Submitted:
04 February 2025
Posted:
05 February 2025
You are already at the latest version
Abstract
Keywords:
Material and Methods
Material
Methods
a) Strain titration
(a.1) Cytopathic Effect (CPE)
(a.2) Vital Dye (Neutral Red)
(a.3) Genomic Detection and Quantification: Real-Time RT-PCR of SARS-Cov2
(b) Inhibition assay
(b.1) Biological Readout Response (DBS/Neutral Red):
(b.2) Molecular Readout Endpoints (RT-PCR):
Experimental Results.
Discussion
References
- a) Hu, B., Guo, H., Zhou, P., & Shi, Z. L. (2021). Characteristics of SARS-CoV-2 and COVID-19. Nature Reviews Microbiology, 19(3), 141-154. b) Suhail, S., Zajac, J., Fossum, C., Lowater, H., McCracken, C., Severson, N., ... & Hati, S. (2020). Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. The protein journal, 39, 644-656. c) Lamers, M. M., & Haagmans, B. L. (2022). SARS-CoV-2 pathogenesis. Nature reviews microbiology, 20(5), 270-284.
- a) Oliveira, B. A., Oliveira, L. C. D., Sabino, E. C., & Okay, T. S. (2020). SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Revista do Instituto de Medicina Tropical de São Paulo, 62, e44. b) Hámilton Forero-Argüello*, A. H.-M.-B.-R. (2021). Caracterización y fisiopatología del Sars-Cov-2, Revisión de literatura actual. Infectopatología, 61-75. c) Cevik M., K. K. (2020). Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ, 371:m3862.
- Valsé Pantellini G (1970): Breve cenno sulla genesi dei tumori e sopra una eventuale terapia dei medesimi con sali di potassio e in particolare con ascorbato di potassio. Rivista di Patologia e Clinica, XXV: 219-225.
- Valsé Pantellini G (1974): Legami idrogeno (H) e salificazione degli stessi da parte del potassio (K) nella strutturazione della materia vivente. Rivista di Patologia e Clinica, XXIX: 193-198.
- Valsé Pantellini G (1984): I nuovi orientamenti sulla terapia dei tumori dal punto di vista biochimico e immunologico. In Atti del Quarto Convegno Nazionale “L’Uomo tra Microcosmo e Macrocosmo”. A cura di Marinucci G. Urbino: 171-176.
- Valsé Pantellini G, Paoli G (1999): Meccanismo d'azione dell'ascorbato di potassio nei sistemi biologici. In LXXXV Congresso Nazionale della Società Italiana di Fisica, Pavia: 108.
- Croci S, Pedrazzi G, Paoli G, Monetti D, Bronzetti G, Ortalli I (2001): Potassium ascorbate as protective agent in oxidation of red cells. Anticancer Research, 21. Abstract of the International Conference on Antioxidant in Cancer Prevention and Therapy: 1571-1572.
- Croci S, Pedrazzi G, Paoli G, Monetti D, Ortalli I (2002): Potassium ascorbate as protective agent in the oxidation of the red blood cells. Hyperfine Interactions (C). Proceedings of the International Conference on the Applications of the Mössbauer effect (ICAME 2001). Thomas MF, Williams JM, Gibb TC Ed.(s), Kluwer Academic Publishers: 241-244.
- Paoli G (2003): The biomagnetic nature of cancer and the role of potassium ascorbate and ribose against cellular degeneration. Journal of New Energy, 7(3): 114-11.
- Croci S, Bruni L, Bussolanti S, Castaldo M, Dondi M (2011): Potassium bicarbonato and D-ribose effects on A72 canine and HTB-126 human cancer cell line proliferation in vitro. Cancer Cell Int, 11:30.
- Bruni L, Babarinde AA, Ortalli I, Croci S (2014): K-D:rib dampens Hs 578T cancer cell chemoinvasion and proliferation. Cancer Cell Int, 14:77.
- Bruni L, Croci S (2014): K-D:rib cancer cell proliferation inibitor and DNAzyme folding promoter. Journal of Biological Research, 87:2135.
- Bruni L (2014): Antitumorigenicità del D-ribosio e KHCO3 sulla linea di carcinoma mammario Hs 578T ed effetti sulla linea d’epitelio mammario umano non tumorale Hs 578BST. Tesi di Dottorato di Ricerca in Biotecnologie. Università degli Studi di Parma, Ciclo XXVI.
- Frajese GV, Benvenuto M et al (2016): Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines “in vitro”. Oncology Letters, 11: 4224-4234.
- Cavicchio C et al. (2017): Potassium Ascorbate with Ribose: Promising Therapeutic Approach for Melanoma Treatment. Oxidative Medicine and Cellular Longevity, Volume 2017. Article ID4256519, 12 pages. [CrossRef]
- Anichini C et al, (2011), Beckwith-Wiedemann Syndrome: Potassium Ascorbate with ribose Therapy in a Syndrome with High Neoplastic Risk. Anticancer Research 31: 3973-3976.
- Anichini C et al, (2012), Antioxidant effects of potassium ascorbate with ribose therapy in a case of Prader Willi Syndrome. Disease Markers 33: 179-183.
- Anichini C et al, (2013), Antioxidant Effects of Potassium Ascorbate with Ribose in Costello Syndrome. Anticancer Research 33: 691-696.
- Anichini C et al, (2014), Antioxidant strategies in genetic syndromes with high neoplastic risk in infant. Tumori 100: 590-599.
- Palmer BF (2015): Regulation of potassium homeostasis. Clin J Am Soc Nephrol, 10(6): 1050-1060.
- Gumz ML, Rabinowitz L, Wingo CS (2015): An Integrated View of Potassium Homeostasis. N Engl J Med, 373(1):60-72.
- Palmer BF, Clegg DJ (2019): Physiology and pathophysiology of potassium homeostasis: Core Curriculum 2019. Am J Kidney Dis, 74(5): 682-695.
- Paoli G (2020): Ascorbato di potassio. La molecola intelligente per regolare le difese dell’organismo. Aam Terra Nuova Ed. Firenze.
- a) Paoli G (2022): Ribosate di potassio e SARS-CoV-2. Le connessioni inattese fra virus e molecole semplici. Natura Docet: la Natura insegna – ND, Anno III, 9:18-22. b) Paoli G (2022): Can a molecule be “intelligent”? Unexpected connections between Physics and Biology. Open Journal of Biophysics, 12(4):234-244.
- S.L., V. R. (2019). STUDY PLAN B-02959 Toxicity Evaluation of the Test Item via Intramuscular Administration in Female Sprague Dawley Rats by the Acute Toxicity-Up-and-Down Procedure. Spain: Nanoimunotech.
- de la Iglesia, P., Melón García, S., López, B., Rodríguez, M., Blanco, M. I., Mellado, P., & de Oña, M. (1998). Rapid screening tests for determining in vitro susceptibility of herpes simplex virus clinical isolates. Journal of clinical microbiology, 36(8), 2389-2391.
- Álvarez ÁL, Habtemariam S, Abdel Moneim AE, Melón S, Dalton KP, Parra F. A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antiviral Res. 2015 Jul;119:8-18. [CrossRef]
- Alvarez AL, Dalton KP, Nicieza I, Diñeiro Y, Picinelli A, Melón S, Roque A, Suárez B, Parra F. Bioactivity-guided fractionation of Phyllanthus orbicularis and identification of the principal anti HSV-2 compounds. Phytother Res. 2012 Oct;26(10):1513-20. [CrossRef]
- Pérez Martínez, Z. (2021) “Efecto de la melatonina en células infectadas con el Herpes Simplex Tipo 1”. Universidad de Oviedo. Asturias-España.
- Martín, G., Rojo-Alba, S., Castello-Abietar, C., Abreu-Salinas, F., Costales González, I., Boga, J. A., Melón, S., & Álvarez-Argüelles, M. E. (2021). Comparison of in-house SARS-CoV-2 genome extraction procedures. A need for COVID-19 pandemic. Research Square preprint. [CrossRef]
- Sandoval Torriente, M., Castello-Abietar, C., Boga Riveiro, J., Álvarez-Argüelles, M. E., Rojo-Alba, S., Abreu-Salinas, F., Costales González, I., Pérez Martínez, Z., Martín Rodríguez, G., Gómez de Oña, J., & Melón García, S. (2021). A novel single nucleotide polymorphism assay for the detection of N501Y SARS-CoV-2 variants. Journal of virological methods, 294, 114143.
- A.E.I.E., B. R. (junio de 2021). Biochemical Research A.E.I.E. Obtenido de Investigación-Estudios: https://www.aeiebiochemical.es/es/content/9-investigacion.
- Van Damme W., D. R. (2021). COVID-19: Does the infectious inoculum dose-response relationship contribute to undertanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses, 146.
- Billah M.A., M. M. (2020). Reproductive number of coronavirus: A systematuc review and meta-analysis based on global level evidence. PLos ONE, 15(11): e0242128.
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.ncbi.nlm.nih.gov/books/NBK570371/.
- Hodgson, S. H., Mansatta, K., Mallet, G., Harris, V., Emary, K., & Pollard, A. J. (2021). What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. The Lancet. Infectious diseases, 21(2), e26-e35.
- Osamu Kanauchi 1 2, Zhao Xuan Low 1, Kenta Jounai 2, Ryohei Tsuji 2, Sazaly AbuBakar 1 Overview of anti-viral effects of probiotics via immune cells in pre-, mid- and post-SARS-CoV2 era. Front Immunol. 2023 Dec 5:14:1280680. [CrossRef]
- K Dhuli 1, C Micheletti 1, P E Maltese 1, B Tanzi 1, S Benedetti 1, S Tezzele 1, C Mareso 2, S T Connelly 3, F Gaffuri 4 5, G M Tartaglia 4 5, S Nodari 6, G Arabia 7, F Fioretti 6, C Calandri 8, M A Perrone 8, M Bertelli 1 2 9 The Role of Olive Tree Polyphenols In The Prevention of COVID-19: A Scoping Review Part 2 Clin Ter. 2023 Nov-Dec;174(Suppl 2(6)):149-153. [CrossRef]
- Jorge Calderón-Parra 1, Andrea Gutiérrez-Villanueva 2, Gerard Ronda-Roca 3, Maria Luisa Martín Jimenez 4, Helena de la Torre 4, María Ródenas-Baquero 5, María Paniura-Pinedo 5, Carla Lozano-Llano 6, Ilduara Pintos-Pascual 5, Ana Fernández-Cruz 7, Antonio Ramos-Martínez 7, Elena Muñez-Rubio 1 Efficacy and safety of antiviral plus anti-spike monoclonal antibody combination therapy vs. monotherapy for high-risk immunocompromised patients with mild-to-moderate SARS-CoV2 infection during the Omicron era. A prospective cohort study. Int J Antimicrob Agents. 2024 Jan 18;63(3):107095. doi: 0.1016/j.ijantimicag.2024.107095. Online ahead of print.
- Saad Alhumaid 1, Abbas Al Mutair 2 3 4 5, Jalal Alali 6, Nourah Al Dossary 7, Sami Hussain Albattat 8, Sarah Mahmoud Al HajjiMohammed 9, Fatimah Saad Almuaiweed 10, Maryam Radhi AlZaid 9, Mohammed Jaber Alomran 11, Zainab Sabri Alqurini 12, Ahmed Abduljalil Alsultan 13, Thamer Saeed Alhajji 13, Sukainah Mohammad Alshaikhnasir 10, Ali Al Motared 14, Koblan M Al Mutared 15, Khalid Hajissa 16, Ali A Rabaan 17 18 19 Efficacy and Safety of Tixagevimab/Cilgavimab to Prevent COVID-19 (Pre-Exposure Prophylaxis): A Systematic Review and Meta-Analysis Diseases2022 Dec 1;10(4):118. [CrossRef]
- Marta Gargantilla, Clara Francés, Anmol Adhav, Alicia Forcada-Nadal, Belén Martínez-Gualda, Olaia Martí-Marí, María Luisa López-Redondo, Roberto Melero, Clara Marco-Marín, Nadine Gougeard, Carolina Espinosa, Antonio Rubio-del-Campo, Rafael Ruiz-Partida, María del Pilar Hernández-Sierra, Laura Villamayor-Belinchón, Jerónimo Bravo, José-Luis Llacer, Alberto Marina, Vicente Rubio, Ana San-Félix, Ron Geller, and María-Jesús Pérez-Pérez. J, Med. Química.2023, 66, 15, 10432-10457.
- Dustin Siegel,† Hon C. Hui,† Edward Doerffler,† Michael O. Clarke,† Kwon Chun,† Lijun Zhang,†Sean Neville,† Ernest Carra,† Willard Lew,† Bruce Ross,† Queenie Wang,† Lydia Wolfe,† Robert Jordan,†Veronica Soloveva,‡ John Knox,† Jason Perry,† Michel Perron,† Kirsten M. Stray,† Ona Barauskas,†Joy Y. Feng,† Yili Xu,† Gary Lee,† Arnold L. Rheingold,§ Adrian S. Ray,† Roy Bannister,† Robert Strickley,†Swami Swaminathan,† William A. Lee,† Sina Bavari,‡ Tomas Cihlar,† Michael K. Lo,∥ Travis K. Warren,‡and Richard L. Mackman*,†J.Med. Química. 2017, 60, 5, 1648-1661.
- Beigel, J. H.; J.H. Beigel, K.M. Tomashek, L.E. Dodd, A.K. Mehta, B.S. Zingman, A.C. Kalil, E. Hohmann, H.Y. Chu, A. Luetkemeyer, S. Kline, D. Lopez de Castilla, R.W. Finberg, K. Dierberg, V. Tapson, L. Hsieh, T.F. Patterson, R. Paredes, D.A. Sweeney, W.R. Short, G. Touloumi, D.C. Lye, N. Ohmagari, M. Oh, G.M. Ruiz-Palacios, T. Benfield, G. Fätkenheuer, M.G. Kortepeter, R.L. Atmar, C.B. Creech, J. Lundgren, A.G. Babiker, S. Pett, J.D. Neaton, T.H. Burgess, T. Bonnett, M. Green, M. Makowski, A. Osinusi, S. Nayak, and H.C. Lane, for the ACTT-1 Study Group Members*N. J. Med. 2020, 383,1813-1826.
- a) Painter, G. R.; Natchus, M. G.; Cohen, O.; Holman, W.; Painter, W. P. Developing a Direct Acting, Orally Available Antiviral Agent in a Pandemic: The Evolution of Molnupiravir as a Potential Treatment for COVID-19. Curr. Opin. Virol. 2021, 50, 17-22. b) Hancioglu B., S. D. (2007). A dynamical model of human immune response to influenza A virus infection. J Theor Biol., 246:70-86.
- Soldà P (2019): Ligands-mediated modulation of G-quadruplex structures within the HIV-1 genome during lytic and latent state of infection. Tesi di Dottorato di Ricerca in Biomedicina. Università degli Studi di Padova, Ciclo XXXII https://www.research.unipd.it/retrieve/e14fb270-0211-3de1-e053-1705fe0ac030/Solda%cc%80_Paola_tesi.pdf.
- Tan J et al (2009): The SARS-Unique Domain (SUD) of SARS Coronavirus contains two macrodomains that bind G-quadruplex. PLoS Pathog, 5(5), e1000428. http://doi.org/10.1371/journal.ppat.1000428.
- Brian W-X et al (2019): Binding of cellular nucleolin with the viral core RNA G-quadruplex structure suppresses HVL replication. Nucleic Acids Res 47(1):56-68 . [CrossRef]
- Abdou K Allayeh 1, Aliaa H El-Boghdady 2, Mohamed A Said 3, Mahmoud G A Saleh 4, Mohammed T Abdel-Aal 2, Mohamed G Abouelenein 2 Discovery of Pyrano [2,3- c]pyrazole Derivatives as Novel Potential Human Coronavirus Inhibitors: Design, Synthesis, In Silico, In Vitro, and ADME Studies. Pharmaceuticals (Basel). 2024 Feb 2;17(2):198. [CrossRef]
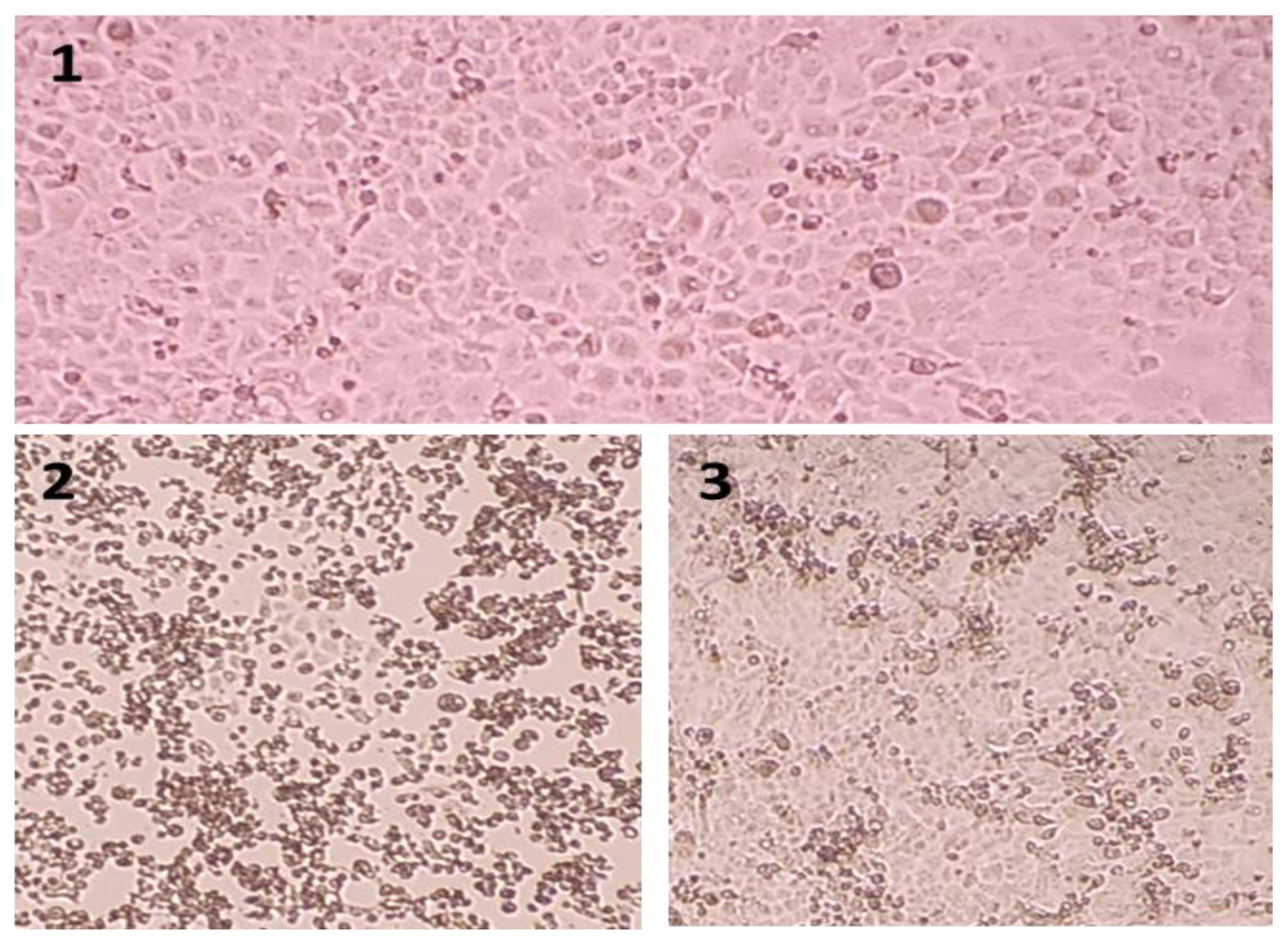
| Titration Strain | Pretreatment with 2021-β | Treatment with 2021-β | ||||||||||
| Experiments | Strains | CPE | Neutral red | PCR Ct / VL1 |
CPE / (% Growth)2 | PCR Ct/VL |
Valuation | CPE (% Growth)2 | PCR Ct / VL |
Valuation | ||
| Test 1 | 1 | - | - | - | - | - | - | - | - | - | ||
| 2 | 4 | - | 22 / 6,7 | - | 22 / 7,0 | NR | - | 35 / 3,13 | VG | |||
| 3 | 4 | - | 21 / 7,0 | - | 24 / 6,4 | R | - | 31 / 4,32 | VG | |||
| 4 | 2 | - | 28 / 5,2 | - | 37 / 2,5 | VG | - | 22 / 7,0 | NR | |||
| 5 | 2 | - | 21 / 6,7 | - | 22 / 7,0 | NR | - | 21 / 7,3 | NR | |||
| 6 | - | - | - | - | - | - | - | - | - | |||
| Test 2 | 1 | 2,5 | - | 25 / 6,1 | - | 30 / 4,6 | R | - | 30 / 4,6 | R | ||
| 2 | 2,8 | - | 25 / 6,1 | - | 28 / 5,2 | R | - | 30 / 4,6 | R | |||
| 3 | 2,5 | - | 27 / 5,5 | - | 40 / 1,5 | VG | - | 40 / 1,5 | VG | |||
| 4 | 2,5 | - | 26/ 5,6 | - | 37 / 2,5 | VG | - | 37 / 2,5 | VG | |||
| 5 | 2,1 | - | 26/ 5,8 | - | 40 / 1,5 | VG | - | 30 / 4,6 | R | |||
| 6 | 2,1 | - | 26 / 5,8 | - | 40 / 1,5 | VG | - | 35 / 3,1 | G | |||
| 7 | 2,8 | - | 27 / 5,5 | - | 40 / 1,5 | VG | - | 40 / 1,5 | VG | |||
| 8 | 2,1 | - | 17 / 8,5 | - | 28 / 5,2 | VG | - | 40 / 1,5 | VG | |||
| Test 3 | 1 | 3,5 | 4,55 | 29,3 / 4,83 | + / Not read | 25,6 / 5,93 | NR | + / Not read | 25,8 / 5,87 | NR | ||
| 2 | 3,5 | 4,00 | 23,7 / 6,49 | + / Not read | 25,6 / 5,93 | NR | + / Not read | 26,5 / 5,66 | R | |||
| 3 | 3,8 | 4,40 | 22,0 / 6,94 | + / Not read | 34,6 / 3,25 | VG | + / Not read | 32,1 / 3,99 | VG | |||
| 4 | 3,5 | 3,20 | 22,6 / 6,82 | + / Not read | 32,7 / 3,81 | VG | + / Not read | 30,9 / 4,35 | G | |||
| 5 | 4,8 | 4,00 | 25,4 / 5,99 | + / Not read | 25,7 / 5,9 | NR | + / Not read | 29,8 / 4,68 | R | |||
| 6 | 3,5 | 3,10 | 20,8 / 7,36 | + / 100 | 20,9 / 7,33 | NR | + / 100 | 20,6 / 7,42 | NR | |||
| 7 | 3,1 | 3,70 | 32,3 / 3,93 | + / 75 | 30,1 / 4,59 | NR | + / 50,50 | 37,9 / 2,27 | VG | |||
| 8 | 5,8 | 4,60 | 20,3 / 7,51 | + / 100 | 18,6 / 8,01 | NR | + / 100 | 18,6 / 8,01 | NR | |||
| 9 | 2,5 | 3,10 | 20,5 / 7,45 | + / 100 | 19,3 / 7,80 | NR | + / 100 | 29,8 / 4,68 | G | |||
| 10 | 3,1 | 3,70 | 35,0 / 3,13 | - | 35,6 / 2,95 | - | - | 34,1 / 3,40 | - | |||
| 11 | 3,5 | 4,50 | 24,3 / 6,31 | + / 91,40 | 22,8 / 6,97 | NR | + / 86,40 | 22,5 / 6,85 | NR | |||
| 12 | 3,5 | 3,20 | 22,6 / 6,82 | ¿? / 100 | 32,7 / 3,81 | VG | + / 100 | 21,7 / 7,09 | NR | |||
| 13 | 4,8 | 4,00 | 25,4 / 5,99 | + / 100 | 25,7 / 5,9 | NR | + / 100 | 19,5 / 7,74 | NR | |||
| 14 | 3,5 | 4,00 | 21,7 / 7,09 | + / 85,50 | 21,3 / 7,21 | NR | + / 89,50 | 20,7 / 7,39 | NR | |||
| 15 | 3,8 | 4,40 | 20,6 / 7,42 | + / 100 | 19,5 / 7,74 | NR | + / 79,30 | 18,5 / 8,04 | NR | |||
| Ct: genomic amplification cycle, VL: viral load, NR: no response, R: normal response, G: good response, VG: very good response. 1In trials 1, 2, 4 and 5, values separated by | refer to the result of infected cultures without 2021-β for the pre-treatment (left) and treatment (right) trials. 2percentage of growth calculated with respect to uninfected cells adjacent to the dilution | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





