1. Introduction
Pollution by organic substances is becoming a global problem not only for the environment but also for human health. Such substances include, for example, pharmaceuticals, pesticides, poly- and perfluorinated substances (PFAS), etc. PFAS are anthropogenic microcontaminants that, due to their abundant occurrence, have an adverse impact not only on the ecosystem, but also on human health. The most studied substances are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) [
1,
2,
3]. Due to its potential multi-organ toxicity and adverse effects on human health, PFOS was the first compound to be regulated by the European Commission in 2006 (EU, 2006) [
4]. It was listed in the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2009 [
5]. PFOA was listed in 2019 [
6] and perfluorohexanesulfonic acid (PFHxS) in 2022 [
7].
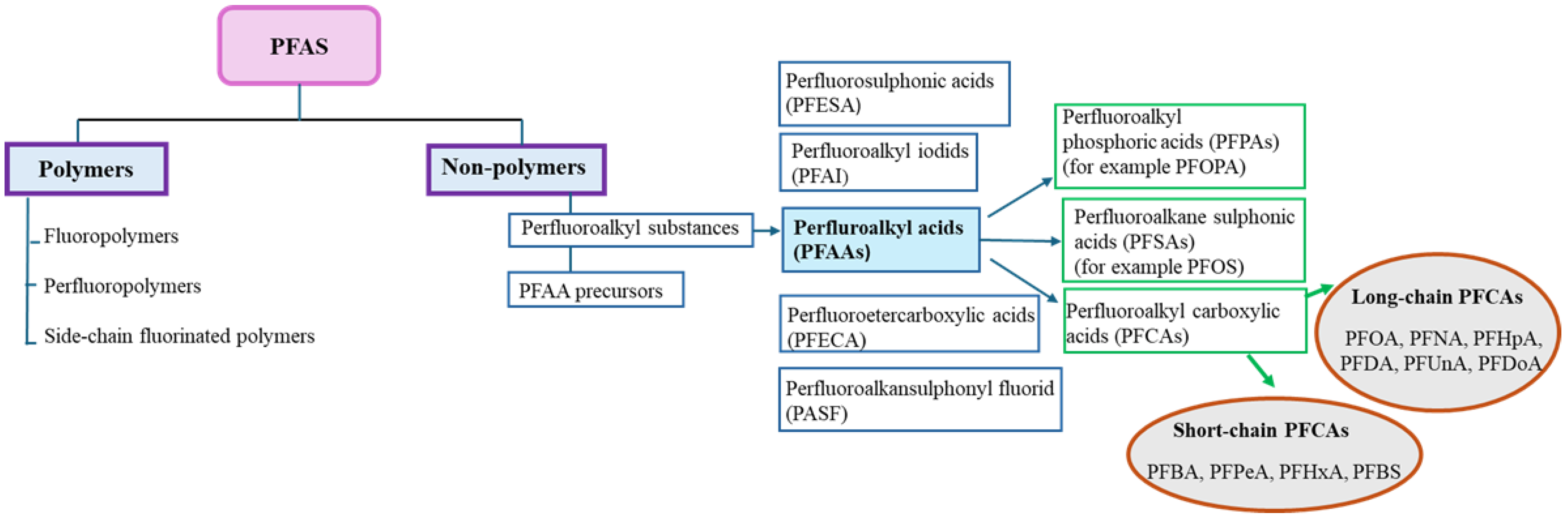
The first classification of PFAS was presented by Buck et al., [
8], who defined them as fluorinated aliphatic substances containing one or more carbon atoms, where the aliphatic hydrogens may be partially or fully replaced by fluorine atoms. In 2018, the Organisation for Economic Co-operation and Development (OECD) defined PFAS as substances that contain -CnF2n+1 or -CnF2n- (n ≥ 1). The existing classification system was further modified by Buck et al. [
9] so that PFAS are divided into two groups: polymers and non-polymers. Non-polymers are divided into per- and polyfluorinated substances, with the perfluorinated ones having polar hydrophilic groups such as carboxylate (COO-), sulfonate (SO
3-) or phosphate (OPO
3-) mostly attached to a hydrophobic carbon chain. Polymers are divided into fluoropolymers, fluorinated side-chain polymers and perfluoropolyether [
10]. They are not bioavailable and do not accumulate in the environment or biological systems. They gradually break down into PFAS. Often, long-chain PFAS are replaced by new short-chain alternatives, such as GenX [
11]. Several alternatives have been proposed to meet the market requirements of PFOS and PFOA. For example, chlorinated polyfluoroethylene ether sulfonic acid (Cl-PFESA) with the trade name F–53 B has been proposed as a replacement for PFOS [
12]; the ammonium salt of 2,3,3,3-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)-propanoic acid under the trade name GenX has been proposed as an alternative to PFOA [
13]. Several studies have shown that alternatives to PFOA and PFOS are found in environment at comparable or higher concentrations than PFOA and PFOS [
14,
15]. For example, concentrations of GenX in surface water samples are three times higher than PFOA in the North Sea [
16]. Concentrations of F–53 B in aquatic and soil environments in China are similar to those of PFOS [
13,
17].
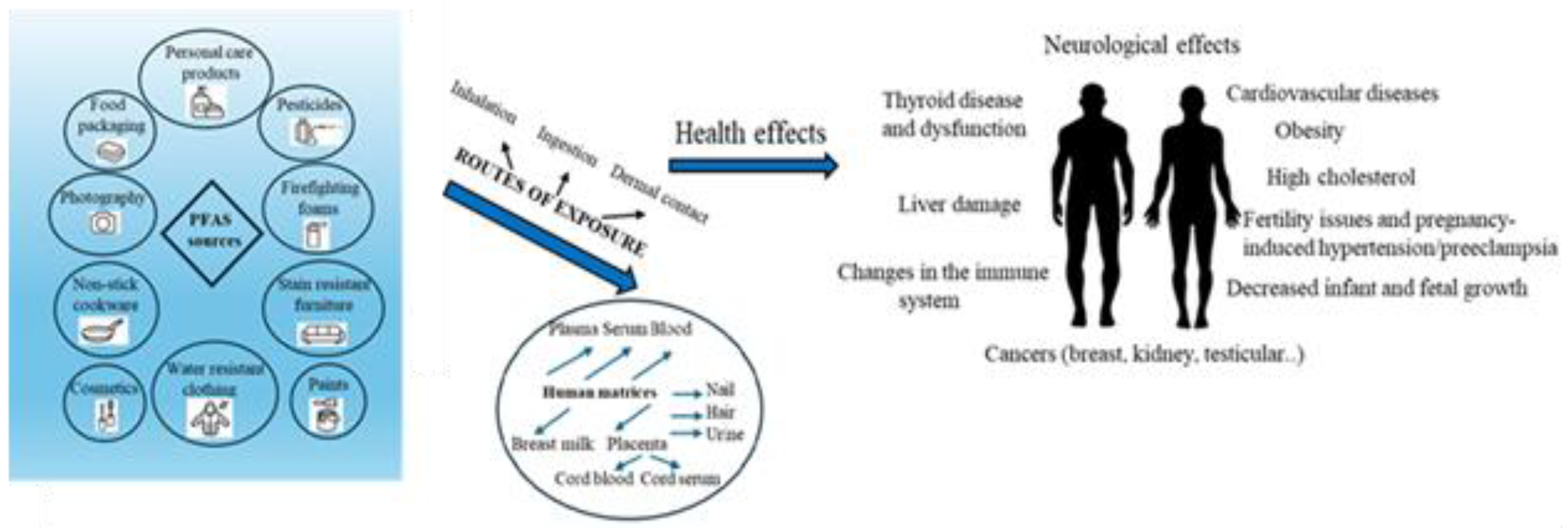
The hydrophobic and oleophobic parts of PFAS compounds cause them to act as surfactants. They are widely used in various industries and consumer products, such as carpets, textile impregnations, fire-fighting foams, electroplating, adhesives, protective coatings, insecticides, household cleaners, cosmetics, electronics, explosives, food packaging, tea bags, and many others from the 1940s to the present. The United States Environmental Protection Agency (US EPA) has set maximum concentrations of PFAS in drinking water at 4 ng/L for PFOA and PFOS and 10 ng/L for PFHxS in 2024. PFAS are ubiquitous substances, found in various environmental compartments, such as air, surface water, drinking water, groundwater, sediment, soil, plants, food, and animals. Routes of exposure to PFAS by inhalation and dermal contact include dietary intake, indoor air, and drinking water [
18,
19]. As a result, PFAS are found in multiple human tissues, such as blood, urine, hair, nails, urine, placenta and breast milk.
2. Properties of PFAS
PFAS contain of a hydrophobic chain of varying lengths made up of carbon and hydrogen atoms and a hydrophilic functional group at the end of the chain. The hydrogens can be fully or partially replaced by fluorine atoms. Accordingly, we divide the compounds into perfluorinated, when all hydrogen atoms are replaced by fluorine atoms, and polyfluorinated, when some hydrogen atoms (attached to at least one carbon) are replaced by fluorine atoms. According to the chemical structure, we divide PFAS into a long-chain group (number of carbons 7-13) called legacy PFAS and a short-chain group (number of carbons 6 or fewer atoms) terms emerging or alternative compounds. Polyfluoroalkyl substances can be chemically modified into perfluoroalkyl substances [
20].
The most studied are the perfluoroalkyl acids (PFAAs), which include long-chain compounds, such as 9-carbon perfluorononanoic acid (PFNA); 8-carbon compounds PFOA and PFOS; 7-carbon perfluoroheptanoic acid (PFHpA), and PFHxS. PFAAs also include short-chain 6-carbon compound perfluorohexanoaic acid (PFHxA); 5-carbon perfluoropentanoic acid (PFPeA); 4-carbon perfluorobutanoic acid (PFBA), and perfluorobutanesulfonic acid (PFBS). The classification of PFAS is presented on
Figure 1.
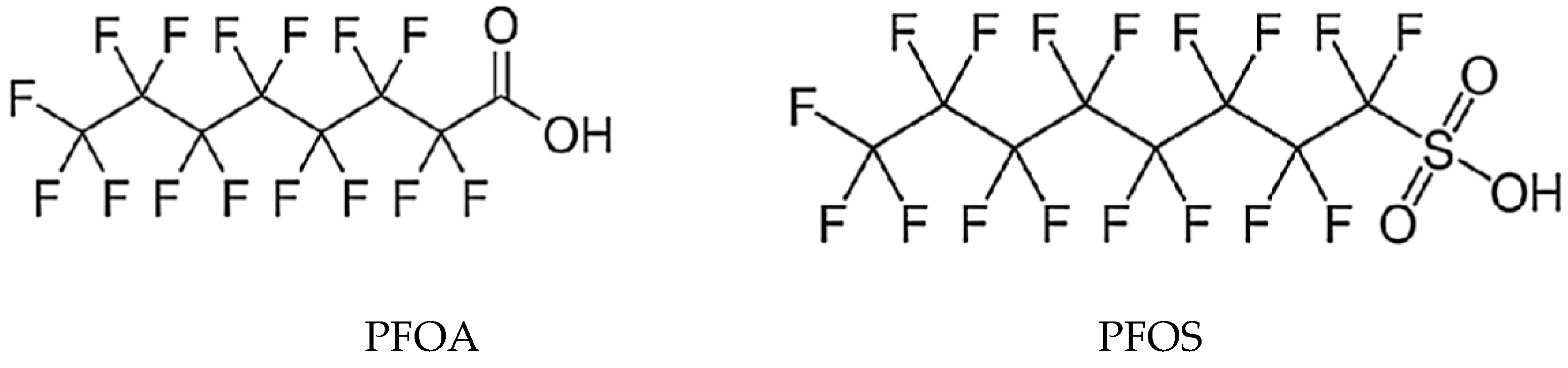
The most commonly monitored PFAS are PFOS and PFOA, which are detectable in up to 90% of the population in the USA. Molecular structures of selected PFAS are shown on
Figure 2.
PFAS consist of two parts, namely: an alkyl chain and a functional group, which can be a carboxylate or sulfonate. The alkyl chain, in which hydrogen atoms are replaced by fluorine, is responsible for the physical and chemical properties of PFAS. Fluorine has the high electronegativity and ionization potential and low polarization. It is the most electronegative element in the periodic table, therefore the bond between carbon and fluorine is among the strongest covalent bonds with a dissociation energy of up to 513.5 kJ.mol
-1. The strength of the bond increases with the number of F atoms bound to carbon atoms. The strong bond between C and F is responsible for the thermal stability of PFAS and their resistance to degradation, hydrolysis, photolysis, which is related to their long-term persistence in various components of the environment, but also in the food chain and in the human body [
21,
22]. They are further divided into polymers and non-polymers, with the polymeric ones degrading to non-polymers over time. Due to the low polarizability of fluorine, PFAS have weak intra- and intermolecular interactions and, as a result, higher volatility and lower boiling point. They have low surface tension and thus good surface wettability. The oleophobic character is a consequence of weak van der Waals forces. The combination of hydrophobic and hydrophilic properties allows PFAS to interact in the environment, they have the ability to combine into micelles, as a result of which they are capable of long-distance transport in air, water, and the atmosphere. Their high mobility provides the possibility of being transported over long distances, for example to remote areas such as the Arctic or Antarctica. PFAS are soluble in both non-polar and polar solvents, and their boiling and melting points increase with chain length. PFAS with higher vapor pressure exist in the gaseous state, with lower vapor pressure they remain solid or liquid [
21,
22].
The most commonly monitored physicochemical parameters include pH, temperature, turbidity, salinity, electrical conductivity (EC), dissolved oxygen (DO) and environmental factors (such as chain length, seasonal variations, organic content, solubility, partition coefficient, redox potential) that affect the fate, mobility and occurrence of PFAS in the aquatic ecosystem [
21,
22].
The study by Ohoro et al. found that at high pH, turbidity and dissolved oxygen, PFOS concentrations are high. Conversely, high electrical conductivity, temperature and salinity cause a decrease in PFOS concentrations in water. The protection of aquatic animals with regard to environmental safety, as well as human safety, is necessary [
23].
3. Sources of PFAS Exposure
There are multiple routes of human exposure to PFAS, with the main sources of exposure being drinking water, air, indoor dust, soil, food, food contact materials, kitchen utensils, mother-to-fetal transmission and breastfeeding to newborns However, it is expected that 98% of the US human population may have detectable levels of PFAS in their blood. PFAS are carcinogens that disrupt the endocrine system, genotoxicity, immunotoxicity, reproductive toxicity, and hepatic toxicity [
24].
In the adult population, food and drinking water are the main sources of exposure, but this is influenced by overall lifestyle and the level of contamination of drinking water. Infants fed formula may be among the most exposed due to high water intake per body weight [
24,
25,
26].
Due to their exceptional properties, such as stability, water resistance, etc., they are widely used in industry and commercial products. They can enter the environment through various routes: industry, emissions, product use, lividation, and the result is insufficiently effective removal of PFAS by wastewater treatment plants, resulting in soil and water contamination. Their degradation and removal from the environment depend on the properties of PFAS, especially the chain length, type of head group, degree of ionization and fluorination. Accumulation in humans occurs through dermal contact, inhalation or ingestion and can cause organ-specific toxicity, such as hepatotoxicity, neurotoxicity, immunotoxicity, etc. Long-chain PFAS accumulate in the body for a longer period of time and can result in health problems such as hormonal imbalance, decreased immune function, liver damage, various developmental and reproductive anomalies, and an increased risk of cancer. The toxicokinetic profile of PFAS in humans consists of absorption, distribution, metabolism, and excretion [
27]. PFAS absorption can occur orally, inhaled, and dermally. The oral route predominates (>90%), mainly through contaminated food and drinking water [
28]. Inhalation can occur when handling consumer products or in work environments where products containing PFAS are manufactured [
29]. Dermal contact occurs when using personal care products or when coming into contact with surfaces contaminated with PFAS [
30]. The rate and extent of absorption in the human body depends on the physicochemical properties of PFAS, namely: molecular weight, chain length, functional groups, hydrophobicity, lipophilicity, solubility, partition coefficient, volatility Short-chain PFAS, such as PFBS, PFHxA are more soluble in water than long-chain PFOS and PFOA, which bioaccumulate in the body over a longer period of time [
31].
Investigating the distribution of PFAS is a critical point for PFAS are known to cross the placenta and PFAS have also been found in the fetus. There have been concerns about adverse effects on fetal development, particularly through disruption of thyroid hormones, which are essential for normal fetal neurobehavioral development [
32].
In a study by Høyer et al. [
33], the association between prenatal exposure to PFHxS, PFHpA, PFNA and PFDA, PFOA and PFOS and behavior and hyperactivity in 5-9 year old children from Greenland and Ukraine was investigated. A country-specific parental standardized questionnaire was used to assess behavior in 1023 children. Parents rated their children’s behavior over the past six months. PFOS exposure levels were higher in Greenland compared to Ukraine. Foods such as fish, seabirds and marine mammals, which have high levels of trace elements, are a major part of the diet. This fact explains the differences in PFAS exposure. The results suggest that some of the associations between PFOS exposure and hyperactivity in some children may be due to postnatal exposure through breastfeeding. In the case of learning disabilities in children, more detailed investigation will be needed as levels of PFHxS and PFNA increase with decreasing PFOS levels [
33].
Bharal 2024 et al. reviewed the persistence of PFAS, exposure routes, and toxicological profiles. They provided a comprehensive review of neurotoxicity studies and their mechanisms induced by PFAS exposure [
34].
4. Analytical Techniques
The chemical structure of PFAS allows them to interact with the environment, which can result in toxic effects on humans and the environment, and therefore monitoring the impact of PFAS on human health is essential. Due to the relatively low concentration of PFAS in biological matrices and the complexity of the sample, continuous improvement of analytical methods is necessary, especially increasing the sensitivity and robustness of the method.
A review article by Kee et al. [
35] describes human sample collection, sample preparation techniques, and instrumental techniques for sample analysis. When collecting, storing, and analyzing samples, the use of sampling material (tubes, sample containers, sampling tools) made of Teflon® (polytetrafluoroethylene, PTFE) should be avoided. Polypropylene (PP) and polyethylene (PE) materials are recommended. Since PFAS have the ability to adsorb to glass surfaces in aqueous environments, the use of glass devices should be avoided [
36].
The most commonly used method for the determination of concentration of PFAS in various human samples (blood, plasma, serum, urine, hair, nails) is the combination of liquid chromatography (mainly UHPLC) with tandem mass spectrometry (MS/MS) using negative electrospray ionization (ESI). In addition, high-resolution mass spectrometry (HRMS) is used for non-targeted analysis for identifying new PFAS [
37,
38,
39]
Less frequently, gas chromatography (GC) combine with a flame ionization detector (FID), an electron capture detector (ECD) or a mass spectrometer (MS) has been used as well [
40]. GC-MS is used, especially for the analysis of volatile PFAS [
41,
42,
43].
Eliminating matrix interferences and simultaneously increasing the detection limit of PFAS in various complex matrices are essential steps in the analysis of PFAS. Matrix effects can cause false signal increases or decreases. Various extraction techniques are used for sample preparation. Review Dhiman et al. deals with different extraction techniques, analysis and detection of PFAS in different matrices [
44].
For accurate assessment of PFAS exposure, proper matrix selection is necessary. Plasma and serum are most commonly used for practical reasons. Non-invasive matrices such as urine, hair, and breast milk are advantageous in terms of ease of collection and efficiency, especially for infants and children. Urine is suitable for monitoring short-chain PFAS. Highly volatile PFAS do not accumulate in hair, and PFAS levels in breast milk may not accurately reflect maternal exposure because breastfeeding may affect the rate of PFAS excretion [
45].
The key task is the pretreatment of the biological matrix. The most commonly pretreatment techniques are solid-phase extraction (SPE) (using mainly hydrophilic-lipophilic balanced (HLB) sorbents; weak anion exchanger (WAX); polymeric cartridges; polyacrylonitrile and divinylbenzene/carboxen/polydimethylsiloxane fibres) [
46]; liquid-liquid extraction (LLE) [
47,
48]; solid-liquid extraction (SLE) [
49]; ultrasound-assisted extraction [
50,
51]; dispersive solid-phase microextraction [
52]; dispersive liquid-liquid microextraction [
53] or accelerated solvent extraction (ASE) [
54]. A review article by Comito et al. [
55] provides an overview of selected analytical methods for the determination of PFAS in various biological matrices as well as sample pretreatment in the period 2003-2022.
In this review,
Table 1 presents selected examples of PFAS determination in conventional biological matrices after 2022.
Nuclear magnetic resonance (NMR) is not sensitive to matrix components and offers clean spectra. It allows PFAS to be quantified without requiring high reproducibility and cost-effectiveness [
76,
77]. Advanced and novel analytical methods include fluorimetric assays, which measure the fluorescence signal of common fluorophores in solution. An interesting technique is molecular imprinting, which combines fluorescent dyes with molecularly imprinted polymers (MIPs). Electrochemical techniques are also used to detect PFAS, which help increase the sensitivity of the method [
78].
5. Human Matrices
Measuring chemicals or their metabolites in different matrices is important for assessing human exposure and associated risks. About 77% of all published articles concerned blood, plasma and serum, as they best reflect internal exposure to PFAS. The disadvantage is the invasiveness of the collection, for example in newborns and children, due to ethical and humanitarian reasons, which limits the number of available samples. Therefore, non-invasive matrices such as hair and nails have several advantages including simple and easy sampling, transport, stable storage, and ethical acceptance. Moreover, hairs contain keratin, which is suitable for monitoring PFAS exposure. Another non-invasive sample is urine. Each matrix has its advantages and disadvantages. Hair and nails require simple collection, but due to external transfer they may contain various impurities that need to be removed [
79].
5.1. Hair
Hair as a non-invasive matrix and contains 15-35% water, 63-95% protein, and 1-9% lipid, making it a suitable reservoir for hydrophobic compounds and their hydrophilic metabolites [
79,
80,
81]. The review article Zhang et al. describes standardized procedures for measuring microorganic contaminants (MOC) in hair, sample collection and preparation, methods for determining chlorinated persistent organic pollutants, brominated flame retardants, non-persistent pesticides, per- and polyfluoroalkyl substances, phthalate esters, bisphenols and polycyclic aromatic hydrocarbons in hair, as well as conclusions from cohort and epidemiological studies. There are several factors that reduce the reliability of hair analysis. Detection of MOC is affected by age, gender, hair treatment and sampling method. Furthermore, it is necessary to distinguish between endogenous and exogenous contamination in hair, either by incorporation via blood or from the environment. Correlations between MOC concentrations in hair and in blood/urine are limited. Since hair has been less used in the past to assess the health risk of contamination with microorganic contaminants compared to blood/urine, there is a need to discuss techniques, experiments, and propose standardized procedures for their measurement [
82].
In a study by Wang et al., concentrations of PFOS, PFOA, PFHxA, PFNA, PFDA, PFUnDA, PFDoA and PFHxS were determined in 39 human serum, urine, hair and nail samples by HPLC-MS/MS. PFHxA was detected only in nails and PFDoA was found in urine, hair and nails at very low concentrations. The concentrations of PFOS were 9.24 ng.mL
-1 in serum, 13.96 ng.L
-1 in urine, 0.58 ng.g
-1 in hair and 0.63 ng.g
-1 in nails and were higher in all matrices compared to other PFAS. A statistically significant difference in serum concentrations of PFOA and PFHxS was found between men and women. However, no statistically significant difference in PFAS concentrations was found between older and younger individuals in all matrices. The results of the study showed that the most suitable matrix for monitoring PFASs, especially PFOS, were nails [
83,
84].
PFAS can enter the hair in three ways: internal passive diffusion through the bloodstream; from the external environment and external transfer into hair shaft from sweat and sebum [
85]. The memory effect of hair due to the accumulation of chemicals allows for retrospective analysis. In the case of hair, it is possible to increase the frequency of sample collection, as well as its amount, which is especially advantageous for toddlers. In an Indian study [
86], e concentrations of 25 PFAS (6 PFSA, 13 PFCA, 6 PFAA precursors) were determined in 39 human hair samples. The effect of gender was also monitored. PFOA, PFOS and PFHxS were the most abundant in the samples. A highly significant correlation was found between PFOS and PFHxS. Women showed a higher incidence of PFAS than men. The article summarizes the results of PFAS analyses in hair obtained around the world. Compared to global results, PFAS values in the Indian study reached lower concentrations [
86].
Review article by Robin et al. presented the development and optimization of the method, including sample collection and preparation, extraction procedures, and instrumental techniques. Regarding hair sampling, the preferred site is the back of the head (posterior vertex, occipital region, or neck) or as close to the scalp as possible. Aluminum, envelopes, polypropylene tubes, or glass vials are used for hair storage [
87].
Samples were mainly stored at room temperature, but storage in a refrigerator or freezer has also been reported. Combinations of aqueous and organic solvents; aqueous solvents; organic solvent and shampoo were used for hair decontamination. Hair samples were dried at room temperature or lyophilized. After external decontamination of the sample, hair was cut into small pieces or ground into powder. The samples were denatured with an organic solvent, most often methanol or by acid hydrolysis (HCl, acetic, formic, nitric acid) and denaturation followed by purification [
88,
89]. In general, LC-MS/MS or GC-MS/MS are applied for determination of polar or non-polar compounds, respectively. Prior to instrumental analysis, matrix interferents have to be removed usually by extraction methods (LLE, SPE or SPME) [
90,
91,
92].
A Spanish study was aimed to determine PFBuA, PFPA, PFHxA, PFHpA, PFOA and PFOS in hair samples from children, women and men, assessing the potential relationship between PFAS concentration and age, gender, smoking and hair dyeing or coloring. The samples came from 42 volunteers, of whom 10 were children, 16 were women and 16 were men. The concentrations of PFAS ranged from 0.6-15.5 ng.g
-1, with PFHpA and PFOS being present in at least 86% and 76% of the analyzed samples, respectively. Longer-chain PFAS, such as PFOA and PFOS, are more commonly detected in hair samples, while shorter-chain PFAS (PFBuA) are more commonly detected in urine [
93].
5.2. Placenta
The placenta is an important organ for the transfer of oxygen and nutrients between the mother and fetus. Various studies have focused on the capacity of the placenta to transfer PFAS and their alternatives. In a Chinese study, Pan et al. included 100 paired samples of human maternal and umbilical cord serum and found that chlorinated polyfluorinated ether sulfonates (6:2 and 8:2 Cl-PFESA) were detected in more than 99% of both matrices. Higher efficiency of placental transfer was associated with older maternal age, higher education, and lower glomerular filtration rate [
94].
Gao et al. presented analysis of 132 paired samples of maternal and umbilical cord serum. The study showed that the extent of placental transfer of short-chain PFAS was in the range of 97-146% [
95].
A review article by Blake et al. [
96] focused on the placenta as a target organ and adverse pregnancy outcomes due to PFAS exposure. Specifically, these include prolonged gestation, pregnancy-induced hypertension, preeclampsia, gestational diabetes, and low birth weight. Studies by Chen [
97] and Wang et al. [
98] in human and animal models have shown that PFAS readily pass from maternal serum through the embryo to the placenta, which in the early stages functions as the liver, lungs, and kidneys for the embryo. Wang analyzed PFHpA, PFOA, PFNA, PFDA, PFUA, PFDoA, PFBS, PFHxS, PFOS and PFOSA in 369 pairs of maternal and cord serum in China. Almost all maternal and cord serum samples were found to contain all PFAS analyzed. All ten PFAS were found in both mothers and cord serum in almost all samples. Maternal and cord levels were closely correlated (r= 0.485-0.908) for all PFAS, except PFBS. The efficiency of transplacental transfer (TTE) was influenced by carbon chain length as well as functional group. Perfluoroalkylsulfonates had a lower maternal-fetal transfer ratio compared to perfluoroalkylcarboxylates [
98].
In a review article, Liu et al. summarized the findings from previously published data on PFAS monitoring in maternal blood, cord blood, breast milk, placenta, amniotic fluid, fetal organs, dried blood spots of newborns, and infant serum; and on PFAS exposure during pregnancy and breastfeeding. PFAS concentrations determined in blood are relatively high, while in breast milk they are relatively low. They emphasized the importance of future research on PFAS isomers and enantiomers [
99].
In Chinese study, there were tested 50 pairs of maternal and umbilical cord serum samples as well as placenta obtained from pregnant women living around the fluorochemical industrial park in Fuxin. 49 target PFAS in 11 classes were identified in human samples by HPLC-MS/MS [
100]. PFBS, PFBA, and PFOA were the main contaminants from 21 target analytes of legacy PFAS in maternal and cord serum samples as well as placentas analyzed by HPLC-MS/MS. PFBS concentrations in maternal serum ranged from 3.6 to 140 ng.mL
-1; PFOA from 0.08 to 123 ng.mL
-1, and PFBA from 0.08 to 87 ng.mL
-1. PFBS, PFOA, and PFBA concentrations in newborns decreased by almost half compared to maternal serum. Similar concentrations were also measured in placenta samples. Using high-resolution MS (HRMS), 49 novel PFAS classified into 11 classes were identified in the primary screening, of which 20 novel congeners in 4 classes were discovered in human blood and placentas for the first time. The concentrations of most novel PFASs were higher in placentas and cord serum compared to maternal serum. In addition, the presence of novel PFAS in cord serum may have affected neonatal birth outcomes and serum thyroid hormone, sex hormone, and glucocorticoid levels [
100].
5.3. Breast Milk
According to the World Health Organization, infants should be exclusively breastfed for the first 6 months [
101], Mothers who cannot or do not want to breastfeed replace milk with formula, either in liquid form or diluted with drinking water. Infant formula manufacturers try to minimize the differences between infant formula and breast milk, and it is important to know that liquid sources of nutrition are safe for the baby. PFAS can be transmitted from mother to child through breastfeeding. Infants can also be exposed to PFAS through infant formula. A review article by LaKind et al. [
102] compared concentrations of PFOA, PFOS, PFNA and PFHxS in breast milk and infant formula with screening values for the listed PFAS in drinking water in children. Results from an earlier study confirmed by more recent data confirm that PFAS concentrations in breast milk are higher than screening levels for drinking water in children [
103]. This is a global problem and it is essential that pregnant women and breastfeeding mothers have the necessary data available to inform their future decisions about breastfeeding.
Lamichhane et al. [
104] studied the effect of maternal PFAS exposure on breast milk lipid composition, as well as the combined effect of exposed breast milk and breast milk lipid composition on infant growth. PFAS and lipid concentrations in maternal serum were measured in 44 mother-infant pairs using UPLC-Q/TOF MS. Lipidomic analysis of breast milk collected 2-4 days after delivery and in 3-month-old infants was also performed. Fecal biomarkers calprotein and beta defensin 2 were measured in the stool of infants aged 3, 6, 9, and 12 months to assess intestinal immunomodulatory function in the infant intestine. High PFAS exposure caused an increase in the ratio of acylated saturated and polyunsaturated fatty acids in triacylglycerols. Altered phospholipid composition due to high PFAS exposure has been linked to slower growth in infants. Maternal exposure to PFAS affects the quality of breast milk, which may affect the health and growth of children [
104].
A Canadian study observed that the use of personal care products (e.g., hairsprays and gels, nailcare products, fragrances and perfumes, makeup, hair dye) may be associated with higher plasma concentrations of PFOA, PFOS, and PFHxS. Similar results were observed in breast milk. The study used prenatal plasma from 1.940 women at 6-13 weeks of gestation and human milk from 664 women 2-10 weeks postpartum. The frequency of use of personal care products was monitored during the 1st and 3rd trimesters, 1 to 2 days postpartum, and 2 to 10 weeks postpartum. Increased use of nail care products, fragrances, makeup, hair dye, hair sprays, and hair gels was observed in the 1st trimester, which caused increased plasma concentrations of PFOA and PFOS. Similar results were obtained in the 3rd trimester in plasma and in milk 2-10 weeks postpartum with the use of personal care products. In addition, the use of colored permanent hair dye 1-2 days postpartum may have caused higher concentrations of PFOA, PFOS, and PFNA in postpartum milk [
105].
6. Health effects
PFAS exposure is associated with adverse health risks such as cancer, steroid hormone disruption, infertility, lipid and insulin dysregulation, higher cholesterol levels, liver and kidney disease, altered immunological and thyroid function, and cardiovascular effects [
28,
106,
107]. In infants and children, PFAS exposure can cause adverse effects on infants and premature babies and can lead to reduced growth parameters, lower visual motor skills and attention deficit/hyperactivity disorder (ADHD) in childhood, lower levels of antibody concentrations against mumps and rubella, reduced lung and respiratory function, along with increased levels of glucocorticoids, progestogens, and uric acid [
108,
109,
110,
111].
The various sources, routes of exposure to PFAS and their adverse health effects on human health are illustrated in
Figure 2.
PFOS and PFOA are representatives of PFAS, which are toxic to humans and animals. Due to their multiple toxicity, they have been banned in many countries and replaced by substitutes, which, however, have shown even higher toxicity. The most pronounced toxicity has been reproductive toxicity. Epidemiological studies have shown that PFOS and PFOA can cause a decrease in testosterone levels in humans, abnormal levels of sex hormones, an increase in the risk of infertility. PFAS can be absorbed through the intestinal or respiratory tract and subsequently cause reproductive disorders such as testicular cancer, testicular dysplasia syndrome and menstrual disorders [
112].
Wee et al. provide a detailed review of the sources, transport routes, and potential toxicological impacts of PFAS. In addition, the article provides an overview of the adverse effects of PFAS on human health [
113].
The Center for Disease Control (Centers for Disease Control (CDC, 2024) and Agency for Toxic Substances and Disease Registry (ATSDR,2021) reported that less consistent data are associated with effects on the immune system, disruption of thyroid hormones, increased risk of cancer [
114,
115].
Lipid Metabolism
The Agency for Toxic Substances and Disease Registry [
115] stated that the most consistent health effect of PFAS is increased cholesterol in the adult population. The relationship between lipid levels and PFAS exposure was also presented in the study by Liu et al. [
66]. In the study, 575 human serum samples were collected. 11 of 18 PFASs were detected in more than 80% of the samples. The highest serum concentrations were measured for PFOA (11.34 ng.mL
-1) and PFOS (7.64 ng.mL
-1). With increased exposure to PFASs, total TC and LDL concentrations also increased, while HDL and TG concentrations were not affected. PFUnDA and PFTrDA had a greater effect on blood lipid concentrations than other PFASs [
66].
In a review article, Ho et al. [
116] comprehensively assessed the effects of PFA exposure on LDL, HDL, total cholesterol TC, and triglyceride TG concentrations in human plasma, serum, and whole blood. The study found a positive relationship between PFOA-LDL, PFOA-TC, PFOS-TC, and PFNA-LDL. The relationships between PFAS, especially perfluoroundecanoic acid (PFUnDA), and triglycerides tended to be negative [
116].
Gardener et al. [
117] investigated the relationship between serum concentrations of PFDA, PFNA, PFOS, PFOA PFHxS and fasting serum concentrations of total cholesterol, triglycerides and insulin. The study included 433 pregnant women whose serum was collected in the third trimester. PFAS were examined in quartiles in relation to serum biomarkers, gestational age at birth and birth weight standardized for gestational age. Results showed that total cholesterol was positively associated with PFDA, PFNA, PFOS and triglycerides, but PFAS were not associated with fasting insulin. PFNA was associated with an increased likelihood of preterm birth (<37 weeks of gestation) [
117].
A large Japanese study by Hasegawa et al. [
65] investigated the association between 28 PFAS and lipid concentrations in both maternal and cord blood. The analysis included 20.960 pregnant women. Plasma samples were collected before 22 weeks of gestation to determine PFAS concentrations. Serum samples collected before, during, after 22 weeks of gestation, at birth, and from cord blood were used to determine total cholesterol and triglycerides. Using linear regression models, 7 PFAS were quantified in more than 80% of the women out of the 28 PFAS analyzed, namely PFOA, PFNA, PFDA, PFOS, PFUnA, PFHxS, PFTrDA. Of these, 6 had positive associations with maternal total cholesterol before 22 weeks of gestation, but no association with cord blood TC. In the case of triglycerides, a negative relationship was shown between 3 PFAS and maternal blood and a positive relationship between 4 PFAS and TG in cord blood [
65].
PFAS exposure may affect lipid metabolism in pregnant women. Elevated plasma triglyceride levels may cause preeclampsia or hypertension. Yang et al. measured 11 PFAS in serum of 436 pregnant women and investigated the association with lipid parameters, namely TC, TG, HDL, LDL, PFOS, PFOA, PFHxS, PFUdA, PFNA, PFDA and PFHpS were detected in serum with a detection rate of more than 70%. The following associations were found positive association of PFHxS with TC, HDL and LDL; negative association of PFUdA with HDL PFDA with LDL; PFOA, PFNA, PFDA and PFUdA with LDL/HDL. The results indicate the possibility of an impact of PFAS exposure on lipid metabolism in pregnant women [
118].
In Italian study, there was conducted a cross-sectional analysis of 319 pregnant women aged 14-48 years, who came from areas with high exposure to PFAS through drinking water. Concentrations of PFOA, PFOS and PFHxS, total cholesterol (TC), HDL-C and LDL-C were determined. Elevated TC, LDL-C and HDL-C values increased gradually and may have adverse effects on both the mother and the fetus. Associations between PFAS and lipid markers varied throughout all trimesters of pregnancy. In the first trimester, the relationship between PFOS and TC and between PFHxS and HDL-C was positive and similar to that in non-pregnant women, with a reversal during the third trimester. There was an inverse relationship between PFOA and PFHxS and TC and LDL-C. The results highlight the importance of the correct timing of PFAS measurements during pregnancy [
119].
Diabetes Mellitus
Exposure to persistent organic pollutants in the environment may be one of the risk factors for the development of diabetes mellitus, which has a growing prevalence worldwide.
In the study [
120], there was investigated the relationship between PFAS exposure and the risk of developing type 2 diabetes mellitus (T2DM). 252 T2DM cases and 252 controls were examined and the results showed that serum PFHxS and PFHpA were significantly positively associated with the risk of developing T2DM at the lower levels [
120].
Xu et al. [
121] tested the hypothesis that PFAS can cause gestational diabetes mellitus (GDM) by modulating glucose metabolism. The study included 171 women with GDM and 169 controls and determined 15 PFASs including homologues of PFOA and PFOS; precursors of PFSA and PFCA and alternatives of PFOS and PFOA, which were detected in more than 70% of maternal serum samples. The highest concentrations in maternal serum were measured for PFOA 7.43 ng/ml, PFOS 4.23 ng/ml and chlorinated polyfluorinated ether sulfonate in the ratio 6:2 (6:2 Cl-PFESA). Exposure to PFOA, PFNA, PFDA, PFUnDA, PFDoA, PFHxS and PFOS was significantly higher in women with GDM compared to controls. Concentrations of PFOA, PFOS, PFUnDA, PFDoA and 6:2Cl-PFESA were associated with impaired glucose homeostasis in pregnancy and an increased risk of GDM, while an inverse association with GDM was found for PFHxS, 4:2FTS and 6:2FTS [
121].
A Korean study [
122] presented the relationship between serum PFAS concentrations and the prevalence of prediabetes and prediagnostic diabetes. This is the first study to describe a strong positive association between serum PFAS concentrations and prediabetes and the prediagnostic stage of diabetes. The study included 2709 participants aged ≥ 19 years. Prediagnostic diabetes and prediabetes were determined based on glycated hemoglobin HbA1c values. Serum PFOA, PFNA, PFDeA, PFHxS and PFOS were determined by HPLC combined with tandem mass spectrometry. Concentrations of individual PFAS compounds as well as mixtures were associated with a higher risk of prediabetes. Significant positive associations were found between serum PFOS and PFHxS and increased HbA1c values and a higher risk of prediagnostic diabetes. The results suggest that PFAS exposure may contribute to impaired glucose homeostasis in the prediabetic stage and thus to the development of diabetes [
122].
A Chinese study [
123] included 495 women, 165 with GDM and 330 controls. GDM was measured between 24-28 weeks of gestation by glucose test. PFOA, PFOS, PFBS, PFDoA, PFUA, PFDA, PFHpS, PFNA,PFHxS, PFDS, PFHpA, PFOSA in serum were determined by UPLC-Q/TOF MS. PFHpA, PFDS and PFOSA were all present in ≤ 80% of samples and were therefore not considered further. PFOS, PFOA, PFBS, PFDoA were measured at significantly higher concentrations in maternal serum in early pregnancy, which may be associated with a higher risk of GDM [
123].
Disorders in Women
There are many studies on PFAS exposure and male fertility, and most of them show that PFAS exposure leads to impaired male fertility [
124]. The available results obtained from studies focusing on women have been quite inconsistent. Therefore, more systematic reviews and meta-analyses have been conducted to examine the evidence on the effect of PFAS exposure during pregnancy on maternal fertility, in addition to quantitative assessment of maternal PFAS concentrations during pregnancy and the risk of fertility and infertility.
Therefore, Wang et al. [
125] conducted a meta-analysis in a review article. A total of 5468 records from 4 databases were searched, and after gradual elimination, 13 articles that fully met the inclusion criteria were left and were used for the meta-analysis. The results showed that exposure to (PFOA) was negatively associated with female fertility and positively associated with the odds ratio for infertility. Exposure to (PFOS) was negatively associated with the odds ratio for fertilization. The obtained effect values for exposure to PFNA), (PFDA) and perfluorohexanesulfonate (PFHxS) did not provide sufficient evidence of an association with female fertility [
125].
Preeclampsia is a condition of hypertensive disorder during pregnancy that can be a cause of maternal mortality or can be associated with low birth weight, prenatal death and preterm birth. Tian et al. [
126] studied the possible association between preeclampsia and serum PFAS concentrations in 82 women with preeclampsia and 169 healthy women. 15 PFAS were analyzed in maternal serum before delivery. Concentrations of PFOA and 6:2Cl-PFESA were associated with an increased risk of developing preeclampsia, especially in primiparous women expecting a girl. Increased PFOA concentrations were significantly associated with increased blood pressure. In relation to neonatal development, a negative relationship was found between exposure to PFOS, PFNA, PFUnDA and 6:2Cl-PFESA and birth weight. Regression models indicated that significantly higher risks of low birth weight occurred in women with preeclampsia compared to normal pregnancies. This observational study considered not only legacy PFAS but also new alternatives and included a low birth weight parameter to better elucidate the possible effects of PFAS exposure on adverse birth outcomes [
126].
Preeclampsia was also addressed in a study by Thompsom et al. [
127] which examined the association between hypertensive disorders of pregnancy (preeclampsia and gestational diabetes) and exposure to 4 PFAS: PFOA, PFOS, PFHxS, and PFNA in a sample of 513 African-American mothers. Serum samples were collected from mothers between 8 and 14 weeks of pregnancy: Regression models were used to assess associations. Mean PFOS and PFHxS levels were lower in patients with preeclampsia compared to patients without hypertensive disorders. PFAS concentrations were not associated with gestational hypertension or preeclampsia. In this study, no association was found between serum PFAS concentrations measured in early pregnancy and hypertensive disorders of pregnancy [
127].
There is an association between PFAS and reduced birth weight, but it may be complicated by glucose status due to the effects of PFAS on fetal growth and placental transport. Wang 2023 analyzed 1405 mother-child pairs and investigated whether maternal glucose levels were associated with prenatal PFAS exposure and birth weight. Plasma concentrations of PFOA, PFOS, PFNA, PFDA, PFUA, and PFHxS were determined in the first trimester. Plasma glucose was measured at 24-28 weeks of gestation. The results suggest that infants born to mothers with hyperglycemia may be sensitive to PFAS exposure [
125].
Zhang et al. investigated whether PFAS exposure is associated with Polycystic ovary syndrome (PCOS). A total of 502 women undergoing reproductive treatment were included in the study and 9 PFAS were measured in serum samples. Higher serum concentrations of PFOS and PFHxS were associated with a higher risk of PCOS. No associations were found for PFOA [
128].
The results of this study were consistent with the results of a case-control study by Zhan et al., which included 366 women with PCOS and 577 controls. Interestingly, the concentrations of PFOA were several times higher than in the discussed article (7.2 versus 1.7 ng.mL
-1), while the concentrations of PFOS (3.9 and 3.5 ng.mL
-1) and PFHxS (0.22 and 0.8 ng.mL
-1) were found. The results support the possible adverse effects of PFAS on female reproductive function, but further studies are needed to confirm the findings [
129].
The review article by Yi et al. summarizes the impact of PFAS exposure on the development of four reproductive health outcomes in women studied to date, namely polycystic ovary syndrome, endometriosis, primary ovarian insufficiency, and diminished ovarian reserve [
130].
Thyroid Hormones
Thyroid hormones play an important role in regulating health, in brain development, depression, and obesity. Thyroid hormone deficiency during pregnancy can cause reduced IQ or neurological damage in children. In addition, it can cause intellectual disability or growth retardation in adolescents. PFAS are potential thyroid disruptor [
131,
132].
He et al. [
133] investigated the relationship between exposure to 30 PFAS and thyroid function in 194 Chinese children aged 3-17 years using multiple statistical models. Free triiodothyronine, free thyroxine (FT4), and thyrotropin were tested as indicators of thyroid function, and subclinical hypothyroidism was diagnosed. PFAS and their alternatives were associated with changes in thyroid hormone levels and subclinical hypothyroidism. Higher concentrations of perfluorohexanoic acid (PFHpA) caused a decrease in FT4 levels and an increased likelihood of subclinical hypothyroidism. The measured PFOA concentration (median) of 23.22 μg.L
-1 was comparable to some previous studies. For example, in America, a concentration of 29.3 μg.L
-1 was found in children aged 1-17 years [
134] in adults 23.1 μg.L
-1 [
135], in pregnant women 23.22 μg.L
-1) [
136]. The PFOS concentrations of 4.31 μg.L
-1 and PFNA 1.79 μg.L
-1 (median) in this study in children were similar to the PFOS and PFNA concentrations in adults (5.17 μg.L
-1 and 1.00 μg.L
-1) [
137] and adolescents (PFOS 7.78 μg.L
-1and PFNA 1.01 μg.L
-1) [
138]. Higher serum PFOA concentrations cause decreased FT4 levels and increased TSH levels. A cohort study in Spain obtained opposite results in children aged 15–17 years [
139].
In a US study [
140] higher serum PFOA levels were associated with lower TSH levels in adolescent females, which are different results from the discussed study in children. These different results may be related to variations in the regions and populations of each study.
In the study by Lebeaux et al. the authors investigated the association between maternal serum concentrations of PFAS (PFOA, PFOS, PFNA, and PFHxS) during pregnancy with levels of thyroid-stimulating hormone (TSH), total thyroxine (TT4), total triiodothyronine (TT3), free thyroxine (FT4), and free triiodothyronine (FT3) measured in maternal and cord serum. The study involved 468 pregnant women and their children. The results show that there is no strong association between maternal serum PFAS concentrations measured in the second trimester and maternal and cord serum thyroid hormones [
132]. A cross-sectional study by Xie et al. examined thyroid function parameters and serum PFAS concentrations in adolescents. Concentrations of 18 PFAS were measured, including 11 PFCAs, 4 PFSAs, and 3 novel PFASs (4:2, 6:2, and 8:2Cl-PFESA). The study included 836 adolescents aged 11-15 years who came from a high-PFCA-exposed area near a fluorochemical industrial plant. Elevated FT3 and decreased FT4 levels were found. Using logistic and linear regression, a significant negative correlation was found between PFOA and FT4 and a positive correlation between PFHxS and FT3, with both PFOA and PFHxS being assessed as risk factors [
141].
Wang et al. conducted a study to investigate the effect of PFAS exposure on serum metabolome and its association with thyroid cancer. The study included 746 thyroid cancer cases and 746 controls. LC-MS/MS was used to determine the concentrations of PFDoA, PFUnDA, PFDA, PFNA, PFOA, PFOS, PFHxS, PFHxA, PFHpA, PFBS, PFPeA. Exposure to PFHxA and PFDoA was associated with an increased risk of thyroid cancer, while PFOA and PFHxS were associated with a decreased risk [
142].
Liver Disorders
General Liver Toxicity
PFAS are known to accumulate in various organs. Many studies have shown an association between PFAS exposure and liver toxicity, as well as the impact of PFAS on liver homeostasis, lipid and bile acid metabolism, and hepatocarcinogenesis. In a review article, Maerten et al discuss the role of PFAS in liver toxicity, the mechanisms underlying the hepatotoxic effects of PFAS, and fill the knowledge gap for a better assessment of PFAS risks [
143].
In humans, the highest concentrations of PFAS were measured in the liver, kidneys, and lungs. PFOSD predominates in the liver, while PFBA predominates in the lungs and kidneys. Many studies [
144,
145,
146,
147] reported that exposure to PFAS (PFOS, PFOA, PFNA, PFHxS, fluorohexanoic acid, perfluoro-3,5,7,9,11-pentaoxadocanoic acid and 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA) can lead to liver damage in humans. Markers of liver damage include alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), alkaline phosphatase and serum bilirubin, the increased values of which cause an increase in inflammatory markers. PFOS, PFOA, PFBA increase ALT activity [
148].
Dyslipidemia, Steatosis and Steatohepatitis
This article summarizes studies that have evaluated the relationship between serum PFAS and steatosis and steatohepatitis as a consequence of dyslipidemia. PFNA correlates with LDL, PFOS with total cholesterol, and PFOA with both LDL and TC [
149,
150,
151,
152].
PFOS, PFOA, and PFHxS have been associated with an increased risk of developing steatohepatitis [
153,
154]. PFAS exposure can lead to hepatic steatosis, which is manifested by increased inflammatory markers and biomarkers of liver injury.
Hepatocarcinogenesis
The International Agency for Research on Cancer considers both PFOS and PFOA to be probable human carcinogens in humans at high exposure and in humans with testicular and kidney tumors. The association of PFOS and PFOA with hepatocarcinogenesis has been demonstrated in laboratory animals, but not in humans. In conclusion, it can be stated that exposure to PFAS can cause: an increase in serum markers of liver damage; an increase in cholesterol and hepatic lipid accumulation in humans; interference with bile acid metabolism by affecting hepatic transporters [
115].
The study by Dai et al. investigated the correlations between PFAS concentrations and biomarkers of liver function. The study included 227 patients, of whom 197 had hepatocellular carcinoma and 30 suffered from severe liver disorders such as cirrhosis and hepatitis. 21 PFAS were determined, with the highest concentrations in serum, then in blood and urine. The concentrations were higher in the group of patients with HCC. Higher concentrations of PFAS are achieved in men compared to women. The highest concentrations in blood and serum were PFOA, PFOS, PFBS and PFHxS. The highest concentration was determined in urine for PFBA and PFPeA. The concentrations of PFBS, PFHpS and PFHxPA correlated with increased values of alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alpha-fetoprotein (AFP) (p < 0.05). The results provide a good basis for further study on the possible hepatotoxicity of PFAS [
155].
Hypertension
PFAS exposure is associated with adverse health effects, such as dyslipidemia, hypertension, and hypertensive disorders of pregnancy (HDP), specifically preeclampsia and gestational hypertension. The link between PFAS and HDP is discussed in a review article by Erinc et al. [
156].
Gestational hypertension (GH) is new-onset hypertension occuring after 20 weeks of pregnancy. A Chinese study evaluated the relationship between 13 PFAS and gestational hypertension and blood pressure (BP) during pregnancy. 826 pregnant women were included, with blood samples collected at 16 weeks of gestation. The highest concentrations of 11.99, 8.81 and 5.43 ng.mL
-1 were measured for PFOA, PFOS and PFHxS respectively. 5.57% of women developed GH. A lower probability of GH was demonstrated for PFOS, PFDA, PFUdA and PFDoA. Associations were found between PFAS and lower systolic and diastolic blood pressure values in the third trimester. PFDA and PFUdA affected systolic blood pressure only in pregnant women whose fetus was female [
157].
The study [
158] investigated the relationship between PFAS exposure and hypertensive disorders of pregnancy (i.e. preeclampsia, gestational hypertension), which can cause maternal and neonatal morbidity and mortality. The study included 1558 pregnant women and measured PFHxS, PFOS, PFOA, PFNA, PFDA, EtFOSAA, MeFOSAA, PFOSA in plasma samples. The parameters examined were preeclampsia, gestational hypertension, and trimester-dependent diastolic and systolic blood pressure. An association was found between PFOS, PFOA, and PFHxS concentrations and gestational hypertension, but not with preeclampsia. Analysis of the PFAS mixture indicated a positive association with gestational hypertension, while the associations for PFOA and PFHxS remained. Furthermore, a positive association was observed between high concentrations of PFOS and PFOA and diastolic blood pressure in the 2nd and 3rd trimesters, as well as for the PFAS mixture in both trimesters. For systolic blood pressure, an association was demonstrated only for PFOA in the second and third trimesters. The results indicate a possible impact of PFAS exposure on blood pressure regulation during pregnancy [
158].
Cancer
The International Agency for Research on Cancer classified PFOA as a Group 1 carcinogen and PFOS as a Group 2B carcinogen in 2023 [
159]. The United States Environmental Protection Agency (EPA) has set a recommended health level of 70 ng.L
-1 for lifetime exposure to PFOS and PFOA [
160]. Given the increasing number of epidemiological studies on PFAS and their adverse effects on humans, it has become imperative to address the carcinogenic mechanisms. The aim of the review by Zheng et al. was to update the evidence on the association between PFAS exposure and cancer. It presents a review of the literature published between 2019-2024 on the potential relationship between PFAS (PFOS, PFOA, PFHxS, PFHxA, PFNA) and various types of cancer, such as breast, testicular, prostate, colorectal, kidney, liver, lung, thyroid, leukemia, melanoma. In addition, the article also addresses potential carcinogenic mechanisms such as endocrine disruption, lipid metabolism, epigenetic alteration, oxidative stress, immunosuppression, and chronic inflammation [
142,
155,
161,
162,
163,
164,
165,
166,
167,
168].
Obesity
Chen et al. 2024 investigated the effects of PFAS exposure (PFOA, PFOS, PFUdA, PFNA, PFHxS, PFDeA, and 2- (N-methyl-perfluorooctane sulfonamide) acetic acid) on obesity. 11,090 individuals were analyzed and linear and logistic regression models were used to evaluate the effects. The role of inflammatory markers (neutrophils, lymphocytes, and alkaline phosphatase) and oxidative stress markers (gamma-glutamyltransferase, total bilirubin, and uric acid) was also examined. Lymphocytes, alkaline phosphatase, and total bilirubin were significantly associated with both obesity and type 2 diabetes mellitus. PFAS exposure was positively associated with the development of obesity and T2DM and mediated by inflammation and oxidative stress. PFNA was positively associated with obesity and T2DM, while PFOA was negatively associated with T2DM. PFDeA was positively associated with hypertension but negatively with obesity and T2DM [
169].
Averina et al. investigated associations between PFAS and dyslipidemia, hypertension and obesity in adolescents. A cross-sectional study was conducted on 940 adolescents with a mean age of 16.4 years who had either hypertension, obesity or dyslipidemia. Serum concentrations of PFHxS, PFOA, PFOS, PFNA, PFDA, PFUnDA were measured by UHPLC-MS/MS. The results showed a positive association of PFOS, PFNA, PFDA and PFUnDA with apolipoprotein B, LDL and total cholesterol. Concentrations of PFOS, PFOA were positively associated with the risk of hypertension, and PFHxS and PFHpS with obesity [
170].
In a study by Geiger et al., the association between PFOA and PFOS and BMI and waist circumference was investigated in a representative sample of 2473 American children. Elevated concentrations of PFOA may indicate an association with overweight and obesity in children. This topic requires further research, which will include, for example, abdominal adiposity, fat distribution, etc. [
171].
Autism
Autism spectrum disorder (ASD) is a complex of neurodevelopmental disorders characterized by deficits in social interaction and communication, as well as the presence of restricted, stereotyped interests and repetitive behaviors [
172]. Although there is a possibility of a potential association between PFAS and the development of ASD, large studies are needed to confirm this. Six case-control studies and one cohort study have evaluated the possible association between PFAS and the development of ASD. The results have been highly inconsistent. The studies by Liew et al. (220 with ASD and 550 controls) and Lyall et al. examined prenatal exposure to PFAS and the association with ASD (533 children with ASD and 433 controls) and found no consistent evidence that maternal plasma concentrations of PFAS were associated with an increased risk of ASD in a Danish population [
173,
174]. Long et al. measured PFAS levels in amniotic fluid of 75 children with ASD and 135 controls and found an inverse association between PFAS and risk of ASD, which may be related to the weak estrogenic activities of PFAS [
175]. Skogheim et al. studied 400 children with ASD and 980 controls and confirmed an association between PFOA and ASD in boys [
176].
Oh et al. investigated the association between prenatal exposure to PFAS and an increased risk of autism spectrum disorders. The study included 173 mother-child pairs, with children aged 3 years confirmed to have autism spectrum disorders. Nine PFAS were measured in maternal serum, namely PFOA, PFOS, PFHxS, PFNA, PFDA, PFUnDA, PFDoDA, MeFOSAA, EtFOSAA. Positive associations were found for PFOA and PFNA and an increased risk of autism spectrum disorders, while PFHxS showed negative associations. Given that exposure to individual and combined PFASs had different effects on the risk of ASD depending on the advanced age of the mother at delivery, further study with a larger sample size is needed [
177].
Respiratory Diseases
The term lung function includes respiratory, metabolic, defense and other functions of the lungs. Changes in lung function indicate the risk and severity of clinical symptoms and their impact on quality of life. Children, as a risk population, are more likely to be exposed to PFAS due to higher respiratory rates, interactions with contaminated surfaces (e.g. crawling on the floor), etc. As a result of environmental exposure to PFAS in children, respiratory burden arises, which raises concerns about the rapid development of pulmonary systems [
178].
It is very important to determine the relationship between PFAS exposure and respiratory diseases, such as asthma. Meta-analysis processed bibliographic data from various studies that were dedicated to diagnosing asthma in children under 17 years of age, revealing a connection between PFOA exposure and a higher risk of developing asthma as well as between PFOS and lung function disorders [
179].
Exposure to PFAS in pregnant women can cause respiratory problems in children after birth. Among the main factors that affect lung development in the prenatal period are, for example, premature birth, placental insufficiency, maternal tobacco smoking, etc.
Kung et al. [
180] in their study investigated the relationship between intrauterine and postnatal PFAS exposure and lung function in children. The study used 165 umbilical cord blood plasma and serum samples from 8-year-old children. The average concentrations of PFOA, PFOS, PFNA and PFUA were determined by HPLC-MS/MS. The monitored lung functions were FEV1 (forced expiratory volume in one second), FVC (forced vital capacity), PEF (peak expiratory flow) and FEV1/FVC. The average concentrations of selected analytes were higher in umbilical cord blood than in serum. PFAS were not shown to significantly affect lung function in children. However, they found that PFOS concentrations were significantly inversely correlated with lung function in children with lower birth weight and suffering from allergic rhinitis [
180].
Kidney Diseases
Chronic kidney disease has a high incidence and mortality rate, especially in patients with diabetes and hypertension. PFAS may be hazardous to kidney function. Liang 2023 et al. investigated the relationship between PFOAS, PFOS and Cl-PFESA, heavy metals (cadmium, lead, arsenic) to glomerular filtration rate and chronic kidney disease. PFOA and heavy metals were positively correlated with chronic kidney disease. PFOA, PFOS, CI-PFESA and arsenic were negatively associated with glomerular filtration rate. The study provided epidemiological evidence that Cl-PFESA alone and together with heavy metals may contribute to kidney damage [
181].
A nationwide cross-sectional study was conducted in 13,979 adults in the US to examine the association of serum PFAS with the risk of uric acid and hyperuricemia. Even at low concentrations, the dominant PFOA may have caused an increase in uric acid and an increased risk of hyperuricemia and contributed to a decline in kidney function [
182].
Cardiovascular Diseases
PFAS can play a role in the development of cardiovascular diseases (myocardial infarction, ischemic stroke, heart failure). A Swedish cohort study examined the association between PFAS exposure and cardiovascular disease in 2278 participants aged 45-75 years. PFOA, PFOS, and PFHxS were measured in plasma. A second independent study measured PFHxS, PFOA, linear isomer of PFOS, PFNA, PFDA, and PFUnDA in plasma in 1016 participants aged 70 years. Neither study found an increased risk of cardiovascular disease at slightly elevated levels of PFAS [
183].
Potential associations between PFAS and values of pre-selected proteomic biomarkers in plasma were observed in a Swedish study by Dunder et al. PFOA, PFOS and PFHxS were measured by untargeted metabolomics and 249 proteomic biomarkers in plasma in 2342 participants were measured by proximity extension assay. Epidermal growth factor receptor (EGFR) and paraoxonase type 3 (PON3) levels were positively associated with all three PFAS, while resistin (RETN) and urokinase surface plasminogen activator receptor (uPAR) showed inverse associations with all three PFAS [
184].
Neurodevelopment in Child
PFAS can cross the placenta, and prenatal exposure to PFAS has been associated with neurodevelopmental disorders in children, increased risk of neuropsychological problems, and abnormal behavior in adulthood. The effects of PFAS on infant neurodevelopment were investigated in a study by Zhou et al., which included 1285 mother-infant pairs. PFOS, PFOA, PFHxS, and 62Cl-PFESA were confirmed in more than 90% of samples. Each increase in PFAS concentration resulted in poorer communication domain scores. The decrease in communication domain scores in 6-month-old infants was mainly attributed to the increase in PFOS concentration. The findings indicated that PFAS may adversely affect neurodevelopment in children [
186].
An association between prenatal exposure to legacy PFAS and children’s intelligence and executive functioning was observed in a Canadian cohort study of mothers and infants. An association with child gender was also examined. Plasma concentrations of PFOA, PFOS and PFHxS were measured in the first trimester, and 522 children were assessed for full-scale, performance and verbal IQ using the Wechsler Preschool and Primary Intelligence Scale. Results indicated that each two-fold increase in prenatal exposure to PFOA, PFOS and PFHxS was inversely associated with performance IQ, but only in males. No significant association was found in females [
187].
Cerebral Palsy
It is known that PFAS can be transported to the fetus across the placenta. Thyroid hormone levels may be disrupted during pregnancy. Insufficient hormone levels during a critical stage of brain development can lead to neurodevelopmental disorders such as cerebral palsy (CP), which is one of the most common physical and motor disabilities in childhood. The author Vilhelmsson et al. [
188] investigated the association between prenatal PFAS exposure and the risk of CP. The study included 322 CP cases, 258 premature infants, and 343 controls. Serum samples collected at 10-14 weeks of gestation were obtained from a biobank, and concentrations of PFOA, PFOS, PFNA, and PFHxS were determined. No associations were observed between prenatal exposure to PFAS and the risk of CP, except in premature infants, but the results were not entirely consistent and therefore require further study.
The Swedish study discussed was preceded by only one study that investigated whether prenatal exposure to PFAS increases the risk of congenital cerebral palsy. This Danish study followed 83,389 newborns and mothers, identified 156 CP cases and randomly selected 550 controls. 6 PFAS were identified in more than 90% of samples and 16 PFAS in maternal plasma collected in early and mid-pregnancy. Higher concentrations of mainly PFOS and PFOA in maternal plasma increased the risk of CP in boys. In girls, no association was found between PFAS and CP [
189].
7. Conclusions
PFAS are characterized by high toxicity, stability and slow degradation, which causes long persistence in various components of the environment, from where they accumulate in the human body through plants and animals and cause adverse health effects. The toxicological properties of PFAS in humans are not sufficiently studied due to the large number of compounds. Our review is based on scientific studies to clarify the presence of per- and polyfluoroalkyl substances in various, conventional and unconventional human matrices, such as hair, nails, urine, placenta and breast milk, but especially to health problems caused by PFAS exposure. Exposure to these toxicants has caused a variety of adverse health consequences, therefore, various biomonitoring approaches in humans are necessary. Exposure data, together with modifiable environmental risk factors, are crucial for risk assessment and facilitate the development of new strategies to prevent and mitigate the adverse effects of PFAS.
Author Contributions
All authors have contributed to this review equally. Conceptualization, C.M., M.V.; methodology, C.M., M.V.; software, C.M., M.V.; validation, C.M.,M.V.; formal analysis, C.M., M.V.; investigation, C.M., M.V.; resources, C.M., M.V.; data curation, C.M., M.V.; writing—original draft preparation, C.M., M.V.; writing—review and editing, C.M., M.V.; visualization, C.M., M.V.; supervision, C.M., M.V.; project administration, C.M., M.V.; funding acquisition, C.M., M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alkhadher, S.A.A.; Sidek, L.M.; Zakaria, M.P.; Al-Garadi, M.A.; Suratman, S. Environmental occurrence and assessment of organic pollutants in surface sediments of South Peninsular Malaysia. Environ. Geochem. Health 2024, 46, 140. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Environ. Geochem. Health 2022, 44, 3409. [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Semerjian, L.; Okoh, A.I.; Okoh, O.O. Occurrence and risk assessment of polybrominated diphenyl ethers in surface water and sediment of Nahoon River Estuary. Molecules 2022, 27, 832. [Google Scholar] [CrossRef] [PubMed]
- EU. Directive 2006/122/EC of the European parliament and of the council of 12. Off. J. Eur. Union 2006, L 372/32, 32–34. [Google Scholar]
- UNEP. Decision SC-4/17. Listing of Perfluorooctane Sulfonic Acid, its Salts and Perfluorooctane Sulfonyl Fluoride. UNEP-POPS-COP.4-SC-4-17. Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland, 2009. [Google Scholar]
- UNEP. Decision: SC-9/12. Listing of Perfluorooctanoic Acid (PFOA), its Salts and PFOA-Related Compounds. UNEP-POPS-COP.9-SC-9-12. Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland, 2019. [Google Scholar]
- UNEP. Decision SC-10/13. Perfluorohexane Sulfonic Acid (PFHxS), its Salts and PFHxS-Related Compounds. United Nations Environment Programme (UNEP), ed. SC-10/13. Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland, 2022. [Google Scholar]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513. [Google Scholar] [PubMed]
- Buck, R.C.; Korzeniowski, S.H.; Laganis, E.; Adamsky, F. Identification and classification of commercially relevant per- and poly-fluoroalkyl substances (PFAS). Integr. Environ. Assess. Manag. 2021, 17, 1045. [Google Scholar] [PubMed]
- Lohmann, R.; Letcher, R.J. The universe of fluorinated polymers and polymeric substances and potential environmental impacts and concerns. Curr. Opin. Green Sustain. Chem. 2023, 41, 100795. [Google Scholar] [PubMed]
- Yuan, W.; Song, S.; Lu, Y.; Shi, Y.; Yang, S.; Wu, Q.; Wu, Y.; Jia, D.; Sun, J. Legacy and alternative per-and polyfluoroalkyl substances (PFASs) in the Bohai Bay Rim: Occurrence, partitioning behavior, risk assessment, and emission scenario analysis. Sci.Total Environ. 2024, 912, 168837. [Google Scholar] [CrossRef]
- Kalyn, M.; Lee, H.; Curry, J.; Tu, W.; Ekker, M.; Mennigen, J.A. Effects of PFOS, F-53B and OBS on locomotor behaviour, the dopaminergic system and mitochondrial function in developing zebrafish (Danio rerio). Environ. Pollut. 2023, 326, 121479. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Niu, Z.; Zhang, Y. Legacy per- and polyfluoroalkyl substances (PFASs) and alternatives (short-chain analogues, F-53B, GenX and FC-98) in residential soils of China: present implications of replacing legacy PFASs. Environ. Int. 2020, 135, 105419. [Google Scholar]
- Shi, Y.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, Ch.; Liang, Y.; Cai, Y. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, W.; Wang, L.; Zhang, Y.; Zhang, Y.; Wang, M.; Wang, Y.; Li, P. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicol.Environ. Saf. 2019, 182, 109402. [Google Scholar] [CrossRef] [PubMed]
- Heydebreck, F.; Tang, J.; Xie, Z.; Ebinghaus, R. Alternative and Legacy Perfluoroalkyl Substances: Differences between European and Chinese River/Estuary Systems. Environ. Sci. Technol. 2015, 49, 8386. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.D.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; Sun, M.; Webster, T.F.; Sunderlandl, E.M. PFAS Exposure Pathways for Humans and Wildlife: A Synthesisof Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, N.M.; Minucci, J.M.; Mullikin, A.; Slover, R.; Hubal, E.A.C. Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: A systematic review. Environ. Int. 2022, 162, 107149. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the Applications, Environmental Release, and Remediation Technologies of Per- and Polyfluoroalkyl Substances. Int J Environ Res Public Health 2020, 17, 8117. [Google Scholar] [CrossRef]
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.B.; Goel, R. Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.Ch.E.; Wanninayake, D.; Chen, D.; Nguyen, N.-T.; Li, Q. Physicochemical properties and interactions of perfluoroalkyl substances (PFAS) - Challenges and opportunities in sensing and remediation. Sci. Total Environ. 2023, 5, 166764. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, Ch.R.; Amaku, J.F.; Conradie, J.; Olisah, Ch.; Akpomie, K.G.; Malloum, A.; Akpotu, S.O.; Adegoke, K.A.; Okeke, E.S.; Omotola, E.O. Effect of physicochemical parameters on the occurrence of per- and polyfluoroalkyl substances (PFAS) in aquatic environment. Mar. Pollut. Bull. 2024, 208, 117040. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (pfass) and present understanding of health effects. J. Expo. Sci. Environ.Epidemiol. 2019, 29, 131. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.R.; Klos, K.S.; Greene, Ch.W.; Huset, C.A.; Barry, K.M.; Goeden, H.M. Per- and polyfluoroalkyl substances (PFAS) in powdered infant formula: potential exposures and health risks. J. Environ. Expo. Assess. 2024, 3, 14. [Google Scholar] [CrossRef]
- LaKind, J.S.; Naiman, J.; Verner, M.-A.; Léveque, L.; Fenton, S. Per- and polyfluoroalkyl substances (PFAS) in breast milk and infant formula: A global issue. Environ. Res. 2023, 219, 115042. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci.Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606. [Google Scholar] [CrossRef] [PubMed]
- Nannaware, N.; Mayilswamy, N.; Kandasubramanian, B. PFAS: exploration of neurotoxicity and environmental impact. Environ. Sci. Pollut. Res. Int. 2024, 31, 12815. [Google Scholar] [CrossRef] [PubMed]
- Namazkar, S.; Ragnarsdottir, O.; Josefsson, A.; Branzell, F.; Abel, S.; Abdallah, M.A.-E.; Harrad, S.; .Benskin, J.P. Characterization and dermal bioaccessibility of residual- and listed PFAS ingredients in cosmetic products. Environ. Sci. 2024, 26, 259. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res. 2014, 50, 318. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Jönsson, B.A.G.; Lindh, Ch.H.; Olesen, R.H.; Larsen, A.; Ernst, E.; Kelsey, T.W.; Andersen, C.Y. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci. Total Environ. 2017, 596-597, 97. [Google Scholar] [CrossRef] [PubMed]
- Høyer, B.B; Bondea, J.P.; Tøttenborga, S.S.; Ramlau-Hansend, S.H.; Lindhe, Ch.; Pedersenf, H.S.; Toft, G. Exposure to perfluoroalkyl substances during pregnancy and child behaviour at 5 to 9 years of age. Hormones and Behavior. 2018, 101, 105. [Google Scholar] [CrossRef]
- Bharal, B.; Ruchitha, Ch.; Kumar, P.; Pandey, R.; Rachamalla, M.; Niyogi, S.; Naidu, R.; Kaundal, R.K. Neurotoxicity of per- and polyfluoroalkyl substances: Evidence and future directions. Sci. Total Environ. 2024, 955, 176941. [Google Scholar] [CrossRef] [PubMed]
- Kee, K.H.; Seo, J.I.; Kim, S.M.; Shiea, J.; Yoo, H.H. Per- and polyfluoroalkyl substances (PFAS): Trends in mass spectrometric analysis for human biomonitoring and exposure patterns from recent global cohort studies. Environ. Int. 2024, 194, 109117. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Zhou, Y.; Nyberg, E.; Namazkar, S.; Yongning, W.; Xiao, Q.; Sun, Y.; Zhu, Z.; Bergman, Å.; Benskin, J.P. Emerging per- and polyfluoroalkyl substances (PFAS) in human milk from Sweden and China. Environ. Sci. Process. Impacts 2020, 22, 2023. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Chen, D.; Li, J.; Zhong, Y.; Zhao, Y.; Wu, Y. On-line solid phase extraction–ultra high performance liquid chromatography–quadrupole/Orbitrap high resolution mass spectrometry determination of per- and polyfluoroalkyl substances in human serum. J. Chromatogr. B 2022, 1212, 123484. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Dai, Y.; Zhang, J.; Wang, Z.; Zhang, L.; Xu, S.; Tan, R.; Guo, J.; Qi, X.; Chang, X.; Wu, C.; Zhou, Z. Associations of perfluoroalkyl substances with adipocytokines in umbilical cord serum: A mixtures approach. Environ. Res. 2023, 216, 114654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ruan, Y.; Yuen, C.N.T.; Lin, H.; Yeung, L.W.Y.; Leung, K.M.Y.; Lam, P.K.S. Tracing per- and polyfluoroalkyl substances (PFASs) in the aquatic environment: Target analysis and beyond. Trends Anal. Chem. 2023, 169, 11735. [Google Scholar] [CrossRef]
- Koch, A.; Aro, R.; Wang, T.; Yeung, L.W.Y. Towards a Comprehensive Analytical Workflow for the Chemical Characterisation of Organofluorine in Consumer Products and Environmental Samples. Trends Anal. Chem. 2020, 123, 115423. [Google Scholar] [CrossRef]
- Li, L.; Yu, N.; Wang, X.; Shi, W.; Liu, H.; Zhang, X.; Yang, L.; Pan, B.; Yu, H.; Wei, S. Comprehensive Exposure Studies of Per- and Polyfluoroalkyl Substances in the General Population: Target, Nontarget Screening, and Toxicity Prediction. Environ. Sci. Technol. 2022, 56, 14617. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Harada, K.H.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Yokoyama, K.; Zhu, J.; Harada Sassa, M.; Tsugane, S.; Iwasaki, M. Serum perfluoroalkyl substances and breast breastrisk in Japanese women: A case-control study. Sci. Total Environ. 2020, 800, 149316. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Harada, K.H.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Yokoyama, K.; Zhu, J.; Harada Sassa, M.; Yoshida, T.; Tsugane, S.; Iwasaki, M. Association between serum concentrations of perfluoroalkyl substances and global DNA methylation levels in peripheral blood leukocytes of Japanese women: A crosssectional study. Sci. Total Environ. 2023, 859, 159923. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Ansari, N.G. A review on extraction, analytical and rapid detection techniques of Per /Poly fluoro alkyl substances in different matrices. Microchem. J. 2024, 196, 109667. [Google Scholar] [CrossRef]
- Di Giorgi, A.; Maida, N.L.; Taoussi, O.; Pichini, S.; Busardó, F.P.B.; Tini, A.; Di Trana, A. Analysis of perfluoroalkyl substances (PFAS) in conventional and unconventional matrices: Clinical outcomes. J. Pharm. Biomed. Anal. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Olomukoro, A.A.; Emmons, R.V.; Godage, N.H.; Cudjoe, E.; Gionfriddo, E. Ion exchange solid phase microextraction coupled to liquid chromatography/laminar flow tandem mass spectrometry for the determination of perfluoroalkyl substances in water samples. J. Chromatogr. A. 2021, 1651, 462335. [Google Scholar] [CrossRef] [PubMed]
- Macheka, L.R.; Olowoyo, J.O.; Mugivhisa, L.L.; Abafe, O.A. Determination and assessment of human dietary intake of per and polyfluoroalkyl substances in retail dairy milk and infant formula from South Africa. Sci. Total Environ. 2021, 755, 142697. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.I.; Becanova, J.; Lohmann, R. A sensitive method for the detection of legacy and emerging per- and polyfluorinated alkyl substances (PFAS) in dairy milk. Anal. Bioanal. Chem. 2022, 414, 1235. [Google Scholar] [CrossRef]
- Timshina, A.S.; Robey, N.M.; Oldnettle, A.; Barron, S.; Mehdi, Q.; Cerlanek, A.; Townsend, T.G.; Bowden, J.A. Investigating the sources and fate of per- and polyfluoroalkyl substances (PFAS) in food waste compost. Waste Manag. 2024, 180, 125. [Google Scholar] [CrossRef] [PubMed]
- Drage, D.S; Sharkey, M.; Berresheim, H.; Coggins, M.; Harrad, S. Rapid determination of selected PFAS in textiles entering the waste stream. Toxics 2023, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Beser, M.I.; Sanchís, Y.; Yusá, V.; Coscollá, C. Determination of 21 per-and poly-fluoroalkyl substances in paper- and cardboard-based food contact materials by ultra-high-performance liquid chromatography coupled to high-resolution mass spectrometry. Anal. Methods 2023, 15, 1559. [Google Scholar] [CrossRef]
- Xian, Y.; Liang, M.; Wu, Y.; Wang, B.; Hou, X.; Dong, H.; Wang, L. Fluorine and nitrogen functionalized magnetic graphene as a novel adsorbent for extraction of perfluoroalkyl and polyfluoroalkyl substances from water and functional beverages followed by HPLC-Orbitrap HRMS determination. Sci. Total Environ. 2020, 723, 138103. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Wang, H.; Liu, Y.; Cao, X. New deep eutectic solvent based superparamagnetic nanofluid for determination of perfluoroalkyl substances in edible oils. Talanta 2021, 228, 122214. [Google Scholar] [CrossRef] [PubMed]
- Androulakakis, N.; Alygizakis, G.; Gkotsis, M.C.; Nika, V.; Nikolopoulou, E.; Bizani, E.; Chadwick, E.; Cincinelli, A.; Claßen, D.; Danielsson, S.; Dekker, R.W.R.J.; Duke, G.; Glowacka, N.; Jansman, H.A.H.; Krone, O.; Martellini, T.; Movalli, P.; Persson, S.; Roos, A.; O'Rourke, E.; Siebert, U.; Treu, G.; van den Brink, N.W.; Walker, L.A.; Deaville, R.; Slobodnik, J.; Thomaidis, N.S. Determination of 56 per- and polyfluoroalkyl substances in top predators and their prey from Northern Europe by LC-MS/MS. Chemosphere, 2022; 287, 131775. [Google Scholar]
- Comito, R.; Porru, E.; Violante, F.S. Analytical methods employed in the identification and quantification of per- and polyfluoroalkyl substances in human matrices - A scoping review. Chemosphere 2023, 345, 140433. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Liu, X.; Xie, X.; Zhou, X.; Pan, Y.; Dai, J. Exposure to per- and polyfluoroalkyl substances in early pregnancy, risk of gestational diabetes mellitus, potential pathways, and influencing factors in pregnant women: A nested case-control study. Environ. Pollut. 2023, 326, 121504. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, B.; Nie, S.; Zhao, N.; He, L.; Cui, J.; Mao, W.; Jin, H. Distribution of per- and poly-fluoroalkyl substances and their precursors in human blood. J. Hazard. Mater. 2023, 441, 129908. [Google Scholar] [CrossRef] [PubMed]
- San Román, A.; Abilleira, E.; Irizar, A.; Santa-Marina, L.; Gonzalez-Gay, B.; Etxebarria, N. Optimization for the analysis of 42 per- and polyfluorinated substances in human plasma: A high-throughput method for epidemiological studies. J. Chromatogr. A. 2023, 1712, 464481. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Duan, L.; Dong, B.; Dong, Q.; Liu, Y.; Yu, W.; Yang, L.; Shi, H. Perfluoroalkyl and polyfluoroalkyl substances in cord serum of newborns and their potential factors. Chemosphere 2023, 313, 137525. [Google Scholar] [CrossRef] [PubMed]
- Rokoff, L.B.; Wallenborn, J.T.; Harris, M.H.; Rifas-Shiman, S.L.; Criswell, R.; Romano, M.E.; Young, J.G.f.; Calafati, A.M.; Oken, E.; Sagiv, S.K.; Fleisch, A.F. Plasma concentrations of per- and polyfluoroalkyl substances in pregnancy and breastfeeding duration in Project Viva. Sci. Total Environ. 2023, 891, 164724. [Google Scholar] [CrossRef] [PubMed]
- Hu, Ch.-Y.; Qiao, J.-Ch.; Gui, S.-Y.; Xu, K.-X.; Dzhambov, A.M.; Zhang, X.-J. Perfluoroalkyl and polyfluoroalkyl substances and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Environ. Res. 2023, 231, 116064. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, Y.; Li, J.; Lyu, B.; Liu, J.; Zhang, J.; Zhao, Y.; Wu, Y. Occurrences of legacy and emerging per- and polyfluoroalkyl substances in human milk in China: Results of the third National Human Milk Survey (2017–2020). J. Hazard. Mat. 2023, 443, 130163. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.; Harmouche-Karaki, M.; Matta, J.; Mahfouz, Y.; Salameh, P.; Younes, H.; Helou, K.; Finan, R.; Abi-Tayeh, G.; Meslimani, M.; Moussa, G.; Chahrour, N.; Osseiran, C.; Skaiki, F.; Narbonne, J.-F. Maternal Serum, Cord and Human Milk Levels of Per- and Polyfluoroalkyl Substances (PFAS), Association with Predictors and Effect on Newborn Anthropometry. Toxics 2023, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, X.; Wang, Y.; Guo, H.; Liu, L.; Liu, L.; Gao, J.; He, M. Association between serum per- and polyfluoroalkyl substances levels and metabolic dysfunction-associated steatotic liver disease. Environ. Pollut. 2024, 363, 125233. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Motoki, N.; Inaba, Y.; Toubou, H.; Shibazaki, T.; Nakayama, S.F.; Kamijima, M.; Tsukahara, T.; Nomiyama, T. Maternal Exposure to Per- and Polyfluoroalkyl Substances and Offspring Chromosomal Abnormalities: The Japan Environment and Children's Study. Environ. Health Perspect. 2024, 132, 97004. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Xu, F.; Zhang, X.; Zhao, N.; Ding, L. Associations between serum per- and polyfluoroalkyl substances as mixtures and lipid levels: A cross-sectional study in Jinan. Sci. Total Environ. 2024, 923, 171305. [Google Scholar] [CrossRef] [PubMed]
- Partington, J.M.; Marchiandi, J.; Szabo, D.; Gooley, A.; Kouremenos, K.; Smith, F.; Clarke, B.O. Validating blood microsampling for per- and polyfluoroalkyl substances quantification in whole blood. J. Chromatogr. A. 2024, 1713, 464522. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.E.; Gallagher, L.G.; Price, G.; Crawford, K.A.; Criswell, R.; Baker, E.; Botelho, J.C.; Calafat, A.M.; Karagas, M.R. Plasma per- and polyfluoroalkyl substance mixtures during pregnancy and duration of breastfeeding in the New Hampshire birth cohort study. Int. J. Hyg. Environ. Health 2024, 258, 114359. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zhang, Q.Q.; Wang, Y.; Wang, Ch.; Hu, Ch.; Wang, L.; Liu, H.; Tian, Z. Associations between per- and polyfluoroalkyl substances exposure and thyroid hormone levels in the elderly. Sci. Total Environ. 2024, 920, 170761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gui, J.G.; Howe, C.G.; Emond, J.A.; Criswell, R.L.; Gallagher, L.G.; Huset, C.A.; Peterson, L.A.; Botelho, J.C.; Calafat, A.M.; Christensen, B.; Karagas, M.R.; Romano, M.E. Association of diet with per- and polyfluoroalkyl substances in plasma and human milk in the New Hampshire Birth Cohort Study. Sci. Total Environ. 2024, 933, 173157. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-Y.; Lin, Z.-Y.; Sun, X.-F.; Feng, J.J.; Mai, L.; Wu, Ch.-Ch.; Huang, G.-L.; Wang, P.; Liu, J.-W.; Liu, L.-Y.; Zeng, E.Y. The effect of per and polyfluoroalkyl substance (PFAS) exposure on gestational diabetes mellitus and its subclinical risk factors: A systematic review and meta-analysis protocol. Environ. Int. 2024, 187, 108719. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, P.; Liu, X.; Zhao, J.; Tian, M.; Huang, Q.; Yan, J.; Tong, Z.; Zhang, Y.; Zhang, J.; Zhang, T.; Guo, J.; Liu, G.; Bian, X.; Li, B.; Wang, T.; Wang, H.; Shen, H. Profiles and transplacental transfer of per- and polyfluoroalkyl substances in maternal and umbilical cord blood: A birth cohort study in Zhoushan, Zhejiang Province, China. J. Hazard. Mater. 2024, 466, 133501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, Q.; Chen, H.; Li, Y.; Hua, Y.; Wang, J.; Chen, F.; Zheng, R. A novel high-throughput quantitative method for the determination of per- and poly-fluoroalkyl substances in human plasma based on UHPLC-Q/ Orbitrap HRMS coupled with isotope internal standard. J. Hazard. Mater. 2024, 480, 136138. [Google Scholar] [CrossRef] [PubMed]
- Belay, M.H.; Robotti, E.; Ghignone, A.; Fabbris, A.; Brandi, J.; Cecconi, D.; Masini, M.A.; Dondero, F.; Marengo, E. Sensitive and accurate determination of 32 PFAS in human serum using online SPE-UHPLC-HRMS. J. Hazard. Mater. 2025, 485, 136780. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Sehgal, N.; Salamova, A.; Fiedler, N.; Hood, R.B.; Yakimavets, V.; Promkam, N.; Prapamontol, T.; Suttiwan, P.; Sittiwang, S.; Mangklabruks, A.; Naksen, W.; Panuwet, P.; Barr, D.B. Per- and polyfluoroalkyl substances in paired serum and breastmilk samples among pregnant farmworkers in Thailand. Int. J. Hyg. Environ. Health 2025, 264, 114509. [Google Scholar] [CrossRef] [PubMed]
- Camdzic, D.; Dickman, R.A.; Joyce, A.S.; Wallace, J.S.; Ferguson, P.L.; Aga, D.S. Quantitation of Total PFAS Including Trifluoroacetic Acid with Fluorine NuclearMagnetic Resonance Spectroscopy. Anal. Chem. 2023, 95, 5484–5488. [Google Scholar] [CrossRef] [PubMed]
- Camdzic, D.; Dickman, R.A.; Aga, D.S. Total and class-specific analysis of per-and polyfluoroalkyl substances in environmental samples using nuclear magnetic resonance spectroscopy. J. Hazard. Mater. Lett. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Rehman, A.U.; Crimi, M.; Andreescu, S. Current and emerging analytical techniques for the determination of PFAS in environmental samples. Tren. Environ. Anal. Chem. 2023, 37, e00198. [Google Scholar] [CrossRef]
- Alves, A.; Jacobs, G.; Vanermen, G.; Covaci, A.; Voorspoels, S. New approach for assessing human perfluoroalkyl exposure via hair. Talanta 2015, 144, 574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, M.; Tang, B.; Luo, W.; Ma, Y.; Ren, M.; Yu, Y.; Luo, X.; Mai, B. Levels, spatial distribution, and impact factors of heavy metals in the hair of metropolitan residents in China and human health implications. Environ. Sci. Technol. 2021, 55, 10578. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.M.; Dereumeaux, C.; Guldner, L.; Briand, O.; Vandentorren, S.; Oleko, A.; Zaros, C.; Appenzeller, B.M.R. Hair versus urine for the biomonitoring of pesticide exposure: results from a pilot cohort study on pregnant women. Environ. Int. 2021, 152, 106481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, X.; Tang, B.; Luo, W.; Chen, S.; Luo, X.; Zheng, J.; Mai, B.; Yu, Y. Human hair as a noninvasive matrix to assess exposure to micro-organic contaminants: State of the art review. Sci.Total Environ. 2023, 892, 164341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Vestergren, R.; Zhou, Z.; Liang, Y.; Cai, Y. Using hair, nail and urine samples for human exposure assessment of legacy and emerging per- and polyfluoroalkyl substances. Sci. Total Environ. 2018, 636, 383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Y.; Li, J.; Zhang, J.; Lyu, B.; Zhao, Y.; Wu, Y. Occurence of perfluoroalkyl substances in matched human serum, urine, hair and nail. J. Environ. Sci. 2018, 67, 191. [Google Scholar] [CrossRef]
- Appenzeller, B.M.; Tsatsakis, A.M. Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: state of the art, critical review and future needs. Toxicol. Lett. 2012, 210, 119. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Lalwani, D.; Kwok, K.Y.; Yamazaki, E.; Taniyasu, S.; Kumar, N.J.I.; Lam, P.K.S.; Yamashita, N. Assessing exposure to legacy and emerging per- and polyfluoroalkyl substances via hair - The first nationwide survey in India. Chemosphere 2019, 229, 366. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.; Lefeuvre, S.; Guihenneuc, J.; Cambien, G.; Dupuis, A.; Venisse, N. Analytical methods and biomonitoring results in hair for the assessment of exposure to endocrine-disrupting chemicals: A literature review. Chemosphere 2024, 353, 141523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yana, X.; Tang, B.; Luoa, W.; Chen, S.; Luoc, X.; Zheng, J.; Mai, B.; Yua, Y. Human hair as a noninvasive matrix to assess exposure to micro-organic contaminants: State of the art review. Sci. Total Environ. 2023, 892, 164341. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-F.; Hsu, J.-Y.; Hsiao, P.-Z.; Chou, T.-Ch.; Liao, P.-Ch. Hair specimens in exposome-health research: Opportunities, challenges, and app lications. Trends Anal. Chem. 2024, 178, 117825. [Google Scholar] [CrossRef]
- Piva, E.; Fais, P.; Cecchetto, G.; Montisci, M.; Viel, G.; Pascali, J.P. Determination of perfluoroalkyl substances (PFAS) in human hair by liquid chromatography-high accurate mass spectrometry (LC-QTOF). J. Chromatogr. B 2021, 1172, 122651. [Google Scholar] [CrossRef]
- Karzi, V.; Tzatzarakis, M.N.; Vakonaki, E.; Alegakis, T.; Katsikantami, I.; Sifakis, S.; Rizos, A.; Tsatsakis, A.M. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 2018, 38, 1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Vestergren, R.; Zhou, Z.; Liang, Y.; Cai, Y. Using hair, nail and urine samples for human exposure assessment of legacy and emerging per- and polyfluoroalkyl substances. Sci. Total Environ. 2018, 636, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Exposure assessment to parabens, bisphenol A and perfluoroalkyl compounds in children, women and men by hair analysis. Sci. Total Environ. 2019, 695, 133864. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, Y.; Zheng, T.; Cui, Q.; Buka, S.L.; Zhang, B.; Guo, Y.; Xia, W.; Yeung, L.W.; Li, Y.; Zhou, A.; Qiu, L.; Liu, H.; Jiang, M.; Wu, C.; Xu, S.; Dai, J. Novel Chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: placental transfer and relationship with serum albumin and glomerular filtration rate. Environ. Sci. Technol. 2017, 51, 634. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhuang, T.; Liu, X.; Fu, J.; Zhang, J.; Fu, J.; Wang, L.; Zhang, A.; Liang, Y.; Song, M.; Jiang, G. Prenatal exposure to per- and polyfluoroalkyl substances (PFASs) and association between the placental transfer efficiencies and dissociation constant of serum proteins-PFAS complexes. Environ. Sci. Technol. 2019, 53, 6529. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicol. 2020, 443, 152565. [Google Scholar] [CrossRef]
- Chen, F.; Yin, S.; Kelly, B.C.; Liu, W. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Matched Maternal, Cord, and Placenta Samples: A Study of Transplacental Transfer. Environ. Sci. Technol. 2017, 51, 6387. [Google Scholar] [CrossRef]
- Wang, Y.; Han, W.; Wang, C.; Zhou, Y.; Shi, R.; Bonefeld-Jørgensen, E.C.; Yao, Q.; Yuan, T.; Gao, Y.; Zhang, J.; Tian, Y. Efficiency of maternal-fetal transfer of perfluoroalkyl and polyfluoroalkyl substances. Environ. Sci. Pollut. Res. 2019, 26, 2691. [Google Scholar] [CrossRef]
- Liu, Y.; Lib, A.; Buchananb, S.; Liu, W. Exposure characteristics for congeners, isomers, and enantiomers of perfluoroalkyl substances in mothers and infants. Environ. Int. 2020, 144, 106012. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.J.; Shao, L.-X.; Liu, Y.; Cui, S.-W.; Wang, X.; Lu, G.-L.; Wang, X.; Jin, J.-H. Target analysis and suspect screening of per- and polyfluoroalkyl substances in paired samples of maternal serum, umbilical cord serum, and placenta near fluorochemical plants in Fuxin, China. Chemosphere 2022, 307, 135731. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Breastfeeding. 2022. Available online: https://www.who.int/health -topics/breastfeeding#tab=tab_1 (accessed on 29 May 2022).
- LaKind, J.S.; Verner, M.A.; Rogers, R.D.; Goeden, H.; Naiman, D.Q.; Marchitti, S.A.; Lehmann, G.M.; Hines, E.P.; Fenton, S.E. Current breast milk PFAS levels in the United States and Canada: after all this time, why don’t we know more? Environ. Health Perspect. 2022, 130, 25002. [Google Scholar] [PubMed]
- Rawn, D.F.K.; Dufresne, G.; Clément, G.; Fraser, W.D.; Arbuckle, T.E. Perfluorinated alkyl substances in Canadian human milk as part of the Maternal- Infant Research on Environmental Chemicals (MIREC) study. Sci. Total Environ. 2022, 831, 154888. [Google Scholar] [PubMed]
- Lamichhane, L.; Siljander, H.; Duberg, D.; Honkanen, J.; Virtanen, S.M.; Orešič, M.; Knip, M.; Hyöyläinen, T. Exposure to per- and polyfluoroalkyl substances associates with an altered lipid composition of breast milk. Environ. Int. 2021, 157, 106855. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.M.; Ashley-Martin, J.; Liang, Ch.L.; Papandonatos, G.D.; Arbuckle, T.E.; Borghese, M.M.; Buckley, J.P.; Cecil, K.M.; Chen, A.; Dodds, L.; Fisher, M.; Lanphear, B.P.; Rawn, D.F.K.; Yolton, K.; Braun, J.M. Personal care product use and per- and polyfluoroalkyl substances in pregnant and lactating people in the Maternal-Infant Research on Environmental Chemicals study. Environ. Int. 2024, 193, 109094. [Google Scholar] [CrossRef]
- Bonato, M.; Corrá, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef] [PubMed]
- Manojkumar, Y.; Pilli, S.; Rao, P.V.; Tyagi, R.D. Sources, occurrence and toxic effects of emerging per- and polyfluoroalkyl substances (PFAS). Neurotoxicol. Teratol. 2023, 97, 107174. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Kim, J.H.; Jung, H.W.; Lim, Y.H; Bae, S.; Kho, Y.; Hong, Y.C.; Shin, C.H.; Yang, S.W. The serum concentrations of perfluoroalkyl compounds were inversely associated with growth parameters in 2-year old children. Sci. Total Environ. 2018, 628–629, 226. [Google Scholar] [CrossRef]
- Harris, M.H.; Oken, E.; Rifas-Shiman, S.L.; Calafat, A.M.; Ye, X.; Bellinger, D.C.; Webster, T.F.; White, R.F.; Sagiv, S.K. Prenatal and childhood exposure to per-and polyfluoroalkyl substances (PFASs) and child cognition. Environ. Int. 2018, 115, 358. [Google Scholar] [CrossRef]
- Li, S.; Wang, Ch.; Yang, Ch.; Chen, Y.; Cheng, Q.; Liu, J.; Zhang, Y.; Jin, L.; Li, Z.; Ren, A.; Wang, L. Prenatal exposure to poly/perfluoroalkyl substances and risk for congenital heart disease in offspring. J. Hazard. Mater. 2024, 469, 134008. [Google Scholar] [CrossRef]
- Huang, H.; Yu, K.; Zeng, X.; Chen, Q.; Liu, Q.; Zhao, Y.; Zhang, J.; Zhang, X.; Huang, L. Association between prenatal exposure to perfluoroalkyl substances and respiratory tract infections in preschool children. Environ. Res. 2020, 191, 110156. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Z.; Li, M.; Dong, H.; Li, J. Reproductive toxicity of PFOA, PFOS and their substitutes: A review based on epidemiological and toxicological evidence. Environ. Res. 2024, 250, 118485. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Environmental impacts, exposure pathways, and health effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. 2024. Available online: https://www.cdc.gov/index.html.
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls. 2021. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 23 June 2022).
- Ho, S.H.; Soha, S.X.H.; Wang, M.X.; Ong, J.; Seah, A.; Wong, Y.; Fang, Z.; Sima, S.; Lim, J.T. Perfluoroalkyl substances and lipid concentrations in the blood: A systematic review of epidemiological studies. Sci. Total Environ. 2022, 850, 158036. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Sun, Q.; Grandjean, P. PFAS concentration during pregnancy in relation to cardiometabolic health and birth outcomes. Environ. Res. 2021, 192, 110287. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.; Du, H.; Fang, H.; Han, M.; Xu, L.; Liu, S.; Yi, J.; Chen, Y.; Jiang, Q.; He, G. Serum perfluoroalkyl substances in relation to lipid metabolism in Chinese pregnant women. Chemosphere 2021, 273, 128566. [Google Scholar] [CrossRef] [PubMed]
- Zuanna, T.D.; Savitz, D.A.; Barbieri, G.; Pitter, G.; Jeddi, M.Z.; Daprá, F.; Fabricio, A.S.C.; Russo, F.; Fletcher, T.; Canova, C. The association between perfluoroalkyl substances and lipid profile in exposed pregnant women in the Veneto region, Italy. Ecotoxicol. Environ. Saf. 2021, 209, 111805. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Sun, H.; Yao, Y.; Li, Y.; Meng, Y.; Lub, Y.; Han, L.; Chenc, L. Serum concentrations of per-/polyfluoroalkyl substances and risk of type 2 diabetes: A case-control study. Sci. Total Environ. 2021, 787, 147476. [Google Scholar] [CrossRef] [PubMed]
- Xu, Ch.; Zhang, L.; Zhoua, Q.; Ding, J.; Yin, S.; Shang, X.; Tian, Y. Exposure to per- and polyfluoroalkyl substances as a risk factor for gestational diabetes mellitus through interference with glucose homeostasis. Sci. Total Environ. 2022, 838, 156561. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.-H. Associations between serum perfluoroalkyl and polyfluoroalkyl concentrations and diabetes mellitus in the Korean general population: Insights from the Korean National Environmental Health Survey 2018–2020. Int. J. Hyg. Environ. Health 2024, 259, 114385. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, Q.; Zhang, J.; Chen, X.; Zhao, H.; Lu, H.; Ma, B.; Wang, Z.; Wu, Ch.; Ying, Ch.; Xiong, Y.; Zhou, Z.; Li, X. Exposure to elevated per- and polyfluoroalkyl substances in early pregnancy is related to increased risk of gestational diabetes mellitus: A nested casecontrol study in Shanghai, China. Environ. Int. 2020, 143, 105952. [Google Scholar] [CrossRef] [PubMed]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hong, X.; Zhao, F.; Wu, J.; Wang, B. The effects of perfluoroalkyl and polyfluoroalkyl substances on female fertility: A systematic review and meta-analysis. Environ. Res. 2023, 216, 114718. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, Q.; Zhang, L.; Li, W.; Yin, S.; Li, F.; Xu, Ch. In utero exposure to per-/polyfluoroalkyl substances (PFASs): Preeclampsia in pregnancy and low birth weight for neonates. Chemosphere 2023, 313, 137490. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Eatman, J.A.; Dunlop, A.L.; Barr, D.B.; Kannan, K.; Corwin, E.K.; Ryan, P.B.; Panuwet, P.; Yakimavets, V.; Taibl, K.R.; Tan, Y.; Liang, D.; Eick, S.M. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with hypertensive disorders of pregnancy in the Atlanta African American Maternal-Child cohort. Chemosphere 2024, 357, 142052. [Google Scholar] [CrossRef]
- Zhang, Y.; Martin, L.; Mustieles, V.; Ghaly, M.; Archer, M.; Sun, Y.; Torres, N.; Coburn-Sanderson, A.; Souter, I.; Petrozza, J.C.; Botelho, J.C; Calafat, A.M.; Wang, Y.-X.; Messerlian, C. Per- and polyfluoroalkyl substances exposure is associated with polycystic ovary syndrome risk among women attending a fertility clinic. Hum. Reprod. 2024, 39, deae108.923. [Google Scholar] [CrossRef]
- Zhan, W.; Qiu, W.; Ao, Y.; Zhou, W.; Sun, Y.; Zhao, H.; Zhang, J. Environmental Exposure to Emerging Alternatives of Per- and Polyfluoroalkyl Substances and Polycystic Ovarian Syndrome in Women Diagnosed with Infertility: A Mixture Analysis. Environ. Health Perspect. 2023, 131, 057001-1. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Feng, Y.; Shi, Y.; Xiao, J.; Liu, M.; Wang, K. Per- and Polyfluoroalkyl Substances (PFASs) and Their Potential Effects on Female Reproductive Diseases. Toxics 2024, 12, 539. [Google Scholar] [CrossRef]
- Du, X.; Wu, Y.; Tao, G.; Xu, J.; Du, Z.; Wu, M.; Gu, T.; Xiong, J.; Xiaoe, S.; Wei, X.; Ruan, Y.; Xiao, P.; Zhang, L.; Zheng, W. Association between PFAS exposure and thyroid health: A systematic review and meta-analysis for adolescents, pregnant women, adults and toxicological evidence. Sci. Total Environ. 2024, 953, 175958. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, L.M.; Doherty, B.T.; Gallagher, L.G.; Zoeller, R.T.; Hoofnagle, A.N.; Calafat, A.M.; Karagas, M.R.; Yolton, K.; Cheng, A.; Lanphearh, B.P.; Braun, J.M.; Romano, M.E. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Environ. Res. 2020, 185, 109395. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, X.; Xu, P.; Sheng, J.; Lou, X.; Chen, Z.; Wu, L.; Xiang, J.; Cheng, P.; Xu, D.; Chen, Y.; Chen, G.; Wang, X. Associations of per- and polyfluoroalkyl substances and alternatives with subclinical hypothyroidism in children: A cross-sectional study in China. Sci. Total Environ. 2024, 957, 177809. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.J.; Mondal, D.; Armstrong, B.; Bloom, M.S.; Fletcher, T. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ. Health Perspect. 2012, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Leonardi, G.; Genser, B.; Lopez-Espinosa, M.J.; Frisbee, S.J.; Karlsson, L.; Ducatman, A.M.; Fletcher, T. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population. Environ. Health Perspect. 2012, 120, 655. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, Z.; Miao, M.; Tian, Y.; Zhou, Y.; Wen, S.; Chen, Y.; Sun, X.; Yuan, W. Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environ. Health 2020, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, Z.; Wang, J.; Qu, Y.; Hu, Q.; Ji, S.; Chang, X.; Zhao, F.; Lv, Y.; Pan, Y.; Shi, X.; Dai, J. Associations between serum per- and polyfluoroalkyl substances and thyroid hormones in Chinese adults: a nationally representative cross-sectional study. Environ. Int. 2024, 184, 108459. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wen, L.L.; Lin, L.Y.; Wen, T.W.; Lien, G.W.; Hsu, S.H.; Chien, K.L.; Liao, C.C.; Sung, F.C.; Chen, P.C.; Su, T.C. The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. J. Hazard. Mater. 2013, 244-245, 637. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Vela-Soria, F.; Castiello, F.; Salamanca-Fernandez, E.; Quesada-Jimenez, R.; Lopez-Alados, M.C.; Fernandez, M.F.; Olea, N. Exposure to perfluoroalkyl substances (PFAS) and association with thyroid hormones in adolescent males. Int. J. Hyg. Environ. Health 2023, 252, 114219. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011-2012. Int. J. Environ. Res. Public Health 2015, 12, 6098. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-N.; Wang, X.-Ch.; Su, L.-Q.; Ji, S.-S.; Gu, W.; Barrett, H.; Dong, X.-J.; Zhu, H.-J.; Hou, S.-S.; Li, Z.-H.; Liu, Y.-L.; Zhang, L.; Zhu, Y. The association between per-/polyfluoroalkyl substances in serum and thyroid function parameters: A cross-sectional study on teenagers living near a Chinese fluorochemical industrial plant. Sci. Total Environ. 2024, 920, 170985. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, Y.; Qin, L.; Zeng, X.; Jiang, H.; Liang, Y.; Wen, S.; Li, X.; Huang, S.; Li, Ch.; Luo, X.; Yang, X. Serum metabolome associated with novel and legacy per- and polyfluoroalkyl substances exposure and thyroid cancer risk: A multi-module integrated analysis based on machine learning. Environ. Int. 2025, 195, 109203. [Google Scholar] [CrossRef] [PubMed]
- Maerten, A.; Callewaert, E.; Sanz-Serrano, J.; Devisscher, L.; Vinken, M. Effects of per- and polyfluoroalkyl substances on the liver: Human-relevant mechanisms of toxicity. Sci. Total Environ. 2024, 954, 176717. [Google Scholar] [CrossRef] [PubMed]
- Omoike, O.E.; Pack, R.P.; Mamudu, H.M.; Liu, Y.; Strasser, S.; Zheng, S.; Okoro, J.; Wang, L. Association between per and polyfluoroalkyl substances and markers of inflammation and oxidative stress. Environ. Res. 2021, 196, 110361. [Google Scholar] [CrossRef]
- Jin, R.; McConnell, R.; Conti, D.; Grazuleviciene, R.; Slama, R.; Thomsen, C.; Wright, J.; Vos, M.; Vrijheid, M.; Chatzi, L. Prenatal exposure to perfluoroalkyl substances and child liver injury. Environ. Epidemiol. 2019, 3, 59. [Google Scholar]
- Liu, J.J.; Cui, X.X.; Tan, Y.W.; Dong, P.X.; Ou, Y.Q.; Li, Q.Q.; Chu, C.; Wu, L.Y.; Liang, L.X.; Qin, S.J.; Zeeshan, M.; Zhou, Y.; Hu, L.W.; Liu, R.Q.; Zeng, X.W.; Dong, G.H.; Zhao, X.M. Per- and perfluoroalkyl substances alternatives, mixtures and liver function in adults: a community-based population study in China. Environ. Int. 2022, 163, 107179. [Google Scholar] [CrossRef] [PubMed]
- Nian, M.; Li, Q.-Q.; Bloom, M.; Qian, Z.; Syberg, K.M.; Vaughn, M.G.; Wang, S.-Q.; Wei, Q.; Zeeshan, M.; Gurram, N.; Chu, C.; Wang, J.; Tian, Y.-P.; Hu, L.-W.; Liu, K.-K.; Yang, B.-Y.; Liu, R.-Q.; Feng, D.; Zeng, X.-W.; Dong, G.-H. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: isomers of C8 Health Project in China. Environ. Res. 2019, 172, 81. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Caligiuri, I.; Sfriso, A.A.; Mauceri, M.; Rotondo, R.; Campagnol, D.; Canzonieri, V.; Rizzolio, F. Early warnings by liver organoids on short- and long-chain PFAS toxicity. Toxics 2022, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Su, W.; Li, H.; Li, L.; An, Z.; Xiao, F.; Liu, Y.; Zhang, X.; Liu, X.; Guo, H.; Li, A. Association of per- and polyfluoroalkyl substances with hepatic steatosis and metabolic dysfunction-associated fatty liver disease among patients with acute coronary syndrome. Ecotoxicol. Environ. Saf. 2023, 264, 115473. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ling, X.; He, S.; Cui, H.; Yang, Z.; An, H.; Wang, L.; Zou, P.; Chen, Q.; Liu, J.; Ao, L.; Cao, J. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: An integrated approach. Environ. Int. 2023, 178, 108138. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Soh, S.X.H.; Wang, M.X.; Ong, J.; Seah, A.; Wong, Y.; Fang, Z.; Sim, S.; Lim, J.T. Perfluoroalkyl substances and lipid concentrations in the blood: a systematic review of epidemiological studies. Sci. Total Environ. 2022, 850, 158036. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ouyang, T.; Liu, H.; Cao, L.; Chen, W. Perfluoroalkyl substance (PFAS) exposure and risk of nonalcoholic fatty liver disease in the elderly: results from NHANES 2003–2014. Environ. Sci. Pollut. Res. 2023, 30, 64342. [Google Scholar] [CrossRef]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; Vos, M.B.; Chatzi, L. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: An untargeted metabolomics approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef] [PubMed]
- Maerten, A.; Callewaert, E.; Sanz-Serrano, J.; Devisscher, L.; Vinken, M. Effects of per- and polyfluoroalkyl substances on the liver: Human-relevant mechanisms of toxicity. Sci. Total Environ. 2024, 954, 176717. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Peng, L.; Li, Y.; Li, Z.; Chen, D.; Wang, F.; Lin, N. Distribution of per- and polyfluoroalkyl substances in blood, serum, and urine of patients with liver cancer and associations with liver function biomarkers. J. Environ. Sci. 2024, 139, 418. [Google Scholar] [CrossRef] [PubMed]
- Erinc, A.; Davis, M.B.; Padmanabhan, V.; Langen, E.; Goodrich, J.M. Considering environmental exposures to per- and polyfluoroalkyl substances (PFAS) as risk factors for hypertensive disorders of pregnancy. Environ. Res. 2021, 197, 111113. [Google Scholar] [CrossRef]
- Yang, L.; Ji, H.; Liang, H.; Yuan, W.; Song, X.; Li, X.; Niu, J.; Shi, H.; Wen, S.; Miao, M. Associations of perfluoroalkyl and polyfluoroalkyl substances with gestational hypertension and blood pressure during pregnancy: A cohort study. Environ. Res. 2022, 215, 114284. [Google Scholar] [CrossRef] [PubMed]
- Preston, E.V.; Hivert, M.-F.; Fleisch, A.F.; Calafat, A.M.; Sagiv, S.K.; Perng, W.; Rifas-Shiman, S.L.; Chavarro, J.E.; Oken, E.; Zota, A.R.; James-Todd, T. Early-pregnancy plasma per- and polyfluoroalkyl substance (PFAS) concentrations and hypertensive disorders of pregnancy in the Project Viva cohort. Environ. Int. 2022, 165, 107335. [Google Scholar] [CrossRef] [PubMed]
- Zahm, S.; Bonde, J.P.; Chiu, W.A.; Hoppin, J.; Kanno, J.; Abdallah, M.; Blystone, C.R.; Calkins, M.M.; Dong, G.H.; Dorman, D.C.; Fry, R.; Guo, H.; Haug, L.S.; Hofmann, J.N.; Iwasaki, M.; Machala, M.; Mancini, F.R.; Maria-Engler, S.S.; Moller, P.; Ng, J.C.; Pallardy, M.; Post, G.B.; Salihovic, S.; Schlezinger, J.; Soshilov, A.; Steenland, K.; Steffensen, I.L.; Tryndyak, V.; White, A.; Woskie, S.; Fletcher, T.; Ahmadi, A.; Ahmadi, N.; Benbrahim-Tallaa, L.; Bijoux, W.; Chittiboyina, S.; de Conti, A.; Facchin, C.; Madia, F.; Mattock, H.; Merdas, M.; Pasqual, E.; Suonio, E.; Viegas, S.; Zupunski, L.; Wedekind, R.; Schubauer-Berigan, M.K. Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid. Lancet Oncol. 2024, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Report on the 2016 U.S. Environmental Protection Agency (EPA) International Decontamination Research and Development Conference. Available online: https://www.epa.gov/laws-regulations/regulations.
- Zheng, J.; Liu, S.; Yang, J.; Zheng, S.; Sun, B. Per- and polyfluoroalkyl substances (PFAS) and cancer: Detection methodologies, epidemiological insights, potential carcinogenic mechanisms, and future perspectives. Sci. Total Environ. 2024, 953, 176158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, N.; Dai, C.; Xu, J.; Zhang, Y.; Xu, M.; Wang, F.; Li, Y.; Chen, D. Occurrence and distribution of per- and polyfluoroalkyl substances (PFASs) in human livers with cancer. Environ. Res. 2021, 202, 111775. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Barry, K.H.; Huang, W.-Y.; Sampson, J.N.; Hofmann, J.N.; Silverman, D.T.; Calafat, A.M.; Botelho, J.C.; Kato, K.; Purdue, M.P.; Berndt, S.I. A prospective nested case-control study of serum concentrations of per- and polyfluoroalkyl substances and aggressive prostate cancer risk. Environ. Res. 2023, 228, 115718. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shi, J.; Gao, X.; He, L.; Huang, H.; Zhao, G.; Wu, G.; Yu, T.; An, Q.; Mai, L.; Chen, G. Associations of exposure to per- and polyfluoroalkyl substances mixture with the numbers of lymph nodes in colorectal cancer patients. Environ. Res. 2024, 240, 117529. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Bai, Y.; Li, Y.; Zheng, S.; Wang, M.; Wang, Z.; Sun, J.; Zhang, D.; Yin, Ch.; Ma, Li.; Lu, Y.; Zhang, L.; Chen, R.; Cheng, Z. Perfluoroalkyl substances exposure and the risk of breast cancer: A nested case-control study in Jinchang Cohort. Environ. Res. 2024, 262, 119909. [Google Scholar] [CrossRef] [PubMed]
- Tateo, V.; Thompson, Z.J.; Gilbert, S.M.; Cortessis, V.K.; Daneshmand, S.; Masterson, T.A.; Feldman, D.R.; Pierorazio, P.M.; Prakash, G.; Heidenreich, A.; Albers, P.; Necchi, A.; Spiess, P.E. Epidemiology and Risk Factors for Testicular Cancer: A Systematic Review. Eur. Urol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-N.; Hu, Y.-H.; Xu, T.-T.; Luan, Y.-L.; Zeng, L.-X.; Zhang, Z.-F.; Guo, Y. Exposure to per- and polyfluoroalkyl substances in lung cancer patients and their associations with clinical health indicators. Environ. Pollut. 2024, 350, 123995. [Google Scholar] [CrossRef]
- Hanvoravongchai, J.; Laochindawat, M.; Kimura, Y.; Mise, N.; Ichihara, S. Clinical, histological, molecular, and toxicokinetic renal outcomes of per-/polyfluoroalkyl substances (PFAS) exposure: Systematic review and meta-analysis. Chemosphere 2024, 368, 143745. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Hu, Y.; Niu, Q.; Yan, Y. Associations between co-exposure to per- and polyfluoroalkyl substances and metabolic diseases: The mediating roles of inflammation and oxidative stress. Sci. Total Environ. 2024, 953, 176187. [Google Scholar] [CrossRef] [PubMed]
- Averina, M.; Brox, J.; Huber, S.; Furberg, A.-S. Exposure to perfluoroalkyl substances (PFAS) and dyslipidemia, hypertension and obesity in adolescents. The Fit Futures study. Environ. Res. 2021, 195, 110740. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.D.; Yao, P.; Vaughn, M.G.; Qian, Z. PFAS exposure and overweight/obesity among children in a nationally representative sample. Chemosphere 2021, 268, 128852. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Liew, Z.; Ritz, B.; von Ehrenstein, O.S.; Bech, B.H.; Nohr, E.A.; Fei, Ch.; Bossi, R.; Henriksen, T.B.; Bonefeld-Jørgensen, E.C.; Olsen, J. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish National Birth Cohort. Environ. Health Perspect. 2015, 123, 367. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Yau, V.M.; Hansen, R.; Kharrazi, M.; Yoshida, C.K.; Calafat, A.M.; Windham, G.; Croen, L.A. Prenatal Maternal Serum Concentrations of Per- and Polyfluoroalkyl Substances in Association with Autism Spectrum Disorder and Intellectual Disability. Environ. Health Persp. 2018, 126, 017001-1. [Google Scholar] [CrossRef]
- Long, M.; Ghisari, M.; Kjeldsen, L.; Wielsøe, M.; Nørgaard-Pedersen, B.; Mortensen, E.L.; Abdallah, M.W.; Bonefeld-Jørgensen, E.C. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol. Autism 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Skogheim, T.S.; Weyde, K.V.F.; Aase, H.; Engel, S.M.; Sur´en, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Brantsæter, A.L.; Haug, L.S.; Sabaredzovic, A.; Auyeung, B.; Villanger, G.D. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environ. Res. 2021, 202, 111692. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Roa, D.L.; Schmidt, R.J.; Hertz-Picciotto, I.; Shin, H.-M. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ. Int. 2021, 147, 106328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, T.; Zhou, R.; Yang, C.; Huang, Q.; Cheng, S. Impact of polyfluoroalkyl chemicals and volatile organic compounds exposure on lung function of American adults. Environ. Pollut. 2024, 363, 125152. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, A.; Faridi, S.; Sly, P.D.; Stone, L.; Kennedy, L.P.; Mahabee-Gittens, E.M. Asthma and decreased lung function in children exposed to perfluoroalkyl and polyfluoroalkyl substances (PFAS): An updated meta-analysis unveiling research gaps. Environ. Res. 2024, 262, 119827. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.-P.; Lin, Ch.-Ch.; Chen, M.-H.; Tsai, M.-S.; Hsieh, W.-S.; Chen, P.-Ch. Intrauterine exposure to per- and polyfluoroalkyl substances may harm children’s lung function development. Environ. Res. 2021, 192, 110178. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-X.; Dong, P.; Zhou, Y.; Zhang, L.; Qian, Z.; Geiger, S.D.; Bingheim, E.; Tang, X.; Wu, Y.; Lv, J.d.; Lin, L.-Z.; Zeeshan, M.; Zeng, X.-W.; Feng, W.; Dong, G.-H. Joint effects of per- and polyfluoroalkyl substance alternatives and heavy metals on renal health: A community-based population study in China. Environ. Res. 2023, 219, 115057. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Duan, Z.; He, W.; Chen, T.; Tang, H.; Du, S.; Sun, J.; Chen, H.; Hu, Y.; Iijima, Y.; Han, S.; Li, J.; Zhao, Z. Kidney function decline mediates the adverse effects of per- and poly-fluoroalkyl substances (PFAS) on uric acid levels and hyperuricemia risk. J. Hazard. Mat. 2024, 471, 134312. [Google Scholar] [CrossRef] [PubMed]
- Dunder, L.; Salihovic, S.; Varotsis, G.; Lind, P.M.; Elmståhl, S.; Lind, L. Plasma levels of per- and polyfluoroalkyl substances (PFAS) and cardiovascular disease – Results from two independent population-based cohorts and a meta-analysis. Environ. Int. 2023, 181, 108250. [Google Scholar] [CrossRef] [PubMed]
- Dunder, L.; Salihovic, S.; Lind, P.M.; Elmståhl, S.; Lind, L. Plasma levels of per- and polyfluoroalkyl substances (PFAS) are associated with altered levels of proteins previously linked to inflammation, metabolism and cardiovascular disease. Environ. Int. 2023, 177, 107979. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Zhou, J.; Marquess, K.M.; Lanphear, B.P.; Cecil, K.M.; Chen, A.; Sears, C.G.; Xu, Y.; Yolton, K.; Kalkwarf, H.J.; Braun, J.M.; Kuiper, J.R. Per- and polyfluoroalkyl substances and bone mineral content in early adolescence: Modification by diet and physical activity. Environ. Res. 2024, 252, 118872. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, O.; Wang, P.; Li, J.; Zhao, W.; Zhang, L.; Wang, H.; Cheng, Y.; Shi, H.; Li, J.; Zhang, Y. Associations of prenatal PFAS exposure and early childhood neurodevelopment: Evidence from the Shanghai Maternal-Child Pairs Cohort. Environ. Int. 2023, 173, 107850. [Google Scholar] [PubMed]
- Goodman, C.V.; Till, Ch.; Green, R.; El-Sabbagh, J.; Arbuckle, T.Y.; Hornung, R.; Lanphear, B.; Seguin, J.R.; Booij, L.; Fisher, M.; Muckle, G.; Bouchard, M.F.; Ashley-Martin, J. Prenatal exposure to legacy PFAS and neurodevelopment in preschool-aged Canadian children: The MIREC cohort. Neurotoxicol. Teratol. 2023, 98, 107181. [Google Scholar] [CrossRef]
- Vilhelmsson, A.; Rylander, L.; Jöud, A.; Lindh, Ch.H.; Mattsson, K.; Liew, Z.; Guo, P.; Ritz, B.; Källén, K.; Thacher, J.D. Exposure to per- and polyfluoroalkyl substances in early pregnancy and risk of cerebral palsy in children. Sci. Total Environ. 2023, 899, 165622. [Google Scholar]
- Liew, Z.; Ritz, B.; Bonefeld-Jørgensen, E.C.; Henriksen, T.B.; Nohr, E.A.; Bech, B.H.; Fei, Ch.; Bossi, R.; von Ehrenstein, O.S.; Streja, E.; Uldall, P.; Olsen, J. Prenatal Exposure to Perfluoroalkyl Substances and the Risk of Congenital Cerebral Palsy in Children. Am J Epidemiol. 2014, 180, 574. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).