1. Introduction
BODIPY dyes, or boron-dipyrromethene compounds, (IUPAC name 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) have shaped the landscape of fluorescent imaging and bioanalytical applications since their inception [
1,
2,
3]. The synthesis of the first BODIPY dye in 1968 by chemists Treibs and Kreuzer marked the beginning of an era characterized by their remarkable optical properties, including high fluorescence quantum yields and strong absorption in the visible and near-infrared spectra [
4]. These characteristics have rendered BODIPY dyes invaluable in biological research, particularly in fluorescence microscopy, cell imaging, and protein labeling [
5,
6,
7]. Over the years, innovative approaches to modify BODIPY structures have expanded their applications, allowing them to serve as effective probes for various biological processes, therapeutic agents, and biosensors. As a result, BODIPY dyes have gained significant attention for their potential in cancer therapies and diagnostic applications due to their tunable properties, photostability, and low toxicity, which are crucial for in vivo imaging and the monitoring of dynamic biological events. The present review will explore the historical development of BODIPY dyes, highlighting their journey from laboratory discovery to pivotal role in advancing biomedicine through their diverse applications in contemporary biological research.
The modification of BODIPY dyes has enhanced their versatility, enabling researchers to customize their properties for specific biological tasks, including sub-cellular imaging and targeted therapy [
7,
8,
9]. A significant breakthrough in this field is the introduction of aza-BODIPYs, which are formed by substituting the meso-carbon atom of the traditional BODIPY structure with a nitrogen atom (aza-N). This substitution markedly improves their photophysical characteristics.
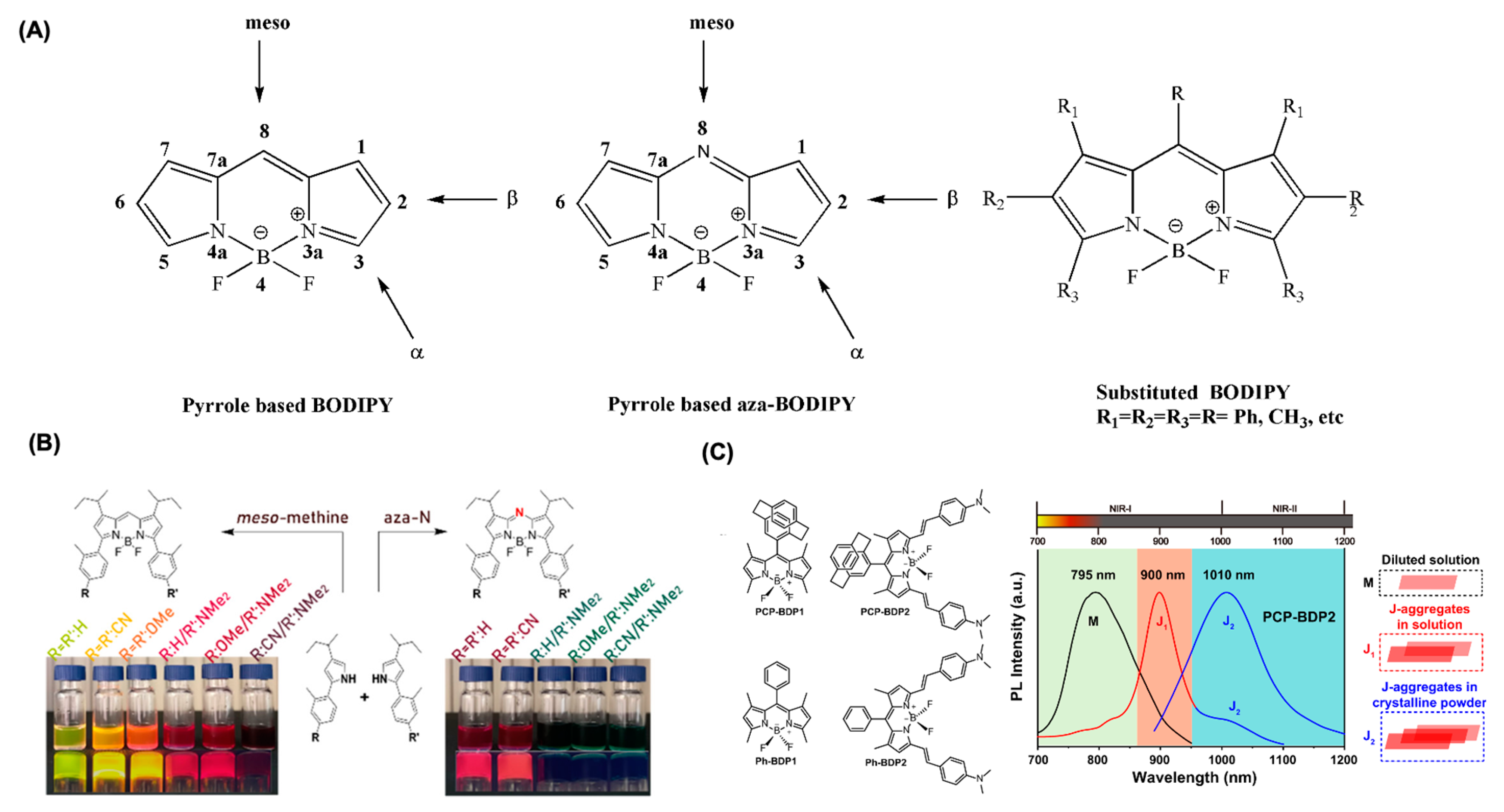
Figure 1A represents a general schematic for the structure of various BODIPY, Aza-BODIPY, and substituted BODIPY dyes. Unlike standard BODIPYs that primarily emit green light, aza-BODIPYs can absorb and emit light in the red to near-infrared spectrum (
Figure 1B) [
10]. Although these compounds generally exhibit lower emission quantum yields, they have an increased propensity for intersystem crossing (ISC) to triplet states, rendering them particularly effective for applications that depend on triplet state processes, such as photodynamic therapy [
11,
12,
13,
14].
Using fluorescent probes that operate within the NIR-II window presents several advantages for bioimaging, including minimized light scattering, enhanced tissue penetration, improved signal-to-background ratios, and better spatiotemporal resolution. BODIPY dyes are particularly advantageous due to their exceptional photostability, elevated quantum yield, narrow absorption profiles, straightforward functionalization, and low cytotoxicity. The integration of these traits into BODIPY probes that are functional in the NIR-II window holds tremendous promise for bioimaging applications (
Figure 1C). As Hu et al. note, pivotal strategies for achieving this goal include electron pumping, conjugation with semiconducting polymers, and the formation of J-aggregates [
16]. Each of these strategies, however, comes with its own set of advantages and challenges. The electron pumping approach utilizes an electron-withdrawing BODIPY core that can effectively incorporate electron-donating groups, thereby enhancing the tunability of the emission wavelength while maintaining photostability. The primary challenge with this method lies in reconciling these emission traits with the overall brightness of the fluorescence [
17,
18,
19,
20,
21]. Conversely, several research teams have investigated the incorporation of BODIPY units into semiconducting polymers, which facilitates the integration of multiple functions in a single system. However, this complexity often leads to limitations in clinical applicability and can reduce brightness [
22,
23,
24]. Similarly, the formation of J-aggregates takes advantage of the specific arrangement of stacked BODIPY molecules, offering substantial benefits such as minimal structural modifications and high fluorescence quantum yields which contribute to superior imaging signals [
15]. Nonetheless, this method faces difficulties with the stability of the aggregates and challenges linked to achieving in situ formation of NIR-II J-aggregates within biological contexts like tumors [
25,
26,
27]. The fluorescence of BODIPY molecules can be controlled by various pathways such as Photoinduced electron transfer (PET), Fo ̈rster resonance energy transfer (FRET), Intramolecular Charge Transfer (ICT) and by various other pathways [
28,
29].
BODIPY dyes have emerged as vital components in the development of small-molecule fluorescent probes, attributed to their remarkable photophysical properties. Unlike fluorescent proteins, which can be bulky and challenging to manipulate, BODIPY dyes are significantly smaller, facilitating their use in biological contexts without interfering with the functionality of target molecules. This unique advantage allows for more effective bioimaging and molecular interactions. Moreover, BODIPY dyes can undergo fine-tuning through chemical modifications to create various derivatives tailored for specific biomolecular interactions or for localization within distinct cellular compartments.
Recent advancements show promise in engineering BODIPY-based probes for targeted localization within cellular organelles such as mitochondria, lysosomes, the Golgi apparatus, lipid droplets, and the endoplasmic reticulum. These probes are designed with high selectivity and specificity, making them particularly suitable for exploring subcellular dynamics and interactions. For instance, probes intended for mitochondrial imaging are formulated to be positively charged, allowing them to navigate into the negatively charged organelle while minimizing background fluorescence, thus enhancing imaging accuracy. Innovations aimed at enhancing the water solubility and membrane permeability of BODIPY derivatives have expanded their applicability in live-cell imaging, overcoming previous limitations related to solubility.
In addition to their utility in imaging, BODIPY dyes show promising applications in therapeutic contexts, particularly in photodynamic therapies (PDT) where BODIPY dye act as photosensitizers that generate cytotoxic reactive oxygen species upon light activation, targeting specific cell types effectively [
30]. By extending the conjugation of BODIPY molecules, researchers have achieved superior photothermal conversion efficiencies exceeding 90%, which is essential for effective energy absorption during PTT [
31]. These attributes position BODIPYs as versatile agents in cancer therapies that utilize light-induced mechanisms to eradicate tumor cells. Recently BODIPY derivatives attached to sodium borocaptate (10B-BSH) for Boron neutron capture therapy for tumors.
BODIPY molecules typically exhibit low water solubility, which poses challenges for in vivo administration and limits their accumulation in solid tumors. Thankfully, advancements in nanotechnology and supramolecular chemistry have introduced strategies to enhance the delivery and tumor-targeting capabilities of BODIPY dyes through nanomedicine formulations [
32,
33]. This progress has established a foundation for ongoing research in cancer theragnostic, offering significant potential for clinical applications.
The present review offers a critical assessment of recent literature reports for the use of BODIPY derivatives for cellular imaging and for therapeutical applications. The use of various targeting conjugates on the backbone of BODIPY dyes allowed localization of the dyes inside tumor and cellular components, which allowed tracking of these cell features using various imaging modalities such as fluorescence microscopy, confocal microscopy, Raman and Photoacoustic imaging. We covered the features of BODIPY dyes as therapeutical drug for PDT, PTT, and BNCT based tumor ablation studies. With the wide application of nanoparticle formulations, the use of BODIPY based nanomedicines were also covered in the review article.
At last, this article offers fresh perspectives on BODIPY dyes, delving into their photophysical characteristics and potential clinical applications. It examines critical clinical factors, such as the regulation of related proteins and pertinent challenges. The review also highlights ongoing and future clinical trials. By providing a detailed exploration, this discussion seeks to enhance the audience’s understanding of the progress in utilizing BODIPY dyes for subcellular imaging and therapeutic innovations. Moreover, these insights emphasize the ongoing role of BODIPY dyes in advancing diagnostic and therapeutic strategies within the biomedical field.
2. Mechanisms of Intracellular Distribution of BODIPY Dyes
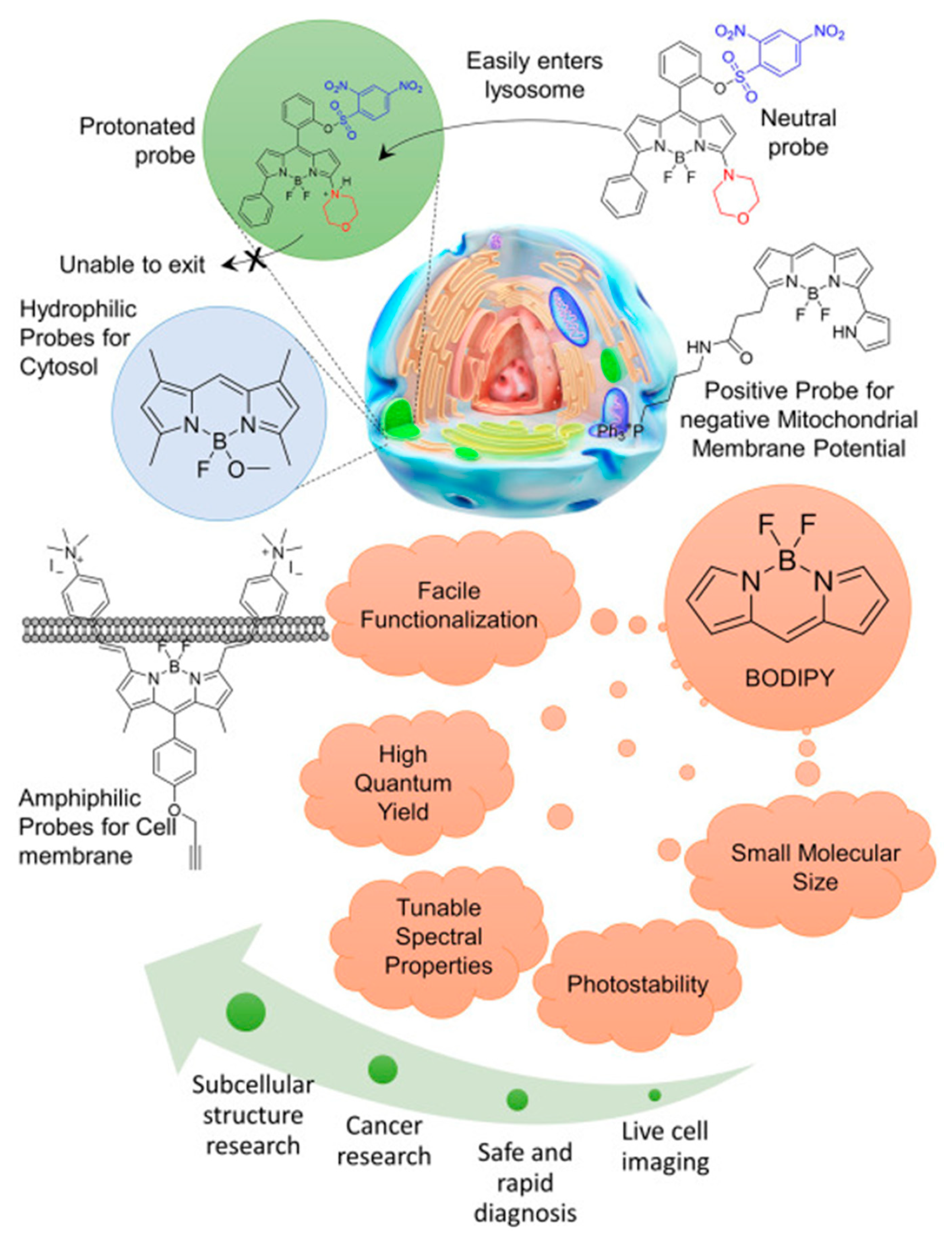
BODIPY compounds’ intracellular distribution plays a vital role in their applications in bioimaging and therapy. Their structural and physicochemical properties influence localization within cells, such as in the plasma membrane, cytoplasm, endoplasmic reticulum, mitochondria, lysosomes, and Golgi apparatus. For instance, unmodified, lipophilic BODIPY dyes integrate into lipid-rich membranes, making them ideal for staining the plasma membrane and endoplasmic reticulum, though they lack specificity for organelles. Functionalizing BODIPY dyes enables targeted subcellular localization; positively charged groups like PPh₃⁺ help target mitochondria due to its negative membrane potential. Lysosomal probes are designed with groups that protonate in acidic environments, ensuring retention. Similarly, amphiphilic modifications facilitate plasma membrane localization, while hydrophilic properties enhance cytoplasmic retention. BODIPY and its unique characteristics enable the visualization of intricate biological processes, including lipid metabolism, membrane dynamics, and protein localization (BODIPY Dye Series—Section 1.4 | Thermo Fisher Scientific - US, 2024). Understanding the intracellular distribution mechanisms of BODIPY dyes is essential for accurately employing these fluorescent probes in various research applications, from cell signaling to cancer biology [
5,
34]. The key mechanisms through which BODIPY dyes distribute intracellularly, is by any of the following pathways such as passive diffusion, endocytosis, and specific interactions with cellular components.
2.1. Passive Diffusion
One of the fundamental mechanisms of intracellular distribution for BODIPY dyes is passive diffusion through cellular membranes. The amphiphilic nature of many BODIPY dyes allows them to readily integrate into lipid bilayers. For instance, BODIPY FL-sphingomyelin analogs are designed to enter cellular membranes spontaneously, enabling the measurement of lipid trafficking and dynamics across various cellular compartments [
35]. This property allows BODIPY dyes to label lipid droplets and enriched regions within the plasma membrane, thus highlighting their utility in studying lipid storage and metabolism [
34]. Furthermore, the design of BODIPY dyes can influence their diffusion characteristics. For example, the addition of different fatty acid chains alters their hydrophobicity, affecting how they partition within the membrane and how readily they can diffuse into cells. Lower concentrations of BODIPY dyes may enhance diffusion efficiency, allowing for their widespread distribution in the cytoplasm and subsequent localization to organelles such as mitochondria [
36].
2.2. Endocytosis
Another critical process governing the intracellular distribution of BODIPY dyes is endocytosis, particularly the clathrin-mediated endocytosis and caveolar uptake mechanisms. Following internalization through these pathways, BODIPY-labeled sphingolipids can be tracked to various organelles, notably lysosomes and the endoplasmic reticulum [
37]. For example, BODIPY-sphingomyelin undergoes distinct trafficking pathways, initially localizing to the plasma membrane and later to the Golgi apparatus and lysosomal compartments. These advancements allow researchers to elucidate the roles of lipid signaling molecules in cellular processes such as differentiation and apoptosis, showcasing the dyes’ capabilities in studying lipid metabolism [
37,
38].
Moreover, the mechanism of endocytosis can vary significantly among different cell types, impacting how BODIPY dyes are taken up and utilized within the cell. For instance, in cell types with higher rates of endocytic activity, such as neural cells, BODIPY dyes might internalize more efficiently, enabling detailed imaging of endosomal pathways. Research indicates that such variability can lead to distinct spatial distributions of BODIPY dyes within cells, influencing the interpretation of intracellular imaging results.
2.3. Specific Interactions with Cellular Components
The interaction between BODIPY dyes and specific cellular components also plays a significant role in determining their intracellular distribution. Functionalization of BODIPY dyes allows for targeted localization within specific organelles or biomolecules. For example, BODIPY dyes can be functionalized to selectively bind to lipid droplets or certain proteins, which directs their accumulation to select sites within the cell [
39,
40]. This specificity is particularly useful when employing BODIPY dyes as indicators of cellular processes, such as monitoring changes in lipid droplet size and density in response to metabolic perturbations [
41,
42,
43].
Additionally, the chemical structure of BODIPY derivatives can affect their spectral properties and the dynamics of their interactions within the cellular environment. For instance, BODIPY dyes can display different fluorescence intensities based on their localization, which can be used to analyze changes in microviscosity in distinct cellular compartments [
36,
44]. Such sensing properties are vital for understanding the physiological and pathological states of cells by offering insights into the biophysical properties of organelles like lipid droplets and membranes [
45,
46,
47].
3. Intracellular Distribution of BODIPY Dyes
BODIPY dyes exhibit versatile partitioning properties, allowing them to distribute within various cellular compartments, including the plasma membrane, cytoplasm, endoplasmic reticulum, mitochondria, lysosomes, and the Golgi apparatus as shown in
Figure 2. This distribution is influenced primarily by the dye’s hydrophilic-hydrophobic balance, charge, and molecular size. For instance, unmodified lipophilic BODIPYs preferentially
integrate into lipid-rich membranes, making them ideal for staining the plasma membrane and endoplasmic reticulum. However, due to their ability to diffuse across membranes, these dyes may stain the cytoplasm without offering specificity for intracellular organelles. Advanced targeting strategies have been developed to enhance the subcellular localization of BODIPY dyes. When aimed at mitochondria, the dye is often functionalized with positively charged groups, such as triphenyl phosphonium (TPP) and quaternary ammonium groups, to exploit the negatively charged mitochondrial membrane potential. Conversely, targeting lysosomes involves using probes that remain neutral at the cytosolic pH, becoming protonated and effectively trapped in the acidic environment of the lysosomes after entry. Similarly, to interact specifically with the plasma membrane, BODIPY probes are designed to be amphiphilic, allowing them to integrate smoothly with the amphiphilic constituents of the membrane. Additionally, increasing the hydrophilicity of these probes can enhance their retention within the cytoplasm, thus improving imaging and biological analysis capabilities. Collectively, these functionalization techniques are crucial for optimizing the bioimaging capabilities and overall application of BODIPY dyes in cell biology research.
4. BODIPY for Bioimaging Applications
4.1 BODIPY Dyes for Membrane Imaging
Design and synthesize of plasma membrane targeted BODIPY dyes are of interest these days due to their less invasive detection methods and they are not affected by most of the biological events due to their versatile and robust designs [
5,
48,
49,
50]. For this the backbone of BODIPY can be easily modified to make the lipophilic balance for the penetration into the plasma membrane.
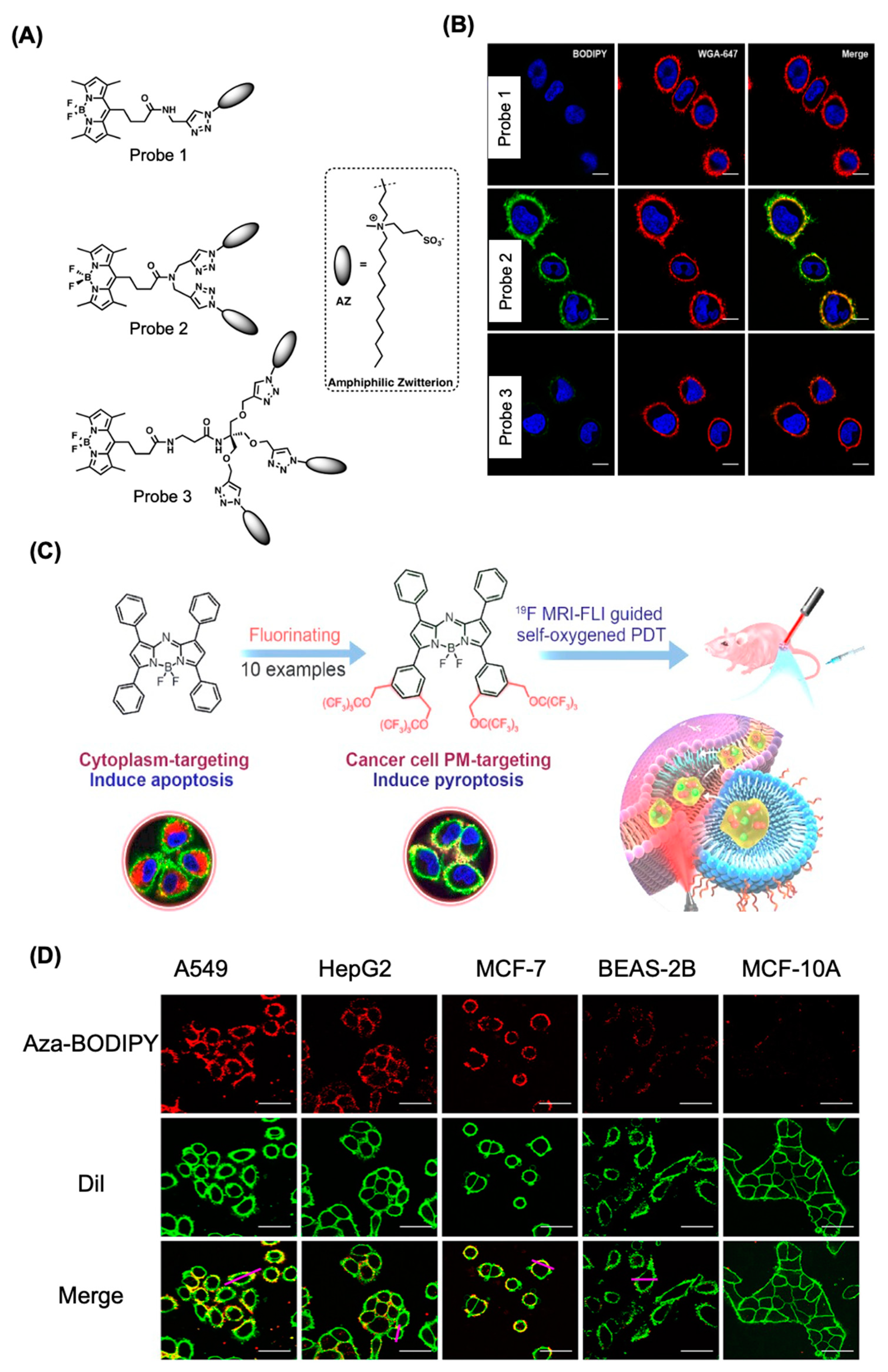
In the pursuit of improving bioimaging techniques, Collot et al. developed a series of BODIPY-based probes specifically designed for efficient staining of plasma membranes [
Figure 3A] [
50]. This novel probe was created by functionalizing a green-emitting BODIPY fluorophore with amphiphilic, zwitterionic, and aliphatic anchors, leading to the generation of three variants possessing one to three anchors. Among these, probe (2) exhibited the highest quantum yield of 0.92, indicating its exceptional fluorescence properties. Probe (2) effectively targets the plasma membrane, existing as nonfluorescent soluble aggregates in aqueous environments that disassemble upon contact with the membrane. This mechanism results in bright fluorescence, yielding high signal-to-noise ratios and enhanced targeting capabilities compared to traditional fluorescently labeled lectins. In various bioimaging applications, including studies on KB cells, HeLa cells, and U87 glioblastoma spheroids, this probe demonstrated robust fluorescence intensity with specific absorption and emission wavelengths near 500 nm [
Figure 3B]. The imaging techniques utilized were confocal microscopy and two-photon excitation, with probe (2) showcasing excellent cellular uptake efficiency and significant overlap with utilized plasma membrane markers. The fluorescence activation acts as a direct response to interactions with the plasma membrane, enabling precise monitoring of cellular components. Importantly, cytotoxicity assessments indicated low toxicity levels, while ex vivo and in vivo studies suggested favorable biodistribution and targetability.
In one of the studies Polita et al. fabricated a viscosity-sensitive s-indacene (BODIPY) probe for plasma membrane viscosity imaging [
49]. Fluorescence lifetime imaging results showed that these probes can stain A549 live lung cancer cells within 5 minutes and the slow internalization allowed to monitor the cytoplasm viscosity. Li et al. fabricated an aza-BODIPY system as a bioimaging agent for the plasma membrane of A549 cells (
Figure 3C) [
51]. To improve the targeting and internalization the BODIPY core is conjugated with fluorine atoms, and this probe is used for both fluorescence and MRI based bioimaging applications (
Figure 3D). The fluorine-19 magnetic resonance imaging allowed detection of oxygen delivery and oxygen partial pressure during PDT studies. Through comparative physicochemical and biological analyses, supported by molecular dynamics simulations, it has been shown that fluorinated BODIPY can act like photosensitizers specifically target and eliminate cancer cells. This occurs through the oxidation of plasma membrane phospholipids by singlet oxygen (¹O₂), triggering pyroptosis. This mechanism effectively inhibits tumor progression when used in self-oxygenating ¹⁹F MRI-FLI-guided photodynamic therapy in mice. The findings highlight a fluorination strategy to develop high-performance, multifunctional materials that precisely target cancer cell membranes, paving the way for advancements in cancer treatment and related applications.
Xiong et al. studied pyrazolone-functionalized probes for bioimaging and therapy, focusing on Nile blue, rhodamine, and BODIPY derivatives [
52]. The BODIPY derivative showed strong potential for subcellular imaging and therapeutic use. It was designed to target cell membranes, with the pyrazolone moiety enabling stable interactions with
membrane proteins through noncovalent bonds with amino acids like arginine. The pyrazolone group’s ability to tautomerize into its enolic form ensured prolonged fluorescence signal retention, making the derivative effective for long-term imaging. The BODIPY-pyrazolone probe exhibits strong fluorescence (excitation at 488 nm, emission 490–540 nm), enabling high-resolution, stable, and photobleaching-resistant imaging of cell membranes for over 96 hours. Its selective binding to membrane proteins, without cell penetration, makes it suitable for long-term monitoring, targeted delivery, and diagnostic applications, particularly in cancer. Among various scaffolds studied, the BODIPY derivative stands out for its robust photophysical properties and potential in subcellular imaging and membrane-targeted therapies, enriching the toolkit of BODIPY-based bioimaging agents.
BODIPY dyes have emerged as indispensable tools in the field of membrane imaging, providing researchers with robust and versatile options for studying cellular membranes. Their exceptional photophysical properties, combined with their ability to be fine-tuned spectrally, make them ideal for a variety of applications in fluorescence microscopy. While challenges such as fluorescence quenching and dye synthesis complexities exist, ongoing advancements in dye development and imaging techniques continue to enhance the efficacy of BODIPY dyes in biological research. As a result, they are likely to remain a fundamental component of molecular imaging strategies aimed at unraveling the intricate dynamics of biological membranes.
4.2. BODIPY Dyes for Cytosol Imaging
BODIPY-based molecular rotors have gained prominence for measuring intracellular microviscosity, a critical parameter influencing cellular behavior and the diffusion of biomolecules. The technique leverages the property that BODIPY dyes respond to varying viscosities in their environment; the intramolecular rotation of the dye is hindered in a highly viscous medium, resulting in increased fluorescence intensity and a longer fluorescence lifetime [
36,
53]. Studies have shown that different BODIPY dyes retain stability in aqueous solutions, allowing for effective monitoring of microviscosity in the cytosol of live cells, including aggressive cancer cell lines such as MDA-MB-231 [
36]. This capability has profound implications for understanding cellular dynamics and disease mechanisms.
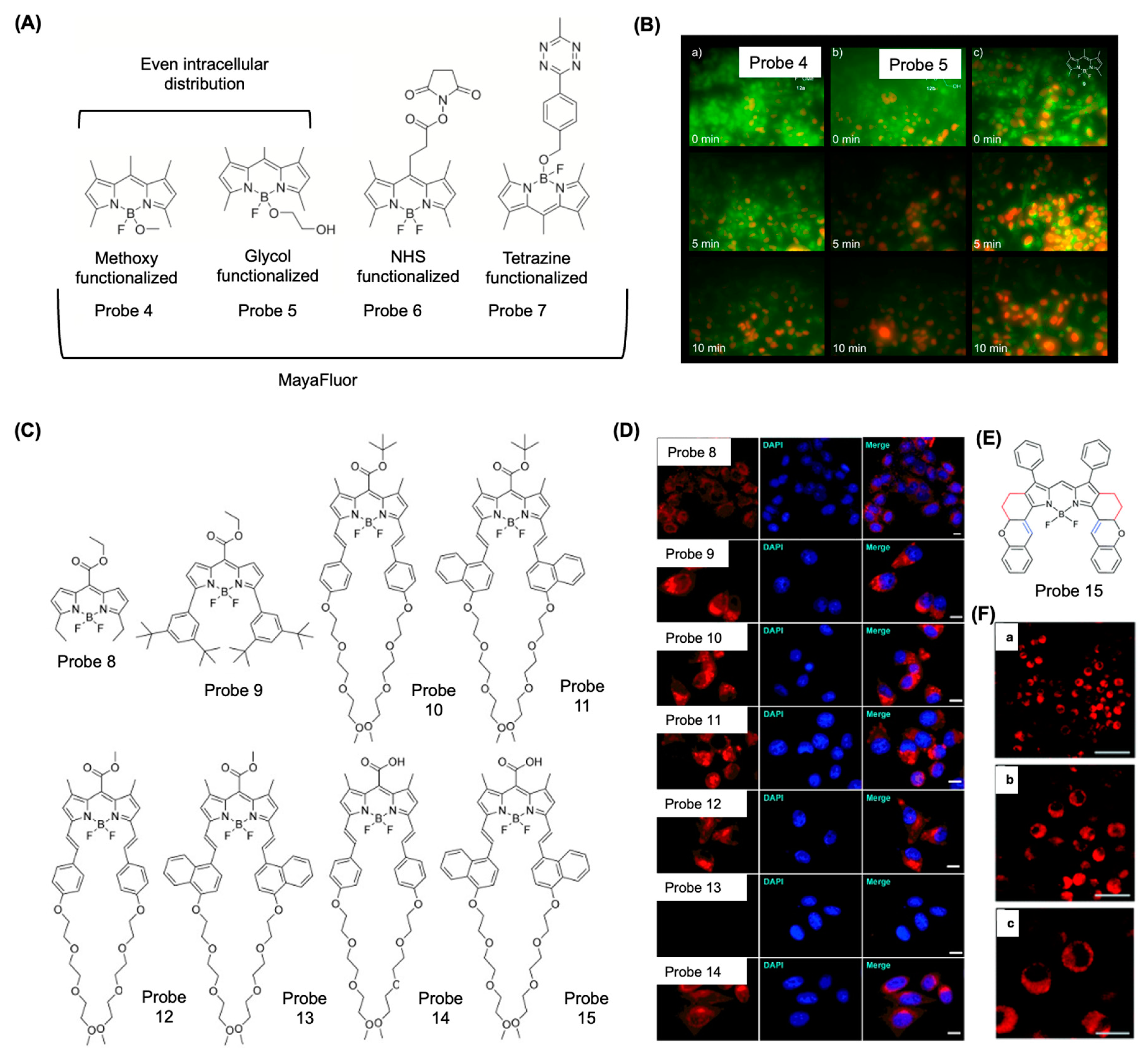
In 2014, Courtis et al. developed monoalkoxy-BODIPY probes (probe 4) known as MayaFluors with improved bio-imaging features (
Figure 4A) [
54]. The synthesis used a simple two-step process, starting with difluoro-BODIPY activation to form a BODIPY-OTf intermediate, followed by alcohol addition. The structure retains one fluorine atom, allowing property fine-tuning and modifications like methoxy (probe 4), glycol (probe 5), NHS-ester (probe 6), and tetrazine (probe 7) to enhance functionality and targetability. MayaFluors (the common name for these dyes) demonstrated an efficient membrane permeability, minimal nonspecific binding, and superior performance compared to other strategies. Tested on MD-MBA-231 and MCF-7 cells, they showed excellent uptake with low background staining (
Figure 4B). Key fluorescence properties include absorption at 490 nm, emission at 512 nm, quantum yields of 0.3–0.5, and dye stability. Colocalization and microscopy studies confirmed their specificity, while FRET-based fluorescence activation via tetrazine-tagged probes makes them ideal for cytosolic targeting.
A series of alkene substituted BODIPY derivatives (probes 8-15) were fabricated by Ni et al. (
Figure 4C) [
55]. The substitution of backbones using methyl ester and carboxylic acid groups improved the solubility, whereas the alkene substitution improved their absorbance and emission to far red region (absorption maxima ranging from 627 nm to 682 nm, emission maxima ranging from 642 nm to 729 nm). The derivatives showed enhanced fluorescence quantum yields from 6 to 36% and showed high sensitivity and specificity
towards Hela cells using fluorescence and confocal microscopy imaging (
Figure 4D). Jiang et al. fabricated a NIR BODIPY probe bearing 3,4,4a-trihydroxanthene moieties for the bioimaging application (
Figure 4E). The probe showed an absorption at 732 nm with an emission at 747 nm [
56]. The targeting mechanism of the probe showed specificity for cellular components, particularly localizing to the cytoplasm of living HepG2 cells (
Figure 4F). The probe showed a turn-on fluorescence response driven by cellular interactions, with changes linked to extended conjugation and rigidification. It holds promise for photodynamic therapy (PDT), leveraging its NIR fluorescence for deep tissue imaging and non-invasive diagnostics. Its stability and low cytotoxicity enhance therapeutic potential, though comparisons with existing treatments are lacking. In vitro studies confirmed low cytotoxicity, but in vivo research on biodistribution and efficacy is limited. While offering stability, non-cytotoxicity, and NIR imaging suitability, further in vivo validation and improved fluorescence intensity and specificity are needed.
Kand et al. developed a highly water-soluble probe with precise cellular localization control and favorable spectroscopic and photoreaction properties [
42]. The synthesis involved sulfonating meso-methyl BODIPY photocages using 2-mercaptoethanesulfonic acid sodium salt, resulting in mono- and di-sulfonated BODIPY variants. These sulfonated groups enhanced water solubility and cellular permeability control. The mono-sulfonated BODIPY could cross the cell membrane, while the di-sulfonated version was restricted to cell-surface receptors. The BODIPY structure, with its hydrophilic sulfonate groups, allowed for regulated accumulation in cells, useful in bioimaging applications for mammalian cells and neurons.
BODIPY dyes represent significant advancements in the field of cellular imaging, especially for cytosol studies. Their unique properties, coupled with the ability to be synthesized with diverse spectral characteristics, make them invaluable tools for researchers seeking to elucidate cellular functions and interactions. By enabling the visualization of microenvironments, tracking biomolecule movements, and measuring cellular viscosity, BODIPY dyes not only enhance our understanding of fundamental biological processes but also pave the way for innovations in disease diagnosis and therapeutic monitoring. As research progresses, the potential applications of BODIPY dyes in cytosol staining and imaging will likely expand, further solidifying their role in modern biological research.
4.3. BODIPY for Mitochondria Imaging
Mitochondrial imaging has gained considerable attention due to the organelle’s critical roles in cellular metabolism, signaling, and overall health. BODIPY dyes, characterized by their unique photophysical properties, have emerged as powerful tools for imaging mitochondria in live cells. These dyes offer high photostability, brightness, and versatility, making them suitable for various imaging techniques, including fluorescence microscopy. The structural versatility of BODIPY dyes allows for modifications that can enhance their specificity and sensitivity toward mitochondria. Their high fluorescence quantum yields, and stability mean that they emit bright signals, which are critical for effective imaging applications. One of the main advantages of BODIPY dyes is their ability to be fine-tuned for specific applications through structural modifications. For example, the introduction of lipophilic cations, such as triaryl phosphonium (TPP+), facilitates the accumulation of these dyes within the mitochondrial matrix, exploiting the negative mitochondrial membrane potential. This characteristic enhances the effectiveness of BODIPY dyes in assessing mitochondrial function and health.
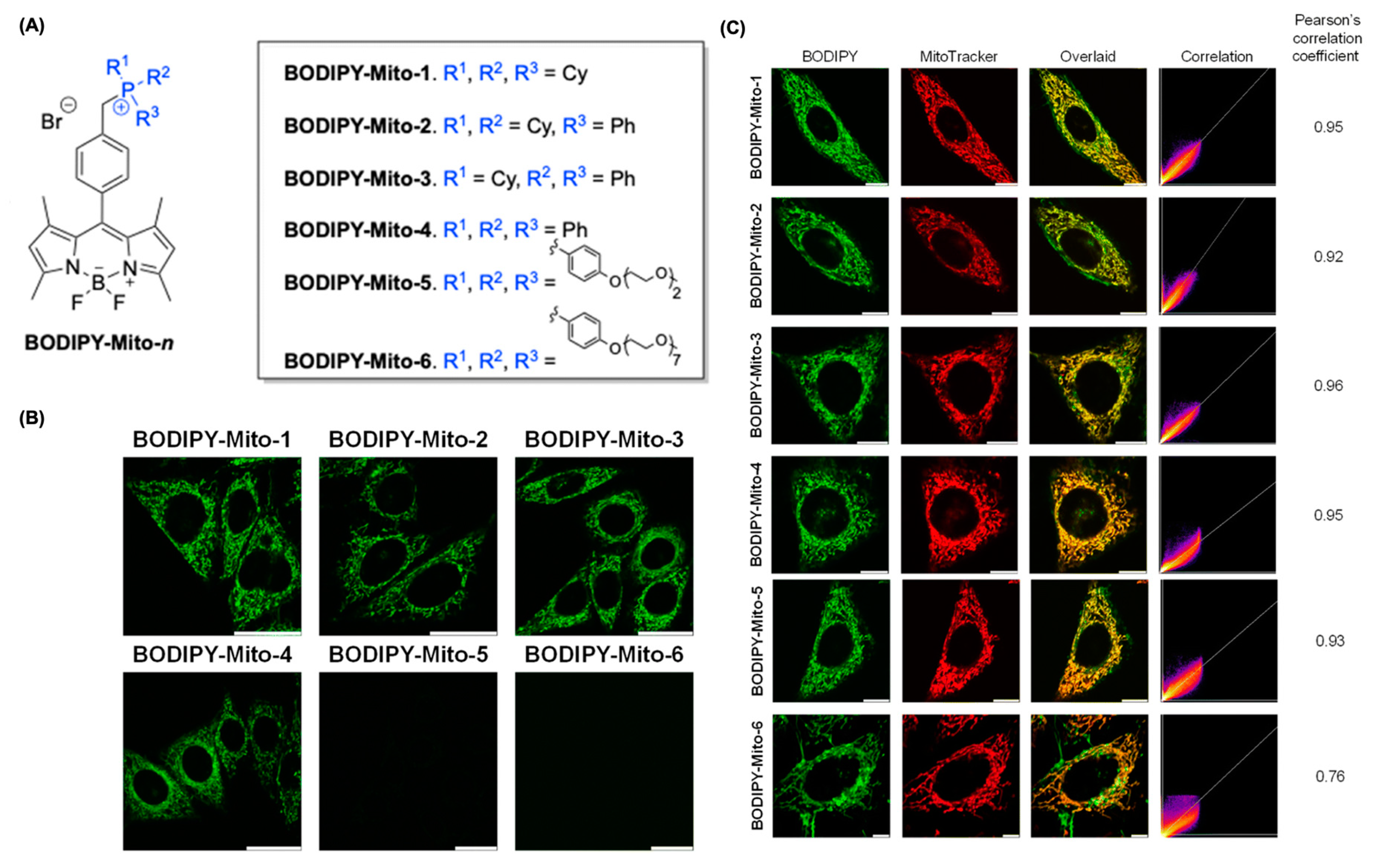
Recent advancements have led to the development of various BODIPY-based probes, specifically designed for mitochondrial imaging. For example, BODIPY-Mito-1 to BODIPY-Mito-6 have been synthesized to exhibit high sensitivity to changes in the mitochondrial membrane potential, allowing for real-time monitoring of mitochondrial dynamics (
Figure 5A) [
48]. Among these, BODIPY-Mito-6 was particularly noted for its efficacy, showing a reduction in emission intensity of up to 75% upon mitochondrial depolarization, indicating its strong potential for studying mitochondrial health and viability. The incorporation of poly(ethylene glycol) chains into BODIPY dyes has been explored to improve biocompatibility. This modification enhances the overall stability of the dyes and reduces cytotoxicity without compromising their ability to target mitochondria (
Figure 5B). Furthermore, these probes have demonstrated excellent localization in mitochondria, as evidenced by high Pearson’s correlation coefficients when co-stained with commercially available mitochondrial markers (
Figure 5C).
Yang et al. developed a BODIPY-based fluorogenic probe (34) that selectively binds to mitochondrial vicinal dithiols proteins [
57]. The probe utilizes phenylarsenicate for vicinal dithiol protein recognition and TPP+ for mitochondrial targeting. It enabled high-resolution mitochondrial imaging using varying beam strengths and time gates, revealing a distinct speckled, filamentous structure at a resolution of around 90 nm with 10% stimulated emission depletion laser power. This advancement is crucial for studying vicinal dithiol proteins in mitochondrial-related diseases and diagnosing conditions linked to redox activities.
Cationic compounds can disrupt cellular function by inducing depolarization of the mitochondrial membrane, leading to unnecessary metabolic processes. To enhance the biocompatibility and cellular penetration of mitochondrial-targeted probes, Singh introduced a neutral BODIPY fluorophore [
58]. This innovative probe enables selective mitochondrial imaging. By incorporating small PEG chains for improved cell penetration and a CF3 group at the meso position to generate a partial positive charge, it effectively directs the probe to the mitochondria. Compared to the commonly used Mitotracker Red CMXRos, this probe exhibited superior mitochondrial selectivity, making it an invaluable tool for studying mitochondrial health. Subsequently, Gupta’s team developed another neutral mitochondrial probe based on thioglycosylated BODIPYs [
59]. This compound, which can also be utilized in anticancer therapies, enhances cellular uptake and exhibits significant cytotoxic effects against HeLa and HaCaT cells. Later Yang and colleagues advanced the field with the creation of a NIR mitochondrial imaging probe [
60]. This probe features piperidinyl groups as the targeting moiety, demonstrating excellent photostability, low cytotoxicity, and high membrane permeability, enabling rapid cellular uptake and targeted accumulation in mitochondria, effectively highlighting these areas.
4.4. BODIPY Dyes for Endoplasmic Reticulum Imaging
Recent studies have developed novel BODIPY derivatives that specifically target the endoplasmic reticulum (ER), improving the accuracy of imaging in live cells. For instance, the ER-Tracker Green dye, a BODIPY derivative, has demonstrated high selectivity for the ER, enabling researchers to visualize ER dynamics in living cells with high fidelity (ER-TrackerTM Green (BODIPYTM FL Glibenclamide, Thermofisher). These targeted probes leverage the unique affinity of BODIPY dyes for specific cellular structures, providing insights into ER functions and stress responses, which are pivotal in understanding various diseases, such as cancer and diabetes. Several imaging techniques effectively utilize BODIPY dyes for visualizing the ER. For example, fluorescence microscopy combined with BODIPY-based dyes allows for detailed studies of ER morphology and dynamics under physiological conditions. Techniques such as fluorescence recovery after photobleaching have demonstrated how BODIPY dyes can be employed to investigate protein mobility within the ER, thereby contributing to the understanding of protein synthesis and folding. Moreover, multicolor and live-cell imaging systems, enabled by different spectrally distinct BODIPY dyes, facilitate simultaneous tracking of multiple organelles, enhancing the overall comprehension of intracellular interactions. The application of BODIPY dyes for ER imaging extends to disease research, especially concerning conditions like pancreatic ductal adenocarcinoma. For instance, a recent study by Chauhan et al. demonstrated a BODIPY-naphtholimine dyad that targets both the ER and lipid droplets within pancreatic ductal adenocarcinoma cells, showing potential for dual imaging and therapeutic applications (
Figure 6A, B)[
61]. This dual approach highlights the role of the ER in lipid metabolism and stress responses associated with cancer, revealing avenues for targeted therapies that exploit ER-specific pathways. Moreover, the ability of BODIPY dyes to cause robust ER stress and cellular lipid peroxidation indicates exciting possibilities for their use in photodynamic therapy. The targeted generation of reactive oxygen species (ROS) via BODIPY dyes can selectively induce apoptosis in malignant cells (
Figure 6C), suggesting a dual role not just as imaging agents but also as therapeutic tools.
Jong et al. fabricated a series of BODIPY-hydroxyquinoline derivatives for targeting ER. Among the eleven synthesized derivatives, the one with quinolin-8-ylpentanoate derivative showed enhanced localization and in vivo labeling for the ER (
Figure 6D) [
62]. This derivative showed a quantum yield of Φ = 55%, with low cytotoxicity, and showed enhanced fluorescence imaging capability than the commercial ER – Tracker Red (
Figure 6E).
The application of aza-BODIPY dyes in live-cell imaging has revolutionized the study of the ER. For example, real-time imaging of cellular processes such as protein folding, lipid synthesis, and stress responses can be achieved due to the persistent fluorescence of these dyes [
11]. Notably, aza-BODIPY dyes can provide insights into abnormal ER dynamics in pathological conditions, including cancer and neurodegenerative diseases. By tracking the distribution of aza-BODIPY dyes within the ER in live cells, researchers can visualize alterations related to disease states and gain insights into cellular stress responses. Hoenke et al. synthesized aza-BODIPY derivative conjugated with 3-O-acetyl-triterpene carboxylic acids using a piperazinyl spacer. The fluorescence microscopy and staining experiments showed that this derivative can be used for the ER imaging of A375 cells without cytotoxicity [
63].
Despite their numerous advantages, the application of BODIPY dyes in ER imaging is not without challenges. The sensitivity of BODIPY dyes to environmental factors such as pH and solvent polarity can affect their performance in live-cell imaging. There is ongoing research to improve the stability and specificity of these dyes, as well as to expand their compatibility with various imaging modalities. Future studies may focus on synthesizing next-generation BODIPY derivatives with enhanced properties for more precise and robust imaging applications.
4.5. BODIPY Dyes for SERS-Based Bioimaging
When combined with SERS, BODIPY dyes serve as effective Raman reporters, enhancing signal detection through the electromagnetic field produced by metallic nanostructures. Upon adsorption onto a nano-roughened metal surface, such as gold or silver, the local electromagnetic field is significantly enhanced, resulting in increased Raman scattering signals from the BODIPY dyes [
64]. This enhancement is pivotal because it allows for the detection of biomolecules at lower concentrations than would be possible with traditional fluorescence techniques. The high specificity of BODIPY dyes for targeted biological molecules, combined with the sensitivity of SERS, positions them as valuable tools in bioimaging applications, particularly in the context of health diagnostics and monitoring disease progression.
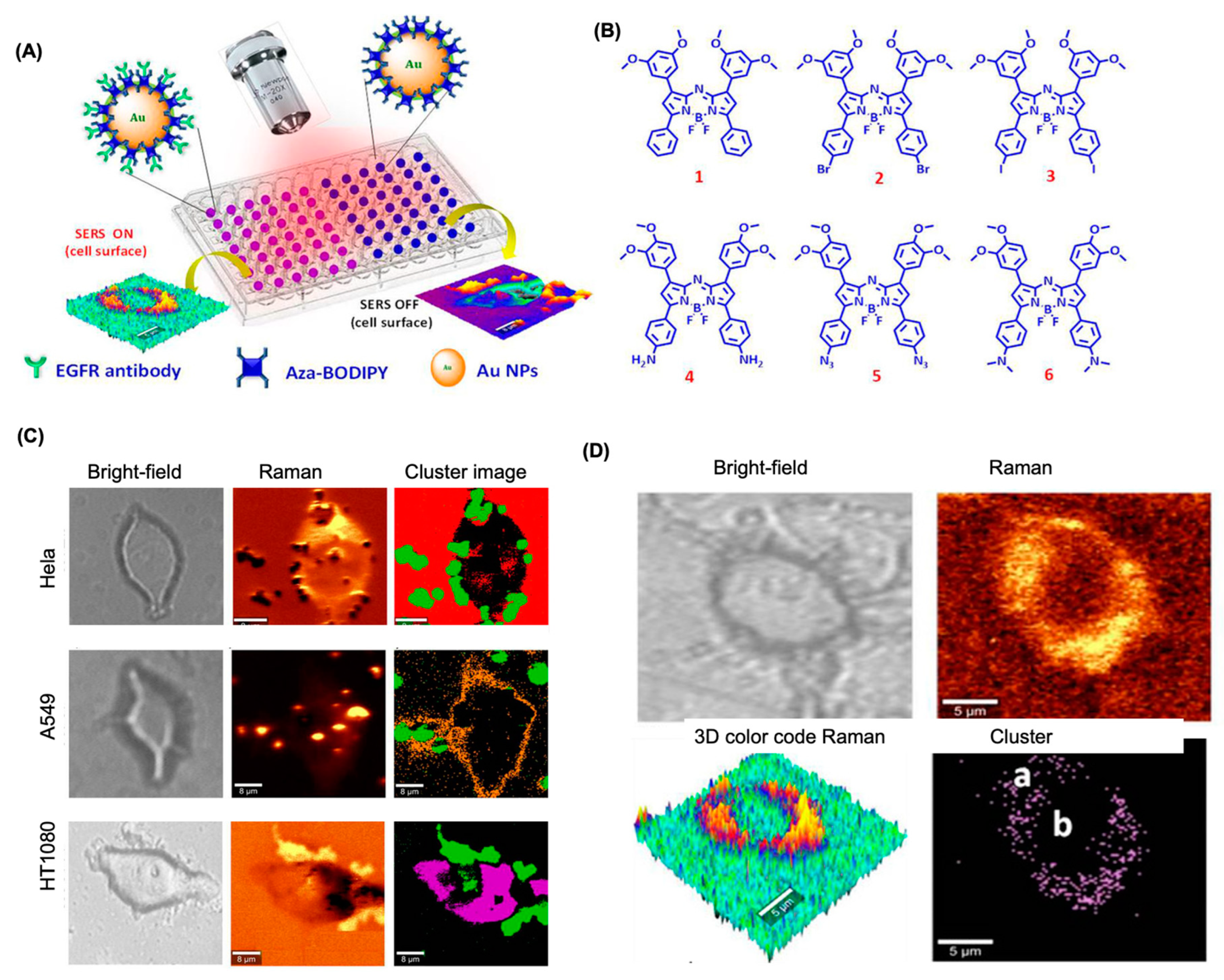
Adarsh et al. fabricated a series of aza-BODIPY derivatives (
1-6), which can adsorb on AuNPs surface for enhanced SERS response for these dyes (
Figure 7A-B) [
65]. Among the series of aza-BODIPY dyes, the derivative
4 showed excellent photophysics in terms of stability, and Raman response under excitation using a laser. Remarkably, it identified three human cancer cell types (A549, HeLa, and HT-1080) without requiring specific surface markers, producing clear Raman mapping and signature peaks (
Figure 7C). A cluster analysis was conducted using characteristic Raman spectral scans for each cancer cell line, under the assumption that the Raman nanotag was likely localized near the cell surface. This proximity of 4-Au@PEG to the cell surface was further validated through three-dimensional Raman imaging and single spectral analysis, which revealed distinctive SERS peaks corresponding to the aza-BODIPY probe 4-Au@PEG. However, no such recognition occurred in normal fibroblast (3T3L1) cells. To validate, the nanoprobe was conjugated with EGFR, a tumor-targeting marker for HT-1080 cells. This enhanced the probe’s specificity for cancer cells, enabling ultrasensitive detection and targeted imaging while sparing normal cells (
Figure 7D). Klapper et al. showed that using Raman spectroscopy the proportions of body fat and fat-free mass can be identified and imaged for metabolic C. elegans [
66]. As shown in
Figure 7C, BODIPY 493/503-labeled structures overlapped with lipid signals as it was measured by Raman spectroscopic measurements.
4.6. BODIPY Dyes for Photoacoustic Based Bioimaging
The field of biomedical imaging has significantly advanced with the integration of photoacoustic imaging (PAI), combining the spatial resolution of optical imaging with the deeper penetration depth of ultrasound. The BODIPY chromophores have been widely used in bioimaging, yet they are not ideal for PAI due to high fluorescence. But the advancement in synthesis and tuning their optical properties, recently BODIPY dyes have been used for PAI applications [
67,
68,
69]. For instance, modifications such as 1H-pyrrole conjugation can lead to increased absorption wavelengths, extending up to 799 nm, which is advantageous for PAI [
70]. A nonfluorescent PyBODIPY dye series was designed through 1H-pyrrole conjugation, and enable PA signal detection at low concentrations, with sensitivity reaching 1 nmol/mL in tubes and 35 pmol/mm³ in tissue phantoms (
Figure 8A-B). In addition to being nonfluorescent, they are non-phototoxic, photostable, and exhibit high molar extinction coefficients and resistance to nucleophilic addition. PEG-400 modification enhances their aqueous solubility, making them suitable for in vivo imaging. PyBODIPY’s properties make it a promising PA contrast agent, with potential for coupling to targeting ligands and further applications in designing near-infrared quenchers for clinical translation.
Ni et al. prepared a naphthalene fused BODIPY dimer Na-BD, which possesses excellent photostability and NIR absorption band between 600–850 nm (
Figure 8C) [
67]. On evaluation of their PA imaging capability the BODIPY dimer showed excellent PAI signals than commercial indocyanine green dye. Na-BD further loaded into bovine serum albumin nanoparticles and were injected into the mice having Hep-G2-tumor. The PAI imaging studies showed the accumulation of these formulations inside the tumor region, with sharp contrasting effect than indocyanine green (
Figure 8D). Similar studies were performed using a meso-ester substituted BODIPY near-infrared dyes for the PAI applications for Hep-G2-tumor bearing mice [
68]. Enhanced PA signals were observed for 72h of post injection. Ma et al. reported an aza-BODIPY derivative for monitoring the variation of ONOO− ions in vivo using PAI [
71]. The PA signal can be controlled by ICT effect and by the electron withdrawing group at 2-posiiton of BODIPY dye. This can be used for studying the arthritis inflammation in the mice, and as shown in
Figure 8E, the left limb injected with λ-carrageenan showed a PA signal three times intense than right limb which is injected with saline. The arthritis causes increased elevation in the level of ONOO− ions, thus resulting in a lesser PA signal from the left limb. In another study a ratiometric signal probe was used for monitoring the oxidative stress and activated neutrophils in plaques [
72]. A BODIPY core attached with neutrophil elastase peptide was fabricated, and under oxidative stress the peptide cleaves enhance the photoinduced electron transfer impacting photoacoustic intensity at 685 and 745 nm as studied by in vivo experiments.
A donor-acceptor BODIPY-Xanthene derivative was fabricated by Xu et al.[
73]. The designed BODIPY dye showed absorption within 700–1000 nm with excellent photostability and photoacoustic signal properties. The PA signal intensity showed distinguishable signal even at a depth of 4 mm using chicken breast and showed a clear distinguishable signal from the ultrasound signals. In vivo experiments showed the BODIPY dye can accumulate in both venous vessels and intestines, thereby dissecting the relevant physiological or pathological events of these two organs.
Thus, the adoption of BODIPY dyes in photoacoustic imaging represents a significant advancement in biomedical diagnostics. Their distinct optical properties, ease of modification, and broad applicability in various medical fields underline their potential as invaluable tools in improving patient care and outcomes in the realm of medical imaging.
5. Therapeutical Application of BODIPY
5.1. BODIPY Dyes in Photodynamic Therapy (PDT)
BODIPY dyes, widely recognized for their exceptional biomarker capabilities, have evolved into crucial tools for photodynamic therapy (PDT) when strategically designed. Photodynamic therapy employs a combination of light, a photosensitizing agent, and molecular oxygen to target and eliminate abnormal cells, particularly cancerous ones. Recently, a novel generation of PDT agents rooted in the BODIPY core has emerged, showcasing significant potential in therapeutic applications over the past decade [
74,
75]. The incorporation of heavy atoms, such as halogens and metal ions, into the BODIPY framework serves as a fundamental strategy to enhance its photosensitizing attributes. This deliberate arrangement of heavy atoms such as iodine, bromine, metal ions within the BODIPY structure promotes spin-orbit coupling (SOC), facilitating intersystem crossing (ISC). These processes are critical to the increased production of reactive oxygen species (ROS), which act as mediators of cell destruction through various programmed cell death pathways. Consequently, the tailored design of BODIPYs as photosensitizers marks a significant advancement in the efficacy of photodynamic therapy for cancer treatment. A list of various BODIPY dyes used for PDT applications are represented in
Figure 9.
For instance, Caruso et al. investigated the photodynamic therapeutical effectiveness of various BODIPYs, specifically the BODIPY-PDT-1a-x and BODIPY-PDT-2a-b compounds. They incorporated an aromatic ring in the meso position along with iodine atoms located in the β-positions, which demonstrated enhanced efficiencies in singlet oxygen production [
76,
77]. Similarly, Kim et al. focused on a BODIPY variant known as BODIPY-PDT-3, which displayed remarkable singlet oxygen sensitization capabilities, effectively inducing necrosis in Lewis lung carcinoma cells during in vitro analyses [
78].
In addition, Cosa and colleagues showcased ROS’s role in activating singlet oxygen photosensitizers [
79]. They found that the inclusion of a trap segment in BODIPY-PDT-4 enhanced its control over singlet oxygen sensitization compared to BODIPY-PDT-5, thereby presenting a substantial improvement in the management of photodynamic reactions. Further investigations by Epelde-Elezcano et al. highlighted the differences in singlet oxygen generation between halogenated BODIPY dyes, illustrating that iodine atoms enhanced ISC more effectively than bromine atoms in the variant BODIPY-PDT-6a-I [
80].
Moreover, Zhao and teams successfully synthesized a near-infrared (NIR) monoiodinated aza-BODIPY photosensitizer, which exhibited significant efficacy in vivo for photodynamic applications [
81]. Yoon et al. contributed by developing highly efficient triplet BODIPY derivatives aimed at photodynamic treatment and bioimaging roles [
82]. Recent advancements also include the work by Qian et al., who reported the development of orthogonally linked donor-acceptor-framed BODIPY variants, such as MA-BOP and Cz-BODIPY, which induced ISC providing promising avenues for intracellular photodynamic therapy [
83,
84].
Furthermore, Wang et al. made strides by introducing a heavy-atom-free helical BODIPY derivative that demonstrated a record low dosage effectiveness in PDT-augmented immunotherapy [
85]. The extensive literature now includes systematic reviews addressing the applications and advancements of heavy-atom-free BODIPY-based photosensitizers in photodynamic therapy, emphasizing their growing significance in therapeutic contexts [
86,
87,
88,
89].
5.2. BODIPY Dyes in Photothermal Therapy (PTT)
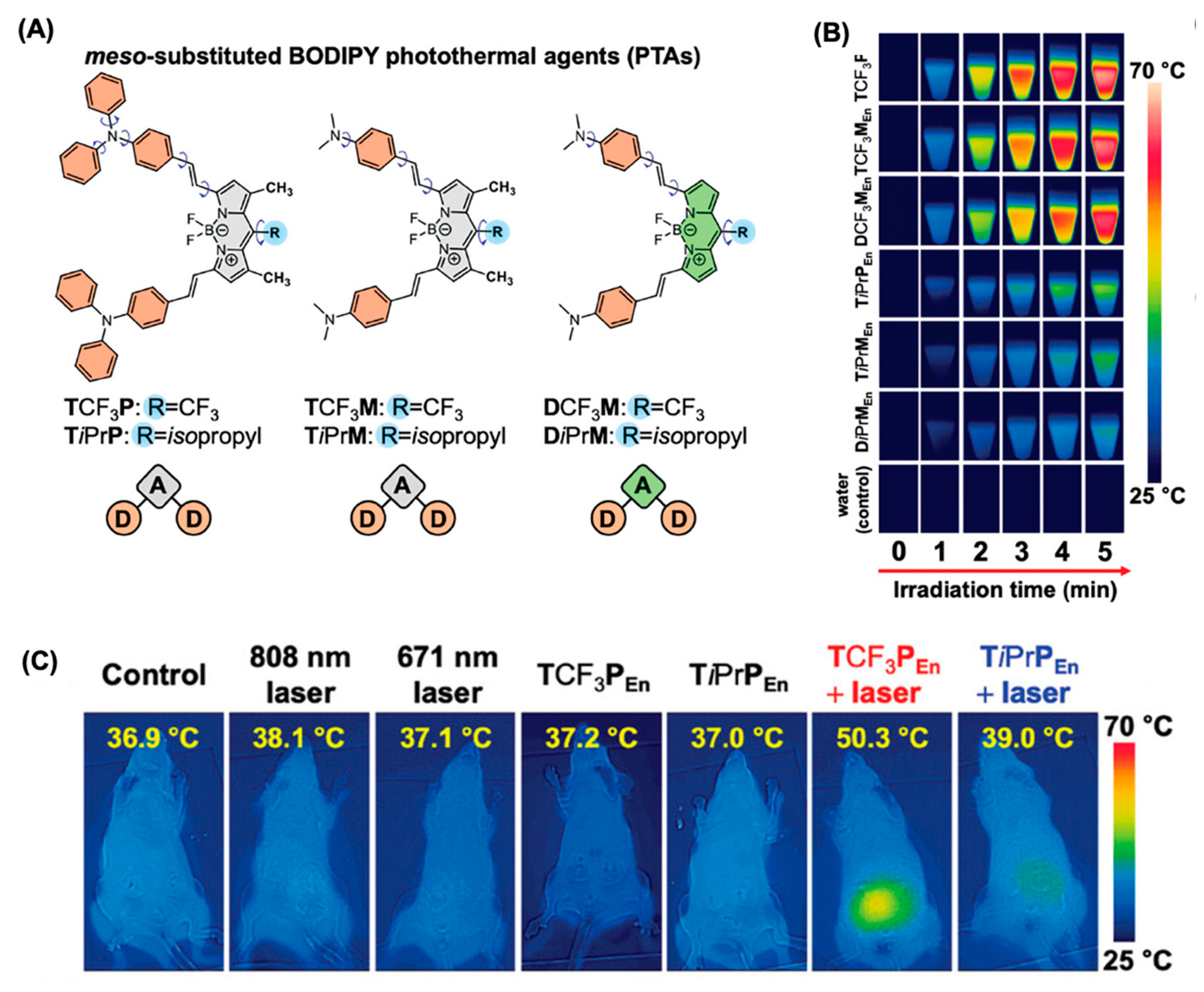
BODIPY dyes possess remarkable photothermal conversion efficiencies, particularly in the near-NIR region, which is advantageous for penetrating biological tissues with minimal damage. Their tunable absorption properties allow for precise customization based on the specific wavelengths required for effective PTT. PTT kills cancer cells by hyperthermia or by generation of heat-shock proteins. BODIPY dyes exhibit strong absorption in the NIR range, making them suitable candidates for applications where deeper tissue penetration is needed. For instance, researchers have developed π-extended BODIPY dyes that achieve over 90% photothermal conversion efficiency, highlighting their effectiveness in converting light energy into heat (
Figure 10A) [
31]. Upon 808 nm laser irradiation (0.75 W cm−2), the temperature of aqueous dispersions of the meso-CF
3-BODIPY NPs (20 µm based on dye) increased with time and reached a maximum at 66.8 °C (ΔT = 43 °C, TCF
3PEn), 64.9 °C (ΔT = 41 °C, TCF
3MEn), and 64.3 °C (ΔT = 41 °C, DCF3MEn) within 5 min (
Figure 10B). The in vitro and in vivo studies showed an enhanced tumor ablation using a MCF-7 Xenograft model with 50% reduction in tumor volume after the treatment. As shown in
Figure 10C, the meso-CF
3 substituted BODIPY (TCF
3M) showed exceptional efficacy in inducing cancer cell apoptosis and in vivo tumor ablation using low-power NIR laser irradiation (0.3 W cm
−2, 808 nm) than the other derivatives studied. In another study Li et al. showed a series of aza-BODIPY derivatives can be used for PTT applications with a photothermal conversion efficiency of 48-50% with an anticancer property towards human colon cancer cells [
90].
Liu et al. fabricated an aza-BODIPY derivative with a strong NIR absorption at 781 nm having excellent photostability and PT conversion efficiency [
91]. PT studied showed the photothermal effect is concentration dependent and a maximum temperature of 74.5 °C was attained for a concentration of 35 μg mL
−1 upon irradiation for 5 min. As shown in
Figure 9D, a dose dependent cell cytotoxicity was observed for the dye after laser irradiation for CT26 cells. The in vivo imaging and simultaneous PTT showed a maximum temperature rise of 44.9 °C after 1 min of irradiation with a suppression in tumor about 96 % for intertumoral injection and 89 % for intravenous injection. The body weight of the mice increased with days indicating no significant toxic effects after PTT. In a similar work Pewklang et al. showed that aza-BODIPY-Pyrazole derivative can show excellent NIR absorption at 900 nm with photothermal conversion efficiency upto 33 % [
92]. The confocal microscopic images showed that these dyes can penetrate the cancer cells less than in 6 h, and PTT studies showed elimination of 70% cancer cells. Moreover, the same dye was used for antibacterial studies towards Escherichia coli 780, and Staphylococcus aureus 1466 with 100 % activity upon laser irradiation.
The application of BODIPY dyes in photothermal therapy highlights their potential as transformative agents in oncological treatments. Their favorable photophysical properties, coupled with the ability to serve multifunctional roles, enable BODIPY dyes to offer targeted and effective thermal ablation of tumors. As research progresses, the incorporation of these dyes into combined therapies may further enhance cancer treatment modalities, paving the way for innovative solutions that address the complexities of cancer management. Continued exploration of BODIPY dyes will undoubtedly solidify their role as critical components in the evolving landscape of photothermal therapy and cancer treatment strategies.
5.3. BODIPY Dyes in Boron Neutron Capture Therapy (BNCT)
Boron Neutron Capture Therapy (BNCT) represents a pioneering strategy in cancer treatment that leverages the unique properties of boron-containing compounds and neutron irradiation to selectively target and destroy tumor cells [
93,
94]. Among the emerging boron carriers, BODIPY derivatives have shown significant promise due to their favorable characteristics, including high photostability, biocompatibility, and the ability to accumulate preferentially in tumor tissues. BNCT relies on the interaction between stable boron-10 isotopes (¹⁰B) and low-energy thermal neutrons to induce a nuclear reaction that produces high-linear energy transfer particles, specifically alpha particles and lithium-7 ions. These particles are cytotoxic and can kill cancer cells within a subcellular range of about 10 micrometers. The effectiveness of BNCT is contingent upon achieving a sufficiently high concentration of boron in tumor tissues while minimizing accumulation in surrounding healthy tissues [
95,
96]. Recent studies have indicated that BODIPYs can serve as efficient boron delivery vectors, as they demonstrate a significant uptake in tumor cells and maintain high tumor-to-normal tissue ratios, making them suitable candidates for BNCT applications [
97,
98,
99].
BCNT shows promising results for glioblastoma treatment, but the major difficulty for this treatment is the passage of drug/Boron through blood brain barrier (BBR). To address this challenge, researchers are investigating innovative solutions to enhance boron delivery to tumor sites. Among these solutions, carborane-BODIPY derivatives have been synthesized as potential agents for BNCT. Specifically, studies conducted by M.G. Vicente et al. focused on assessing the permeability of these derivatives, BDP-Me-oCB-20 and BDP-Me-oCB-21, using human brain endothelial cell lines, such as hCMEC/D3 [
97]. Their findings revealed that BDP-Me-oCB-20 exhibited a permeability coefficient (Pe) of 0.05 × 10−6 cm/s, while BDP-Me-oCB-21 demonstrated a Pe of 0.13 × 10−6 cm/s, indicating that both derivatives were inadequately internalized. The poor uptake is likely attributed to their high hydrophobicity, as evidenced by their log P values of 1.41 and 1.35, respectively. Consequently, enhancing the properties of carborane-BODIPY derivatives to improve their BBB penetration remains crucial for their application in BNCT. Continued research in optimizing these compounds is anticipated, aiming to increase their therapeutic efficacy against GBM while mitigating the limitations imposed by the BBB.
One of the primary advantages of using BODIPY derivatives in BNCT is their versatile photophysical properties, which can be tailored through chemical modifications. These optoelectronic characteristics allow BODIPYs to be engineered for enhanced solubility, stability, and tumor-targeting capabilities. For example, aza-BODIPY, a BODIPY variant, has been developed that emits in the near-infrared (NIR) region, which is advantageous for in vivo optical imaging due to its deeper tissue penetration and lower autofluorescence of biological tissues. Additionally, aza-BODIPY can form stable conjugates with boron-containing compounds such as sodium borocaptate (10B-BSH), improving the delivery of boron into tumors and facilitating real-time imaging of its biodistribution within the body [
100]. The newly developed aza-SWIR-BSH-01 compound, for instance, has been demonstrated to achieve high tumor accumulation due to its favorable physicochemical properties, while simultaneously conveying imaging capabilities for monitoring its delivery and distribution. Clinical trials exploring the potential of BODIPY-based BNCT have yielded promising results, particularly for the treatment of solid tumors such as glioblastoma and head and neck cancers. In preclinical models, the use of aza-BODIPY/10B-BSH conjugates has shown improved therapeutic efficacy as compared to conventional boron carriers like 10B-BSH alone. Studies reveal that tumor growth was significantly reduced when treated with the aza-BODIPY-conjugated boron agents combined with neutron irradiation. This advancement not only underscores the effectiveness of BODIPY in enhancing BNCT but also positions it as a potential frontrunner in the search for targeted therapies against challenging cancers that are resistant to conventional treatments [
100].
Bellomo et al. fabricated a library of 2,6- and 3,5-distyrenyl-substituted carborane-BODIPY dyes with excellent NIR absorption and photostability [
101]. The attachment of BODIPY unit to carborane enhanced the boron content for BNCT as suggested.
6. BODIPY Based Nanomedicines for Theragnostic Applications
Owing to good photostability, fluorescence and therapeutical applications use of BODIPY for biological applications are still challenging due to hydrophobicity of most of the dyes, or special synthetic routes needed for making them water soluble. Advancement in nanotechnology overcomes these issues and various BODIPY based nanoparticles (BDPNPs) have been prepared recently for biomedical applications [
32,
102,
103]. Controlling the aggregation property of BODIPY (J or H-aggregates) using nanoprecipitation method allows fabrication of a library of BDPNPs for bioimaging and therapeutical applications [
104,
105,
106,
107]. The aggregation enhances the emission and photosensitization property for such dyes for biomedical applications. Another strategy is BODIPY dyes can directly conjugate with metallic NPs for various biomedical applications [
108,
109,
110,
111,
112,
113].
6.1. Sel-Assembled BDPNPs for Bioimaging and Therapeutical Applications
For preparing self-assembled BDPNPs mostly nanoprecipitation technique is used, in which a miscible solution of dye in acetone, methanol, tetrahydrofuran (THF) etc. were added dropwise to water under stirring condition and the organic solvent is evaporated slowly, followed by dialysis, centrifuge, the precipitated NPs were collected and redisperse them in water for various applications. As shown in
Figure 11A the self-assembled NPs can be used for various biomedical applications.
In recent developments within the field of photodynamic therapy and bioimaging, BDPNPs have emerged as significant agents due to their impressive optical properties and biocompatibility. A series of BDPNPs were prepared from various BODIPY dyes as shown in
Figure 11B.
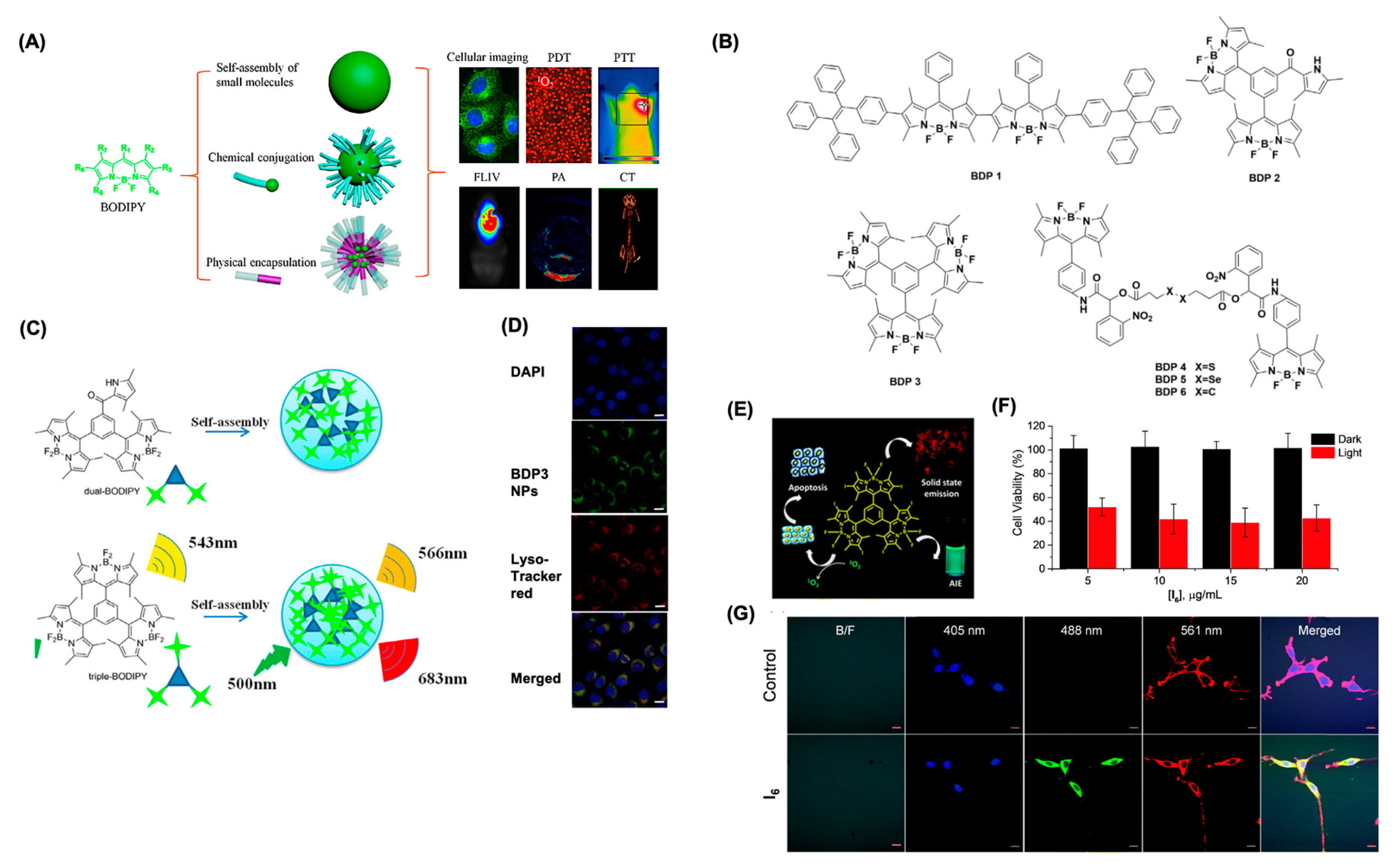
A stable nanoparticle composed of a BODIPY dimer, specifically BDP 1, was reported by Z. Li et al. [
115]. These nanoparticles were synthesized using a structure that combined the BODIPY dye with tetraphenylethene, resulting in nanoparticles with an approximate diameter of 110 nm, as measured by both transmission electron microscopy and scanning electron microscopy. Importantly, the photostability of BDP 1 nanoparticles showed noteworthy enhancements compared to their monomeric counterparts when assessed under UV light irradiation in tetrahydrofuran. This photostability is a crucial factor for their practical application in cellular environments. The cellular imaging capabilities of BDP 1 nanoparticles were evaluated using confocal laser scanning microscopy on human cervical carcinoma (HeLa) cells, revealing bright red fluorescence localized in the cytoplasm. This highlighted the potential of BDP 1 nanoparticles for effective imaging in biological systems, paving the way for further research and applications. Building upon these findings, Liu et al. synthesized two additional BODIPY derivatives, BDP 2 and BDP 3, which also demonstrated the ability to self-assemble into nanoparticles, achieving diameters of 112 nm and 98 nm, respectively (
Figure 11C) [
114]. A notable observation regarding BDP 2 NPs was their sensitivity to temperature, as their diameter expanded significantly from 112 nm to 2,000 nm upon storage at room temperature for one month, indicating a potential instability issue. In contrast, BDP 3 NPs exhibited enhanced stability in aqueous environments. Of particular interest is the dual-emission characteristic of BDP 3 NPs, which displayed peaks at 566 nm and 683 nm upon excitation at 500 nm, contrasting with a maximum emission wavelength of 543 nm in THF. When THF was introduced to BDP 3 NPs in water, fluorescence spectroscopic analysis indicated an increase in intensity at 566 nm alongside a decrease at 683 nm, suggesting a transition from nanoparticle aggregates to individual molecules. According to dynamic light measurements, BDP 3 NPs were eliminated from the medium with the addition of just 30% THF, underscoring their transformation dynamics in varying solvent conditions. Remarkably, BDP 3 NPs retained their proficiency for cellular imaging, with fluorescence localization confirmed within lysosomal compartments, thus indicating their effectiveness as imaging agents in live-cell applications (
Figure 11D).
Kumar et al. fabricated a hexaiodo BODIPY with efficient photostability and self-assembling property (
Figure 11E) [
107]. Incorporation of iodine atoms quenched the fluorescence of BODIPY dye in solution, but the self-assembled nanoaggregates enhanced the emission and photosensitization property of this BODIPY dye. The nanoaggregates showed a singlet oxygen quantum yield of 0.82 which is higher than commercially available methylene blue. Owing to the high singlet oxygen quantum yield the nanoaggregates used for PDT studies and results showed ∼60% cell death for C6 cells after irradiation of NPs with laser (
Figure 11F). Owing to the enhanced emission property the nanoaggregates were used for cell imaging using different laser excitations such as 405/488/561 nm simultaneously (
Figure 11G).
These findings collectively depict the advancement in BODIPY nanoparticle technology, demonstrating their significance as versatile tools in both imaging and therapeutic modalities in cancer treatment.
6.2. Physical Encapsulation of BODIPY to Form BDNPs
Due to the hydrophobicity and extended pi conjugated backbones BPODIPY dyes can be easily entrapped into various liposomes, polymers, etc for the fabrication of BDPNPs. In of the studies carbazole-BODIPY dye was entrapped into PLGA-Folic acid conjugate for the fabrication of BDPNPs. The NPs showed an average size of 20 nm with excellent stability for days with enhanced NIR emission [
116]. PDT studies on subcutaneous 4T1 tumor xenograft model showed that this NPS can suppress the growth of 8 mm sized tumors with efficient singlet oxygen generations and showed enhanced bioimaging capability. In another study Huang, Li, et al., showed that conjugating two BODIPY units using a triazole link can act as a FRET pair with 1.9-fold enhanced in singlet oxygen generation than the monoderivative. Amphiphilic pluronic surfactant with folic acid conjugate was used as matrix to improve the solubility of the dye, and in vitro and in vivo studies showed the NPs can kill HeLa cells than normal cells [
117]. Naim et al. reported a supramolecular nanocomposite from BODIPY, tetra iodoethylene, embedded into chitosan matrix for enchanted singlet oxygen generation [
118]. Nanocomposite showed excellent photostability, as compared to individual components, and reduced cytotoxicity for bioapplications. The use of tetra iodoethylene is crucial since it enhances the intersystem crossing of the nanomaterial for photosensitization. The water-soluble nanomaterial showed an average particle size between 250-330 nm, with a singlet oxygen quantum yield of 0.12. In vitro tumor studies (C6 cells) showed 39% cell viability after irradiating the nanomaterial with white light (80 mW cm
−2) for 30 minutes.
BODIPY compounds have garnered significant attention due to their responsiveness to the tumor microenvironment, particularly those that are pH sensitive. Among these, the aza-BODIPY reported by Dong’s group features a dimethylaminophenyl group, enhancing its utility in biomedical applications [
119]. The NPs formed from this compound, BDP 13, exhibit a diameter of approximately 30 nm and are created using a combination of hydrophobic BDP 13 and amphiphilic DSPE-mPEG2000, an agent that facilitates better solubility and stability in physiological conditions. As the pH of the surrounding environment decreases from 7.4 to 5.0, BDP 13 NPs display enhanced capabilities in photoacoustic imaging, as well as photodynamic and photothermal effects. These improvements were confirmed through both in vitro and in vivo studies, demonstrating the potential of these NPs for effective tumor visualization and treatment.
Furthermore, research by Lin et al. has expanded the applications of BODIPY compounds by integrating them with PEG–PLA for NIR fluorescence imaging, which boasts a high signal-to-noise ratio, along with PA and computed tomography functionalities [
120]. This series of pH-sensitive BODIPY nanoparticles offers a great promise for simultaneous imaging and therapeutic interventions.
Dong’s team has also explored variations in the number of diethylaminophenyl groups attached to BODIPY, which further heighten its pH sensitivity compared to compounds with dimethylaminophenyl groups [
121]. Notably, the introduction of two diethylaminophenyl groups in BDP 14 resulted in a marked red shift of the absorption and fluorescence emission wavelengths, enhancing tissue penetration. Following a similar preparation with DSPE-mPEG2000, BDP 14 NPs achieved an absorption peak in the NIR range at 735 nm. Additionally, under illumination with a 730 nm laser, BDP 13 NPs demonstrated an increase in temperature from 19.5 to 22.3 °C, accompanied by a pH drop from 7.4 to 5.5, indicative of an enhanced photothermal response. This pH/ROS-activated strategy highlights the dual utility of these BODIPY NPs, making them promising candidates for fluorescence-guided photodynamic therapy (PDT) and photothermal therapy (PTT) in cancer management. Through these advancements, pH-sensitive BODIPY nanoparticles not only facilitate improved imaging and therapeutic efficacy but also pave the way for more targeted and effective cancer therapies.
6.3. Supramolecular Approach Using Metallic Nanoparticles
The integration of metallic nanoparticles with BODIPY dyes has opened promising avenues in the fields of cancer imaging and therapy. Suitable designs from BODIPY dyes which can anchor with metallic NPs can exhibit enhanced properties, including improved localization in tumor tissues and increased efficiency in light-activated therapeutic mechanisms [
32].
Metallic nanoparticles, such as gold and silver, offer unique optical properties due to their surface plasmon resonance, which can be exploited to enhance the photodynamic effects of BODIPY dyes. These nanoparticles can accumulate preferentially in tumor tissues due to the EPR effect, thereby facilitating targeted delivery of the BODIPY dyes directly to malignant cells [
122]. Studies have shown that gold nanoparticles fused with BODIPY derivatives can act both as imaging agents and therapeutic agents, providing dual functionality [
88,
123]. For instance, BODIPY-conjugated gold nanoparticles have been effectively used as fluorescent sensors in in vitro models of cancer, enabling real-time imaging of biological processes specific to tumor development [
124]. Uniform polyethyleneimine-coated magnetic Fe₃O₄ nanoparticles were synthesized via a modified co-precipitation method and covalently conjugated with the BODIPY fluorophore using DCC/DMAP coupling reactions. The NPs showed imaging capability was tested for the first time on human cancer cells (A549 and Ishikawa), while cytotoxicity was assessed on both cancer and healthy HUVEC cells using the MTT assay. Additionally, the nanoparticles’ in vitro activities were evaluated and suggest they can be used for future MRI applications.
Kim and colleagues designed an innovative drug delivery platform utilizing gold monolayer nanoparticles [
125]. Their approach incorporated “hydrophobic pockets,” previously identified by Lucarini and Pasquato, to facilitate effective payload encapsulation. Zwitterionic chains were grafted onto the gold surface, creating spaces to load BODIPY molecules as fluorescent markers. The study revealed that the dye transfer occurs via a monolayer-membrane interaction mechanism. Additionally, the nanoparticles exhibited minimal uptake in MCF-7 human breast cancer cells. However, significant cytosolic delivery was observed within two hours of incubation. Notably, the BODIPY-loaded nanoparticles demonstrated remarkable stability, retaining their integrity for over a month.
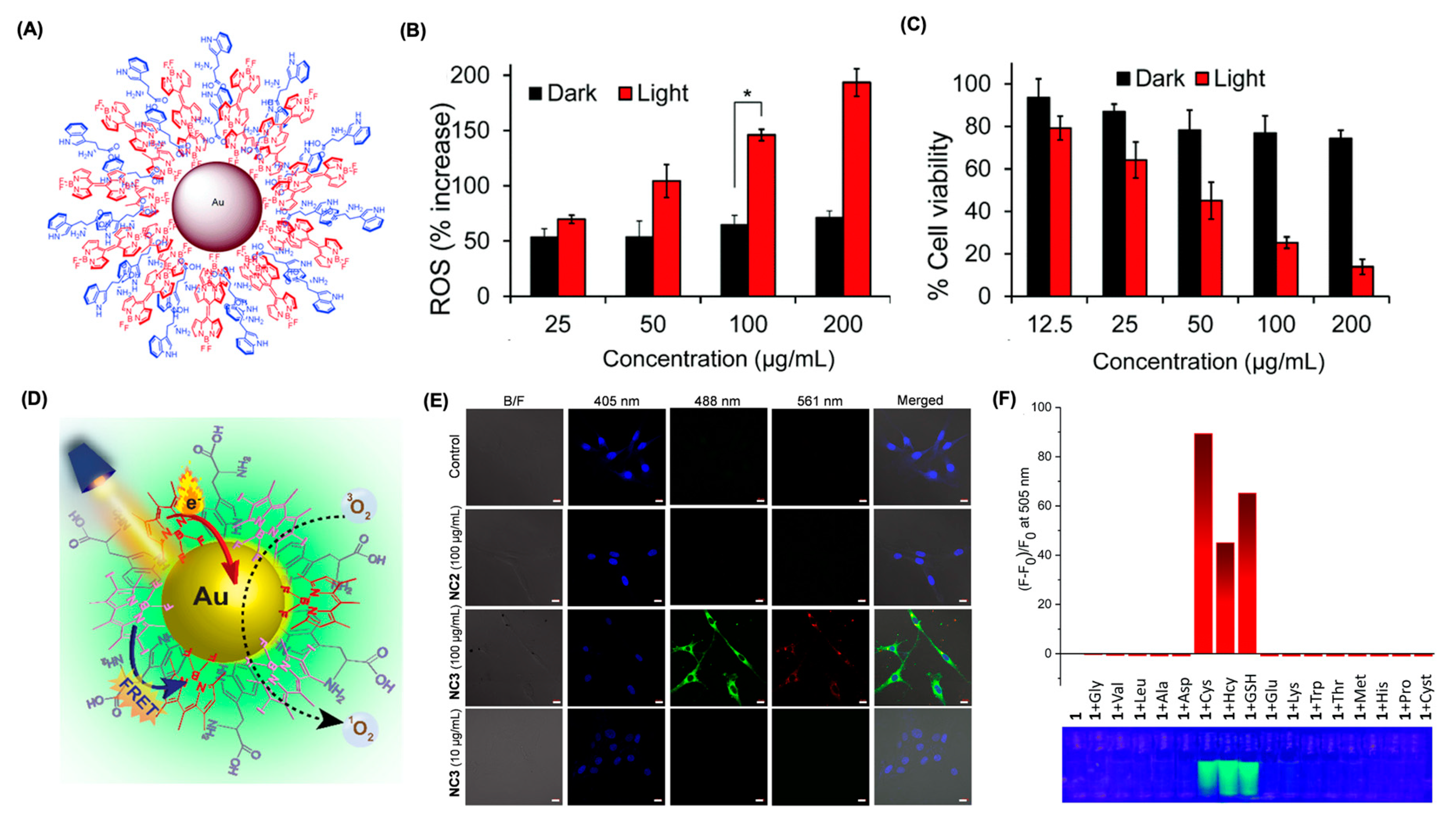
In PDT studies incorporation of AuNPs with BODIPY dye showed enhanced therapeutical and bioimaging applications. For instance, Kumar et al. showed a series of design strategy in which AuNPs can be incorporated with BODIPY dyes and controlling their structure activity relationships the photophysics and fluorescence of these nanomaterials can be controlled for various applications [
109]. Use of tryptophan reduced AuNPs as a matrix the simple BODIPY dye can be incorporated into the AuNPs via various supramolecular interactions (
Figure 12A). The nanomaterial showed enhanced photostability, and quenched fluorescence from BODIPY with a singlet oxygen quantum yield of ΦΔ = 0.46. These nanomaterials showed enhanced PDT effect towards C6 cells with a phototoxicity of 85% without cytotoxic to normal cells (
Figure 12B-C) [
112]. This work has been extended using interplay between BODIPY structures and a FRET based nanocomposite is prepared. Under light, these nanocomposites showed enhanced luminescence on the surface of AuNPs via distinct radio pathway, and this property used for simultaneous cell imaging and PDT studies (
Figure 12D). The nanocomposite showed singlet oxygen quantum yield of ΦΔ = 0.68, which is comparable with known dye methylene blue, but the nanocomposite showed excellent photostability than methylene blue [
109]. Detection biothiols in tumor cells is important since an elevated biothiol concentration is present in tumor cells. Using an indicator displacement approach, in which the BODIPY dyes were non-fluorescent on the surface of AuNPs, displace the BODIPY from metallic surface after interaction with biothiols, and shows a fluorescent turn-on for BODIPY dyes (
Figure 12E). Such design strategy is used for the cancer cell imaging and quantification of biothiols in tumor cells [
111,
113].
Overall, the combination of metallic nanoparticles and BODIPY dyes represents a powerful strategy for advancing cancer imaging and therapy, leveraging their complementary strengths to improve diagnostic capabilities and therapeutic efficacy in oncological applications. This innovative approach not only facilitates early detection and precise monitoring of cancers but also enhances therapeutic responses, thereby paving the way for more effective cancer treatment modalities.
7. Summary and Conclusions
The utilization of BODIPY dyes and their nanoparticles in cancer imaging and therapeutics represents a promising frontier in modern oncology, driven by the inherent optical properties and versatility of these compounds. BODIPY derivatives have been recognized for their strong absorption and emission characteristics in both the visible and near-infrared regions, making them highly effective fluorescent probes for imaging applications. Their excellent photostability and high quantum yield are pivotal for enhancing imaging fidelity, thus facilitating accurate diagnosis and treatment monitoring. Furthermore, BODIPY dyes can be engineered to target specific cancer cells, minimizing damage to surrounding healthy tissues, which is a crucial advancement over traditional therapies that often lack precision.
In recent developments, BODIPY nanoparticles have emerged as a powerful platform for photodynamic therapy (PDT), providing a synergistic approach to cancer treatment. By generating reactive oxygen species (ROS) upon light activation, these nanoparticles can effectively induce cell death in cancerous tissues while maintaining a favorable safety profile. Their ability to enhance cellular uptake and selectively accumulate in tumor microenvironments, aided by the Enhanced Permeability and Retention effect, significantly augments their therapeutic efficacy. Despite their potential, challenges remain, including the need for improved water solubility and selectivity of BODIPY photosensitizers. Ongoing research focuses on addressing these limitations through innovative modifications and the development of multifunctional nanoparticles that can cater to both diagnostic and therapeutic needs simultaneously.
In conclusion, BODIPY dyes and their nanoparticles hold great promise in revolutionizing cancer imaging and therapies, offering novel approaches that may enhance patient outcomes by improving diagnosis accuracy and treatment precision. As research continues to uncover the full potential of these compounds, they are likely to play an increasingly significant role in the future of cancer care, paving the way for more effective, targeted, and less invasive treatment modalities.
8. Future Outlook for BODIPY Dyes and Nanoparticles in Cancer Imaging and Therapy
The field of cancer treatment has seen significant advancements, but challenges remain in accurately detecting and effectively treating various types of cancer. BODIPY dyes, known for their superior photophysical properties, and BODIPY nanoparticles present great promise in enhancing cancer imaging and therapeutic applications. This essay discusses the outlook and potential developments regarding the use of BODIPY dyes and nanoparticles in tackling cancer challenges.
8.1. Enhanced Targeting and Delivery Mechanisms
One of the primary areas of advancement for BODIPY-based nanoparticles lies in enhancing targeting mechanisms and drug delivery systems. The unique properties of BODIPY dyes, such as their strong absorption in the visible and near-infrared regions, can be effectively exploited using nanoparticles that can preferentially accumulate in tumor tissues. Future research should focus on engineering nanoparticles that are responsive to specific biological stimuli within the tumor environment, including pH, enzymatic activity, or hypoxic conditions. This will enhance the targeted delivery of BODIPY-based therapeutics, minimizing adverse effects on healthy tissues while improving therapeutic efficacy [
5,
126].
8.2. Integration of Theranostic Applications
The integration of therapeutic and diagnostic functionalities into a single platform, termed theragnostic, represents a promising direction for BODIPY dyes and nanoparticles. The development of BODIPY conjugates that exhibit both imaging capabilities and therapeutic action can significantly speed up the clinical translation of cancer therapies. The ability to perform real-time monitoring of drug effectiveness using fluorescence imaging while administering treatment could allow for more personalized and timely interventions. Recent advancements in BODIPY-based theragnostic systems are encouraging, with preliminary studies showing their potential to provide dual functionality in cancer imaging and therapy [
30].
8.3. Innovations in Photodynamic Therapy (PDT)
BODIPY dyes have emerged as highly efficient photosensitizers for photodynamic therapy (PDT). Future advancements will likely focus on improving the efficacy of BODIPY-based PDT by optimizing their chemical structures to enhance singlet oxygen generation, thereby increasing their phototoxicity towards cancer cells [
75]. Furthermore, combining BODIPY dyes with other therapeutic modalities, such as chemotherapy or immunotherapy, could lead to synergistic effects, providing a multifaceted approach to cancer treatment [
127]. For instance, incorporating BODIPY dyes into existing chemotherapy protocols has demonstrated improved selectivity and reduced side effects due to their targeting capabilities [
128] Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation.
8.4. Development of Novel BODIPY Derivatives
Ongoing research into modifying the core structure of BODIPY molecules has the potential to revolutionize their applications in cancer therapy. Structural modifications can yield new derivatives with carefully tailored properties, such as altered solubility profiles, emission wavelengths, and enhanced stability under biological conditions. Innovations in synthetic techniques, such as click chemistry, can facilitate the creation of libraries of BODIPY derivatives, leading to the identification of optimal candidates for specific applications.
8.5. Utilization of BODIPY in Combination with Nanotechnology
The intersection of BODIPY chemistry and nanotechnology opens new avenues for cancer imaging and treatment. Nanotechnology not only enables enhanced stability and solubility of BODIPY dyes but also aids in the creation of multifunctional nanocarriers capable of delivering multiple therapeutic agents. Additionally, the development of targeted nanosystems that can respond to specific tumor markers offers a promising pathway for the precise delivery of BODIPY dyes, enhancing their role in both imaging and therapy.
8.6. Addressing Challenges
Despite the significant potential of BODIPY dyes and nanoparticles, several challenges remain to be addressed. These include concerns over biocompatibility, long-term stability in biological systems, and the scalability of synthesis techniques for clinical applications. Future research must focus on overcoming these challenges to facilitate the translation of BODIPY-based technologies from the laboratory to the clinic. The establishment of clear regulatory pathways and guidelines for the use of nanoparticles in medical applications will be critical in this regard [
129].
The future of BODIPY dyes and nanoparticles in cancer imaging and therapeutic applications looks promising. The ongoing development of advanced targeting mechanisms, innovative theragnostic platforms, and novel chemical derivatives will likely enhance their efficacy and applicability. By addressing current challenges and optimizing their properties, BODIPY-based technologies could play a transformative role in the fight against cancer, leading to improved diagnostic capabilities and more effective therapies for patients worldwide. As research continues to advance, BODIPY dyes are poised to become integral components of the next generation of cancer treatments.
Author Contributions
Conceptualization PPK.; resources, PPK.; data curation, SS, RJ and PPK.; writing—original draft preparation PPK.; writing—review and editing, SS, RJ and PPK.; supervision, PPK. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
PPK shows sincere gratitude to Department of Biomedical Engineering, Michigan State university, for the facilities and use of resources for the literature collections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [CrossRef]
- Samanta, S.; Lai, K.; Wu, F.; Liu, Y.; Cai, S.; Yang, X.; Qu, J.; Yang, Z. Xanthene, Cyanine, Oxazine and BODIPY: The Four Pillars of the Fluorophore Empire for Super-Resolution Bioimaging. Chem. Soc. Rev. 2023, 52, 7197–7261. [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural Modification Strategies for the Rational Design of Red/NIR Region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [CrossRef]
- Treibs, A.; Kreuzer, F. Difluorboryl-Komplexe von Di- Und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [CrossRef]
- Das, S.; Dey, S.; Patra, S.; Bera, A.; Ghosh, T.; Prasad, B.; Sayala, K.D.; Maji, K.; Bedi, A.; Debnath, S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules 2023, 13, 1723. [CrossRef]
- Kaur, P.; Singh, K. Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [CrossRef]
- Ahmad, H.; Muhammad, S.; Mazhar, M.; Farhan, A.; Iqbal, M.S.; Hiria, H.; Yu, C.; Zhang, Y.; Guo, B. Unveiling Cellular Mysteries: Advances in BODIPY Dyes for Subcellular Imaging. Coordination Chemistry Reviews 2025, 526, 216383. [CrossRef]
- Yadav, I.S.; Misra, R. Design, Synthesis and Functionalization of BODIPY Dyes: Applications in Dye-Sensitized Solar Cells (DSSCs) and Photodynamic Therapy (PDT). J. Mater. Chem. C 2023, 11, 8688–8723. [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [CrossRef]
- Schäfer, C.; Mony, J.; Olsson, T.; Börjesson, K. Effect of the Aza-N-Bridge and Push–Pull Moieties: A Comparative Study between BODIPYs and Aza-BODIPYs. J. Org. Chem. 2022, 87, 2569–2579. [CrossRef]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of Small Molecule Aza-BODIPY: From Rational Structural Design to in Vivo Investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [CrossRef]
- Dutta, D.; Nair, R.R.; Mangalath, S.; Nair, S.A.; Joseph, J.; Gogoi, P.; Ramaiah, D. Biocompatible Aza-BODIPY-Biotin Conjugates for Photodynamic Therapy of Cancer. ACS Omega 2023, 8, 26180–26190. [CrossRef]
- Elgun, T.; Yurttas, A.G.; Cinar, K.; Ozcelik, S.; Gul, A. Effect of Aza-BODIPY-Photodynamic Therapy on the Expression of Carcinoma-Associated Genes and Cell Death Mode. Photodiagnosis and Photodynamic Therapy 2023, 44, 103849. [CrossRef]
- Hlogyik, T.; Laczkó-Rigó, R.; Bakos, É.; Poór, M.; Kele, Z.; Özvegy-Laczka, C.; Mernyák, E. Synthesis and in Vitro Photodynamic Activity of Aza-BODIPY-Based Photosensitizers. Org. Biomol. Chem. 2023, 21, 6018–6027. [CrossRef]
- Li, K.; Duan, X.; Jiang, Z.; Ding, D.; Chen, Y.; Zhang, G.-Q.; Liu, Z. J-Aggregates of Meso-[2.2]Paracyclophanyl-BODIPY Dye for NIR-II Imaging. Nat Commun 2021, 12, 2376. [CrossRef]
- Hu, X.; Fang, Z.; Zhu, C.; Yang, Y.; Yang, Z.; Huang, W. Crucial Breakthrough of BODIPY-Based NIR-II Fluorescent Emitters for Advanced Biomedical Theranostics. Adv Funct Materials 2024, 34, 2401325. [CrossRef]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel Aza-BODIPY Based Small Molecular NIR-II Fluorophores for in Vivo Imaging. Chem. Commun. 2019, 55, 10920–10923. [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; Yetisen, A.K.; et al. Near-Infrared-II Nanoparticles for Cancer Imaging of Immune Checkpoint Programmed Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano 2021, 15, 515–525. [CrossRef]
- Yang, N.; Song, S.; Liu, C.; Ren, J.; Wang, X.; Zhu, S.; Yu, C. An Aza-BODIPY-Based NIR-II Luminogen Enables Efficient Phototheranostics. Biomater. Sci. 2022, 10, 4815–4821. [CrossRef]
- Tian, Y.; Zhou, H.; Cheng, Q.; Dang, H.; Qian, H.; Teng, C.; Xie, K.; Yan, L. Stable Twisted Conformation Aza-BODIPY NIR-II Fluorescent Nanoparticles with Ultra-Large Stokes Shift for Imaging-Guided Phototherapy. J. Mater. Chem. B 2022, 10, 707–716. [CrossRef]
- Bian, S.; Zheng, X.; Liu, W.; Li, J.; Gao, Z.; Ren, H.; Zhang, W.; Lee, C.-S.; Wang, P. Pyrrolopyrrole Aza-BODIPY-Based NIR-II Fluorophores for in Vivo Dynamic Vascular Dysfunction Visualization of Vascular-Targeted Photodynamic Therapy. Biomaterials 2023, 298, 122130. [CrossRef]
- Tang, D.; Yu, Y.; Zhang, J.; Dong, X.; Liu, C.; Xiao, H. Self-Sacrificially Degradable Pseudo-Semiconducting Polymer Nanoparticles That Integrate NIR-II Fluorescence Bioimaging, Photodynamic Immunotherapy, and Photo-Activated Chemotherapy. Advanced Materials 2022, 34, 2203820. [CrossRef]
- Wan, J.; Zhang, X.; Tang, D.; Liu, T.; Xiao, H. Biodegradable NIR-II Pseudo Conjugate Polymeric Nanoparticles Amplify Photodynamic Immunotherapy via Alleviation of Tumor Hypoxia and Tumor-Associated Macrophage Reprogramming. Advanced Materials 2023, 35, 2209799. [CrossRef]
- Bu, F.; Kang, X.; Tang, D.; Liu, F.; Chen, L.; Zhang, P.; Feng, W.; Yu, Y.; Li, G.; Xiao, H.; et al. Enhancing Near-Infrared II Photodynamic Therapy with Nitric Oxide for Eradicating Multidrug-Resistant Biofilms in Deep Tissues. Bioactive Materials 2024, 33, 341–354. [CrossRef]
- Tian, Y.; Yin, D.; Cheng, Q.; Dang, H.; Teng, C.; Yan, L. Supramolecular J-Aggregates of Aza-BODIPY by Steric and π–π Nteractions for NIR-II Phototheranostics. J. Mater. Chem. B 2022, 10, 1650–1662. [CrossRef]
- Zhu, Y.; Wu, P.; Liu, S.; Yang, J.; Wu, F.; Cao, W.; Yang, Y.; Zheng, B.; Xiong, H. Electron-Withdrawing Substituents Allow Boosted NIR-II Fluorescence in J-Type Aggregates for Bioimaging and Information Encryption. Angew Chem Int Ed 2023, 62, e202313166. [CrossRef]
- Dang, H.; Yin, D.; Tian, Y.; Cheng, Q.; Teng, C.; Xu, Y.; Yan, L. In Situ Formation of J-Aggregate in the Tumor Microenvironment Using Acidity Responsive Polypeptide Nanoparticle Encapsulating Galactose-Conjugated BODIPY Dye for NIR-II Phototheranostics. J. Mater. Chem. B 2022, 10, 5279–5290. [CrossRef]
- Li, F.-Z.; Wu, Z.; Lin, C.; Wang, Q.; Kuang, G.-C. Photophysical Properties Regulation and Applications of BODIPY-Based Derivatives with Electron Donor-Acceptor System. Results in Chemistry 2022, 4, 100384. [CrossRef]
- Bumagina, N.A.; Antina, E.V. Review of Advances in Development of Fluorescent BODIPY Probes (Chemosensors and Chemodosimeters) for Cation Recognition. Coordination Chemistry Reviews 2024, 505, 215688. [CrossRef]
- Mao, Z.; Kim, J.H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J.S. Engineering of BODIPY-Based Theranostics for Cancer Therapy. Coordination Chemistry Reviews 2023, 476, 214908. [CrossRef]
- Kim, G.; Luo, Y.; Shin, M.; Bouffard, J.; Bae, J.; Kim, Y. Making the Brightest Ones Dim: Maximizing the Photothermal Conversion Efficiency of BODIPY-Based Photothermal Agents. Adv Healthcare Materials 2024, 13, 2400885. [CrossRef]
- Koczorowski, T.; Glowacka-Sobotta, A.; Sysak, S.; Mlynarczyk, D.T.; Lesyk, R.; Goslinski, T.; Sobotta, L. BODIPY-Based Nanomaterials—Sensing and Biomedical Applications. Applied Sciences 2022, 12, 7815. [CrossRef]
- Chen, D.; Zhang, T.; Dong, X.; Mou, X. BODIPY Based Nanomedicine for Cancer Imaging and Phototherapy. Colloid and Interface Science Communications 2025, 64, 100816. [CrossRef]
- Zhu, J.; Tan, N.K.; Kikuchi, K.; Kaur, A.; New, E.J. BODIPY-based Fluorescent Indicators for Lipid Droplets. Analysis & Sensing 2024, 4, e202300049. [CrossRef]
- Fan, W.; Li, X. Using BODIPY FL-Sphingolipid Analogs to Study Sphingolipid Metabolism in Mouse Embryonic Stem Cells. BIO-PROTOCOL 2022, 12. [CrossRef]
- Jurgutis, D.; Jarockyte, G.; Poderys, V.; Dodonova-Vaitkuniene, J.; Tumkevicius, S.; Vysniauskas, A.; Rotomskis, R.; Karabanovas, V. Exploring BODIPY-Based Sensor for Imaging of Intracellular Microviscosity in Human Breast Cancer Cells. IJMS 2022, 23, 5687. [CrossRef]
- Nusshold, C.; Uellen, A.; Bernhart, E.; Hammer, A.; Damm, S.; Wintersperger, A.; Reicher, H.; Hermetter, A.; Malle, E.; Sattler, W. Endocytosis and Intracellular Processing of BODIPY-Sphingomyelin by Murine CATH.a Neurons. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2013, 1831, 1665–1678. [CrossRef]
- Qiu, B.; Simon, M. BODIPY 493/503 Staining of Neutral Lipid Droplets for Microscopy and Quantification by Flow Cytometry. BIO-PROTOCOL 2016, 6. [CrossRef]
- Listenberger, L.L.; Brown, D.A. Fluorescent Detection of Lipid Droplets and Associated Proteins. CP Cell Biology 2007, 35. [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Medicine 2021, 9, 20503121211034366. [CrossRef]
- Lin, Q.; Buccella, D. Highly Selective, Red Emitting BODIPY-Based Fluorescent Indicators for Intracellular Mg2+ Imaging. J. Mater. Chem. B 2018, 6, 7247–7256. [CrossRef]
- Kand, D.; Liu, P.; Navarro, M.X.; Fischer, L.J.; Rousso-Noori, L.; Friedmann-Morvinski, D.; Winter, A.H.; Miller, E.W.; Weinstain, R. Water-Soluble BODIPY Photocages with Tunable Cellular Localization. J. Am. Chem. Soc. 2020, 142, 4970–4974. [CrossRef]
- Spangenburg, E.E.; Pratt, S.J.P.; Wohlers, L.M.; Lovering, R.M. Use of BODIPY (493/503) to Visualize Intramuscular Lipid Dropletsin Skeletal Muscle. BioMed Research International 2011, 2011, 598358. [CrossRef]
- Miao, W.; Yu, C.; Hao, E.; Jiao, L. Functionalized BODIPYs as Fluorescent Molecular Rotors for Viscosity Detection. Front. Chem. 2019, 7, 825. [CrossRef]
- Ordóñez-Hernández, J.; Ferrusca-Martínez, F.; Jiménez-Sánchez, A. BODIPY-Derived Fluorescent Probes for Targeting and Tracking Lipid Droplets Dynamics. Tetrahedron 2024, 168, 134354. [CrossRef]
- Tatenaka, Y.; Kato, H.; Ishiyama, M.; Sasamoto, K.; Shiga, M.; Nishitoh, H.; Ueno, Y. Monitoring Lipid Droplet Dynamics in Living Cells by Using Fluorescent Probes. Biochemistry 2019, 58, 499–503. [CrossRef]
- Chen, J.; Yue, F.; Kuang, S. Labeling and Analyzing Lipid Droplets in Mouse Muscle Stem Cells. STAR Protocols 2022, 3, 101849. [CrossRef]
- Walter, E.R.H.; Lee, L.C.-C.; Leung, P.K.-K.; Lo, K.K.-W.; Long, N.J. Mitochondria-Targeting Biocompatible Fluorescent BODIPY Probes. Chem. Sci. 2024, 15, 4846–4852. [CrossRef]
- Polita, A.; Stancikaitė, M.; Žvirblis, R.; Maleckaitė, K.; Dodonova-Vaitkūnienė, J.; Tumkevičius, S.; Shivabalan, A.P.; Valinčius, G. Designing a Green-Emitting Viscosity-Sensitive 4,4-Difluoro-4-Bora-3a,4a-Diaza- s -Indacene (BODIPY) Probe for Plasma Membrane Viscosity Imaging. RSC Adv. 2023, 13, 19257–19264. [CrossRef]
- Collot, M.; Boutant, E.; Lehmann, M.; Klymchenko, A.S. BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjugate Chem. 2019, 30, 192–199. [CrossRef]
- Li, A.; Wang, F.; Li, Y.; Peng, X.; Liu, Y.; Zhu, L.; He, P.; Yu, T.; Chen, D.; Duan, M.; et al. Fluorination of Aza-BODIPY for Cancer Cell Plasma Membrane-Targeted Imaging and Therapy. ACS Appl. Mater. Interfaces 2025, acsami.4c17943. [CrossRef]
- Xiong, T.; Chen, Y.; Peng, Q.; Li, M.; Lu, S.; Chen, X.; Fan, J.; Wang, L.; Peng, X. Pyrazolone-Protein Interaction Enables Long-Term Retention Staining and Facile Artificial Biorecognition on Cell Membranes. J. Am. Chem. Soc. 2024, 146, 24158–24166. [CrossRef]
- Melo, R.C.N.; D’Avila, H.; Wan, H.-C.; Bozza, P.T.; Dvorak, A.M.; Weller, P.F. Lipid Bodies in Inflammatory Cells: Structure, Function, and Current Imaging Techniques. J Histochem Cytochem. 2011, 59, 540–556. [CrossRef]
- Courtis, A.M.; Santos, S.A.; Guan, Y.; Hendricks, J.A.; Ghosh, B.; Szantai-Kis, D.M.; Reis, S.A.; Shah, J.V.; Mazitschek, R. Monoalkoxy BODIPYs—A Fluorophore Class for Bioimaging. Bioconjugate Chem. 2014, 25, 1043–1051. [CrossRef]
- Ni, Y.; Zeng, L.; Kang, N.; Huang, K.; Wang, L.; Zeng, Z.; Chang, Y.; Wu, J. Meso -Ester and Carboxylic Acid Substituted BODIPYs with Far-Red and Near-Infrared Emission for Bioimaging Applications. Chemistry A European J 2014, 20, 2301–2310. [CrossRef]
- Jiang, X.-D.; Gao, R.; Yue, Y.; Sun, G.-T.; Zhao, W. A NIR BODIPY Dye Bearing 3,4,4a-Trihydroxanthene Moieties. Org. Biomol. Chem. 2012, 10, 6861. [CrossRef]
- Yang, Z.; Kang, D.H.; Lee, H.; Shin, J.; Yan, W.; Rathore, B.; Kim, H.-R.; Kim, S.J.; Singh, H.; Liu, L.; et al. A Fluorescent Probe for Stimulated Emission Depletion Super-Resolution Imaging of Vicinal-Dithiol-Proteins on Mitochondrial Membrane. Bioconjugate Chem. 2018, 29, 1446–1453. [CrossRef]
- Gayathri, T.; Karnewar, S.; Kotamraju, S.; Singh, S.P. High Affinity Neutral Bodipy Fluorophores for Mitochondrial Tracking. ACS Med. Chem. Lett. 2018, 9, 618–622. [CrossRef]
- Kesavan, P.E.; Pandey, V.; Raza, M.K.; Mori, S.; Gupta, I. Water Soluble Thioglycosylated BODIPYs for Mitochondria Targeted Cytotoxicity. Bioorganic Chemistry 2019, 91, 103139. [CrossRef]
- Yang, J.; Zhang, R.; Zhao, Y.; Tian, J.; Wang, S.; Gros, C.P.; Xu, H. Red/NIR Neutral BODIPY-Based Fluorescent Probes for Lighting up Mitochondria. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 248, 119199. [CrossRef]
- Chauhan, N.; Koli, M.; Ghosh, R.; Majumdar, A.G.; Ghosh, A.; Ghanty, T.K.; Mula, S.; Patro, B.S. A BODIPY-Naphtholimine-BF2 Dyad for Precision Photodynamic Therapy, Targeting, and Dual Imaging of Endoplasmic Reticulum and Lipid Droplets in Cancer. JACS Au 2024, 4, 2838–2852. [CrossRef]
- De Jong, F.; Pokorny, J.; Manshian, B.; Daelemans, B.; Vandaele, J.; Startek, J.B.; Soenen, S.; Van Der Auweraer, M.; Dehaen, W.; Rocha, S.; et al. Development and Characterization of BODIPY-Derived Tracers for Fluorescent Labeling of the Endoplasmic Reticulum. Dyes and Pigments 2020, 176, 108200. [CrossRef]
- Hoenke, S.; Brandes, B.; Csuk, R. Non-Cytotoxic Aza-BODIPY Triterpene Conjugates to Target the Endoplasmic Reticulum. European Journal of Medicinal Chemistry Reports 2023, 7, 100099. [CrossRef]
- Faulds, K.; McKenzie, F.; Smith, W.E.; Graham, D. Quantitative Simultaneous Multianalyte Detection of DNA by Dual-Wavelength Surface-Enhanced Resonance Raman Scattering. Angew Chem Int Ed 2007, 46, 1829–1831. [CrossRef]
- Adarsh, N.; Ramya, A.N.; Maiti, K.K.; Ramaiah, D. Unveiling NIR Aza-Boron-Dipyrromethene (BODIPY) Dyes as Raman Probes: Surface-Enhanced Raman Scattering (SERS)-Guided Selective Detection and Imaging of Human Cancer Cells. Chemistry A European J 2017, 23, 14286–14291. [CrossRef]
- Klapper, M.; Ehmke, M.; Palgunow, D.; Böhme, M.; Matthäus, C.; Bergner, G.; Dietzek, B.; Popp, J.; Döring, F. Fluorescence-Based Fixative and Vital Staining of Lipid Droplets in Caenorhabditis Elegans Reveal Fat Stores Using Microscopy and Flow Cytometry Approaches. Journal of Lipid Research 2011, 52, 1281–1293. [CrossRef]
- Ni, Y.; Kannadorai, R.K.; Peng, J.; Yu, S.W.-K.; Chang, Y.-T.; Wu, J. Naphthalene-Fused BODIPY near-Infrared Dye as a Stable Contrast Agent for in Vivo Photoacoustic Imaging. Chem. Commun. 2016, 52, 11504–11507. [CrossRef]
- Ni, Y.; Kannadorai, R.K.; Yu, S.W.-K.; Chang, Y.-T.; Wu, J. Push–Pull Type Meso-Ester Substituted BODIPY near-Infrared Dyes as Contrast Agents for Photoacoustic Imaging. Org. Biomol. Chem. 2017, 15, 4531–4535. [CrossRef]
- Miao, Q.; Lyu, Y.; Ding, D.; Pu, K. Semiconducting Oligomer Nanoparticles as an Activatable Photoacoustic Probe with Amplified Brightness for In Vivo Imaging of pH. Advanced Materials 2016, 28, 3662–3668. [CrossRef]
- Merkes, J.M.; Lammers, T.; Kancherla, R.; Rueping, M.; Kiessling, F.; Banala, S. Tuning Optical Properties of BODIPY Dyes by Pyrrole Conjugation for Photoacoustic Imaging. Advanced Optical Materials 2020, 8, 1902115. [CrossRef]
- Ma, D.; Hou, S.; Bae, C.; Pham, T.C.; Lee, S.; Zhou, X. Aza-BODIPY Based Probe for Photoacoustic Imaging of ONOO− in Vivo. Chinese Chemical Letters 2021, 32, 3886–3889. [CrossRef]
- Ma, Y.; Cao, H.; Chen, B.; Xu, X.; Zhang, Q.; Chen, H.; Zhang, X.; Song, G. Simultaneous In Vivo Imaging of Neutrophil Elastase and Oxidative Stress in Atherosclerotic Plaques Using a Unimolecular Photoacoustic Probe. Angew Chem Int Ed 2024, 63, e202411840. [CrossRef]
- Xu, M.; Sun, Q.; Wang, X.; Gao, H.; Liu, Z. Near-Infrared Absorbing BODIPY-Xanthene Hybrids for Multiplexed Photoacoustic Imaging. Org. Lett. 2024, 26, 3750–3755. [CrossRef]
- Awuah, S.G.; You, Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Adv. 2012, 2, 11169. [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [CrossRef]
- Caruso, E.; Gariboldi, M.; Sangion, A.; Gramatica, P.; Banfi, S. Synthesis, Photodynamic Activity, and Quantitative Structure-Activity Relationship Modelling of a Series of BODIPYs. Journal of Photochemistry and Photobiology B: Biology 2017, 167, 269–281. [CrossRef]
- Caruso, E.; Malacarne, M.C.; Marras, E.; Papa, E.; Bertato, L.; Banfi, S.; Gariboldi, M.B. New BODIPYs for Photodynamic Therapy (PDT): Synthesis and Activity on Human Cancer Cell Lines. Bioorganic & Medicinal Chemistry 2020, 28, 115737. [CrossRef]
- Kim, B.; Sui, B.; Yue, X.; Tang, S.; Tichy, M.G.; Belfield, K.D. In Vitro Photodynamic Studies of a BODIPY-Based Photosensitizer. Eur J Org Chem 2017, 2017, 25–28. [CrossRef]
- Durantini, A.M.; Greene, L.E.; Lincoln, R.; Martínez, S.R.; Cosa, G. Reactive Oxygen Species Mediated Activation of a Dormant Singlet Oxygen Photosensitizer: From Autocatalytic Singlet Oxygen Amplification to Chemicontrolled Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 1215–1225. [CrossRef]
- Epelde-Elezcano, N.; Martínez-Martínez, V.; Peña-Cabrera, E.; Gómez-Durán, C.F.A.; Arbeloa, I.L.; Lacombe, S. Modulation of Singlet Oxygen Generation in Halogenated BODIPY Dyes by Substitution at Their Meso Position: Towards a Solvent-Independent Standard in the Vis Region. RSC Adv. 2016, 6, 41991–41998. [CrossRef]
- Yu, Z.; Zhou, J.; Ji, X.; Lin, G.; Xu, S.; Dong, X.; Zhao, W. Discovery of a Monoiodo Aza-BODIPY Near-Infrared Photosensitizer: In Vitro and in Vivo Evaluation for Photodynamic Therapy. J. Med. Chem. 2020, 63, 9950–9964. [CrossRef]
- Nguyen, V.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew Chem Int Ed 2020, 59, 8957–8962. [CrossRef]
- Wang, L.; Bai, J.; Qian, Y. Synthesis of a Triphenylamine BODIPY Photosensitizer with D–A Configuration and Its Application in Intracellular Simulated Photodynamic Therapy. New J. Chem. 2019, 43, 16829–16834. [CrossRef]
- Wang, C.; Qian, Y. A Water Soluble Carbazolyl-BODIPY Photosensitizer with an Orthogonal D–A Structure for Photodynamic Therapy in Living Cells and Zebrafish. Biomater. Sci. 2020, 8, 830–836. [CrossRef]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angewandte Chemie 2020, 132, 16248–16255. [CrossRef]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [CrossRef]
- Filatov, M.A. Heavy-Atom-Free BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer. Org. Biomol. Chem. 2020, 18, 10–27. [CrossRef]
- Zhang, W.; Ahmed, A.; Cong, H.; Wang, S.; Shen, Y.; Yu, B. Application of Multifunctional BODIPY in Photodynamic Therapy. Dyes and Pigments 2021, 185, 108937. [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. IJMS 2022, 23, 10198. [CrossRef]
- Li, J.; Wang, J.; Zhang, D.; Cui, T.; Xu, Z.; Jiang, X.-D. Synthesis, Structure and Photochemical Properties of Asymmetric NMe2-Bearing Aza-BODIPYs as Novel Photothermal Agents. Dyes and Pigments 2022, 199, 110092. [CrossRef]
- Liu, Y.; Song, N.; Chen, L.; Liu, S.; Xie, Z. Synthesis of a Near-Infrared BODIPY Dye for Bioimaging and Photothermal Therapy. Chemistry An Asian Journal 2018, 13, 989–995. [CrossRef]
- Pewklang, T.; Saiyasombat, W.; Chueakwon, P.; Ouengwanarat, B.; Chansaenpak, K.; Kampaengsri, S.; Lai, R.; Kamkaew, A. Revolutionary Pyrazole-based Aza-BODIPY: Harnessing Photothermal Power Against Cancer Cells and Bacteria. ChemBioChem 2024, 25, e202300653. [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 601820. [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A Realistic Appraisal of Boron Neutron Capture Therapy as a Cancer Treatment Modality. Cancer Communications 2018, 38, 1–7. [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. International Journal of Particle Therapy 2022, 9, 71–82. [CrossRef]
- Li, X.; Golberg, D. Boron Nitride Nanotubes as Drug Carriers. In Boron Nitride Nanotubes in Nanomedicine; Elsevier, 2016; pp. 79–94 ISBN 978-0-323-38945-7.
- Xuan, S.; Zhao, N.; Zhou, Z.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and in Vitro Studies of a Series of Carborane-Containing Boron Dipyrromethenes (BODIPYs). J. Med. Chem. 2016, 59, 2109–2117. [CrossRef]
- Gibbs, J.H.; Wang, H.; Bhupathiraju, N.V.S.D.K.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis and Properties of a Series of Carboranyl-BODIPYs. Journal of Organometallic Chemistry 2015, 798, 209–213. [CrossRef]
- Nakata, E.; Koizumi, M.; Yamashita, Y.; Onaka, K.; Sakurai, Y.; Kondo, N.; Ono, K.; Uto, Y.; Hori, H. Design, Synthesis and Destructive Dynamic Effects of BODIPY-Containing and Curcuminoid Boron Tracedrugs for Neutron Dynamic Therapy. Anticancer Res 2011, 31, 2477–2481.
- Kalot, G.; Godard, A.; Busser, B.; Pliquett, J.; Broekgaarden, M.; Motto-Ros, V.; Wegner, K.D.; Resch-Genger, U.; Köster, U.; Denat, F.; et al. Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications. Cells 2020, 9, 1953. [CrossRef]
- Bellomo, C.; Zanetti, D.; Cardano, F.; Sinha, S.; Chaari, M.; Fin, A.; Maranzana, A.; Núñez, R.; Blangetti, M.; Prandi, C. Red Light-Emitting Carborane-BODIPY Dyes: Synthesis and Properties of Visible-Light Tuned Fluorophores with Enhanced Boron Content. Dyes and Pigments 2021, 194, 109644. [CrossRef]
- Zhang, T.; Ma, C.; Sun, T.; Xie, Z. Unadulterated BODIPY Nanoparticles for Biomedical Applications. Coordination Chemistry Reviews 2019, 390, 76–85. [CrossRef]
- Lin, W.; Colombani-Garay, D.; Huang, L.; Duan, C.; Han, G. Tailoring Nanoparticles Based on Boron Dipyrromethene for Cancer Imaging and Therapy. WIREs Nanomed Nanobiotechnol 2020, 12, e1627. [CrossRef]
- Wang, X.; Jiang, Z.; Liang, Z.; Wang, T.; Chen, Y.; Liu, Z. Discovery of BODIPY J-Aggregates with Absorption Maxima beyond 1200 Nm for Biophotonics. Sci. Adv. 2022, 8, eadd5660. [CrossRef]
- Liu, Z.; Jiang, Z.; Yan, M.; Wang, X. Recent Progress of BODIPY Dyes With Aggregation-Induced Emission. Front. Chem. 2019, 7, 712. [CrossRef]
- Descalzo, A.B.; Ashokkumar, P.; Shen, Z.; Rurack, K. On the Aggregation Behaviour and Spectroscopic Properties of Alkylated and Annelated Boron-Dipyrromethene (BODIPY) Dyes in Aqueous Solution. ChemPhotoChem 2020, 4, 120–131. [CrossRef]
- Kumar, P.P.P.; Yadav, P.; Shanavas, A.; Neelakandan, P.P. Aggregation Enhances Luminescence and Photosensitization Properties of a Hexaiodo-BODIPY. Mater. Chem. Front. 2020, 4, 965–972. [CrossRef]
- Salis, F.; Descalzo, A.B.; Benito-Peña, E.; Moreno-Bondi, M.C.; Orellana, G. Highly Fluorescent Magnetic Nanobeads with a Remarkable Stokes Shift as Labels for Enhanced Detection in Immunoassays. Small 2018, 14, 1703810. [CrossRef]
- Rahman, A.; Praveen Kumar, P.P.; Yadav, P.; Goswami, T.; Shanavas, A.; Ghosh, H.N.; Neelakandan, P.P. Gold–BODIPY Nanoparticles with Luminescence and Photosensitization Properties for Photodynamic Therapy and Cell Imaging. ACS Appl. Nano Mater. 2022, 5, 6532–6542. [CrossRef]
- Kumar, P.P.P.; Rahman, A.; Goswami, T.; Ghosh, H.N.; Neelakandan, P.P. Fine-Tuning Plasmon-Molecule Interactions in Gold-BODIPY Nanocomposites: The Role of Chemical Structure and Noncovalent Interactions. ChemPlusChem 2021, 86, 87–94. [CrossRef]
- Kumar, P.P.P. A Multimode Detection Platform for Biothiols Using BODIPY Dye-Conjugated Gold Nanoparticles. Colorants 2024, 3, 214–228. [CrossRef]
- Kumar, P.P.P.; Yadav, P.; Shanavas, A.; Thurakkal, S.; Joseph, J.; Neelakandan, P.P. A Three-Component Supramolecular Nanocomposite as a Heavy-Atom-Free Photosensitizer. Chem. Commun. 2019, 55, 5623–5626. [CrossRef]
- Praveen Kumar, P.P.; Kaur, N.; Shanavas, A.; Neelakandan, P.P. Nanomolar Detection of Biothiols via Turn-ON Fluorescent Indicator Displacement. Analyst 2020, 145, 851–857. [CrossRef]
- Liu, Y.; Song, N.; Chen, L.; Xie, Z. Triple-BODIPY Organic Nanoparticles with Particular Fluorescence Emission. Dyes and Pigments 2017, 147, 241–245. [CrossRef]
- Li, Z.; Zheng, M.; Guan, X.; Xie, Z.; Huang, Y.; Jing, X. Unadulterated BODIPY-Dimer Nanoparticles with High Stability and Good Biocompatibility for Cellular Imaging. Nanoscale 2014, 6, 5662–5665. [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Zhang, Y.; Wu, S.; Zhao, J.; Han, G. Ultralow-Power Near Infrared Lamp Light Operable Targeted Organic Nanoparticle Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 14586–14591. [CrossRef]
- Huang, L.; Li, Z.; Zhao, Y.; Yang, J.; Yang, Y.; Pendharkar, A.I.; Zhang, Y.; Kelmar, S.; Chen, L.; Wu, W.; et al. Enhancing Photodynamic Therapy through Resonance Energy Transfer Constructed Near-Infrared Photosensitized Nanoparticles. Advanced Materials 2017, 29, 1604789. [CrossRef]
- Naim, K.; Nair, S.T.; Yadav, P.; Shanavas, A.; Neelakandan, P.P. Supramolecular Confinement within Chitosan Nanocomposites Enhances Singlet Oxygen Generation. ChemPlusChem 2018, 83, 418–422. [CrossRef]
- Tang, Q.; Xiao, W.; Huang, C.; Si, W.; Shao, J.; Huang, W.; Chen, P.; Zhang, Q.; Dong, X. pH-Triggered and Enhanced Simultaneous Photodynamic and Photothermal Therapy Guided by Photoacoustic and Photothermal Imaging. Chem. Mater. 2017, 29, 5216–5224. [CrossRef]
- Lin, W.; Zhang, W.; Liu, S.; Li, Z.; Hu, X.; Xie, Z.; Duan, C.; Han, G. Engineering pH-Responsive BODIPY Nanoparticles for Tumor Selective Multimodal Imaging and Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 43928–43935. [CrossRef]
- Zou, J.; Wang, P.; Wang, Y.; Liu, G.; Zhang, Y.; Zhang, Q.; Shao, J.; Si, W.; Huang, W.; Dong, X. Penetration Depth Tunable BODIPY Derivatives for pH Triggered Enhanced Photothermal/Photodynamic Synergistic Therapy. Chem. Sci. 2019, 10, 268–276. [CrossRef]
- Villalobos Gutiérrez, P.; Muñoz Carrillo, J.; Sandoval Salazar, C.; Viveros Paredes, J.; Gutiérrez Coronado, O. Functionalized Metal Nanoparticles in Cancer Therapy. Pharmaceutics 2023, 15, 1932. [CrossRef]
- Malacarne, M.C.; Caruso, E.; Gariboldi, M.B.; Marras, E.; Della Bitta, G.; Santoro, O.; Simm, A.; Li, R.; Ferguson, C.T.J. Evaluation of Nanoparticles Covalently Bound with BODIPY for Their Photodynamic Therapy Applicability. IJMS 2024, 25, 3187. [CrossRef]
- Topel, S.D.; Topel, Ö.; Bostancıoğlu, R.B.; Koparal, A.T. Synthesis and Characterization of Bodipy Functionalized Magnetic Iron Oxide Nanoparticles for Potential Bioimaging Applications. Colloids and Surfaces B: Biointerfaces 2015, 128, 245–253. [CrossRef]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Sig Transduct Target Ther 2023, 8, 418. [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current Advance of Nanotechnology in Diagnosis and Treatment for Malignant Tumors. Sig Transduct Target Ther 2024, 9, 200. [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol Cancer 2023, 22, 169. [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res Lett 2021, 16, 173. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).