Introduction
Dandruff, a prevalent scalp condition caused by the overgrowth of
Malassezia species, particularly
Malassezia furfur, affects individuals worldwide [
1,
2]. Current treatments rely heavily on antifungal agents such as ketoconazole, climbazole, zinc pyrithione, and selenium sulphide [
3]. These pharmaceutical ingredients are commonly formulated in shampoos to alleviate dandruff by reducing flakes and oiliness. However, regular use of these products often leads to side effects, including scalp dryness, sensitivity, microbiome disruption, and even antimicrobial resistance [
4,
5]. Such drawbacks have driven consumer interest toward natural, holistic scalp care solutions. The global anti-dandruff market reflects this shift, with projections indicating a compound annual growth rate (CAGR) exceeding 5% between 2024 and 2033. Consumers increasingly demand products that align with wellness trends and environmental values, creating opportunities for innovative cosmeceutical formulations derived from natural ingredients [
6].
Natural alternatives, such as herbal extracts and essential oils, have been extensively researched for their anti-dandruff properties [
7]. Plant-based bioactives are prized for their low toxicity and cost-effectiveness but face challenges such as degradation, limited stability, and reduced shelf life due to their high water content. Traditional extraction methods, like solvent and hydro extraction, have been used to isolate these bioactives but often pose concerns regarding toxicity, environmental impact, and efficacy [
8]. To address these issues, eco-friendly extraction techniques, including microwave-assisted and supercritical fluid extraction, have emerged. While these methods are environmentally sustainable, their complexity and high cost limit widespread adoption [
9].
Fermentation offers a promising alternative for developing cosmeceuticals. This process utilizes microorganisms to break down complex compounds, enhancing the safety, stability, and bioavailability of bioactives [
10]. Fermentation also improves the therapeutic efficacy of natural ingredients and produces new active compounds like organic acids, peptides, and antioxidants, making it a sustainable and cost-effective option [
11,
13]. The global market for fermented cosmeceuticals is growing rapidly [
12], with an estimated CAGR of 8-10% from 2023 to 2030. While most fermented cosmeceuticals target skincare, the hair care segment, particularly in India, remains untapped.
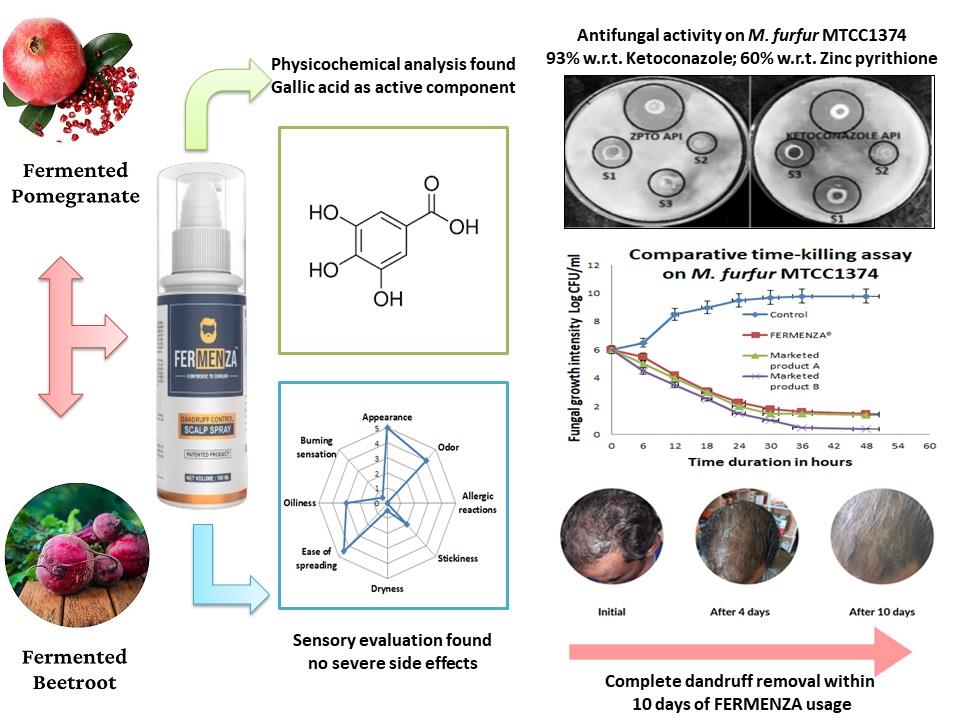
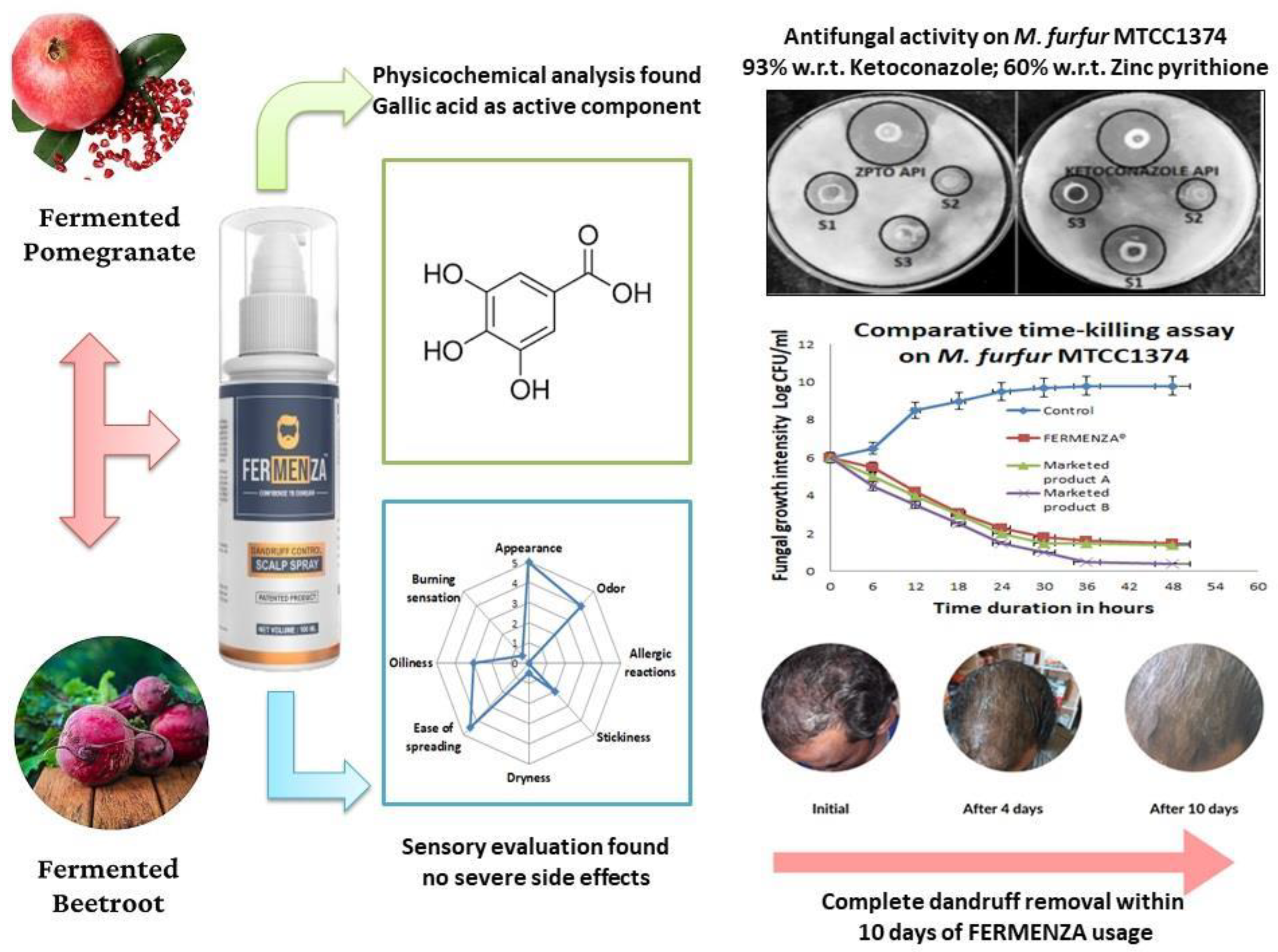
To address this gap, our research team developed FERMENZA
®, a patented formulation [
14] derived from the fermentation of fruits and vegetables. FERMENZA
® demonstrated enhanced antioxidant activity, reducing power, and total phenolic content compared to conventional extracts [
15]. It also exhibited strong antimicrobial efficacy against
M. furfur (MTCC 1374), suggesting its potential as a natural and cost-effective anti-dandruff solution. To further validate its effectiveness, FERMENZA
® was tested against two pharmaceutical agents (ketoconazole and zinc pyrithione) and two leading anti-dandruff products in the Indian market. The formulation with the best inhibitory activity underwent additional testing, including time-dependent growth inhibition assays to understand its mechanism of action, physicochemical characterization, and sensory evaluation.
This innovative research aligns with growing consumer demand for natural, sustainable, and effective scalp care solutions. By offering proven results, FERMENZA® addresses Malassezia-associated skin conditions while meeting the expectations of a rapidly evolving anti-dandruff market.
2. Methods
2.1. Preparation of Fermented Extracts and Their Formulation
Whole fruits and vegetables were washed thoroughly with clean water to remove surface impurities. Three substrate combinations were prepared:
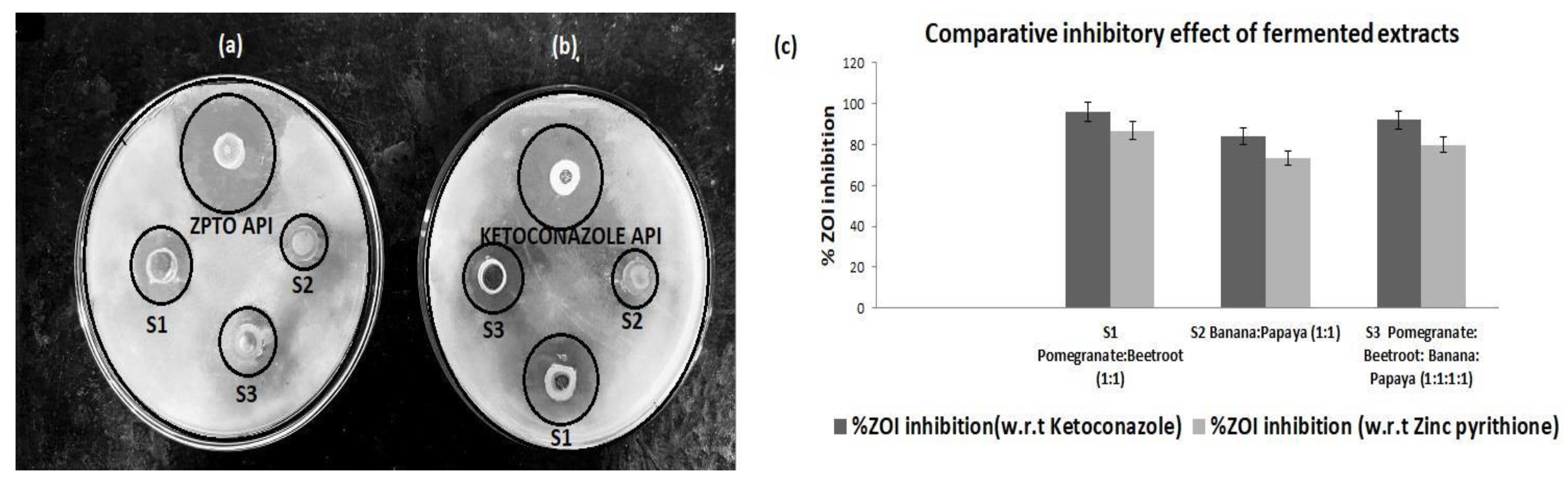
S1: pomegranate and beetroot (1:1),
S2: banana and papaya (1:1),
S3: pomegranate, beetroot, banana, and papaya (1:1:1:1).
These combinations were chopped and blended into uniform pastes. Each substrate mix was subjected to fermentation using either a fermentor/bioreactor or a shake flask setup. Fermentation was carried out with Saccharomyces cerevisiae under optimized conditions: 35°C for 72 hours. The resulting fermented extracts were labeled as S1, S2, and S3.
FERMENZA®, a water-based formulation, was developed at Breww Therapeutics Pvt. Ltd. Mohali, Punjab, India, as disclosed in the granted Indian Patent No. 459674. Formulations containing the fermented extracts were prepared using a consistent excipient composition. Formulations with extracts S1, S2, and S3 were labeled S4, S5, and S6, respectively. Ingredients were weighed accurately, and the aqueous base, comprising water, propylene glycol, and glycerin, was heated to 80-90°C. Fermented extracts and sodium benzoate were then added and stirred for one hour to ensure uniform mixing. The resulting formulations were cooled and packaged in bottles. These formulations were designed for use as hair care products, including but not limited to hair oils, tonics, and serums.
2.2. Preparation of Test Microorganisms
Malassezia furfur (MTCC 1374) was cultured on SDA supplemented with 1% (v/v) olive oil, which provided essential lipids for fungal growth. Cultures were incubated at 32°C for 48 hours. A standardized fungal suspension was prepared in sterile saline, adjusting the optical density to match the 0.5 McFarland standard (approximately 1 × 10⁶ CFU/mL). Sterile SDA plates with 1% olive oil were inoculated with this suspension using a sterile cotton swab. Plates were dried under aseptic conditions for 10 minutes before introducing test samples.
2.3. Antifungal Susceptibility Assay of Fermented Extracts
The antifungal activity of the fermented extracts (S1, S2, S3) was evaluated using the agar well diffusion method. This method was chosen for its effectiveness against M. furfur (MTCC 1374). Wells of 6 mm diameter were punched into SDA plates using a sterile cork borer. Each well received 50 µL of the test sample (fermented extracts S1, S2, or S3). Positive controls included 50 µL of standard antifungal agents (2% ketoconazole or zinc pyrithione). Plates were incubated at 32°C for 48 hours in a humidified chamber. Zones of inhibition (ZOI) were measured using a digital caliper, and the growth inhibition percentage was calculated using the formula:
% Growth Inhibition = [(Zc - Zt) / Zc] × 100
Where Zc is the ZOI for the control and Zt is the ZOI for the test sample.
2.4. Comparative Inhibition of FERMENZA® with Marketed Products
The inhibitory activity of FERMENZA® formulations (S4, S5, S6) against M. furfur (MTCC 1374) was compared with two marketed products: Product A and Product B. Agar well diffusion was used, as described previously. 50 µL of each formulation and marketed product were introduced into the wells. Growth inhibition percentages were calculated for the formulations relative to the marketed products using the same formula mentioned above.
2.5. Evaluation of Physico-Chemical and Microbial Properties of FERMENZA®
Based on optimal growth inhibition against
M. furfur, the formulation S4, containing fermented pomegranate and beetroot extracts, was selected for further studies and developed into the final product, FERMENZA
® hair tonic (
Table 1). Physico-chemical and microbial evaluations were conducted at DN Laboratory, Panchkula, Haryana, India, an FDA-approved and ISO 9001:2015, GLP-certified facility. Using pharmacopoeial and in-house methods, the product was analyzed for pH, specific gravity, polyphenol and gallic acid content, preservative levels, synthetic color, heavy metals, and microbial counts (total aerobic and fungal). The results affirm FERMENZA
® as a safe and effective therapeutic hair care solution.
2.6. Minimum Inhibitory Concentration (MIC) Assay
The formulation S4 (pomegranate and beetroot extract) was selected for further development into the final product, FERMENZA
®, based on its superior growth inhibition. MIC values against
M. furfur (MTCC 1374) were determined using a modified method based on Gonelimali et al [
16]. Serial dilutions of FERMENZA
® (2-20 mg/mL) were tested. Ketoconazole (2% w/v in propylene glycol) was used as a positive control, and propylene glycol alone served as a negative control. Wells of 6 mm diameter were loaded with varying concentrations of FERMENZA
®, and plates were incubated at 32°C for 48 hours. The MIC
50 and MIC
90 values were defined as the lowest concentrations achieving >50% and >90% growth inhibition, respectively.
2.7. Time-Kill Assay
The time-kill assay assessed the fungistatic or fungicidal effects of FERMENZA® on M. furfur (MTCC 1374). A fungal suspension (1 × 10⁶ CFU/mL) was exposed to FERMENZA® at 2× MIC50 concentrations. Samples were collected at intervals (0, 6, 12, 18, 24, 30, 36, and 48 hours), serially diluted, and plated on SDA plates, which were incubated at 32°C for 48 hours, and colony counts (CFU/mL) were recorded. Growth reduction over time was plotted to determine the antifungal kinetics.
2.8. FERMENZA® Efficacy Assessment
A consumer trial under expert guidance, involved 25 volunteers aged between 25-45 years with moderate to severe dandruff and hair fall. Participants received 100 mL of FERMENZA
® hair tonic and were instructed to apply it daily, avoiding other anti-dandruff products. Over 10 days, volunteers were reviewed every two days. Efficacy was scored on a 0-5 scale for parameters such as dandruff removal, itch reduction, flaking, scaling, and hair fall reduction. Scoring criteria ranged from [0] (no improvement) to [
5] (complete resolution).
2.9. FERMENZA® Sensory and Safety Assessment
FERMENZA
® was evaluated for sensory attributes and safety using an eight-parameter scoring system. Parameters included appearance, odor, stickiness, oiliness, ease of spreading, dryness, allergic reactions, and burning sensation. Each parameter was rated on a 0-5 scale, where [0] indicated no effect and [
5] indicated the highest intensity. Sensory assessment focused on product color, consistency, and user experience, while safety evaluation captured adverse reactions such as redness, swelling, or discomfort.
3. Results
3.1. Inhibitory Activity of Fermented Extracts Against M. furfur(MTCC 1374)
The antifungal potential of the fermented extracts was evaluated using the Zone of Inhibition (ZOI) in comparison to marketed antifungal agents Ketoconazole and Zinc Pyrithione. The results are presented in
Table 2. Among the tested samples, Sample S1 (Pomegranate and Beetroot fermented extract) exhibited the highest activity, with ZOI values of 24 mm against Ketoconazole and 26 mm against Zinc Pyrithione. Sample S2 (Banana and Papaya fermented extract) demonstrated moderate activity, with ZOI values of 21 mm and 22 mm, respectively. Sample S3 (a combination of Pomegranate, Beetroot, Banana, and Papaya extracts) showed strong but slightly reduced activity compared to S1, with ZOI values of 23 mm and 24 mm. The percent inhibition of Sample S1 was 96% and 86.7% relative to Ketoconazole and Zinc Pyrithione, respectively (
Figure 1).
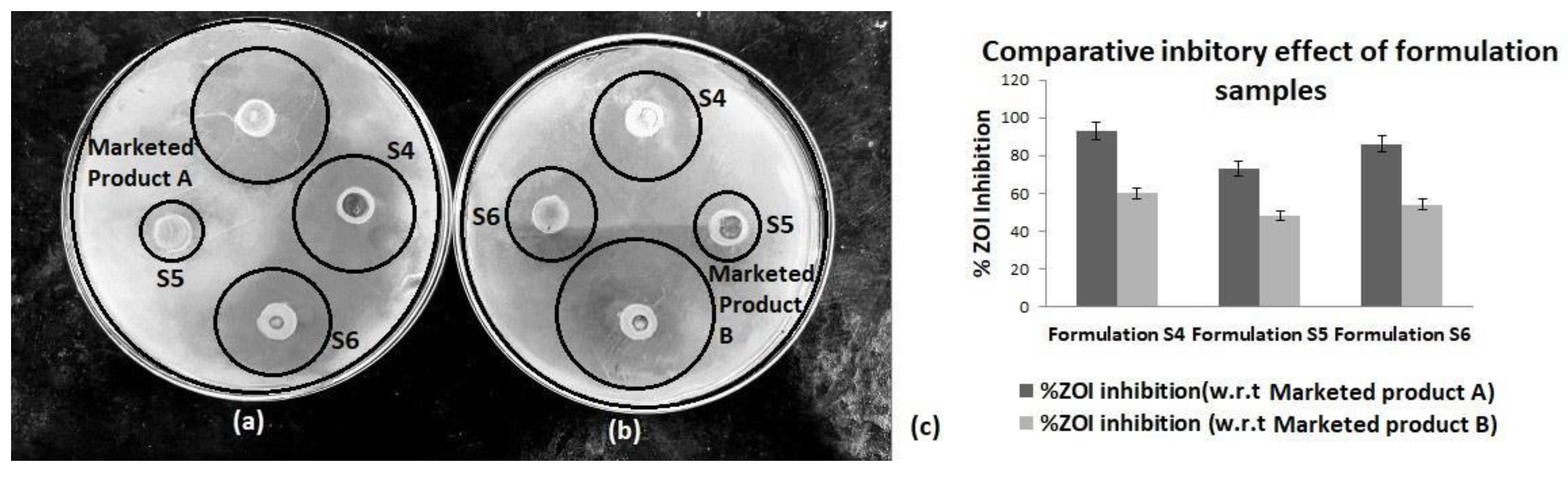
3.2. Comparative Activity of of Investigational Formulation on M. furfur(MTCC 1374) Growth Inhibition
The investigational formulations (S4, S5, and S6) exhibited varied antifungal efficacy represented in
Table 3. S4 (derived from Pomegranate and Beetroot) displayed the highest ZOI of 28 ± 0.12 mm, achieving a 93 ± 0.04% inhibition compared to Marketed Product A lotion. S5 (Banana and Papaya-based) and S6 (combination of all four extracts) showed ZOIs of 22 ± 0.05 mm and 26 ± 0.15 mm, with 73 ± 0.09% and 86 ± 0.06% inhibition, respectively (
Figure 2). When benchmarked against Marketed Product B lotion, S4 again demonstrated superior activity with a ZOI of 27 ± 0.14 mm, followed by S6 (24 ± 0.05 mm) and S5 (21 ± 0.08 mm).
3.3. Physico-Chemical Properties of FERMENZA® Hair Tonic
The FERMENZA
® hair tonic appeared as a transparent, light brownish, viscous liquid with a mild and pleasant fragrance. The pH and specific gravity were measured at 6.7 and 1.14, respectively. High levels of total polyphenols (5.05% w/w) and gallic acid (19.90 ppm) were determined using an HPLC method (in-house). No traces of artificial color or heavy metals (lead, arsenic, mercury, and cadmium) were detected in the tonic, making it a safe and effective choice for hair care with minimal risk of adverse effects. The total viable aerobic count and fungal count were recorded as <10 cfu/ml, which is well within the permissible limits set by official pharmacopoeial standards (
Table 5).
Table 4.
FERMENZA® Minimum inhibitory concentration (MIC) assay findings.
Table 4.
FERMENZA® Minimum inhibitory concentration (MIC) assay findings.
| MIC assay parameters |
Fermented extract concentration (mg/ml) in FERMENZA®
|
| 2 |
4 |
6 |
8 |
10 |
12 |
14 |
16 |
18 |
20 |
| ZOI (mm) |
3.0±0.14 |
3.7±0.02 |
6.1±0.05 |
9.2±0.11 |
12.7±0.08 |
18.2±0.04 |
20.2±0.15 |
21.0±0.15 |
22.5±0.03 |
23.0±0.02 |
| % ZOI (w.r.t. 2% Ketoconazole API) |
12±0.02 |
14±0.15 |
24±0.04 |
36±0.08 |
51±0.08 |
72±0.14 |
80±0.12 |
84±0.03 |
90±0.04 |
92±0.01 |
Table 5.
Physicochemical evaluation of FERMENZA® hair tonic.
Table 5.
Physicochemical evaluation of FERMENZA® hair tonic.
| Parameter |
Result |
Limit |
| Description |
Pale yellow colour oily liquid filled in plastic bottle |
- |
| pH Value |
6.71 |
- |
| Specific Gravity |
1.136841 gm/ml |
- |
| Polyphenol |
5.05% |
- |
| Sodium Benzoate |
0.73 ppm |
- |
| Gallic Acid |
19.90 ppm |
- |
| Ethyl Alcohol |
0.0078% |
- |
| Heavy Metals |
|
|
| - Lead (as Pb) |
<10 ppm (LDL 0.4) |
NMT 10 ppm |
| - Arsenic (as As) |
Not Detected (LDL 0.4) |
NMT 3 ppm |
| - Mercury (as Hg) |
Not Detected (LDL 0.4) |
NMT 1 ppm |
| - Cadmium (as Cd) |
Not Detected (LDL 0.4) |
NMT 0.3 ppm |
| Total Parabens |
Not Detected |
- |
| Microbiological Test |
|
|
| - Total Viable Aerobic Count |
<10 cfu/ml |
NMT 10 cfu/ml |
| - Total Fungal Count |
<10 cfu/ml |
NMT 10 cfu/ml |
| - E. Coli/ml |
Absent |
Should be absent |
| - Salmonella/ml |
Absent |
Should be absent |
| - S. Aureus/ml |
Absent |
Should be absent |
| - P. Aeruginosa/ml |
Absent |
Should be absent |
|
Key: LDL: Lower Detectable Limit; NMT: Not More Than |
3.4. FERMENZA® Potency Findings from MIC
The Minimum Inhibitory Concentration (MIC) of FERMENZA
® demonstrated a concentration-dependent antifungal activity against
M. furfur (MTCC 1374). At 8 mg/mL, the MIC threshold was observed with a ZOI of 9.2 ± 0.11 mm (36 ± 0.08% inhibition relative to Ketoconazole) as represented in
Table 4. Higher concentrations further increased activity, with the MIC
50 value observed at 10 mg/mL (ZOI: 12.7 ± 0.08 mm, 51 ± 0.08% inhibition) and the MIC
90 value at 20 mg/mL (ZOI: 23.0 ± 0.02 mm, 92 ± 0.01% inhibition).
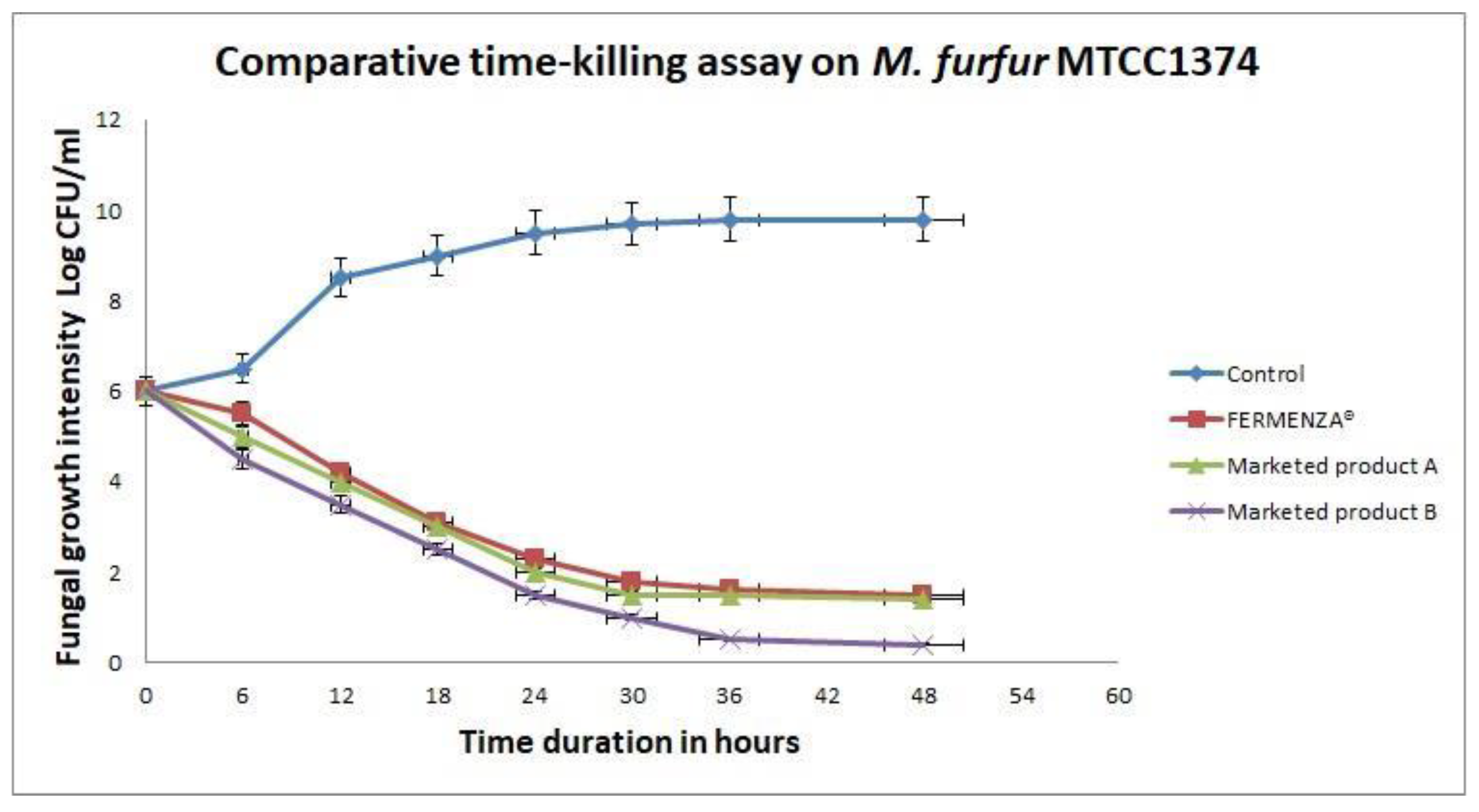
3.5. FERMENZA® Mechanism of Action
Time-kill assays (
Figure 3) revealed a rapid and sustained reduction in fungal growth by FERMENZA
®. Starting at 6 log CFU/mL, fungal growth was significantly reduced to 1.5 log CFU/mL within 48 hours. Compared to Marketed Products A and B, FERMENZA
® consistently outperformed the former while showing comparable efficacy to the later.
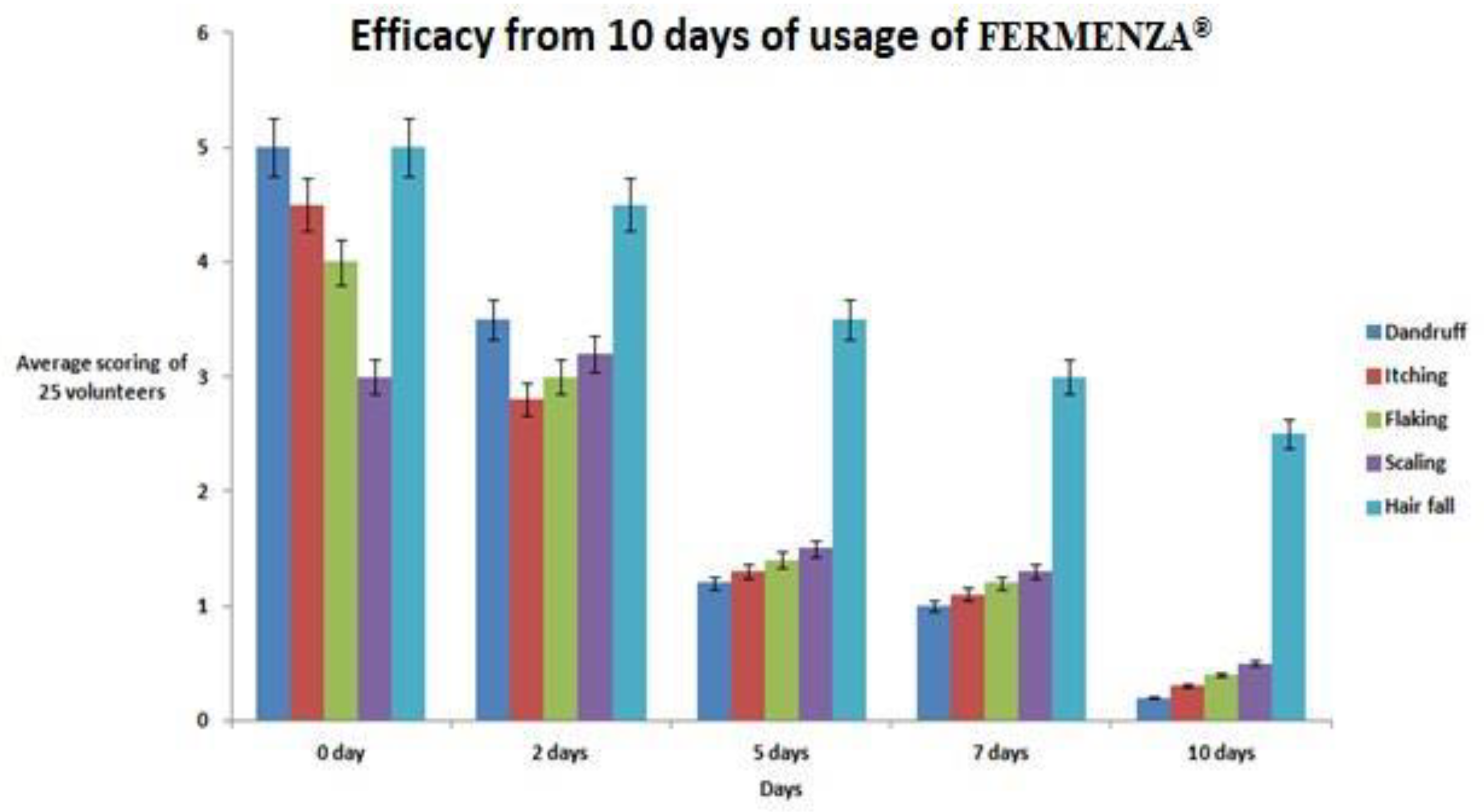
3.6. Efficacy of FERMENZA® in Addressing Dandruff and Scalp Concerns
Consumer trial evaluation of FERMENZA
® demonstrated significant improvements in dandruff and related scalp issues. Dandruff severity scores decreased from 5 (Day 0) to 0.2 (Day 10), reflecting a 96% reduction. Itching severity dropped from 4.5 to 0.4 (91% improvement), while flaking and scaling were reduced by 85% and 77%, respectively. Hairfall showed a moderate improvement, decreasing by 50% over the study period. These findings (
Figure 4) highlight FERMENZA
®’s effectiveness in promoting scalp health and addressing multiple concerns within a short duration.
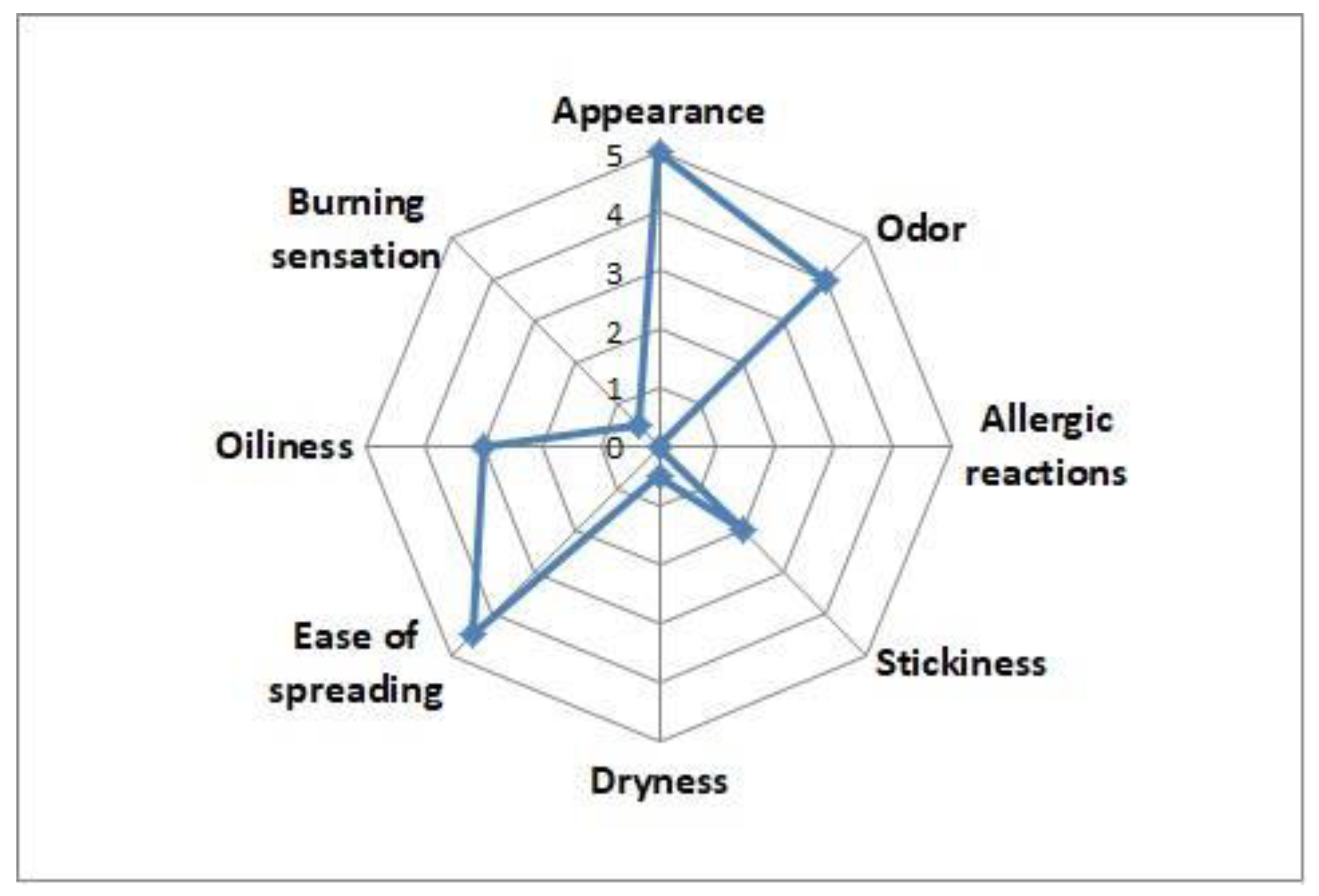
3.7. Sensory and Safety Profile of FERMENZA®
FERMENZA
® scored highly in sensory evaluations (
Figure 5), with excellent ratings for appearance (5/5) and spreadability (4.5/5). Minimal stickiness (2/5) and oiliness (3/5) were noted, while dryness and burning sensation were negligible (scores of 0.5/5). No allergic reactions were reported, confirming its safety for regular use. These results affirm FERMENZA
®’s suitability as a user-friendly product.
4. Discussion
The results of this study demonstrate the potent antifungal activity of fermented botanical extracts, particularly those derived from Pomegranate and Beetroot. Sample S1’s superior ZOI values and percent inhibition underscore the therapeutic potential of fermentation-derived bioactives, which enhance the bioavailability of polyphenols and other antifungal compounds. This highlights the therapeutic potential of fermented botanicals as effective antifungal agents, in alignment with studies by Ziemlewska et al. [
23], which emphasized the enhanced bioactivity of fermentation-derived products. The superior activity observed in Sample S1 could be attributed to its unique mixed substrate composition pomegranate and beetroot, which are potentially antioxidant rich and fermentation, further led to the production of even more bioavailable therapeutically active compounds with enhanced antifungal potency. Li et al. [
22] also demonstrated synergistic effect of plant extracts against
M. fufur in their recent study.
The comparative analysis of investigational formulations highlights the efficacy of S4, suggesting that targeted substrate selection and fermentation processes can optimize antifungal potency. Notably, S4 derived from pomegranate and beetroot ferment consistently outperformed the other formulations, emphasizing its potential as a robust candidate in antifungal treatment with its high total phenolic content and gallic acid content as reported in
Table 5 and also in earlier studies [
14,
15] . The moderate activity of S5 reflects its comparatively lower polyphenolic content, while S6’s performance suggests potential antagonistic interactions among the combined extracts.
The minimum inhibitory concentration of FERMENZA
® was measured using the produced inhibition zones of minimum 7 mm or larger in the agar well diffusion assay [
21]. The MIC findings reveal the dose-dependent activity of FERMENZA
®, with significant inhibition achieved at relatively low concentrations. These results align with earlier studies emphasizing the role of bioactive secondary metabolites, such as gallic acid, in disrupting fungal cell walls and inhibiting growth [
17,
18]. The dose-dependent antifungal activity of FERMENZA
® highlights its potential as a natural alternative to synthetic antifungal agents. The presence of gallic acid and other phenolic compounds in FERMENZA
® likely contributes to its effectiveness, corroborating previous research on the antifungal mechanisms of plant-derived metabolites [
19,
20]. Its antioxidant properties as reported in earlier study [
15] induce oxidative stress, compromising fungal cell walls and membranes. This mechanism is consistent with its observed fungistatic and fungicidal effects, making FERMENZA® a promising therapeutic agent.
FERMENZA®’s consumer trial performance further validates its potential as a comprehensive solution for scalp health. The observed reductions in dandruff, itching, and flaking are consistent with its antifungal properties, while the moderate improvement in hairfall suggests additional benefits beyond fungal inhibition. Sensory evaluations confirm its user acceptability, with minimal side effects and high satisfaction ratings.
This study supports the development of FERMENZA® as natural, effective alternatives to synthetic antifungals. Future research could explore the synergistic effects of combined extracts and optimize formulations to address a broader range of dermatological concerns.
5. Limitations of the Study
The limitation of the study was assessed based on the data which mainly focused on in-vitro experiments with a limited sample size of Malassezia furfur MTCC 1374. Hence, further studies on clinical isolates and larger human samples are needed for long-term clinical data generation for better understanding of its clinical efficacy and safety.
6. Conclusion
This study highlights FERMENZA® as a groundbreaking natural alternative to synthetic antifungal agents like ketoconazole and zinc pyrithione for managing dandruff and scalp disorders. The formulation showcased superior antifungal efficacy, effectively inhibiting the growth of Malassezia furfur and outperforming widely used commercial products. Its potent activity stems from its unique composition of pomegranate and beetroot fermented extracts, rich in gallic acid, which disrupts fungal cell integrity and metabolic pathways. FERMENZA® also demonstrated remarkable effectiveness in addressing scalp issues such as dandruff, itching, flaking, and scaling, alongside noticeable hairfall reduction. With strong antifungal properties, excellent safety, and high user satisfaction, FERMENZA® offers a holistic and natural solution for scalp health and hair care. This study underscores its potential as a dependable alternative to conventional treatments, meeting the growing demand for safer, sustainable, and more effective therapeutic options in modern dermatological care.
Acknowledgments
The authors would like to acknowledge Technology Business Incubator, IISER Mohali, Punjab, India for incubation support.
Conflicting Interest: The authors declare they have no competing financial interests that could have appeared to influence the work reported in this paper.
Declaration statement: During the preparation of this work the author(s) used ChatGPT (
https://openai.com/index/chatgpt/) in order to improve the language quality of the manuscript. After using this tool, the author(s) reviewed and edited the content as needed and take full responsibility for the content of the published article. The authors have seen and approved the manuscript and it has not been published or considered for publication elsewhere.
Ethics approval and consent to participate: This study was conducted in accordance with the written informed consent obtained from all participants prior to their inclusion in the study.
References
- Saunte DML, Gaitanis G, Hay RJ. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front Cell Infect Microbiol 2020; 10:112. [CrossRef]
- Rhimi W, Theelen B, Boekhout T, Otranto D, Cafarchia C. Malassezia spp. Yeasts of Emerging Concern in Fungemia. Front Cel. Infect Microbiol 2020; 10:370. [CrossRef]
- Billamboz M, Jawhara S. Anti-Malassezia Drug Candidates Based on Virulence Factors of Malassezia-Associated Diseases. Microorganisms 2023; 11, 2599. [CrossRef]
- Schwartz, JR. Zinc Pyrithione: a topical antimicrobial with complex pharmaceutics. J Drugs Dermatol 2016; 15, 140–144.
- Chen G, Miao M, Hoptroff M, Fei X, Collins LZ, Jones A, Janssen HG. Sensitive and simultaneous quantification of zinc pyrithione and climbazole deposition from anti-dandruff shampoos onto human scalp. J Chromatogr B Analyt Technol Biomed Life Sci 2015; 1003:22-6. [CrossRef] [PubMed]
- Deb, T. Deb T. Pharmaceutical Market Research Reports- Dandruff Treatment Market. Published August 2024. https://marketresearch.biz/report/dandruff-treatment-market/.
- Ayatollahi A, Firooz A, Lotfali E, Mojab F, Fattahi M. Herbal therapy for the management of seborrheic dermatitis: a narrative review. Recent Adv Antiinfect Drug Discov 2021; 16, 209–226. [CrossRef]
- Khwaza V, Aderibigbe BA. Antifungal Activities of Natural Products and Their Hybrid Molecules. Pharmaceutics 2023; 15(12):2673. [CrossRef] [PubMed] [PubMed Central]
- Chemat F, Abert-Vian M, Fabiano-Tixier AS. Green extraction of natural products: Concept and principles. Int J Mol Sci 2017; 18(7): 1179. [CrossRef]
- Majchrzak W, Motyl I, Śmigielski K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022; 27(15):4845. [CrossRef] [PubMed] [PubMed Central]
- Pérez-Rivero C, López-Gómez JP. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023; 9(5):463. [CrossRef]
- Fermented Ingredients Global Market Report 2024 https://www.thebusinessresearchcompany.
- Chaiyana W, Punyoyai C, Sriyab S, Prommaban A, Sirilun S, Maiti J, Chantawannakul P, Neimkhum W, Anuchapreeda S. Anti-Inflammatory and Antimicrobial Activities of Fermented Ocimum sanctum Linn. Extracts against Skin and Scalp Microorganisms. Chem Biodiversity 2022; 19, e202100799.
- Ghosh S, Bhattacharya M. Fermented Cider compositions and method of preparation thereof. 2023. (Indian patent No. 459674) https://www.ipindia.gov.in/.
- Ghosh S, Bhattacharya M. FERMENZA®: A patented bioactive fermented product developed through process optimization. [CrossRef]
- Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M and Hatab SR. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front Microbiol 2018; 9:1639. [CrossRef]
- Rhimi W, Aneke CI, Annoscia G, Otranto D, Boekhout T, Cafarchia C. Effect of chlorogenic and gallic acids combined with azoles on antifungal susceptibility and virulence of multidrug-resistant Candida spp. and Malassezia furfur isolates. Med Mycol 2020; 58(8):1091-1101. [CrossRef] [PubMed]
- Nayeem N, Asdaq SMB, Salem H, Ahel-Alfqy S. Gallic acid: a promising lead molecule for drug development. J Appl Pharm 2016; 8: 1–4.
- Borges A, Ferreira C, Saavedra MJ, Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 2013; 19(4):256-65. [CrossRef] [PubMed]
- Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, Momtaz S, Abbasabadi Z, Rahimi R, Farzaei MH, Bishayee A. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci 2019; 22(3):225-237. [CrossRef] [PubMed] [PubMed Central]
- Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed 2011; 1(5):370-5. [CrossRef] [PubMed] [PubMed Central]
- Li L, He Y, Zou Q, Chen W, Liu Y, He H, Zhang J. In vitro and in vivo synergistic inhibition of Malassezia furfur targeting cell membranes by Rosa rugosa Thunb. and Coptidis Rhizoma extracts. Front Microbiol 2024; 15:1456240. [CrossRef]
- Ziemlewska A, Nizioł-Łukaszewska Z, Bujak T. Effect of fermentation time on the content of bioactive compounds with cosmetic and dermatological properties in Kombucha Yerba Mate extracts. Sci Rep 2021; 11: 18792. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).