1. Introduction
Parkinson's disease (PD) is characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc), leading to motor impairments. The number of PD cases has increased significantly from 1990 to 2019, likely due to aging populations and improved diagnostic methods [
1]. PD is a neurodegenerative disease that presents with a wide range of symptoms in both the motor and nonmotor domains. The disease is most commonly associated with motor symptoms, such as bradykinesia, rigidity, tremors, and gait disturbances. However, nonmotor symptoms are also common and can significantly impact a person's quality of life. These nonmotor symptoms can be caused by factors such as neurotransmitter imbalances, neuroinflammation, and the widespread distribution of Lewy bodies [
2].
Fatigue is a diverse and intricate symptom that is typified by an intense sense of tiredness, depletion, or low energy. It might show itself as a physical, mental, or emotional condition that affects a person's capacity to go about their everyday life normally [
3]. Fatigue associated with PD is also known as “Park fatigue.” This unique symptom needs special consideration in diagnosis and treatment as it can seriously impair a person's quality of life [
4]. Fatigue is a prevalent non-motor symptom of PD, affecting half of the patients, according to a meta-analysis of 44 studies including 7,427 individuals. This research emphasizes how crucial it is to identify and treat fatigue in treating PD [
5].
In 2016, Kluger et al. established diagnostic criteria for fatigue associated with Parkinson's disease (
Table 1). These criteria require patients to report a significant decline in energy levels or an excessive perception of effort that is disproportionate to their level of activity. Symptoms must persist for most of the day, occurring daily or nearly every day over the past month. The criteria are divided into four sections (A–D), with a diagnosis of PD-related fatigue requiring the presence of at least four out of nine symptoms in Section A, along with the fulfillment of Sections B–D [
6].
In PD, depression is a prevalent comorbidity that can cause fatigue on its own. Effective care necessitates determining the underlying cause of fatigue, as depression-induced fatigue cannot be treated with the same approaches as PD-related fatigue. Understanding the many causes of weariness enables the development of customized therapies to enhance patients' quality of life [
7]. Friedman et al. followed 51 patients with PD for nine years, finding that 21 continued to experience significant fatigue. This suggests that fatigue is a persistent problem for many PD patients, even over a long period [
8]. When compared to PD patients with mild fatigue, those with severe fatigue showed a reduced proportion of REM sleep and a higher likelihood of REM sleep behavior disorder (RBD). Even after controlling for many other variables, these components remained independent predictors of fatigue. Furthermore, even after controlling for factors such as age, sex, length, body mass index, illness severity, depression, anxiety, and other sleep disorders, the percentage of REM sleep and the amount of REM sleep without atonia continued to be linked to fatigue severity ratings [
9]. High levels of anxiety and depression, as determined by the Hospital Anxiety and Depression Scale (HADS) depression and anxiety subscales and the Non-Motor Symptoms Scale (NMSS) for PD assessing mood domain, were substantially correlated with fatigue [
10]. A lower standard of living, a higher chance of falls, and damaged relationships are just a few of the effects that can drastically affect their day-to-day existence. It is important to address these many facets in order to enhance the general well-being and quality of life of people with PD [
11].
Certain medications used to treat PD, such as dopamine agonists and anticholinergics, can contribute to fatigue as a side effect, potentially due to their effects on neurotransmitter systems and brain circuits involved in energy regulation and motivation. Careful monitoring and adjustment of medication regimens or exploring alternative medications or non-pharmacological interventions may be necessary to minimize medication-induced fatigue in PD patients [
12]. Compared to patients with lower LED (levodopa equivalent dosage), those with higher LED exhibited much more intense weariness. So, increasing levodopa dosages could play a role in PD-related fatigue severity. When changing a patient's levodopa dose for PD, clinicians should be aware of this possible adverse effect [
13]. Even though understanding and treating fatigue in PD has advanced significantly, more work must be done before the condition is effectively managed. Methylphenidate and other medications provide some optimism, but further studies are needed to verify their long-term effectiveness and investigate other possible pharmacological treatments. While there is currently no clear evidence linking exercise to fatigue, leading an active lifestyle is recommended for general health reasons and its ability to alleviate other symptoms of PD. Establishing a commonly acknowledged definition of fatigue in PD is still a crucial first step since it will open the door to more precise diagnostic instruments and focused therapies. We can enable people with PD to recover their energy and quality of life by investigating various management techniques and further researching the intricate nature of tiredness [
14].
This review aims to provide a comprehensive understanding of fatigue in PD, encompassing its pathophysiology, prevalence, clinical manifestations, assessment, and management strategies. We aim to delve into the underlying mechanisms of fatigue in PD, exploring the roles of dopaminergic and non-dopaminergic pathways, inflammation, genetic factors, and neuroendocrine dysregulation. Investigate the prevalence and epidemiology of fatigue in PD, providing insights into its impact on the PD population. Describe the clinical manifestations of fatigue in PD, including its symptoms, impact on quality of life, and differentiation from other PD symptoms. Discuss the various assessment tools used to evaluate fatigue in PD and identify factors contributing to its severity.
2. Pathophysiology
2.1. Overview of Fatigue In Neurological Disorders
Fatigue is one of the nonmotor symptoms that usually accompany many neurological conditions, such as PD, multiple sclerosis, myasthenia gravis, stroke, and wide autonomic dysfunctions. It significantly affects the quality of life [
15]. Different classifications of fatigues have been introduced across the literature. Some papers classified fatigue into physical and pathological fatigue. The first one results from the high duration of intense activities, while the chronic one appears regardless of the degree of the activities [
16,
17]. Fatigue can also be classified as physical fatigue, which represents the physical exhaustion from exercise, and mental fatigue, which covers cognitive abilities [
18,
19]. One of the widest-used classifications in the literature is fatigue as central and peripheral fatigue.
Central fatigue represents continuous tiredness from a chronic condition, while peripheral definition represents the inability to sustain muscle contraction [
20]. Multiple studies have been conducted in the context of sleep in neurological disorders. A new classification has been introduced due to the discovered association between fatigue and some non-motor symptoms, such as mood and sleep disorders, which classify fatigue into primary and secondary [
15,
21]. Fatigue is considered secondary if it is accompanied by a mood or sleep disorder, while isolated fatigue is considered primary [
22]. Fatigue was also found to correlate with the severity of PD in multiple studies [
10,
21]. The association between fatigue and sleep disorders in PD has been studied across a wide spectrum of studies. It is believed that PD can cause circadian dysfunction, and the opposite sequence can also occur with PD, resulting from circadian problems [
23]. A study conducted by Skorvanek et al. reported a significantly higher prevalence of fatigue among the secondary fatigue group compared to the primary fatigue group, demonstrating a possible correlation between mood and sleep disorders with fatigue [
22]. Fatigued PD patients tend to present with more sleep problems, more cognitive problems, pain, urinary symptoms, depression, anxiety, and subsequent bad quality of life [
24,
25]. Some studies suggest that fatigue can be one of the important predictors of sleep problems [
26], while other studies in neurological disorders proposed a separate pathophysiological mechanism for sleep disturbance in PD by tackling CNS abnormalities and circadian rhythm disorders [
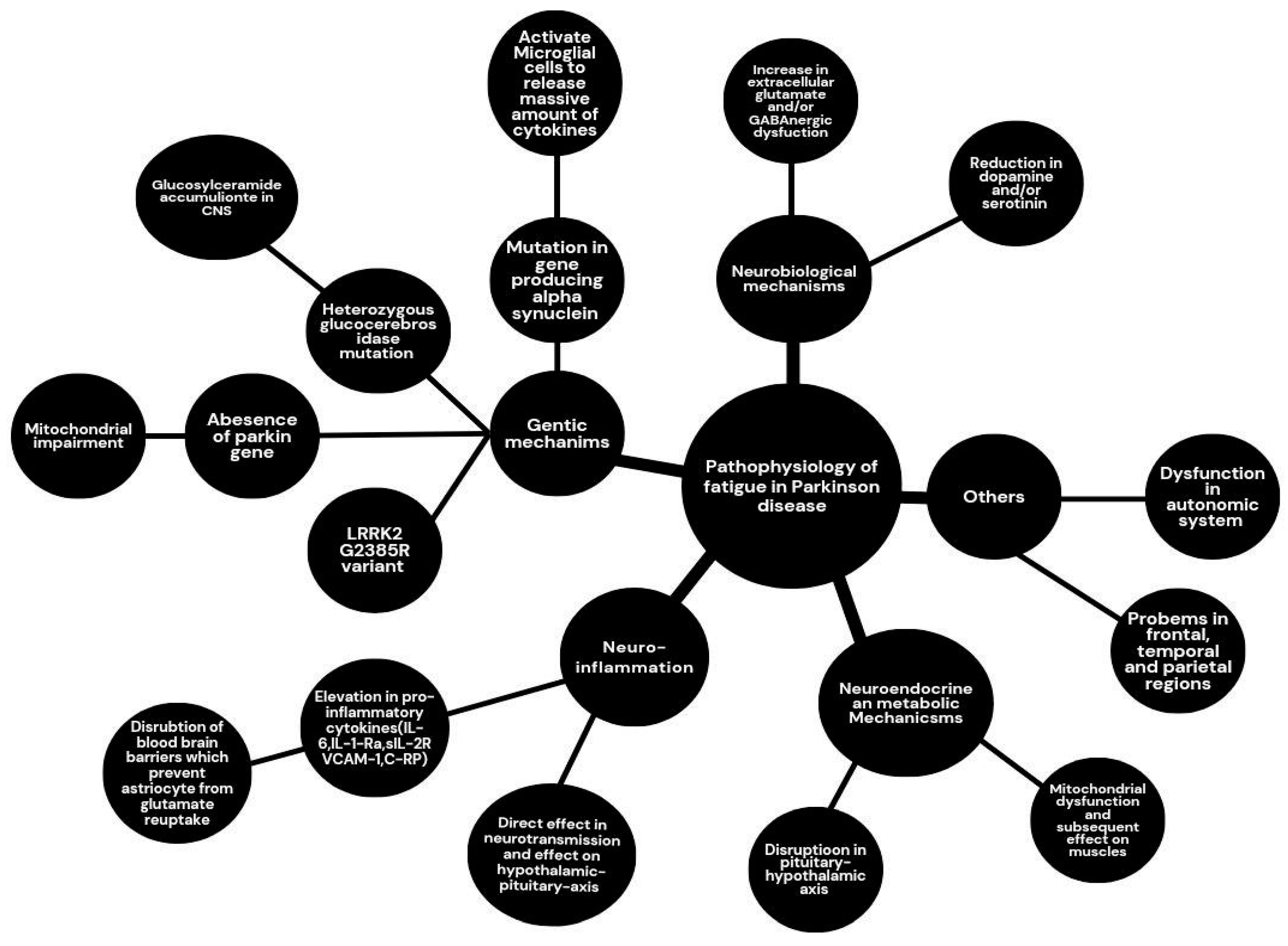
27]. So, more studies need to be conducted to determine whether sleep disturbances are just a consequence of fatigue in patients with PD. Multiple mechanisms and pathophysiology have been introduced in the literature for fatigue in PD (
Figure 1). These mechanisms vary widely in anatomical and physiological pathophysiology.
2.2. Neurobiological Mechanisms
2.2.1. Dopaminergic Pathways
Dopamine is one of the most common catecholamine synthesized in the CNS, and it is synthesized in the substantia nigra-affected in PD- and ventral tegmental area [
28]. The role of dopamine in the pathophysiology of fatigue remains controversial. Some studies found good dopamine effects and reported less fatigue progression among patients with PD who took levodopa [
12]. Also, two studies on rasagiline (a MAO inhibitor drug that increases dopamine concertation in the striatum) reported its benefits in decreasing fatigue scale [
29,
30]. Moreover, another clinical trial was conducted using methylphenidate, which blocks the reuptake of dopamine and norepinephrine. The trial concluded that the drug significantly lowered the fatigue scale [
31]. On the contrary, multiple studies have found no effect of dopamine agonists on fatigue [
32,
33].
The most important of the opposite studies is a meta-analysis conducted in 2023 on pharmacological and non-pharmacological interventions in patients with PD. Due to a lack of studies in the pharmacological part, only modafinil, which inhibits the reuptake of dopamine and increases extracellular dopamine, was included in the meta-analysis. The study revealed a non-significant effect of the drug on patients' fatigue with PD (SMD = -0.21, 95% CI -0.74–0.31, p = 0.43) [
34].
Several structural and functional imaging studies have been conducted among multiple neurological disorders. These studies showed abnormalities in the prefrontal cortex—which receives dopamine projections—and frontal lobe in fatigued patients [
35]. Moreover, striatal infarcts were found to be associated with fatigue in stroke patients [
36]. In addition, reduced perfusion to the frontal lobe was significantly associated with fatigue in patients with PD [
37].
2.2.2. Non-Dopaminergic Systems
Another neurotransmitter that is hypothesized to be related to fatigue in PD is serotonin. The main reason for the hypothesis is that fatigue is one of depression symptoms, and it is treated by increasing serotonin levels [
38]. Serotonin pathways are also believed to be affected by PD as Lewy bodies accumulate in median raphe nuclei, which contain serotonergic neurons [
39]. A study by Pavesa et al. reported a significant reduction in serotonin transporter binding in the striatum and thalamus of fatigued patients with PD, which supports the role of serotonin in fatigue [
40]. Moreover, another study by Yamamoto et al. on chronic fatigue reported a reduction in serotonin transporter density using radioactive material ([11C](+)McN5652), which may also be noticed in patients with PD [
41]. However, a study by Pauletti et al. mentioned some concerning findings due to a significant reduction in serotonergic central tone between the patients with PD and healthy controls but no difference between patients with PD with fatigue and those without it. They used loudness dependence of auditory evoked potentials to measure the central tone, and they concluded that fatigue may result from serotonin/dopamine imbalance rather than serotonin deficiency by itself. More research is required to identify the intercorrelation between serotonin and dopamine in causing fatigue [
42].
Other than dopamine and serotonin, other neurotransmitters and chemicals have also been examined for their effect on fatigue in patients with PD. Caffeine, an adenosine antagonist, was reduced in patients with PD complaining of fatigue, but the effect was insignificant. This requires more studies on the involvement of adenosine [
43]. Moreover, glutamate was also found to precipitate fatigue in these patients. A clinical trial conducted in 2022 and a review in 2021 reflected the effect of safinamide (which inhibits abnormal glutamate release) in eliminating and preventing fatigue episodes. However, this may be due to an additional mechanism by the drug, which also can affect MAO and cause subsequent elevation in dopamine concentration, so further investigations are required [
44]. Few studies have studied the effect of GABAergic dysfunction on fatigue, but it was not in PD, such as Versace et al., who reported an increase in fatigue due to a reduction in GABAergic inhibition in the primary motor cortex [
45].
2.3. Role Of Inflammation
Neuroinflammation was found to play a role in the pathophysiology of neurological diseases and even contribute to the progression of PD [
46]. The role of inflammation has been examined in many neurological and muscular diseases before, such as multiple sclerosis and PD [
47]. The role of inflammation in fatigue among patients with PD has been indirectly discovered by measuring inflammatory markers. According to a review conducted in 2019, higher serum IL-6, IL-1-Ra, sIL-2R, and VCAM-1 were found to be associated with higher fatigue levels among patients with PD. Surprisingly, the review reported a lower level of fatigue associated with higher serum uric, an indicator of oxidative stress [
48]. This can be justified as serum uric acid was reported not to have a significant correlation with fatigue scores in multiple sclerosis [
47]. A study by Lindqvist et al. reported the elevation of another inflammatory marker among fatigue patients whose C-reactive protein was elevated (p = 0.008) [
49]. However, sTNFR (TNF-a antagonist) was not to be different between fatigued patients with PD and non-fatigued patients [
50]. While TNF-a was found to have a positive association with fatigue scale [
51]. Different mechanisms have been presented for the role of these cytokines and chemokines in causing fatigue, such as microglial activation, recruitment of leukocytes, direct effect in neurotransmission, and effect on hypothalamic-pituitary axis [
52]. Another suggested mechanism is elevation in proinflammatory cytokines due to chronic inflammation. These elevated levels disrupt the blood-brain barrier by combining with its endothelium. These cytokines work in the brain to prevent the astrocytes from re-uptaking glutamate, which can lead to an increase in glutamate concentration and then precipitation of fatigue [
53].
2.4. Genetic Factors
Genetic factors also affect the fatigue mechanism, as several mutations have been linked with fatigue in patients with PD. One of these mutations is the heterozygous glucocerebrosidase mutation, which was reported to be associated with more fatigue in Parkinson's patients (p = 0.001) [
54]. Mutation in this enzyme also increases the risk of PD itself, as glucosylceramide can accumulate in different visceral organs, including CNS [
55]. Another gene studied is the LRRK2 G2385R variant, a known PD risk factor [
56]. People with the LRRK2 G2385R variant were found to have significantly higher levels of fatigue than the non-carriers (p = 0.002) [
57]. Interestingly, the carriers of this gene were also found to have an increased prevalence of autonomic dysfunction and sleep disorder [
58]. Moreover, the absence of the Parkin gene, as in PD, was found to promote mitochondrial impairment, a known source of cell energy, and can be a possible mechanism of fatigue [
59]. Another important genetic factor is a mutation in gene-producing alpha-synuclein, which was found to cause familial PD [
60]. A hypothesis that illustrates the ability of elevation in alpha-synuclein to cause fatigue in Parkinson's patients is presented. Firstly, abnormal aggregation of this protein can occur due to inflammation or just mutations in amino acid sequences. Then conformational change shifts the molecule to insoluble B sheets [
53,
61]. Alpha-synuclein then activates toll-like receptors (TLR4) on microglial cells, releasing many pro-inflammatory cytokines. These cytokines, as mentioned previously, can inhibit the reuptake of glutamate by astrocytes, promoting fatigue in Parkinson's patients [
53]. What supports this mechanism is the growing evidence of a significant association between elevated levels of CSF alpha-synuclein and fatigue in Parkinson's patients [
51,
62].
2.5. Neuroendocrine And Metabolic Dysregulation
Hypothalamic-pituitary axis has also been shown to contribute to the pathophysiology of fatigue in PD. This axis has vast connections to wide brain areas such as basal ganglia, amygdala, thalamus, and frontal cortex, which may precipitate the pathology in case of axis dysfunction [
63]. Several neurological studies have addressed the endocrine system's role in fatigue induction. A study conducted by Gottschalk et al. reported significantly higher levels of adrenocorticotropic hormone (ACTH) in multiple sclerosis patients with fatigue [
64]. On the contrary, Schaefer et al. reported decreased cortisol levels among fatigued patients with CNS infection [
65]. This illustrates the need for more studies to understand the role of ACTH and cortisol in fatigue induction in patients with PD. Other hormones, such as testosterone, have also been tested in patients with fatigue but have not been found to correlate with fatigue [
66].
From a muscular system point of view, multiple mechanisms have been studied. In the case of parkin gene absence, electron transport chain genes are down-regulated. This disrupts oxidative phosphorylation and increases the oxidative stress. The subsequent mechanism leads to mitochondrial dysfunction and damage, which may affect the cells' energy level [
59]. On the contrary, a study conducted by Stevens-Lapsley et al. concluded that less fatigue in the patients with PD group was due to a reduction in central activation, preventing muscle overload and the subsequent metabolic fatigue [
67]. The musculoskeletal system's role remains controversial, as studies show a variable range of findings. In a meta-analysis conducted in 2023, physical exercise was found to be a good treatment for fatigue, which is inconsistent with the lack of energy and central activation mentioned in other studies [
34].
2.6. Other Mechanisms
The autonomic system has also been linked to fatigue, as fatigue was found to be correlated with autonomic dysfunction [
15]. In addition, the management of autonomic symptoms and depression in PD is quite helpful in fatigue treatment [
68]. Multiple brain areas, such as frontal, temporal, and parietal regions, have been found to correlate to fatigue [
48]. Imaging studies in patients with PD reflected increased activity in areas other than basal ganglia, such as the premotor cortex and cerebellum, as a compensatory mechanism. These areas lead to differences in corticospinal excitability, and further studies must address their role in fatigue [
69].
Cardiac sympathetic innervation plays a crucial role in modulating cardiovascular responses to physical exertion, including changes in heart contractility and chronotropic regulation. Studies analyzing cardiac metaiodobenzylguanidine (MIBG) heart-to-mediastinum uptake in fatigued and non-fatigued individuals with Parkinson’s disease identified a possible link between fatigue and cardiac sympathetic denervation [
70]. While typically asymptomatic in PD, this denervation may impair autonomic cardiovascular regulation, potentially leading to reduced cardiac contractility during exercise. This dysfunction could contribute to exercise intolerance, manifesting as shortness of breath and an increased perception of fatigue. Moreover, the dysregulation of catecholaminergic signaling in PD may further exacerbate these symptoms, limiting the body's ability to adapt to exertional demands, and even impairment in cognition [
68]. Understanding the relationship between fatigue and cardiovascular dysautonomia in PD could provide new insights into therapeutic strategies aimed at mitigating fatigue-related disability.
3. Prevalence And Epidemiology
One of the most common non-motor symptoms of PD that affects a sizable percentage of patients is fatigue. Numerous studies have revealed prevalence percentages ranging from 36.8% to 59.46%, underscoring the diversity of research populations and evaluation techniques. Several studies included in this table provide valuable insights into the prevalence of fatigue in PD (
Table 2).
These studies show that between around 36% and 60% of people with PD experience tiredness. It is probable that variations in research populations, evaluation techniques, and definitions of weariness account for the fluctuations in prevalence rates. It is important to note that fatigue can significantly impact the quality of life of PD patients, affecting their daily activities, social interactions, and overall well-being. Therefore, recognizing and addressing fatigue is crucial for comprehensive PD management.
Mukadam et al. studied how the COVID-19 pandemic affected subjective cognition and social functioning in people with Parkinson’s disease. A longitudinal analysis of 123 patients revealed that declines in these areas were linked to increased anxiety, fatigue, and motor symptoms. Fatigue, in particular, was a significant factor in worsening quality of life during the pandemic. Additionally, subjective cognitive decline correlated with depression and social functioning decline. Women reported a greater perceived impact of COVID-19, while in men, personal COVID-19 experience was associated with cognitive decline [
79].
4. Clinical Manifestations
PD patients describe their fatigue as a “feeling of abnormal and overwhelming tiredness and lack of energy that is distinct both qualitatively and quantitatively from normal tiredness [
80].” Fatigue was considered to be related to the pathology of PD. It has a pre-motor feature as it can occur early in the course of the disease, preceding the onset of other motor symptoms related to PD [
81]. Moreover, a 16-year UK study indicated that experiencing fatigue five years prior to a PD diagnosis elevated the relative risk of developing the disease to 1.56 [
82]. In early PD, non-motor symptoms such as fatigue may be more disabling than motor symptoms [
83]. Several studies reported that fatigue exerts higher negative effects on PD patients compared to other non-motor symptoms [
84].
In the context of PD, fatigue should be precisely defined, as there are several confounding symptoms. To address this, a unifying taxonomy has been proposed by Kluger et al. to define fatigue in clinical and research contexts [
16]. This taxonomy is based on five main criteria: 1) Differentiating fatigue from related phenomena, such as sleepiness. 2) Recognizing the perception of fatigue, which is a sensation of exhaustion or increased effort, as phenomenologically distinct from performance fatigue, which is a decline in performance caused by continuous effort on a demanding task. 3) Distinguishing clinically significant (pathological) fatigue from normal physiological fatigue. 4) Identifying potential causal factors based on their function (e.g., homeostatic, psychological) or neuroanatomical location (e.g., central, peripheral). 5) Specifying the domain(s) of performance affected, such as motor or cognitive functions.
Fatigue in Parkinson's disease (PD) can be classified into two types: peripheral fatigue, which is a measurable condition that occurs when a muscle loses strength after repeated contractions, and central fatigue, which is a subjective feeling that cannot yet be objectively measured [
85].
Fatigue represents a considerably debilitating experience for PD patients. Patients with PD who experience fatigue are more likely to develop apathy [
86], and this association is also linked to other important factors such as depression [
30], cognitive dysfunction, and reduced quality of life [
87]. Patients experiencing fatigue had a longer duration of their illness and more severe motor symptoms, except for tremors. They also showed more depressive symptoms and experienced greater sleep disturbances than those without fatigue. Additionally, the severity of sleep disturbances was found to be an independent factor contributing to fatigue [
78]. Due to fatigue, some patients were forced to decrease their working hours, besides reducing their participation in social activities [
88]. Interestingly, in contrast to fatigue in the general population, a study reported that PD fatigue is commonly alleviated through physical exercise [
89].
PD fatigue showed a pronounced impact on quality of life. A prospective study demonstrated a significant association between PD fatigue and overall HRQOL [
90]. Moreover, another study reported fatigue as the most important non-motor symptom affecting HRQOL [
91], a finding that is supported by another study [
92]. The latter study revealed the presence of a relationship between fatigue and depression, as well as fatigue and sleep disturbances among IPD patients. Additionally, depression and sleep disturbances were more prevalent in the IPD group with fatigue compared to the IPD group without fatigue [
92]. One of the challenges in addressing and managing fatigue in PD is the high prevalence of potentially confounding symptoms such as daytime sleepiness, apathy, and depression [
87]. Like depression, fatigue is often linked with daytime sleepiness and sleep disorders. However, it can be distinguished by the fact that sleep does not provide restoration, and it may also occur in PD patients who have normal sleep patterns [
93]. A recent case-control study revealed that PD patients with fatigue had fewer night sleep hours compared to those without fatigue. Moreover, patients with fatigue experienced more pronounced pain, anxiety, and depression. Additionally, restlessness legs syndrome was more prominent in patients with fatigue compared to those without fatigue (IRLSSG score 5.61 ± 7.98 vs. 2.49 ± 6.26, p = 0.013). At the same time, higher worry and less happiness were recorded in patients with fatigue. On the other hand, this study also showed the fatigue impact on cognitive capacity, as lower scores in Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scales were detected among PD patients with fatigue, indicating greater cognitive dysfunction compared to patients without fatigue [
25].
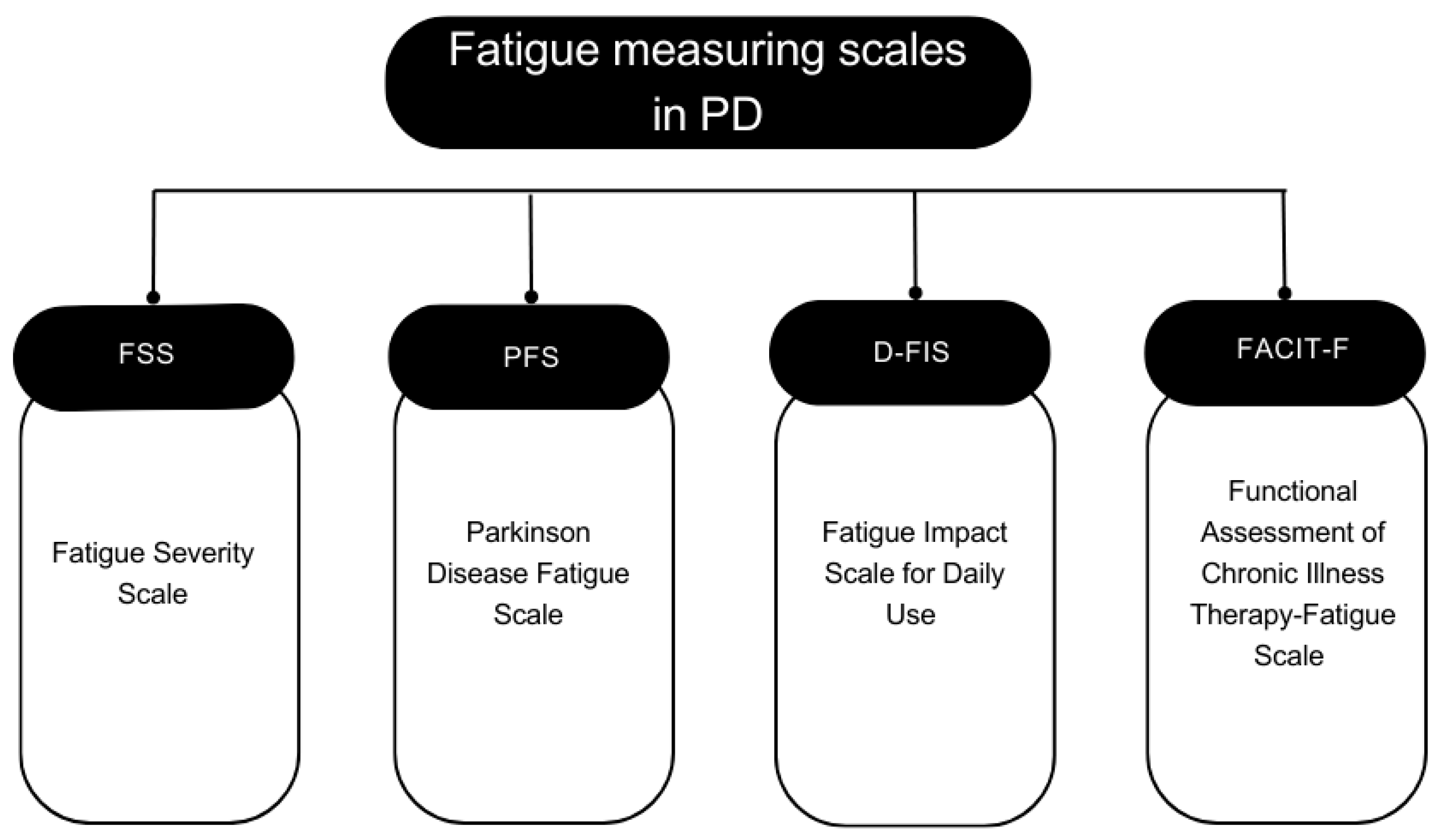
4.1. Assessment Of Fatigue In Parkinson's Disease
Since fatigue is a subjective symptom, patient self-reported questionnaire remains the primary method for either diagnosing or measuring fatigue severity [
94]. Fatigue Severity Scale (FSS) is a nine-item scale used to assess the physical aspects of fatigue and its impact on quality of life (QOL) [
95]. It evaluates how fatigue affects routine activities such as exercise, physical functioning, work duties, responsibilities, and family and social life. Each item is expressed as a statement in the form of a concise declaration that is evaluated using a Likert scale ranging from one to seven. Grade 1 represents a complete disagreement, while Grade 7 represents a complete agreement. The FSS total score, which is the average of the nine items' scores, indicates the severity of fatigue, with higher scores indicating a more severe level of fatigue. This scale is recommended by the International Movement Disorder Society and has been validated in different languages [
96].
In 2005, Brown et al. developed a scale known as the Parkinson’s Disease Fatigue Scale (PFS) and confirmed its validity and reliability [
80]. This scale is intended to be used mainly for PD patients. PFS is a 16-item self-reported questionnaire designed to evaluate the physical aspects of fatigue in patients with PD. The PFS offers two scoring methods. The original method features response options ranging from 1 (strongly disagree) to 5 (strongly agree), with the total PFS-16 score ranging from 1 to 5, obtained by dividing the sum of all item scores by 16. The binary scoring method, on the other hand, transforms item responses into 1s and 0s, with agree and strongly agree being scored as 1s, and all other responses as 0s. This method yields a total score between 0 and 16, with 16 representing higher levels of fatigue. Another scale called the Fatigue Impact Scale for Daily Use (D-FIS) is an 8-item scale developed to measure subjective daily experience of fatigue [
97]. Additionally, another generic fatigue questionnaire has been used to assess fatigue in PD; it’s called the Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F) [
98].
Figure 2 summarizes the different scales used to assess fatigue in Parkinson's disease.
Fatigue is a prevalent and debilitating non-motor symptom in Parkinson’s disease (PD), requiring accurate assessment tools for both clinical and research purposes. Several fatigue scales are available, each differing in their structure, scoring system, and clinical utility [
99]. The Fatigue Severity Scale (FSS) and Parkinson’s Fatigue Scale (PFS) are among the most widely recommended by the Movement Disorders Society (MDS) for screening and severity assessment. The FSS, consisting of nine items scored on a 7-point Likert scale, evaluates the impact of fatigue over a two-week period, with a total score range of 9 to 63 [
100]. The PFS, specifically designed for PD, includes 16 items scored from 16 to 80 and focuses on the presence and impact of fatigue over the same timeframe [
101]. Both scales have established cutoff values and are considered reliable measures for assessing fatigue burden in PD. Other scales, such as the Fatigue Assessment Inventory (FAI) and Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F), offer broader evaluations but with some limitations. The FAI, with 29 items scored on a 7-point scale, includes a definition of fatigue and assesses symptoms over two weeks, though it is only suggested by the MDS for screening. The FACIT-F, comprising 13 items on a 5-point scale, assesses fatigue over one week with a total score range of 0 to 52. While it has a defined cutoff for clinical significance, it is not specific to PD, which may limit its sensitivity in this population [
102]. The Multidimensional Fatigue Inventory (MFI), a 20-item scale evaluating fatigue across multiple domains, lacks established cutoff values and has not been strongly recommended for PD-related fatigue assessment. Additional fatigue scales, such as the Fatigue Severity Inventory (FSI), Daily Fatigue Impact Scale (D-FIS), and Fatigue Visual Analog Scale (FVAS), are also used but with varying degrees of validation in PD. The D-FIS, an 8-item scale assessing fatigue on a single day, is suggested for use but lacks defined cutoff values. The FSI, with 33 items scored on a 7-point scale, and the FVAS, a simple visual scale, are listed by the MDS but not strongly recommended. The Clinical Global Impression Scale (CGIS), which provides a variable and subjective assessment, is also listed but lacks a standardized scoring system [
103]. Future studies are needed to refine these tools and determine the most effective scale for capturing PD-related fatigue and its impact on daily functioning.
4.2. Factors Contributing To Fatigue In Parkinson’s Disease
Although fatigue in PD is primarily caused by the disease's pathological disturbances, several other factors may contribute to the onset and increasing severity of fatigue in PD. Fatigue in PD was associated with the severity of depressive symptoms [
104]. The association between depression and fatigue has been addressed in several studies [
21]. On the other hand, anxiety has been shown to have an association with fatigue in PD [
105]. In general, strong associations between levels of depression, anxiety, and fatigue in PD were demonstrated in several studies [
5,
106].
Poor sleep is indicated as a major contributor to fatigue in PD [
107]. For this reason, careful attention to nighttime sleep is required. The association between sleep disturbances and fatigue has been addressed in several studies [
108]. Additionally, a recent longitudinal study revealed that poor baseline sleep was associated with greater fatigue and depression after one year [
109]. At the same time, a cross-sectional study reported that anxiety is a significant predictor of poor sleep quality [
110]. The correlation between fatigue severity and the Non-Motor Symptoms Scale (NMSS) was significant [
111]. This was evident in the presence and frequency of disturbances in the affective sphere (anhedonia, loss of interest, apathy, and anxiety). Additionally, sleep disorders, such as daytime sleepiness and sleep difficulties, were also strongly associated with fatigue severity. Furthermore, gastrointestinal and sexual dysfunctions were linked to the severity of fatigue [
111]. These findings support the idea that both the affective sphere and sleep disorders are closely related to the development of fatigue. This is consistent with Metta et al., who found a connection between fatigue and sleep dysfunction, as well as anxiety and depression [
10]. A recent study reported that individuals diagnosed with de novo Parkinson's disease and experiencing distressing fatigue displayed elevated levels of sleepiness, depression, anxiety, and apathy [
11]. Such finding suggested that the presence of distressing fatigue, as assessed by PFS, is linked to a higher load of non-motor symptoms [
111]. Further analysis in the same study using logistic regression revealed that only sleepiness, cognitive apathy, and episodic anxiety were the key independent factors with the greatest explanatory power in relation to distressing fatigue. Kwon et al. study explores the relationship between motor and non-motor symptoms in patients with de novo Parkinson’s disease. Among 105 analyzed patients, those with non-tremor-dominant PD exhibited more severe fatigue and dysautonomia compared to tremor-dominant patients, while other non-motor symptoms, such as cognition, depression, and anxiety, showed no significant differences. Further analysis revealed that postural instability and gait difficulty were significantly associated with fatigue (r = 0.3659, P = 0.0002), suggesting a potential link between motor impairment and fatigue severity. These findings highlight the impact of motor subtypes on fatigue, emphasizing the need for targeted interventions in PD management [
112].
In terms of the relationship between fatigue and cognitive apathy, several studies reported the association between fatigue and cognitive apathy in PD [
11,
89,
104]. Moreover, fatigue was demonstrated to be associated with poor decision-making [
113]. On the other hand, fatigue is associated with the severity of motor symptoms, as indicated by the Unified Parkinson's Disease Rating Scale Part III (UPDRS III), as well as with disease disability [
114]. A prospective longitudinal study by Alves et al. [
115] demonstrated a correlation between fatigue and Parkinson's disease (PD) severity. A recently conducted study indicated that non-motor symptoms tend to have a greater impact on fatigue than motor symptoms [
116]. The study showed that there is a link between fatigue and higher BMI scores, increased levels of anxiety and depression, lower daily equivalent levodopa dose LEDD, and more severe non-motor symptoms of daily life activities (DLAs).
Differences in non-motor symptoms have been observed between genders for Parkinson's disease (PD) [
117]. Few studies investigated the relationship between fatigue and gender in PD, but these studies are contradictory. Some studies indicated that gender was associated with fatigue [
118]. At the same time, a recent review of 22 studies revealed that only one study showed less fatigue in males [
5]. Additionally, females with concurrent chronic illness in the general population have been described to experience higher levels of fatigue [
119]. Therefore, the relationship between fatigue and gender is more likely influenced by general factors rather than PD-specific factors. Therefore, this issue needs to be further investigated.
In terms of the relationship between fatigue and medication, longitudinal studies on fatigue in early PD have produced contradictory findings regarding the relationship between dopamine and fatigue. While Ongre et al. demonstrated that de novo PD patients using dopamine agonists experienced lower levels of fatigue compared to those using levodopa [
120], Ou et al. found higher fatigue prevalence in patients with higher LEDD at the 2 and 3-year follow-up marks [
121].
4.3. Overlap Of Fatigue with Other Conditions
Fatigue is a prevalent non-motor symptom in Parkinson's disease (PD), affecting approximately 44.2% of patients, compared to 18% in healthy elderly individuals [
122]. This symptom often coexists with conditions such as depression, dementia, and sleep disturbances. However, studies indicate that even PD patients without these comorbidities report significant levels of fatigue, suggesting that fatigue may be an independent manifestation of PD rather than solely a consequence of overlapping conditions.
Moreover, there is a notable overlap between fatigue and excessive daytime sleepiness in PD patients. Research demonstrates that while both symptoms are prevalent, they exhibit different correlations with disease characteristics. For instance, excessive daytime sleepiness is more closely associated with disease duration and the type of dopaminergic treatment, whereas fatigue shows a stronger correlation with depressive symptoms. This distinction underscores the complex interplay between various non-motor symptoms in PD and highlights the necessity for comprehensive assessment and tailored management strategies for affected individuals [
123].
4.3.1. Sleep Disorders
Fatigue and sleep disturbances are prevalent non-motor symptoms in Parkinson's disease (PD), significantly impacting patients' quality of life. Over 75% of individuals with PD experience sleep-related issues, including insomnia, excessive daytime sleepiness (EDS), restless legs syndrome (RLS), and REM sleep behavior disorder (RBD). Fatigue often correlates with these sleep disturbances, creating a cyclical pattern where poor nighttime sleep exacerbates daytime fatigue, and vice versa. Addressing sleep disorders through proper management and treatment is crucial in alleviating fatigue and enhancing the overall well-being of PD patients [
9].
4.3.2. Depressive Disorders
Fatigue and depression are common and often overlapping non-motor symptoms in Parkinson’s disease (PD), contributing to significant impairment in daily functioning. Research suggests that depression affects around 36% of PD patients, while fatigue is reported by nearly 40%, with both conditions frequently coexisting [
124]. This overlap complicates diagnosis and treatment, as fatigue may be influenced by both psychological and neurological factors inherent to PD. Studies also indicate that individuals with both symptoms experience a more severe disease burden, including increased disability and diminished quality of life.
The relationship between fatigue and depression in PD is complex, with evidence suggesting a bidirectional influence. Depression can intensify perceptions of fatigue, while persistent fatigue may contribute to the development or worsening of depressive symptoms. Additionally, neurobiological mechanisms, including disruptions in dopaminergic and serotonergic pathways, are implicated in both conditions, highlighting the need for targeted therapeutic strategies. Addressing fatigue in PD requires a multidimensional approach, incorporating pharmacological treatments, behavioral interventions, and lifestyle modifications to improve overall patient well-being.
4.3.3. Cognitive Deficits
Fatigue and cognitive deficits are prevalent non-motor symptoms in Parkinson's disease (PD), often presenting concurrently and significantly impacting patients' quality of life. Research indicates that cognitive impairments, particularly in executive functions, may be associated with cognitive fatigue, as patients struggle with tasks requiring sustained mental effort. Additionally, attention deficits have been linked to fatigue in PD, suggesting that difficulties in maintaining focus may contribute to the perception of fatigue [
125].
The interplay between fatigue and cognitive deficits in PD is complex, with evidence pointing towards a bidirectional relationship. Cognitive impairments can exacerbate feelings of fatigue, while persistent fatigue may further impair cognitive performance, creating a vicious cycle that hinders daily functioning. Understanding this relationship is crucial for developing effective interventions aimed at alleviating both fatigue and cognitive deficits in PD patients [
126].
4.4. Deep Brain Stimulation And Parkinson’s Disease Fatigue
Bilateral subthalamic deep brain stimulation (STN-DBS), a widely recognized treatment for motor symptoms in Parkinson’s disease, has also shown potential benefits for non-motor symptoms. However, its effects on fatigue remain insufficiently studied, with small-scale investigations yielding inconsistent results [
127].
The exact mechanisms driving this improvement remain uncertain but are likely linked to STN-DBS modulation of the basal ganglia–thalamo–cortical and limbic circuits [
128]. Nevertheless, the studies assessing DBS in fatigue had small sample size, lack of a control group, and absence of a PD-specific fatigue scale limit the generalizability of the results. Future research using larger cohorts and PD-specific fatigue assessments is needed to clarify the effects of STN-DBS and identify potential predictors of treatment response.
A recent retrospective cross-sectional study reviewed the medical records of 50 individuals with PD who underwent STN-DBS, with an average follow-up of 1.98 ± 1.36 years. Fatigue was evaluated using the Non-Motor Symptoms Scale, revealing a significant median improvement of 41.5%. However, no baseline or follow-up factors were identified as significant predictors of fatigue improvement [
129]. These findings suggest that STN-DBS may help alleviate PD-related fatigue.
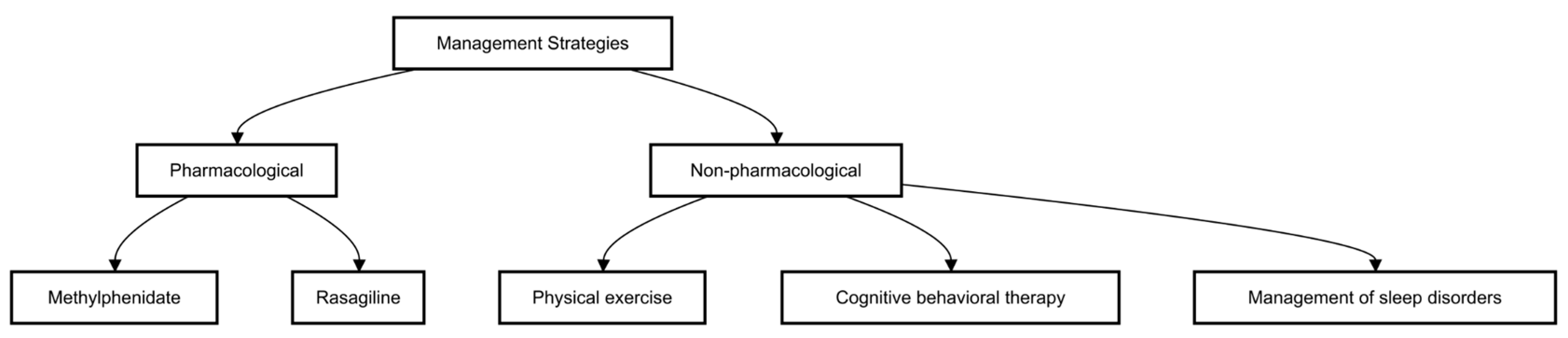
5. Management
Managing fatigue in Parkinson’s disease (PD) requires a multifaceted approach tailored to address both its physiological and psychological contributors. Non-pharmacological strategies, such as structured exercise programs, cognitive behavioral therapy (CBT), and sleep optimization, have shown benefits in reducing fatigue severity. Additionally, lifestyle modifications, including maintaining a balanced diet, managing stress, and ensuring proper hydration, can help improve energy levels. Pharmacological options, such as dopaminergic medications, stimulants, or antidepressants, may be considered in cases where fatigue significantly impacts daily functioning. However, individualized treatment plans are essential, as responses to therapy vary among patients. A comprehensive management approach that combines behavioral, pharmacological, and rehabilitative strategies can enhance overall well-being and quality of life for individuals with PD (
Figure 3).
5.1. Pharmacological Management
Fatigue is a prevalent and debilitating non-motor symptom in Parkinson's disease (PD), yet its pharmacological management remains challenging due to limited and inconclusive evidence. Levodopa, the cornerstone of PD motor symptom treatment, does not consistently alleviate fatigue, although some studies suggest it may slow its progression. This inconsistency underscores the need for alternative therapeutic options.
Stimulant medications, such as modafinil and methylphenidate, have been explored for their potential to reduce fatigue in PD patients. Methylphenidate is a dopamine transporter blocker. In a placebo-controlled study, it showed a statistically significant reduction in Fatigue Severity Scale [
31]. However, clinical trials have yielded mixed results, with some individuals reporting benefits while others experience minimal improvement. Notably, these stimulants are not currently approved for treating PD-related fatigue, highlighting the necessity for further rigorous studies to establish their efficacy and safety in this context.
Another pharmacological agent, rasagiline, a monoamine oxidase-B (MAO-B) inhibitor, has been investigated for its effects on fatigue in PD. It proved to have statistically significant reduction in Parkinson Fatigue Scale [
30]. While some studies indicate that rasagiline may reduce fatigue symptoms, the observed effect sizes are generally small and may not translate into clinically meaningful improvements. Consequently, more comprehensive research is required to determine the true benefit of rasagiline in managing PD-related fatigue.
Intrajejunal levodopa infusion (IJLI) has been investigated for its effects on non-motor symptoms, including fatigue, in Parkinson's disease (PD) patients. A prospective open-label observational study involving 22 advanced PD patients demonstrated significant improvements in six of the nine domains of the Non-Motor Symptoms Scale (NMSS), notably including the sleep/fatigue domain, following six months of IJLI therapy [
130]. These findings suggest that IJLI may effectively alleviate fatigue in PD patients, potentially enhancing their overall quality of life. However, further large-scale, randomized controlled trials are necessary to confirm these benefits and to better understand the long-term impact of IJLI on fatigue management in PD.
Doxepin, a tricyclic antidepressant, has been investigated for its potential to alleviate fatigue in Parkinson's disease (PD). A study by Rios Romenets et al. found that doxepin reduced fatigue severity and its impact on activities of daily living in PD patients [
131]. However, the authors noted that these findings require confirmation through larger, high-quality randomized controlled trials. Regarding dopaminergic medications, research indicates that levodopa may improve physical fatigue in PD patients. A study by Lou et al. demonstrated that levodopa administration led to significant improvements in physical fatigue during finger tapping and force generation tasks [
12]. However, the authors emphasized the need for further studies to confirm these results and understand the underlying mechanisms. Despite these potential pharmacological interventions, there is currently no definitive treatment for PD-related fatigue.
5.2. Non-Pharmacological Interventions
A study conducted in 2014 reported that high-intensity exercise in PD patients resulted in a significant improvement in fatigue by 17% [
132]. Moreover, a clinical trial showed that aerobic exercise accounted for a 0.5-point reduction in fatigue severity scale in PD [
133]. Adding to this, a recent meta-analysis investigating physical exercise as an intervention to treat fatigue in PD reported a standardized mean difference (SMD) of -0.37 (95% CI - 0.69- 0.05) when compared to the placebo group [
34], demonstrating a significant small effect of physical exercise on fatigue. At the same time, a randomized-controlled trial showed that home-based treadmill training is effective in reducing fatigue in patients with mild PD [
134]. In the latter study, treadmill training was associated with a 1.2-point reduction in the 7-point fatigue scale. Despite this, further research is still required to examine the effectiveness of exercise in improving fatigue in PD.
Another possible intervention is cognitive behavioral therapy (CBT). It has been demonstrated that CBT, a non-pharmacological approach to alter psychological states and ameliorate psychological issues, is beneficial for mental illnesses [
135]. A recent meta-analysis investigating the efficacy of CBT on mood disorders, sleep, and fatigue in PD showed a significant impact of CBT on depression, anxiety, and sleep disorders, yet no significant effect on fatigue [
136]. However, since fatigue is linked to several contributing factors, especially neuropsychiatric ones, as has been discussed before, CBT remains a potential choice, and its efficacy warrants further investigation.
The impact of sleep quality on fatigue progression is pivotal, as a positive correlation was indicated between sleep disorders and fatigue severity in PD [
10]. Moreover, it was found that PD patients with severe sleep disturbances are more likely to experience fatigue [
78]. Excessive Daytime Sleepiness (EDS), defined as undesired sleepiness during waking hours, has been found to influence fatigue in PD [
137]. In this regard, sleep quality is considered to play a critical role in the management plan. Addressing the factors associated with poor sleep quality in PD, such as depression, represents a detrimental approach to improving the quality of sleep [
138]. On a related note, a recent clinical trial showed that acupuncture therapy is beneficial in improving the quality of sleep in PD and thus quality of life [
139]. Tan et al. study aimed to systematically assess the effectiveness of traditional Chinese exercises in managing neuropsychiatric symptoms in individuals with Parkinson's disease. While some benefits were observed, the analysis revealed no statistically significant improvements in fatigue-related measures [
140].
6. Specialist Recommendations And Future Studies
Many biomarkers have been suggested to indicate fatigue in Parkinson's patients. As previously mentioned, neuroinflammation has a role in fatigue in Parkinson's patients. So multiple inflammatory markers can be used to detect fatigue, such as IL-6, IL1-Ra, sIL-2R, and VCAM-1 [
48]. A study by Crichton and the team in traumatic brain injury reported also IL-8 can also serve as a possible indicator of fatigue in these patients [
141]. This illustrates the importance of further studies on IL-8 and other inflammatory markers to identify fatigue in Parkinson's disease.
A recent exploratory study examined the cerebrospinal fluid of individuals with Parkinson’s disease who had low versus high fatigue scores. Using label-free liquid chromatography and tandem mass spectrometry, researchers identified 20 differentially expressed proteins. Supervised partial least squares discriminant analysis (PLS-DA) revealed that these proteins were associated with innate immunity, cellular stress responses, and sickness behavior, suggesting that these biological processes contribute to fatigue in PD. While the study’s small sample size limited the ability to control for confounding variables, the findings highlight the need for larger-scale investigations to further clarify the molecular mechanisms underlying PD-related fatigue [
142].
The latest systematic reviews in Parkinson's patients' fatigue (2018 and 2023) included only cross-sectional studies with a great lack of exploring longitudinal studies of fatigue [
5,
143]. Another systematic review conducted in 2015 concluded that there are no clear recommendations for fatigue in Parkinson's patients. Interestingly, it also highlighted the importance of further studies on behavioral or cognitive aspects of fatigue in Parkinson's patients, which was also supported by another study in 2018 [
144]. Moreover, most of the conducted studies focused on fatigue as one group with the same contributing factors. That is why Skorvanek et al. recommended studying fatigue in Parkinson's patients as two separate groups: primary and secondary fatigue due to differences in their clinical and psychosocial factors [
22]. Even the recent meta-analysis in interventions for fatigue in Parkinson's disease has its shortcomings as it only covered one pharmacological option in the analysis, which is modafinil. Also, the authors suggested further studies on the efficacy of physical exercises in the treatment of fatigue. This demands more studies to cover a wide range of pharmacological and non-pharmacological options [
34].
Kluger et al. suggested some recommendations in 2015 to improve the clinical research in this area, such as distinguishing fatigue from other related disorders (apathy, depression, etc.) [
6]. This was also suggested by Siciliano and colleagues, who in addition strengthened the importance of identifying fatigue biomarkers and proper fatigue management protocol [
5]. Kluger et al. lists continue as they also suggested differentiation in studies between subjective fatigue and objective fatigue and also suggested the specification of domains and causal factors affected by either type of fatigue [
6]. In addition to areas of intervention, further exploring of the pathophysiology of Parkinson's is still required. Wasson et al. conducted a study about fatigue in older adults. They noticed that higher mental fatigability is associated with smaller basal ganglia and limbic systems. The same basal ganglia play a central role in the pathophysiology of Parkinson's. That is why this hypothesis needs further studies in the Parkinson's population [
145].
7. Conclusions
Fatigue in Parkinson's disease presents one of the greatest challenges to the patient and clinicians alike, and its complexity characterizes a very multifactorial phenomenon (
Figure 4). That means it is not the result of a simple consequence of the illness but of an interaction among neurobiological, inflammatory, genetic, and psychosocial factors. The hallmark dopaminergic pathway dysfunction of PD must also necessarily be causally involved since studies have shown that there is some improvement with levodopa and dopamine agonists. However, variable results and the limited efficacy of dopaminergic therapies suggest that non-dopaminergic systems also contribute to fatigue, related to serotonin, norepinephrine, and adenosine. The role of chronic inflammation is substantiated by a growing body of evidence in PD, whereby raised inflammatory markers, such as IL-6 and TNF-α, have been seen in association with greater severity of fatigue. Genetic predispositions to heightened fatigue risk in PD patients are also attributed to genes such as LRRK2 and GBA mutations. These get further complicated by psychosocial factors, which include depression, anxiety, sleep disturbances, and medication side effects, having an Albany effect to further undermine fatigue and its impact on overall well-being and QOL. So intricately interwoven are the causative factors that not only motor symptoms but also, importantly, the non-motor burden of this most debilitating disease makes compelling arguments for a multifaced-like assessment and management.
Author Contributions
: J.P.R., I.K., A.D., W.A.F.E., M.H.E., R.S. and A.L.F.C. conceived and designed the methodology of the literature review. J.P.R., I.K., A.D., W.A.F.E., M.H.E., and R.S. extracted and collected the relevant information and drafted the manuscript. A.L.F.C. supervised the article selection and reviewed and edited the manuscript. J.P.R. and A.L.F.C. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACTH |
Adrenocorticotropic Hormone |
| CBT |
Cognitive Behavioral Therapy |
| CNS |
Central Nervous System |
| CSF |
Cerebrospinal Fluid |
| EDS |
Excessive Daytime Sleepiness |
| FACIT-F |
Functional Assessment of Chronic Illness Therapy-Fatigue Scale |
| FSS |
Fatigue Severity Scale |
| HADS |
Hospital Anxiety and Depression Scale |
| HRQoL |
Health-Related Quality of Life |
| IL-1Ra |
Interleukin 1 Receptor Antagonist |
| IL-6 |
Interleukin 6 |
| LEDD |
Levodopa Equivalent Daily Dose |
| MAO |
Monoamine Oxidase |
| MoCA |
Montreal Cognitive Assessment |
| NMSS |
Non-Motor Symptoms Scale |
| PFS |
Parkinson's Disease Fatigue Scale |
| PD |
Parkinson's Disease |
| REM |
Rapid Eye Movement |
| RBD |
REM Sleep Behavior Disorder |
| SNpc |
Substantia Nigra pars compacta |
| TLR4 |
Toll-Like Receptor 4 |
| TNF-α |
Tumor Necrosis Factor-alpha |
| VCAM-1 |
Vascular Cell Adhesion Molecule 1 |
References
- Hindeya Gebreyesus, H.; Gebrehiwot Gebremichael, T. The Potential Role of Astrocytes in Parkinson’s Disease (PD). Med Sci (Basel) 2020, 8. [Google Scholar] [CrossRef]
- Garcia Ruiz, P.J.; Catalán, M.J.; Fernández Carril, J.M. Initial Motor Symptoms of Parkinson Disease. Neurologist 2011, 17, S18–20. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Pollina, D.A. Mechanisms and Management of Fatigue in Progressive Neurological Disorders. Curr Opin Neurol 1996, 9, 456–460. [Google Scholar] [CrossRef]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Non Motor Subtypes and Parkinson’s Disease. Parkinsonism Relat Disord 2016, 22 Suppl 1, S41–46. [Google Scholar] [CrossRef]
- Siciliano, M.; Trojano, L.; Santangelo, G.; De Micco, R.; Tedeschi, G.; Tessitore, A. Fatigue in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov Disord 2018, 33, 1712–1723. [Google Scholar] [CrossRef]
- Kluger, B.M.; Herlofson, K.; Chou, K.L.; Lou, J.-S.; Goetz, C.G.; Lang, A.E.; Weintraub, D.; Friedman, J. Parkinson’s Disease-Related Fatigue: A Case Definition and Recommendations for Clinical Research. Mov Disord 2016, 31, 625–631. [Google Scholar] [CrossRef]
- Prakash, K.M.; Nadkarni, N.V.; Lye, W.-K.; Yong, M.-H.; Tan, E.-K. The Impact of Non-Motor Symptoms on the Quality of Life of Parkinson’s Disease Patients: A Longitudinal Study. Eur J Neurol 2016, 23, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H.; Friedman, H. Fatigue in Parkinson’s Disease: A Nine-Year Follow-Up. Mov Disord 2001, 16, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-Y.; Zhang, J.-R.; Shen, Y.; Mao, C.-J.; Shen, Y.-B.; Cao, Y.-L.; Gu, H.-Y.; Wang, F.; Liu, C.-F. Fatigue Correlates with Sleep Disturbances in Parkinson Disease. Chin Med J (Engl) 2020, 134, 668–674. [Google Scholar] [CrossRef]
- Metta, V.; Logishetty, K.; Martinez-Martin, P.; Gage, H.M.; Schartau, P.E.S.; Kaluarachchi, T.K.; Martin, A.; Odin, P.; Barone, P.; Stocchi, F.; et al. The Possible Clinical Predictors of Fatigue in Parkinson’s Disease: A Study of 135 Patients as Part of International Nonmotor Scale Validation Project. Parkinsons Dis 2011, 2011, 125271. [Google Scholar] [CrossRef]
- Siciliano, M.; Trojano, L.; De Micco, R.; De Mase, A.; Garramone, F.; Russo, A.; Tedeschi, G.; Tessitore, A. Motor, Behavioural, and Cognitive Correlates of Fatigue in Early, de Novo Parkinson Disease Patients. Parkinsonism Relat Disord 2017, 45, 63–68. [Google Scholar] [CrossRef]
- Lou, J.-S.; Kearns, G.; Benice, T.; Oken, B.; Sexton, G.; Nutt, J. Levodopa Improves Physical Fatigue in Parkinson’s Disease: A Double-Blind, Placebo-Controlled, Crossover Study. Mov Disord 2003, 18, 1108–1114. [Google Scholar] [CrossRef]
- Gołąb-Janowska, M.; Kotlęga, D.; Safranow, K.; Meller, A.; Budzianowska, A.; Honczarenko, K. Risk Factors of Fatigue in Idiopathic Parkinson’s Disease in a Polish Population. Parkinsons Dis 2016, 2016, 2835945. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.E.; Sethares, K.A. Fatigue in Parkinson Disease: An Integrative Review. J Neurosci Nurs 2015, 47, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.L.; Gilman, S.; Bohnen, N.I. Association between Autonomic Dysfunction and Fatigue in Parkinson Disease. J Neurol Sci 2017, 377, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and Fatigability in Neurologic Illnesses: Proposal for a Unified Taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in Healthy and Diseased Individuals. Am J Hosp Palliat Care 2014, 31, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue and Basal Ganglia. J Neurol Sci 2000, 179, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.S.; Kearns, G.; Oken, B.; Sexton, G.; Nutt, J. Exacerbated Physical Fatigue and Mental Fatigue in Parkinson’s Disease. Mov Disord 2001, 16, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H.; Beck, J.C.; Chou, K.L.; Clark, G.; Fagundes, C.P.; Goetz, C.G.; Herlofson, K.; Kluger, B.; Krupp, L.B.; Lang, A.E.; et al. Fatigue in Parkinson’s Disease: Report from a Mutidisciplinary Symposium. NPJ Parkinsons Dis 2016, 2, 15025. [Google Scholar] [CrossRef] [PubMed]
- Havlikova, E.; Rosenberger, J.; Nagyova, I.; Middel, B.; Dubayova, T.; Gdovinova, Z.; W Groothoff, J.; P van Dijk, J. Clinical and Psychosocial Factors Associated with Fatigue in Patients with Parkinson’s Disease. Parkinsonism Relat Disord 2008, 14, 187–192. [Google Scholar] [CrossRef]
- Skorvanek, M.; Nagyova, I.; Rosenberger, J.; Krokavcova, M.; Ghorbani Saeedian, R.; Groothoff, J.W.; Gdovinova, Z.; van Dijk, J.P. Clinical Determinants of Primary and Secondary Fatigue in Patients with Parkinson’s Disease. J Neurol 2013, 260, 1554–1561. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wang, W.-S.; Lewis, S.J.G.; Wu, S.-L. Fighting Against the Clock: Circadian Disruption and Parkinson’s Disease. J Mov Disord 2024, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Barone, P.; Marconi, R.; Morgante, L.; Zappulla, S.; Pontieri, F.E.; Ramat, S.; Ceravolo, M.G.; Meco, G.; Cicarelli, G.; et al. The Progression of Non-Motor Symptoms in Parkinson’s Disease and Their Contribution to Motor Disability and Quality of Life. J Neurol 2012, 259, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, S.; Monescu, V.; Filip, R.; Marian, L.; Kakucs, C.; Murasan, I.; Chaudhuri, K.R.; Jianu, D.C.; Falup-Pecurariu, C.; Opritoiu, B. The Impact of Fatigue on Sleep and Other Non-Motor Symptoms in Parkinson’s Disease. Brain Sci 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Sun, L.; Liu, Z.; Huang, X.-Y.; Zuo, L.-J.; Cao, C.-J.; Zhang, W.; Wang, X.-M. Sleep Disorders in Parkinson’s Disease: Clinical Features, Iron Metabolism and Related Mechanism. PLoS One 2013, 8, e82924. [Google Scholar] [CrossRef]
- Maestri, M.; Romigi, A.; Schirru, A.; Fabbrini, M.; Gori, S.; Bonuccelli, U.; Bonanni, E. Excessive Daytime Sleepiness and Fatigue in Neurological Disorders. Sleep Breath 2020, 24, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Vallone, D.; Picetti, R.; Borrelli, E. Structure and Function of Dopamine Receptors. Neuroscience & biobehavioral reviews 2000, 24, 125–132. [Google Scholar]
- Rascol, O.; Fitzer-Attas, C.J.; Hauser, R.; Jankovic, J.; Lang, A.; Langston, J.W.; Melamed, E.; Poewe, W.; Stocchi, F.; Tolosa, E.; et al. A Double-Blind, Delayed-Start Trial of Rasagiline in Parkinson’s Disease (the ADAGIO Study): Prespecified and Post-Hoc Analyses of the Need for Additional Therapies, Changes in UPDRS Scores, and Non-Motor Outcomes. Lancet Neurol 2011, 10, 415–423. [Google Scholar] [CrossRef]
- Stocchi, F. Benefits of Treatment with Rasagiline for Fatigue Symptoms in Patients with Early Parkinson’s Disease. Eur J Neurol 2014, 21, 357–360. [Google Scholar] [CrossRef]
- Mendonça, D.A.; Menezes, K.; Jog, M.S. Methylphenidate Improves Fatigue Scores in Parkinson Disease: A Randomized Controlled Trial. Mov Disord 2007, 22, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.J.; Lin, T.S. Evaluation of Fatigue in Parkinson’s Disease Patients with Stimulated Single Fiber Electromyography. Acta Neurol Scand 2001, 104, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Oved, D.; Ziv, I.; Treves, T.A.; Paleacu, D.; Melamed, E.; Djaldetti, R. Effect of Dopamine Agonists on Fatigue and Somnolence in Parkinson’s Disease. Mov Disord 2006, 21, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, A.-K.; Nielsen, J.; Gollan, R.; Lansu, A.; Solfronk, D.; Monsef, I.; Ernst, M.; Skoetz, N.; Zeuner, K.E.; Kalbe, E. Physical Exercise as a Potential Treatment for Fatigue in Parkinson’s Disease? A Systematic Review and Meta-Analysis of Pharmacological and Non-Pharmacological Interventions. J Parkinsons Dis 2023, 13, 659–679. [Google Scholar] [CrossRef] [PubMed]
- Pardini, M.; Bonzano, L.; Mancardi, G.L.; Roccatagliata, L. Frontal Networks Play a Role in Fatigue Perception in Multiple Sclerosis. Behav Neurosci 2010, 124, 329–336. [Google Scholar] [CrossRef]
- Tang, W.K.; Chen, Y.K.; Mok, V.; Chu, W.C.W.; Ungvari, G.S.; Ahuja, A.T.; Wong, K.S. Acute Basal Ganglia Infarcts in Poststroke Fatigue: An MRI Study. J Neurol 2010, 257, 178–182. [Google Scholar] [CrossRef]
- Abe, K.; Takanashi, M.; Yanagihara, T. Fatigue in Patients with Parkinson’s Disease. Behav Neurol 2000, 12, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central Fatigue: The Serotonin Hypothesis and Beyond. Sports Med 2006, 36, 881–909. [Google Scholar] [CrossRef] [PubMed]
- Hornung, J.-P. The Human Raphe Nuclei and the Serotonergic System. J Chem Neuroanat 2003, 26, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Pavese, N.; Metta, V.; Bose, S.K.; Chaudhuri, K.R.; Brooks, D.J. Fatigue in Parkinson’s Disease Is Linked to Striatal and Limbic Serotonergic Dysfunction. Brain 2010, 133, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ouchi, Y.; Onoe, H.; Yoshikawa, E.; Tsukada, H.; Takahashi, H.; Iwase, M.; Yamaguti, K.; Kuratsune, H.; Watanabe, Y. Reduction of Serotonin Transporters of Patients with Chronic Fatigue Syndrome. Neuroreport 2004, 15, 2571–2574. [Google Scholar] [CrossRef]
- Pauletti, C.; Mannarelli, D.; Locuratolo, N.; Maffucci, A.; Currà, A.; Marinelli, L.; Fattapposta, F. Serotonergic Central Tone in Parkinson’s Disease with Fatigue: Evidence from the Loudness Dependence of Auditory Evoked Potentials (LDAEP). Neurosci Lett 2021, 764, 136242. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R.; et al. Caffeine for Treatment of Parkinson Disease: A Randomized Controlled Trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Pagonabarraga, J.; Tinazzi, M.; Caccia, C.; Jost, W.H. The Role of Glutamatergic Neurotransmission in the Motor and Non-Motor Symptoms in Parkinson’s Disease: Clinical Cases and a Review of the Literature. J Clin Neurosci 2021, 90, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Versace, V.; Sebastianelli, L.; Ferrazzoli, D.; Romanello, R.; Ortelli, P.; Saltuari, L.; D’Acunto, A.; Porrazzini, F.; Ajello, V.; Oliviero, A.; et al. Intracortical GABAergic Dysfunction in Patients with Fatigue and Dysexecutive Syndrome after COVID-19. Clin Neurophysiol 2021, 132, 1138–1143. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Valitabar, Z.; Ashraf-Ganjouei, A.; Mojtahed Zadeh, M.; Ghazi Sherbaf, F.; Aarabi, M.H. Cerebrospinal Fluid C-Reactive Protein in Parkinson’s Disease: Associations with Motor and Non-Motor Symptoms. Neuromolecular Med 2018, 20, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Katarina, V.; Gordana, T.; Svetlana, M.D.; Milica, B. Oxidative Stress and Neuroinflammation Should Be Both Considered in the Occurrence of Fatigue and Depression in Multiple Sclerosis. Acta Neurol Belg 2020, 120, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Witte, O.W.; Grosskreutz, J. Biomarkers for Dementia, Fatigue, and Depression in Parkinson’s Disease. Front Neurol 2019, 10, 195. [Google Scholar] [CrossRef]
- Lindqvist, D.; Hall, S.; Surova, Y.; Nielsen, H.M.; Janelidze, S.; Brundin, L.; Hansson, O. Cerebrospinal Fluid Inflammatory Markers in Parkinson’s Disease--Associations with Depression, Fatigue, and Cognitive Impairment. Brain Behav Immun 2013, 33, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.R.; Santos, L.V.D.; Santos, R.M.S.; Campos, A.L.F.; Pimenta, A.L.; de Oliveira, M.S.; Bacheti, G.G.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; et al. IL-6 Serum Levels Are Elevated in Parkinson’s Disease Patients with Fatigue Compared to Patients without Fatigue. J Neurol Sci 2016, 370, 153–156. [Google Scholar] [CrossRef]
- Wang, L.; Yi, H.; Liang, X.; Xu, F.; Li, T.; Yang, X.; Wei, M.; Ou, Z.; Tong, Q. Plasma TNF-α and Phosphorylated α-Syn Are Associated with Fatigue in Patients with Parkinson’s Disease. J Neuroimmunol 2023, 385, 578222. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory Mechanisms in Parkinson’s Disease: Potential Environmental Triggers, Pathways, and Targets for Early Therapeutic Intervention. Exp Neurol 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Zhao, J.; Guo, X.; Hu, M.; Chen, Y. Possible Inflammatory Mechanisms and Predictors of Parkinson’s Disease Patients with Fatigue (Brief Review). Clin Neurol Neurosurg 2021, 208, 106844. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Duran, R.; Hughes, D.A.; Mehta, A.; Schapira, A.H.V. A Clinical and Family History Study of Parkinson’s Disease in Heterozygous Glucocerebrosidase Mutation Carriers. J Neurol Neurosurg Psychiatry 2012, 83, 853–854. [Google Scholar] [CrossRef]
- Grabowski, G.A.; Zimran, A.; Ida, H. Gaucher Disease Types 1 and 3: Phenotypic Characterization of Large Populations from the ICGG Gaucher Registry. Am J Hematol 2015, 90 Suppl 1, S12–18. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, J.-Y.; Kim, H.J.; Kim, J.S.; Shin, E.-S.; Cho, J.-H.; Park, S.S.; Jeon, B.S. The LRRK2 G2385R Variant Is a Risk Factor for Sporadic Parkinson’s Disease in the Korean Population. Parkinsonism Relat Disord 2010, 16, 85–88. [Google Scholar] [CrossRef]
- Fu, R.; Cui, S.-S.; Du, J.-J.; He, Y.-C.; Gao, C.; Huang, P.; Qian, Y.-W.; Luo, X.-G.; Chen, S.-D. Fatigue Correlates with LRRK2 G2385R Variant in Chinese Parkinson’s Disease Patients. Parkinsonism Relat Disord 2017, 44, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, H.; Yuan, Y.; Fan, S.; Li, L.; Jiang, C.; Mao, C.; Shi, C.; Xu, Y. Peripheral Synucleinopathy in Parkinson Disease with LRRK2 G2385R Variants. Ann Clin Transl Neurol 2021, 8, 592–602. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; Loos, B.; Swart, C.; Kinnear, C.; Henning, F.; van der Merwe, L.; Pillay, K.; Muller, N.; Zaharie, D.; Engelbrecht, L.; et al. Mitochondrial Impairment Observed in Fibroblasts from South African Parkinson’s Disease Patients with Parkin Mutations. Biochem Biophys Res Commun 2014, 447, 334–340. [Google Scholar] [CrossRef]
- Ross, O.A.; Braithwaite, A.T.; Skipper, L.M.; Kachergus, J.; Hulihan, M.M.; Middleton, F.A.; Nishioka, K.; Fuchs, J.; Gasser, T.; Maraganore, D.M.; et al. Genomic Investigation of Alpha-Synuclein Multiplication and Parkinsonism. Ann Neurol 2008, 63, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G. Alpha Synuclein: Neurodegeneration and Inflammation. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Zuo, L.J.; Yu, S.Y.; Wang, F.; Hu, Y.; Piao, Y.S.; Du, Y.; Lian, T.H.; Wang, R.D.; Yu, Q.J.; Wang, Y.J.; et al. Parkinson’s Disease with Fatigue: Clinical Characteristics and Potential Mechanisms Relevant to α-Synuclein Oligomer. J Clin Neurol 2016, 12, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue in Neurological Disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Kümpfel, T.; Flachenecker, P.; Uhr, M.; Trenkwalder, C.; Holsboer, F.; Weber, F. Fatigue and Regulation of the Hypothalamo-Pituitary-Adrenal Axis in Multiple Sclerosis. Arch Neurol 2005, 62, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.; Boegershausen, N.; Meyer, S.; Ivan, D.; Schepelmann, K.; Kann, P.H. Hypothalamic-Pituitary Insufficiency Following Infectious Diseases of the Central Nervous System. Eur J Endocrinol 2008, 158, 3–9. [Google Scholar] [CrossRef]
- Kenangil, G.; Orken, D.N.; Ur, E.; Forta, H.; Celik, M. The Relation of Testosterone Levels with Fatigue and Apathy in Parkinson’s Disease. Clin Neurol Neurosurg 2009, 111, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Stevens-Lapsley, J.; Kluger, B.M.; Schenkman, M. Quadriceps Muscle Weakness, Activation Deficits, and Fatigue with Parkinson Disease. Neurorehabil Neural Repair 2012, 26, 533–541. [Google Scholar] [CrossRef]
- Khalil, I.; Sayad, R.; Kedwany, A.M.; Sayed, H.H.; Caprara, A.L.F.; Rissardo, J.P. Cardiovascular Dysautonomia and Cognitive Impairment in Parkinson’s Disease (Review). Med Int (Lond) 2024, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Shawky, O.A.; Kamel, N.F.; Rothwell, J.C.; Ahmed, M.A.; Hamdy, A. Dopamine Release after Repetitive Transcranial Magnetic Stimulation of Motor Cortex in Parkinson’s Disease Patients. Egypt J Neurol Psychiat Neurosurg 2007, 44, 323–331. [Google Scholar]
- Pitton Rissardo, J.; Fornari Caprara, A.L. Cardiac 123I-Metaiodobenzylguanidine (MIBG) Scintigraphy in Parkinson’s Disease: A Comprehensive Review. Brain Sci 2023, 13. [Google Scholar] [CrossRef]
- Souza, B.R.A.; Nóbrega, K.C.C.; Silva, B.E. de A. da; Gonçalves, R.A.; Martins, T.S.; Santos, G.F.; Frazão, A.H.; Roque, A.C.; Nascimento, I.A.P. da S.; Piemonte, M.E.P. The Impact of Motor, Non-Motor, and Social Aspects on the Sexual Health of Men Living with Parkinson’s Disease. J Parkinsons Dis 2024, 14, 565–574. [Google Scholar] [CrossRef]
- Minibajeva, O.; Zeltiņa, E.; Karelis, G.; Kurjāne, N.; Ķēniņa, V. Clinical Symptoms Influencing Parkinson’s Patients’ Quality of Life in Latvia: A Single-Center Cohort Study. Medicina (Kaunas) 2023, 59. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Song, T.; Zhao, Y.; Pan, H.; Xu, Q.; Chen, Y.; Sun, Q.; Wu, X.; Yan, X.; et al. Characteristics of Fatigue in Parkinson’s Disease: A Longitudinal Cohort Study. Front Aging Neurosci 2023, 15, 1133705. [Google Scholar] [CrossRef]
- Nassif, D.V.; Pereira, J.S. Fatigue in Brazilian Patients with Parkinson’s Disease. Dement Neuropsychol 2022, 16, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Güler, S.; Caylan, A.; Turan, F.N.; Dağdeviren, N. Prevalence and Clinical Features of Idiopathic Parkinson’s Disease in Western Turkey. Noro Psikiyatr Ars 2022, 59, 98–104. [Google Scholar] [CrossRef]
- Ineichen, C.; Baumann-Vogel, H. Deconstructing Apathy in Parkinson’s Disease: Challenges in Isolating Core Components of Apathy From Depression, Anxiety, and Fatigue. Front Neurol 2021, 12, 720921. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; Trojano, L.; De Micco, R.; Giordano, A.; Russo, A.; Tedeschi, G.; Chiorri, C.; Tessitore, A. Predictors of Fatigue Severity in Early, de Novo Parkinson Disease Patients: A 1-Year Longitudinal Study. Parkinsonism Relat Disord 2020, 79, 3–8. [Google Scholar] [CrossRef]
- Fu, R.; Luo, X.-G.; Ren, Y.; He, Z.-Y.; Lv, H. Clinical Characteristics of Fatigued Parkinson’s Patients and the Response to Dopaminergic Treatment. Transl Neurodegener 2016, 5, 9. [Google Scholar] [CrossRef]
- Mukadam, N.; Kinger, S.B.; Neargarder, S.; Salazar, R.D.; McDowell, C.P.; Wall, J.; Kaplan, R.I.; Cronin-Golomb, A. Changes in Subjective Cognitive and Social Functioning in Parkinson’s Disease from Before to During the COVID-19 Pandemic. Healthcare (Basel) 2025, 13. [Google Scholar] [CrossRef]
- Brown, R.G.; Dittner, A.; Findley, L.; Wessely, S.C. The Parkinson Fatigue Scale. Parkinsonism Relat Disord 2005, 11, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pont-Sunyer, C.; Hotter, A.; Gaig, C.; Seppi, K.; Compta, Y.; Katzenschlager, R.; Mas, N.; Hofeneder, D.; Brücke, T.; Bayés, A.; et al. The Onset of Nonmotor Symptoms in Parkinson’s Disease (the ONSET PD Study). Mov Disord 2015, 30, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Horsfall, L.; Walters, K.; Noyce, A.; Petersen, I. Prediagnostic Presentations of Parkinson’s Disease in Primary Care: A Case-Control Study. Lancet Neurol 2015, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Assmus, J.; Herlofson, K.; Larsen, J.P.; Tysnes, O.-B. Importance of Motor vs. Non-Motor Symptoms for Health-Related Quality of Life in Early Parkinson’s Disease. Parkinsonism Relat Disord 2013, 19, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Miwa, T. Fatigue in Patients with Parkinson’s Disease: Impact on Quality of Life. Intern Med 2011, 50, 1553–1558. [Google Scholar] [CrossRef]
- Friedman, J.H.; Abrantes, A.; Sweet, L.H. Fatigue in Parkinson’s Disease. Expert Opin Pharmacother 2011, 12, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Qi, Y.; He, J.; Zheng, X.; Ren, W.; Chang, Y. Analysis of Influencing Factors of Apathy in Patients with Parkinson’s Disease. Brain Sci 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M. Fatigue in Parkinson’s Disease. Int Rev Neurobiol 2017, 133, 743–768. [Google Scholar] [CrossRef]
- Zesiewicz, T.A.; Patel-Larson, A.; Hauser, R.A.; Sullivan, K.L. Social Security Disability Insurance (SSDI) in Parkinson’s Disease. Disabil Rehabil 2007, 29, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, G.D.; Rizvi, S.; Abrantes, A.M.; Crabtree, B.; Cahill, J.; Friedman, J.H. The Association between Fatigue and Apathy in Patients with Either Parkinson’s Disease or Multiple Sclerosis. Parkinsonism Relat Disord 2015, 21, 1093–1095. [Google Scholar] [CrossRef]
- Elbers, R.G.; van Wegen, E.E.; Verhoef, J.; Kwakkel, G. Impact of Fatigue on Health-Related Quality of Life in Patients with Parkinson’s Disease: A Prospective Study. Clin Rehabil 2014, 28, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, X.; Chen, K.; Chen, X.; Cao, B.; Wei, Q.; Huang, R.; Zhao, B.; Wu, Y.; Shang, H.-F. The Impact of Non-Motor Symptoms on the Health-Related Quality of Life of Parkinson’s Disease Patients from Southwest China. Parkinsonism Relat Disord 2014, 20, 149–152. [Google Scholar] [CrossRef]
- Dogan, V.B.; Koksal, A.; Dirican, A.; Baybas, S.; Dirican, A.; Dogan, G.B. Independent Effect of Fatigue on Health-Related Quality of Life in Patients with Idiopathic Parkinson’s Disease. Neurol Sci 2015, 36, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Friedman, H. Fatigue in Parkinson’s Disease. Neurology 1993, 43, 2016–2018. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The Fatigue Severity Scale. Application to Patients with Multiple Sclerosis and Systemic Lupus Erythematosus. Arch Neurol 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.E.; Jandorf, L.; Krupp, L.B. The Measurement of Fatigue: A New Instrument. J Psychosom Res 1993, 37, 753–762. [Google Scholar] [CrossRef]
- Schiehser, D.M.; Ayers, C.R.; Liu, L.; Lessig, S.; Song, D.S.; Filoteo, J.V. Validation of the Modified Fatigue Impact Scale in Parkinson’s Disease. Parkinsonism Relat Disord 2013, 19, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Catalan, M.J.; Benito-Leon, J.; Moreno, A.O.; Zamarbide, I.; Cubo, E.; van Blercon, N.; Arillo, V.C.; Pondal, M.; Linazasoro, G.; et al. Impact of Fatigue in Parkinson’s Disease: The Fatigue Impact Scale for Daily Use (D-FIS). Qual Life Res 2006, 15, 597–606. [Google Scholar] [CrossRef]
- Hagell, P.; Höglund, A.; Reimer, J.; Eriksson, B.; Knutsson, I.; Widner, H.; Cella, D. Measuring Fatigue in Parkinson’s Disease: A Psychometric Study of Two Brief Generic Fatigue Questionnaires. J Pain Symptom Manage 2006, 32, 420–432. [Google Scholar] [CrossRef]
- Pitton Rissardo, J.; Fornari Caprara, A.L. Parkinson’s Disease Rating Scales: A Literature Review. Annals of Movement Disorders 2020, 3. [Google Scholar] [CrossRef]
- Huether, A.X.A.; Pottinger, T.; Lou, J.-S. Screening Cut-off Scores for Clinically Significant Fatigue in Early Parkinson’s Disease. Clin Park Relat Disord 2023, 9, 100228. [Google Scholar] [CrossRef] [PubMed]
- Niimi, Y.; Shima, S.; Mizutani, Y.; Ueda, A.; Ito, S.; Mutoh, T. Fatigue Evaluated Using the 16-Item Parkinson Fatigue Scale (PFS-16) Predicts Parkinson’s Disease Prognosis. Fujita Med J 2019, 5, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Chong, R.; Albor, L.; Wakade, C.; Morgan, J. The Dimensionality of Fatigue in Parkinson’s Disease. J Transl Med 2018, 16, 192. [Google Scholar] [CrossRef]
- Panigrahi, B.; Pillai, K.S.; Radhakrishnan, D.M.; Rajan, R.; Srivastava, A.K. Fatigue in Parkinson’s Disease—A Narrative Review. Annals of Movement Disorders 2024, 7, 157–171. [Google Scholar] [CrossRef]
- Sáez-Francàs, N.; Hernández-Vara, J.; Corominas Roso, M.; Alegre Martín, J.; Casas Brugué, M. The Association of Apathy with Central Fatigue Perception in Patients with Parkinson’s Disease. Behav Neurosci 2013, 127, 237–244. [Google Scholar] [CrossRef]
- Hagell, P.; Brundin, L. Towards an Understanding of Fatigue in Parkinson Disease. J Neurol Neurosurg Psychiatry 2009, 80, 489–492. [Google Scholar] [CrossRef]
- Spirgi, S.; Meyer, A.; Calabrese, P.; Gschwandtner, U.; Fuhr, P. Effects of Cognitive Performance and Affective Status on Fatigue in Parkinson’s Disease. Dement Geriatr Cogn Dis Extra 2019, 9, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.; Edison, B.; Mantri, S.; Albert, S.; Daeschler, M.; Kopil, C.; Marras, C.; Chahine, L.M. Triggers and Alleviating Factors for Fatigue in Parkinson’s Disease. PLoS One 2021, 16, e0245285. [Google Scholar] [CrossRef]
- Okuma, Y.; Kamei, S.; Morita, A.; Yoshii, F.; Yamamoto, T.; Hashimoto, S.; Utsumi, H.; Hatano, T.; Hattori, N.; Matsumura, M.; et al. Fatigue in Japanese Patients with Parkinson’s Disease: A Study Using Parkinson Fatigue Scale. Mov Disord 2009, 24, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.R.E.; Chua, C.Y.; Ng, S.Y.-E.; Chia, N.S.-Y.; Saffari, S.E.; Chen, R.Y.-Y.; Choi, X.; Heng, D.L.; Neo, S.X.; Tay, K.Y.; et al. Poor Sleep Quality Is Associated with Fatigue and Depression in Early Parkinson’s Disease: A Longitudinal Study in the PALS Cohort. Front Neurol 2022, 13, 998103. [Google Scholar] [CrossRef]
- Junho, B.T.; Kummer, A.; Cardoso, F.E.; Teixeira, A.L.; Rocha, N.P. Sleep Quality Is Associated with the Severity of Clinical Symptoms in Parkinson’s Disease. Acta Neurol Belg 2018, 118, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Solla, P.; Cannas, A.; Mulas, C.S.; Perra, S.; Corona, A.; Bassareo, P.P.; Marrosu, F. Association between Fatigue and Other Motor and Non-Motor Symptoms in Parkinson’s Disease Patients. J Neurol 2014, 261, 382–391. [Google Scholar] [CrossRef]
- Kwon, K.-Y.; Joo, B.-E.; You, J.; Kim, R.O. Impact of Motor Features on Non-Motor Symptoms in Patients with de Novo Parkinson’s Disease: Cognition, Depression, Anxiety, Fatigue, and Dysautonomia. Geriatr Gerontol Int 2025. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Francàs, N.; Hernández-Vara, J.; Corominas-Roso, M.; Alegre, J.; Jacas, C.; Casas, M. Relationship between Poor Decision-Making Process and Fatigue Perception in Parkinson’s Disease Patients. J Neurol Sci 2014, 337, 167–172. [Google Scholar] [CrossRef]
- Yu, H.-X.; Guo, M.-R.; Li, G.; Zhang, B. Association between Fatigue and Motor Progression in Parkinson’s Disease in Southern Chinese. Neurol Sci 2020, 41, 161–164. [Google Scholar] [CrossRef]
- Alves, G.; Wentzel-Larsen, T.; Larsen, J.P. Is Fatigue an Independent and Persistent Symptom in Patients with Parkinson Disease? Neurology 2004, 63, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Terra, M.B.; Lopes, J.; Bueno, M.E.B.; Trinca, L.A.; Smaili, S.M. Association between Fatigue and MDS-UPDRS in Individuals with Parkinson’s Disease: Cross-Sectional Study. Neurol Sci 2024, 45, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Falup Pecurariu, C.; Odin, P.; van Hilten, J.J.; Antonini, A.; Rojo-Abuin, J.M.; Borges, V.; Trenkwalder, C.; Aarsland, D.; Brooks, D.J.; et al. Gender-Related Differences in the Burden of Non-Motor Symptoms in Parkinson’s Disease. J Neurol 2012, 259, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Beiske, A.G.; Loge, J.H.; Hjermstad, M.J.; Svensson, E. Fatigue in Parkinson’s Disease: Prevalence and Associated Factors. Mov Disord 2010, 25, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Lerdal, A.; Wahl, A.; Rustøen, T.; Hanestad, B.R.; Moum, T. Fatigue in the General Population: A Translation and Test of the Psychometric Properties of the Norwegian Version of the Fatigue Severity Scale. Scand J Public Health 2005, 33, 123–130. [Google Scholar] [CrossRef]
- Ongre, S.O.; Dalen, I.; Tysnes, O.-B.; Alves, G.; Herlofson, K. Progression of Fatigue in Parkinson’s Disease - a 9-Year Follow-Up. Eur J Neurol 2021, 28, 108–116. [Google Scholar] [CrossRef]
- Ou, R.; Hou, Y.; Liu, K.; Lin, J.; Jiang, Z.; Wei, Q.; Zhang, L.; Cao, B.; Zhao, B.; Song, W.; et al. Progression of Fatigue in Early Parkinson’s Disease: A 3-Year Prospective Cohort Study. Front Aging Neurosci 2021, 13, 701906. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, K.; Larsen, J.P.; Tandberg, E.; Jørgensen, K. Fatigue in Patients with Parkinson’s Disease. Mov Disord 1999, 14, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Valko, P.O.; Waldvogel, D.; Weller, M.; Bassetti, C.L.; Held, U.; Baumann, C.R. Fatigue and Excessive Daytime Sleepiness in Idiopathic Parkinson’s Disease Differently Correlate with Motor Symptoms, Depression and Dopaminergic Treatment. Eur J Neurol 2010, 17, 1428–1436. [Google Scholar] [CrossRef]
- Shulman, L.M.; Taback, R.L.; Bean, J.; Weiner, W.J. Comorbidity of the Nonmotor Symptoms of Parkinson’s Disease. Mov Disord 2001, 16, 507–510. [Google Scholar] [CrossRef]
- Ortelli, P.; Versace, V.; Saltuari, L.; Randi, A.; Stolz, J.; Dezi, S.; Maestri, R.; Buechner, S.; Giladi, N.; Oliviero, A.; et al. Looking Deeper: Does a Connection Exist between Fatigue and Attentional Deficits in Parkinson’s Disease? A Conceptual Framework. Front Neurol 2023, 14, 1212876. [Google Scholar] [CrossRef] [PubMed]
- Di Vico, I.A.; Cirillo, G.; Tessitore, A.; Siciliano, M.; Venturelli, M.; Falup-Pecurariu, C.; Tedeschi, G.; Morgante, F.; Tinazzi, M. Fatigue in Hypokinetic, Hyperkinetic, and Functional Movement Disorders. Parkinsonism Relat Disord 2021, 86, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M.; Parra, V.; Jacobson, C.; Garvan, C.W.; Rodriguez, R.L.; Fernandez, H.H.; Fogel, A.; Skoblar, B.M.; Bowers, D.; Okun, M.S. The Prevalence of Fatigue Following Deep Brain Stimulation Surgery in Parkinson’s Disease and Association with Quality of Life. Parkinsons Dis 2012, 2012, 769506. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Vora, N.M.; Tariq, I.; Mujtaba, A.; Caprara, A.L.F. Deep Brain Stimulation for the Management of Refractory Neurological Disorders: A Comprehensive Review. Medicina (Kaunas) 2023, 59, 1991. [Google Scholar] [CrossRef]
- Lazcano-Ocampo, C.; van Wamelen, D.; Samuel, M.; Silverdale, M.; Rizos, A.; Sauerbier, A.; Koch, J.; Podlewska, A.; Leta, V.; Dafsari, H.S.; et al. Evaluation of the Effect of Bilateral Subthalamic Nucleus Deep Brain Stimulation on Fatigue in Parkinson’s Disease as Measured by the Non-Motor Symptoms Scale. Br J Neurosurg 2024, 38, 712–715. [Google Scholar] [CrossRef]
- Honig, H.; Antonini, A.; Martinez-Martin, P.; Forgacs, I.; Faye, G.C.; Fox, T.; Fox, K.; Mancini, F.; Canesi, M.; Odin, P.; et al. Intrajejunal Levodopa Infusion in Parkinson’s Disease: A Pilot Multicenter Study of Effects on Nonmotor Symptoms and Quality of Life. Mov Disord 2009, 24, 1468–1474. [Google Scholar] [CrossRef]
- Rios Romenets, S.; Creti, L.; Fichten, C.; Bailes, S.; Libman, E.; Pelletier, A.; Postuma, R.B. Doxepin and Cognitive Behavioural Therapy for Insomnia in Patients with Parkinson’s Disease -- a Randomized Study. Parkinsonism Relat Disord 2013, 19, 670–675. [Google Scholar] [CrossRef]
- Kelly, N.A.; Ford, M.P.; Standaert, D.G.; Watts, R.L.; Bickel, C.S.; Moellering, D.R.; Tuggle, S.C.; Williams, J.Y.; Lieb, L.; Windham, S.T.; et al. Novel, High-Intensity Exercise Prescription Improves Muscle Mass, Mitochondrial Function, and Physical Capacity in Individuals with Parkinson’s Disease. J Appl Physiol (1985) 2014, 116, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Uc, E.Y.; Doerschug, K.C.; Magnotta, V.; Dawson, J.D.; Thomsen, T.R.; Kline, J.N.; Rizzo, M.; Newman, S.R.; Mehta, S.; Grabowski, T.J.; et al. Phase I/II Randomized Trial of Aerobic Exercise in Parkinson Disease in a Community Setting. Neurology 2014, 83, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.G.; Allen, N.E.; Dean, C.M.; Goh, L.; Fung, V.S.C. Home-Based Treadmill Training for Individuals with Parkinson’s Disease: A Randomized Controlled Pilot Trial. Clin Rehabil 2012, 26, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.K.; Andrews, L.A.; Witcraft, S.M.; Powers, M.B.; Smits, J.A.J.; Hofmann, S.G. Cognitive Behavioral Therapy for Anxiety and Related Disorders: A Meta-Analysis of Randomized Placebo-Controlled Trials. Depress Anxiety 2018, 35, 502–514. [Google Scholar] [CrossRef]
- Luo, F.; Ye, M.; Lv, T.; Hu, B.; Chen, J.; Yan, J.; Wang, A.; Chen, F.; He, Z.; Ding, Z.; et al. Efficacy of Cognitive Behavioral Therapy on Mood Disorders, Sleep, Fatigue, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Psychiatry 2021, 12, 793804. [Google Scholar] [CrossRef] [PubMed]
- Knie, B.; Mitra, M.T.; Logishetty, K.; Chaudhuri, K.R. Excessive Daytime Sleepiness in Patients with Parkinson’s Disease. CNS Drugs 2011, 25, 203–212. [Google Scholar] [CrossRef]
- Wade, R.; Pachana, N.A.; Mellick, G.; Dissanayaka, N. Factors Related to Sleep Disturbances for Individuals with Parkinson’s Disease: A Regional Perspective. Int Psychogeriatr 2020, 32, 827–838. [Google Scholar] [CrossRef]
- Yan, M.; Fan, J.; Liu, X.; Li, Y.; Wang, Y.; Tan, W.; Chen, Y.; He, J.; Zhuang, L. Acupuncture and Sleep Quality Among Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Netw Open 2024, 7, e2417862. [Google Scholar] [CrossRef]
- Tan, W.; Pan, Z.; He, J.; Wu, T.; Wu, F.; Xu, Y.; Liu, L.; Yang, Z.; Li, C.; Hu, Y.; et al. Traditional Chinese Exercises for the Treatment of Neuropsychiatric Symptoms in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Complement Ther Med 2025, 103134. [Google Scholar] [CrossRef]
- Crichton, A.; Ignjatovic, V.; Babl, F.E.; Oakley, E.; Greenham, M.; Hearps, S.; Delzoppo, C.; Beauchamp, M.H.; Guerguerian, A.-M.; Boutis, K.; et al. Interleukin-8 Predicts Fatigue at 12 Months Post-Injury in Children with Traumatic Brain Injury. J Neurotrauma 2021, 38, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Eidem, L.E.; Birkeland, E.; Austdal, M.; Bårdsen, K.; Lange, J.; Alves, G.; Berven, F.; Nilsen, M.M.; Herlofson, K.; Tysnes, O.-B.; et al. Fatigue in Parkinson’s Disease: A Proteomic Study of Cerebrospinal Fluid. Mov Disord 2024, 39, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, Y.; Qu, Y.; Wang, C.; Li, Z.; Zhou, J.; Xu, Z. Pharmacological and Behavioral Interventions for Fatigue in Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials. J Geriatr Psychiatry Neurol 2023, 36, 487–495. [Google Scholar] [CrossRef]
- Coe, S.; Franssen, M.; Collett, J.; Boyle, D.; Meaney, A.; Chantry, R.; Esser, P.; Izadi, H.; Dawes, H. Physical Activity, Fatigue, and Sleep in People with Parkinson’s Disease: A Secondary per Protocol Analysis from an Intervention Trial. Parkinsons Dis 2018, 2018, 1517807. [Google Scholar] [CrossRef] [PubMed]
- Wasson, E.; Rosso, A.L.; Santanasto, A.J.; Rosano, C.; Butters, M.A.; Rejeski, W.J.; Boudreau, R.M.; Aizenstein, H.; Gmelin, T.; Glynn, N.W. Neural Correlates of Perceived Physical and Mental Fatigability in Older Adults: A Pilot Study. Exp Gerontol 2019, 115, 139–147. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).