Submitted:
25 January 2025
Posted:
27 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Quality Assessment
- Study identification data and study characteristics: First author’s name and year of publication, country of origin of first author, trial register ID number, RCT design type, study time frame-duration, funding sources, number of participants randomized
- Number of study arms included, number of patients per arm, inclusion/exclusion criteria, study quality, type of analysis, deviation from the initial protocol and selective reporting, follow-up period)
- Baseline characteristics of participants (mean age and standard deviations (SD), gender, BMI, primary indication for stoma creation, type of ostomy, technique of stoma-skin closure, administration of antibiotics)
- Data for the intervention (type of NPWT, duration of application, NPWT brand, frequency of dressing changes, duration of follow-up)
- Data for the comparator (type of non-pressure dressings, duration of application, frequency of dressing changes, duration of follow-up)
- Outcome data (outcome title, outcome definition and diagnostic criteria, SSI rate, rate of other wound healing complications, days of hospital stay, time to complete wound healing, patient-reported wound cosmesis)
2.5. Outcomes
2.6. Statistical Analysis
2.7. Quality Assessment
3. Results
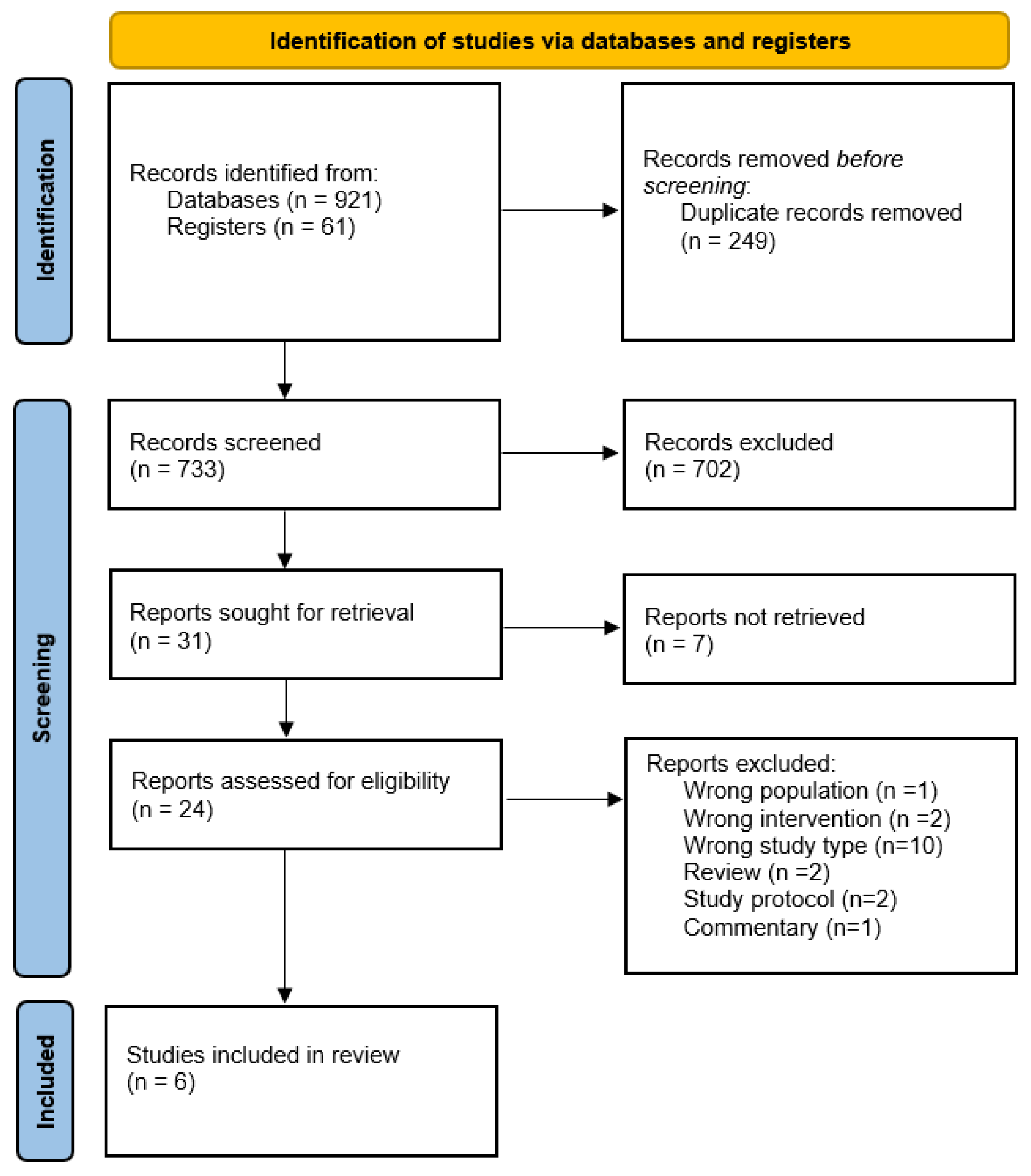
3.1. Study Selection Process
3.2. Baseline Study Characteristics
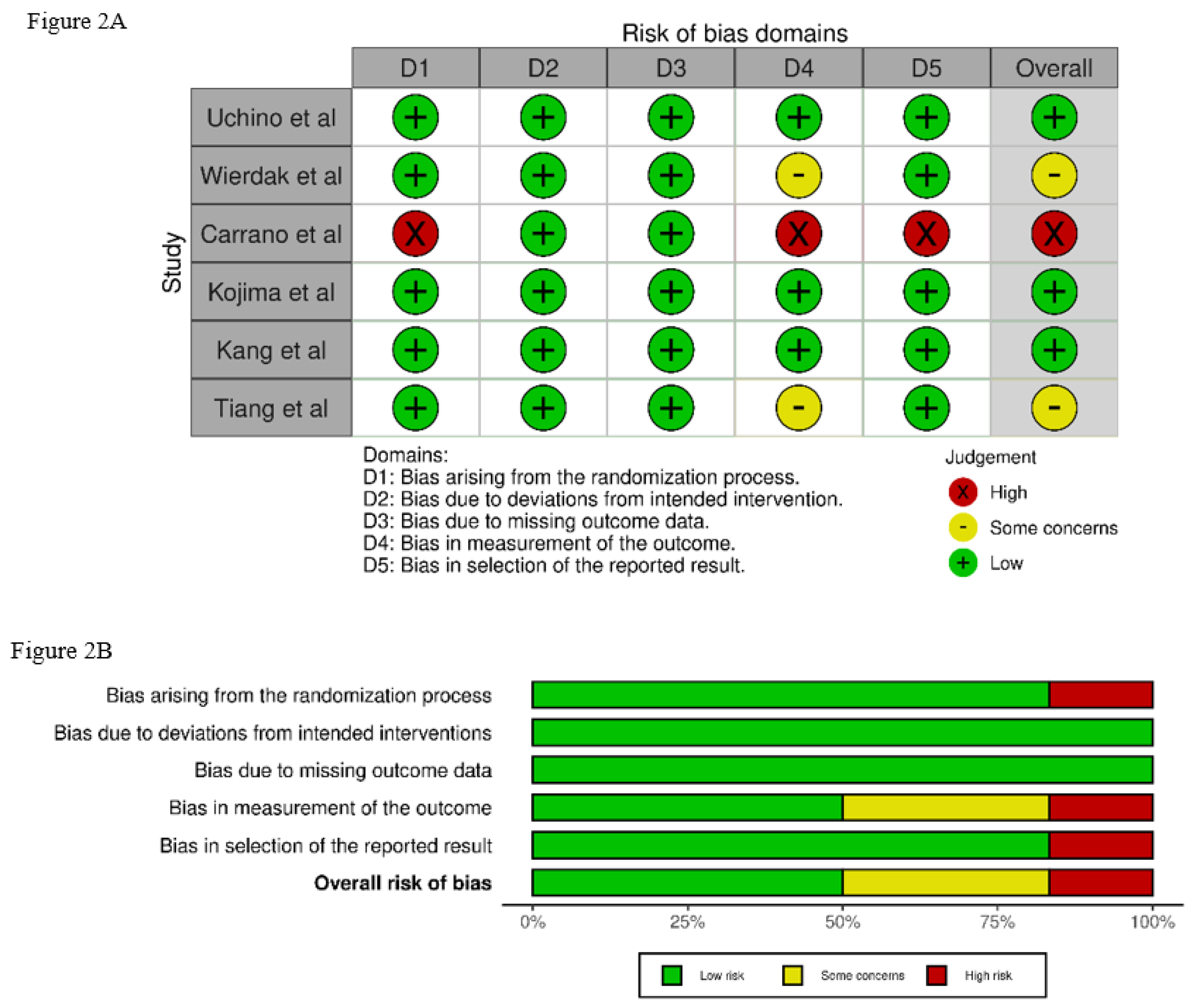
3.3. Quality Assessment
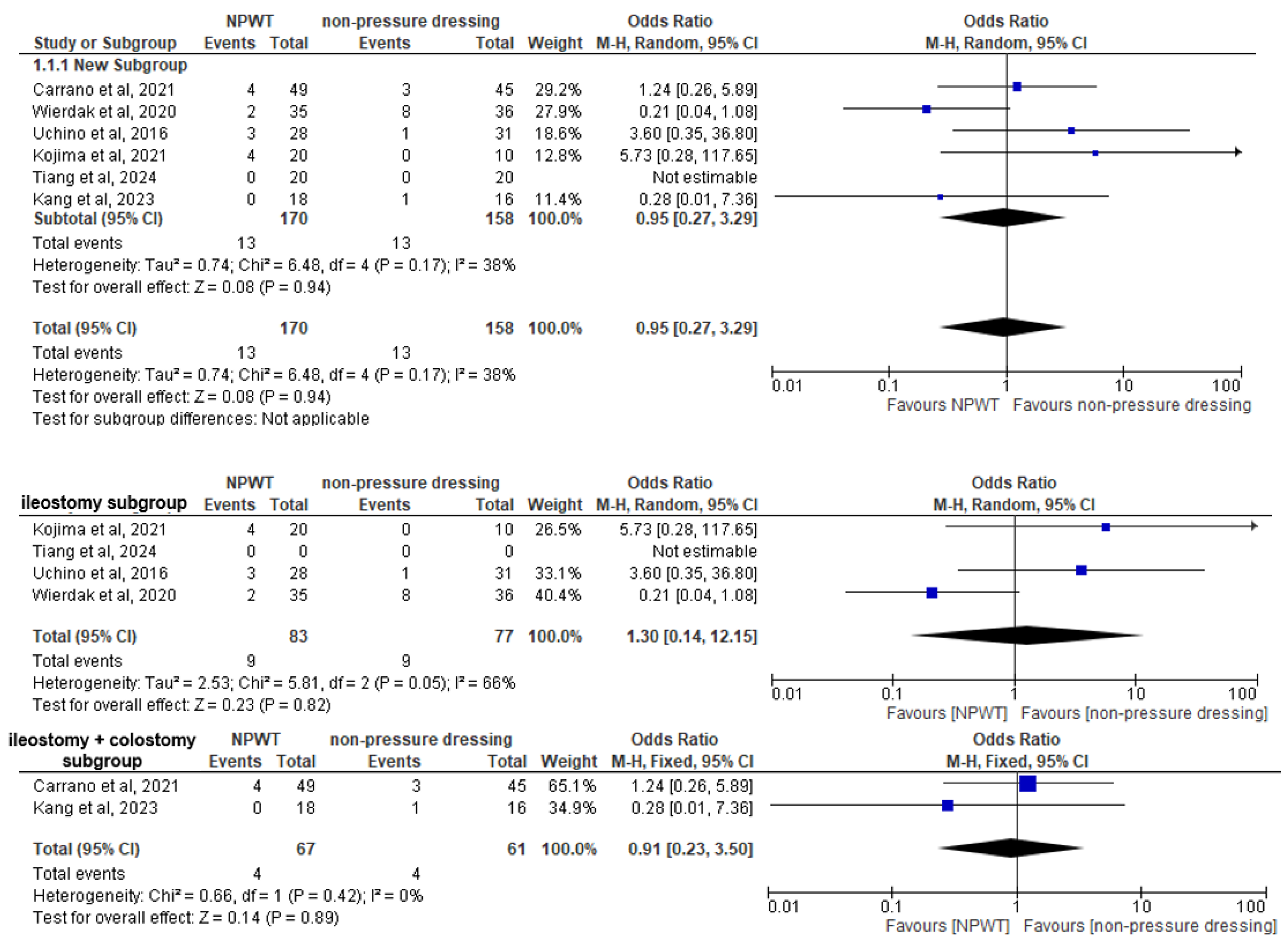
3.4. Primary Outcome
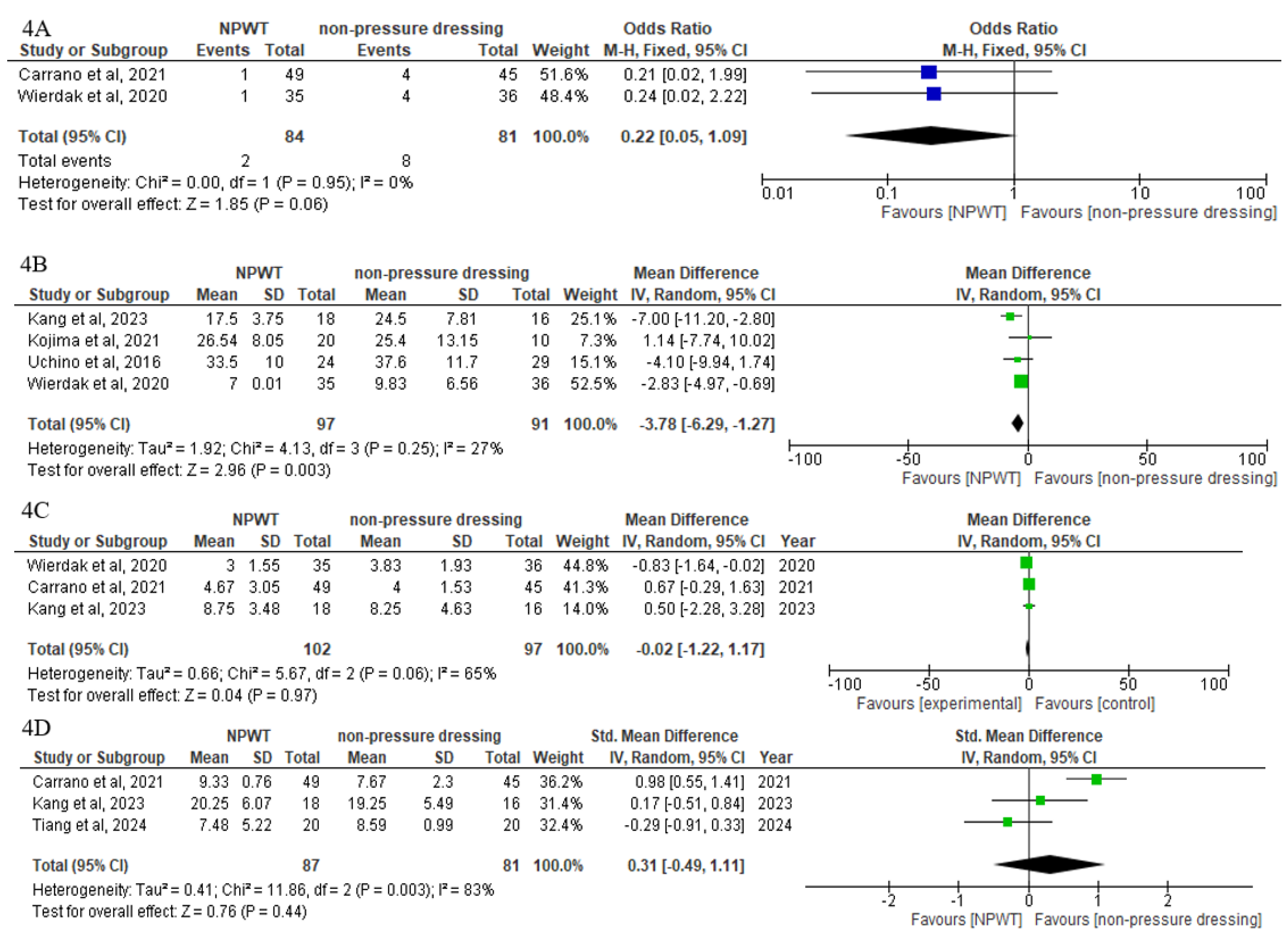
3.5. Secondary Outcomes
3.6. Subgroup Analysis
3.7. Sensitivity Analysis
4. Discussion
5. Strengths of the Study
6. Limitations and Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| CI | Confidence Interval |
| ECDC | European Centre for Disease Prevention and Control |
| LOS | Length of Hospital Stay |
| MD | Mean Difference |
| NPWT | Negative Pressure Wound Therapy |
| NPD | Non-Pressure Dressing |
| OR | Odds Ratio |
| PICO | Patient, Intervention, Comparison, and Outcome |
| POSAS | Patient and Observer Scar Assessment Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trial |
| SD | Standard Deviation |
| SMD | Standardized Mean Difference |
| SR-SSI | Stoma Reversal Surgical Site Infection |
| SSI | Surgical Site Infection |
| VAS | Visual Analog Scale |
References
- Winter, K.; Dziki, A. Volume of Surgical Interventions for Benign Colorectal Tumors – an Analysis of 3510 Surgical and Endoscopic Resections in the Single Colorectal Center in Poland. 2021, 93, 11–19. [CrossRef]
- Daghmouri, M.A.; Chaouch, M.A.; Oueslati, M.; Rebai, L.; Oweira, H. Regional Techniques for Pain Management Following Laparoscopic Elective Colonic Resection: A Systematic Review. Ann. Med. Surg. 2021, 72, 103124. [CrossRef]
- Krishnamurty, D.M.; Blatnik, J.; Mutch, M. Stoma Complications. Clin. Colon Rectal Surg. 2017, 30, 193–200. [CrossRef]
- Malik, T.; Lee, M.J.; Harikrishnan, A.B. The Incidence of Stoma Related Morbidity - a Systematic Review of Randomised Controlled Trials. Ann. R. Coll. Surg. Engl. 2018, 100, 501–508. [CrossRef]
- Pandiaraja, J.; Chakkarapani, R.; Arumugam, S. A Study on Patterns, Indications, and Complications of an Enteric Stoma. J. Fam. Med. Prim. care 2021, 10, 3277–3282. [CrossRef]
- Zafar, S.N.; Changoor, N.R.; Williams, K.; Acosta, R.D.; Greene, W.R.; Fullum, T.M.; Haider, A.H.; Cornwell, E.E.; Tran, D.D. Race and Socioeconomic Disparities in National Stoma Reversal Rates. Am. J. Surg. 2016, 211, 710–715. [CrossRef]
- Owens, C.D.; Stoessel, K. Surgical Site Infections: Epidemiology, Microbiology and Prevention. J. Hosp. Infect. 2008, 70, 3–10. [CrossRef]
- Papadopoulos, A.; Machairas, N.; Tsourouflis, G.; Chouliaras, C.; Manioti, E.; Broutas, D.; Kykalos, S.; Daikos, G.L.; Samarkos, M.; Vagianos, C. Risk Factors for Surgical Site Infections in Patients Undergoing Emergency Surgery: A Single-Centre Experience. In Vivo 2021, 35, 3569–3574. [CrossRef]
- Gillespie, B.M.; Harbeck, E.; Rattray, M.; Liang, R.; Walker, R.; Latimer, S.; Thalib, L.; Andersson, A.E.; Griffin, B.; Ware, R.; et al. Worldwide Incidence of Surgical Site Infections in General Surgical Patients: A Systematic Review and Meta-Analysis of 488,594 Patients. Int. J. Surg. 2021, 95, 106136. [CrossRef]
- Dixon, L.K.; Biggs, S.; Messenger, D.; Shabbir, J. Surgical Site Infection Prevention Bundle in Elective Colorectal Surgery. J. Hosp. Infect. 2022, 122, 162–167. [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [CrossRef]
- Li, L.T.; Brahmbhatt, R.; Hicks, S.C.; Davila, J.A.; Berger, D.H.; Liang, M.K. Prevalence of Surgical Site Infection at the Stoma Site Following Four Skin Closure Techniques: A Retrospective Cohort Study. Dig. Surg. 2014, 31, 73–78. [CrossRef]
- Mirande, M.D.; McKenna, N.P.; Bews, K.A.; Shawki, S.F.; Cima, R.R.; Brady, J.T.; Colibaseanu, D.T.; Mathis, K.L.; Kelley, S.R. Risk Factors for Surgical Site Infections and Trends in Skin Closure Technique after Diverting Loop Ileostomy Reversal: A Multi-Institutional Analysis. Am. J. Surg. 2023, 226, 703–708. [CrossRef]
- Liang, M.K.; Li, L.T.; Avellaneda, A.; Moffett, J.M.; Hicks, S.C.; Awad, S.S. Outcomes and Predictors of Incisional Surgical Site Infection in Stoma Reversal. JAMA Surg. 2013, 148, 183–189. [CrossRef]
- Khan, M.A.; Niaz, K.; Asghar, S.; Yusufi, M.A.; Nazir, M.; Muhammad Ali, S.; Ahmed, A.; Salahudeen, A.A.; Kareem, T. Surgical Site Infection After Stoma Reversal: A Comparison Between Linear and Purse-String Closure. Cureus 2023, 15, e50057. [CrossRef]
- Normandin, S.; Safran, T.; Winocour, S.; Chu, C.K.; Vorstenbosch, J.; Murphy, A.M.; Davison, P.G. Negative Pressure Wound Therapy: Mechanism of Action and Clinical Applications. Semin. Plast. Surg. 2021, 35, 164–170. [CrossRef]
- Cooper, H.J.; Singh, D.P.; Gabriel, A.; Mantyh, C.; Silverman, R.; Griffin, L. Closed Incision Negative Pressure Therapy versus Standard of Care in Reduction of Surgical Site Complications: A Systematic Review and Meta-Analysis. Plast. Reconstr. surgery. Glob. open 2023, 11, e4722. [CrossRef]
- Kim, P.J.; Attinger, C.E.; Constantine, T.; Crist, B.D.; Faust, E.; Hirche, C.R.; Lavery, L.A.; Messina, V.J.; Ohura, N.; Punch, L.J.; et al. Negative Pressure Wound Therapy with Instillation: International Consensus Guidelines Update. Int. Wound J. 2020, 17, 174–186. [CrossRef]
- O’Leary, D.P.; Peirce, C.; Anglim, B.; Burton, M.; Concannon, E.; Carter, M.; Hickey, K.; Coffey, J.C. Prophylactic Negative Pressure Dressing Use in Closed Laparotomy Wounds Following Abdominal Operations: A Randomized, Controlled, Open-Label Trial: The P.I.C.O. Trial. Ann. Surg. 2017, 265, 1082–1086. [CrossRef]
- Groenen, H.; Jalalzadeh, H.; Buis, D.R.; Dreissen, Y.E.M.; Goosen, J.H.M.; Griekspoor, M.; Harmsen, W.J.; IJpma, F.F.A.; van der Laan, M.J.; Schaad, R.R.; et al. Incisional Negative Pressure Wound Therapy for the Prevention of Surgical Site Infection: An up-to-Date Meta-Analysis and Trial Sequential Analysis. eClinicalMedicine 2023, 62. [CrossRef]
- Singh, P.K.; Sethi, M.K.; Mishra, T.S.; Kumar, P.; Ali, S.M.; Sasmal, P.K.; Mishra, S.S. Comparison of Surgical Site Infection (SSI) between Negative Pressure Wound Therapy (NPWT) Assisted Delayed Primary Closure and Conventional Delayed Primary Closure in Grossly Contaminated Emergency Abdominal Surgeries: A Randomized Controlled Trial. Langenbeck’s Arch. Surg. 2023, 409, 19. [CrossRef]
- Borejsza-Wysocki, M.; Bobkiewicz, A.; Ledwosiński, W.; Szmyt, K.; Banasiewicz, T.; Krokowicz, Ł. Stoma Close to the Abdominal Wound - A Real Technical Problem: A Description of a Novel Care Strategy. Pol. Prz. Chir. Polish J. Surg. 2023, 95, 31–38. [CrossRef]
- Curchod, P.; Clerc, D.; Jurt, J.; Hubner, M.; Hahnloser, D.; Demartines, N.; Grass, F. Closed-Wound Negative Pressure Therapy Dressing after Loop Ostomy Closure: A Retrospective Comparative Study. Sci. Rep. 2022, 12, 7790. [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372. [CrossRef]
- Choong, M.K.; Galgani, F.; Dunn, A.G.; Tsafnat, G. Automatic Evidence Retrieval for Systematic Reviews. J. Med. Internet Res. 2014, 16, e223. [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [CrossRef]
- Tiang, T.; Behrenbruch, C.; Noori, J.; Lam, D.; Bhamidipaty, M.; Johnston, M.; Woods, R.; D’Souza, B. Prophylactic Negative Pressure Wound Therapy to Improve Wound Healing Rates Following Ileostomy Closure: A Randomized Controlled Trial. ANZ J. Surg. 2024. [CrossRef]
- Wierdak, M.; Pisarska-Adamczyk, M.; Wysocki, M.; Major, P.; Kołodziejska, K.; Nowakowski, M.; Vongsurbchart, T.; Pędziwiatr, M. Prophylactic Negative-Pressure Wound Therapy after Ileostomy Reversal for the Prevention of Wound Healing Complications in Colorectal Cancer Patients: A Randomized Controlled Trial. Tech. Coloproctol. 2021, 25, 185–193. [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; 2019; pp. 205–228 ISBN 9781119536604.
- Kojima, K.; Goto, M.; Nagashima, Y.; Saito, Y.; Kawai, M.; Takebe, S.; Egawa, A.; Tanba, M.; Ishikawa, K.; Matsuoka, H.; et al. Effectiveness of Negative Pressure Wound Therapy for the Wound of Ileostomy Closure : A Multicenter , Phase II Randomized Controlled Trial. BMC Surg. 2021, 1–10. [CrossRef]
- Carrano, F.M.; Maroli, A.; Carvello, M.; Foppa, C.; Sacchi, M.; Crippa, J.; Clerico, G.; De Lucia, F.; Coppola, E.; Ben David, N.; et al. Negative-Pressure Wound Therapy after Stoma Reversal in Colorectal Surgery: A Randomized Controlled Trial. BJS Open 2021, 5. [CrossRef]
- Uchino, M.; Hirose, K.; Bando, T.; Chohno, T.; Takesue, Y.; Ikeuchi, H. Randomized Controlled Trial of Prophylactic Negative-Pressure Wound Therapy at Ostomy Closure for the Prevention of Delayed Wound Healing and Surgical Site Infection in Patients with Ulcerative Colitis. Dig. Surg. 2016, 33, 449–454. [CrossRef]
- Kang, S. Il; Kim, S. The Effectiveness of Negative-Pressure Wound Therapy for Wound Healing after Stoma Reversal: A Randomized Control Study. Ann. Surg. Treat. Res. 2023, 105, 126–132. [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect. Control Hosp. Epidemiol. 1999, 20, 250–280. [CrossRef]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC Definitions of Nosocomial Surgical Site Infections, 1992: A Modification of CDC Definitions of Surgical Wound Infections. Infect. Control Hosp. Epidemiol. 1992, 13, 606–608.
- Yang, S.; Tang, G.; Zhang, Y.; Wei, Z.; Du, D. Meta-Analysis: Loop Ileostomy versus Colostomy to Prevent Complications of Anterior Resection for Rectal Cancer. Int. J. Colorectal Dis. 2024, 39, 68. [CrossRef]
- Alkaaki, A.; Al-Radi, O.O.; Khoja, A.; Alnawawi, A.; Alnawawi, A.; Maghrabi, A.; Altaf, A.; Aljiffry, M. Surgical Site Infection Following Abdominal Surgery: A Prospective Cohort Study. Can. J. Surg. 2019, 62, 111–117. [CrossRef]
- Lopez, M.P.J.; Melendres, M.F.A.; Maglangit, S.A.C.A.; Roxas, M.F.T.; Monroy, H.J.; Crisostomo, A.C. A Randomized Controlled Clinical Trial Comparing the Outcomes of Circumferential Subcuticular Wound Approximation ( CSWA ) with Conventional Wound Closure after Stoma Reversal. Tech. Coloproctol. 2015, 19, 461–468. [CrossRef]
- Shambhu, S.; Gordon, A.S.; Liu, Y.; Pany, M.; Padula, W. V; Pronovost, P.J.; Hsu, E. The Burden of Health Care Utilization, Cost, and Mortality Associated with Select Surgical Site Infections. Jt. Comm. J. Qual. Patient Saf. 2024, 50, 857–866. [CrossRef]
- Miller, C. The History of Negative Pressure Wound Therapy (NPWT): From “Lip Service” to the Modern Vacuum System. J. Am. Coll. Clin. Wound Spec. 2012, 4, 61–62. [CrossRef]
- Jeong, Y.S.; Cho, S.H.; Park, B.-S.; Son, G.M.; Kim, H.S. Role of Subcutaneous Closed Suction Drain in the Prevention of Incisional Surgical Site Infection after Loop Ileostomy Reversal with Purse-String Skin Closure: A Retrospective Observational Study. BMC Surg. 2024, 24, 252. [CrossRef]
- Hsieh, M.-C.; Kuo, L.-T.; Chi, C.-C.; Huang, W.-S.; Chin, C.-C. Pursestring Closure versus Conventional Primary Closure Following Stoma Reversal to Reduce Surgical Site Infection Rate: A Meta-Analysis of Randomized Controlled Trials. Dis. Colon Rectum 2015, 58, 808–815. [CrossRef]
- Zaver, V.; Kankanalu, P. Negative Pressure Wound Therapy. In; Treasure Island (FL), 2024.
- Zhu, Y.; Dai, L.; Luo, B.; Zhang, L. Meta-Analysis of Prophylactic Negative Pressure Wound Therapy for Surgical Site Infections (SSI) in Caesarean Section Surgery. Wideochirurgia i inne Tech. maloinwazyjne = Videosurgery other miniinvasive Tech. 2023, 18, 224–234. [CrossRef]
- Meyer, J.; Roos, E.; Justin, R.; Nicolas, D.; Buchs, C.; Meyer, J. Does Prophylactic Negative-Pressure Wound Therapy Prevent Surgical Site Infection After Laparotomy ? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. 2023, 1464–1474. [CrossRef]
- Yoon, S. Il; Bae, S.M.; Namgung, H.; Park, D.G. Clinical Trial on the Incidence of Wound Infection and Patient Satisfaction after Stoma Closure: Comparison of Two Skin Closure Techniques. Ann. Coloproctol. 2015, 31, 29–33. [CrossRef]
- Zhu, J.; Sun, Q.; Xu, W.; Geng, J.; Feng, Q.; Zhao, Z.; Li, S. Effect of Negative Pressure Wound Therapy on Surgical Site Infections Following Stoma Reversal in Colorectal Surgery: A Meta-Analysis. J. Investig. Surg. 2023. [CrossRef]
- Kisielewski, M.; Richter, K.; Pisarska-Adamczyk, M.; Wysocki, M.; Kłos, N.; Stefura, T.; Wojewoda, T.; Wysocki, W.M. Incisional Negative Pressure Wound Therapy Versus Primary Wound Suturing after Intestinal Ostomy Closure: A Systematic Review and Meta-Analysis. Adv. Wound Care 2024. [CrossRef]
- Norman, G.; Shi, C.; Goh, E.L.; Murphy, E.M.; Reid, A.; Chiverton, L.; Stankiewicz, M.; Dumville, J.C. Negative Pressure Wound Therapy for Surgical Wounds Healing by Primary Closure. Cochrane database Syst. Rev. 2022, 4, CD009261. [CrossRef]
- Strugala, V.; Martin, R. Meta-Analysis of Comparative Trials Evaluating a Prophylactic Single-Use Negative Pressure Wound Therapy System for the Prevention of Surgical Site Complications. Surg. Infect. (Larchmt). 2017, 18, 810–819. [CrossRef]
- Banaszkiewicz, Z.; Cierzniakowska, K.; Tojek, K. Surgical Site Infection among Patients after Colorectal Cancer Surgery. [CrossRef]
- Hajibandeh, S.; Hajibandeh, S.; Maw, A. Purse-String Skin Closure versus Linear Skin Closure in People Undergoing Stoma Reversal. Cochrane database Syst. Rev. 2024, 3, CD014763. [CrossRef]
- Carannante, F.; Costa, G.; Miacci, V.; Bianco, G.; Masciana, G.; Lauricella, S.; Caricato, M.; Capolupo, G.T. Comparison of Purse-String Technique vs Linear Suture for Skin Closure after Ileostomy Reversal. A Randomized Controlled Trial. Langenbeck’s Arch. Surg. 2024, 409, 141. [CrossRef]
- Jung, K.; Covington, S.; Sen, C.K.; Januszyk, M.; Kirsner, R.S.; Gurtner, G.C.; Shah, N.H. Rapid Identification of Slow Healing Wounds. Wound repair Regen. Off. Publ. Wound Heal. Soc. [and] Eur. Tissue Repair Soc. 2016, 24, 181–188. [CrossRef]
- Langer, V.; Bhandari, P.S.; Rajagopalan, S.; Mukherjee, M.K. Negative Pressure Wound Therapy as an Adjunct in Healing of Chronic Wounds. Int. Wound J. 2015, 12, 436–442. [CrossRef]
- Nicolazzo, D.; Rusin, E.; Varese, A.; Galassi, M. Negative Pressure Wound Therapy and Traditional Dressing: An Italian Health Technology Assessment Evaluation. Int. J. Environ. Res. Public Health 2023, 20. [CrossRef]
- Antonio, S. Effect of Negative Pressure Wound Therapy on Wound Healing. 2014, 51, 301–331. [CrossRef]
- Gupta, S.; Gabriel, A.; Lantis, J.; Téot, L. Clinical Recommendations and Practical Guide for Negative Pressure Wound Therapy with Instillation. Int. Wound J. 2016, 13, 159–174. [CrossRef]
- Doctoroff, L.; Herzig, S.J.; Israel, B.; Medical, D. HHS Public Access. 2021, 58, 778–784. [CrossRef]
- Eskandari, M.; Hossein, A.; Bahmani, A.; Ali, H.; Fard, M.; Karimzadeh, I. Evaluation of Factors That Influenced the Length of Hospital Stay Using Data Mining Techniques. BMC Med. Inform. Decis. Mak. 2022, 1–11. [CrossRef]
- Carrière, M.E.; Mokkink, L.B.; Tyack, Z.; Westerman, M.J.; Pijpe, A.; Pleat, J.; van de Kar, A.L.; Brown, J.; de Vet, H.C.W.; van Zuijlen, P.P.M. Development of the Patient Scale of the Patient and Observer Scar Assessment Scale (POSAS) 3.0: A Qualitative Study. Qual. life Res. an Int. J. Qual. life Asp. Treat. care Rehabil. 2023, 32, 583–592. [CrossRef]
- Duncan, J.A.L.; Bond, J.S.; Mason, T.; Ludlow, A.; Cridland, P.; O’Kane, S.; Ferguson, M.W.J. Visual Analogue Scale Scoring and Ranking: A Suitable and Sensitive Method for Assessing Scar Quality? Plast. Reconstr. Surg. 2006, 118, 909–918. [CrossRef]
| Study | Journal | Country | Study period | Number of arms | Number of participants | Lost in follow-up |
Follow-up (days) |
SSI Diagnosis Criteria | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | NPWT | NPD | |||||||||
| Uchino et al., 2016 [32] |
Digestive Surgery | Japan | Nov 2014 - Sep 2015 | 2 | 59 | 28 | 31 | 2 (1 per arm) | 28 | CDC | Duration of complete wound healing incisional SSI prevalence |
| Wierdak et al., 2020 [28] | Tech Coloproct | Poland | Jan 2016 - Dec 2018 | 2 | 71 | 35 | 36 | 4 (1 from non-pressure dressing group and 3 from in NPWT group) | 30 | CDC+ECDC | Healing complications, incidence of SSI, LOS, time to complete wound healing |
| Kojima et al., 2021 [30] | BMC Surgery | Japan | March 2018- March 2019 | 3 A: NPD B:NPWT for 7d, C:NPWT for 14d |
30 | 20 | 10 | Not specified | 1,3,7,10,14 | Presence of pus in the wound | Rate of wound reduction, SSI incidence, wound size healing time, and complication rate (excluding SSI) |
| Carrano et al., 2021 [31] | BJS Open | Italy | June 2019 -January 2021 | 2 | 98 | 50 (finally analyzed 49) | 48 (finally analyzed 45) | 4 (3 from the non-pressure dressing group and 1 from the NPWT group) | 30 | CDC | Rate of SSIs and other wound complication SSIs, postoperative wound pain, rate of wound healing after 30 days, wound aesthetic satisfaction |
| Kang et al., 2023 [33] | Ann Surg Treat Res | Korea | June 2019 - May 2021 | 2 | 34 | 18 (finally analyzed 16) | 18 | 2 from the non-pressure dressing group | twice a week until complete wound healing | CDC | Complete wound-healing period, SSI rate, LOS, total cost, and the patient and observer scar assessment |
| Tiang et al., 2024 [27] | ANZ Journal of Surgery | Australia | June 2018 -December 2021 | 2 | 40 | 20 (finally analyzed 19) | 20 (finally analyzed 19) | 1 per arm |
7, 14, 42 | Not specified | Complete wound healing at day 42, patient-reported wound cosmesis, SSI rate |
| Study | Age (years) | BMI (kg/m2) | Sex (F/M) |
Type of stoma |
Stoma indication |
Stoma closure technique | Perioperative antibiotics | |||
|---|---|---|---|---|---|---|---|---|---|---|
| NPWT group | NPD group | NPWT group | NPD group | NPWT group | NPD group | |||||
| Uchino et al., 2016 [32] | 48.1±14. 9 |
40.4±15. 9 |
19.8±4.3 | 19.7±3.8 | 11/17 | 8/23 | ileostomy | Ulcerative colitis, with a 2-stage procedure of after restorative proctocolectomy with ileal pouch anal anastomosis | PSC | second-generation cephalosporin (20 mg/kg) |
| Wierdak et al., 2020 [28] |
61.6±11.3 |
62.4±11.3 |
26.2±4.5 |
26.2±4.3 |
11/24 | 16/20 | ileostomy | Colorectal cancer |
LC | second-generation cephalosporin (20 mg/kg) |
| Kojima et al. 2021 [30] | 66±10 | 64.5 ±9.81 | 20.96±2.3 9 |
22.72±3.95 | 8/12 | 4/6 | ileostomy | Not specified | PSC | Not specified |
| Carrano et al., 2021 [31] | 56.32±12.92 | 55.08±16.25 | 23.81±3.38 | 23.45±3.66 | 15/35 | 17/31 | NPWT group: 41 ileostomy, 9 colostomy NPD group: 38 ileostomy, 10 colostomy |
NPWT group: Malignant disease 28/ Benign disease 22 Non-pressure dressing group: Malignant disease 26/ Benign disease 22 |
PSC | Cefazolin (20 mg/kg) |
| Kang et al., 2023 [33] | 65.75±15.85 | 61.5±12.97 | 23.45±21.27 | 25.35±14.56 | 9/9 | 8/8 | NPWT group: 10 ileostomy, 8 colostomy NPD group: 12 ileostomy, 4 colostomy |
Not specified | PSC | 2nd generation cephalosporin |
| Tiang et al., 2024 [27] | 55.67±17.1 | 62±15.1 | 27.62±6.21 | 26.12±4.50 | equal number of males and females in each group | ileostomy | NPWT group: Malignant disease 11, Benign disease 9Non-pressure dressing group: Malignant disease 14, Benign disease 6 |
PSC | cefazolin and metronidazole | |
| Study | NPWT group (Intervention) | Non-pressure dressing group (Comparator) | |||
|---|---|---|---|---|---|
| Type | Pressure settings | Details and duration | Type of dressing | Application protocol | |
| Uchino et al., 2016 [32] | PICO Single Use Negative Pressure Wound Therapy System (Smith and Nephew Healthcare, Hull, UK) |
–80 ± 20 mm Hg | 24 h after surgery, continued for 2 weeks, with changes every 3–4 days | simple adhesive plaster |
Not specified |
| Wierdak et al., 2020 [28] | NANOVA negative-pressure dressing | Not specified | Removal of the NANOVA dressing at 72 h, placement of Steri-Strips and a standard sterile dressing, which was then changed every 24 h until the removal of sutures | sterile wound dressing | 1st dressing change at 48 h, and thereafter every 24 h until the removal of sutures |
| Kojima et al., 2021 [30] | PICO Single Use Negative Pressure Wound Therapy System (Smith and Nephew Healthcare, Hull, UK) |
–80 mm Hg | Insertion of foam and PREVENA system on POD 1 day. On POD 3, removal of dressing and foam, washing of the wound and application of NPWT without inserting a foam piece. Continuation up to POD 7/POD 14 and thereafter daily gauze changes until epithelialization | simple gauze | Removal on POD 1 daily washing of the wound and gauze changes until epithelialization |
| Carrano et al., 2021 [31] | PICO Single Use Negative Pressure Wound Therapy System (Smith and Nephew Healthcare, Hull, UK) |
–80 ± 20 mm Hg | Direct application on the wound, maintenance for 7 days | iodoform gauze | Packing with iodoform gauze for 48 h, thereafter no packing |
| Kang et al., 2023 [33] | PICO Single Use Negative Pressure Wound Therapy System (Smith and Nephew Healthcare, Hull, UK) |
Not specified | Changes twice a week | Simple transparent waterproof dressing (Allevin, Smith & Nephew Health Care) | Daily changes |
| Tiang et al., 2024 [27] |
SNaP™ (Smart Negative Pressure) wound care system (Spiracur, Inc., Sunnyvale, CA), | -125 mmHg | A gauze interface wick dressing, cut into a 2.5 cm width strip, with a length 4 times the depth of the wound, was placed in the wound bed, followed by a hydrocolloid dressing connected to 60 mL cartridge. First change of the NPWT system at 3rd post-operative day, complete removal at 7th day | simple, non-woven, absorbent cotton dressing | Daily changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





