1. Introduction
HIV remains a major global public health challenge, with approximately 39.9 million people living with HIV (PLWH), 1.3 million new infections, and 630,000 deaths reported in 2023 [
1]. The Joint United Nations Programme on HIV/AIDS (UNAIDS) aims to end AIDS by 2030 through the 95-95-95 targets: 95% of PLWH identified, 95% on ART, and 95% achieving viral suppression [
2]. Despite advancements in treatment and prevention through highly active antiretroviral therapy (ART), its effectiveness relies heavily on patient adherence [
3,
4,
5]. High adherence to ART is essential for achieving undetectable viral loads, improving immune function, and reducing HIV transmission [
6,
7,
8]. Research indicates that Black or African Americans face more significant barriers to adherence and HIV care compared to White counterparts[
9,
10], further compounding health disparities. In addition, a critical factor in the success of ART is ensuring appropriate drug exposure, which can be influenced by various factors, including drug-drug interactions, physiological changes during pregnancy, altered drug absorption in certain populations such as children, adolescents, and individuals with malabsorption disorders, as well as organ dysfunction [
11,
12,
13,
14,
15]. Maintaining appropriate drug levels is essential for ART effectiveness. Supratherapeutic drug levels can lead to toxic reactions, while subtherapeutic levels may compromise treatment efficacy and contribute to viral resistance. Inadequate ART exposure, whether due to poor adherence or altered drug metabolism, not only harms individual health but also facilitates the development and transmission of drug-resistant HIV strains, posing risks to public health [
16,
17]. Therapeutic drug monitoring (TDM), which involves the measurement of medication concentrations in biological fluids, offers a promising approach to optimize ART by guiding dose adjustments in these populations and ensuring treatment outcomes and toxic reaction management in PLWH [
18,
19,
20].
Despite TDM’s potential benefits in improving treatment outcome and toxic reaction management, its implementation in clinical practice encounters several significant challenges. Healthcare providers often face time constraints and limited resources and facility capabilities, which impede their ability to effectively incorporate TDM into patient care [
21]. Additionally, there is a recognized need for increased education and training among providers regarding managing medication adherence, including the utilization of TDM. Recent studies have highlighted these barriers, for instance, technological and logistical constraints in adopting TDM [
22,
23], critical gaps in provider training [
23,
24] and guideline availability [
25]. However, concerns about the accurate interpretation of the results underscore the need for standard TDM protocol and training [
26,
27]. Despite these advancements, there remains a limited understanding of HIV providers’ attitudes and perceived barriers toward ART TDM. Assessing provider perspectives is essential for informing the development of effective research initiatives and implementation strategies.
Therefore, this study aims to investigate the attitudes and perceived barriers of HIV care providers toward the implementation of TDM for ART treatment. We hope to provide actionable insights for developing targeted interventions, such as protocols, provider training, and technological advancements. Ultimately, the findings aim to guide the integration of TDM into routine clinical practice, fostering improved adherence outcomes and advancing the personalized care of individuals living with HIV.
2. Materials and Methods
This study was a cross-sectional study consisting of a one-time electronic survey conducted in February 2024 among infectious disease providers caring for people living with HIV in Houston, Texas.
2.1. Study Design
A 15-question survey was adapted from similar studies based on a literature review [
28,
29] and conducted by an online survey (Qualtrics survey tool) [
30]. The questionnaire was first piloted among two pharmacy students at Texas Southern University. After 1 round of modification, the questions were finalized. An email invitation to complete the survey was distributed to infectious disease providers in the Greater Houston Area, including physicians, pharmacists, and mid-level practitioners involved in HIV patient care. The survey was disseminated via the Houston Citywide Infectious Diseases Provider Network and the Houston AIDS Education and Training Center. The survey employed close-ended questions to evaluate providers’ attitudes, perceptions, practices, and barriers related to medical research. A Likert 5-point scale ranging from strongly disagree to strongly agree was used to specify the level of agreement or disagreement with attitudes and barriers to using TDM. Additionally, demographic characteristics and information regarding research involvement were collected to contextualize the findings. All responses were anonymous. The study was registered with and approved by the Texas Southern University Institutional Review Board (#1821A). Consent for participation was obtained through the online survey tool’s starting page, where individuals received background information on the survey.
2.2. Statistical Analysis (Cecilia: Please Review and Revise This Section)
The overall survey results are presented using descriptive statistics. All variables were categorical and expressed as frequencies and percentages. The data collected using a 5-point Likert scale were analyzed quantitatively by assigning numerical values to each point on the scale (e.g., 5 for "strongly agree" and 1 for "strongly disagree"). When the field "other" provided the option to enter free text, answers were coded and sorted where possible. Only completed responses were analyzed, and the number of answers to each question was counted. The data were analyzed using SAS software (version 9.4).
3. Results
3.1. Demographic Characteristics
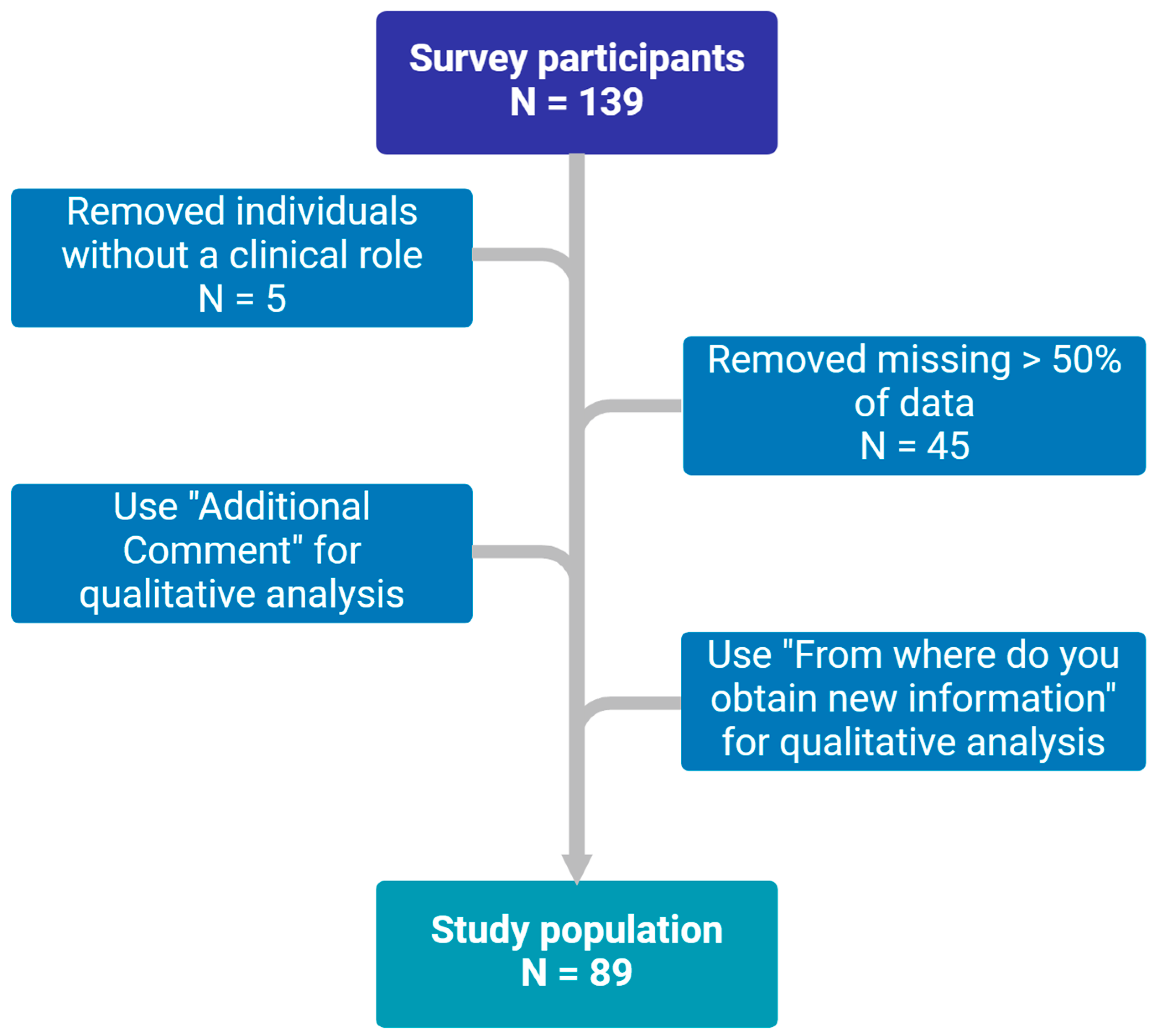
Responses were received from 139 participants, of whom 89 met the inclusion criteria, as summarized in
Figure 1. The baseline characteristics of the participants are presented in
Table 1. Most were female (62.9%), and nearly half (53.4%) were 34 or younger. More than half of the participants (52.3%) worked in hospitals, 35.2% in clinics, 10.2% in academia, and 2.3% in public health departments. The sample reflected diverse professional roles, with 50% physicians, 36.4% pharmacists, and 10.2% mid-level practitioners such as nurse practitioners and physician assistants.
In terms of experience in infectious disease practice, approximately one-third of participants (34.8%) were still in training, 36.0% had 0–5 years of experience, 11.2% had 6–10 years, 10.1% had 11–19 years, and 7.9% had over 20 years of experience. Regarding their patient populations, 56.2% reported that more than 10% of their patients were living with HIV, with 27.0% indicating that over 50% of their patient population consisted of individuals with HIV. Monthly encounters with HIV patients varied, with 60.7% seeing more than five HIV patients and 21.4% managing more than 30. Viral suppression rates among patients on ART were noteworthy, with 21.4% achieving suppression rates exceeding 95%. However, 67.4% of participants lacked access to TDM for antiretrovirals.
3.2. Attitude About TDM
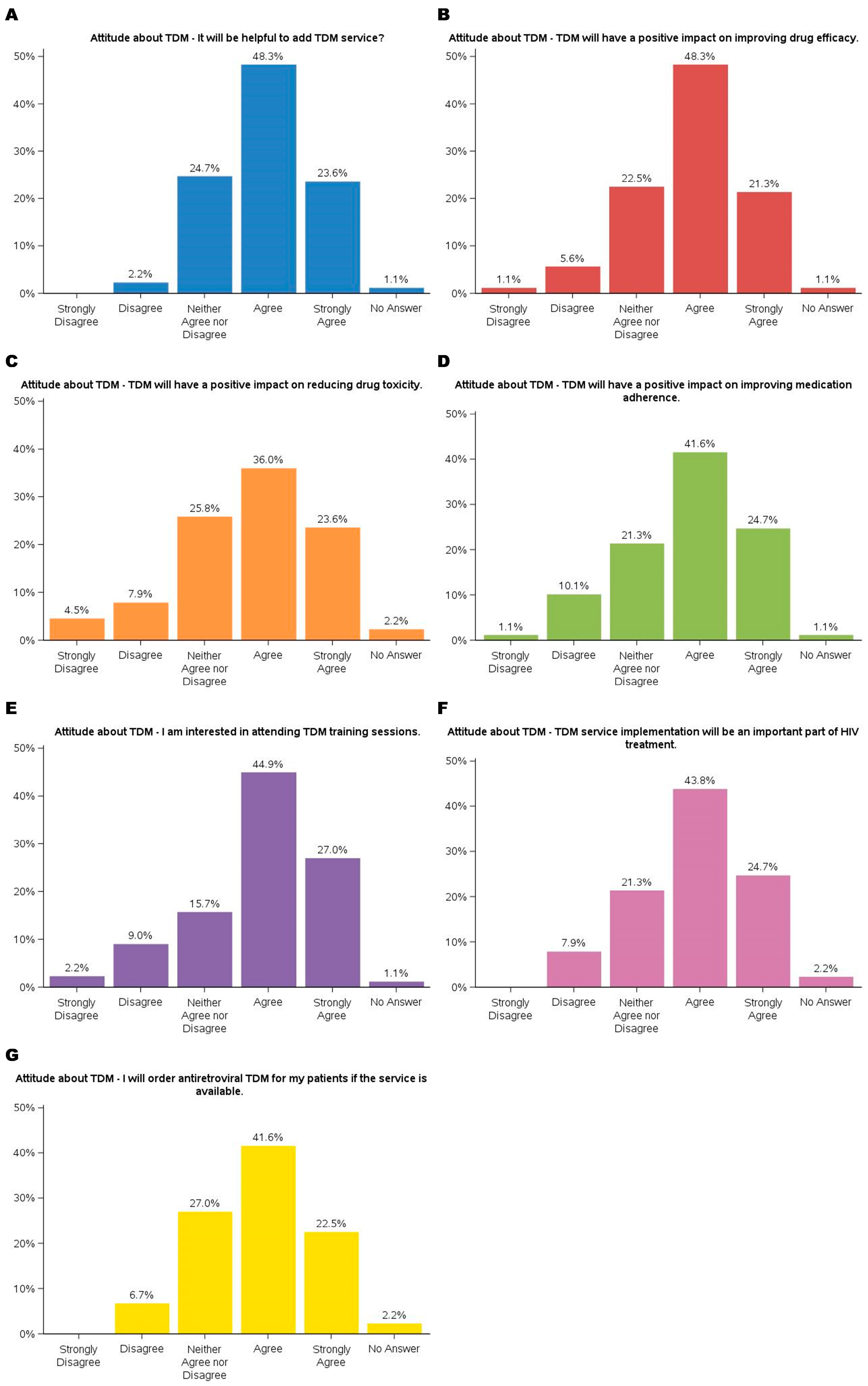
The findings demonstrated participants’ predominantly positive attitudes toward TDM. The results are summarized in
Figure 2. Nearly 72% agreed (agreed or strongly agreed) that adding TDM services would be helpful. Similarly, the majority acknowledged TDM’s potential to enhance clinical outcomes, with 48.3% agreeing and 21.3% strongly agreeing that it could improve drug efficacy, 59.6% (36.0% agreed and 23.6% strongly agreed) recognizing its role in reducing drug toxicity, and 66.3% (41.6% agreed and 24.7% strongly agreed) believing it could positively impact medication adherence. These results suggested that TDM is a valuable tool for optimizing drug therapy and addressing challenges in patient management, particularly for complex treatment regimens such as ART.
Participants also expressed enthusiasm for integrating TDM into their practice and professional development. A large portion (44.9% agreed, 27.0% strongly agreed) showed interest in attending TDM training sessions, highlighting the need for education on implementing this service. Additionally, most of the participants (43.8% agreed, 24.7% strongly agreed) agreed that TDM would play an essential role in HIV treatment, and 64.1% (41.6% agreed, 22.5% strongly agreed) indicated they would order TDM for their patients if the service were available. While a minority expressed neutral or dissenting views, these findings underscore a strong inclination toward adopting TDM as a clinical practice component, emphasizing its potential to improve patient outcomes.
3.3. Barries to TDM
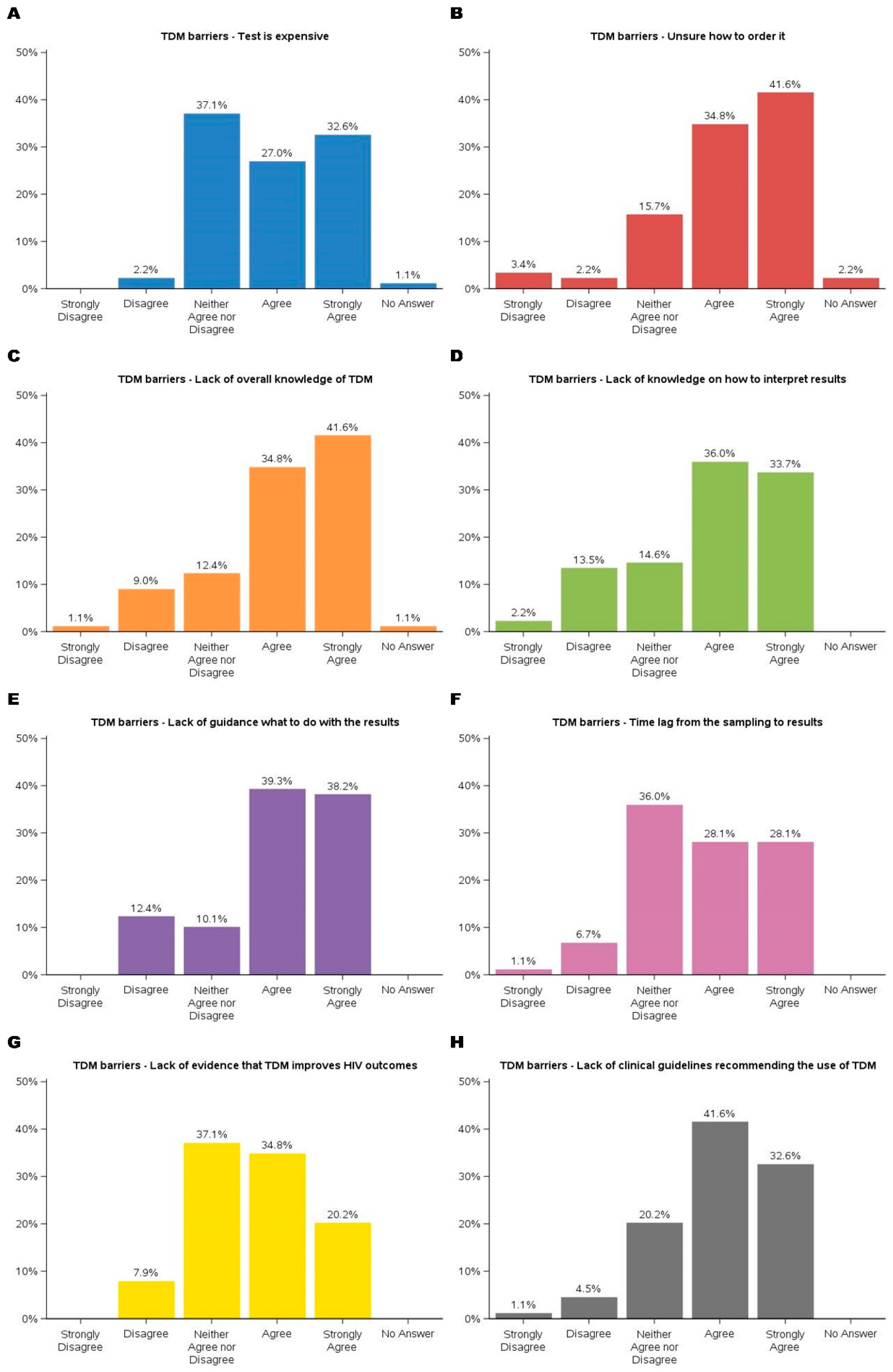
Barriers to implementing TDM were multifaceted, as shown in
Figure 3. The key barriers were the lack of evidence and guidelines supporting TDM in improving HIV treatment outcomes. Participants expressed concerns about the limited availability of evidence demonstrating TDM’s impact on HIV outcomes, with over 90% either ambivalent or agreeing that there wasn’t enough evidence to support its use. Similarly, nearly 95% participants recognized that existing guidelines either did not endorse TDM or were uncertain about their stance. These challenges underscore the necessity for robust clinical data and guideline development to support the integration of TDM into routine HIV care.
Financial constraints were among the most frequently cited challenges, with 27.0% agreeing and 32.6% strongly agreeing that cost posed a significant barrier. Participants expressed concerns about the affordability of TDM services, particularly in resource-limited settings, which could limit accessibility and sustainability. Additionally, procedural uncertainties emerged as a prominent barrier, with 76.4% agreeing (34.8% agreed, 41.6% strongly agreed) that a lack of knowledge about ordering TDM services hindered its implementation. These findings highlighted the need for financial strategies and accessible procedural resources to support TDM adoption.
Knowledge gaps also presented substantial challenges. Approximately 33.7% strongly agreed and 36.0% agreed that interpreting TDM results was difficult. Nearly 80% of participants agreed that there is a lack of guidance on using TDM results, such as addressing medication adherence, pharmacogenetic differences that affect metabolism, pharmacokinetics changes in special populations, and significant drug-drug interactions. These findings align with previous barriers related to the lack of evidence and guidelines supporting TDM. To address these concerns, further research is necessary to generate high-quality evidence, develop evidence-based guidelines, and enhance provider training to facilitate the effective implementation of TDM in HIV care.
4. Discussion
TDM may be a valuable tool in the management of antiretroviral treatment. The findings of this study shed light on the significant barriers and overall positive attitudes towards TDM in the management of ART for individuals living with HIV. Consistent with prior research, procedural uncertainties emerged as a prominent barrier [
22,
23,
24]. The financial concerns are compounded by procedural barriers, including knowledge gaps in ordering, interpreting, and acting on TDM results. This underscores the need for targeted educational initiatives and resources to support healthcare providers in effectively integrating TDM into clinical practice. Furthermore, the time delays associated with obtaining TDM results were cited as a significant logistical challenge, potentially impacting timely clinical decision-making and patient care. This may change quickly because many clinical labs are now equipped with LC-MS/MS and data will be generated much faster.
A notable finding was the lack of evidence supporting TDM use in HIV management, which many providers identified as a critical impediment. These findings highlight the varied practice experiences and underscore the importance of targeted support for managing HIV patients effectively. This aligns with earlier studies emphasizing the necessity of standardized protocols to ensure the appropriate and consistent application of TDM [
31,
32]. The findings of this study highlight the critical gaps in evidence and guidelines supporting the use of therapeutic drug monitoring (TDM) in HIV treatment. While TDM has demonstrated utility in managing drug exposure and toxicity in specific populations, such as pregnant individuals, children, and patients with complex drug interactions [
11,
12,
13,
14,
15]., its role in optimizing ART remains uncertain. The survey results underscore the prevailing uncertainty among providers, with a majority recognizing the potential benefits of TDM but also acknowledging the lack of definitive clinical outcomes supporting its widespread use.
The lack of robust clinical trials evaluating the impact of TDM on HIV outcomes further complicates its adoption in clinical guidelines. While small-scale studies suggest potential benefits [
33,
34], large-scale, longitudinal research is needed to establish its efficacy in improving viral suppression, reducing drug resistance, and enhancing overall treatment success. At present, the World Health Organization (WHO) has not issued formal recommendations on the role of TDM in HIV treatment [
35]. Both the European AIDS Clinical Society (EACS) and U.S. Department of Health and Human Services (DHHS) guidelines acknowledge that TDM may be considered in patients with virologic failure [
36,
37]. The EACS guidelines further extend the role of TDM to special populations, adherence assessment, second-line ART, and very young children [
37]. While routine TDM is not currently recommended, it may be beneficial in specific cases such as suspected malabsorption, drug-drug interactions, toxicity management, and in special populations including children, pregnant individuals, and the elderly, as well as for patients with good adherence but suboptimal treatment responses [
38]. The results of our study emphasize the urgent need for further research to generate high-quality evidence that can inform guideline development and support the integration of TDM into routine HIV care. Until such evidence is available, the adoption of TDM will likely remain limited, constrained by provider uncertainty and the absence of clear clinical recommendations.
Despite these barriers, the overall attitudes toward TDM were predominantly positive. Most participants recognized its potential to enhance drug efficacy, reduce toxicity, and improve medication adherence. However, an aspect arose from our data: while participants showed enthusiasm for TDM’s potential benefits, a substantial proportion also agreed that there is a lack of robust evidence demonstrating TDM’s positive impact on clinical outcomes. This dichotomy highlights the need for further research to substantiate TDM’s efficacy and ensure its credibility among healthcare providers.
This study has several strengths, including its sample population of clinicians and pharmacists from a large metropolitan area with a high number of HIV patients and well-established healthcare facilities, such as those within the Texas Medical Center. The diverse professional backgrounds of respondents enhance the generalizability of the findings. However, there are also limitations. The study was a preliminary survey rather than a controlled study comparing the effectiveness of TDM, limiting the ability to draw causal inferences. Additionally, the sample size was relatively small and included a limited percentage of infectious disease specialists. The sample size also did not allow for stratified analyses based on clinical roles (e.g., physicians, advanced practice providers, pharmacists) or practice settings (e.g., inpatient vs. outpatient), which may have provided further insights into the variation in attitudes toward TDM.
In conclusion, the findings of this study suggest that addressing the outlined barriers could foster broader adoption of TDM, ultimately optimizing HIV treatment outcomes and toxicity management. Future efforts should focus on determining the impact of TDM on clinical outcomes so that evidence-based guidelines can address TDM.
Author Contributions
Conceptualization, Hongmei Wang and Thomas Giordano; Data curation, Cecilia Torres; Formal analysis, Cecilia Torres; Funding acquisition, Hongmei Wang and Dong Liang; Investigation, Hongmei Wang; Methodology, Bich Dang; Project administration, Hongmei Wang; Resources, Thomas Giordano; Software, Cecilia Torres; Supervision, Hongmei Wang; Validation, Dong Liang; Visualization, Cecilia Torres; Writing – original draft, Hongmei Wang; Writing – review & editing, Cecilia Torres, Thomas Giordano and Dong Liang.
Funding
This research was funded by the National Institute on Minority Health and Health Disparities [grant number U54MD007605] and by the National Institute of Allergy and Infectious Diseases [grant number P30AI161943]. The APC was funded by U54MD007605.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Texas Southern University (protocol code #1821A and date of approval 01/31/2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available at the request of the corresponding author due to the potential risks of identifying individuals in the sensitive demographic categories included in the survey.
Acknowledgments
We thank Dr. Shital Patel and the Houston AIDS Education and Training Center for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- HIV.gov. Global HIV/AIDS Overview. 17 December 2024. [Google Scholar]
- UNAIDS. The path that ends AIDS: UNAIDS Global AIDS Update. UNAIDS.Org, 2023. [Google Scholar]
- Giordano, T.P.; Suarez-Almazor, M.E.; Grimes, R.M. The population effectiveness of highly active antiretroviral therapy: are good drugs good enough? Curr. HIV/AIDS Rep. 2005, 2, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bangsberg, D.R.; Perry, S.; Charlebois, E.D.; Clark, R.A.; Roberston, M.; Zolopa, A.R.; Moss, A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001, 15, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Parienti, J.; Fournier, A.L.; Cotte, L.; Schneider, M.; Etienne, M.; Unal, G.; Perré, P.; Dutheil, J.; Morilland-Lecoq, E.; Chaillot, F.; Bangsberg, D.R.; Gagneux-Brunon, A.; Prazuck, T.; Cavassini, M.; Verdon, R.; Hocqueloux, L. Forgiveness of Dolutegravir-Based Triple Therapy Compared With Older Antiretroviral Regimens: A Prospective Multicenter Cohort of Adherence Patterns and HIV-RNA Replication. Open Forum Infectious Diseases 2021, 8, ofab316. [Google Scholar] [CrossRef]
- Eisinger, R.W.; Dieffenbach, C.W.; Fauci, A.S. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019, 321, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Manalel, J.A.; Kaufman, J.E.; Wu, Y.; Fusaris, E.; Correa, A.; Ernst, J.; Brennan-Ing, M. Association of ART regimen and adherence to viral suppression: an observational study of a clinical population of people with HIV. AIDS Research and Therapy 2024, 21, 68. [Google Scholar] [CrossRef]
- Komandt, M.; Canfield, S.; Lengel, M.; Gilmore, V.; Kilcrease, C. Correlation between medication adherence using proportion of days covered and achieving viral suppression in patients living with HIV. JMCP 2023, 29, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.L.; Ritchwood, T.D.; Metzger, I.W. Structural Racism and Racial Trauma Among African Americans at Elevated Risk for HIV Infection. Am. J. Public Health 2023, 113, S102–S106. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Murdock, C.J.; Laurencin, L.; Christensen, D.M. HIV/AIDS and the African-American Community 2018: a Decade Call to Action. J. Racial Ethn. Health. Disparities 2018, 5, 449–458. [Google Scholar] [CrossRef]
- Goodlet, K.J.; Zmarlicka, M.T.; Peckham, A.M. Drug–drug interactions and clinical considerations with co-administration of antiretrovirals and psychotropic drugs. CNS Spectrums 2019, 24, 287–312. [Google Scholar] [CrossRef]
- Nhean, S.; Tseng, A.; Back, D. The intersection of drug interactions and adverse reactions in contemporary antiretroviral therapy. Current Opinion in HIV and AIDS 2021, 16, 292–302. [Google Scholar] [CrossRef]
- Lim, S.Y.; Pettit, R.S. Pharmacokinetic considerations in pediatric pharmacotherapy. Am. J. Health. Syst. Pharm. 2019, 76, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Collins, I.J.; Turkova, A. A step closer to optimal ART for all children. The Lancet HIV 2023, 10, e487–e489. [Google Scholar] [CrossRef]
- Jhajra, S.; Singh, S.; Holm, K.A.; Faqi, A.S.; Sahi, J. Influence of changes in physiology, transporters, and enzyme expression on disposition and metabolism of drugs during pregnancy and clinical implications. In Encyclopedia of Drug Metabolism and Interactions; 2012; pp. 1–29. [Google Scholar] [CrossRef]
- Kim, J.; Lee, E.; Park, B.; Bang, J.H.; Lee, J.Y. Adherence to antiretroviral therapy and factors affecting low medication adherence among incident HIV-infected individuals during 2009–2016: A nationwide study. Scientific Reports 2018, 8, 3133. [Google Scholar] [CrossRef]
- Ekstrand, M.L.; Shet, A.; Chandy, S.; Singh, G.; Shamsundar, R.; Madhavan, V.; Saravanan, S.; Heylen, E.; Kumarasamy, N. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int. Health 2011, 3, 27–34. [Google Scholar] [CrossRef]
- Scibona, P.; Cruz, C.V.; Lopardo, G.; Gimenez, M.I.; Frecha, C.; Rodriguez, L.F.; Rolon, M.J.; Cecchini, D.; Cordova, E.; Abela, C.; Sanchez, M.; Sierra, M.D.P.; Jaume, M.; Belloso, W. Individualized Antiretroviral Therapy. Impact of pharmacogenetic and therapeutic drug monitoring in the safety and efficacy of first line antirretroviral therapy in patients with HIV infection. Preliminary report. International Journal of Infectious Diseases 2018, 73, 243–244. [Google Scholar] [CrossRef]
- Aarnoutse, R.E.; Schapiro, J.M.; Boucher, C.A.B.; Hekster, Y.A.; Burger, D.M. Therapeutic Drug Monitoring. Drugs 2003, 63, 741–753. [Google Scholar] [CrossRef]
- Burger, D.M. Criteria for therapeutic drug monitoring in HIV. 2002. Available online: https://hospitalpharmacyeurope.com/news/editors-pick/criteria-for-therapeutic-drug-monitoring-in-hiv/.
- Perry, C. Monitoring Drug Therapy: Importance, Methods, and Challenges. Journal of Developing Drugs 2023, 12, 1–2. [Google Scholar]
- Milone, M.C.; Shaw, L.M. Breaking the Therapeutic Drug Monitoring Logistics Barrier. Clinical Chemistry 2014, 60, 1471–1472. [Google Scholar] [CrossRef]
- Chatjaroenpat, S.; Chuenmueang, C.; Jaisue, S. A cross-sectional study of the current situation with therapeutic drug monitoring in Thailand: Requirements, challenges and the role of educational institutions. Pharmacy Education 2024, 24, 154–163. [Google Scholar] [CrossRef]
- Bjørlykke, K.H.; Jahnsen, J.; Brynskov, J.; Molander, P.; Eberhardson, M.; Davidsdottir, L.G.; Sipponen, T.; Hjortswang, H.; Goll, G.L.; Syversen, S.W.; Langholz, E.; Jørgensen, K.K.; Steenholdt, C. Therapeutic drug monitoring in inflammatory bowel disease: implementation, utilization, and barriers in clinical practice in Scandinavia. Scandinavian Journal of Gastroenterology 2023, 58, 25–33. [Google Scholar] [CrossRef]
- DeArmond, P.D.; Bunch, D.R. Dasgupta, A., Ed.; Chapter 10 - therapeutic drug monitoring of antiretroviral drugs for the management of human immunodeficiency infection. In Therapeutic Drug Monitoring (Second Edition); Academic Press, 2024; pp. 241–264. [Google Scholar] [CrossRef]
- Preskorn, S.H. Charting and Handling Therapeutic Drug Monitoring Results: How they Differ From Most Laboratory Results. Journal of Psychiatric Practice® 2021, 27, 283. [Google Scholar] [CrossRef]
- Hazarika, I. Therapeutic Drug Monitoring (TDM): An Aspect of Clinical Pharmacology and Pharmacy Practice. Research & Reviews: Journal of Pharmacology 2015, 5, 27–34. [Google Scholar]
- Selinger, C.; Carbonell, J.; Kane, J.; Omer, M.; Ford, A.C. Acceptability of a ‘treat to target’ approach in inflammatory bowel disease to patients in clinical remission. Frontline Gastroenterology 2021, 12, 30–38. [Google Scholar] [CrossRef]
- Grossberg, L.B.; Papamichael, K.; Feuerstein, J.D.; Siegel, C.A.; Ullman, T.A.; Cheifetz, A.S. A Survey Study of Gastroenterologists’ Attitudes and Barriers Toward Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease. Inflamm Bowel Dis 2017, 24, 191–197. [Google Scholar] [CrossRef]
- Citing Qualtrics in Academic Research. Qualtrics, 5 May 2018.
- Archibald, T.L.; Murrell, D.E.; Brown, S.D. Chromatographic methods in HIV medicine: Application to therapeutic drug monitoring. Biomedical Chromatography 2018, 32, e4170. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, T. The Appropriately Designed TDM Clinical Trial: Endpoints, Pitfalls, and Perspectives. Therapeutic Drug Monitoring 2023, 45, 6. [Google Scholar] [CrossRef] [PubMed]
- Buzibye, A.; Musaazi, J.; von Braun, A.; Nanzigu, S.; Sekaggya-Wiltshire, C.; Kambugu, A.; Fehr, J.; Lamorde, M.; Gutteck, U.; Muller, D.; Sowinski, S.; Reynolds, S.J.; Castelnuovo, B. Antiretroviral concentration measurements as an additional tool to manage virologic failure in resource limited settings: a case control study. AIDS Research and Therapy 2019, 16, 39. [Google Scholar] [CrossRef]
- Li, N.; Zheng, H.; He, W.; He, X.; Li, R.; Cui, W.; Yang, W.; Dong, X.; Shen, Z.; Zheng, Y. Treatment outcomes amongst older people with HIV infection receiving antiretroviral therapy. AIDS 2024, 38, 803–812. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. 2021. Available online: https://www.who.int/publications/i/item/9789240031593.
- HIV.gov. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents With HIV. 2024. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new.
- European AIDS Clinical Society (EACS). EACS Guidelines for the Management of People Living with HIV in Europe. 2023. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/.
- Rindi, L.V.; Zaçe, D.; Compagno, M.; Colagrossi, L.; Santoro, M.M.; Andreoni, M.; Perno, C.F.; Sarmati, L. Management of low-level HIV viremia during antiretroviral therapy: Delphi consensus statement and appraisal of the evidence. Sex Transm Infect 2024, 100, 442–449. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).