Submitted:
15 February 2025
Posted:
18 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ti₃C₂Tₓ MXene and Synthesis Methods
2.1. Introduction to Ti₃C₂Tₓ MXenes
2.2. Synthesis of Ti₃C₂Tₓ MXenes
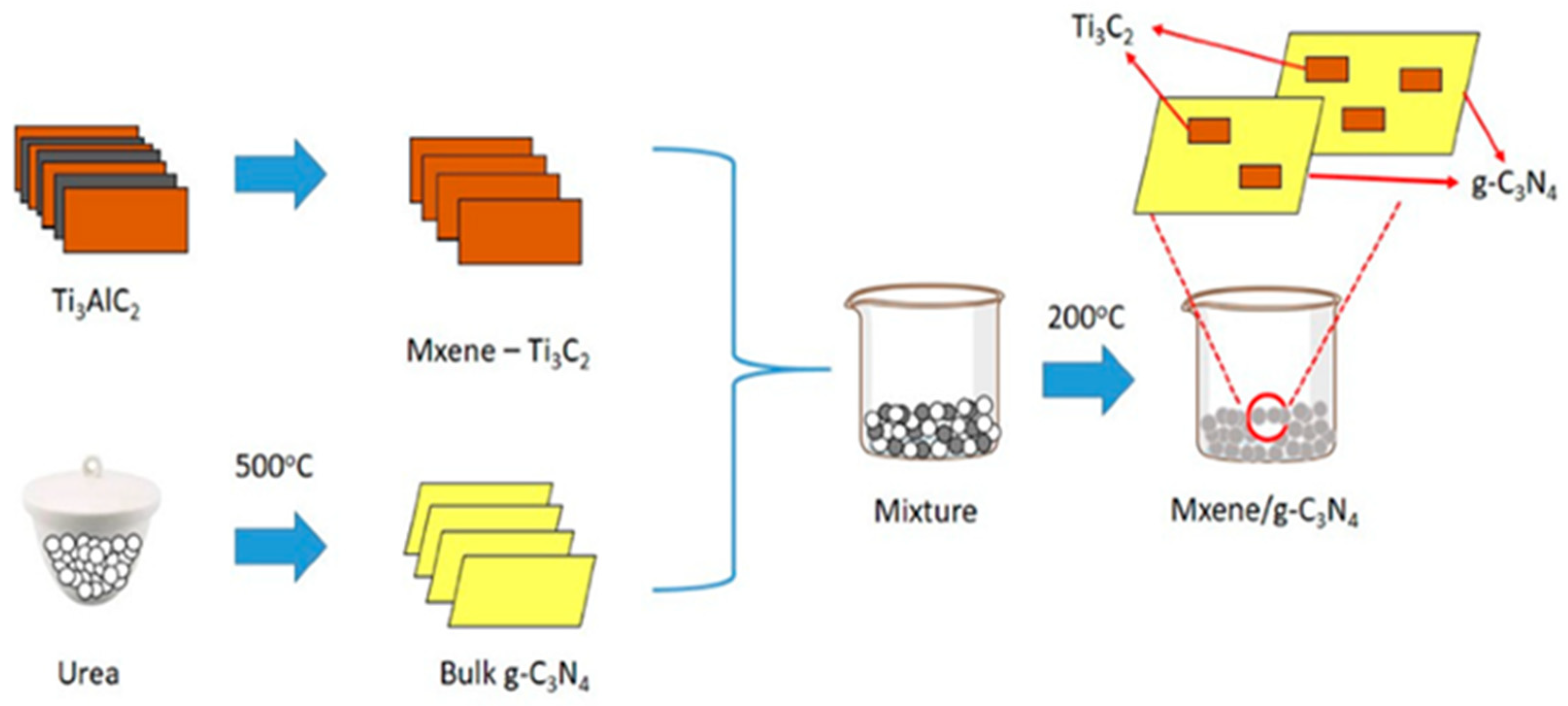
3. Design and Fabrication of Ti₃C₂Tₓ MXene-Based Hybrid Photocatalysts
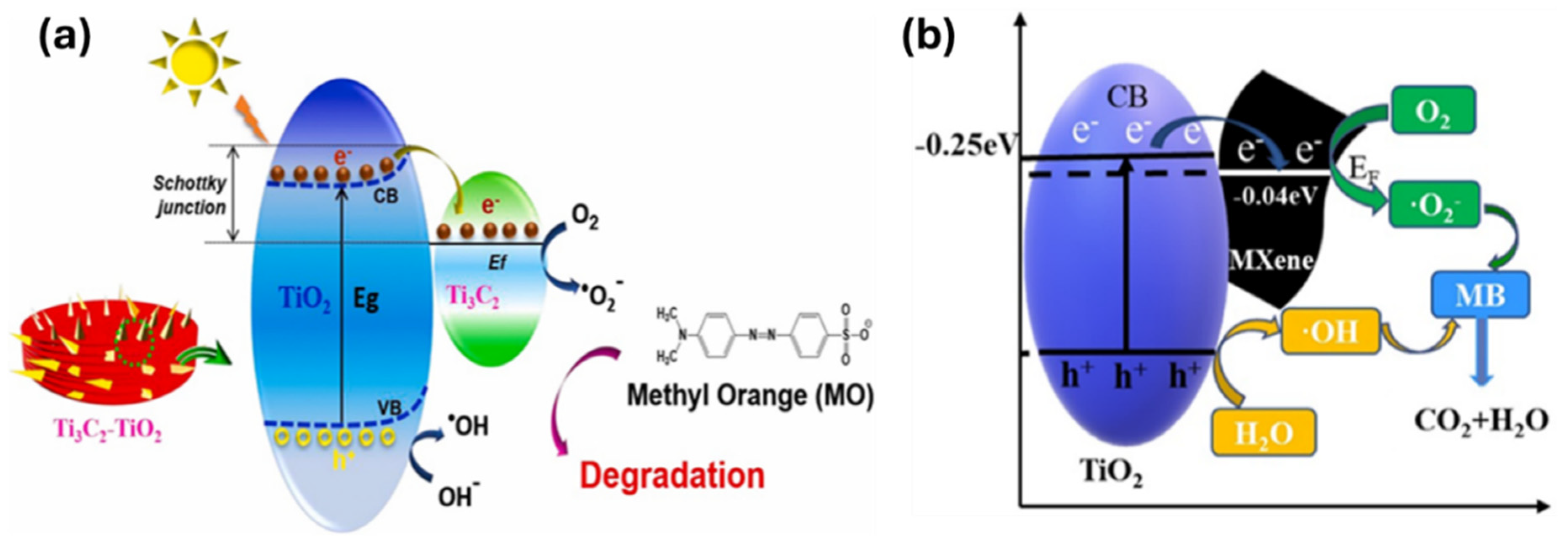
4. Photocatalytic Degradation of Dyes Using Ti₃C₂Tₓ MXene Hybrids
| Nonhybrid photocatalysts | Dyes | Type of dyes | Degradation percentage (%) | References |
|---|---|---|---|---|
| CdS | MO | Anionic | 95 (300 min) | Ref[81]. |
| δ-Bi2O3 | MO | ’’ | 98 (180 min) | Ref[82]. |
| TiO2 NPs | MO | ’’ | ~95 (~120 min) | Ref[83]. |
| ZnO | MB | Cationic | 40.88 (21h) | Ref[84]. |
| TiO2 Hollow Nanofiber | MB | ’’ | 95.2 (4h) | Ref[85]. |
| CuO | MB | ’’ | 62 (270 min) | Ref[86]. |
| Bi2S2O3 | CR | Anionic | 82 (75 min) | Ref[87]. |
| ZnO | CR | ’’ | 97.6(75 min) | Ref[88]. |
| MoSe2 | CR | ’’ | 8.44(120 min) | Ref[89]. |
| g-C3N4 | RhB | Cationic | 75 (180 min) | Ref[90]. |
| BiMnO3 | RhB | ’’ | 68 (75 min) | Ref[91]. |
| MXene-based hybrid photocatalysts | Dyes | Type of dyes (Based on charge) |
Degradation Percentage/ (Time) | References |
|---|---|---|---|---|
| TiO2/Ti3C2Tx |
MO | Anionic | 92(50 min) | Ref.[55] |
| MoS2@Ti3C2 | MO | Anionic | 98(60 min) | Ref.[69] |
| Ti3C2/TiO2/CuO | MO | Anionic | 99 (80 min) | Ref.[99] |
| ZnO/Ti3C2Tx | MO | Anionic | 99.7(50 | Ref.[56] |
| Ti3C2Tx /Bi4Ti3O12 | MO | Anionic | 100(60 min) | Ref[98] |
| TiO2/Ti3C2 Mxene | MB | Cationic | 96.44(60) | Ref[100] |
| AgNPs/TiO2/Ti3C2Tx | MB | Cationic | 99 (30 min) | Ref[95] |
| Ti3C2/g-C3N4 | MB | Cationic | 100(180 min) | Ref[101] |
| NiMnO3/NiMn2O4 -Ti3C2Tx MXene | MB | Cationic | 100 (50 min) | Ref[97] |
| 1D Mn2O3-Ti3C2Tx | MB | Cationic | 100(25 min) | Ref [96] |
| Mn-codoped bismuth ferrite/Ti3C2 |
CR | Anionic | 93(30 min) | Ref[65] |
| CoFe2O4 @MXene | CR | Anionic | 98.9(30 min) | Ref[102] |
| BiVO4/Ti3C2 | CR | Anionic | 99.5(60 min) | Ref[68] |
| BGFO-20Sn/MXene | CR | Anionic | 100(120 min) | Ref[103] |
| BiFeO3/Ti3C2 | CR | Anionic | 100(42min) | Ref[64] |
| TiO2@Ti3C2 | RhB | Cationic | 97(40 min) | Ref[54] |
| BiOBr/TiO2/ Ti3C2Tx |
RhB | Cationic | 99.8(30 min) | Ref[104] |
| Bi2WO6/Ti3C2 | RhB | Cationic | 99.9(20 min) | Ref[92] |
| ZnS/MXene | RhB | Cationic | 100(100 min) | Ref [105] |
| Ti3C2Tx/Bi4Ti3O12 | RhB | Cationic | 100 (50 min) | Ref [98] |
5. Computational Studies and Simulations
6. Other Applications of Ti3C2Tx MXene-Based Hybrid Photocatalysts
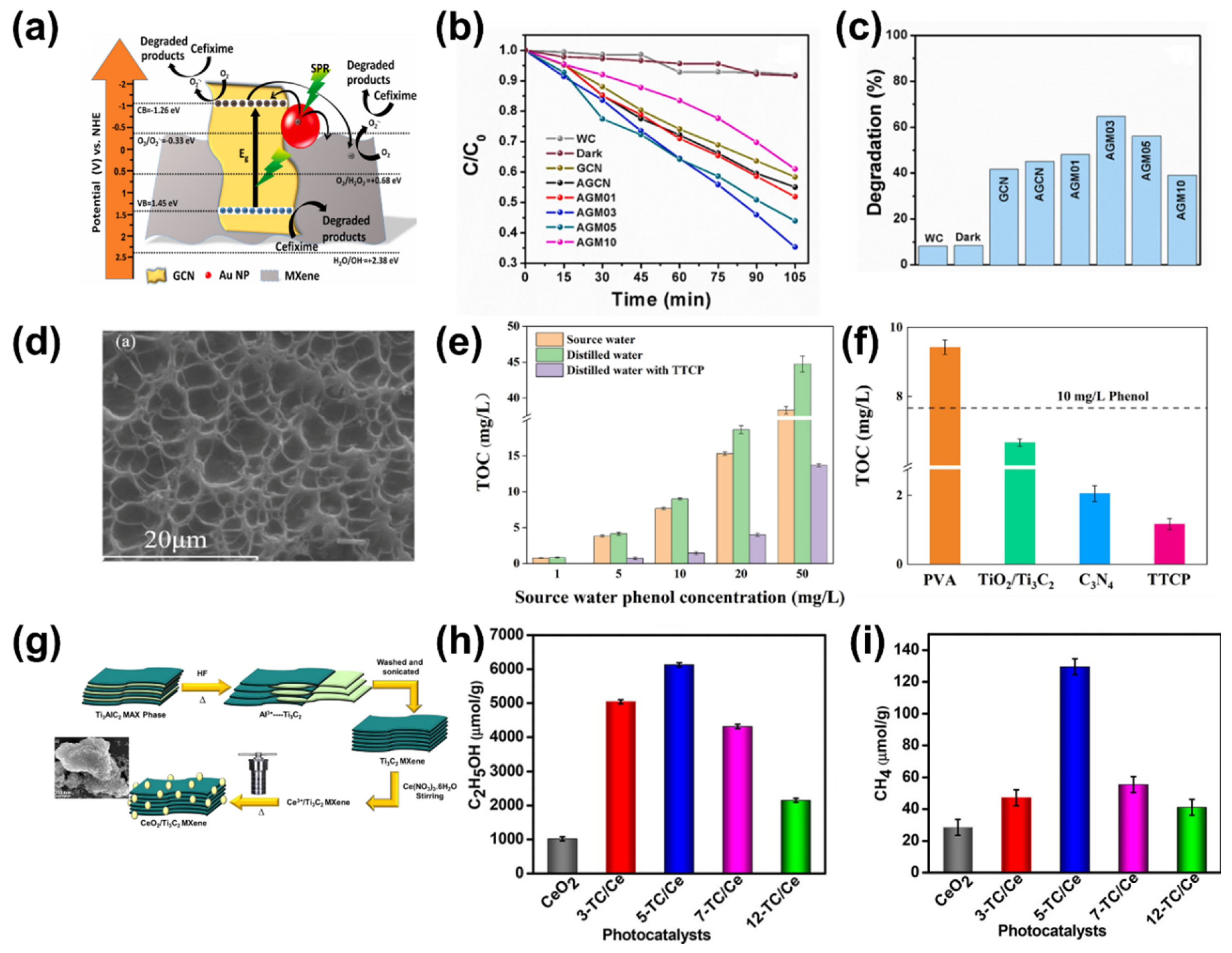
7. Working Mechanism of Ti3C2Tx MXene-Based Hybrid Photocatalyst
8. Challenges and Future Directions
9. Conclusion
Acknowledgments
References
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A Systematic Review of Industrial Wastewater Management: Evaluating Challenges and Enablers. J. Environ. Manage. 2023, 348, 119230. [Google Scholar] [CrossRef]
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; Shah, M.P., Ed.; The Royal Society of Chemistry, 2021; pp. 1–14 ISBN 978-1-83916-279-4.
- López-Ahumada, E.; Salazar-Hernández, M.; Talavera-López, A.; Solis-Marcial, O.J.; Hernández-Soto, R.; Ruelas-Leyva, J.P.; Hernández, J.A. Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia Crassipes as Bioadsorbent. Molecules 2022, 27, 6442. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, Y.; Zhang, G. Two-Dimensional MXene-Based and MXene-Derived Photocatalysts: Recent Developments and Perspectives. Chem. Eng. J. 2021, 409, 128099. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on G-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Lee, Q.Y.; Li, H. Photocatalytic Degradation of Plastic Waste: A Mini Review Micromachines. 2021, 12, 907. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Gupta, T.; Chauhan, R.P. Photocatalytic Degradation of Water Pollutants Using II-VI Semiconducting Catalysts: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 106734. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Bi, Z.; Chen, X.; Hu, X.; Pan, W. Recent Advances and Perspectives of g–C3N4–Based Materials for Photocatalytic Dyes Degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef]
- Yadav, S.; Shakya, K.; Gupta, A.; Singh, D.; Chandran, A.R.; Varayil Aanappalli, A.; Goyal, K.; Rani, N.; Saini, K. A Review on Degradation of Organic Dyes by Using Metal Oxide Semiconductors. Environ. Sci. Pollut. Res. 2022, 30, 71912–71932. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Q.; Yang, C.; Ma, X.; Zhang, Z.; Cui, X. Constructing a Novel TiO2 /γ-Graphyne Heterojunction for Enhanced Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2018, 6, 20947–20955. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A Review of Graphene-Based Semiconductors for Photocatalytic Degradation of Pollutants in Wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef]
- Zhou, W.; Pan, K.; Qu, Y.; Sun, F.; Tian, C.; Ren, Z.; Tian, G.; Fu, H. Photodegradation of Organic Contamination in Wastewaters by Bonding TiO2/Single-Walled Carbon Nanotube Composites with Enhanced Photocatalytic Activity. Chemosphere 2010, 81, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon Quantum Dot Supported Semiconductor Photocatalysts for Efficient Degradation of Organic Pollutants in Water: A Review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, M.; Chen, W.; Huo, X.; Xu, F.; Yan, H.; Lai, C.; Wang, W.; Hu, S.; Qin, L.; et al. Recent Advances in Carbon-Based Material/Semiconductor Composite Photoelectrocatalysts: Synthesis, Improvement Strategy, and Organic Pollutant Removal. Coord. Chem. Rev. 2024, 500, 215498. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3 C2 Tx ) in Photocatalysis: A Review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Im, J.K.; Sohn, E.J.; Kim, S.; Jang, M.; Son, A.; Zoh, K.-D.; Yoon, Y. Review of MXene-Based Nanocomposites for Photocatalysis. Chemosphere 2021, 270, 129478. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Hao, A.; Cao, Y.; Chen, R.; Xie, J.; Lu, Z.; Hu, J.; Jia, D. Construction of MXene/Bi2 WO6 Schottky Junction for Highly Efficient Piezocatalytic Hydrogen Evolution and Unraveling Mechanism. Nano Lett. 2024, 24, 3361–3368. [Google Scholar] [CrossRef]
- Sinopoli, A.; Othman, Z.; Rasool, K.; Mahmoud, K.A. Electrocatalytic/Photocatalytic Properties and Aqueous Media Applications of 2D Transition Metal Carbides (MXenes). Curr. Opin. Solid State Mater. Sci. 2019, 23, 100760. [Google Scholar] [CrossRef]
- Cui, L.; Wen, J.; Deng, Q.; Du, X.; Tang, T.; Li, M.; Xiao, J.; Jiang, L.; Hu, G.; Cao, X.; et al. Improving the Photocatalytic Activity of Ti3C2 MXene by Surface Modification of N Doped. Materials 2023, 16, 2836. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Li, H.; Ren, G.; Wang, L.; Wang, W.; Meng, X. Photocatalytic Applications of Two-Dimensional Ti3 C2 MXenes: A Review. ACS Appl. Nano Mater. 2020, 3, 9581–9603. [Google Scholar] [CrossRef]
- Tang, R.; Xiong, S.; Gong, D.; Deng, Y.; Wang, Y.; Su, L.; Ding, C.; Yang, L.; Liao, C. Ti3 C2 2D MXene: Recent Progress and Perspectives in Photocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 56663–56680. [Google Scholar] [CrossRef]
- Illahi, C.; Hutabarat, W.E.F.; Nurdini, N.; Failamani, F.; Kadja, G.T.M. Photocatalytic Degradation of Azo Dyes over MXene-Based Catalyst: Recent Developments and Future Prospects. Nanotechnol. 2024, 6, 100055. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3 C2 Tx ) in Photocatalysis: A Review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Goel, N.; Kushwaha, A.; Kumar, M. Two-Dimensional MXenes: Recent Emerging Applications. RSC Adv. 2022, 12, 25172–25193. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; De Barros, A.L.F. Recent Advances in MXenes Based Composites as Photocatalysts: Synthesis, Properties and Photocatalytic Removal of Organic Contaminants from Wastewater. ChemCatChem 2023, 15, e202300690. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, T.; Wei, P.; Xu, W.; Pan, H.; Peng, F.; Jia, J.; Zhang, K.; Yu, H. Photocatalysis over MXene-Based Hybrids: Synthesis, Surface Chemistry, and Interfacial Charge Kinetics. APL Mater. 2021, 9, 070703. [Google Scholar] [CrossRef]
- Javaid, A.; Latif, S.; Imran, M.; Hussain, N.; Bilal, M.; Iqbal, H.M.N. MXene-Based Hybrid Composites as Photocatalyst for the Mitigation of Pharmaceuticals. Chemosphere 2022, 291, 133062. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXene-Based Photocatalysts in Degradation of Organic and Pharmaceutical Pollutants. Molecules 2022, 27, 6939. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3 AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Ayad, M.M.; El-Nasr, A.A. Anionic Dye (Acid Green 25) Adsorption from Water by Using Polyaniline Nanotubes Salt/Silica Composite. J. Nanostructure Chem. 2012, 3, 3. [Google Scholar] [CrossRef]
- Limbu, T.B.; Chitara, B.; Orlando, J.D.; Garcia Cervantes, M.Y.; Kumari, S.; Li, Q.; Tang, Y.; Yan, F. Green Synthesis of Reduced Ti3 C2 Tx MXene Nanosheets with Enhanced Conductivity, Oxidation Stability, and SERS Activity. J. Mater. Chem. C 2020, 8, 4722–4731. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, C.; Filatov, A.S.; Cho, W.; Lagunas, F.; Wang, M.; Vaikuntanathan, S.; Liu, C.; Klie, R.F.; Talapin, D.V. Direct Synthesis and Chemical Vapor Deposition of 2D Carbide and Nitride MXenes. Science 2023, 379, 1242–1247. [Google Scholar] [CrossRef]
- Ayodhya, D. A Review of Recent Progress in 2D MXenes: Synthesis, Properties, and Applications. Diam. Relat. Mater. 2023, 132, 109634. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent Advances in MXene: Preparation, Properties, and Applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Yin, J.; Smajic, J.; Bahabri, M.; Lei, Y.; Hedhili, M.N.; Hota, M.K.; Shi, L.; Guo, T.; et al. Anisotropic Superconducting Nb2 CT x MXene Processed by Atomic Exchange at the Wafer Scale. Adv. Mater. 2024, 36, 2305326. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent Surface Modifications and Superconductivity of Two-Dimensional Metal Carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the Synthesis of MXenes and Other Ultrathin 2D Transition Metal Carbides and Nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Gao, Z.; Pan, H.; Zhen, J.; Linghu, J.; Li, Z. Applications of Artificial Intelligence in Materials Research for Fuel Cells. AI Mater. 2025. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3 C2 Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Koh, S.W.; Rekhi, L.; Arramel; Birowosuto, M.D.; Trinh, Q.T.; Ge, J.; Yu, W.; Wee, A.T.S.; Choksi, T.S.; Li, H.Tuning the Work Function of MXene via Surface Functionalization. ACS Appl. Mater. Interfaces 2024, 16, 66826–66836. [CrossRef]
- Guo, L.; Jiang, W.-Y.; Shen, M.; Xu, C.; Ding, C.-X.; Zhao, S.-F.; Yuan, T.-T.; Wang, C.-Y.; Zhang, X.-Q.; Wang, J.-Q. High Capacitance of MXene (Ti3C2T ) through Intercalation and Surface Modification in Molten Salt. Electrochimica Acta 2022, 401, 139476. [Google Scholar] [CrossRef]
- Cao, Z.; Yin, Q.; Zhang, Y.; Li, Y.; Yu, C.; Zhang, M.; Fan, B.; Shao, G.; Wang, H.; Xu, H.; et al. Heterostructure Composites of TiO2 and CdZnS Nanoparticles Decorated on Ti3C2Tx Nanosheets and Their Enhanced Photocatalytic Performance by Microwave Hydrothermal Method. J. Alloys Compd. 2022, 918, 165681. [Google Scholar] [CrossRef]
- Purbayanto, M.A.K.; Bury, D.; Chandel, M.; Shahrak, Z.D.; Mochalin, V.N.; Wójcik, A.; Moszczyńska, D.; Wojciechowska, A.; Tabassum, A.; Naguib, M.; et al. Ambient Processed rGO/Ti3 CNTx MXene Thin Film with High Oxidation Stability, Photosensitivity, and Self-Cleaning Potential. ACS Appl. Mater. Interfaces 2023, 15, 44075–44086. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2 WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Latif, F.E.A.; Khalid, M.; Numan, A.; Manaf, N.A.; Mubarak, N.M.; Zaharin, H.A.; Abdullah, E.C. Microwave-Assisted Hydrothermal Synthesis of Ti3C2Tx MXene: A Sustainable and Scalable Approach Using Alkaline Etchant. J. Mol. Struct. 2025, 1329, 141407. [Google Scholar] [CrossRef]
- My Tran, N.; Thanh Hoai Ta, Q.; Noh, J.-S. Unusual Synthesis of Safflower-Shaped TiO2/Ti3C2 Heterostructures Initiated from Two-Dimensional Ti3C2 MXene. Appl. Surf. Sci. 2021, 538, 148023. [Google Scholar] [CrossRef]
- Quyen, V.T.; Ha, L.T.T.; Thanh, D.M.; Le, Q.V.; Viet, N.M.; Nham, N.T.; Thang, P.Q. Advanced Synthesis of MXene-Derived Nanoflower-Shaped TiO2@Ti 3 C 2 Heterojunction to Enhance Photocatalytic Degradation of Rhodamine B. Environ. Technol. Innov. 2021, 21, 101286. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, H.; Zhao, Y.; Que, M.; Lei, X.; Zhang, K.; Luo, Y. Preparation of Facet Exposed TiO2/Ti3C2T Composites with Enhanced Photocatalytic Activity. J. Phys. Chem. Solids 2020, 145, 109565. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Tran, N.M.; Noh, J.-S. Rice Crust-Like ZnO/Ti3C2Tx MXene Hybrid Structures for Improved Photocatalytic Activity. Catalysts 2020, 10, 1140. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on G-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene Interface Materials as a Schottky Catalyst with Enhanced Photocatalytic Activities and Anti-Photocorrosion Performance. Appl. Catal. B Environ. 2018, 239, 545–554. [Google Scholar] [CrossRef]
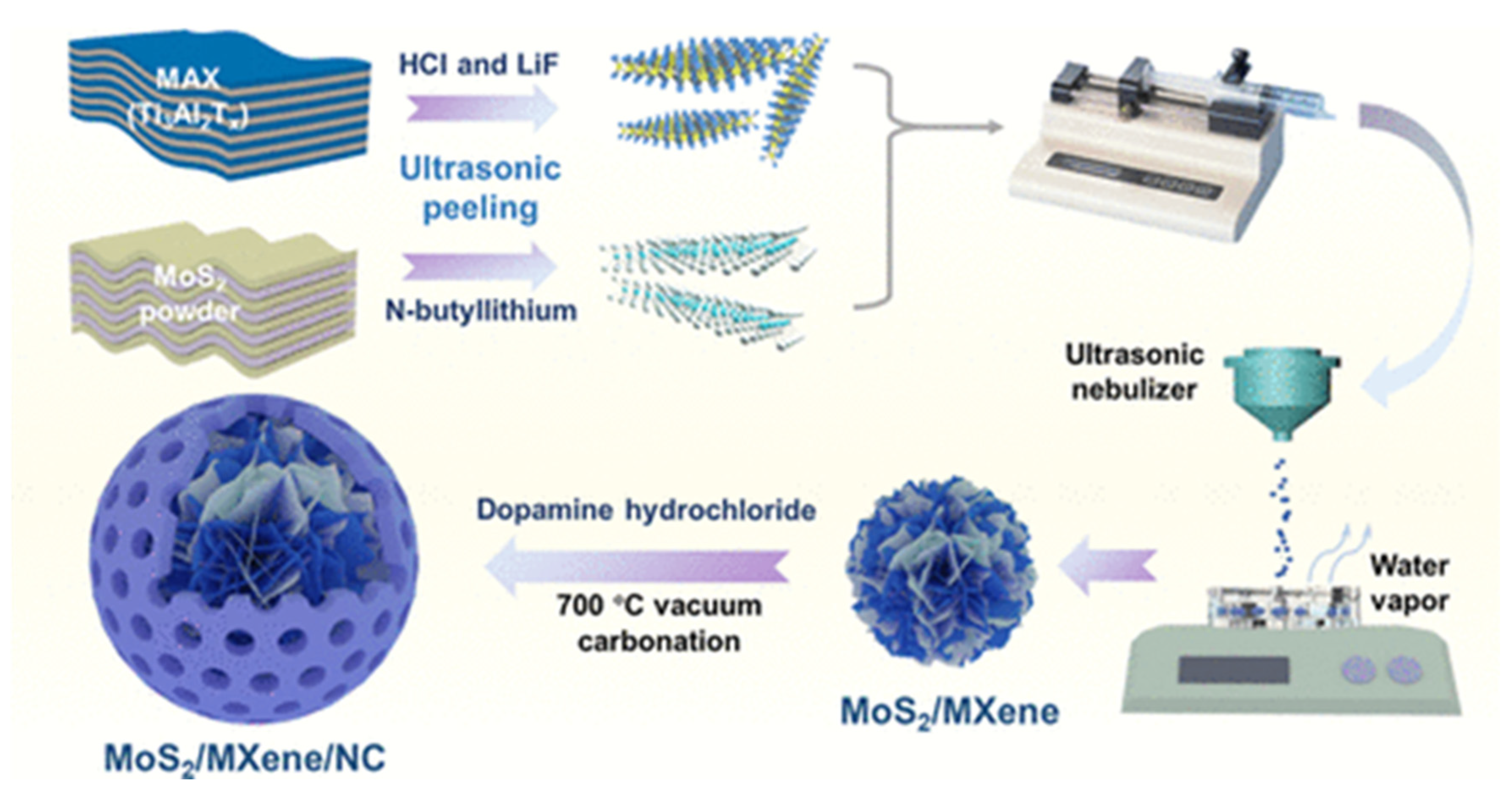
- Fan, Y.; Liu, Z.; Li, Q.; Zhao, K.; Ahmad, M.; Liu, P.; Zhang, Q.; Zhang, B. Preparation of MoS2 /MXene/NC Porous Composite Microspheres with Wrinkled Surface and Their Microwave Absorption Performances. ACS Appl. Mater. Interfaces 2023, 15, 41720–41731. [Google Scholar] [CrossRef]
- Jin Lee, D.; Mohan Kumar, G.; Sekar, S.; Chang Jeon, H.; Young Kim, D.; Ilanchezhiyan, P. Ultrasonic Processing of WO3 Nanosheets Integrated Ti3C2 MXene 2D-2D Based Heterojunctions with Synergistic Effects for Enhanced Water Splitting and Environmental Remediation. Ultrason. Sonochem. 2023, 101, 106681. [Google Scholar] [CrossRef]
- Alsafari, I.A.; Munir, S.; Zulfiqar, S.; Saif, M.S.; Warsi, M.F.; Shahid, M. Synthesis, Characterization, Photocatalytic and Antibacterial Properties of Copper Ferrite/MXene (CuFe2O4/Ti3C2) Nanohybrids. Ceram. Int. 2021, 47, 28874–28883. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Chen, C. Ultrasonic Oscillation Synthesized ZnS Nanoparticles/Layered MXene Sheet with Outstanding Photocatalytic Activity under Visible Light. Vacuum 2021, 183, 109834. [Google Scholar] [CrossRef]
- Ishfaq, M.; Rasheed, A.; Ajmal, S.; Dastgeer, G.; Naz, T.; Baig, M.M.; Lee, S.G. Synthesis and Characterization of a Novel MnO2@MXene-Based 2D/3D Hierarchical Z-Scheme for Sustainable Environmental Applications. Ceram. Int. 2024, 50, 9801–9810. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Iqbal, M.Z.; Akinwande, D.; Rizwan, S. Ti3 C2 -MXene/Bismuth Ferrite Nanohybrids for Efficient Degradation of Organic Dyes and Colorless Pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.A.; Ali, S.I.; Amin, F.; Tariq, A.; Iqbal, M.Z.; Rizwan, S. La- and Mn-Codoped Bismuth Ferrite/Ti3 C2 MXene Composites for Efficient Photocatalytic Degradation of Congo Red Dye. ACS Omega 2019, 4, 8661–8668. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, P.; Tian, W.; Wang, Y.; Zhang, Y.; Chen, J.; Sun, Z. Microwave-Assisted Synthesis of SnO2-Ti3C2 Nanocomposite for Enhanced Supercapacitive Performance. Mater. Lett. 2017, 209, 122–125. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, J.; Wang, F.; Cao, M.; Zhao, T. One-Step Synthesis of Ceria/Ti 3C 2 Nanocomposites with Enhanced Photocatalytic Activity. Mater. Lett. 2017, 206, 237–240. [Google Scholar] [CrossRef]
- Sajid, M.M.; Khan, S.B.; Javed, Y.; Amin, N.; Zhang, Z.; Shad, N.A.; Zhai, H. Bismuth Vanadate/MXene ( BiVO4 /Ti3C2) Heterojunction Composite: Enhanced Interfacial Control Charge Transfer for Highly Efficient Visible Light Photocatalytic Activity. Environ. Sci. Pollut. Res. 2021, 28, 35911–35923. [Google Scholar] [CrossRef]
- Jiao, S.; Liu, L. Friction-Induced Enhancements for Photocatalytic Degradation of MoS2 @Ti3 C2 Nanohybrid. Ind. Eng. Chem. Res. 2019, 58, 18141–18148. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, M.; Wu, J.; Wang, Z.; Lu, X.; Li, K.; Lee, J.-M.; Min, Y. Photocatalytic Degradation of TiO2 via Incorporating Ti3C2 MXene for Methylene Blue Removal from Water. Catal. Commun. 2023, 174, 106594. [Google Scholar] [CrossRef]
- Othman, Z.; Sinopoli, A.; Mackey, H.R.; Mahmoud, K.A. Efficient Photocatalytic Degradation of Organic Dyes by AgNPs/TiO2 /Ti3 C2 Tx MXene Composites under UV and Solar Light. ACS Omega 2021, 6, 33325–33338. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Smith, M.L.; Mayyahi, A.A.; Amama, P.B. MXenes in Photocatalytic NOx Abatement: Current Innovations, Opportunities, and Challenges. Appl. Catal. B Environ. Energy 2024, 358, 124352. [Google Scholar] [CrossRef]
- Lei, J.-C.; Zhang, X.; Zhou, Z. Recent Advances in MXene: Preparation, Properties, and Applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Ahmaruzzaman, Md. MXenes and MXene-Supported Nanocomposites: A Novel Materials for Aqueous Environmental Remediation. RSC Adv. 2022, 12, 34766–34789. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, Md. MXene-Based Novel Nanomaterials for Remediation of Aqueous Environmental Pollutants. Inorg. Chem. Commun. 2022, 143, 109705. [Google Scholar] [CrossRef]
- Atri, S.; Loni, E.; Zazimal, F.; Hensel, K.; Caplovicova, M.; Plesch, G.; Lu, X.; Nagarajan, R.; Naguib, M.; Monfort, O. MXene-Derived Oxide Nanoheterostructures for Photocatalytic Sulfamethoxazole Degradation. ACS Appl. Nano Mater. 2024, 7, 16506–16515. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, U.; Singh, K.; Prakash Gupta, S.; S. Jamal Beg, M. Structure and Properties of Dyes and Pigments. In Dyes and Pigments - Novel Applications and Waste Treatment; Papadakis, R., Ed.; IntechOpen, 2021 ISBN 978-1-83968-614-6.
- Javaid, A.; Latif, S.; Imran, M.; Hussain, N.; Bilal, M.; Iqbal, H.M.N. MXene-Based Hybrid Composites as Photocatalyst for the Mitigation of Pharmaceuticals. Chemosphere 2022, 291, 133062. [Google Scholar] [CrossRef]
- Wang, C.; Ye, J.; Liang, L.; Cui, X.; Kong, L.; Li, N.; Cheng, Z.; Peng, W.; Yan, B.; Chen, G. Application of MXene-Based Materials in Fenton-like Systems for Organic Wastewater Treatment: A Review. Sci. Total Environ. 2023, 862, 160539. [Google Scholar] [CrossRef]
- Amrillah, T.; Supandi, A.R.; Puspasari, V.; Hermawan, A.; Seh, Z.W. MXene-Based Photocatalysts and Electrocatalysts for CO2 Conversion to Chemicals. Trans. Tianjin Univ. 2022, 28, 307–322. [Google Scholar] [CrossRef]
- Dey, P.Ch.; Das, R. Enhanced Photocatalytic Degradation of Methyl Orange Dye on Interaction with Synthesized Ligand Free CdS Nanocrystals under Visible Light Illumination. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 231, 118122. [Google Scholar] [CrossRef]
- Medina, J.C.; Bizarro, M.; Silva-Bermudez, P.; Giorcelli, M.; Tagliaferro, A.; Rodil, S.E. Photocatalytic Discoloration of Methyl Orange Dye by δ-Bi2O3 Thin Films. Thin Solid Films 2016, 612, 72–81. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Srisai, J.; Muangnapoh, T.; Vas-Umnuay, P. Comparative Study on Photocatalytic Degradation of Methylene Blue Using Pristine ZnO and Ni/ZnO Composite Films. Mater. Today Proc. 2022, 66, 3168–3173. [Google Scholar] [CrossRef]
- Mohammad Jafri, N.; Jaafar, J.; Alias, N.; Samitsu, S.; Aziz, F.; Wan Salleh, W.; Mohd Yusop, M.; Othman, M.; Rahman, M.; Ismail, A.; et al. Synthesis and Characterization of Titanium Dioxide Hollow Nanofiber for Photocatalytic Degradation of Methylene Blue Dye. Membranes 2021, 11, 581. [Google Scholar] [CrossRef]
- Seling, T.R.; Katzbaer, R.R.; Thompson, K.L.; Aksoy, S.E.; Chitara, B.; Shringi, A.K.; Schaak, R.E.; Riaz, U.; Yan, F. Transition Metal-Doped CuO Nanosheets for Enhanced Visible-Light Photocatalysis. J. Photochem. Photobiol. Chem. 2024, 448, 115356. [Google Scholar] [CrossRef]
- Seling, T.R.; Kumar Shringi, A.; Wang, K.; Riaz, U.; Yan, F. Bi2O2S Nanosheets for Effective Visible Light Photocatalysis of Anionic Dye Degradation. Mater. Lett. 2024, 361, 136136. [Google Scholar] [CrossRef]
- Mary A, S.; Norbert, A.; Shaji, S.; Philip, R.R. Electrochemically Anodized Solid and Stable ZnO Nanorods as an Adsorbent/ Nanophotocatalyst: ROS Mediated Degradation of Azo Dyes Congo Red and Methyl Orange. J. Clean. Prod. 2023, 428, 139466. [Google Scholar] [CrossRef]
- Mittal, H.; Khanuja, M. Optimization of MoSe2 Nanostructure by Surface Modification Using Conducting Polymer for Degradation of Cationic and Anionic Dye: Photocatalysis Mechanism, Reaction Kinetics and Intermediate Product Study. Dyes Pigments 2020, 175, 108109. [Google Scholar] [CrossRef]
- Fang, S.; Lv, K.; Li, Q.; Ye, H.; Du, D.; Li, M. Effect of Acid on the Photocatalytic Degradation of Rhodamine B over G-C3N4. Appl. Surf. Sci. 2015, 358, 336–342. [Google Scholar] [CrossRef]
- Revathi, B.; Balakrishnan, L.; Pichaimuthu, S.; Nirmala Grace, A.; Krishna Chandar, N. Photocatalytic Degradation of Rhodamine B Using BiMnO3 Nanoparticles under UV and Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 2020, 31, 22487–22497. [Google Scholar] [CrossRef]
- Zhao, D.; Cai, C. Layered Ti3 C2 MXene Modified Two-Dimensional Bi2 WO6 Composites with Enhanced Visible Light Photocatalytic Performance. Mater. Chem. Front. 2019, 3, 2521–2528. [Google Scholar] [CrossRef]
- Akbari, M.; Rasouli, J.; Rasouli, K.; Ghaedi, S.; Mohammadi, M.; Rajabi, H.; Sabbaghi, S. MXene-Based Composite Photocatalysts for Efficient Degradation of Antibiotics in Wastewater. Sci. Rep. 2024, 14, 31498. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, C. Decolorization of Methylene Blue with TiO 2 Sol via UV Irradiation Photocatalytic Degradation. Int. J. Photoenergy 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Othman, Z.; Sinopoli, A.; Mackey, H.R.; Mahmoud, K.A. Efficient Photocatalytic Degradation of Organic Dyes by AgNPs/TiO2 /Ti3 C2 Tx MXene Composites under UV and Solar Light. ACS Omega 2021, 6, 33325–33338. [Google Scholar] [CrossRef]
- Kalaiselvi, C.; Krishna Chandar, N. Accordion-like Multilayer Ti3C2Tx MXene Sheets Decorated 1D Mn2O3 Nanorods-Based Nanocomposites: An Efficient Catalyst for Swift Removal of Single and Mixed Dyes. J. Phys. Chem. Solids 2023, 182, 111591. [Google Scholar] [CrossRef]
- Chandiran, K.; Pandian, M.S.; Balakrishnan, S.; Pitchaimuthu, S.; Chen, Y.-S.; Nagamuthu Raja, K.C. Ti3C2Tx MXene Decorated with NiMnO3 / NiMn2O4 Nanoparticles for Simultaneous Photocatalytic Degradation of Mixed Cationic and Anionic Dyes. Colloids Surf. Physicochem. Eng. Asp. 2024, 692, 133888. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Que, M.; Wu, Q.; Wang, X.; Zhou, Y.; Ma, Y.; Li, Y.; Yang, X. MXene-Derived Ti3C2Tx /Bi4Ti3O12 Heterojunction Photocatalyst for Enhanced Degradation of Tetracycline Hydrochloride, Rhodamine B, and Methyl Orange under Visible-Light Irradiation. Appl. Surf. Sci. 2023, 639, 158270. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, M.; Zhou, A.; Hu, Q.; Wang, L. Preparation and Photocatalytic Performance of Ti3 C2 /TiO2 /CuO Ternary Nanocomposites. J. Nanomater. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Chen, L.; Ye, X.; Chen, S.; Ma, L.; Wang, Z.; Wang, Q.; Hua, N.; Xiao, X.; Cai, S.; Liu, X. Ti3C2 MXene Nanosheet/TiO2 Composites for Efficient Visible Light Photocatalytic Activity. Ceram. Int. 2020, 46, 25895–25904. [Google Scholar] [CrossRef]
- Nasri, M.S.I.; Samsudin, M.F.R.; Tahir, A.A.; Sufian, S. Effect of MXene Loaded on G-C3N4 Photocatalyst for the Photocatalytic Degradation of Methylene Blue. Energies 2022, 15, 955. [Google Scholar] [CrossRef]
- Ayub, A.; Kim, B.; Lim, Y.; Devarayapalli, K.C.; Kim, G.; Lee, D.S. Hydrothermal Synthesis of Cobalt Ferrite-Functionalized Ti3C2Tx MXene for the Degradation of Congo Red via Peroxymonosulfate Activation System. J. Alloys Compd. 2023, 963, 171294. [Google Scholar] [CrossRef]
- Tariq, A.; Ali, S.I.; Akinwande, D.; Rizwan, S. Efficient Visible-Light Photocatalysis of 2D-MXene Nanohybrids with Gd3+ - and Sn4+ -Codoped Bismuth Ferrite. ACS Omega 2018, 3, 13828–13836. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, J.; Cong, Y.; Jiang, S.; Zhang, Q.; Zhu, H.; Li, Y.; Li, X. Ternary BiOBr/TiO2/Ti3C2T MXene Nanocomposites with Heterojunction Structure and Improved Photocatalysis Performance. Chin. Chem. Lett. 2020, 31, 1022–1025. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Q.; Chen, C. Ultrasonic Oscillation Synthesized ZnS Nanoparticles/Layered MXene Sheet with Outstanding Photocatalytic Activity under Visible Light. Vacuum 2021, 183, 109834. [Google Scholar] [CrossRef]
- Chen, L.; Ye, X.; Chen, S.; Ma, L.; Wang, Z.; Wang, Q.; Hua, N.; Xiao, X.; Cai, S.; Liu, X. Ti3C2 MXene Nanosheet/TiO2 Composites for Efficient Visible Light Photocatalytic Activity. Ceram. Int. 2020, 46, 25895–25904. [Google Scholar] [CrossRef]
- Lemos, H.G.; Ronchi, R.M.; Portugal, G.R.; Rossato, J.H.H.; Selopal, G.S.; Barba, D.; Venancio, E.C.; Rosei, F.; Arantes, J.T.; F. Santos, S. Efficient Ti3 C2 Tx MXene/TiO2 Hybrid Photoanodes for Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5, 15928–15938. [CrossRef]
- Yang, J.; Zhu, Q.; Xie, Z.; Wang, Y.; Wang, J.; Peng, Y.; Fang, Y.; Deng, L.; Xie, T.; Xu, L. Enhancement Mechanism of Photocatalytic Activity for MoS2/Ti3C2 Schottky Junction: Experiment and DFT Calculation. J. Alloys Compd. 2021, 887, 161411. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Ge, J.; Zhao, C.; Zhao, Q.; Zhang, F.; Ni, T.; Wu, W. 3D Interconnected G-C3N4 Hybridized with 2D Ti3C2 MXene Nanosheets for Enhancing Visible Light Photocatalytic Hydrogen Evolution and Dye Contaminant Elimination. Appl. Surf. Sci. 2022, 579, 152180. [Google Scholar] [CrossRef]
- Cheng, X.; Liao, J.; Xue, Y.; Lin, Q.; Yang, Z.; Yan, G.; Zeng, G.; Sengupta, A. Ultrahigh-Flux and Self-Cleaning Composite Membrane Based on BiOCl-PPy Modified MXene Nanosheets for Contaminants Removal from Wastewater. J. Membr. Sci. 2022, 644, 120188. [Google Scholar] [CrossRef]
- Wang, B.; Lin, L.; Chen, Y.; Yang, Q.; Xiong, Y.; Zhang, L.; Dai, X.; Jiang, Y.; Zhong, C.; Liao, J.; et al. Rational Construction of S-Scheme Pt-MnO2/TiO2@Ti3C2Tx via Ti-O-Mn Bond for Distinguished Charge Transfer in Photocatalytic Wastewater Environmental Governance and Hydrogen Production. Compos. Sci. Technol. 2023, 241, 110137. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Yan, G.; Luo, J.; Cheng, X.; Zhao, Z.; Li, H. Self-Cleaning Photocatalytic MXene Composite Membrane for Synergistically Enhanced Water Treatment: Oil/Water Separation and Dyes Removal. Chem. Eng. J. 2022, 427, 131668. [Google Scholar] [CrossRef]
- Khadidja, M.F.; Fan, J.; Li, S.; Li, S.; Cui, K.; Wu, J.; Zeng, W.; Wei, H.; Jin, H.-G.; Naik, N.; et al. Hierarchical ZnO/MXene Composites and Their Photocatalytic Performances. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127230. [Google Scholar] [CrossRef]
- Xue, H.; Yan, Q.; Chen, L.; Wang, Y.; Xie, X.; Sun, J. Ti3C2 MXene Assembled with TiO2 for Efficient Photocatalytic Mineralization of Gaseous O-Xylene. Appl. Surf. Sci. 2023, 608, 155136. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Phung, T.K.; Nguyen, T.-Q.; Khan, A.; Doan, V.D.; Tran, V.A.; Le, V.T. Photocatalytic Degradation of Methyl Orange Dye by Ti3C2–TiO2 Heterojunction under Solar Light. Chemosphere 2021, 276, 130154. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Mohtaram, M.S.; Rasouli, K.; Mohtaram, S.; Rajabi, H.; Sabbaghi, S. Efficient Visible-Light-Driven Photocatalytic Degradation of Antibiotics in Water by MXene-Derived TiO2-Supported SiO2 /Ti3C2 Composites: Optimisation, Mechanism and Toxicity Evaluation. Environ. Pollut. 2025, 367, 125624. [Google Scholar] [CrossRef]
- Huang, G.; Li, S.; Liu, L.; Zhu, L.; Wang, Q. Ti3C2 MXene-Modified Bi2WO6 Nanoplates for Efficient Photodegradation of Volatile Organic Compounds. Appl. Surf. Sci. 2020, 503, 144183. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, G.; Yu, J.; Fan, W. Surface Plasmon Resonance-Mediated Photocatalysis by Noble Metal-Based Composites under Visible Light. J. Mater. Chem. 2012, 22, 21337. [Google Scholar] [CrossRef]
- Qin, J.; Hu, X.; Li, X.; Yin, Z.; Liu, B.; Lam, K. 0D/2D AgInS2/MXene Z-Scheme Heterojunction Nanosheets for Improved Ammonia Photosynthesis of N2. Nano Energy 2019, 61, 27–35. [Google Scholar] [CrossRef]
- Yusuf, B.O.; Umar, M.; Aliyu, M.; Alhassan, A.M.; Awad, M.M.; Taialla, O.A.; Abdullahi, A.S.; Musa, J.N.; Alhooshani, K.R.; Ganiyu, S.A. Recent Advances and Future Prospects of MXene-Based Photocatalysts in Environmental Remediations. J. Environ. Chem. Eng. 2024, 12, 114812. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J. MXene-Based Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 18–44. [Google Scholar] [CrossRef]
- Parwaiz, S.; Sohn, Y.; Khan, M.M. Insights into MXenes and MXene-Based Heterostructures for Various Photocatalytic Applications. Mater. Sci. Semicond. Process. 2025, 186, 109099. [Google Scholar] [CrossRef]
- You, Z.; Liao, Y.; Li, X.; Fan, J.; Xiang, Q. State-of-the-Art Recent Progress in MXene-Based Photocatalysts: A Comprehensive Review. Nanoscale 2021, 13, 9463–9504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Majithia, P.; Choudhary, P.; Mabbett, I.; Kuehnel, M.F.; Pitchaimuthu, S.; Krishnan, V. MXene Coupled Graphitic Carbon Nitride Nanosheets Based Plasmonic Photocatalysts for Removal of Pharmaceutical Pollutant. Chemosphere 2022, 308, 136297. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Yan, M.; Li, X.; Zhou, C.; Peng, B.; Chen, H.; Zhang, H. In-Situ Grown of g-C3N4 /Ti3C2/TiO2 Nanotube Arrays on Ti Meshes for Efficient Degradation of Organic Pollutants under Visible Light Irradiation. Colloids Surf. Physicochem. Eng. Asp. 2020, 594, 124511. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, M.; Zhan, R.; Wang, X.; Peng, G.; Niu, J. Ti3C2 MXene-Induced Interface Electron Separation in g-C3N4/Ti3C2 MXene/MoSe2 Z-Scheme Heterojunction for Enhancing Visible Light-Irradiated Enoxacin Degradation. Sep. Purif. Technol. 2021, 275, 119194. [Google Scholar] [CrossRef]
- Rdewi, E.H.; Abbas, K.K.; AbdulkadhimAl-Ghaban, A.M.H. Removal Pharmaceutical Carbamazepine from Wastewater Using ZnO-TiO2-MXene Heterostructural Nanophotocatalyst under Solar Light Irradiation. Mater. Today Proc. 2022, 60, 1702–1711. [Google Scholar] [CrossRef]
- Abbas, K.K.; AbdulkadhimAl-Ghaban, A.M.H.; Rdewi, E.H. Synthesis of a Novel ZnO/TiO2-Nanorod MXene Heterostructured Nanophotocatalyst for the Removal Pharmaceutical Ceftriaxone Sodium from Aqueous Solution under Simulated Sunlight. J. Environ. Chem. Eng. 2022, 10, 108111. [Google Scholar] [CrossRef]
- Sukidpaneenid, S.; Chawengkijwanich, C.; Pokhum, C.; Isobe, T.; Opaprakasit, P.; Sreearunothai, P. Multi-Function Adsorbent-Photocatalyst MXene-TiO2 Composites for Removal of Enrofloxacin Antibiotic from Water. J. Environ. Sci. 2023, 124, 414–428. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for Photocatalytic Degradation of Antiepileptic Drug Carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Mohanty, S.; Sharma, M.; Kumar, A.; Krishnan, V. Hot Electron-Mediated Photocatalytic Degradation of Ciprofloxacin Using Au-Decorated SrTiO3 - and Ti3 C2 MXene-Based Interfacial Heterostructure Nanoarchitectonics. J. Phys. Chem. C 2023, 127, 17711–17722. [Google Scholar] [CrossRef]
- Du, X.; Ye, L.; Zhu, J.; Ye, Y.; Wang, A.; Zhang, H.; Xu, Z.; Dai, L.; Wang, Y. Novel Cerium (IV) Oxide -Ti3C2- Titanium Dioxide Heterostructure Photocatalyst for Pharmaceutical Pollutants Removal: Photocatalyst Characterization, Process Optimization and Transformation Pathways. Surf. Interfaces 2024, 46, 103892. [Google Scholar] [CrossRef]
- Sergiienko, S.A.; Tobaldi, D.M.; Lajaunie, L.; Lopes, D.V.; Constantinescu, G.; Shaula, A.L.; Shcherban, N.D.; Shkepu, V.I.; Calvino, J.J.; Frade, J.R.; et al. Correction: Photocatalytic Removal of Benzene over Ti3 C2 Tx MXene and TiO2 –MXene Composite Materials under Solar and NIR Irradiation. J. Mater. Chem. C 2023, 11, 5225–5225. [Google Scholar] [CrossRef]
- Mo, H.; Wang, Y. A Bionic Solar-Driven Interfacial Evaporation System with a Photothermal-Photocatalytic Hydrogel for VOC Removal during Solar Distillation. Water Res. 2022, 226, 119276. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.P.; Mrinalini, M.; Kumar, N.; Bastia, S.; Chaudhary, Y.S. Efficient Photocatalytic CO2 Reduction with High Selectivity for Ethanol by Synergistically Coupled MXene-Ceria and the Charge Carrier Dynamics. Langmuir 2023, 39, 14189–14203. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.-F. 2D MXenes as Co-Catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2 WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, Y.; Guo, Y.; Zhang, S.; Song, H.; Li, X.; Gao, Q.; Shang, W.; Hu, S.; et al. MXene Ti3C2 Decorated G-C3N4/ZnO Photocatalysts with Improved Photocatalytic Performance for CO2 Reduction. Nano Mater. Sci. 2023, 5, 237–245. [Google Scholar] [CrossRef]
- Irfan, M.; Ahmad, I.; Shukrullah, S.; Hussain, H.; Atif, M.; Legutko, S.; Petru, J.; Hatala, M.; Naz, M.Y.; Rahman, S. Construction of 0D/2D Schottky Heterojunctions of ZnO and Ti3C2 Nanosheets with the Enriched Transfer of Interfacial Charges for Photocatalytic Hydrogen Evolution. Materials 2022, 15, 4557. [Google Scholar] [CrossRef]
- Colmenares, J.; Kuna, E. Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview. Molecules 2017, 22, 790. [Google Scholar] [CrossRef]
- Peng, Y.; Cai, P.; Yang, L.; Liu, Y.; Zhu, L.; Zhang, Q.; Liu, J.; Huang, Z.; Yang, Y. Theoretical and Experimental Studies of Ti3 C2 MXene for Surface-Enhanced Raman Spectroscopy-Based Sensing. ACS Omega 2020, 5, 26486–26496. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Seyed Dorraji, M.S.; Rasoulifard, M.H. Boosting Photo-Charge Transfer in 3D/2D TiO2@Ti3C2 MXene/Bi2S3 Schottky/Z-Scheme Heterojunction for Photocatalytic Antibiotic Degradation and H2 Evolution. Compos. Part B Eng. 2023, 262, 110820. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Phung, T.K.; Nguyen, T.-Q.; Khan, A.; Doan, V.D.; Tran, V.A.; Le, V.T. Photocatalytic Degradation of Methyl Orange Dye by Ti3C2–TiO2 Heterojunction under Solar Light. Chemosphere 2021, 276, 130154. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, L.; Song, D.; Qiu, J.; Kong, F.; Guo, L.-H. A Formation Model of Superoxide Radicals Photogenerated in Nano-TiO2 Suspensions. RSC Adv. 2019, 9, 29429–29432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, M.; Wu, J.; Wang, Z.; Lu, X.; Li, K.; Lee, J.-M.; Min, Y. Photocatalytic Degradation of TiO2 via Incorporating Ti3C2 MXene for Methylene Blue Removal from Water. Catal. Commun. 2023, 174, 106594. [Google Scholar] [CrossRef]
- Farghaly, A.; Maher, E.; Gad, A.; El-Bery, H. Synergistic Photocatalytic Degradation of Methylene Blue Using TiO2 Composites with Activated Carbon and Reduced Graphene Oxide: A Kinetic and Mechanistic Study. Appl. Water Sci. 2024, 14, 228. [Google Scholar] [CrossRef]
- Islam Molla, Md.A.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Evaluation of Reaction Mechanism for Photocatalytic Degradation of Dye with Self-Sensitized TiO<Sub>2</Sub> under Visible Light Irradiation. Open J. Inorg. Non-Met. Mater. 2017, 07, 1–7. [Google Scholar] [CrossRef]
- Kumar Mandal, R.; Ghosh, S.; Pal Majumder, T. Comparative Study between Degradation of Dyes (MB, MO) in Monotonous and Binary Solution Employing Synthesized Bimetallic (Fe-CdO) NPs Having Antioxidant Property. Results Chem. 2023, 5, 100788. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, Y.; Wang, H.; Khan, K.; Tareen, A.K.; Qian, W.; Zhang, H.; Ågren, H. Recent Advances in Oxidation Stable Chemistry of 2D MXenes. Adv. Mater. 2022, 34, 2107554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).