1. Introduction
Skin aging is a multifaceted process based on two major underlying intrinsic and extrinsic mechanisms. While intrinsic skin aging is associated with chronological changes and cellular senescence, extrinsic skin aging is the accumulation of environmental factors, such as ultraviolet (UV) radiation and pollution. Extrinsic aging, also referred to as photoaging, implicates the primary role of UV rays in skin aging [
1]. Among the various signaling pathways involved in photoaging, the transforming growth factor-β (TGF-β) signaling, mitogen-activated protein kinase (MAPK)/activator protein-1 (AP-1) signaling, nuclear factor kappa B (NF-κB), and nuclear factor erythroid 2-related factor (Nrf2) pathways have been suggested as the major signaling pathways [
2]. While most of these signaling mediators are released from epidermal keratinocytes and dermal fibroblasts, the potential roles of adipose tissue in skin aging have recently been proposed [
3]. In addition to the mechanical and physical changes associated with subcutaneous white adipose tissue, especially in the facial area, physiological interaction of adipocytes with dermal fibroblasts modulating the biological activities of fibroblasts has also been reported [
4].
Adipokines are biologically active peptides mainly produced by adipocytes and include leptin, tumor necrosis factor (TNF)-⍺, plasminogen activator inhibitor-1 (PAI-1), and adiponectin [
5]. However, many adipokines are also expressed and secreted by nonadipocytes. Adiponectin, an important adipokine, plays a critical role in metabolic regulation and exhibits potential protective effects in various physiological systems including the skin [
6]. While it is primarily secreted by adipose tissue, epidermal keratinocytes and dermal fibroblasts also express adiponectin receptors, AdipoR1 and AdipoR2, suggesting potential paracrine effects of adiponectin in skin cells [
7]. In addition to its potential roles in cutaneous inflammatory responses [
8,
9] and collagen production and degradation [
10], adiponectin is involved in UV-induced skin damage. Specifically, attenuation of adiponectin expression was observed in UV-exposed skin area, and experimental ablation of adiponectin induced the photoaging-like symptoms of stimulating matrix metalloproteinase-1 (MMP-1) expression and inhibiting procollagen synthesis in cultured human dermal fibroblasts [
11]. Similar photoprotective effects of adiponectin were observed in UVA-exposed dermal fibroblasts. Increased expression of MMP-1, MMP-3, and cyclooxygenase (COX)-2, as well as decreased expression of type I and III collagens, following UVA exposure were partially rescued by treatment with adiponectin [
12]. Despite the potential anti-UV efficacies of adiponectin, the clinical efficacy of adiponectin or adiponectin modulators in skin care has not been well studied.
Previously, we reported that heptasodium hexacarboxymethyl dipeptide-12 and its derivatives have autophagy-stimulating activities in the skin. Autophagy is a lysosome-dependent cellular recycling process essential for maintaining proper cellular homeostasis, and by stimulating autophagy signaling, various cosmetic benefits, including the reduction of cellular senescence [
13] and improvement of skin barrier function [
14], were observed. Recently, we also reported that autophagy-stimulating peptide derivatives can provide soothing effects on sensitive skin [
15]. Based on the close interrelationship between autophagy and adiponectin [
16,
17], we screened an autophagy activator library for potential adiponectin-stimulating candidates. We identified a new peptide derivative, pentasodium tetracarboxymethyl hexanoyl dipeptide-12 (PTHD-12), that stimulates adiponectin expression in dermal fibroblasts.
In this study, using the adiponectin stimulating peptide (PTHD-12), we explored the effects of adiponectin on UV-induced skin damages using in vitro and ex vivo testing. The clinical efficacy of adiponectin stimulating peptide in UV-exposed skin was further examined in a double-blind, randomized, placebo-controlled study.
2. Materials and Methods
2.1. Materials
The test peptide pentasodium tetracarboxymethyl hexanoyl dipeptide-12 (PTHD-12) and its precursor heptasodium hexacarboxymethyl dipeptide-12 were prepared using solid-phase peptide synthesis with fluorenylmethyloxycarbonyl chloride. After synthesis, the test material was purified by reverse-phase high-performance liquid chromatography (RP-HPLC).
2.2. In Vitro Studies
Normal human dermal fibroblasts (hDF) and the culture media used in this study were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). hDF was maintained using human fibroblast expansion basal medium with low serum growth supplement and gentamicin/amphotericin purchased from Thermo Fisher Scientific Inc. Cells were cultured and maintained at 37 °C in a 5% CO
2 atmosphere. For quantitative real-time polymerase chain reaction (qRT-PCR), HDF were seeded in 6 well (1 × 10
5 cells/well) and cultured for 24 h. After removing the culture medium and washing, the cells were treated with either serum-free medium or the test peptide at a concentration of 40 ppm and further incubated for 24 h. After washing the cells with phosphate-buffered saline solution (pH 7.4), 50mJ/cm2 of UVB was irradiated, and cells were further cultured for 24 h. Then, 30 ng of cDNA was prepared from the total RNA fraction of cell lysates, as described previously [
18], and PCRs were performed using the QuantStudio™ 3 system (Thermo Fisher Scientific Inc.) according to the manufacturer’s instructions. The following primer sets were used for quantitation: adiponectin, 5’-CAGATGCCCCAGCAAGTGTA-3’ and 5’ 5’-TCAGAAACAGGCACACAACTCA-3’; MMP-1, 5’- CTCGCTGGGAGCAAACACA-3’ and 5’-CTTGGCAAATCTGGCGTGTA-3; and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5’-GGAGTCAACGGATTTGGTCGTA-3’ and 5’-GCAACAATATCCACTTTACCAGAGTTAA-3.’ Gene expression levels were calculated as cycle threshold (Ct) values using the 2
−ΔΔCT quantification method and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
To measure protein quantity, HDF were seeded in 6 well (2 × 104 cells/well) and cultured for 24 h. After removing the culture medium and washing, the cells were treated with the test peptide for 24 h and cell lysates were harvested. Cell lysates were then separated by SDS-PAGE and proteins were blotted onto nitrocellulose membranes. After blocking with 0.5% bovine serum albumin solution containing 0.1% Tween-20, the membranes were incubated with primary antibodies overnight at 4 °C. Secondary antibodies conjugated with horseradish peroxidase were incubated for one hour at room temperature, and protein quantity was measured using ECL detection reagent (Amersham Biosciences Corp., Little Chalfont, UK) using the ChemiDoc system (Alliance Mini HD 9, Uvitec, Cambridge, UK).
To observe adiponectin expression in dermal fibroblasts, the cells were seeded on a 2-well cell culture slide and cultured for 24 h. After UVB exposure and sample treatment, cells were fixed with 4% paraformaldehyde solution for 20 min. After rinsing, the cells were permeabilized with 0.1% Triton X-100 in PBS solution for 10 min at room temperature and treated with 5% bovine serum albumin solution for 60 min. The cells were then incubated with mouse anti-human adiponectin antibody (ab22554; Abcam, Cambridge, MA, USA) overnight at 4°C. After rinsing, the cells were treated with goat anti-mouse IgG H&L (Alexa Fluor® 568, ab175473, Abcam) for 60 min at room temperature and examined under a conformal LASER scanning microscope (LSM 880 with Airyscan, Carl Zeiss, Oberkochen, Germany).
Expression of interleukin-8 (IL-8) was measured using an ELISA kit (R&D Systems, Cat. No. DY208) according to the manufacturer’s instructions, as previously described [
19].
2.3. Ex Vivo Studies
NativeSkin® ex vivo human skin explant model was procured from GeneSkin SAS (Toulouse, France). A 24-year-old female Caucasian donor with type 3 Fitzpatrick skin type, with no history of allergy aordermatological disorder, underwent abdominoplasty, and anonymized human skin samples were retrieved. Ethical compliance was enacted through official authorization from the French Ministry of Research (Protocol AC-2022-4863, October 14, 2022), and the study was performed in accordance with the principles of the Declaration of Helsinki. After surgery, the skin tissues were harvested and the subcutaneous adipose tissues were carefully excised from the skin. 11 mm diameter punch biopsies were taken, and tissues were embedded in a proprietary biological matrix in Transwell inserts (Millicell, Merck KGaA, Darmstadt, Germany), following the proprietary protocol developed by Genoskin. The epidermal surface of the skin tissue was maintained in direct contact with air, and the dermal side was immersed in the biological matrix. The tissue was cultured in a chemically defined, serum-free medium with 100 µg/mL penicillin and 100 µg/mL streptomycin, and cultivated in a humidified chamber with 5% CO2 at 37 °C. After receiving the tissues, the skin models were stabilized in a 12-well culture plate containing 1 mL of maintenance medium (Genoskin) in a 5% CO2 humidified chamber. After 2 h of stabilization, the culture medium was replaced with a fresh maintenance medium and maintained until the next step. After irradiation with 50mJ/cm2 of UVB on the epidermal surface of the skin tissue, either 25 μL of test sample solution or blank solution was topically applied onto the skin surface once a day for 2 days. At 24 hours after the second application, tissues were harvested, fixed with 4% neutral buffered formalin solution overnight at 4°C, and embedded in paraffin. For immunohistochemical staining of the tissue, 5μM thick tissue sections were deparaffinized and rehydrated before heat-induced epitope revival treatment in an antigen retrieval solution (ab937, Abcam) at pH 6.0, for 10 min at 97 °C, followed by cooling for 10 min in a cooling chamber. After blocking non-specific antibody binding using a blocking reagent (X0909, Agilent, Santa Clara, CA, USA), mouse anti-human anti-adiponectin (Abcam, ab22554) antibody was applied to the tissue and incubated in a humidified chamber overnight at 4°C. Epidermal expression of interleukin-6 and tumor necrosis factor-α was observed using mouse anti-human TNF-⍺ antibody (ab9579, Abcam) and rabbit anti-human IL-6 antibody (ab6672, Abcam), respectively. Either goat anti-rabbit immunoglobulin (IgG) H&L (Alexa Fluor® 488, ab150077, abcam) or goat anti-mouse IgG H&L (Alexa Fluor® 568, ab175473, abcam) were added to the tissues and fluorescence intensity was analyzed under a fluorescence microscope (Eclipse Ni-U, Intenslight C-HGFI, DS-Ri2, Nikon, Tokyo, Japan) at 400 × magnification. All experiments were performed in triplicate.
2.4. Clinical Efficacy Testing
To assess the clinical efficacy of the test peptide against UV-induced skin damage, a double-blind, randomized, placebo-controlled study was performed. 20 healthy female volunteers (46.7 ± 4.4 years old: mean ± standard deviation) were enrolled. The following exclusion criteria were adopted to select participants.
Pregnant or lactating women, and women with potential for pregnancy
Individuals who used topical steroid-containing dermatological drugs within the past one month for skin disease treatment
Participants who have engaged in the similar clinical testing within the past 6 months
Individuals who have dermatological abnormalities such as pigmentation spots, acne, erythema, and/or telangiectasia
Individuals who have used cosmetics or pharmaceutical products with identical or similar efficacy on the test site within the past 3 months
Individuals who received aesthetic procedures on the test site within the past 6 months
Other individuals who were considered unsuitable for testing at the principal investigator’s discretion.
This study was approved by the Institutional Review Board of the Mariedm Skin Research Center (approval number: MDSRC-2400PR-168) (8th, November 2024). All the studies complied with the World Medical Association’s Declaration of Helsinki (2013) concerning biomedical research involving human subjects. All volunteers signed an informed consent form and participated in the study after the purpose and protocol were explained to them. UV rays of 60.2 mJ/cm
2, which was equivalent to 2 minimal erythema dose (MED) energies, were irradiated to the dorsal areas. Before and immediately after irradiation, and at 3 days and 7 days after irradiation, trans-epidermal water loss (TEWL) and skin redness were measured in air-conditioned environments (temperature 22 ± 2 °C; relative humidity 50% ± 10%) after an acclimatization period of at least 30 min. TEWL, which represents the epidermal permeability barrier function, was measured using a Vapometer (Delfin, Kuopio, Finland), and skin redness was measured using a Spectrophotometer CM-2600d (Konica Minolta, Inc., Osaka, Japan). Skin images were also acquired using an Antera 3D CS (Miravex, Dublin, Ireland). After the baseline measurement, participants were administered either the test product or placebo product at randomly chosen sites twice a day for 7 days. The complete information regarding the test products is provided in
Table 1.
2.5. Statistical Analysis
Values are expressed as the arithmetic mean ± standard deviation. Data normality was verified using the Shapiro-Wilk test and Kurtosis/Skewness analysis, while inter-group homogeneity was verified using one-way ANOVA and Tukey’s HSD post-hoc test. To compare the pre- and post-usage results and between-group differences, Repeated Measures Analysis of Variance (RM-ANOVA) was used when normality was satisfied, and the Friedman test was used when normality was not satisfied. Statistical analyses were performed using SPSS ver. 20 (IBM, New York, USA) and significance level was set at p<0.05 for all statistical results, and the improvement rate (%) based on changes before and after product use was calculated using the mean values of all subjects’ measurements, according to the following formula.
3. Results
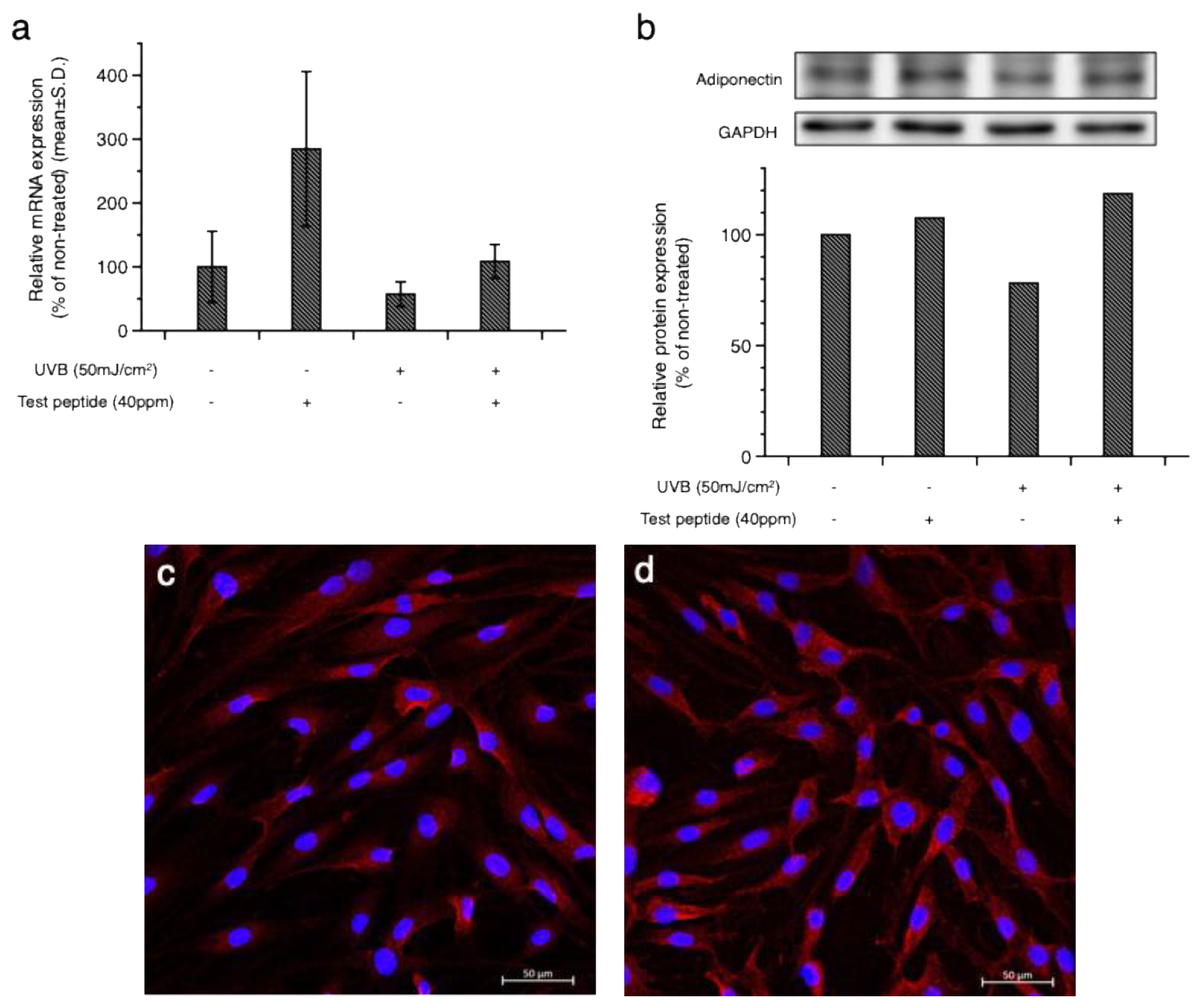
To address the change in adiponectin expression in dermal fibroblasts after UV exposure, cultured human dermal fibroblasts were exposed to 50 mJ/cm
2 UVB, and the mRNA and protein expression of adiponectin were measured by quantitative RT-PCR and Western blotting, respectively. As shown in
Figure 1, a slight but significant reduction in both mRNA and protein levels was observed in the UVB-exposed cells. Based on a preliminary cytotoxicity assessment, the concentration of the test peptide for
the in vitro studies was decided as 40ppm, which did not show any cytotoxicity. Co-treatment with the test peptide restored adiponectin expression (
Figure 1a and b), and confocal microscopic observation further confirmed the increased expression of adiponectin in the cytoplasmic region by the test peptide (
Figure 1d) compared to the non-treated cells (
Figure 1c).
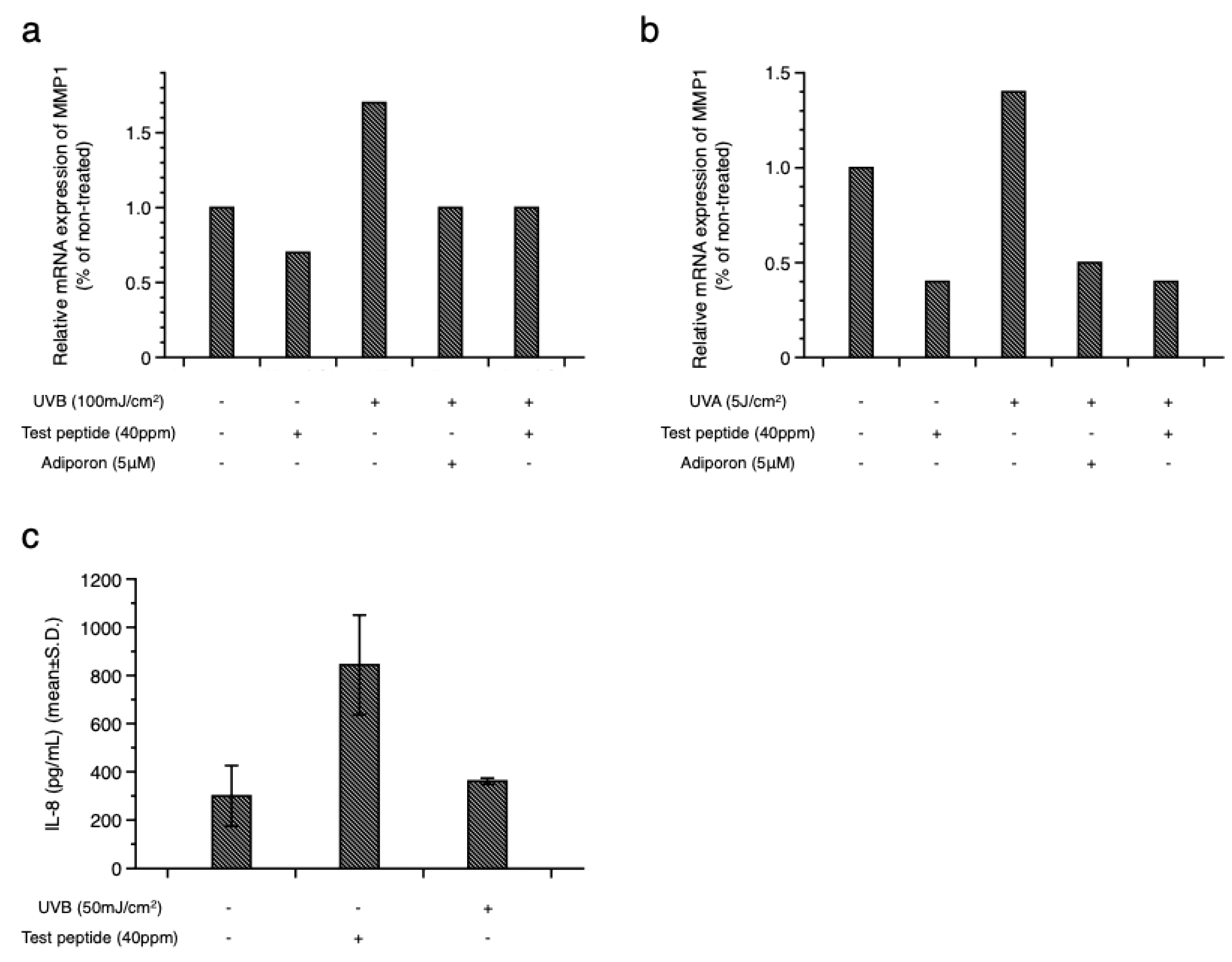
To investigate whether the increased expression of adiponectin could alleviate UV-induced cellular damage, the expression of inflammatory mediators was examined. As shown in
Figure 2, the increased expression of matrix metalloproteinase (MMP)-1 induced by UVB (
Figure 2a) and UVA (
Figure 2b) was downregulated by the test peptide. Treatment with AdipoRon (AR), an adiponectin receptor agonist, showed a similar inhibitory activity on MMP-1 expression, suggesting the potential involvement of adiponectin signaling in UV-induced skin damage. Interestingly, treatment of normal cells with the test peptides also decreased the expression of MMP-1 in dermal fibroblasts, suggesting a potential application of adiponectin stimulating peptide for anti-wrinkle purposes. Measurement of interleukin (IL)-8, an inflammatory cytokine, in cultured human epidermal keratinocytes further confirmed the anti-inflammatory activity of the test peptide (
Figure 2c).
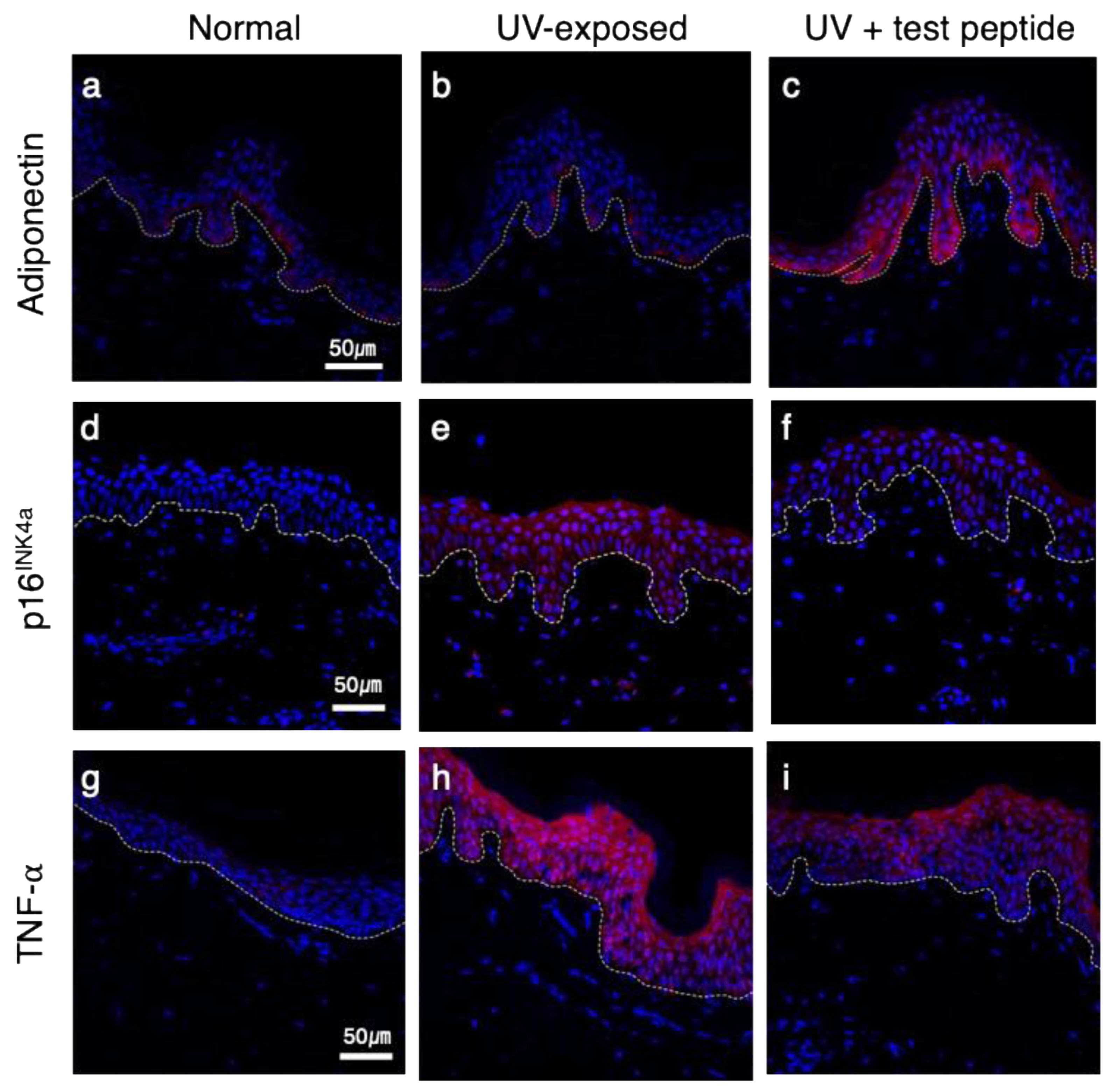
Next, we evaluated the biological activity of the test peptide using an ex vivo human skin explant model. Skin tissues obtained from healthy donor were exposed to UVB rays, and changes in adiponectin and other inflammatory markers were assessed by immunohistochemical staining. As shown in
Figure 3, a modest expression of adiponectin in the basal layer was observed in normal skin (
Figure 3a), and UVB exposure did not result in significant changes (
Figure 3b). However, topical application of the test peptide after UVB exposure significantly upregulated adiponectin expression in both basal and spinous layers (
Figure 3c). UVB exposure also resulted in increased expression of p16
INK4a protein (
Figure 3e), as a marker of senescent cells [
20], compared to non-exposed tissue (
Figure 3d), which is consistent with previous reports [
8,
21]. Interestingly, topical application of adiponectin stimulating peptide also prevented the expression of p16
INK4a after UVB exposure (
Figure 3f). Epidermal expression of tumor necrosis factor-⍺ (TNF-⍺) also showed similar changes, which were significantly increased by UV exposure (
Figure 3h) and decreased by the test peptide application (
Figure 3i). Adiponectin upregulates the
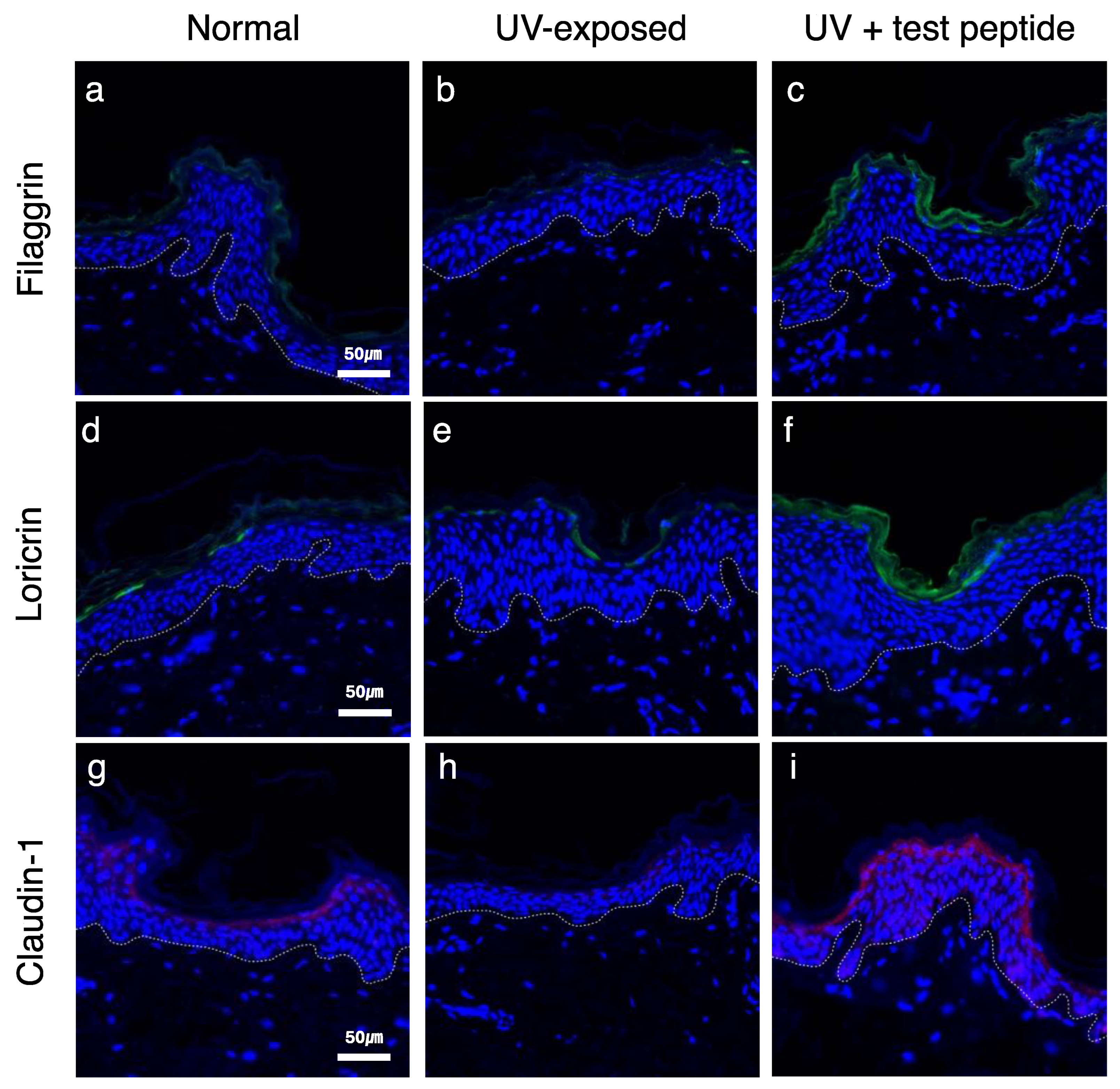
filaggrin (
FLG) gene expression in cultured human epidermal keratinocytes [
22]. Considering the crucial role of filaggrin protein in skin barrier function, the beneficial effects of adiponectin on the skin barrier were further examined. Filaggrin and loricrin were expressed in suprabasal layer in normal tissue (
Figure 4a and d) and significantly attenuated by UVB exposure (
Figure 4b and e). Topical application of adiponectin stimulating peptide restored barrier protein expression (
Figure 4c and f), and similar changes were also observed for claudin-1, which constitutes the tight junction. Collectively, these results suggest that stimulation of adiponectin expression can provide potential anti-aging and skin barrier-enhancing effects on UVB-induced photoaging.
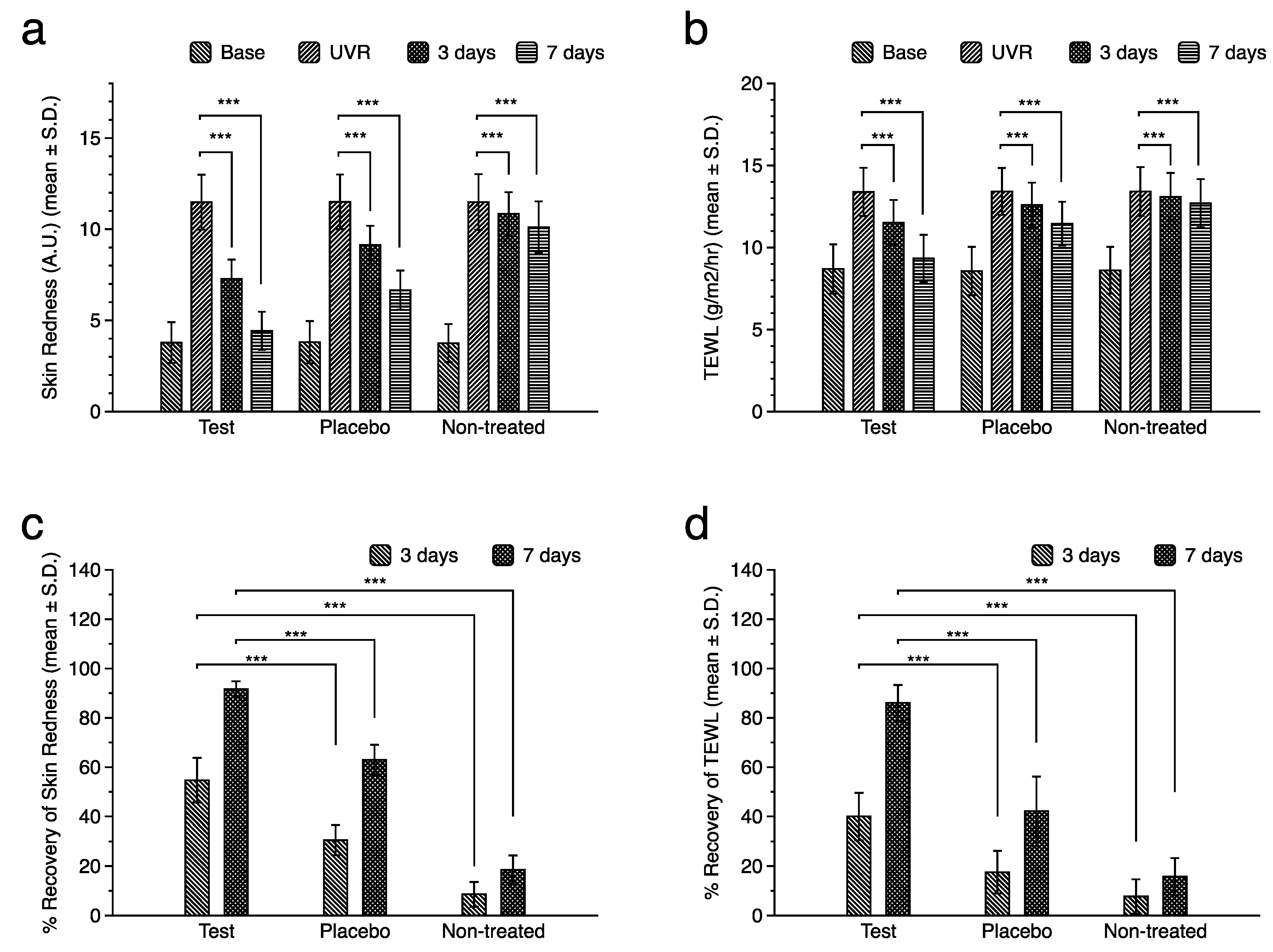
To verify whether the observed effects manifested clinically, a double-blind, randomized, placebo-controlled clinical study was performed. Twenty healthy female volunteers (mean age, 46.7 years old ± standard deviation of 4.4 years) participated and UVB radiation of 2 minimal erythema dose (MED) energy was irradiated on the dorsal area. Skin redness and trans-epidermal water loss (TEWL) were measured to examine their effects on skin irritation and barrier function. Significant increases in both skin redness, represented by a-values and TEWL values, were observed after UVB irradiation (
Figure 5a and b). While spontaneous restoration of skin redness and skin barrier function was observed in the non-treated site, topical application of the test product significantly accelerated the recovery rate (
Figure 5c and d) compared to the placebo-treated site. These results substantiated the clinical efficacy of adiponectin stimulating peptide in UVB-induced skin damage.
4. Discussion
Various signaling molecules, including transforming growth factor-beta (TGF-β), mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), and nuclear factor erythroid 2–related factor 2 (Nrf2), have been reported to be important factors in skin photoaging. While most signaling molecules originate from either epidermal or dermal tissues, the potential involvement of adipose tissue and adipocytes in skin aging has been recently proposed [
23]. In addition to the important roles of adipose tissue in providing the structural integrity and mechanical properties of the skin, the signaling molecules generated from the adipose tissue, including adipokines, also play crucial roles in the regenerative capacity and immune functions of the skin [
3]. Previous studies have reported that adiponectin suppresses the production of pro-inflammatory cytokines such as interleukin (IL)-17, tumor necrosis factor (TNF)-α, and IL-6, and genetic ablation of adiponectin exacerbates the inflammatory responses in an animal model of psoriasis by enhancing the infiltration of IL-17-producing T cells [
24]. In this study, potential anti-inflammatory and senopreventive activities of adiponectin stimulating peptide were observed. The increased expression of p16
INK4a, a well-known senescent cell marker, and TNF-α induced by UVB exposure was significantly prevented by PTHD-12 application. A similar skin soothing activity has also been observed in a clinical study. By irradiating UVB with 2 MED energy, skin irritation, represented by increased skin redness, can be induced. Use of PTHD-12 containing product significantly accelerated the restoration of skin redness, suggesting that the stimulation of adiponectin expression can provide potential soothing effects.
Adiponectin also promotes wound healing, mainly by upregulating epidermal keratinocyte proliferation and migration [
7]. The cosmetic benefits expected from adiponectin include anti-wrinkle activities by boosting the production of hyaluronic acid (HA) and collagen by dermal fibroblasts, contributing to the maintenance of the extracellular matrix and structural integrity of the skin [
10]. The potential protection of UV-induced skin damage by reducing the oxidative stress and inflammatory responses by adiponectin [
25], which leads to the reduction of matrix metalloproteinases (MMPs), also helps to mitigate photoaging. From in vitro experiments, we also observed that UVA- and UVB-induced MMP-1 expression was prevented by adiponectin stimulating peptide PTHD-12 treatment. However, owing to its short testing period, we could not assess the clinical anti-aging effects of PTHD-12, which guarantees the need for further long-term studies.
Another important activity of adiponectin in the skin is the modulation of skin barrier function. Previous studies have reported that adiponectin treatment upregulated the synthesis of epidermal lipids, including ceramides [
26] and filaggrin [
27], which constitute the intercellular lipids and corneocytes of the stratum corneum. Stimulation of lipid and protein synthesis by adiponectin can enhance skin barrier function. In this study, we also observed increased expression of barrier marker proteins filaggrin and loricrin, as well as claudin-1, following PTHD-12 treatment. Claudin-1 is a major component of epidermal tight junctions, which create a dynamic paracellular barrier that regulates epidermal permeability [
28]. In clinical efficacy testing, accelerated recovery of trans-epidermal water loss, a functional parameter representing epidermal permeability barrier function, was also observed.
Although several studies have suggested the potential beneficial effects of adiponectin on skin aging, little has been reported regarding its clinical efficacy. In this study, we observed that UV-induced skin damage could be attenuated by adiponectin stimulating peptide treatment. While this study is the first report of the clinical effects of adiponectin stimulating peptide for cosmetic applications, it has a few limitations. First, the study was performed over a short period, and only acute effects were examined. The relatively small number of participants also precludes drawing broad generalizations from the results. Large-scale and long-term studies are required to confirm the anti-aging effects of these test peptides. Additional investigations of the effects of adiponectin on intrinsic aging are necessary. However, despite its limitations, this study suggests that targeting adiponectin may be a plausible strategy for developing new skin anti-aging ingredients.
Author Contributions
Conceptualization, H.K. and S.J.; methodology, Yongwoo K., S.K. and S.J.; investigation, Yongwoo K., S.Y., S.K. and Yeonjae K.; writing—original draft preparation, Yongwoo K. and S.J.; writing—review and editing, S.J., K.W., and H.K.; supervision, H.K; project administration, S.J. and H.K.; funding acquisition, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Mariedm Skin Research Center (approval number: MDSRC-2400PR-168) (8th, November 2024) for studies involving humans. The collection, manufacture, and use of skin models for research purposes were formally authorized by the French Ministry of Research (AC-2022-4863, October 14, 2022).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article and further inquiries can be directed to the corresponding author.
Conflicts of Interest
Yongwoo K. was employed by the company Kolmar Korea, S.Y., S.K., Yeonjae .K. and S.J. were employed by the company Incospharm Corp.. The remaining author declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
References
- Shin, S.H.; Lee, Y.H.; Rho, N.-K.; Park, K.Y. Skin Aging from Mechanisms to Interventions: Focusing on Dermal Aging. Front. Physiol. 2023, 14, 1195272, doi:10.3389/fphys.2023.1195272. [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268, doi:10.3390/antiox4020248. [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Skin Aging: Are Adipocytes the next Target? Aging 2016, 8, 1457–1469, doi:10.18632/aging.100999. [CrossRef]
- Wollina, U.; Wetzker, R.; Abdel-Naser, M.B.; Kruglikov, I.L. Role of Adipose Tissue in Facial Aging. Clin. Interv. Aging 2017, Volume 12, 2069–2076, doi:10.2147/CIA.S151599. [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556, doi:10.1210/jc.2004-0395. [CrossRef]
- Oh, J.; Lee, Y.; Oh, S.-W.; Li, T.; Shin, J.; Park, S.-H.; Lee, J. The Role of Adiponectin in the Skin. Biomol. Ther. 2022, 30, 221–231, doi:10.4062/biomolther.2021.089. [CrossRef]
- Shibata, S.; Tada, Y.; Asano, Y.; Hau, C.S.; Kato, T.; Saeki, H.; Yamauchi, T.; Kubota, N.; Kadowaki, T.; Sato, S. Adiponectin Regulates Cutaneous Wound Healing by Promoting Keratinocyte Proliferation and Migration via the ERK Signaling Pathway. J. Immunol. 2012, 189, 3231–3241, doi:10.4049/jimmunol.1101739. [CrossRef]
- Seo, H.-S.; Seong, K.H.; Kim, C.-D.; Seo, S.J.; Park, B.C.; Kim, M.H.; Hong, S.-P. Adiponectin Attenuates the Inflammation in Atopic Dermatitis-Like Reconstructed Human Epidermis. Ann. Dermatol. 2019, 31, 186–195, doi:10.5021/ad.2019.31.2.186. [CrossRef]
- Suh, J.H.; Lee, Y.; Jin, S.-P.; Kim, E.J.; Seo, E.Y.; Li, N.; Oh, J.-H.; Kim, S.J.; Lee, S.-H.; Lee, D.H.; et al. Adiponectin Prevents Skin Inflammation in Rosacea by Suppressing S6 Phosphorylation in Keratinocytes. J. Invest. Dermatol. 2024, S0022202X24019821, doi:10.1016/j.jid.2024.07.018. [CrossRef]
- Ezure, T.; Amano, S. Adiponectin and Leptin Up-regulate Extracellular Matrix Production by Dermal Fibroblasts. BioFactors 2007, 31, 229–236, doi:10.1002/biof.5520310310. [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, M.-K.; Kim, S.; Kim, J.Y.; Lee, D.H.; Chung, J.H. UV-Induced Inhibition of Adipokine Production in Subcutaneous Fat Aggravates Dermal Matrix Degradation in Human Skin. Sci. Rep. 2016, 6, 25616, doi:10.1038/srep25616. [CrossRef]
- Fang, C.-L.; Huang, L.-H.; Tsai, H.-Y.; Chang, H.-I. Dermal Lipogenesis Inhibits Adiponectin Production in Human Dermal Fibroblasts While Exogenous Adiponectin Administration Prevents against UVA-Induced Dermal Matrix Degradation in Hu-man Skin. Int. J. Mol. Sci. 2016, 17, 1129, doi:10.3390/ijms17071129. [CrossRef]
- Lim, C.J.; Lee, Y.-M.; Kang, S.G.; Lim, H.W.; Shin, K.-O.; Jeong, S.K.; Huh, Y.H.; Choi, S.; Kor, M.; Seo, H.S.; et al. Aquatide Activation of SIRT1 Reduces Cellular Senescence through a SIRT1-FOXO1-Autophagy Axis. Biomol. Ther. 2017, 25, 511–518, doi:10.4062/biomolther.2017.119. [CrossRef]
- Shin, K.O.; Lim, C.j.; Park, H. Y.; Kim, S.; Kim, B.; Lee, Y.; Chung, H.; Jeong, S.; Park, K.; K. Park. Activation of SIRT1 Enhances Epidermal Permeability Barrier Formation through Ceramide Synthases 2 and 3-Dependent Mechanisms. J Invest Dermatol 2020, 140, 1435–1438, doi:https://doi.org/10.1016/j.jid.2019.12.021. [CrossRef]
- Eun, S.; Lim, M.; Jung, J.; Shin, K.; Kim, S.; Kim, Y.; Nam, G.; Jeong, S.; Kim, H. Skin Barrier-Improving and Skin-Soothing Effects of Autophagy-Activating Peptide on Sensitive Skin. Cosmetics 2024, 11, 223, doi:10.3390/cosmetics11060223. [CrossRef]
- Xu, A.; Sweeney, G. Emerging Role of Autophagy in Mediating Widespread Actions of ADIPOQ/Adiponectin. Autophagy 2015, 11, 723–724, doi:10.1080/15548627.2015.1034418. [CrossRef]
- Zhou, L.; Liu, F. Autophagy Roles in Obesity-Induced ER Stress and Adiponectin Downregulation in Adipocytes. Autophagy 2010, 6, 1196–1197, doi:10.4161/auto.6.8.13478. [CrossRef]
- Lee, J.O.; Kim, Y.; Jang, Y.N.; Lee, J.M.; Shin, K.; Jeong, S.; Chung, H.-J.; Kim, B.J. ICP5249 Promotes Hair Growth by Activating the AMPK-Autophagy Signaling Pathway. J. Microbiol. Biotechnol. 2024, 34, 1810–1818, doi:10.4014/jmb.2406.06015. [CrossRef]
- Kim, S.; Kim, Y.; Jung, J.; Kim, H.-J.; Jeong, S.; Shin, H.; Ryu, W.-S.; Sohn, J.-H.; Nam, G. Clinical Efficacy of Endocannabinoid-Mimetic Fatty Acid Amide as a Skin-Soothing Ingredient. Cosmetics 2024, 11, 225, doi:10.3390/cosmetics11060225. [CrossRef]
- Safwan-Zaiter, H.; Wagner, N.; Wagner, K.-D. P16INK4A—More Than a Senescence Marker. Life 2022, 12, 1332, doi:10.3390/life12091332. [CrossRef]
- Ahmed, N. Induced Expression of P16 and P21 Proteins in UVB-Irradiated Human Epidermis and Cultured Keratinocytes. J. Dermatol. Sci. 1999, 19, 175–181, doi:10.1016/S0923-1811(98)00068-1. [CrossRef]
- Jin, T.; Park, K.Y.; Seo, S.J. Adiponectin Upregulates Filaggrin Expression via SIRT1-Mediated Signaling in Human Normal Keratinocytes. Ann. Dermatol. 2017, 29, 407, doi:10.5021/ad.2017.29.4.407. [CrossRef]
- Liu, M.; Lu, F.; Feng, J. Aging and Homeostasis of the Hypodermis in the Age-Related Deterioration of Skin Function. Cell Death Dis. 2024, 15, 1–11, doi:10.1038/s41419-024-06818-z. [CrossRef]
- Shibata, S.; Tada, Y.; Hau, C.S.; Mitsui, A.; Kamata, M.; Asano, Y.; Sugaya, M.; Kadono, T.; Masamoto, Y.; Kurokawa, M.; et al. Adiponectin Regulates Psoriasiform Skin Inflammation by Suppressing IL-17 Production from γδ-T Cells. Nat. Commun. 2015, 6, 7687, doi:10.1038/ncomms8687. [CrossRef]
- Liu, Y.; Palanivel, R.; Rai, E.; Park, M.; Gabor, T.V.; Scheid, M.P.; Xu, A.; Sweeney, G. Adiponectin Stimulates Autophagy and Reduces Oxidative Stress to Enhance Insulin Sensitivity During High-Fat Diet Feeding in Mice. Diabetes 2015, 64, 36–48, doi:10.2337/db14-0267. [CrossRef]
- Hong, S.-P.; Seo, H.-S.; Shin, K.-O.; Park, K.; Park, B.C.; Kim, M.H.; Park, M.; Kim, C.-D.; Seo, S.J. Adiponectin Enhances Hu-man Keratinocyte Lipid Synthesis via SIRT1 and Nuclear Hormone Receptor Signaling. J. Invest. Dermatol. 2019, 139, 573–582, doi:10.1016/j.jid.2018.08.032. [CrossRef]
- Choi, S.Y.; Kim, M.J.; Ahn, G.R.; Park, K.Y.; Lee, M.-K.; Seo, S.J. The Effect of Adiponectin on the Regulation of Filaggrin Ex-pression in Normal Human Epidermal Keratinocytes. Ann. Dermatol. 2018, 30, 645, doi:10.5021/ad.2018.30.6.645. [CrossRef]
- Brandner, J.M. Importance of Tight Junctions in Relation to Skin Barrier Function. In Current Problems in Dermatology; Agner, T., Ed.; S. Karger AG, 2016; Vol. 49, pp. 27–37 ISBN 978-3-318-05585-6.
Figure 1.
Stimulation of adiponectin expression by the test peptides in human dermal fibroblasts. UVB exposure-induced attenuation of adiponectin expression was restored by test peptide treatment, both in mRNA and protein levels (a and b). Confocal microscopic observation further confirmed the increased expression of adiponectin by the test peptide in hDF cells (d), compared to non-treated hDF cells (c). (magnification: X400).
Figure 1.
Stimulation of adiponectin expression by the test peptides in human dermal fibroblasts. UVB exposure-induced attenuation of adiponectin expression was restored by test peptide treatment, both in mRNA and protein levels (a and b). Confocal microscopic observation further confirmed the increased expression of adiponectin by the test peptide in hDF cells (d), compared to non-treated hDF cells (c). (magnification: X400).
Figure 2.
Alleviation of UV-induced cellular damage by adiponectin stimulating peptide. Increased expression of MMP-1 by UVB (a) and UVA (b) was downregulated by both the test peptide and AdipoRon, an adiponectin receptor agonist, in cultured human dermal fibroblasts. The increased expression of interleukin (IL)-8 in UVB-exposed human epidermal keratinocytes was also decreased by test peptide treatment (c).
Figure 2.
Alleviation of UV-induced cellular damage by adiponectin stimulating peptide. Increased expression of MMP-1 by UVB (a) and UVA (b) was downregulated by both the test peptide and AdipoRon, an adiponectin receptor agonist, in cultured human dermal fibroblasts. The increased expression of interleukin (IL)-8 in UVB-exposed human epidermal keratinocytes was also decreased by test peptide treatment (c).
Figure 3.
Stimulation of adiponectin expression by test peptide in an ex vivo human skin explant model. Significantly increased expression of adiponectin was observed in test peptide-treated tissue (c) compared to non-irradiated (a) and UVB-exposed tissues (b). Epidermal expressions of p16INK4a, a marker of senescence cell, was also increased by UVB exposure (e), compared to non-irradiated tissue (d), which was prevented by test peptide application (f). [red: adiponection (a, b, c), p16INK4a (d, e, f) and TNF-⍺ (g, h, i); blue: DAPI; dotted line: dermal-epidermal junction] (magnification: X400).
Figure 3.
Stimulation of adiponectin expression by test peptide in an ex vivo human skin explant model. Significantly increased expression of adiponectin was observed in test peptide-treated tissue (c) compared to non-irradiated (a) and UVB-exposed tissues (b). Epidermal expressions of p16INK4a, a marker of senescence cell, was also increased by UVB exposure (e), compared to non-irradiated tissue (d), which was prevented by test peptide application (f). [red: adiponection (a, b, c), p16INK4a (d, e, f) and TNF-⍺ (g, h, i); blue: DAPI; dotted line: dermal-epidermal junction] (magnification: X400).
Figure 4.
Restoration of skin barrier marker proteins by adiponectin stimulating treatment UVB exposure-induced attenuation of protein expressions (b, e, h) were rescued by test peptide application (c, f, i). [green: filaggrin (a, b, c), loricrin (d, e, f) and claudin-1 (g, h, i); blue: DAPI; dotted line: dermal-epidermal junction] (magnification: X400).
Figure 4.
Restoration of skin barrier marker proteins by adiponectin stimulating treatment UVB exposure-induced attenuation of protein expressions (b, e, h) were rescued by test peptide application (c, f, i). [green: filaggrin (a, b, c), loricrin (d, e, f) and claudin-1 (g, h, i); blue: DAPI; dotted line: dermal-epidermal junction] (magnification: X400).
Figure 5.
Changes in skin redness and trans-epidermal water loss (TEWL) after UVB irradiation. Significant increases in skin redness (a) and TEWL (b) were observed after UVB irradiation, and topical application of either placebo product or test product significantly accelerated the recovery rate at 3 and 7 days after irradiation (c, d). The test group showed the highest recovery rate compared with the placebo and non-treated groups.
Figure 5.
Changes in skin redness and trans-epidermal water loss (TEWL) after UVB irradiation. Significant increases in skin redness (a) and TEWL (b) were observed after UVB irradiation, and topical application of either placebo product or test product significantly accelerated the recovery rate at 3 and 7 days after irradiation (c, d). The test group showed the highest recovery rate compared with the placebo and non-treated groups.
Table 1.
Clinical efficacy testing product information.
Table 1.
Clinical efficacy testing product information.
| Test Product |
Control Product |
| Aqua |
Aqua |
| Propanediol |
Propanediol |
| Diisobutyl adipate |
Diisobutyl adipate |
| Ethylhexyl triazone |
Ethylhexyl triazone |
| Diisopropyl sebacate |
Diisopropyl sebacate |
| Butyloctyl salicylate |
Butyloctyl salicylate |
| Polymethylsilsesquioxane |
Polymethylsilsesquioxane |
| Diethylamino Hydroxybenzoyl Hexyl Benzoate |
Diethylamino Hydroxybenzoyl Hexyl Benzoate |
| Niacinamide |
Niacinamide |
| Drometrizole Trisiloxane |
Drometrizole Trisiloxane |
| Methylene Bis-Benzotriazolyl Tetramethylbutylphenol |
Methylene Bis-Benzotriazolyl Tetramethylbutylphenol |
| Caprylyl Methicone |
Caprylyl Methicone |
| 1,2-hexanediol |
1,2-hexanediol |
| Butylene glycol |
Butylene glycol |
| Panthenol |
Panthenol |
| Pentylene Glycol |
Pentylene Glycol |
| Behenyl Alcohol |
Behenyl Alcohol |
| Polyglyceryl-3 Methylglucose Distearate |
Polyglyceryl-3 Methylglucose Distearate |
| Polyglyceryl-6 Distearate |
Polyglyceryl-6 Distearate |
| Decyl Glucoside |
Decyl Glucoside |
| Tromethamine |
Tromethamine |
| Candelilla/Jojoba/Rice Bran Polyglyceryl-3 Esters |
Candelilla/Jojoba/Rice Bran Polyglyceryl-3 Esters |
| Carbomer |
Carbomer |
| Acrylates/C10-30 Alkyl Acrylate Crosspolymer |
Acrylates/C10-30 Alkyl Acrylate Crosspolymer |
| Sodium Stearoyl Glutamate |
Sodium Stearoyl Glutamate |
| Polyacrylate Crosspolymer-6 |
Polyacrylate Crosspolymer-6 |
| Ethylhexylglycerin |
Ethylhexylglycerin |
| Adenosine |
Adenosine |
| Glycerin |
Glycerin |
| Xanthan Gum |
Xanthan Gum |
| Tocopherol |
Tocopherol |
|
Pentasodium Tetracarboxymethyl Hexanoyl Dipeptide-12(100 ppm)
|
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).