Introduction
Schistosomiasis is a snail-borne acute and chronic parasitic disease that is caused by trematode blood flukes of the genus Schistosoma [
1,
2]. Globally, it is one of the World Health Organization’s (WHO) Neglected Tropical Diseases (NTDs) that has increasingly drawn attention of public health experts over the past decade and a half. Transmission has been reported from 78 countries, the majority of which are classified as low- or middle- income countries [
3]. An estimated 207 million people in 74 countries are infected with the bulk of the global prevalence (90%) occurring in sub-Saharan Africa [
2,
3,
4,
5]. There are 2 major forms of schistosomiasis—intestinal (due to
Schistosoma mansoni and
S. japonicum) and urogenital (predominantly due to
S. haematobium). Both the intestinal form (caused by S.
mansoni) and urogenital form (caused by
S. haematobium) are known to occur in Nigeria, which has the largest number of people in the world in need of treatment for schistosomiasis (> 25 million) [
5] and the fourth-largest number of children in need of treatment of STHs (> 48 million) [
6].
Common signs and symptoms of urogenital S. haematobium include a swollen belly, blood in the urine, stunted growth, cognitive impairment in children and infertility among adults of childbearing age. Advanced disease may sometimes be present with fibrosis of the bladder and ureter, kidney damage and bladder cancer. People are often infected during routine agricultural, domestic, occupational (e.g., car washing, sand harvesting, fishing), and recreational activities, which expose them to contaminated waters.
On the other hand, helminths transmitted by contact with soil are known as Soil Transmitted Helminths (STH) or intestinal parasites. They are the most common infections worldwide and affect the poorest and most vulnerable populations. The causative agents are
Ascaris lumbricoides,
Trichuris trichiura, and hookworms. According to the WHO, approximately 1.5 billion individuals worldwide are believed to be infected with at least one species of STHs, leading to the loss of approximately 5 million disability-adjusted life years (DALYs) [
7,
8]. Moreover, severe morbidity associated with STHs affects around 300 million people, resulting in an estimated annual death toll ranging from 12,000 to 135,000 [
9].
The cornerstone of current schistosomiasis and STH control is preventive chemotherapy (PC) with praziquantel and Albendazole/Mebendazole for schistosomiasis and STH respectively, targeted towards school-age children. The frequency of treatment is determined by the disease endemicity within a subset of surveyed schools, which are classified using parasitological prevalence and intensity of infections [
6,
10,
11].
Prevalence for schistosomiasis is classified as Low (1-<10%), Moderate (10-<50%) and High (>50%) while that of STH is classified as Low (1-<20%), Moderate (20-<50%) and High (>50%). All classification for schistosomiasis were recommended to receive Mass Drug Administration (MDA) either annually or every other year while in the case of STH, classes with high, moderate and low were recommended for twice per annual, annual and no treatment respectively
Problem Statement
Based on the prevalence results in the 2008-2015 survey (
Supplemental Table S1), LGAs (the administrative units for treatment decisions) were stratified to receive MDA for schistosomiasis and STH either annually or every other year between 2015 and 2022. The 15 endemic LGAs received Albendazole/Mebendazole for STH annually from 2015 to 2022 except in 2021 where only Ado Ekiti, Ilejemeje and Irepodun/Ifelodun received treatment. For schistosomiasis, Ado Ekiti, Ekiti West and Ise/Orun, Ekiti South West and Gbonyi LGAs were targeted for annual treatment with Praziquantel while others were targeted for every other year except Irepodun/Ifelodun that was not endemic. All targeted LGAs received treatment in 2015, 2017, 2019 and 2022. More detailed treatment histories are available in
Supplemental Table S2. Administratively, reported treatment coverage among school-age children was skeletal and sub optimal from 2010-2015 but improved generally to above 75% from 2016. Although coverage dropped in one round because of the shortage of Praziquantel and mebendazole tablets, all LGAs have achieved between 3 & 5 rounds of effective treatment
In view of the difficulty in meeting requirements, a more targeted approach based on better data would allow more efficient use of the limited resources available. This granular assessment presents the results of the 2008–2015 surveys along with the 2023 surveys and offers conclusions regarding the impact of MDA during those years and the potential to improve targeting overall outcomes in Ekiti States, Southwest Nigeria.
Ethical Clearance
The Ethical Approval was received from the Research Ethics Committee (Ekiti State Ministry of Health and Human Services, HREC) under approval number: MOH/EKHREC/EA/P/59 and all research was performed in accordance with the relevant guidelines and regulations.
Methodology
Study Site and Population
Ekiti State is in the South-western region of the country. It has a population of 3,480,006 (Male-1,774,803 and Female-1,705,203) as at 2023 when projected from the 2006 population census and covers a land area of 5,434 square kilometers. It has 16 Local Government Areas, 176 wards and lies between latitude 70 151 and 80 51 N and longitude 40 451 E. Ekiti has a population growth rate of 3.1% per annum.
Study Design: Site Selection and Sample Size
This comparative cross-sectional assessment was conducted in one selected site across each of the 166 out of the 177 wards of Ekiti State and results aggregated at LGA level to measure any changes in disease burden. It was conducted at least 6 months after the 2022 round of SCH+STH MDA. During the 2008-2015 SCH+STH baseline survey, 5 communities were randomly selected from each of the 16 LGAs and prevalence was based on the mean of the 5 sites. However, during this follow up survey in 2023, a more granular approach which involves an average of 10 communities per LGA with 1 community selected from each political ward was assessed. This was necessary to obtain more refined data due on heterogeneity in the distribution of schistosomiasis. The population for this study was drawn from school-age children attending selected primary schools in each of the 166 wards across the State. Selection of schools was purposive and was guided by previous knowledge of the areas where transmission is known, suspected or more likely near water bodies; lakes, streams, dams and irrigation areas, including sites where the 2014 baseline data were collected.
A total of 9,027 respondents were expected to be assessed across 177 schools in the 177 wards based on WHO guidelines on impact assessment which recommends 50-55 children per school [
11], inclusive of 10% non-response rate, but security issues and local belief prevented sampling at the 177 schools; we therefore surveyed 7,670 pupils across 166 schools. This surveyed population was far more than the 4,000 respondents across the 80 schools from the 2008-2015 survey. In each of the selected schools, 50 participants, age ranging from 5 to 14 years were systematically selected. Selection was stratified based on age. A two-stage cluster sampling was undertaken such that one school and 50 school-age children, aged 5–14 years were selected in each ward. The samples were collected between the hours of 10:00 am–2:00 pm. The sample collection process included participants whose parents/guardian provided written informed consent and who were willing to provide assent. Children with severe disease requiring urgent medical intervention, or residing in the area for less than 6 months, were also excluded.
Data Collection
Data was collected manually and stored electronically. The biodata form was used to collect information on the participants’ unique identifiers, name, year of birth and gender. The laboratory reporting form contained information on the participants unique identifier (ID) and the infection screening results. All forms were cross-checked to maintain quality control. The completed forms were digitized and stored in appropriate databases.
Data Processing and Analysis
To determine the prevalence/intensity at 95% confidence intervals (CIs), descriptive statistics such as percentages, frequencies etc were computed to estimate the prevalences.
Parasitological data were entered using Microsoft Excel and subsequently analyzed with statistical functions in MATLAB®, IBM SPSS Statistics software version 23 and Data tab accordingly.
The prevalence of infection was estimated as the number of those who test positive out of the total number of participants surveyed. The intensity of any of the parasite ova (
Ascaris lumbriciodes, Hookworm,
Tricuris tricuira,
S. mansoni and
S. haematobium infections were enumerated and expressed as eggs per gram (epg) and eggs/10mL of urine and categorized according to WHO- thresholds for the classification of individuals with helminth infections as shown in
Table 2.
Results
Demographic Data
The samples were collected from 7712 pupils within the age group of 5-14 years. However, only 7670 samples were analyzed as 42 respondents later changed their mind after submission of their samples and demanded that their samples be returned. Of the samples analysed, 3823 (49.8%) were males and 3847 (50.1%) were females. Ado Ekiti LGA has the highest number of participants with 658 School-Age Children (SAC) across the 13 wards while Emure recorded the lowest number of participants with 320 SAC across 9 out of 10 wards.
Table 1.
Demography of the studied population.
Table 1.
Demography of the studied population.
| S/N |
LGA |
No of wards where samples were collected |
No of wards where samples were NOT collected/returned |
No of Participants per LGA |
No of participants whose samples were returned |
| 1. |
Ado |
13 |
0 |
658 |
0 |
| 2. |
Efon |
9 |
1 |
416 |
0 |
| 3. |
Ekiti East |
12 |
0 |
618 |
0 |
| 4. |
Ekiti South West |
11 |
0 |
556 |
0 |
| 5. |
Ekiti West |
9 |
2 |
469 |
0 |
| 6. |
Emure |
9 |
0/1 |
320 |
9 |
| 7. |
Gbonyin |
9 |
1 |
447 |
0 |
| 8. |
Ido/Osi |
11 |
0 |
509 |
0 |
| 9. |
Ijero |
10 |
2 |
303 |
0 |
| 10. |
Ikere |
11 |
0 |
550 |
0 |
| 11. |
Ikole |
12 |
0 |
527 |
0 |
| 12. |
Ilejemeje |
10 |
0 |
546 |
0 |
| 13. |
Irepodun/Ifelodun |
10 |
1 |
396 |
0 |
| 14. |
Ise/Orun |
8 |
1/1 |
517 |
33 |
| 15. |
Moba |
11 |
0 |
420 |
0 |
| 16. |
Oye |
11 |
1 |
418 |
0 |
| Total |
166 |
9/2 |
7670 |
42 |
Prevalence of Schistosomiasis
58 (0.76%) out of 7670 pupils were infected with S. haematobium. No S. mansoni infection was recorded in any of the 7670 analyzed samples. In the 16 LGAs assessed, Ekiti West had the highest S. haematobium prevalence of 4.26%. Ise/Orun and Oye ranked 2nd and 3rd with a prevalence of 3.48% & 2.40% respectively. No prevalence was recorded in Efon, Ekiti-East, Ekiti South-west, Ido/Osi & Moba as no parasites were detected in any of the respondents.
Table 2.
Overall prevalence of Schistosomiasis.
Table 2.
Overall prevalence of Schistosomiasis.
| S/N |
LGA |
No. of respondents |
Number positive |
Prevalence |
| 1 |
Ado |
658 |
4 |
0.6 |
| 2 |
Efon |
416 |
0 |
0.0 |
| 3 |
Ekiti East |
618 |
0 |
0.0 |
| 4 |
Ekiti South West |
556 |
0 |
0.0 |
| 5 |
Ekiti West |
469 |
20 |
4.3 |
| 6 |
Emure |
320 |
0 |
0.0 |
| 7 |
Gbonyin |
447 |
1 |
0.2 |
| 8 |
Ido/Osi |
509 |
0 |
0.0 |
| 9 |
Ijero |
303 |
1 |
0.3 |
| 10 |
Ikere |
550 |
8 |
1.5 |
| 11 |
Ikole |
527 |
2 |
0.4 |
| 12 |
Ilejemeje |
546 |
2 |
0.4 |
| 13 |
Irepodun/Ifelodun |
396 |
1 |
0.3 |
| 14 |
Ise/Orun |
517 |
18 |
3.5 |
| 15 |
Moba |
420 |
0 |
0.0 |
| 16 |
Oye |
418 |
1 |
0.2 |
| Total |
|
7670 |
58 |
0.8 |
Prevalence of Schistosomiasis by Sex
Of all the 7670 participants, 3828 (49.84%) were male and 3847 (50.16%) were female. Similar prevalence level of schistosomiasis was recorded among both male and female participants across all the LGAs with an overall prevalence of 0.76% and 0.75% respectively representing a difference of 0.01% as shown in
supplemental Table 3
Prevalence of Soil-Transmitted Helminths (STH)
Two hundred and ninety-six (3.86%) of the examined 7670 pupils had STHs as shown in
Table 6 and Table 7. When aggregated at LGA level, the prevalence was highest in Ekiti-West (10.45%), while Gbonyin & Isle/Orun had the prevalence of 9.62 & 8.9% respectively, making them 2nd and 3rd in ranking accordingly. Emure, Ikole and Irepodun Local Governments had the lowest prevalence of 0.31%, 0.38% & 1.01% respectively.
Table 3.
Overall prevalence of STH.
Table 3.
Overall prevalence of STH.
| S/N |
LGA |
No. of respondents |
Number positive |
Prevalence |
| 1 |
Ado |
658 |
9 |
1.4 |
| 2 |
Efon |
416 |
22 |
5.3 |
| 3 |
Ekiti East |
618 |
9 |
1.5 |
| 4 |
Ekiti South West |
556 |
15 |
2.7 |
| 5 |
Ekiti West |
469 |
49 |
10.4 |
| 6 |
Emure |
320 |
1 |
0.3 |
| 7 |
Gbonyin |
447 |
43 |
9.6 |
| 8 |
Ido/Osi |
509 |
16 |
3.1 |
| 9 |
Ijero |
303 |
7 |
2.3 |
| 10 |
Ikere |
550 |
26 |
4.7 |
| 11 |
Ikole |
527 |
2 |
0.4 |
| 12 |
Ilejemeje |
546 |
26 |
4.8 |
| 13 |
Irepodun/Ifelodun |
396 |
4 |
1.0 |
| 14 |
Ise/Orun |
517 |
46 |
8.9 |
| 15 |
Moba |
420 |
8 |
1.9 |
| 16 |
Oye |
418 |
13 |
3.1 |
|
Total |
|
7670 |
296 |
3.9 |
Distribution of Parasites by Species Across the LGA
While 58 (0.76%) out of 7670 pupils were infected with S. haematobium, no S. mansoni ova were detected in any of the 7670 samples analyzed. Any form of the STHs species: A Lumbricoides (AL), T. Trichuria (TT) and Hookworm (HW) were observed in 75 of the 166 wards assessed. A. lumbricoides was detected in 255 (3.2%) of all the samples analyzed with highest prevalence of 9.59% observed in Ekiti West. TT & HW were observed in 17 (0.22%) & 33 (0.42%) of examined stool samples respectively. The highest prevalence of TT (2.46%) was reported in Gbonyin and HW had the highest prevalence of 2.13% in Ise/Orun LGA.
Table 4.
Prevalence of parasite by species.
Table 4.
Prevalence of parasite by species.
| |
Schistosomiasis |
STH |
| LGA |
S. Hae |
S. Man |
Ascaris |
TT |
HW |
| Ado |
4 (0.61) |
0 (0.00) |
8 (1.22) |
0 (0.00) |
1 (0.15) |
| Efon |
0 (0.00) |
0 (0.00) |
22 (5.29) |
0 (0.00) |
0 (0.00) |
| Ekiti East |
0 (0.00) |
0 (0.00) |
9 (1.46) |
1 (0.16) |
0 (0.00) |
| Ekiti South West |
0 (0.00) |
0 (0.00) |
15 (2.70) |
0 (0.00) |
0 (0.00) |
| Ekiti West |
20 (4.24) |
0 (0.00) |
45 (9.59) |
1 (0.21) |
4 (0.85) |
| Emure |
0 (0.00) |
0 (0.00) |
1 (0.31) |
0 (0.00) |
0 (0.00) |
| Gbonyin |
1 (0.22) |
0 (0.00) |
29 (6.49) |
11 (2.46) |
6 (1.34) |
| Ido/Osi |
0 (0.00) |
0 (0.00) |
12 (2.36) |
0 (0.00) |
4 (0.79) |
| Ijero |
1 (0.33) |
0 (0.00) |
6 (1.98) |
0 (0.00) |
1 (0.33) |
| Ikere |
8 (1.45) |
0 (0.00) |
24 (4.36) |
0 (0.00) |
2 (0.36) |
| Ikole |
2 (0.38) |
0 (0.00) |
1 (0.19) |
1 (0.19) |
0 (0.00) |
| Ilejemeje |
2 (0.37) |
0 (0.00) |
23 (4.21) |
2 (0.37) |
1 (0.18) |
| Irepodun/Ifelodun |
1 (0.25) |
0 (0.00) |
4 (1.01) |
0 (0.00) |
0 (0.00) |
| Ise/Orun |
18 (3.48) |
0 (0.00) |
37 (7.16) |
0 (0.00) |
11 (2.13) |
| Moba |
0 (0.00) |
0 (0.00) |
8 (1.90) |
0 (0.00) |
0 (0.00) |
| Oye |
1 (0.24) |
0 (0.00) |
11 (2.63) |
1 (0.24) |
3 (0.72) |
| Total |
58 (0.76) |
0 (0.00) |
255 (3.32) |
17 (0.22) |
33 (0.43) |
| Total |
0.76 |
3.86 |
Infection Load Estimation
The overall mean intensity for Schistosoma haematobium was 28 eggs per 10mL of urine, 611 eggs per gram of stool for Ascaris lumbricoides, 137 eggs per gram of stool for Tricuris tricuira and 129 eggs per gram of stool for Hookworm in the assessed population. While the intensity was high for schistosomiasis in Irepodun/Ifelodun (119 eggs/10 mL of urine) and Ado (97 eggs/10 mls of urine), it was low in all the other LGAs as they all recorded average eggs of less than 50/10mls of urine. All the endemic LGAs recorded light intensity for all the species of STH except Irepodu/Ifelodun that recorded a moderate intensity for Ascaris lumbricoides. Of all the 7670 participants, 3828 (49.84%) are male and 3847 (50.16%) are female. The prevalences of STH among the male and female participants are almost the same. A slight difference of 0.18 was computed.
Table 5.
Parasite Intensity.
Table 5.
Parasite Intensity.
| LGA |
Average Intensity of Infection Load |
| S. haematobium |
Intensity |
A. lumbricoides |
Intensity |
T. tricuira |
Intensity |
Hookworm |
Intensity |
| Ado |
78 |
High |
213 |
Light |
0 |
None |
24 |
Light |
| Efon |
0 |
None |
862 |
Light |
0 |
None |
0 |
None |
| Ekiti East |
0 |
None |
187 |
Light |
408 |
Light |
0 |
None |
| Ekiti South West |
0 |
None |
294 |
Light |
0 |
None |
0 |
None |
| Ekiti West |
14 |
Low |
751 |
Light |
336 |
Light |
24 |
Light |
| Emure |
0 |
None |
4080 |
Light |
0 |
None |
0 |
None |
| Gbonyin |
1 |
Low |
778 |
Light |
111 |
Light |
56 |
Light |
| Ido/Osi |
0 |
None |
170 |
Light |
0 |
None |
30 |
Light |
| Ijero |
16 |
Low |
24 |
Light |
0 |
None |
24 |
Light |
| Ikere |
24 |
Low |
911 |
Light |
0 |
None |
36 |
Light |
| Ikole |
2 |
Low |
264 |
Light |
264 |
Light |
0 |
None |
| Ilejemeje |
1 |
Low |
279 |
Light |
36 |
Light |
24 |
Light |
| Irepodun/Ifelodun |
119 |
High |
5886 |
Moderate |
0 |
None |
0 |
None |
| Ise/Orun |
39 |
Low |
322 |
Light |
0 |
None |
244 |
Light |
| Moba |
0 |
None |
78 |
Light |
0 |
None |
0 |
None |
| Oye |
1 |
Low |
168 |
Light |
24 |
Light |
288 |
Light |
| Total (State) |
28 |
Low |
611 |
Light |
137 |
Light |
129 |
Light |
Table 6.
Prevalence of STH by sex.
Table 6.
Prevalence of STH by sex.
| S/N |
LGA |
No. of Participants (Positive) |
No. of Male (Positive) |
No. of Female (Positive) |
Prevalence of STH among Male |
Prevalence of STH among Female |
Prevalence of STH among the participants |
| 1. |
Ado |
658 (9) |
324 (2) |
334 (7) |
0.62 |
2.10 |
1.37 |
| 2. |
Efon |
416 (22) |
199 (11) |
217 (11) |
5.53 |
5.07 |
5.29 |
| 3. |
Ekiti East |
618 (9) |
313 (6) |
305 (3) |
1.92 |
0.98 |
1.46 |
| 4. |
Ekiti South West |
556 (15) |
250 (4) |
306 (11) |
1.60 |
3.59 |
2.70 |
| 5. |
Ekiti West |
469 (49) |
220 (21) |
249 (28) |
9.55 |
11.24 |
10.45 |
| 6. |
Emure |
320 (1) |
161 (0) |
159 (1) |
0.00 |
0.63 |
0.31 |
| 7. |
Gbonyin |
447 (43) |
222 (24) |
225 (19) |
10.81 |
8.44 |
9.62 |
| 8. |
Ido/Osi |
509 (16) |
260 (8) |
249 (8) |
3.08 |
3.21 |
3.14 |
| 9. |
Ijero |
303 (7) |
169 (4) |
134 (3) |
2.37 |
2.24 |
2.31 |
| 10. |
Ikere |
550 (26) |
265 (13) |
285 (13) |
4.91 |
4.56 |
4.73 |
| 11. |
Ikole |
527 (2) |
261 (2) |
266 (0) |
0.77 |
0.00 |
0.38 |
| 12. |
Ilejemeje |
546 (26) |
258 (13) |
288 (13) |
5.04 |
4.51 |
4.76 |
| 13. |
Irepodun/Ifelodun |
396 (4) |
207 (1) |
189 (3]) |
0.48 |
1.59 |
1.01 |
| 14 |
Ise/Orun |
517 (46) |
291 (26) |
226 (20) |
8.93 |
8.85 |
8.90 |
| 15. |
Moba |
420 (8) |
213 (5) |
207 (3) |
2.35 |
1.45 |
1.90 |
| 16. |
Oye |
418 (13) |
210 (11) |
208 (2) |
5.24 |
0.96 |
3.11 |
| Total |
7670 (296) |
3823 (151) |
3847 (145) |
3.95 |
3.77 |
3.86 |
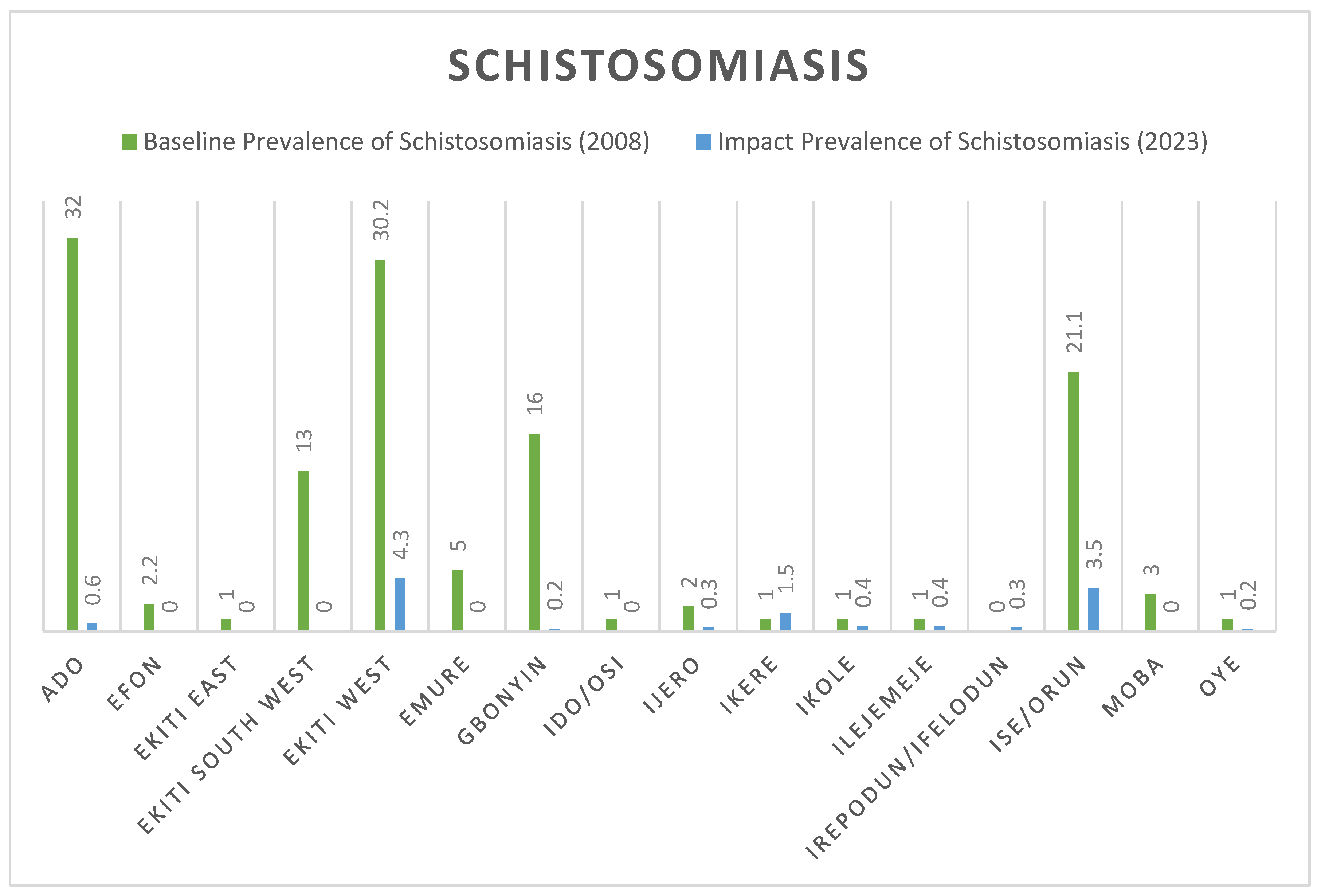
Measured Prevalence Compared with Baseline Prevalence
Figure 1 below show the comparative analysis between the baseline prevalence of SCH in 2008 and the impact prevalence in 2023. Ado Ekiti and Ekiti West demonstrates a clear decline from 32% and 30.2% prevalence level in 2008 to 0.6% and 4.6% respectively in 2023 for Schistosoma infection. A sharp decline to 0% was recorded in 6 LGAs, including: Efon, Ekiti East, Ekiti South West, Emure, Ido/Osi and Moba as shown in
Figure 1 below
A marginal increase was observed in the prevalence of schistosomiasis from 0% in 2008 to 0.3% in this 2023 impact survey in Irepodun/Ifelodun LGA. All the communities in this LGA demonstrated 0% prevalence except Igbemo with a prevalence of 2.9% leading to a cumulative prevalence of 0.3%. Similar increase in prevalence was observed in Ikere LGA where prevalence has increased from 0% to 0.5%. Again, one community (Ugele) with a prevalence of 13.1% was responsible for the jump in prevalence
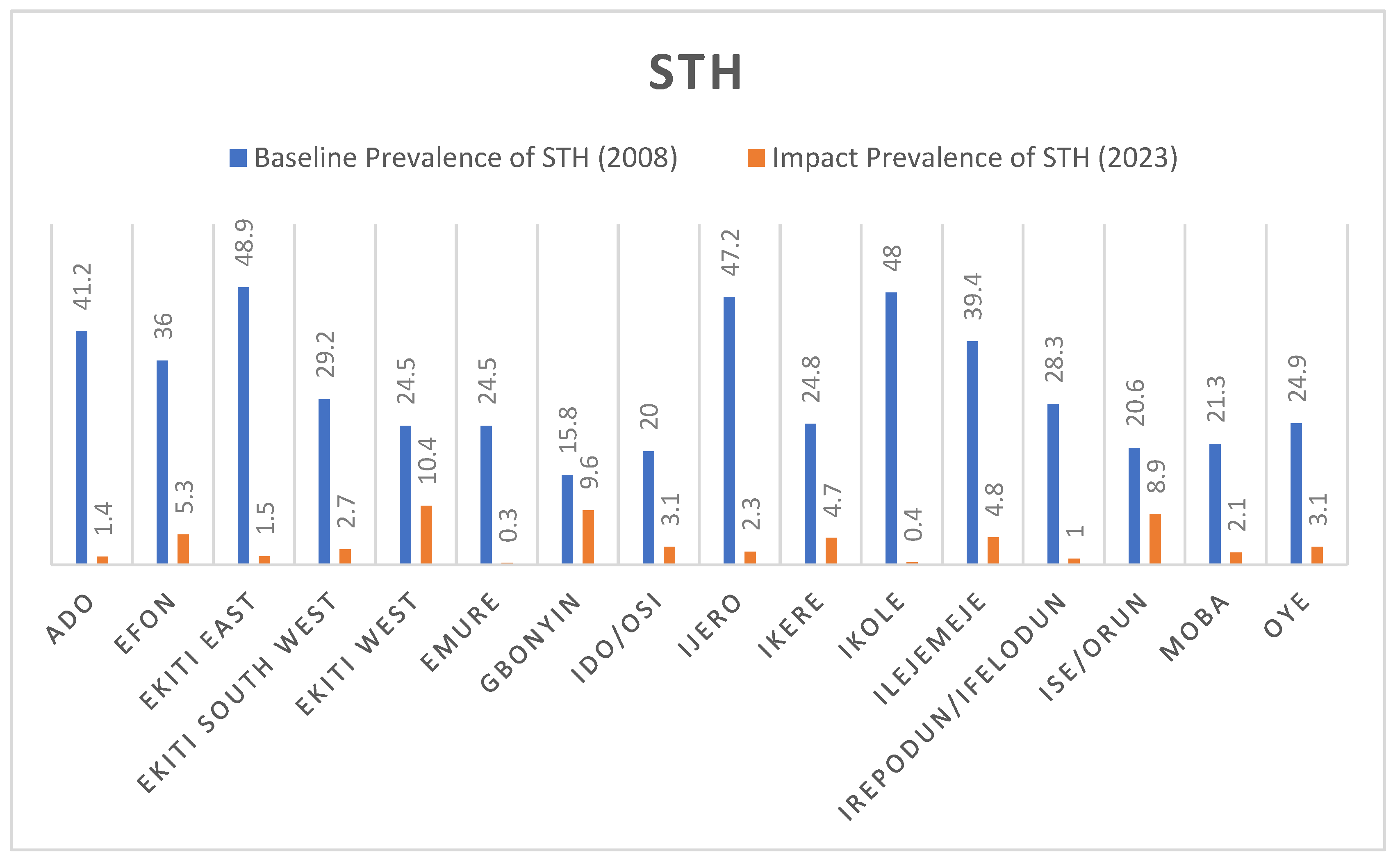
The plots in the
Figure 2 below show the comparative analysis between the baseline prevalence in 2015 and the impact prevalence in 2023 for STH. All the LGAs recorded a significant reduction in prevalence compared to the 2014 baseline. Ekiti East and Ikole LGAs with prevalence of 48.9% and 48% at baseline were reduced drastically to 1.5% and 0.4% respectively.
Discussion
We observed a significant decline in the prevalence and intensity of Schistosomiasis and STH among school-aged children after 3–5 years of effective interventions across LGAs and schools in Ekiti State.
The overall prevalence of schistosomiasis in Ekiti State (0.8%) is below the 1% WHO threshold required for treatment and qualifies the State to move into surveillance phase. However, this adjustment in treatment decision will be applied based on each current LGA specific prevalence. Other than Ekiti West (4.3%), Ise/Orun (3.5%) and Ikere (1.5%), all other LGAs now have prevalences below the 1% threshold required to proceed to surveillance phase and will no longer require any form of mass treatment. The 3 LGAs with prevalence greater than 1% will still require one round of mass treatment every 2-3 years as shown in (
Supplemental Figure S3) and as per the WHO decision tree for SCH treatment frequency.
The observed low prevalence indicates effectiveness of the deworming program in the States despite imperfect implementation. Ado-Ekiti (State Capital) with a baseline prevalence of 32% in 2014 is now reduced to 0.6%. Although 6 rounds of MDA were conducted, only 3 were classified as effective based on implementation metrics of 100% geographic coverage and at least 75% therapeutic coverages. This reduction in endemicity supports findings of [
15,
16] that Praziquantel administered at a single oral dose of 40 mg/kg should achieve a cure rate of 89.8%–91.7% and in individuals not cured, the drug should cause egg excretion to be reduced by 86.8%–91%.
A marginal increase in the prevalence of Schistosomiasis was observed in some LGAs Although no mass treatment was intended for these non-endemic LGAs at baseline, MDA was erroneously conducted across the LGAs in 2010. The impact of this one-off treatment may have worn-out over the past 13 years. The introduction of schistosomiasis into these LGAs may be attributed to their proximity to other previously endemic LGAs. With migration of people or animals across LGAs and from one local community, diseases are easily imported/transported across communities if there are no instituted surveillance system. Since the endemicity is still below the 1% treatment threshold, no mass administration of praziquantel is required for the LGAs but identified hotspot communities with prevalence greater than 1% should be targeted for mass treatment.
The prevalence by species is consistent with the results reported in 2015 [
17]. From this result, it is evident that
S. haematobium was more prevalent than
S. mansoni in Ekiti State.
The prevalence of STHs has significantly reduced across the 16 LGAs.
Ascaris lumbricoides is more prevalent than
Trichuris trichuira and hookworms. Comparative analysis of the computed and baseline prevalence shows that 6 LGAs now have prevalence below the 2% threshold and qualifies them to move into surveillance phase where mass treatment is no longer required as shown in
Supplemental Figure S4. Prevalence for the other 10 LGAs now lies between 2 and 10% and will continue with 1 round of MDA annually. When disaggregated to the granular level, Soil-transmitted helminths (STH) surveillance is required in 105 wards while 1 round of MDA is required in 61 wards annually.
Overall, the results indicate considerable heterogeneity in the prevalence of nematode infection within State and the LGAs. This illustrates the need for methods to improve the granularity of mapping. It has been difficult to maintain broad mass treatment on schedule across the wide areas previously designated as having high prevalence. If the foci where infections are truly a problem can be efficiently delineated, then more effective management should be possible at lower cost in financial and human resources. This should result in more effective programs. Methods to improve the efficiency of surveys through automated analysis of digital microscopy are currently being assessed to make such surveys feasible [
12].
Conclusion
Significant progress has been achieved in Ekiti State MDA program over the years. Continued SCH and STH MDAs in required areas could enable Ekiti State to reach its SCH and STH control and elimination goal. However, further effort is required including investments in vector control, clean water and sanitation (WASH) interventions to achieve elimination. Both human and material resources can be concentrated more effectively in areas that requires treatment and deploy monitoring and evaluation programs for disease surveillance to meet the 2030 elimination target.
Acknowledgements
Ekiti State Ministry of Health
Ekiti State University Teaching Hospital
Ekiti State University, College of Medicine
Federal Ministry of Health
Delft Global Initiative - Delft University of Technology
INSPiRED - Delft University of Technology/Leiden University Medical Centrum
MITOSATH
AiDx Medical BV
THE END FUND
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethical Approval was received from the Research Ethics Committee (Ekiti State Ministry of Health and Human Services, HREC) under approval number: MOH/EKHREC/EA/P/59 and all research was performed in accordance with the relevant guidelines and regulations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J, 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6: 411–425.
- Hotez PJ, Yamey G, 2009. The evolving scope of PLoS Neglected Tropical Diseases. PLoS Negl Trop Dis 3: e379. [CrossRef]
- WHO, 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: Report of a WHO expert committee. WHO Technical Report Series No. 912. Geneva: World Health Organization, 1–57.
- Anderson RM, May RM. Infectious diseases of humans. 1991. Oxford: Oxford University Press.
- Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, Dunne DW, et al. 1999. Adult resistance to schistosomiasis mansoni: age-dependence to reinfection remains constant in communities with diverse exposure patterns. Parasitology; 118:101–5. [CrossRef]
- WHO, 2006. World Health Organization. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization Press.
- World Health Organization (WHO). PCT databank—Soil-Transmitted Helminthiases. World Health Organization, Geneva, Switzerland (2016a). Available online: http://www.who.int/neglected_diseases/preventive_chemotherapy/sth/en/ (accessed on 23 May 2023).
- Hotez, P. J. et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 8, e2865 (2014). [CrossRef]
- World Health Organization (WHO). Soil-transmitted Helminth INFECTIONS—Fact sheet. World Health Organization, Geneva, Switzerland (2016b). Available online: http://www.who.int/mediacentre/factsheets/fs366/en/ (accessed on 23 May 2023).
- Tchuem Tchuente L-A, Stothard JR, Rollinson D, Reinhard-Rupp J. 2018. Precision mapping: An innovative tool and way forward to shrink the map, better target interventions, and accelerate toward the elimination of schistosomiasis. PLoS Negl Trop Dis 12(8): e0006563. [CrossRef]
- WHO-AFRO, 2014. Guide for Mapping Neglected Tropical Diseases Targeted by Preventive Chemotherapy in the African Region. World Health Organization-Regional Office for Africa.
- Makau-Barasa, L., Assefa, L., Aderogba, M., Bell, D., Solomon, J., Urude, R. O., Nebe, O. J., J, A. E., Damen, J. G., Popoola, S., Diehl, J. C., Vdovine, G. & Agbana, T. (2023). Performance evaluation of the AiDx multi-diagnostic automated microscope for the detection of schistosomiasis in Abuja, Nigeria. Sci Rep, 13, 14833. [CrossRef]
- Onasanya A, Bengtson M, Agbana T, Oladunni O, van Engelen J, Oladepo O, Diehl JC. Towards Inclusive Diagnostics for Neglected Tropical Diseases: User Experience of a New Digital Diagnostic Device in Low-Income Settings. Tropical Medicine and Infectious Disease. 2023; 8(3):176. [CrossRef]
- National Protocol for Integrated Epidemilogogical Mapping and Baseline Survey of Schistosomiasis and Soil Transmitted Helminths. Federal Ministry of Health, Nigeria (2013).
- Hailegebriel T, Nibret E, Munshea A. Efficacy of Praziquantel for the Treatment of Human Schistosomiasis in Ethiopia: A Systematic Review and Meta-Analysis. J Trop Med. 2021 Dec 20;2021:2625255. [CrossRef] [PubMed] [PubMed Central]
- Berhanu MS, Atnafie SA, Ali TE, Chekol AA, Kebede HB. Efficacy of Praziquantel Treatment and Schistosoma Mansoni Infection among Primary School Children in Kemisse Town, Northeast Ethiopia. Ethiop J Health Sci. 2022 May;32(3):631-640. [CrossRef] [PubMed] [PubMed Central]
- Report on Epidemiologibal Mapping of Schistosomiasis and Soil Transmitted Helminthiasisin 19 States and the FCT, Nigeria. May 2015.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).