Introduction
Symptomatic neuromas are a well-described and difficult complication of peripheral nerve injuries. Following nerve trauma, disorganized axonal growth between the proximal and distal nerve ends can lead to the formation of a painful nerve stump [
1]. Patients may report pain, hyperalgesia, paresthesia, or loss of function in the affected region. Furthermore, significant mental and physical disabilities are associated with upper extremity neuromas [
2]. Various treatment algorithms currently exist for the management of symptomatic neuromas [
2]. Nonsurgical interventions such as therapy, pharmacologic management, desensitization, and ablation are possible interventions performed before surgical modalities. Surgical management is considered after failure of conservative care. Although various surgical strategies have been proposed, management typically consists of resection of the neuroma stump and adjunctive treatment to minimize the risk of neuroma recurrence.
The surgical management of neuromas can be classified as ablative (passive) or reconstructive (active) [
2]. Passive techniques involve nerve relocation such as neurectomy, nerve capping, or implantation into the muscle or bone. Active techniques aim to achieve nerve regeneration, including neuroma resection, primary repair, nerve reconstruction, end-to-side neurorrhaphy, regenerative peripheral nerve interfaces (RPNI), vascularized denervated muscle targets (VDMT), and targeted muscle reinnervation (TMR). TMR provides a physiologically favorable environment for axonal regeneration by implanting the proximal nerve stump into the intact motor end target of an innervated muscle. Initially developed to amplify myoelectric signals for upper-extremity prostheses, TMR has recently shown success in reducing the rate of neuroma pain among amputees [
3,
4].
Radial sensory nerve (RSN) neuromas are particularly challenging for both patients and surgeons [
2]. Nonsurgical modalities are often ineffective, and surgical treatment has a high rate of recurrence. Literature has shown that surgical management of RSN neuromas results in a 20% rate of secondary surgery, with only 68% of patients reporting improvement after surgical intervention [
5]. There are various surgical techniques to effectively manage RSN; however, the success rates of these techniques vary [
6,
7,
8]. More recently, TMR of the anterior interosseous nerve (AIN) has emerged as a viable and clinically favorable treatment option for RSN neuroma in the distal forearm [
9,
10,
11].
In this study, we present additional cadaveric and clinical outcome data for TMR treatment of symptomatic RSN neuromas with the transfer of the RSN to the AIN. We propose a classification system for this type of transfer related to various levels of RSN injury.
Materials and Methods
Surgical Technique
We used five below-elbow cadaveric specimens to determine the feasibility of RSN to AIN transfer at different levels of injury. An “S” shaped incision was created with the longitudinal limbs over the RSN distally and the AIN proximally. On average, the RSN pierced the forearm fascia at the brachioradialis (BR), approximately 9 cm proximal to the radial styloid. The RSN split into radial and ulnar divisions in all specimens prior to arborizing over the dorsal and radial hand distally.
The AIN branched distal to the flexor digitorum superficialis (FDS) arch and entered the proximal edge of the pronator quadratus (PQ) after the innervation of the flexor digitorum profundus (FDP) and flexor pollicis longus (FPL). The AIN coursed radial to the anterior interosseous artery and dorsal to the PQ in all specimens. On average, the terminal motor branch of the AIN was found approximately 2 cm proximal to the ulnar head in the mid-substance of the PQ, prior to continuing as its terminal sensory branch.
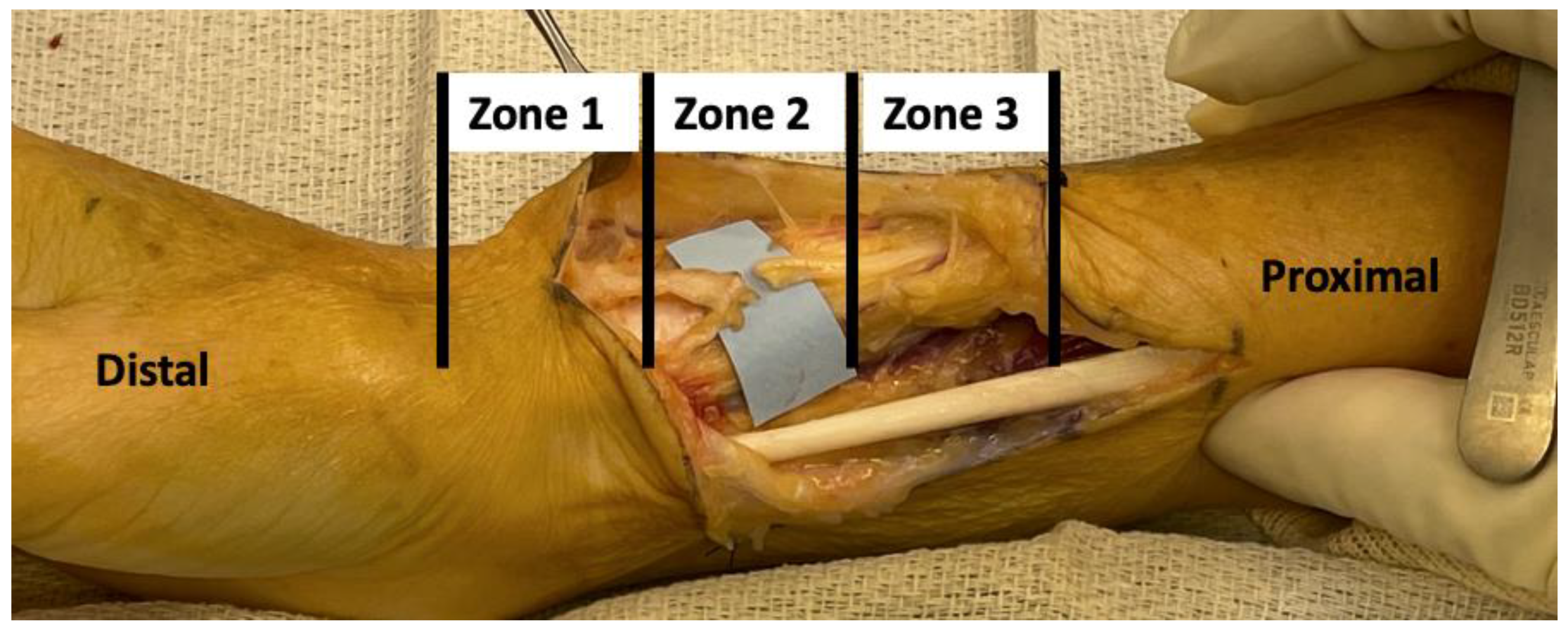
We simulated sequential RSN injury in a distal-to-proximal fashion. We classified the simulated RSN injuries into three zones. Zone one injuries comprised those up to 4 cm distal to the bifurcation of the RSN after piercing the BR. Zone two injuries comprised those at the bifurcation level. Zone three injuries comprised injuries up to 4 cm proximal to the bifurcation (
Figure 1).
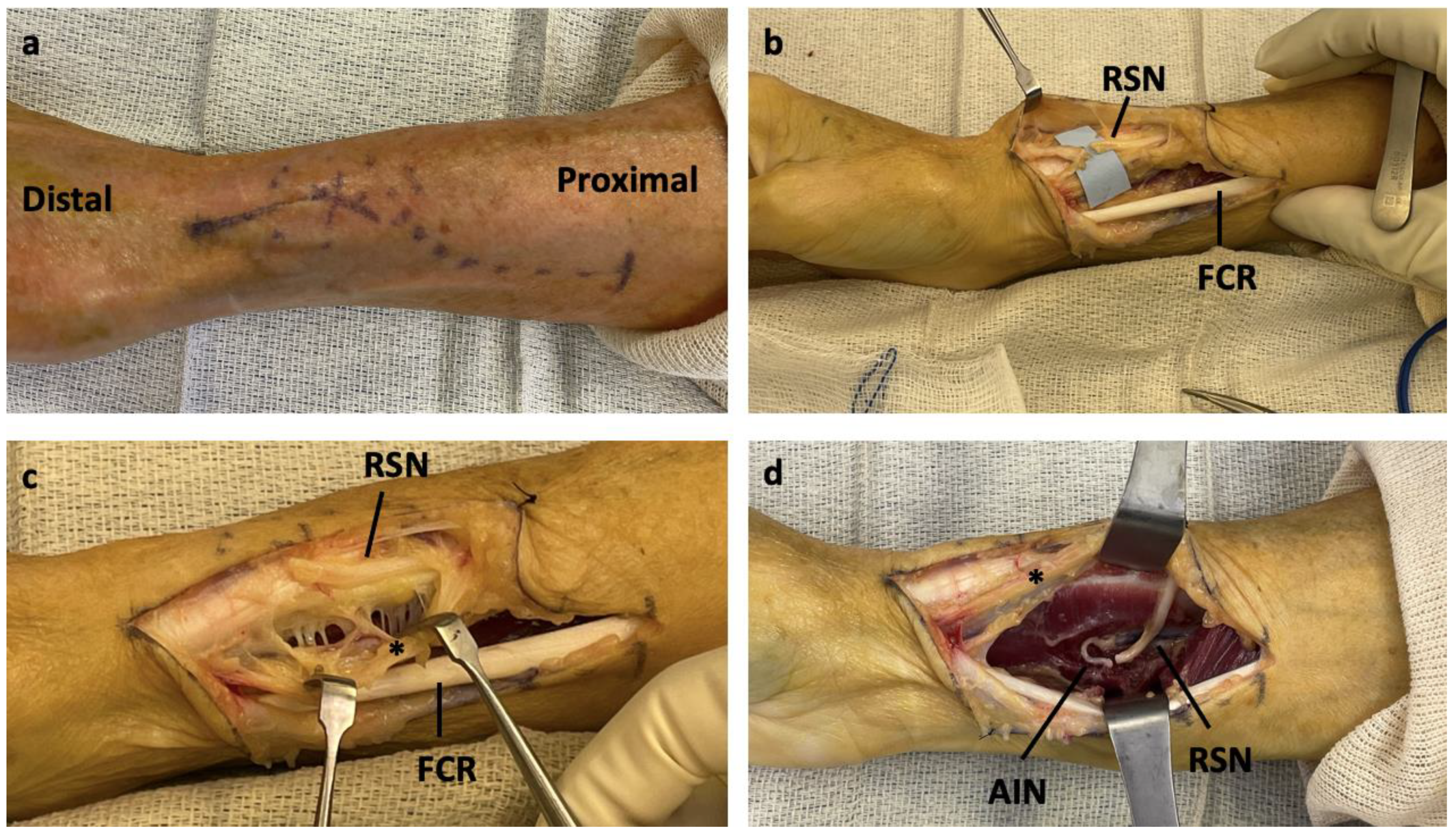
An S-shaped incision was made from the volar forearm to the radial styloid (
Figure 2A). The zone of injury for the RSN was identified (
Figure 2B). The radial artery was identified and protected while the dissection proceeded ulnar to the vessel (
Figure 2C). The deep fascia of the flexor carpi radialis (FCR) was incised, and the FPL was retracted in the ulnar direction. AIN was identified at the proximal edge of the PQ. After mobilization of the AIN, the proximal limb of the transected RSN was mobilized. Passage of the RSN to the AIN was feasible, either under or above the radial artery. In our opinion, passage deep to the radial artery resulted in a more favorable position of the RSN for the transfer. For each level of nerve transection, coaptation of the RSN to the AIN was completed (
Figure 2D).
Clinical Outcomes
Five cases of RSN to AIN nerve transfer were performed by a single fellowship-trained hand surgeon in patients with symptomatic RSN neuromas who failed prior treatment. Over the course of one-year, clinical outcomes were prospectively collected. The primary outcomes of interest were the visual analog scale (VAS) pain scores and Quick Disabilities of Arm, Shoulder, & Hand (DASH) scores. Other outcomes of interest included wrist flexion-extension arc, wrist radial-ulnar deviation arc, and complications, such as changes in sensorimotor function. All data were collected in accordance with the institutional review board approval, and statistical analysis was performed using paired two-tailed Student’s t-tests. Specifically, the mean preoperative outcomes were compared to the mean one-year post-operative outcomes.
Patient Characteristics
Patient 1 -Zone 1 Injury
Patient 1 was a 56-year-old female who had sustained a sharp RSN laceration 3 years prior to presentation. She underwent an attempted primary repair at an outside institution, followed by subsequent nerve reconstruction with an allograft for persistent pain 1 year later. On presentation to our clinic, she reported ongoing pain, hyperesthesia, and an inability to return to her occupation.
Patient 2 -Zone 2 Injury
Patient 2 was a 37-year-old male who sustained iatrogenic RSN injury during a 1st dorsal compartment release 1 year prior to presentation. Primary repair was attempted at the time of the injury. Six months later, he underwent a secondary neuroma resection with nerve burial 6 months later for persistent pain. He presented to our clinic 6 months postoperatively after secondary nerve burial with persistent pain and dysfunction.
Patient 3 -Zone 2 Injury
Patient 3 was a 45-year-old female who sustained a crush injury to her wrist two years prior to presentation. Her post-injury course included initial treatment for complex regional pain syndrome (CRPS), followed by surgical exploration, nerve resection, and allograft reconstruction for a neuroma in situ 6 months after injury. She presented to our clinic 18 months after allograft reconstruction with persistent wrist pain and dysesthesia.
Patient 4 -Zone 3 Injury
Patient 4 was a 58 year old female with a sharp laceration of her wrist from a kitchen knife. She underwent primary RSN repair with persistent pain and paresthesia following her repair. She underwent six months of occupational therapy and presented to our clinic one year postoperatively.
Patient 5 -Zone 3 Injury
Patient 5 was a 22 year old male who had sustained a laceration from a metal canister 2 years prior to presentation. The patient underwent primary nerve reconstruction with an allograft. He underwent subcutaneous neurolysis and nerve wrapping 6 months after the initial surgery for persistent pain. He presented to our clinic 15 months after the first surgery for ongoing pain and dysesthesia.
Results
Surgical Technique
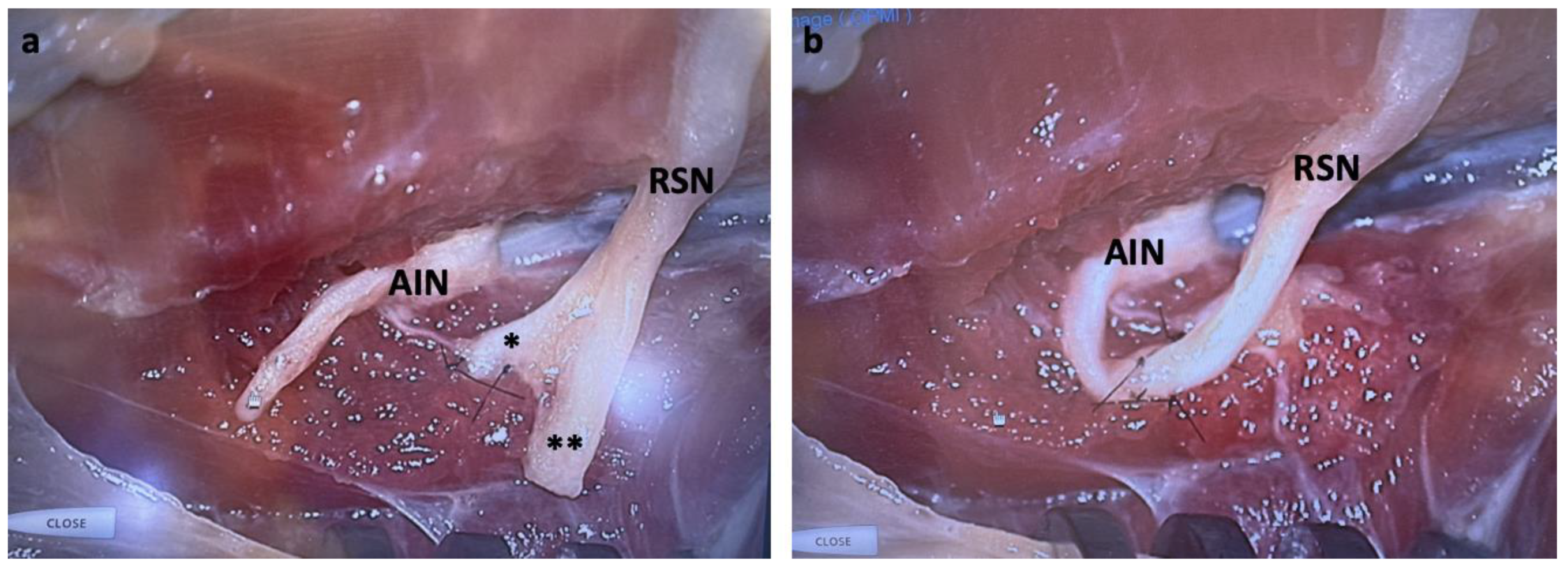
All cadaveric specimens underwent successful nerve transfer. For zone one injuries, the length of the RSN limb was sufficient to allow coaptation of the radial or ulnar branch to the terminal branch of the AIN after innervation of the terminal motor branch in the PQ. We then buried the remaining radial or ulnar branches in the PQ muscle. For zone two injuries, we coapted the radial or ulnar branch of the RSN to the AIN at the midpoint of the PQ, with the remaining branch again buried in the muscle (
Figure 3A). For zone three injuries, we coapted the RSN to the AIN prior to PQ innervation to allow sufficient length for the transfer (
Figure 3B). As zone three was proximal to the bifurcation, no supplemental nerve burial was required for the remaining branch. Simulated RSN injuries > 4 cm proximal to the bifurcation created excessive tension on the transfer and was not feasible.
Clinical Outcomes
RSN to AIN transfer was successfully performed in five patients with symptomatic RSN neuroma who had failed prior primary repair and secondary reconstruction. The transfer was performed successfully for all identified zones. All patients had a minimum follow-up of one-year. No postoperative complications occurred in any patient, and none of the patients required revision surgery. As expected, all the patients had persistent radial sensory nerve distribution numbness. All patients reported satisfaction with their outcomes and stated that they would recommend surgery to other patients. The mean VAS pain score significantly improved by 6±2 points (range 4-8, p < 0.05). The mean QuickDASH scores significantly improved by 37±11points (range 25-58, p < 0.05). The wrist flexion-extension arc of motion significantly improved by 30±14 degrees (range 20-55, p < 0.05). The radial–ulnar deviation arc of motion significantly improved by 10±3 °(range 5-15, p < 0.05).
Discussion
TMR is a promising approach for the treatment of neuromas. TMR has been shown to successfully reduce recurrent neuroma pain [
3,
4,
12]. Our study supports that AIN is a viable motor target for painful sensory neuromas of the wrist [
9,
13]. The anatomic and technical feasibility of RSN to AIN coaptation has been successfully performed in cadaveric models, and recent studies have reported clinical applications with favorable outcomes as well [
9,
10,
11]. Grome et al. were the first to propose an RSN TMR with the most distal extent of the AIN to the PQ. In a cadaveric model, they divided the AIN just distal to the final branch to the FPL and mobilized it for coaptation to the sensory nerves of the wrist, including the RSN [
9]. They reported only one successful clinical application involving a painful neuroma of the palmar cutaneous branch of the median nerve (PCBMN), and not the RSN. More recently, Langeveld et al. published a detailed cadaveric investigation of RSN and its potential motor targets for TMR. In seven forearms, they found that the distal AIN was a suitable donor target for neuromas of the distal third of the forearm and hand but not the proximal two-thirds of the forearm [
10]. Furthermore, Muneer et al. were the first to report the clinical outcomes of TMR for RSN neuromas with AIN as the motor target. They reported the outcomes of six cases involving RSN. They concluded that AIN was a first-choice motor target for peripheral sensory neuromas around the wrist [
11]. However, similar to Grome et al. and Langeveld et al., their surgical techniques involved mobilization of the AIN distal to the FPL branch with denervation of the PQ. All previous investigators felt that PQ was expendable in patients with intact pronator teres (PT), but did not provide options if this was not the case.
In our study, variations in treatment were dictated by the RSN injury zone, which has not been previously reported. As shown in
Table 1, we categorized the location of RSN injury into three zones: zone 1 injuries occur distal to the bifurcation, zone 2 injuries occur at the level of the bifurcation, and zone 3 injuries occur proximal to the bifurcation. In our cohort, zone 3 injuries were treated using isolated RSN to AIN end-to-end coaptation. However, Zone 1 and 2 injuries were treated with RSN to AIN end-to-end coaptation, in addition to burial of the ulnar or radial limb in the PQ. Importantly, all transfers produced a tension-free repair with preserved range of motion postoperatively. This novel classification system establishes an anatomic treatment-based approach to characterize RSN neuromas. Additionally, our surgical technique does not involve denervation of the PQ, which will be useful in the treatment armamentarium for patients without a functional PT.
Furthermore, this study, compared to previous studies, describes more in-depth clinical outcomes of successful RSN to AIN transfer performed for five patients with symptomatic RSN neuromas who failed prior primary and secondary surgeries. The literature on outcomes following the surgical management of RSN neuromas for comparison is limited. Stokvis et al. reported a 33% patient satisfaction rate after the surgical management of 18 primary RSN neuromas [
14]. In comparison, all patients in our study were satisfied with their outcomes and recommended surgery. Gottlieb et al. evaluated the outcomes following surgical treatment of 54 primary RSN neuromas. In their study, 68% of the patients reported improvement after primary repair, and 20% of the patients required secondary surgery [
5]. Muneer et al. offered the first clinical outcomes for RSN TMR to AIN and observed an improvement in VAS pain scores from seven to two [
11]. However, their findings were grouped with outcomes of PCBMN neuromas and subsequent MN to AIN TMR without a subgroup analysis. In comparison, our study reported outcomes of only RSN to AIN TMR, and we found statistically significant improvements in mean VAS pain scores and QuickDASH scores (p < 0.05). The degree of improvement in both measures exceeded the established thresholds for substantial clinical benefit (SCB). Specifically, the SCB for VAS pain score established by Randall et al is 2.4 (range 2.2-2.6) and the SCB for QuickDASH score established by Hubbard et al is 19.9 (range 16.9-22.8) [
15,
16]. The knowledge of this degree of improvement provides useful context when discussing the risks and benefits of the surgery with patients. Our results are promising but insufficient to change the standard of surgical care for primary RSN neuroma from neuroma excision and grafting. However, as TMR continues to be implemented and studied, the standard can change its favor.
This study was limited by the small sample size. Additional larger studies with longer follow-up periods are needed to better assess the effectiveness of this emerging technique and usefulness of our classification system. We classified the feasibility of this transfer for the zones of RSN injury in cadaveric models. More importantly, when our system was applied, we observed statistically significant improvements in function, pain, and wrist range of motion. We consider this technique to be a viable option for the treatment of refractory, symptomatic RSN neuromas that warrant further study.
Financial Disclosure Statement
No conflicts of interest for all authors.
References
- Watson, J.; Gonzalez, M.; Romero, A.; Kerns, J. Neuromas of the hand and upper extremity. J Hand Surg Am 2010, 35, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, K.R.; Ducic, I. Surgical Algorithm for Neuroma Management: A Changing Treatment Paradigm. Plast Reconstr Surg Glob Open 2018, 6, e1952. [Google Scholar] [CrossRef]
- Bowen, J.B.; Wee, C.E.; Kalik, J.; Valerio, I.L. Targeted Muscle Reinnervation to Improve Pain, Prosthetic Tolerance, and Bioprosthetic Outcomes in the Amputee. Adv Wound Care (New Rochelle) 2017, 6, 261–267. [Google Scholar] [CrossRef]
- Souza, J.M.; Cheesborough, J.E.; Ko, J.H.; Cho, M.S.; Kuiken, T.A.; Dumanian, G.A. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res 2014, 472, 2984–2990. [Google Scholar] [CrossRef]
- Gottlieb, R.W.; Westenberg, R.F.; Chen, N.C.; Coert, J.H.; Eberlin, K.R. Long-Term Outcomes after Surgical Treatment of Radial Sensory Nerve Neuromas: Patient-Reported Outcomes and Rate of Secondary Surgery. Plast Reconstr Surg 2021, 147, 101–111. [Google Scholar] [CrossRef]
- Al-Qattan, M.M. Prevention and treatment of painful neuromas of the superficial radial nerve by the end-to-side nerve repair concept: an experimental study and preliminary clinical experience. Microsurgery 2000, 20, 99–104. [Google Scholar] [CrossRef]
- Aszmann, O.C.; Korak, K.J.; Rab, M.; Grunbeck, M.; Lassmann, H.; Frey, M. Neuroma prevention by end-to-side neurorraphy: an experimental study in rats. J Hand Surg Am 2003, 28, 1022–1028. [Google Scholar] [CrossRef]
- Konofaos, P.; Bassilios Habre, S.; Wallace, R.D. End-to-Side Nerve Repair: Current Concepts and Future Perspectives. Ann Plast Surg 2018, 81, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Grome, L.J.; Agrawal, N.A.; Wang, E.; Netscher, D.T. Targeted Muscle Reinnervation for Symptomatic Neuromas Utilizing the Terminal Anterior Interosseous Nerve. Plast Reconstr Surg Glob Open 2020, 8, e2979. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, M.; Bruin, L.L.; Hundepool, C.A.; Power, D.; Duraku, L.S.; Zuidam, J.M. Anatomy of the Superficial Radial Nerve and Its Target Nerves for Targeted Muscle Reinnervation: An Anatomical Cadaver Study. Plast Reconstr Surg 2024, 153, 95e–100e. [Google Scholar] [CrossRef] [PubMed]
- Muneer, M. TMR for Peripheral Sensory Nerve Neuroma around the Wrist Utilizing the Distal Anterior Interosseous Nerve. Plast Reconstr Surg Glob Open 2024, 12, e5531. [Google Scholar] [CrossRef] [PubMed]
- Pierrie, S.N.; Gaston, R.G.; Loeffler, B.J. Targeted Muscle Reinnervation for Prosthesis Optimization and Neuroma Management in the Setting of Transradial Amputation. J Hand Surg Am 2019, 44, 525–e521. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M.; Rose, A.; King, W.; Engelman, K.; Bednarz, B. Anterior Interosseous Nerve to Ulnar Nerve Transfer: A Systematic Review. JPRAS Open 2022, 32, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Stokvis, A.; Coert, J.H.; van Neck, J.W. Insufficient pain relief after surgical neuroma treatment: Prognostic factors and central sensitisation. J Plast Reconstr Aesthet Surg 2010, 63, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.C.; Zhang, Y.; Qiu, Y.; Yoo, M.; Stephens, A.R.; Zeidan, M.; Kazmers, N.H. Establishing the Substantial Clinical Benefit in a Non-Shoulder Hand and Upper Extremity Population for the QuickDASH and PROMIS Upper Extremity and Physical Function Computer Adaptive Tests. J Hand Surg Am 2022, 47, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.J.; Zhang, Y.; Li, H.; Hubbard, J.C.; Kazmers, N.H. Establishing the Minimal Clinically Important Difference and Substantial Clinical Benefit for the Pain Visual Analog Scale in a Postoperative Hand Surgery Population. J Hand Surg Am 2022, 47, 645–653. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).