Submitted:
26 January 2025
Posted:
27 January 2025
You are already at the latest version
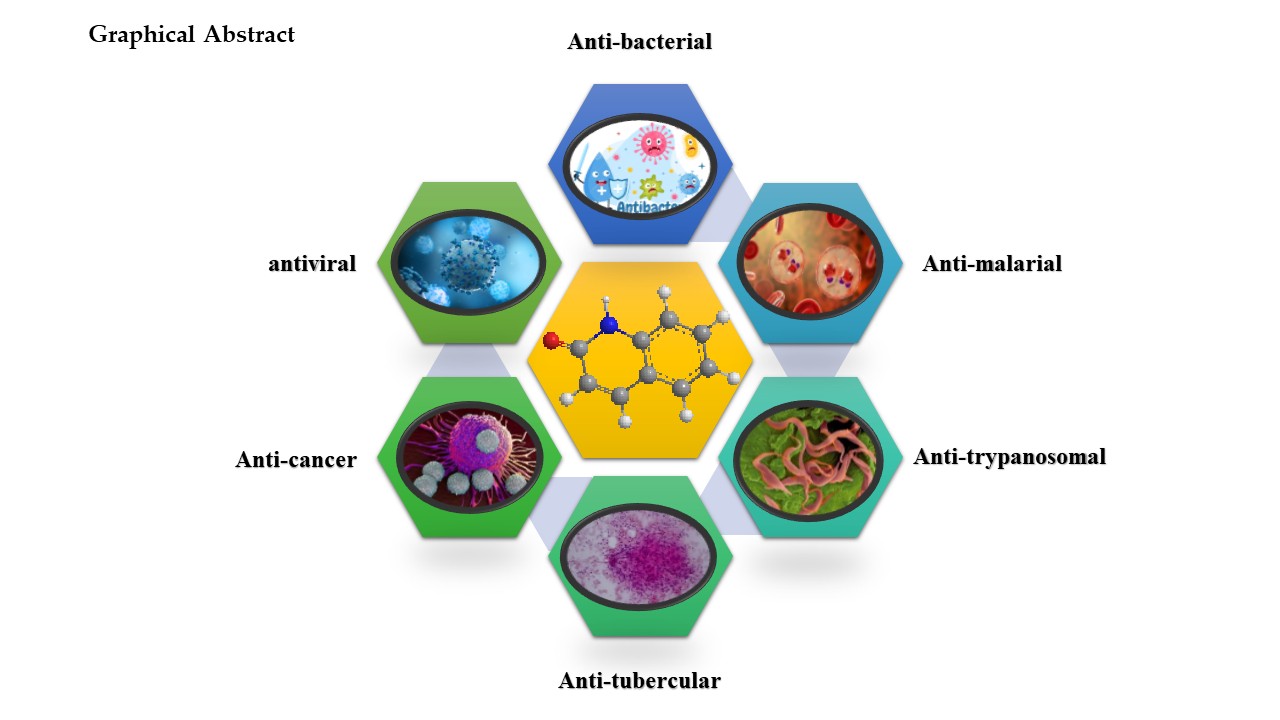
Abstract
Keywords:
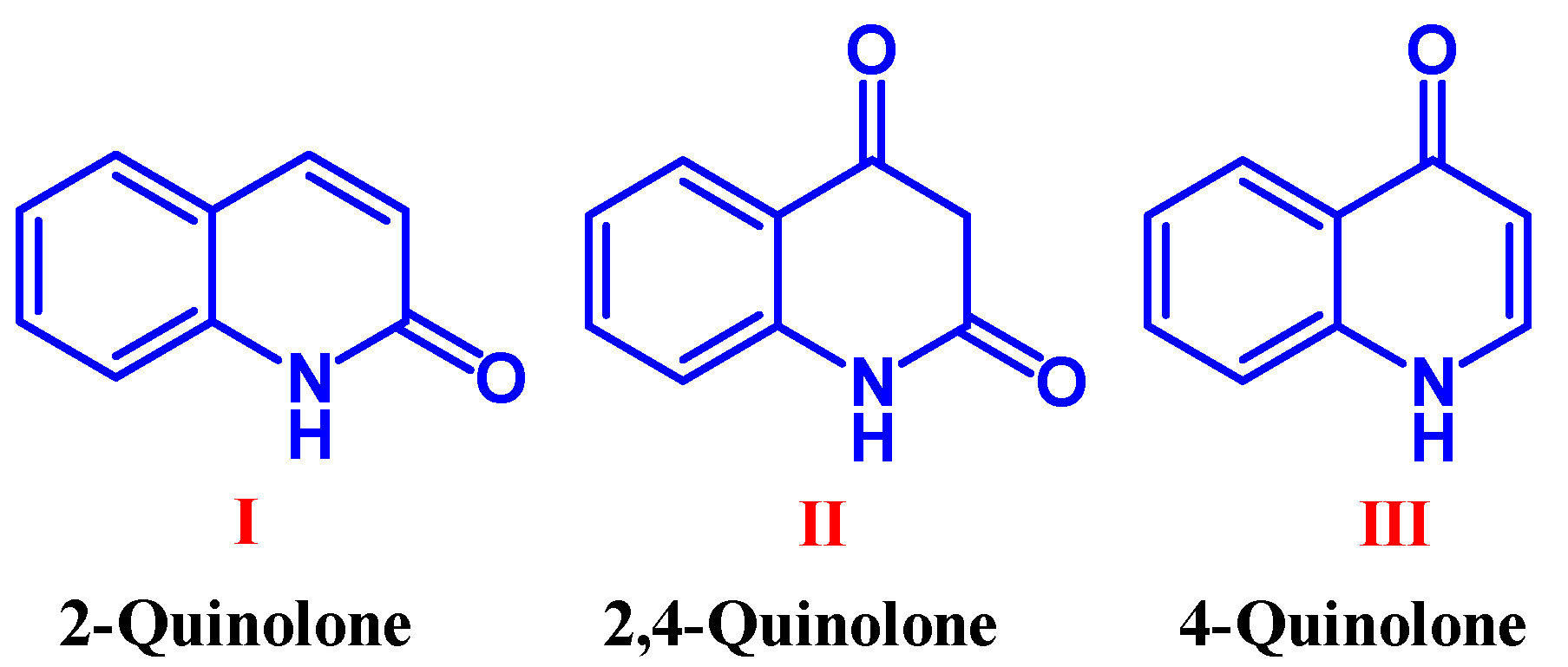
1. Introduction
2. Methods of Synthesis of Quinolone Derivatives
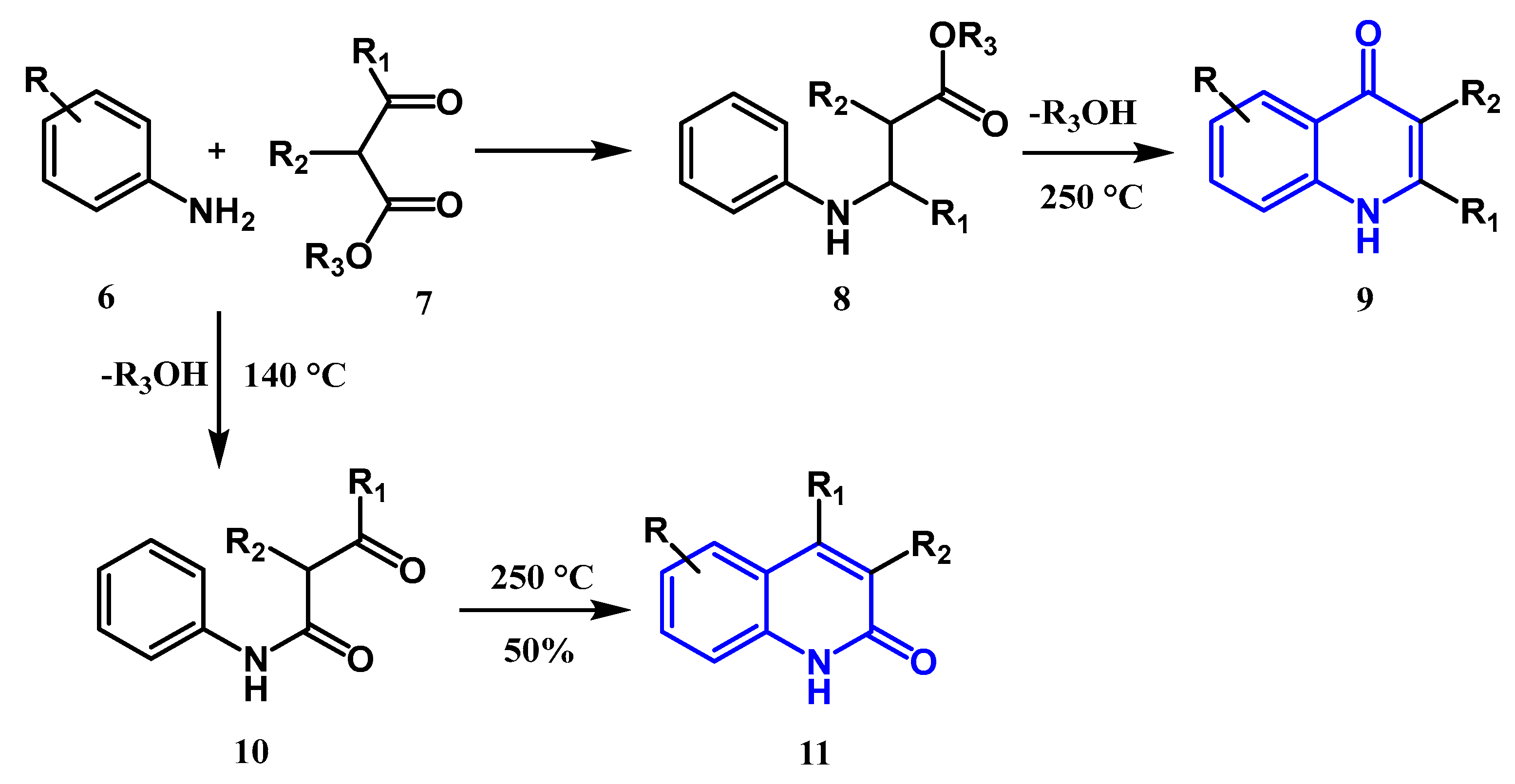
2.1. Synthesis from Derivatives of Aniline and an Arylamine
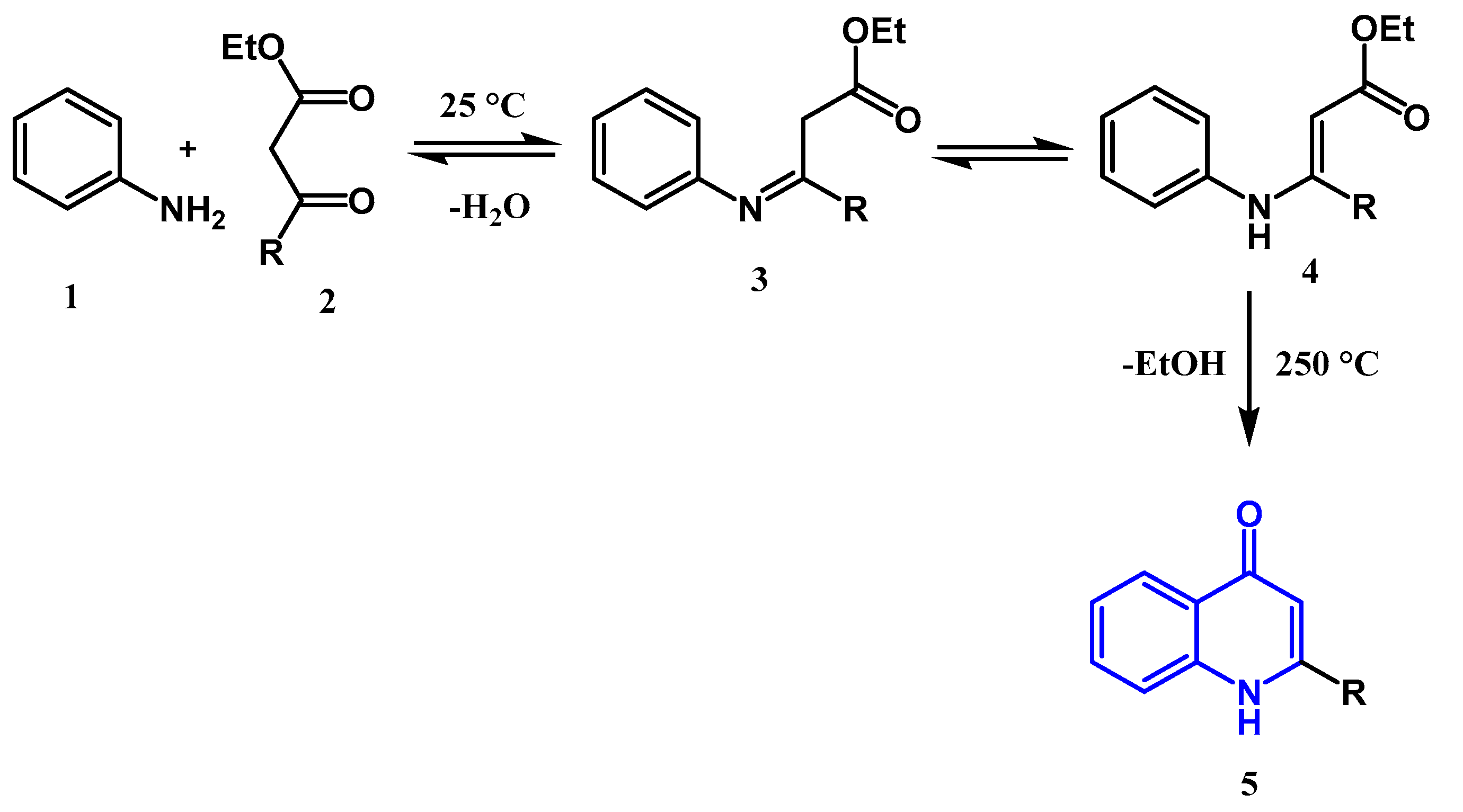
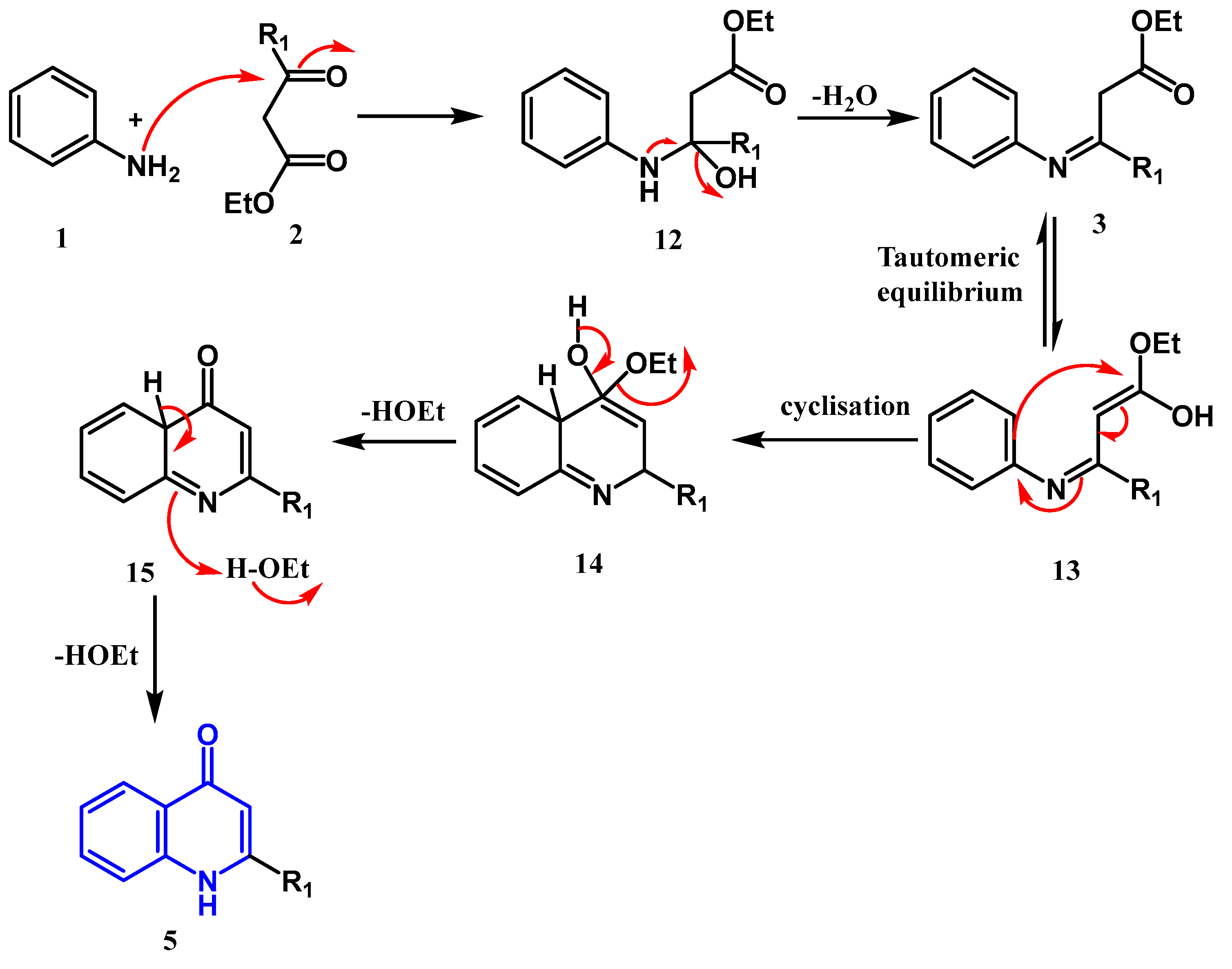
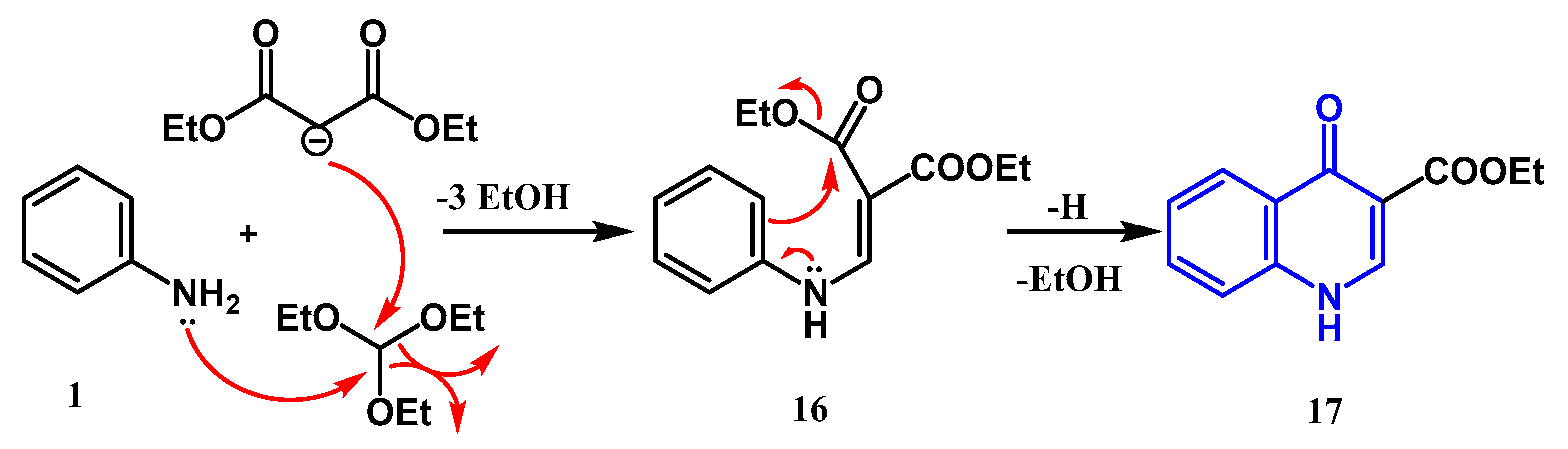
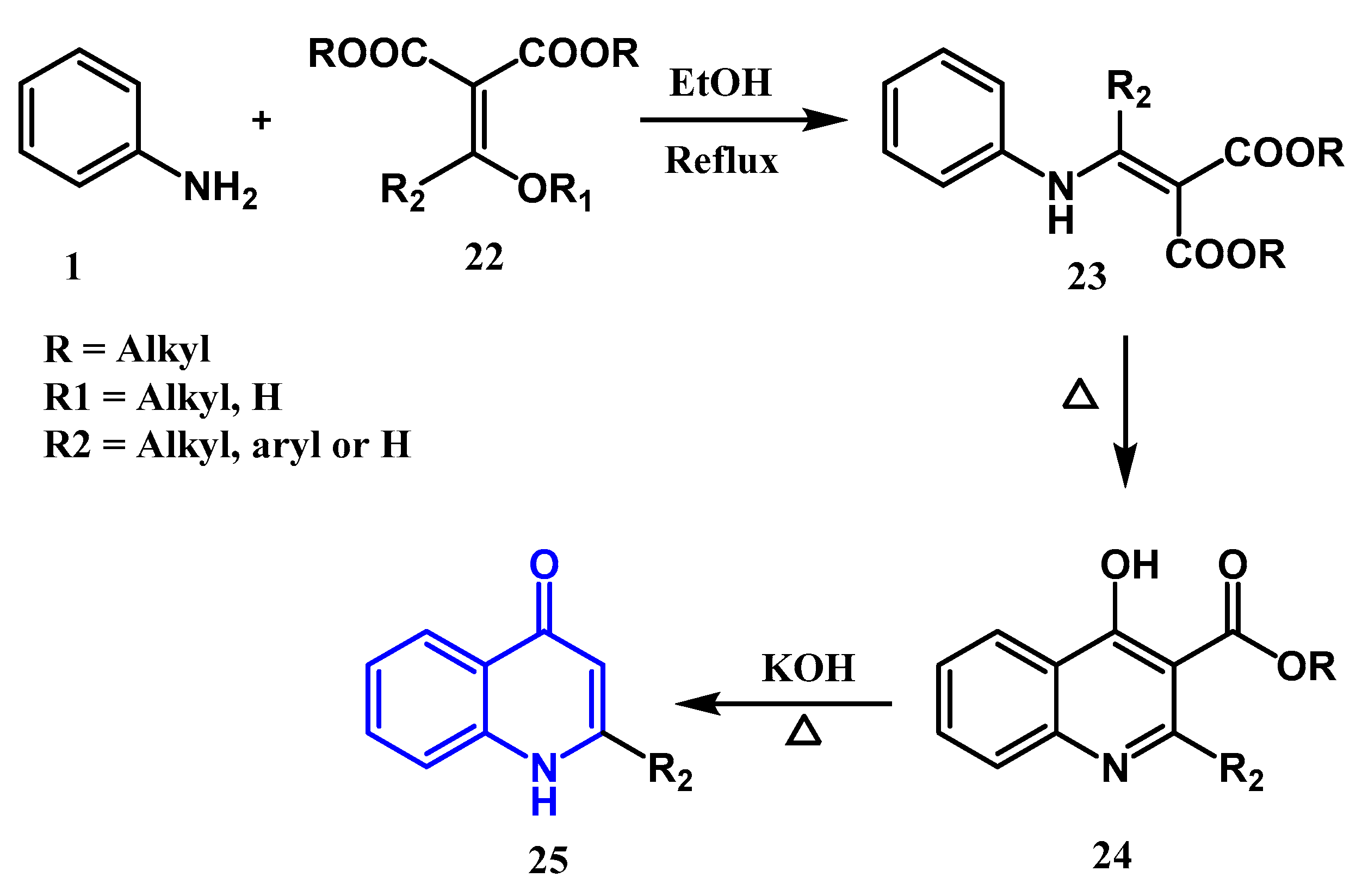
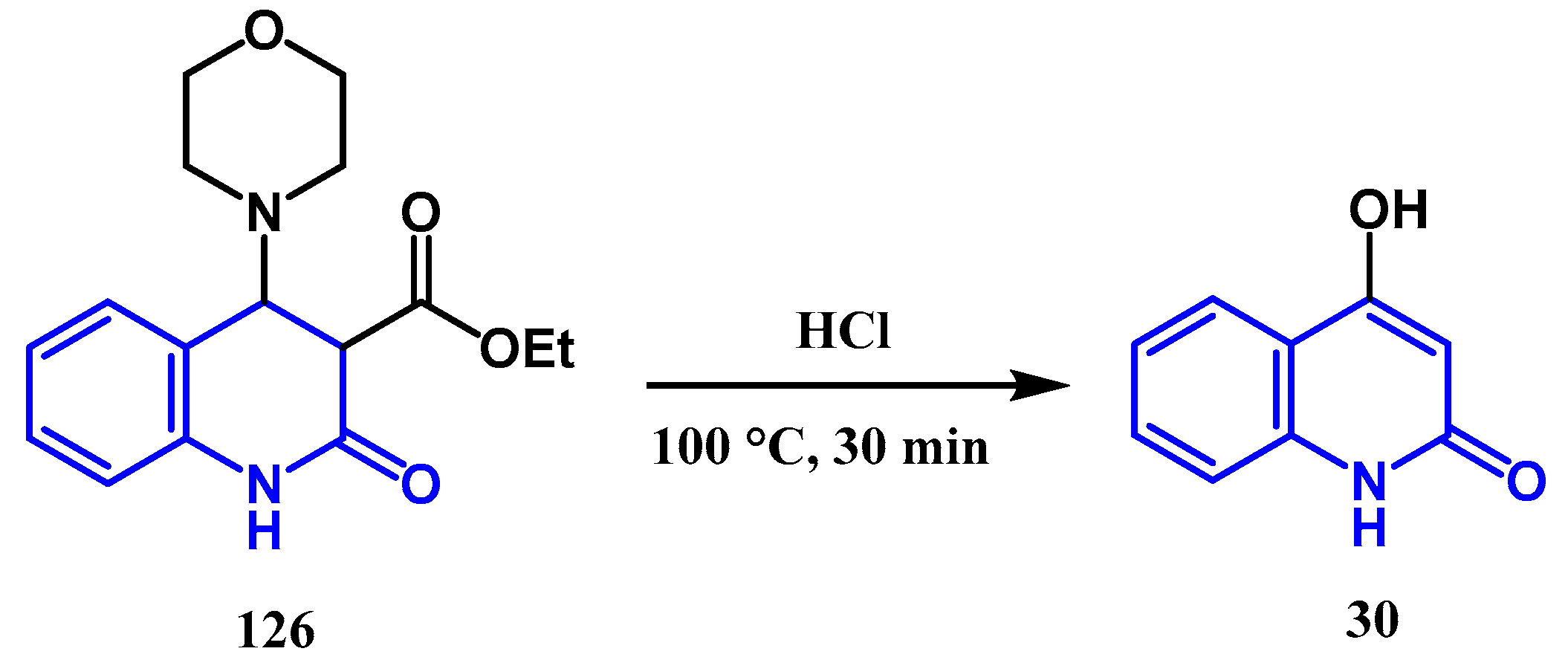
2.1.1. Conrad Limpach Knorr's Reaction
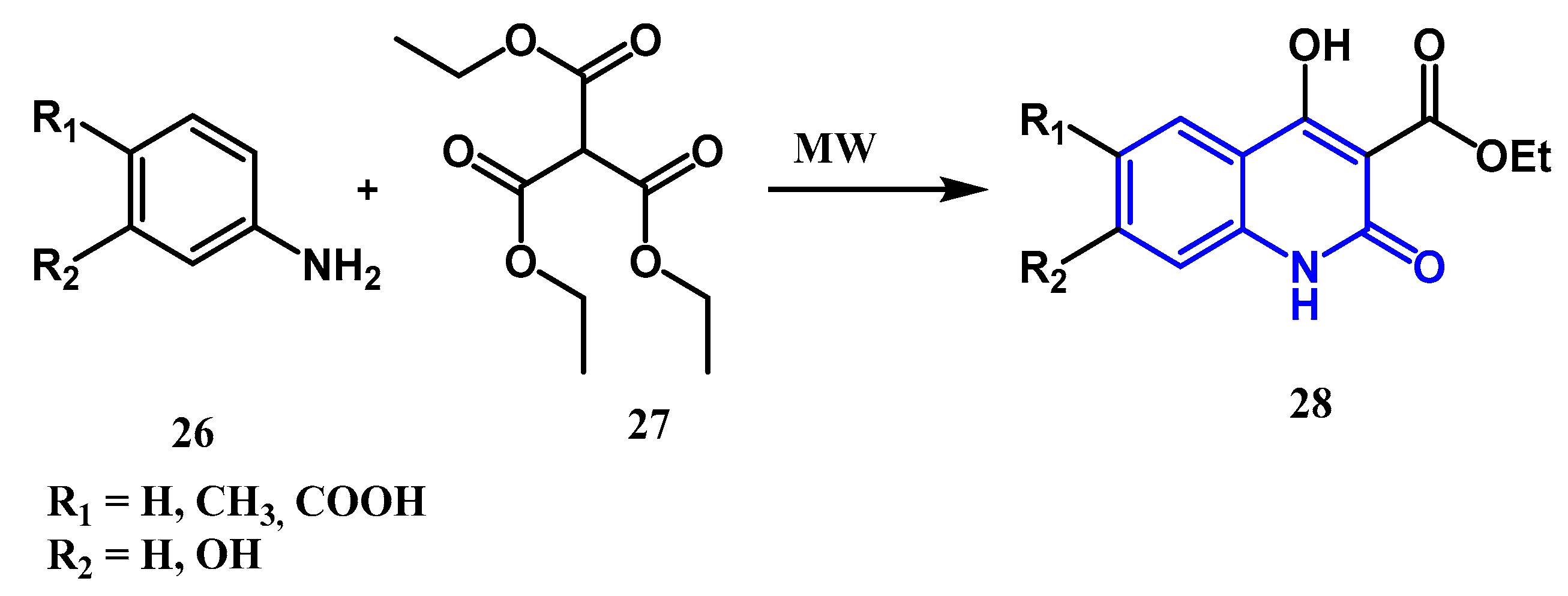
2.1.2. Doebner Multicomponent Reaction
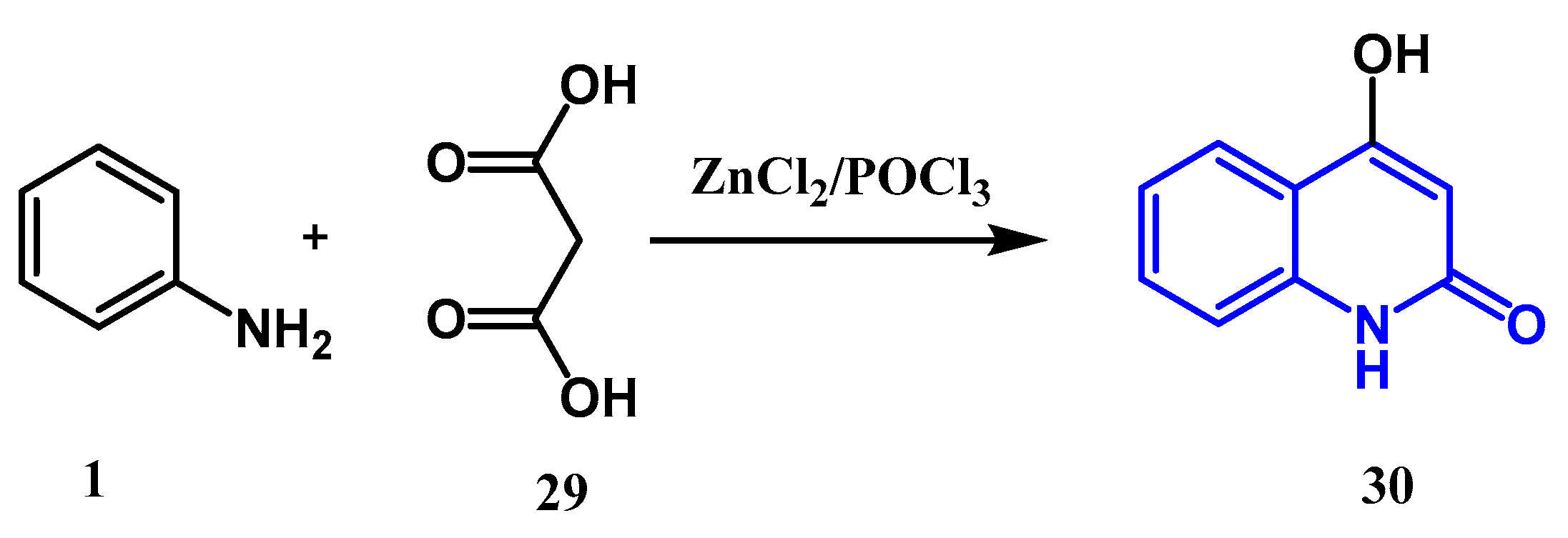
2.1.3. Gould-Jacobs Reactions
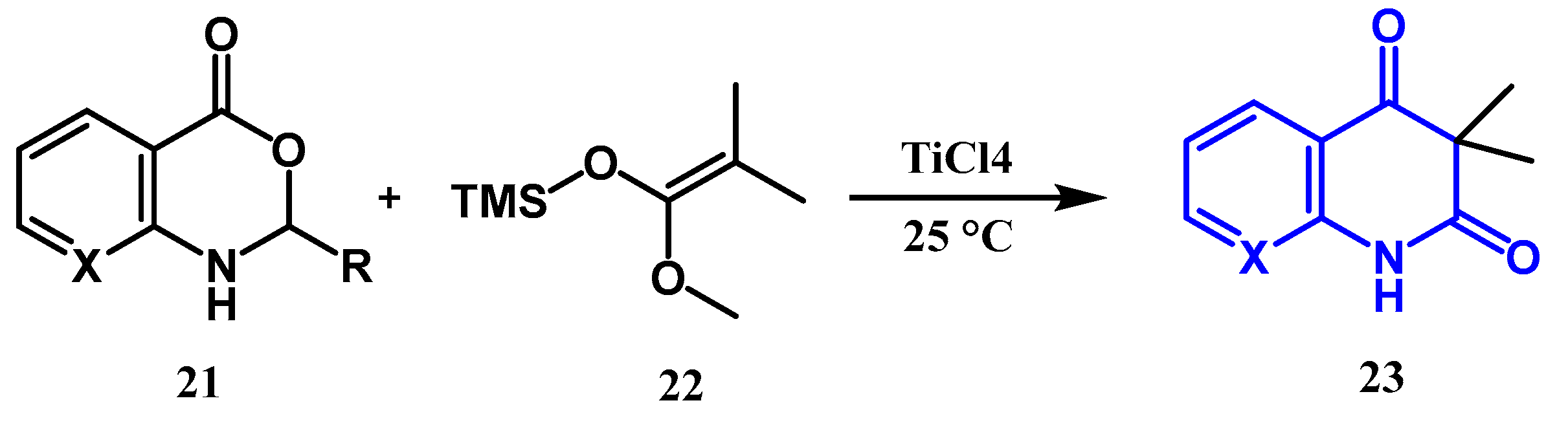
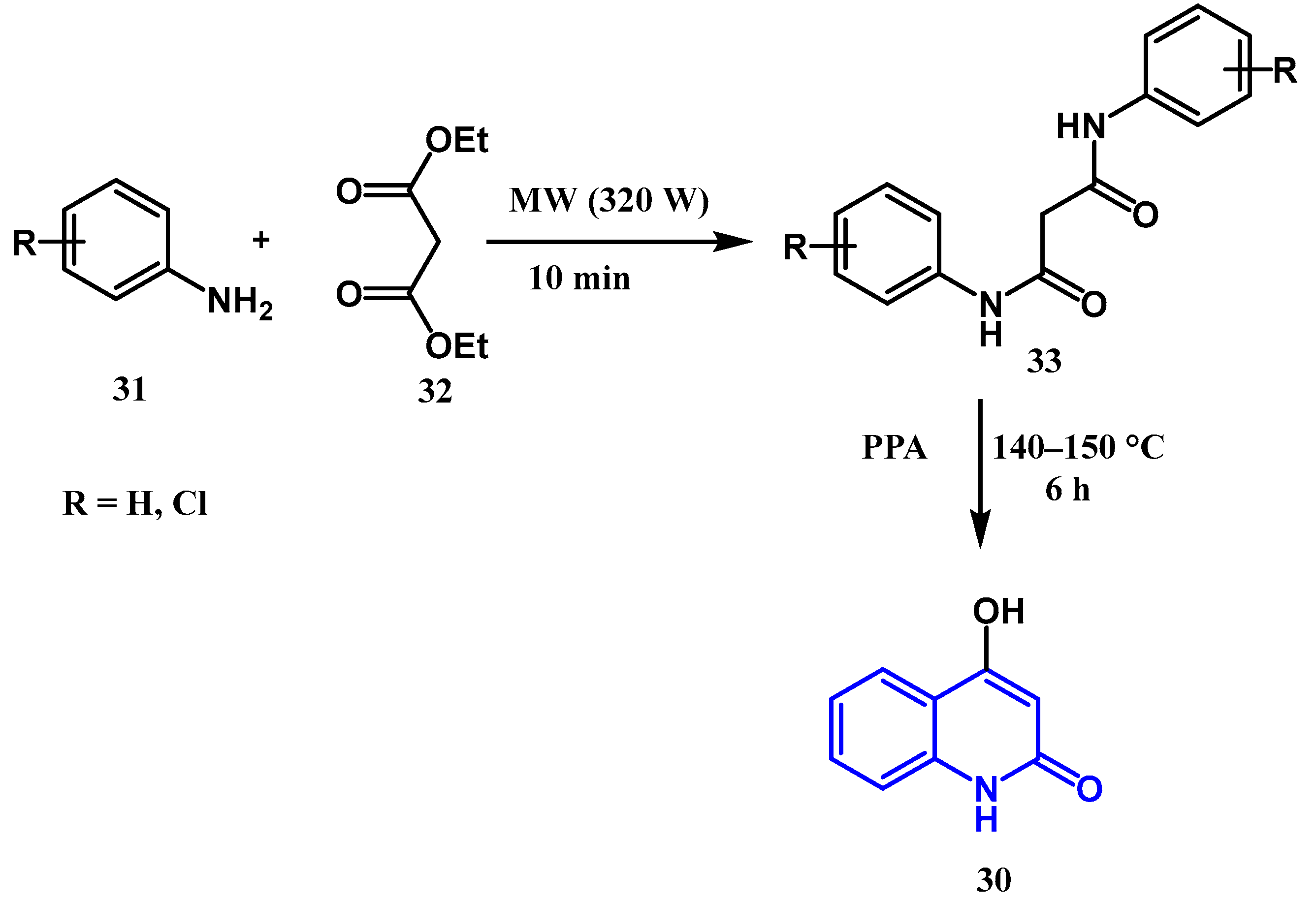
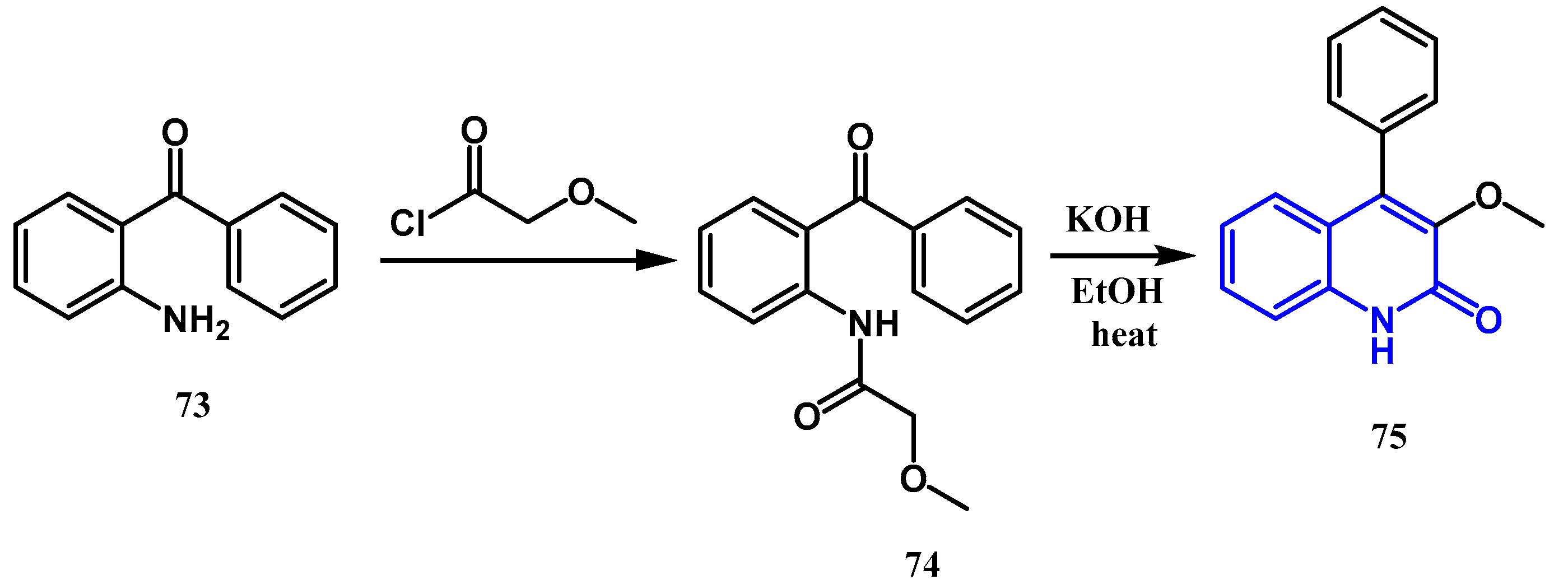
2.1.4. Jampilek Reaction:
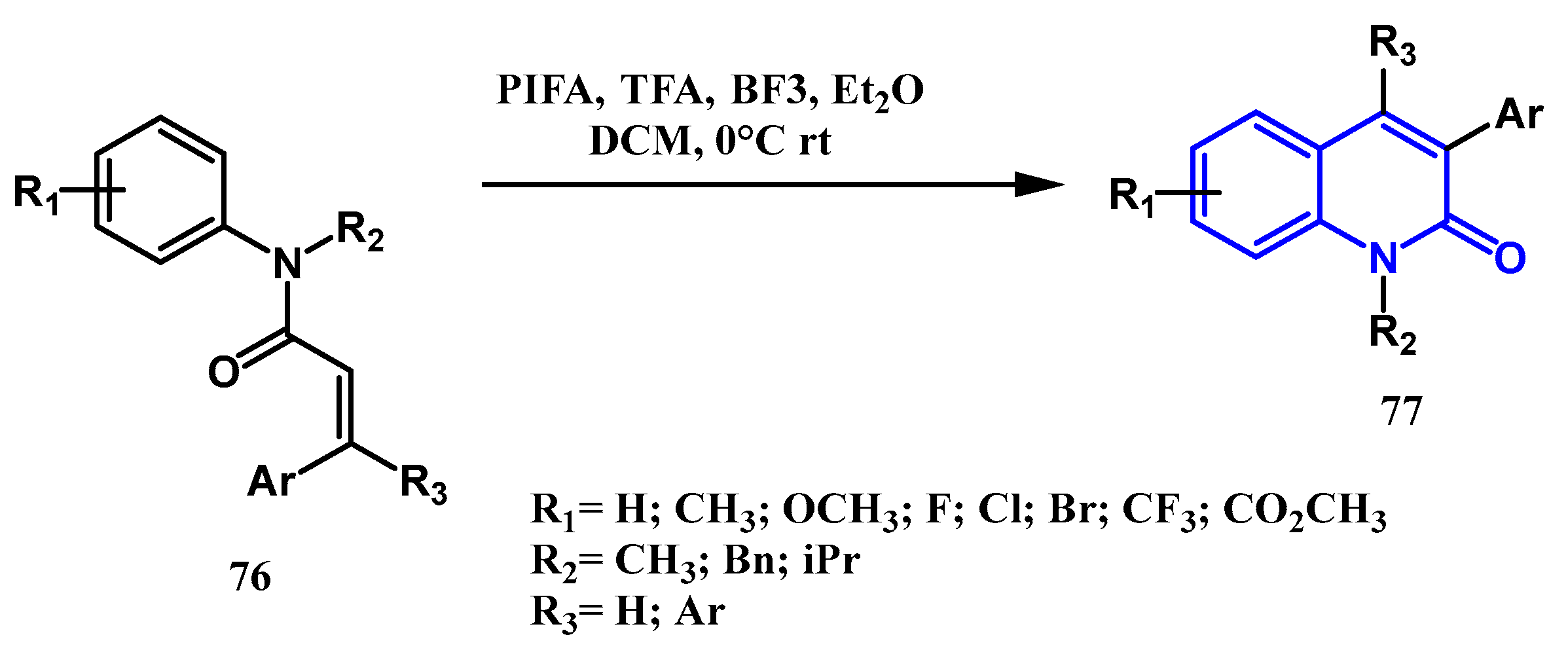
2.1.5. Cheng Reaction
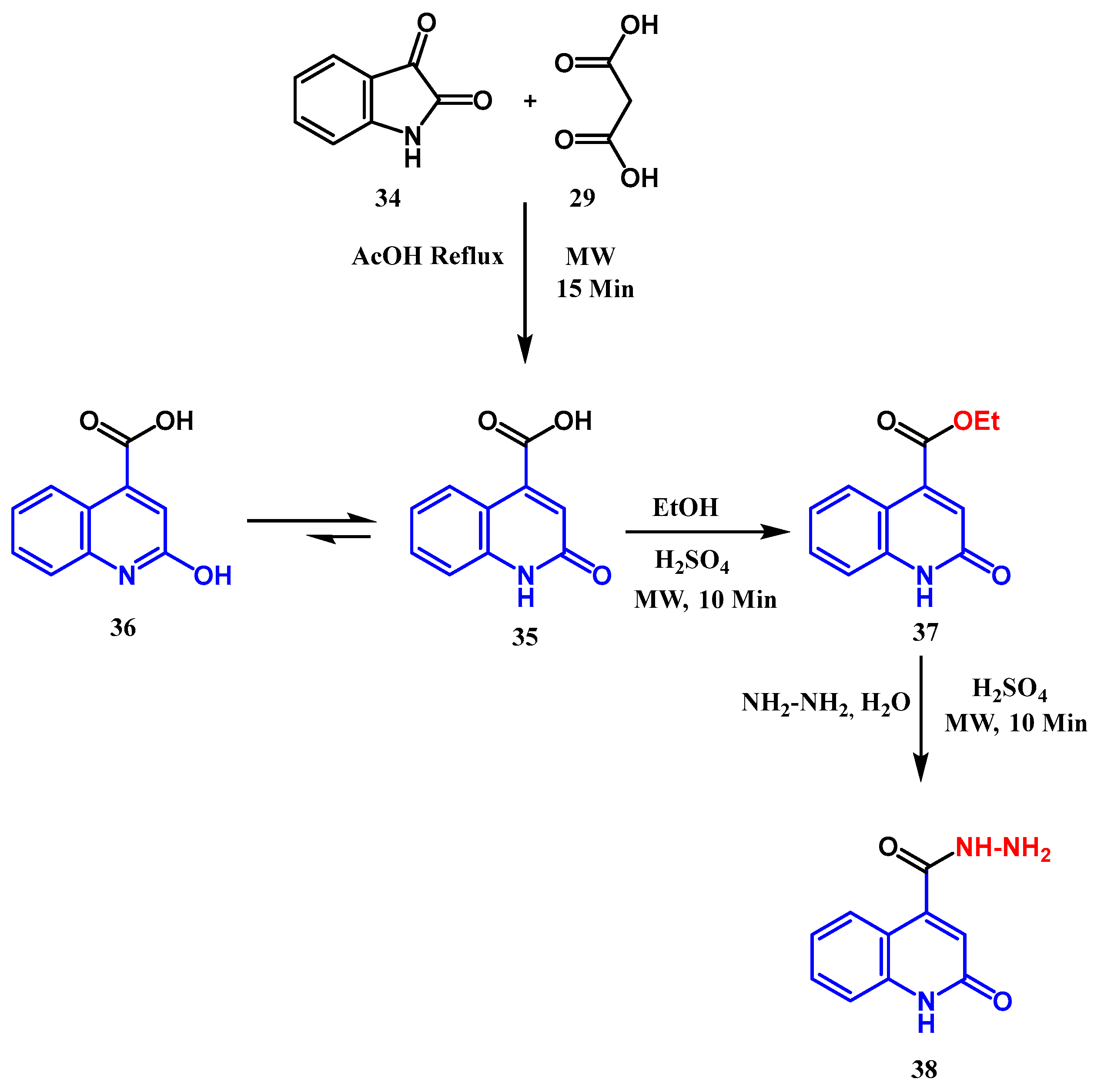
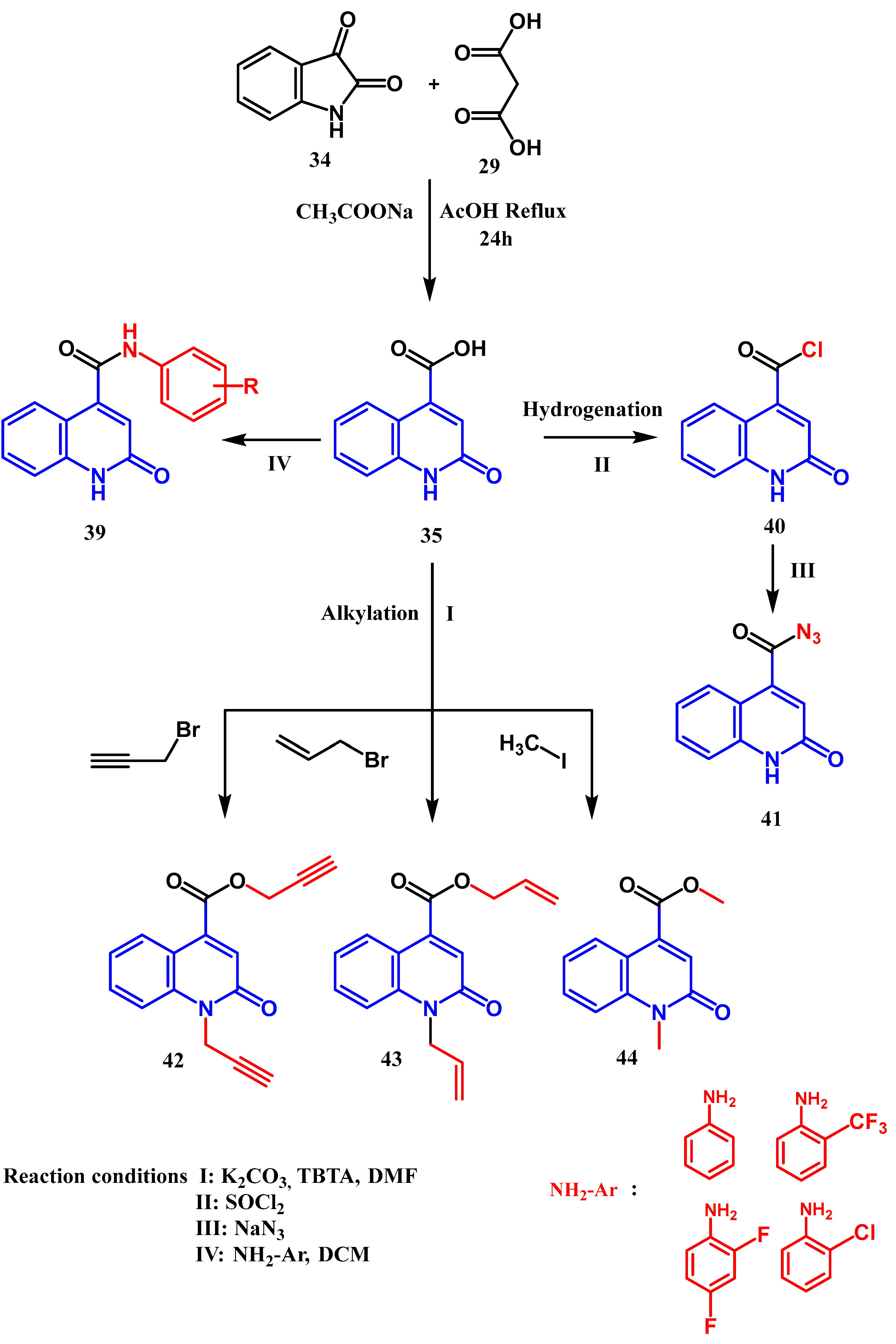
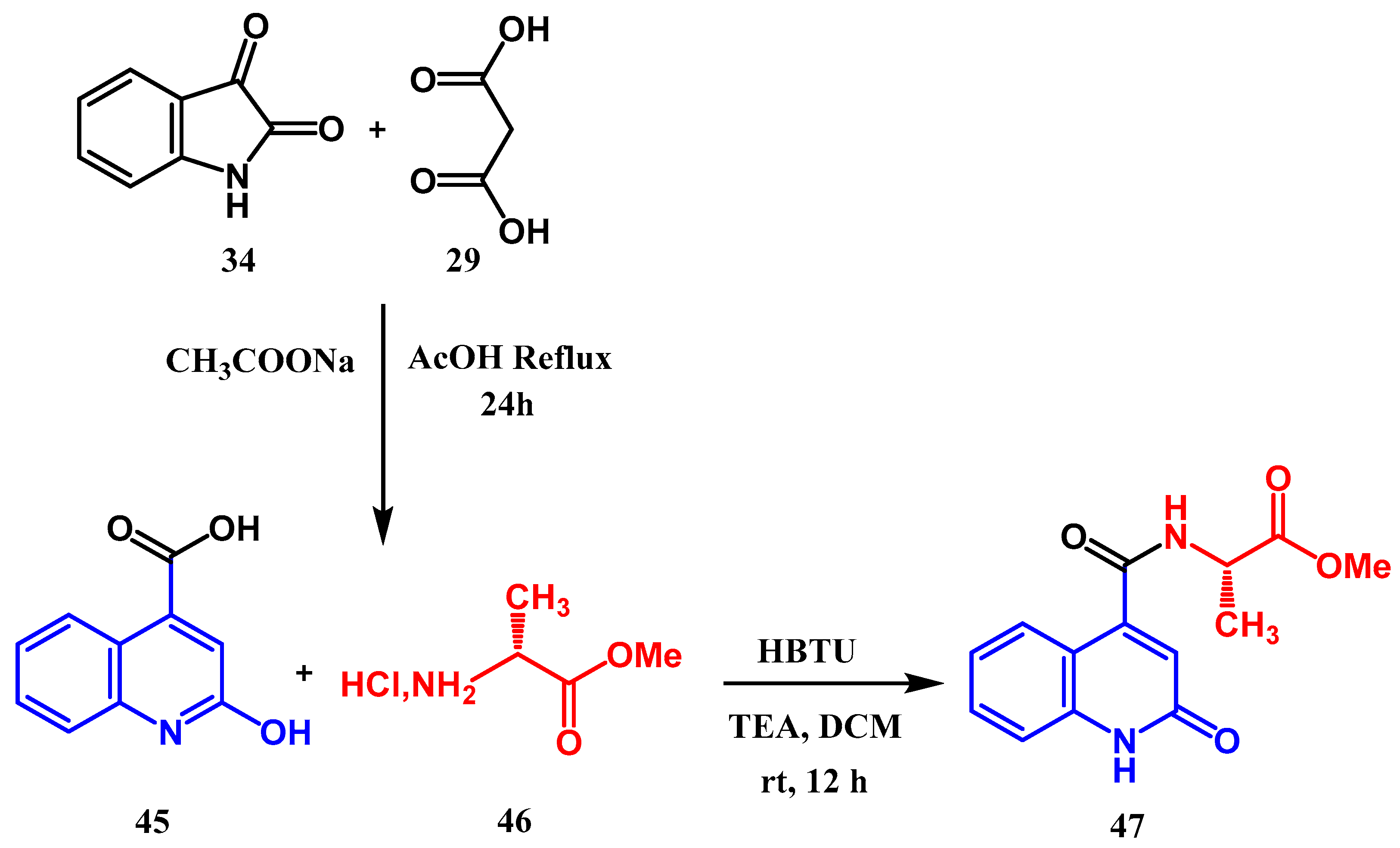
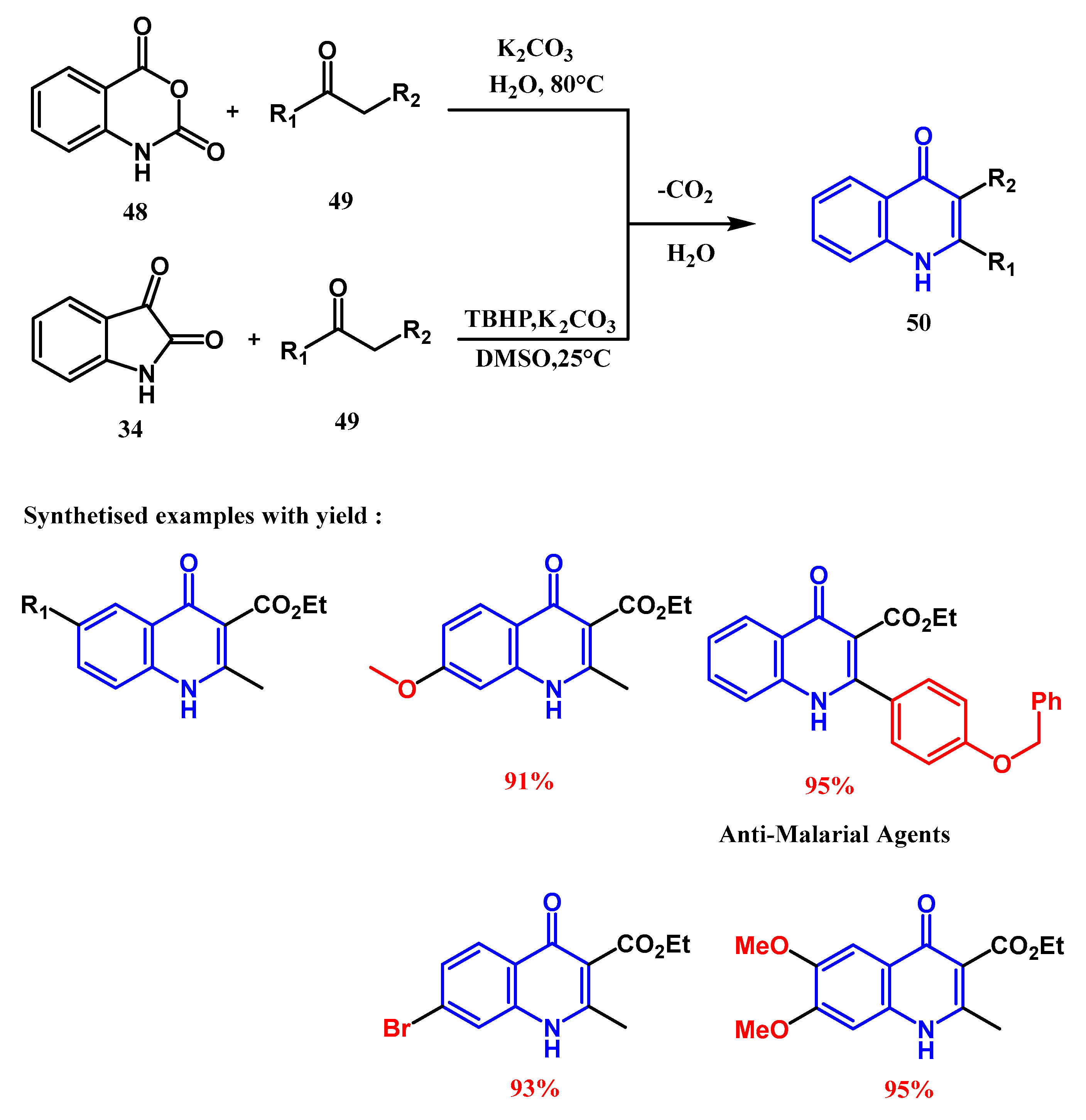
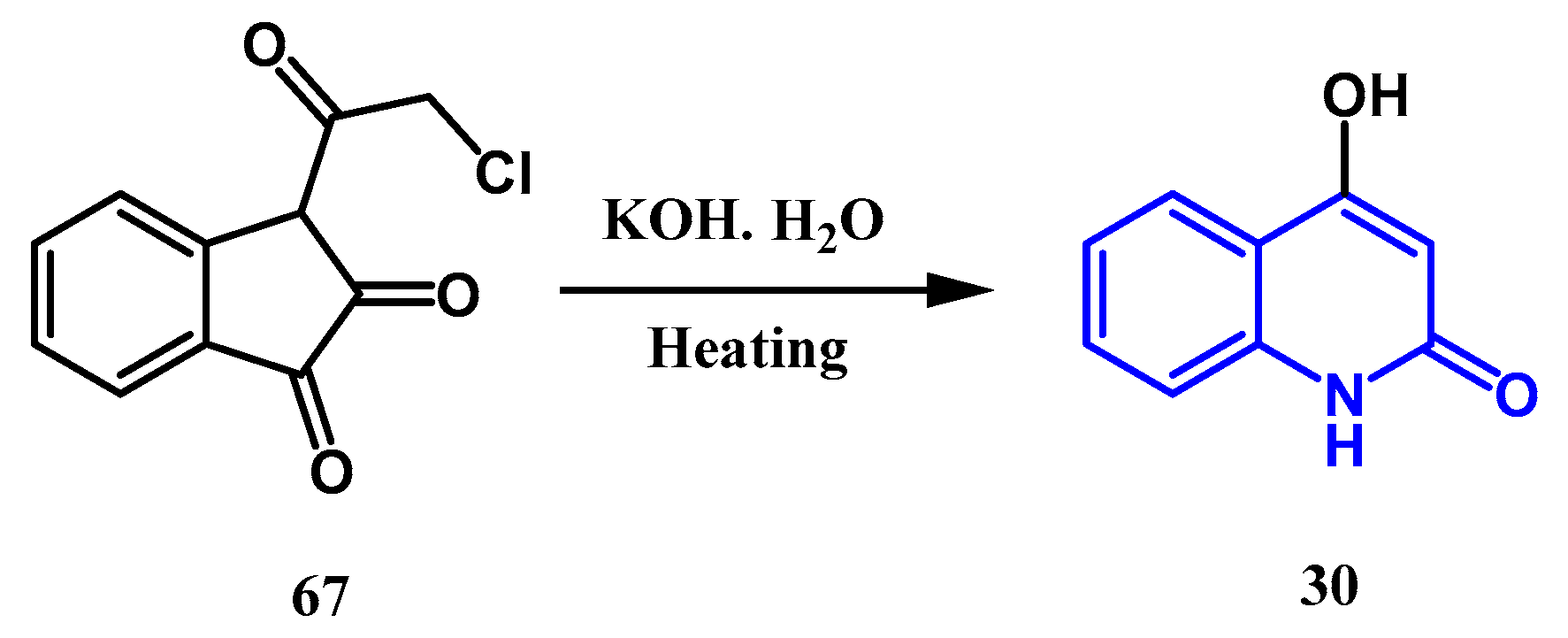
2.2. Syntheses from the Indole-2,3-Dione Ring
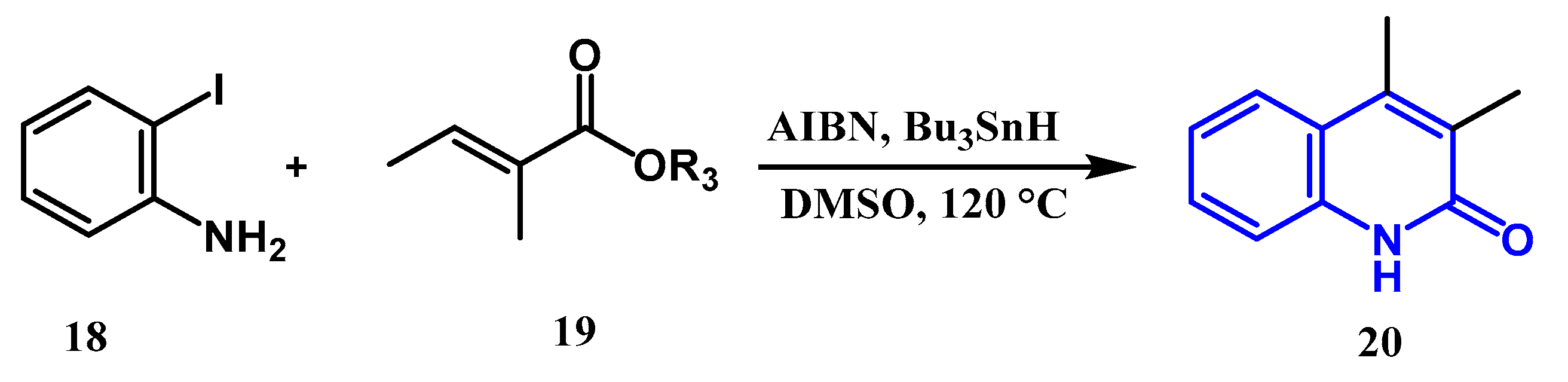
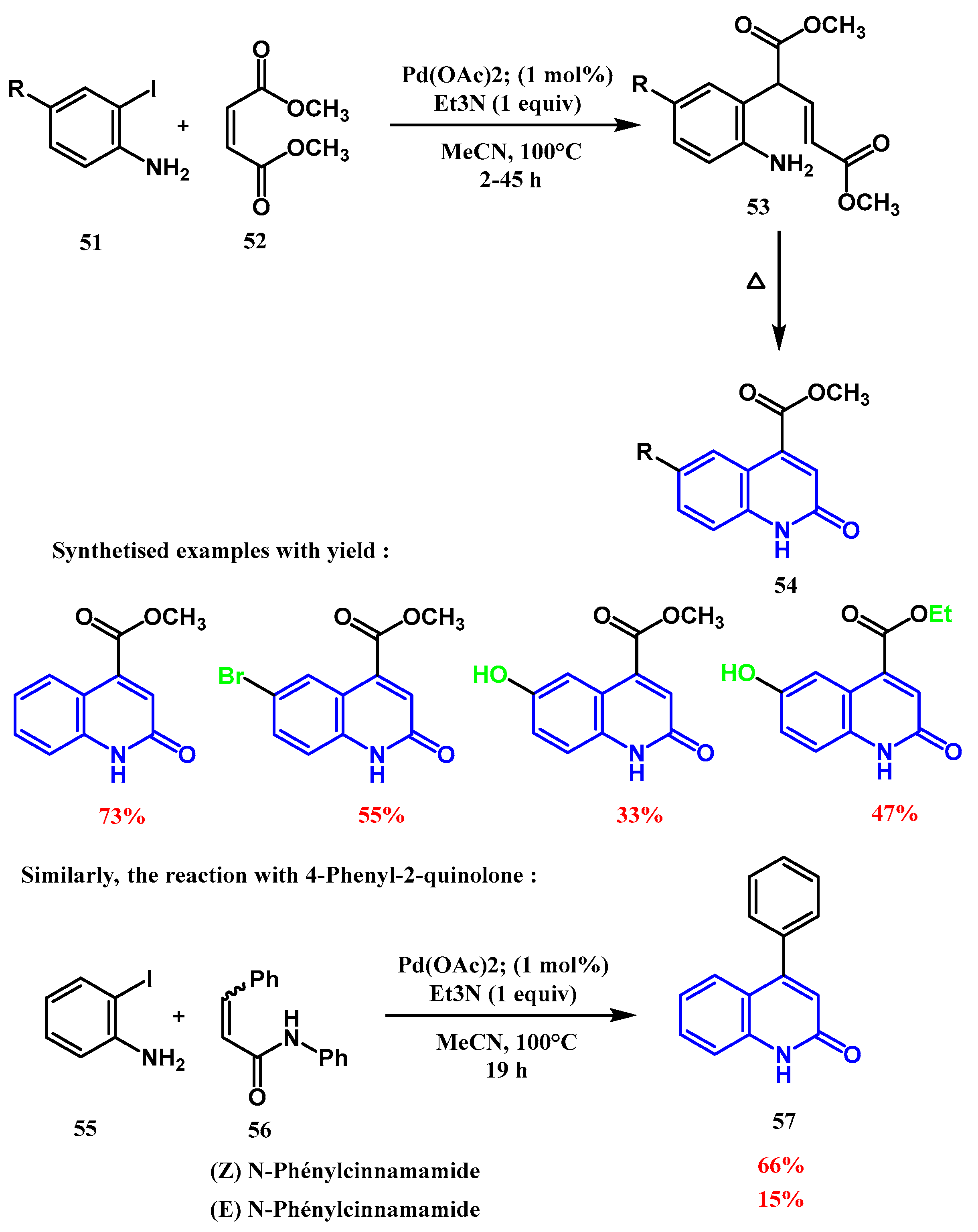
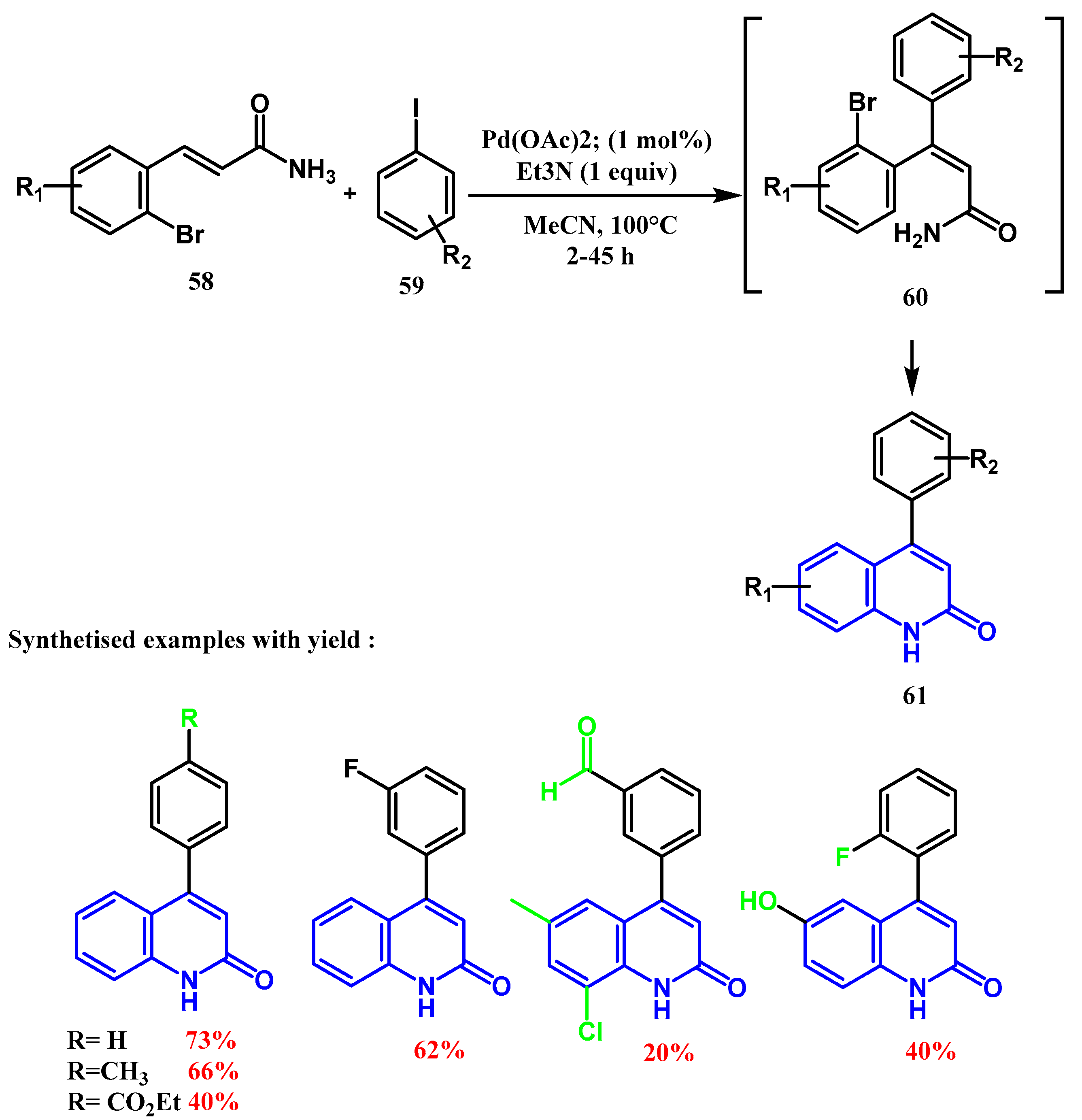
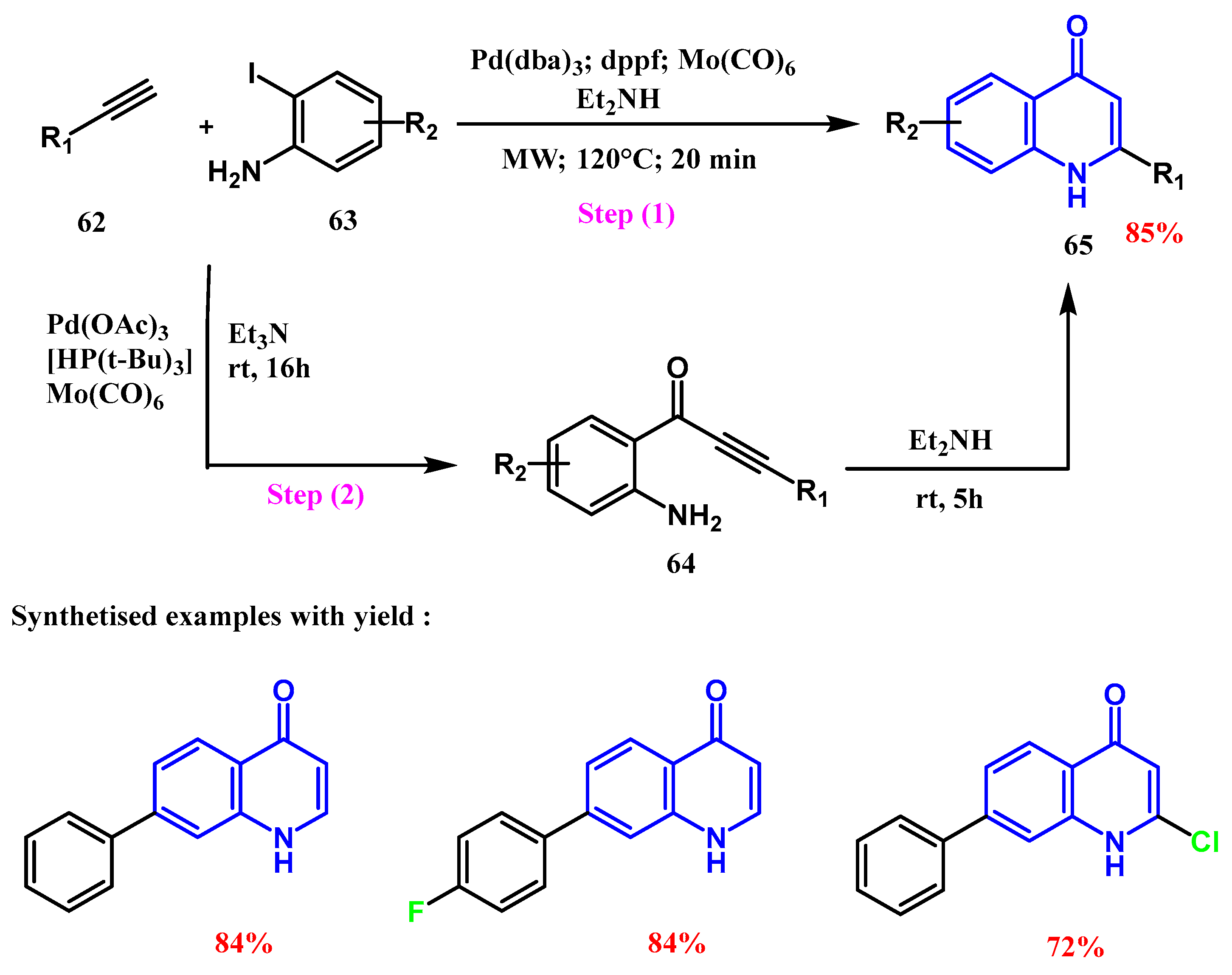
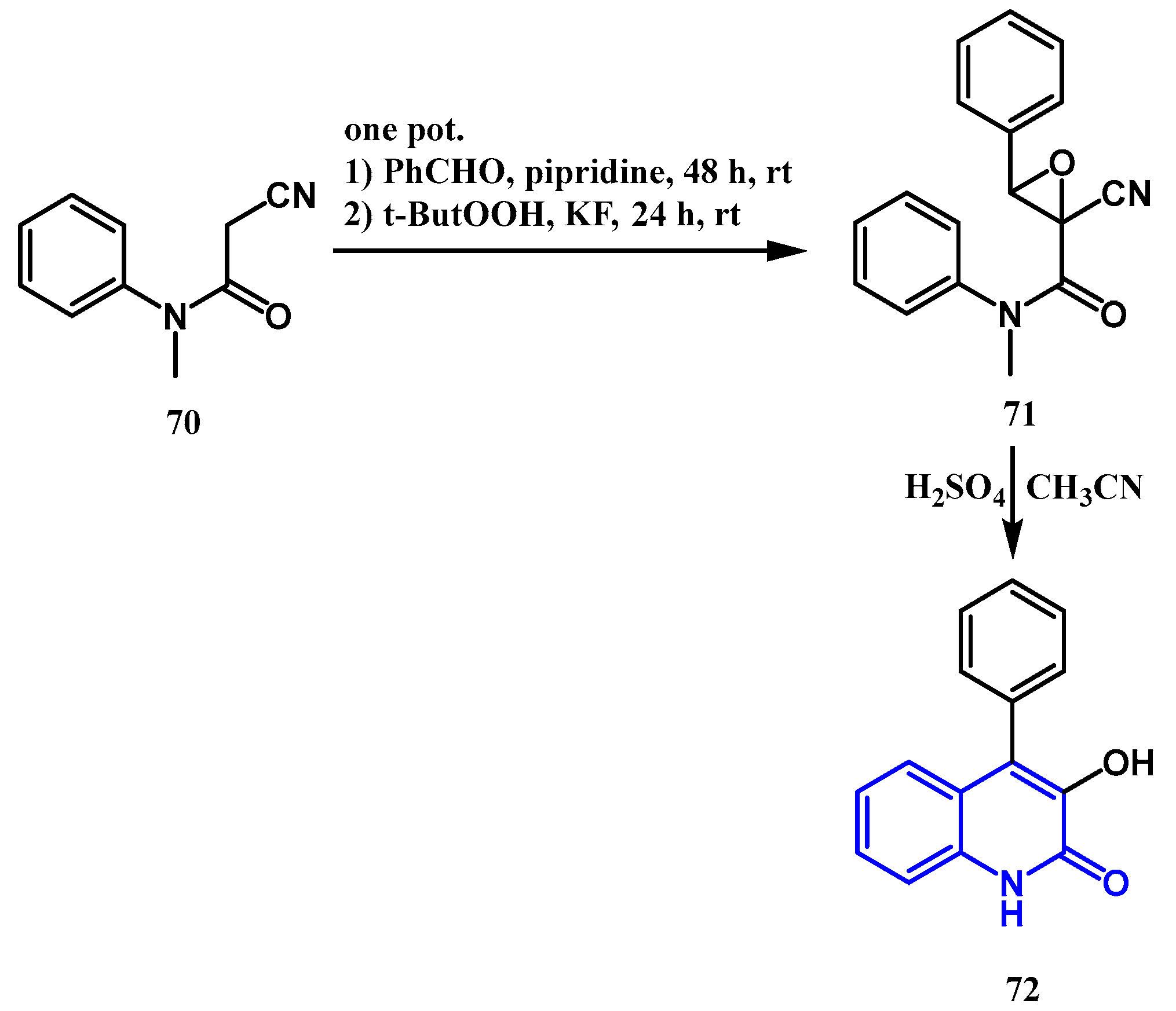
2.3. Reactions Catalyzed by Pd
2.4. Catalytic Reduction:
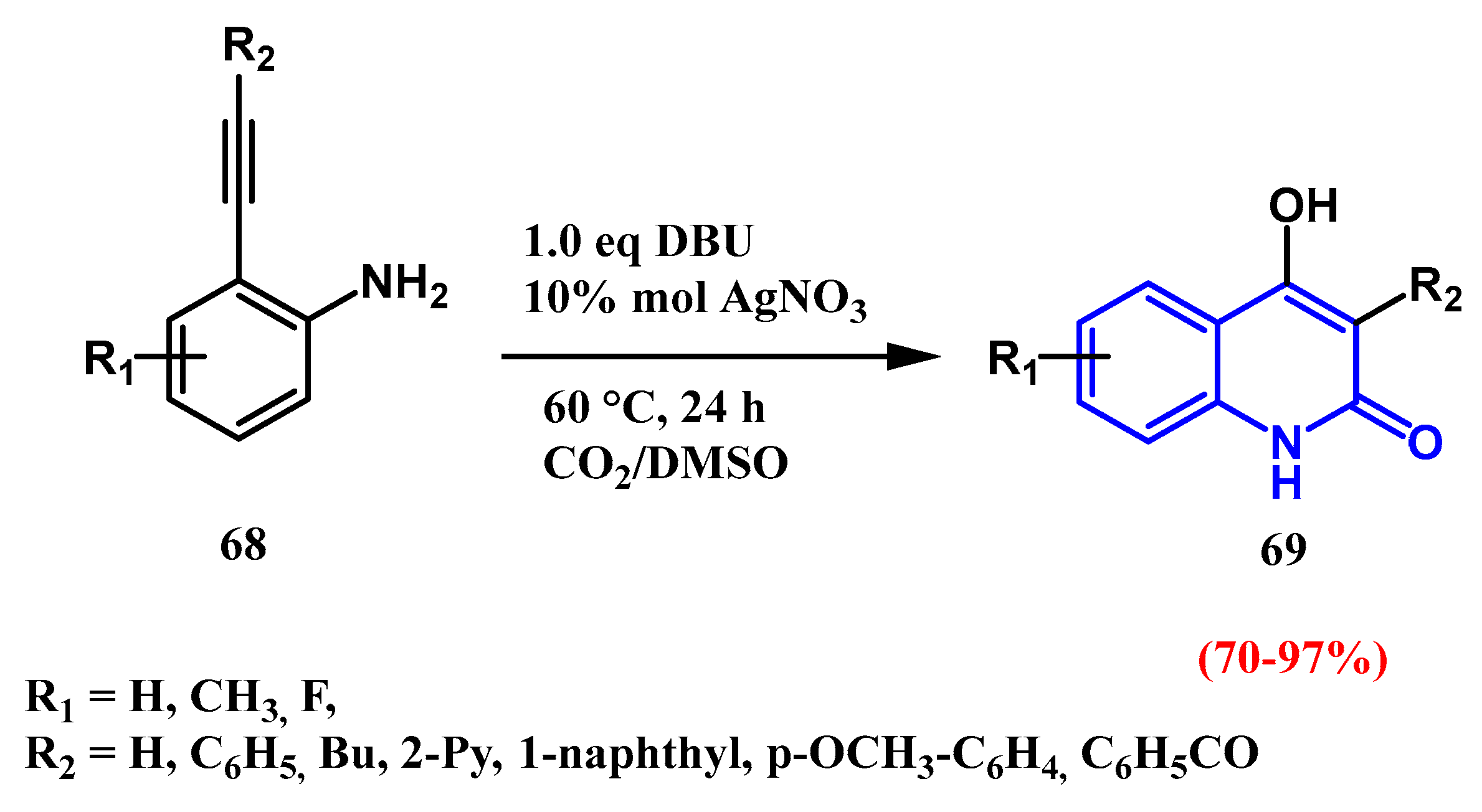
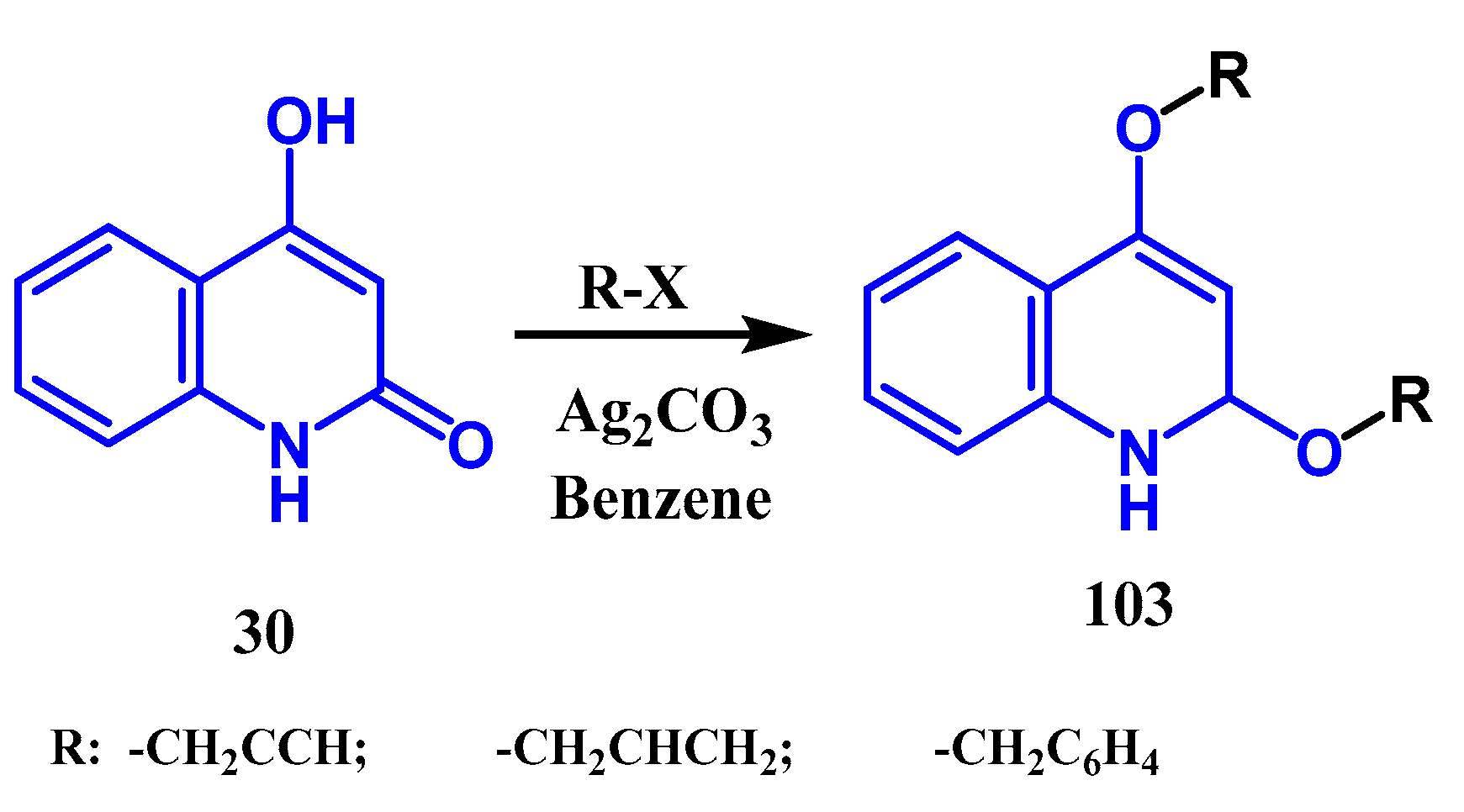
2.5. Silver-Catalyzed Carboxylation:
3. Reactivity of Quinolones Derivatives
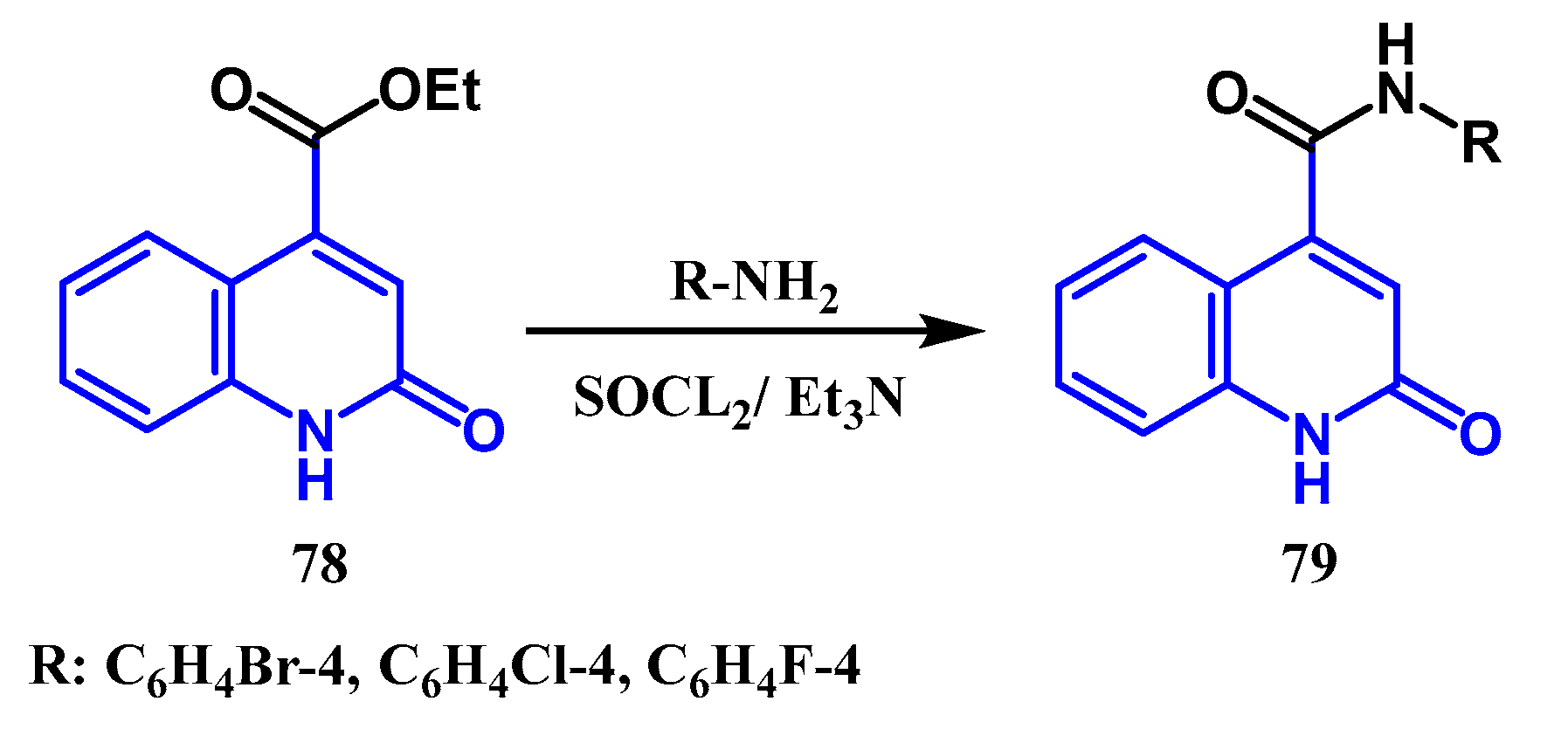
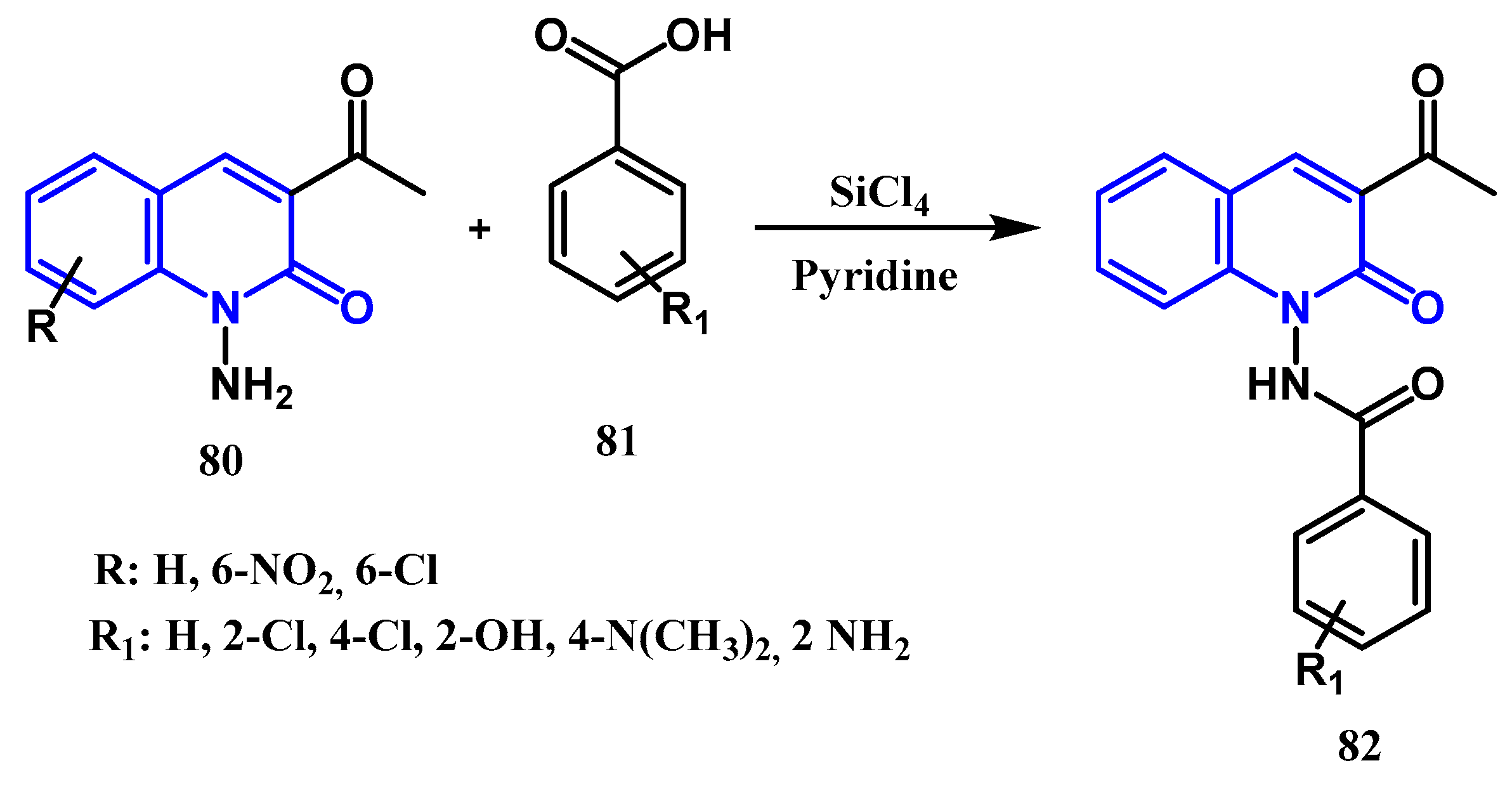
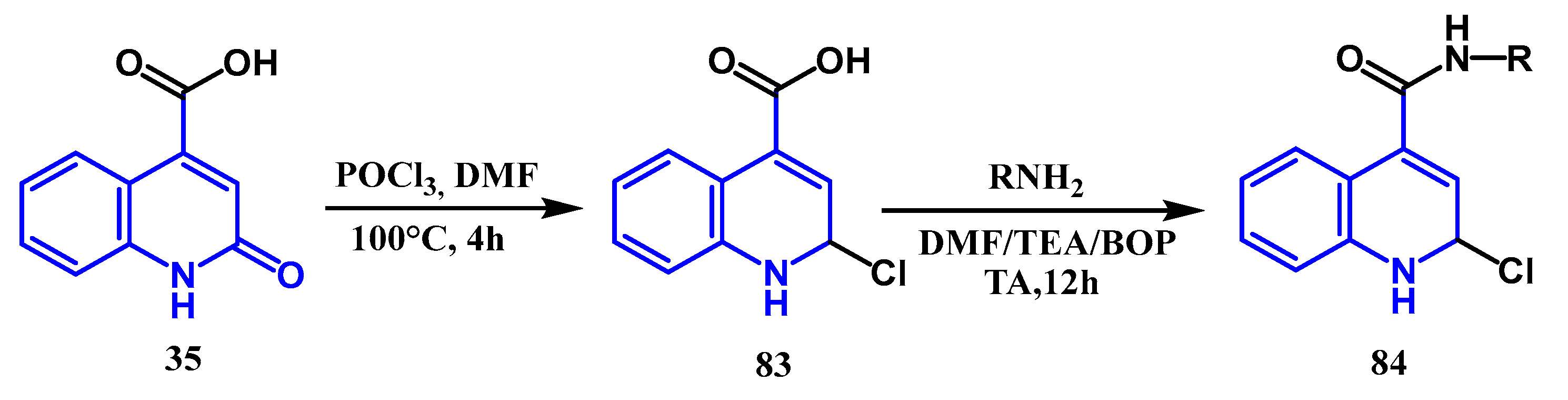
3.1. Amidation and Amination
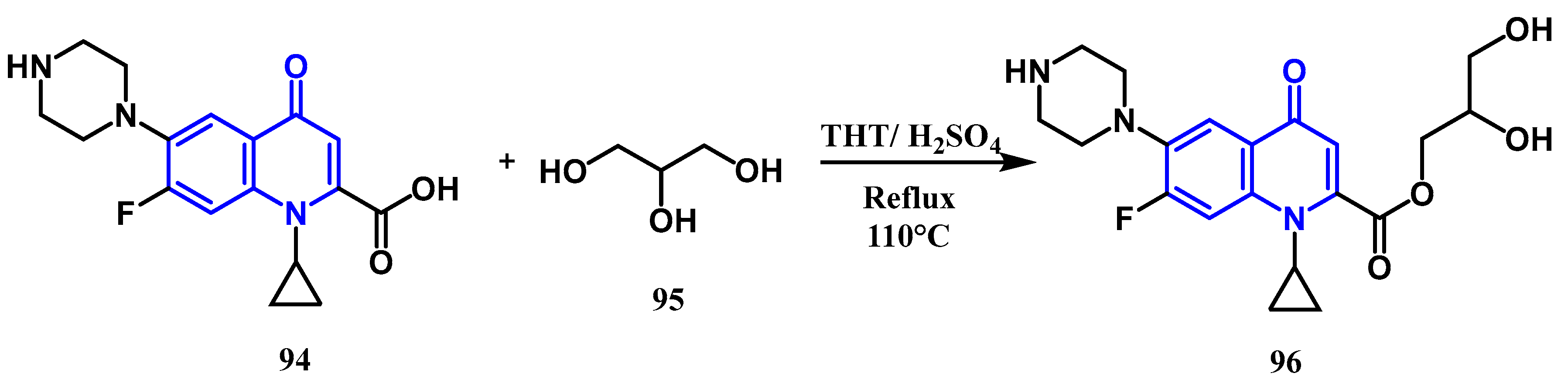
3.2. Esterification
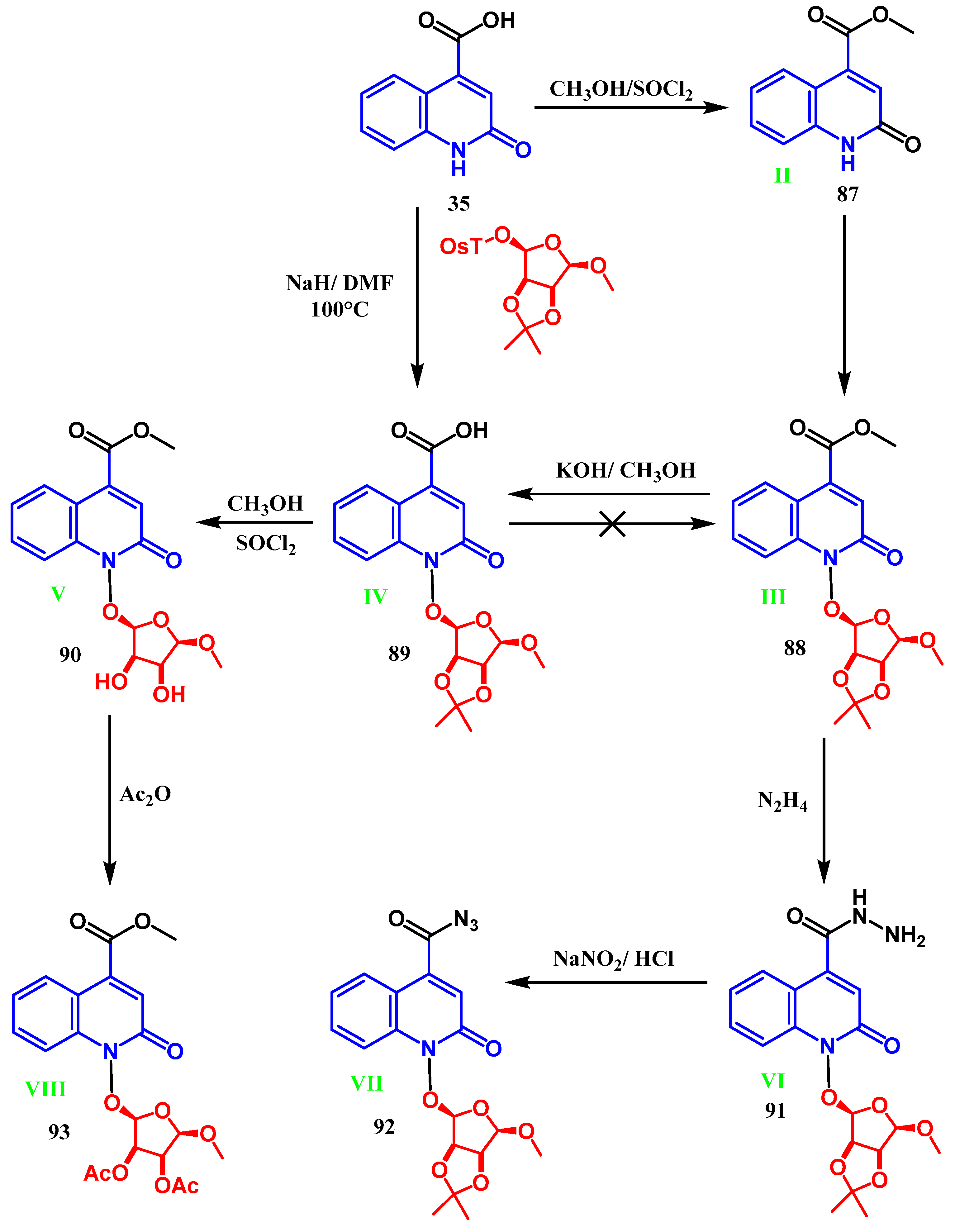
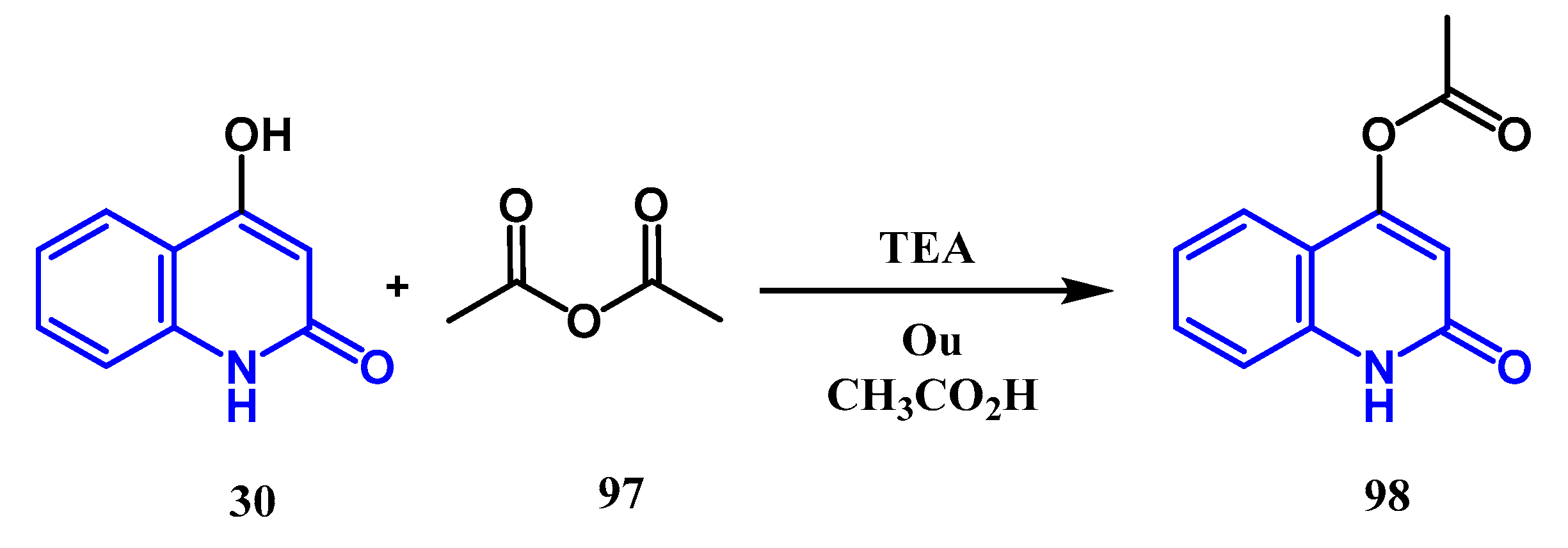
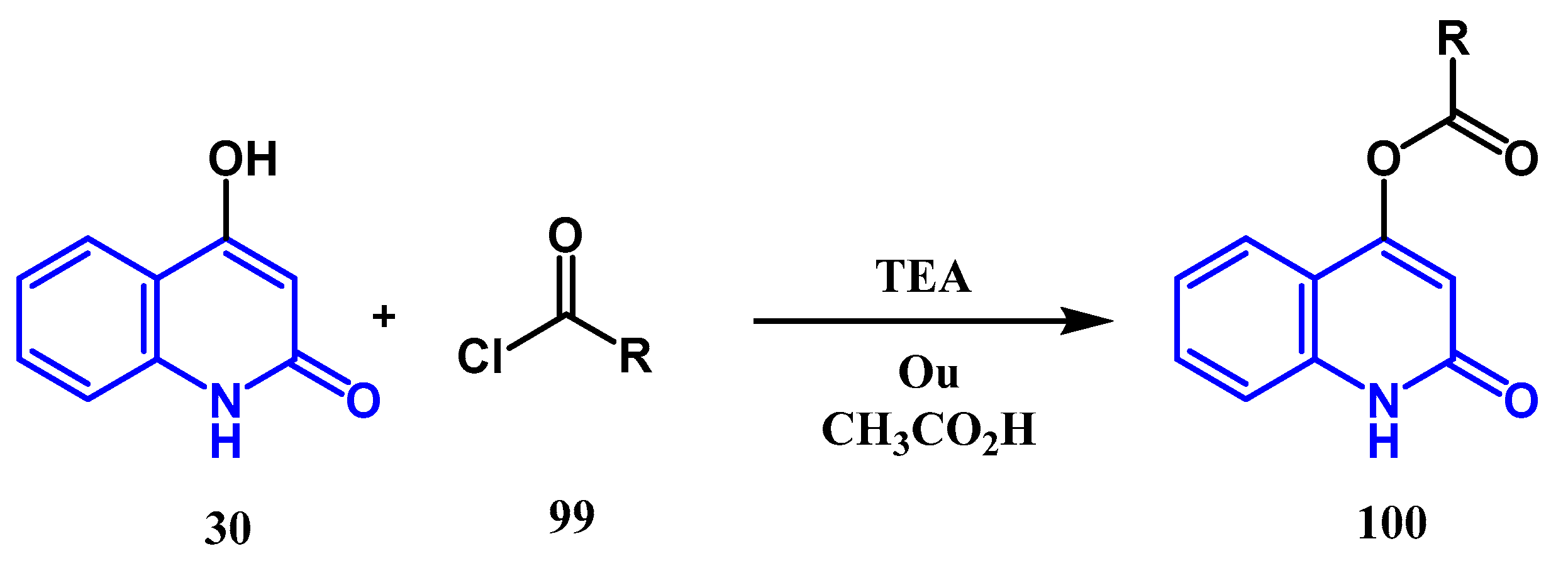
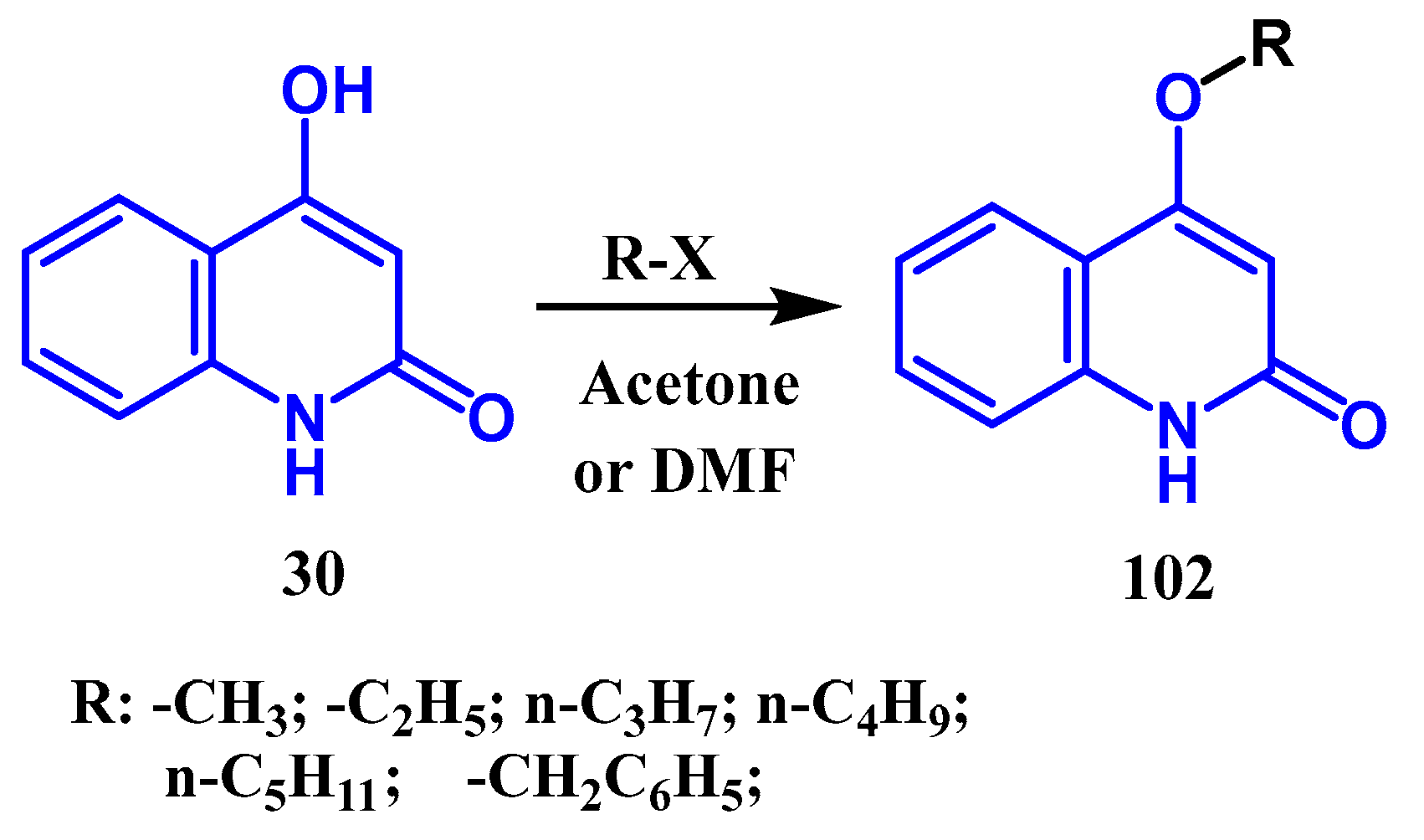
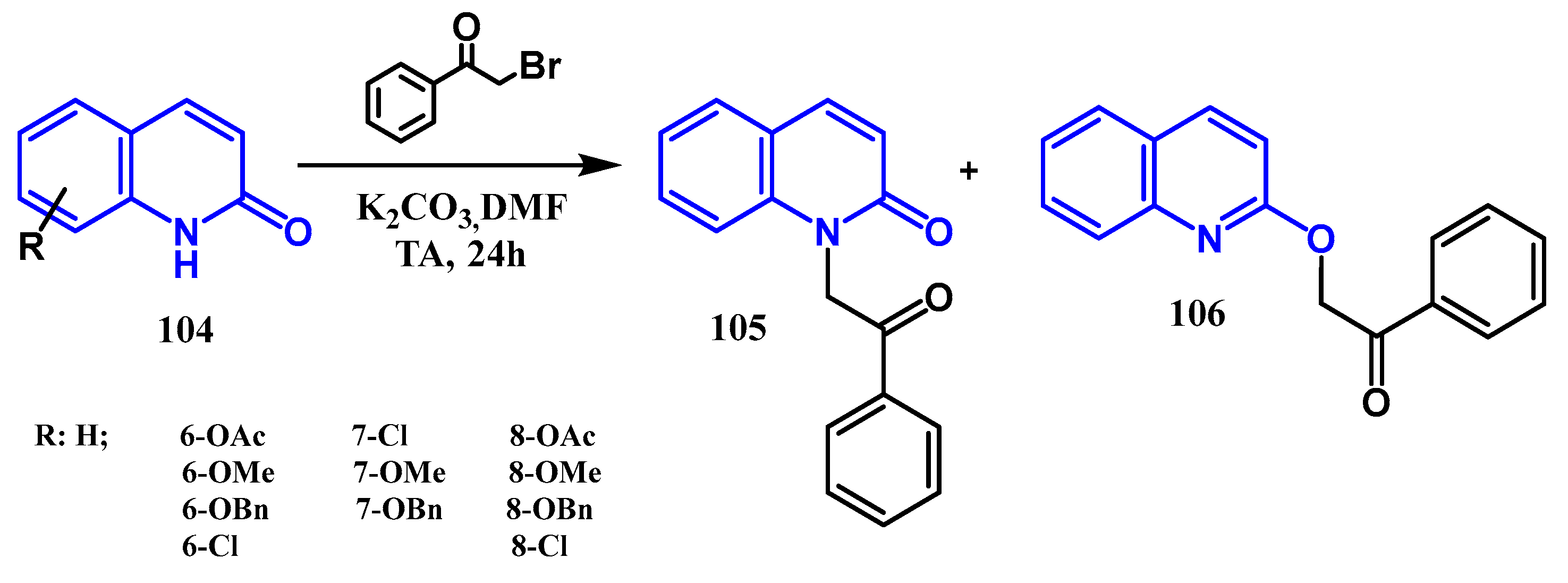
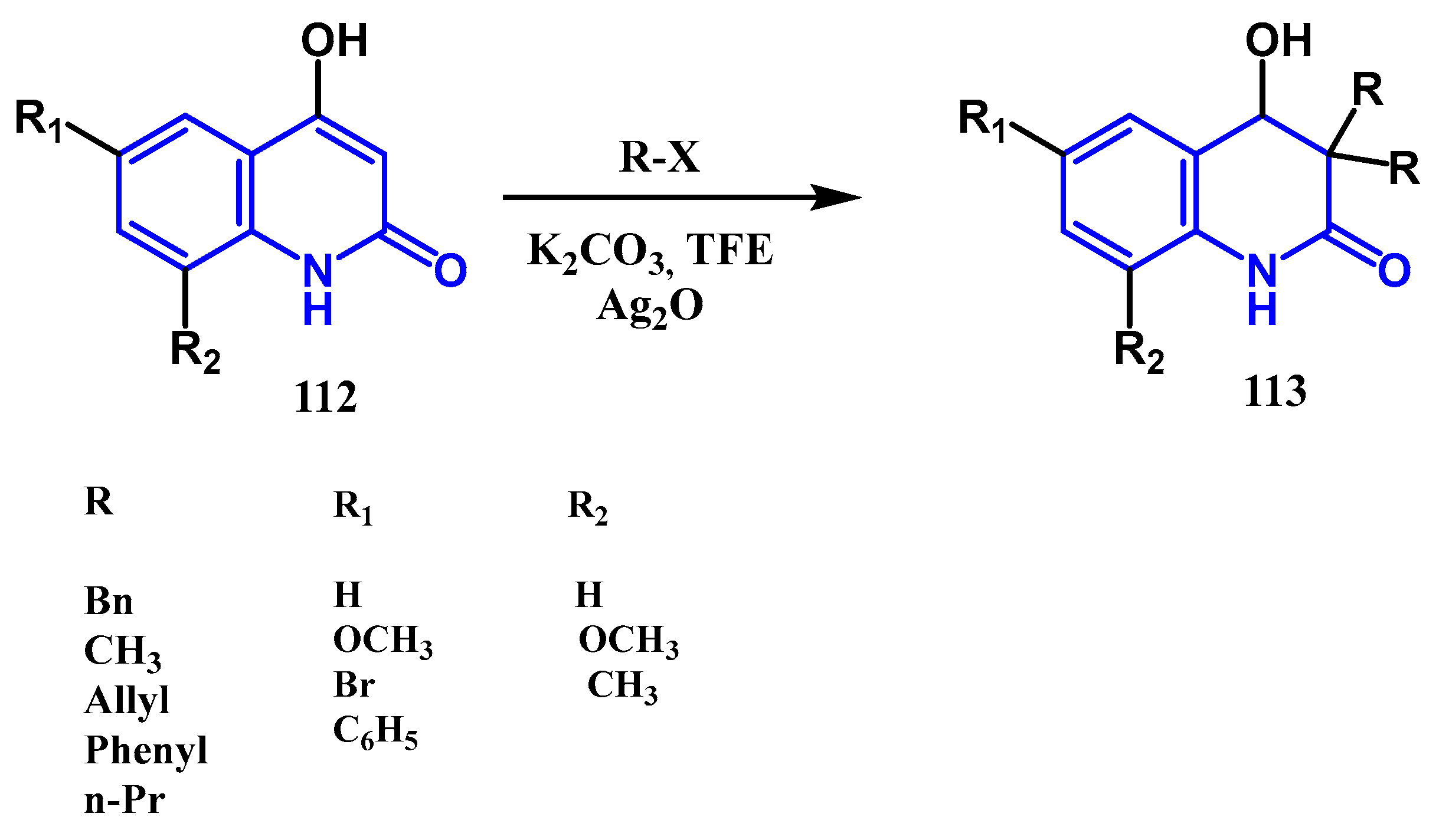
3.3. N-, O- and C-Alkylation
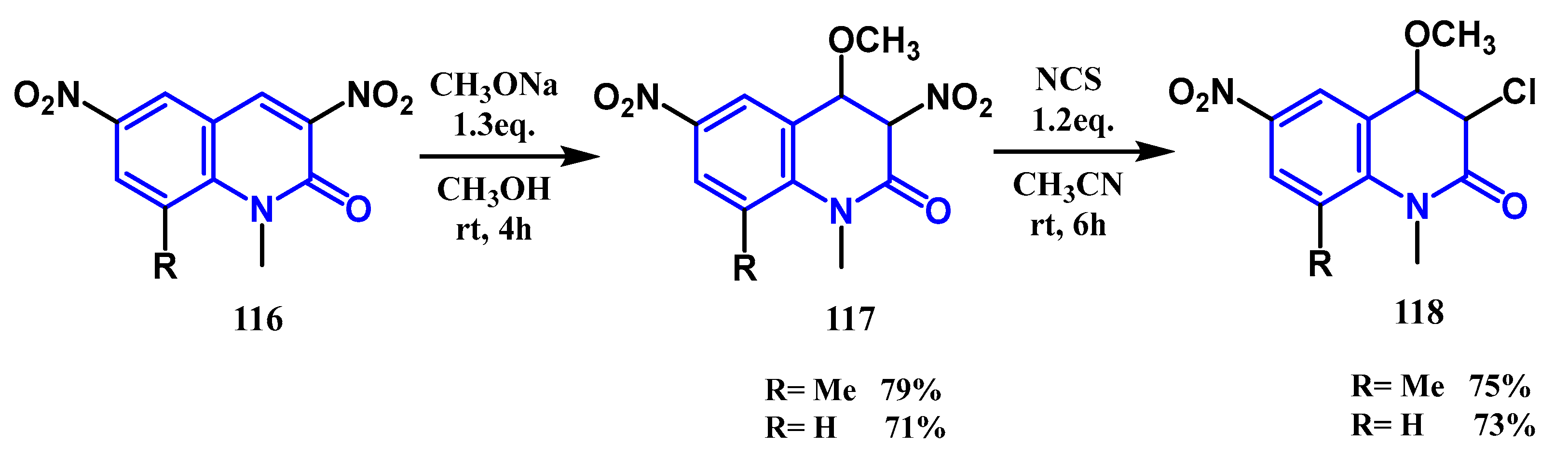
3.4. Halogenation
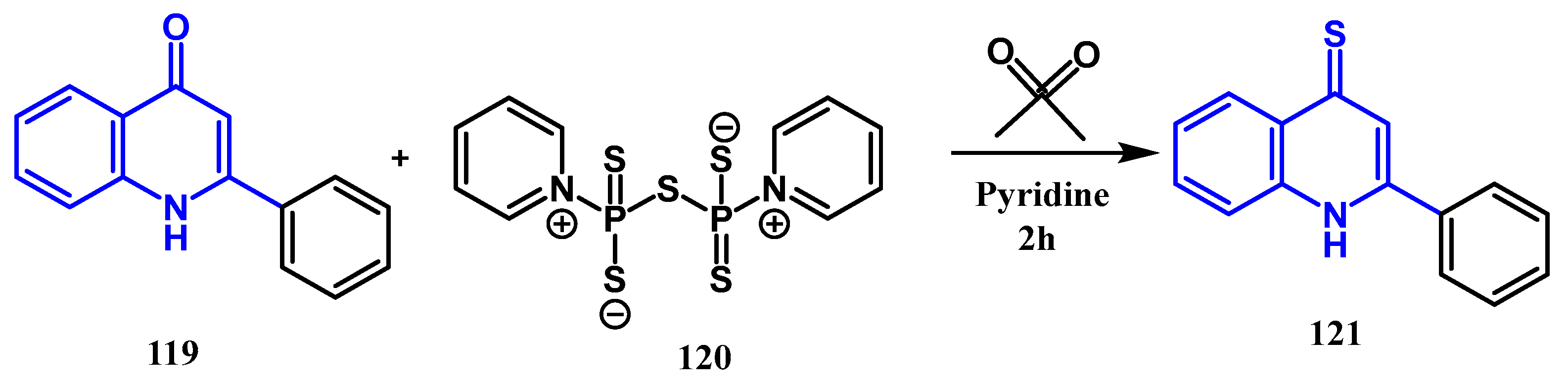
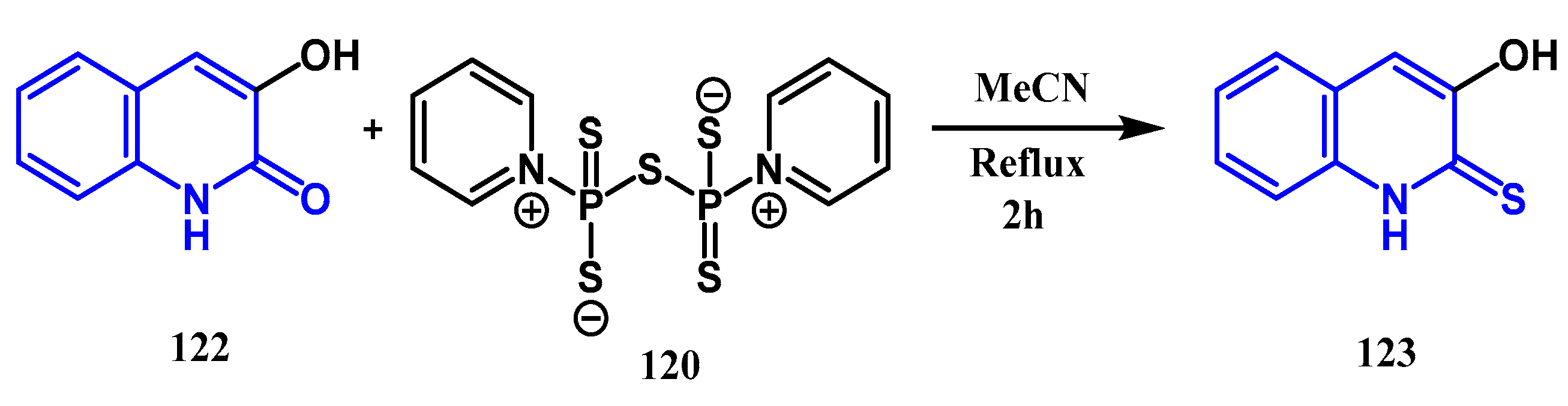
3.5. Thionation
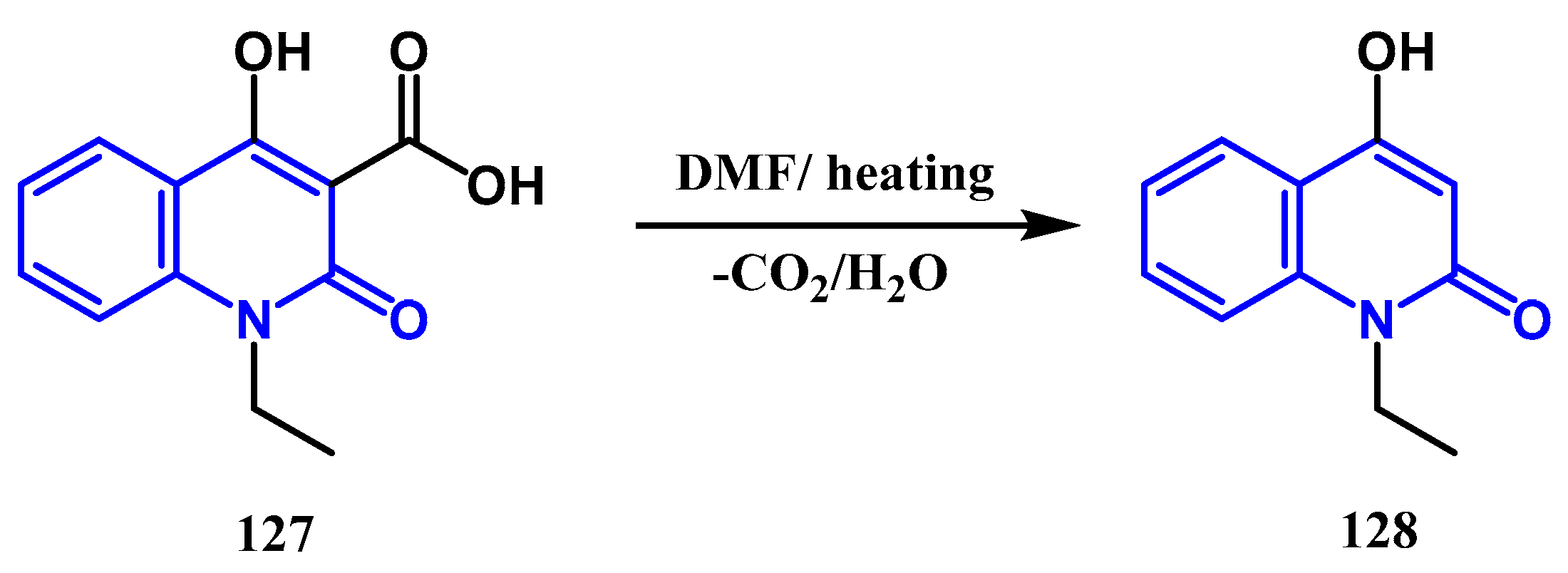
3.6. Hydrolyse and Decarboxylation
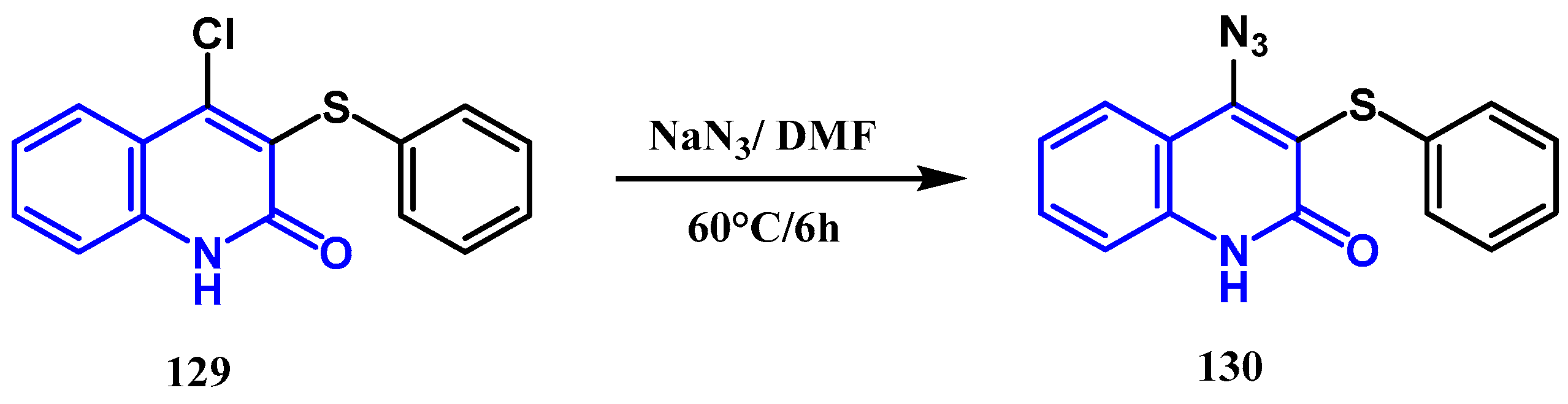
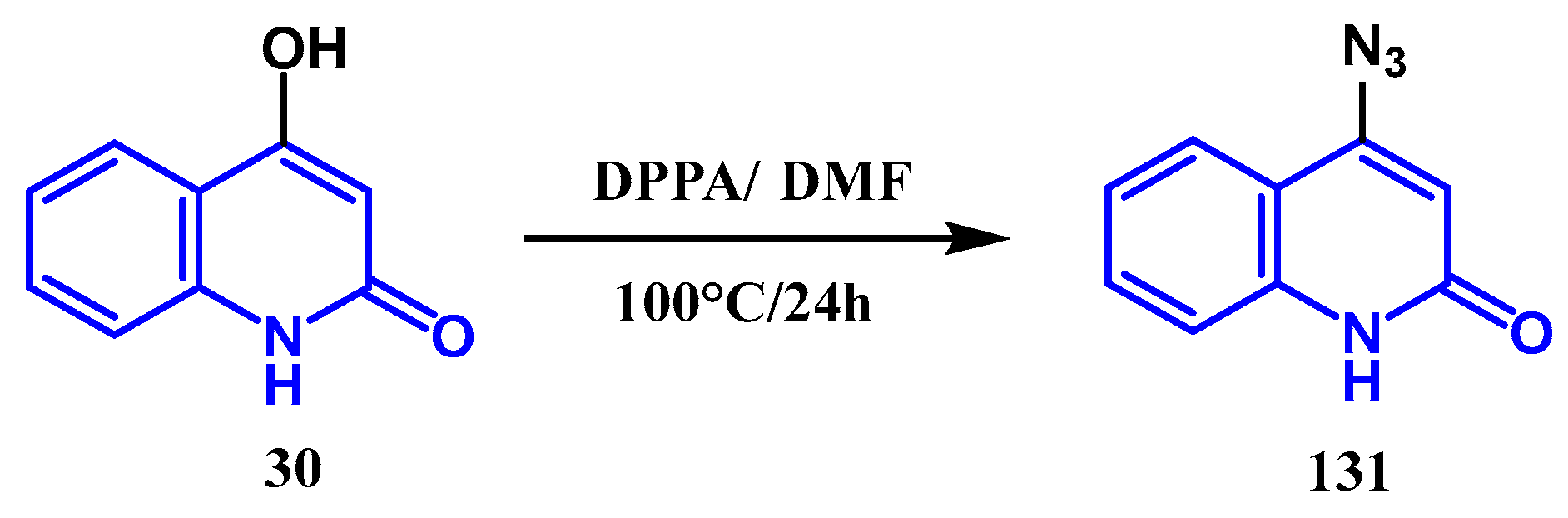
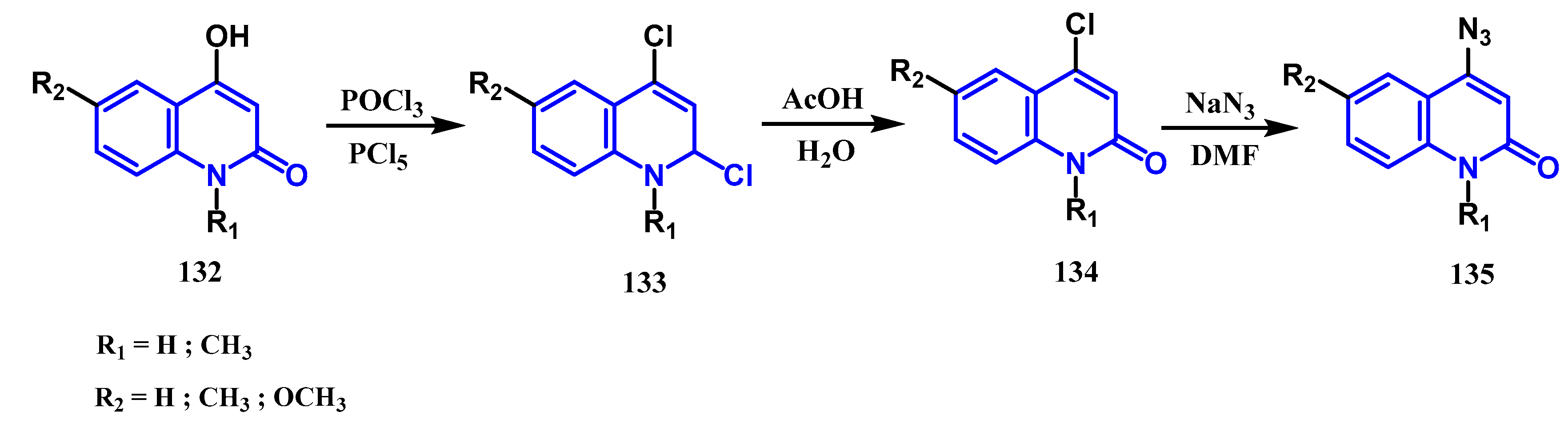
3.7. Azidation
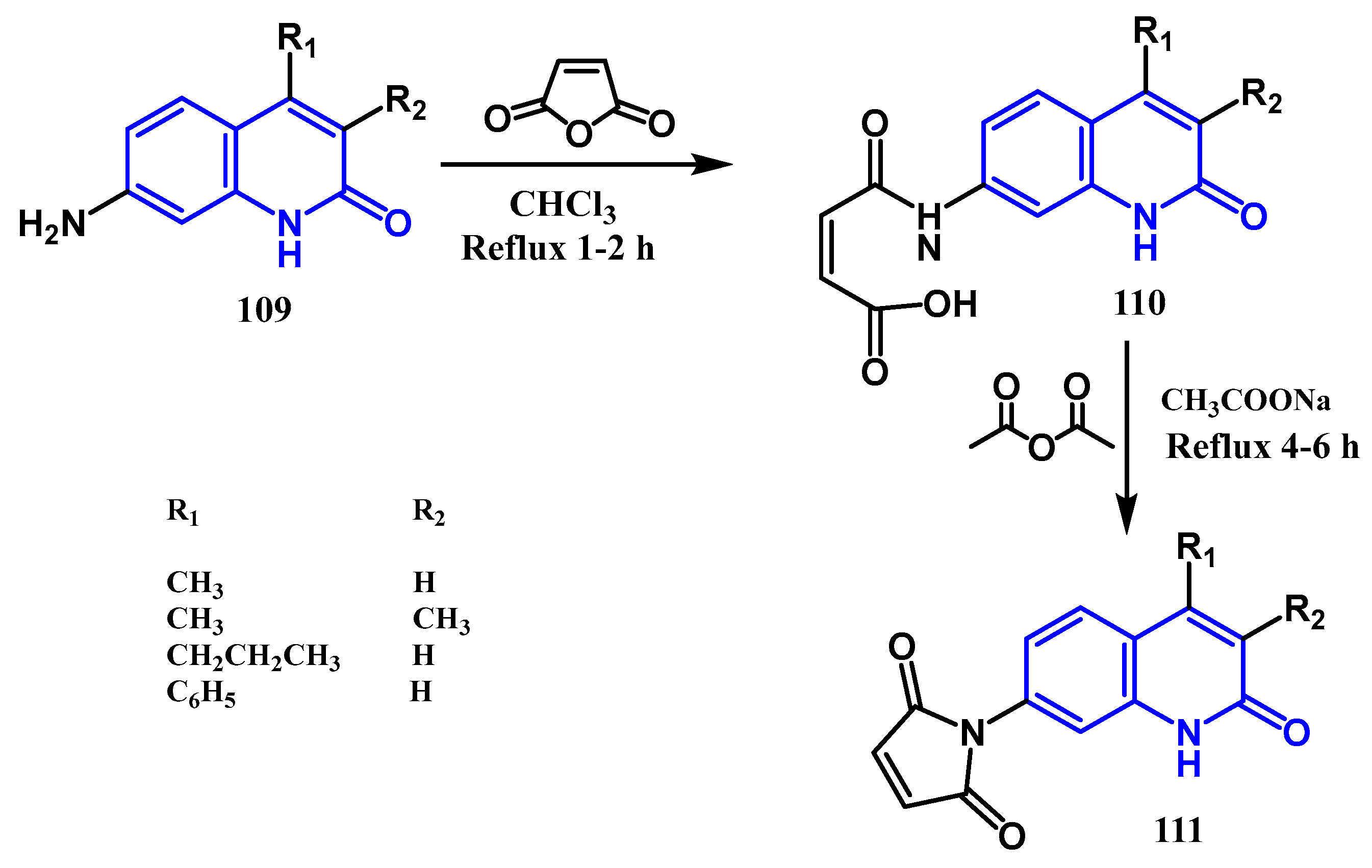
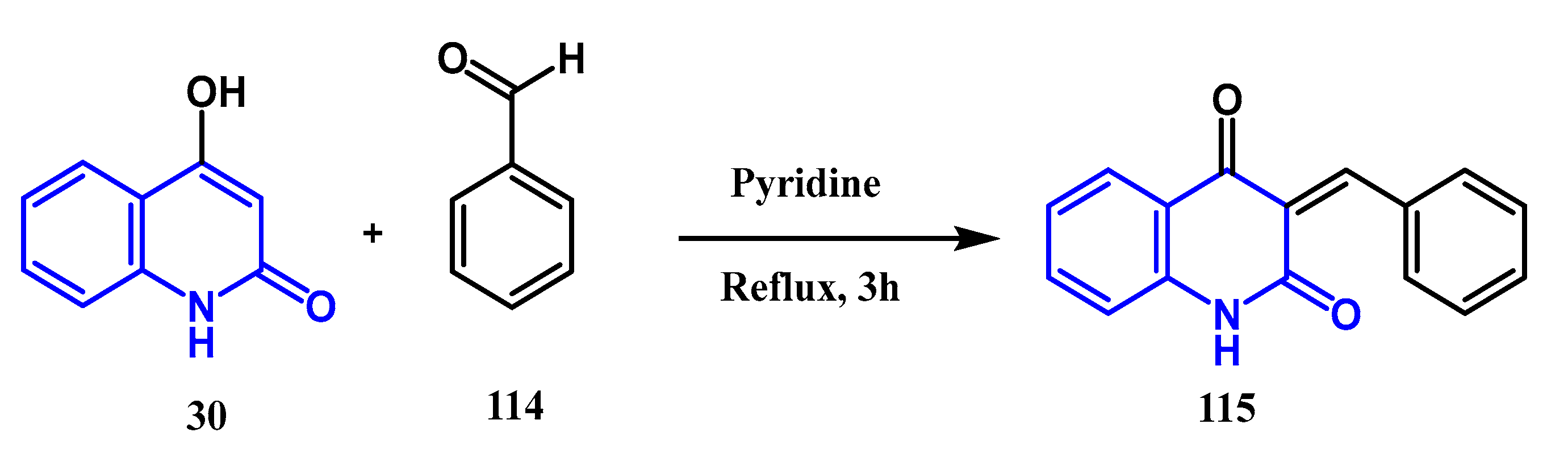
3.8. Cycloaddition
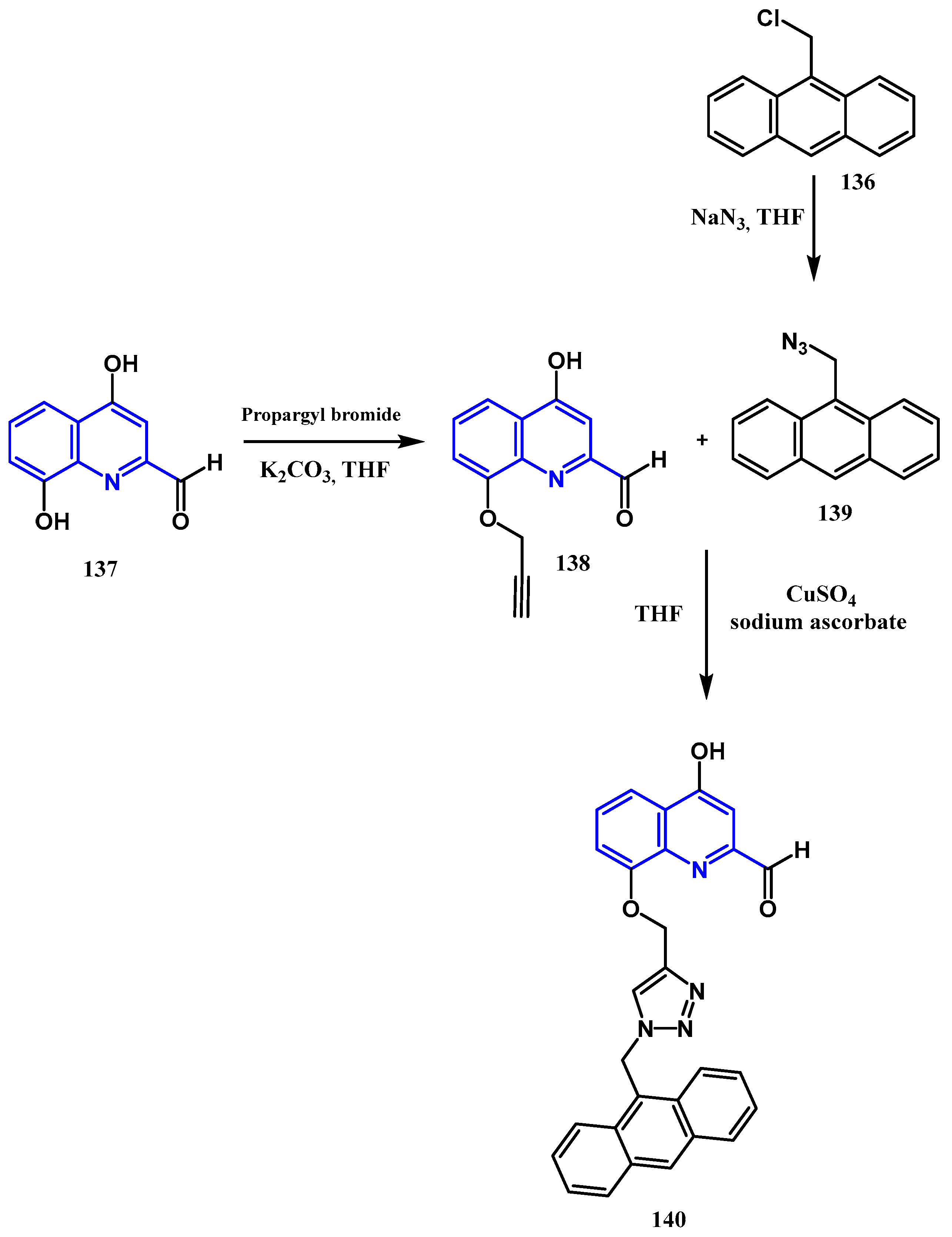
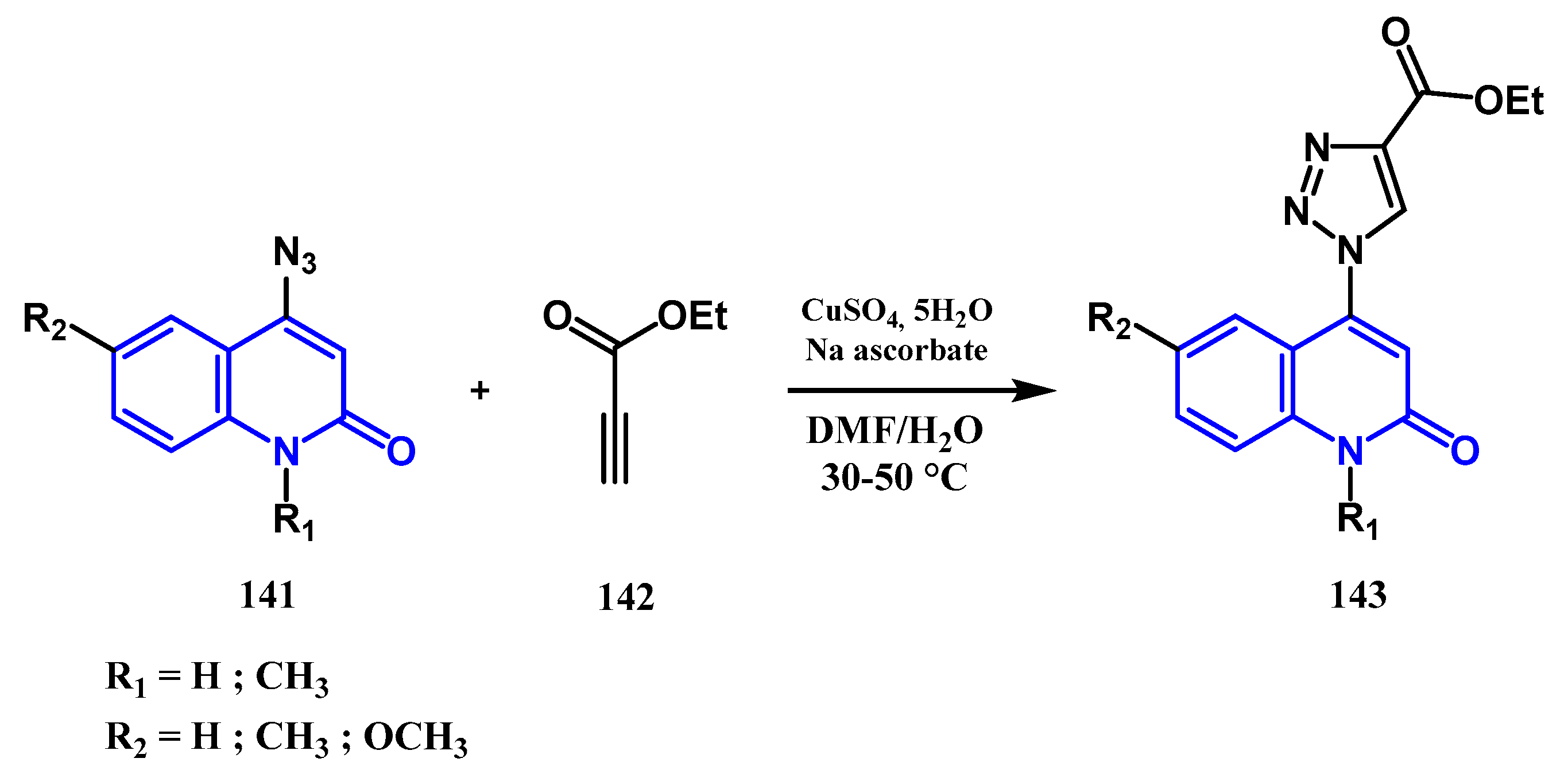
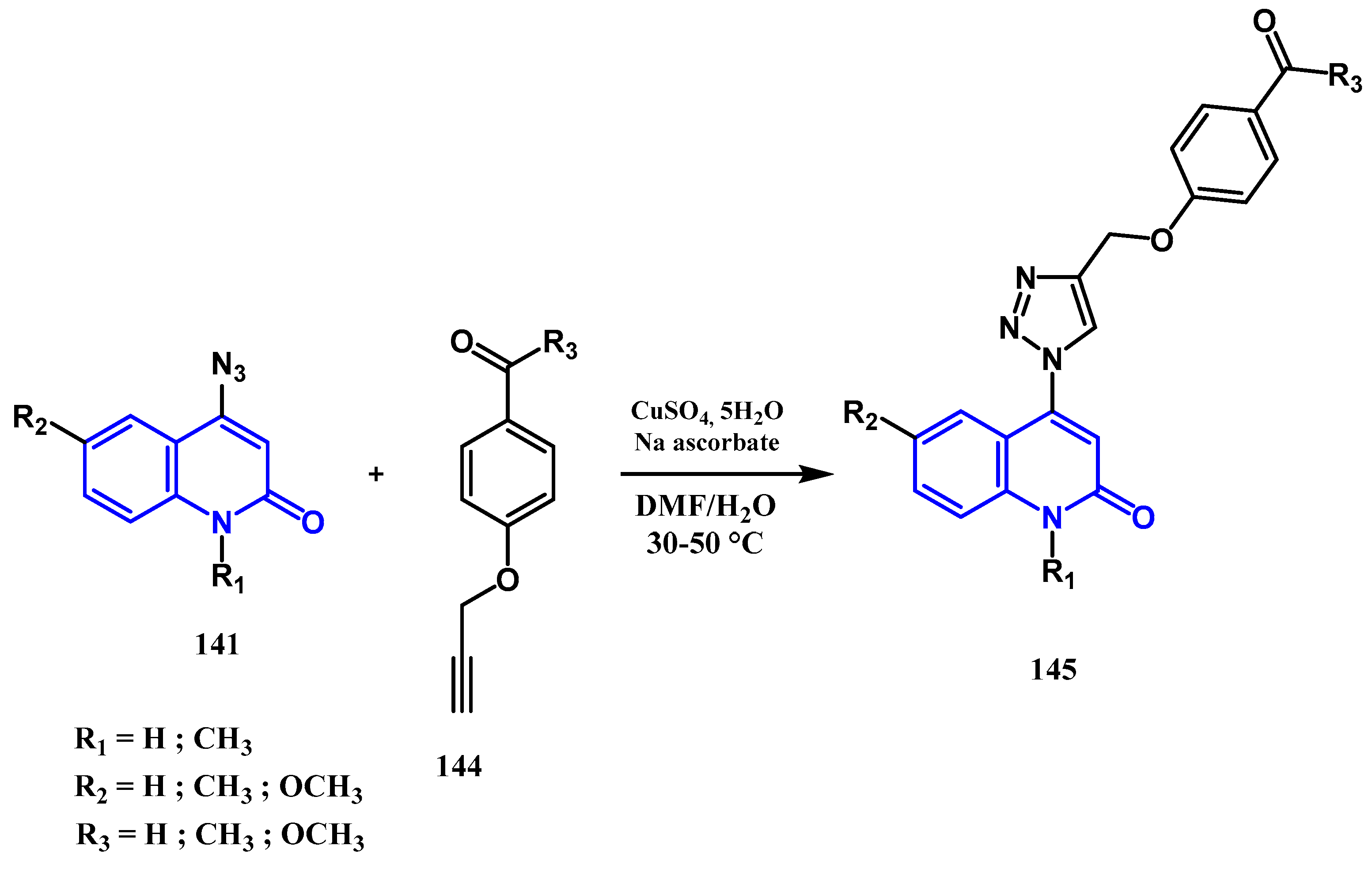
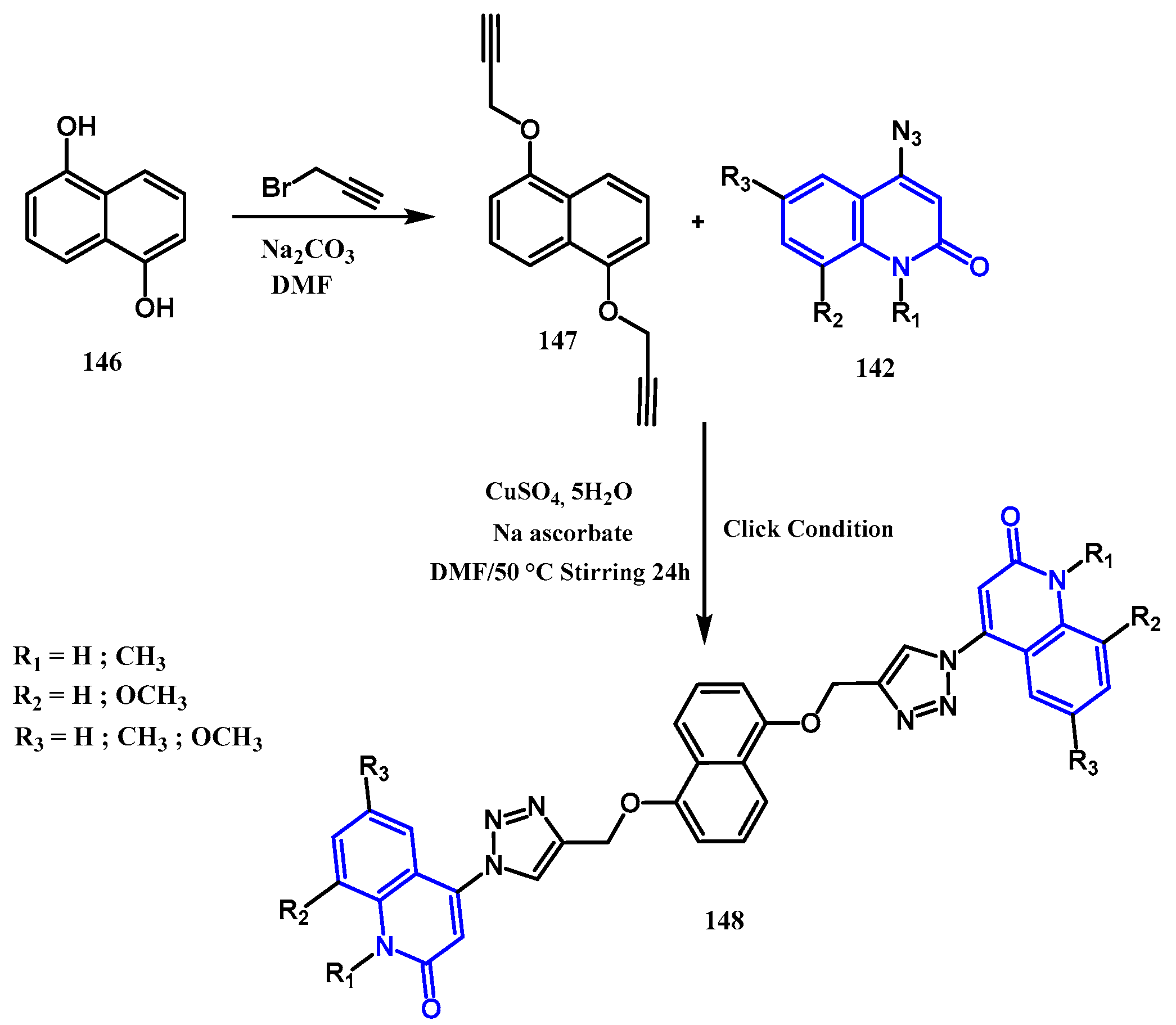
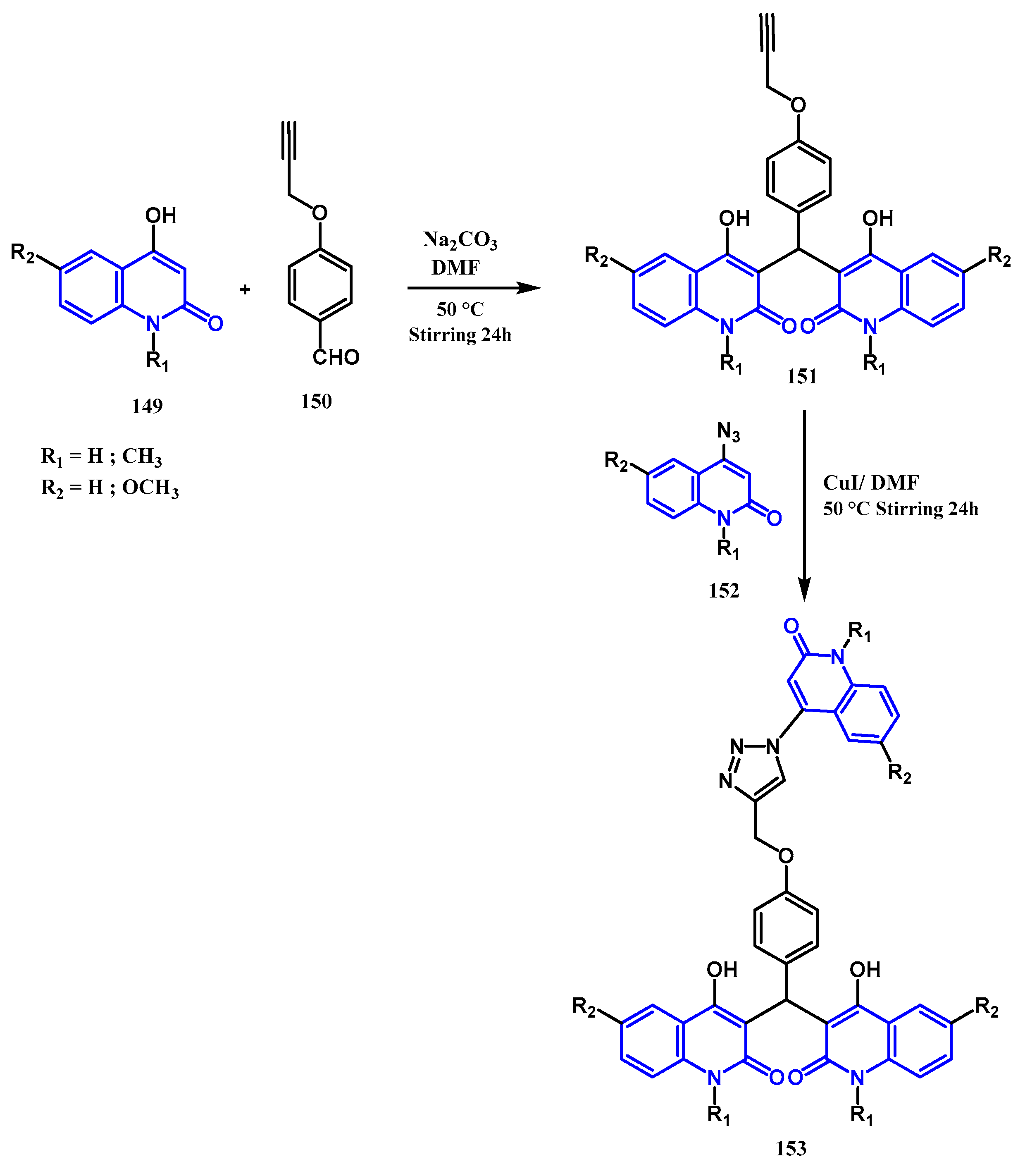
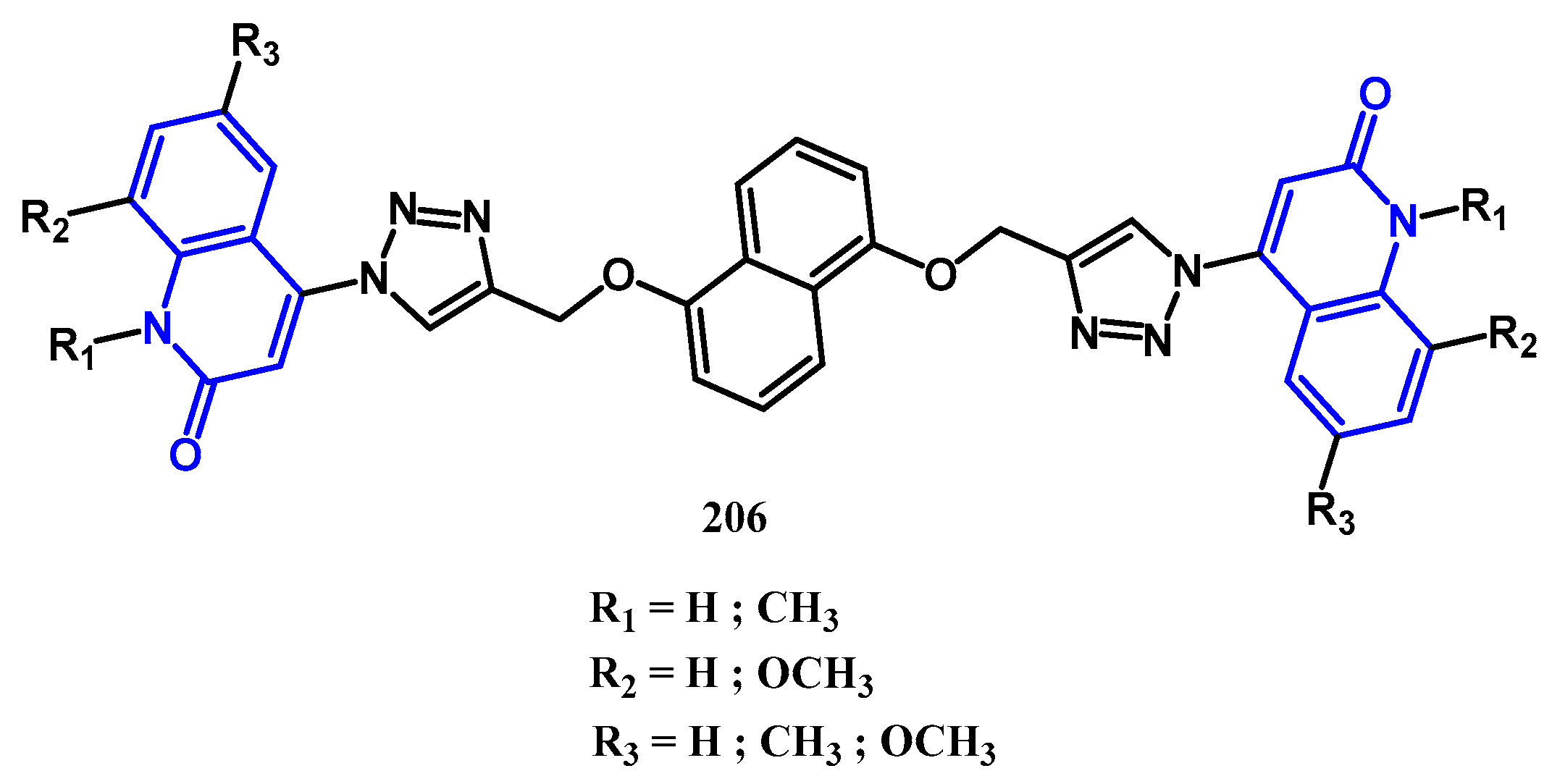
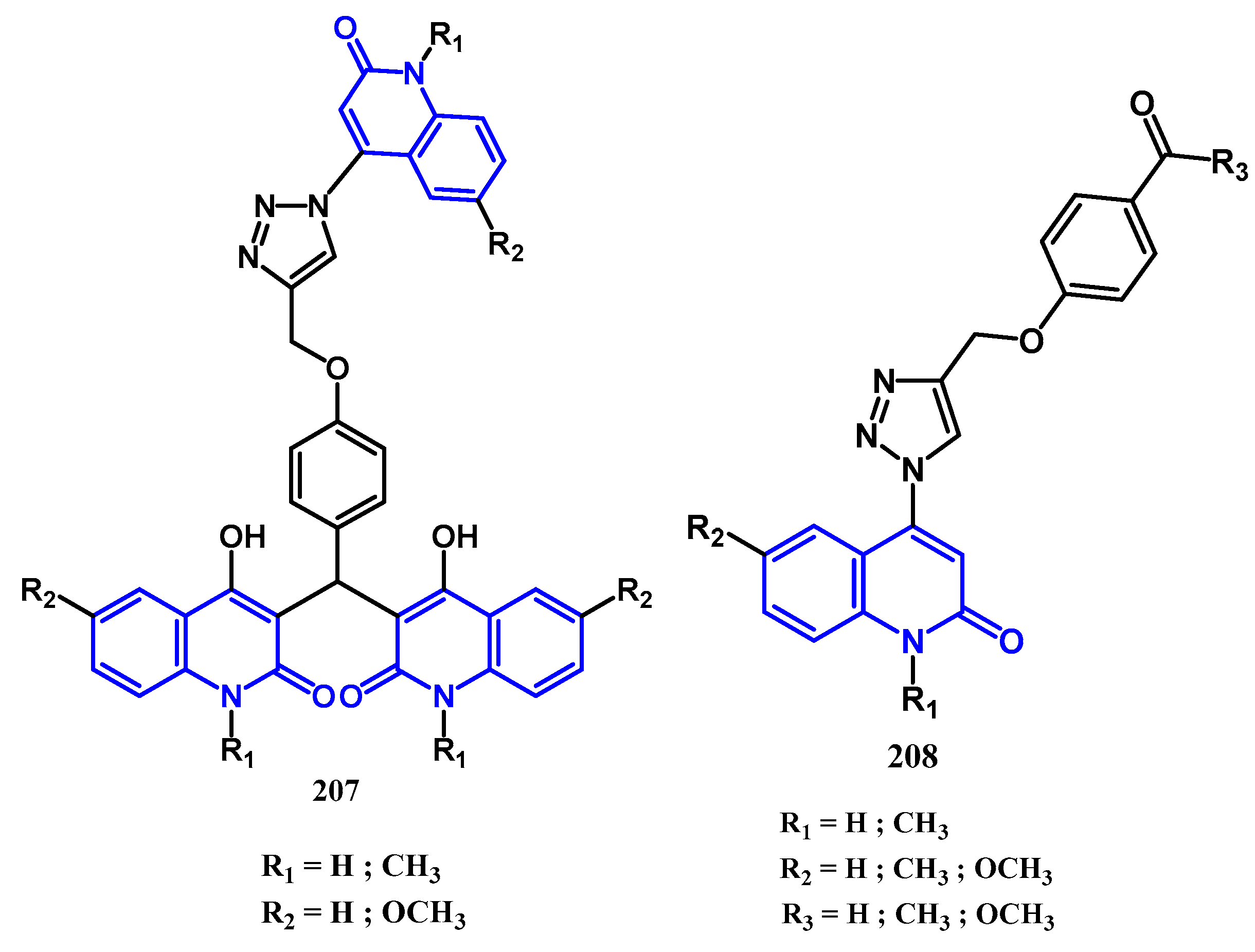
3.8.1. Preparation of Triazoles-Quinolone:
3.8.2. Preparation of Furanoquinolones and Pyranoquinolones
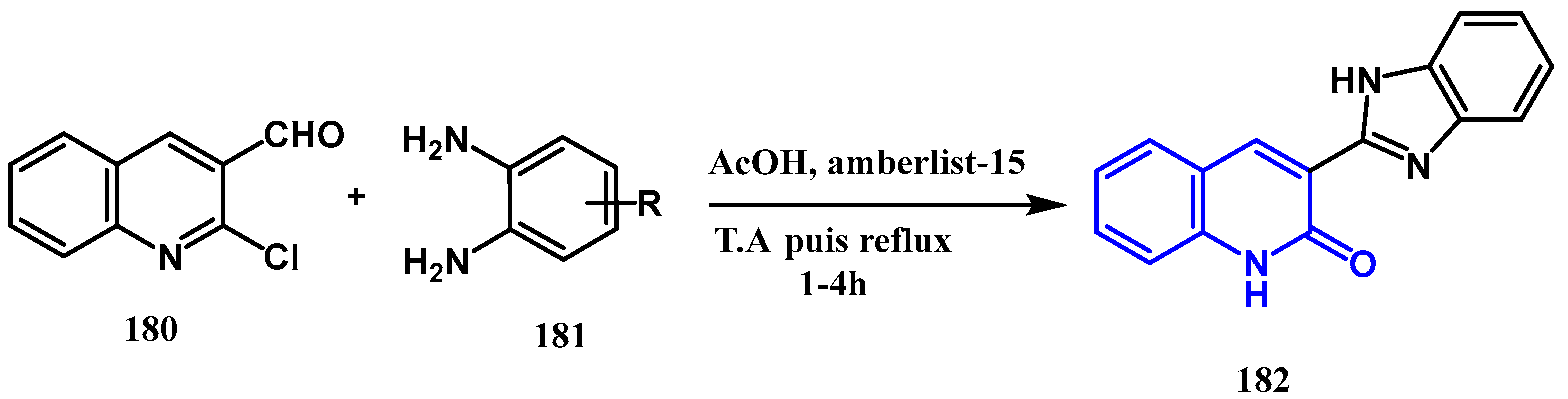
3.8.3. Preparation of Imidazole Quinolones
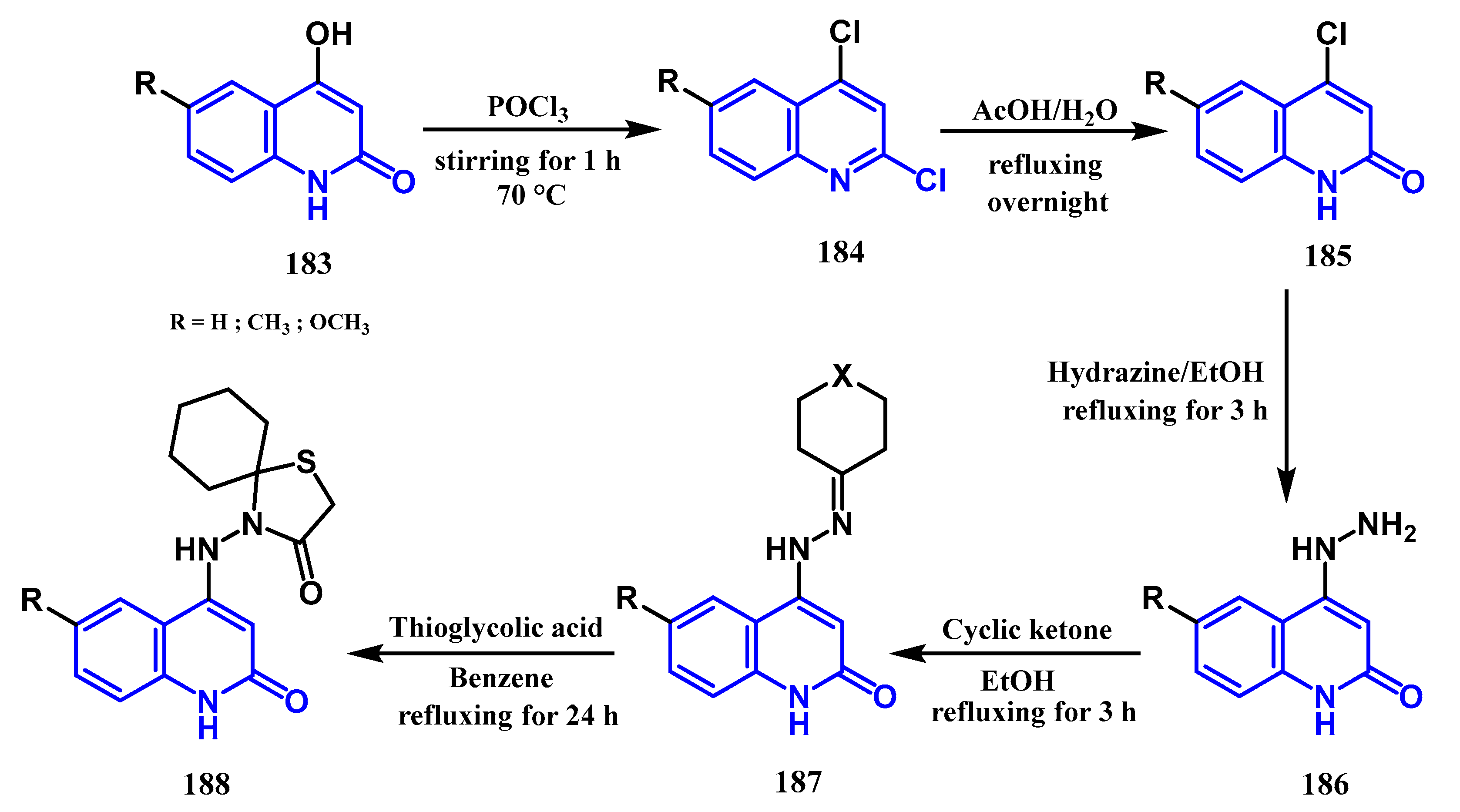
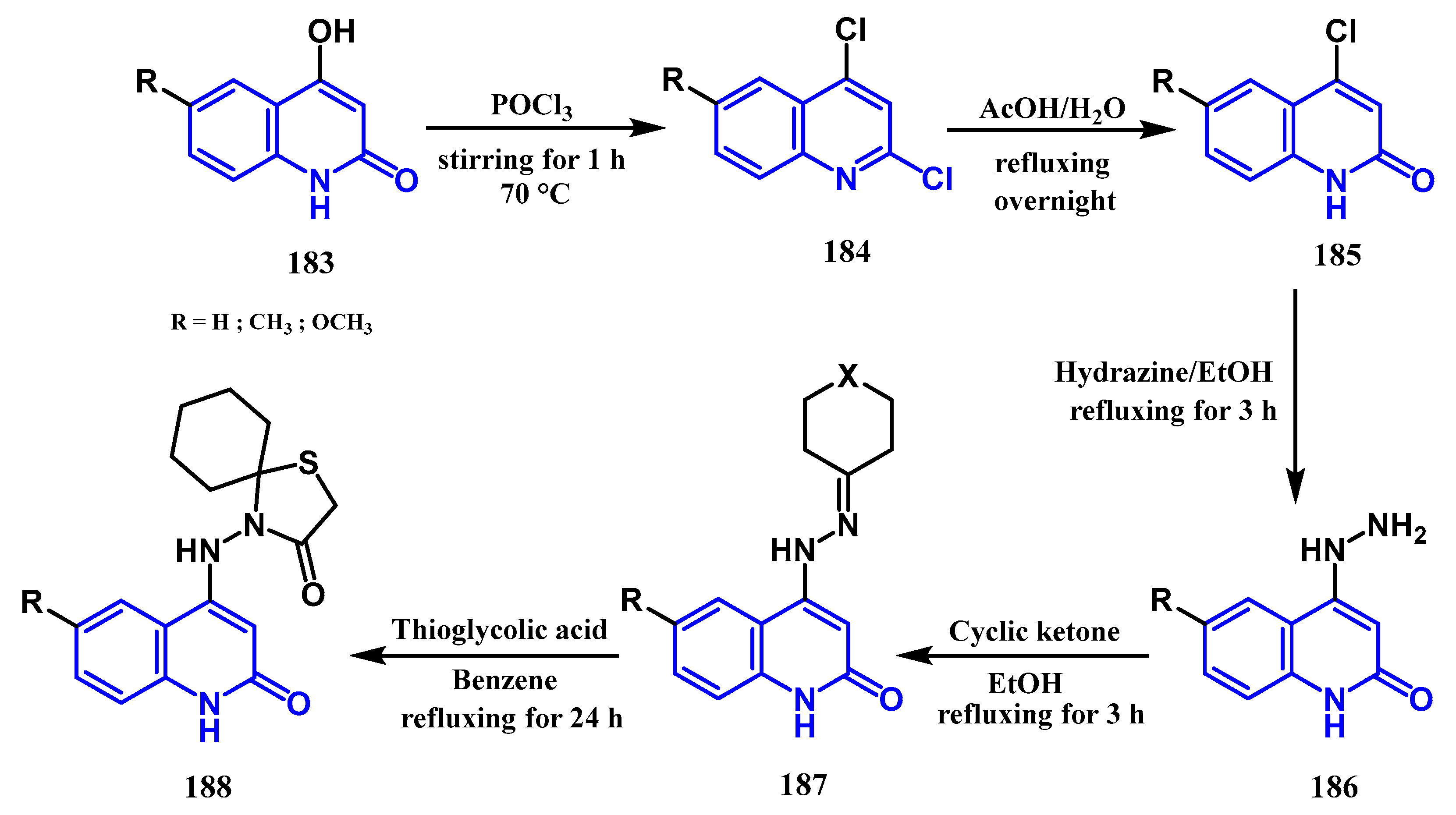
3.8.4. Preparation of Thiazolidin-4-One Quinolones:
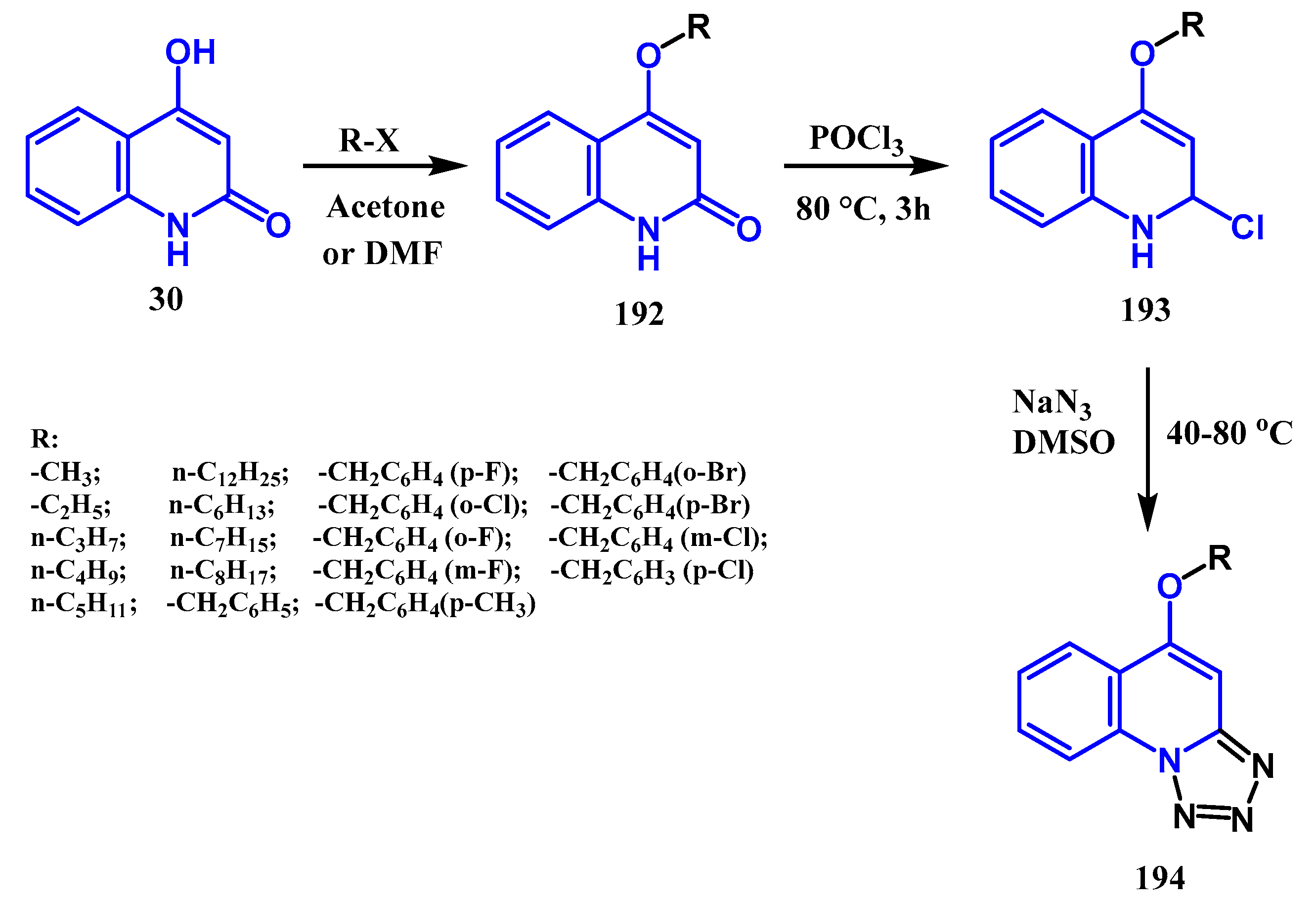
3.8.5. Preparation of Tetrazole Quinolones
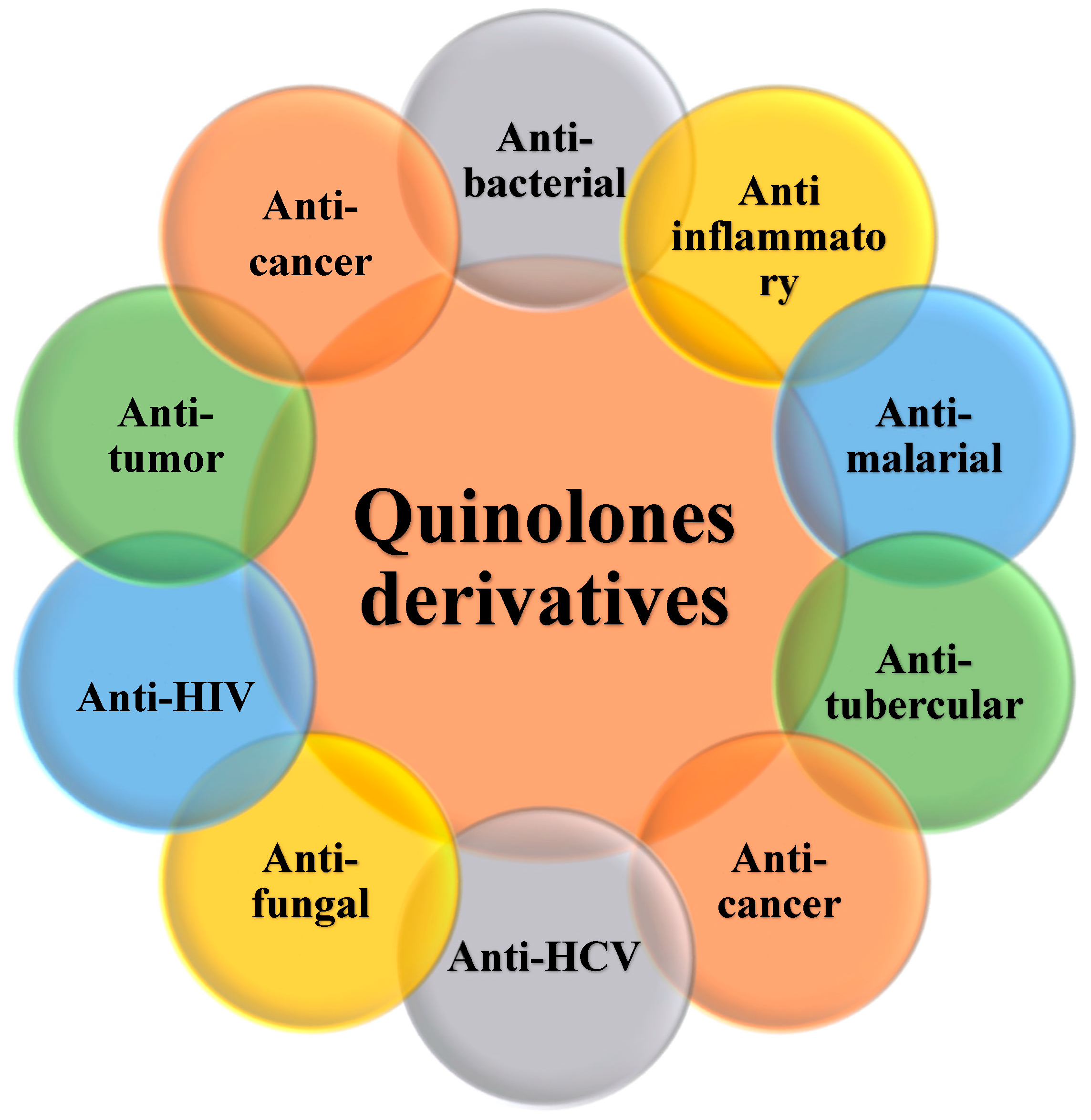
4. Biological Activities of Quinolone Derivatives
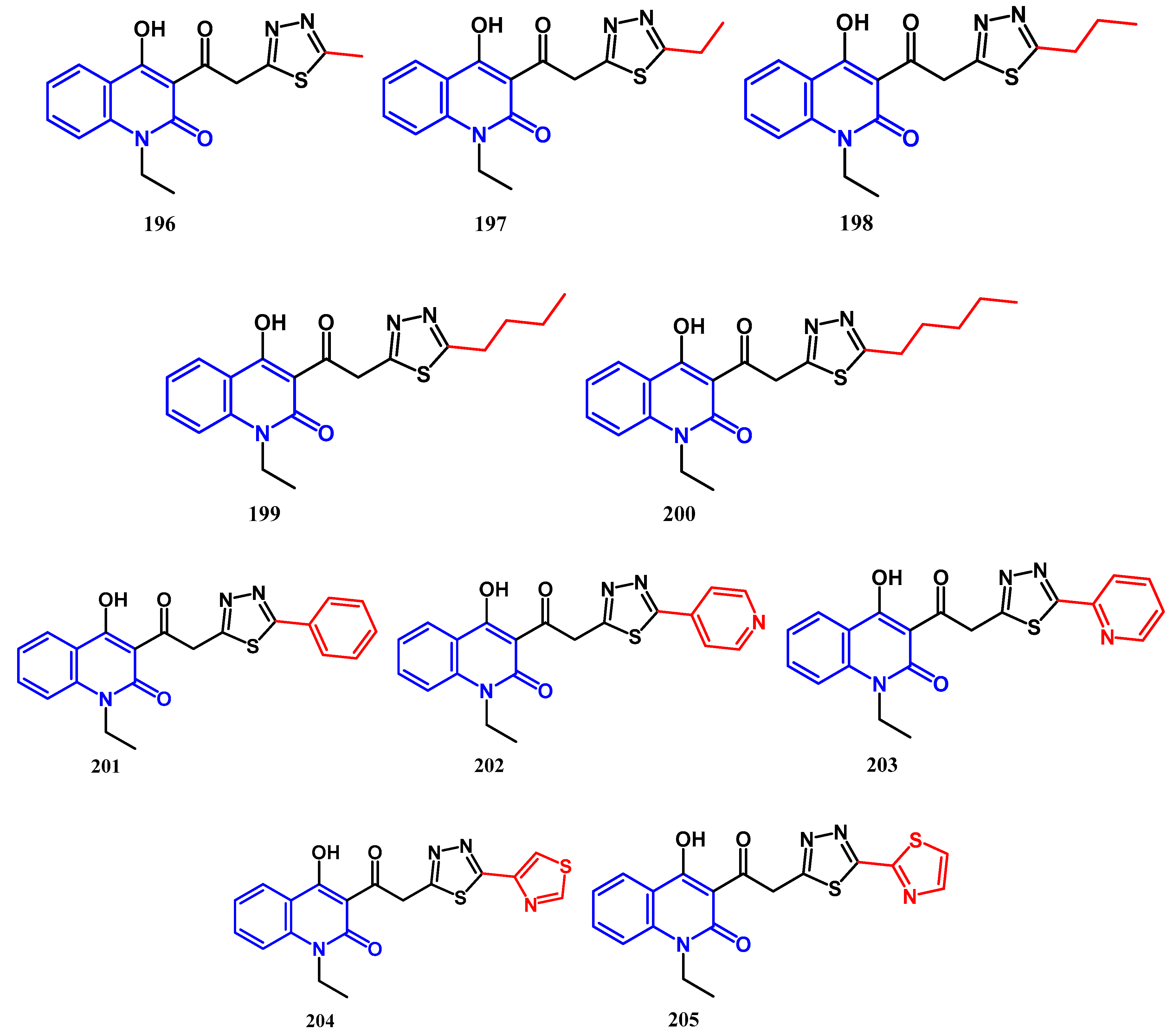
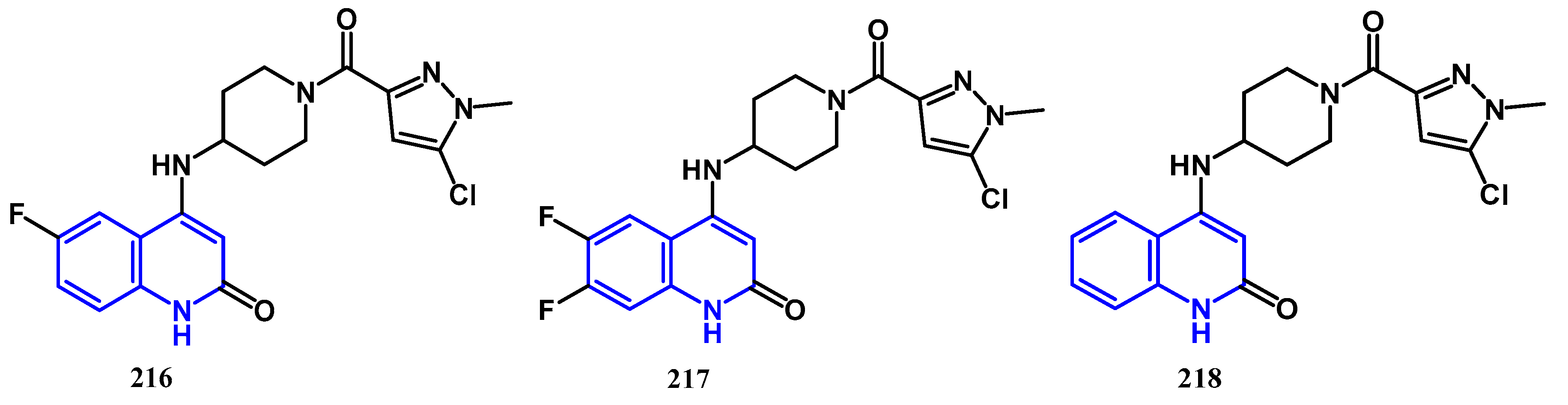
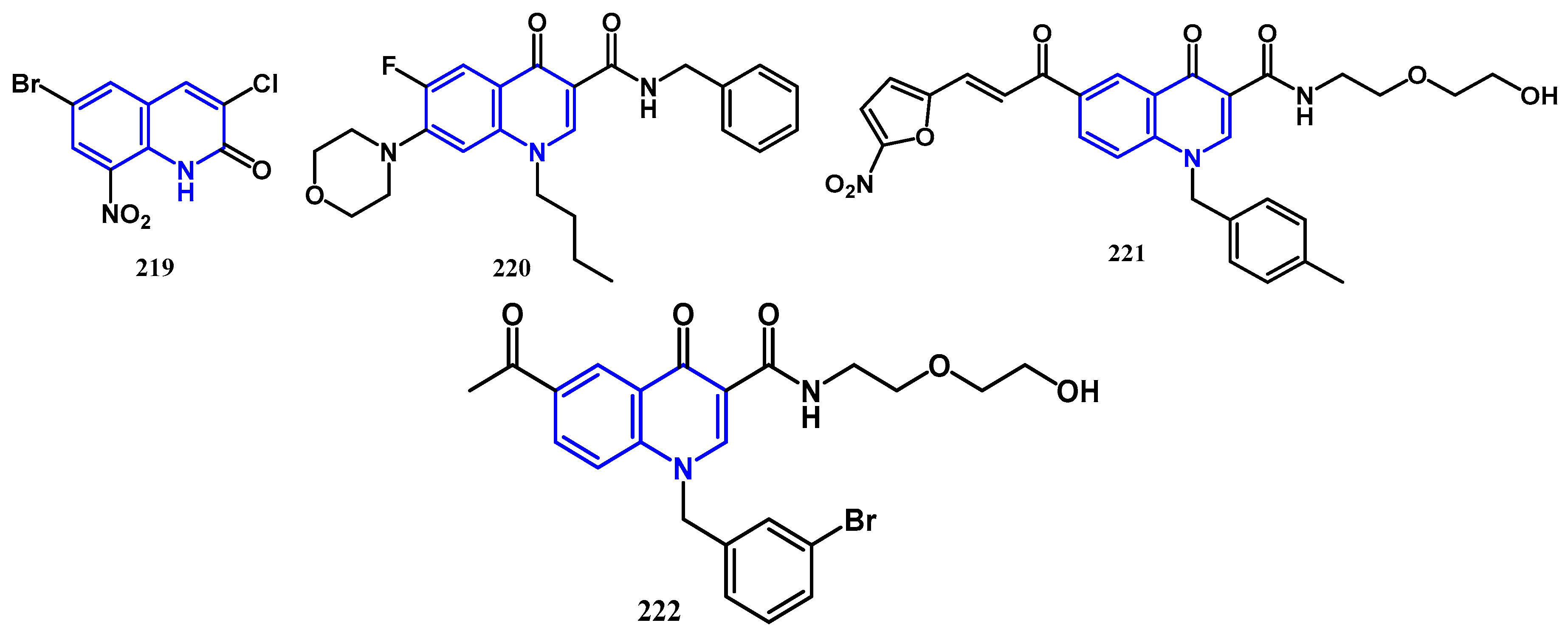
4.1. Antibacterial Activity
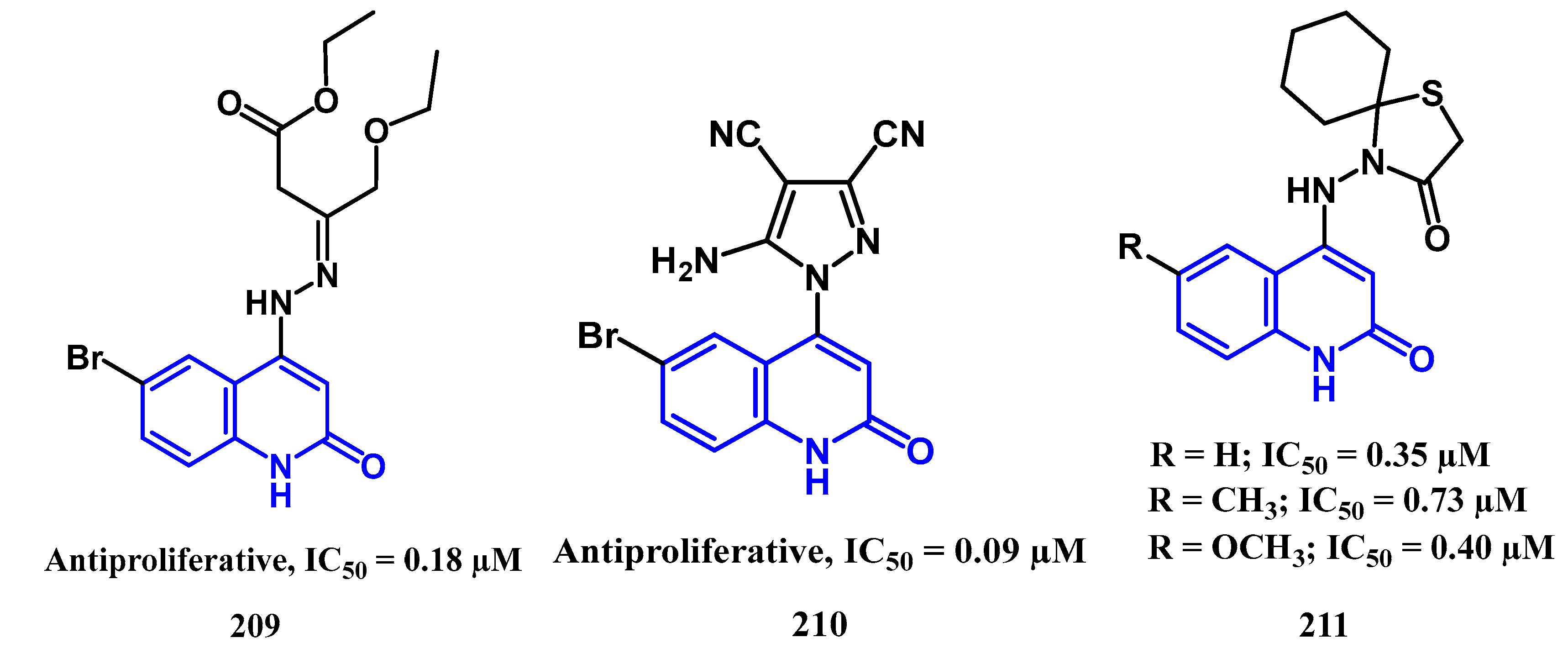
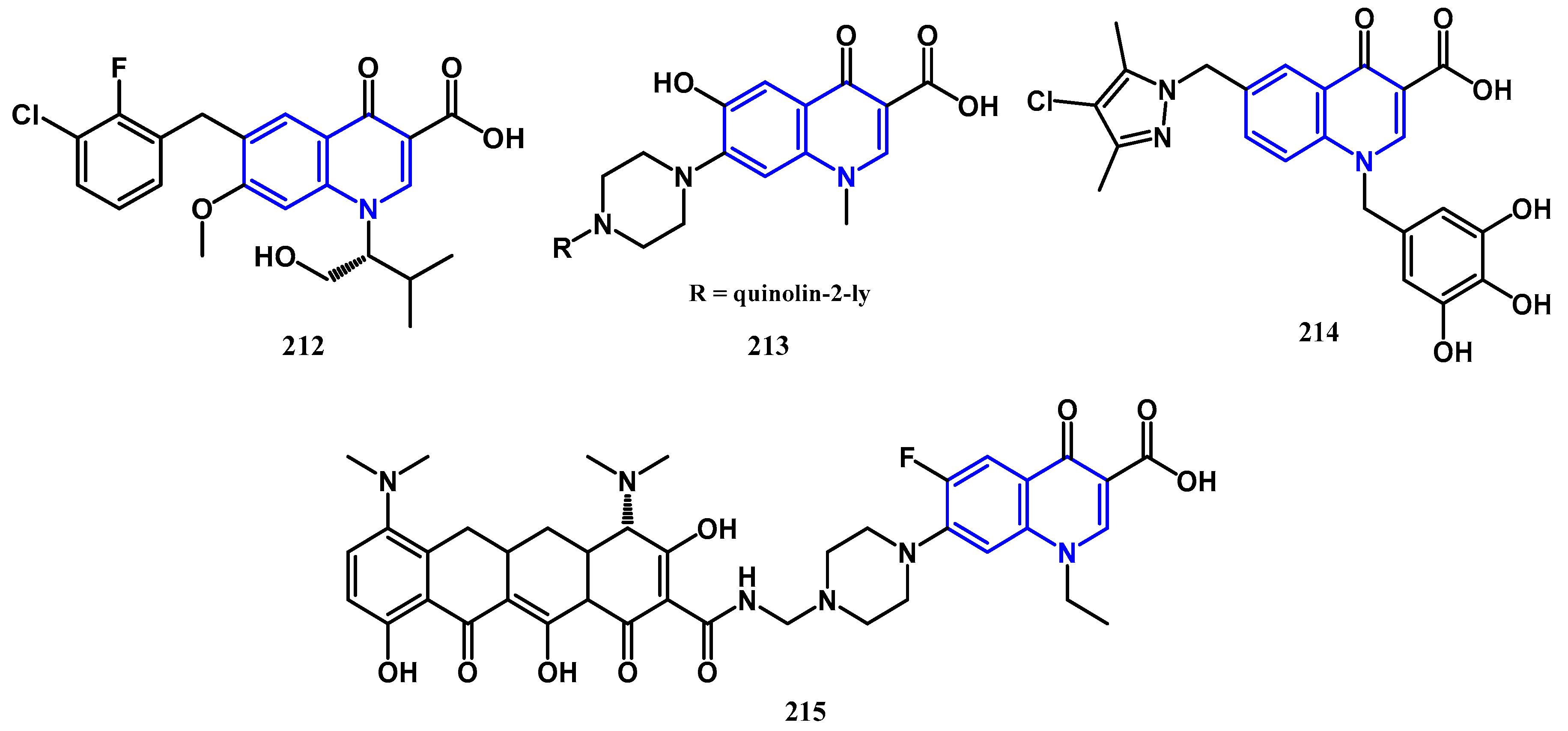
4.2. Antiproliferative Activity:
4.3. Antiviral Activity:
4.4. Antitrypanosomal and Antileishmaniasis Activity:
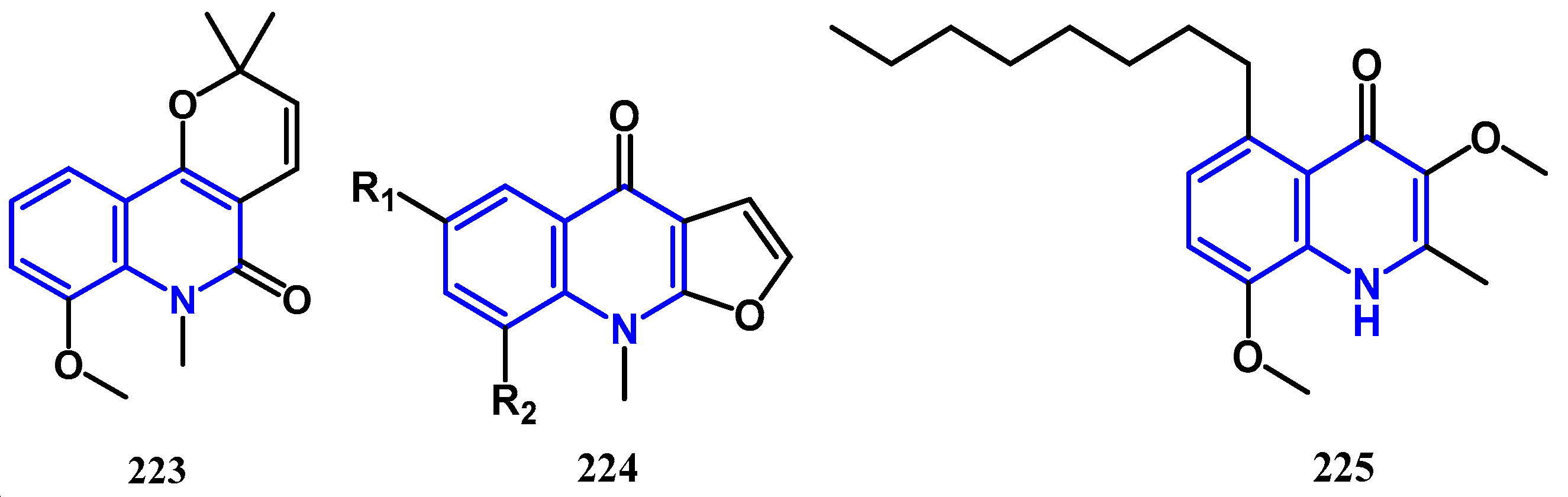
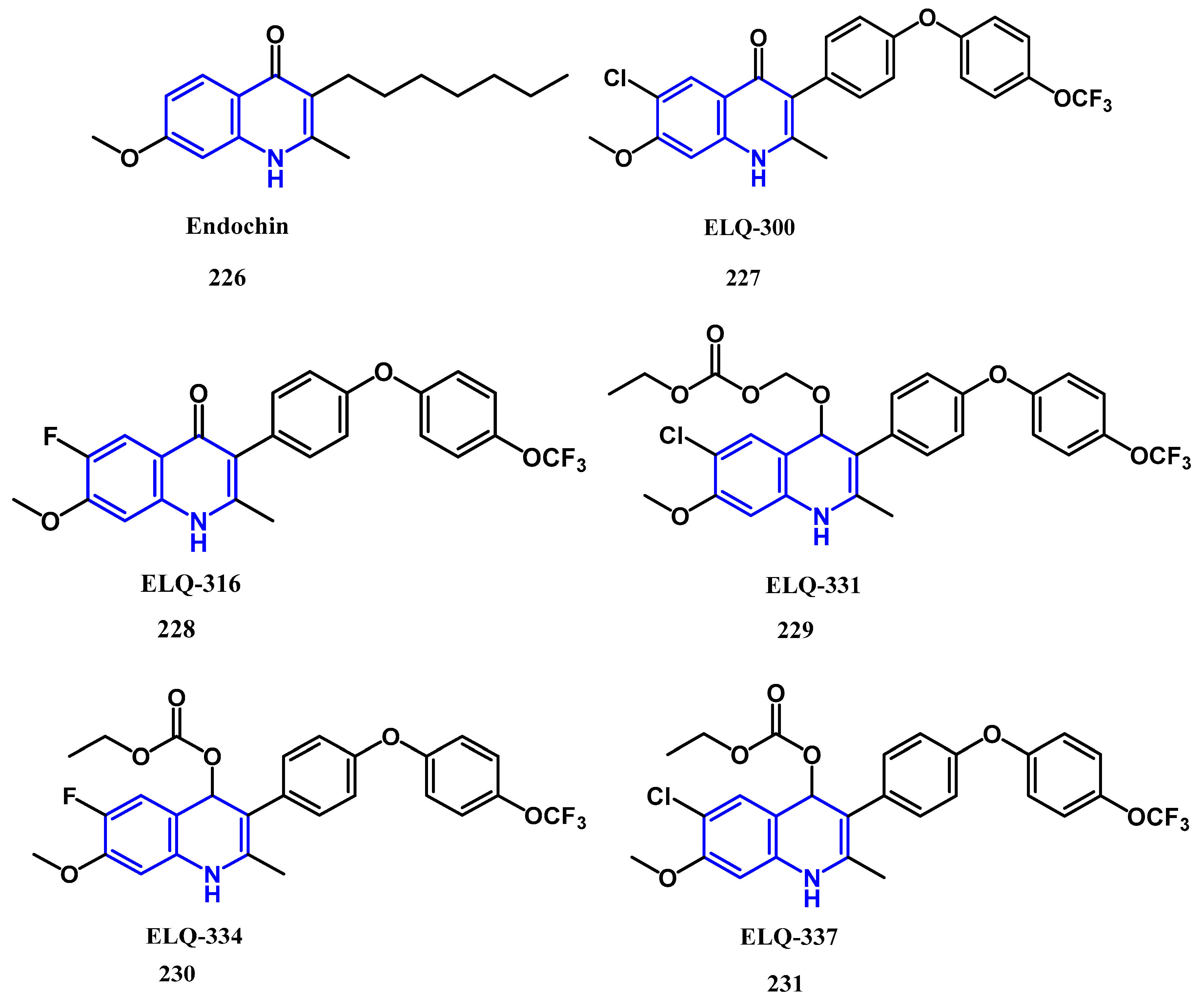
4.5. Anti-Malaria Activity:
5. Conclusion and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barros, A.I.; Nunes, F.M.; Gonçalves, B.; Bennett, R.N.; Silva, A.P. Effect of cooking on total vitamin C contents and antioxidant activity of sweet chestnuts (Castanea sativa Mill.). Food Chemistry. 2011, 128, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1, 3-dipolar cycloaddition reactions. Chemical Reviews. 1998, 98, 863–910. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- Xing, G.; Pan, L.; Yi, C.; Li, X.; Ge, X.; Zhao, Y.; et al. Design, synthesis and biological evaluation of 5-(2-amino-1-hydroxyethyl)-8-hydroxyquinolin-2 (1H)-one derivatives as potent β2-adrenoceptor agonists. Bioorganic & Medicinal Chemistry. 2019, 27, 2306–2314. [Google Scholar]
- Batista, V.F.; Pinto, D.C.G.A.; Silva, A.M.S. Synthesis of Quinolines: A Green Perspective. ACS Sustainable Chem Eng. 1 août 2016, 4, 4064–4078. [Google Scholar] [CrossRef]
- Diaz, G.; Miranda, I.L.; Diaz, M.A.N. Quinolines, isoquinolines, angustureine, and congeneric alkaloids—Occurrence, chemistry, and biological activity. Phytochemicals-isolation, characterisation and role in human health 2015, 141–162. [Google Scholar]
- Pranger, A.D.; Van Der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.W.C. The Role of Fluoroquinolones in the Treatment of Tuberculosis in 2019. Drugs. févr 2019, 79, 161–171. [Google Scholar] [CrossRef]
- Fan, Y.L.; Cheng, X.W.; Wu, J.B.; Liu, M.; Zhang, F.Z.; Xu, Z.; et al. Antiplasmodial and antimalarial activities of quinolone derivatives: An overview. European Journal of Medicinal Chemistry. 2018, 146, 1–14. [Google Scholar] [CrossRef]
- Sood, D.; Kumar, N.; Singh, A.; Sakharkar, M.K.; Tomar, V.; Chandra, R. Antibacterial and pharmacological evaluation of fluoroquinolones: A chemoinformatics approach. Genomics & informatics. 2018, 16, 44. [Google Scholar]
- Chokkar, N.; Kalra, S.; Chauhan, M.; Kumar, R. A review on quinoline derived scaffolds as anti-hiv agents. Mini reviews in medicinal chemistry. 2019, 19, 510–526. [Google Scholar] [CrossRef]
- Kojima, H.; Kaita, K.D.E.; Hawkins, K.; Uhanova, J.; Minuk, G.Y. Use of Fluoroquinolones in Patients with Chronic Hepatitis C Virus-Induced Liver Failure. Antimicrob Agents Chemother. oct 2002, 46, 3280–3282. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.Y.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the discovery and development of quinolones and analogs as antitumor agents. Current medicinal chemistry. 1999, 6, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, T.c.; Jin, Y.; Xu, J.g.; Yu, J.g.; Lv, Y.w. Synthesis, molecular docking and biological evaluation of quinolone derivatives as novel anticancer agents. Chemical and Pharmaceutical Bulletin. 2018, 66, 55–60. [Google Scholar] [CrossRef]
- Sharma, V.; Das, R.; Mehta, D.K.; Gupta, S.; Venugopala, K.N.; Mailavaram, R.; et al. Recent insight into the biological activities and SAR of quinolone derivatives as multifunctional scaffold. Bioorganic & Medicinal Chemistry. 2022, 59, 116674. [Google Scholar]
- Daneshtalab, M.; Ahmed, A. Nonclassical biological activities of quinolone derivatives. Journal of Pharmacy & Pharmaceutical Sciences. 2012, 15, 52–72. [Google Scholar]
- Dhiman, P.; Arora, N.; Thanikachalam, P.V.; Monga, V. Recent advances in the synthetic and medicinal perspective of quinolones: A review. Bioorganic Chemistry. 2019, 92, 103291. [Google Scholar] [CrossRef]
- Sales, E.M.; Figueroa-Villar, J.D. Recent studies about synthesis and biological activity of quinolones and derivatives: A Review. World J Pharm Pharm Sci. 2016, 5, 253–268. [Google Scholar]
- Lungu, I.A.; Moldovan, O.L.; Biriș, V.; Rusu, A. Fluoroquinolones hybrid molecules as promising antibacterial agents in the fight against antibacterial resistance. Pharmaceutics. 2022, 14, 1749. [Google Scholar] [CrossRef]
- Dube, P.S.; Legoabe, L.J.; Beteck, R.M. Quinolone: A versatile therapeutic compound class. Mol Divers. juin 2023, 27, 1501–1526. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry. 18 mars 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules. 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. Journal of infection and public health. 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, J.; Xu, Z.; Lv, Z.S. Recent advances of 2-Quinolone-based derivatives as anti-tubercular agents. Anti-Infective Agents. 2018, 16, 4–10. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X.; Wang, T.; Xiao, J. Quinolone hybrids and their anti-cancer activities: An overview. European journal of medicinal chemistry. 2019, 165, 59–79. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.A. Recent updates of fluoroquinolones as antibacterial agents. Archiv der Pharmazie https://onlinelibrary.wiley.com/doi/10.1002/ardp.201800141. 2018, 351, 9. [Google Scholar] [CrossRef]
- Fan, Y.L.; Wu, J.B.; Cheng, X.W.; Zhang, F.Z.; Feng, L.S. Fluoroquinolone derivatives and their anti-tubercular activities. European journal of medicinal chemistry. 2018, 146, 554–563. [Google Scholar] [CrossRef]
- Chu, X.M.; Wang, C.; Liu, W.; Liang, L.L.; Gong, K.K.; Zhao, C.Y.; et al. Quinoline and quinolone dimers and their biological activities: An overview. European journal of medicinal chemistry. 2019, 161, 101–117. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Lv, Z.S.; Gao, F.; Wang, Y.; Zhang, F.; et al. Fluoroquinolone-isatin hybrids and their biological activities. European Journal of Medicinal Chemistry. 2019, 162, 396–406. [Google Scholar] [CrossRef]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The current case of quinolones: Synthetic approaches and antibacterial activity. Molecules. 2016, 21, 268. [Google Scholar] [CrossRef]
- Conrad, M.; Limpach, L. Ueber das γ-Oxychinaldin und dessen Derivate. Berichte der deutschen chemischen Gesellschaft. 1887, 20, 948–959. [Google Scholar] [CrossRef]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC advances. 2020, 10, 20784–20793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, L.; Jiao, N. The tandem reaction combining radical and ionic processes: An efficient approach to substituted 3, 4-dihydroquinolin-2-ones. Tetrahedron. 2009, 65, 1982–1987. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Chronaki, K.; Prousis, K.C.; Pontiki, E.; Hadjiplavlou-Litina, D.; et al. Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules. 2023, 29, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications [Internet]. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. https://link.springer.com/10.1007/978-3-642-01053-8.
- Wang, H. Comprehensive Organic Name Reactions [Internet]. Vol. 2. Wiley New York, NY, USA:; 2010. http://sutlib2.sut.ac.th/sut_contents/H124526_V2.pdf.
- Jampilek, J.; Musiol, R.; Pesko, M.; Kralova, K.; Vejsova, M.; Carroll, J.; et al. Ring-substituted 4-hydroxy-1 H-quinolin-2-ones: Preparation and biological activity. Molecules. 2009, 14, 1145–1159. [Google Scholar] [CrossRef]
- Aly, A.A.; Ramadan, M.; Abuo-Rahma, G.E.D.A.; Elshaier, Y.A.; Elbastawesy, M.A.; Brown, A.B.; et al. Quinolones as prospective drugs: Their syntheses and biological applications. In: Advances in heterocyclic chemistry [Internet]. Elsevier; 2021. p. 147-96.
- Abdou, M.M. Chemistry of 4-Hydroxy-2 (1H)-quinolone. Part 1: Synthesis and reactions. Arabian Journal of Chemistry. 2017, 10, S3324–S3337. [Google Scholar] [CrossRef]
- Ahmed, N.; Brahmbhatt, K.G.; Singh, I.P.; Bhutani, K.K. Efficient chemoselective alkylation of quinoline 2,4-diol derivatives in water. Journal of Heterocyclic Chem. janv 2011, 48, 237–240. [Google Scholar] [CrossRef]
- Ahmed, N.; Brahmbhatt, K.G.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and anti-HIV activity of alkylated quinoline 2, 4-diols. Bioorganic & medicinal chemistry. 2010, 18, 2872–2879. [Google Scholar]
- Arya, K.; Agarwal, M. Microwave prompted multigram synthesis, structural determination, and photo-antiproliferative activity of fluorinated 4-hydroxyquinolinones. Bioorganic & Medicinal Chemistry Letters. 2007, 17, 86–93. [Google Scholar]
- Cheng, P.; Gu, Q.; Liu, W.; Zou, J.F.; Ou, Y.Y.; Luo, Z.Y.; et al. Synthesis of quinolin-2-one alkaloid derivatives and their inhibitory activities against HIV-1 reverse transcriptase. Molecules. 2011, 16, 7649–7661. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Ramadan, E.S.; Abdel Hamid, H.; Hagar, M. Microwave-Assisted Synthesis of Quinoline Derivatives from Isatin. Synthetic Communications. 1 sept 2005, 35, 2243–2250. [Google Scholar] [CrossRef]
- Baba, Y.F.; Gökce, H.; Rodi, Y.K.; Hayani, S.; Chahdi, F.O.; Boukir, A.; et al. Syntheses of novel 2-oxo-1, 2-dihydroquinoline derivatives: Molecular and crystal structures, spectroscopic characterizations, Hirshfeld surface analyses, molecular docking studies and density functional theory calculations. Journal of Molecular Structure. 2020, 1217, 128461. [Google Scholar] [CrossRef]
- Baba, Y.F.; Misbahi, K.; Chahdi, F.O.; Kerbal, A. SYNTHESE ET REACTIVITE DE NOUVEAUX SYSTEMES HETEROCYLIQUES DERIVES DE LA QUINOLEINE. Moroccan Journal of Heterocyclic Chemistry [Internet]. 2014 ;13(1).
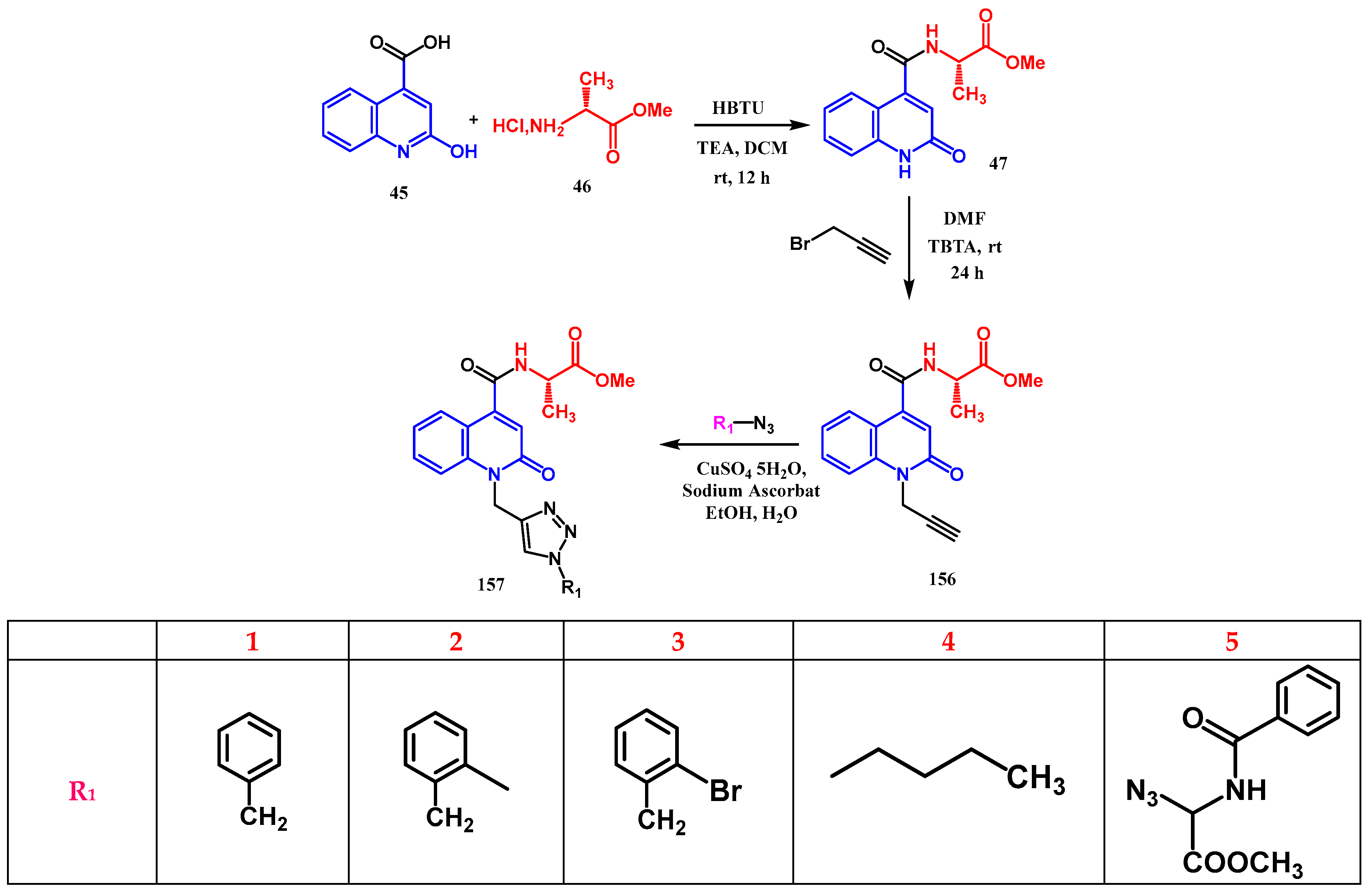
- Moussaoui, O.; Bhadane, R.; Sghyar, R.; Ilaš, J.; El Hadrami, E.M.; Chakroune, S.; et al. Design, Synthesis, in vitro and in silico Characterization of 2-Quinolone-L-alaninate-1,2,3-triazoles as Antimicrobial Agents. ChemMedChem. 4 mars 2022, 17, e202100714. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, H.; Rouhani, A.; Nejabat, M.; Hadizadeh, F.; Mirzaei, S.; Nadri, H.; et al. Synthesis and molecular dynamic simulation studies of novel N-(1-benzylpiperidin-4-yl) quinoline-4-carboxamides as potential acetylcholinesterase inhibitors. Journal of Molecular Structure. 2021, 1244, 130919. [Google Scholar] [CrossRef]
- Slania, S.L.; Das, D.; Lisok, A.; Du, Y.; Jiang, Z.; Mease, R.C.; et al. Imaging of Fibroblast Activation Protein in Cancer Xenografts Using Novel (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine-Based Small Molecules. J Med Chem. 8 avr 2021, 64, 4059–4070. [Google Scholar] [CrossRef]
- Baragaña, B.; Norcross, N.R.; Wilson, C.; Porzelle, A.; Hallyburton, I.; Grimaldi, R.; et al. Discovery of a Quinoline-4-carboxamide Derivative with a Novel Mechanism of Action, Multistage Antimalarial Activity, and Potent in Vivo Efficacy. J Med Chem. 10 nov 2016, 59, 9672–9685. [Google Scholar] [CrossRef]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; et al. Selective Inhibitors of Fibroblast Activation Protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine Scaffold. ACS Med Chem Lett. 9 mai 2013, 4, 491–496. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, Y.; Zhang, D.; Meng, Y.; Tang, T.; Wang, K.; et al. Eco-friendly decarboxylative cyclization in water: Practical access to the anti-malarial 4-quinolones. Green Chemistry. 2019, 21, 478–482. [Google Scholar] [CrossRef]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Sferrazza, A. 4-Aryl-2-quinolones via a domino Heck reaction/cyclization process. Heterocycles. 2006, 69, 99. [Google Scholar] [CrossRef]
- Cortese, N.A.; Ziegler, C.B.; Hrnjez, B.J.; Heck, R.F. Palladium-catalyzed synthesis of 2-quinolone derivatives from 2-iodoanilines. J Org Chem. juill 1978, 43, 2952–2958. [Google Scholar] [CrossRef]
- Cho, C.S.; Kim, J.U. An approach for quinolines via palladium-catalyzed Heck coupling followed by cyclization. Tetrahedron letters. 2007, 48, 3775–3778. [Google Scholar] [CrossRef]
- Åkerbladh, L.; Nordeman, P.; Wejdemar, M.; Odell, L.R.; Larhed, M. Synthesis of 4-Quinolones via a Carbonylative Sonogashira Cross-Coupling Using Molybdenum Hexacarbonyl as a CO Source. J Org Chem. 6 févr 2015, 80, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Bunce, R.A.; Nammalwar, B. 4(1 H )-Quinolinones by a Tandem Reduction-Addition-Elimination Reaction. Organic Preparations and Procedures International. 12 nov 2010, 42, 557–563. [Google Scholar] [CrossRef]
- Flipo, M.; Beghyn, T.; Leroux, V.; Florent, I.; Deprez, B.P.; Deprez-Poulain, R.F. Novel Selective Inhibitors of the Zinc Plasmodial Aminopeptidase PfA-M1 as Potential Antimalarial Agents. J Med Chem. 2007, 50, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Kamel, M.M.; Mohamed, L.W.; Faggal, S.I. Synthesis of new indole derivatives structurally related to donepezil and their biological evaluation as acetylcholinesterase inhibitors. Molecules. 2012, 17, 4811–4823. [Google Scholar] [CrossRef]
- Kikuchi, S.; Yamada, T. Carbon Dioxide Incorporation into Alkyne Compounds Mediated by Silver Catalysts. The Chemical Record. févr 2014, 14, 62–69. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Harayama, T. A Concise and Versatile Synthesis of Viridicatin Alkaloids from Cyanoacetanilides. Org Lett. 2 avr 2009, 11, 1603–1606. [Google Scholar] [CrossRef]
- Ribeiro, N.; Tabaka, H.; Peluso, J.; Fetzer, L.; Nebigil, C.; Dumont, S.; et al. Synthesis of 3-O-methylviridicatin analogues with improved anti-TNF-α properties. Bioorganic & medicinal chemistry letters. 2007, 17, 5523–5524. [Google Scholar]
- Liu, L.; Lu, H.; Wang, H.; Yang, C.; Zhang, X.; Zhang-Negrerie, D.; et al. PhI(OCOCF 3 ) 2 -Mediated C–C Bond Formation Concomitant with a 1,2-Aryl Shift in a Metal-Free Synthesis of 3-Arylquinolin-2-ones. Org Lett. 2013, 15, 2906–2909. [Google Scholar] [CrossRef]
- Schlummer, B.; Scholz, U. Palladium-Catalyzed CN and CO Coupling–A Practical Guide from an Industrial Vantage Point †. Adv Synth Catal. déc 2004, 346, 1599–1626. [Google Scholar] [CrossRef]
- Dubrovin, A.N.; Mikhalev, A.I.; Ukhov, S.V.; Goldshtein, A.G.; Novikova, V.V.; Odegova, T.F.; et al. Synthesis, Properties, and Biological Activities of 2-Methyl- and 2-Styrylquinoline-4-Carboxylic Acids. Pharm Chem J. août 2015, 49, 309–312. [Google Scholar] [CrossRef]
- Kumar, A.; Fernandes, J.; Kumar, P. Synthesis, antimicrobial and antitubercular activities of some novel carboxamide derivatives of 2-quinolones. Orient J Chem. 2014, 4, 1993–1997. [Google Scholar] [CrossRef]
- Shivaraj, Y.; Naveen, M.H.; Vijayakumar, G.R.; Kumar, D.B.A. Design, synthesis and antibacterial activity studies of novel quinoline carboxamide derivatives. 대한화학회지. 2013, 57, 241–245. [Google Scholar] [CrossRef]
- Suthar, S.K.; Jaiswal, V.; Lohan, S.; Bansal, S.; Chaudhary, A.; Tiwari, A.; et al. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: Design, synthesis and biological screening. European journal of medicinal chemistry. 2013, 63, 589–602. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Rizk, H.F.; El-Borai, M.A.; Sadek, M.E. Green routes for the synthesis of new pyrazole bearing biologically active imidiazolyl, pyridine and quinoxaline derivatives as promising antimicrobial and antioxidant agents. J IRAN CHEM SOC. juin 2021, 18, 1391–1404. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia. 2018, 129, 317–365. [Google Scholar] [CrossRef]
- Donaire-Arias, A.; Montagut, A.M.; Puig de la Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1 H-Pyrazolo [3, 4-b] pyridines: Synthesis and biomedical applications. Molecules. 2022, 27, 2237. [Google Scholar] [CrossRef]
- Polo-Cuadrado, E.; Rojas-Peña, C.; Acosta-Quiroga, K.; Camargo-Ayala, L.; Brito, I.; Cisterna, J.; et al. Design, synthesis, theoretical study, antioxidant, and anticholinesterase activities of new pyrazolo-fused phenanthrolines. RSC advances. 2022, 12, 33032–33048. [Google Scholar] [CrossRef]
- Noser, A.A.; Baren, M.H.; Ibrahim, S.A.; Rekaby, M.; Salem, M.M. New Pyrazolothiazole as Potential Wnt/β-Catenin Inhibitors: Green Synthesis, Characterization, Antimicrobial, Antioxidant, Antineoplastic Evaluation, and Molecular Docking Study. ChemistrySelect. 28 mars 2023, 8, e202204670. [Google Scholar] [CrossRef]
- Aljaar, N.; Ibrahim, M.M.; Younes, E.A.; Al-Noaimi, M.; Abu-Safieh, K.A.; Ali, B.F.; et al. Strategies towards the Synthesis of 2-Ketoaryl Azole Derivatives using C-H Functionalization Approach and 1,2-Bis-Nucleophile Precursors. Asian J Org Chem. avr 2023, 12, e202300036. [Google Scholar] [CrossRef]
- Okada, K.; Sakuma, H.; Kondo, M.; Inoue, S. DIELS–ALDER REACTION OF 3-METHOXYCARBONYLMETHYLENE-2-OXOINDOLINE DERIVATIVES WITH UNSYMMETRICAL BUTADIENES. Chemistry Letters. 1979, 8, 213–216. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.H.; Abdel-Megied, A.E.S.; Goda, A.E.S.; Zeid, I.F.; El Ashry, E.S.H. Synthesis and Anti-HBV Activity of Thiouracils Linked via S and N-1 to the 5-Position of Methyl β-D-Ribofuranoside. Nucleosides, Nucleotides and Nucleic Acids. nov 2003, 22, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Kuş, N.S. The synthesis of ribose and nucleoside derivatives. Madridge J Nov Drug Res. 2018, 2, 37–56. [Google Scholar] [CrossRef]
- Khwaza, V.; Mlala, S.; Aderibigbe, B.A. Advancements in Synthetic Strategies and Biological Effects of Ciprofloxacin Derivatives: A Review. International Journal of Molecular Sciences. 2024, 25, 4919. [Google Scholar] [CrossRef]
- Alasadi, G.M.; Al-Obaidi, Z. Synthesis of novel acylated and esterified ciprofloxacin derivatives as efficient anticancer and antimicrobial agents. Frontiers in Materials. 2023, 10, 1255955. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Wang, J.; Guo, Y.; Sun, B.; Liu, W.; et al. Synthesis and Biological Evaluation of Quinolinone Compounds as SARS CoV 3CL pro Inhibitors. Chin J Chem. sept 2013, 31, 1199–1206. [Google Scholar] [CrossRef]
- Priya, N.; Gupta, A.; Chand, K.; Singh, P.; Kathuria, A.; Raj, H.G.; et al. Characterization of 4-methyl-2-oxo-1, 2-dihydroquinolin-6-yl acetate as an effective antiplatelet agent. Bioorganic & medicinal chemistry. 2010, 18, 4085–4094. [Google Scholar]
- El-Mrabet, A.; Haoudi, A.; Dalbouha, S.; Skalli, M.K.; Hökelek, T.; Capet, F.; et al. Crystal structure, Hirshfeld surface analysis, interaction energy and energy framework calculations, as well as density functional theory (DFT) computation, of methyl 2-oxo-1-(prop-2-ynyl)-1, 2-dihydroquinoline-4-carboxylate. Acta Crystallographica Section E: Crystallographic Communications. 2023, 79, 883–889. [Google Scholar] [CrossRef]
- Deng, X.Q.; Wei, C.X.; Song, M.X.; Chai, K.Y.; Sun, Z.G.; Quan, Z.S. Synthesis and studies on anticonvulsant and antidepressant activities of 5-alkoxy-tetrazolo [1, 5-a] quinolines. Bulletin of the Korean Chemical Society. 2010, 31, 447–452. [Google Scholar] [CrossRef]
- Morel, A.F.; Larghi, E.L.; Selvero, M.M. Mild, Efficient and Selective Silver Carbonate Mediated O-Alkylation of 4-Hydroxy-2-quinolones: Synthesis of 2,4-Dialkoxyquinolines. Synlett. 2005, 18, 2755–2758. [Google Scholar] [CrossRef]
- Chen C,l. ; Chen, I.l.; Chen, J.j.; Wei, D.c.; Hsieh, H.j.; Chang, K.M.; et al. Studies on the alkylation of quinolin-2 (1H)-one derivatives. Journal of the Chilean Chemical Society. 2015, 60, 2812–2816. [Google Scholar]
- Grzelakowska, A.; Kolińska, J.; Mąkiewicz, M. The synthesis and spectroscopic characterisation of 3-formyl-2-quinolones in the presence of biothiols. Coloration Technology. déc 2018, 134, 440–449. [Google Scholar] [CrossRef]
- Kolińska, J.; Grzelakowska, A.; Sokołowska, J. Novel 7-maleimido-2(1 H )-quinolones as potential fluorescent sensors for the detection of sulphydryl groups. Coloration Technology. avr 2018, 134, 148–155. [Google Scholar] [CrossRef]
- Selvero, M.M.; Ledesma, G.N.; Abram, U.; Schulz-Lang, E.; Morel, A.F.; Larghi, E.L. 2, 2, 2-trifluoroethanol-promoted access to symmetrically 3, 3-disubstituted quinoline-2, 4-diones. Journal of Fluorine Chemistry. 2020, 234, 109520. [Google Scholar] [CrossRef]
- Refouvelet, B.; Guyon, C.; Jacquot, Y.; Girard, C.; Fein, H.; Bévalot, F.; et al. Synthesis of 4-hydroxycoumarin and 2, 4-quinolinediol derivatives and evaluation of their effects on the viability of HepG2 cells and human hepatocytes culture. European journal of medicinal chemistry. 2004, 39, 931–937. [Google Scholar] [CrossRef]
- Hao, F.; Asahara, H.; Nishiwaki, N. A direct and vicinal functionalization of the 1-methyl-2-quinolone framework: 4-alkoxylation and 3-chlorination. Organic & Biomolecular Chemistry. 2016, 14, 5128–5135. [Google Scholar]
- Bergman, J.; Pettersson, B.; Hasimbegovic, V.; Svensson, P.H. Thionations Using a P 4 S 10 −Pyridine Complex in Solvents Such as Acetonitrile and Dimethyl Sulfone. J Org Chem. 18 mars 2011, 76, 1546–1553. [Google Scholar] [CrossRef]
- de Macedo, M.B.; Kimmel, R.; Urankar, D.; Gazvoda, M.; Peixoto, A.; Cools, F.; et al. Design, synthesis and antitubercular potency of 4-hydroxyquinolin-2 (1H)-ones. European Journal of Medicinal Chemistry. 2017, 138, 491–500. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Sidorenko, L.V.; Gorokhova, O.V.; Slobodzyan, S.V. 4-Hydroxy-2-quinolones. 97. Simple synthesis of the esters of 4-halo-substituted 2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem Heterocycl Compd. juill 2006, 42, 882–885. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Sidorenko, L.V.; Slobodzyan, S.V.; Rybakov, V.B.; Chernyshev, V.V. 4-Hydroxyquinol-2-ones. 87. Unusual Synthesis of 1-R-4-Hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylic Acid Pyridylamides. Chem Heterocycl Compd. sept 2005, 41, 1158–1166. [Google Scholar] [CrossRef]
- Täubl, A.E.; Langhans, K.; Kappe, T.; Stadlbauer, W. Thermolytic ring closure reactions of 4-azido-3-phenylsulfanyl-and 4-azido-3-phenylsulfonyl-2-quinolones to 12 H -quinolino-[3,4- b ][1,4]benzothiazin-6(5 H )-ones. Journal of Heterocyclic Chem. nov 2002, 39, 1259–1264. [Google Scholar] [CrossRef]
- Aizikovich, A.; Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Byk, G.; et al. A new application of diphenylphosphorylazide (DPPA) reagent: Convenient transformations of quinolin-4-one, pyridin-4-one and quinazolin-4-one derivatives into the 4-azido and 4-amino counterparts. Tetrahedron letters. 2004, 45, 4241–4243. [Google Scholar] [CrossRef]
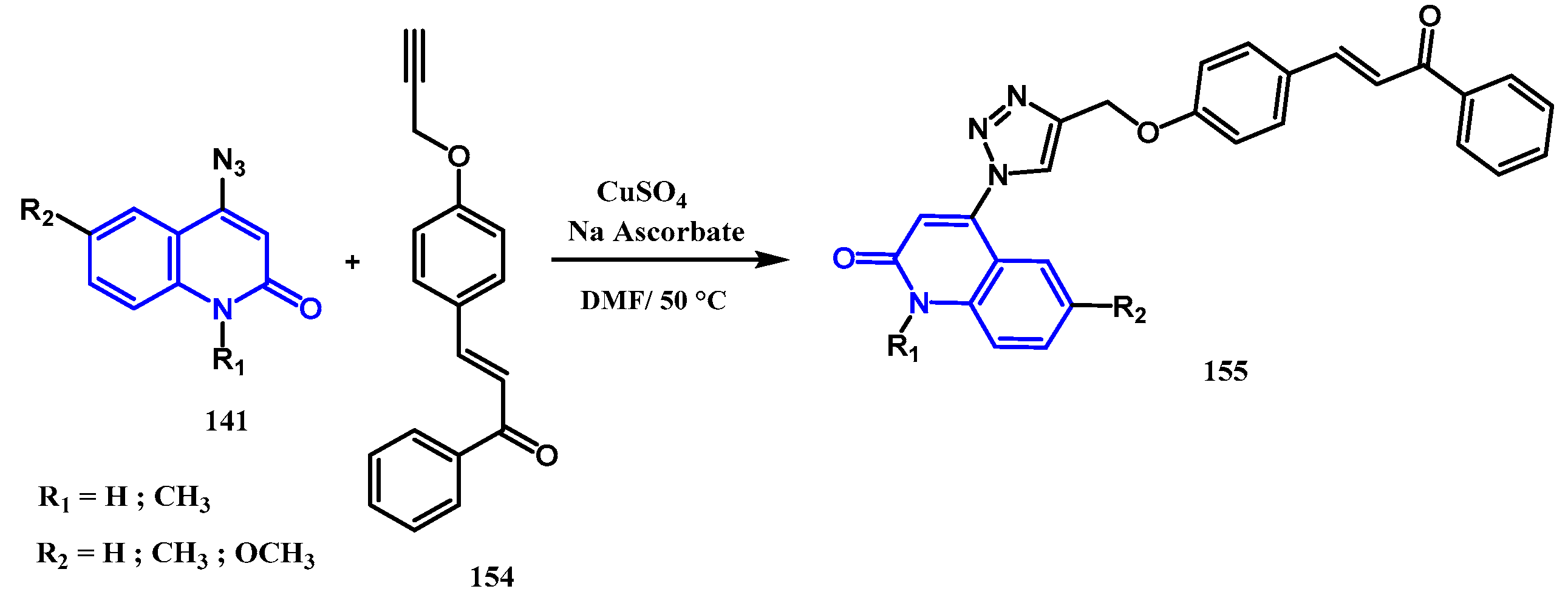
- Alshammari, M.B.; Aly, A.A.; Brown, A.B.; Bakht, M.A.; Shawky, A.M.; Abdelhakem, A.M.; et al. An efficient click synthesis of chalcones derivatized with two 1-(2-quinolon-4-yl)-1,2,3-triazoles. Zeitschrift für Naturforschung B 2021, 76, 395–403. [Google Scholar] [CrossRef]
- Chung, P.Y.; Bian, Z.X.; Pun, H.Y.; Chan, D.; Chan, A.S.C.; Chui, C.H.; et al. Recent Advances in Research of Natural and Synthetic Bioactive Quinolines. Future Med Chem. juin 2015, 7, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Faraz, K.M.; Garima, V.; Wasim, A.; Akranth, M.; Mumtaz, A.M.; Mymoona, A.; et al. Synthetic trends followed for the development of 1, 2, 3-triazole derivatives. Int J Drug Dev. 2017, 9, 22–25. [Google Scholar]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chemistry An Asian Journal. 4 oct 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Chen, Y.L.; Fang, K.C.; Sheu, J.Y.; Hsu, S.L.; Tzeng, C.C. Synthesis and Antibacterial Evaluation of Certain Quinolone Derivatives. J Med Chem. 1 juill 2001, 44, 2374–2377. [Google Scholar] [CrossRef]
- Abass, M.; Hassanin, H.M.; Allimony, H.A.; Hassan, H. Substituted quinolinones 27.* Regioselective synthesis of pyrazolo-, oxazolo-, and triazepinoquinoline derivatives. Chem Heterocycl Comp, 1023; 51. [Google Scholar]
- Abass, M.; Mostafa, B.B. Synthesis and evaluation of molluscicidal and larvicidal activities of some novel enaminones derived from 4-hydroxyquinolinones: Part IX. Bioorganic & medicinal chemistry. 2005, 13, 6133–6144. [Google Scholar]
- Ganesh, I. Conversion of carbon dioxide into several potential chemical commodities following different pathways-a review. In: Materials Science Forum [Internet]. Trans Tech Publ; 2013. p. 1-82.
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. [No title found]. Angew Chem. 15 juill 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J Org Chem. 1 mai 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Goddard-Borger, E.D.; Stick, R.V. An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride. Org Lett. 2007, 9, 3797–3800. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; Van Maarseveen, J.H. Cu I -Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur J Org Chem. 2006, 2006, 51–68. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed. 1 juin 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Gumus, A.; Gumus, S. Synthesis of Triazole-Coupled Quinoline-Based Fluorescent Sensor. The Eurasia Proceedings of Science Technology Engineering and Mathematics. 2019, 7, 58–62. [Google Scholar]
- El-Sheref, E.M.; Aly, A.A.; Ameen, M.A.; Brown, A.B. Synthesis of new 4-(1,2,3-triazolo)quinolin-2(1H)-ones via Cu-catalyzed [3 + 2] cycloaddition. Monatsh Chem. avr 2019, 150, 747–756. [Google Scholar] [CrossRef]
- El-Sheref, E.M.; Ameen, M.A.; El-Shaieb, K.M.; Abdel-Latif, F.F.; Abdel-Naser, A.I.; Brown, A.B.; et al. Design, synthesis and biological evaluation of syn and anti-like double warhead quinolinones bearing dihydroxy naphthalene moiety as epidermal growth factor receptor inhibitors with potential apoptotic antiproliferative action. Molecules. 2022, 27, 8765. [Google Scholar] [CrossRef]
- El-Sheref, E.M.; Elbastawesy, M.A.; Brown, A.B.; Shawky, A.M.; Gomaa, H.A.; Bräse, S.; et al. Design and synthesis of (2-oxo-1, 2-dihydroquinolin-4-yl)-1, 2, 3-triazole derivatives via click reaction: Potential apoptotic antiproliferative agents. Molecules. 2021, 26, 6798. [Google Scholar] [CrossRef]
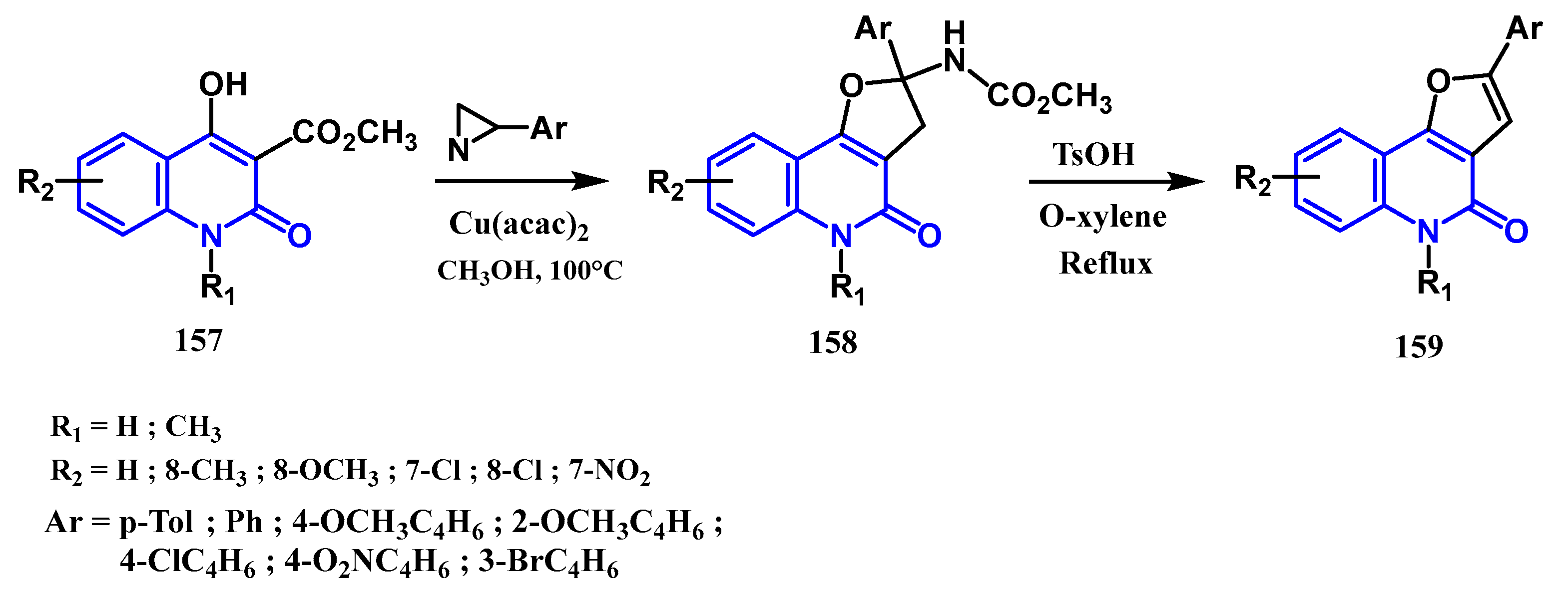
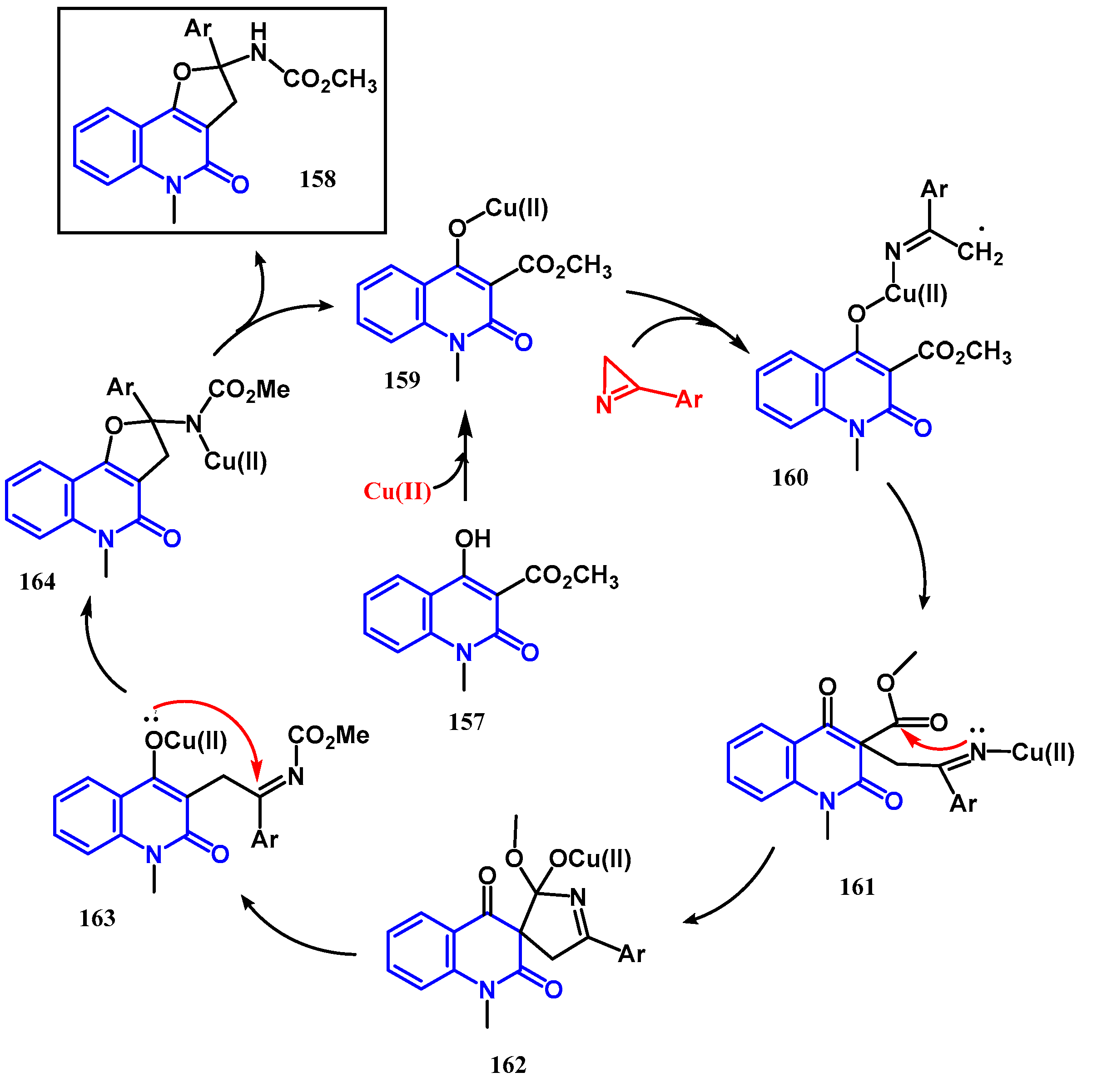
- Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Panikorovskii, T.L.; Novikov, M.S. 2 H -Azirines as C–C Annulation Reagents in Cu-Catalyzed Synthesis of Furo[3,2- c ]quinolone Derivatives. Org Lett. 2019, 21, 3615–3619. [Google Scholar] [CrossRef]
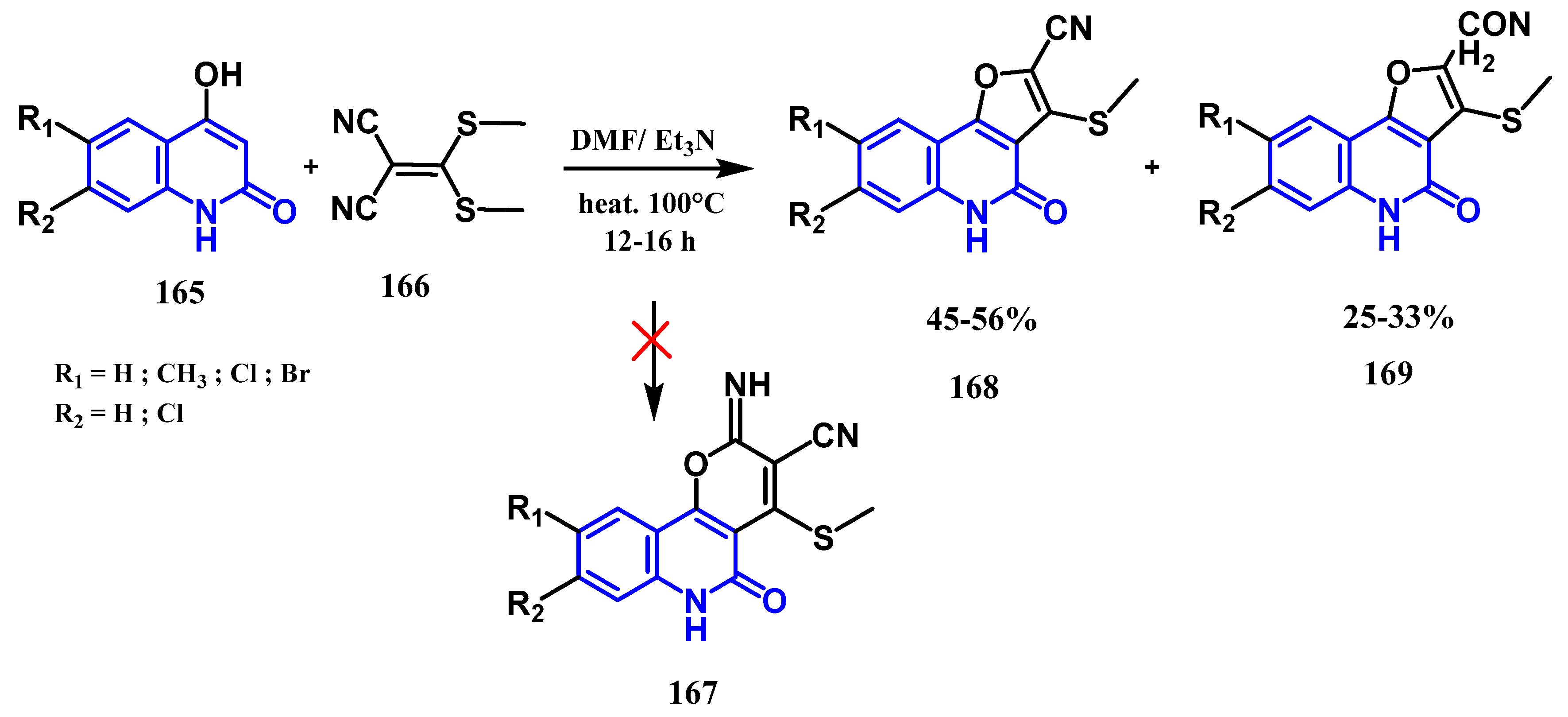
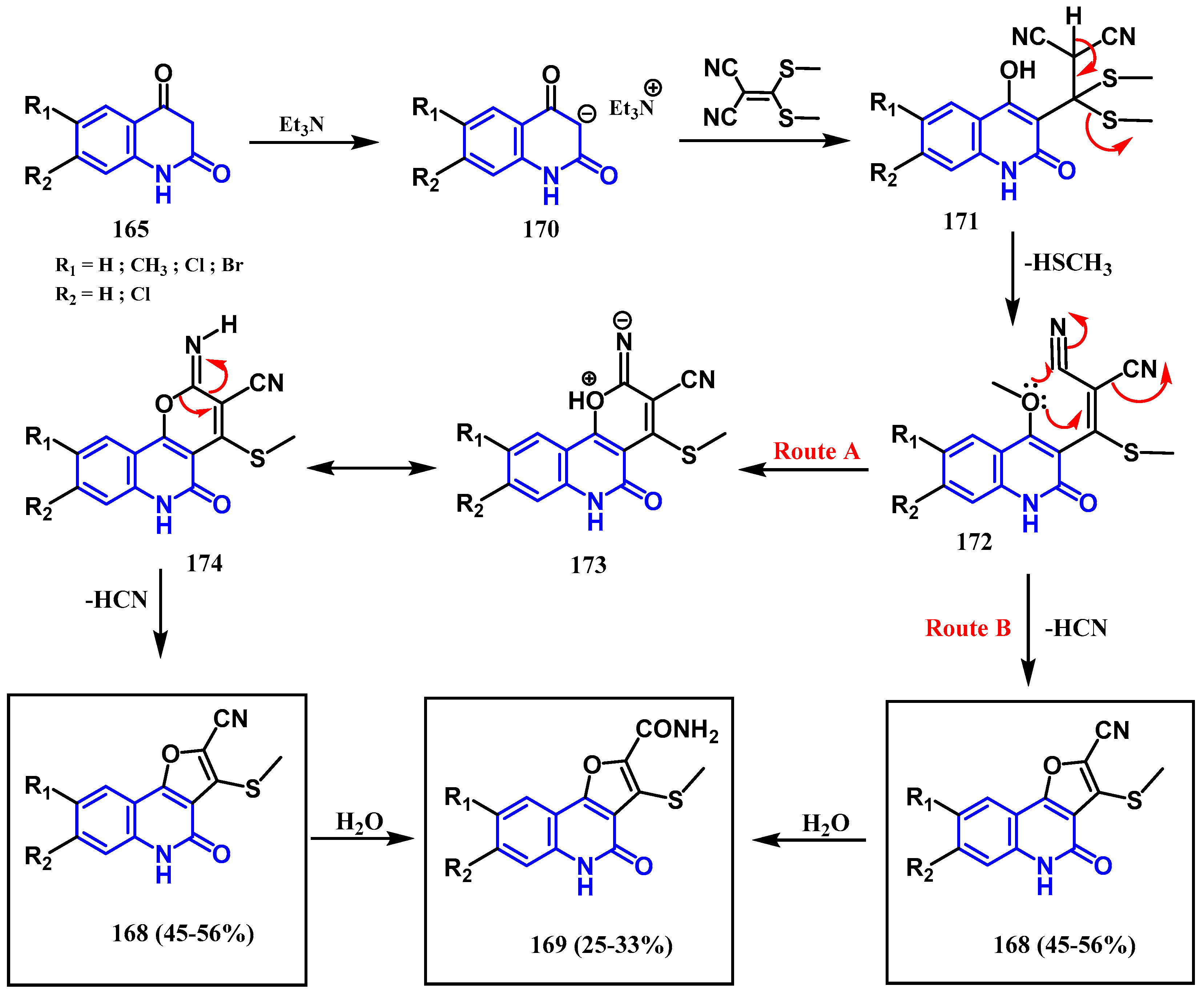
- Aly, A.A.; Ishak, E.A.; Shwaky, A.M.; Mohamed, A.H. Formation of furo[3,2-c]quinolone-2-carbonitriles and 4-oxo-4,5-dihydrofuro[3,2-c]quinolone-2-carboxamides from reaction of quinoline-2,4-diones with 2-[bis(methylthio)methylene]malononitrile. Monatsh Chem. févr 2020, 151, 223–229. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Natural Product Reports. 2002, 19, 742–760. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Natural product reports. 2007, 24, 223–246. [Google Scholar] [CrossRef]
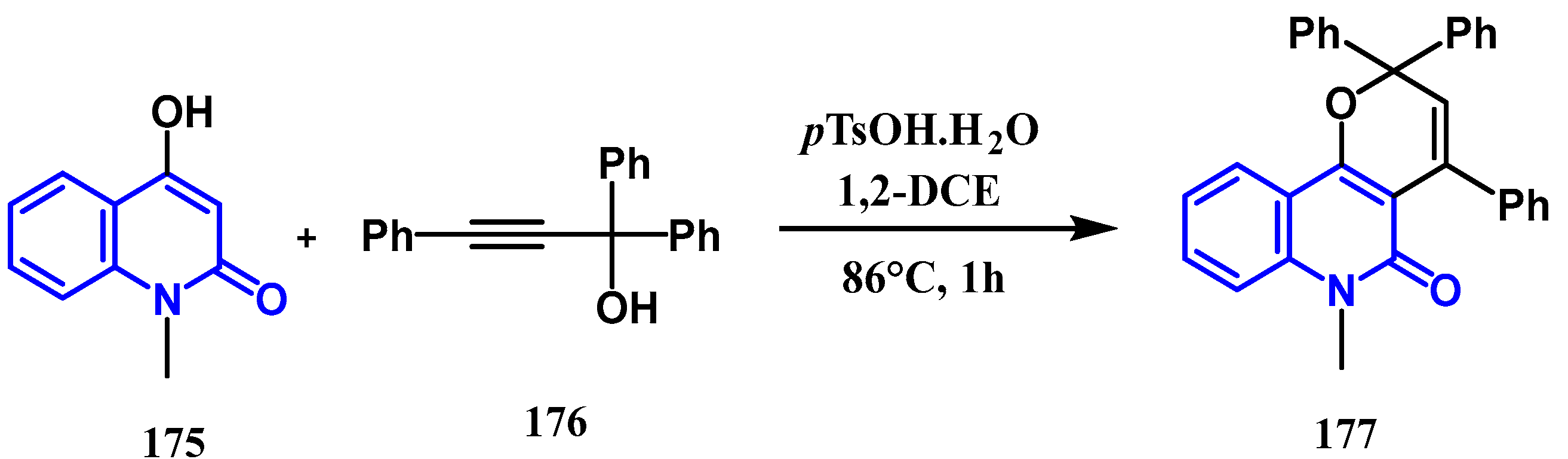
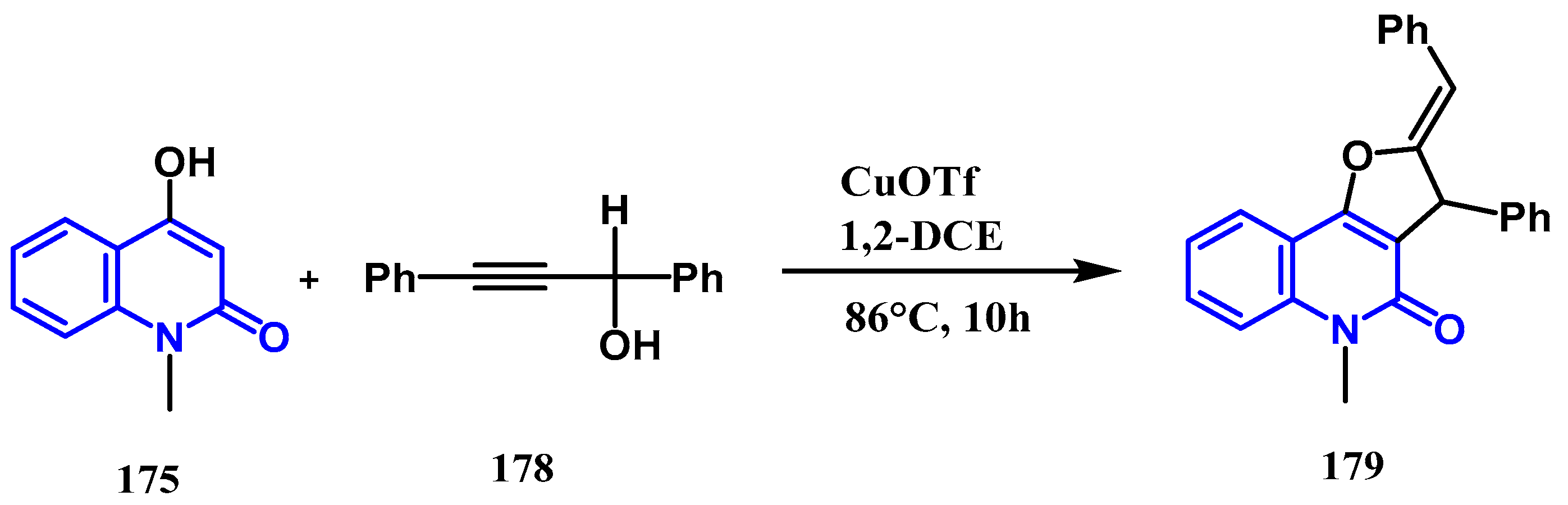
- Yin, H.; Wu, Y.; Gu, X.; Feng, Z.; Wang, M.; Feng, D.; et al. Synthesis of pyrano [3, 2-c] quinolones and furo [3, 2-c] quinolones via acid-catalyzed tandem reaction of 4-hydroxy-1-methylquinolin-2 (1 H)-one and propargylic alcohols. RSC advances. 2022, 12, 21066–21078. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Castillo, J.; Cuervo, P.; Insuasty, B.; Quiroga, J.; Ortíz, A.; et al. A Simple One-Pot Synthesis of New Imidazol-2-yl-1 H -quinolin-2-ones from the Direct Reaction of 2-Chloroquinolin-3-carbaldehyde with Aromatic o -Diamines. Eur J Org Chem. 2010, 2010, 317–325. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; El-Sheref, E.M.; Hammouda, M.M.; Youssif, B.G. One-pot synthesis of 1-Thia-4-azaspiro [4.4/5] alkan-3-ones via schiff base: Design, synthesis, and apoptotic antiproliferative properties of dual EGFR/BRAFV600E inhibitors. Pharmaceuticals. 2023, 16, 467. [Google Scholar] [CrossRef]
- Pais, J.P.; Policarpo, M.; Pires, D.; Francisco, A.P.; Madureira, A.M.; Testa, B.; et al. Fluoroquinolone derivatives in the treatment of mycobacterium tuberculosis infection. Pharmaceuticals. 2022, 15, 1213. [Google Scholar] [CrossRef]
- Tan, Y.M.; Li, D.; Li, F.F.; Ansari, M.F.; Fang, B.; Zhou, C.H. Pyrimidine-conjugated fluoroquinolones as new potential broad-spectrum antibacterial agents. Bioorganic & Medicinal Chemistry Letters. 2022, 73, 128885. [Google Scholar]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, lethality and their contributions to antibiotic resistance. Molecules. 2020, 25, 5662. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry. 18 mars 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Szulczyk, D.; Woziński, M.; Koliński, M.; Kmiecik, S.; Głogowska, A.; Augustynowicz-Kopeć, E.; et al. Menthol- and thymol-based ciprofloxacin derivatives against Mycobacterium tuberculosis: In vitro activity, lipophilicity, and computational studies. Sci Rep. 2023, 13, 16328. [Google Scholar] [CrossRef]
- Xue, W.; Li, X.; Ma, G.; Zhang, H.; Chen, Y.; Kirchmair, J.; et al. N-thiadiazole-4-hydroxy-2-quinolone-3-carboxamides bearing heteroaromatic rings as novel antibacterial agents: Design, synthesis, biological evaluation and target identification. European Journal of Medicinal Chemistry. 2020, 188, 112022. [Google Scholar] [CrossRef]
- Berhan, A.; Bayleyegn, B.; Getaneh, Z. HIV/AIDS Associated Lymphoma: Review. BLCTT. 2022, 12, 31–45. [Google Scholar] [CrossRef]
- He, N. Research progress in the epidemiology of HIV/AIDS in China. China CDC weekly. 2021, 3, 1022. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Amekpor, F.; Scott, G.Y. An update of human immunodeficiency virus infection: Bleeding disorders. J Pub Health Nutri [Internet]. 2023.
- Wang, R.; Xu, K.; Shi, W. Quinolone derivatives: Potential anti-HIV agent—Development and application. Archiv der Pharmazie. sept 2019, 352, 1900045. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yan, S.; Luo, Z.; Han, X.; Wang, Y.; Wang, Z.; et al. Design, practical synthesis, and biological evaluation of novel 6-(pyrazolylmethyl)-4-quinoline-3-carboxylic acid derivatives as HIV-1 integrase inhibitors. Molecules. 2012, 17, 10652–10666. [Google Scholar] [CrossRef] [PubMed]
- Dube, P.S.; Legoabe, L.J.; Beteck, R.M. Quinolone: A versatile therapeutic compound class. Mol Divers. juin 2023, 27, 1501–1526. [Google Scholar] [CrossRef]
- Papagni, R.; Novara, R.; Minardi, M.L.; Frallonardo, L.; Panico, G.G.; Pallara, E.; et al. Human African Trypanosomiasis (sleeping sickness): Current knowledge and future challenges. Frontiers in Tropical Diseases. 2023, 4, 1087003. [Google Scholar] [CrossRef]
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African Trypanosomiasis (Sleeping Sickness)—Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr Trop Med Rep. 25 oct 2023, 10, 222–234. [Google Scholar] [CrossRef]
- Jamabo, M.; Mahlalela, M.; Edkins, A.L.; Boshoff, A. Tackling sleeping sickness: Current and promising therapeutics and treatment strategies. International Journal of Molecular Sciences. 2023, 24, 12529. [Google Scholar] [CrossRef]
- Pham, T.D.; Ziora, Z.M.; Blaskovich, M.A. Quinolone antibiotics. Medchemcomm. 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Dine, I.; Mulugeta, E.; Melaku, Y.; Belete, M. Recent advances in the synthesis of pharmaceutically active 4-quinolone and its analogues: A review. RSC advances. 2023, 13, 8657–8682. [Google Scholar] [CrossRef]
- Pedron, J.; Boudot, C.; Brossas, J.Y.; Pinault, E.; Bourgeade-Delmas, S.; Sournia-Saquet, A.; et al. New 8-Nitroquinolinone Derivative Displaying Submicromolar in Vitro Activities against Both Trypanosoma brucei and cruzi. ACS Med Chem Lett. 2020, 11, 464–472. [Google Scholar] [CrossRef]
- Hiltensperger, G.; Jones, N.G.; Niedermeier, S.; Stich, A.; Kaiser, M.; Jung, J.; et al. Synthesis and Structure–Activity Relationships of New Quinolone-Type Molecules against Trypanosoma brucei. J Med Chem. 22 mars 2012, 55, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Beteck, R.M.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Synthesis, in vitro cytotoxicity and trypanocidal evaluation of novel 1, 3, 6-substituted non-fluoroquinolones. South African Journal of Chemistry. 2018, 71, 188–195. [Google Scholar] [CrossRef]
- Angula, K.T.; Legoabe, L.J.; Swart, T.; Hoppe, H.C.; Beteck, R.M. Synthesis and in vitro antitrypanosomal evaluation of novel 6-heteroarylidene-substituted quinolone derivatives. European Journal of Medicinal Chemistry. 2022, 227, 113913. [Google Scholar] [CrossRef] [PubMed]
- Chanquia, S.N.; Larregui, F.; Puente, V.; Labriola, C.; Lombardo, E.; Liñares, G.G. Synthesis and biological evaluation of new quinoline derivatives as antileishmanial and antitrypanosomal agents. Bioorganic Chemistry. 2019, 83, 526–534. [Google Scholar] [CrossRef]
- Arora, G.; Chuang, Y.M.; Sinnis, P.; Dimopoulos, G.; Fikrig, E. Malaria: Influence of Anopheles mosquito saliva on Plasmodium infection. Trends in immunology. 2023, 44, 256–265. [Google Scholar] [CrossRef]
- Patel, P.; Bagada, A.; Vadia, N. Epidemiology and Current Trends in Malaria. In: Amponsah SK, Shegokar R, Pathak YV, éditeurs. Rising Contagious Diseases [Internet]. 1re éd. Wiley; 2024. p. 261-82.
- Shaw, W.R.; Marcenac, P.; Catteruccia, F. Plasmodium development in Anopheles: A tale of shared resources. Trends in Parasitology. 1 févr 2022, 38, 124–135. [Google Scholar] [CrossRef]
- Balogun, T.A.; Omoboyowa, D.A.; Saibu, O.A. In silico Anti-malaria Activity of Quinolone Compounds against Plasmodium falciparum Dihydrofolate Reductase (pfDHFR). International Journal of Biochemistry Research & Review. 1 août 2020, 29, 10–17. [Google Scholar]
- Van Schalkwyk, D.A.; Riscoe, M.K.; Pou, S.; Winter, R.W.; Nilsen, A.; Duffey, M.; et al. Novel Endochin-Like Quinolones Exhibit Potent In Vitro Activity against Plasmodium knowlesi but Do Not Synergize with Proguanil. Antimicrob Agents Chemother. 2020, 64, e02549–e19. [Google Scholar] [CrossRef]
- Pou, S.; Dodean, R.A.; Frueh, L.; Liebman, K.M.; Gallagher, R.T.; Jin, H.; et al. New Scalable Synthetic Routes to ELQ-300, ELQ-316, and Other Antiparasitic Quinolones. Org Process Res Dev. 2021, 25, 1841–1852. [Google Scholar] [CrossRef]
- Doggett, J.S.; Schultz, T.; Miller, A.J.; Bruzual, I.; Pou, S.; Winter, R.; et al. Orally Bioavailable Endochin-Like Quinolone Carbonate Ester Prodrug Reduces Toxoplasma gondii Brain Cysts. Antimicrob Agents Chemother. 20 août 2020, 64, e00535–e20. [Google Scholar] [CrossRef]
- Miley, G.P.; Pou, S.; Winter, R.; Nilsen, A.; Li, Y.; Kelly, J.X.; et al. ELQ-300 Prodrugs for Enhanced Delivery and Single-Dose Cure of Malaria. Antimicrob Agents Chemother. 2015, 59, 5555–5560. [Google Scholar] [CrossRef] [PubMed]
- Frueh, L.; Li, Y.; Mather, M.W.; Li, Q.; Pou, S.; Nilsen, A.; et al. Alkoxycarbonate Ester Prodrugs of Preclinical Drug Candidate ELQ-300 for Prophylaxis and Treatment of Malaria. ACS Infect Dis. 2017, 3, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Balbino, L.S.; Bernardes, J.C.; Ladeia, W.A.; Martins, F.D.C.; Nino, B.D.S.L.; Mitsuka-Breganó, R.; et al. Epidemiological study of toxoplasmosis outbreaks in Brazil. Transbounding Emerging Dis. juill 2022, 69, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Mesa-Valle, C.; Manzano-Agugliaro, F. Human parasitology worldwide research. Parasitology. mai 2018, 145, 699–712. [Google Scholar] [CrossRef]
- Eberhard, N.; Balmer, V.; Müller, J.; Müller, N.; Winter, R.; Pou, S.; et al. Activities of Endochin-Like Quinolones Against in vitro Cultured Besnoitia besnoiti Tachyzoites. Front Vet Sci 2020, 7. [Google Scholar] [CrossRef]
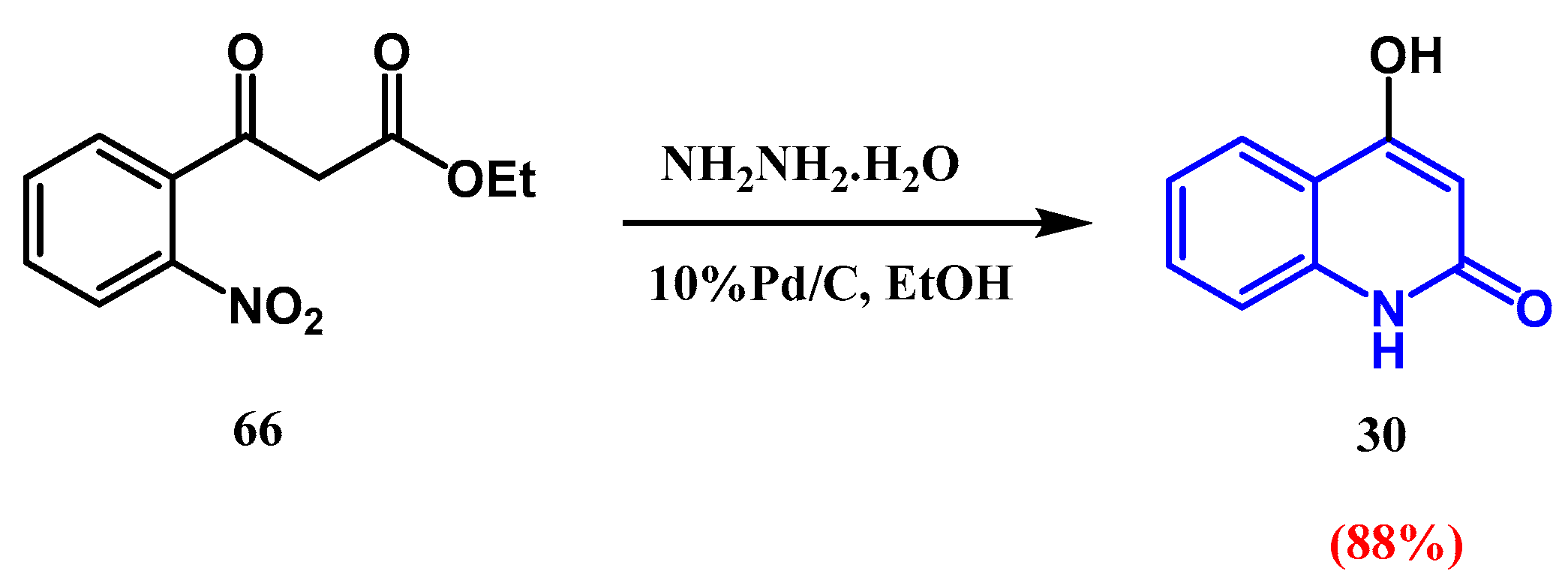
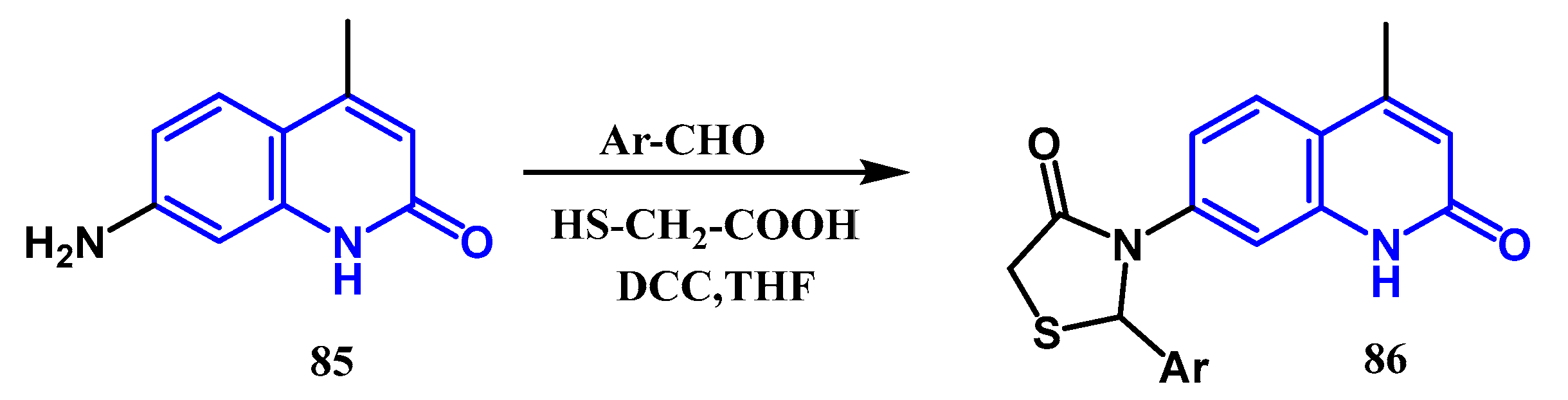
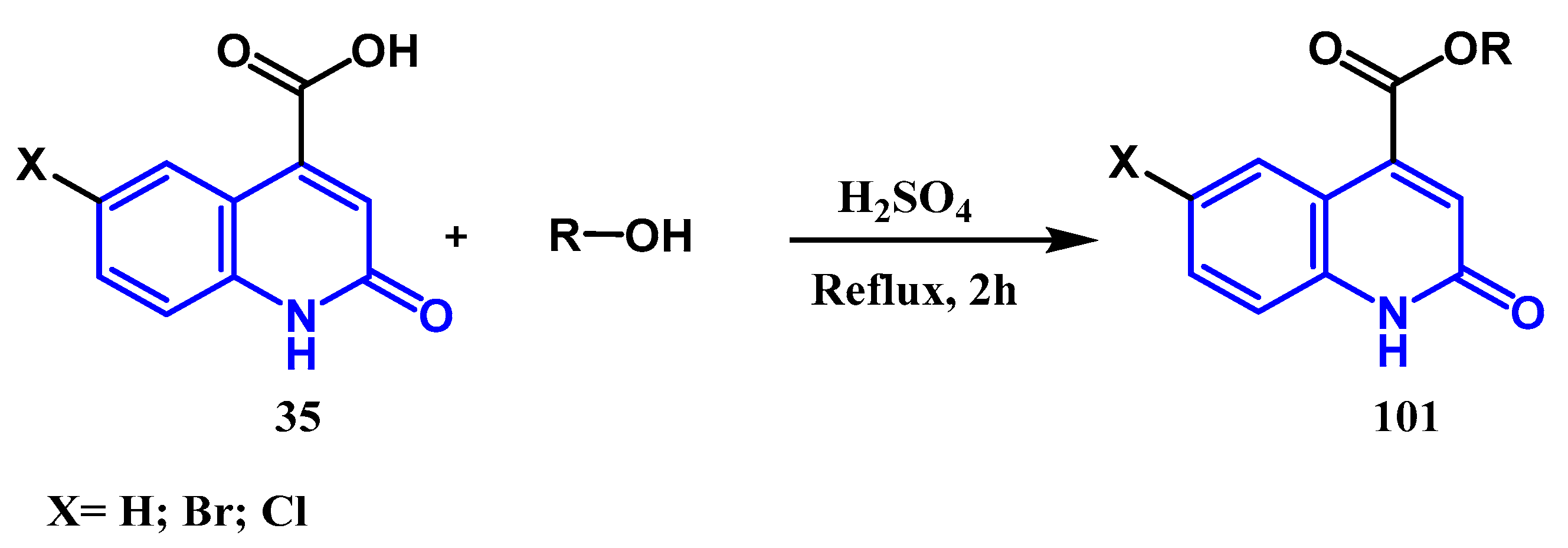
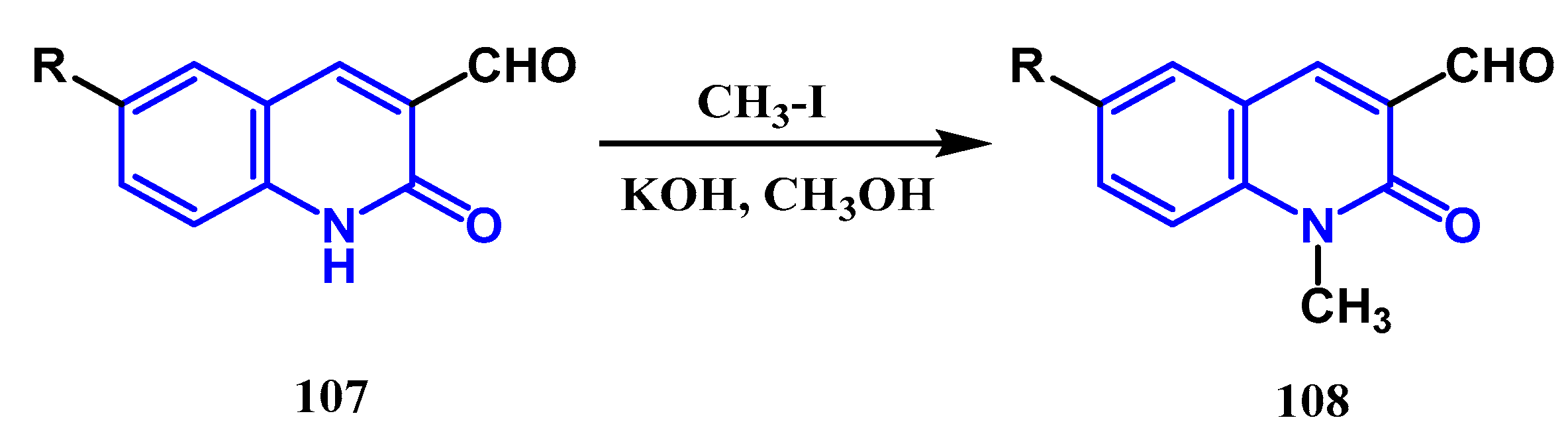
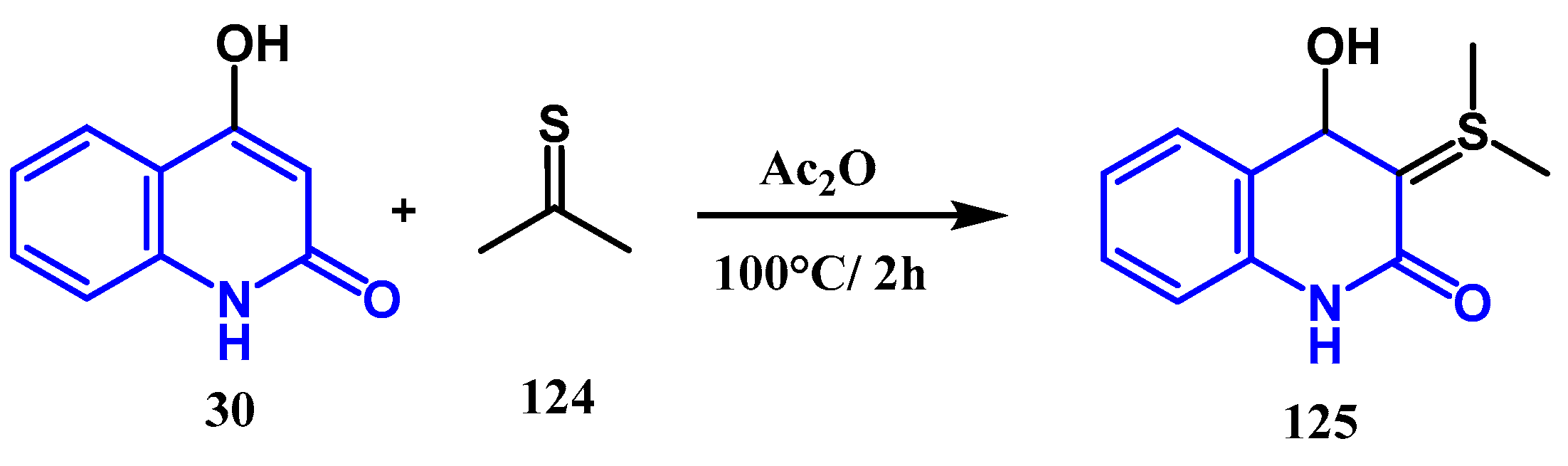
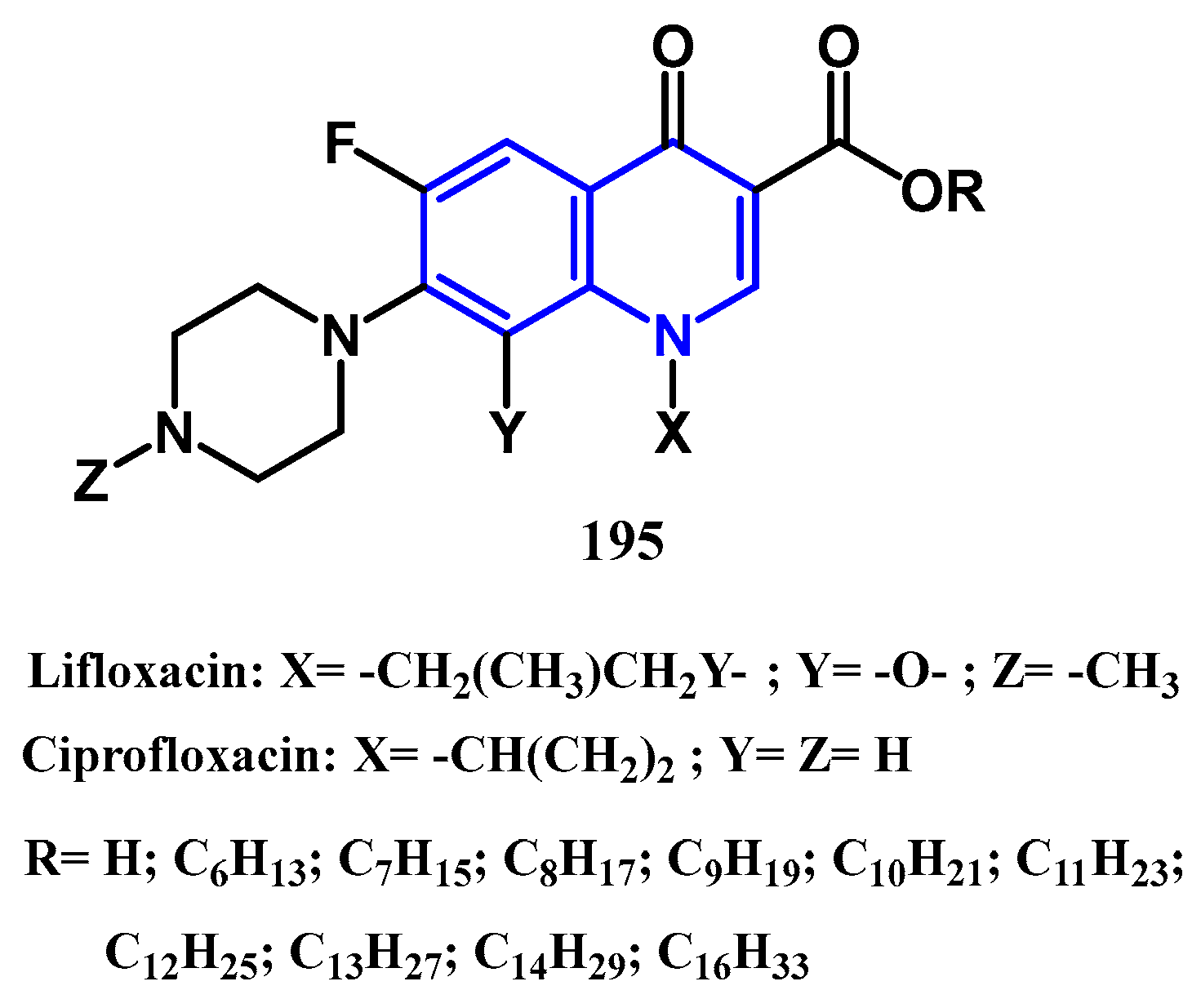
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




