Submitted:
28 January 2025
Posted:
28 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
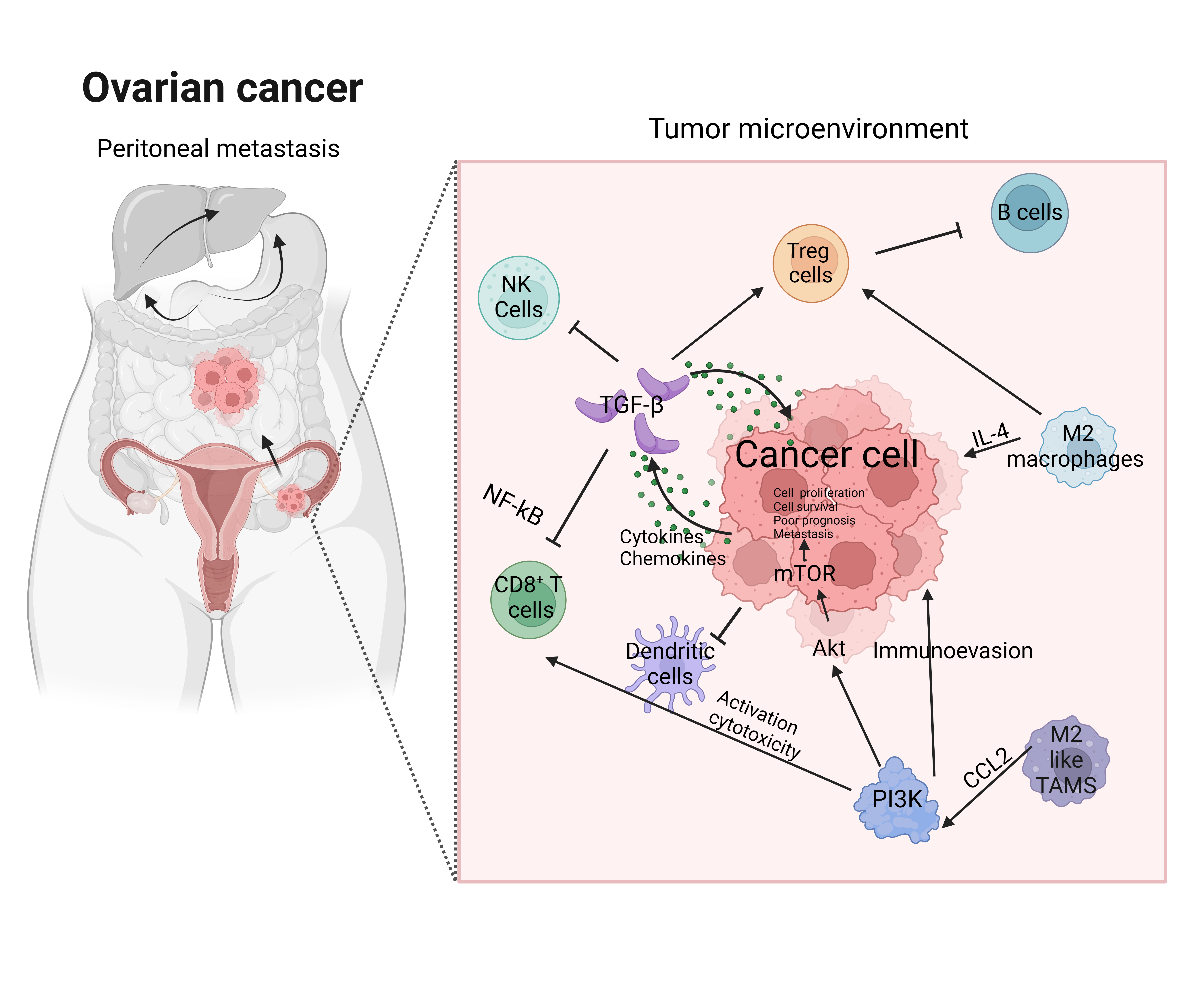
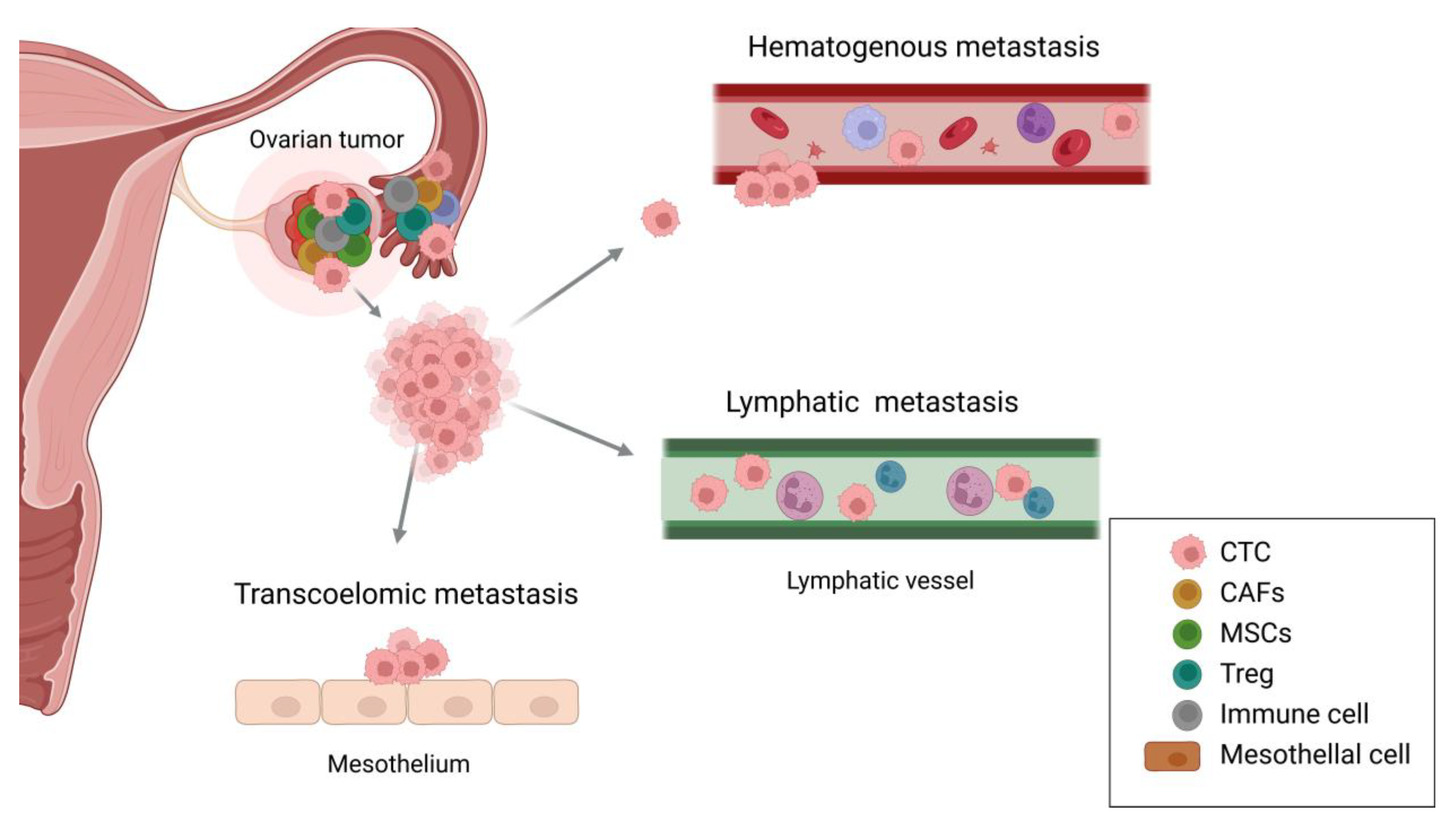
2. Ovarian Cancer Tropism
3. Metastatic Signaling Pathways in Ovarian Cancer
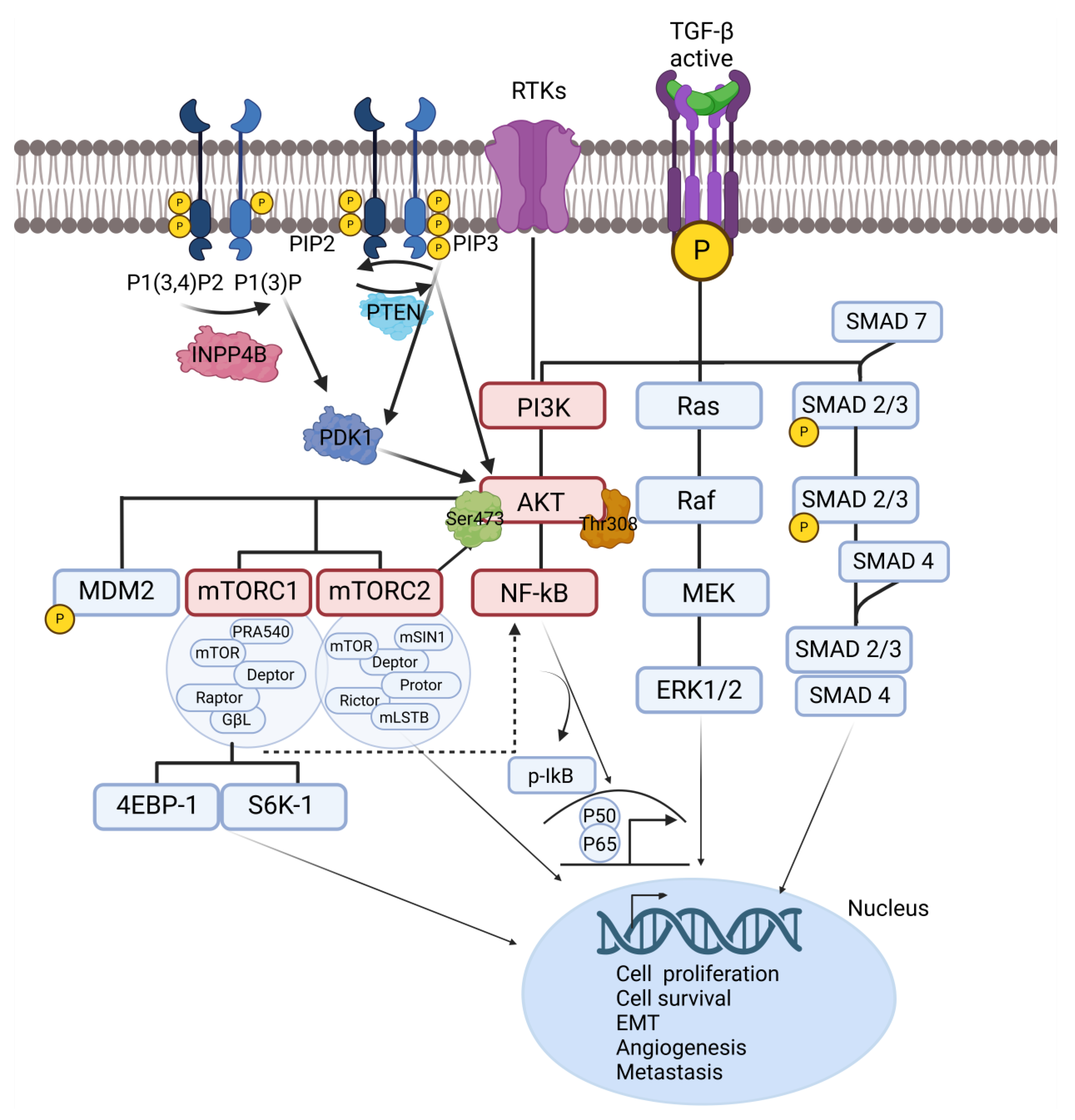
3.1. TGF-β Signaling Pathway
3.2. NF-κB Signaling Pathway
3.3. PI3K/AKT/mTOR Signaling Pathway
4. Therapeutic Applications for Ovarian Cancer Metastasis
4.1. Recent Advanced Combination Immunotherapy in Ovarian Cancer Metastasis
4.2. Signaling Pathway Targeted Therapy in Ovarian Cancer Metastasis
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TME | Tumor microenvironment |
| ECM | Extracellular matrix |
| TGF-β | Transforming growth factor-β |
| EMT | Epithelial-to-mesenchymal transition |
| SMAD | Suppressor of mothers against decapentaplegic |
| PI3K | Phosphoinositide 3-kinase |
| mTOR | Mammalian target of rapamycin |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| NF-κB | Nuclear factor of kappa-light chain of enhancer-activated B cells |
| TAMs | Tumor-associated macrophages |
| TNF-α | Tumor necrosis factor-alpha |
| PMCs | Peritoneal mesothelial cells |
| CAFs | Cancer-associated fibroblasts |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-derived suppressor cells |
| NK | Natural killer |
| CTLs | Cytotoxic T lymphocytes |
| MMPs | Matrix metalloproteinases |
| VEGF | Vascular endothelial growth factor |
| HGF | Hepatocyte growth factor |
| CTLA-4 | Cytotoxic T lymphocyte-associated protein 4 |
| PD-1 | Programmed cell death |
| PD-L1 | Programmed cell death ligand |
| VSV | Vesicular stomatitis virus |
| TβRI | TGF-β receptor type I |
| BA | Bintrafusp alfa |
| PARP | Poly (ADP-ribose) polymerase |
| ROS | Reactive oxygen species |
| CTC | Circulating tumor cell |
| MSCs | Mesenchymal stem cells |
| RTK | Receptor tyrosine kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol (3,4,5)-triphosphate |
| PTEN | Phosphatase and tensin homolog |
| INPP4B | Inositol polyphosphate 4-phosphatase B |
| PDK1 | Phosphoinositide-dependent protein kinase 1 |
References
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Chen, X.; Wang, K.; Chen, Y. Tumor microenvironment in ovarian cancer peritoneal metastasis. Cancer cell international 2023, 23((1)), 11. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Iyoshi, S.; Yoshihara, M.; Kitami, K.; Mogi, K.; Fujimoto, H.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Nawa, A. Metastatic voyage of ovarian cancer cells in ascites with the assistance of various cellular components. International Journal of Molecular Sciences 2022, 23((8)), 4383. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, A.; Semba, T.; Zhang, J.; Fan, Y.; Yasuda-Yoshihara, N.; Wang, H.; Uchihara, T.; Yasuda, T.; Nishimura, A.; Fu, L., Mesothelial cells with mesenchymal features enhance peritoneal dissemination by forming a protumorigenic microenvironment. Cell Reports 2024, 43, (1).
- Laurent-Issartel, C.; Landras, A.; Agniel, R.; Giffard, F.; Blanc-Fournier, C.; Cruz, E. D. S.; Habes, C.; Leroy-Dudal, J.; Carreiras, F.; Kellouche, S. Ascites microenvironment conditions the peritoneal pre-metastatic niche to promote the implantation of ovarian tumor spheroids: Involvement of fibrinogen/fibrin and αV and α5β1 integrins. Experimental Cell Research 2024, 441((1)), 114155. [Google Scholar] [CrossRef]
- Gutic, B.; Bozanovic, T.; Mandic, A.; Dugalic, S.; Todorovic, J.; Dugalic, M. G.; Sengul, D.; Detanac, D. A.; Sengul, I.; Detanac, D. Preliminary outcomes of five-year survival for ovarian malignancies in profiled Serbian Oncology Centre. Clinics 2023, 78, 100204. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A. S.; Casarin, J.; Raffaelli, R.; Cromi, A.; Franchi, M.; Barra, F.; Alkatout, I.; Ferrero, S.; Ghezzi, F. Secondary and tertiary ovarian cancer recurrence: what is the best management? Gland Surg 2020, 9((4)), 1118–1129. [Google Scholar] [CrossRef]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur J Med Chem 2022, 232, 114205. [Google Scholar] [CrossRef]
- Elies, A.; Rivière, S.; Pouget, N.; Becette, V.; Dubot, C.; Donnadieu, A.; Rouzier, R.; Bonneau, C. The role of neoadjuvant chemotherapy in ovarian cancer. Expert Rev Anticancer Ther 2018, 18((6)), 555–566. [Google Scholar] [CrossRef]
- Chen, C.; Ge, X.; Zhao, Y.; Wang, D.; Ling, L.; Zheng, S.; Ding, K.; Wang, J.; Sun, L. Molecular Alterations in Metastatic Ovarian Cancer From Gastrointestinal Cancer. Front Oncol 2020, 10, 605349. [Google Scholar] [CrossRef]
- Cui, M.; Liu, Y.; Cheng, L.; Li, T.; Deng, Y.; Liu, D. Research progress on anti-ovarian cancer mechanism of miRNA regulating tumor microenvironment. Frontiers in Immunology 2022, 13, 1050917. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, J.; Chen, T. Unraveling the extracellular vesicle network: insights into ovarian cancer metastasis and chemoresistance. Molecular Cancer 2024, 23((1)), 201. [Google Scholar] [CrossRef] [PubMed]
- Baba, A. B.; Rah, B.; Bhat, G. R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming growth factor-beta (TGF-β) signaling in cancer-A betrayal within. Frontiers in pharmacology 2022, 13, 791272. [Google Scholar] [CrossRef] [PubMed]
- Jinesh, G. G.; Brohl, A. S. Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis. Signal transduction and targeted therapy 2022, 7((1)), 296. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A. A.; Dao, T.; Suarez, N. G.; Annabi, B. Diet-Derived Gallated Catechins Prevent TGF-β-Mediated Epithelial-Mesenchymal Transition, Cell Migration and Vasculogenic Mimicry in Chemosensitive ES-2 Ovarian Cancer Cells. Nutr Cancer 2021, 73((1)), 169–180. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, W.; Huang, W.; Ye, M.; Zhu, X. Prognostic Values of Transforming Growth Factor-Beta Subtypes in Ovarian Cancer. BioMed Research International 2020, 2020((1)), 2170606. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease, and therapeutics. Signal transduction and targeted therapy 2024, 9((1)), 61. [Google Scholar]
- Ali, S.; Rehman, M. U.; Yatoo, A. M.; Arafah, A.; Khan, A.; Rashid, S.; Majid, S.; Ali, A.; Ali, M. N. TGF-β signaling pathway: Therapeutic targeting and potential for anti-cancer immunity. European Journal of Pharmacology 2023, 947, 175678. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R. K.; Azizov, S.; Raza, A. S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal transduction and targeted therapy 2023, 8((1)), 375. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A. S.; Lam, H. Y.; Yap, K. C.; Jacot, W.; Jones, R. H.; Eng, H.; Nair, M. G.; Makvandi, P.; Geoerger, B. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Molecular cancer 2023, 22((1)), 138. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling 2020, 18, 1–19. [Google Scholar] [CrossRef]
- Harrington, B. S.; Annunziata, C. M. NF-κB signaling in ovarian cancer. Cancers 2019, 11((8)), 1182. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Witte, K. E.; Greiner, J. F.; Weissinger, F.; Kaltschmidt, C. Targeting NF-κB signaling in cancer stem cells: a narrative review. Biomedicines 2022, 10((2)), 261. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Li, X.; Zhang, X.; Xue, S.; Cao, Y.; Niedermann, G.; Lu, Y.; Xue, J. Development of pharmacological immunoregulatory anti-cancer therapeutics: current mechanistic studies and clinical opportunities. Signal Transduction and Targeted Therapy 2024, 9((1)), 126. [Google Scholar] [CrossRef]
- Siminiak, N.; Czepczyński, R.; Zaborowski, M. P.; Iżycki, D. Immunotherapy in ovarian cancer. Archivum Immunologiae et Therapiae Experimentalis 2022, 70((1)), 19. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism insights and therapeutic intervention of tumor metastasis: latest developments and perspectives. Signal transduction and targeted therapy 2024, 9((1)), 192. [Google Scholar] [CrossRef]
- Dunbar, K. J.; Efe, G.; Cunningham, K.; Esquea, E.; Navaridas, R.; Rustgi, A. K. Regulation of metastatic organotropism. Trends in Cancer 2024. [Google Scholar] [CrossRef]
- Gao, Y.; Bado, I.; Wang, H.; Zhang, W.; Rosen, J. M.; Zhang, X. H.-F. Metastasis organotropism: redefining the congenial soil. Developmental cell 2019, 49((3)), 375–391. [Google Scholar] [CrossRef]
- Ford, C. E.; Werner, B.; Hacker, N. F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. British journal of cancer 2020, 123((1)), 9–16. [Google Scholar] [CrossRef]
- Pal, S.; Bhowmick, S.; Sharma, A.; Sierra-Fonseca, J. A.; Mondal, S.; Afolabi, F.; Roy, D., Lymphatic vasculature in ovarian cancer. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2023, 188950.
- Szczerba, A.; Śliwa, A.; Pieta, P. P.; Jankowska, A. The role of circulating Tumor cells in Ovarian Cancer Dissemination. Cancers 2022, 14((24)), 6030. [Google Scholar] [CrossRef]
- Yousefi, M.; Dehghani, S.; Nosrati, R.; Ghanei, M.; Salmaninejad, A.; Rajaie, S.; Hasanzadeh, M.; Pasdar, A. Current insights into the metastasis of epithelial ovarian cancer-hopes and hurdles. Cellular Oncology 2020, 43, 515–538. [Google Scholar] [CrossRef]
- Pascual-Antón, L.; Cardeñes, B.; Sainz de la Cuesta, R.; González-Cortijo, L.; López-Cabrera, M.; Cabañas, C.; Sandoval, P. Mesothelial-to-mesenchymal transition and exosomes in peritoneal metastasis of ovarian cancer. International journal of molecular sciences 2021, 22((21)), 11496. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Gerakopoulos, V.; Oehler, R. Metastasis-associated fibroblasts in peritoneal surface malignancies. British Journal of Cancer 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal transduction and targeted therapy 2021, 6((1)), 362. [Google Scholar] [CrossRef] [PubMed]
- Rakina, M.; Kazakova, A.; Villert, A.; Kolomiets, L.; Larionova, I. Spheroid formation and peritoneal metastasis in ovarian cancer: the role of stromal and immune components. International journal of molecular sciences 2022, 23((11)), 6215. [Google Scholar] [CrossRef]
- Xu, T.; Yu, S.; Zhang, J.; Wu, S., Dysregulated tumor-associated macrophages in carcinogenesis, progression and targeted therapy of gynecological and breast cancers. Journal of hematology & oncology 2021, 14, 1-20.
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Molecular cancer 2020, 19, 1–23. [Google Scholar] [CrossRef]
- Wang, X.; Xue, X.; Pang, M.; Yu, L.; Qian, J.; Li, X.; Tian, M.; Lyu, A.; Lu, C.; Liu, Y. Epithelial–mesenchymal plasticity in cancer: signaling pathways and therapeutic targets. MedComm 2024, 5((8)), e659. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X., The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. Journal of hematology & oncology 2022, 15, (1), 129.
- Chou, M.-Y.; Yang, M.-H. Interplay of immunometabolism and epithelial–mesenchymal transition in the tumor microenvironment. International Journal of Molecular Sciences 2021, 22((18)), 9878. [Google Scholar] [CrossRef]
- Wang, X.; Eichhorn, P. J. A.; Thiery, J. P. In TGF-β, EMT, and resistance to anti-cancer treatment, Seminars in Cancer Biology, 2023; Elsevier: 2023; pp 1-11.
- Sun, L.; Xing, J.; Zhou, X.; Song, X.; Gao, S., Wnt/β-catenin signalling, epithelial-mesenchymal transition and crosslink signalling in colorectal cancer cells. Biomedicine & Pharmacotherapy 2024, 175, 116685.
- Yang, Y.; Ye, W.-L.; Zhang, R.-N.; He, X.-S.; Wang, J.-R.; Liu, Y.-X.; Wang, Y.; Yang, X.-M.; Zhang, Y.-J.; Gan, W.-J., The role of TGF-β signaling pathways in cancer and its potential as a therapeutic target. Evidence-Based Complementary and Alternative Medicine 2021, 2021, (1), 6675208.
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Molecular cancer 2023, 22((1)), 48. [Google Scholar] [CrossRef]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Molecular cancer 2019, 18((1)), 63. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-mediated epithelial-mesenchymal transition and cancer metastasis. International journal of molecular sciences 2019, 20((11)), 2767. [Google Scholar] [CrossRef]
- Xue, V. W.; Chung, J. Y.-F.; Córdoba, C. A. G.; Cheung, A. H.-K.; Kang, W.; Lam, E. W.-F.; Leung, K.-T.; To, K.-F.; Lan, H.-Y.; Tang, P. M.-K. Transforming growth factor-β: a multifunctional regulator of cancer immunity. Cancers 2020, 12((11)), 3099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2((4)), 618–653. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G. R.; Sethi, I.; Sadida, H. Q.; Rah, B.; Mir, R.; Algehainy, N.; Albalawi, I. A.; Masoodi, T.; Subbaraj, G. K.; Jamal, F. Cancer cell plasticity: From cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance. Cancer and Metastasis Reviews 2024, 43((1)), 197–228. [Google Scholar] [CrossRef]
- Oh, A.; Pardo, M.; Rodriguez, A.; Yu, C.; Nguyen, L.; Liang, O.; Chorzalska, A.; Dubielecka, P. M. NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype. Cell Communication and Signaling 2023, 21((1)), 291. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J. C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M. A.; Alvarez-Sánchez, M. E. Role of matrix metalloproteinases in angiogenesis and cancer. Frontiers in oncology 2019, 9, 1370. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Zhong, Z.; Wei, C.; Liu, Y.; Zhu, X. Peritoneal immune microenvironment of endometriosis: Role and therapeutic perspectives. Frontiers in Immunology 2023, 14, 1134663. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Molecular cancer 2021, 20, 1–30. [Google Scholar] [CrossRef]
- Han, J.; Dong, L.; Wu, M.; Ma, F. Dynamic polarization of tumor-associated macrophages and their interaction with intratumoral T cells in an inflamed tumor microenvironment: from mechanistic insights to therapeutic opportunities. Frontiers in Immunology 2023, 14, 1160340. [Google Scholar] [CrossRef]
- Rinne, N.; Christie, E. L.; Ardasheva, A.; Kwok, C. H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resistance 2021, 4((3)), 573. [Google Scholar] [CrossRef]
- Ediriweera, M. K.; Tennekoon, K. H.; Samarakoon, S. R. In Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance, Seminars in cancer biology, 2019; Elsevier: 2019; pp 147-160.
- Mafi, S.; Mansoori, B.; Taeb, S.; Sadeghi, H.; Abbasi, R.; Cho, W. C.; Rostamzadeh, D. mTOR-mediated regulation of immune responses in cancer and tumor microenvironment. Frontiers in immunology 2022, 12, 774103. [Google Scholar] [CrossRef]
- Ritch, S. J.; Telleria, C. M. The transcoelomic ecosystem and epithelial ovarian cancer dissemination. Frontiers in Endocrinology 2022, 13, 886533. [Google Scholar] [CrossRef] [PubMed]
- van Baal, J. O.; van Noorden, C. J.; Nieuwland, R.; Van de Vijver, K. K.; Sturk, A.; van Driel, W. J.; Kenter, G. G.; Lok, C. A., Development of peritoneal carcinomatosis in epithelial ovarian cancer: a review. Journal of Histochemistry & Cytochemistry 2018, 66, (2), 67-83.
- Maioru, O.-V.; Radoi, V.-E.; Coman, M.-C.; Hotinceanu, I.-A.; Dan, A.; Eftenoiu, A.-E.; Burtavel, L.-M.; Bohiltea, L.-C.; Severin, E.-M. Developments in genetics: better management of ovarian cancer patients. International Journal of Molecular Sciences 2023, 24((21)), 15987. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B. L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 2021, 10((5)), 1056. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P. J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2((1)), 27–59. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered metabolism in cancer: Insights into energy pathways and therapeutic targets. Molecular Cancer 2024, 23((1)), 203. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhanghuang, C.; Mi, T.; Jin, L.; Liu, J.; Li, M.; Wu, X.; Wang, J.; Li, M.; Wang, Z., The PI3K-AKT-mTOR signaling pathway mediates the cytoskeletal remodeling and epithelial-mesenchymal transition in bladder outlet obstruction. Heliyon 2023, 9, (11).
- Yang, C.; Xia, B.-R.; Zhang, Z.-C.; Zhang, Y.-J.; Lou, G.; Jin, W.-L. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Frontiers in immunology 2020, 11, 577869. [Google Scholar] [CrossRef]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian cancer immunotherapy: turning up the heat. International journal of molecular sciences 2019, 20((12)), 2927. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Nelson, A.; Walker, B.; Oliphant, T.; Lobert, L.; Mahoney, D.; Johnston, B., Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. Journal for immunotherapy of cancer 2021, 9, (3).
- Hoogstad-van Evert, J. S.; Bekkers, R.; Ottevanger, N.; Jansen, J. H.; Massuger, L.; Dolstra, H. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecologic oncology 2020, 157((3)), 810–816. [Google Scholar] [CrossRef]
- Apolonio, J. S.; de Souza Gonçalves, V. L.; Santos, M. L. C.; Luz, M. S.; Souza, J. V. S.; Pinheiro, S. L. R.; de Souza, W. R.; Loureiro, M. S.; de Melo, F. F. Oncolytic virus therapy in cancer: A current review. World journal of virology 2021, 10((5)), 229. [Google Scholar] [CrossRef]
- Pignata, S.; Bookman, M.; Sehouli, J.; Miller, A.; Penson, R. T.; Taskiran, C.; Anderson, C.; Hietanen, S.; Myers, T.; Madry, R. Overall survival and patient-reported outcome results from the placebo-controlled randomized phase III IMagyn050/GOG 3015/ENGOT-OV39 trial of atezolizumab for newly diagnosed stage III/IV ovarian cancer. Gynecologic oncology 2023, 177, 20–31. [Google Scholar] [CrossRef]
- Moore, K.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Maenpaa, J. LBA31 Primary results from IMagyn050/GOG 3015/ENGOT-OV39, a double-blind placebo (pbo)-controlled randomised phase III trial of bevacizumab (bev)-containing therapy+/-atezolizumab (atezo) for newly diagnosed stage III/IV ovarian cancer (OC). Annals of Oncology 2020, 31, S1161–S1162. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chung, C.-L.; Hu, T.-H.; Chen, J.-J.; Liu, P.-F.; Chen, C.-L., Recent progress in TGF-β inhibitors for cancer therapy. Biomedicine & Pharmacotherapy 2021, 134, 111046.
- Makker, V.; Green, A. K.; Wenham, R. M.; Mutch, D.; Davidson, B.; Miller, D. S. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecologic oncology research and practice 2017, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kment, J. L.; Newsted, D.; Young, S.; Vermeulen, M.; Craig, A. W., 544 Coordinated blockade of TGF-β and PD-L1 by bintrafusp alfa promotes survival in preclinical ovarian cancer models by promoting T effector memory responses. In BMJ Specialist Journals: 2023.
- Kment, J.; Newsted, D.; Young, S.; Vermeulen, M. C.; Laight, B. J.; Greer, P. A.; Lan, Y.; Craig, A. W. Blockade of TGF-β and PD-L1 by bintrafusp alfa promotes survival in preclinical ovarian cancer models by promoting T effector and NK cell responses. British Journal of Cancer 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Matsumura, N.; Mandai, M.; Huang, Z.; Oura, T.; Baba, T.; Hamanishi, J.; Yamaguchi, K.; Kang, H. S.; Okamoto, T. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. International Journal of Cancer 2012, 130((1)), 20–28. [Google Scholar] [CrossRef]
- Guo, Y.; Cui, W.; Pei, Y.; Xu, D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β signaling pathway. Gynecologic oncology 2019, 153((3)), 639–650. [Google Scholar] [CrossRef]
- Cui, Z. Y.; Park, S. J.; Jo, E.; Hwang, I.-H.; Lee, K.-B.; Kim, S.-W.; Kim, D. J.; Joo, J. C.; Hong, S. H.; Lee, M.-G. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-κB signaling pathway. Cell death discovery 2018, 4((1)), 62. [Google Scholar] [CrossRef]
- Jang, H.-J.; Yang, K. E.; Hwang, I.-H.; Huh, Y. H.; Kim, D. J.; Yoo, H.-S.; Park, S. J.; Jang, I.-S. Cordycepin inhibits human ovarian cancer by inducing autophagy and apoptosis through Dickkopf-related protein 1/β-catenin signaling. American journal of translational research 2019, 11((11)), 6890. [Google Scholar]
- Choi, H. J.; Heo, J. H.; Park, J. Y.; Jeong, J. Y.; Cho, H. J.; Park, K. S.; Kim, S. H.; Moon, Y. W.; Kim, J. S.; An, H. J. A novel PI3K/mTOR dual inhibitor, CMG002, overcomes the chemoresistance in ovarian cancer. Gynecologic Oncology 2019, 153((1)), 135–148. [Google Scholar] [CrossRef]
- Ghoneum, A.; Said, N. PI3K-AKT-mTOR and NFκB pathways in ovarian cancer: implications for targeted therapeutics. Cancers 2019, 11((7)), 949. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Jiang, N.; Zhang, Y.; Bian, X.; Wang, X.; Roberts, T. M.; Zhao, J. J.; Liu, P.; Cheng, H. Effective use of PI3K inhibitor BKM120 and PARP inhibitor Olaparib to treat PIK3CA mutant ovarian cancer. Oncotarget 2016, 7((11)), 13153. [Google Scholar] [CrossRef]
- Jin, Z.; Niu, H.; Wang, X.; Zhang, L.; Wang, Q.; Yang, A. Preclinical study of CC223 as a potential anti-ovarian cancer agent. Oncotarget 2017, 8((35)), 58469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Acharya, S.; Karthikeyan, M.; Biswas, P.; Kumari, S. Limitations and potential of immunotherapy in ovarian cancer. Frontiers in Immunology 2024, 14, 1292166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. L.; Cummings, M.; Thangavelu, A.; Theophilou, G.; de Jong, D.; Orsi, N. M. Barriers to immunotherapy in ovarian cancer: metabolic, genomic, and immune perturbations in the tumour microenvironment. Cancers 2021, ((24)), 6231. [Google Scholar] [CrossRef] [PubMed]
- Moore, K. N.; Pignata, S., Trials in progress: IMagyn050/GOG 3015/ENGOT-OV39. A Phase III, multicenter, randomized study of atezolizumab versus placebo administered in combination with paclitaxel, carboplatin, and bevacizumab to patients with newly-diagnosed stage III or stage IV ovarian, fallopian tube, or primary peritoneal cancer. International journal of gynecologic cancer 2019, 29, (2).
| Type of Therapy | Related Signaling pathway | Drug | Function | Reference |
|---|---|---|---|---|
| Combination immunotherapy |
- | NK cell therapy with oncolytic virus |
Infect cancer cells to enhance immune response and anti-tumor effect | [68] |
| - | IMAgyn050 | Target PD-L1 (atezolizumab) with bevacizumab | [87] | |
| TGF-β | LY2157299 | Small molecule TGF-β inhibitor with paclitaxel and carboplatin | [73] | |
| TGF-β | Bintrafusp alfa | Block both TGF-β and PD-L1 to promote immune reaction including CD4, CD8, and NK T cells | [75] | |
| Signaling pathway targeted therapy |
TGF-β | A-83-01 | Suppress EMT, MMP2, and pSMAD2 to regulate cell invasion and adhesion | [77,78] |
| AKT/NF-κB | Cordycepin | Modulate cell proliferation, inflammation, anti-cancer effect as a polyadenylation inhibitor | [79] | |
| PI3K/mTOR | CMG002 | Induce apoptosis and G1 cell cycle arrest to address chemoresistance | [82] | |
| PI3K | BKM120 | Repress cell growth, migration, and invasion with PARP inhibitor Olaparib | [83] | |
| mTOR | CC223 | Degrade mTORC complexes to inhibit cell proliferation and upregulate ROS generation | [82,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





