1. Introduction
Colorectal surgeries (CRS) are becoming increasingly frequent today compared to previous years. As with all gastrointestinal surgeries, anastomotic leakage (AL) remains one of the most common and severe postoperative complications following CRS, significantly impacting morbidity, mortality, and healthcare costs [
1]. Despite continuous advancements in surgical techniques and perioperative management, the incidence of AL varies widely, ranging between 3% and 19%, posing a persistent challenge for colorectal surgeons worldwide [
2]. Given the clinical and economic burden of AL, identifying risk factors that predispose patients to this complication is critical for optimizing surgical outcomes [
1].
One potential risk factor that has gained attention in recent years is aortic calcification (AC) [
2,
3]. AC is a manifestation of systemic atherosclerosis, reflecting widespread vascular disease and chronic inflammation. Since adequate tissue perfusion is essential for proper anastomotic healing, it has been hypothesized that severe AC may impair anastomotic integrity by contributing to microvascular dysfunction and ischemic insult at the anastomotic site [
3]. Several studies have investigated the association between AC and AL in various gastrointestinal surgeries, including esophagectomy and colorectal resections, reporting conflicting findings [
3,
4,
5,
6,
7].
In addition to vascular factors such as AC, tumor-related characteristics and patient comorbidities may also influence AL risk. Tumor histology, TNM stage, tumor localization, and neoadjuvant oncological therapy have been implicated as potential contributors to anastomotic healing failure [
8]. Furthermore, comorbid conditions such as hypertension, diabetes mellitus, and cardiovascular disease are known to affect vascular integrity and wound healing, which may further compound the risk of AL [
9]. However, limited studies have systematically evaluated these variables in conjunction with AC as risk factors for AL.
Understanding the interplay between vascular calcifications, oncological parameters, and systemic comorbidities is essential for improving preoperative risk stratification and perioperative management strategies. The objective of this study is to investigate the relationship between AC and AL in patients undergoing CRS, while also evaluating the role of tumor histology, TNM staging, neoadjuvant therapy, and patient comorbidities in influencing this association. By providing a comprehensive analysis of these factors, this study aims to enhance clinical decision-making in colorectal surgery and potentially contribute to risk reduction strategies.
2. Materials and Methods
The study period spanned from January 2020 to October 2023. During this time, patients diagnosed with colorectal carcinoma and operated on by the Department of General Surgery at Erol Olçok Training and Research Hospital were evaluated systematically for this study. Individuals under the age of 18 and above the age of 90, those with known hematological and oncological diseases, and those with vascular and endothelial diseases other than AC were excluded from the study.
A power analysis was conducted to determine the statistical power of the study. Based on a significance level of p<0.05, 80% power (1-β), and the expected effect size, at least 86 patients were required. The final sample size of 151 patients met this requirement and was included in the study after applying the aforementioned exclusion criteria.
2.1. Data Collection
In the retrospective analysis, data regarding patient demographics (age, gender), tumor localization, aortic calcification (AC) measurements, and anastomotic leak (AL) incidents were systematically collected. Additionally, tumor histology (adenocarcinoma, mucinous carcinoma, other), TNM staging (I, II, III, IV), neoadjuvant oncological therapy (chemotherapy and/or radiotherapy), and comorbidities (hypertension, diabetes mellitus, cardiovascular disease, smoking history) were recorded from patient medical records.
2.2. Ethical Approval and Patient Consent
This study was designed retrospectively and received ethical approval from the Institutional Review Board of the Hitit University Faculty of Medicine Clinical Research Ethics Committee (Protocol Number: 2023-143, date: 01/11/2023). Since this was a retrospective study with anonymized patient data, informed consent was waived.
2.3. Imaging Protocol and Calcification Measurement
Preoperative computed tomography (CT) scans were performed using a 64-multidetector scanner (LightSpeed VCT; GE Healthcare, Waukesha, WI, USA). The imaging parameters were set as follows: tube current of 150 mA, tube voltage of 120 kVp, section thickness of 5 mm, and section interval of 5 mm.
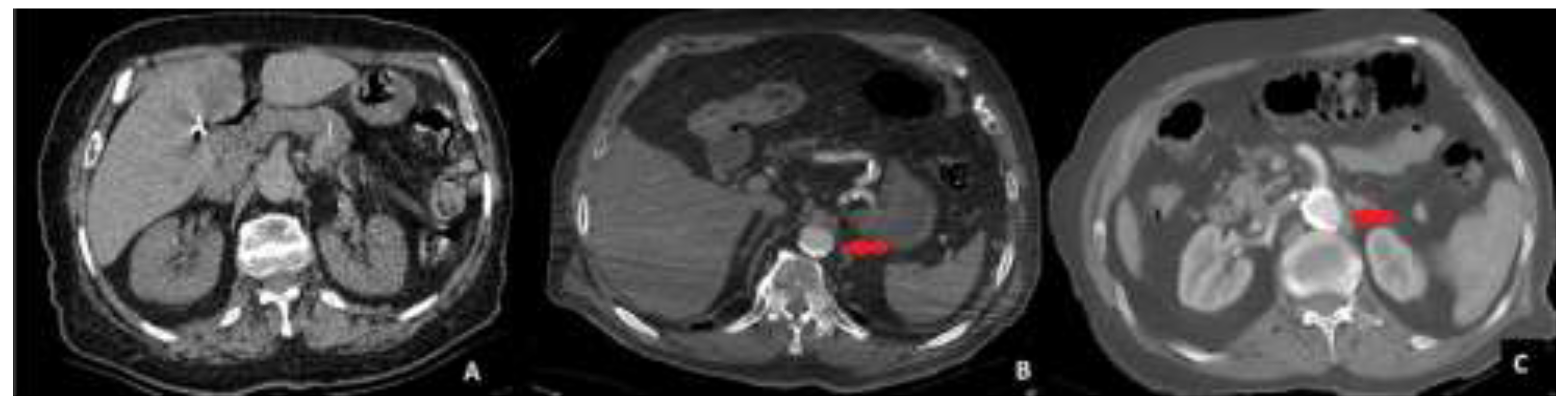
The abdominal CT scans were evaluated for aortic calcification (AC) in the sections between the celiac trunk and the aortic bifurcation. Aortic calcification was classified into three stages based on the extent of calcified plaques encircling the aortic lumen. Stage 0 was defined as the absence of any detectable calcification. Stage 1 was assigned when calcification occupied less than 50% of the aortic circumference. Stage 2 was defined as calcification occupying more than 50% of the aortic circumference.
To ensure consistency in classification, all imaging evaluations were performed by two independent radiologists who were blinded to the surgical outcomes. Any discrepancies in the assessment were resolved by consensus discussion. A visual example of each calcification stage is provided in
Figure 1.
2.4. Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows software (version 26; IBM Corp., Armonk, N.Y., USA). Descriptive statistics were reported for categorical variables as counts and percentages and for numerical variables as mean ± standard deviation or median ± interquartile range based on data distribution.
The normality of data distribution was assessed using the Shapiro-Wilk test. Correlations between variables were evaluated using Pearson and Spearman correlation coefficients depending on data distribution. Comparisons of numerical variables between groups, such as age, were made using the Student's t-test or Mann-Whitney U test, based on the normality assumption. Categorical variables, including gender, tumor localization, histology, TNM stage, comorbidities, neoadjuvant therapy, and AC incidence, were evaluated for differences between groups using the Chi-square test or Fisher's exact test.
A multivariate logistic regression analysis was performed to adjust for potential confounding factors. The model included variables such as age, sex, TNM stage, tumor histology, tumor localization, neoadjuvant therapy, hypertension, diabetes mellitus, cardiovascular disease, smoking, and intraoperative hypotension. The interaction between AC and AL was assessed using univariate binomial logistic regression analysis with bootstrapping. The odds ratio (OR) of aortic calcification for developing anastomotic leakage was calculated, and a p-value of <0.05 was considered statistically significant in all analyses.
3. Results
A total of 151 patients were included in the study cohort, with a mean age of 65.39 ± 10.97 years. Among them, 93 (61.59%) were male and 58 (38.41%) were female. The most prevalent tumor localization was the rectum (32.45%), followed by the ascending colon (31.13%) (
Table 1).
Among the patients, 126 (83.44%) had adenocarcinoma, while 19 (12.58%) had mucinous carcinoma, and 6 (3.97%) had other histological subtypes. Based on TNM staging, 17 (11.25%) patients were Stage I, 52 (34.44%) were Stage II, 61 (40.39%) were Stage III, and 21 (13.91%) were Stage IV. No significant differences were observed in histological subtypes between the AL and non-AL groups (p=0.438), whereas higher TNM stages (Stage III/IV) were slightly more frequent in the AL group, but the difference did not reach statistical significance (p=0.089) (
Table 2).
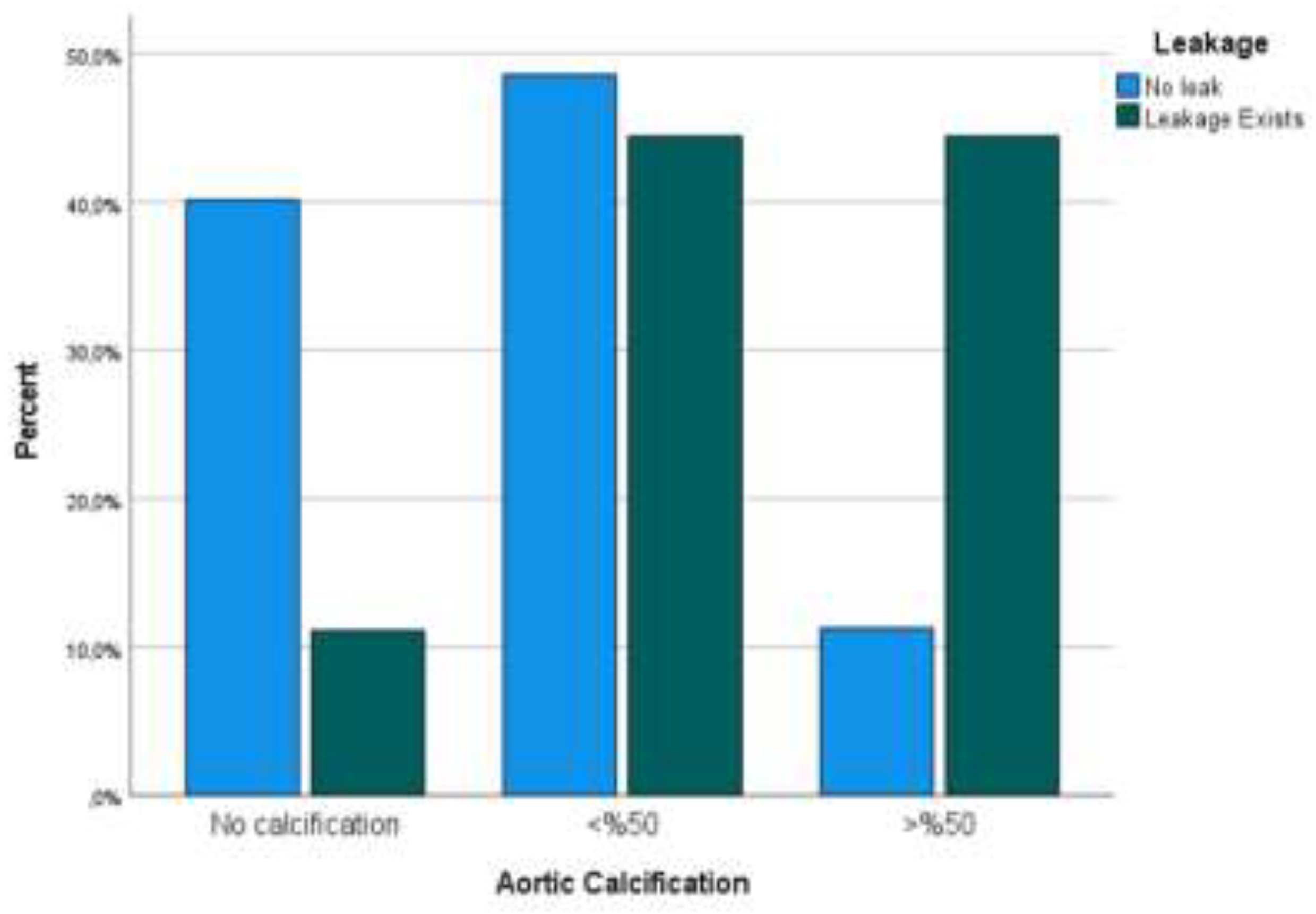
Comorbidities were prevalent among the study population, with hypertension (HT) in 68 patients (45.03%), diabetes mellitus (DM) in 42 (27.81%), cardiovascular disease (CVD) in 31 (20.53%), and smoking history in 47 (31.12%) patients. Patients in the AL group had a higher prevalence of HT (66.67% vs. 43.66%, p=0.027) and CVD (44.44% vs. 18.30%, p=0.014) compared to the non-AL group. Additionally, 38 (25.17%) patients received neoadjuvant oncological therapy (chemotherapy and/or radiotherapy), and AL incidence was higher in patients who received neoadjuvant treatment (10.52% vs. 4.18%, p=0.045). Anastomotic leakage percentages are displayed in
Figure 2.
38.41% of the patients exhibited no calcification (Stage 0), 48.34% had calcification occupying less than half of the aortic circumference (Stage 1), and 13.25% had calcification occupying more than half of the aortic circumference (Stage 2). Anastomotic leaks were observed in 9 out of 151 patients (5.96%) postoperatively. When patients were stratified based on the presence or absence of AL, there were no significant differences in mean age (p=0.543) or gender distribution (p=0.747). However, a notable discrepancy was observed in tumor localization, as rectal tumors had a significantly higher AL incidence compared to colonic tumors (8.3% vs 4.2%, p=0.038). Additionally, a significant difference in AC prevalence was observed between the leakage and no-leakage groups, with a higher prevalence of >50% AC in the leakage group (44.47%) compared to the no-leakage group (11.27%, p=0.012).
Further analysis employing binomial logistic regression with bootstrapping revealed that patients with >50% AC exhibited a substantially elevated risk of AL. The odds ratio (OR) for developing AL in patients with >50% AC was 14.25 [95% CI: 1.486-136.611], with a p-value of 0.021, indicating statistical significance (
Table 3)
Conversely, multivariate analysis adjusting for TNM stage, hypertension, cardiovascular disease, and neoadjuvant therapy showed that AC remained an independent risk factor for AL (OR = 10.38, 95% CI: 1.243-92.118, p=0.032). These findings underscore the potential association between extensive AC and an increased risk of AL following CRS. Additionally, rectal tumor localization, hypertension, cardiovascular disease, and neoadjuvant therapy were associated with increased AL risk. The significant odds ratio for patients with >50% AC highlights the importance of preoperative vascular health assessment to improve surgical outcomes and mitigate this complication.
4. Discussion
Despite advances in clinical experience and the development of advanced surgical tools, anastomotic leakage (AL) remains one of the most serious complications in colorectal cancer surgery. Although various studies have aimed to elucidate the relationship between arteriosclerosis and AL in CRS, the associations remain unclear. Our study demonstrates a significant correlation between the extent of aortic calcification (AC), as assessed by preoperative CT, and the risk of AL. Specifically, patients with >50% AC were found to have a markedly higher risk of AL, highlighting the clinical importance of preoperative vascular assessments in colorectal surgeries.
Several previous studies have analyzed the predictive value of AC for AL. Deguelte et al. reported that abdominal aortic calcification ≥5% should be indicative of a higher risk of AL [
10]. Eveno et al.'s study also determined that aortic artery calcification was an independent determinant of postoperative anastomotic fistula in patients undergoing left colon or rectum resection [
11]. These findings parallel our results and suggest that measuring aortic artery calcification in risk assessment before CRS may enhance risk prediction accuracy. Knight KA and Morita S also confirmed that abdominal aortic calcification could be an indicator of AL [
12,
13]. Previous studies have reported that abdominal aortic calcification (AC) may increase the risk of AL development [
10,
11]. Our study supports this literature, demonstrating that severe AC is an independent risk factor for AL. However, the study by Pochhammer et al. reported that AC is not directly associated with AL. These conflicting results highlight the need for larger-scale studies [
14]. However, Pochhammer et al. argued that impairment of visceral circulation did not affect CRS outcomes, especially in the presence of AL [
14]. The discrepancies between our findings and those of Pochhammer et al. [
14] may be attributed to differences in study design, patient selection criteria, and AC measurement methods. While our study utilized a systematic classification of AC severity based on preoperative CT scans, Pochhammer et al. primarily assessed iliac artery calcifications, which may have different implications for colonic microcirculation. Furthermore, variations in surgical techniques, patient demographics, and perioperative care protocols across studies may contribute to these conflicting results.
Our study suggests that the association between AC and AL may be due to atherosclerosis and its impact on tissue perfusion. Arteriosclerosis, even in asymptomatic patients, is a major contributor to anastomotic ischemia [
15,
16]. When AC occurs, arterial narrowing limits blood flow to the anastomotic site, thereby increasing the risk of AL due to compromised tissue healing [
17,
18,
19]. Ischemic changes, as predictors of AL, have also been reported by Vignali et al., supporting our hypothesis [
20].
In addition to AC, our study identified several other factors associated with an increased risk of AL. Rectal tumor localization was found to have a higher incidence of AL compared to colonic tumors. This is consistent with previous studies suggesting that rectal anastomoses may be more vulnerable due to lower blood supply and increased technical complexity [
21]. Furthermore, patients with hypertension and cardiovascular disease exhibited significantly elevated AL rates, suggesting that systemic vascular health plays a crucial role in anastomotic healing.
The strong association between extensive AC and AL emphasizes the necessity for comprehensive vascular assessments in patients scheduled for CRS. Identifying high-risk patients with significant AC enables the adoption of tailored perioperative strategies to potentially mitigate AL risk. For instance, enhanced cardiovascular interventions and vigilant postoperative monitoring could be beneficial in this patient subset [
22]. Given the multifactorial nature of AL, integrating AC severity, TNM staging, tumor localization, and patient comorbidities into a comprehensive risk stratification model could enhance preoperative planning. A predictive scoring system incorporating these variables may help identify high-risk patients who could benefit from targeted interventions, such as preoperative vascular optimization, perioperative hemodynamic monitoring, and modified anastomotic techniques.
Our study has several limitations. First, due to its retrospective design, there is a potential risk of selection bias and information bias. As data collection relies on medical records, there is a possibility that some clinical variables were misreported or missing. To mitigate this limitation, patient medical records were systematically reviewed, and missing data were minimized. However, prospective studies are needed to validate these findings more robustly. Second, the assessment of aortic calcification (AC) was performed manually, which may introduce interobserver variability. Manual measurements may lead to subjective differences between observers, potentially affecting the consistency of the results. To minimize this bias, future studies should consider using automated image analysis systems or artificial intelligence-assisted measurement techniques to improve accuracy and reproducibility. Third, the relatively small sample size may limit the statistical power of the study. The low incidence of anastomotic leak (AL) could influence the significance level of certain variables in statistical analyses. Particularly in multivariate analyses, larger patient cohorts are required to better establish independent risk factors. Future multi-center prospective studies will be essential to validate these associations. Lastly, there may be potential confounding factors that were not accounted for in this study. Factors such as smoking status, diabetes, and hypertension are known to influence vascular health and could have a direct impact on AL risk. While our study attempted to control for major variables, we acknowledge that additional multivariate analyses with a broader range of variables could further clarify whether AC is an independent risk factor.
Despite these limitations, this study has several strengths. Firstly, it addresses an innovative and clinically relevant topic by examining a potential risk factor for AL in colorectal surgery. The comprehensive data analysis and use of advanced statistical methods improve the reliability of the findings. Additionally, the detailed methodology allows for accurate interpretation and understanding of the results. The study contributes valuable insights for preoperative planning and risk stratification, which could aid in reducing AL rates in clinical practice.
5. Conclusions
In conclusion, our study highlights a significant association between extensive aortic calcification and an increased risk of AL following CRS. Additionally, rectal tumor localization, hypertension, cardiovascular disease, and neoadjuvant therapy were associated with increased AL risk. Preoperative vascular assessments, along with comprehensive risk stratification models incorporating oncological and systemic factors, could help identify patients at higher risk, allowing for the implementation of tailored perioperative strategies. Future studies with larger sample sizes and a prospective design are needed to further elucidate the role of AC in AL and validate these findings.
Author Contributions
Conceptualization, V.B.T., O.K., B.K.; methodology, V.B.T.; software, M.B.T.; validation, V.B.T., O.K., E.G.A., B.K.; formal analysis, V.B.T., M.B.T.; investigation, V.B.T., O.K., M.B.T.; resources, V.B.T., O.K., B.K., E.G.A., M.K.; data curation, V.B.T., B.K., F.Ş.; writing—original draft preparation, V.B.T., O.K., M.B.T., B.K., F.Ş., M.K., E.G.A.; writing—review and editing, V.B.T., O.K., M.B.T., B.K., F.Ş., M.K., E.G.A.; visualization, M.B.T.; supervision, V.B.T.; project administration, V.B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Hitit University Faculty of Medicine Clinical Research Ethics Committee (Protocol Number: 2023-143, date: 01/11/2023).
Informed Consent Statement
Since this was a retrospective study with anonymized patient data, informed consent was waived.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AL |
Anastomotic leakage |
| AC |
Aortic calcification |
| CRS |
Colorectal surgery |
| CT |
Computerized tomography |
| CI |
Confidence interval |
| OR |
Odds ratio |
References
- Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol. 2011;104:734-40. [CrossRef]
- Ma L, Pang X, Ji G, Sun H, Fan Q, Ma C. The impact of anastomotic leakage on oncology after curative anterior resection for rectal cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e22139. [CrossRef]
- Hoek VT, Edomskis PP, Menon AG, Kleinrensink GJ, Lagarde SM, Lange JF, et al. Arterial calcification is a risk factor for anastomotic leakage after esophagectomy: A systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:1975-88. [CrossRef]
- Goense L, van Rossum PSN, Weijs TJ, van Det MJ, Nieuwenhuijzen GA, Luyer MD, et al. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann Thorac Surg. 2016;102:247-52. [CrossRef]
- van Rossum PSN, Haverkamp L, Verkooijen HM, van Leeuwen MS, van Hillegersberg R, Ruurda JP. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology. 2015;274:124-32. [CrossRef]
- Tao W, Cheng YX, Zou YY, Peng D, Zhang W. Aorta Calcification Increases the Risk of Anastomotic Leakage After Gastrectomy in Gastric Cancer Patients. Cancer Manag Res. 2021;13:3857-65. [CrossRef]
- Shen Z, An Y, Shi Y, Yin M, Xie Q, Gao Z, et al. The Aortic Calcification Index is a risk factor associated with anastomotic leakage after anterior resection of rectal cancer. Colorectal Dis. 2019;21:1397-404. [CrossRef]
- Kryzauskas M, Bausys A, Degutyte AE, Abeciunas V, Poskus E, Bausys R, et al. Risk factors for anastomotic leakage and its impact on long-term survival in left-sided colorectal cancer surgery. World J Surg Oncol. 2020;18:205. [CrossRef]
- Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J Gastroenterol. 2006;12:2721-9. [CrossRef]
- Deguelte S, Besson R, Job L, Hoeffel C, Jolly D, Kianmanesh R. Assessing abdominal aortic calcifications before performing colocolic or colorectal anastomoses: A case-control study. J Res Med Sci. 2021;26:110.
- Eveno C, Latrasse V, Gayat É, Lo Dico R, Dohan A, Pocard M. Colorectal anastomotic leakage can be predicted by abdominal aortic calcification on preoperative CT scans: A pilot study. J Visc Surg. 2016;153:253-7. [CrossRef]
- Knight KA, Fei CH, Boland KF, Dolan DR, Golder AM, McMillan DC, et al. Aortic calcification is associated with non-infective rather than infective postoperative complications following colorectal cancer resection: an observational cohort study. Eur Radiol. 2021;31:4319-29.
- Morita S, Tsuruta M, Okabayashi K, Shigeta K, Seishima R, Monno M, et al. Evaluation of abdominal aortic calcification by plain CT predicts anastomotic leakage in laparoscopic surgery for colorectal cancer. Jpn J Clin Oncol. 2022;52:122-7. [CrossRef]
- Pochhammer J, Tröster F, Blumenstock G, Closset J, Lang S, Weller MP, et al. Calcification of the iliac arteries: a marker for leakage risk in rectal anastomosis-a blinded clinical trial. Int J Colorectal Dis. 2018;33:163-70. [CrossRef]
- Scharff JR, Longo WE, Vartanian SM, Jacobs DL, Bahadursingh AN, Kaminski DL. Ischemic colitis: spectrum of disease and outcome. Surgery. 2003;134:624-9; discussion 9-30.
- Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am. 2001;30:445-73. [CrossRef]
- Braunschmid T, Hartig N, Baumann L, Dauser B, Herbst F. Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc. 2017;31:5318-26. [CrossRef]
- Chung RS. Blood flow in colonic anastomoses. Effect of stapling and suturing. Ann Surg. 1987;206:335-9. [CrossRef]
- Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, Momiyama M, et al. Evaluation of the intestinal blood flow near the rectosigmoid junction using the indocyanine green fluorescence method in a colorectal cancer surgery. Int J Colorectal Dis. 2015;30:329-35. [CrossRef]
- Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43:76-82. [CrossRef]
- Brisinda G, Chiarello MM, Pepe G, Cariati M, Fico V, Mirco P, et al. Anastomotic leakage in rectal cancer surgery: Retrospective analysis of risk factors. World J Clin Cases. 2022;10:13321-36. [CrossRef]
- Knight KA, Horgan PG, McMillan DC, Roxburgh CSD, Park JH. The relationship between aortic calcification and anastomotic leak following gastrointestinal resection: A systematic review. Int J Surg. 2020;73:42-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).