Introduction
Automated reactors have revolutionized chemical and nanomaterial synthesis by increasing efficiency, precision, and reproducibility.[
1,
2,
3,
4,
5] These platforms allow researchers to focus on process optimization and creative problem-solving rather than repetitive manual labour.[
6] Plasmonic nanoparticles (PNPs), such as gold and silver, play a crucial role in biomedical engineering, optics, and catalysis, necessitating precise control over their size, shape, and surface properties.[
7,
8,
9] While batch synthesis remains widely used, continuous flow platforms offer several advantages, including enhanced reproducibility, scalability, and process optimization.[
10] The global nanomaterials market is expected to grow significantly, driven by increasing demand for precision-engineered nanoparticles in various fields such as healthcare, electronics, and sustainable energy solutions.[
11,
12,
13] Autonomous self-optimization of nanoparticle synthesis has emerged as a crucial area of development, allowing researchers to achieve user-defined nanoscale geometries that are otherwise challenging to fabricate.[
14] By integrating continuous flow synthesis with advanced automation techniques, researchers can unlock novel synthetic methodologies, ensuring safety, reproducibility and scalability in nanoparticle manufacturing.[
10,
15]
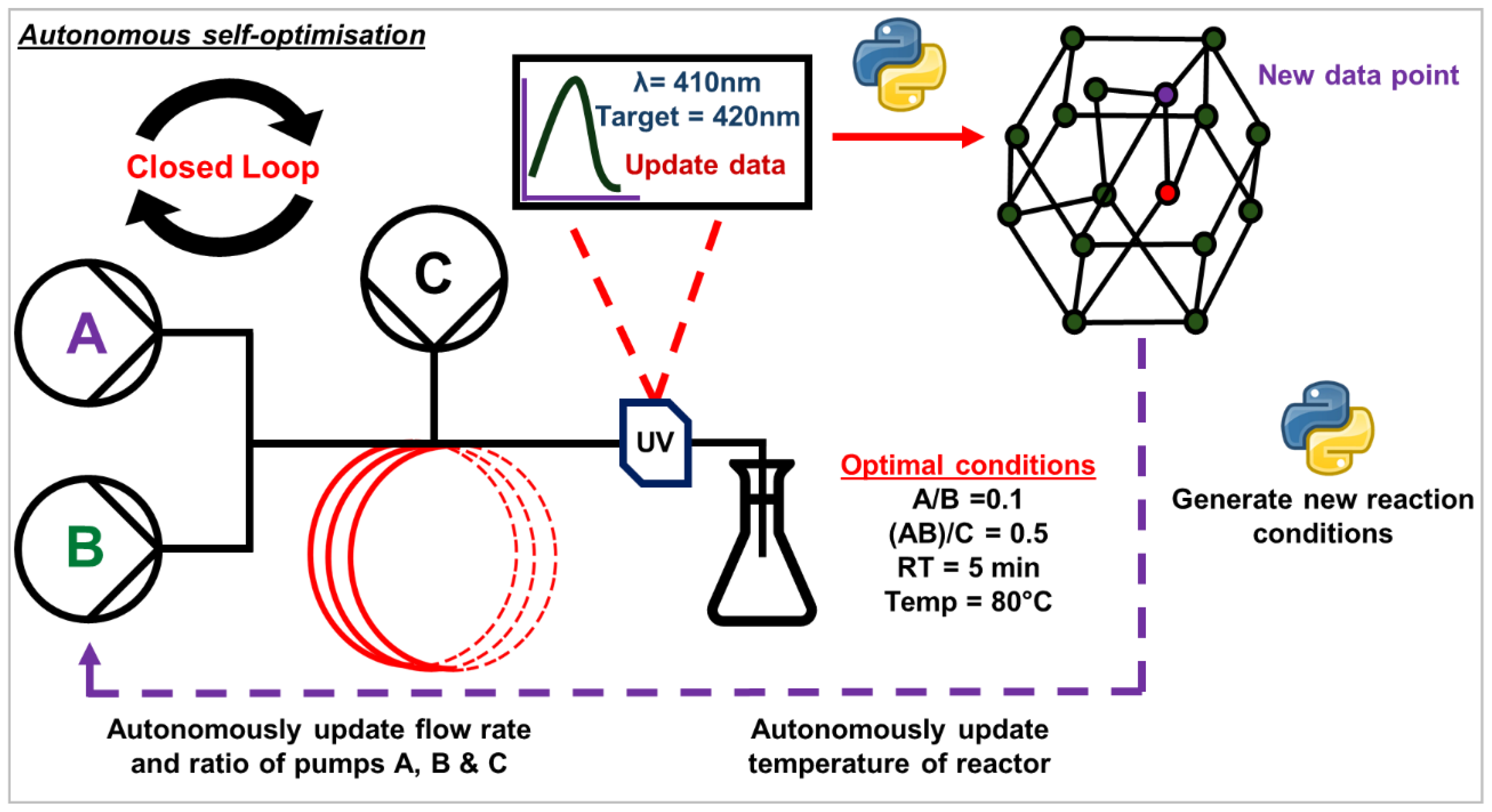
Figure 1.
Pictographic representation of a simple autonomous self-optimising platform using UV-Vis as the inline analysis.
Figure 1.
Pictographic representation of a simple autonomous self-optimising platform using UV-Vis as the inline analysis.
Recent advancements in continuous flow reactors have demonstrated their potential in optimizing the synthesis of plasmonic nanoparticles.[
16] These platforms enable real-time monitoring, adaptive feedback control, and rapid fine-tuning of reaction parameters to achieve high-quality nanoparticles with tailored optical, electrical, and catalytic properties. Additionally, integrating microfluidic reactors into continuous flow synthesis has significantly improved reaction kinetics, mixing efficiency, and heat transfer, allowing for superior control over nanoparticle morphology and composition.[
17,
18,
19,
20] Furthermore, autonomous self-optimization enables safer and more efficient nanoparticle synthesis by incorporating real-time toxicity assessment and Bayesian optimization algorithms.[
21] These algorithms iteratively refine reaction conditions based on real-time feedback, enhancing reproducibility while reducing experimentation time.[
22,
23] This capability is particularly beneficial in biomedical applications, where precise control over nanoparticle properties is essential for drug delivery, biosensing, and imaging applications.[
24] As the field continues to evolve, the integration of AI-driven predictive models and machine learning algorithms will further enhance the efficiency and adaptability of automated reactors.[
25] By addressing key challenges such as reactor fouling, process scalability, and inline characterization limitations, future advancements will pave the way for next-generation nanofabrication technologies.
This mini-review explores recent developments in automated reactor technologies, highlighting their impact on plasmonic nanomaterial synthesis and potential applications in diverse industries.
Continuous Flow Platforms
Continuous flow synthesis has revolutionized nanofabrication by providing enhanced control over reaction conditions, enabling inline analysis, and ensuring precise reagent handling.[
29,
30] Unlike batch processing, which often faces challenges with inconsistencies in particle size, morphology, and reproducibility, continuous flow methods utilize controlled flow rates, residence times, and reaction environments to achieve superior uniformity and scalability.[
31,
32,
33] Despite these advantages, challenges such as energy consumption, reactor clogging, and the integration of real-time analytical techniques persist. This section critically evaluates key studies that have advanced the field of continuous flow nanomaterial synthesis.
Pinho and Torrente-Murciano (2021) introduced the "Dial-a-Particle" system, a microfluidic reactor platform designed for the precise manufacturing of plasmonic nanoparticles.[
34] This innovative system combines fast, integrated multipoint particle sizing with a modular "plug-n-play" platform, featuring reactors in series and distributed feed capabilities. The real-time early growth information obtained allows for accurate prediction and control of particle properties, enabling automated synthesis of nanoparticles with tunable sizes ranging from approximately 4 to 100 nm. This approach represents a significant advancement toward reproducible nanomaterial production. However, the study primarily utilized UV-Vis spectroscopy for characterization, which offers limited insight into detailed morphological features. Future work could benefit from integrating advanced analytical techniques, such as electron microscopy or dynamic light scattering, to enhance characterization accuracy.
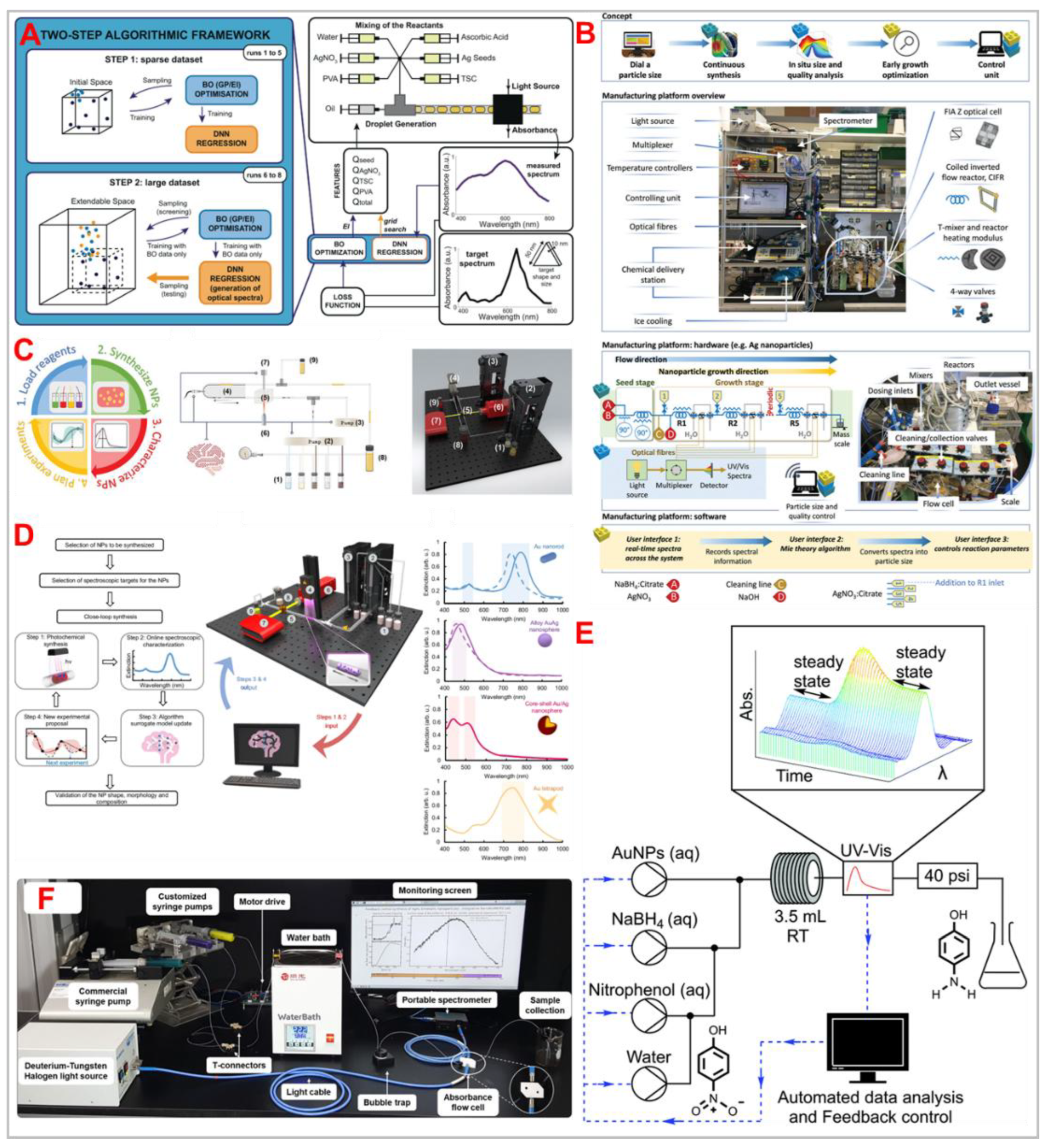
Mekki-Berrada
et al. (2021) proposed a two-step machine learning framework for the high-throughput microfluidic synthesis of silver nanoparticles with desired optical properties.[
35] The approach combines Gaussian process-based Bayesian optimization with a deep neural network, enabling the rapid production of silver nanoparticles tailored to specific absorbance spectra. While this method effectively optimized particle shape and size, it required extensive data acquisition prior to model training, presenting a considerable drawback. This study highlights the classic trade-off in machine learning-based synthesis: large datasets improve predictive accuracy but can slow down the optimization process. Future advancements could focus on transfer learning or active learning strategies to reduce the data acquisition burden while maintaining model performance.
Hall
et al. (2021) demonstrated the integration of autonomous optimization within a continuous flow system for nanoparticle-catalyzed reactions.[
36] They developed an automated continuous flow reactor equipped with inline analysis, applying it to the self-optimization of a gold nanoparticle-catalyzed 4-nitrophenol reduction reaction. The system optimized experimental conditions to achieve maximum conversion in under 2.5 hours. Data obtained from this optimization facilitated the generation of a kinetic model, allowing for the prediction of reaction outcomes under varying conditions. This study exemplifies the potential of AI-driven synthesis for catalytic applications, particularly in dynamically optimizing reaction conditions. However, it also underscores a critical bottleneck: the necessity for advanced inline analytical techniques to complement AI-driven decision-making. Without robust real-time monitoring, the system's ability to make precise adjustments is constrained, limiting its broader applicability.
Wu et al. (2025) introduced a self-driving laboratory designed for the photochemical synthesis of plasmonic nanoparticles with specific structural and optical characteristics.[
37] This autonomous system integrates real-time monitoring and adaptive feedback mechanisms to fine-tune reaction parameters, ensuring the production of nanoparticles that meet predefined criteria. The study highlights the potential of combining artificial intelligence with photochemical processes to achieve precise control over nanoparticle synthesis, paving the way for advancements in materials science and nanotechnology. However, the implementation of such autonomous systems necessitates sophisticated inline analytical tools capable of providing accurate, real-time data to inform the AI-driven adjustments. The development and integration of these advanced analytical techniques remain a significant challenge, critical for the broader application of self-optimizing synthetic platforms.
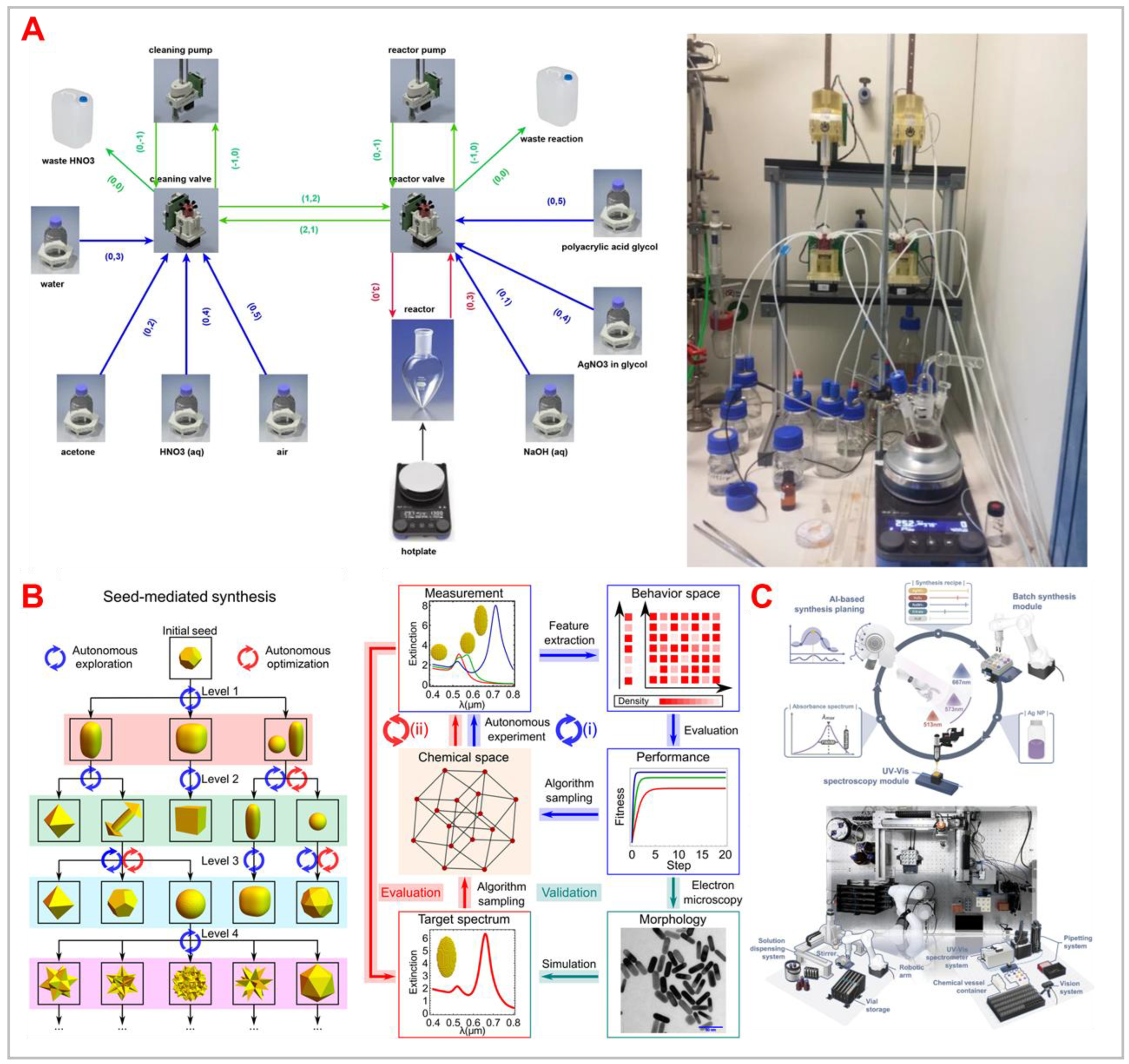
Figure 3.
A) Autonomous self-optimisation of AgNPs through a two-step optimization algorithmic framework. Reproduced from [
35] under a Creative Commons 4.0 CC BY license. B) Autonomous self-optimisation of specific size Au and AgNPs. Reproduced from [
34] under a Creative Commons 4.0 CC BY license. C) Self-driving platform for different size AuNPs. Reproduced and adapted from [
38] under the terms of CC BY-NC 4.0 with permission from the Authors. D) Autonomous Fluidic Identification and Optimization Nanochemistry (AFION) self-driving lab showing the workflow and synthesised NPs. Reproduced from [
37] under a Creative Commons 4.0 CC BY license. E) Closed loop self-optimising platform for AuNP catalysis. Reproduced from [
36] under a Creative Commons 4.0 CC BY license. F) A digital image showing the automated flow system for the synthesis of AgAu alloy nanoboxes with tailored optical properties. Reproduced from [
39] under a Creative Commons 4.0 CC BY license.[
34,
35,
36,
37,
38,
39].
Figure 3.
A) Autonomous self-optimisation of AgNPs through a two-step optimization algorithmic framework. Reproduced from [
35] under a Creative Commons 4.0 CC BY license. B) Autonomous self-optimisation of specific size Au and AgNPs. Reproduced from [
34] under a Creative Commons 4.0 CC BY license. C) Self-driving platform for different size AuNPs. Reproduced and adapted from [
38] under the terms of CC BY-NC 4.0 with permission from the Authors. D) Autonomous Fluidic Identification and Optimization Nanochemistry (AFION) self-driving lab showing the workflow and synthesised NPs. Reproduced from [
37] under a Creative Commons 4.0 CC BY license. E) Closed loop self-optimising platform for AuNP catalysis. Reproduced from [
36] under a Creative Commons 4.0 CC BY license. F) A digital image showing the automated flow system for the synthesis of AgAu alloy nanoboxes with tailored optical properties. Reproduced from [
39] under a Creative Commons 4.0 CC BY license.[
34,
35,
36,
37,
38,
39].
Tao
et al. (2021) developed a self-driving platform that integrates oscillatory microfluidics, online spectroscopy, and machine learning for the autonomous synthesis of metal nanoparticles.[
38] This innovative system employs machine learning algorithms to analyse real-time spectroscopic data, enabling the dynamic adjustment of synthesis parameters to achieve desired nanoparticle properties without human intervention. The study demonstrates the platform's capability to efficiently navigate complex reaction spaces, optimizing conditions to produce nanoparticles with specific characteristics. This approach not only accelerates the discovery and development of new nanomaterials but also enhances reproducibility in nanoparticle synthesis. However, the successful implementation of such autonomous systems relies heavily on the integration of advanced inline analytical techniques that provide accurate, real-time data. Ensuring the precision and reliability of these analytical components is crucial for the system's ability to make informed decisions during the synthesis process.
Bui
et al. (2024) introduced an automated flow chemistry system employing proportional–integral (PI) feedback control to synthesize silver–gold (AgAu) alloy nanoboxes with precise optical properties.[
39] This system utilizes a PI control algorithm based on a first-order plus dead-time model, correlating precursor flow rates with the maximum absorbance peaks of the resulting nanoboxes. By iteratively adjusting the flow rate in response to real-time UV–vis absorbance measurements, the system achieves the target optical characteristics of the AgAu nanoboxes. This approach enhances the consistency and reliability of nanoparticle synthesis, minimizing human intervention. However, the effectiveness of this automated system depends on the accuracy of real-time analytical measurements and the robustness of the feedback control algorithm, which are critical for maintaining the desired product specification.
Collectively, these studies underscore the transformative potential of continuous flow synthesis in nanomaterial fabrication. The integration of real-time monitoring, machine learning, and autonomous optimization not only enhances precision and reproducibility but also addresses scalability and efficiency challenges. Ongoing research focusing on overcoming existing limitations, such as reactor design optimization and advanced inline analytical integration, will be pivotal in fully realizing the capabilities of continuous flow nanomanufacturing.
Challenges and Future Outlook
The future of automated plasmonic nanoparticle synthesis lies in the integration of advanced machine learning algorithms and real-time adaptive control mechanisms. AI-driven predictive models will enable researchers to fine-tune reaction conditions dynamically, leading to improved process efficiency and reduced material wastage. Additionally, hybrid systems that combine the strengths of batch and continuous flow platforms could provide greater flexibility for complex nanoparticle synthesis. Another promising area is the expansion of inline characterization techniques. While UV-Vis spectroscopy is widely used, the incorporation of complementary methods such as Raman spectroscopy, mass spectrometry, and electron microscopy (
in situ liquid TEM) will allow for a more comprehensive understanding of nanoparticle properties.[
40] This will enhance the ability to produce nanoparticles with tailored optical, electronic, and catalytic properties. Scalability remains a significant challenge for both batch and continuous flow synthesis. While continuous flow systems offer inherent scalability advantages, further advancements in modular reactor design and process standardization will be required for industrial-scale implementation. Collaborative efforts between academia and industry will be crucial in bridging this gap, ensuring that automated nanoparticle synthesis technologies are both practical and commercially viable.
Conclusion
In conclusion automated reactors are transforming the field of plasmonic nanoparticle synthesis, with batch and continuous flow systems offering unique advantages and challenges. While batch platforms have demonstrated high-throughput capabilities, continuous flow methods provide superior reproducibility and real-time optimization. Integrating AI, machine learning, and advanced analytical tools will further enhance the potential of these systems, paving the way for next-generation nanofabrication technologies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Ahrberg, C. D.; Wook Choi, J.; Geun Chung, B. Automated droplet reactor for the synthesis of iron oxide/gold core-shell nanoparticles. Scientific Reports 2020, 10, 1737. [Google Scholar] [CrossRef]
- Clayton, A. D.; Pyzer-Knapp, E. O.; Purdie, M.; Jones, M. F.; Barthelme, A.; Pavey, J.; Kapur, N.; Chamberlain, T. W.; Blacker, A. J.; Bourne, R. A. Bayesian Self-Optimization for Telescoped Continuous Flow Synthesis. Angew. chem. 2023, 135, e202214511. [Google Scholar] [CrossRef]
- Clayton, A. D. Recent Developments in Reactor Automation for Multistep Chemical Synthesis. Chemistry–Methods 2023, 3, e202300021. [Google Scholar] [CrossRef]
- Pittaway, P. M.; Chingono, K. E.; Knox, S. T.; Martin, E.; Bourne, R. A.; Cayre, O. J.; Kapur, N.; Booth, J.; Capomaccio, R.; Pedge, N.; Warren, N. J. Exploiting Online Spatially Resolved Dynamic Light Scattering and Flow-NMR for Automated Size Targeting of PISA-Synthesized Block Copolymer Nanoparticles. ACS Poly. Au 2025, 5, 1–9. [Google Scholar] [CrossRef]
- Taylor, C. J.; Pomberger, A.; Felton, K. C.; Grainger, R.; Barecka, M.; Chamberlain, T. W.; Bourne, R. A.; Johnson, C. N.; Lapkin, A. A. A Brief Introduction to Chemical Reaction Optimization. Chem. Rev. 2023, 123, 3089–3126. [Google Scholar] [CrossRef]
- Kioumourtzoglou, S.; Hof, S.; Kalk, C.; Toth, V.; Görlin, M.; Nováková, J.; Sá, J. Nanomaterials as a Service (NaaS) concept: on-demand protocols for volume synthesis of nanomaterials. Nanoscale Horizons 2024, 9, 1364–1371. [Google Scholar] [CrossRef]
- Mitchell, M. J.; Billingsley, M. M.; Haley, R. M.; Wechsler, M. E.; Peppas, N. A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J. S.; Nees, N.; Khan, H.; Traoré, N. E.; Drobek, D.; Peukert, W.; Apeleo Zubiri, B.; Spiecker, E.; Stingl, M.; Pflug, L.; Klupp Taylor, R. N. Continuous flow synthesis and simulation-supported investigation of tunable plasmonic gold patchy nanoparticles. Nanoscale 2024, 16, 19284–19297. [Google Scholar] [CrossRef]
- (9) Patrizia Di, P.; Gaetano, S.; Lidia, Z.; Cristina, S. Gold and Silver Nanoparticles for Applications in Theranostics. Current Topics in Medicinal Chemistry 2016, 16, 3069–3102. [Google Scholar] [CrossRef]
- Jellicoe, M. Flowing into the future 2025.
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G. M.; Eggleton, B. J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Truong, T. T.; Mondal, S.; Doan, V. H. M.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T. D.; Misra, M.; Lee, B.; Oh, J. Precision-engineered metal and metal-oxide nanoparticles for biomedical imaging and healthcare applications. Advances in Colloid and Interface Science 2024, 332, 103263. [Google Scholar] [CrossRef]
- Jiang, Y.; Salley, D.; Sharma, A.; Keenan, G.; Mullin, M.; Cronin, L. An artificial intelligence enabled chemical synthesis robot for exploration and optimization of nanomaterials. Sci. Adv. 8, eabo2626. [CrossRef] [PubMed]
- Preprint. [CrossRef]
- Khositanon, C.; Adpakpang, K.; Bureekaew, S.; Weeranoppanant, N. Continuous-flow purification of silver nanoparticles and its integration with flow synthesis. J. Flow Chem. 2020, 10, 353–362. [Google Scholar] [CrossRef]
- Jellicoe, M.; Igder, A.; Chuah, C.; Jones, D. B.; Luo, X.; Stubbs, K. A.; Crawley, E. M.; Pye, S. J.; Joseph, N.; Vimalananthan, K.; et al. Vortex fluidic induced mass transfer across immiscible phases. Chem Sci. 2022, 13, 3375–3385. [Google Scholar] [CrossRef]
- Jellicoe, M.; Yang, Y.; Stokes, W.; Simmons, M.; Yang, L.; Foster, S.; Aslam, Z.; Cohen, J.; Rashid, A.; Nelson, A. L.; et al. Continuous Flow Synthesis of Copper Oxide Nanoparticles Enabling Rapid Screening of Synthesis-Structure-Property Relationships. Small 2025, 21, 2403529. [Google Scholar] [CrossRef]
- Alharbi, T. M. D.; Jellicoe, M.; Luo, X.; Vimalanathan, K.; Alsulami, I. K.; Al Harbi, B. S.; Igder, A.; Alrashaidi, F. A. J.; Chen, X.; Stubbs, K. A.; et al. Sub-micron moulding topological mass transport regimes in angled vortex fluidic flow. Nanoscale Adv. 2021, 3, 3064–3075. [Google Scholar] [CrossRef]
- Jackson, C.; Robertson, K.; Sechenyh, V.; Chamberlain, T. W.; Bourne, R. A.; Lester, E. Self-optimising continuous-flow hydrothermal reactor for nanoparticle synthesis. React. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- Karthik, V.; Karuna, B.; Kumar, P. S.; Saravanan, A.; Hemavathy, R. V. Development of lab-on-chip biosensor for the detection of toxic heavy metals: A review. Chemosphere 2022, 299, 134427. [Google Scholar] [CrossRef]
- Schweidtmann, A. M.; Clayton, A. D.; Holmes, N.; Bradford, E.; Bourne, R. A.; Lapkin, A. A. Machine learning meets continuous flow chemistry: Automated optimization towards the Pareto front of multiple objectives. Chemical Engineering Journal 2018, 352, 277–282. [Google Scholar] [CrossRef]
- Müller, P.; Clayton, A. D.; Manson, J.; Riley, S.; May, O. S.; Govan, N.; Notman, S.; Ley, S. V.; Chamberlain, T. W.; Bourne, R. A. Automated multi-objective reaction optimisation: which algorithm should I use? React. Chem. Eng. 2022, 7, 987–993. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.-X. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef] [PubMed]
- Manson, J. A.; Chamberlain, T. W.; Bourne, R. A. MVMOO: Mixed variable multi-objective optimisation. J. Glob. Optim. 2021, 80, 865–886. [Google Scholar] [CrossRef]
- Wolf, J. B.; Stawski, T. M.; Smales, G. J.; Thünemann, A. F.; Emmerling, F. Towards automation of the polyol process for the synthesis of silver nanoparticles. Sci Rep 2022, 12, 5769. [Google Scholar] [CrossRef]
- Yoo, H. J.; Kim, N.; Lee, H.; Kim, D.; Ow, L. T. C.; Nam, H.; Kim, C.; Lee, S. Y.; Lee, K.-Y.; Kim, D.; Han, S. S. Bespoke Metal Nanoparticle Synthesis at Room Temperature and Discovery of Chemical Knowledge on Nanoparticle Growth via Autonomous Experimentations. Advanced Functional Materials 2024, 34, 2312561. [Google Scholar] [CrossRef]
- Salley, D.; Keenan, G.; Grizou, J.; Sharma, A.; Martín, S.; Cronin, L. A nanomaterials discovery robot for the Darwinian evolution of shape programmable gold nanoparticles. Nat Commun 2020, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Mirzamani, M.; Abudiyah, T.; Al-Antaki, A. H. M.; Jellicoe, M.; Harvey, D. P.; Crawley, E.; Chuah, C.; Whitten, A. E.; Gilbert, E. P.; et al. Vortex fluidic regulated phospholipid equilibria involving liposomes down to sub-micelle size assemblies. Nanoscale Adv. 2024, 6, 1202–1212. [Google Scholar] [CrossRef]
- Adamo, A.; Beingessner, R. L.; Behnam, M.; Chen, J.; Jamison, T. F.; Jensen, K. F.; Monbaliu, J.-C. M.; Myerson, A. S.; Revalor, E. M.; Snead, D. R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef]
- Jellicoe, M.; Gibson, C. T.; Quinton, J. S.; Raston, C. L. Coiling of Single-Walled Carbon Nanotubes via Selective Topological Fluid Flow: Implications for Sensors. ACS Applied Nano Materials 2022, 5, 11586–11594. [Google Scholar] [CrossRef]
- Britton, J.; Stubbs, K. A.; Weiss, G. A.; Raston, C. L. Vortex Fluidic Chemical Transformations. Chemistry – A European Journal 2017, 23, 13270–13278. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C. L. Multi-step continuous-flow synthesis. Chemical Society Reviews 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.; Torrente-Murciano, L. Dial-A-Particle: Precise Manufacturing of Plasmonic Nanoparticles Based on Early Growth Information—Redefining Automation for Slow Material Synthesis. Advanced Energy Materials 2021, 11, 2100918. [Google Scholar] [CrossRef]
- Mekki-Berrada, F.; Ren, Z.; Huang, T.; Wong, W. K.; Zheng, F.; Xie, J.; Tian, I. P. S.; Jayavelu, S.; Mahfoud, Z.; Bash, D.; et al. Two-step machine learning enables optimized nanoparticle synthesis. Npj Comput. Mater. 2021, 7, 55. [Google Scholar] [CrossRef]
- Hall, B. L.; Taylor, C. J.; Labes, R.; Massey, A. F.; Menzel, R.; Bourne, R. A.; Chamberlain, T. W. Autonomous optimisation of a nanoparticle catalysed reduction reaction in continuous flow. Chemical Communications 2021, 57, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Kheiri, S.; Hickman, R. J.; Tao, H.; Wu, T. C.; Yang, Z.-B.; Ge, X.; Zhang, W.; Abolhasani, M.; Liu, K.; et al. Self-driving lab for the photochemical synthesis of plasmonic nanoparticles with targeted structural and optical properties. Nature Communications 2025, 16, 1473. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Kheiri, S.; Aldeghi, M.; Aspuru-Guzik, A.; Kumacheva, E. Self-Driving Platform for Metal Nanoparticle Synthesis: Combining Microfluidics and Machine Learning. Advanced Functional Materials 2021, 31, 2106725. [Google Scholar] [CrossRef]
- Bui, H. K.; Nguyen, T. T. H.; Dao, T. D.; Seo, T. S. A Proportional–Integral Feedback Controlled Automatic Flow Chemistry System to Produce On-Demand AgAu Alloy Nanoboxes. Small Structures 2024, 5, 2300397. [Google Scholar] [CrossRef]
- Pu, S.; Gong, C.; Robertson, A. W. Liquid cell transmission electron microscopy and its applications. Royal Society Open Science 2020, 7, 191204. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).