, resulting in further DNA damage and protein oxidation. The group treated both Y-79 and ARPE-19 cell lines, the latter being healthy RPE cells, to magnetic hyperthermia and determined that in the presence of DCIONS, cell apoptosis was selectively triggered in retinoblastoma cells depending both on dose and time following irradiation, with minimal cytotoxicity to healthy cells [
64].
Ultrasonic hyperthermia is another heat-depositing treatment that has been implemented in retinoblastoma, where high-frequency sound waves (ultrasound) is used to generate localised heat in a tissue. Moradi et al. hypothesised that gold nanoparticles can enhance the efficacy of ultrasonic hyperthermia against retinoblastoma cells. Indeed, their in vitro experiments showed that cell apoptosis of 50% was achieved in just 4.5 minutes of radiation for Y97 cells under the presence of gold nanoparticles instead of the 9 minutes that were required with ultrasonic radiation alone. In addition, the cytotoxic effect was proportional to the concentration of gold nanoparticles, proving that Y97 cells are more susceptible to hyperthermia in the presence of gold NPs in a dose dependent manner [
65].
Laser-induced hyperthermia is another concept that has been explored and successfully implemented for cancer cell apoptosis, especially with the involvement of gold nanoparticles. With a similar concept to SLT (Selective Laser Trabeculoplasty) that is routinely used in clinical practice for the treatment of open angle glaucoma by targeting the pigmented cells of the trabecular meshwork, Pitsillides et al. developed a method that used short laser pulses to ensure that heat does not have time to flow away and is confined at the target, a phenomenon known as thermal confinement. Their technique relied solely on light absorption, specifically the interaction of light with nanoparticles. The nanoparticles of choice were gold nanoparticles which were conjugated to different antibodies for targeted delivery. The group experimented with laser parameters, such as wavelength and duration, as well as nanoparticle size and concluded that gold nanoparticles could render the plasma membranes of target cells transiently permeable and inactivate their proteins, resulting in their selective death upon treatment with nanolaser [
66].
Katchinskiy et al. explored a similar technique for the treatment of RB. Specifically, they used PEG-ylated gold nanorods coated with EpCAM antibodies for targeted delivery. They also utilised femtosecond lasers, which have an even shorter pulse duration than nanolasers and can achieve higher peak power, leading to very high temperatures for the gold nanorods, beyond the melting point of bulk gold. The nanorods can then exert their thermal properties to the environment which are confined to the cell they are in, without affecting neighbouring cells, leading to targeted apoptosis of tumour cells. Their choice of wavelength for the laser pulses at 800 nm was also key to the optimal effect of the laser, because it falls within the "tissue transparency window" of the eye. This means that light at this wavelength can pass through the cornea, lens, and other eye structures with minimal absorption before reaching the retina [
67].
The following year, Darviot et al. used a 527 nm nanosecond laser, which is conventionally used for vitreolysis in higher fluences. For their experiment, they used AuNP and AuAgNPs with lower fluences to irradiate Y79 cell cultures in a vitreous phantom model made of hyaluronan to reproduce the viscosity of the vitreous. AuNP and AuAgNPs accomplished similar results, so they settled on AuNPs as they are considered less toxic. They proved that the presence of gold nanoparticles can lower the fluence required for apoptosis as they managed a cellular death rate of 80% with fluence of 20 J cm-2 cell clusters. Another concern is that Y79 cells tend to form aggregates which are harder to reach by irradiation alone but gold nanoparticles succeeded in diffusing within the clusters even in a viscous medium, achieving cell death despite the aggregates [
68].
4.2. Light-Activated NPs / Photosensitizers
For the treatment of retinoblastoma, there are instances of nanoparticle-based photosensitizers designed for PDT from over a decade ago. For example, Makky et al., in 2011, created liposomes to carry glycodendrimeric porphyrins, which are commonly used for their photodynamic activity. They experimented with three different types of porphyrin compounds, the first without mannose residues, the second and third with mannose units attached to their dendrimer structures, with the third compound having a slightly longer spacer between the porphyrin core and the mannose units than the second - triethylene glycol (TEG) spacer as opposed to diethylene glycol (DEG). The liposomes with the porphyrin compounds were tested in an artificial Rb cell membrane model and it was concluded that mannosylated porphyrins, especially the group with the TEG spacer, could bind more effectively to Rb cells and be potentially used in PDT [
69].
Another example involves the use of titanium dioxide (TiO2) nanoparticles which have been used in the past as photosensitizers and have exhibited notable photocatalytic activity. Balachadran et al. tested both pure TiO2 and cerium (Ce)- doped TiO2 nanoparticles against Y-79 cells for their photodynamic capacity. It was determined that the Ce - TiO2 were significantly more cytotoxic against cancer cells upon UV radiation exposure, which was attributed to the light responsive capability and strong redox properties of Cerium [
70].
For the treatment of uveal melanoma, Normand et al. created light sensitive nanoparticles, called vectosomes, formed by complexing the C-terminal half of the VP22 protein with antisense oligonucleotides (ODNs). They used the antisense c- raf ODN to target and inhibit the expression of the c- raf kinase gene which is crucial to cancer cell proliferation and prevention of cell death. The vectosomes were tested both in vitro and in vivo, in a mice model, and were found to successfully reach the retina when intravitreally injected and were internalised by both the OCM-1 (uveal melanoma) and ARPE-19 (normal RPE) cells lines, where they remained stable unless illuminated. Upon transscleral illumination with either cold white light or Argon laser, the ODNs were released and quickly reached the nucleus, inhibiting OCM-1 cell proliferation by up to 60% compared to untreated or unilluminated groups [
71].
Perhaps the most ambitious and promising treatment currently investigated for the treatment of uveal melanoma is Belzupacap sarotalocan (AU-011), a light-activated nanoparticle. It consists of a human papillomavirus (HPV) virus-like particle (VPL) conjugated with IRDye® 700, a phthalocyanine photosensitizer that responds to NIR irradiation. The VPL can deliver over 40 times the amount of dye molecules compared to the alternative, antibody-drug conjugates [
72]. It is also designed to specifically target heparan-sulfate proteoglycans (HSPGs), which are often overexpressed and mutated in tumour cells [
73]. In vitro studies have demonstrated that, when activated by NIR light, AU-011 can induce immunogenic cell death in uveal melanoma cell lines [
74]. Subsequent in vivo studies in rabbits explored both intravitreal and suprachoroidal administration to determine optimal drug distribution and penetration into the choroid [
75]. Suprachoroidal administration proved superior and advanced to a Phase 2 clinical trial of AU-011 for patients with primary indeterminate lesions and small choroidal melanoma (NCT04417530)[
76].
Visudyne®, as it has already been mentioned, is a lipid-based nanoformulation of the photosensitizer verteporfin and the only commercially available formulation of verteporfin. It has been clinically approved for PDT therapy in AMD and choroidal neovascularization and it is now being explored for PDT therapy in uveal melanoma. Multiple case series with verteporfin-mediated PDT have been conducted for the treatment of small choroidal melanoma, including 3 studies of 45 patients in total, that showed tumour regression for most patients, stable or improved visual acuity and depletion of subretinal fluid, which is an indicator of abnormal leaking vessels. One of the most recent case series of 12 patients showed a 67% rate of complete tumour regression following 3 sessions of PDT after an average of 5 years. However, another study with a 5-year follow-up time and 26 patients suggests the possibility of recurrent malignancy and proposes other therapies be prioritised [
77]. Nonetheless, all four studies demonstrated that PDT with Visudyne was well tolerated and without significant complications. [
78,
79,
80] It is also worth noting that another clinical study was conducted by Sun Yat-sen University to evaluate the safety and effectiveness of Visudyne-mediated PDT in retinoblastoma, though the results have yet to be made available. (NCT04429139)[
81]
5. Multimodal Applications
A revolutionary aspect of using nanoparticles for photothermal therapies is that they can be combined with in vivo imaging, targeted chemotherapy as well as with each other. While some examples of imaging or photo-based therapies separately have been mentioned, most studies capitalise on the multimodal capabilities of nanoparticles.
5.1. PTT in Retinoblastoma
Lipid-based nanoparticles are at the forefront of current research for combining PTT of retinoblastoma with other modalities, due to their versatility and ability to integrate different types of molecules. Liu et al. utilised liposomes to encapsulate and stabilise indocyanine green (ICG), a NIR agent that is FDA approved for PTT but whose use often suffers due to issues of aggregation, instability and fast clearance. In vitro and in vivo experiments in mice with retinoblastoma showed that its conjugation to liposomes can not only prevent its premature clearance through systemic circulation but also improve delivery to the target-site with better tissue penetration. As a result, the ICG-liposomes greatly enhanced fluorescence and photoacoustic imaging in vivo and boosted the efficacy of PTT, advancing the development of image-guided phototherapy [
82].
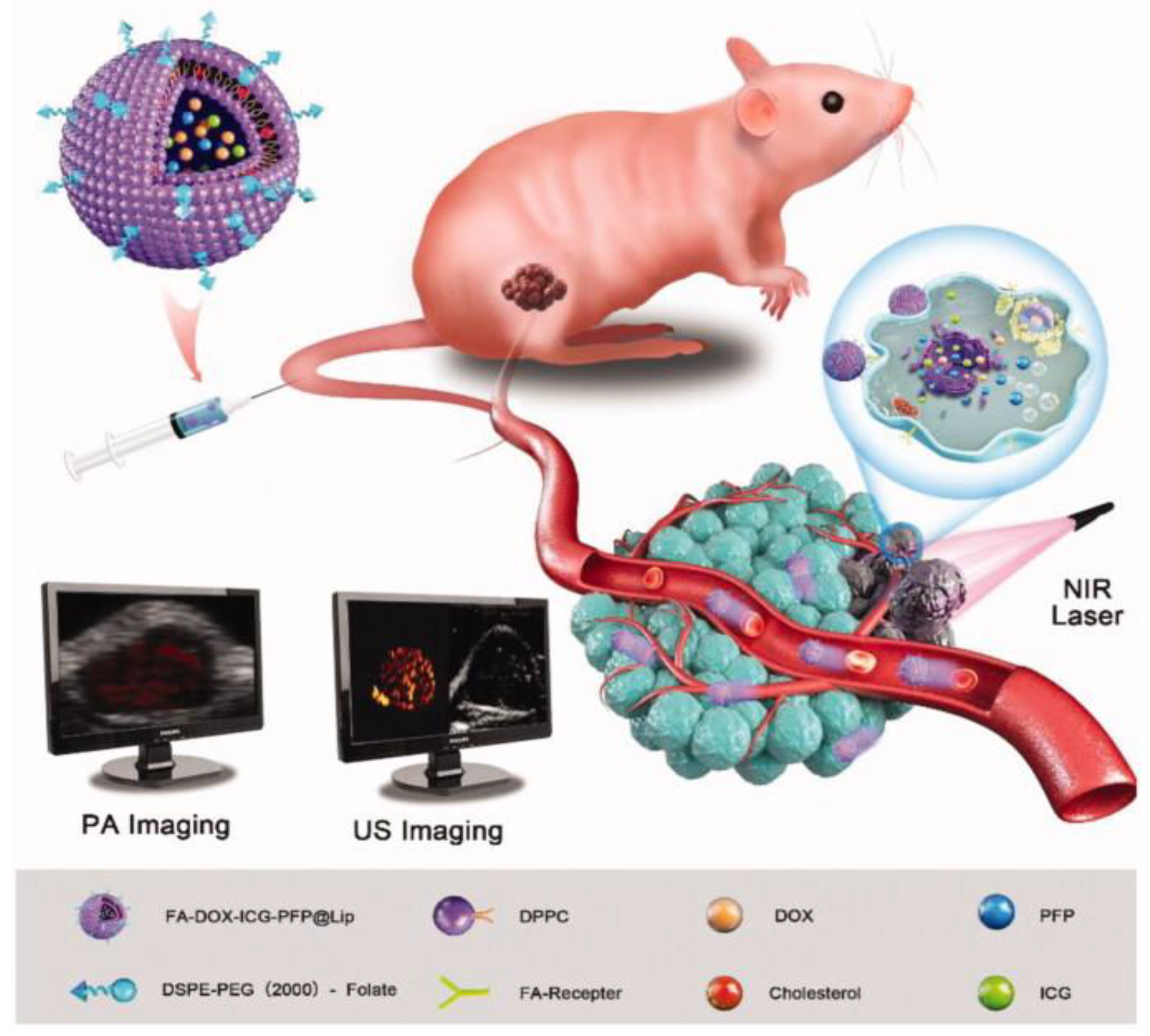
In the same year, Li et al. also developed ICG-liposomes, additionally incorporating doxorubicin for combined chemotherapy, along with liquid perfluoropentane (PFP) to further enhance imaging. Perfluoropentane is a fluorocarbon that is liquid at room temperature but has a relatively low boiling point, vaporising to form microbubbles that improve the clarity of US imaging with slight increases in temperature. Furthermore, they decorated the nanoplatform with folate acid (FA) to specifically target the folate receptors of Rb cells. The resulting FA-DOX-ICG-PFP@Lip could be monitored in real time with PAI and upon irradiation with 808nm NIR laser in vitro, they could quickly achieve high temperatures that induced apoptosis of Y-79 cells. Simultaneously they would transition from liquid to gas, serving as highly effective contrast agents for ultrasound imaging, while a burst of doxorubicin release would also occur. Subsequently, the group proceeded to in vivo experiments in Y-79 tumour bearing mice to assess the distribution and biocompatibility of the nanoparticles, while monitoring them with dual PA/US imaging (
Figure 3). They found their size allowed them to be internalised by tumour cells through the EPR effect in addition to folate receptor-mediated endocytosis. Healthy cells and the hematopoietic cell lines (WBC, RBC, platelets) remained largely unaffected, deeming the liposomal formulation safe in this regard, as opposed to conventional chemotherapy. Finally, the cancer killing efficacy of the FA-DOX-ICG-PFP@Lip was evaluated and proved to be higher than all the other treatment groups, such as free doxorubicin alone, irradiation alone, non-targeted DOX-ICG-PFP@Lip or FA-DOX-ICG-PFP@Lip without laser. This is a pioneering study in retinoblastoma that demonstrates the multi-functionality of nanoparticles in theranostics, as well as the synergistic effect of multiple therapeutic approaches as opposed to individual treatments [
83].
Lipid based nanoparticles were also explored by Wang et al. for combined chemotherapy and PTT of retinoblastoma. More specifically, the group encapsulated melphalan and black phosphorus quantum dots (BPQD) in lipid nanoparticles. The LNP@Me&BP exhibited good photothermal properties due to the BPQD both in vitro and in vivo, and enhanced the cytotoxic effect of melphalan on Rb cells. Surprisingly, the in vivo experiments in mice revealed that BPQDs alone had a slightly stronger inhibitory effect on tumour cell proliferation and greater reduction in ki67 level than LNP@Me&BP, which the team attributes to greater concentration of BPQDs than what was used in the lipid nanoformulation. Nonetheless, high doses of BPQDs are associated with side effects, such as nephrotoxicity, inciting the necessity of lower doses. The LNP@Me&BP system accomplished this by combining melphalan with a lower concentration of BPQD resulting in comparable tumour-killing efficacy and with a better safety profile [
84].
Other types of organic nanoparticles have also been investigated for the same purpose. Mudigunda et al. designed polymeric nanoparticles (PNP) from PLGA and PCL, which they conjugated with a NIR dye, IR820 (IR), and the chemotherapeutic drug Palbociclib (PCB), a CDK4/CDK6 inhibitor. The PCB/IR PNPs were excellent photothermal agents as well as contrast agents for PA imaging and the synergistic effect of PTT and chemotherapic compared to individual therapies was proven in vitro against Y-79 cells. Biocompatibility and toxicity studies were also performed in vivo in mice, where the PCB/IR PNPs were shown to be safe, making them a viable option for imaging-guided dual cancer treatment [
85].
5.2. PTT in Uveal Melanoma
Polymer based nanoformulations have also contributed in uveal melanoma theranostics, along with inorganic nanoparticles. Li et al. created a Poly(N-isopropylacrylamide) (PNIPAM) hydrogel based on ultrasmall rare-earth nanoparticles (RENP), which have luminescent properties on their own but were further decorated with ICG. They were then loaded with FA for target specificity as well as doxorubicin. The resulting nanogel (RENP-ICG@PNIPAM:Dox-FA) was dually responsive to both temperature changes and to glutathione (GSH), an antioxidant found in abundance in the tumour microenvironment due to its altered metabolism. Following in vitro experiments, PTT with RENP-ICG@PNIPAM:Dox-FA was shown to have the highest antitumor effect on OCM-1 cells compared to all other treatments, such as independent chemotherapy, PTT with ICG alone or RENP-ICG@PNIPAM:Dox hydrogel without FA. Further experiments in vivo in a choroidal melanoma mouse model, confirmed the synergy of combined treatment opposite plain doxorubicin and non targeted nanogel, with significant reduction in tumour volume after 21 days of treatment. NIR-II imaging was also performed both in vitro and in vivo, confirming the excellent photothermal properties of the hydrogel while monitoring its distribution and biodegradability [
11].
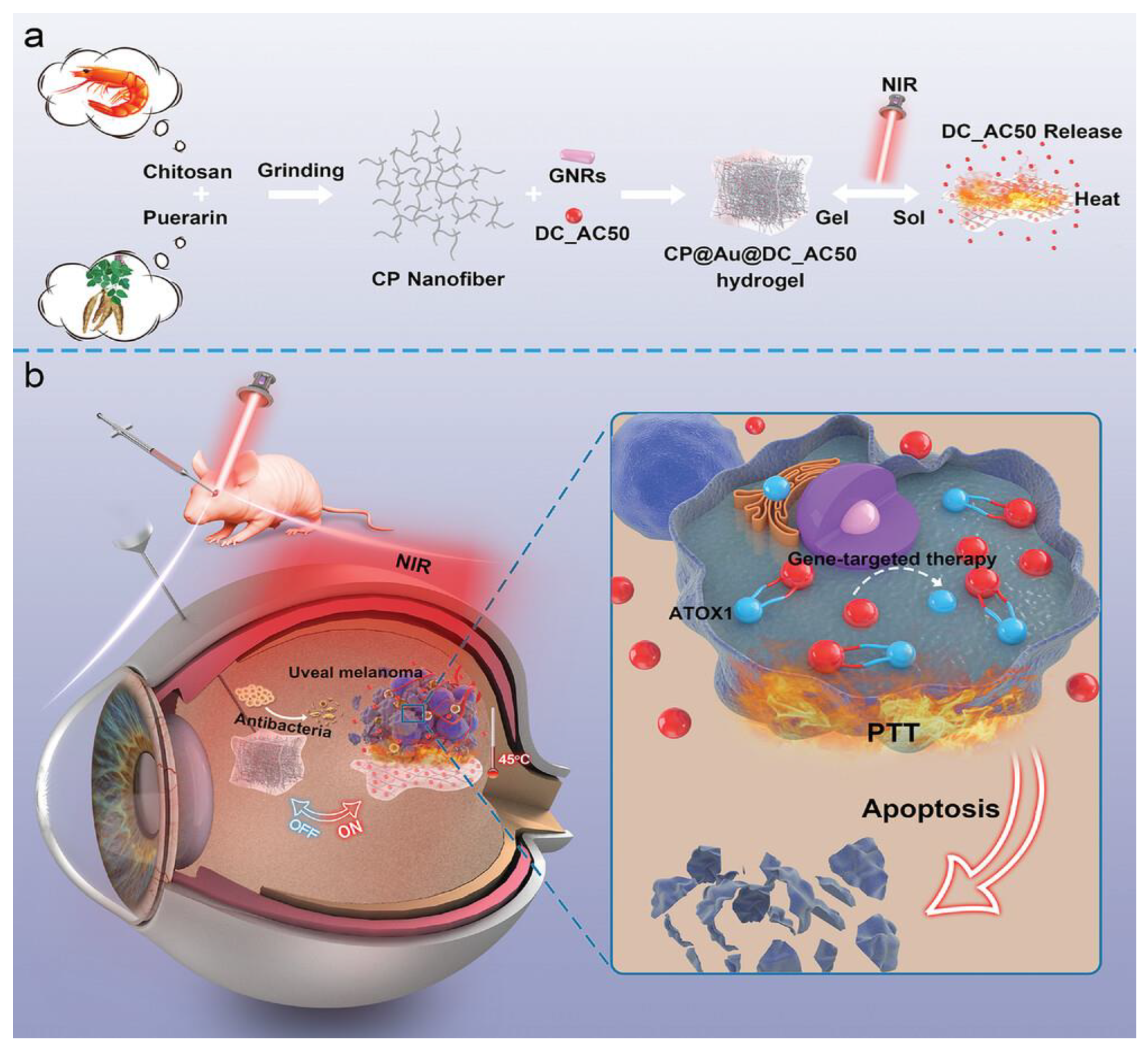
Shortly after the promising results of this research, another thermoresponsive hydrogel was presented by Wang et al.. This self-assembling chitosan@puerarin(CP) gel incorporated gold nanoparticles and DC_AC50, a gene-targeted drug which inhibits ATOX1, an antioxidant chaperone protein that is upregulated in UM cells. Upon irradiation with a 808 nm laser, the light responsive gold nanorods would heat up, causing the nanogel to undergo a sol-gel transition, releasing the DC_AC50. Experiments with living mice confirmed the excellent photothermal and conversion properties of the hydrogel, as well as an “on-off” phenomenon through NIR light triggers, allowing for further control of drug release and for multiple treatment sessions following a single intravitreal injection. The synergistic effect of PTT and the gene-targeted drug was also demonstrated against OCM-1 tumour bearing mice where the combination therapy almost completely eradicated the tumour whereas the two treatments individually showed only partial reduction. Lastly, the hydrogel was shown to be resistant to infections, which was attributed to the combination of PTT and the antibacterial properties of chitosan (
Figure 4) [
86].
Gold nanoparticles and polymers, specifically fucoidan, were also utilised by Kim et al. to conjugate Doxorubicin for dual chemo-photothermal therapy. Fucoidan, a polysaccharide known for its anti-tumor and anti-inflammatory properties, was essential to ensure the successful conjunction of gold nanoparticles and doxorubicin, since they were both positively charged. The final product of Dox-Fu@AuNPs was used both for PTT in vitro and in vivo, in tumour - bearing rabbits’ eyes, where the tumour was eliminated in its entirety within a few days. In contrast, other treatments—Dox-Fu@AuNPs without laser, free doxorubicin, and PTT without nanoparticles—only temporarily slowed tumour growth before it resumed increasing. As an additional benefit, photoacoustic imaging (PAI) was enhanced and better visualisation of the tumoral margins was achieved due to the light absorbing properties of gold nanoparticles. Therefore, this study not only harnessed the synergistic effects of chemotherapy and PTT but also advanced in vivo image-guided treatment [
87].
5.3. PDT in Retinoblastoma
Similarly to PTT, nanoparticles used in PDT aim to be multi-functional. For the treatment of retinoblastoma specifically, mesoporous silica nanoparticles have been involved in multiple experiments by the research group of the University of Montpellier. The group had already demonstrated how MSNPs could be used for simultaneous PDT and chemotherapy in various types of cancer cells, by encapsulating a water soluble photosensitizer and camptothecin, a topoisomerase 1 inhibitor that works by destabilising the S phase of the cell cycle and causing potentially lethal double-strand DNA breaks during DNA replication [
88].
For retinoblastoma, they used the same method, adding mannose or galactose to target the receptors on Y-79 cells while also fluorescently labelling the MSNPs so that they could be monitored with confocal microscopy. Both groups performed significantly better at inducing cell death upon irradiation compared to MSNPs without camptothecin, or MSNPs with camptothecin but without the photosensitizer. The group then proceeded to experiments with biphotonic PDT by using a different photosensitizer. Biphotonic PDT, often referred to as two-photon PDT, utilises the simultaneous absorption of two lower-energy photons rather than one higher-energy photon to activate the photosensitizers. This approach allows for deeper tissue penetration, as it uses NIR light instead of visible or ultraviolet light that is used in traditional PDT. It also offers increased precision and three dimensional spatial resolution, as only the small area where the two photons converge is activated. Moreover, because the photons are of lower energy, there are fewer scattering losses and reduced photodamage to surrounding tissues. [
89] Indeed, biphotonic PDT proved to be more efficient, leading the group to adopt it for subsequent experiments where they focused on improving targeting specificity. They identified two subtypes of mannose receptors, MRC2 and CD209, that are overexpressed by Y-79 cells, and the MSNPs functionalized with those were the ones that achieved the highest cell death rates. Rhodamine was used to label the MSNPs so the cellular uptake through both endocytosis and co-location into the lysosomes could be tracked in real time with confocal microscopy, and the potential cytotoxic effect of PDT could also be monitored and adjusted. Overall, these experiments demonstrated promising outcomes, paving the way for simultaneous imaging, PDT, targeting, and chemotherapy [
90].
N’Diaye et al. experimented with hybrid nanoparticles, composed of a polymer core and a phospholipid bilayer coating. This allowed them to encapsulate beta-lapachone (β-Lap) and the photosensitizer m-THPC, which are both lipophilic molecules, simultaneously in separate compartments. β-Lap is a naturally occurring quinone compound derived from the bark of the Lapacho tree that is activated by the enzyme NAD(P)H:quinone oxidoreductase (NQO1) which is often overexpressed in cancer cells. Once activated, it exerts its anti-cancer effects by inducing the generation of ROS. It was also hypothesised that the presence of ROS itself can further lead to overexpression of NQO1, so that PDT-induced ROS could enhance the activation of β-Lap. Unfortunately, no such synergistic effect was made apparent by the experiments, but the combined therapies still had an additive cytotoxicity on Y-79 cells, which allows for the use of lower, safer doses while maintaining the same level of effectiveness as single treatments [
91].
5.4. Combined PTT/PDT
Although PTT and PDT have been at the forefront of current anticancer research, both photothermal techniques have certain limitations when applied separately. On one hand, the overexpression of heat shock proteins (HSP) in PTT can significantly suppress its cytotoxic effect, as their role is to protect other proteins from heat damage. On the other hand, PDT is overly dependent on oxygen which can hinder its efficacy in hypoxic environments. By combining PTT and PDT, these limitations can be addressed, resulting in improved therapeutic outcomes. Given the pivotal role of nanoparticles in both methods, it was a logical step to employ them in the integration of these two modalities.
With this in mind, Zheng et al. formulated superparamagnetic cationic nanoliposomes loaded with indocyanine (ICG), a photoacoustic (PA) imaging agent with excellent photoexcitation properties, and perfluorohexane(PFH) creating a versatile platform for the treatment of retinoblastoma. PFH is a perfluorocarbon that has been widely used in nanomedicine due to its phase changing ability from liquid to gas upon absorption of enough energy, and its oxygen- carrying and releasing function which can reinforce PDT in hypoxic regions. Superparamagnetic Iron Oxide Nanoparticles (SPIONS) were embedded within the lipid bilayer, along with folate for targeting, while the ICG and PFH were encapsulated in the core. SPIONS served not only as contrast agents for MRI but also for targeting through the application of an external magnet. Similarly, ICG was utilised for PAI as well as a photosensitizer for PTT and PDT. This approach requires a single excitation source, a 808 nm laser, which largely simplifies the process of combining the two photothermal therapies. Although admittedly ICG-based PDT was challenging in the hypoxic tumour environment, PFH’s oxygen supply compensated for it. PFH’s phase change upon irradiation and the resulting microbubbles, facilitated US imaging. The dual-targeted (folate/magnetic) nanoliposomes were tested in vitro as well as in Y-79 tumour-bearing mice and demonstrated the synergy of combined PTT/PDT along with successful multimodal US/PA/MR imaging (
Figure 5) [
92].
A similar nanoplatform was introduced by Huang et al. for uveal melanoma theranostics. They chose PLGA nanoparticles which they loaded with chlorin e6 (Ce6) and coated with FeIII-tannic acid (FeIII-TA). Ce6 is a chlorophyll-derivative that serves as a PDT photosensitizer, but as is often the case with photosensitizers, its use is limited due to its tendency to aggregate in aqueous solutions. FeIII-TA is a complex formed between iron in its ferric state (FeIII) and tannic acid, a natural polyphenol, that exhibits excellent photothermal and paramagnetic properties, enabling its use in PAI and PTT as well as MRI. Since Ce6 and FeIII-TA are optimally excited at different wavelengths, the use of both a 660 nm laser for PDT and an 808 nm laser for PTT was imperative. Following in vitro experiments in C918 cells that confirmed the synergistic effect of dual photothermal therapy, the PLGA nanoparticles were tested in a mouse model. The accumulation of nanoparticles in the tumour region was confirmed through dual PA/MR imaging, which helped define the therapeutic window by pinpointing the moment of peak accumulation. Photothermal imaging was employed as well to monitor the temperature during PTT and ensure no damage was inflicted to the surrounding tissues. The combination of PTT and PDT using the nanoparticles resulted in a remarkable reduction in tumour volume in mice compared to either treatment alone or the control group, even resulting in complete tumour eradication in some cases, while showing good compatibility and a favourable safety profile [
93].
6. Nanoparticles in Brachytherapy
Brachytherapy, also known as episcleral plaque radiotherapy, is a specialised form of radiation therapy used to treat intraocular tumours that involves suturing a radioactive plaque on the sclera. This permits the delivery of highly concentrated radiation to the tumour site, the dose of which depends on the type and amount of the radioactive seeds and can be further adjusted by length of time. It is the most commonly used eye sparing treatment for uveal melanoma, with a survival rate similar to enucleation. It can also be used for retinoblastoma as long as there is no vitreous seeding [
3].
Several isotopes have been used in brachytherapy, such as cobalt-60, iodine-125 (
125I), iridium-192, palladium-103 (
103Pd), and ruthenium-106 (
106Ru), but clinical practice has mainly settled on
125I and
106Ru due to their good dosage distribution and availability. Additionally, the emission of gamma rays from
125I offers an advantage in treating larger tumours, as it provides greater tissue penetration compared to the beta-emitting
106Ru [
94].
Nanotechnology can improve brachytherapy by enhancing the radiation dose and improving targeting to minimise the damage inflicted on surrounding healthy tissues. Gold nanoparticles act as potent radiosensitizers by leveraging their high atomic number (Z = 79) that allows them to efficiently absorb and scatter the radiation, and their strong photoelectric effect that produces secondary photo/Auger electrons to cause further damage to the cancer cells. They are also stable, can easily be functionalized with drugs and targeting ligands and their small size permits intratumoral accumulation.
Gold nanoparticles have been previously proven to sensitise cutaneous melanoma B16F10 cells to radiation in vivo [
95] and to disrupt the tumour vasculature when combined with brachytherapy [
96]. Building on these principles, gold nanoparticles have been investigated as a treatment for uveal melanoma. Asadi et al. utilised them in a Monte Carlo model of the human eye, demonstrating that the tumour site absorbed significantly more radiation in the presence of gold nanoparticles than with
125I irradiation alone. This finding suggests that the required therapeutic dose could be achieved with shorter brachytherapy sessions, reducing potential damage to surrounding healthy tissues [
97].
Further evidence of dose enhancement with gold nanospheres came from another Monte Carlo model using
103Pd as the radioisotope, where higher concentrations of gold nanoparticles directly correlated to higher doses intratumorally and shorter time required to administer the desirable dose. [
98] However, a comparative study evaluating both
103Pd and
125I in nanoparticle-assisted brachytherapy revealed a more pronounced dose enhancement factor with
125I, solidifying its position as the preferred isotope [
99].
Monte Carlo models were also used to study the parameters that affect dosimetry, such as the size and concentrations of AuNPs and low or high energy photon sources. It was concluded that higher concentrations and AuNPs of larger diameters were optimal for dose enhancement, while the efficacy of low energy sources was highlighted [
100].
The AuNP-assisted brachytherapy approach was subsequently escalated to in vitro experiments with choroidal melanoma cells. After being exposed to various concentrations of AuNPs, as well as radiation alone, the cell viability was assessed throughout a 7-day period. While lower concentrations had no significant cytotoxic effect over the first three days compared to radiation without AuNPs, cell death reached 30% by day 7 as opposed to merely 10% with plain radiation. Higher concentrations achieved even more impressive results, with over 70% of cell apoptosis by the seventh day [
101].
The concern of whether nanoparticles are indeed sufficiently selective was also addressed by Kanavi et al. who investigated the distribution of gold nanoparticles ex vivo in an enucleated human eye with choroidal melanoma. They found that the AuNPs did not reach extratumoral areas but instead dispersed within the tumour and highly infiltrated the intratumoral vascular endothelial lining which is on par with their ability to disrupt the malignant vasculature. [
102] The dose distribution itself in brachytherapy with
125I seeds was also measured in a model eyeball and the presence of AuNPs resulted in higher dose within the tumour and lower in the healthy tissues compared to plain brachytherapy [
103].
However, in a later study with a
106Ru plaque in a Monte Carlo model, optimal dose enhancement was achieved for short distances from the plaque surface, while for more than 2mm the dose enhancement factor dropped, and more radiation was accumulated in the sclera. The group hypothesised that this phenomenon may be due to high energy electrons interacting with the heavy gold nuclei and releasing Bremsstrahlung photons that stray from the intended target and can reach healthy tissues. They concluded that while AuNPs can ameliorate dose distribution for superficial tumours, they may not be a wise choice for deeper reaching tumours [
104].
Other ways in which nanoparticles can contribute to brachytherapy apart from dose enhancers, are by acting as radiation shields. In a recent study, magnetite nanoparticles suspended in silicon polymer were used to formulate high-density ferrofluids. A modified magnetic plaque was then used to attract ferrofluids to the tumour surface, directing the radiation to the target while minimising the exposure of the neighbouring tissues. [
105] Nanoparticle assisted brachytherapy also opens the opportunity for combination therapy. For example,
125I brachytherapy with AuNps and simultaneous ultrasonic hyperthermia was tested on a rabbit model by Moradi et al. for the treatment of retinoblastoma. All different combinations of brachytherapy, phototherapy and AuNPs as well as the respective monotherapies were tested and the group that involved all three performed the best, thus proving their synergistic effect [
106].
Alternatively to brachytherapy, external beam radiotherapy has been used in conjunction with nanoparticles, which delivers a lower total dose and has lower dose enhancement, but has the advantage of being noninvasive [
107]. Finally, Altundal et al. experimented not only with gold nanoparticles for dose enhancement, but also with carboplatin nanoparticles for adjuvant chemotherapy in both choroidal melanoma and retinoblastoma [
108].
7. Nanoparticles in Gene Therapy
Nanoparticle-mediated gene delivery has been explored as an alternative to viral carriers for the treatment of ocular diseases. While viral vectors, such as adeno-associated viruses (AAV), have demonstrated high efficiency in gene transduction and integration, they present several significant disadvantages. One major concern is the triggering of immune responses, which can lead to inflammation and the production of neutralising antibodies, particularly with repeated treatments. These immune reactions not only reduce the efficacy of the therapy but may also result in serious side effects. Additionally, viral vectors are constrained by their limited cargo capacity, with recombinant AAV vectors reportedly being able to accommodate inserts up to 4.7 kb, making them unsuitable for larger genes [
109,
110]. Other limitations include cell tropism, which restricts the types of cells that can be targeted, and the random integration of viral DNA into the host genome, raising concerns about potential oncogenesis. In some clinical trials, the use of viral vectors has indeed led to severe outcomes, such as cancer or mortality due to systemic inflammatory response [
111,
112]. Despite their promise, these drawbacks necessitate careful consideration when using viral vectors in gene therapy and highlight the need for safer alternatives [
113,
114,
115].
In search of non-viral methods for gene therapy, different types of nanoparticles have been studied, with the most prominent being liposomes, polymeric nanoparticles and combinations of those. The qualities that are sought after for an ideal vector are high transduction efficiency, capacity to carry large sequences of nucleic acids, targeted transgenesis and a safe profile at a reasonable cost. The main physiological challenge when selecting a vector is the penetration of the cell membrane. As discussed, key factors for permeating the cell membrane are size (ideally less than 200 nm), surface charge and surface chemistry. Positively charged nanoparticles can enter the negatively charged cell-membrane through endocytosis, mainly macropinocytosis or clathrin-mediated endocytosis. Functionalizing their surface with ligands that bind to membrane receptors also promotes endocytosis. For directly acting nucleic acids, such as messenger RNA (mRNA) or small interfering RNA (siRNA) it suffices to enter the intracellular space while DNA sequences have the additional challenge of bypassing the nuclear membrane [
110,
116].
7.1. Lipid Nanoparticles in Gene Therapy
Lipid nanoparticles have several advantages and can be modified to accommodate the needs of an effective gene carrier. They can be composed from cationic or ionisable lipids, PEGylated lipids and structural lipids that self-assemble. Apart from facilitating the entry inside the cell, ionisable lipids are responsible for binding nucleic acid and protecting it from endosomal lysis, while PEG ensures the stability of the formulation [
115]. Such LNPs were used for delivery of mRNA to the posterior segment of the eye in vivo, by Patel et al. to treat retinal degeneration [
117].
Further gene transfer has also been achieved with DNA plasmids in Rs1h-deficient mice suffering from juvenile retinoschisis, a genetic degenerative disorder of the retina. Apart from the RS1 gene, the EGFP plasmid was also loaded in the LNPs to induce the expression of the Enhanced Green Fluorescent Protein (EGFP) for visualisation and tracking purposes [
118,
119,
120]. The successful gene transduction in retinal cells along with studies that employ gene therapy for cancer treatment [
121] and even the simultaneous encapsulation of chemotherapeutic agents [
122], are very promising for the use of LNPs as gene carriers in the treatment of retinoblastoma. In fact, in vivo testing has already started, as Tabatabaei et al. (2019) formulated cationic LNPs to co-deliver melphalan and miR-181a, a microRNA that downregulates the anti-proliferative gene MAPK1 and the anti-apoptotic gene Bcl-2, while boosting the expression of pro-apoptotic gene BAX in RB cells [
123]. Another multimodal approach was executed by Wu et al. for simultaneous imaging and laser-activated gene release. They introduced cationic nanoparticles composed of a lipid shell modified with FA for targeting, inside which they encapsulated liquid PFP and ICG for PA and US imaging. The cationic surface of the nanoparticles facilitated the attachment of the HSV-TK/GCV suicide gene system, a set of genes that encode the herpes simplex virus type thymidine kinase (HSV-TK) and its prodrug ganciclovir (GCV), the combination of which is highly cytotoxic. The recombinant pDNA of GFR was also added for tracking. Laser irradiation was then employed to increase cell membrane permeability, enhancing DNA uptake. Thus, upon NIR laser exposure, Rb cells demonstrated efficient transfection, as evidenced by the strong GFP signal and a significant increase in apoptotic cell death [
124].
Of course, LNPs do not come without some disadvantages. Due to their charge, when used repetitively they can induce significant immune and inflammatory responses compared to neutral nanoparticles or interact with negatively charged extracellular components and form aggregates. To avoid this, PEG or polysaccharides such as dextran or hyaluronic acid can be added and they have been shown to facilitate their course through the vitreous to better reach the retinal layers [
119,
120,
125].
Moreover, condensation of the nucleic acid is desirable in order to achieve a bigger load, but it can lead to problematic transfection if done at an excessive degree, as it inhibits its release from the complex. [
126] To address the low transfection capacity, the use of peptides, such as the TAT2 peptide or protamine, has been implemented in vivo experiments. Rudolph et al. showed that the compaction of pDNA with TAT2 before loading it to the SNL vectors, improved gene transfer efficiency to bronchial epithelial cells. Delgado et al. who experimented with ARPE-19 cells, specifically for the treatment of X-linked juvenile retinoschisis, opted for protamine to stabilise the pDNA. This method, along with the addition of dextran coating, significantly enhanced the expression of retinoschisin and EGFP in vitro and subsequently in vivo [
120,
127].
7.2. Polymeric Nanoparticles in Gene Therapy
Another promising non-viral vector is compacted DNA/RNA - polymeric nanoparticles. Those nanoformulations that typically range from 1 to 100 nm are usually composed of a nucleic acid segment compacted with a polycationic polymer. [
113] Polymers have been studied since the beginning of the milenia and different types of polymers, such as carbohydrate polymers and dendrimers have been used as gene delivery carriers in cancer therapy [
128].
For ophthalmic cancers, the main focus is gene delivery to the retina, which has also been widely studied. For example, plasmid DNA (pDNA) for Enhanced Green Fluorescent Protein (EGFP) compacted with PEG-substituted lysine peptides was successfully transferred to the retina by Farjo et al. in 2006 [
114]. Other early studies explored co-polymers, such as PLA and PLGA for the same purpose and observed the greatest uptake by the RPE [
129,
130]. PLGA is negatively charged in neutral pH and only becomes positively charged in the acid environment of the endolysosomal intracellular sites. Therefore, it typically needs to be enhanced with other cationic molecules such as chitosan or PEI, which have excellent compatibility [
131]. Such chitosan-PLGA nanoparticles have already been applied to the treatment of RB by delivering etoposide to Y-79 cells as mentioned earlier [
17], while they have also successfully delivered pDNA to the retina for inhibition of neovascularization [
132,
133]. Taking those into consideration, PLGA-assisted gene delivery in retinoblastoma can be envisioned in the future.
The other categories of polymers, such as polysaccharides, e.g. chitosan, dextran and hyaluronan, as well as dendrimers, can also be used in gene delivery independently of PLGA. Polysaccharides are naturally occurring molecules, so they stand out for their biocompatibility, and they have various functional groups which makes them easily modifiable [
134]. They have already been employed for chemotherapy delivery in retinoblastoma so it is auspicious that their use can be extended in gene delivery [
37]. For example, compacted pDNA of green fluorescent protein (GFP) was loaded in glycol chitosan as early as 2014 by Mitra et al. and was shown to reach the RPE in vivo [
135].
As far as dendrimers are concerned, PEI holds a pivotal position in gene delivery, boasting notable advantages, such as a cationic charge that facilitates binding and condensing the negatively charged amino acids, and the ability to escape the endosome due to the proton sponge effect. The amino groups of PEI act as buffers to the acidic environment of the endosome by absorbing protons, leading to the osmotic swelling of the endosome and eventually its rupture, so that the cargo can escape to the cytoplasm [
136].
In ophthalmology, there are instances of formulations consisting of PEI that have been used for DNA delivery to retinal ganglion cells [
137], as well as other dendrimers, such as PLL-LAA for reaching the RPE [
138]. PEGylation of PEI has been previously discussed as a method of neutralising its charge, thus rendering it less cytotoxic. Wang et al. applied this technique and created the hyperbranched-star PEI, grafted with PEG (PEG-g-PEI), that can effectively condense and carry genes. Specifically, pDNA encoding enhanced red fluorescent protein (RFP) and GFP were used to study cellular uptake of the vectors by retinoblastoma cells and gene transfection efficiency in vitro. Compared to other, non-PEGylated PEI-based vectors which failed to induce RFP expression, the hyperbranched-star PEG-g-PEI was successful in RFP transfection, accomplished higher GFP expression, and had a markedly improved biocompatibility and safety profile [
139].
In another study pDNA was compacted in dendrimer nanoparticles to transfect human choroidal melanoma OCM-1 cell line. The recombinant pDNA that was used coexpressed the tumour necrosis factor-α (TNF-α) and the herpes simplex virus thymidine kinase (HSV-TK), that leads to cancer cell apoptosis while sparing healthy cells through a process called suicide gene therapy, first described by Moolten [
140]. After the transfection, OCM-1 were also irradiated with 2 Gy
125I which led to increased gene expression [
141].
Last but not least, combinations of the different types of NPs, such as lipid and polymeric NPs, are possible. Tan et al. experimented with PAMAM dendrimers, which functionalized with three different amino acids to improve biocompatibility and cellular uptake. DNA and a nuclear localization signal (NLS), which is a short sequence of amino acids that facilitates the delivery of DNA to the cell nucleus, were integrated to the dendrimers and the entire complex was encapsulated in a lipid bilayer. The lipids chosen were pH-sensitive, so that the lipid bilayer could fuse with the endosomal membrane upon exposure to the acidic environment of the endosome, releasing the cargo to the cytoplasm. Finally, hyaluronic acid-1,2-dioleoylphosphatidylethanolamine (HA-DOPE) was used for the outer coating, as HA targets the CD44 receptor of the retinal cells. In vitro studies with ARPE-19 cells, the nanocarriers demonstrated good endosomal escape ability, penetration of the nucleus and transfection efficiency. Similar results were accomplished in vivo with minimal cytotoxicity [
142].
7.3. Inorganic Nanoparticles in Gene Therapy
Gene therapy research is not limited to organic nanoparticles alone. Inorganic NPs are also gaining popularity, with AuNPs having been extensively studied as carriers for nucleic acids. [
143,
144,
145,
146] AuNPs have been established as highly versatile nanoparticles, one of their key advantages being the ease of modifying their surface. In fact, it has been shown that surface modification of AuNPs with cationic NPs, such as PEI, creates a mutually beneficial relationship: the PEI enhances the binding ability of nucleic acid to the AuNPs, while the AuNPs mitigate PEI's inherent cytotoxicity. Such complexes have shown promise in anti-oncogenic gene therapy [
147].
In extent of those principles, Mitra et al. used PEI - capped AuNPs to deliver siRNA to retinoblastoma cells. The AuNP-PEI nanocarriers were conjugated with an EpCAM antibody to target EpCAM expressing cells. The EpCAM not only serves as a target to detect malignant cells; it also plays a crucial role in tumour cell proliferation, metastasis, and survival. [
148] Thus, the group used EpCAM siRNA to silence the EpCAM gene and disrupt its signalling pathway in vitro in Y-79 cells, while proving that siRNA loaded in EpCAM-AuNP-PEI nanoconjugates was effective in half the amount of naked siRNA required for the same result. They also demonstrated that EpCAM-AuNP-PEI were safe for healthy cells [
149].
Posch et al. focused on the GNAQ mutations that are an oncogenic and metastatic driver in a great portion of UM cases. They designed AuNPs to both detect mutated GNAQ mRNA and to silence them using siRNA. For detection, the AuNPs were functionalized with oligonucleotides that would structurally change upon binding to the mutated mRNA, resulting in a detectable fluorescent signal in confocal microscopy. For siRNA delivery, they developed a novel release system through the modification of siRNA-AuNPs with a dithiolane moiety and disulfide groups, that could release the siRNAs faster and more effectively in the presence of the antioxidant GSH. The efficacy and selectivity of the siRNA-AuNPs was proved in the UM cell lines OMM1.3, whose viability steeply declined due to disruption of the GNAQ signalling, whereas minimal impact was observed in healthy cells or cells without the specific mutation [
150].
It is also worth mentioning that inorganic nanoparticles have been used for molecular therapy that falls outside the strict definition of gene therapy, as carriers of peptides and even multimodal applications. Kalmodia et al. designed AuNPs conjugated with the anti-HDM2 peptide, a peptide that inhibits the interaction between the tumor suppressor protein p53 and its negative regulator, human double minute 2 (HDM2), that degrades the p53 and limits its tumor-suppressing activities. In vitro experiments in Y-79 cells demonstrated that AuNP-HDM2 could restore the functions of p53, and identified p53-mediated tumor suppressor miRNAs as another potential target for future research [
151].
Wang et al. also used AuNPs which they conjugated with iron oxide to create magnetic hollow mesoporous gold nanocages (AuNCs–Fe3O4) to deliver the muramyl dipeptide (MDP) to Rb cells. Apart from the MDP, which is an immunomodulator used in cancer therapy, they added PFP to enhance US imaging, as well as low-intensity focused ultrasound (LIFU) therapy. The resulting AuNC–Fe3O4/MDP/PFP nanoparticles could be used for combined LIFU/immunotherapy while being guided simultaneously by multimodal PA/US/MR imaging [
152].
Finally, other types of inorganic nanoparticles, such as MSNPs and iron oxide NPs have been used for the delivery of nucleic acids, on their own, [
153,
154,
155,
156] or in combination with organic NPs [
157,
158,
159,
160]. With their applications extending in oncology [
158,
161], there is potential that they will be adapted for ophthalmic uses, and ocular cancers in particular, in the near future.
8. Safety and Regulatory Concerns
As with every emerging and rapidly growing field in medicine, safety and toxicity issues are of primary concern. As materials shrink to nano-dimensions, they acquire a new set of physicochemical properties, very different from their bulk counterparts, which are not yet completely understood. Therefore, although the material itself is an important factor of toxicity, there are also other determining factors, most notably size, shape and charge. Smaller sized nanoparticles are generally considered more toxic due to their higher surface area to volume ratio that allows them to interact more readily with biological systems and their increased ability to bypass biological barriers, including the cellular membrane. They also pose a greater risk of genotoxicity, which refers to their potential to cause damage to genetic material [
162]. As far as surface charge is concerned, charged NPs are more likely to elicit an immune response, induce ROS production or interfere with the mitochondria. [
163] Positively charged NPs in particular, are more attracted to the negatively charged cellular membrane, which increases cellular uptake, while some can even escape the lysosomes and enter the nucleus, making them more likely to induce DNA damage [
164].
Nonetheless, the mere endeavor to study nanotoxicity can be challenging. The ADME (absorption, distribution, metabolism, excretion) profile of nanomedicines, which is the main tool for evaluating safety, remains unclear due to the difficulty in detecting molecules at nanosize to monitor biodistribution, accumulation and excretion pathways. [
165] For example, increased mobility and permeation of biological barriers can be detrimental in the case of crossing the blood-brain barrier, while renal and hepatic accumulation are equally alarming. ADME profiles can also vary significantly with slight changes in nanoparticle parameters, requiring extensive studies for different combinations. Nanoparticle characterization is further complicated by changes in morphology during storage or in vivo administration, lack of standardized methods, and unpredictable interactions like protein corona formation, which alters their behavior in vivo [
166,
167]. Poor batch-to-batch reproducibility, aggregation, and instability add to manufacturing and regulatory challenges, especially for multifunctional and hybrid nanoparticles [
165,
168,
169,
170].
All the above factors contribute to the difficulty of setting clear regulatory mechanisms in place and delay the translation of nanomedicine from preclinical research to clinical use. In addition, nanoparticles are not well-defined and there is currently no global consensus on their classification. The issue is magnified with multifunctional nanoparticles, which may combine diagnostic, therapeutic, and targeting capabilities within a single platform, blurring the boundaries between drugs, devices, and biologics, and requiring evaluation under multiple regulatory frameworks. A comprehensive table of studies on nanoparticle applications in ocular cancer management is provided in supplementary material.
9. Conclusions
Nanomedicine has made significant contributions to ophthalmology and is now expanding into the field of ocular oncology, transforming both diagnosis and treatment. Over the past two decades, advancements in nanoparticle-based drug delivery, imaging, and theranostics have provided promising alternatives to conventional therapies, overcoming key challenges such as ocular barriers and systemic toxicity. With several pre-clinical studies, as well as some transitioning into clinical studies, nanomedicine paves a new era in the management of ophthalmic cancers, with multimodal applications and targeted and personalised treatment being at the forefront. Nevertheless, further research, cautious and comprehensive regulations and frameworks are essential to ensure patient safety and optimal quality of care. With continued innovation and rigorous scientific validation, nanomedicine holds the promise of transforming the future of ocular oncology.
Author Contributions
“Conceptualization, ES and E.P.E; methodology, M.T, E.S; writing—original draft preparation, M.T, A.D, M.Z and E.S; and writing—review and editing, E.P.E., M.Z, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This review was conducted in the Frame of Master Program entitled “Nanomedicine” for the academic year 2024–2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EPR |
Enhanced Permeability and Retention |
| PLGA |
Poly(lactic-co-glycolic acid) |
| PAMAM |
Poly(amidoamine) |
| NP |
Nanoparticle |
| MSNP |
Mesoporous Silica Nanoparticle |
| CE-MIP |
Carboplatin and Etoposide Molecularly Imprinted Polymer |
| VCR |
Vincristine |
| ROS |
Reactive Oxygen Species |
| PEG |
Polyethylene Glycol |
| NIR |
Near-Infrared |
| MDR |
Multidrug Resistance |
| MAPK |
Mitogen-Activated Protein Kinase |
| p53 |
Tumor Protein p53 |
| SLT |
Selective Laser Trabeculoplasty |
| OCT |
Optical Coherence Tomography |
| PAI |
Photoacoustic Imaging |
| SPIONS |
Superparamagnetic Iron Oxide Nanoparticles |
| TA |
Tannic Acid |
| Ce6 |
Chlorin e6 |
| PDT |
Photodynamic Therapy |
| PTT |
Photothermal Therapy |
| GFP |
Green Fluorescent Protein |
| HSV-TK |
Herpes Simplex Virus Thymidine Kinase |
| GCV |
Ganciclovir |
| BAX |
Bcl-2 Associated X Protein |
| MAPK1 |
Mitogen-Activated Protein Kinase 1 |
| NLS |
Nuclear Localization Signal |
| TNF-α |
Tumor Necrosis Factor-alpha |
| TK |
Thymidine Kinase |
|
125I |
Iodine-125 |
|
106Ru |
Ruthenium-106 |
|
103Pd |
Palladium-103 |
| AuNP |
Gold Nanoparticle |
| LIFU |
Low Intensity Focused Ultrasound |
| MDP |
Muramyl dipeptide |
References
- Krantz, B. A.; Dave, N.; Komatsubara, K. M.; Marr, B. P.; Carvajal, R. D. Uveal Melanoma: Epidemiology, Etiology, and Treatment of Primary Disease. Clin. Ophthalmol. Auckl. NZ 2017, 11, 279–289. [Google Scholar] [CrossRef]
- Lohmann, D. R.; Gallie, B. L. Adam, M. P., Feldman, J., Mirzaa, G. M., Pagon, R. A., Wallace, S. E., Amemiya, A., Eds.; Retinoblastoma. In GeneReviews®; University of Washington, Seattle: Seattle (WA), 1993. [Google Scholar]
- Salmon, J. F.; Kanski, J. J. Kanski’s Clinical Ophthalmology: A Systematic Approach, Ninth edition.; Elsevier: Edinburgh, 2020. [Google Scholar]
- Cholkar, K.; Dasari, S. R.; Pal, D.; Mitra, A. K. 1 - Eye: Anatomy, Physiology and Barriers to Drug Delivery. In Ocular Transporters and Receptors; Mitra, A. K., Ed.; Woodhead Publishing Series in Biomedicine; Woodhead Publishing, 2013; pp 1–36. [CrossRef]
- Akhter, M. H.; Ahmad, I.; Alshahrani, M. Y.; Al-Harbi, A. I.; Khalilullah, H.; Afzal, O.; Altamimi, A. S. A.; Najib Ullah, S. N. M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels Basel Switz. 2022, 8, 82. [Google Scholar] [CrossRef]
- Kumar, N.; Kumbhat, S. Essentials in Nanoscience and Nanotechnology; John Wiley & Sons, 2016.
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-Based Strategies for Treatment of Ocular Disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Figueiró Longo, J. P.; Muehlmann, L. A. Nanomedicine Beyond Tumor Passive Targeting: What Next? Nanomed. 2020, 15, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A. K.; O’Reilly, R. K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjug. Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Alili, L.; Sack, M.; Karakoti, A. S.; Teuber, S.; Puschmann, K.; Hirst, S. M.; Reilly, C. M.; Zanger, K.; Stahl, W.; Das, S.; Seal, S.; Brenneisen, P. Combined Cytotoxic and Anti-Invasive Properties of Redox-Active Nanoparticles in Tumor–Stroma Interactions. Biomaterials 2011, 32, 2918–2929. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Z.; Chen, Z.; Gao, R.; Pan, L.; Deng, J.; Ye, X.; Zhang, J.; Zhang, S.; Mei, C.; Yu, J.; Feng, Y.; Wang, Q.; Yu, A.-Y.; Yang, M.; Huang, J. Microenvironment-Triggered Degradable Hydrogel for Imaging Diagnosis and Combined Treatment of Intraocular Choroidal Melanoma. ACS Nano 2020, 14, 15403–15416. [Google Scholar] [CrossRef]
- Afarid, M.; Mahmoodi, S.; Baghban, R. Recent Achievements in Nano-Based Technologies for Ocular Disease Diagnosis and Treatment, Review and Update. J. Nanobiotechnology 2022, 20, 361. [Google Scholar] [CrossRef]
- Wang, C.; Pang, Y. Nano-Based Eye Drop: Topical and Noninvasive Therapy for Ocular Diseases. Adv. Drug Deliv. Rev. 2023, 194, 114721. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Tasso, B.; Villa, C.; Brullo, C. Nanotechnology for Pediatric Retinoblastoma Therapy. Pharmaceuticals 2022, 15, 1087. [Google Scholar] [CrossRef]
- Tang, J.-Q.; Hou, X.-Y.; Yang, C.-S.; Li, Y.-X.; Xin, Y.; Guo, W.-W.; Wei, Z.-P.; Liu, Y.-Q.; Jiang, G. Recent Developments in Nanomedicine for Melanoma Treatment. Int. J. Cancer 2017, 141, 646–653. [Google Scholar] [CrossRef]
- Mitra, M.; Dilnawaz, F.; Misra, R.; Harilal, A.; Verma, R. S.; Sahoo, S. K.; Krishnakumar, S. Toxicogenomics of Nanoparticulate Delivery of Etoposide: Potential Impact on Nanotechnology in Retinoblastoma Therapy. Cancer Nanotechnol. 2011, 2, 21–36. [Google Scholar] [CrossRef]
- Godse, R.; Rathod, M.; De, A.; Shinde, U. Intravitreal Galactose Conjugated Polymeric Nanoparticles of Etoposide for Retinoblastoma. J. Drug Deliv. Sci. Technol. 2021, 61, 102259. [Google Scholar] [CrossRef]
- Ahmad, I.; Pandit, J.; Sultana, Y.; Mishra, A. K.; Hazari, P. P.; Aqil, M. Optimization by Design of Etoposide Loaded Solid Lipid Nanoparticles for Ocular Delivery: Characterization, Pharmacokinetic and Deposition Study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Kang, S. J.; Durairaj, C.; Kompella, U. B.; O’Brien, J. M.; Grossniklaus, H. E. Subconjunctival Nanoparticle Carboplatin in the Treatment of Murine Retinoblastoma. Arch. Ophthalmol. 2009, 127, 1043–1047. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. EpCAM Antibody-Conjugated Mesoporous Silica Nanoparticles to Enhance the Anticancer Efficacy of Carboplatin in Retinoblastoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ali, M. J.; Kondapi, A. K. Carboplatin Loaded Protein Nanoparticles Exhibit Improve Anti-Proliferative Activity in Retinoblastoma Cells. Int. J. Biol. Macromol. 2014, 70, 572–582. [Google Scholar] [CrossRef]
- Narayana, R. V. L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R. M.; Manukonda, R.; Kaliki, S.; Coupland, S. E.; Alexander, J.; Kalirai, H.; Kondapi, A. K.; Vemuganti, G. K. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Invest. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef]
- Shinde, U. A.; De, A.; Godse, R. D.; Rathod, M.; Thorat, R.; Sharma, N.; Ansari, M.; Singh, K. Efficacy Evaluation of Carboplatin -Etoposide Co-Loaded PLGA Microparticles for Subconjunctival Chemotherapy in Retinoblastoma. J. Drug Deliv. Sci. Technol. 2024, 92, 105333. [Google Scholar] [CrossRef]
- Bedikian, A. Y.; Silverman, J. A.; Papadopoulos, N. E.; Kim, K. B.; Hagey, A. E.; Vardeleon, A.; Hwu, W.-J.; Homsi, J.; Davies, M.; Hwu, P. Pharmacokinetics and Safety of Marqibo (Vincristine Sulfate Liposomes Injection) in Cancer Patients With Impaired Liver Function. J. Clin. Pharmacol. 2011, 51, 1205–1212. [Google Scholar] [CrossRef]
- Spectrum Pharmaceuticals, Inc. A Phase 2 Study of Marqibo in Patients With Metastatic Uveal Melanoma; Clinical trial registration NCT00506142; clinicaltrials.gov, 2019. https://clinicaltrials.gov/study/NCT00506142 (accessed 2024-10-31).
- Children’s Oncology Group. A Study of Unilateral Retinoblastoma With and Without Histopathologic High-Risk Features and the Role of Adjuvant Chemotherapy; Clinical trial registration NCT00335738; clinicaltrials.gov, 2021.
- Children’s Oncology Group. A Single Arm Trial of Systemic And Subtenon Chemotherapy For Groups C And D Intraocular Retinoblastoma; Clinical trial registration NCT00072384; clinicaltrials.gov, 2021.
- Sadri, E.; Khoee, S.; Moayeri, S.; Haji Ali, B.; Pirhajati Mahabadi, V.; Shirvalilou, S.; Khoei, S. Enhanced Anti-Tumor Activity of Transferrin/Folate Dual-Targeting Magnetic Nanoparticles Using Chemo-Thermo Therapy on Retinoblastoma Cancer Cells Y79. Sci. Rep. 2023, 13, 22358. [Google Scholar] [CrossRef]
- Park, H.; Yang, J.; Lee, J.; Haam, S.; Choi, I.-H.; Yoo, K.-H. Multifunctional Nanoparticles for Combined Doxorubicin and Photothermal Treatments. ACS Nano 2009, 3, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Boddu, S. H. S.; Jwala, J.; Chowdhury, M. R.; Mitra, A. K. In Vitro Evaluation of a Targeted and Sustained Release System for Retinoblastoma Cells Using Doxorubicin as a Model Drug. J. Ocul. Pharmacol. Ther. 2010, 26, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S. K. Evaluation of Cytotoxicity and Mechanism of Apoptosis of Doxorubicin Using Folate-Decorated Chitosan Nanoparticles for Targeted Delivery to Retinoblastoma. Cancer Nanotechnol. 2010, 1, (1–6). [Google Scholar] [CrossRef]
- Gao, R.; Mitra, R. N.; Zheng, M.; Wang, K.; Dahringer, J. C.; Han, Z. Developing Nanoceria-Based pH-Dependent Cancer-Directed Drug Delivery System for Retinoblastoma. Adv. Funct. Mater. 2018, 28, 1806248. [Google Scholar] [CrossRef]
- Sims, L. B.; Tyo, K. M.; Stocke, S.; Mahmoud, M. Y.; Ramasubramanian, A.; Steinbach-Rankins, J. M. Surface-Modified Melphalan Nanoparticles for Intravitreal Chemotherapy of Retinoblastoma. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1696–1705. [Google Scholar] [CrossRef]
- Mohseni, M.; Shojaei, Y.; Naseripour, M.; Delavar, F.; Mirzaei, M.; Mehravi, B. Lauric Acid-Grafted Biopolymeric Nanoparticles for Efficient Melphalan Delivery across the Corneal Layers for Retinoblastoma: Ex Vivo and in Vivo Permeation Study. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 035005. [Google Scholar] [CrossRef]
- Bogan, C. M.; Kaczmarek, J. V.; Pierce, J. M.; Chen, S.; Boyd, K. L.; Calcutt, M. W.; Bridges, T. M.; Lindsley, C. W.; Nadelmann, J. B.; Liao, A.; Hsieh, T.; Abramson, D. H.; Francis, J. H.; Friedman, D. L.; Richmond, A.; Daniels, A. B. Evaluation of Intravitreal Topotecan Dose Levels, Toxicity and Efficacy for Retinoblastoma Vitreous Seeds: A Preclinical and Clinical Study. Br. J. Ophthalmol. 2022, 106, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Delrish, E.; Jabbarvand, M.; Ghassemi, F.; Amoli, F. A.; Atyabi, F.; Lashay, A.; Soleimani, M.; Aghajanpour, L.; Dinarvand, R. Efficacy of Topotecan Nanoparticles for Intravitreal Chemotherapy of Retinoblastoma. Exp. Eye Res. 2021, 204, 108423. [Google Scholar] [CrossRef]
- Delrish, E.; Ghassemi, F.; Jabbarvand, M.; Lashay, A.; Atyabi, F.; Soleimani, M.; Dinarvand, R. Biodistribution of Cy5-Labeled Thiolated and Methylated Chitosan-Carboxymethyl Dextran Nanoparticles in an Animal Model of Retinoblastoma. J. Ophthalmic Vis. Res. 2022, 17, 58. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic Acid-Conjugated Mesoporous Silica Nanoparticles for Enhanced Therapeutic Efficacy of Topotecan in Retina Cancers. Int. J. Nanomedicine 2018, 13, 4379–4389. [Google Scholar] [CrossRef]
- Algazi, A. P.; Weber, J. S.; Andrews, S. C.; Urbas, P.; Munster, P. N.; DeConti, R. C.; Hwang, J.; Sondak, V. K.; Messina, J. L.; McCalmont, T.; Daud, A. I. Phase I Clinical Trial of the Src Inhibitor Dasatinib with Dacarbazine in Metastatic Melanoma. Br. J. Cancer 2012, 106, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Versluis, M.; El Filali, M.; Bronkhorst, I.; Baghat, A.; Luyten, G.; Jager, M.; Van Der Velden, P. ERK Activation and Monosomy 3 Are Associated with Src Expression in Uveal Melanoma and May Serve as Biomarkers for Dasatinib Treatment. Acta Ophthalmol. (Copenh.) 2011, 89, 0–0. [Google Scholar] [CrossRef]
- Wu, J.; Liao, X.; Yu, B.; Su, B. Dasatinib Inhibits Primary Melanoma Cell Proliferation through Morphology-dependent Disruption of Src-ERK Signaling. Oncol. Lett. 2013, 5, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qian, X.; Li, H. Y.; Lai, K. L.; Gao, Q.; Lee, W. Y. T. Safety Assessment of Polymeric Micelles as an Ophthalmic Drug Delivery System for Intravitreal Administration of Dasatinib. Int. J. Pharm. 2021, 596, 120226. [Google Scholar] [CrossRef]
- Saha, S.; Adhikary, A.; Bhattacharyya, P.; Das, T.; Sa, G. Death by Design: Where Curcumin Sensitizes Drug-Resistant Tumours. Anticancer Res. 2012, 32, 2567–2584. [Google Scholar] [PubMed]
- Farghadani, R.; Naidu, R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers 2021, 13, 3427. [Google Scholar] [CrossRef]
- Choi, B. H.; Kim, C. G.; Lim, Y.; Shin, S. Y.; Lee, Y. H. Curcumin Down-Regulates the Multidrug-Resistance Mdr1b Gene by Inhibiting the PI3K/Akt/NFκB Pathway. Cancer Lett. 2008, 259, 111–118. [Google Scholar] [CrossRef]
- Das, M.; Sahoo, S. K. Folate Decorated Dual Drug Loaded Nanoparticle: Role of Curcumin in Enhancing Therapeutic Potential of Nutlin-3a by Reversing Multidrug Resistance. PLoS ONE 2012, 7, e32920. [Google Scholar] [CrossRef]
- Xie, L.; Yue, W.; Ibrahim, K.; Shen, J. A Long-Acting Curcumin Nanoparticle/In Situ Hydrogel Composite for the Treatment of Uveal Melanoma. Pharmaceutics 2021, 13, 1335. [Google Scholar] [CrossRef]
- Latorre, A.; Latorre, A.; Castellanos, M.; Lafuente-Gómez, N.; Diaz, C. R.; Crespo-Barreda, A.; Lecea, M.; Cordani, M.; Martín-Duque, P.; Somoza, Á. Albumin-Based Nanostructures for Uveal Melanoma Treatment. Nanomedicine Nanotechnol. Biol. Med. 2021, 35, 102391. [Google Scholar] [CrossRef]
- Valdés, K.; Morales, J.; Rodríguez, L.; Günther, G. Potential Use of Nanocarriers with Pentacyclic Triterpenes in Cancer Treatments. Nanomed. 2016, 11, 3139–3156. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid--A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Mol. Basel Switz. 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Silva, A. M.; Alvarado, H. L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M. L.; García, M. L.; Calpena, A. C.; Souto, E. B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Soto-Castro, D.; Cruz-Morales, J. A.; Apan, M. T. R.; Guadarrama, P. Solubilization and Anticancer-Activity Enhancement of Methotrexate by Novel Dendrimeric Nanodevices Synthesized in One-Step Reaction. Bioorganic Chem. 2012, 41–42, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J. F.; Candido, K. A.; Cao, Z.; Nigavekar, S. S.; Majoros, I. J.; Thomas, T. P.; Balogh, L. P.; Khan, M. K.; Baker, J. R., Jr. Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human Epithelial Cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Kavalaraki, A.; Spyratou, E.; Kouri, M. A.; Efstathopoulos, E. P. Gold Nanoparticles as Contrast Agents in Ophthalmic Imaging. Optics 2023, 4, 74–99. [Google Scholar] [CrossRef]
- Chen, F.; Si, P.; Zerda, A. de la; Jokerst, J. V.; Myung, D. Gold Nanoparticles to Enhance Ophthalmic Imaging. Biomater. Sci. 2021, 9, 367–390. [Google Scholar] [CrossRef]
- Prabhulkar, S.; Matthews, J.; Rawal, S.; Awdeh, R. M. Molecular Histopathology Using Gold Nanorods and Optical Coherence Tomography. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1192. [Google Scholar] [CrossRef]
- Raveendran, S.; Lim, H.-T.; Maekawa, T.; Matham, M. V.; Kumar, D. S. Gold Nanocages Entering into the Realm of High-Contrast Photoacoustic Ocular Imaging. Nanoscale 2018, 10, 13959–13968. [Google Scholar] [CrossRef]
- Warther, D.; Jimenez, C. M.; Raehm, L.; Gérardin, C.; Durand, J.-O.; Morère, A.; Cheikh, K. E.; Gallud, A.; Gary-Bobo, M.; Maynadier, M.; Garcia, M. Small Sized Mesoporous Silica Nanoparticles Functionalized with Mannose for Retinoblastoma Cell Imaging. RSC Adv. 2014, 4, 37171–37179. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I. H.; Qian, W.; El-Sayed, M. A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D. P.; Hirsch, L. R.; Halas, N. J.; Payne, J. D.; West, J. L. Photo-Thermal Tumor Ablation in Mice Using near Infrared-Absorbing Nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chang, Y.-T.; Cho, K.-C.; Chiu, K.-C.; Lien, C.-H.; Yeh, C.-S.; Chen, S.-J. Gold Nanomaterials Conjugated with Indocyanine Green for Dual-Modality Photodynamic and Photothermal Therapy. Biomaterials 2012, 33, 3270–3278. [Google Scholar] [CrossRef]
- Lara-Vega, I.; Vega-López, A. Combinational Photodynamic and Photothermal - Based Therapies for Melanoma in Mouse Models. Photodiagnosis Photodyn. Ther. 2023, 43, 103596. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R. Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89. [Google Scholar] [CrossRef]
- Demirci, H.; Slimani, N.; Pawar, M.; Kumon, R. E.; Vaishnava, P.; Besirli, C. G. Magnetic Hyperthermia in Y79 Retinoblastoma and ARPE-19 Retinal Epithelial Cells: Tumor Selective Apoptotic Activity of Iron Oxide Nanoparticle. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef]
- Moradi, S.; Mokhtari-Dizaji, M.; Ghassemi, F.; Sheibani, S.; Amoli, F. A. The Effect of Ultrasound Hyperthermia with Gold Nanoparticles on Retinoblastoma Y79 Cells. Gold Bull. 2020, 53, 111–120. [Google Scholar] [CrossRef]
- Pitsillides, C. M.; Joe, E. K.; Wei, X.; Anderson, R. R.; Lin, C. P. Selective Cell Targeting with Light-Absorbing Microparticles and Nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef]
- Katchinskiy, N.; Godbout, R.; Hatef, A.; Elezzabi, A. Y. Anti-EpCAM Gold Nanorods and Femtosecond Laser Pulses for Targeted Lysis of Retinoblastoma. Adv. Ther. 2018, 1, 1800009. [Google Scholar] [CrossRef]
- Darviot, C.; Hardy, P.; Meunier, M. Laser-Induced Plasmon-Mediated Treatment of Retinoblastoma in Viscous Vitreous Phantom. J. Biophotonics 2019, 12, e201900193. [Google Scholar] [CrossRef]
- Makky, A.; Michel, J. P.; Maillard, P.; Rosilio, V. Biomimetic Liposomes and Planar Supported Bilayers for the Assessment of Glycodendrimeric Porphyrins Interaction with an Immobilized Lectin. Biochim. Biophys. Acta 2011, 1808, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Kartha, B.; Thanikachalam, K.; Vijayakumar, N.; Alharbi, N. S.; Kadaikunnan, S.; Khaled, J. M.; Gopinath, K.; Govindarajan, M. Synthesis and Characterization of Ce-Doped TiO2 Nanoparticles and Their Enhanced Anticancer Activity in Y79 Retinoblastoma Cancer Cells. Green Process. Synth. 2022, 11, 143–149. [Google Scholar] [CrossRef]
- Normand, N.; Valamanesh, F.; Savoldelli, M.; Mascarelli, F.; BenEzra, D.; Courtois, Y.; Behar-Cohen, F. VP22 Light Controlled Delivery of Oligonucleotides to Ocular Cells in Vitro and in Vivo. Mol. Vis. 2005, 11, 184–191. [Google Scholar]
- Chawla, B. V.; Aronow, M. E. Global Perspectives in Ocular Oncology; Springer Nature, 2023.
- Kines, R. C.; Varsavsky, I.; Choudhary, S.; Bhattacharya, D.; Spring, S.; McLaughlin, R.; Kang, S. J.; Grossniklaus, H. E.; Vavvas, D.; Monks, S.; MacDougall, J. R.; de Los Pinos, E.; Schiller, J. T. An Infrared Dye-Conjugated Virus-like Particle for the Treatment of Primary Uveal Melanoma. Mol. Cancer Ther. 2018, 17, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huis In’t Veld, R. V.; Houy, A.; Stern, M.-H.; Rich, C.; Ossendorp, F. A.; Jager, M. J. In Vitro Testing of the Virus-Like Drug Conjugate Belzupacap Sarotalocan (AU-011) on Uveal Melanoma Suggests BAP1-Related Immunostimulatory Capacity. Invest. Ophthalmol. Vis. Sci. 2023, 64, 10. [Google Scholar] [CrossRef]
- Savinainen, A.; Grossniklaus, H. E.; King, S.; Wicks, J.; Rich, C. C. Ocular Distribution and Exposure of AU-011 after Suprachoroidal or Intravitreal Administration in an Orthotopic Rabbit Model of Human Uveal Melanoma. Invest. Ophthalmol. Vis. Sci. 2021, 62, 2861. [Google Scholar]
- Aura Biosciences. A Phase 2 Open-Label, Ascending Single and Repeat Dose Escalation Trial of Belzupacap Sarotalocan (AU-011) Via Suprachoroidal Administration in Subjects With Primary Indeterminate Lesions and Small Choroidal Melanoma; Clinical trial registration NCT04417530; clinicaltrials.gov, 2023. https://clinicaltrials.gov/study/NCT04417530 (accessed 2024-01-01).
- Roelofs, K. A.; Fabian, I. D.; Arora, A. K.; Cohen, V. M. L.; Sagoo, M. S. Long-Term Outcomes of Small Pigmented Choroidal Melanoma Treated with Primary Photodynamic Therapy. Ophthalmol. Retina 2021, 5, 468–478. [Google Scholar] [CrossRef]
- Rundle, P. Treatment of Posterior Uveal Melanoma with Multi-Dose Photodynamic Therapy. Br. J. Ophthalmol. 2014, 98, 494–497. [Google Scholar] [CrossRef]
- Fabian, I. D.; Stacey, A. W.; Papastefanou, V.; Al Harby, L.; Arora, A. K.; Sagoo, M. S.; Cohen, V. M. L. Primary Photodynamic Therapy with Verteporfin for Small Pigmented Posterior Pole Choroidal Melanoma. Eye 2017, 31, 519–528. [Google Scholar] [CrossRef]
- Turkoglu, E. B.; Pointdujour-Lim, R.; Mashayekhi, A.; Shields, C. L. PHOTODYNAMIC THERAPY AS PRIMARY TREATMENT FOR SMALL CHOROIDAL MELANOMA. Retina Phila. Pa 2019, 39, 1319–1325. [Google Scholar] [CrossRef]
- Chen-jin, J. Photodynamic Therapy With Visudyne for Human Retinoblastoma: A Preliminary Study; Clinical trial registration NCT04429139; clinicaltrials.gov, 2020. https://clinicaltrials.gov/study/NCT04429139 (accessed 2025-02-19).
- Liu, Y.; Han, Y.; Chen, S.; Liu, J.; Wang, D.; Huang, Y. Liposome-Based Multifunctional Nanoplatform as Effective Therapeutics for the Treatment of Retinoblastoma. Acta Pharm. Sin. B 2022, 12, 2731–2739. [Google Scholar] [CrossRef]
- Li, M.; Bian, X.; Chen, X.; Fan, N.; Zou, H.; Bao, Y.; Zhou, Y. Multifunctional Liposome for Photoacoustic/Ultrasound Imaging-Guided Chemo/Photothermal Retinoblastoma Therapy. Drug Deliv. 2022, 29, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, P.; Zhang, Y.; Zhang, S. Lipid-Nanoparticles-Based Co-Delivery of Black Phosphorus Quantum Dots and Melphalan by Photothermal Therapy Combined with Chemotherapy for Retinoblastoma. Polym. Test. 2023, 129, 108292. [Google Scholar] [CrossRef]
- Mudigunda, S. V.; Pemmaraju, D. B.; Paradkar, S.; Puppala, E. R.; Gawali, B.; Upadhyayula, S. M.; Vegi Gangamodi, N.; Rengan, A. K. Multifunctional Polymeric Nanoparticles for Chemo/Phototheranostics of Retinoblastoma. ACS Biomater. Sci. Eng. 2022, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, B.; Ouyang, L.; Wang, D.; Tan, J.; Qiao, Y.; Ge, S.; Ruan, J.; Zhuang, A.; Liu, X.; Jia, R. A Novel Stimuli-Responsive Injectable Antibacterial Hydrogel to Achieve Synergetic Photothermal/Gene-Targeted Therapy towards Uveal Melanoma. Adv. Sci. 2021, 8, 2004721. [Google Scholar] [CrossRef]
- Kim, H.; Nguyen, V. P.; Manivasagan, P.; Jung, M. J.; Kim, S. W.; Oh, J.; Kang, H. W. Doxorubicin-Fucoidan-Gold Nanoparticles Composite for Dual-Chemo-Photothermal Treatment on Eye Tumors. Oncotarget 2017, 8, 113719–113733. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Hocine, O.; Brevet, D.; Maynadier, M.; Raehm, L.; Richeter, S.; Charasson, V.; Loock, B.; Morère, A.; Maillard, P.; Garcia, M.; Durand, J.-O. Cancer Therapy Improvement with Mesoporous Silica Nanoparticles Combining Targeting, Drug Delivery and PDT. Int. J. Pharm. 2012, 423, 509–515. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Hocine, O.; Maynadier, M.; Gallud, A.; Da Silva, A.; Mongin, O.; Blanchard-Desce, M.; Richeter, S.; Loock, B.; Maillard, P.; Morère, A.; Garcia, M.; Raehm, L.; Durand, J.-O. Multifunctionalized Mesoporous Silica Nanoparticles for the in Vitro Treatment of Retinoblastoma: Drug Delivery, One and Two-Photon Photodynamic Therapy. Int. J. Pharm. 2012, 432, 99–104. [Google Scholar] [CrossRef]
- Gallud, A.; Warther, D.; Maynadier, M.; Sefta, M.; Poyer, F.; Thomas, C. D.; Rouxel, C.; Mongin, O.; Blanchard-Desce, M.; Morère, A.; Raehm, L.; Maillard, P.; Durand, J. O.; Garcia, M.; Gary-Bobo, M. Identification of MRC2 and CD209 Receptors as Targets for Photodynamic Therapy of Retinoblastoma Using Mesoporous Silica Nanoparticles. RSC Adv. 2015, 5, 75167–75172. [Google Scholar] [CrossRef]
- N’Diaye, M.; Vergnaud-Gauduchon, J.; Nicolas, V.; Faure, V.; Denis, S.; Abreu, S.; Chaminade, P.; Rosilio, V. Hybrid Lipid Polymer Nanoparticles for Combined Chemo- and Photodynamic Therapy. Mol. Pharm. 2019, 16, 4045–4058. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, X.; Zou, H.; Xu, Y.; Li, P.; Zhou, X.; Wu, M. Dual-Target Multifunctional Superparamagnetic Cationic Nanoliposomes for Multimodal Imaging-Guided Synergistic Photothermal/Photodynamic Therapy of Retinoblastoma. Int. J. Nanomedicine 2022, 17, 3217–3237. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xu, X.; Cheng, C.; Wang, J.; Yang, L. Cooperative Phototherapy Based on Bimodal Imaging Guidance for the Treatment of Uveal Melanoma. J. Nanobiotechnology 2023, 21, 146. [Google Scholar] [CrossRef]
- Bilmin, K.; Synoradzki, K. J.; Czarnecka, A. M.; Spałek, M. J.; Kujawska, T.; Solnik, M.; Merks, P.; Toro, M. D.; Rejdak, R.; Fiedorowicz, M. New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma. Cancers 2022, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Shiau, A.-L.; Chen, Y.-H.; Chang, C.-J.; Chen, H. H.-W.; Wu, C.-L. Increased Apoptotic Potential and Dose-Enhancing Effect of Gold Nanoparticles in Combination with Single-Dose Clinical Electron Beams on Tumor-Bearing Mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar] [CrossRef]
- Ngwa, W.; Makrigiorgos, G. M.; Berbeco, R. I. Applying Gold Nanoparticles as Tumor-Vascular Disrupting Agents during Brachytherapy: Estimation of Endothelial Dose Enhancement. Phys. Med. Biol. 2010, 55, 6533–6548. [Google Scholar] [CrossRef]
- Asadi, S.; Vaez-zadeh, M.; Masoudi, S. F.; Rahmani, F.; Knaup, C.; Meigooni, A. S. Gold Nanoparticle-Based Brachytherapy Enhancement in Choroidal Melanoma Using a Full Monte Carlo Model of the Human Eye. J. Appl. Clin. Med. Phys. 2015, 16, 344–357. [Google Scholar] [CrossRef]
- Rezaei, H.; Zabihzadeh, M.; Ghorbani, M.; Goli Ahmadabad, F.; Mostaghimi, H. Evaluation of Dose Enhancement in Presence of Gold Nanoparticles in Eye Brachytherapy by 103Pd Source. Australas. Phys. Eng. Sci. Med. 2017, 40, 545–553. [Google Scholar] [CrossRef]
- Asadi, S.; Vaez-zadeh, M.; Vahidian, M.; Marghchouei, M.; Masoudi, S. F. Ocular Brachytherapy Dosimetry for and in the Presence of Gold Nanoparticles: A Monte Carlo Study. J. Appl. Clin. Med. Phys. 2016, 17, 90–99. [Google Scholar] [CrossRef]
- Sharabiani, M.; Asadi, S.; Barghi, A. R.; Vaezzadeh, M. Comparison of Parameters Affecting GNP-Loaded Choroidal Melanoma Dosimetry; Monte Carlo Study. Radiat. Phys. Chem. 2018, 145, 180–183. [Google Scholar] [CrossRef]
- Kanavi, M. R.; Asadi, S.; Balagholi, S.; Alikarami, F.; Nosrati, H.; Ahmadieh, H. Gamma Irradiation of Ocular Melanoma and Lymphoma Cells in the Presence of Gold Nanoparticles: In Vitro Study. J. Appl. Clin. Med. Phys. 2018, 19, 268–275. [Google Scholar] [CrossRef]
- Kanavi, M. R.; Asadi, S.; Ahmadieh, H. Ex Vivo Distribution of Gold Nanoparticles in Choroidal Melanoma. Int. J. Nanomedicine 2017, 12, 8527–8529. [Google Scholar] [CrossRef]
- Zabihzadeh, M.; Rezaee, H.; Hosseini, S. M.; Feghhi, M.; Danyaei, A.; Hoseini-Ghahfarokhi, M. Improvement of Dose Distribution in Ocular Brachytherapy with 125I Seeds 20-Mm COMS Plaque Followed to Loading of Choroidal Tumor by Gold Nanoparticles. J. Cancer Res. Ther. 2019, 15, 504. [Google Scholar] [CrossRef]
- Hashemi, S.; Aghamiri, M.; Kahani, M.; Jaberi, R. Investigation of Gold Nanoparticle Effects in Brachytherapy by an Electron Emitter Ophthalmic Plaque. Int. J. Nanomedicine 2019, 14, 4157–4165. [Google Scholar] [CrossRef] [PubMed]
- Oare, C. C.; Dailey, J. P.; Gerbi, B.; Ferreira, C. Novel Intraocular Shielding Device for Eye Plaque Brachytherapy Using Magnetite Nanoparticles: A Proof-of-Concept Study Using Radiochromic Film and Monte Carlo Simulations. Brachytherapy 2023, 22, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mokhtari-Dizaji, M.; Ghassemi, F.; Sheibani, S.; Asadi Amoli, F. Increasing the Efficiency of the Retinoblastoma Brachytherapy Protocol with Ultrasonic Hyperthermia and Gold Nanoparticles: A Rabbit Model. Int. J. Radiat. Biol. 2020, 96, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ma, X.; Sun, W.; Mendez, S.; Stryker, S.; Starr-Baier, S.; Delliturri, G.; Zhu, D.; Nath, R.; Chen, Z.; Roberts, K.; MacDonald, C. A.; Liu, W. Monte Carlo Dosimetry Modeling of Focused kV X-Ray Radiotherapy of Eye Diseases with Potential Nanoparticle Dose Enhancement. Med. Phys. 2018, 45, 4720–4733. [Google Scholar] [CrossRef]
- Altundal, Y.; Sajo, E.; Makrigiorgos, G. M.; Berbeco, R. I.; Ngwa, W. Nanoparticle-Aided Radiotherapy for Retinoblastoma and Choroidal Melanoma. IFMBE Proc. 2015, 51, 907–910. [Google Scholar] [CrossRef]
- Flotte, T. R. Size Does Matter: Overcoming the Adeno-Associated Virus Packaging Limit. Respir. Res. 2000, 1, 16–18. [Google Scholar] [CrossRef]
- Adijanto, J.; Naash, M. I. Nanoparticle-Based Technologies for Retinal Gene Therapy. Eur. J. Pharm. Biopharm. 2015, 95, 353–367. [Google Scholar] [CrossRef]
- Baum, C.; Kustikova, O.; Modlich, U.; Li, Z.; Fehse, B. Mutagenesis and Oncogenesis by Chromosomal Insertion of Gene Transfer Vectors. Hum. Gene Ther. 2006, 17, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Raper, S. E.; Chirmule, N.; Lee, F. S.; Wivel, N. A.; Bagg, A.; Gao, G.; Wilson, J. M.; Batshaw, M. L. Fatal Systemic Inflammatory Response Syndrome in a Ornithine Transcarbamylase Deficient Patient Following Adenoviral Gene Transfer. Mol. Genet. Metab. 2003, 80, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Conley, S.; Naash, M. Nanoparticle Applications in Ocular Gene Therapy. Vision Res. 2008, 48, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Farjo, R.; Skaggs, J.; Quiambao, A. B.; Cooper, M. J.; Naash, M. I. Efficient Non-Viral Ocular Gene Transfer with Compacted DNA Nanoparticles. PLOS ONE 2006, 1, e38. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Rajala, R. V. S. Lipid Nanoparticles for Ocular Gene Delivery. J. Funct. Biomater. 2015, 6, 379–394. [Google Scholar] [CrossRef]
- Mandal, M.; Banerjee, I.; Mandal, M. Nanoparticle-Mediated Gene Therapy as a Novel Strategy for the Treatment of Retinoblastoma. Colloids Surf. B Biointerfaces 2022, 220, 112899. [Google Scholar] [CrossRef]
- Patel, S.; Ryals, R. C.; Weller, K. K.; Pennesi, M. E.; Sahay, G. Lipid Nanoparticles for Delivery of Messenger RNA to the Back of the Eye. J. Controlled Release 2019, 303, 91–100. [Google Scholar] [CrossRef]
- Del Pozo-Rodríguez, A.; Solinís, M. Á.; Rodríguez-Gascón, A. Applications of Lipid Nanoparticles in Gene Therapy. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2016, 109, 184–193. [Google Scholar] [CrossRef]
- Apaolaza, P. S.; Del Pozo-Rodríguez, A.; Torrecilla, J.; Rodríguez-Gascón, A.; Rodríguez, J. M.; Friedrich, U.; Weber, B. H. F.; Solinís, M. A. Solid Lipid Nanoparticle-Based Vectors Intended for the Treatment of X-Linked Juvenile Retinoschisis by Gene Therapy: In Vivo Approaches in Rs1h-Deficient Mouse Model. J. Control. Release Off. J. Control. Release Soc. 2015, 217, 273–283. [Google Scholar] [CrossRef]
- Delgado, D.; del Pozo-Rodríguez, A.; Solinís, M. Á.; Avilés-Triqueros, M.; Weber, B. H. F.; Fernández, E.; Gascón, A. R. Dextran and Protamine-Based Solid Lipid Nanoparticles as Potential Vectors for the Treatment of X-Linked Juvenile Retinoschisis. Hum. Gene Ther. 2012, 23, 345–355. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Xu, X.; Shen, L.; Yao, Y.; Yang, Z.; Liu, P. STAT3 Decoy Oligodeoxynucleotides-Loaded Solid Lipid Nanoparticles Induce Cell Death and Inhibit Invasion in Ovarian Cancer Cells. PloS One 2015, 10, e0124924. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, P.; Chen, Y.; Sun, J.; Kong, F. Co-Delivery of Plasmid DNA and Doxorubicin by Solid Lipid Nanoparticles for Lung Cancer Therapy. Int. J. Mol. Med. 2014, 34, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S. N.; Derbali, R. M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J. L.; Hardy, P. Co-Delivery of miR-181a and Melphalan by Lipid Nanoparticles for Treatment of Seeded Retinoblastoma. J. Controlled Release 2019, 298, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiong, H.; Zou, H.; Li, M.; Li, P.; Zhou, Y.; Xu, Y.; Jian, J.; Liu, F.; Zhao, H.; Wang, Z.; Zhou, X. A Laser-Activated Multifunctional Targeted Nanoagent for Imaging and Gene Therapy in a Mouse Xenograft Model with Retinoblastoma Y79 Cells. Acta Biomater. 2018, 70, 211–226. [Google Scholar] [CrossRef]
- Martens, T. F.; Peynshaert, K.; Nascimento, T. L.; Fattal, E.; Karlstetter, M.; Langmann, T.; Picaud, S.; Demeester, J.; De Smedt, S. C.; Remaut, K.; Braeckmans, K. Effect of Hyaluronic Acid-Binding to Lipoplexes on Intravitreal Drug Delivery for Retinal Gene Therapy. Eur. J. Pharm. Sci. 2017, 103, 27–35. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M. A.; Gascón, A. R.; Pedraz, J. L. Solid Lipid Nanoparticles: Formulation Factors Affecting Cell Transfection Capacity. Int. J. Pharm. 2007, 339, 261–268. [Google Scholar] [CrossRef]
- Rudolph, C.; Schillinger, U.; Ortiz, A.; Tabatt, K.; Plank, C.; Müller, R. H.; Rosenecker, J. Application of Novel Solid Lipid Nanoparticle (SLN)-Gene Vector Formulations Based on a Dimeric HIV-1 TAT-Peptide in Vitro and in Vivo. Pharm. Res. 2004, 21, 1662–1669. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Zarghami, N. Cyclodextrin Based Natural Nanostructured Carbohydrate Polymers as Effective Non-Viral siRNA Delivery Systems for Cancer Gene Therapy. J. Controlled Release 2021, 330, 1046–1070. [Google Scholar] [CrossRef]
- Bejjani, R. A.; BenEzra, D.; Cohen, H.; Rieger, J.; Andrieu, C.; Jeanny, J.-C.; Gollomb, G.; Behar-Cohen, F. F. Nanoparticles for Gene Delivery to Retinal Pigment Epithelial Cells. Mol. Vis. 2005, 11, 124–132. [Google Scholar]
- Bourges, J.-L.; Gautier, S. E.; Delie, F.; Bejjani, R. A.; Jeanny, J.-C.; Gurny, R.; BenEzra, D.; Behar-Cohen, F. F. Ocular Drug Delivery Targeting the Retina and Retinal Pigment Epithelium Using Polylactide Nanoparticles. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3562–3569. [Google Scholar] [CrossRef]
- Tahara, K.; Sakai, T.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Establishing Chitosan Coated PLGA Nanosphere Platform Loaded with Wide Variety of Nucleic Acid by Complexation with Cationic Compound for Gene Delivery. Int. J. Pharm. 2008, 354, 210–216. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.-S.; Wu, H.; Zhang, Z.-X.; Cai, Y.; Hou, H.-Y.; Zhao, W.; Yang, X.-M.; Ma, J.-X. Inhibitory Efficacy of Hypoxia-Inducible Factor 1α Short Hairpin RNA Plasmid DNA-Loaded Poly (D, L-Lactide-Co-Glycolide) Nanoparticles on Choroidal Neovascularization in a Laser-Induced Rat Model. Gene Ther. 2010, 17, 338–351. [Google Scholar] [CrossRef]
- Jin, J.; Zhou, K. K.; Park, K.; Hu, Y.; Xu, X.; Zheng, Z.; Tyagi, P.; Kompella, U. B.; Ma, J.-X. Anti-Inflammatory and Antiangiogenic Effects of Nanoparticle-Mediated Delivery of a Natural Angiogenic Inhibitor. Invest. Ophthalmol. Vis. Sci. 2011, 52, 6230–6237. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Martens, T. F.; Braeckmans, K.; Demeester, J.; De Smedt, S. C. Polysaccharide-Based Nucleic Acid Nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef]
- Mitra, R. N.; Han, Z.; Merwin, M.; Al Taai, M.; Conley, S. M.; Naash, M. I. Synthesis and Characterization of Glycol Chitosan DNA Nanoparticles for Retinal Gene Delivery. ChemMedChem 2014, 9, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Thomas, M.; Klibanov, A. M.; Langer, R. Exploring Polyethylenimine-Mediated DNA Transfection and the Proton Sponge Hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-W.; Yau, K.-W. In Vivo Gene Delivery in the Retina Using Polyethylenimine. BioTechniques 2007, 42, 285–288. [Google Scholar] [CrossRef]
- Marano, R. J.; Toth, I.; Wimmer, N.; Brankov, M.; Rakoczy, P. E. Dendrimer Delivery of an Anti-VEGF Oligonucleotide into the Eye: A Long-Term Study into Inhibition of Laser-Induced CNV, Distribution, Uptake and Toxicity. Gene Ther. 2005, 12, 1544–1550. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhao, P.; Chen, Z.; Lin, Q. Hyperbranched-Star PEI-g-PEG as a Nonviral Vector with Efficient Uptake and Hypotoxicity for Retinoblastoma Gene Therapy Application. Colloid Interface Sci. Commun. 2022, 50, 100647. [Google Scholar] [CrossRef]
- Moolten, F. L. Tumor Chemosensitivity Conferred by Inserted Herpes Thymidine Kinase Genes: Paradigm for a Prospective Cancer Control Strategy. Cancer Res. 1986, 46, 5276–5281. [Google Scholar]
- Wang, Y.; Mo, L.; Wei, W.; Shi, X. Efficacy and Safety of Dendrimer Nanoparticles with Coexpression of Tumor Necrosis Factor-α and Herpes Simplex Virus Thymidine Kinase in Gene Radiotherapy of the Human Uveal Melanoma OCM-1 Cell Line. Int. J. Nanomedicine 2013, 8, 3805–3816. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Liu, D.; Zhu, R.; Pan, H.; Li, J.; Pan, W. A Core-Shell Nanoplatform as a Nonviral Vector for Targeted Delivery of Genes to the Retina. Acta Biomater. 2021, 134, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C. S.; Kim, S. T.; Landis, R. F.; Rotello, V. M. Gold Nanoparticles for Nucleic Acid Delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, A.; Pawlowska, R.; Jedrzejczyk, D.; Chworos, A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules 2020, 25, 204. [Google Scholar] [CrossRef]
- Guo, J.; J. Armstrong, M.; M. O’Driscoll, C.; D. Holmes, J.; Rahme, K. Positively Charged, Surfactant-Free Gold Nanoparticles for Nucleic Acid Delivery. RSC Adv. 2015, 5, 17862–17871. [Google Scholar] [CrossRef]
- Mendes, B. B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; Shi, J.; Schroeder, A.; Conde, J. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Primer 2022, 2, 1–21. [Google Scholar] [CrossRef]
- Song, W.-J.; Du, J.-Z.; Sun, T.-M.; Zhang, P.-Z.; Wang, J. Gold Nanoparticles Capped with Polyethyleneimine for Enhanced siRNA Delivery. Small 2010, 6, 239–246. [Google Scholar] [CrossRef]
- Baeuerle, P. A.; Gires, O. EpCAM (CD326) Finding Its Role in Cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef]
- Mitra, M.; Kandalam, M.; Rangasamy, J.; Shankar, B.; Maheswari, U. K.; Swaminathan, S.; Krishnakumar, S. Novel Epithelial Cell Adhesion Molecule Antibody Conjugated Polyethyleneimine-Capped Gold Nanoparticles for Enhanced and Targeted Small Interfering RNA Delivery to Retinoblastoma Cells. Mol. Vis. 2013, 19, 1029–1038. [Google Scholar]
- Posch, C.; Latorre, A.; Crosby, M. B.; Celli, A.; Latorre, A.; Vujic, I.; Sanlorenzo, M.; Green, G. A.; Weier, J.; Zekhtser, M.; Ma, J.; Monico, G.; Char, D. H.; Jusufbegovic, D.; Rappersberger, K.; Somoza, Á.; Ortiz-Urda, S. Detection of GNAQ Mutations and Reduction of Cell Viability in Uveal Melanoma Cells with Functionalized Gold Nanoparticles. Biomed. Microdevices 2015, 17, 15. [Google Scholar] [CrossRef]
- Kalmodia, S.; Parameswaran, S.; Ganapathy, K.; Yang, W.; Barrow, C. J.; Kanwar, J. R.; Roy, K.; Vasudevan, M.; Kulkarni, K.; Elchuri, S. V.; Krishnakumar, S. Characterization and Molecular Mechanism of Peptide-Conjugated Gold Nanoparticle Inhibiting P53-HDM2 Interaction in Retinoblastoma. Mol. Ther. Nucleic Acids 2017, 9, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Q.; Li, M.; Zou, H.; Wang, Z.; Ran, H.; Zheng, Y.; Jian, J.; Zhou, Y.; Luo, Y.; Ran, Y.; Jiang, S.; Zhou, X. Multifunctional Nanoparticles for Multimodal Imaging-Guided Low-Intensity Focused Ultrasound/Immunosynergistic Retinoblastoma Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5642–5657. [Google Scholar] [CrossRef]
- Keasberry, N. A.; Yapp, C. W.; Idris, A. Mesoporous Silica Nanoparticles as a Carrier Platform for Intracellular Delivery of Nucleic Acids. Biochem. Mosc. 2017, 82, 655–662. [Google Scholar] [CrossRef]
- Xiong, L.; Bi, J.; Tang, Y.; Qiao, S.-Z. Magnetic Core–Shell Silica Nanoparticles with Large Radial Mesopores for siRNA Delivery. Small 2016, 12, 4735–4742. [Google Scholar] [CrossRef]
- Galli, M.; Guerrini, A.; Cauteruccio, S.; Thakare, P.; Dova, D.; Orsini, F.; Arosio, P.; Carrara, C.; Sangregorio, C.; Lascialfari, A.; Maggioni, D.; Licandro, E. Superparamagnetic Iron Oxide Nanoparticles Functionalized by Peptide Nucleic Acids. RSC Adv. 2017, 7, 15500–15512. [Google Scholar] [CrossRef]
- Magro, M.; Martinello, T.; Bonaiuto, E.; Gomiero, C.; Baratella, D.; Zoppellaro, G.; Cozza, G.; Patruno, M.; Zboril, R.; Vianello, F. Covalently Bound DNA on Naked Iron Oxide Nanoparticles: Intelligent Colloidal Nano-Vector for Cell Transfection. Biochim. Biophys. Acta BBA - Gen. Subj. 2017, 1861, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Kapilov-Buchman, Y.; Lellouche, E.; Michaeli, S.; Lellouche, J.-P. Unique Surface Modification of Silica Nanoparticles with Polyethylenimine (PEI) for siRNA Delivery Using Cerium Cation Coordination Chemistry. Bioconjug. Chem. 2015, 26, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Liu, T.; Zhang, D. S.; Wang, Y.; Gu, H.; Di, W. Highly Effective Antiangiogenesis via Magnetic Mesoporous Silica-Based siRNA Vehicle Targeting the VEGF Gene for Orthotopic Ovarian Cancer Therapy. Int. J. Nanomedicine 2015, 10, 2579–2594. [Google Scholar] [CrossRef]
- Kievit, F. M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J. W.; Lee, D.; Ellenbogen, R. G.; Olson, J. M.; Zhang, M. PEI–PEG–Chitosan-Copolymer-Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, Complexation, and Transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, J.; Wang, J.; Liu, Q.; Shang, R.; Yang, X.; Lu, P.; Xia, C.; Wang, L.; Dou, K. Superparamagnetic Iron Oxide Nanoparticles Modified with Polyethylenimine and Galactose for siRNA Targeted Delivery in Hepatocellular Carcinoma Therapy. Int. J. Nanomedicine 2018, 13, 1851–1865. [Google Scholar] [CrossRef]
- Paris, J. L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- D, R.; Rao, P. Nanoparticles: Is Toxicity a Concern? EJIFCC 2011, 22, 92–101. [Google Scholar] [PubMed]
- Fu, Y.; Fan, M.; Xu, L.; Wang, H.; Hu, Q.; Jin, Y. Amino-Functionalized Polystyrene Nano-Plastics Induce Mitochondria Damage in Human Umbilical Vein Endothelial Cells. Toxics 2022, 10, 215. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Azadmanesh, K.; Shokrgozar, M. A.; Journeay, W. S.; Laurent, S. Effect of Nanoparticles on the Cell Life Cycle. Chem. Rev. 2011, 111, 3407–3432. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef]
- Leong, H. S.; Butler, K. S.; Brinker, C. J.; Azzawi, M.; Conlan, S.; Dufés, C.; Owen, A.; Rannard, S.; Scott, C.; Chen, C.; Dobrovolskaia, M. A.; Kozlov, S. V.; Prina-Mello, A.; Schmid, R.; Wick, P.; Caputo, F.; Boisseau, P.; Crist, R. M.; McNeil, S. E.; Fadeel, B.; Tran, L.; Hansen, S. F.; Hartmann, N. B.; Clausen, L. P. W.; Skjolding, L. M.; Baun, A.; Ågerstrand, M.; Gu, Z.; Lamprou, D. A.; Hoskins, C.; Huang, L.; Song, W.; Cao, H.; Liu, X.; Jandt, K. D.; Jiang, W.; Kim, B. Y. S.; Wheeler, K. E.; Chetwynd, A. J.; Lynch, I.; Moghimi, S. M.; Nel, A.; Xia, T.; Weiss, P. S.; Sarmento, B.; Neves, J. das; Santos, H. A.; Santos, L.; Mitragotri, S.; Little, S.; Peer, D.; Amiji, M. M.; Alonso, M. J.; Petri-Fink, A.; Balog, S.; Lee, A.; Drasler, B.; Rothen-Rutishauser, B.; Wilhelm, S.; Acar, H.; Harrison, R. G.; Mao, C.; Mukherjee, P.; Ramesh, R.; McNally, L. R.; Busatto, S.; Wolfram, J.; Bergese, P.; Ferrari, M.; Fang, R. H.; Zhang, L.; Zheng, J.; Peng, C.; Du, B.; Yu, M.; Charron, D. M.; Zheng, G.; Pastore, C. On the Issue of Transparency and Reproducibility in Nanomedicine. Nat. Nanotechnol. 2019, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Landry, M. P.; Moore, A.; Coreas, R. The Protein Corona from Nanomedicine to Environmental Science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in Ocular Applications and Their Potential Toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).