1. Introduction
In Kazakhstan, there are huge reserves of unconditional phosphate raw materials for obtaining mineral fertilizers and feed monocalcium phosphate. The Karatau phosphorite-bearing basin belongs to a group of large phosphorite deposits in the south of Kazakhstan, in Zhambyl and Turkestan regions [
1,
2].In the Karatau basin there are such deposits as Aksay, Koksu, Kokzhon, Cholaktau, the reserves of which are 15.0 billion tons with a content of 15.2-25.1% Р
2О
5. In addition, phosphorite ores are found in the Chilisay deposit, the reserves of which are 1.13 billion tons with a content of 16.51-19.36% P
2O
5 [
3,
4]. Currently, out of 12 known sites of the deposit, three are being developed: Chulaktau, Aksay and Dzhanatas, the total reserves of which reach 1.5 billion tons [
5].
In the future, concentrated mineral fertilizers may be obtained from such ores using the complex-circulation method. In this area, we are developing highly effective production technologies for obtaining complex mineral fertilizer and pure fluoride-free feed monocalcium phosphate based on unconditional phosphate raw materials and technogenic waste.
“Mineral Fertilizers” plant by Kazphosphate LLP produces mineral fertilizers: simple superphosphate, ammophos, sulfammophos, nitroammophos, feed defluorinated phosphates (feed tricalcium phosphate 27% P2O5) – for agriculture by processing conditioned phosphate ores (21-25%). At the same time, the reserves of conditioned phosphate mineral raw materials are depleted.
Kazakhstan has large reserves of unconditional phosphate raw materials in the Chilisay deposit, Aktobe region. The total volume of explored and established resources for the entire area of the Chilisay deposit is 484 million tons of ore with a content of 10.53% P2O5 or, in terms of the useful component, 51 million tons of P2O5. The estimated volume of resources is 1.128 billion tons of ore with a content of 10.28% P2O5 (or 116 million tons of P2O5).
The Chilisay phosphorites are characterized by variable mineral composition. Their quality varies widely depending on the content of harmful impurities that reduce the technological parameters of the products.
Due to the depletion of rich phosphate ore reserves in Karatau in recent years, the mineral fertilizer industry receives raw materials with an increasingly low content of 10-19% P
2O
5. Much attention is paid to the research of phosphate ore beneficiation issues in the literature. The results of researches on ore beneficiation in accordance with various schemes, as well as laboratory experiments and semi-factory tests on the processing of the Chilisay phosphorites are considered [
6,
7,
8].
However, due to high costs and inefficiency of phosphorite beneficiation, this development is not used in production. To improve efficiency, we propose a circulation technology for processing the Chilisay deposit phosphorites without preliminary ore beneficiation, which allows eliminating the costs of beneficiation and obtaining pure mineral fertilizer and fluoride-free feed monocalcium phosphate.
Recently, an effective method [
9] for phosphorite ore beneficiation was developed. This method is most suitable for sandy and shell phosphorites. However, for phosphorites in which even with fine grinding the amount of monomineral grains is small and ineffective [
10].
The work [
11] researches the decomposition of the Karatau and Chilisay deposit phosphorites by potassium bisulfate. The experimental data obtained characterize the kinetics of phosphorite decomposition by 5-25% KHSO
4 solutions at temperatures of 25, 50, 70°C. Bisulfate was introduced in large excess compared to the stoichiometry of the reaction:
However, expensive potassium salt reagent is used to decompose phosphate raw materials.
The paper [
12] proposes the possibility of obtaining feed defluorinated phosphates from low-quality Karatau and Chilisay phosphorites using corrective additives in the form of acidic phosphates. The use of acidic phosphates promotes a deeper process of fluorapatite defluorination under hydrothermal conditions and an increase in the solubility of products in a 0.4% hydrochloric acid solution. The possibility of decarbonizing phosphorite placed in the anode chamber of an electrodialyzer during neutralization of wastewater from an extraction phosphoric acid shop is presented [
13]. The maximum degree of phosphorite decarbonization is achieved at a 50% phosphorite rate and a mixing speed of 600-800 rpm. With an increase in the concentration of the initial solution of 0.11-0.4% by P
2O
5, the phosphorite decarbonization degree decreases.
A mechanochemical method for obtaining fertilizers with a high content (up to 100%) of the citrate-soluble form of P
2O
5 and the use of humic compounds were proposed [
14]. The results of experimental researches of the selective destruction of crystalline phases of the Chilisay phosphorite ingredients with varying acidity of the extract solutions were considered. When researching the dynamics of calcium compound extraction from phosphorite into solution, the limiting values of acidity of the extract solutions providing calcium solutions from impurity minerals (pH>3) were identified. Kinetic and technological dependencies were found that can be used to select optimal conditions for chemical enrichment, methods for combating price formation and for the extraction processing of low-grade phosphate raw materials [
15,
16].
Currently, researches are conducted into the technology of processing low-grade phosphorites using humate compounds that promote rapid plant growth and increase soil fertility [
17,
18].
Particular attention is paid to the comprehensive processing of technogenic waste. Over 800 million tons of industrial waste are generated annually in the Republic of Kazakhstan, such as overburden and ash (70%), waste from the manufacturing industry (10%) [
19,
20]. Only in Kazphosphate LLP at Novo-Dzhambul Phosphorus Plant and Mineral Fertilizer Plant, about 7.0 million tons of industrial waste – slag, dust and phosphogypsum – are accumulated. Such waste increases by 10% annually, pollutes the atmosphere and causes environmental damage to the regions where the plants are located [
21].
In this regard, the problems of recycling solid and liquid industrial waste into mineral fertilizers are relevant.
Waste from the production of phosphorus, phosphoric acid and phosphorus fertilizers are the most high-tonnage waste of the chemical industrial complex. The largest share in the phosphorus industry falls on the production of phosphorus fertilizers – superphosphate. The raw materials for obtaining these products are ores containing phosphoritesСа
3(РО
4)
2 and apatites – fluoroapatite Са
3(РО
4)
2⋅CaF
2 and hydroxyapatite Са
5(РО
4)
3ОН. Phosphorus ores are sedimentary rocks cemented by calcium phosphates [
22,
23,
24].
In addition, the existing technology of electrothermal phosphorus production is applicable to the processing of only lump phosphorites, during the preliminary preparation of which (crushing, grinding, screening, transportation, etc.) losses are more than 40%. Small fractions of phosphate raw materials accumulate in dumps. The yield of these fractions is 35-44% of the mined ore, in some areas 46-48% [
25,
26,
27,
28].
One of the main reasons for the formation of waste is the low quality of the original raw material – the Karatau basin phosphorites. It is known that unstable in chemical and mineralogical composition, prone to depletion in phosphorus, containing a significant amount of ballast rocks, phosphorites are difficult to enrich raw materials. Enriched apatite and phosphorite concentrates are used to obtain mineral fertilizer and phosphorus [
29,
30,
31]. The authors of [
32] researched the possibilities of using poor phosphorite ores of the Dzhanatas deposit and phosphate-siliceous shales in agglomeration roasting. Thermodynamic features of obtaining phosphorite agglomerate using phosphate-siliceous shales and oil refining waste were researched.
The production of mineral fertilizers is one of the key sectors of the Kazakhstan chemical complex, traditionally occupying a leading position in non-hydrocarbon non-raw material exports. Kazakhstani producers are fully provided with the main raw materials for the production of all types of mineral fertilizers. Due to the demand for mineral fertilizers from potential foreign consumers, they are ready to increase the volume of production [
33,
34]. In addition, by improving the physical and chemical properties of mineral fertilizers, it is possible to significantly increase the efficiency of their use and avoid losses at the application stage [
35,
36]. Consumer properties of granulated mineral fertilizers – static strength of granules, caking, dust emission – are interconnected and depend on the chemical composition and structure of the granules, which are largely determined by the technological parameters of the production process [
37,
38].
Particular attention is paid to the quality of phosphorus-containing fertilizers. The proportion of fertilizer application largely depends on the composition and structure of the soil: on semi-sandy and sandy soils 85-95 g/m
2, on clay soils 45-75 g/m
2. Fertilizer application rates for vegetable crops are 10-40 g for each hole, trees and bushes 40-60 g/m
2 [
39]. In such cases, encapsulation of fertilizers allows for effective control of the release of nutrients. This is especially important in unfavorable agroclimatic conditions, when plants are stressed and need additional nutrition. Microspheres regulate the release of fertilizers in accordance with plant needs, delivering the necessary substances at the optimal time [
40,
41].
A number of scientific papers are devoted to the production and processing of phosphorus-containing sludge into complex mineral fertilizers [
42,
43,
44,
45]. The work established that phosphorus sludge is a colloidal system (gel) consisting of the smallest mineral and carbon particles firmly bound to phosphorus [
46,
47,
48,
49,
50], from the vapor-gas phase and the capture of dust remaining after the gases pass through electric precipitators [
51,
52], which is successfully processed (burned) in a thermal acid production plant [
48,
49,
50,
51,
53,
54], and the solid residue containing oxidized phosphorus and potassium, after the addition of organic polymers promotes structure formation in the system and the formation of a polymer-fertilizer complex, the preservation of assimilable P
2O in the fertilizer composition and, when used later, leads to the aggregation of soil aggregates, these aggregates retain moisture, which has a beneficial effect on maintaining soil moisture for a long time. Thus, organic polymers contribute to the improvement of melioration due to soil aggregation, but also the agronomic properties of saline and gray soils [
55,
56,
57,
58,
59].
The use of modified derivatives of polyelectrolytes based on polyacrylonitrile (PAN) in the production of superphosphate and double superphosphate both improves the performance properties of mineral fertilizers and increases the yield of agricultural crops [
57,
58,
59,
60,
61,
62]. In addition, they form a thin film on the surface of complex fertilizer particles [
63,
64,
65,
66].
The work determined the standard thermal effects during changes in the enthalpy of the system, the standard change in the entropy of the system and the Gibbs energy in the range of 333-363 K during the decomposition of cottrel dust with a solution of sulfuric acid, entering into a chemical reaction of potassium-calcium phosphate with sulfuric acid [
67,
68,
69,
70,
71].
The possibility of chemical reactions occurring during the decomposition of phosphorus sludge with a sulfuric acid solution was researched. During the researches, it was established that fluorapatite, potassium-calcium phosphate and sodium dihydrogen phosphate, which characterize the main composition of phosphorus sludge, are decomposed by a sulfuric acid solution in the temperature range of 333-363K [
72,
73,
74,
75]. The results of researches on the production of modified and encapsulated mineral fertilizers are presented in [
76,
77,
78,
79,
80], the kinetics of the process and the data obtained are recommended for conducting an experimental test.
Thus, from the above data, obtaining high-quality monocalcium phosphate from unconditional phosphate raw materials has not been researched and there is no information in scientific sources. The research and obtaining of high-quality fluoride-free monocalcium phosphate from unconditional phosphate raw materials is relevant and of scientific interest.
The objective of the work is to research the process of obtaining high-quality monocalcium phosphate, as well as to determine the optimal decomposition parameters of low-grade phosphate raw materials and to increase the product yield. To achieve the objective, the following tasks were defined: development of a circulation technology for processing phosphate raw materials; foaming reduction; determination of the optimal process parameter, concentration and norm of phosphoric acid. Based on the results of the researches, the following parameters of monocalcium phosphate (MCP) obtaining from the Chilisay deposit phosphorites were determined: phosphoric acid concentration 36-42% P2O5; norm of recycled phosphoric acid 540-560% of the stoichiometric amount for MCP formation; decomposition temperature 95-100°C; decomposition duration 40-50 min.; filtration temperature of insoluble residue 85-90°C; crystallization temperature of MCP 40-45°C; crystallization duration 85-90 min; concentration of sulfuric acid for sulfation of the mother solution 86-93% H2SO4; norm of sulfuric acid 95-100% of the stoichiometric for Ca(H2PO4)2 decomposition.
2. Research methodology
In accordance with the logic of scientific search on the topic of the work, the authors have chosen the methodology for conducting the study. It is a complex of theoretical and experimental methods, the combination of which makes it possible to research with the greatest reliability such a complex problem of processing low-grade phosphate raw materials by the circulation method using phosphoric acid.
Phosphorite samples No. 1 and No. 2 were used to research the elemental and mineralogical composition. Elemental analysis was performed on the scanning electron microscope JSM-6490LV with INCAPentaFET-x3 energy dispersive X-ray microanalysis system. X-ray diffraction analysis was carried out on the automated diffractometer DRON-3 with CuКα – radiation, β-filter. Conditions for recording diffraction patterns: U=35 kV; I=20 mA; recording θ-2θ; detector 2 deg/min. Semi-quantitative X-ray phase analysis was performed based on diffraction patterns of powder samples using the method of equal weighed portions and artificial mixtures. Quantitative ratios of crystalline phases were determined. Diffraction patterns were interpreted using data from the ICDD card index: powder diffraction data base PDF2 (Powder Diffraction File) Release 2022 and diffraction patterns of minerals free of impurities.
The processes of acid decomposition of phosphate raw materials, dissolution and crystallization of monocalcium phosphate under the influence of temperatures and excess phosphoric acid were researced. The experiments were carried out on a laboratory thermostatted installation. The Chilisay deposit phosphorites and phosphoric acid were used in the researches.
During the experiment, the calculated phosphorite sample was decomposed with the calculated amount of phosphoric acid. To obtain monocalcium phosphate from the Chilisay phosphorite, a mixture of extraction and thermal phosphoric acid was used in a 2:1 ratio, with a concentration of Н3РО4 50-58% (36.2-42.0% Р2О5). Sulfuric acid with a concentration of 93% was used to sulfate the solution and obtain gypsum.
The consumption rate of phosphoric acid with a 5-fold excess was calculated based on the content of CaO, MgO, Fe2O3, AI2O3 in the phosphorite and amounted to 80.16 g H3PO4 per 100 g of the phosphorite.
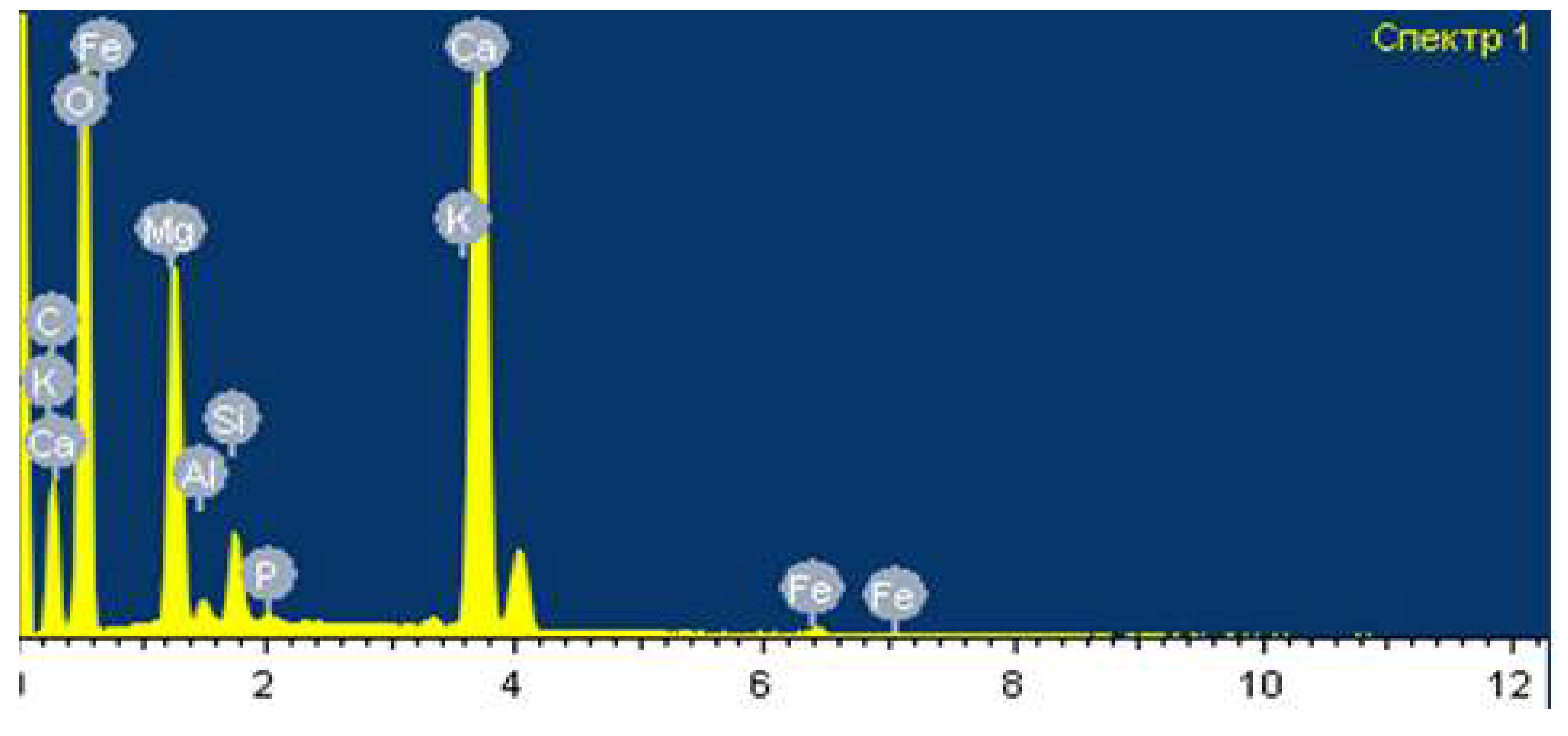
Figure 1 shows the spectral analysis of the Chilisay deposit phosphorites. it is evident that the elemental analysis of phosphorite differs in the content (sample No. 1) of the following elements: P, Ca, Mg, Fe, Si, C.
Table 1 shows the results of the spectral analysis of sample No. 1.
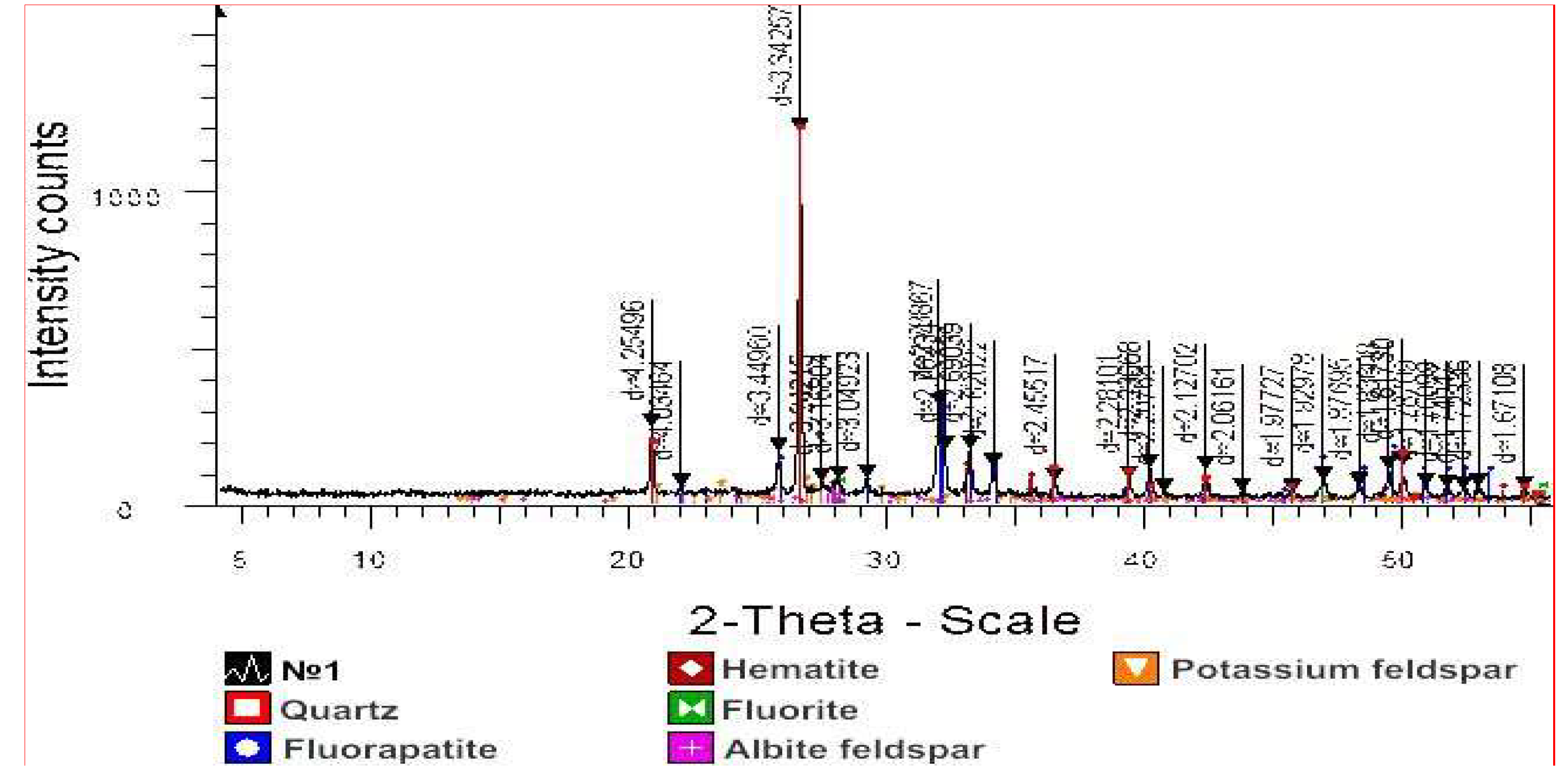
From
Figure 2 it is evident that the intensity of the sample No. 1 confirms the presence of SiO
2 (quartz) in the composition with characteristic diffraction maxima d/n=4.25496, 4.03464, 3.34257 Å, diffraction maxima d/n=3.34257, 3.04923 Å correspond to CaF
2 (fluorite), diffraction maxima d/n=2.78867, 2.76934 Å correspond to Ca
5(PO
4)3F/CaF
2•3Ca
3(PO
4)
2 (fluoroapatite), diffraction maxima d/n=2.69039 Å correspond to Fe
2O
3 and diffraction maxima d/n=3.24215 Å correspond to KAlSi
3O
8 (potassium feldspar).
The content was calculated for the main phases. Possible impurities, the identification of which cannot be unambiguous due to low contents and the presence of only 1-2 diffraction reflections or poor crystallization of compounds. The results of semi-quantitative X-ray phase analysis of crystalline phases of sample No. 1 are shown in
Table 2.
From
Figure 3 it is evident that the intensity of the sample No. 2 confirms the presence in the composition of diffraction maxima d/n=4.2526; 3.3411 Å which correspond to SiO
2, diffraction maxima d/n=3.4476 Å which correspond to FePO
4, diffraction maxima d/n=3.0372 Å which correspond to CaCO
3, diffraction maxima d/n=2.7897 Å which correspond to Ca
5(PO
4)
3F.
The results of semi-quantitative X-ray phase analysis of crystalline phases of sample No. 2 are shown in
Table 3.
From the data in
Table 3, it can be seen that the phosphate raw material mainly consists of quartz, fluorapatite, calcite and iron phosphate.
The Chilisay deposit phosphorites are of the sandy type. They are mainly represented by quartz grains of various sizes (0.002-15 mm), cemented by calcium and iron phosphate. Feldspars, gypsum and carbonates are present in smaller quantities.
The Chilisay phosphorites are characterized by variable mineral composition. Their quality varies widely depending on the content of harmful impurities that reduce the technological indicators of the products. Quartz is present in the form of well-rounded pebbles, large and angular small grains. The grains are mainly composed of pure quartz, the surface of some of them is covered with limonite or hydromica films.
Limonite is a product of oxidation of iron-containing minerals (pyrite, glauconite, etc.), found in the pebble layer in the form of large accumulations or fine dust on the surface of glauconite, pyrite, quartz. Organic matter in the Chilisay phosphorites is up to 0.8% and is associated mainly with phosphate matter and clay material.
The Chilisay deposit phosphorite (sample No. 1) was used for the experiment; its average composition is presented in
Table 4.
From
Table 4 it can be seen that the phosphate raw materials contain mainly P
2O
5, CaO, SiO
2, and also carbonate compounds.
The Chilisay phosphorites, due to the peculiarities of their composition, contain a large amount of carbonates, in terms of carbon dioxide reaching 4.56%, as well as glauconites. When decomposing this phosphorite, the formation of large volumes of very stable, difficult to destroy foam is observed, due to the release of carbon dioxide. The solution to the foaming problem is supposed to be due to the preliminary treatment of phosphate raw materials in bulk mass, phosphoric acid, i.e. a decarbonization stage is introduced, preceding the stage of phosphorite decomposition, at which a significant part of carbon dioxide is released. At the decarbonization stage, the selection of optimal conditions (temperature value, norm, concentration of phosphoric acid) is carried out, under which it is possible to achieve the maximum decarbonization degree of the Chilisay phosphorite. At the next decomposition stage of decarbonized phosphorite, it is necessary to select the norm, concentration of phosphoric acid, as well as the decomposition time, which must be established by researching the decomposition process kinetics. The hot filtration stage (100 °C), where the insoluble residue is separated from the total mass of the monocalcium phosphate solution, is complicated by the organic matter content in the phosphorite. The oily, finely dispersed layer of organic matter greatly complicates and increases the filtration time.
After monocalcium phosphate crystallization from the mother solution, the sediment is filtered, and the mother solution is returned to the head of the sulfation process to obtain phosphoric acid. When neutralizing free acidity in monocalcium phosphate, a neutralizing reagent is required. Calcium carbonate is used as a neutralizer according to GOST 5331-63, containing 88% СаСО3.
At the first stage, decomposition was carried out in a thermostatted vessel equipped with a reflux condenser and a paddle stirrer, with a rotation speed of 200 rpm. The phosphorite was introduced in small portions into phosphoric acid heated to 95 °C, then the reagents were mixed at this temperature for a specified time.
At the second stage, after the decomposition was complete, the pulp was filtered on a heated vacuum filter through filter cloth (2 layers) at a negative pressure of 70 kPa, and the filtration time was measured. The sediment was washed with a certain amount of water heated to 75-80 °C. The washed sediment was dried, weighed and analyzed for the content of Р2О5gen. and Р2О5assim. The phosphate decomposition coefficient and the residue filtration rate were determined.
The filtrate after separation of the insoluble residue was placed in a thermostatted reactor equipped with a stirrer, cooled to a temperature of 40-45 °C and maintained with slow stirring for 60-70 min. Then the formed crystals were filtered on a vacuum filter. The sediment was weighed, analyzed for the content of Р2О5gen, Ca(H2PO4)2 and Н3РО4 and treated with limestone to neutralize the free acid, dried, weighed and analyzed for the content of all forms of Р2О5.
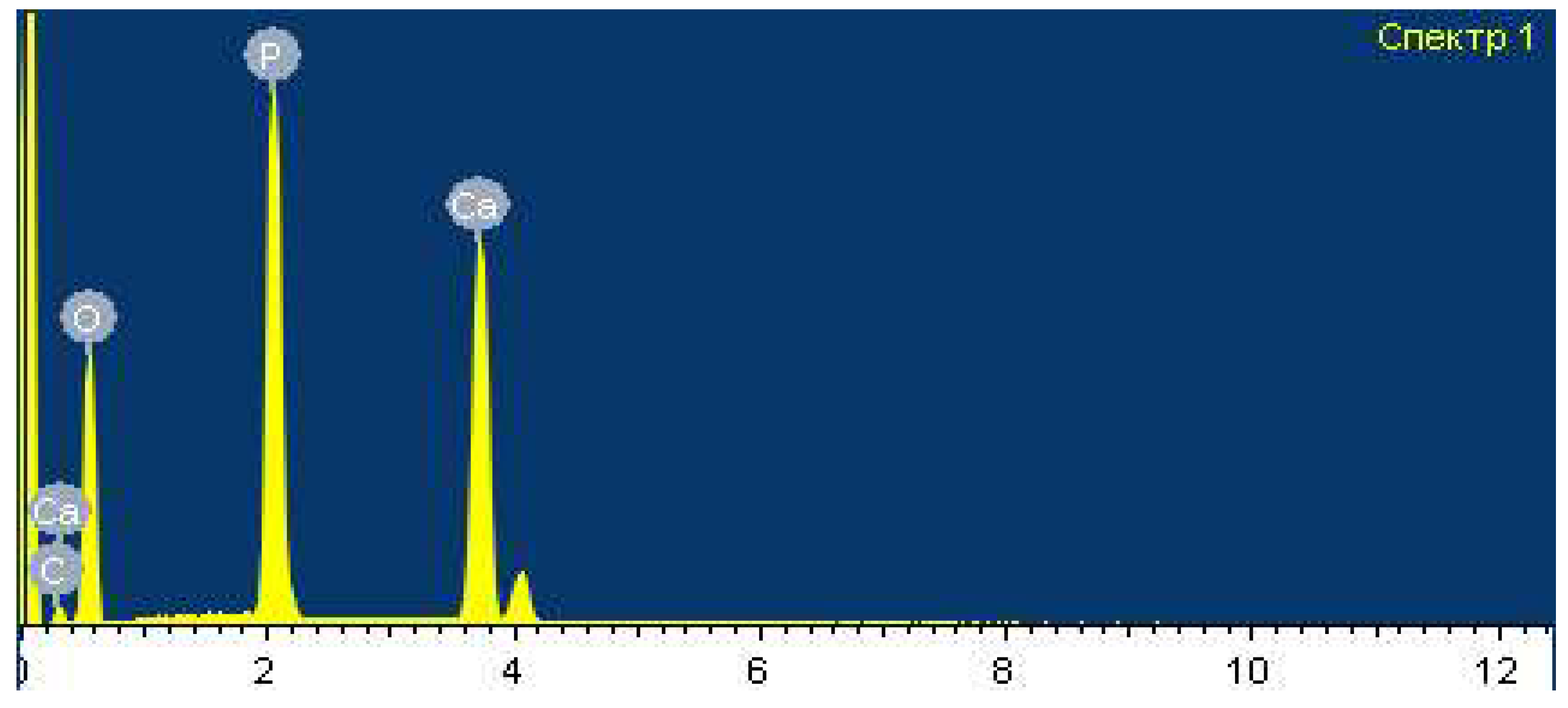
Spectral analysis of the obtained dry monocalcium phosphate was carried out using the scanning electron microscope JSM-6490LV (
Figure 4). Results shown in
Table 5.
From
Table 5 it is clear that monocalcium phosphate contains mainly calcium, phosphorus and is practically free of fluorine and other impurities.
Sulfation of the mother solution of sulfuric acid was also carried out in a thermostatted glass reactor equipped with a stirrer. The temperature in the solution did not exceed 60 °C. 20 minutes after mixing the reagents, the formed crystals of calcium sulfate were filtered on a vacuum filter, the precipitate was washed with hot (75-80 °C) water, weighed, dried to a constant weight and also analyzed for the content of Р2О5gen., Р2О5water, F, Fe2O3, Al2O3, CaO, SO3.
The filtrate obtained after separation of calcium sulfate was weighed, the content of Н3РО4free, Н2SО4, МgО, Аl2О3, F was determined and it was returned to the decomposition stage in the next cycle. Losses of Р2О5 with insoluble residue and gypsum were compensated by introducing an additional amount of phosphoric acid into the cycle.
The washing of the insoluble residue and gypsum in the second cycle was carried out first with the filtrates obtained after washing the insoluble residue and gypsum in the first cycle, and then with water. In the third and subsequent cycles, three-fold washing of the insoluble residue and gypsum was carried out. Moreover, in the third cycle, the first and second washings were carried out with the filtrates obtained during the first and second washings in the second cycle, and the third – with water. In subsequent cycles, the first and second washings of the insoluble residue and gypsum were carried out with the filtrates obtained after the second and third washings of the insoluble residue and gypsum in the previous cycle, and the third – with water. The filtrate obtained after the first washing of the insoluble residue was added to the mother solution obtained after the separation of the crystals in the corresponding cycle, and the filtrate obtained after the first washing of the gypsum was mixed with the recycled phosphoric acid (the main filtrate) obtained after the separation of the calcium sulfate crystals in the same cycle. In this way, it is possible to regenerate the phosphoric acid fed to the phosphorite decomposition.
3. Results and Discussion
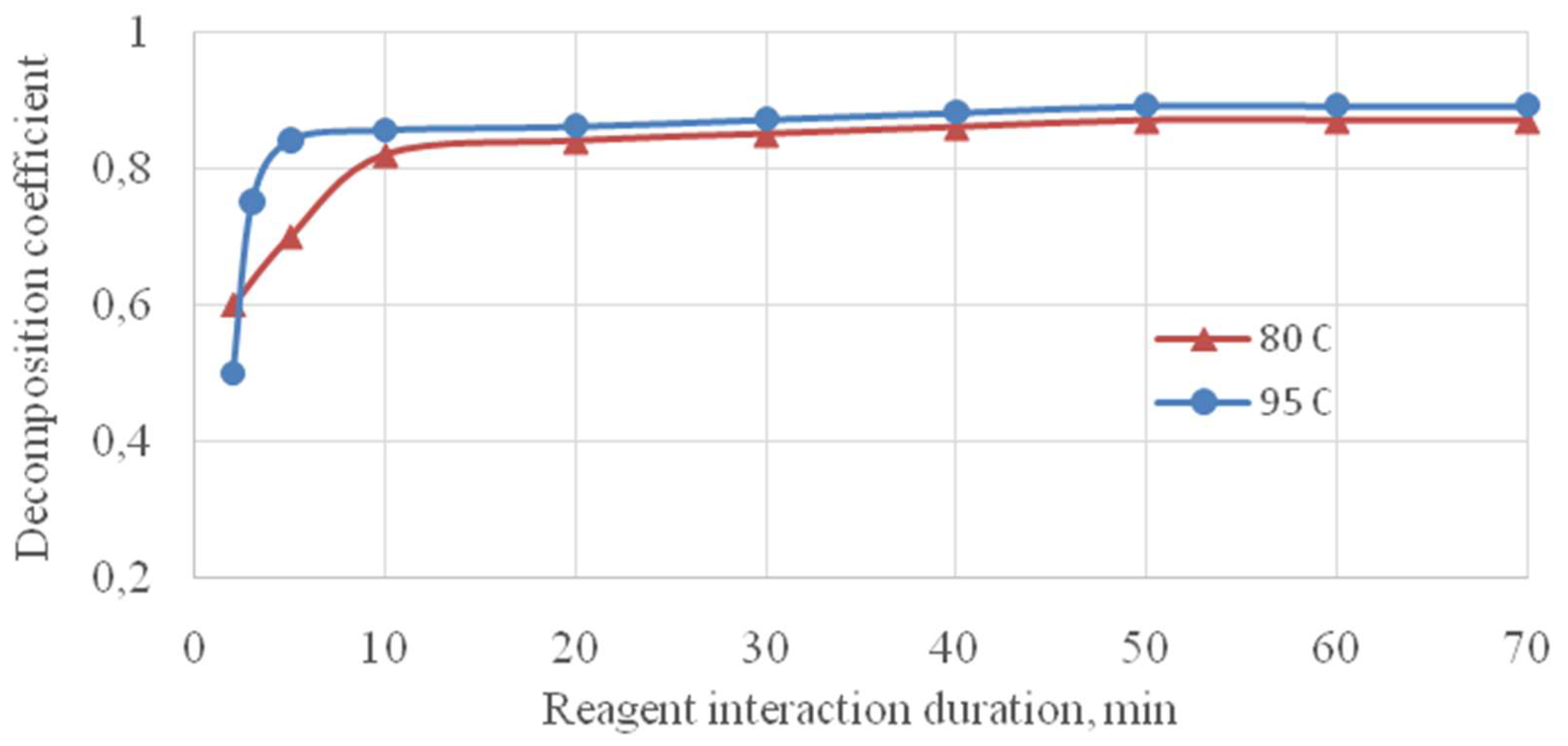
The phosphorite decomposition kinetics at known rates and concentrations of phosphoric acid was researched under the following parameters: temperature 80.95 °C, duration of reagent interaction 1-50 min.
Figure 5 shows the effect of duration on the decomposition coefficient of the phosphate raw materials.
From the results of the researches shown in
Figure 5 it follows that with an increase in the reagent interaction duration in the range from 1 to 50 minutes, the decomposition coefficient increases and the main part of the phosphorite decomposes in the first 10-15 minutes. With a further increase in time, additional decomposition of the raw material occurs, and by 40 minutes, almost complete decomposition of the phosphorites of 98% is achieved. An increase in temperature from 80 to 95°C causes an increase in the decomposition coefficient by only 0.05-0.07%. At a temperature of 95°C with the process duration of 30 minutes, the maximum achievable degree of decomposition is 98% and with the process duration of 40 minutes it is the same – 98%.
Almost complete opening of the phosphorite is provided by using a 5-fold excess of phosphoric acid (540-560% of stoichiometry). Excess phosphoric acid allows monocalcium phosphate to be dissolved in acid and for complete precipitation of silicon into the sediment. In this case, a solution of monocalcium phosphate is formed, unsaturated by CaO, the sediment is mainly silica-containing compound.
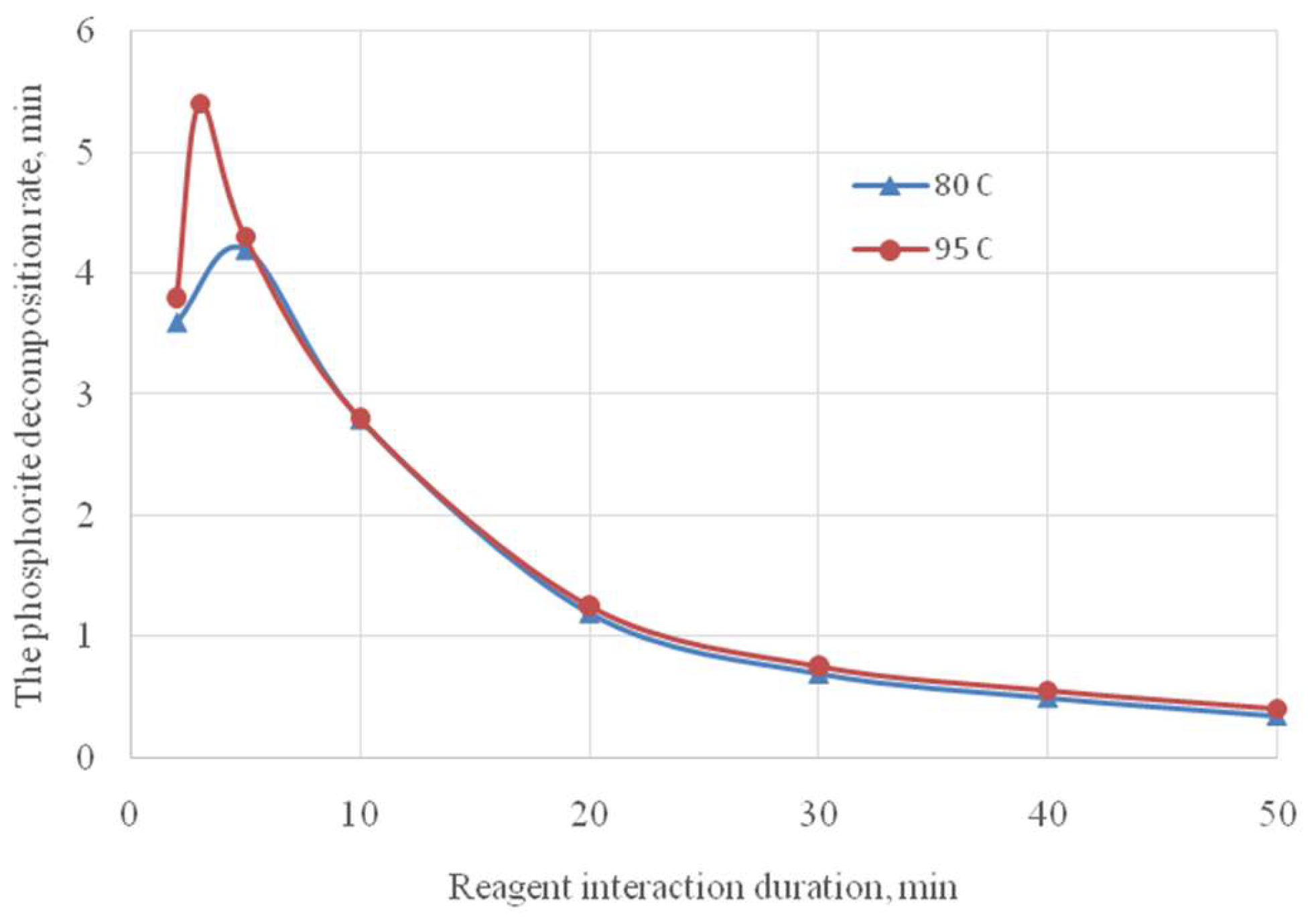
Figure 6 shows the phosphorite decomposition rate from the process duration.
The highest decomposition rate is achieved in the first 5 minutes (4-5 g phosphorite/min). The sharp decrease in the ore decomposition rate after 5 minutes is apparently due to a decrease in the activity of hydrogen ions in the liquid phase due to the neutralization of the first hydrogen ion. At the same time, the temperature dependence of the degree and rate of decomposition is completely absent in the time interval from 10 to 50 minutes (
Figure 6). The high rate of the process is ensured by the selected theoretically substantiated conditions – decomposition with phosphoric acid containing 36.0% P
2O
5.
Thus, it was established that almost complete decomposition of the phosphorites is achieved with the reagent interaction duration of 40 minutes and the process temperature of 95 °C.
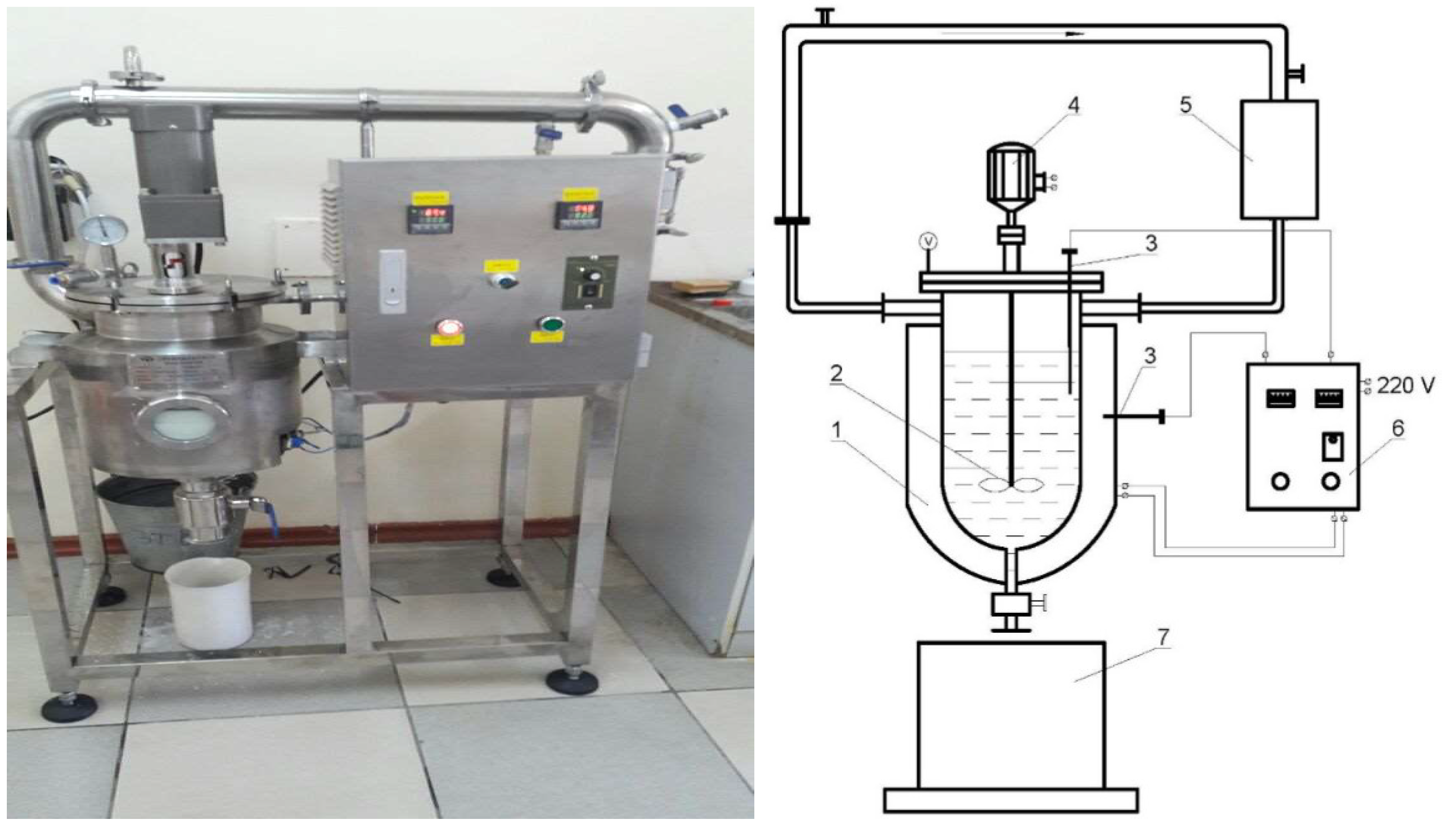
Based on the obtained research data, laboratory tests were carried out on the installation shown in
Figure 7.
Laboratory device (1) consists of a 0.01 m3 reactor-mixer, where phosphorite and phosphoric acid are mixed with a stirrer (2). The reactor-mixer has a jacket, the temperature of which is regulated by a control panel (6). The temperature is measured by thermocouple (3). The stirrer is rotated by an electric motor (4). The evaporated liquid from the reactor is condensed in the condenser (5) and the condensate return into the reactor (1). After experiments, the pulp enters into the collector (7) and fed for filtration to separate the sediment from the mother liquor Ca(H2PO4)2, H3PO4.
To conduct laboratory tests, the phosphorite of the composition given above in
Table 4 was prepared. To obtain monocalcium phosphate from the Chilisay phosphorite, a mixture of extraction and thermal phosphoric acid in a ratio of 2:1, a concentration of 50-58% Н
3РО
4, and for sulfation of the mother solution 93% H
2SO
4 were used. The decomposition temperature was 95-100 °C; the decomposition duration was 40-50 minutes.
Based on the results of the tests, the main parameters of the process for obtaining monocalcium phosphate from the Chilisay deposit phosphorites were determined:
1. Concentration of phosphoric acid: 42% Р2О5
2. Norm of recycled phosphoric acid: 540-560% of the stoichiometric amount for the formation of MCP
3. Decomposition temperature: 98-100 °C
4. Decomposition duration: 45-50 minutes
5. Filtration temperature of the insoluble residue: 85-90 °C
6. Crystallization temperature of the mineral fertilizer (MCP): 40-45 °C
7. Crystallization duration: 85-90 minutes
8. Concentration of sulfuric acid for sulfation of the mother solution: 93% H2SO4
9. Norm of sulfuric acid: 100% of the stoichiometric amount for the decomposition of Ca(H2PO4)2 contained in the solution with the formation of gypsum and phosphoric acid.
4. Conclusions
1. The chemical and mineralogical composition of the Chilisay deposit unconditional phosphate raw materials was researched. It was established that the Chilisay deposit phosphorites belong to the sandy type. They are mainly represented by quartz grains of various sizes (0.002-15 mm), cemented by calcium and iron phosphate. Feldspars, gypsum and carbonates are present in smaller quantities. Quartz is present in the form of well-rounded pebbles, large and angular small grains. The grains are mainly composed of pure quartz, the surface of some of them is covered with limonite or hydromica films.
2. The process of obtaining high-quality monocalcium phosphate was researched, and the optimal parameters of the decomposition process of low-grade phosphate raw materials were determined. Based on the results of the researches, the following parameters of monocalcium phosphate (MCP) obtaining from the Chilisay deposit phosphorites were determined: phosphoric acid concentration 36-42% P2O5; norm of recycled phosphoric acid 540-560% of the stoichiometric amount for MCP formation; decomposition temperature 95-100°C; decomposition duration 40-50 min.; filtration temperature of the insoluble residue 85-90°C; crystallization temperature of MCP 40-45°C; crystallization duration 85-90 min; concentration of sulfuric acid for sulfation of the mother solution 86-93% H2SO4; norm of sulfuric acid 95-100% of the stoichiometric for Ca(H2PO4)2 decomposition. The following composition of monocalcium phosphate was obtained: Р2О5 – 55%, Са – 18.01%, Н2О – 4.0%, F – 0.01%, As – 0.004%, Pb – 0.002%, which meets the requirements of GOST 23999-80. The results of the work are recommended for commercialization of projects and organizations for production of feed monocalcium phosphate and mineral fertilizer. The research work was carried out on the topic BR21882181 “Development of technology for production of highly effective materials based on mineral raw materials and technogenic waste”.
Author Contributions
A.A. Anarbayev: Writing – review and editing, Writing – original draft, Validation, Supervision, Methodology, Data processing, Conceptualization, Project administration. B.N. Kabylbekova: Writing – review and editing, Writing – original draft, Validation, Methodology, Formal analysis. Zh.Ye. Khussanov: Writing – review and editing, Author’s supervision, Formal analysis, Data curation. B.M. Smailov: Writing – review and editing, Visualization, Validation. N.A. Anarbayev: Writing – review and editing, Visualization, Validation. Y.G. Kulikov: Writing - review and editing, Data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant No. BR21882181).
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
- Karatau phosphorite basin. In Kazakhstan. National Encyclopedia. – Kazakh Encyclopedia: Almaty, Kazakhstan, 2005; Volume III. ISBN 9965-9389-8-9.
- Petrov, V.P. Karatau phosphorite-bearing basin. In Great Soviet Encyclopedia. Volume 11. Italy-Kvarkush; Soviet Encyclopedia: Moscow, USSR, p.608.
- Karatau phosphorite-bearing basin. In Mining Encyclopedia; Chief Editor Kozlovsky, E.A.; Soviet Encyclopedia: Moscow, USSR, 1985; Volume 2, p. 575.
- Shubin, A.P. Phosphate ores of Kazakhstan. Science: Alma-Ata, USSR, 1990; 320 p.
- Eganov, E.A.; Sovetov, Yu K.; Yanshin, A.L. Proterozoic and Cambrian phosphorites deposits: Karatau, southern Kazakhstan, USSR. In Phosphate Deposits of the World. 1st ed.; Cook, P.J., Shergold, J.H.; Cambridge University Press, 2005; Volume 1.
- Klassen, P.V.; Samigullina, L.I.; Kharitonov, A.B. Enrichment and processing of the Chilisay deposit phosphorites. Review Information. Series “Mineral fertilizers and sulfuric acid”. 1988, pp. 37-39.
- Levin, B.V.; Dovidenko, V.V.; Suschev S.V. et al. Relevance and practical steps for involving low-grade phosphate raw materials in processing into complex fertilizers. Chemical Industry Developments. 2006, Volume 11, pp. 11-18.
- Lygach, A.V.; Ignatkina, V.A. Flotation enrichment of poor nodular phosphorite ores. Proceedings of International Conference “Resource conservation and environmental protection in the enrichment and processing of mineral raw materials”. 2016, pp. 529-532.
- Perkovich, S.G.; Kaitmazova, T.I.; Protasov, V.D.; Brazhnik; I.S. Method for enriching phosphate ores. Patent1585004, IPC B03B 7/00, Russian Federation. Applied on 25 April 1987; Published on 15 August 1990, Bulletin No. 30.
- Lygach, A.V. Development of technology for complex enrichment of nodule phosphorites using multifunctional reagents, Title of Candidate of Technical Sciences, National University of Science and Technology «MISIS», Moscow, 2019.
- Bazhina, N.L., Ondar, E.E., Deryabina, Yu.M. Specificity of light absorption in the visible and ultraviolet spectral range by humic acids. Bulletin of Orenburg State University. 2014. Volume 6 (167), pp. 189-194.
- 1Khil’ko, S.L., Taperko, G.V., Rogatko M.I. Interaction of modified humic acids salts with transition metal salts. Vestnik NovSU. Issue: Engineering Sciences. 2021, Volume 4(125), pp.68-71. [CrossRef]
- Chen X., Zhang P., Wang Y., Peng W., Ren Z., Li Y., Chu B., Zhu Q. Research progress on synthesis of zeolites from coal fly ash and environmental applications. Frontiers of Environmental Science and Engineering, 2023, Volume 17. [CrossRef]
- Luсаkovа, M.; Kolajovа, R. Dissociation ability of humic acids: Spectroscopic determination of pKa and comparison with multi-step mechanism. Reactive and Functional Polymers. 2014, Volume 78 (1), pp. 1-6. [CrossRef]
- Zhu, K.; Liu, Q.; Xiong, X.; Zhang, Y.; Wang, M.; Liu, H. Carbon footprint and embodied carbon emission transfer network obtained using the multi–regional input–output model and social network analysis method: A case of the Hanjiang River basin, China. Frontiers in Ecology and Evolution. 2022, Volume 10. [CrossRef]
- Dyachenko, E.N.; Shevelev, A.T. Influence of the aftereffect of mineral and lime fertilizers on the yield and grain quality of spring wheat in the Baikal region. Agrochemical Bulletin. 2020, Volume 3, pp. 45-48.
- Bezuglova, O.S.; Polienko, E.A.; Gorovtsov, A.V. Humic preparations as growth stimulants for plants and microorganisms. News of Universities. 2016, Volume 2, p. 11.
- Lygach, A.V. et al. Study of the material composition of phosphorites of the Egoryevsk deposit. In Collection of materials of the XI Congress of the CIS countries. Moscow, Russia, 2017, pp. 305-309.
- Unece Available online: https://unece.org/sites/default/files/2021-03/3.1_Kazakhstan_Mustafina_0.pdf. (accessed on 04.02.2025).
- Mining industry. Central Asia. https://dprom.kz/pererabotka/tyehnogyenniye-meenyeralniye-obrazovaneeya-rk/. (accessed on 25.10.2023).
- Nurpeisova, M.B.; Yestemesov, Z.A.; Bekbasarov, Sh.S. Waste recycling is one of the key areas of business development. Collection of works of ISC “Innovative Technologies in Geoinformation Digital Engineering”. KazNITU: Almaty, Kazakhstan, 2022; pp. 191-198.
- Online Student Library https://studbooks.net/781040/ekonomika/analiz_obrazovaniya_nakopleniya_promyshlennyh_othodov_regionov (accessed on 30.10.2023).
- Pozin, M.E. Technology of Mineral Salts; Chemistry: Leningrad. USSR, 1990; p. 156.
- Selivanovskaya, S.Yu.; Latypova, V.Z. On the issue of experimental assessment of waste toxicity class. Ecological expertise: Review information. 2001, Volume 1, pp. 67-74.
- Tleuova, S.T.; Zhuldyzbayeva, S.Ye.; Tleuov, A.S.; Sikhymbayeva, Zh. Waste-free Technology. In Study guide. Nuray Print Service: Almaty, Kazakhstan, 2015; p. 195.
- Arbuzov, S.I.; Rikhvanov, L.P.; Volostnov, A.V.; Ershov, V.V. et al. Comprehensive geochemical assessment of coals and coal-bearing rocks of the Chalpan section of the Bey coal deposit. Research report, Tomsk, Russia, 2001.
- Yazikov, E.G.; Rikhvanov, L.P.; Arkhangelsky, V.V. Assessment of the toxicity class of industrial waste of JSC Teiskoe Mine Administration. Report on economic operations No. 54 dated 27.04.1999; MGP Ecogeos: Tomsk, Russia, 2001.
- Unified ecological internet resource of the Ministry of Ecology and Natural Resources of the Republic of Kazakhstan. https://ecogosfond.kz/wp-content/uploads/2018/03/NDSOS_2011-2014.pdf (accessed on 29.10.2023).
- Adilet. Legal information system of Regulatory Legal Acts of the Republic of Kazakhstan. https://adilet.zan.kz/rus/docs/U1300000577 (accessed on 15.08.2023).
- Scientific articles Kazakhstan. https://articlekz.com/article/27891 (accessed on 15.08.2023).
- Online Student Library. https://studbooks.net/2538980/tovarovedenie/pererabotka_othodov_obrazuyuschihsya_protsesse_polucheniya_fosfornoy_kisloty (accessed on 20.08.2023).
- Tleuova, S.; Tileuberdi, A.; Pazylova, D.; Ulbekova, M.; Sagyndykova, N.; Lavrov, B.; Turishbekov Z. Investigation of theProcess of Agglomeration of Phosphorites Using Phosphate-Siliceous Shales and Oil Sludge. Open Chem Engineering Journal. 2024, Volume 18. [CrossRef]
- Ibiyev, G.Z.; Savoskina, O.A.; Chebanenko, S.I. The global market of mineral fertilizers and its impact on the grain industry. Agricultural Economics of Kazakhstan. 2021, Volume 12, pp. 97-102. [CrossRef]
- Sergeev, I.B.; Ponomarenko, T.V. Strategies of mineral and chemical companies in the context of the modern global market. Journal of Siberian Federal University. Humanities and Social Sciences. 2015, Volume 5, pp. 958-971.
- Falina, N.V.; Dyukarev, D.O. The global market of mineral fertilizers. Economics, 2016. Volume 1(10), pp. 83-86.
- Bogachev, A.I.; Dorofeeva, L.N. Russian market of mineral fertilizers: features of functioning in new realities and development metamorphoses. Bulletin of Agrarian Science. 2022, Volume (96), pp. 78-92. [CrossRef]
- Kruchinina, V.M.; Ryzhkova, S.M. Fertilizer market in Russia: state and directions of development. Bulletin of Voronezh State University of Engineering Technologies. 2021, Volume 1 (87), pp. 375-384. [CrossRef]
- Ilkiv, N. Russian market of mineral fertilizers. AgroForum. 2021, Volume 7, pp. 44-48.
- IFASTAT. https://api.ifastat.org/reports/download/13140 (accessed on 16.11.2023).
- World Integrated Trade Solution. https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2021/tradeflow/Exports/ (accessed on 16.11.2023).
- Beisenbayev, O.K.; Tleuov, A.S.; Smailov, B.M. et al. Method for producing phosphorus-containing microfertilizers. Patent. No. 4122 Republic of Kazakhstan, IPC C05F11/02. Published on 25.06.2019.
- Beisenbayev, O.K.; Tleuov, A.S.; Smailov, B.M. et al. Method for producing polymer-containing microfertilizers. 42 Patent No. 4380 Republic of Kazakhstan, IPC C05F11/02. Published on 23.10.2019.
- Beisenbayev, O.K.; Tleuov, A.S.; Smailov, B.M. et al. Method for producing phosphorus-containing microfertilizers. Patent No. 4353 Republic of Kazakhstan, IPC C05F11/02. Published on 09.10.2019.
- Batkayev, R.I.; Beisenbayev, O.K.; Kholoshenko, L.Kh.; Dygay, L.V.; Batkayeva, L.R. Method for obtaining phosphorus fertilizer from technogenic waste. Author’s certificate No. 81786 dated 04.03.2013.
- Bishimbayev, V.K.; Batkayev, R.I. Complex use of technogenic waste from phosphorus production. Bulletin of the Academy of Sciences. 2010, Volume 2, pp.12-16.
- Beisenbayev, O.K.; Beloborodova, A.Ye.; Dygay, L.V. Production of polymer-containing organomineral fertilizers based on technogenic waste. Proceedings of International Symposium “Modern Problems of Higher Education and Science in Chemistry and Chemical Engineering”. 2013, Almaty, Kazakhstan, pp. 182-185.
- Lotosh, V.E. Recycling of nature management waste, Polygraphist: Yekaterinburg, Russia, 2007, 503 p.
- Batkayev, R.I.; Nugmanov, A.A.; Batkayev, I.I.; Shevchenko, V.A.; Gorbunova, T.A. Method for extracting phosphorus from phosphorus production sludge. Patent No. 21852 Republic of Kazakhstan. 2007.
- Batkayev, R.I.; Nugmanov, A.A.; Batkayev, I.I.; Shevchenko, V.A. Method of extracting phosphorus from sludge. Patent No. 212790 Republic of Kazakhstan. 2007.
- Batkayev, R.I.; Nugmanov, A.A.; Batkayev, I.I.; Shevchenko, V.A. Method of extracting phosphorus from technogenic waste. Patent No. 21853 Republic of Kazakhstan. 2007.
- Batkayev, R.I. Development of technology for obtaining marketable products from technogenic waste of phosphorus production. Title of Doctor of Technical Sciences, South Kazakhstan State University, Shymkent, Kazakhstan, 2010.
- Beisenbayev, O.K.; Beloborodova, A.Ye.; Dygay, L.V. Production of polymer-containing organomineral fertilizers based on technogenic waste. Proceedings of International Symposium “Modern Problems of Higher Education and Science in Chemistry and Chemical Engineering”. 2013, pp. 182-185.
- Lotosh, V.E. Recycling of nature management waste. Polygraphist: Yekaterinburg, Russia, 2007; 503 p.
- Nadirov, K.S. Obtaining gossypol and its derivatives during processing of cotton seeds and oil; M. Auezov South Kazakhstan State University: Shymkent, Kazakhstan, 2012; 115 p.
- Bakyt Smailov and Usha Aravind. Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan. Green Processing and Synthesis. 2024. Volume 13. [CrossRef]
- Smailov, B.M.; Kydyralyeva, А.Sh.; Beisenbayev, O.K.; Issabayev, N.N.; Azimov, А.M.; Issa, A.B.; Assanova, A.R. Study of modification of sodium montmorillonite from the Darbazinsk deposit. Rasayan Journal of Chemistry. 2022, Volume 15(3), рр. 1787-1791. [CrossRef]
- Beysenbayev, О. К.; Ahmedov, U.K.; Issa, A.B.; Smailov, В.М.; Esirkepova, M.M.; Artykova, Zh.K. Receiving and research of the mechanism of capsulation of superphosphate and double superphosphate for giving of strength properties. News of the National Academy of Sciences of the Republic of Kazakhstan, series of Geology and technical sciences. 2019, Volume 6 (438), pp.36-45. [CrossRef]
- Artykova, Zh.K.; Beisenbayev, O.K.; Kadyrov, A.A.; Sakibayeva, S.A.; Smailov, B.M.. Synthesis and preparation of polyacrylonitrile and vinyl sulfonic acid in the presence of gossypol resin for drilling fluids. Rasayan Journal of Chemistry. 2023, Volume 16(4), 2313-2320. [CrossRef]
- Dzhakipbekova, N.; Sakibayeva, S.; Dzhakipbekov, E. and etc. The study of physical and chemical properties of the water-soluble polymer reagents and their application as an ointment. Oriental J. Chem. 2018, Volume 34(4), 1779–1786. [CrossRef]
- Romana Kratochvílová, Milan Kráˇcalík, Marcela Smilková and etc. Functional Hydrogels for Agricultural Application. Gels. 2023, Volume 9(7), p.590. [CrossRef]
- Timilsena, Y. Haque, M.; Adhikari, B. Encapsulation in the food industry: A brief historical overview to recent developments. Food Nutr Sci. 2020, Volume 11, pp.481–508. [CrossRef]
- Jarosiewicz, A.; Tomaszewska, M. Controlled release NPK fertilizer encapsulated by polymeric membranes. J Agric Food Chem. 2003, Volume 51(2), 413–417. [CrossRef]
- Smailov, B.M.; Aravind Usha; Zakirov, B.S.; Azimov, А.M.; Tleuov, A.S.; Beisenbayev, O.K.; Aimenov, Zh.T. and Issabayev NN. Technology for obtaining chelated organic and mineral microfertilizers based on humate-containing components. Rasayan J. Chem. 2023, Volume 16(1), pp.428-433. [CrossRef]
- Issa, A.B.; Beisenbayev, O.K.; Akhmedov, U.K. and etc. Polymeric compositions to increase oil recovery. Rasayan J. Chem. 2023, Volume 16(2), pp.876-883. [CrossRef]
- Beysenbayev, O.К., Tleuov A.S.; Smailov, B.M.; Zakirov, B.S. Obtaining and research of physical and chemical properties of chelated polymer-containing microfertilizers on the basis of technogenic waste for rice seed biofortification. News Nat Acad Sci Repub Kazakhstan. 2019, Volume 1(433), pp.80–89. [CrossRef]
- Smailov, B.M.; Beisenbayev, O.K.; Tleuov, A.S.; Kadirbaeva, A.A.; Zakirov, B.S.; Mirzoyev, B. Production of chelate polymer-containing microfertilizers based on humic acid and ammophos. Rasayan J. Chem. 2020, Volume 13(3), pp.1372-1378. [CrossRef]
- Lipin, A.G.; Nebukin, V.O.; Lipin, A.A. The encapsulation of granules in a polymer shells as a method of creation of mineral fertilizers with controlled speed of liberation of nutrients. Modern high technology. 2017, Volume 3(51), pp. 86-91.
- Temirov, U.S.; Namazov, S.S.; Usanbayev, N.K. Intensive technology for processing bird litter in organic and mineral fertilizers. ChemTech. 2020, Volume 63(12), pp.85-94. [CrossRef]
- Raiymbekov, Y.; Besterekov, U.; Abdurazova, P.; Nazarbek, U. Review of methods and technologies for the enrichment of low-grade phosphorites. Reviews in Inorganic Chemistry. 2022, Volume 42(4), pp.385-395. [CrossRef]
- Li Lv; Dongyao Zheng; Shengwei Tang; Tao Zhang; Weizao Liu. Phosphate ore Particles Dissolution Kinetics in Hydrochloric Acid Based on a Structure-Related Segmented Model. Powder Technology. 2021, Volume 392, pp.141–49. [CrossRef]
- Karataev, S.S.; Kholoshenko, L.K.; Batkaev, L.V.; Beisenbayev, O.K. Method of Obtaining Organomineral Fertilizer. Patent. No. 30648. Committee on Intellectual Property Rights of the Ministry of Justice of the RK. 15 December 2015.
- Sevim, F.; Sarac, H.; Kocakerim, M.M.; Yartasi, A. Dissolution Kinetics of Phosphate Ore in H2SO4 Solutions. Ind. Eng. Chem. Res. 2003, Volume 42(10), pp.2052–2057. [CrossRef]
- Zholmaganbetova, M.A.; Usmanov, S.; Mussina, A.S.; Smailov, B.M. Synthesis of copper phytocompound and polyfunctional drug based on dicalcium phosphate. Rasayan J Chem. 2024, Volume 17(2), pp.562–566. [CrossRef]
- Ismailov, B.A.; Zakirov, B.S.; Kadirbayeva, A.A.; Koshkarbayeva, Sh.T.; Smailov, B.M.; Azimov, A.M.; et al. Methods for obtaining phosphorus-containing fertilizers based on industrial waste. Inorganics. 2023, Volume 11(6), p.224.
- Smailov, B.M.; Ismailov, B.A.; Zakirov, B.S.; Turakulov, B.B.; Tursynbay, L.M.; Aimenova, Zh. Study and mechanism of formation of phosphorus production waste in Kazakhstan. Green Processing and Synthesis. 2024, Volume 13(1). [CrossRef]
- Smailov, B.M.; Beisenbayev, O.K.; Anarbayev, A.A.; Zakirov, B.S.; Aravind, U.K. Influence of granule structure mineral fertilizers for their physical and chemical properties. Complex Use of Mineral Resources. 2025, Volume 335(4), pp.26-33. [CrossRef]
- Beisenbayev, O.K.; Smailov, B.M.; Sakibayeva, S.A.; Issa, A.B.; Kydyralieva, A.Sh. Рroduction and research of high-strength structured fertilizers based on technogenic waste. News of the national academy of sciences of the Republic of Kazakhstan. series chemistry and technology. 2024, Volume 460(3), pp.27–41. [CrossRef]
- Bakyt Smailov; Usha Aravind; Almagul Kadirbayeva; Nursulu Sarypbekova; Abdugani Azimov; Nurpeis Issabayev. Synthesis of EPAN and applications in the encapsulation of potassium humate. Green Processing and Synthesis. 2024, Volume 13(1). [CrossRef]
- Smailov, B.M.; Zakirov, B.S.; Beisenbayev, O.K.; Tleuov, A.S.; Issa, A.B.; Azimov, А.M.; Issabayev, N.N.; Bolyssova G.S.; Toktabek, A.A. Thermodynamic-kinetic research and mathematical planning on the obtaining of phosphorus-containing components based on cottrel dust from phosphorus production waste. Rasayan J. Chem., 2022, Volume 15(4), pp.2274-2279. [CrossRef]
- Smailov, B.M.; Zharkinbekov, M.A.; Tuleshova, K.T.; Issabayev, N.N.; Tleuov, A.S.; Beisenbayev, O.K.; Esirkepova, M.M.; Azimov, А.M. Kinetic research and mathematical planning on the obtaining of potassium humate from brown coal of the Lenger deposit. Rasayan J Chem, 2021, Volume 14(3), pp.1899-1905. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).