1. Introduction
In South America, the average cheese production was 1.14 million tons per year in the period 2011-2021. In 2021, Ecuador was the fourth largest producer, with 94,422 tons of cheese [
1]. The main by-product/waste produced during cheesemaking is cheese whey (CW), with around 9 L of CW produced for each kilogram of cheese [
2]. Therefore, in 2021, the CW production in Ecuador was approximately 850,000 m
3 [
1,
2]. This liquid residue comprises mainly milk water, lactose, proteins, and mineral salts, and the concentration of these components depends on the origin of the milk and the process used to make the cheese. There are two types of CW: sweet CW (pH = 6.0–7.0; produced from casein coagulation by the enzymatic action of rennet) and acid CW (pH = 4.5–5.8; obtained from milk coagulation by the acid action of lactobacilli or inorganic acids) [
3,
4].
CW has different uses in the food and non-food industries, such as the production of fermented or non-fermented dairy beverages [
5] and the development of a wide range of food products (whey cheese, ice cream, confectionery products, soups, processed meats, salad dressings, etc.) [
4]. Bioactive peptides, sugars, oligosaccharides, and sugar alcohols derived from CW have physiological properties beneficial to human health and are used as a functional ingredient in foods, dietary supplements, food for special medical purposes, and pharmaceutical products, as well as in toiletries and cosmetics [
6,
7,
8]. Moreover, CW has been used for animal feed [
9], as a biofertilizer [
10], and to obtain biopolymers through the biological transformation of lactose, enabling the production of biodegradable plastic components [
11]. CW is considered a promising resource for biofuel production, such as biomethane, bioethanol, biohydrogen, and biodiesel derived from microbial lipids obtained by fermenting the sugars contained in CW by highly oleaginous microorganisms [
12].
However, in Ecuador, only 1.5% of CW is incorporated in the production process of dairy products (unfermented and fermented beverages, yogurt, fresh cheese, spreadable cheese, etc.) [
13]. This low percentage of recovered CW is mainly because the cheese industry in most Latin American countries, including Ecuador, is made up of small family companies, which cannot make the necessary investments to transform CW into value-added products [
4,
14]. Therefore, most of the CW is discharged into effluents or the soil [
15]. These practices have adverse environmental impacts since they damage aquatic life by reducing the dissolved oxygen caused by the degradation of the high organic load of this waste [
4]. They also provoke eutrophication processes [
16] and the acidification, salinization, and reduction of the redox potential of the soil, as well as altering its physical structure [
17].
Hence, co-composting CW with solid organic waste can be an appropriate way to treat it within the environment of small companies due to its effectiveness and low technology and cost [
18]. Several investigations have focused on recycling liquid wastes through composting, such as the co-composting of liquid manure with vegetable wastes [
19] or municipal solid wastes [
20], where this liquid waste has been used as a source of moisture or easily degradable soluble organic matter, achieving final composts with notable nutrient content and stabilized organic matter in less time. The liquid residue from olive oil extraction has been co-composted with olive mill sludge from evaporation ponds, grape marc, and green wastes [
21] or with olive mill pomace, olive mill solid husk, poultry manure, and green wastes [
22]. Both studies confirmed the success of using the co-composting process to recycle this liquid waste and stabilize and transform the other wastes into mature and hygienic composts that can be used as biofertilizer. Moreover, Ghasemzadeh et al. [
23] co-composted rice straw with poultry manure or sugarcane vinasse as cheap and accessible C/N reducers to accelerate the composting process of a highly lignocellulosic waste such as rice straw. However, these authors did not find that applying vinasse had positive effects on accelerating the composting process or on the agricultural quality of the compost obtained. Studies have also been found on co-composting liquid wastes from wine-processing with other wine-processing solid wastes and urban wastes [
24] or with wastes from olive mill, grape marc, and green wastes [
21]. In these studies, it was observed that adding these liquid wastes did not negatively affect the composting process, and composts rich in nutrients, without phytotoxicity, and with stabilized and humified organic matter were obtained.
However, there is little information related to treating CW through composting. Alfonzo et al. [
25] evaluated the effects of adding CW to the composting of grape pomace mixed with green herbaceous crop and pruning residues, using this liquid waste as a source of moisture and nutrients. These authors observed that adding CW resulted in a more substantial reduction of total and dissolved organic carbon content and more accelerated microbial carbon mineralization than the pile where the moisture content was maintained by water irrigation due to the stimulation of microbial activity by the nitrogen compounds in this liquid waste. CW has been co-composted with woodchips and biochar as a water-soluble carbon source to extract the heat generated during composting through a hydraulic circuit [
26]. Considering that the efficiency and effectiveness of the composting process for the treatment of CW have been little explored, the objective of this work was to determine the composition of CW generated in different small cheese companies in Canton Mocha (Tungurahua-Ecuador) to evaluate its recycling potential through co-composting with agro-livestock residues.
2. Materials and Methods
2.1. Surveys on the Dairy and Agro-Livestock Sectors of the Study Area
The study was conducted in the Mocha canton, in the southwestern part of the province of Tungurahua, Ecuador. This canton is located at altitudes ranging from 2,500 to 4,965 m above sea level (a.s.l.), with a total area of 85.8 km
2. The climate varies widely, but the high mountain equatorial climate covers the largest area. Average annual temperatures vary between 2-10°C, and the average annual rainfall ranges between 1,000-500 mm. The main economic activities in the Mocha canton are agriculture and cattle raising. Livestock farming in this canton focuses on milk production, which is mostly used to make cheese [
27].
In this study, ten small dairy companies were surveyed to obtain information on the type of dairy products produced, the daily milk processing volume, and the use or disposal of CW in the Mocha canton. Twenty-five farmers and eleven randomly selected livestock breeders were surveyed to determine the types of crops and cattle species produced in the canton, along with the type of management carried out on the waste generated by these activities.
2.2. Sampling of the Cheese Whey
Ten samples of CW were collected from the cheese companies participating in the survey. Approximately 1,000 mL of CW were taken directly from the refrigeration tanks, buckets, or collection containers of each participating company. The samples were transported in containers with coolants, maintaining a temperature between 2-6°C throughout the transportation process until they arrived at the laboratory, where they were stored at 6°C for later analysis. All determinations were made in triplicate.
2.3. Design of Co-Composting Experiment
To evaluate the feasibility of treating the CW through composting, a waste pile weighing approximately 1000 kg was formed, with the dimensions of 2 × 3 m at the base and a height of 1.5 m. The pile composition consisted of a mixture of wastes from corn and bean crops, cow manure, and CW as a source of moisture. The specific proportions of these wastes (on a fresh weight basis) were 60% corn residues + 5% bean residues + 35% cow manure. The main characteristics of these wastes were: for corn residues, pH 4.84 ± 0.02, 3.42 ± 0.35 dS/m electrical conductivity (EC), 91.3 ± 0.1 % organic matter (OM), 43.7 ± 0.2 % total organic carbon (Corg), 0.74 ± 0.04 % total nitrogen (Nt) and 59.1 ± 3.1 Corg/Nt ratio; for bean residues, pH 4.96 ± 0.05, 5.52 ± 0.13 dS/m EC, 88.6 ± 0.1 % OM, 41.1 ± 1.4 % Corg, 3.32 ± 0.18 % Nt and 12.4 ± 0.3 Corg/Nt ratio; and for cow manure, pH 7.79 ± 0.06, 6.06 ± 0.10 dS/m EC, 77.7 ± 0.9 % OM, 41.0 ± 0.2 % Corg, 2.16 ± 0.16 % Nt and 19.0 ± 1.4 Corg/Nt ratio. Throughout the bio-oxidative phase, the moisture content was controlled by adding CW (total volume = 0.35 L CW/kg fresh weight of the initial waste mixture), ensuring appropriate values in the 40-60% range. The characteristics of this CW are those shown for sample CW-2, indicated below.
During the composting process, daily average temperatures were recorded at five different points, at a depth of 30 cm, using a portable probe. Daily ambient temperatures were also measured. A total of two turnings were conducted on the pile to homogenize the mixture and provide the oxygen necessary for the aerobic degradation of the organic matter. The bio-oxidative phase lasted for 80 days, followed by a 30-day maturation period, during which the moisture content was continuously maintained at between 40-60% by irrigation with water. The mixture was sampled four times during the bio-oxidative phase and at the end of maturation by collecting sub-samples from seven randomly selected points covering the entire profile of the pile. These subsamples were then mixed to obtain a representative sample of 2 kg. These samples were dried at 60°C, then ground and sieved to 0.5 mm for subsequent analysis. All determinations were performed in triplicate.
2.4. Analytical Methods
In the evaluation of the CW, physical-chemical and chemical analyses were conducted, addressing parameters such as pH, electrical conductivity (EC), total and suspended solids (TS and SS, respectively), biochemical oxygen demand (BOD
5), chemical oxygen demand (COD), macro and micronutrients, and heavy metals determined according to the Standard Methods for the Examination of Water and Wastewater [
28]. Fat, protein, and lactose contents of the CW were also analyzed using the methods described in Standard Methods for the Examination of Dairy Products [
29].
In the analysis of the initial materials and samples from the composting pile, pH, EC, OM, Corg, water-soluble anions and polyphenols, cation exchange capacity (CEC), germination index (GI), macro and micronutrients, and heavy metals were measured following the analytical techniques used by Idrovo-Novillo et al. [
30].
2.5. Statistical Methods
Regarding the results of the physicochemical and chemical analyses of the CW, the mean value, range of values, and coefficient of variation (CV) of each parameter were calculated. A principal component analysis (PCA) was applied to the different parameters using the Varimax rotational method with normalization to identify groups of interrelated variables.
For the initial materials and samples from the composting pile, the standard deviation was determined for all the mean values of the parameters analyzed. The LSD (least significant difference; p < 0.05) method was used to determine the significant differences in the evolution of each parameter in the pile during the composting process. The IBM SPSS 27 statistical program was used to perform the statistical treatment of all the results.
3. Results and Discussion
3.1. Situation of the Dairy and Agro-livestock Sectors Studied and Destinations of the Generated Wastes
The results derived from the surveys conducted in the dairy industry of the Mocha canton revealed that the dairy products with the highest production were cheese and yogurt, with all the companies producing cheese and 20% of them also producing yogurt. Seventy percent of the companies processed between 600 and 1000 liters of milk daily, compared to the other companies processing quantities of 300 to 500 liters (20%) and 50 to 200 liters (10%). Milk processing for cheese production produced CW as a by-product, which was generally considered waste by most of the companies. It was not incorporated into the production process of dairy products mainly due to the lack of knowledge about the nutritional benefits of this by-product and the difficulties small family companies have acquiring the appropriate technologies for its handling and processing in the dairy industry. Regarding the destination of the CW, 50% of the producers used it as feed for cattle and pigs, 20% allocated it for pasture irrigation, 10% sold it at a very low price (0.01 US dollar/L), and the remaining 20% did not make any use of this liquid waste, dumping it into the environment (ravines, surface water bodies, etc.). It is important to highlight that pouring this liquid residue into the soil can affect the physical structure and chemical composition of the soil, as well as negatively influencing crop yields. The suspended solids contained in CW can clog soil pores, reducing its infiltration rate, and discharging it into the soil can also produce acidification and salinization and reduce the soil’s redox potential [
17]. Continuously dumping large amounts of CW into the soil or surface water bodies can lead to groundwater and effluent contamination [
4,
16].
The surveys conducted in the agricultural sector indicated that in the Mocha canton, potatoes represented 24.3% of the predominant crops, followed by corn at 20.3%. Other significant crops included red and white onions, at 13.5% and 8.1%, respectively. Percentages of 8.1, 6.8, and 5.4 were obtained for peas, beans, and carrots, respectively. Vegetables such as quinoa, cilantro, and tomatoes, among others, were also grown, each accounting for less than 5% of the total crops produced. Crops such as corn, potatoes, peas, beans, quinoa, and tomatoes produce higher amounts of waste because only the edible part of the plant is marketed. However, crops such as carrots, cilantro, and white and red onions create less waste since most of their plant structure is marketed. Regarding the destination of harvest residues, 51% of producers used them as feed for livestock, whereas 14% incinerated them, emitting aerosols and greenhouse gases [
31,
32], as well as decreasing carbon storage in the soil and reducing nutrients through runoff during rainfall [
33]. The remaining 35% of farmers applied these residues directly to the soil without prior stabilization. Applying fresh crop residues to the soil can modify its redox potential due to oxygen consumption in the mineralization of unstabilized organic matter, increase soil microbial activity, which can mineralize endogenous soil organic carbon, modify soil pH [
34], and reduce crop germination and development due to the presence of phytotoxic compounds such as polyphenols [
35].
Regarding the livestock sector, the data indicated that 56.8% of participants bred native-breed cattle, compared to 43.2% who opted for improved breeds. Native-breed cattle are able to adapt to extreme conditions, including low-quality forage, low temperatures, and reduced humidity, which are characteristics of the study area. Furthermore, they are very versatile, as they are used for milk and meat production and work tasks [
36]. Among the surveyed farmers, 71.6% identified manure as a waste; of this group, 58.0% dumped it directly on the soil, while 35.0% used it as organic amendment after drying. The rest of the respondents preferred to leave it where the cattle excrete. These practices cause significant environmental impacts, such as soil and water contamination by pathogens, excess nutrients and emerging contaminants [
37,
38], and greenhouse gas emissions [
39].
This scenario highlights the need to seek environmentally friendly alternatives, such as composting, to treat dairy, agricultural, and livestock wastes. This approach will not only favor the sustainability of the dairy and agricultural sectors in this region but will also address existing environmental issues, thereby contributing to improving the profitability of the activities of these sectors.
3.2. Cheese Whey Characterization
The physicochemical characteristics of the CW samples are shown in
Table 1. The acidity levels recorded in these samples ranged from 4.88 to 6.77, with sweet whey (pH around 6.0-7.0) represented in CW-2, CW-6, CW-7, CW-8, and CW-9, and the rest were acid whey (pH around 4.5-5.8) [
4]. The variability in pH values is due to the process used for coagulating milk casein by adding rennet, by the action of lactobacillus, or by adding lactic acid or mineral acids [
4]. Adding large volumes of CW, especially acidic CW, during solid organic waste co-composting can be a limiting factor for adequate composting since the optimal pH range for microbiota growth during this process is 5.5-9.0 [
40]. Considerable salt concentrations were evident in all the samples evaluated through EC, especially in the case of CW-6 (EC = 8.19 dS/m). This high salt content was possibly due to the solubilization of minerals from the milk and salts added to the cheese during its production, such as CaCl
2 to promote rennet coagulation, HCl for milk coagulation through acidification, NaCl for salting, etc. [
3]. The variability of salts that can be solubilized in CW could also explain the variation in the EC values of the whey samples studied (CV = 20). The elevated concentrations of mineral salts may limit the addition of this liquid residue to small quantities during the composting of other organic waste because there is an increase in salts as a consequence of OM mineralization and the concentration effect caused by the lost mass during composting [
40]. This could result in an excessively saline final compost whose agricultural use could adversely impact soil quality and crops.
The elevated levels of BOD
5 and COD reflected the high organic load in the CW samples (
Table 1), indicating the environmental pollution problem that its disposal creates. The BOD
5 and COD values were within the range found by Carvalho et al. [
41] in a review study of the characteristics of cheese effluents (27000-60000 mg/L and 50000-102000mg/L for BOD
5 and COD, respectively). According to these authors, lactose is the main whey component responsible for its high organic load, representing 70-75% of the ST. In the present study, these solids were in the range of 59155-78700 mg/L, whereas the SS ranged between 2851 and 3733 mg/L, both parameters having values within the intervals reported by other studies [
41].
The contents of the main organic components and macronutrients of the CW samples are shown in
Table 2. Lactose, proteins, and fats are the most abundant organic constituents of whey, considered the primary solids that are not incorporated into the cheese and remain in the CW (about 90% of the lactose, 20% of the protein, and 10% fat of the milk are present in the whey) [
3]. In the CW samples analyzed, the lactose content ranged from 34.5 to 42.1 g/L, below the range observed by other authors (42.6-60.0 g/L) [
3,
4,
41]. Regarding the protein content, CW-2 and CW-9 had the lowest and highest concentrations of this organic compound, respectively, and this range of values was within that reported by Carvalho et al. [
41] in a review study of the characteristics of cheese effluents (1.42-8.00 g/L). Most of the CW samples presented fat concentrations within the values found by previous authors (0.99-9.44 g/L). However, substantial variability was found in the values of this parameter among the samples analyzed (CV = 108), probably because the fat content of the CW depends on factors such as the fat concentration of the milk used to make the cheese. In addition, the extent of some operations carried out during the process (milk pasteurization and pumping and cutting and cooking the curd) can favor free fat formation (non-globular) that is lost in the whey [
3].
The values of the macronutrient contents were in the ranges 0.62-0.75 g/L, 0.06-0.11 g/L, 0.11-0.27 g/L, and 2.03-2.94 mg/L for N, P, Ca, and K, respectively (
Table 2). These data were within or below the scale of values found by other authors (0.20-1.76 g/L for N and 0.12-0.5 g/L for P [
41] and 0.4-1.6 g/L for Ca [
3,
42]). Ca was the macronutrient with the greatest variability among the samples analyzed (CV = 31), probably because this element can be transferred from milk to whey and added in cheese making as CaCl
2 to promote rennet coagulation [
3].
According to the chemical analyses of the micronutrients and heavy metals in the CW samples, most of these elements had values below the detection limits of the equipment used to measure them, with only very low concentrations of Fe and Zn found (
Table 3). The CW samples presented notable variability in Fe content, probably due to factors such as the breed of cattle, dietary intake, and, especially, the process used for milk casein coagulation (by adding rennet or by acidification) and the type of cheese that is produced [
3]. From an agronomic perspective, the presence of macro and micronutrients in CW suggests that its co-composting with other organic solid wastes can improve the fertilizing value of the final compost. Furthermore, its low heavy metal content guarantees the safe agricultural use of this compost.
3.3. Relationships Among the Cheese Whey Characteristics
PCA was applied to the following parameters analyzed in the CWs: pH, EC, TS, SS, COD, BOD5, lactose, protein, fat, N, P, Ca, K, Fe, and Zn (n=15). The model obtained through PCA explains > 70.0% of the variability by establishing three principal components, with the following contribution of each principal component: component 1, 26.4%; component 2, 24.0%; and component 3, 20.6%. In the proposed model, the KMO (Kaiser-Meyer-Olkin's measure of adequacy) value was > 0.5 (KMO = 0.539), and the P-value was 0.000 for Barlett's test of sphericity. None of the variables presented an extraction value < 0.5. All this indicates that the model is adequate.
In principal component 1, the variables TS, SS, lactose, and fat were grouped and directly correlated (
Table 4). This indicated that the TS and SS were mainly composed of the lactose and fats contained in the CW samples, agreeing with Fox et al. [
3] that most of the lactose and part of the milk fat are present in the whey.
Principal component 2 was associated with COD, Ca, pH, Zn, BOD
5, EC, K, Fe, and P, with COD, BOD
5, pH, and P negatively correlated with the rest of the variables. However, the factorial loading of P was low, indicating the least association of this variable with this principal component. The direct relationship between the variables COD, BOD
5, and pH indicated that in this study, the reduction of CW acidity favored the content of the organic load of this liquid waste. The negative correlation between the pH and the determined macro and micronutrients has also been observed by other authors, where the acidic CW samples had a higher mineral content [
3,
4,
41]. EC was positively correlated with the determined mineral elements, corroborating the relationship between CW salinity and its macro and micronutrient content.
Significant variables for principal component 3 included protein and N, which correlated positively due to their chemical relationship and their determination (total protein = N × 6.38).
3.4. Co-Composting Cheese Whey with Agro-livestock Wastes
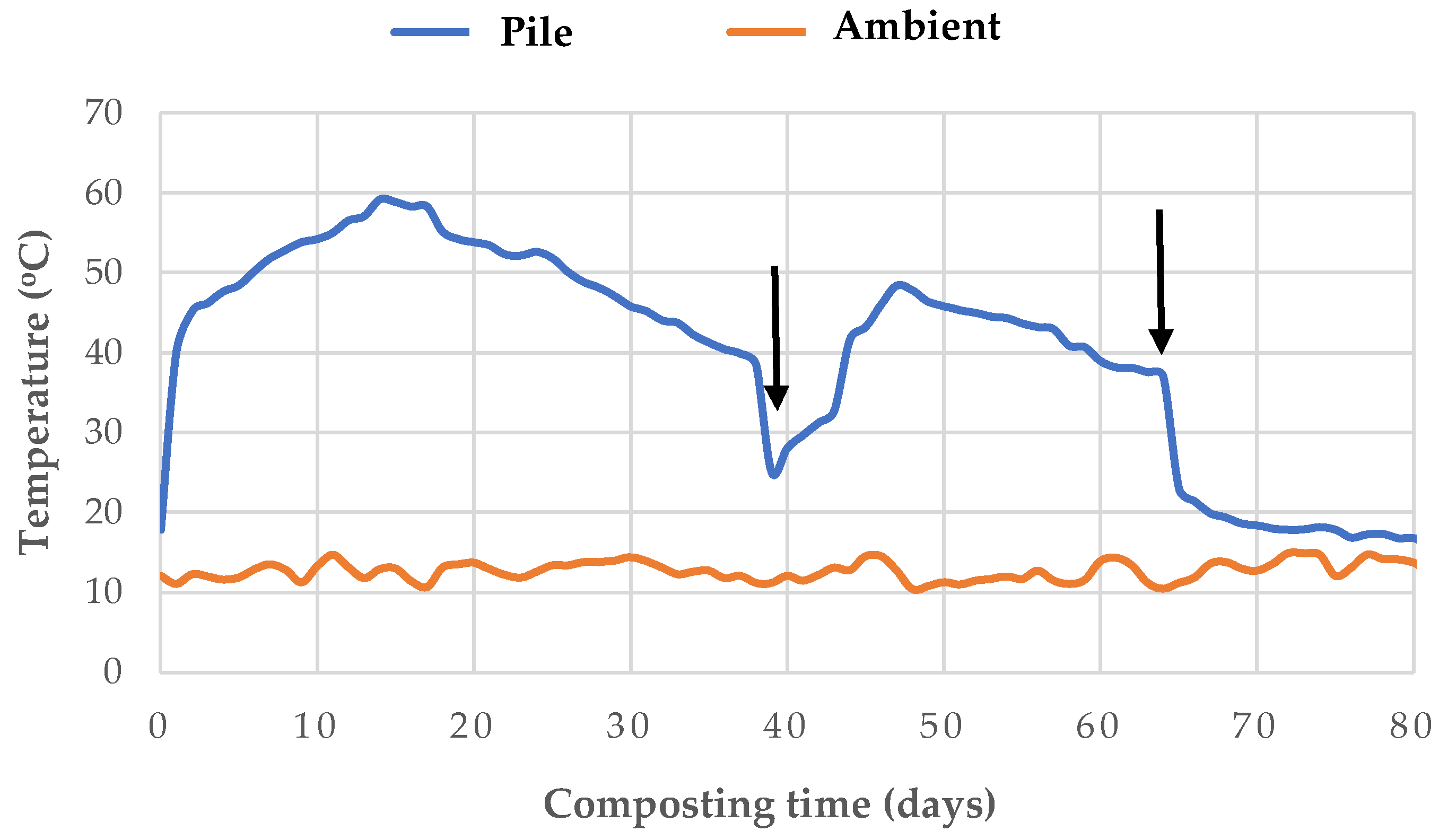
The temperature in the pile rose rapidly at the onset of the process, reaching thermophilic values (> 40°C) within the first 72 hours. These values were maintained for approximately 37 days until the first turning (
Figure 1). The maximum average temperature was recorded on day 15, at 59.2°C. Other researchers also observed a rapid increase in temperature early in the composting process when co-composting plant residues with CW as a source of moisture and nutrients [
25,
26]. The supply of oxygen and non-degraded materials was controlled by turnings, which were carried out when the temperature dropped below 40°C. After the first turning, the temperature increased to above 40°C for 16 days, while no thermophilic temperature values were observed after the second turning. The high temperatures recorded during the thermophilic stage, together with their duration, were not adequate to ensure effective sanitization of the composted material. According to the requirements of the United States Environmental Protection Agency [
43], in piles with aeration by turning, the temperature has to be maintained at ≥ 55°C for 15 consecutive days. These requirements were not met in the present composting experiment, as temperatures were ≥ 55°C for only seven consecutive days. The bio-oxidative phase of composting was considered complete when the pile temperature stabilized and remained close to ambient [
44]. After day 80, the composting process entered the maturation phase for one month, evidenced by a stabilization of the pile temperature.
The initial pH value in the pile (7.85;
Table 5) was within the range of 5.5-8.0, suggesting it is suitable for composting [
44]. This parameter increased significantly during the first 65 days, which could be attributed to acid-type compound degradation and the mineralization of organic nitrogen to ammonia [
44]. However, a reduction in pH was observed at the end of the bio-oxidative and maturity stages, probably due to the release of H
+ from nitrification processes [
40], as explained below.
The EC values increased continuously throughout the composting process (
Table 5), possibly due to soluble salts provided by adding CW during composting and OM mineralization, as well as the concentration effect of ions caused by the mass loss of the pile [
40]. This progressive increase in salt content throughout the composting process has also been observed in other experiments where liquid wastes have been used as a moisture source, such as winery wastewater [
21] and olive mill wastewater [
22].
OM degradation during the process led to its content being reduced from 80.9% to 67.6% (
Table 5). This degradation produced a loss of mass in the pile that contributed to a significant increase in Nt throughout the process [
44]. However, an accumulation of nitrogen due to the contribution of this nutrient with the CW applied during the composting process cannot be excluded. The degradation of Corg and increase of Nt caused the Corg/Nt ratio to decrease from an initial value of 35.7 to a final value of 10.3, indicating the evolution of OM maturity [
44].
Soluble polyphenol content is one of the main parameters used to monitor phytotoxicity reduction in vegetable residues during composting. Plants synthesize these compounds to defend themselves from attack by phytopathogens and the damaging effects of solar UV rays [
45]. At the same time, it has been observed that these compounds have phytotoxic effects on seed germination and seedling development [
46]. Therefore, their degradation throughout the process is closely related to the detoxification of the material to be composted, as can be observed in an increase in GI as polyphenolic compounds were degraded in the composting process, showing an absence of phytotoxicity from day 39 of composting (GI > 50% [
47]) (
Table 5).
Soluble anions contributed to the salinity of the composted materials. Most of these ions increased throughout the process as a consequence of their production when the OM degraded and the ion concentration effect of pile weight loss [
40], as well as through the CW applied during the process (
Table 6). Only in the case of sulfates was no significant increase in their concentration observed during the composting process. However, the rest of the anions studied increased their content from initial values of 0.53 g/kg, 0.86 g/kg, and 4.94 g/kg to final values of 0.89 g/kg, 15.99 g/kg, and 6.43 g/kg for Cl
-, NO
3-, and PO
43-, respectively. Notably, the highest nitrate concentrations were observed at the end of the bio-oxidative and maturation stages, when temperatures were < 40
oC and NH
3 concentrations were probably low. This could be because the nitrification process is inhibited at thermophilic temperatures and when there is an excessive amount of NH
3 from organic N degradation in the initial stages of the process, as reported by Cáceres et al. [
48] in a review study on nitrification in composting.
Table 7 shows the values of the parameters determined in the final compost. In this compost, the range of pH and EC values established as suitable in compost for different agricultural applications and field conditions by the American guidelines of the US Composting Council [
49] was exceeded. Despite this, this compost did not show phytotoxic effects on germination and root elongation of Lepidium sativum L. seeds, as corroborated by the GI value (GI > 50%, absence of phytotoxicity [
47]). The compost presented an OM content within the range indicated by the American guidelines, while the Nt and P contents were above the recommended values (OM = 50-60% and Nt and P ≥ 10 g/kg [
49]), showing the remarkable fertilizing capacity of the compost obtained. In addition, the compost presented an adequate degree of maturity, as can be observed from the values of the Corg/Nt ratio and CEC (Corg/Nt < 20 and CEC > 67 meq/100 g OM [
50]). Finally, the contents of potentially toxic elements were below the limit values recomended by the US Composting Council [
49] for safe agricultural compost use.
4. Conclusions
According to the results obtained from the characterization of the CW produced in the Mocha canton (Tungurahua-Ecuador), this liquid waste presented a high organic load, consisting mainly of lactose, proteins, and fats, and a notable content of macronutrients, as well as low concentrations of potentially toxic elements. All these characteristics are beneficial for co-composting CW with other organic solid wastes. However, the low pH values of whey, especially in the case of CW with milk casein coagulation by acid action (pH = 4.88-5.78), and its high salt content can be limiting factors for developing the composting process and obtaining a compost of agricultural quality.
Based on the results of treating CW by composting, we can infer that co-composting this liquid waste with plant residues and cow manure can be an effective way to recycle these residues in remote areas with a low-income population and limited access to expensive technologies. The quantities of residues used (60% corn residues + 5% bean residues + 35% cow manure—on a fresh weight basis—and 0.35 L CW/kg fresh weight of initial waste mixture) were adequate to achieve the thermophilic stage of the composting process, which is necessary to reduce phytotoxic compounds and pathogenic microorganisms. However, it was observed that irrigation with CW increased salinity levels, so caution should be taken with the amounts supplied to the composting process to avoid compost with excess salts. Nevertheless, the overall results were compatible with plant growth due to the significant OM, Nt, and P contents of the final compost and the final values of Corg/Nt ratio (< 20), CEC (> 67 meq/100 g OM) and GI (>50%), indicating OM stabilization and humification and phytotoxicity reduction during the composting process.
Therefore, co-composting agro-livestock wastes with CW can contribute to synergies between small and medium-sized cheese, agricultural, and livestock farms, which could benefit from centralized composting facilities to manage their wastes and mitigate environmental impacts. However, further studies are needed to optimize the amount or dilution of CW to reduce the salt content of the final compost, as this was the main limiting factor of using this liquid waste as a moisture source during the co-composting of solid organic wastes.
Author Contributions
Conceptualization. C.P. and I.G.-T.; Methodology. S.R.-R., I.G.-T., J.I.-N., A.I.-G., V.V.-O. and C.P.; Data curation. C.P., J.I.-N., and S.R.-R.; Validation. C.P. and I.G.-T.; Formal analysis. S.R.-R., I.G.-T., J.I.-N., and C.P.; Investigation. C.P. and I.G.-T.; Writing—original draft preparation. S.R.-R., C.P., and I.G.-T.; Writing—review and editing. C.P.; Visualization. C.P., I.G.-T., and J.I.-N.; Supervision. C.P. and I.G.-T.; Project administration. I.G.-T.; Funding acquisition. I.G.-T and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Higher Polytechnic School of Chimborazo (Ecuador), in the framework of the 1824.IDI.ESPOCH.2021 project.
Acknowledgments
This study is part of the research project entitled: “Estudio de biorremediación de suelos contaminados con la ceniza volcánica empleando compost proveniente de residuos agroindustriales en la provincia de Chimborazo”, carried out between the Associated Research Group in Biotechnology, Environment and Chemistry (GAIBAQ) of the Higher Polytechnic School of Chimborazo and the Environmental Research Group of Agrochemistry and Environment (GIAAMA) of the Miguel Hernández University of Elche, for which the authors appreciate their financial support and scientific contribution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 8 May 2024).
- Mazorra-Manzano, M.A.; Moreno-Hernández, J.M. Propiedades y opciones para valorizar el lactosuero de la quesería artesanal. Ciencia UAT 2019, 14, 133–144. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2rd ed.; Springer: New York, USA, 2017. [Google Scholar]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Panghal, A.; Patidar, R.; Jaglan, S.; Chhikara, N.; Khatkar, S.K.; Gat, Y.; Sindhu, N. Whey valorization: current options and future scenario – a critical review. Nutr. Food Sci. 2018, 48, 520–535. [Google Scholar] [CrossRef]
- Ostertag, F.; Schmidt, C.M.; Berensmeier, S.; Hinrichs, J. Development and validation of an RP-HPLC DAD method for the simultaneous quantification of minor and major whey proteins. Food Chem. 2021, 342, 128176. [Google Scholar] [CrossRef]
- de Almeida, M.P.G.; Mockaitis, G.; Weissbrodt, D.G. Got whey? Sustainability endpoints for the dairy industry through resource biorecovery. Fermentation 2023, 9, 897. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Chen, Q.; Wu, H.; Mu, W. Sugar alcohols derived from lactose: lactitol, galactitol, and sorbitol. Appl. Microbiol. Biotechnol. 2020, 104, 9487–9495. [Google Scholar] [CrossRef]
- Arshad, U.-e.; Hassan, A.; Ahmad, T.; Naeem, M.; Chaudhary, M.T.; Abbas, S.Q.; Randhawa, M.A.; Pimentel, T.C.; da Cruz, A.G.; Aadil, R.M. A recent glance on the valorisation of cheese whey for industrial prerogative: high-value-added products development and integrated reutilising strategies. Int. J. Food Sci. Technol. 2023, 58, 2001–2013. [Google Scholar] [CrossRef]
- Ketterings, Q.; Czymmek, K.; Gami, S.; Godwin, G.; Ganoe, K. Guidelines for Land Application of Acid Whey; Department of Animal Science, College of Agriculture & Life Sciences, Cornell University: Ithaca, NY, USA, 2017. [Google Scholar]
- Chalermthai, B.; Giwa, A.; Schmidt, J.E.; Taher, H. Life cycle assessment of bioplastic production from whey protein obtained from dairy residues. Bioresour. Technol. Rep. 2021, 15, 100695. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Gómez-Falcon, N.; Brar, S.K.; Ramírez, A.A. Cheese whey as a potential feedstock for producing renewable biofuels: A review. Energies 2022, 15, 6828. [Google Scholar] [CrossRef]
- Estudio de Mercado. Sector lácteo. 2021. Available online: https://www.sce.gob.ec/sitio/wp-content/uploads/2021/04/estudio_de_mercado_sector_lacteo_SCPM-IGT-INAC-002-2019.pdf (accessed on 23 July 2024).
- Acosta, A.; Galetto, A.; Valdés, A.; Londinsky, A. Más allá de la finca lechera - Enmarcando el diálogo de política lechera en América Latina; FAO and FEPALE: Rome, Italy, 2022. [Google Scholar]
- Poveda, E. Suero lácteo, generalidades y potencial uso como fuente de calcio de alta biodisponibilidad. Rev. Chil. Nutr. 2013, 40, 397–403. [Google Scholar]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Caballero, P.; Rodríguez-Morgado, B.; Sandra, M.; Manuel, T.; Juan, P. Obtaining plant and soil biostimulants bywaste whey fermentation. Waste Biomass Valoriz. 2020, 11, 3281–3292. [Google Scholar] [CrossRef]
- Ayilara, M.; Olanrewaju, O.; Babalola, O.; Odeyemi, O. Waste management through composting: challenges and potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Vázquez, M.A.; de la Varga, D.; Plana, R.; Soto, M. Integrating liquid fraction of pig manure in the composting process for nutrient recovery and water re-use. J. Clean. Prod. 2015, 104, 80–89. [Google Scholar] [CrossRef]
- Rastogi, M.; Nandal, M.; Nain, L. Additive effect of cow dung slurry and cellulolytic bacterial inoculation on humic fractions during composting of municipal solid waste. Int. J. Recycl. Org. Waste Agricult. 2019, 8, 325–332. [Google Scholar] [CrossRef]
- Majbar, Z.; Lahlou, K.; Ben Abbou, M.; Ammar, E.; Triki, A.; Abid, W.; Nawdali, M.; Bouka, H.; Taleb, M.; El Haji, M.; Rais, Z. Co-composting of olive mill waste and wine-processing waste: An application of compost as soil amendment. J. Chem. 2018, 7918583. [Google Scholar] [CrossRef]
- Bargougui, L.; Guergueb, Z.; Chaieb, M.; Mekki, A. Co-composting of olive industry wastes with poultry manure and evaluation of the obtained compost maturity. Waste Biomass Valor. 2020, 11, 6235–6247. [Google Scholar] [CrossRef]
- Ghasemzadeh, S.; Sharafi, R.; Salehi Jouzani, G.; Karimi, E.; Ardakani, M.R.; Vazan, S. Efficient lignocellulose degradation during rice straw composting with native effective microorganisms and chicken manure. Org. Agr. 2022, 12, 397–409. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Moreno-Caselles, J.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Bernal, M.P. Co-composting of distillery and winery wastes with sewage sludge. Water Sci. Technol. 2007, 56, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Laudicina, V.A.; Muscarella, S.M.; Badalucco, L.; Moschetti, G.; Spanò, G.M.; Francesca, N. Cellulolytic bacteria joined with deproteinized whey decrease carbon to nitrogen ratio and improve stability of compost from wine production chain by-products. J. Environ. Manag. 2022, 304, 114194. [Google Scholar] [CrossRef] [PubMed]
- Pivato, A.; Malesani, R.; Bocchi, S.; Rafieenia, R.; Schievano, A. Biochar addition to compost heat recovery systems improves heat conversion yields. Front. Energy Res. 2024, 11, 1327136. [Google Scholar] [CrossRef]
- Memoria técnica cantón Mocha/bloque 1.1. Available online: http://metadatos.sigtierras.gob.ec/pdf/Memoria_tecnica_Coberturas_MOCHA_20150306.pdf (accessed on 29 July 2024).
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard methods for the examination of water and wastewater, Volume 10; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Wehr, H.M.; Frank, J.F. Standard methods for the examination of dairy products, 17th ed.; American Public Health Association: Washington, DC, USA, 2004. [Google Scholar]
- Idrovo-Novillo, J.; Gavilanes-Terán, I.; Bustamante, M.A.; Paredes, C. Composting as a method to recycle renewable plant resources back to the ornamental plant industry: Agronomic and economic assessment of composts. Process Saf. Environ. Protect. 2018, 116, 388–395. [Google Scholar] [CrossRef]
- Rahman, M.H.; Singh, N.; Kundu, S.; Datta, A. Potential areas of crop residue burning contributing to hazardous air pollution in Delhi during the post-monsoon season. J. Environ. Qual. 2022, 51, 181–192. [Google Scholar] [CrossRef]
- Mgalula, M.E.; Wasonga, O.V.; Hülsebusch, C.; Richter, U.; Hensel, O. Greenhouse gas emissions and carbon sink potential inEastern Africa rangeland ecosystems: A review. Pastoralism 2021, 11, 19. [Google Scholar] [CrossRef]
- Snyman, H.A. Short-term responses of Southern African semi-arid rangelands to fire: A review of impact on soils. Arid Land Res. Manag. 2015, 29, 222–236. [Google Scholar] [CrossRef]
- Medina, J.; Monreal, C.; Barea, J.M.; Arriagada, C.; Borie, F.; Cornejo, P. Crop residue stabilization and application to agricultural and degraded soils: A review. Waste Manag. 2015, 42, 41–54. [Google Scholar] [CrossRef]
- Fu, B.; Chen, L.; Huang, H.; Qu, P.; Wei, Z. Impacts of crop residues on soil health: A review. Environ. Pollut. Bioavailab. 2021, 33, 164–173. [Google Scholar] [CrossRef]
- Mendoza, D.; Marini, P.; Zambrano, J. Los bovinos criollos un recurso zoogenético de seguridad alimentaria para Ecuador y Latinoamérica. Revista Científica Arbitrada Multidisciplinaria PENTACIENCIAS 2022, 4, 175–185. [Google Scholar]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure-Environmental risk due to antibiotics after application to soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef] [PubMed]
- van derWeerden, T.J.; Noble, A.; de Klein, C.A.M.; Hutchings, N.; Thorman, R.E.; Alfaro, M.A.; Amon, B.; Beltran, I.; Grace, P.; Hassouna, M.; et al. Ammonia and nitrous oxide emission factors for excreta deposited by livestock and land-applied manure. J. Environ. Qual. 2021, 50, 1005–1023. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.; Iroh, I.N.; Ezeogu, L.I. Composting technology in waste stabilization: On the methods, challenges and future prospects. J. Environ. Manag. 2017, 190, 140–157. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Nicolás, J.L.; González-Olivares, L.G.; Vázquez-Rodríguez, G.A.; Lucho-Constatino, C.; Castañeda-Ovando, A.; Cruz-Guerrero, A.E. Valorization of whey using a biorefinery. Biofuels, Bioprod. Bioref. 2020, 14, 1010–1027. [Google Scholar] [CrossRef]
- EPA. United States Environment Protection Agency. Environmental Regulations and Technology Control of Pathogens and Vector Attraction in Sewage Sludge; EPA625-/R-92/-103; EPA: Cincinnati, OH, USA, 2003. [Google Scholar]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C., Jr. Current approaches and future trends in compost quality criteria for agronomic, environmental, and human health benefits. Adv. Agron. 2017, 144, 143–233. [Google Scholar]
- Fereidoon, S.; Vamadevan, V.; Won Young, O.; Han, P. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar]
- Hegab, M.M.; Abdelgawad, H.; Abdelhamed, M.S.; Hammouda, O.; Pandey, R.; Kumar, V.; Zinta, G. Effects of tricin isolated from jungle rice (Echinochloa colona L.) on amylase activity and oxidative stress in wild oat (Avena fatua L.). Allelopath. J. 2013, 31, 345–354. [Google Scholar]
- Zucconi, F.; Pera, A.; Forte, M.; de Bertoldi, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- US Composting Council. Field Guide to Compost Use. 2001. Available online: http://www.mncompostingcouncil.org/uploads/1/5/6/0/15602762/fgcu.pdf (accessed on 16 December 2024).
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).