New & Noteworthy:
This rat study of severe intestinal ischemia reperfusion injury demonstrates a novel role for Tranilast as a potential therapy. Administration of Tranilast led to a marked reduction in mortality, inflammation and intestinal permeability and damage. The study proved that Tranilast functions through upregulation of Heme oxygenase-1.
1. Introduction
The intestinal tract is a highly complex structure that permits safe absorption of nutrients necessary to sustain life(1). A single layer of tightly linked epithelial cells form a barrier between the intestinal lumen and underlying innate immune cells(2). Damage to its integrity, like intestinal ischemia, can lead to bacterial translocation, sepsis and multiple organ failure(3). Intestinal ischemia is caused by both acute arterial occlusions(4,5) - such as embolism or atherosclerotic plaque ruptures - and ‘low flow’ ischemia due to sepsis, major vascular surgery or cardiopulmonary bypass(6,7). Due to its insidious initial presentation and lack of specific treatment, mortality rates of up to 80% have been reported (8,9). International guidelines recommend rapid revascularization and resection of all necrotic bowel(10,11). However, restoring circulation to the bowel is not harmless, but will lead to additional injury through a process known as intestinal Ischemia reperfusion injury (IRI)(8,12). In this process reactive oxygen species (ROS) are produced which cause additional tissue damage(13). As a consequence, IRI disrupts the mucosal barrier, leading to bacterial translocation and sepsis(3,8,12). In Intestinal transplantation (ITx), a life-saving procedure for patients with complicated irreversible intestinal failure (14), there is an inevitable period of cold and warm ischemia followed by reperfusion that causes damage and can potentially compromise the graft function(15). Specifically, the production of ‘danger signals’ caused by IRI prime the innate and adaptive immune system of the graft, provoking rejection(16). Reducing this damage is critical to improve both short- and long-term survival.
Tranilast (N-[3,4-dimethoxycinnamoyl]-anthranilic acid)(TL) is a commercially available drug with strong anti-inflammatory properties(17). It induces heme oxygenase-1 (HO-1) upregulation, a well-conserved anti-inflammatory pathway in all cells(18). It blocks production of pro-inflammatory cytokines (interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF- α) by downregulating the NF-κB pathway(19). Secondly, it is also a powerful anti-oxidant(20), which can help to reduce ROS that are formed during intestinal IRI (13,21). Furthermore, it has been shown to have immunomodulatory effects, by inducing regulatory T cells (T-regs)(22). TL pretreatment has already been shown to reduce IRI in different organs such as kidneys and brains(23,24). However, the effect of TL pretreatment on intestinal IRI has not been evaluated.
We hypothesized that oral TL pretreatment could attenuate the damage caused by intestinal IRI in a pre-clinical rat model of intestinal IRI, partly through the up-regulation of HO-1.
2. Methods
2.1. Animal Model
We used the validated rat model of intestinal IRI previously described by our group(25). Briefly, intestinal IRI was induced in male Sprague Dawley rats (n=54, weight 275-325 grams) (Janvier Labs, Saint Berthevin Cedex, France) by clamping the superior mesenteric artery for 60 minutes. Clamping was achieved through a midline laparotomy after general anesthesia delivered through intraperitoneal injection with a mixture of xylazine (Xyl-M2%, Van Miert & Dams Chemie, Belgium) and ketamin (Anesketin, Eurovet, the Netherlands). The abdomen was temporarily closed and after 60 minutes the clamp was removed leading to reperfusion. The sham group underwent the same procedure as the other groups (including anesthesia and surgery) with the exception of clamping the superior mesenteric artery. At the end of the experiments, animals were sacrificed through exsanguination under general anesthesia. All animals underwent necropsy at time of death or sacrifice. Animals were housed in a dedicated facility following European Union guidelines on animal welfare, which included daily monitoring and postoperative analgesia using buprenorphine (Vetergesic). Animals were allowed full access to water and food prior and after surgery. A standardized morbidity score was utilized registering signs of distress and resulting in euthanasia if too high(25). None of the animals had to be euthanized in this study. Treated animals received either Tranilast (Sigma-Aldrich, Belgium) at 650mg/kg (dissolved in 1.4% NaHCO3 solution) or equivalent volume of vehicle only (1.4% NaHCO3 solution). Administration was performed through oral gavage at 24 and 2 hours prior to start of ischemia.
The experiments were approved by the University of Leuven Ethics Committee for Animal Experimentation (EC P141/2012).
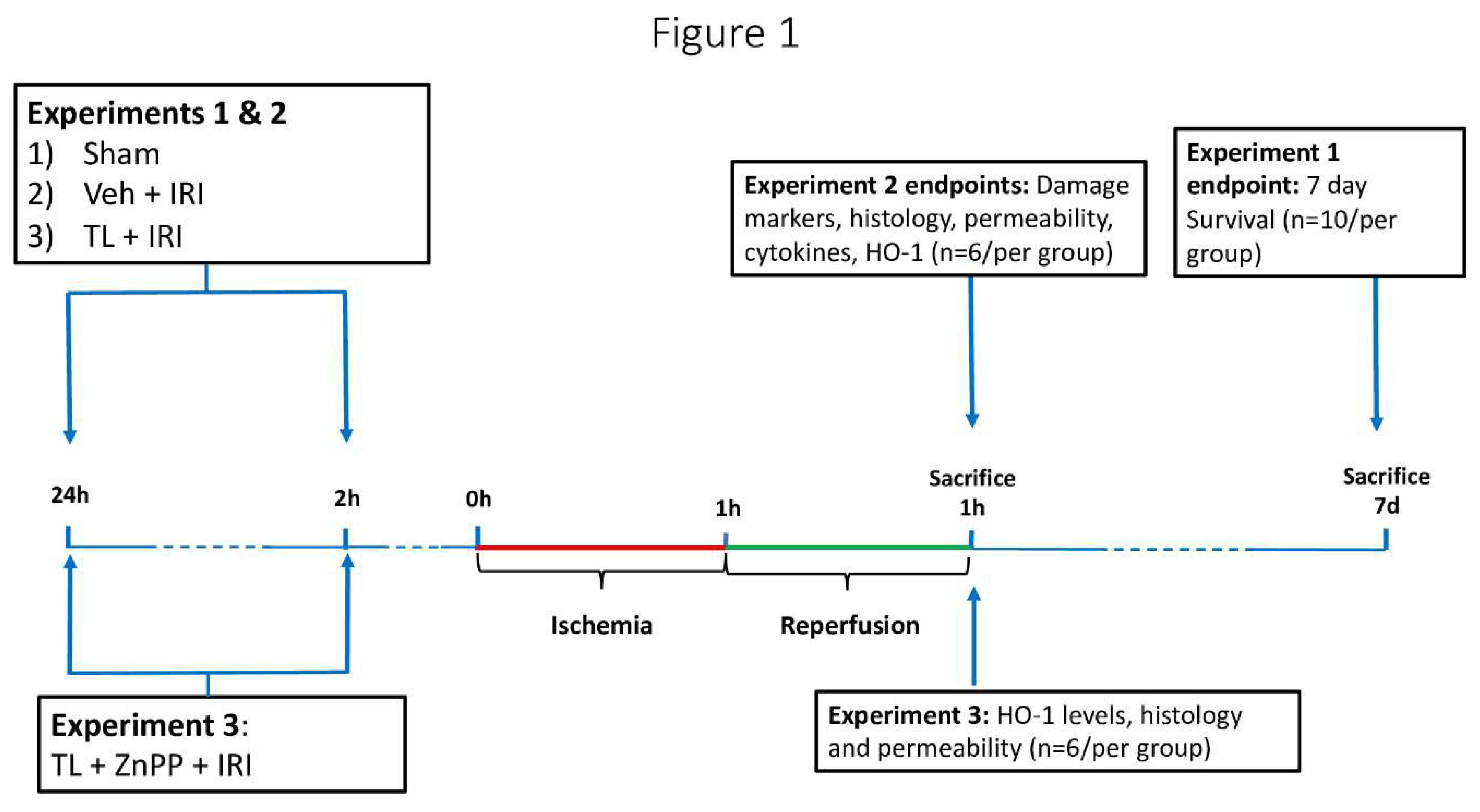
2.2. Experimental design
This study involved three separate experimental set-ups (
Figure 1).
Experiment 1: The effect of TL pretreatment (administration before induction of ischemia) on survival was studied. Three groups were studied (n=10 per group): 1/ sham, 2/Vehicle + IRI and 3/TL + IRI. After IRI, rats were observed for 7 days. In these animals, the abdomen was closed in two layers using non-resorbable sutures. The 7-day survivors were sacrificed for analysis. If an animal died during these 7 days, the cause was ascertained through necropsy.
Experiment 2: In these experiments, the effect of TL pretreatment on various endpoints related to intestinal wall structure and inflammation was assessed. Three groups were studied (n=6 per group): 1/ sham: anesthesia and surgery, without ischemia; 2/Vehicle + IRI: oral administration of vehicle (1.4% NaHCO3) 24 and 2 hours prior to start of intestinal ischemia, 60 minutes ischemia and 60 minutes reperfusion; and 3/ TL + IRI: oral administration of TL (650mg/kg) 24 and 2 hours prior to start of intestinal ischemia, 60 minutes ischemia and 60 minutes reperfusion. Dosing and timing of administration of TL were based on previously published data(17,26). Animals were sacrificed by exsanguination under general anesthesia after 60 minutes of reperfusion. The studied end-points were plasma biomarkers for enterocyte damage (L-lactate, Intestinal fatty acid-binding protein (I-FABP)), histology (Park-Chiu score), epithelial barrier function (Ussing chambers technique), endotoxin translocation (Limulus Amebocyte Lysate Pyrogent kit), expression of tissue pro- and anti-inflammatory cytokines (quantitative reverse-transcription polymerase chain reaction (qRT-PCR)), and expression of HO-1 levels (Western blot (WB)).
Experiment 3: Finally, the role of TL induced HO-1 upregulation was studied. In an additional group of rats, Zinc protoporphyrin (ZnPP) (Sigma-Aldrich, Belgium) - a powerful inhibitor of HO-1 upregulation was administered in addition to TL. ZnPP was administered via intraperitoneal injection at 20mg/kg, 24 hours prior to IRI. This dosing and timing were based on previous studies(27).
2.3. Sample Collection
After sacrifice, arterial blood was spun at 3500 rpm for 10 minutes and the isolated serum was stored at -80°C after snap freezing in liquid nitrogen. Following a standardized protocol, 5 segments of distal ileum were collected (one segment of 5 cm and 4 segments of 1 cm long, starting from the ileo-cecal valve). The long segment was stored in 10mM Glucose in Krebs-Ringer bicarbonate buffer and mounted in an Ussing chamber to measure permeability(28,29). Three segments were snap frozen for qRT-PCR and WB analysis. Finally, one segment was fixed in 4% neutral-buffered formalin for histological analysis.
2.4. Damage Biomarkers
A sample (100 µL) of arterial blood was analyzed for L-Lactate using a blood gas analyzer (ABL-815, Radiometer, Denmark). I-FABP, a marker of enterocyte damage, was measured by WB in the serum (see below)(30).
2.5. Histology
Ileal tissue samples were cut in 1 longitudinal and 2 transverse sections after fixation, properly oriented and embedded in paraffin. The tissue blocks were then cut into 5 µm-thick sections which were mounted on glass slides, stained with hematoxylin and eosin following the standard lab protocol, and coverslipped. The samples were analyzed, in a blinded fashion, by an experienced pathologist (GDH). The standardized analysis involved inspecting 4 separate fields as previously described(25). Each field was independently graded for IRI related damage by measuring the average villus length (from tip of the villi to the mouths of the crypts, measured in µm) and the validated Park-Chiu IRI scoring system (0-8)(25,31).
2.6. Epithelial Barrier Assessment
In order to evaluate the effect of IRI on intestinal wall integrity, three ileal segments were mounted in an Ussing chamber setup (Mussler Scientific Instruments, Aachen, Germany). The aim was to measure the trans-epithelial electrical resistance (TEER) as an objective parameter of epithelial intestinal permeability to ions (28,29). Briefly, the bowel segment was opened longitudinally and mounted in the setup with exposure to 10mM Mannitol and 10 mM Glucose in Krebs-Ringer bicarbonate buffers on the mucosal and serosal sides respectively.
The tissue was kept oxygenated and at 37°C. After 30 minutes stabilization, the average TEER was measured over a period of 90 minutes. TEER was corrected for villus length to compensate for areas of patchy necrosis. This was done by multiplying the original TEER by the ratio of the corresponding villus length by the mean sham villus length(25).
2.7. Bacterial Translocation
The plasma level of lipopolysaccharide (LPS) endotoxin levels was measured using a colorimetric Limulus Amebocyte Lysate (LAL QCL1000) Enzyme-Linked Immunosorbent Assay (ELISA) (Lonza, Switserland). Plasma LPS is a reflection of the transcellular epithelial passage of large molecules from the intestinal lumen to the blood.(32)
2.8. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
In these analyses, the relative expression of both pro-inflammatory cytokines (Interleukin (IL)-1β, IL-6, interferon (IFN)-γ), tumor necrosis factor (TNF)-α) and anti-inflammatory cytokines (IL-10 and IL-13) was determined. Stored ileal tissue was homogenized in TRIzol reagent (Life Technologies, CA, USA) and total RNA was isolated using RNeasy Minikit (Qiagen, Antwerp, Belgium) per instructions of the manufacturer. Next, c-DNA was created from 200ng RNA using M-MLV transcriptase (Life-Technologies, CA, USA). Finally, the relative expression of pro- and anti-inflammatory cytokines were determined by qRT-PCR using a LightCycler 96W (Roche, Vilvoorde, Belgium) with Taqman Fast Universal PCR Master Mix and Taqman Gene Expression Assays (Life-Technologies, CA, USA) (IL-6 (Rn01410330_m1), IL-1β (Rn00580432_m1), TNF-α, (Rn00562055) IFN-γ (Rn00594078), IL-10 (Rn00563409) and IL-13 (Rn00587615). Amplification was performed in three steps: 95 ̊C for 10 min followed by 45 cycles of amplification (95 ̊C for 10 sec, 60 ̊C for 15 sec, 72 ̊C for 10 sec) and finally a melting curve program. Target messenger RNA (mRNA) expression was quantified relative to the house- keeping gene GAPDH (Rn01775763_g1; Life Technologies, CA, USA).
Cytokine data are shown as fold changes compared to the sham data of their respective series (i.e. mean sham value is 1).
Western blot
Ileal (HO-1) and plasma samples (I-FABP) were used for these analyses. Ileal samples were first homogenized using RIPA buffer (50mM Tris/HCl, 150mM sodium chloride, 1% IPEGAL, 1 mM ethylenediamine tetra-acetic acid, 1% Protease Inhibitor Cocktail (Sigma- Aldrich, MO, USA), 1% Phosphatase Inhibitor Cocktail 2 and 3 (Sigma-Aldrich, MO, USA).
Protein concentration was measured by a standard Bradford assay (Sigma-Aldrich, MO, USA). Samples (50 μg protein) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using Any kDa Mini-Protean TGX Precast Gels (Bio-Rad, CA, USA) and proteins were blotted on polyvinylidene difluoride (PVDF) membranes using the Transblot Turbo system (Bio-Rad, CA, USA).
For the plasma samples, the membranes were incubated with 0.1% Ponceau S staining solution. The membranes were then blocked for 1 hour at room temperature with PBS-Tween (0.1%) containing 5% milk powder and incubated with the primary antibody (FABP2 (21252-1-AP, Proteintech Europe); HO-1; GAPDH (G8795, Sigma-Aldrich, MO, USA) antibody overnight at 4 ̊C. After a 1-hour incubation with the secondary antibody (anti-rabbit IgG HRP-linked antibody for FABP2 and HO-1 and anti-mouse IgG HRP-linked antibody (all Cell Signaling Technologies, MA, USA), the proteins were detected using enhanced chemiluminescence (Pierce ECL Western Blotting Substrate) and digital detection with the Chemidoc MP system. Quantification of relative band intensity was then performed with the associated ImageLab software (Bio-Rad, CA, USA).
2.9. Statistical Analysis
The data in the text is shown as mean + standard deviation. All data were tested for normality using the Kolmogorov-Smirnov test. Multiple group comparisons were performed using One-Way Anova and post-hoc Tukey test in case of normal distribution or Kruskal-Wallis with post-hoc Dunn test for non-normal distribution. Kaplan-Meier estimator was used for survival analysis (log-rank test). A p value <0.05 was considered statistically significant. All data sets were screened for outliers using the Robust regression and OUTlier removal (ROUT) technique using a coefficient of 5%(33). In graphs, the middle line represents the mean and the whiskers indicate the standard error of the mean. GraphPAD Prism version 9.0 (San Diego, CA, USA) was used for all analyses and graphs.
3. Results
3.1. Experiment 1: Effect of Tranilast Pretreatment on Survival
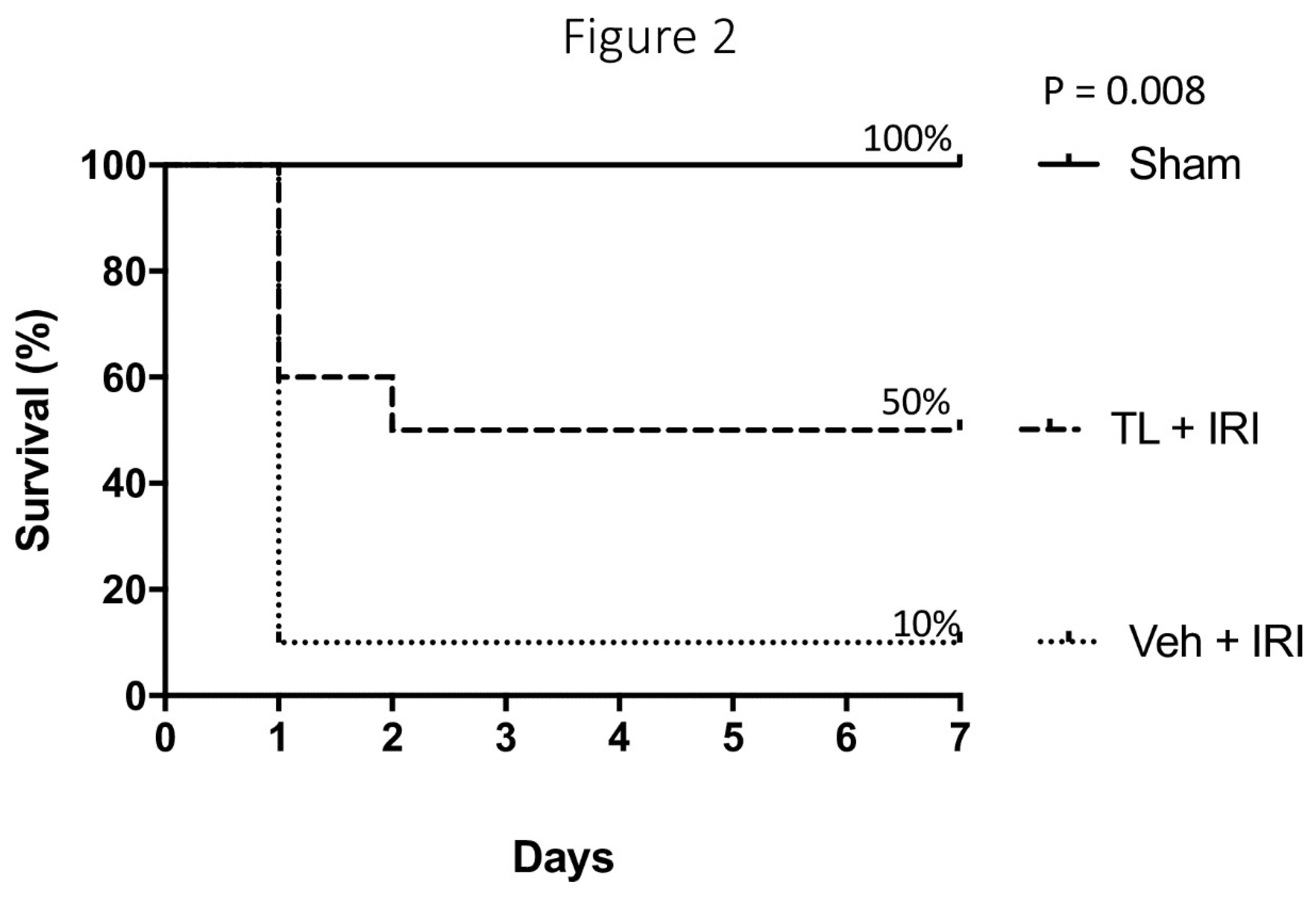
TL Pretreatment Improved Seven-Day Survival After IRI
All sham rats survived the surgery and promptly recovered. IRI led to severe sepsis and early death (<24 hours) in 9 out of 10 rats (
Figure 2). All these animals succumbed to multi-organ failure and sepsis. In contrast, 5 rats pre-treated with TL survived for 7 days (p=0.008). Four died within 24 hours (severe sepsis) and one died at day 2 (intestinal perforation). Necropsy revealed a profoundly necrotic bowel with several perforations in all rats that died before 7 days. This extreme damage of the bowel wall led to endotoxin translocation and death. At sacrifice after 7 days, all surviving rats from the sham group had a normal aspect of the bowel. The surviving rat from the IRI-group presented with a generally normal aspect of the bowel but with severe adhesions.
3.2. Experiment 2: Effect of Tranilast Pretreatment on Morphology, Barrier Function and Immune Activation
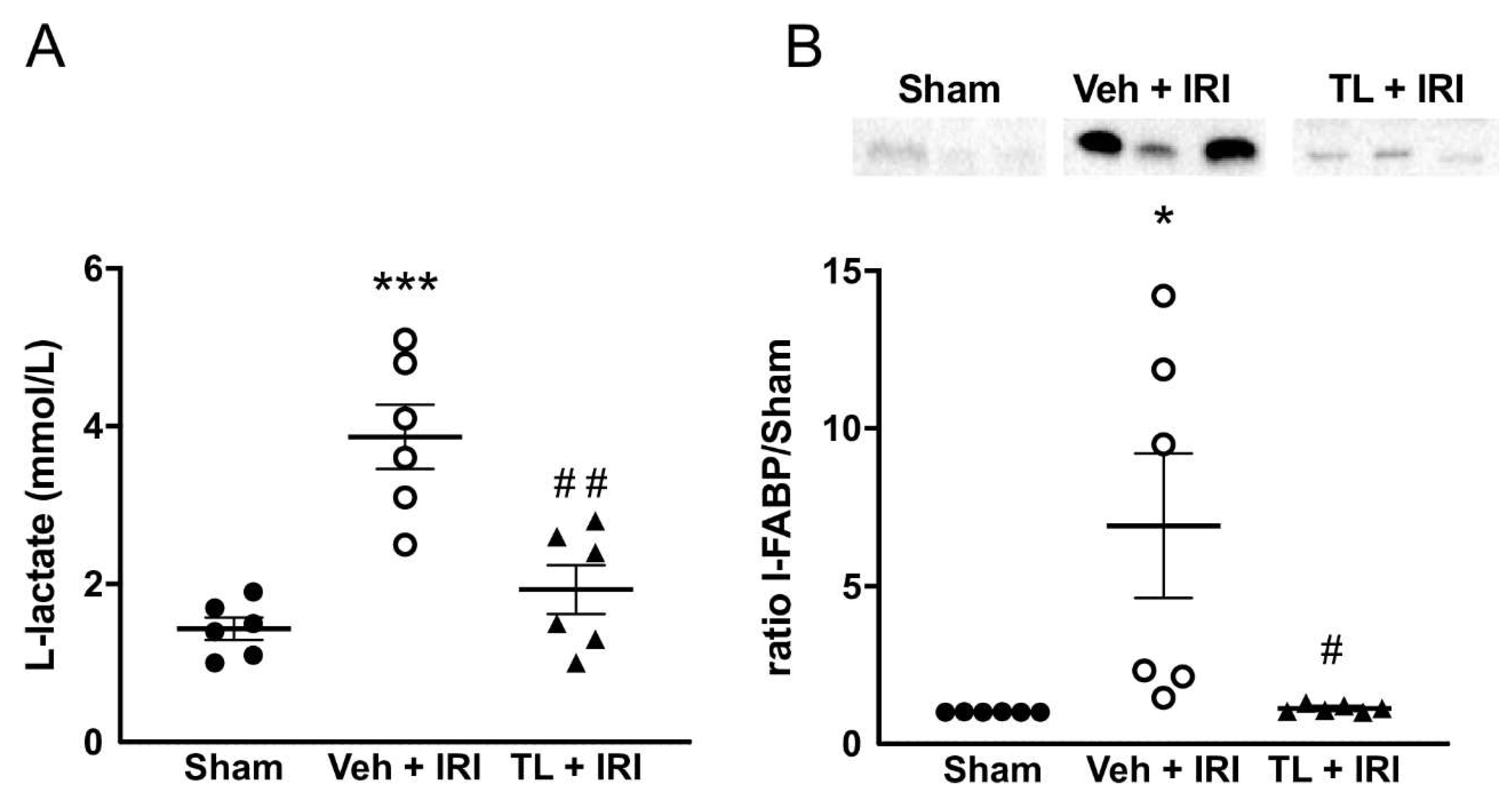
3.2.1. TL Pretreatment Reduced IRI Induced Epithelial Damage
L-lactate levels were increased after IRI compared to the sham group (3.87mmol/L ±1.00 vs. 1.43±0.34 mmol/L, p<0.0005) (
Figure 3A). Pretreatment with TL reduced circulating L-lactate levels to near-to-normal values (1.93±0.76 mmol/L, p=0.012).
I-FABP showed a similar pattern as L-lactate. Its expression was very low in the sham rats, but it was higher after IRI (6.91±5.62 fold increased, p=0.0171). TL pretreatment led to a marked reduction to levels equal to the sham group (1.13±0.11, p=0.0192 compared IRI group) (
Figure 3B).
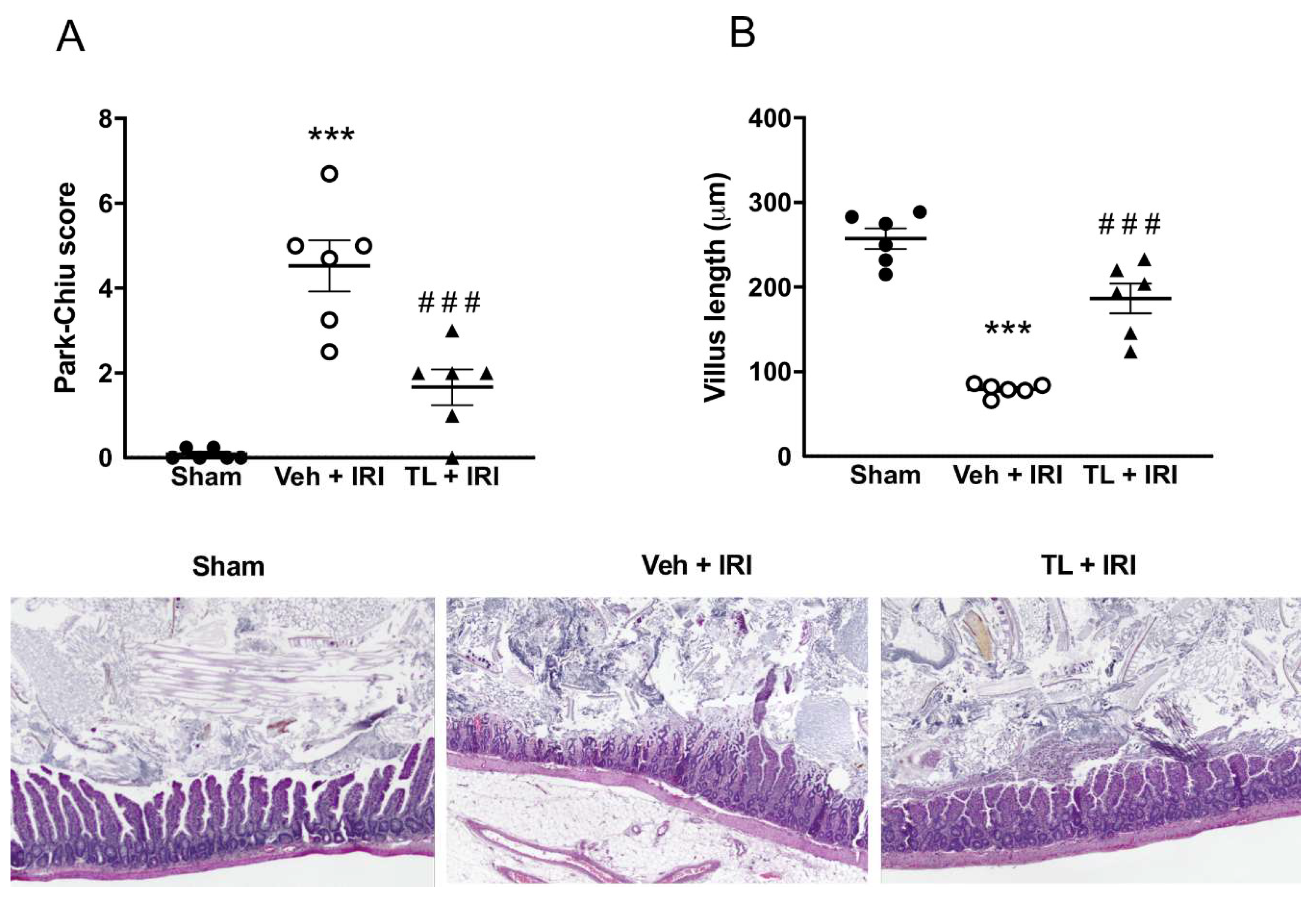
3.2.2. TL Pretreatment Reduced IRI Induced Intestinal Wall Damage
Sham rats had normal intestinal histology, with a Park-Chiu score of 0 and a villus length of 257μm±30 (
Figure 4A-B). Intestinal IRI led to pronounced damage to the intestinal wall, resulting in a Park-Chiu score of 4.53±1.48 which was reduced in the TL+IRI group (1.67±1.03, p=0.0007). Similarly, IRI induced damage which resulted in a marked reduction in villus length (79μm±7, p<0.0005 compared to sham). Villus length was preserved in TL pretreated rats (187μm ±43, p<0.0005 compared to vehicle). On classic Hematoxylin and Eosin staining, IRI resulted in loss of villus integrity and severe interstitial edema (
Figure 4C). This was attenuated in the TL TL+IRI group.
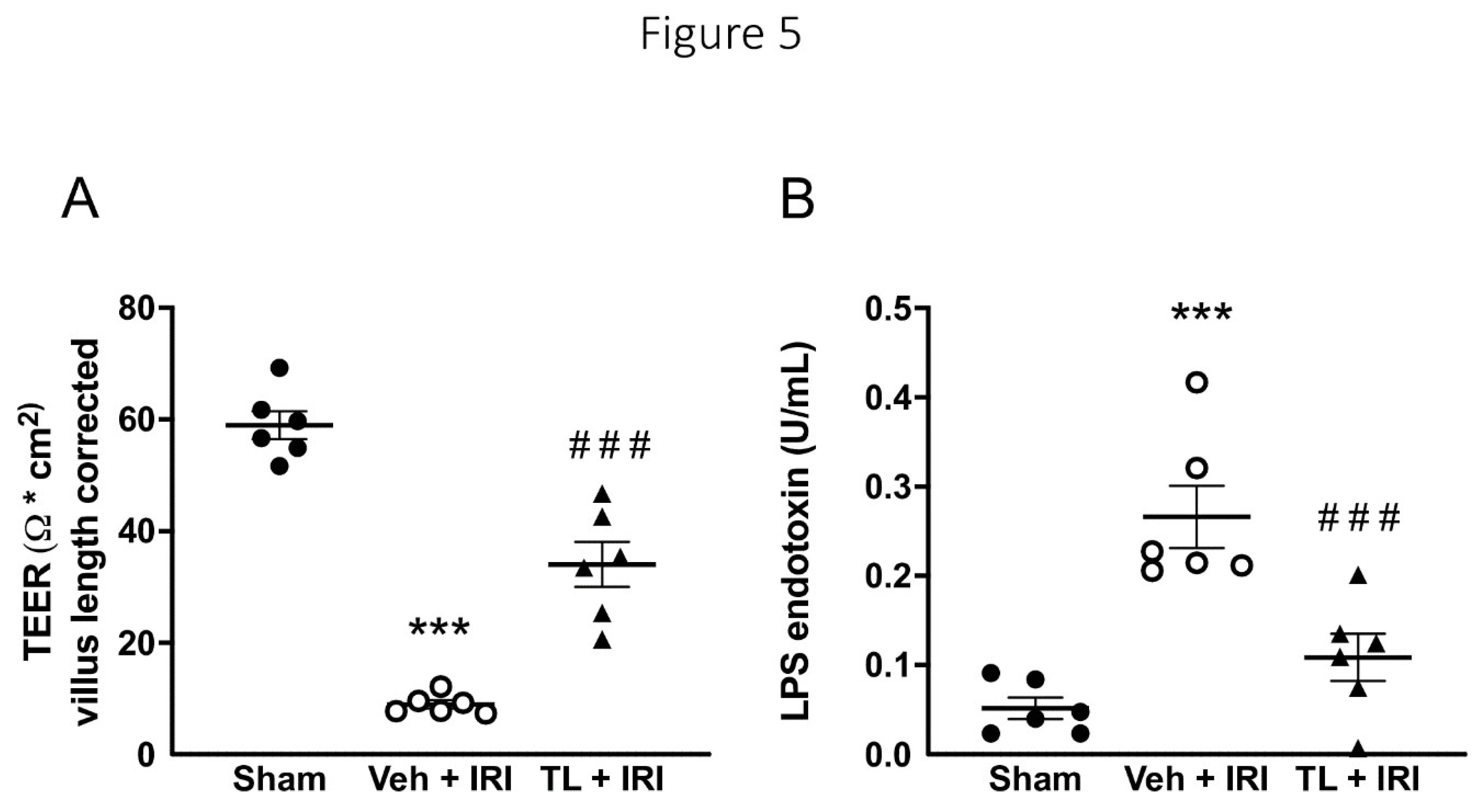
3.2.3. TL Pretreatment Reduced Epithelial Permeability of the Intestine
Mirroring the morphological results, IRI also increased the epithelial paracellular permeability as shown by a reduced TEER. TEER was lower in the vehicle group compared to sham (9 ±2 vs. 59 ±6 Ω*cm
2, p<0.0005) (
Figure 5A). TL pretreatment led to an increase in TEER (34Ω*cm
2 ±10, p<0.0005
vs. vehicle). Moreover, we assessed the transcellular permeability by measuring circulating endotoxin. Translocation of endotoxin increased after IRI (0.27U/L ±0.09
vs. 0.05U/L±0.03 in the sham group, p<0.0005). TL pretreatment led to lower endotoxin levels (0.11U/L ±0.06, p=0.0019) compared to vehicle (
Figure 5B).
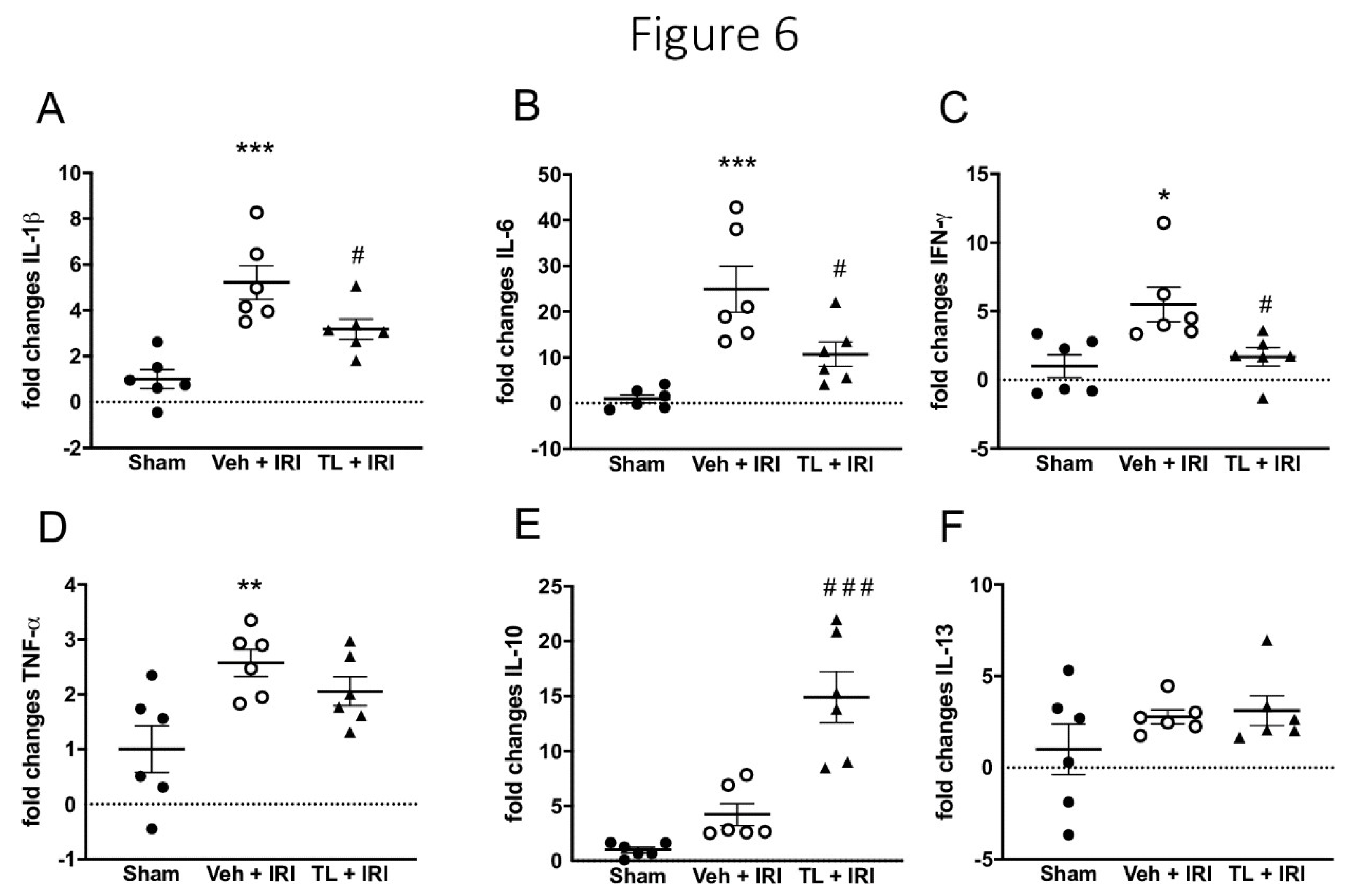
3.2.4. TL Pretreatment Reduced Pro-Inflammatory and Increased Anti-Inflammatory Cytokines
Intestinal IRI led to increased inflammation as evidenced by an upregulation of i) IL-1β in ileal samples (5.22±1.82 vs.3.18±1.07 fold; p<0.0005), and ii) plasmatic IL-6 (24.94±12.37 vs. 10.71±6.57) when compared to the sham groups (1±0.93 and 1±2,22 respectively, p<0.0005 for both). TL pretreatment led to reduction in both IL-1β (3.18±1.07, p=0.049) and IL-6 (9.71±6.57, p=0.023) (
Figure 6A and B).
Pro-inflammatory IFN-γ was also increased in the IRI group (5.51±3.1 vs.1.0±2.02; p=0.011) and TL reduced this to 1.67±1.64 (p=0.031 compared to vehicle) (
Figure 6C). TNF-α was increased in the IRI group (2.57±0.60) compared to sham (1±1.05, p=0.01). However, there was no significant difference between TL group (1.06±0.64) and both the vehicle (p=0.51) or the sham group (p=0.085) (
Figure 6D).
The expression of the anti-inflammatory cytokine IL-10 was higher in the TL+IRI group (14.91±5.71) while levels remained low in the IRI group (4.22±2.45, p<0.0005) (
Figure 6E). IL-13 was not different in any of the groups (Sham: 1±3.38; IRI: 2.78±0.93 and TL+IRI; 3.12±1.98) (
Figure 6F).
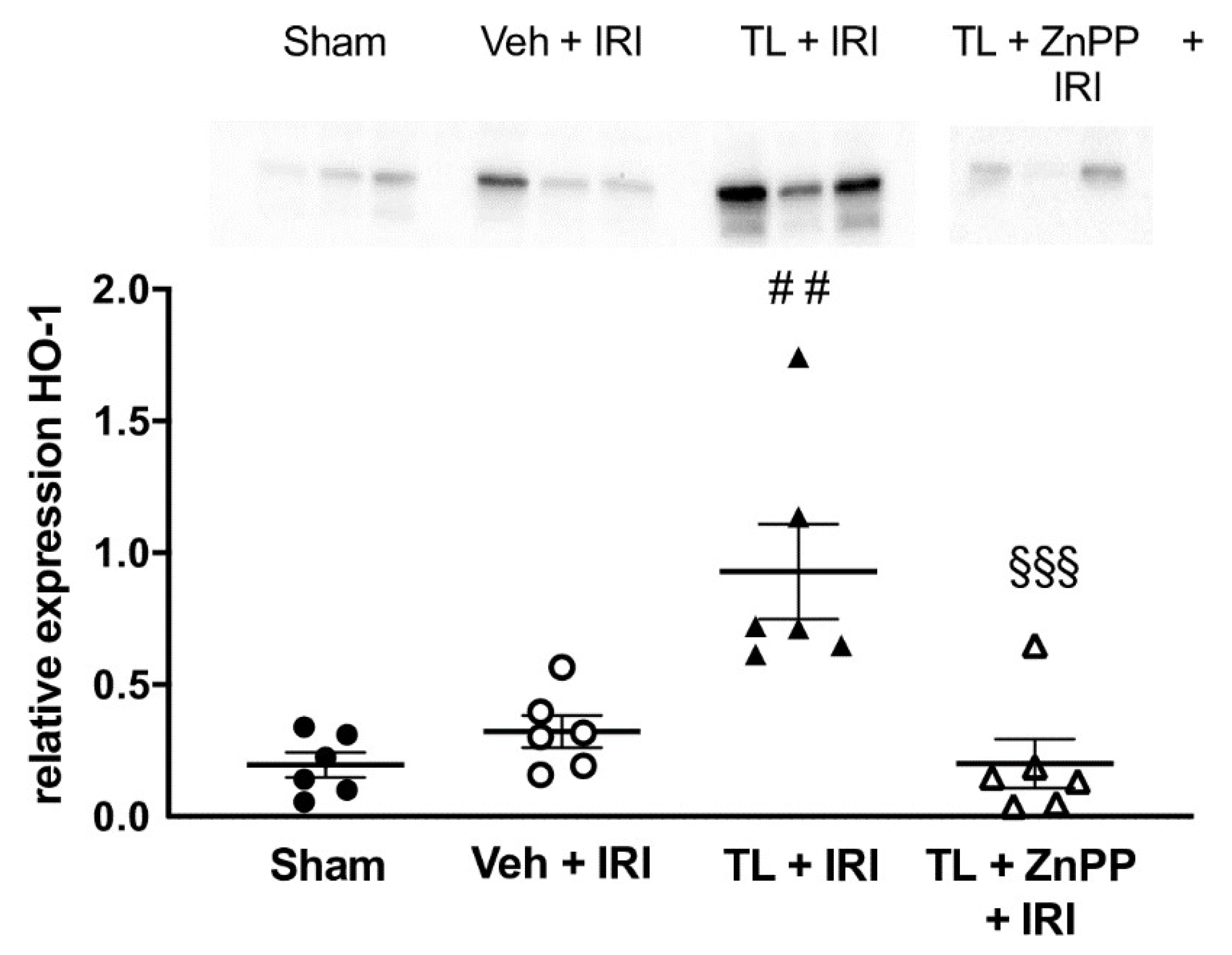
3.2.3. TL Led to Upregulation of HO-1 in the Ileal Tissue
In the vehicle-treated IRI group, HO-1 levels were comparable to the results in the sham operated animals (0.34±0.18 vs. 0.20±0.12; p=0.66). Pretreatment with TL led to an increased expression of HO-1 in the ileum (0.93±0.44) compared to both the sham (p=0.001) and the vehicle-treated IRI group (p=0.005) (
Figure 7).
3.3. Experiment 3: Effect of HO-1 Inhibition
ZnPP abolishes the TL-Induced Upregulation of HO-1
Administration of ZnPP in addition to Tranilast (TL + ZnPP + IRI) blocked the upregulation of HO-1 to 0.20 ± 0.23 which was not different compared to IRI while at the same time being lower than the TL + IRI group (0.93±0.44, p<0.001) (
Figure 7).
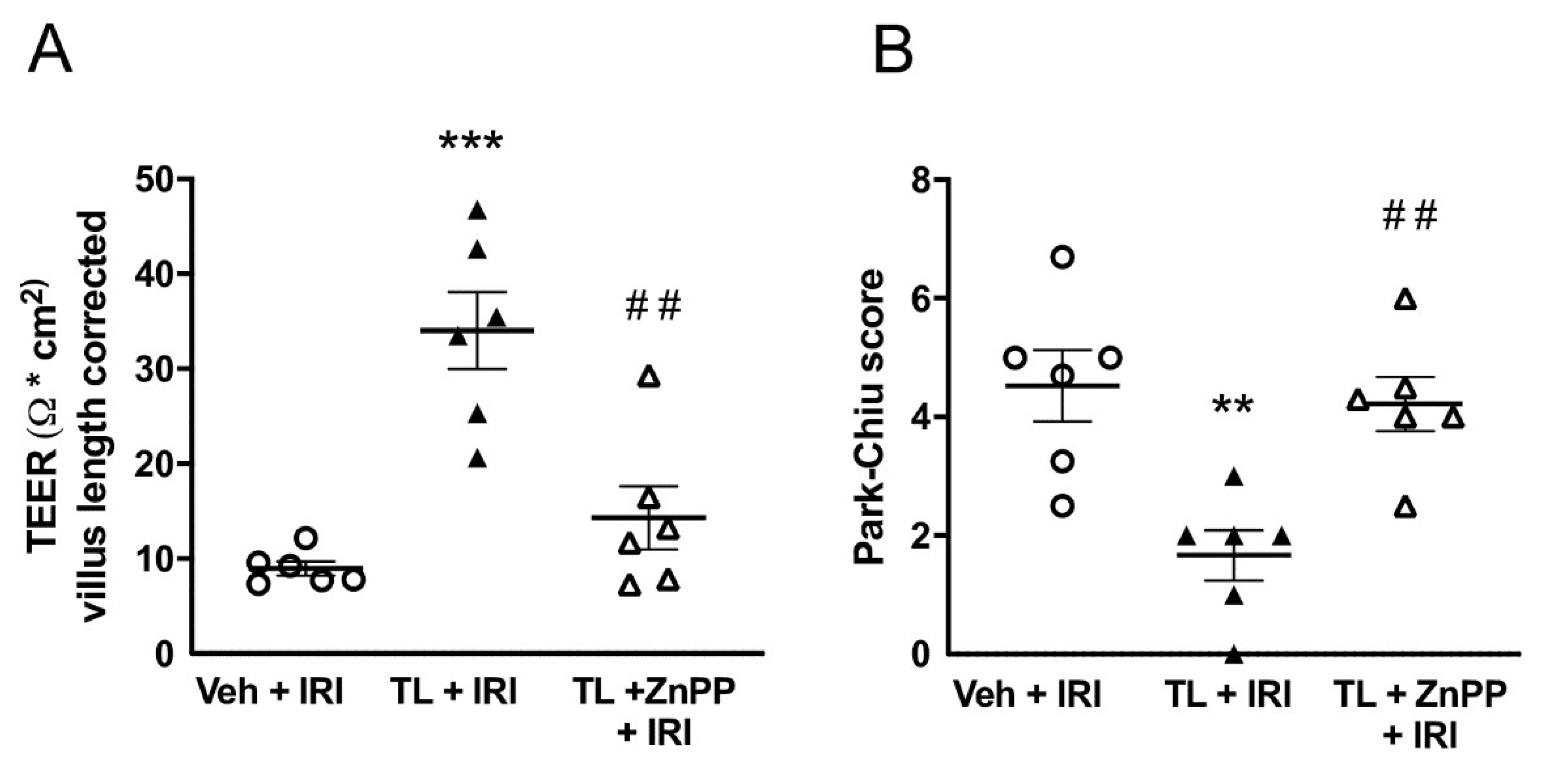
3.4. Inhibiting HO-1 Upregulation aBolished the Protective Effect of TL Pretreatment
ZnPP administration limited the protective effect of TL on the intestinal barrier function by reducing the TEER to the same levels as in the IRI group (14±8 Ω*cm
2 vs. 9±2 Ω*cm
2; p=0.45) (
Figure 8A). Similarly, the histological damage, illustrated by the Park-Chiu score, became comparable to the vehicle-treated IRI group (5.22±1.82 vs. 4.53±1.48; p=0.90) (
Figure 8B).
4. Discussion
Intestinal ischemia is a devastating pathology, with an increasing prevalence due to an aging population(34). Damage to the intestine leads to increased permeability, translocation of luminal contents and ultimately fatal sepsis(35). For this reason, intestinal IRI is more dangerous compared to IRI in other organs. This is also the reason why ITx remains very challenging, as IRI is inevitable during the transplantation process where the resulting inflammation primes the recipient immune system, increasing the risk of rejection(36).
Our data show that TL pretreatment protects the intestine against IRI. TL consistently improves the 7-days survival from 10% to 50%. This improvement is accompanied by a reduction of both intestinal damage and the ensuing inflammation, both structurally and functionally. Moreover, our data strongly suggests that the enzyme HO-1 mediates partly the protective effect of TL.
TL was first described in 1975 as an anti-allergic drug(37). This synthetic analog was derived from anthranilic acid, a metabolite of the indoleamine-pyrrole 2,3-dioxygenase induced tryptophan degradation(38). TL is used in the clinic for the treatment of allergic dermatitis, rheumatoid arthritis and structuring Crohn’s disease(39–41). More recently, its anti-inflammatory properties have been recognized. However, until now its potential protective effect in intestinal ischemia has not been investigated. In this study, TL reduced the levels of pro-inflammatory cytokines (especially IL-1β, IL-6 and IFN-γ) and upregulated the anti-inflammatory cytokine IL-10. We demonstrated a significant amelioration of the epithelial barrier function of the small intestine resulting in reduced endotoxin translocation and improved survival.
HO-1 upregulation seems to be the most likely protective mechanism of TL. This was demonstrated in this study, as blocking HO-1 with ZnPP prevented the protective effect of TL.
Firstly, HO-1 is a highly conserved anti-inflammatory mediator present in all cells and protects against IRI injury in several tissues, including kidney, heart and lung(27,42,43). HO-1 is an enzyme that catalyzes the degradation of heme to biliverdin, ferrous iron, and carbon monoxide (CO). HO-1 is the primary source for endogenous CO production. Low concentrations of CO have anti-oxidant, anti-inflammatory and anti-apoptotic properties(44). HO-1 blocks the pro-inflammatory NF-κB pathway, crucial in systemic inflammation and IRI(19). Specifically, TL activates the mitogen-activated protein kinase (MAPK) pathways which leads to HO-1 production. In vivo and in vitro studies have shown that HO-1, along with its major byproduct CO, inhibits pro-inflammatory cytokine expression and upregulates IL-10 (45).
Secondly, both HO-1 and CO are powerful anti-oxidants, able to counter the effects of IRI induced ROS formation(46). Furthermore, endotoxin and bacterial translocation leading to sepsis causes mortality after IRI. Chung et al.(47) induced fecal peritonitis in mice through a standardized cecal puncture. They showed that HO-1 deficient mice were much less resistant to the resulting peritonitis. In contrast, overexpression of HO-1 improved the ability of macrophages to clear the invading bacteria and to counteract the infection. The ability of CO, produced by the enzymatic action of HO-1, to improve phagocytosis has also been demonstrated by other groups(48,49). This could add a ‘second line of defense’ to overcome the effects of IRI if the intestinal barrier is breached.
Finally, TL is currently primarily used as a mast cell stabilizer for anti-allergic therapy(17). However, mast cell degranulation also plays an important role in IRI as the release of histamine, leukotrienes, and platelet-activating factors initiate part of the damaging cascade. In a rat model of intestinal IRI, mast cell deficient knock-out rats had significantly improved histology and mucosal permeability compared to wild type controls(50). In this model, we did not measure mast cell activation which could be investigated in future experiments.
The protective role of HO-1 upregulation in intestinal IRI has previously been demonstrated in two rat models (51,52). The first used a subcutaneous hemin (an HO-1 inducing agent) injection 2 hours prior to ischemia and showed improved histology and recovery of transit after 6 hours. However, no difference in myeloperoxidase activity (a marker of neutrophil activation) was noted between the experimental groups. No survival or inflammatory markers were reported. In the second study, another HO-1 inducing agent (cobalt protoporphyrin) was administered intraperitoneally 24 hours prior to intestinal IRI. This resulted in reduced IL-6 production, histological damage and myeloperoxidase activity. In our experiment we showed that TL-induced HO-1 upregulation also positively influenced survival, permeability, other cytokines (both pro- and anti-inflammatory) and endotoxin translocation. Furthermore, in contrast to hemin and cobalt protoporphyrin, TL has been used clinically for more than 40 years, making the use of an HO-1 therapy more feasible in the clinical practice.
Having shown that TL is effective as a pre-treatment option, the next question to address will be its efficacy as a treatment approach when given after the onset of ischemia. For this, intravenous administration of TL will be mandatory and an important hurdle is the relatively low solubility of TL in water and its poor bio-availability, requiring high dosing(17). However, this has been addressed by a wet-milling technique leading to a new TL formulation based on nano-crystalline solid dispersion which resulted in a significant improvement in both solubility and bio-availability(53). In future studies, this highly soluble version of TL could be used to further explore its potential as an actual treatment drug for intestinal ischemia.
TL could also be of particular interest in ITx, not only by reducing IRI induced damage in the transplanted bowel but also by promoting CD4+ T-regs. T cells play a crucial role in intestinal graft rejection. The activation and infiltration of cytotoxic T cells into the graft causes rejection(54). In contrast, our group showed that expansion of T-regs (subtype: CD4+-CD25+-FoxP3) can limit intestinal graft rejection in clinical ITx(55). The ability of TL to induce CD4+ T-regs production and reduce rejection demonstrated in rat models of skin and cornea transplantation(56). TL induced T-cell cycle arrest by stimulating the cell cycle-specific inhibitors p21 and p15. However, T-regs were spared from this effect and their number actually increased which may be due to differential stimulation of the aryl-hydrocarbon receptor by TL(57,58). This immunomodulatory effect of TL has also been shown in other pre-transplant clinical models, including liver and heart(26,59) and could be of particular interest in ITx.
In conclusion, we demonstrated that pretreatment with TL improves survival in a rat model of intestinal IRI and that this was accompanied by a substantial containment of inflammation, preservation of the intestinal barrier function, and reduced endotoxin translocation. We also showed that the protective effect of TL can be attributed to HO-1 upregulation. Future studies should investigate the efficacy of intravenously administered TL as a treatment approach for intestinal ischemia.
Conflicts of Interest Statement:
There are no conflicts of interest to report from any of the authors.
Disclosures:
EC is supported by an unrestricted ESOT (European Society of Transplantation) Transplant Fellowship Grant. TV is a senior clinical researcher supported the Flanders Research Foundation (FWO Vlaanderen). JP holds named chairs at the KU Leuven from the Institut Georges Lopez and from the “Centrale Afdeling voor Fractionering” (DGF-CAF). LJC holds a named chair at KU Leuven sponsored by Medtronic and is supported by a post-doctoral research grant from the University Hospitals Leuven (KOOR).
Author Contributions
Conception and design of the work: EC, RF, LC, Literature review: EC, RF, JP, LC Assistance in writing: EC, RF, GDH, AD, TV, JP, LC, Critical revision for important intellectual content: EC, RF, GDH, AD, TV, JP, LC. Approval final draft: EC, RF, GDH, AD, TV, JP, LC
Acknowledgments
We would like to acknowledge lab technicians Veerle Heedfeld and Tine Wylin for their technical support.
Abbreviations
| CO |
carbon monoxide |
| HO-1 |
heme oxygenase-1 |
| I-FABP |
intestinal fatty acid-binding protein |
| IFN- γ |
interferon- γ |
| IL |
interleukin |
| IRI |
ischemia reperfusion injury |
| ITx |
intestinal transplantation |
| LPS |
lipopolysaccharide |
| qRT-PCR |
quantitative reverse-transcription polymerase chain reaction |
| ROS |
reactive oxygen species |
| T-regs |
regulatory T cells |
| TEER |
trans-epithelial electrical resistance |
| TL |
tranilast |
| TNF-α |
tumor necrosis factor-α |
| WB |
western blot |
| ZnPP |
zinc protoporphyrin |
References
- Duggan, C.; Gannon, J.; Walker, W.A. Protective nutrients and functional foods for the gastrointestinal tract. Am. J. Clin. Nutr. 2002, 75, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Alterations in intestinal permeability. Gut 2006, 55, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. REVIEW: Ischemia–Reperfusion Injury of the Intestine and Protective Strategies Against Injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef] [PubMed]
- Acosta S, Ögren M, Sternby NH, Bergqvist D, Björck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: Autopsy findings in 213 patients. Ann Surg [Internet]. 2005 Mar [cited 2020 Aug 14];241(3):516–22.
- Acosta, S. Epidemiology of Mesenteric Vascular Disease: Clinical Implications. Semin. Vasc. Surg. 2010, 23, 4–8. [Google Scholar] [CrossRef]
- Howard, T.J.; Plaskon, L.A.; Wiebke, E.A.; Wilcox, M.G.; Madura, J.A. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am. J. Surg. 1996, 171, 405–408. [Google Scholar] [CrossRef]
- Acosta, S.; Ögren, M.; Sternby, N.; Bergqvist, D.; Björck, M. Fatal nonocclusive mesenteric ischaemia: population-based incidence and risk factors. J. Intern. Med. 2006, 259, 305–313. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Schoots, I.G.; I Koffeman, G.; A Legemate, D.; Levi, M.; van Gulik, T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br. J. Surg. 2003, 91, 17–27. [Google Scholar] [CrossRef]
- Björck, M.; Koelemay, M.; Acosta, S.; Goncalves, F.B.; Kölbel, T.; Kolkman, J.; Lees, T.; Lefevre, J.; Menyhei, G.; Oderich, G.; et al. Editor's Choice – Management of the Diseases of Mesenteric Arteries and Veins. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 460–510. [Google Scholar] [CrossRef]
- Roussel, A.; Castier, Y.; Nuzzo, A.; Pellenc, Q.; Sibert, A.; Panis, Y.; Bouhnik, Y.; Corcos, O. Revascularization of acute mesenteric ischemia after creation of a dedicated multidisciplinary center. J. Vasc. Surg. 2015, 62, 1251–1256. [Google Scholar] [CrossRef]
- Kong, S.; Blennerhassett, L.R.; Heel, K.A.; McCauley, R.D.; Hall, J.C. ISCHAEMIA-REPERFUSION INJURY TO THE INTESTINE. ANZ J. Surg. 1998, 68, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Ogura, J.; Tsujimoto, T.; Kuwayama, K.; Koizumi, T.; Sasaki, S.; Maruyama, H.; Kobayashi, M.; Yamaguchi, H.; Iseki, K. Intestinal P-glycoprotein Expression is Multimodally Regulated by Intestinal Ischemia-Reperfusion. J. Pharm. Pharm. Sci. 2014, 17, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.; Abu-Elmagd, K.; Mazariegos, G.; Vianna, R.; Langnas, A.; Mangus, R.; Farmer, D.G.; Lacaille, F.; Iyer, K.; Fishbein, T. Intestinal Transplant Registry Report: Global Activity and Trends. Am. J. Transplant. 2014, 15, 210–219. [Google Scholar] [CrossRef]
- Grootjans J, Lenaerts K, Derikx JPM, Matthijsen R a, de Bruïne AP, van Bijnen A a, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol [Internet]. 2010 May [cited 2014 Dec 6];176(5):2283–91.
- Lenaerts, K.; Ceulemans, L.J.; Hundscheid, I.H.; Grootjans, J.; Dejong, C.H.; Damink, S.W.O. New insights in intestinal ischemia–reperfusion injury. Curr. Opin. Organ Transplant. 2013, 18, 298–303. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and Clinical Aspects of Heme Oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Pae, H.-O.; Jeong, S.-O.; Koo, B.S.; Ha, H.-Y.; Lee, K.-M.; Chung, H.-T. Tranilast, an orally active anti-allergic drug, up-regulates the anti-inflammatory heme oxygenase-1 expression but down-regulates the pro-inflammatory cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW264.7 macrophages. Biochem. Biophys. Res. Commun. 2008, 371, 361–365. [Google Scholar] [CrossRef]
- Tan, S.M.; Zhang, Y.; Cox, A.J.; Kelly, D.J.; Qi, W. Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol. Dial. Transplant. 2010, 26, 100–110. [Google Scholar] [CrossRef]
- Şener, G.; Akgün; Şatıroğlu, H.; Topaloğlu, U.; Uysal, K. The effect of pentoxifylline on intestinal ischemia/reperfusion injury. Fundam. Clin. Pharmacol. 2001, 15, 19–22. [CrossRef]
- Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity [Internet]. Vol. 23, Seminars in Immunology. NIH Public Access; 2011 [cited 2020 Aug 17]. p. 202–8.
- Wang CH, Shao K, Wang XH, Xu D, Zhou PJ, Zhan JM, et al. Effects of Tranilast on renal structure and function in mice with ischemic reperfusion injury. Vol. 32, Journal of Shanghai Jiaotong University (Medical Science). 2012. p. 446–51.
- Zhuo Y, Zhuo J. Tranilast Treatment Attenuates Cerebral Ischemia-Reperfusion Injury in Rats Through the Inhibition of Inflammatory Responses Mediated by NF-κB and PPARs. Clin Transl Sci. 2019;12(2):196–202.
- Ceulemans, L.J.; Verbeke, L.; Decuypere, J.-P.; Farré, R.; De Hertogh, G.; Lenaerts, K.; Jochmans, I.; Monbaliu, D.; Nevens, F.; Tack, J.; et al. Farnesoid X Receptor Activation Attenuates Intestinal Ischemia Reperfusion Injury in Rats. PLOS ONE 2017, 12, e0169331. [Google Scholar] [CrossRef]
- Ménoret, S.; Bézie, S.; Li, X.-L.; Usal, C.; Caron, L.; Anegon, I. Tranilast, an analogue of tryptophan catabolites, induces allograft tolerance by CD161+ cells. J. Transl. Med. 2011, 9, P27–P27. [Google Scholar] [CrossRef]
- Xia, Z.-Y.; Gao, J.; Ancharaz, A.K. Protective effect of ischemic postconditioning on lung ischemia-reperfusion injury in rats and the role of heme oxygenase-1. . 2009, 12, 162–6. [Google Scholar] [PubMed]
- Clarke, LL. A guide to Ussing chamber studies of mouse intestine. Vol. 296, American Journal of Physiology - Gastrointestinal and Liver Physiology. 2009.
- Thomson, A.; Smart, K.; Somerville, M.S.; Lauder, S.N.; Appanna, G.; Horwood, J.; Raj, L.S.; Srivastava, B.; Durai, D.; Scurr, M.J.; et al. The Ussing chamber system for measuring intestinal permeability in health and disease. BMC Gastroenterol. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thuijls G, Wijck K Van, Grootjans J, Joep P. Early Diagnosis of Intestinal Ischemia Using Urinary and Plasma Fatty Acid Binding Proteins. Ann Surg. 2011;253(2):303–8.
- Park, P.; Haglund, U.; Bulkley, G.; Falt, K. THE SEQUENCE OF DEVELOPMENT OF INTESTINAL TISSUE-INJURY AFTER STRANGULATION ISCHEMIA AND REPERFUSION. 1990, 107, 574–580.
- Farré, R.; Vicario, M. Abnormal barrier function in gastrointestinal disorders. Handb Exp Pharmacol [Internet]. 2017 Jan 1 [cited 2020 Dec 13];239:193–217.
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Kärkkäinen, J.M.; Lehtimäki, T.T.; Manninen, H.; Paajanen, H. Acute Mesenteric Ischemia Is a More Common Cause than Expected of Acute Abdomen in the Elderly. J. Gastrointest. Surg. 2015, 19, 1407–1414. [Google Scholar] [CrossRef]
- Nadatani Y, Watanabe T, Shimada S, Otani K, Tanigawa T, Fujiwara Y. Microbiome and intestinal ischemia/reperfusion injury [Internet]. Vol. 63, Journal of Clinical Biochemistry and Nutrition. The Society for Free Radical Research Japan; 2018 [cited 2020 Aug 14]. p. 26–32.
- Lenaerts K, Ceulemans LJ, Hundscheid IHR, Grootjans J, Dejong CHC, Damink SWMO. New insights in intestinal ischemia-reperfusion injury: Implications for intestinal transplantation. Curr Opin Organ Transplant [Internet]. 2013 Jun [cited 2015 Oct 29];18(3):298–303.
- Koda, A,.; Nagai, H,.; Watanabe S, Yanagihara Y, Sakamoto K. Inhibition of hypersensitivity reactions by a new drug, N(3′,4′-dimethoxycinnamoyl) anthranilic acid (N-5′). J Allergy Clin Immunol. 1976 May 1;57(5):396–407.
- Bauer, T.M.; Jiga, L.P.; Chuang, J.-J.; Randazzo, M.; Opelz, G.; Terness, P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 2005, 18, 95–100. [Google Scholar] [CrossRef]
- Azuma H, Banno K, Yoshimura T. Pharmacological properties of N-(3’,4’-dimethoxycinnamoyl) anthranilic acid (N-5’), a new anti-atopic agent. Br J Pharmacol [Internet]. 1976;58(4):483–8.
- Shiota, N.; Kovanen, P.; Eklund, K.; Shibata, N.; Shimoura, K.; Niibayashi, T.; Shimbori, C.; Okunishi, H. The anti-allergic compound tranilast attenuates inflammation and inhibits bone destruction in collagen-induced arthritis in mice. Br. J. Pharmacol. 2010, 159, 626–635. [Google Scholar] [CrossRef]
- Oshitani, N.; Yamagami, H.; Watanabe, K.; Higuchi, K.; Arakawa, T. Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn's disease. Gut 2007, 56, 599–600. [Google Scholar] [CrossRef]
- Wagner, M.; Cadetg, P.; Ruf, R.; Mazzucchelli, L.; Ferrari, P.; Redaelli, C.A. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003, 63, 1564–1573. [Google Scholar] [CrossRef]
- Liu, X.; Wei, J.; Peng, D.H.; Layne, M.D.; Yet, S.-F. Absence of Heme Oxygenase-1 Exacerbates Myocardial Ischemia/Reperfusion Injury in Diabetic Mice. Diabetes 2005, 54, 778–784. [Google Scholar] [CrossRef]
- Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications [Internet]. Vol. 86, Physiological Reviews. Physiol Rev; 2006 [cited 2020 Dec 13]. p. 583–650.
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Cheng Y, Rong J. Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury. Curr Pharm Des [Internet]. 2017 Oct 3 [cited 2020 Aug 15];23(26).
- Chung, S.W.; Liu, X.; Macias, A.A.; Baron, R.M.; Perrella, M.A. Heme oxygenase-1–derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Investig. 2008, 118, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.-J.; Coronata, A.A.; Fredenburgh, L.E.; Chung, S.W.; Perrella, M.A.; Nakahira, K.; Ryter, S.W.; Choi, A.M. Carbon Monoxide Confers Protection in Sepsis by Enhancing Beclin 1-Dependent Autophagy and Phagocytosis. Antioxidants Redox Signal. 2014, 20, 432–442. [Google Scholar] [CrossRef] [PubMed]
- E Otterbein, L.; May, A.; Chin, B.Y. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR)4 expression. Cell Mol Biol (Noisy-le-grand) 2005, 51, 433–40. [Google Scholar]
- Andoh, A.; Kimura, T.; Fukuda, M.; Araki, Y.; Fujiyama, Y.; Bamba, T. Rapid intestinal ischaemia-reperfusion injury is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin. Exp. Immunol. 1999, 116, 90–93. [Google Scholar] [CrossRef]
- Attuwaybi, B.; Kozar, R.; Moore-Olufemi, S.; Sato, N.; Hassoun, H.; Weisbrodt, N.; Moore, F. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury1,2. J. Surg. Res. 2004, 118, 53–57. [Google Scholar] [CrossRef]
- Wasserberg, N.; Pileggi, A.; Salgar, S.K.; Ruiz, P.; Ricordi, C.; Inverardi, L.; Tzakis, A.G. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury: A laboratory based study. Int. J. Surg. 2007, 5, 216–224. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamamoto, K.; Debari, K.; Onoue, S.; Yamada, S. Novel crystalline solid dispersion of tranilast with high photostability and improved oral bioavailability. Eur. J. Pharm. Sci. 2010, 39, 256–262. [Google Scholar] [CrossRef]
- Mathew, J.M.; Tryphonopoulos, P.; DeFaria, W.; Ruiz, P.; Miller, J.; Barrett, T.A.; Tzakis, A.G.; Kato, T. Role of Innate and Acquired Immune Mechanisms in Clinical Intestinal Transplant Rejection. Transplantation 2015, 99, 1273–1281. [Google Scholar] [CrossRef]
- Ceulemans, L.J.; Braza, F.; Monbaliu, D.; Jochmans, I.; De Hertogh, G.; Du Plessis, J.; Emonds, M.; Kitade, H.; Kawai, M.; Li, Y.; et al. The Leuven Immunomodulatory Protocol Promotes T-Regulatory Cells and Substantially Prolongs Survival After First Intestinal Transplantation. Am. J. Transplant. 2016, 16, 2973–2985. [Google Scholar] [CrossRef]
- Zaher SS, Coe D, Chai JG, Larkin DFP, George AJT. Suppression of the allogeneic response by the anti-allergy drug N-(3,4-dimethoxycinnamonyl) anthranilic acid results from T-cell cycle arrest. Immunology. 2013;138(2):157–64.
- Prud'Homme, G.J.; Glinka, Y.; Toulina, A.; Ace, O.; Subramaniam, V.; Jothy, S. Breast Cancer Stem-Like Cells Are Inhibited by a Non-Toxic Aryl Hydrocarbon Receptor Agonist. PLOS ONE 2010, 5, e13831. [Google Scholar] [CrossRef]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.; Jobin, C.; Chen, Z.-M.E.; Zhou, L. The Aryl Hydrocarbon Receptor Preferentially Marks and Promotes Gut Regulatory T Cells. Cell Rep. 2017, 21, 2277–2290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-F.; Ding, J.-G.; Sheng, J.-F.; Zhu, M.-H.; Li, J.-J.; Sheng, Z.-K.; Tang, X.-F. Novel action of 3,4-DAA ameliorating acute liver allograft injury. Cell Biochem. Funct. 2011, 29, 673–678. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).