1. Introduction
Gastrin-releasing peptide receptor (GRPR) is a member of G-protein coupled receptors and is expressed in human body including pancreas, gastrointestinal tract, and central nervous system [
1,
2]. It is involved in regulating many physiological functions, such as hormone secretion, smooth muscle contraction, and synaptic plasticity [
1,
2]. Furthermore, GRPR is found significantly overexpressed in various solid malignancies, including breast, prostate, lung, and colon cancers, where it functions as a tumor growth factor [
3,
4,
5,
6,
7,
8]. Such characteristics of GRPR makes it a promising target for the design of targeted radiopharmaceuticals for diagnosis and radioligand therapy of GRPR-expressing cancer.
Bombesin (BBN) is a natural peptide showing very potent binding affinity to GRPR. Its
C-terminal heptapeptide, bombesin(8-14), has been identified as the sequence needed for binding to GRPR and is widely utilized for the design of GRPR-targeted pharmaceuticals [
9,
10,
11,
12,
13,
14,
15,
16]. However, most clinically evaluated GRPR-targeted radiopharmaceuticals derived from BBN showed extremely high pancreas uptake, which not only limits the detection of GRPR-expressing tumor lesions in and near pancreas but also lowers the maximum tolerated dose for radiotherapeutic applications [
9,
10,
11,
12,
13,
14,
17]. Moreover, enzymatic degradation by neutral endopeptidase 24.11 (NEP, EC 3.4.24.11, neprilysin) results in low in vivo stability for most of reported GRPR-targeted radiopharmaceuticals derived from the BBN sequence [
18,
19].
To address these two major limitations, our group previously developed a series of GRPR-targeted radiopharmaceuticals [
20,
21]. These ligands were derived from RC-3950-II ([D-Phe
6,Leu
13(ψ)Thz
14]Bombesin(6-14)), which has a Thz
14 (thiazoline-4-carboxylic acid) substitution and a reduced peptide bond (CH
2-N) between residues 13-14 (Leu
13(ψ)Thz
14) [
15,
16]. All the
68Ga-labeled GRPR antagonists derived from the [Leu
13(ψ)Thz
14]Bombesin(6-14) pharmacophore and all the
68Ga-labeled GRPR agonists derived from the [Thz
14]Bombesin(6-14) pharmacophore showed low pancreas uptake (0.78 - 7.26%ID/g at 1 h post-injection (pi)) [
20,
21]. Subsequently, our group improved in vivo stability of the lead candidates by systematically substituting natural amino acids at potential cleavage sites (Gln
7, Trp
8, Ala
9, Val
10, Gly
11 and His
12) with unnatural amino acids. This led to the discoveries of several GRPR-targeted radiopharmaceuticals with significantly improved in vivo stability [
22,
23]. We discovered that Tle
10 and NMe-His
12 substitutions either in combination or alone can significantly enhance the in vivo stability and improve the tumor uptake of
68Ga-labeled GRPR agonists derived from the [Thz
14]Bombesin(6-14) pharmacophore [
22]. We also identified that NMe-Gly
11 substitution can improve the tumor uptake and tumor-to-organ uptake ratios of
68Ga-labeled GRPR antagonists derived from the [Leu
13(ψ)Thz
14]Bombesin(6-14) pharmacophore [
20,
23]. Recently, we reported two novel GRPR-targeted ligands, LW02056 and ProBOMB5 (
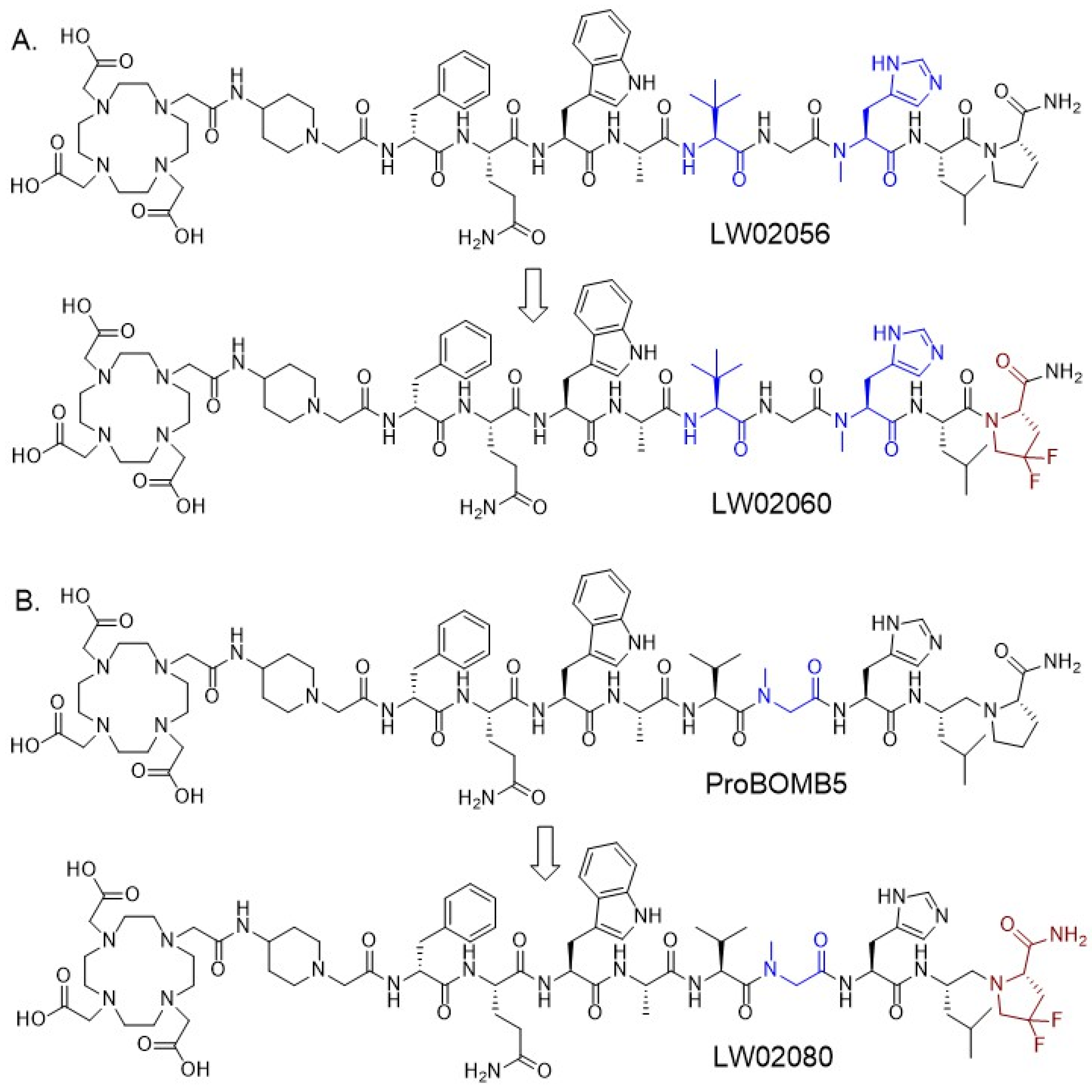
Figure 1), by replacing the Thz
14 in our previously identified LW01110 and TacsBOMB5, respectively, with Pro
14 to avoid oxidation of Thz in the product formulation [
24]. Though a slight reduction was observed for the tumor uptake of [
68Ga]Ga-LW02056 (8.93 ± 1.96 %ID/g at 1 h pi), it had very low pancreas uptake (1.31 ± 0.42 %ID/g at 1 h pi) and good tumor-to-background imaging contrast [
24]. The Pro
14-derived antagonist, [
68Ga]Ga-ProBOMB5, showed good tumor uptake (12.4 ± 1.35 %ID/g), low pancreas uptake (1.37 ± 0.40 %ID/g), and excellent tumor-to-background imaging contrast at 1 h pi [
24].
Fluorinated proline derivatives play a crucial role in peptide engineering and drug discovery due to their resistance to degradation by proteases and their ability to modulate polarity and lipophilicity [
25,
26]. A 4,4-difluoroproline (diF-Pro) derivative, 4,4-difluoropyrrolidine-2-carbonitrile, has been successfully introduced in the design of potent fibroblast activation protein (FAP) inhibitors to mimic Pro [
27], and now 4,4-difluoropyrrolidine-2-carbonitrile is widely used for the design of FAP-targeted radiopharmaceuticals [
28]. In this study, we synthesized two GRPR-targeted ligands, LW02060 and LW02080, by replacing Pro
14 of LW02056 and ProBOMB5, respectively, with diF-Pro
14 (
Figure 1). Subsequently, we characterized the antagonist/agonist characteristics of these two ligands and their Ga/Lu-complexed analogs. We also assessed their potential for PET imaging and targeted radiotherapy using a preclinical tumor model derived from GRPR-expressing PC-3 prostate cancer cells. We hypothesized that (1) diF-Pro
14 substitution would retain GRPR agonist/antagonist characteristics and good binding affinity to GRPR; (2) compared with their Pro
14 analogs, the diF-Pro
14-derived radioligands would have comparable or even higher in vivo stability, and could be promising for detection and radioligand therapy of GRPR-expressing malignancies.
3. Discussion
Based on our previously reported LW02056 and ProBOMB5, we designed two novel DOTA-conjugated GRPR-targeted ligands, LW02060 and LW02080, by replacing Pro
14 in LW02056 and ProBOMB5, respectively, with diF-Pro
14 (
Figure 1) [
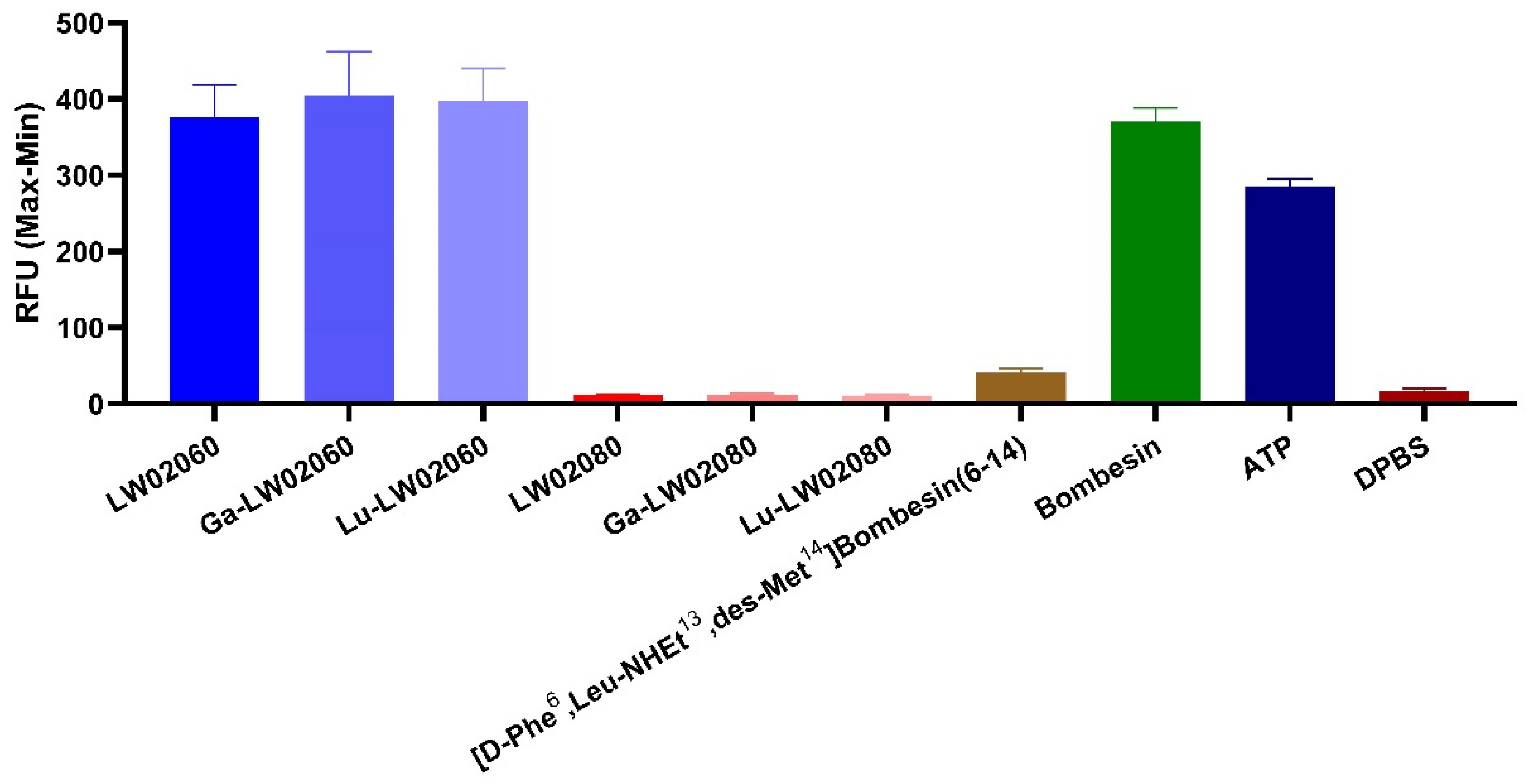
24]. The data from intracellular calcium efflux assay confirm the agonist characteristics of LW02060 and its Ga/Lu-complexed analogs, and the antagonist characteristics of LW02080 and its Ga/Lu-complexed analogs (
Figure 2). The agonist/antagonist characteristics of both diF-Pro
14 derivatives are aligned with their Pro
14 analogs (LW02056 and ProBOMB5). This validates our hypothesis that diF-Pro
14 substitution on previously reported LW02056 and ProBOMB5 would retain their GRPR agonist/antagonist characteristics
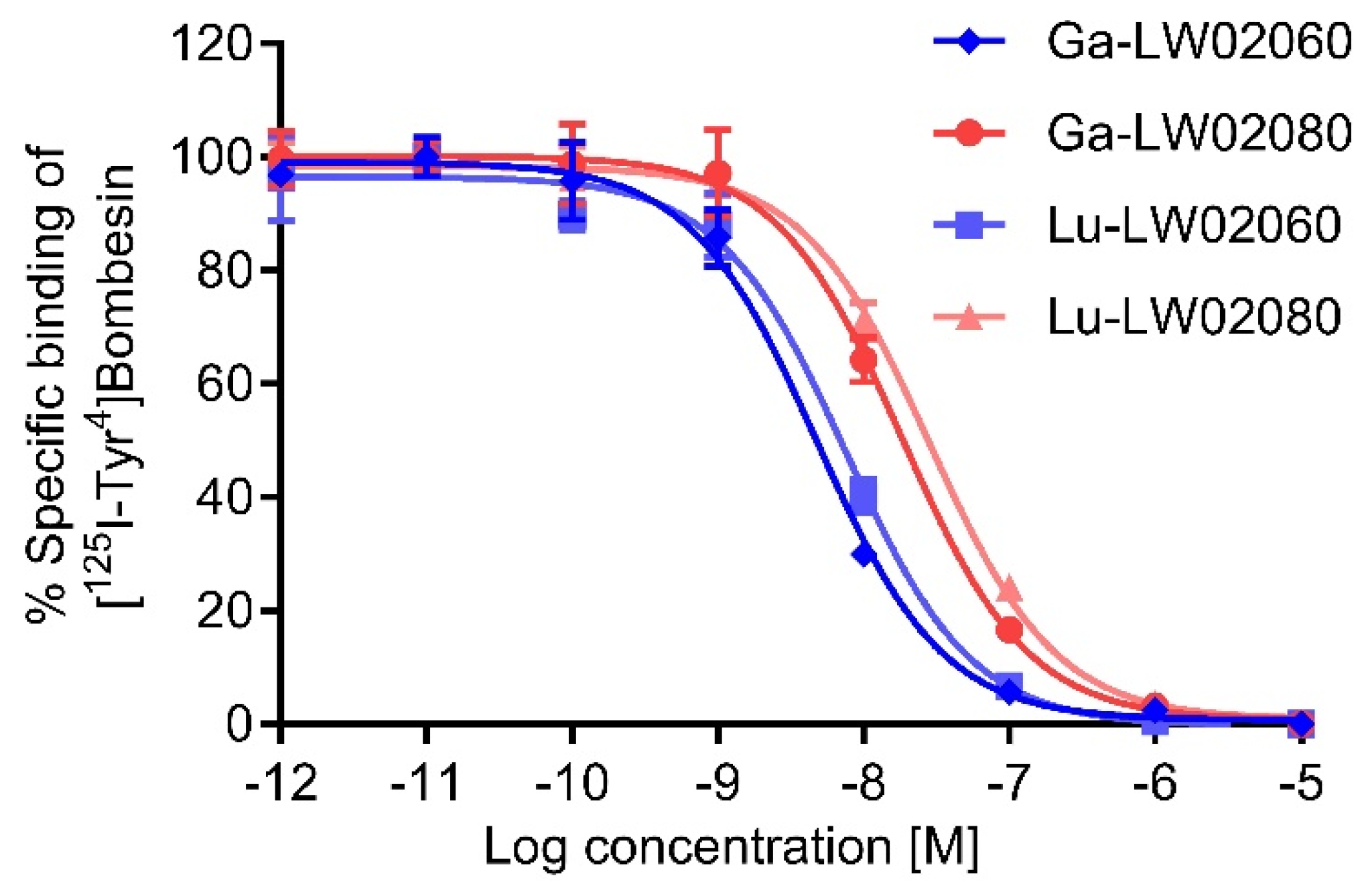
The binding affinities of the Ga/Lu-complexed LW02060 analogs were better than that of LW02080, respectively (
Figure 3). Compared to its Pro
14 analog Ga-LW02056, Ga-LW02060 showed a significantly higher binding affinity to GRPR (K
i = 14.7 ± 4.81 vs 5.57 ± 2.47 nM,
p < 0.05) [
24]. In contrast, the binding affinities of the GRPR antagonists, Ga-LW02080 and Lu-LW02080, were found to be lower than that of Ga-ProBOMB5 and Lu-ProBOMB5 (K
i = 21.7 ± 6.69 and 32.1 ± 8.14 nM vs 12.2 ± 1.89 and 13.6 ± 0.25 nM, respectively) [
24]. This observation suggests that the diF-Pro
14 substitution enhances the binding affinity of GRPR agonists (Ga-LW02060), while reducing the binding affinity of GRPR antagonists (Ga-LW02080 and Lu-LW02080). Previously, we also observed that NMe-Gly
11 substitution was tolerable for antagonists, but decreased the binding affinity of agonists [
20,
23]. Similarly, NMe-His
12 substitution was found to improve binding affinity for only agonists, but decrease the binding affinity for antagonists [
22]. Our accumulated data suggest that agonists and antagonists bind to GRPR in different configurations.
The hydrophilic nature of [68Ga]Ga-LW02060, [68Ga]Ga-LW02080, [177Lu]Lu-LW02060, and [177Lu]Lu-LW02080 were confirmed via the logD7.4 measurements with logD7.4 values all ≤ -2.33. Compared with their Pro14 analogs ([68Ga]Ga-LW02056 and [68Ga]Ga-ProBOMB5), both [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 had significantly higher logD7.4 values (–2.93 ± 0.08 and –2.74 ± 0.04 vs –2.57 ± 0.04 and –2.60 ± 0.03, respectively; p < 0.01). This suggests that replacing Pro14 with diF-Pro14 slightly increases the lipophilicity of two tracers.
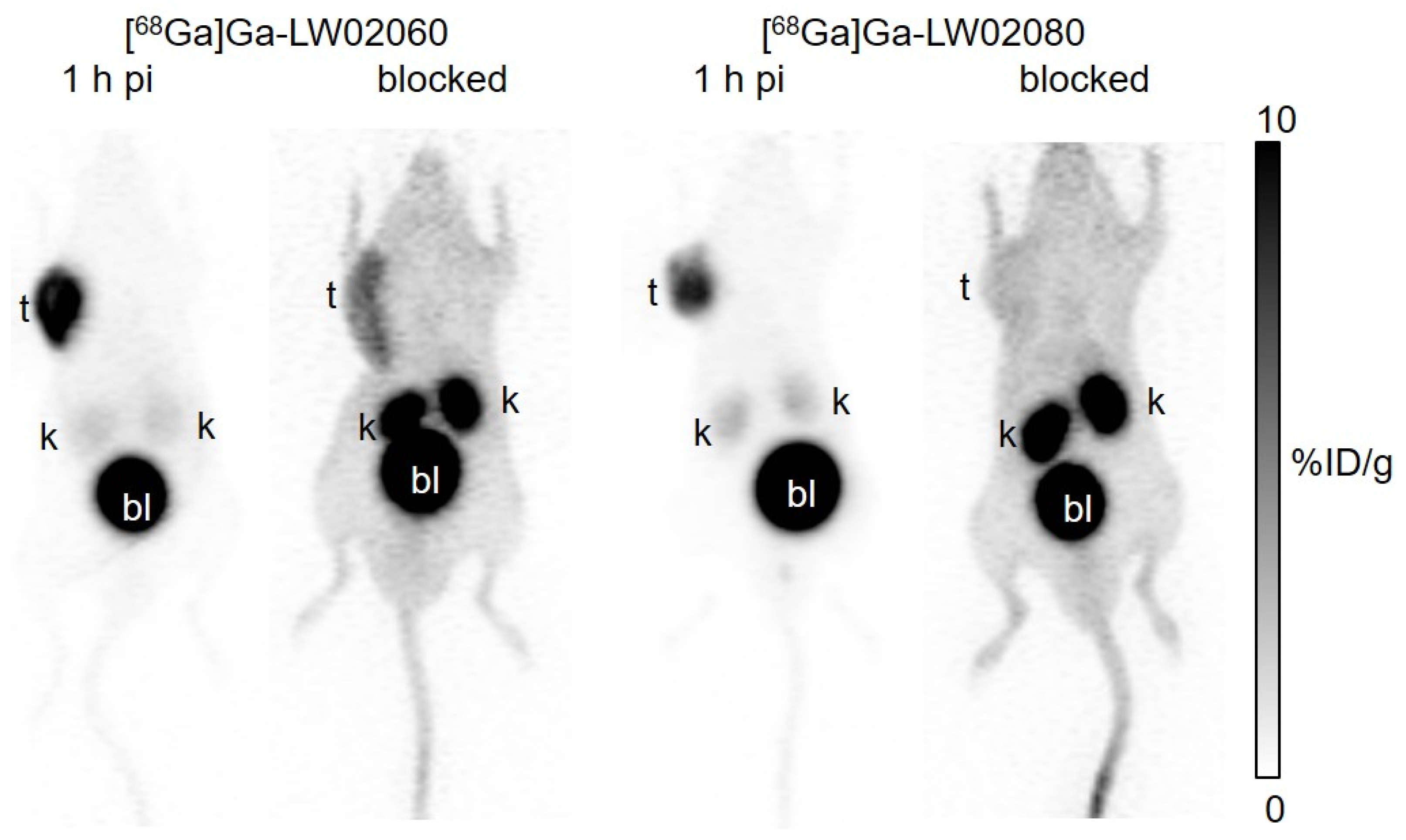
Both [
68Ga]Ga-LW02060 and [
68Ga]Ga-LW02080 enabled clear visualization of the PC-3 tumor xenografts in PET images at 1 h pi (
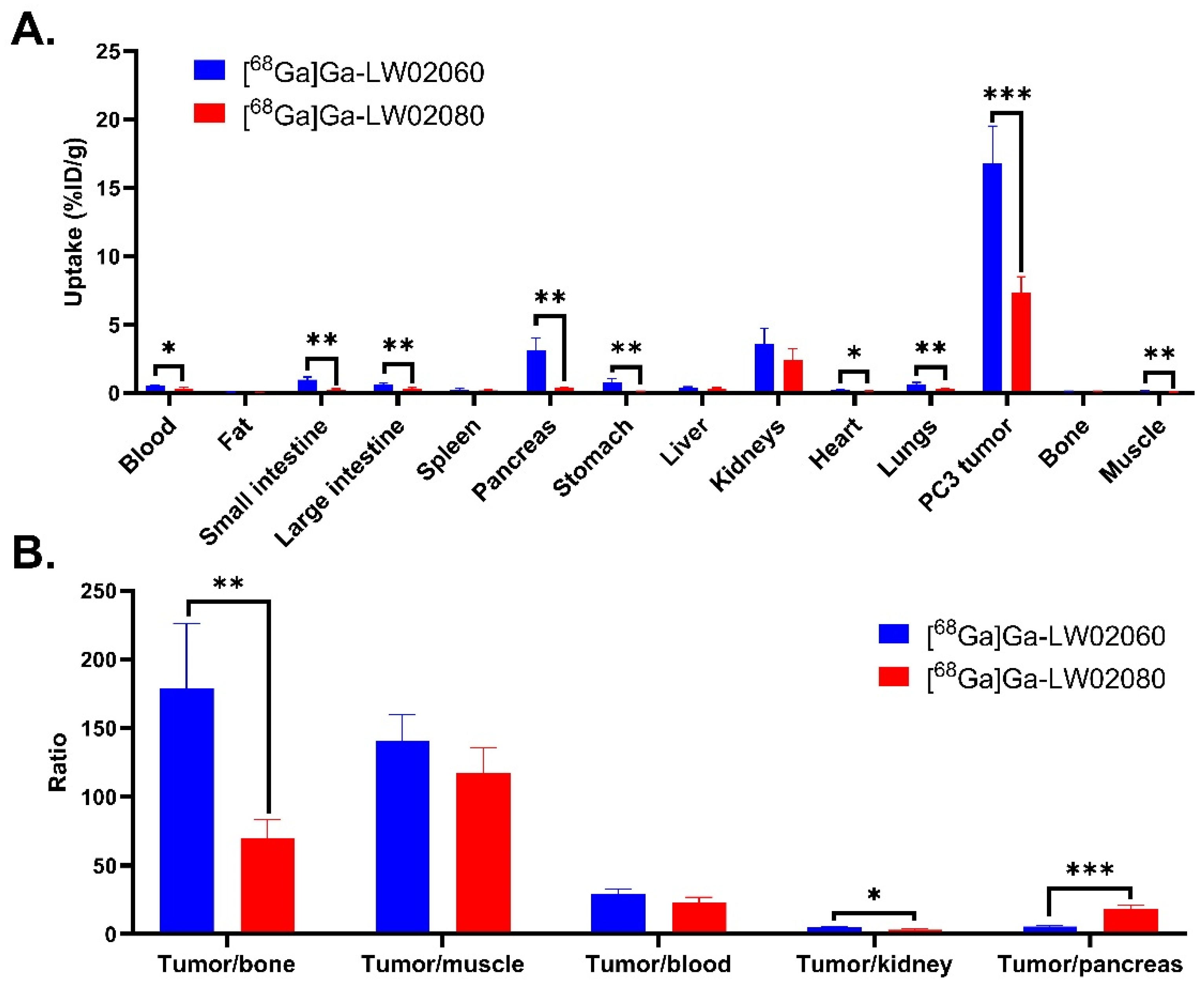
Figure 4). The extremely high accumulation of both tracers in the urinary bladders seen in PET images indicates that their main excretion was via the renal pathway, likely resulting from the highly hydrophilic nature of both tracers. The ex vivo biodistribution data of [
68Ga]Ga-LW02060 and [
68Ga]Ga-LW02080 were consistent with the observations from their PET images (
Figure 4,
Figure 5 and
Figure 6 and
Table S4). Possibly owing to its higher binding affinity, [
68Ga]Ga-LW02060 exhibited 2.3-fold uptake in PC-3 tumor xenografts when compared to that of [
68Ga]Ga-LW02080 (16.8 ± 2.70 vs 7.36 ± 1.13 %ID/g), and 1.9-fold uptake when compared to the previously reported [
68Ga]Ga-LW02056 (16.8 ± 2.70 vs 8.93 ± 1.96 %ID/g) [
24]. On the other hand, compared with [
68Ga]Ga-ProBOMB5, the diF-Pro
14-derived [
68Ga]Ga-LW02080 showed a significantly lower tumor uptake (7.36 ± 1.13 vs 12.4 ± 1.35 %ID/g,
p < 0.005), likely due to the inferior GRPR binding affinity of Ga-LW02080 [
24].
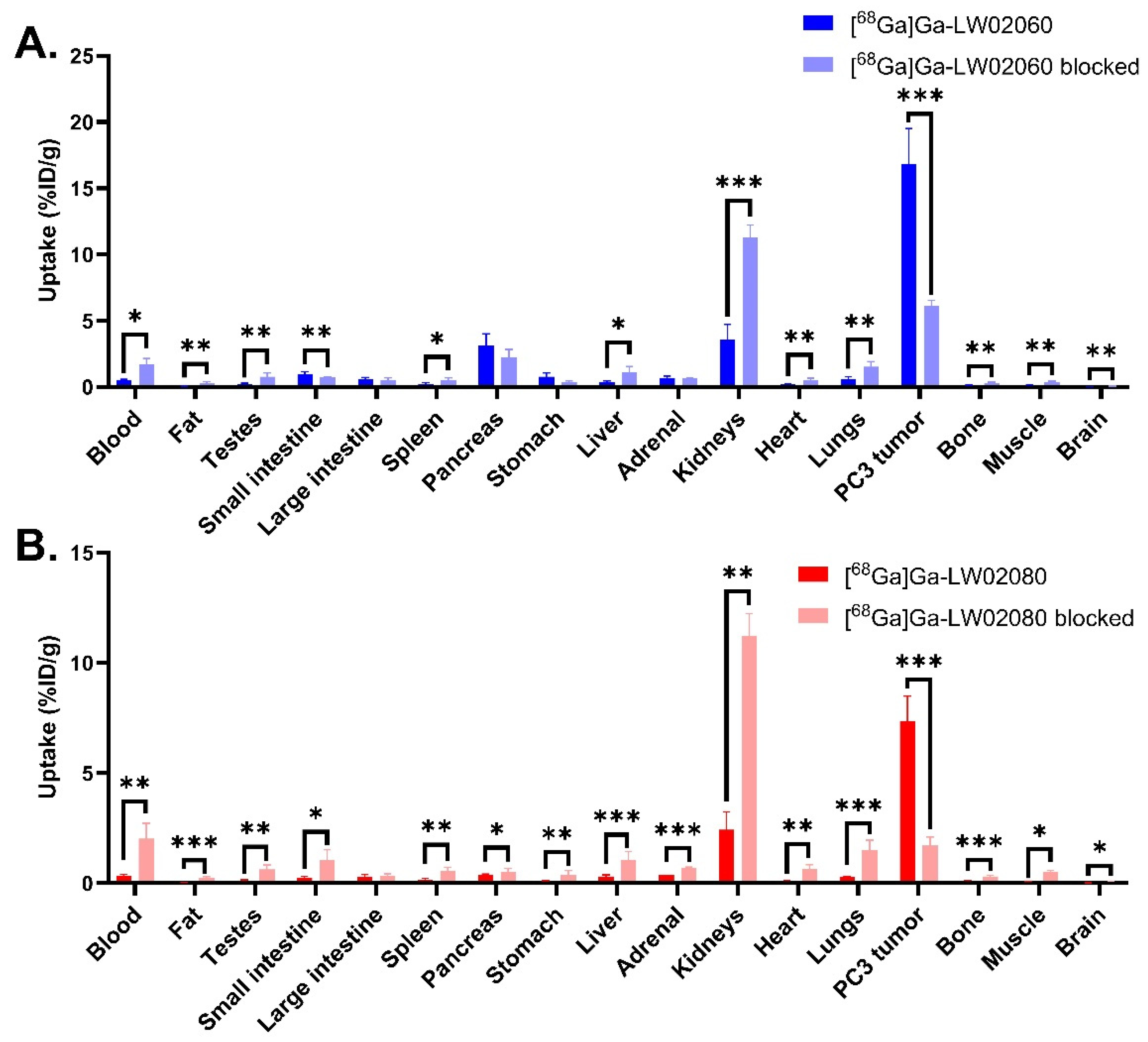
To avoid the potential toxicity caused by injecting an excessive amount of a GRPR agonist, we conducted the blocking study of the agonist tracer, [
68Ga]Ga-LW02060, by co-injection with 100 μg of a GRPR antagonist, [D-Phe
6,Leu-NHEt
13,des-Met
14]Bombesin(6-14) (
Figure 4 and
Figure 6, and
Table S4). For the antagonist tracer, [
68Ga]Ga-LW02080, the blocking study was conducted with the co-injection of its nonradioactive standard, Ga-LW02080 (100 μg) (
Figure 4 and
Figure 6, and
Table S4). With a significantly reduced tumor uptake in the blocked mice, both blocking studies confirmed the specific uptake of [
68Ga]Ga-LW02060 and [
68Ga]Ga-LW02080 in the GRPR-expressing PC-3 tumor xenografts. The increased background uptake especially in the kidneys of blocked mice was mainly resulted from competitive binding of blocking agents to the GRPR in PC-3 tumor xenografts, leading to more unbound [
68Ga]Ga-LW02060 and [
68Ga]Ga-LW02080 in the blood pool and to be excreted through the renal pathway.
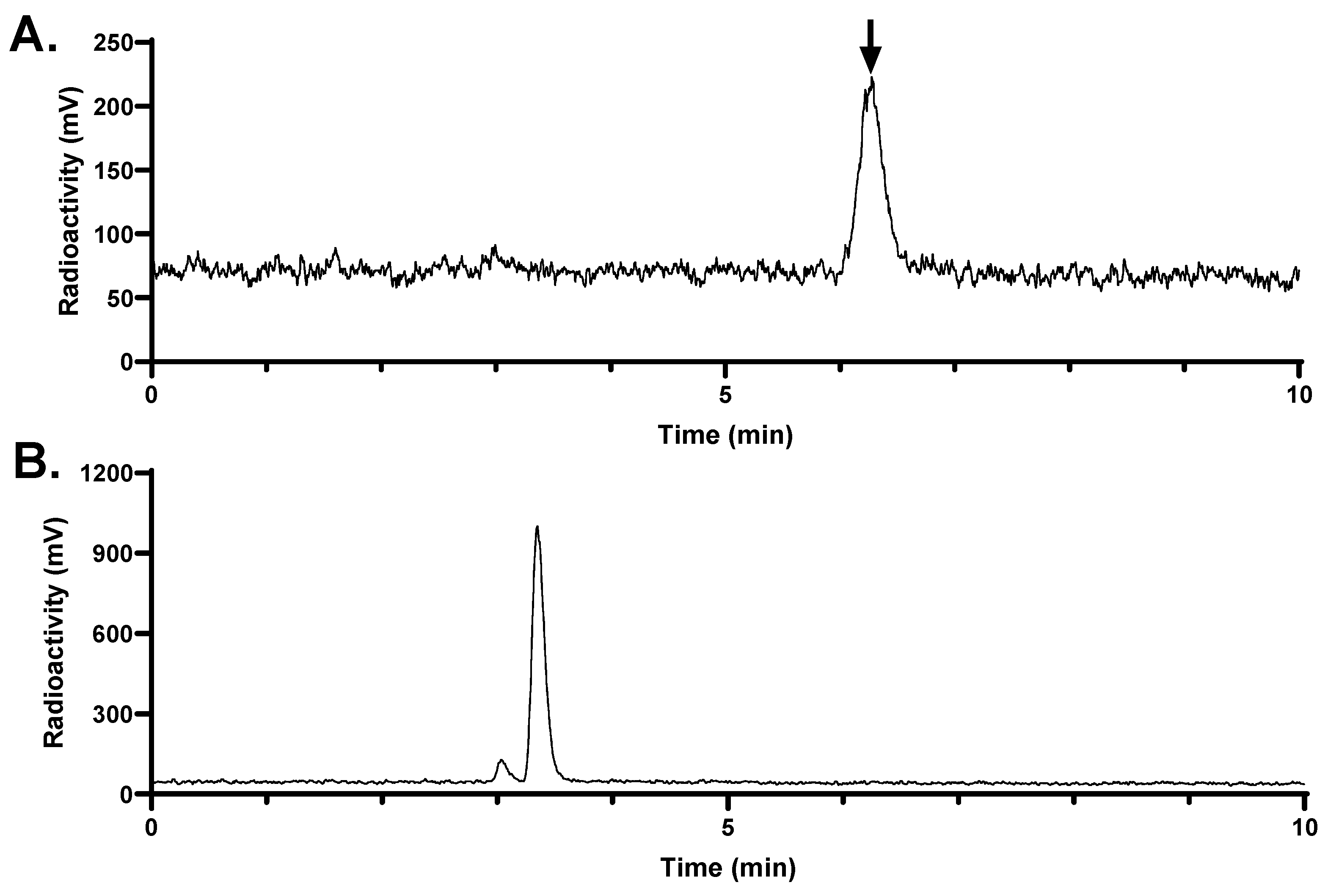
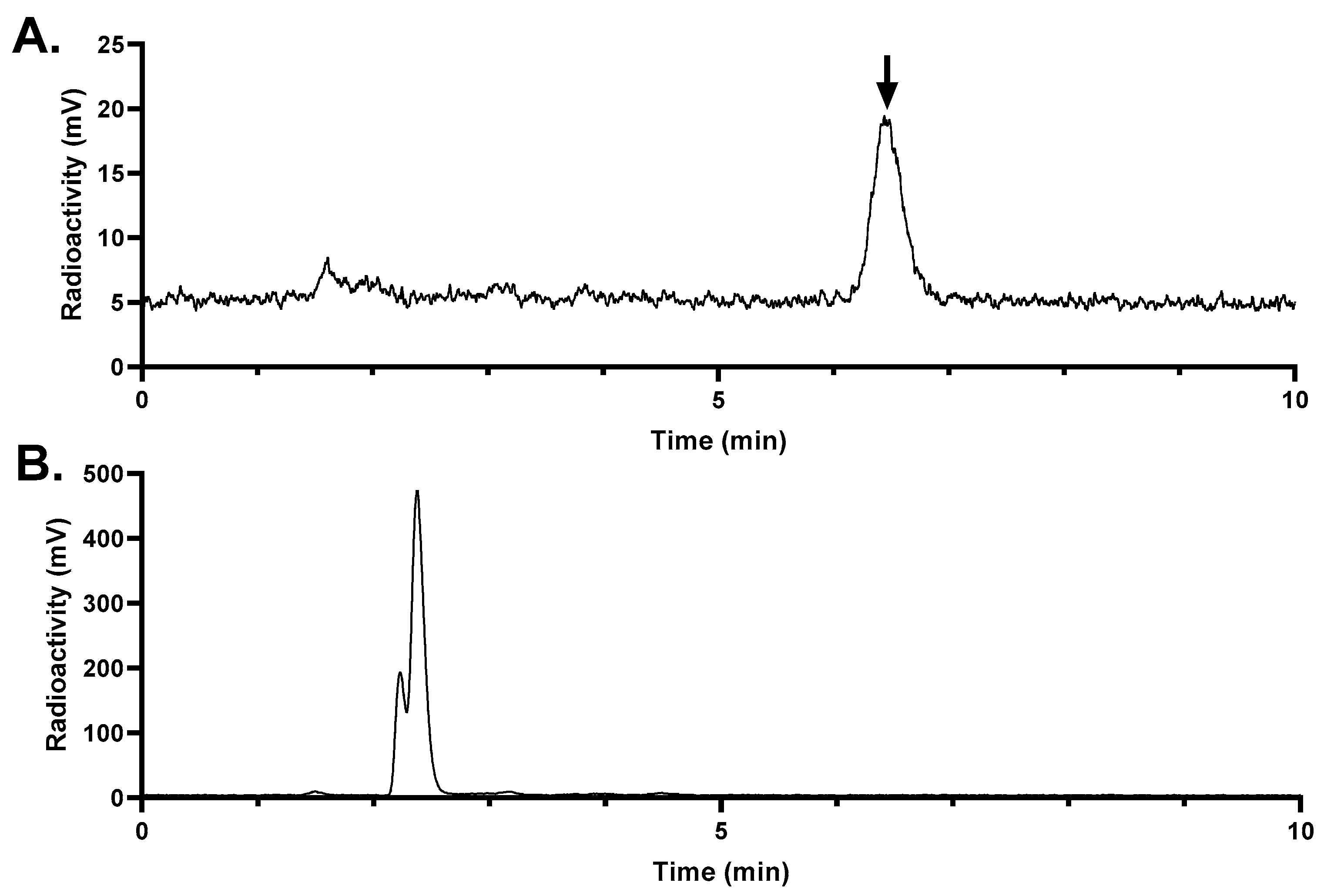
The in vivo stability of [
68Ga]Ga-LW02060 and [
68Ga]Ga-LW02080 was assessed at 15 min pi (
Figure 7 and
Figure 8). More than 99% of [
68Ga]Ga-LW02060 remained intact in mouse plasma, which was significantly higher than that of [
68Ga]Ga-LW02080 (87.4 ± 5.34%) and our previously reported GRPR-targeted tracers (12.7% - 92.9%) [
20,
21,
22,
23,
24]. The excellent in vivo stability of [
68Ga]Ga-LW02060 likely contributes to its high tumor uptake (16.8 ± 2.70 at 1 h pi). Same as most of the previously reported GRPR-targeted radioligands, no intact tracer was observed in mouse urine samples, likely due to degradation by NEP which is expressed abundantly in the kidneys [
18,
19,
20,
21,
22,
23,
24].
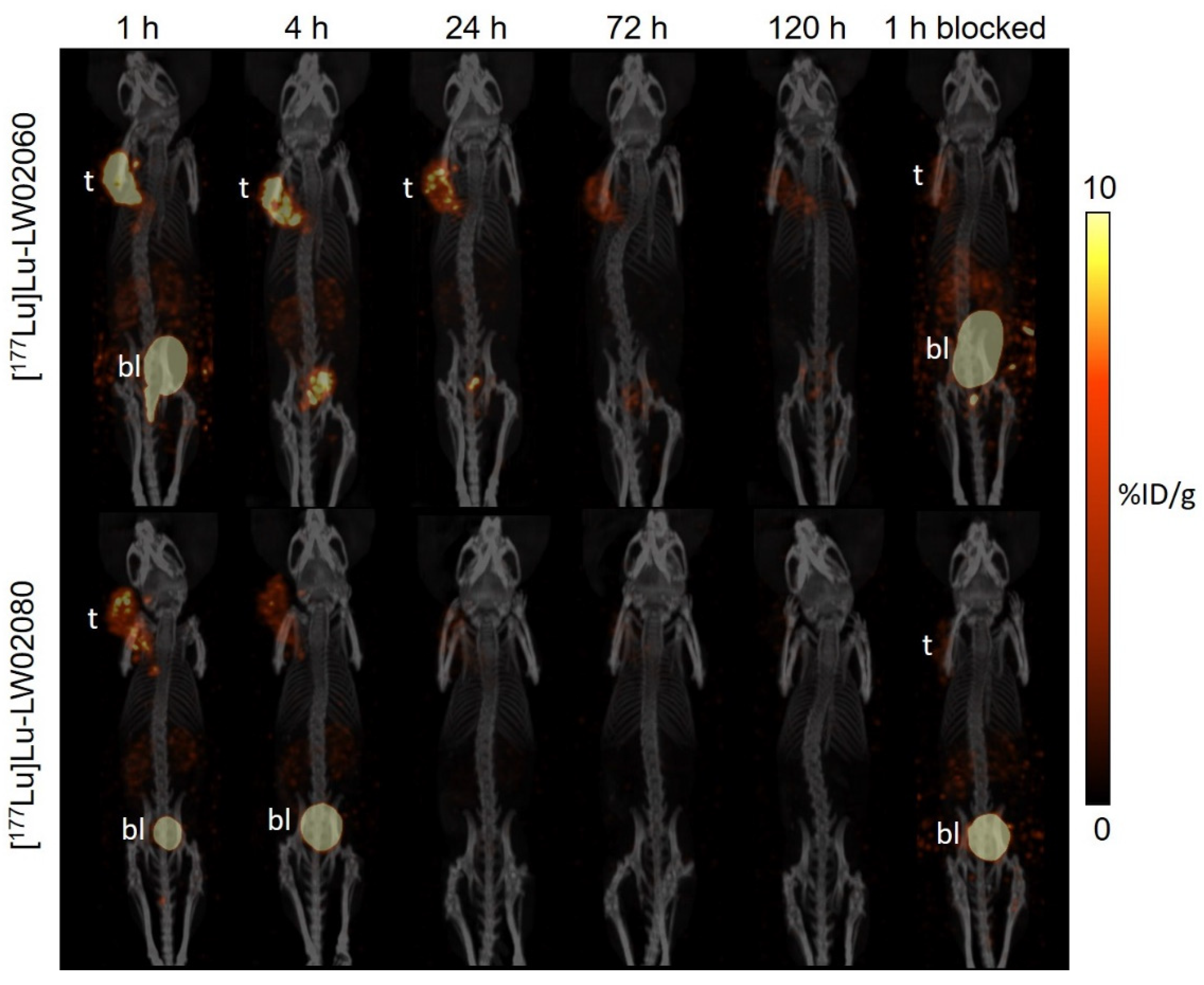
Subsequently, we labeled LW02060 and LW02080 with Lu-177 and evaluated the therapeutic potential of their
177Lu-labeled analogs via SPECT/CT imaging, ex vivo biodistribution studies, and dosimetry calculations. The longitudinal SPECT/CT images showed that compared with [
177Lu]Lu-LW02060, [
177Lu]Lu-LW02080 had a lower tumor uptake and was cleared faster from the PC-3 tumor xenograft (
Figure 9). This is likely due to the combination of its inferior GRPR binding affinity and being an antagonist (lack of internalization upon binding to GRPR) [
1,
29,
30]. The observations from the SPECT/CT images were confirmed by data from ex vivo biodistribution studies (
Figure 10 and
Tables S5-S6). The tumor uptake of [
177Lu]Lu-LW02060 and [
177Lu]Lu-LW02080 at 1 h pi were lower than that of their
68Ga-labeled analogs, [
68Ga]Ga-LW02060 and [
68Ga]Ga-LW02080 (9.59 ± 3.37 and 5.67 ± 1.02 %ID/g vs 16.8 ± 2.70 and 7.36 ± 1.13 %ID/g, respectively). The inferior tumor uptake for the
177Lu-labeled analogs could be due to their slightly inferior GRPR binding affinity, possibly resulting from variation in charge distribution of different DOTA-metal complexes. It has been previously reported by Fani et al. that complexation with a different metal could have a high impact on the binding affinity of a somatostatin receptor 2 (Sstr2)-targeted ligand, DOTA-JR11 [
31]. While the IC
50 values of Y-DOTA-JR11 and Lu-DOTA-JR11 were 0.47 and 0.73 nM, respectively, the IC
50 value of Ga-DOTA-JR11 was only 29 nM. Surprisingly, with a different chelator, Ga-NODAGA-JR11 was much more potent and had IC
50 value at 1.2 nM, close to that of Y-DOTA-JR11 and Lu-DOTA-JR11. Therefore, we are currently investigating the effect of different chelators on the binding affinity of Ga/Lu-complexed GRPR-targeted ligands.
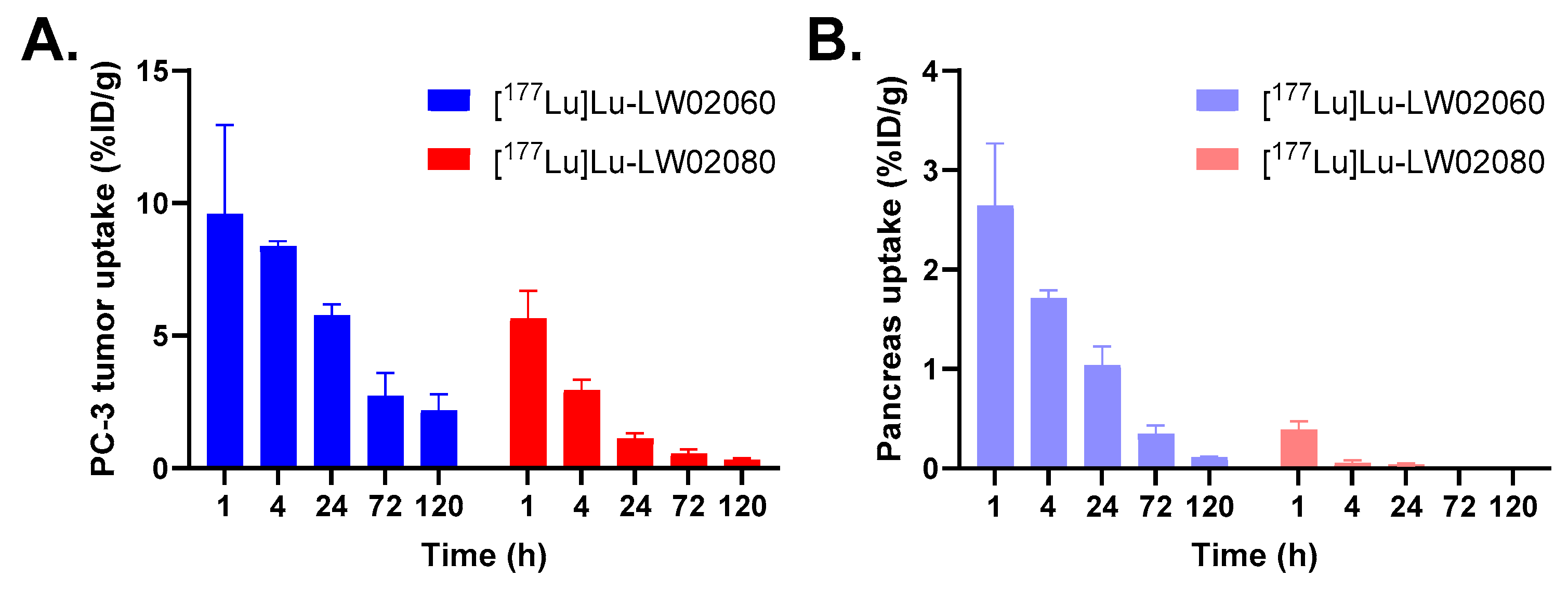
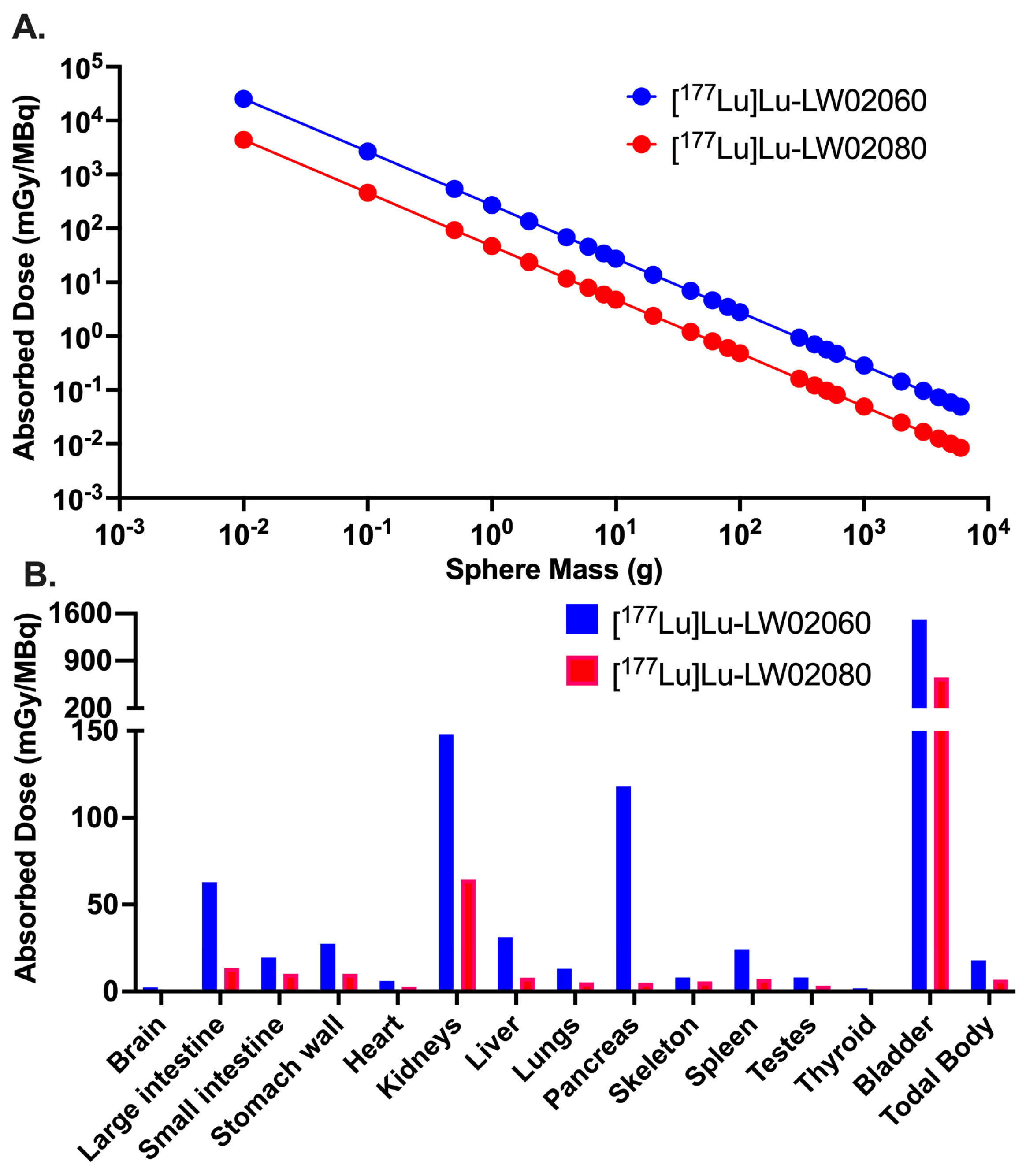
Owing to their dominantly renal excretion, the highest absorbed dose for both [
177Lu]Lu-LW02060 and [
177Lu]Lu-LW02080 was received by the urinary bladder in the mouse model. The calculated absorbed dose of [
177Lu]Lu-LW02060 in a 1-g PC-3 tumor xenograft was 272 mGy/MBq, which was 5.8-fold of that of [
177Lu]Lu-LW02080 (47.0 mGy/MBq) and 4.7-fold of the tumor absorbed dose of our previously reported [
177Lu]Lu-ProBOMB5 (57.3 mGy/MBq) [
24]. However, the absorbed dose of the clinical validated GRPR antagonist [
177Lu]Lu-RM2 in the same tumor model was 429 mGy/MBq, which is 1.6-fold of that of [
177Lu]Lu-LW02060. This is due to the longer tumor retention of [
177Lu]Lu-RM2, likely resulted from its higher GRPR binding affinity (K
i = 1.19 ± 0.16 nM) [
24]. The radiation absorbed doses delivered to the pancreas by [
177Lu]Lu-LW02060 and [
177Lu]Lu-LW02080 (118 and 5.03 mGy/MBq) were markedly lower than that of [
177Lu]Lu-RM2 (316 mGy/MBq) [
24]. Additionally, the overall lower absorbed doses of [
177Lu]Lu-LW02060 and [
177Lu]Lu-LW02080 in most selected organs/tissues compared to that of [
177Lu]Lu-RM2 indicate less off-target binding of [
177Lu]Lu-LW02060 and [
177Lu]Lu-LW02080 in the mouse model.
Though compared to [177Lu]Lu-LW02080, [177Lu]Lu-LW02060 has a higher tumor uptake and a longer tumor retention, it is still not ideal for therapeutic application due to its insufficient radiation absorbed dose deposited in tumors. Further optimizations are needed to enhance tumor uptake and extend tumor retention, thereby achieving a potentially better therapeutic efficacy.
4. Materials and Methods
4.1. General Methods
All chemicals and solvents used in this study were purchased from commercial sources, and used without further purification. GRPR-targeting peptides were synthesized using solid phase approach on an AAPPTec (Louisville, KY, USA) Endeavor 90 peptide synthesizer. Purification and quality control of synthesized peptides were performed on Agilent (Santa Clara, CA, USA) HPLC systems equipped with a model 1200 quaternary pump, a model 1200 UV absorbance detector (220 nm), and a Bioscan (Washington, DC, USA) NaI scintillation detector. The operation of Agilent HPLC systems was controlled using the Agilent ChemStation software. The HPLC columns used were a semi-preparative column (Luna C18, 5 µm, 250 × 10 mm) and an analytical column (Luna C18, 5 µm, 250 × 4.6 mm) purchased from Phenomenex (Torrance, CA, USA). The collected HPLC eluates were lyophilized using a Labconco (Kansas City, MO, USA) FreeZone 4.5 Plus freeze-drier. MS spectra were acquired on an AB SCIEX (Framingham, MA, USA) 4000 QTRAP mass spectrometer system with an ESI ion source. C18 Sep-Pak cartridges (1 cm
3, 50 mg) were purchased from Waters (Milford, MA, USA).
68Ga was eluted from an ITM Medical Isotopes GmbH (Munich, Germany) generator, and purified according to the previously published procedures using a DGA resin column from Eichrom Technologies LLC (Lisle, IL, USA) [
32].
177LuCl
3 was purchased from Isotopia Molecular Imaging Ltd (Petah Tikva, Israel) and ITM
Medical Isotopes GmbH (Munich, Germany). Radioactivity of
68Ga/
177Lu-labeled peptides was measured using a Capintec (Ramsey, NJ, USA) CRC
®-25R/W dose calibrator. The radioactivity of samples collected from biodistribution studies, binding assays, and logD
7.4 measurements were counted using a Perkin Elmer (Waltham, MA, USA) Wizard2 2480 automatic gamma counter.
4.2. Synthesis of Fmoc-Leu(ψ)diF-Pro-OH
Fmoc-Leu(ψ)diF-Pro-OH (
5) was synthesized following the reaction steps depicted in Scheme S1. Detailed synthesis procedures and characterizations for Fmoc-Leu(ψ)diF-Pro-OH (
5) and its intermediates are provided in
Supplementary Materials (
Figures S1-S16).
4.3. Synthesis of DOTA-Conjugated Peptides
Both LW02060 and LW02080 were synthesized on solid phase using the Fmoc peptide chemistry. For the synthesis of LW02060, Rink Amide MBHA resin (0.1 mmol) was treated with 20% piperidine in N,N-dimethylformamide (DMF) to remove the Fmoc protecting group. Fmoc-protected amino acids (5 eq.) and Fmoc-4-amino-(1-carboxymethyl)piperidine (5 eq.) were pre-activated with HATU (5 eq.), HOAt (5 eq.), and N,N-diisopropylethylamine (DIEA, 15 eq.) and then sequentially coupled to the resin. For the synthesis of LW02080, Sieber resin (0.1 mmol) was treated with 20% piperidine in DMF to remove the Fmoc protecting group. Fmoc-Leu(ψ)diF-Pro-OH (5) (5 eq.), Fmoc-protected amino acids (5 eq.), and Fmoc-4-amino-(1-carboxymethyl)piperidine (5 eq.) were pre-activated with HATU (5 eq.), HOAt (5 eq.), and DIEA (15 eq.), and then sequentially coupled to the resin. At the end, DOTA(tBu)3 (5 eq.) pre-activated with HATU (5 eq.) and DIEA (25 eq.) was coupled to the resin for both LW02060 and LW02080.
The peptides were deprotected and simultaneously cleaved from the resin with a mixture of trifluoroacetic acid (TFA, 81.5%), triisopropylsilane (TIS 1.0%), water (5%), 2,2′-(ethylenedioxy)diethanethiol (DODT, 2.5%), thioanisole (5%), and phenol (5%) for 4 h at room temperature. The cleaved peptides were filtered and precipitated by the addition of cold diethyl ether. The crude peptides were collected after centrifugation and purified with HPLC (semi-preparative column; flow rate: 4.5 mL/min). The eluates containing the desired peptides were collected and lyophilized. The HPLC conditions, retention times, isolated yields and MS confirmations of LW02060 and LW02080 are provided in
Table S1 and
Figures S17-S18.
4.4. Synthesis of Nonradioactive Ga/Lu-Complexed Standards
The nonradioactive Ga-complexed standards of LW02060 and LW02080 were prepared by incubating a solution of the DOTA-conjugated precursor with excess GaCl
3 (5 eq.) in NaOAc buffer (0.1 M, 500 µL, pH 4.5) at 80 °C for 15 min. The nonradioactive Lu-complexed standards of LW02060 and LW02080 were synthesized by incubating a solution of the precursor with excess LuCl
3 (10 eq.) in NaOAc buffer (0.1 M, 500 µL, pH 4.5) at 90 °C for 30 min. After that, the reaction mixture was purified via HPLC (semi-preparative column, flow rate: 4.5 mL/min). The HPLC eluates containing the desired peptide were collected and lyophilized. The HPLC conditions, retention times, isolated yields and MS confirmations of these nonradioactive Ga/Lu-complexed standards are provided in
Table S2 and
Figures S19-S22.
4.5. Synthesis of 68Ga/177Lu-Labeled Ligands
The Ga-68 radiolabeling experiments were performed following previously published procedures [
32,
33,
34]. Briefly, purified
68Ga in 0.5 mL water was added into a 4-mL glass vial preloaded with 0.7 mL of HEPES buffer (2 M, pH 5.0) and 10 μL precursor solution (1 mM). The radiolabeling reaction was carried out under microwave heating for 1 min at 100 ℃, followed by HPLC purification using the semi-preparative column.
The Lu-177 radiolabeling experiments were conducted following literature procedures [
35].
177LuCl
3 was added into a 4-mL glass vial preloaded with 0.7 mL of NaOAc buffer (0.1 M, pH 4.5) and 10 μL precursor solution (1 mM), and incubated at 95 ℃ for 15 min with a heating block.
177Lu-labeled ligands were purified by HPLC using the semi-preparative column.
The eluate fraction containing the radiolabeled product was collected, diluted with water (50 mL), and passed through a C18 Sep-Pak cartridge that was pre-washed with ethanol (10 mL) and water (10 mL). The trapped
68Ga/
177Lu-labeled product on the cartridge was eluted off with ethanol (0.4 mL), and diluted with PBS for imaging and biodistribution studies. The HPLC conditions and retention times for the purification and quality control of
68Ga/
177Lu-labeled ligands are provided in
Table S3.
4.6. The LogD7.4 Measurement
The logD
7.4 values of
68Ga/
177Lu-labeled ligands were measured using the previously reported shake flask method [
32]. An aliquot of the
68Ga/
177Lu-labeled ligand was added into a 15 mL falcon tube containing a mixture of n-octanol (3 mL) and DPBS (3 mL, pH 7.4). The mixture was mixed by 1-min vortexing, followed by 15-min centrifugation at 3,000 rpm. Samples of the n-octanol (1 mL) and DPBS (1 mL) layers were collected and measured in a gamma counter (n = 3). The logD
7.4 value was calculated using the following equation:
4.7. Fluorometric Calcium Release Assay
The agonist/antagonist characteristics of LW02060, Ga-LW02060, Lu-LW02060, LW02080, Ga-LW02080, and Lu-LW02080 were determined following previously published procedures [
35]. A 96-well clear bottom black plate was seeded with 5 × 10
4 PC-3 cells in 100 μL growth media per well 24 h prior to the assay. The loading buffer (100 μL/well) containing a calcium-sensitive dye (FLIPR Calcium 6 assay kit from Molecular Devices, San Jose, CA, USA) was added into the 96-well plate, followed by 1 h incubation at 37 °C. The plate was then transferred into a FlexStation 3 microplate reader (Molecular Devices, San Jose, CA, USA). LW02060 (50 nM), Ga-LW02060 (50 nM), Lu-LW02060 (50 nM), LW02080 (50 nM), Ga-LW02080 (50 nM), Lu-LW02080 (50 nM), [D-Phe
6,Leu-NHEt
13,des-Met
14]Bombesin(6-14) (50 nM, antagonist control), bombesin (50 nM, agonist control), adenosine triphosphate (ATP, 50 nM, positive control), or DPBS (blank control) was added, and the fluorescent signals were acquired for 2 min (λ
Ex = 485 nm; λ
Em = 525 nm; n = 4). The relative fluorescent units (RFU = max – min) were calculated to determine the agonistic/antagonistic characteristics of the tested ligands.
4.8. In Vitro Competition Binding Assay
Inhibition constants (K
i) of GRPR-targeted ligands to GRPR were measured via in vitro competition binding assay using PC-3 cells and [
125I-Tyr
4]Bombesin as the radioligand following previously reported method [
36]. PC-3 cells were seeded in 24-well poly-D-lysine plates at 2 × 10
5 cells/well 48 h prior to the assay. The growth medium was replaced with 400 μL of reaction medium (RPMI 1640 containing 2 mg/mL BSA, and 20 mM HEPES). After 1 h incubation at 37 °C, Ga-LW02060, Ga-LW02080, Lu-LW02060, and Lu-LW02080 in 50 μL reaction medium with decreasing concentrations (10 μM to 1 pM) and 50 μL of 0.01 nM [
125I-Tyr
4]Bombesin were added into the wells followed by incubation with moderate agitation for 1 h at 37 °C. Cells were gently washed with ice-cold PBS twice, harvested by trypsinization, and counted for radioactivity on a Perkin Elmer (Waltham, MA) Wizard2 2480 automatic gamma counter. Data were analyzed using nonlinear regression (one binding site model for competition assay) with GraphPad (San Diego, CA, USA) Prism 10 software (Version 10.1.1).
4.9. PET/CT Imaging and Ex Vivo Biodistribution
PET/CT imaging, biodistribution, and in vivo stability studies were conducted on male NOD.Cg-Rag1
tm1Mom Il2rg
tm1Wjl/SzJ (NRG) mice following previously published procedures [
32,
37]. The experiments were conducted according to the guidelines established by the Canadian Council on Animal Care and approved by Animal Ethics Committee of the University of British Columbia (protocol number A20-0113, approved on September 30th, 2022). The mice were anaesthetized and subcutaneously implanted with 5 × 10
6 PC-3 cells (100 µL; 1:1 PBS/Matrigel) behind the left shoulder. PET/CT imaging and biodistribution studies were performed once the tumors reached a diameter of 5-8 mm, typically after approximately 4 weeks of growth.
PET/CT imaging experiments were performed using a Siemens (Knoxville, TN, USA) Inveon micro PET/CT scanner. The mice were injected with 3-5 MBq of the 68Ga-labeled tracer through a lateral caudal tail vein under anaesthesia, followed by recovery and roaming freely in their cages. At 50 min post-injection, a 10-min CT scan was conducted first for localization, attenuation correction, and reconstructing the PET images, followed by a 10-min static PET imaging acquisition.
For ex vivo biodistribution studies, the mice were injected with the 68Ga-labeled tracer (2-4 MBq) via a lateral caudal tail vein. For blocking [68Ga]Ga-LW02060, the mice were co-injected with 100 μg of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14). For blocking [68Ga]Ga-LW02080, the mice were co-injected with 100 μg of its nonradioactive standard Ga-LW02080. At 1 h pi, the mice were euthanized by CO2 inhalation. Blood and organs/tissues of interest were collected, weighed, and counted using a gamma counter.
4.10. SPECT/CT Imaging and Ex Vivo Biodistribution
SPECT/CT imaging was conducted using an MI Labs (Houten, The Netherlands) U-SPECT-II/CT scanner with a custom-made ultra-high sensitivity big mouse collimator (2 mm pinhole size). PC-3 tumor-bearing mice were sedated (2.5% isoflurane in O2) and injected with the 177Lu-labeled ligand through a lateral caudal tail vein. The mouse was imaged at 1, 4, 24, 72, and 120 h pi. At each time point, a 5-min CT scan was obtained using 615 μA and 60 kV parameters for localization and attenuation, followed by 2 × 30-min static SPECT scans acquired in list mode with an energy window centered around 208 keV. The U-SPECT II software was used to reconstruct data, and the images were decay corrected to the time of injection with PMOD v 3.402 (PMOD Technologies GmbH, Fallanden, Switzerland).
For ex vivo biodistribution studies, the mice were injected with ~3-5 MBq of the 177Lu-labeled ligand (n = 5). At 1, 4, 24, 72, and 120 h post-injection, the mice were anesthetized with 2% isoflurane, euthanized by CO2 inhalation, and the tissues/organs of interest were corrected for radioactivity counting. The blocking study was performed at 1 h pi via co-injection of the 177Lu-labeled ligand with 100 μg of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14).
4.11. In Vivo Stability Study
For in vivo stability studies, 200 μL of [68Ga]Ga-LW02060 or [68Ga]Ga -LW02080 was injected into healthy male NRG mice (n = 3) tough a lateral caudal tail vein. At 15 min pi, mice were anaesthetized and euthanized followed by collection of the urine and blood samples. The plasma was extracted from whole blood by adding an equal volume of CH3CN, followed by vortexing, centrifugation, and collection of the supernatant. The plasma and urine samples were analyzed via radio-HPLC.
4.12. Dosimetry Analysis
The uptake values (%ID/g) obtained from ex vivo biodistribution studies (n = 5) were decayed corrected to the appropriate time point and fitted to mono- or bi-exponential equations using SciPy library integrated into an in-house Python script (Python Software Foundation v.3.10.12) [
38]. The best fit was selected based on maximizing the coefficient of determination (R
2) and minimizing the residuals. Time–activity curves calculated from the parameters obtained from the best fit for each organ were then integrated and normalized to injected activity to acquire time-integrated activity coefficients (TIACs) per unit gram, and subsequently multiplied by the mass of model tissue (30-g mouse phantom) [
39]. The TIACs were corrected for tumor sink effect following formula adopted in the report by Cicone et al. [
40] as shown below:
The TIAC values were input into OLINDA (Hermes Medical Solutions, v2.2.3) software [
41] which has pre-calculated dose factors for mouse models. Mouse biodistribution data were extrapolated to humans using the method proposed by Kirschner, et al. using the following equation [
42]:
where m(organ)
H and m(organ)
M are masses of human and mouse organs, respectively, and
WB represents total-body mass. Human TIACs calculated with the above equation were input into OLINDA and dosimetry results were assessed for ICRP 89 Adult Male Model [
43]. The %ID/g value for the blood was assumed to be that for the heart contents of the phantom. Lastly, the TIAC for the tumor was also calculated on the basis of the biodistribution data, and the values were input into the sphere model available in OLINDA [
44].
4.13. Statistical Analysis
Statistical analyses were performed via Student’s t-test using the Microsoft (Redmond, WA, USA) Excel software. The unpaired two-tailed test was used to compare biodistribution data, binding affinities, and logD7.4 values of two radioligands. The unpaired one-tailed test was used to compare the biodistribution data of unblocked mice with that of blocked mice. Statistically significant difference was considered when the adjusted p value was < 0.05.
Author Contributions
Conceptualization, K.-S.L.; methodology, L.W., C.-C.C., D.C., A.A.W.L.W., S.K., and W.S.L.; validation, L.W., C.-C.C., D.C., A.A.W.L.W., S.K. and W.S.L.; formal analysis, L.W., C.-C.C., D.C., A.A.W.L.W., S.K. and W.S.L.; investigation, L.W., C.-C.C., D.C., A.A.W.L.W., S.K., and K.-S.L.; resources, C.U., F.B., and K.-S.L.; data curation, L.W. and K.-S.L.; writing—original draft preparation, L.W.; writing—review and editing, K.-S.L.; visualization, L.W.; supervision, C.U., K.-S.L., and F.B.; project administration, K.-S.L.; funding acquisition, C.U., K.-S.L. and F.B. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Chemical structures of (A) LW02056 and LW02060, (B) ProBOMB5 and LW02080. Unnatural amino acid substitutions (Tle10, NMe-Gly11 and NMe-His12) are in blue and diF-Pro14 is in brown.
Figure 1.
Chemical structures of (A) LW02056 and LW02060, (B) ProBOMB5 and LW02080. Unnatural amino acid substitutions (Tle10, NMe-Gly11 and NMe-His12) are in blue and diF-Pro14 is in brown.
Figure 2.
Intracellular calcium efflux in PC-3 cells induced by various tested ligands. Error bars indicate standard deviation (n = 4).
Figure 2.
Intracellular calcium efflux in PC-3 cells induced by various tested ligands. Error bars indicate standard deviation (n = 4).
Figure 3.
Displacement curves of [125I-Tyr4]Bombesin by Ga-LW02060, Ga-LW02080, Lu-LW02060, and Lu-LW02080 generated using GRPR-expressing PC-3 cells. Error bars indicate standard deviation (n = 3).
Figure 3.
Displacement curves of [125I-Tyr4]Bombesin by Ga-LW02060, Ga-LW02080, Lu-LW02060, and Lu-LW02080 generated using GRPR-expressing PC-3 cells. Error bars indicate standard deviation (n = 3).
Figure 4.
Representative PET images of [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 acquired at 1 h pi in PC-3 tumor-bearing mice. The blocked mouse of [68Ga]Ga-LW02060 was co-injected with 100 μg of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14), and the blocked mouse of [68Ga]Ga-LW02080 was co-injected with 100 μg of nonradioactive Ga-LW02080. t: tumor; k: kidney; bl: urinary bladder.
Figure 4.
Representative PET images of [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 acquired at 1 h pi in PC-3 tumor-bearing mice. The blocked mouse of [68Ga]Ga-LW02060 was co-injected with 100 μg of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14), and the blocked mouse of [68Ga]Ga-LW02080 was co-injected with 100 μg of nonradioactive Ga-LW02080. t: tumor; k: kidney; bl: urinary bladder.
Figure 5.
(A) Uptake of [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 in PC-3 tumor xenografts and major organs/tissues of NRG mice at 1 h pi. Error bars indicate standard deviation; (B) Tumor-to-organ uptake ratios of [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 obtained from PC-3 tumor-bearing mice at 1 h pi. Error bars indicate standard deviation. *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 5.
(A) Uptake of [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 in PC-3 tumor xenografts and major organs/tissues of NRG mice at 1 h pi. Error bars indicate standard deviation; (B) Tumor-to-organ uptake ratios of [68Ga]Ga-LW02060 and [68Ga]Ga-LW02080 obtained from PC-3 tumor-bearing mice at 1 h pi. Error bars indicate standard deviation. *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 6.
(A) Comparison of [68Ga]Ga-LW02060 with/without co-injection of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14) on the uptake in PC-3 tumor xenografts and major organs/tissues in mice at 1 h pi; (B) comparison of [68Ga]Ga-LW02080 with/without co-injection of its nonradioactive standard on the uptake in PC-3 tumor xenografts and major organs/tissues in mice at 1 h pi. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6.
(A) Comparison of [68Ga]Ga-LW02060 with/without co-injection of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14) on the uptake in PC-3 tumor xenografts and major organs/tissues in mice at 1 h pi; (B) comparison of [68Ga]Ga-LW02080 with/without co-injection of its nonradioactive standard on the uptake in PC-3 tumor xenografts and major organs/tissues in mice at 1 h pi. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 7.
Representative radio-HPLC chromatograms from analysis of intact fraction of [68Ga]Ga-LW02060 in mouse (A) plasma and (B) urine samples collected at 15 min pi. The black arrow points to the peak of intact [68Ga]Ga-LW02060.
Figure 7.
Representative radio-HPLC chromatograms from analysis of intact fraction of [68Ga]Ga-LW02060 in mouse (A) plasma and (B) urine samples collected at 15 min pi. The black arrow points to the peak of intact [68Ga]Ga-LW02060.
Figure 8.
Representative radio-HPLC chromatograms from analysis of intact fraction of [68Ga]Ga-LW02080 in mouse (A) plasma and (B) urine samples collected at 15 min pi. The black arrow points to the peak of intact [68Ga]Ga-LW02080.
Figure 8.
Representative radio-HPLC chromatograms from analysis of intact fraction of [68Ga]Ga-LW02080 in mouse (A) plasma and (B) urine samples collected at 15 min pi. The black arrow points to the peak of intact [68Ga]Ga-LW02080.
Figure 9.
Longitudinal SPECT/CT images of (A) [177Lu]Lu-LW02060 and (B) [177Lu]Lu-LW02080 in PC-3 tumor-bearing NRG mice. Acquisition time points were 1, 4, 24, 72, and 120 h pi. The blocked mice were co-injected with 100 μg of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14). t: tumor; bl: urinary bladder.
Figure 9.
Longitudinal SPECT/CT images of (A) [177Lu]Lu-LW02060 and (B) [177Lu]Lu-LW02080 in PC-3 tumor-bearing NRG mice. Acquisition time points were 1, 4, 24, 72, and 120 h pi. The blocked mice were co-injected with 100 μg of [D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6-14). t: tumor; bl: urinary bladder.
Figure 10.
Uptake of [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080 in (A) PC-3 tumor xenografts and (B) pancreas at 1, 4, 24, 72, and 120 h pi. Error bars indicate standard deviation (n = 5).
Figure 10.
Uptake of [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080 in (A) PC-3 tumor xenografts and (B) pancreas at 1, 4, 24, 72, and 120 h pi. Error bars indicate standard deviation (n = 5).
Figure 11.
(A) Radiation absorbed doses (mGy/MBq) of [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080 to PC-3 tumor xenografts, obtained with various tumor masses but assuming the same tumor uptake (%ID) and residence time for [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080; (B) Comparison of radiation absorbed doses with the tumor sink effect correction for selected mouse organs/tissues per unit of injected activity (mGy/MBq) from [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080.
Figure 11.
(A) Radiation absorbed doses (mGy/MBq) of [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080 to PC-3 tumor xenografts, obtained with various tumor masses but assuming the same tumor uptake (%ID) and residence time for [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080; (B) Comparison of radiation absorbed doses with the tumor sink effect correction for selected mouse organs/tissues per unit of injected activity (mGy/MBq) from [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080.
Table 1.
Estimated radiation absorbed doses (mGy/MBq) in adult human males for [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080.
Table 1.
Estimated radiation absorbed doses (mGy/MBq) in adult human males for [177Lu]Lu-LW02060 and [177Lu]Lu-LW02080.
| Target Organ |
[177Lu]Lu-LW02060 |
[177Lu]Lu-LW02080 |
| Adrenals |
2.45E-02 |
4.24E-03 |
| Brain |
2.85E-04 |
1.04E-04 |
| Esophagus |
8.10E-04 |
3.14E-04 |
| Eyes |
5.72E-04 |
2.45E-04 |
| Gallbladder wall |
1.20E-03 |
4.15E-04 |
| Left colon |
2.59E-02 |
5.58E-03 |
| Small intestine |
7.60E-03 |
3.97E-03 |
| Stomach wall |
3.70E-03 |
1.45E-03 |
| Right colon |
1.35E-02 |
2.98E-03 |
| Rectum |
1.30E-02 |
3.00E-03 |
| Heart |
2.66E-03 |
1.17E-03 |
| Kidneys |
6.36E-02 |
2.78E-02 |
| Liver |
1.29E-02 |
3.17E-03 |
| Lungs |
5.31E-03 |
2.11E-03 |
| Pancreas |
5.08E-02 |
1.69E-03 |
| Prostate |
1.64E-03 |
6.94E-04 |
| Salivary glands |
5.91E-04 |
2.52E-04 |
| Red marrow |
7.68E-04 |
3.23E-04 |
| Skeleton |
1.04E-03 |
4.73E-04 |
| Spleen |
9.47E-03 |
2.92E-03 |
| Testes |
2.49E-03 |
1.05E-03 |
| Thymus |
6.92E-04 |
2.85E-04 |
| Thyroid |
6.38E-04 |
2.68E-04 |
| Urinary bladder |
1.50E-01 |
6.51E-02 |
| Total body |
2.55E-03 |
9.77E-04 |
| Effective dose (mSv/MBq) |
1.16E-02 |
4.15E-03 |