1. Introduction
The importance of H
2S (hydrogen sulfide) in organisms manifests in multiple ways[
1,
2,
3]. It is not only a toxic, flammable, and corrosive gas within organisms but also a vital gaseous signaling molecule involved in regulating various physiological and pathological processes. The H
2S exerts a bidirectional regulatory effect on vascular tone, capable of both relaxing and contracting blood vessels[
4,
5,
6]. Additionally, H
2S can influence the proliferation and apoptosis of vascular smooth muscle cells, as well as vascular autophagy, thereby affecting cardiovascular diseases such as atherosclerosis[
7,
8]. H
2S also regulates bronchial tone, participates in gas exchange within the lungs, modulates respiration, and is associated with the occurrence of diseases such as asthma, wheezing, pneumonia, and lung injury. As research on H
2S continues to deepen, scientists have discovered its broad application prospects in the medical field. For instance, exogenous delivery of H
2S or modulation of endogenous H
2S can improve cardiac function, reduce ischemia-reperfusion injury, and mitigate heart complications of various other cardiac diseases, including arrhythmia, heart failure, myocardial hypertrophy, myocardial fibrosis, and myocardial infarction. Furthermore, H
2S-related therapeutic agents also hold promise in the treatment of neurodegenerative diseases[
9]. Therefore, the development of novel nanoplatforms for controlled H
2S release holds significant importance for the treatment of several major diseases.
Extensive research has been conducted on the design of probes for H
2S release. For example, the Pry-Ps@CP-PEG multifunctional hydrogen sulfide nanoregulator designed by the research team can accurately locate tumor areas, achieve precise photothermal therapy, and rapidly release hydrogen sulfide to alleviate inflammation[
10]. However, the majority of H
2S donors often release inadequate amounts, necessitating an increase in dosage during treatment, which subsequently heightens drug toxicity. Furthermore, the swift release kinetics of numerous H
2S donors not only hinders prolonged therapeutic effectiveness but also poses a risk of intensifying bodily injury.[
11,
12]. Another approach involves loading both the H
2S prodrug ADT and magnetic nanoparticles into liposomes to construct targeted controlled-release nanosystems (AMLs) for tumor-targeted therapy. This system can release H
2S at the tumor site to achieve the purpose of tumor treatment. In summary, although various nanomaterials capable of releasing hydrogen sulfide have been developed, they still have limitations in terms of biocompatibility, controllability, preparation cost, targeting ability, and efficiency. Therefore, it is urgently needed to overcome these limitations and develop safer, more efficient, and controllable hydrogen sulfide nano-release materials.
Therefore, in order to enhance the performance of H2S releasing materials and expand their applications, we successfully synthesized iron sulfide (Fe3S4) nanoparticles with a particle size of approximately 16 nanometers using the hydrothermal method. Subsequently, we further modified these nanoparticles with liposomes, obtaining a composite material with superior bioavailability, which we named Fe3S4@Lip. The Fe3S4 nanoparticles can slowly and persistently release H2S gas under weak acidic conditions, a characteristic that renders them potentially valuable for biological applications. More importantly, Fe3S4@Lip, after modification with liposomes, not only retains its original release characteristics but also acquires magnetic field-guided functionality. This means that under the action of an external magnetic field, Fe3S4@Lip can accurately target the lesion area and release H2S gas in response to the weak acidic inflammatory microenvironment, thereby effectively regulating this microenvironment. The Fe3S4@Lip probe we designed not only achieves precise release and targeted regulation of H2S but also provides a new approach for the detection and imaging of major H2S-related diseases (such as inflammatory diseases, tumors, etc.). This innovative achievement is expected to bring revolutionary breakthroughs in the diagnosis and treatment of related diseases.
2. Experimental Details
2.1. Preparation of Fe3S4@Lip Nanomedicine
Firstly, biodegradable Fe
3S
4 nanoparticles were prepared by a hydrothermal method[
13]. 1 mmoL of FeSO
4·7H
2O, 1 mmol of L-Cysteine, and poly(vinyl pyrrolidone) (PVP, K30) were combined and dissolved in 30 mL of deionized (DI) water while undergoing vigorous magnetic stirring. Subsequently, 100 μL of ethylenediamine was added to the solution. The resultant mixture was then transferred to a stainless steel autoclave, sealed tightly, and heated to 200 °C for a duration of 24 hours. A black precipitate was obtained through centrifugation and subsequently washed multiple times with ethanol and DI water. To encapsulate Fe
3S
4 within liposomes, thin liposomes were prepared using the thin-film hydration method. Then, the thin liposomes were hydrated with Fe
3S
4 in PBS solution through vortexing and ultrasonication. The final suspension (Fe
3S
4@Lip) was further purified by centrifugation. It was then filtered through a membrane with a pore size of 0.22 μm to discard any larger residues.
2.2. Structural Characterization and Biosafety Evaluation of Fe3S4@Lip Nanomedicine
The composition and structure of the nanocomposite were analyzed using Transmission Electron Microscopy (TEM), X-ray Photoelectron Spectroscopy (XPS), and Scanning Electron Microscopy (SEM). The nanoparticle size of the nanocomposite was detected using a Litesizer particle analyzer. The cellular compatibility of the nanomaterial was evaluated based on hemolysis experiments, while cytotoxicity assessment and direct cell contact culture methods were employed to evaluate the cytotoxicity of the nanocomposite.
2.3. Evaluation of the Performance of Releasing H2S
The H2S release performance was evaluated using a methylene blue standard curve. Firstly, Na2S standard solutions with concentrations of 5, 10, 20, 40, 60, 80, and 100 μM were prepared using Na2S and distilled water. 1 mL of each concentration of Na2S standard solution was taken, and the process was repeated three times. The reaction solution was allowed to fully react with methylene blue reagent at room temperature for 30 minutes. The absorption spectrum was detected using a UV-Vis spectrophotometer. Then, a standard curve was plotted for comparison. Subsequently, Fe3S4@Lip was mixed with 10 mL of deionized water, and 1 mL was taken each time to react with methylene blue reagent at room temperature for 30 minutes. The maximum absorbance was detected at 670 nm. The concentration of H2S was calculated based on the aforementioned standard curve.
2.4. CCK-8 Assay
For the in vitro cytotoxicity assessment, the standard Cell Counting Kit-8 (CCK-8) assay was employed. Various concentrations of the Fe3S4@Lip nanocomposite were introduced into 96-well plates containing different populations of L02 cells at specified time points (100 μL per well). Subsequently, 10 μL of CCK-8 solution was added to each well, followed by a 1-hour incubation period. The plate was then analyzed at a wavelength of 450 nm.
3. Results and Discussion
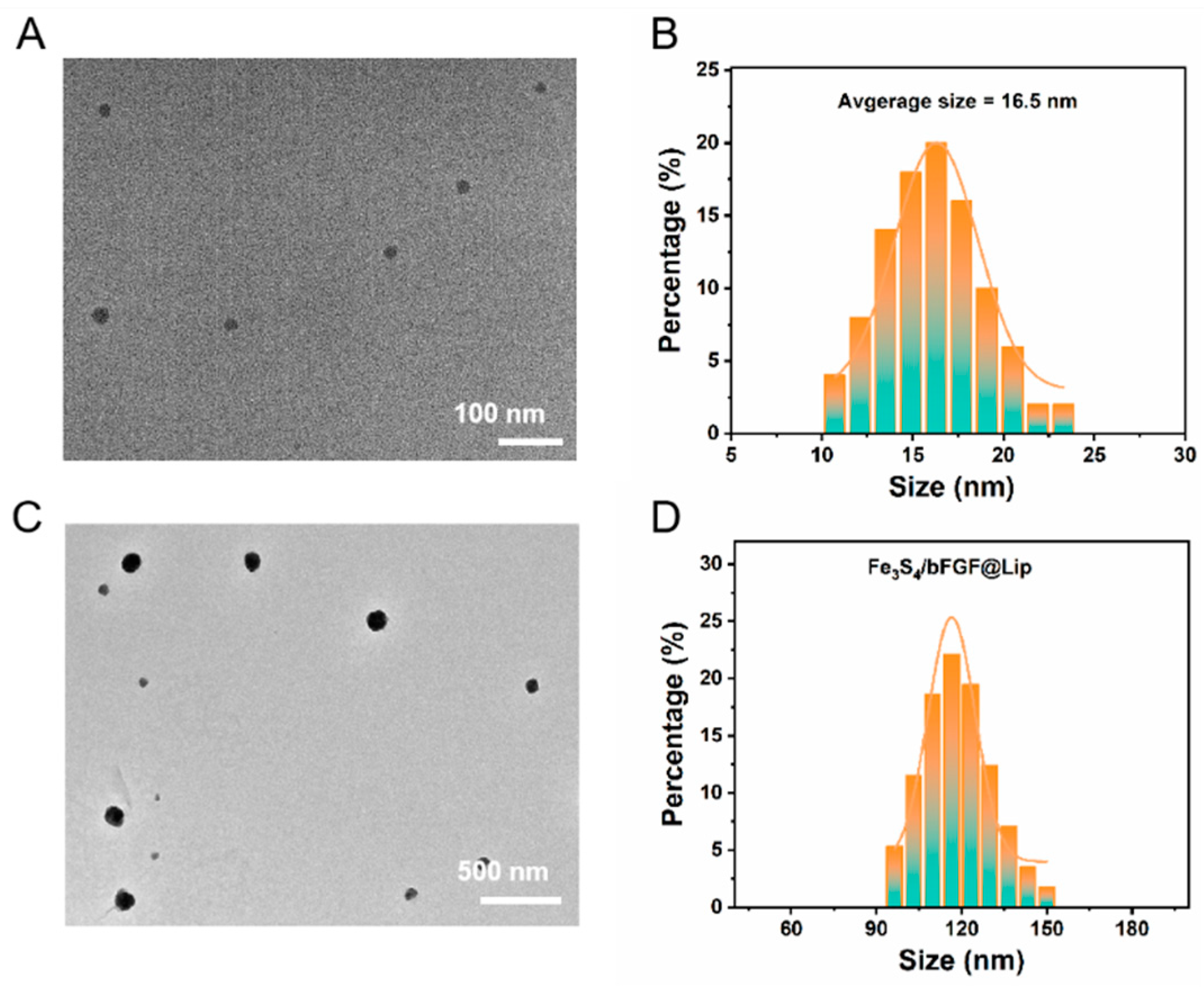
To construct a nanoplatform capable of effectively releasing H
2S, we employed a hydrothermal synthesis method to successfully prepare Fe
3S
4 nanoparticles with uniform size and good dispersion. As shown in
Figure 1A-B, these Fe
3S
4 nanoparticles exhibit an average size of approximately 16.5 nanometers and display excellent dispersion in water. To further enhance the biosecurity and application potential of these nanoparticles, we utilized the liposome thin-film hydration method to encapsulate the biodegradable and H
2S-releasing paramagnetic inorganic nanomaterial, Fe
3S
4 nanoparticles, thereby creating the biosecure Fe
3S
4@Lip nanocomposite. Through characterization using Transmission Electron Microscopy (TEM), we can clearly observe that after encapsulation with liposomes, the particle size of the nanoparticles increases to about 120 nm (
Figure 1C, 1D). This result not only further confirms the successful synthesis of liposome-encapsulated nanoparticles but also reveals the effective encapsulation of the nanoparticles by the lipid layer, providing a solid foundation for subsequent biological applications. In summary, we have successfully prepared Fe
3S
4@Lip nanocomposites with hydrogen sulfide release functionality. This material not only possesses uniform nanoparticle size and good dispersion but also achieves enhanced biosecurity through liposome encapsulation, providing powerful support for subsequent biological experiments and medical applications.
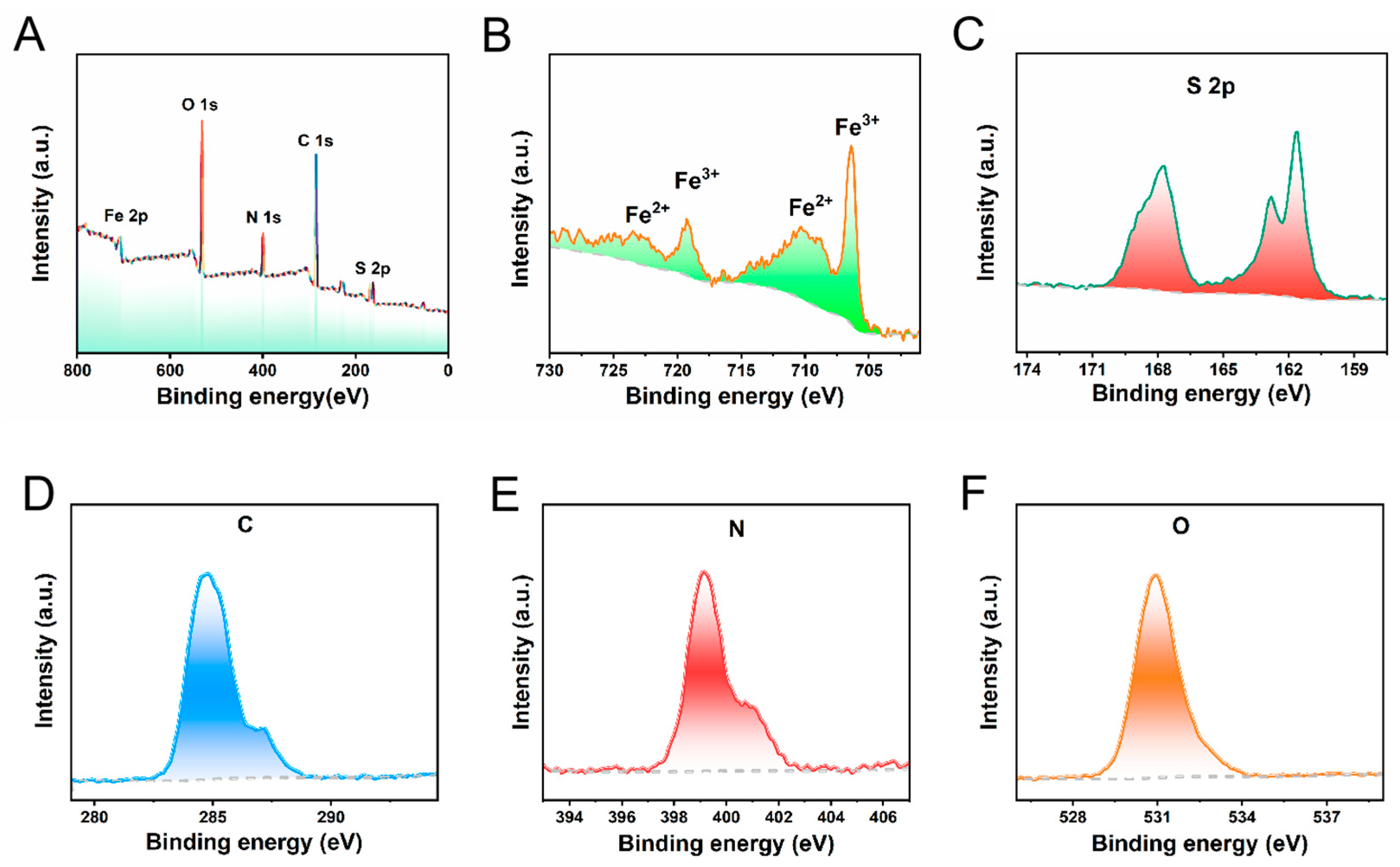
The valence states of Fe and S were analyzed by X-ray electron spectroscopy (XPS). Typical Fe and S peaks were detected in the full-scan XPS spectrum (
Figure 2A). In the Fe 2p region, two peaks at 707.6 eV and 710.3 eV are assigned to Fe(ii). The Fe peak at 2p
3/2 = 720.2 eV which can be assigned to Fe0. The binding energies of Fe 2p
3/2 and Fe 2p
1/2 are at 713.3 eV and 724.4 eV, respectively, proving of the existence of Fe(iii)(
Figure 2B). As shown in
Figure 2C, the binding energies observed at 161.08 eV and 162.18 eV correspond to S
2- 2p3/2, 2p1/2, respectively, which suggests that Fe
3S
4 NPs contain sulfur to allow the release of H
2S. At the same time, during our comprehensive analysis, we also distinctly detected the presence of signals corresponding to the elements C, H, and O. These signals are predominantly attributed to the unique modification of iron sulfide that has been carried out through the utilization of liposomes, indicating a successful integration of these components within the experimental setup(
Figure 2D-F).
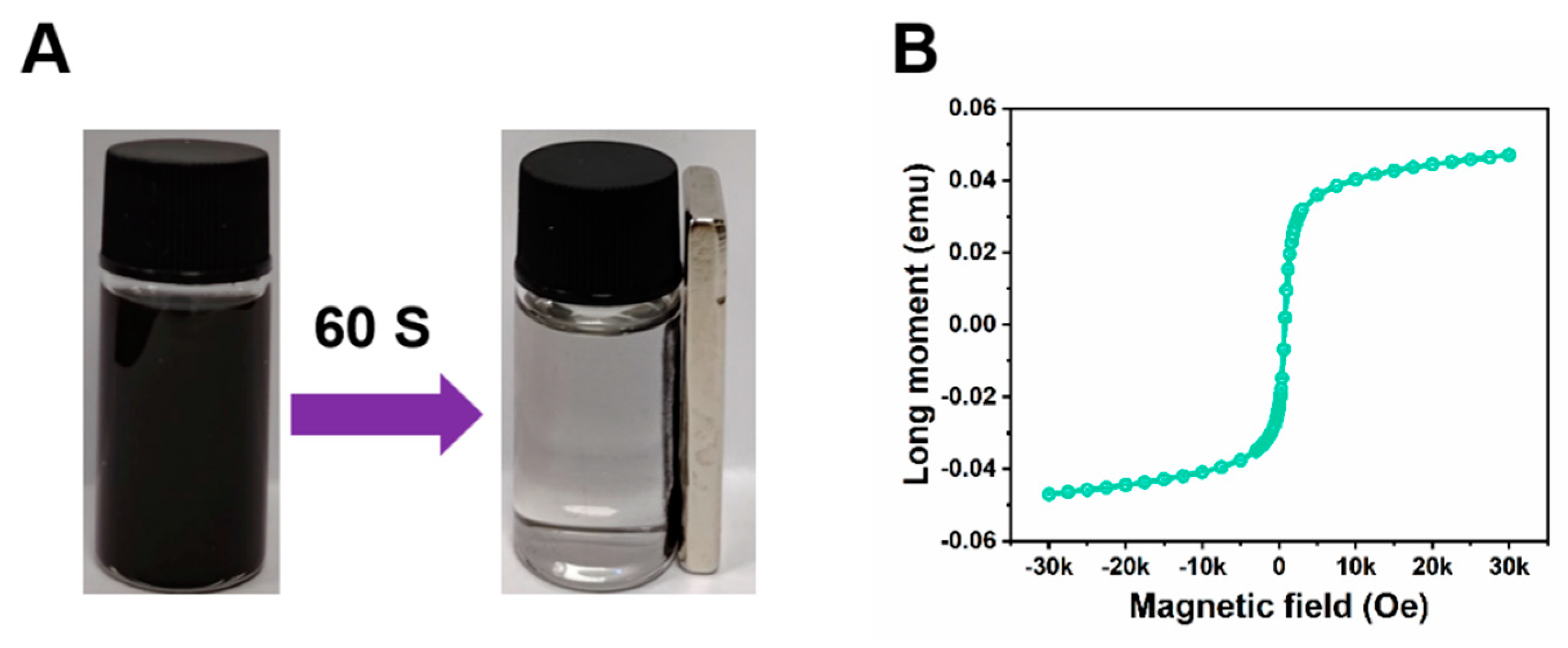
As shown in
Figure 3A, before the application of a magnet, the nanoparticles exhibit a uniform dispersion state, scattered irregularly in the solution, each independent and non-interfering with each other. However, when a magnet is introduced and allowed to adsorb for 60 seconds, these nanoparticles seem to be drawn by an invisible force, beginning to move orderly towards the side where the magnet is placed, and ultimately adsorbing uniformly on that side to form a dense layer of nanoparticles. It is noteworthy that the magnetic saturation value of these nanoparticles reaches up to 0.05 emu(
Figure 3B), a figure that strongly demonstrates their superparamagnetic properties. This implies that, under the influence of an external magnetic field, these nanoparticles can rapidly respond and adjust their magnetization state, thereby exhibiting strong magnetism and magnetic responsiveness. This superparamagnetism not only facilitates the manipulation of nanoparticles in magnetic fields but also opens up new possibilities for their applications in fields such as biomedicine and materials science.
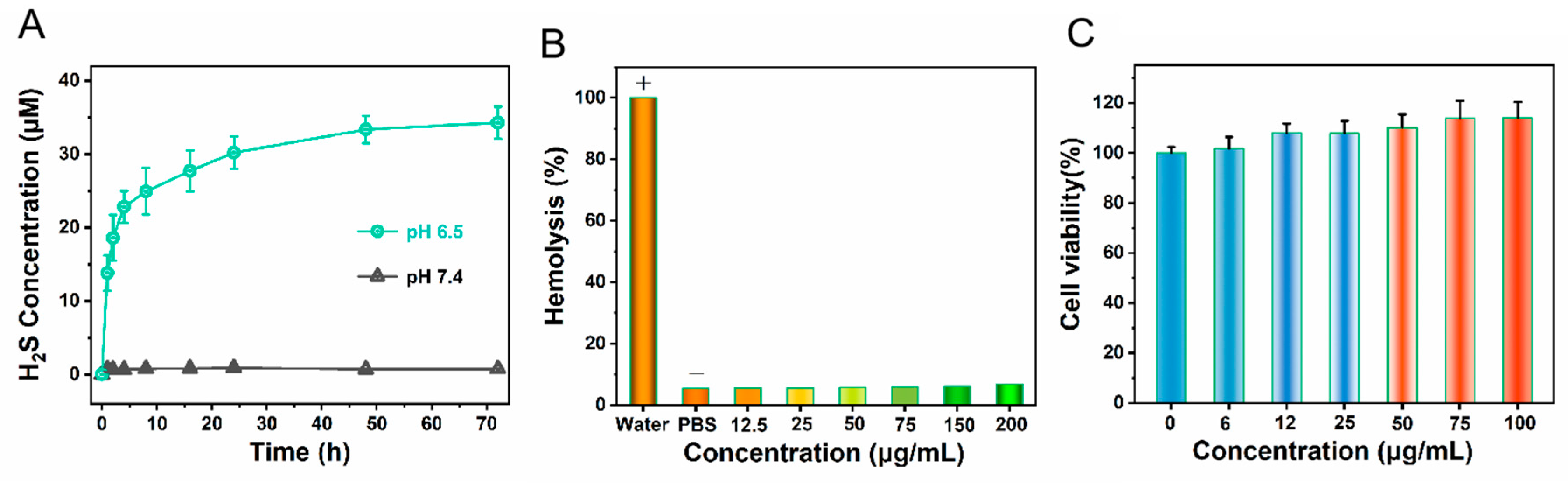
Certain disease tissues create an environment with a reduced pH level, presenting a weakly acidic condition. Therefore, we investigated the H₂S release from Fe
3S
4@Lip nanocomposites in solutions of different pH values. The results indicate that Fe
3S
4@Lip nanocomposites release more H₂S under acidic conditions (as shown in
Figure 4A). Consequently, the Fe
3S
4@Lip nanocomposites can serve as effective slow-release gas reactors with the capability to efficiently scavenge reactive oxygen species (ROS) and reactive nitrogen species (RNS). Meanwhile, for in vivo applications, materials with good blood biocompatibility are fundamental and crucial. To assess the blood biocompatibility of the nanomaterial, we conducted hemolysis tests. The test results show that the nanomaterial exhibits excellent blood compatibility(
Figure 4B), with a hemolysis rate below 5% at a concentration of 200 μg/mL, which is a very desirable indicator. Further observation reveals that the structure of red blood cells remains intact during this process, further confirming the good biocompatibility of the nanomaterial. Based on these test results, to ensure the safety and effectiveness of the experiments, we strictly stipulated in subsequent in vivo experiments that the maximum concentration of nanoprobes in mouse blood must be strictly controlled below 200 μg/mL. This concentration setting aims to fully safeguard the life and health of the experimental mice while ensuring that the nanoprobes can achieve the best detection effect in vivo. Additionally, we tested the toxicity of the nanomaterial using the CCK8 assay(
Figure 4C). The CCK8 test results showed that as the material concentration increased, the cell survival rate remained stable, proving that our designed material, after being encapsulated with liposomes, exhibits excellent biocompatibility, which provides an opportunity for subsequent delivery of hydrogen sulfide to deep tissues in living organisms.
4. Conclusions
Herein, we have designed a nanoplatform capable of H₂S release. The gas releaser we designed possesses the ability to selectively release H₂S based on pH levels. Notably, with the assistance of a magnetic field, it can achieve efficient H₂S release through magnetic force. Furthermore, encapsulated in liposomes, the nanoprobes exhibit excellent biocompatibility. In conclusion, our findings suggest that Fe₃S₄@Lip can serve as an H₂S slow-release bioreactor, offering a potentially innovative approach for programmed H₂S release in deep tissues of living organisms and the detection and treatment of major diseases.
Conflict of interests
The authors declare no competing financial interest.
Acknowledgements
This work was supported by the Research Foundation of Education Bureau of Hunan Province, China (22A0628), China Postdoctoral Science Foundation (2023M731063).
References
- Shaik, R.; Kishore, R.; Kumar, A.; Shekhar, C.; Kumar, M. Metal oxide nanofibers based chemiresistive H2S gas sensors. Coord. Chem. Rev. 2022, 471, 214752. [Google Scholar] [CrossRef]
- Zhao, Z. N.; Guo, W. Y.; Xu, C. W.; Wang, Q.; Mao, C.; Wan, M. M. Physiological functions and donor design of hydrogen sulffde and its application in central nervous system diseases, Chem. Eng. J. 2023, 452, 139089. [Google Scholar]
- Kimura, H. ; Signaling by hydrogen sulffde (H2S) and polysulffdes (H2Sn) in the central nervous system. Neurochem. Int. 2019, 126, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. H.; Wu, Y. L.; Chen, J. J.; Zhong, J.; Zeng, F.; Wu, S. Z. A Turn-On Optoacoustic Probe for Imaging Metformin-Induced Upregulation of Hepatic Hydrogen Sulfide and Subsequent Liver Injury. Theranostics 2019, 9, 77–89. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signaling 2014, 20, 770–782. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Wilinś ki, B.; Wilinś ki, J.; Somogyi, E.; Piotrowska, J.; Opoka, W. Metformin Raises Hydrogen Sulfide Tissue Concentrations in Various Mouse Organs. Pharmacol. Rep. 2013, 65, 737–742. [Google Scholar] [CrossRef]
- Zhou, L. N.; Wang, Q. Advances of H2S in Regulating Neurodegenerative Diseases by Preserving Mitochondria Function. Antioxidants 2023, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, L. S.; Li, B.; Yin, C.; Wang, G. H.; Sang, W.; Li, W. X.; Tian, H.; Zhang, Z.; Zhang, X. J.; Fan, Q. L. Dai, Y. L. Engineering a Hydrogen-Sulfide-Based Nanomodulator to Normalize Hyperactive Photothermal Immunogenicity for Combination Cancer Therapy. Adv. Mater. 2021, 33, 2008481. [Google Scholar] [CrossRef] [PubMed]
- He, Q. J. Precision gas therapy using intelligent nanomedicine. Biomater. Sci. 2017, 5, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chang, J. H.; He, F.; Gai, S. L.; Yang, P. P. Hydrogen sulffde: an emerging precision strategy for gas therapy, Adv. Healthcare Mater. 2022, 11, 2101984. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. C.; Guo, X.; Zhao, Z.; Li, B.; Qin, J. B.; Peng, Z. Y.; He, G. J.; Brett, Dan J. L.; Wang, R. H.; Lu, X. W. Fe3S4 nanoparticles for arterial inflammation therapy: Integration of magnetic hyperthermia and photothermal treatment. Applied Materials Today 2020, 18, 100457. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).