1. Introduction
The coordination chemistry of nickel (II) dichlorides with bidentate phosphines is one of the most studied areas of co-ordination chemistry with the variation in coordination geometries [
1,
2,
3] between square planar and tetrahedral [
4,
5] being used as a vehicle to teach the differences between high and low spin complexes.[
6,
7] In most cases the colour of a metal complex is enough to assign whether the complex is low or high spin: larger energy gap (low spin) means the colour of the complex is the complementary one i.e. blue or green wavelengths missing therefore a yellow orange or red. Some complexes such as [Ni(PPh
3)
2Cl
2] exist in both coordination modes depending on the solvent it was prepared in. In rare cases a complex may be observed as a Zwitterion which is the subject of this work. The ligand we used was 1,2-bis-(di-
tbutylphosphinomethyl)benzene, [
8,
9,
10]
1, which is the classic so-called alpha ligand.[
11] This ligand was first reported and developed by Pringle’s group and its synthesis and use remains seminal in the field of catalysis.[
12] We have extensive experience in the coordination of these ligands particularly the ferrocene class.[
13,
14,
15] The palladium coordinates to the phosphorus atoms of the ferrocene alpha ligand,
butphos,
2, in a square planar fashion, Figure 1. [
13] The side on views indicate the ligand arms flank the palladium, in a sterically crowded environment.
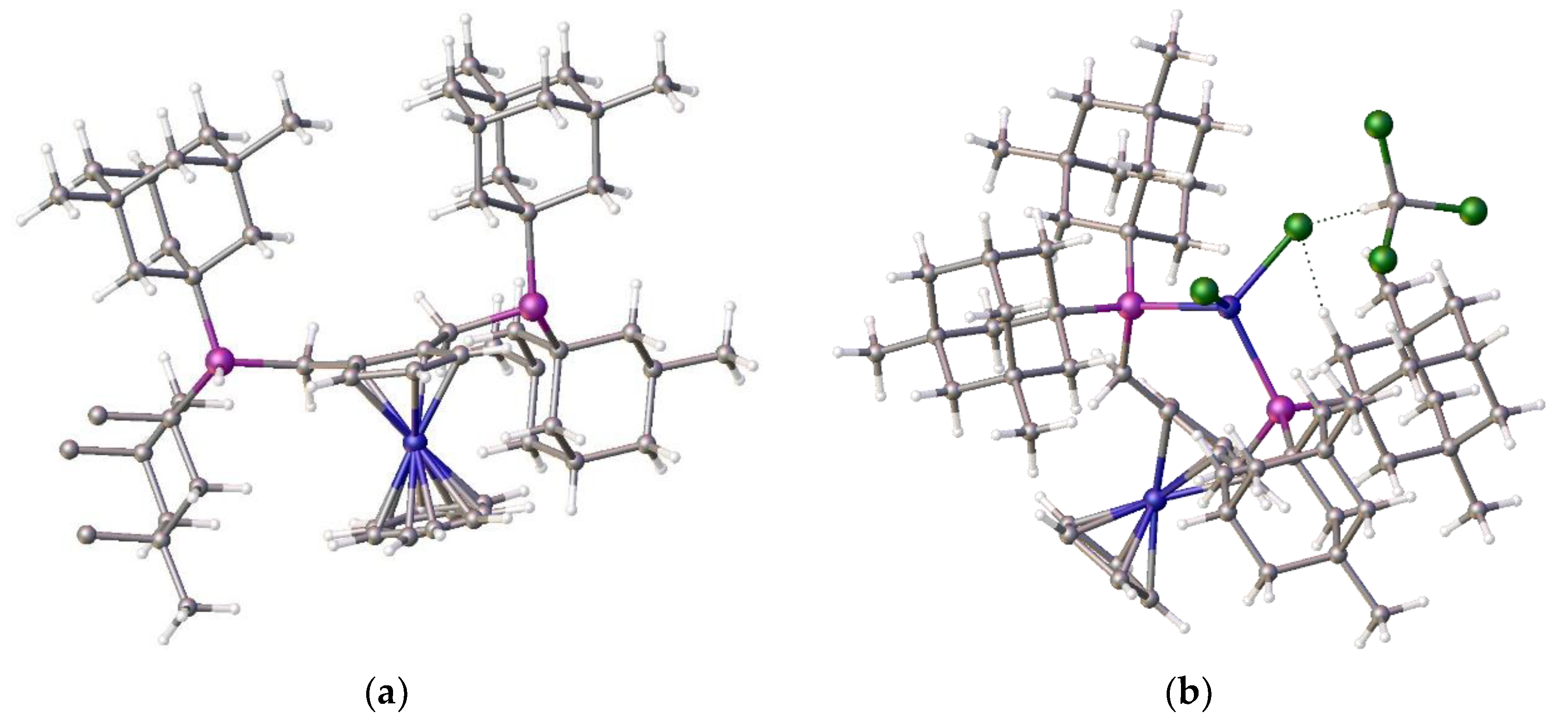
Figure 1.
Side and top views of the crystal structure of [{1,2-ƞ
5-C
5H
3(CH
2P
tBu
2)
2}Fe(ƞ
5-C
5H
5)PdCl
2], [(
butphos)PdCl
2]* showing the square planar coordination mode, [
13]. (*solvent omitted).
Figure 1.
Side and top views of the crystal structure of [{1,2-ƞ
5-C
5H
3(CH
2P
tBu
2)
2}Fe(ƞ
5-C
5H
5)PdCl
2], [(
butphos)PdCl
2]* showing the square planar coordination mode, [
13]. (*solvent omitted).
As part of a model study, it was decided to initially examine the complexation reaction of nickel with the simple alpha ligand. Clearly this is a very important research activity simply because the development of a nickel-based catalyst of either the
alpha ligand or the
butphos ligand for acrylic production would be much cheaper than the current technology. Much is known about the similar square planar coordination chemistry of ligand
1 with palladium and platinum as these complexes are useful industrial catalysts, [
16,
17], but less so with nickel, [
18].
2. Results and Discussion
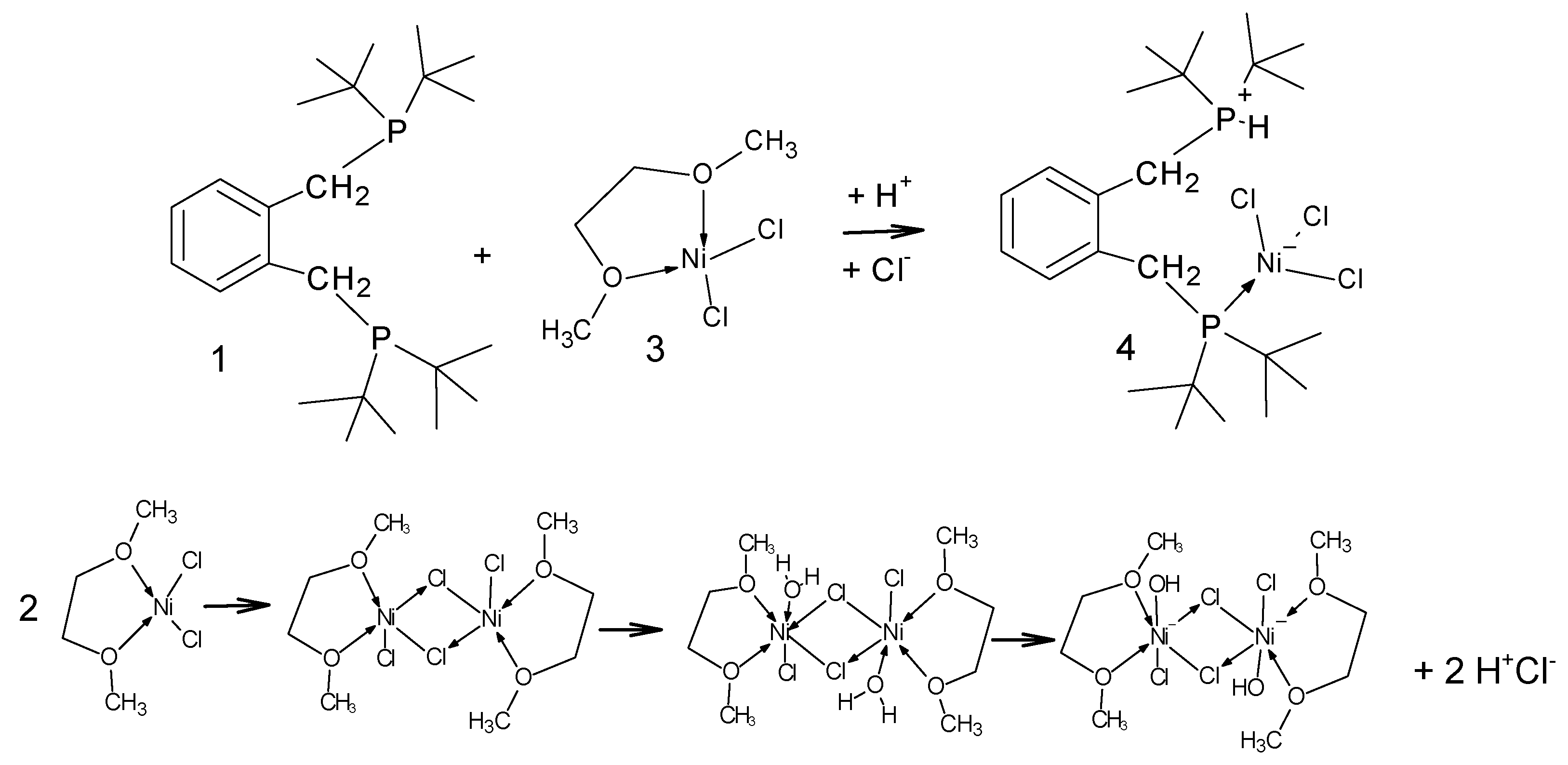
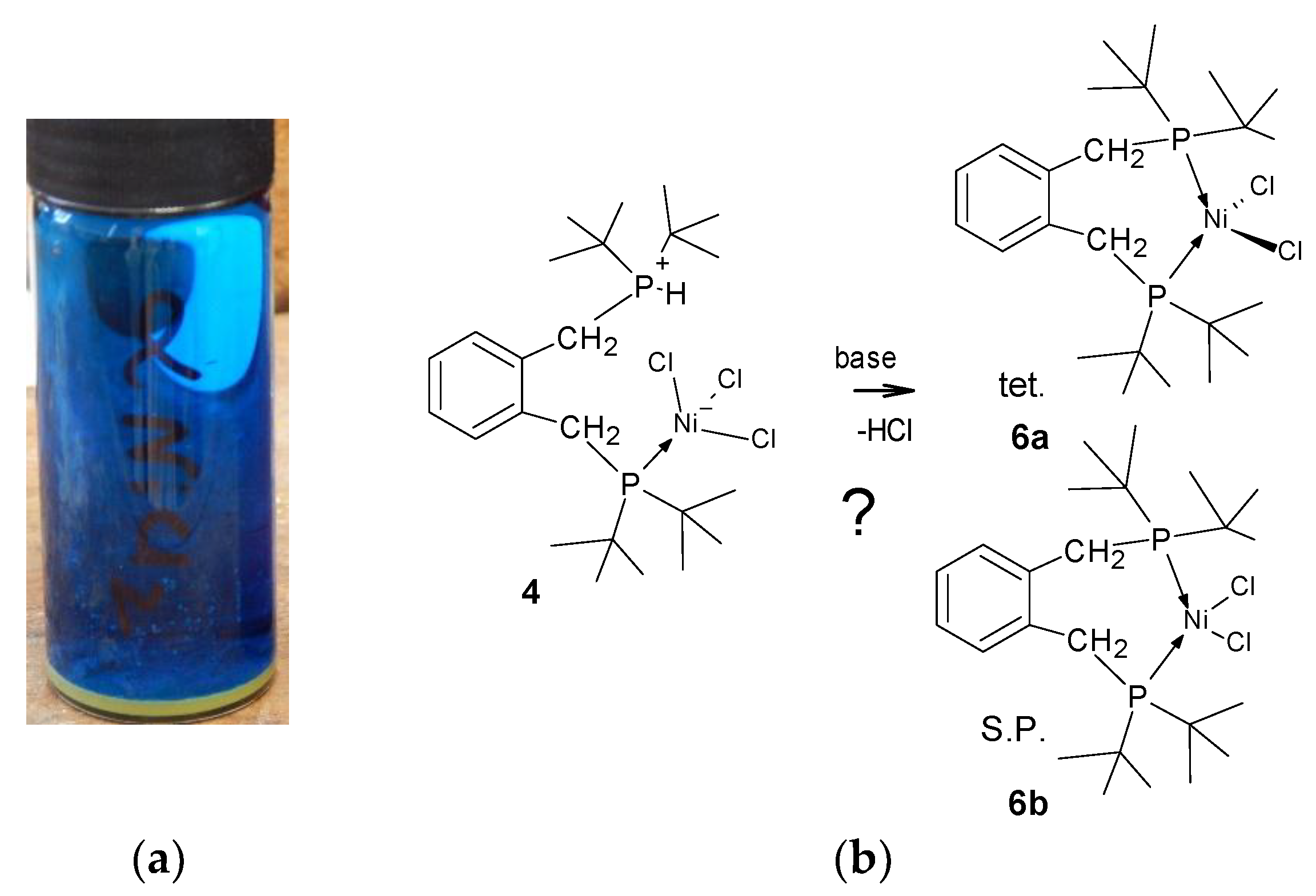
With this information in hand the reaction of 1,2-bis-(di-
tert-butylphosphinomethyl)benzene,
1 with hydrated nickel chloride in alcoholic solvents, under reflux, was attempted but this led only to amorphous yellow powders. However, when a solution of the ligand is added to the [Ni(DME)Cl
2],
3, (DME = dimethoxyethane), complex [
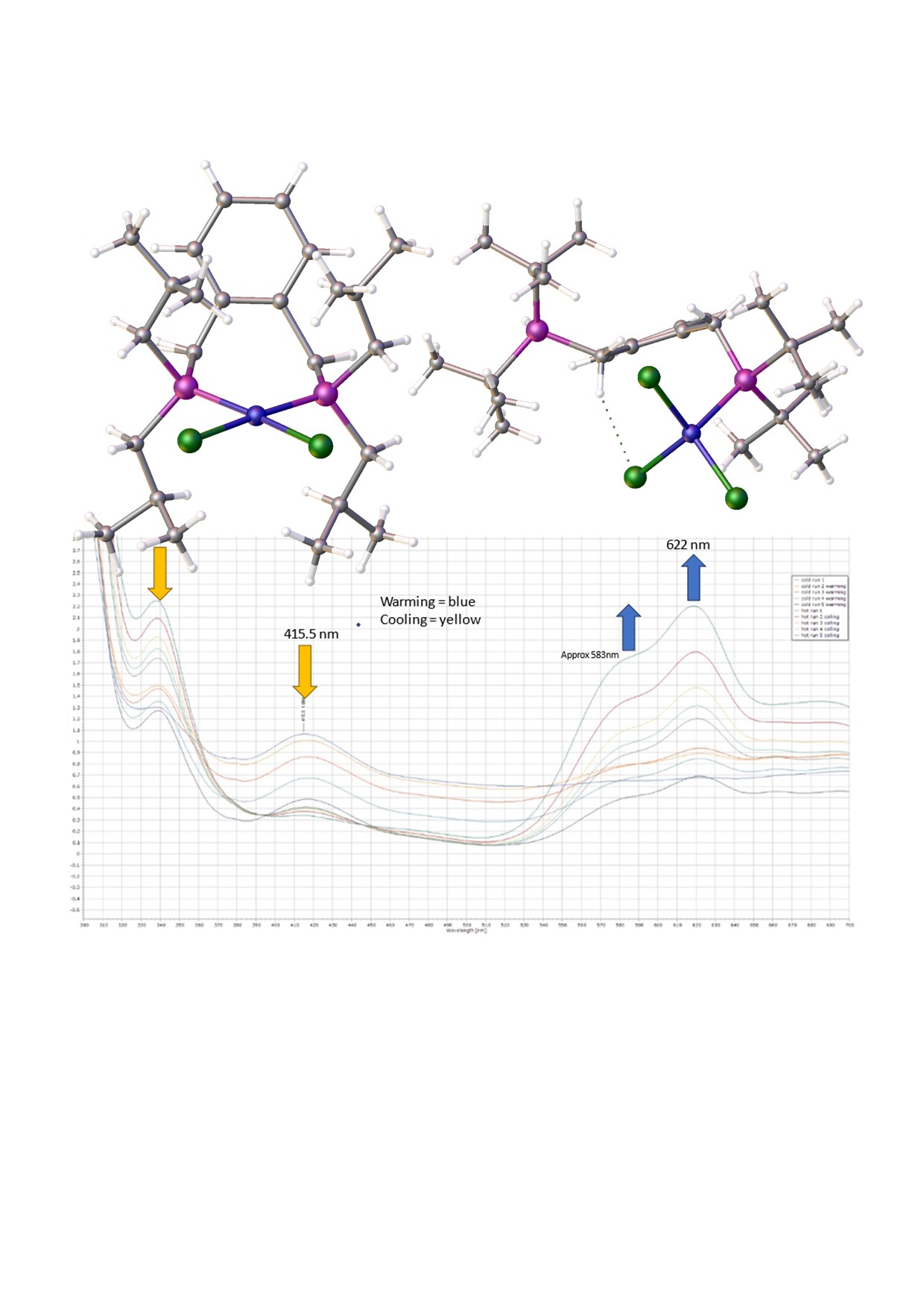
19] in dichloromethane the solution develops a blue colour and when left for several days blue crystals begin to grow. We had anticipated the formation of red crystals which would have indicated the formation of the square planar complex or deep blue/green crystals which would indicate a tetrahedral complex. The pale blue crystals were collected after a minimum of 2 weeks and examined by single crystal diffraction; it is a rare Zwitterionic compound which accounts for its poor solubility in organic solvents. The product complex was identified as compound
4, Figure 1.
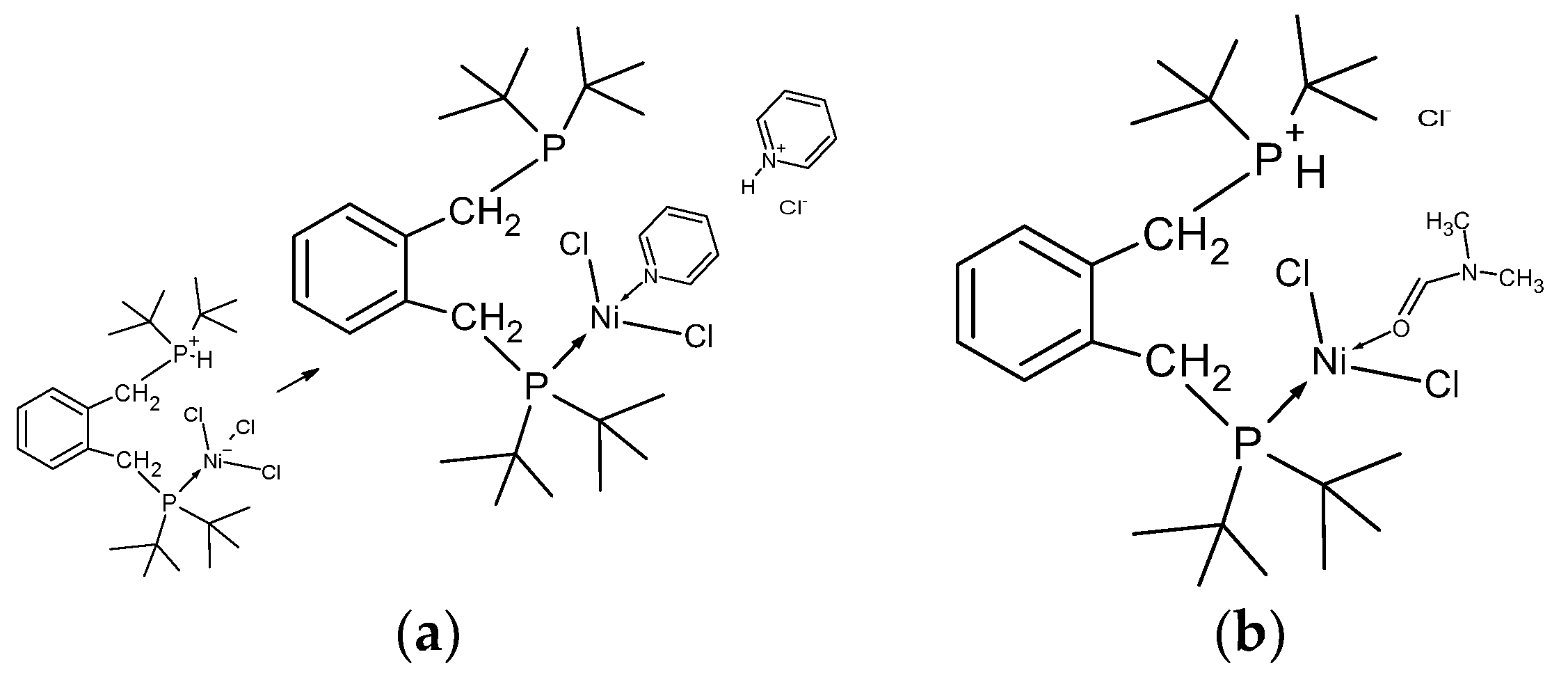
Figure 1.
Schematic showing the formation of [C6H4-CH2PtBu2-2-C6H4-CH2P(H)tBu2)2NiCl3], 4. Crucial to this complexation is the addition of a proton and a chloride ion, (HCl). In the lower graphic one of the many possible modes of formation of HCl is shown; the key to this is the reaction of water with [Ni(DME)Cl2], 3.
Figure 1.
Schematic showing the formation of [C6H4-CH2PtBu2-2-C6H4-CH2P(H)tBu2)2NiCl3], 4. Crucial to this complexation is the addition of a proton and a chloride ion, (HCl). In the lower graphic one of the many possible modes of formation of HCl is shown; the key to this is the reaction of water with [Ni(DME)Cl2], 3.
Only one phosphine is bound to nickel which has 3 chloride ligands attached in pseudo tetrahedral coordination,
Figure 2, while the other phosphorus is protonated, forming a phosphonium pendant arm.
The Cl-Ni-Cl angles are 101.011(32), 120.882(32), 109.770(29) degrees respectively, while there are two close contacts between a chloride and proton on the phosphorus (2.7091(3) Å) and a chloride and a proton on the phenyl ring (2.794(7) Å), along with the internal close contact (fig 2a) of a chloride and methylene proton (2.4747(9) Å). In the structure the pendant arms of the ligand lie above and below the benzene ring, which is the same orientation observed in both the free ligand and its disulfide which is shown, for comparison, in
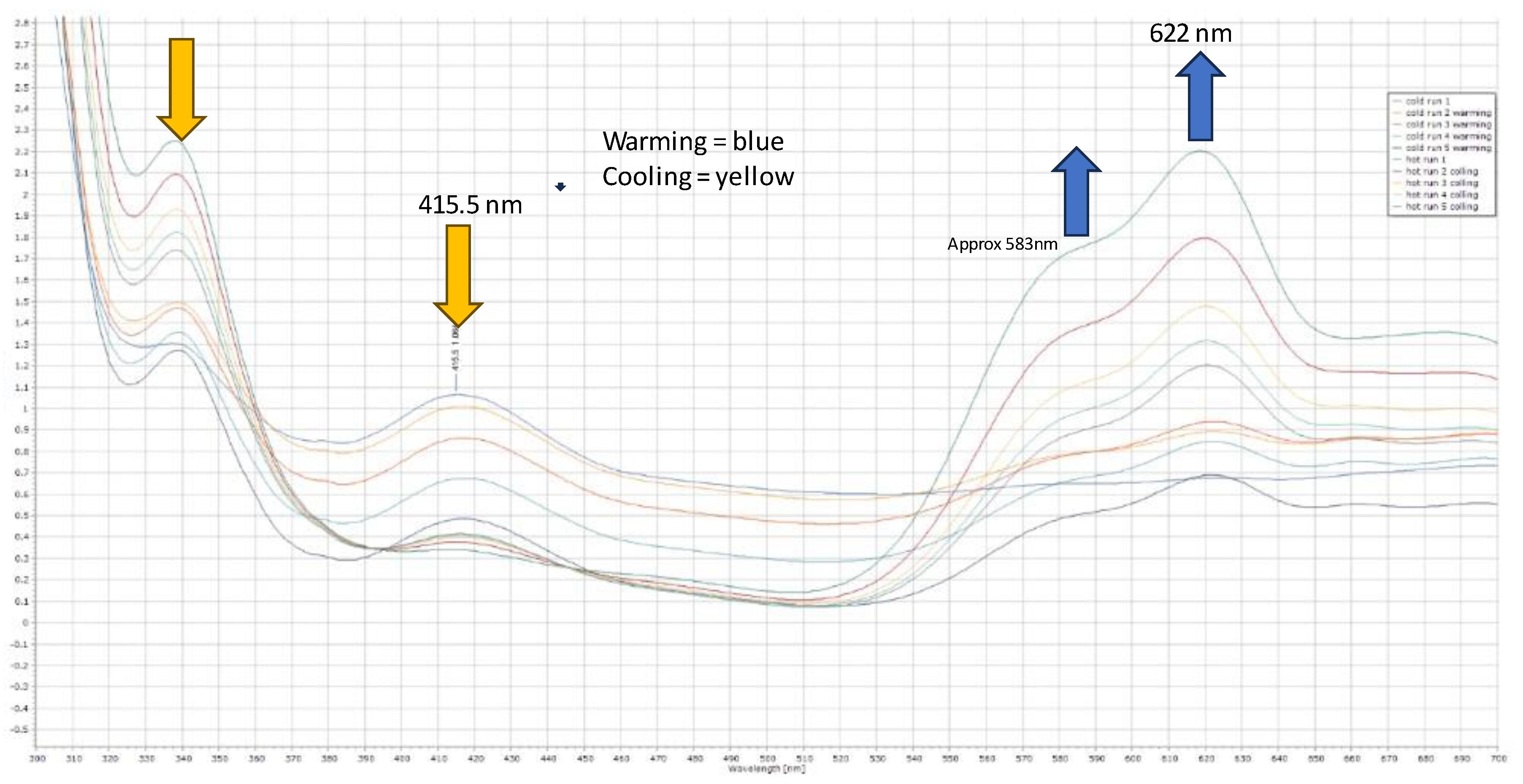
Figure 2c. The origin of the additional proton and the additional chloride on nickel is unclear, but we propose that the chloride ion though is likely to originate from the excess nickel complex, and that the proton probably originates from trace water as the starting nickel complex is highly hygroscopic. The product complex decomposes in methanol and forms a pale-yellow solution/slurry. It is sparingly soluble in chloroform, dichloromethane, tetrachloroethane and DMF and forms a pale blue solution at room temperature. On cooling the solution in DMF to -20
oC, the blue colour (λ
max,
~ 621nm) changes to yellow (λ
max,
~ 417nm) reversibly. (see
Figure 3). It is likely that DMF reacts reversibly with the nickel centre as it is a hard Lewis base with a low pKa. Interestingly [(C
6H
4-CH
2PtBu
2-2-C
6H
4-CH
2P(H)
tBu
2)
2NiCl
3], is a complex which crystallised well in regimented fashion in local magnetic fields, which was examined in a separate study we were involved in. [
20]
By looking at the structure it might be envisaged that compound
4 would react under basic conditions to produce either the square planar or tetrahedral complex,
Figure 4, hence closer examination by NMR is warranted. Excepting the obvious steric crowding, it is not immediately clear why one of these chelate complexes does not form under the conditions used since we have successfully used this synthetic method for the preparation of related nickel phosphine complexes. [
21]
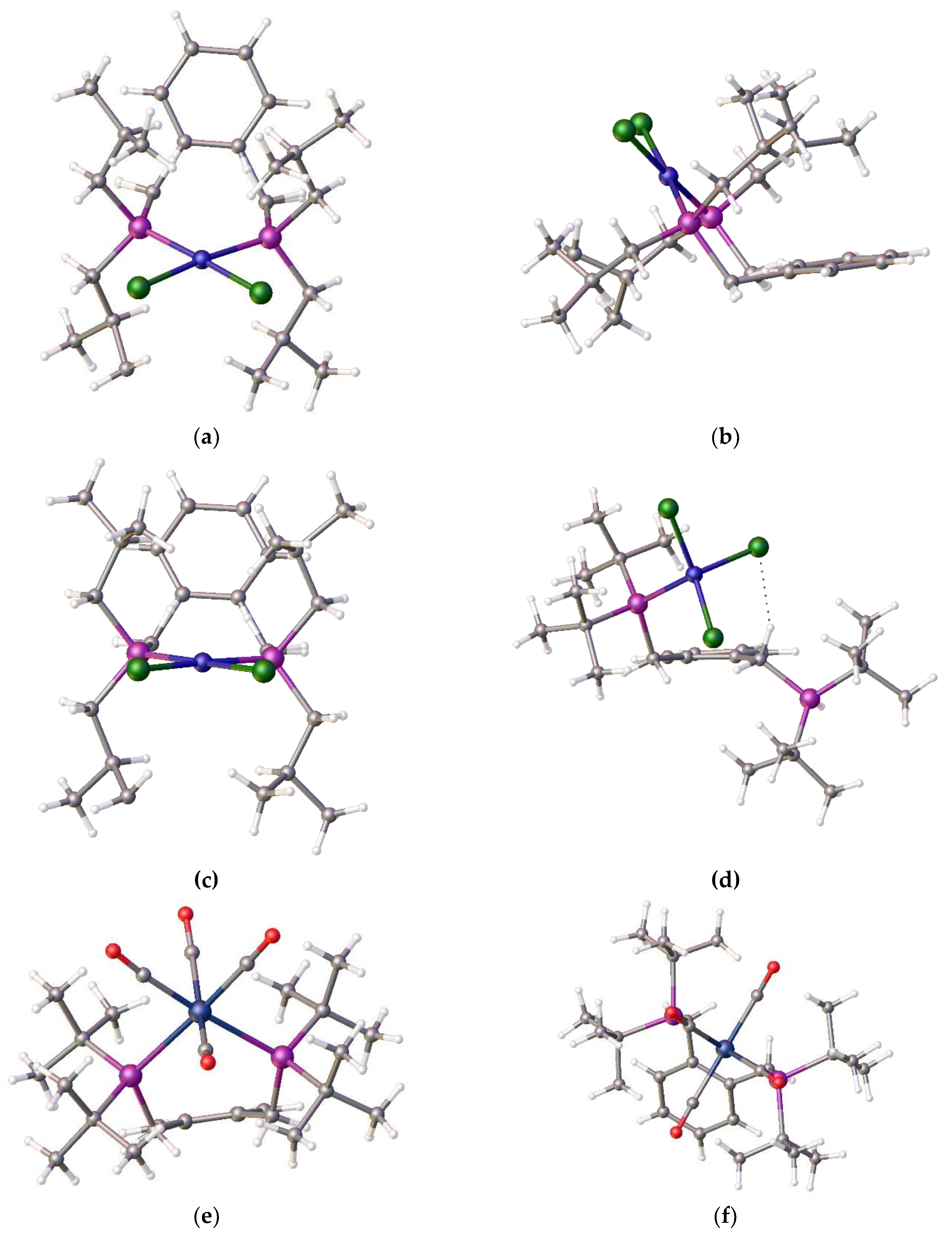
2.1. Structural Comparisons
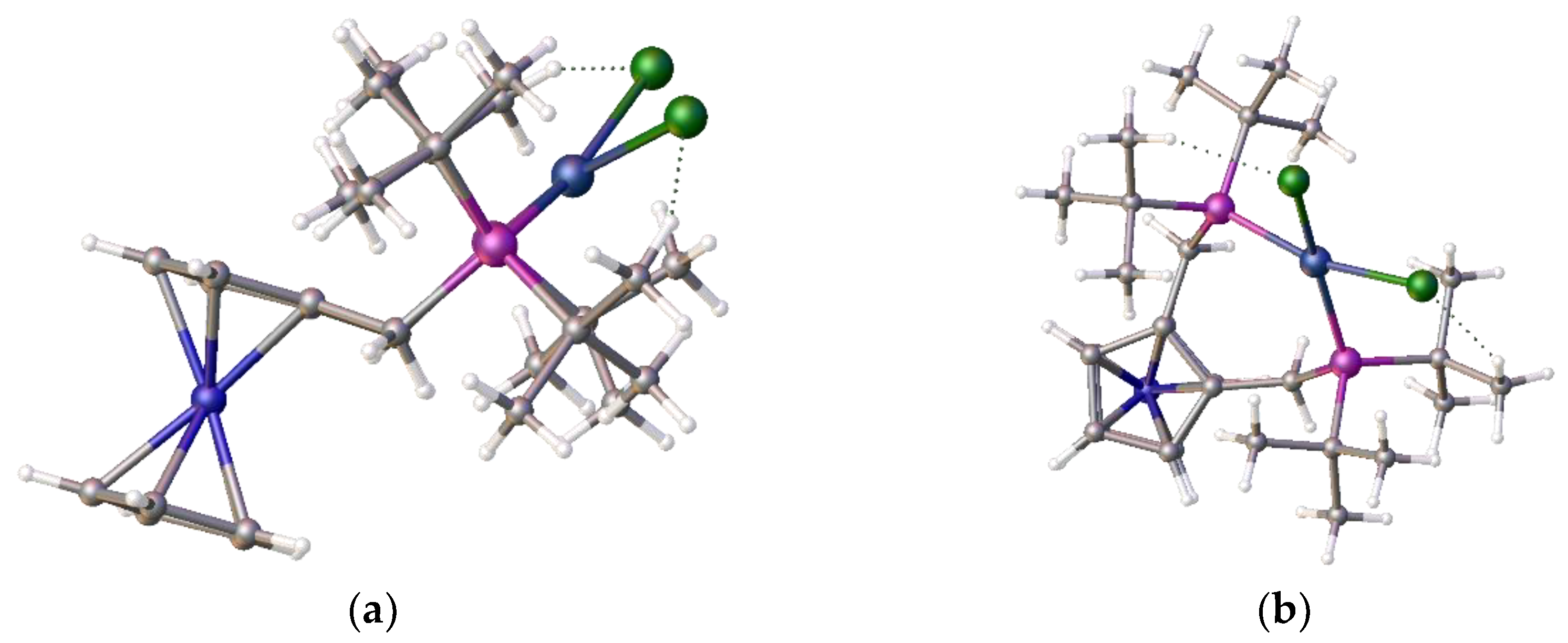
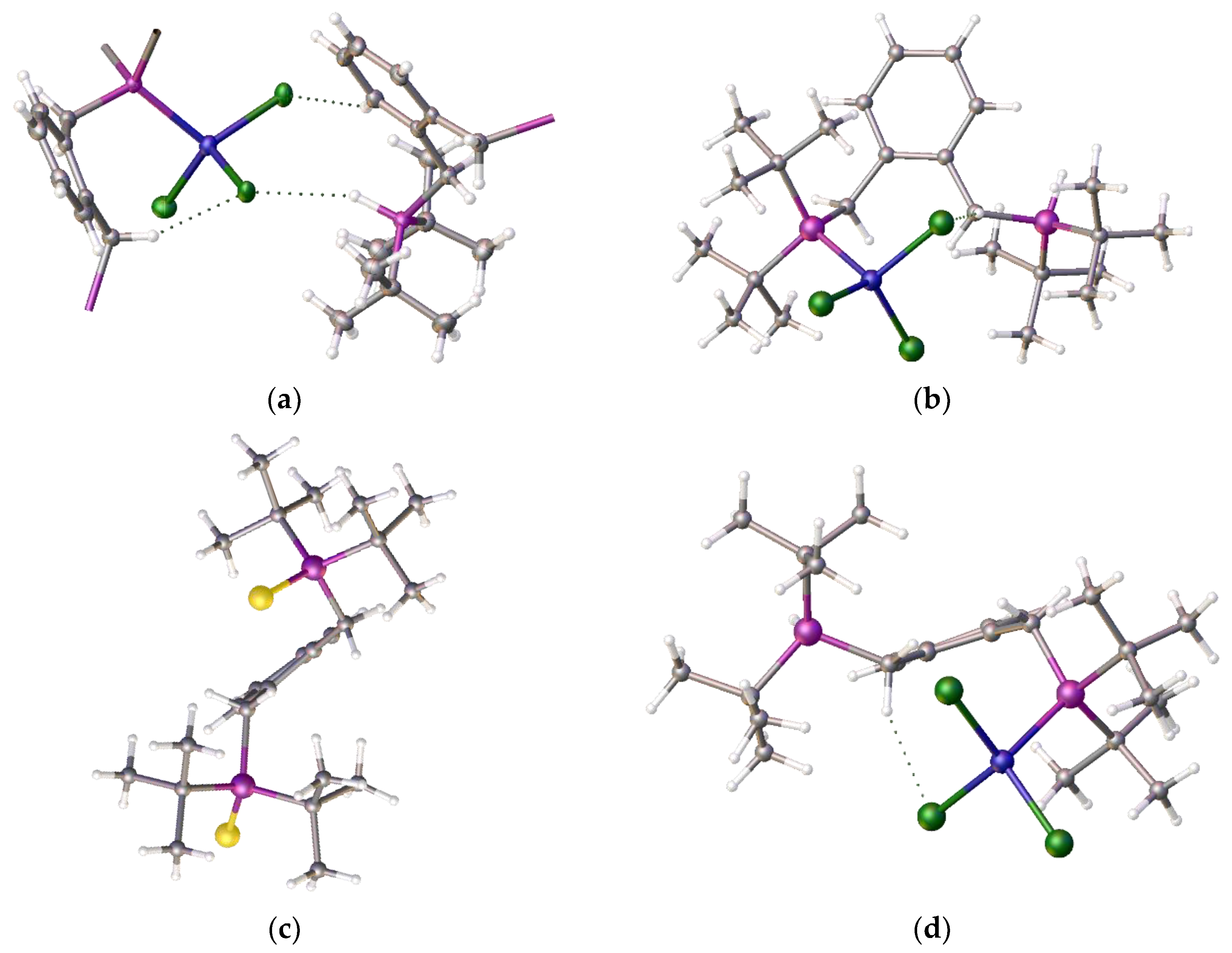
To understand the reason for the formation of compound
4 and not the chelate complex we decided to compare the product structure with related structures within our portfolio. During these coordination studies we have non-definitive evidence for the formation of the anticipated square-planar Ni-alpha complex as small red crystals which were isolated during one of our room temperature coordination reactions, however the initial crystallographic results indicated a disordered structure. However, we note that we have been able to determine the structure of the isomeric red nickel dichloride complex,
7, of the related isomeric ligand [1,2-C
6H
4-(CH
2P
iBu
2)
2],
8. The structure is disordered but it clearly shows that the nickel adopts a square planar coordination mode,
Figure 5, (a)-(c).
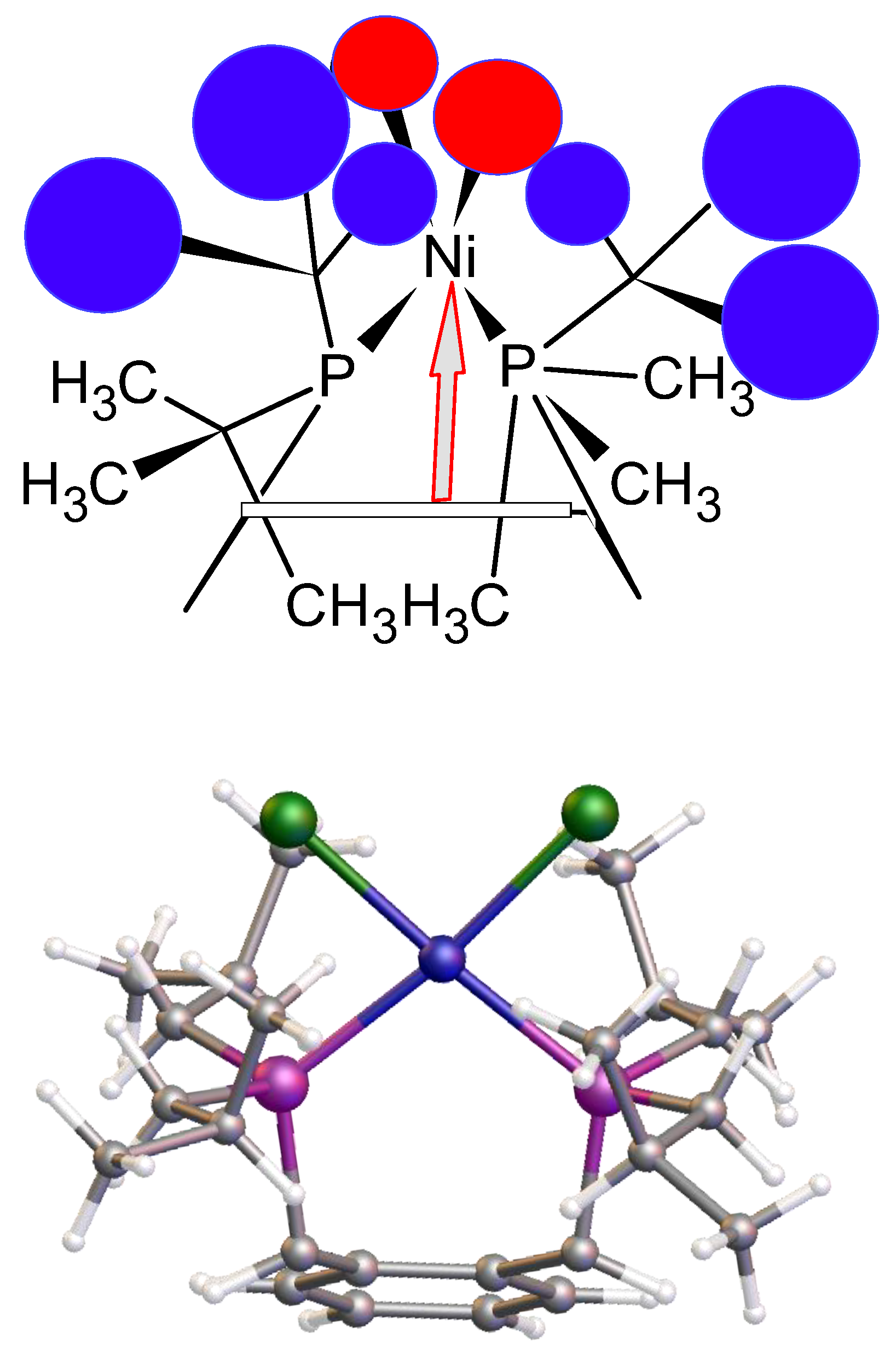
This ligand with its pendant iso-butylphosphine arms is less hindered than its tert-butylphosphine isomer which partially accounts for the difference in coordination properties and therefore its relative ease of formation. However, this cannot be the only reason for the observed differences. An examination of the structure of the tungsten tetracarbonyl complex of ligand
1, [
22] see (e) and (f)
Figure 5 visually highlights a possible reason. It is simply that the shorter bond length of the nickel-phosphine bond compared to the those of the palladium-P or tungsten-P and consequently the tighter chelate bond angles of the P-Ni-P prevents coordination. Interestingly, we had previously observed structural disorder in the chloroform solvate of the nickel dichloride complex of the
bis-1,2-[(1,3-dimethyladamantyl)phosphino]ferrocene,
8, which exhibited tetrahedral coordination, compared with its palladium analogue, presumably, in part, because of the shorter bond length with the very sterically bulky ligand,
Figure 6.
This of course leads to the question why doesn’t tetrahedral coordination occur if square planar coordination is not possible due to steric crowding? The answer is not immediately obvious but maybe simply due to the mild reaction conditions used or that the shorter length of the nickel phosphine bond hinders the formation of the bidentate product. Structural comparisons between nickel and palladium complexes are complex [
23,
24] and clearly an extended study of a range of related ligands is warranted. Clearly the importance of the geometric parameters around the metal are important and will affect any catalytic reaction [
25]. To highlight the importance of these structures a recent study using a chiral variant of this ligand class has recently been used in asymmetric hydrogenation of α-substituted acrylic acids to give chiral alpha-substituted propionic acids. In this case the catalyst was prepared in situ using nickel acetate as a precursor. [
26] The next question we need to address is to confirm the source of trace water required in the synthesis, which is examined next.
2.2. Examination of the Reaction of DMF with in [Ni(DME)Cl2], Looking for Source of Trace Water
To this point we have not proven that there is trace water present in the precursor, [Ni(DME)Cl
2], which could act as a source of the proton in the Zwitterionic complex. Therefore, we decided to examine the reaction of DMF with [Ni(DME)Cl
2] alone. It is known that complex equilibria exist in DMF solutions of nickel chlorides and thus DMF binding to nickel may occur. This will also have a bearing on the observed thermochromic properties of the product. Two complexes were formed in this room temperature reaction using anhydrous DMF. The first was the previously reported octahedral hexadimethylformaldehydo-nickel tetrachloronickelate, [Ni(DMF)
6)]
2+[NiCl
4]
2-,
8, [
27] which we crystallographically characterized (CCD Deposition Number: 2421634, full data given in the
Supplementary Materials). This confirms that DMF readily coordinates to the nickel centre (to give [Ni(DMF)
6)]
2+), and it also confirms that chloro- ligands are transferred, under ambient conditions, between nickel centres (to give [NiCl
4]
2-) in DMF thus confirming the source of the additional chloride. The second complex which was identified was the all
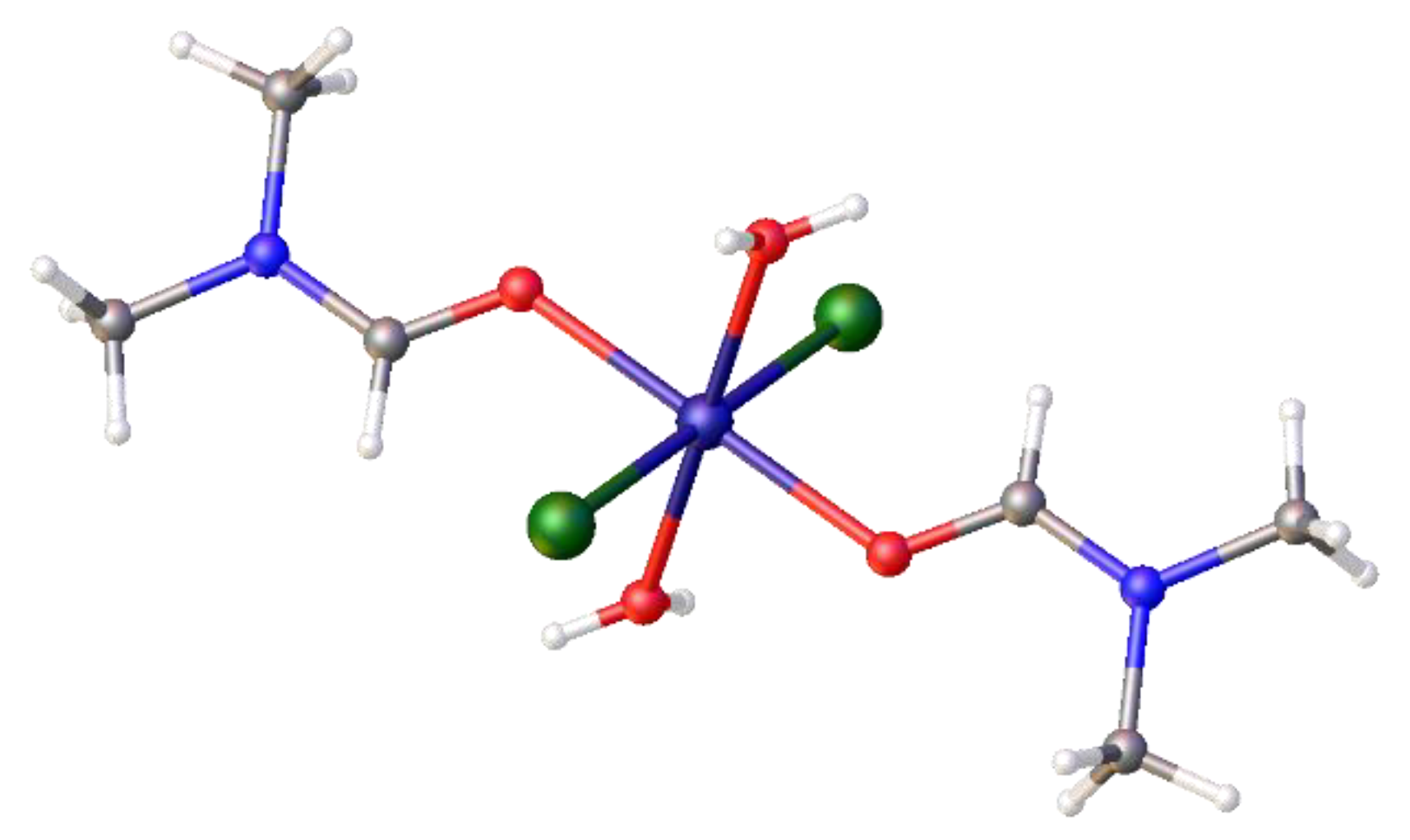
trans-di-aqua-bis-dimethylformaldehydo-dichloro nickel (II), [Ni(H
2O)
2(DMF)
2 Cl
2],
9,
Figure 5. (2017ncs0366c; CCDC 2421635). A detailed literature search indicated this complex has since been isolated elsewhere, together with [Ni(DMF)
2(H
2O)
4]Cl
2·2H
2O [
28]. This complex exists as hydrogen bonded ribbons and cutaway sections of a packing diagram are also shown in
Figure 5. This complex completes a series of complexes of aqua nickel complexes as the related nickel(II), trans-[NiCl
2(H
2O)
4]·2H
2O had been previously reported, [
29]. More importantly for this work though, it confirms the presence of trace water in the precursor. Thus, we have established the likely sources of the additional H
+ and Cl
-.
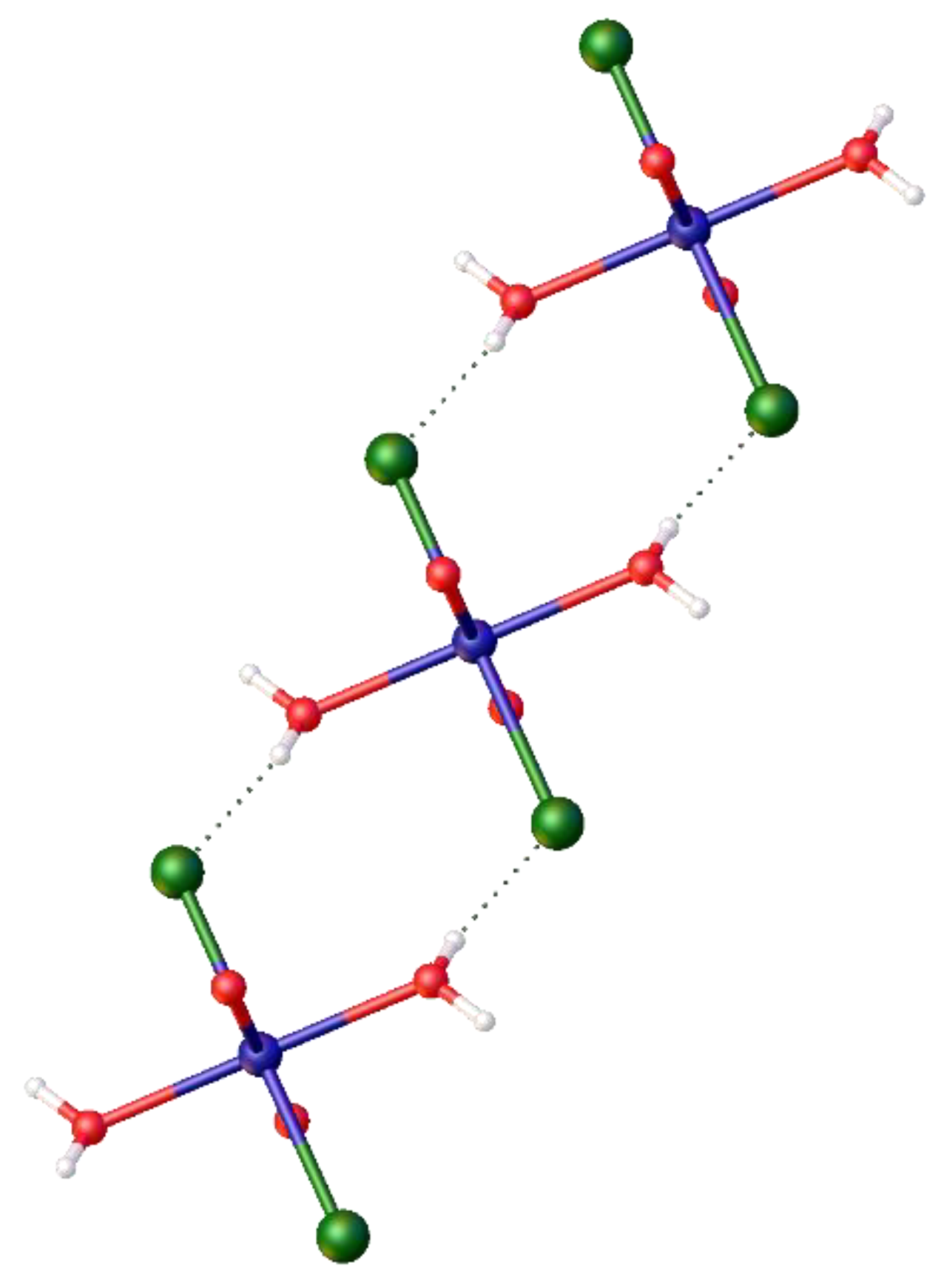
Figure 8.
The crystal structure of the diaqua- complex, trans-[Ni(Cl)2(H2O)2(DMF)2], 9, formed from [Ni(DME)2Cl2], and DMF at room temperature and its partial packing diagram showing the hydrogen bonding between molecules.
Figure 8.
The crystal structure of the diaqua- complex, trans-[Ni(Cl)2(H2O)2(DMF)2], 9, formed from [Ni(DME)2Cl2], and DMF at room temperature and its partial packing diagram showing the hydrogen bonding between molecules.
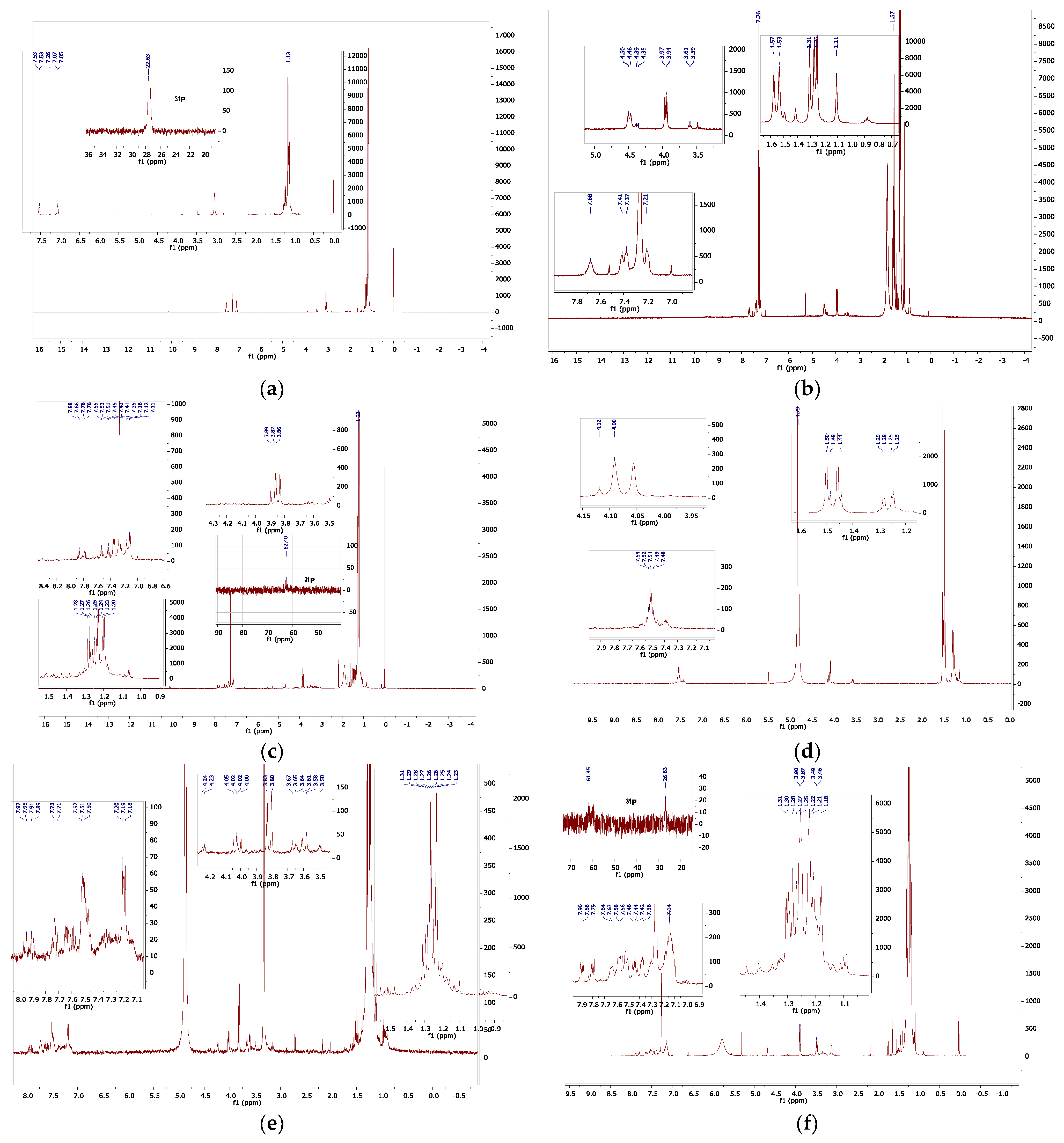
2.3. NMR Spectroscopic Examination of Compound 4
To obtain more clarification proton and phosphorus NMR spectra of the complex were run in a range of solvents. In some solvents only broad resonance were observed, which generally meant that there were equilibria involving the solvent, and in other solvents there was clearly complex decomposition, nevertheless some useful data were obtained which are visually summarized in
Figure 9. The low solubility in all solvents studied hinders the measurements but general conclusions may be drawn. Solvents which themselves can act as bases or ligands interact with the compound
4, in a more complex fashion. This is not surprising given we have already observed thermochromic behaviour in DMF. In all cases there is considerable resonance overlap in the aryl region and in the alkyl-methyl regions of the spectra, however the methylene region (approx. 2.5-4.5 ppm) is relatively clear of additional resonances and is a useful diagnostic. The protons of the -CH
2- spacer in the ligand are observed at ca 3.4 ppm, while that of the mono-protonated ligand, which we generated independently, (ligand
1 was treated with 1,1,2,2-tetrabromoethane, which generates HBr in situ in the presence of alcohols, amine and phosphines [
30]) is observed at ca 4.5 ppm. In chloroform-
d, two groups of methylene proton are observed, the first at 3.96 ppm which we assign to those on the nickel bonded arm and the second at 4.48 ppm, (slightly broader) which we assign to those on the protonated phosphorus arm. The tert-butyl resonances of these are seen at 1.29 and 1.55 ppm respectively. The four aryl resonances are observed between 7.2 and 7.7 ppm. To observe the decomposition a sample in chloroform was treated with D
2O. In this case, there are two methylene resonances which are overlapped at 3.86 and 3.88 ppm. In addition, there are two sets of very low intensity multiplets at ca 3.3 and 4.3 ppm. All methyl resonances are overlapped between 1.1-1.3 ppm. In the aryl region there appear to be two groups of resonance, one set from an unsymmetrically substituted benzene ring (2 triplets, 2 doublets), and a second set of multiplet resonances at 7.12 and 7.35 ppm while in the phosphorus NMR there are again two sets of resonances one corresponding to that of the ligand at ca 27ppm and one set at ca 61.5 ppm. It is clear there is partial delegation on addition of D
2O but also there are new products formed. In neat D
2O the spectra are complex: there are at least 6 methylene resonances (therefore at least 3 compounds) and several overlapped aryl resonances, 7.1-7.6 ppm, while all tert-butyl resonances are overlapped. In deuterated methanol there is similar decomposition. In this case the one weak phosphorus resonance was observed at 65.4 ppm. In the case of the diagnostic methylene resonances; there is one large doublet (3.82 ppm, symmetrical compound), two smaller pairs of doublets (3.62, 4.01 ppm; 3.66,4.04 ppm, both unsymmetrical compounds and very low intensity resonance (dd, 4.23 ppm). In the
31P NMR spectrum only very low intensity resonances were observed therefore a separate long acquisition time experiment was conducted,
This showed a high intensity singlet resonance at 66.6 ppm, a lower intensity 1:1:1 triplet at 64.36 ppm (J
P-D = 182 Hz), and several low intensity singlets at 65.04, 64.20, 58.46 and 42.17ppm respectively. These were all sharp resonances, whereas a broader, yet significant, 1:1:1 triplet was observed at 38.79 ppm (J
P-D = 70 Hz). These data show that significant proton/deuterium exchange is occurring in the sample. In one last sample pyridine-
d5 was added to sample of compound
4 in chloroform-
d; this was because the pyridine should deprotonate the phosphonium phosphorus. Two large methylene resonances were observed at 3.47 and 3.89 ppm respectively together with a series of low intensity multiplets which are centred at 3.3 and 4.2 ppm. Thus, although the deprotonation occurs a complex spectrum is the result. From these data it may be concluded that the compound
4 reacts readily with solvents which themselves may act as ligands. The chloro-ligands may be replaced or augmented to with solvent. In DMF it is likely that the DMF completely solvates the nickel centre reversibly akin to the preparation of compound
8, shown earlier. Thus, the observed thermochromic behaviour in DMF is almost certainly a solvent related phenomenon with the chloro- ligands being displaced by DMF. [
31] The first step is shown in
Figure 10. Clearly it would be interesting to examine the behaviour of similar solvents towards the nickel centre. Additional NMR spectra in additional solvents may be seen in the
Supplementary Materials as well as a comparison with the ferrocenyl alpha ligand. In conclusion the complex
4 is highly sensitive to solvent reactions and therefore it exhibits a high degree of lability. An examination of compound
4 using mass spectrometry (APCI on Orbitrap Instrument) was carried out; mass ions of the oxidised phosphine ligands were apparent; however, no parent mass ion was found. Interestingly however, a mass ion of (M
++3) with the anticipated isotopic pattern was observed. Also present were higher mass ions with isotopic patterns centred at of m/z 938 and 971 respectively. Clearly, it would be interesting to further investigate the sold state properties of this compound.
3. Materials and Methods
The protocols used in this work were essentially standard coordination and crystallization at room temperature, conditions which were developed with a view to keeping the methodology as simple as possible. We could add that crystallization is one of the main methods of self-assembly. All metal complex crystallizations were carried out with the compound dissolved in a solution of reagent grade dichloromethane with a top layer of diethyl ether added carefully. Crystals formed in all cases after the solvents co-diffused over several days. All NMR experiments were conducted at 400MHz for proton on a Bruker WH-400 instrument. Additional experimental detail is given in the Supporting Materials Section.
4. Conclusions
Although the coordination of the alpha ligand with palladium and platinum is relatively straightforward the coordination with nickel is less so- the natural propensity towards square-planar coordination may be difficult because of the steric bulk of the ligand in a relatively small coordination sphere. However, it is interesting to observe the formation of a new Zwitterionic complex. Given that there is a readily available supply of related ligands it will be of interest to observe the general trends in coordination chemistry when the synthetic method described here is applied. Clearly, given that these nickel complexes are so much cheaper to prepare than the corresponding palladium and platinum derivatives and the former of these is used in such large quantities in industrial acrylic formation, it is clearly imperative to test them in carboxy-alkylation reactions. We would encourage interested research groups to contact us to develop such work. Also, by performing the reaction in 1,1,2,2-tetrabromoethane it may be possible to add a bromine instead of chlorine to the nickel center as this compound acts as a source of HBr in the presence of phosphines. At the suggestion of reviewers, it would be extremely interesting to examine the temperature variant magnetic properties of compound 4 and its congeners. We thank the reviewers for their positive suggestions.
Supplementary Materials
Detailed NMR spectra in a range of solvents are presented. Crystallographic data for [1,2-(C
6H
4-CH
2PtBu
2)
2Mo(CO)
4], [1,2-(C
6H
4-H
2P(S)
tBu
2)
2], [2-(C
6H
4-CH
2P(H)tBu
2-1-(CH
2PtBu
2NiCl
3)], [1,2-(C
6H
4-CH
2PiBu2)
2NiCl
2], [Ni(DMF)
6)]
2+[NiCl
4]
2-, [Ni(Cl)
2(H
2O)
2(DMF)
2] are available as supplementary materials. CCDC 2421627 and 2421631-5 respectively also contain the supplementary crystallographic data for this paper. These CCDC data can be obtained free of charge via
http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from CCDC, 12 Union Road, Cambridge, CB2 1EZ. Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). Total 114 pages.
Author Contributions
I.R.B.: investigations, experimental work, writing, reviewing and editing, supervision, methodology, conception. P.N.H.: crystallography, data deposition, writing; W.C.: proofing, crystallography work, director of crystallographic facilities; S.J.C.: Director of National Crystallographic Services and project overseer; K.M.F. synthesis, thesis work; M.D. early crystallography (2004); K. S.: synthetic work and experimental work. All authors have read and agreed to the published version of the manuscript.
Funding
We thank EPSRC for funding the National Crystallography Service (both Southampton and Daresbury: grant number for synchrotron work was EP/D07746X/1). No additional external funding was used for the synthetic work.
Data Availability Statement
All requisite data is available in the Supplementary Information.
Acknowledgments
The authors would like to thank Dr Mohammed Al-mashhadani who helped us with the many thermochromic data runs. You are a star. We all thank the EPSRC National Crystallographic Services for grants and facilities and the EPSRC National Mass Spectrometry Centre and their staff based at Swansea University. Crystallographic work was carried out at the EPSRC National Crystallography Centre based at the University of Southampton and at the Daresbury Laboratory Synchrotron Radiation Source. We thank the staff of both these institutions for their excellent and painstaking work. Additional mass spectroscopic data was also obtained in house, and we thank the technical staff based in Bangor. IRB thanks Patrick Murphy for his reliable support and lab provision which enabled this work to be done.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicholls, D. The Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic Chemistry, Pergamon texts in Inorg. Chem. 2013, 24, Elsevier, 2013. (199 pages). eBook ISBN: 9781483146430.
- Blanchard, S.; Neese, F.; Bothe, E.; Weyhermüllerb E. B.T.; Wieghardt, Square planar vs tetrahedral coordination in diamagnetic complexes of nickel(II) containing two bidentate pi-radical monoanions. K. Inorg. Chem., 2005, 44, 3636–3656. [CrossRef]
- Collinson, S.R.; Schröder, M. Nickel: Inorganic & Coordination Chemistry, Encyclopaedia of Inorganic Chemistry, 2006. Wiley. [CrossRef]
- Lomjanský, D.; Rajnák, C.; Titiš, J.; Moncoľ, J.; Smolko, L.; Boča, R. Impact of tetrahedral and square planar geometry of Ni(II) complexes with (pseudo)halide ligands to magnetic properties, Inorg. Chim. Acta, 2018, 483, 352-358. [CrossRef]
- Cope, J.D.; Denny, J.A.; Lamb, R.W.; McNamara, L.E.; Hammer, N.I.; Webster, C.E.; Hollis, T.K. Electrocatalytic reduction of CO2 with CCC-NHC pincer nickel complexes, J. Organometal. Chem., 2017, 845, 258-265. And references therein. [CrossRef]
- Venanzi, L.M. Tetrahedral complexes of nickel (II) and the factors determining their formation, J. Inorg. Nucl. Chem., 1958, 8, 137-142. [CrossRef]
- Balakrishnan, K.P. Preparation and properties of nickel(II) complex dyes, J. Chem. Eng. Data, 1980, 25, 186-187. [CrossRef]
- W. Clegg, W.; Eastham, G.R.; Elsegood, M.R.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R.; Zacchini, S. Highly active and selective catalysts for the production of methyl propanoate via the methoxycarbonylation of ethene, Organometallics, 2002, 21, 1832-1840. [CrossRef]
- Bellabarba, R.M.; Tooze, R.P.; Slawin, A.M.Z. Synthesis, X-ray characterisation and reactions of a trigonal planar palladium(0) carbonyl complex, (tbpx)PdCO, Chem Commun., 2003, 15, 1916-1917. [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R.; Zacchini,S. Synthesis and reactivity of palladium hydrido-solvento complexes, including a key intermediate in the catalytic methoxycarbonylation of ethene to methyl propanoate, J. Chem. Soc. Dalton Trans., 2002, 17, 3300-3308. [CrossRef]
- Fanjul, T.; Eastham, G.; Floure, J.; Forrest S.J.; Haddow, M.F.; Hamilton, A.; Pringle, P.G.; Orpen, A.G.; Waugh, M. Interplay of bite angle and cone angle effects. A comparison between o-C6H4(CH2PR2)(PR'2) and o-C6H4(CH2PR2)(CH2PR'2) as ligands for Pd-catalysed ethene hydromethoxycarbonylation. Dalton Trans., 2013, 42,100-115. [CrossRef]
- Fanjul, T; Eastham, G.; Fey, N.; Hamilton, A.; Orpen, A.G.; Pringle, P.G.; Waugh, M. Palladium complexes of the heterodiphosphine o-C6H4 (CH2PtBu2)(CH2PPh2) are highly selective and robust catalysts for the hydromethoxycarbonylation of ethene. Organometallics, 2010, 29,2292-305. [CrossRef]
- Fortune, K.M; Castel, C.; Robertson, C.M.; Horton, P.N.; Light, M.; Coles, S.J.; Waugh, M.; Clegg, W.; Harrington, R.W.; Butler, I.R. Ferrocenylmethylphosphanes and the Alpha Process for Methoxycarbonylation: The Original Story, Inorganics, 2021, 9, 57. [CrossRef]
- Butler, I.R.; Baker, P.K.; Eastham, G.R.; Fortune, K.M.; Horton, P.N.; Hursthouse, M.B. Ferrocenylmethylphosphines ligands in the palladium-catalysed synthesis of methyl propionate. Inorg. Chem. Commun., 2004, 7, 1049-1052. [CrossRef]
- Butler, I.R.; Horton, P.N.; Fortune, K.M.; Morris, K.; Greenwell, C.H.; Eastham, G.R.; Hursthouse, M.B. The first 1,2,3-tris(phosphinomethyl)ferrocene. Inorg. Chem. Commun., 2004, 7, 923-928. [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Tooze, R.P.; Wang, L. Highly active and selective catalysts for the production of methyl propanoate via the methoxycarbonylation of ethene. Chem. Commun., 1999, 1877-1878. [CrossRef]
- Knight, J.G.; Doherty, S.; Harriman, A.; Robins, E.G.; Berham, M.; Eastham, G.R.; Tooze, R.P.; Elsegood, M.R.J.; Champkin, P.; Clegg, W. Remarkable Differences in Catalyst Activity and Selectivity fo the Production of Methyl Propanoate versus CO−Ethylene Copolymer by a Series of Palladium Complexes of Related C4-Bridged Diphosphines. Organometallics, 2000, 19, 4957-4967. [CrossRef]
- Bowen Li, B.; Wang, Z.; Luo, Y.; Wei, H.; Chen, J.; Liu, D.; Zhang, W, Nickel-catalyzed asymmetric hydrogenation for the preparation of α-substituted propionic acids. Nat Commun. 2020, 15, 5482- . [CrossRef]
- Ward, L.G.L. Anhydrous nickel(II) halides and their tetrakis(ethanol) and 1,2-dimethoxyethane complexes, Inorganic Syntheses, 1971, 13, 154-64. https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470132449.ch30?msockid=2387c07414a862d13e0ed482153763cf.
- Butler, I.R.; Williams, R.M.; Heeroma, A.; Horton, P.N.; Coles, S.J.; Jones, L.F. The Effect of Localized Magnetic Fields on the Spatially Controlled Crystallization of Transition Metal Complexes. Inorganics 2025, 13, 117. [CrossRef]
- Horton, P.N.; Coles, S.J.; Clegg, W.; Harrington, R.W.; Butler, I.R. A Rapid General Synthesis and the Spectroscopic Data of 2,2′-Bis-(di-isopropylphosphino)-1,1′-dibromoferrocene, (bpdbf), 1,1′,2,2′-Tetrakis-(di-isopropylphosphino) Ferrocene, (tdipf) and Related Ligands: Taking dppf into the Future. Inorganics, 2025, 13, 10. [CrossRef]
- Fortune, K.; PhD thesis, Bangor University, 2004. Nitrogen donor complexes of molybdenum and tungsten and new routes to bis-1,2 & tris-1,2,3 substituted ferrocenes. https://research.bangor.ac.uk/portal/files/50696506/K_M_FORTUNE_PhD_2004_OCR.pdf.
- Oberhauser, W.; Bachmann, C.; Stampfl, T.; Haid, R.; Brüggeller, P. Structural differences in nickel(II) and palladium(II) complexes containing cis-1,2-bis(diphenylphosphino)ethene or 1,2-bis(diphenylphosphino)ethane, Polyhedron, 1997, 16, 2827-2835. [CrossRef]
- Donahue C.M.; McCollom, S.P.; Forrest C.M.; Blake A.V.; Bellott, B.J.; Keith, J.M.; Daly, S.R. Impact of Coordination Geometry, Bite Angle, and Trans Influence on Metal-Ligand Covalency in Phenyl-Substituted Phosphine Complexes of Ni and Pd. Inorg Chem., 2015, 54, 5646-59. Epub 2015 May 21. Erratum in: Inorg Chem. 2015 Sep 8;54(17):8857. doi: 10.1021/acs.inorgchem.5b01794. [CrossRef]
- Wu, K., Doyle, A. Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects. Nature Chem., 2017, 9, 779–784. [CrossRef]
- Li, B.; Wang, Z.; Luo, Y.; Wei, H.; Chen, J.; Liu, D.; Zhang, W. Nickel-catalyzed asymmetric hydrogenation for the preparation of α-substituted propionic acids. Nat. Commun., 2024, 15, 5482-. [CrossRef]
- Hay, R.W.; Albedyhl, S.; Lightfoot, P. The crystal structure of [Ni(dmf)6][NiCl4] and comments on the hydrolysis of coordinated amides and peptides in metal complexes. Transition Metal Chemistry, 1998, 23, 257–260. [CrossRef]
- Du, J.-L.; Li, L.-J.; Synthesis And Crystal Structure Of Nickel Complex, Ni[(DMF)4(H2O)2 ]·Br2. Inorg. Chem., An Indian Journal, 2007, 2, 88-90. https://www.tsijournals.com/articles/synthesis-and-crystal-structure-of-nickel-complex-nidmf4h2o2br2.pdf.
- Bogachev, N.A., Tsyrul’nikov, N.A., Makarova, A.A.; Tolmachev, M.V.; Starova, L.; Yu, M.; Skripkin, Yu.M.; Nikol’skiiet, A.B. Solubility of d-Element Salts in Organic and Aqueous-Organic Solvents: VII. Structure of Nickel Chloride Solvatocomplexes. Russ. J. Gen. Chem., 2019, 89, 859–864. [CrossRef]
- Butler, I.R.; Coles, S.J.; Horton, P.N. On the self-assembly of a trans-dibromo-bis-(dppfo2) iron (III), a ferrocene-ligand complex, dppfo2 = [(ƞ5-C5H4P(O)Ph2)2Fe]: Letting nature do the work, Inorg. Chem. Commun., 2018, 97, 166-170. [CrossRef]
- Pathania, A.; Bagchi, S. Slowdown of solvent structural dynamics in aqueous DMF solutions, Chemical Physics Impact, 2024, 9, 100711. [CrossRef]
- Additional useful general refs for readers and reviewers.
- Keskin, S.G. Julie M. Stanley, J.M; Alan H. Cowley, A.H. Synthesis, characterization and theoretical investigations of molybdenum carbonyl complexes with phosphorus/nitrogen/phosphorus ligand as bidentate and tridentate modes, Polyhedron, 2017, 138, 206-217. [CrossRef]
- Laine, T.V.; Klinga, M.; Leskela, M. Pyridinylimine-based nickel(II) and palladium(II) complexes: preparation, structural characterization and use as alkene polymerization catalysts. Eur. J. Inorg. Chem., 1999, 6, 959-964. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).