Submitted:
13 February 2025
Posted:
13 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Unmet Needs
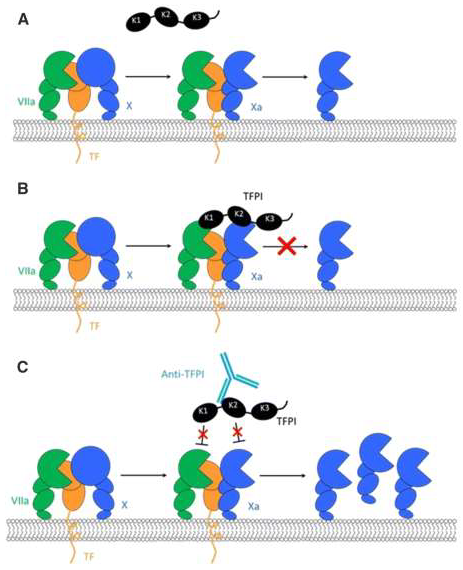
3. Rationale for Inhibiting TFPI
4. Concizumab: Mechanism of Action
5. Efficacy and Safety of Concizumab in Persons with HA or HB with Inhibitors
6. Adherence to Treatment
7. Outcome Measures
8. Discussion
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | Annualized bleeding rate |
| AE | Adverse event |
| aPCC | Activated prothrombin complex concentrate |
| CI | Confidence interval |
| ELISA | Enzyme-linked immunosorbent assay |
| FIX | Factor IX |
| FVIIa | Activated factor VII |
| FVIII | Factor VIII |
| FX | Factor X |
| HA | Hemophilia A |
| HB | Hemophilia B |
| IQR | Interquartile range |
| rFIX | Recombinant factor IX |
| rFVIII | Recombinant factor VIII |
| TF | Tissue factor |
| TFPI | Tissue factor pathway inhibitor |
| TMDD | Target-mediated drug disposition |
References
- Berntorp, E.; Fischer, K.; Hart, D.P.; Mancuso, M.E.; Stephensen, D.; Shapiro, A.D.; Blanchette, V. Haemophilia. Nat Rev Dis Primers 2021, 7, 45. [CrossRef]
- Troisi, R.; Balasco, N.; Autiero, I.; Sica, F.; Vitagliano, L. New insight into the traditional model of the coagulation cascade and its regulation: illustrated review of a three-dimensional view. Res. Pr. Thromb. Haemost. 2023, 7, 102160. [CrossRef]
- van den Berg, H.M.; Srivastava, A. Hemostasis – a balancing act. N Engl J Med 2023, 389, 853-856. [CrossRef]
- Leuci, A.; Dargaud, Y. Blood-Induced Arthropathy: A Major Disabling Complication of Haemophilia. J. Clin. Med. 2023, 13, 225. [CrossRef]
- Nogami, K.; Shima, M. Current and future therapies for haemophilia—Beyond factor replacement therapies. Br. J. Haematol. 2022, 200, 23–34. [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. 6), 1–158. [CrossRef]
- Swan, D.; Mahlangu, J.; Thachil, J. Non-factor therapies for bleeding disorders: A primer for the general haematologist. eJHaem 2022, 3, 584–595. [CrossRef]
- Keam, S.J. Concizumab: First Approval. Drugs 2023, 83, 1053–1059. [CrossRef]
- Crescioli, S.; Kaplon, H.; Chenoweth, A.; Wang, L.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2024. mAbs 2024, 16, 2297450. [CrossRef]
- Núñez, R.; Álvarez-Román, M.T.; Bonanad, S.; González-Porras, J.R.; De La Corte-Rodriguez, H.; Berrueco, R.; Jiménez-Yuste, V. The Limitations and Unmet Needs of the Five Cornerstones to Guarantee Lifelong Optimization of Prophylaxis in Hemophilia Patients. TH Open 2022, 06, e365–e377. [CrossRef]
- Ozelo, M.C.; Yamaguti-Hayakawa, G.G. Impact of novel hemophilia therapies around the world. Res. Pr. Thromb. Haemost. 2022, 6, e12695. [CrossRef]
- Tischer, B.; Marino, R.; Napolitano, M. Patient preferences in the treatment of hemophilia A: impact of storage conditions on product choice. Patient Preference Adherence 2018, ume 12, 431–441. [CrossRef]
- Giangrande, P.L.F.; Hermans, C.; O'Mahony, B.; de Kleijn, P.; Bedford, M.; Batorova, A.; Blatny, J.; Jansone, K.; European Haemophilia Consortium (EHC); the European Association for Haemophilia and Allied Disorders. European principles of inhibitor management in patients with haemophilia. Orphanet J Rare Dis 2018, 13, 66. [CrossRef]
- Male, C.; Andersson, N.G.; Rafowicz, A.; Liesner, R.; Kurnik, K.; Fischer, K.; Platokouki, H.; Santagostino, E.; Chambost, H.; Nolan, B.; et al. Inhibitor incidence in an unselected cohort of previously untreated patients with severe haemophilia B: a PedNet study. Haematologica 2020, 106, 123–129. [CrossRef]
- Puetz, J.; Soucie, J.M.; Kempton, C.L.; Monahan, P.E.; Hemophilia Treatment Center Network (HTCN) Investigators Prevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection database. Haemophilia 2013, 20, 25–31. [CrossRef]
- Berg, H.M.v.D.; Fischer, K.; Carcao, M.; Chambost, H.; Kenet, G.; Kurnik, K.; Königs, C.; Male, C.; Santagostino, E.; Ljung, R. Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood 2019, 134, 317–320. [CrossRef]
- Wight, J.; Paisley, S.; Knight, C. Immune tolerance induction in patients with haemophilia A with inhibitors: a systematic review. Haemophilia 2003, 9, 436–463. [CrossRef]
- D'Angiolella, L.S.; Cortesi, P.A.; Rocino, A.; Coppola, A.; Hassan, H.J.; Giampaolo, A.; Solimeno, L.P.; Lafranconi, A.; Micale, M.; Mangano, S.; et al. The socioeconomic burden of patients affected by hemophilia with inhibitors. Eur. J. Haematol. 2018, 101, 435–456. [CrossRef]
- Dolan, G. Partnering to change the world for people with haemophilia: 7th Haemophilia Global Summit, Madrid, Spain 22–24 September 2016. Eur. J. Haematol. 2017, 99, 3–9. [CrossRef]
- Oladapo, A.O.; Lu, M.; Walsh, S.; O’hara, J.; Kauf, T.L. Inhibitor clinical burden of disease: a comparative analysis of the CHESS data. Orphanet J. Rare Dis. 2018, 13, 198. [CrossRef]
- Walsh, C.E.; Soucie, J.M.; Miller, C.H.; United States Hemophilia Treatment Center Network. Impact of inhibitors on hemophilia a mortality in the United States. Am. J. Hematol. 2015, 90, 400–405. [CrossRef]
- Berntorp, E.; Hermans, C.; Solms, A.; Poulsen, L.; Mancuso, M.E. Optimising prophylaxis in haemophilia A: The ups and downs of treatment. Blood Rev. 2021, 50, 100852. [CrossRef]
- Brackmann, H.H.; Schramm, W.; Oldenburg, J.; Cano, V.; Turecek, P.L.; Négrier, C. Origins, Development, Current Challenges and Future Directions with Activated Prothrombin Complex Concentrate for the Treatment of Patients with Congenital Haemophilia with Inhibitors. Hamostaseologie 2020, 40, 606–620. [CrossRef]
- Hermans, C.; Giangrande, P.L.F.; O'Mahony, B.; de Kleijn, P.; Bedford, M.; Batorova, A.; Blatny, J.; Jansone, K.; European Haemophilia, C.; the European Association for, H., et al. European principles of inhibitor management in patients with haemophilia: implications of new treatment options. Orphanet J Rare Dis 2020, 15, 219. [CrossRef]
- Hay, C.R.M.; DiMichele, D.M.; International Immune Tolerance, S. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012, 119, 1335–1344. [CrossRef]
- Ljung, R.; Auerswald, G.; Benson, G.; Dolan, G.; Duffy, A.; Hermans, C.; Jimenez-Yuste, V.; Lambert, T.; Morfini, M.; Zupancic-Salek, S., et al. Inhibitors in haemophilia A and B: Management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients. Eur J Haematol 2019, 102, 111-122. [CrossRef]
- Doshi, B.S.; Arruda, V.R. Gene therapy for hemophilia: what does the future hold? Ther Adv Hematol 2018, 9, 273-293. [CrossRef]
- Mancuso, M.E.; Mahlangu, J.N.; Pipe, S.W. The changing treatment landscape in haemophilia: from standard half-life clotting factor concentrates to gene editing. Lancet 2021, 397, 630–640. [CrossRef]
- Miesbach, W.; Klamroth, R.; Oldenburg, J.; Tiede, A. Gene therapy for hemophilia – opportunities and risks. Dtsch Arztebl Int 2022, 119, 887-894. [CrossRef]
- Miesbach, W.; O’mahony, B.; Key, N.S.; Makris, M. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia 2019, 25, 545–557. [CrossRef]
- Nowrouzi, A.; Penaud-Budloo, M.; Kaeppel, C.; Appelt, U.; Le Guiner, C.; Moullier, P.; von Kalle, C.; O Snyder, R.; Schmidt, M. Integration Frequency and Intermolecular Recombination of rAAV Vectors in Non-human Primate Skeletal Muscle and Liver. Mol. Ther. 2012, 20, 1177–1186. [CrossRef]
- Jiménez-Yuste, V.; Auerswald, G.; Benson, G.; Dolan, G.; Hermans, C.; Lambert, T.; Ljung, R.; Morfini, M.; Santagostino, E.; Šalek, S.Z. Practical considerations for nonfactor-replacement therapies in the treatment of haemophilia with inhibitors. Haemophilia 2021, 27, 340–350. [CrossRef]
- Mahlangu, J.; Iorio, A.; Kenet, G. Emicizumab state-of-the-art update. Haemophilia 2022, 28 Suppl 4, 103-110. [CrossRef]
- European Medicines Agency. Hemlibra. Summary of product characteristics. 2018. Availabe online: https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_en.pdf (accessed on 23 May 2024).
- Abbattista, M.; Ciavarella, A.; Noone, D.; Peyvandi, F. Hemorrhagic and thrombotic adverse events associated with emicizumab and extended half-life factor VIII replacement drugs: EudraVigilance data of 2021. J. Thromb. Haemost. 2023, 21, 546–552. [CrossRef]
- Arcudi, S.; Gualtierotti, R.; Scalambrino, E.; Clerici, M.; Hassan, S.; Begnozzi, V.; Boccalandro, E.A.; Novembrino, C.; Valsecchi, C.; Palla, R.; et al. Predictive parameters for spontaneous joint bleeding during emicizumab prophylaxis. Blood Adv. 2024, 8, 2901–2907. [CrossRef]
- Batsuli, G.; Wheeler, A.P.; Weyand, A.C.; Sidonio, R.F., Jr.; Young, G. Severe muscle bleeds in children and young adults with hemophilia A on emicizumab prophylaxis: Real-world retrospective multi-institutional cohort. Am. J. Hematol. 2023, 98, E285–E287. [CrossRef]
- Levy-Mendelovich, S.; Brutman-Barazani, T.; Budnik, I.; Avishai, E.; Barg, A.A.; Levy, T.; Misgav, M.; Livnat, T.; Kenet, G. Real-World Data on Bleeding Patterns of Hemophilia A Patients Treated with Emicizumab. J. Clin. Med. 2021, 10, 4303. [CrossRef]
- Warren, B.B.; Chan, A.; Manco-Johnson, M.; Branchford, B.R.; Buckner, T.W.; Moyer, G.; Gibson, E.; Thornhill, D.; Wang, M.; Ng, C.J. Emicizumab initiation and bleeding outcomes in people with hemophilia A with and without inhibitors: A single-center report. Res. Pract. Thromb. Haemost. 2021, 5, e12571. [CrossRef]
- Kizilocak, H.; Guerrera, M.F.; Young, G. Neutralizing antidrug antibody to emicizumab in patients with severe hemophilia A: Case report of a first noninhibitor patient and review of the literature. Res. Pract. Thromb. Haemost. 2023, 7, 102194. [CrossRef]
- Chaudhry, R.; Usama, S.M.; Babiker, H.M. Physiology, coagulation pathways. In StatPearls, Treasure Island (FL), 2023.
- Zaidi, A.; Green, L. Physiology of haemostasis. Anaesth Intensive Care Med 2022, 23, 111-117.
- Mehic, D.; Colling, M.; Pabinger, I.; Gebhart, J. Natural anticoagulants: A missing link in mild to moderate bleeding tendencies. Haemophilia 2021, 27, 701–709. [CrossRef]
- Shetty, S.; Vora, S.; Kulkarni, B.; Mota, L.; Vijapurkar, M.; Quadros, L.; Ghosh, K. Contribution of natural anticoagulant and fibrinolytic factors in modulating the clinical severity of haemophilia patients. Br. J. Haematol. 2007, 138, 541–544. [CrossRef]
- Lane, D.A. Correcting the hemophilic imbalance. Blood 2017, 129, 10–11. [CrossRef]
- Kato, H. Tissue factor pathway inhibitor; its structure, function and clinical significance. Pol J Pharmacol. 1996, 48, 67–72.
- Chowdary, P. Anti-tissue factor pathway inhibitor (TFPI) therapy: a novel approach to the treatment of haemophilia. Int. J. Hematol. 2018, 111, 42–50. [CrossRef]
- Chowdary, P. Inhibition of Tissue Factor Pathway Inhibitor (TFPI) as a Treatment for Haemophilia: Rationale with Focus on Concizumab. Drugs 2018, 78, 881–890. [CrossRef]
- Hilden, I.; Lauritzen, B.; Sørensen, B.B.; Clausen, J.T.; Jespersgaard, C.; Krogh, B.O.; Bowler, A.N.; Breinholt, J.; Gruhler, A.; Svensson, L.A.; et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood 2012, 119, 5871–5878. [CrossRef]
- Chowdary, P.; Lethagen, S.; Friedrich, U.; Brand, B.; Hay, C.; Karim, F.A.; Klamroth, R.; Knoebl, P.; Laffan, M.; Mahlangu, J.; et al. Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial. J. Thromb. Haemost. 2015, 13, 743–754. [CrossRef]
- An, G. Concept of pharmacologic target-mediated drug disposition in large-molecule and small-molecule compounds. J Clin Pharmacol 2020, 60, 149-163. [CrossRef]
- Agersø, H.; Overgaard, R.V.; Petersen, M.B.; Hansen, L.; Hermit, M.B.; Sørensen, M.H.; Petersen, L.C.; Hilden, I. Pharmacokinetics of an anti-TFPI monoclonal antibody (concizumab) blocking the TFPI interaction with the active site of FXa in Cynomolgus monkeys after iv and sc administration. Eur. J. Pharm. Sci. 2014, 56, 65–69. [CrossRef]
- Novo Nordisk Canada. AlhemoTM (concizumab injection): Product Monograph. 2023. Availabe online: https://www.novonordisk.ca/content/dam/nncorp/ca/en/products/alhemo-en-product-monograph.pdf (accessed on 23 May 2024).
- Favresse, J.; Lippi, G.; Roy, P.M.; Chatelain, B.; Jacqmin, H.; Ten Cate, H.; Mullier, F. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci 2018, 55, 548-577.
- Capecchi, M.; Scalambrino, E.; Griffini, S.; Grovetti, E.; Clerici, M.; Merati, G.; Chantarangkul, V.; Cugno, M.; Peyvandi, F.; Tripodi, A. Relationship between thrombin generation parameters and prothrombin fragment 1+2 plasma levels. Int. J. Lab. Hematol. 2021, 43, E248–E251. [CrossRef]
- Matsushita, T.; Shapiro, A.; Abraham, A.; Angchaisuksiri, P.; Castaman, G.; Cepo, K.; D’oiron, R.; Frei-Jones, M.; Goh, A.-S.; Haaning, J.; et al. Phase 3 Trial of Concizumab in Hemophilia with Inhibitors. New Engl. J. Med. 2023, 389, 783–794. [CrossRef]
- Shapiro, A.D.; Angchaisuksiri, P.; Astermark, J.; Benson, G.; Castaman, G.; Eichler, H.; Jiménez-Yuste, V.; Kavakli, K.; Matsushita, T.; Poulsen, L.H.; et al. Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors. Blood Adv. 2022, 6, 3422–3432. [CrossRef]
- Shapiro, A.D. Concizumab: a novel anti-TFPI therapeutic for hemophilia. Blood Adv. 2021, 5, 279–279. [CrossRef]
- Kjalke, M.; Kjelgaard-Hansen, M.; Andersen, S.; Hilden, I. Thrombin generation potential in the presence of concizumab and rFVIIa, APCC, rFVIII, or rFIX: In vitro and ex vivo analyses. J. Thromb. Haemost. 2021, 19, 1687–1696. [CrossRef]
- Young, G. Nonfactor Therapies for Hemophilia. HemaSphere 2023, 7, e911. [CrossRef]
- Anandani, G.; Patel, T.; Parmar, R. The Implication of New Developments in Hemophilia Treatment on Its Laboratory Evaluation. Cureus 2022, 14, e30212. [CrossRef]
- Shapiro, A.D.; Angchaisuksiri, P.; Astermark, J.; Benson, G.; Castaman, G.; Chowdary, P.; Eichler, H.; Jiménez-Yuste, V.; Kavakli, K.; Matsushita, T.; et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood 2019, 134, 1973–1982. [CrossRef]
- Castaman, G.; Abraham, A.; Angchaisuksiri, P.; Martinez, L.V.; Nogami, K.; Sathar, J.; Shen, C.; Zaw, J.J.T.; Young, G. The Effect of Concizumab Prophylaxis on Target Joints, Resolution and Joint Bleeds in Patients With Hemophilia A or B With or Without Inhibitors in Phase 3 Clinical Trials. Blood 2023, 142, 284–284. [CrossRef]
- Chan, A.K.; Barnes, C.; Mathias, M.; Linari, S.; Jaime, F.J.L.; Poulsen, L.H.; Bovet, J.; Odgaard-Jensen, J.; Matsushita, T. Surgical Procedures and Hemostatic Outcome in Patients with Hemophilia Receiving Concizumab Prophylaxis during the Phase 3 explorer7 and explorer8 Trials. Blood 2023, 142, 30–30. [CrossRef]
- Eichler, H.; Angchaisuksiri, P.; Kavakli, K.; Knoebl, P.; Windyga, J.; Jiménez-Yuste, V.; Hyseni, A.; Friedrich, U.; Chowdary, P. A randomized trial of safety, pharmacokinetics and pharmacodynamics of concizumab in people with hemophilia A. J. Thromb. Haemost. 2018, 16, 2184–2195. [CrossRef]
- Waters, E.K.; Sigh, J.; Friedrich, U.; Hilden, I.; Sørensen, B.B. Concizumab, an anti-tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay. Haemophilia 2017, 23, 769–776. [CrossRef]
- Seremetis, S.V.; Cepo, K.; Rasmussen, J.S.; Rose, T.H.; Tamer, S.; Porstmann, T.; Haaning, J. Risk Mitigation Strategy for Concizumab Clinical Trials after Pause Due to Non-Fatal Thrombotic Events. Blood 2020, 136, 40–40. [CrossRef]
- Peyvandi, F.; Garagiola, I.; Mannucci, P.M. Post-authorization pharmacovigilance for hemophilia in Europe and the USA: Independence and transparency are keys. Blood Rev. 2021, 49, 100828. [CrossRef]
- Thornburg, C.D.; Duncan, N. Treatment adherence in hemophilia. Patient Preference Adherence 2017, ume 11, 1677–1686. [CrossRef]
- Johnston, K.; Stoffman, /.J.M.; Mickle, A.T.; Klaassen, R.J.; Diles, D.; Olatunde, S.; Eliasson, L.; Bahar, R. Preferences and Health-Related Quality-of-Life Related to Disease and Treatment Features for Patients with Hemophilia A in a Canadian General Population Sample. Patient Preference Adherence 2021, ume 15, 1407–1417. [CrossRef]
- Coleman, C.I.; Limone, B.; Sobieraj, D.M.; Lee, S.; Roberts, M.S.; Kaur, R.; Alam, T. Dosing Frequency and Medication Adherence in Chronic Disease. J. Manag. Care Pharm. 2012, 18, 527–539. [CrossRef]
- Coyne, M.; Rinaldi, A.; Brigham, K.; Hawthorne, J.; Katsaros, D.; Perich, M.; Carrara, N.; Pericaud, F.; Franzese, C.; Jones, G. Impact of Routines and Rituals on Burden of Treatment, Patient Training, Cognitive Load, and Anxiety in Self-Injected Biologic Therapy. Patient Preference Adherence 2022, ume 16, 2593–2607. [CrossRef]
- Rasmussen, N.K.; Berg, B.; Christiansen, A.S.L.; Neergaard, J.S.; Ter-Borch, G.; Hildebrand, E.; Gonczi, M.; Sparre, T. The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference. Patient Preference Adherence 2024, ume 18, 1713–1727. [CrossRef]
- Stoner, K.L.; Harder, H.; Fallowfield, L.J.; Jenkins, V.A. Intravenous versus Subcutaneous Drug Administration. Which Do Patients Prefer? A Systematic Review. Patient - Patient-Centered Outcomes Res. 2014, 8, 145–153. [CrossRef]
- Roszkiewicz, J.; Swacha, Z.; Smolewska, E. Prefilled pen versus prefilled syringe: a pilot study evaluating two different methods of methotrexate subcutaneous injection in patients with JIA. Pediatr Rheumatol Online J 2020, 18, 1–8. [CrossRef]
- Vermeire, S.; D'Heygere, F.; Nakad, A.; Franchimont, D.; Fontaine, F.; Louis, E.; Van Hootegem, P.; Dewit, O.; Lambrecht, G.; Strubbe, B.; et al. Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Preference Adherence 2018, ume 12, 1193–1202. [CrossRef]
- Young, G. The dosing conundrum of emicizumab: to waste product or not? Res Pract Thromb Haemost 2023, 7, 100087. [CrossRef]
- Hermans, C.; Noone, D.; Benson, G.; Dolan, G.; Eichler, H.; Jiménez-Yuste, V.; Königs, C.; Lobet, S.; Pollard, D.; Zupančić-Šalek, S.; et al. Hemophilia treatment in 2021: Choosing the”optimal” treatment using an integrative, patient-oriented approach to shared decision-making between patients and clinicians. Blood Rev. 2021, 52, 100890. [CrossRef]
- Castaman, G.; Jimenez-Yuste, V.; Gouw, S.; D'Oiron, R. Outcomes and outcome measures. Haemophilia 2024, 30, 112–119. [CrossRef]
- Di Minno, M.N.D.; Martinoli, C.; Pasta, G.; la Corte-Rodriguez, H.; Samy, I.; Stephensen, D.; Timmer, M.A.; Winburn, I. How to assess, detect, and manage joint involvement in the era of transformational therapies: role of point-of-care ultrasound. Haemophilia 2023, 29, 1-10. [CrossRef]
- Dutreil, S. Physical and psychosocial challenges in adult hemophilia patients with inhibitors. J. Blood Med. 2014, 5, 115–122. [CrossRef]
- Brod, M.; Bushnell, D.M.; Neergaard, J.S.; Waldman, L.T.; Busk, A.K. Understanding treatment burden in hemophilia: development and validation of the Hemophilia Treatment Experience Measure (Hemo-TEM). J. Patient-Reported Outcomes 2023, 7, 1–23. [CrossRef]
- Thachil, J.; Connors, J.M.; Mahlangu, J.; Sholzberg, M. Reclassifying hemophilia to include the definition of outcomes and phenotype as new targets. J. Thromb. Haemost. 2023, 21, 1737–1740. [CrossRef]
- Tran, H.; von Mackensen, S.; Abraham, A.; Castaman, G.; Hampton, K.; Knoebl, P.; Linari, S.; Odgaard-Jensen, J.; Neergaard, J.S.; Stasyshyn, O.; et al. Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: patient-reported outcome results from the phase 3 explorer7 study. Res. Pr. Thromb. Haemost. 2024, 8, 102476. [CrossRef]
- Mannucci, P.M. Hemophilia treatment innovation: 50 years of progress and more to come. J. Thromb. Haemost. 2023, 21, 403–412. [CrossRef]
- Arruda, V.R.; Samelson-Jones, B.J. Gene therapy for immune tolerance induction in hemophilia with inhibitors. J. Thromb. Haemost. 2016, 14, 1121–1134. [CrossRef]
- Baas, L.; van der Graaf, R.; van Hoorn, E.S.; Bredenoord, A.L.; Meijer, K.; consortium, S. The ethics of gene therapy for hemophilia: a narrative review. J. Thromb. Haemost. 2023, 21, 413–420. [CrossRef]
- Spadarella, G.; Di Minno, A.; Milan, G.; Franco, N.; Polimeno, M.; Castaldo, F.; Di Minno, G. Paradigm shift for the treatment of hereditary haemophilia: Towards precision medicine. Blood Rev. 2020, 39, 100618. [CrossRef]
- Haute Autorite de Sante. ALHEMO (concizumab) - hemophilia A and B with inhibitors - early access decision. Posted on 6 Oct 2023 [in French]. 2023. Availabe online: https://www.has-sante.fr/jcms/p_3466237/fr/alhemo-concizumab-hemophilie-a-et-b-avec-inhibiteurs (accessed on 2 Oct 2024).
- Pharmaceutical Evaluation Division Pharmaceutical Safety and Environmental Health Bureau. Report on the deliberation results - Alhemo. 2023. Availabe online: https://www.pmda.go.jp/files/000268789.pdf (accessed on 2 Oct 2024).
- Swissmedic. Alhemo® (active substance: concizumab). 2023. Availabe online: https://www.swissmedic.ch/swissmedic/en/home/about-us/publications/public-summary-swiss-par/public-summary-swiss-par-alhemo.html (accessed on 2 Oct 2024).
- Therapeutic Goods Administration. Alhemo. 2023. Availabe online: https://www.tga.gov.au/resources/auspmd/alhemo (accessed on 2 Oct 2024).
| Characteristic | Description |
|---|---|
| Mechanism of action [48] | Concizumab binds to the Kunitz-2 domain of the TFPI protein and prevents TFPI from binding to FXa and to the TF/FVIIa complex; the inhibition of TFPI increases thrombin generation |
| Administration [53] | Subcutaneous using a prefilled multidose pen |
| Half-life [53] | 38 h |
| Frequency of administration [53] | Once daily |
| Dose calculation [53] | Patient bodyweight (kg) × dose (1.00, 0.15, 0.20 or 0.25 mg/kg) = total amount (mg) of concizumab to be administered in a single daily injection |
| Antidote [60] | None, but quick washout |
| Laboratory monitoringa [61] |
Monitoring drug concentration: Measurement of TFPI levels using ELISA Measurement of residual TFPI activity using specific activity assays, e.g., diluted PT-based assay or TF-dependent chromogenic assays Monitoring drug efficacy: Thrombin generation, thromboelastography, clot waveform analysis before and after treatment commencement |
| Breakthrough bleed treatment | No concizumab dose adjustment needed Bypassing agents (rFVIIa, aPCC, plasma-derived FVIIa/FX), factor concentrates |
| Laboratory monitoring during concomitant treatment with concizumab and bypassing agents | Thrombin generation |
| Treatment management during surgery |
Minor surgery: No concizumab dose adjustment needed Major surgery: Concizumab should be paused 4 days prior to surgery and resumed at the normal daily maintenance dose (either 0.15, 0.20, or 0.25 mg/kg) 10–14 days after surgery, considering each patient’s overall clinical pictureb |
| Immunogenicity [57] | In the explorer4 and 5 trials [57], 25% of patients developed mostly low-titer and transient neutralizing anti-concizumab antibodies |
| AEs (frequency in the explorer7 trial) [56] |
Common AEs (occurring in ≥5% of patients): injection-site reactions (22.8%), arthralgia (11.4%), upper respiratory tract infections (7.0%), headache (5.3%), pyrexia (5.3%) Less common AEs: hypersensitivity (2.6%), thromboembolic events (0.9%), pruritus (0.9%) |
| Trial ID | Study type | Intervention | Number of participants | Findings |
|---|---|---|---|---|
| explorer1 (NCT01228669) Phase 1 |
A multicenter, randomized, double-blind, placebo-controlled, single-dose, dose-escalation trial investigating safety, PK and PD of NNC 0172-0000-2021 administered intravenously and SC to healthy male subjects and persons with HA or HB | Concizumab or placebo | 52 (28 healthy volunteers, 24 persons with HA or HB) | Primary endpoint: safety 76 AEs (75% mild) |
| explorer2 (NCT01631942) Phase 1 |
A multicenter, open-label, multiple-dosing trial investigating safety, PK and PD of NNC 0172-2021 administered SC to healthy male subjects and persons with HA or HB | Low, medium, or high dose of concizumab | 22 (4 healthy volunteers, 18 persons with HA or HB) | Primary endpoint: safety No severe or unexpected AEs Increased thrombin generation with concizumab in thrombin generation assay ex vivo and in vivo |
| explorer3 (NCT02490787) Phase 1b |
A multicenter, randomized, placebo-controlled, double-blind, multiple-dose trial investigating safety, PK and PD of concizumab administered SC to persons with HA | Placebo, or five escalating doses of concizumab | 24 | 56 AEs in 19 persons (54 mild and 2 moderate); 91 bleeds (almost all mild) |
| explorer4 (NCT03196284) Phase 2 |
A multicenter, randomized, open-label, controlled trial evaluating the efficacy and safety of prophylactic administration of concizumab in persons with HA or HB with inhibitors | Concizumab (main and extension phases), with eptacog alfa administered on-demand during bleeding episodes | 26 | Estimated ABR 4.5 (95% CI: 3.2–6.4) in the concizumab arm vs. 20.4 (95% CI: 14.4–29.1) in the rFVIIa on-demand arm Low AE rates, no severe AEs reported, no AE-related withdrawals, no thromboembolic events, and no deaths |
| explorer6 (NCT03741881) Phase 3 |
A prospective, multinational, non-interventional study in persons with HA or HB with or without inhibitors treated according to routine clinical practice | No treatment given | 231* | No published results |
| explorer7 (NCT04083781) Phase 3 |
Efficacy and safety of concizumab prophylaxis in persons with HA or HB with inhibitors | No prophylaxis for ≥24 weeks (group 1), or prophylaxis with concizumab for ≥32 weeks (group 2), or nonrandomly assigned to prophylaxis with concizumab for ≥24 weeks (groups 3 and 4) | 133(19 in group 1; 33 in group 2; 21 in group; and 60 in group 4) | Median ABR was 9.8 (IQR 6.5–20.2) in group 1 vs. 0.0 (IQR 0.0–3.3) in group 2 Overall median ABR in the concizumab groups was 0.0 No thromboembolic events after resuming the therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





