1. Introduction
Temporal lobe epilepsy (TLE) is the most common and medically refractory form of focal epilepsy in adults [
1]. TLE is characterized by recurrent seizures with an onset involving the amygdalohippocampal complex and parahippocampal place area (PHA) [
2,
3]. Approximately 30–50% of patients with TLE develop drug resistance [
4,
5]. For those patients in whom seizures persist despite maximum medical management, epilepsy surgery is a potentially curative treatment. Surgical treatment of TLE may include ablation or resection of temporal lobe epileptic tissue, including stereotactic laser amygdalohippocampotomy (SLAH) or amygdalohippocampectomy (AH) with or without anterior temporal lobectomy (ATL), respectively [
6]. Seizure-freedom rates are ~60% for patients treated with ablative surgery (i.e. SLAH) and up to 80% for patients treated with resective surgery (i.e. AH with or without ATL) [
7,
8].
The extent of resection, pathology type, epilepsy duration, and localization pattern may be important determinants of post-surgical seizure control [
9,
10,
11,
12,
13]. However, it has remained challenging to predict postoperative seizure freedom solely based on these features. Other factors may extend beyond traditional clinical variables, such as those involving cellular or molecular processes that lower seizure threshold [
14]. An important unanswered question is to what extent can differences in gene expression in epileptic hippocampal tissue predict outcome after resective epilepsy surgery. There is still limited direct evidence on specific cellular or signaling pathway differences in resected brain tissue that correlate with seizure freedom
versus seizure recurrence after temporal lobe epilepsy surgery.
Transcriptome studies have identified altered gene expression patterns in tissues resected from patients with TLE [
6,
14,
15,
16,
17]. These observations suggest that recurrences after epilepsy surgery may be influenced partly by differences in neuroinflammatory and/or neuronal healing/remodeling pathways. Here we perform RNAseq on hippocampal tissue from eight patients, four of whom that remained seizure-free (SF) and four of whom that experienced seizure recurrence (NSF) after surgery. Analyses of the altered genome-wide patterns of transcript abundance reveal several commonalities, as well as stark differences between these two cohorts. Our results suggest that resected tissue exhibiting strong proinflammatory processes are associated with better post-surgery seizure outcomes than patients exhibiting cellular signaling processes related to ECM reorganization, autoantibody production and neural circuit formation.
2. Materials and Methods
2.1. Patient Samples
The research protocol and consents for all human subjects studied were approved by the University of Arizona Institutional Review Board. Hippocampal tissue was obtained from 8 selected patients diagnosed with medically intractable TLE during anterior temporal lobectomy with amygalohippocampectomy (ATL/AH). All patients experienced complex partial seizures and underwent ATL/AH on the right-side temporal lobe. The surgical procedure of ATL/AH involved up to 5.5 cm right-lateral temporal lobe resection. In all patients, the hippocampus was resected
en bloc posteriorly to at least the level of the cerebral peduncle and preserved for analysis as previously described [
18].
2.2. RNAseq and Statistical Analyses
Results of statistical summaries were generally expressed as mean ± SD. We used a “perturbation signature” approach to identify genome-wide differences in transcript abundance between patients that were not seizure free (NSF) and those that were seizure free (SF) after surgery. We compare RNA-sequencing findings in our non-seizure free cohort (NSF) with those of the seizure-free (SF) cohort in order to filter out common alterations due to having epilepsy per se. RNAseq and differential expression analyses were performed as previously described [
15,
19]. Briefly, libraries were constructed using a stranded mRNA-Seq Kit and average fragment size was assessed. Sequencing was performed using Rapid-Run SBS 2x100bp chemistry on the HiSeq2500 (Illumina, San Diego, CA, USA). RNA-seq data from controls came from five healthy post-mortem human hippocampal tissues [
20]. Gene expression counts were obtained using htseq-count version 0.6.1 [
21]. We utilized the exactTest function in edgeR and gene expression counts were first normalized using the calcNormFactors function. Approximately 20 million high quality sequencing reads were obtained per sequencing run, and >90% of these reads aligned to the reference genome. All reads were considered for analysis, leading to a final set of 63,677 transcripts for differential expression analysis.
All significant differentially expressed genes (DEGs) including false discovery rate (FDR) ≤ 0.05 for NSF versus SF and FDR ≤0.01 for NSF and SF versus controls were analyzed with Ingenuity® Pathway Analysis (IPA) to identify biological pathways that were significantly activated or deactivated involving putative upstream transcriptional regulators (Qiagen, Hilden, Germany). Rather than focusing on any single gene, bioinformatic analyses of our RNAseq data identified the most statistically significant biological pathways that were enriched given the set of differentially expressed genes (DEGs) in each experiment. We performed principal component analysis (PCA) using DESeq2’s plotPCA function on a set of log2-transformed counts calculated by the rlog function in DESeq2 [
22]. PCA plots were generated based on read counts for the 500 genes with the greatest variance in expression.
To predict whether pathways were activated or deactivated relative to baseline, we also included comparisons of NSF and SF samples versus a set of controls derived from RNA-seq data of five healthy post-mortem human hippocampal tissues [
20]. To ensure that our results were not overly biased by altered gene expression due solely to post-mortem processes, we reported results for pathways that were shown to be significantly altered only in the NSF versus SF comparison (i.e., the intersection of all three comparisons).
2.3. Pathway Literature Searches and Feature Selection
“Claude” AI assistant was used to aid in literature searches on the role of canonical pathways identified by IPA (Anthropic, San Francisco, CA, USA). Initially, the question was posed: “what does activation/deactivation of ‘canonical pathway name’ have to do with brain injury?” Several pathway “effects” commonly reported in pathological conditions (e.g., neuroinflammation, Blood-brain barrier, etc.) were identified in the NSF and SF individuals. For each canonical pathway, a score of 1 or 2 was assigned to each effect depending on whether it was ‘detrimental’ or ‘beneficial’, respectively, in association with an activated or deactivated state as appropriate. For example, in the case of the pathway effect “neuroinflammation”, a ‘1’ or ‘2’ was assigned if pro- or anti-inflammatory cytokines were inferred to be produced early and/or late in the injury process, respectively. A score of 1/2 was assigned if the effect was beneficial in one context and detrimental in another. If an effect was not involved in a given pathway it was assigned “0”. An underlying assumption in the interpretation of whether an effect was beneficial or detrimental was that tissue samples were obtained from patients in the chronic stages of epileptogenesis. Finally, a list of cited references in each search was compiled and inspected to verify results for the pathways that were deemed to be most relevant in the study.
3. Results
3.1. Cohort Characteristics
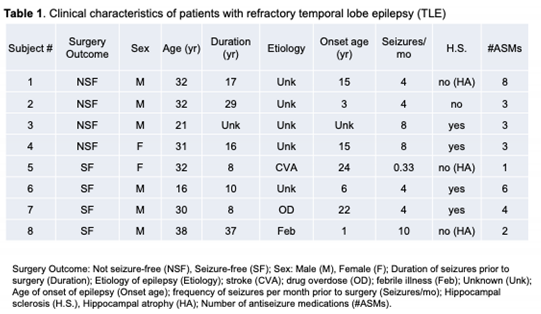
The ages of the 6 males and 2 females spanned 16 to 38 years (mean= 29.0 ± 7.0). The NSF and SF cohorts each had 3 males and 1 female, with mean ages of 29.0 ± 5.4 and 29.0 ± 9.3 years, respectively (Table 1). The etiology of seizures included drug overdose, stroke, and febrile seizures, with 5 of unspecified origin. An equal number of patients in the NSF and SF cohorts had signs of hippocampal sclerosis and hippocampal atrophy. All patients recorded seizure activity for at least 8 years prior to surgery (mean seizure duration = 17.9 ± 11.2 years), with NSF and SF averaging 20.7 ± 7.2 and 15.8 ± 14.2 years, respectively. Pre-surgical seizure frequency data was collected for each patient, which ranged from 0.33 to 10 seizures per month (mean= 5.3 ± 3.1), with NSF and SF averaging 6.0 ± 2.3 and 4.6 ± 4.0 seizures/month, respectively. Patients were taking an average of 3.8 ± 2.3 anti-seizure medications (ASMs), with NSF and SF averaging 4.3 ± 2.5 and 3.3 ± 2.2 ASMs, respectively. Patients were followed clinically for 34.5 ± 27.9 months post-operatively; while SF patients were followed for a minimum of 12 months (mean= 23.5 ± 10.2 months). There were no statistically significant differences in these features between the NSF and SF cohorts.
3.2. Principal Component Analysis
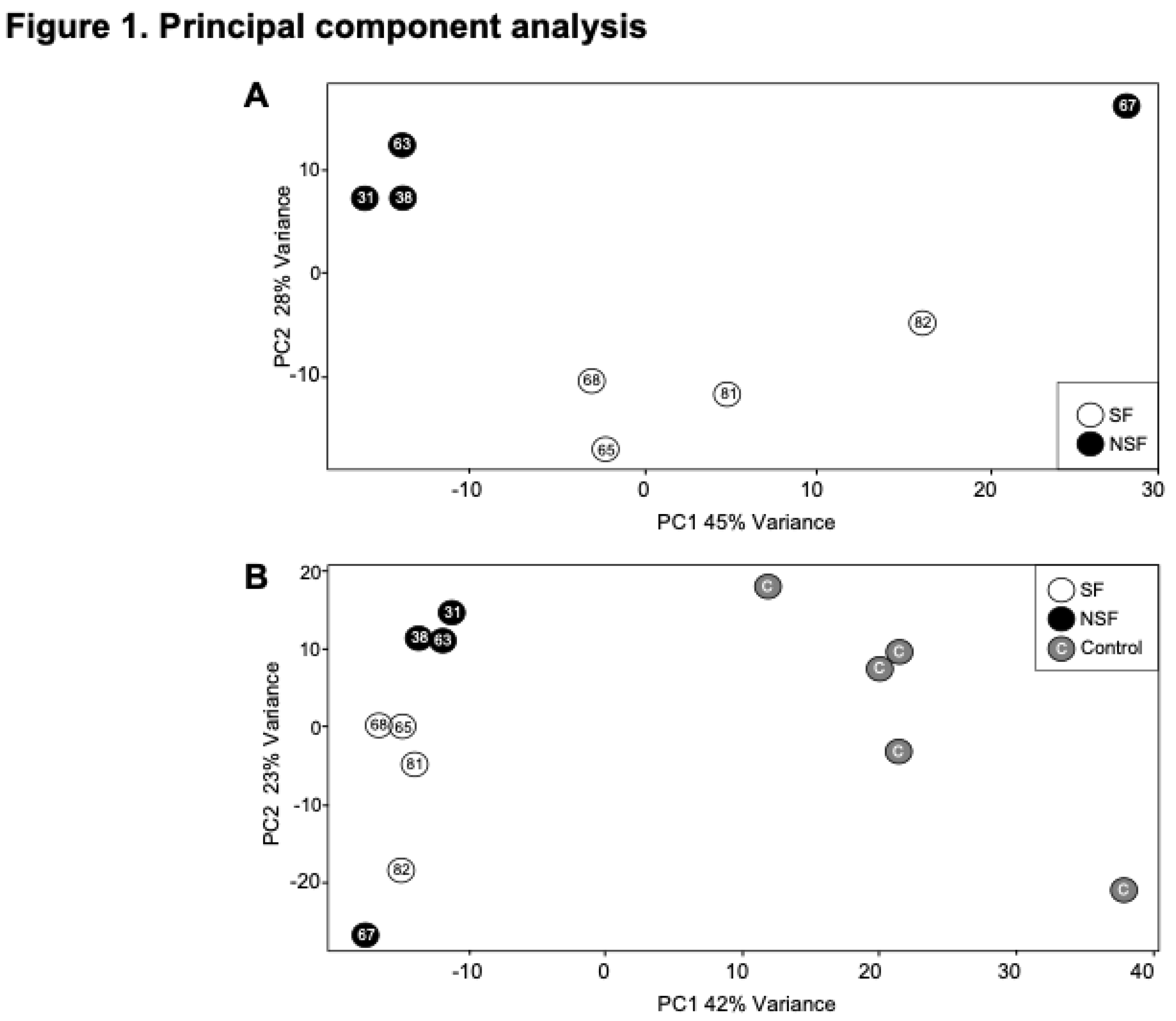
Figure 1 shows the results of a principal component analysis that was conducted using the 500 transcripts with the greatest variance to determine the largest source of variation in the data for the NSF versus SF comparison. The first two principal components explain ~63% of the total variation in gene expression. Three of the four NSF subjects cluster tightly on the upper left portion of the plot with one subject as an outlier in the upper right side. The four SF subjects span the central portions of the plot (
Figure 1A).
We then performed a similar analysis after including RNA-seq data from five “controls” that derive from five adult autopsy samples from hippocampal tissue [
20]. In this case, a total of 65% of the variance was explained in the first two dimensions, with the five controls positioned on the right side of the plot (
Figure 1B). A similar pattern was obtained for NSF and SF, with 3 of the 4 NSF subjects clustering in the upper left and a single outlier at the bottom left. The four SF subjects clustered between these two points. These results suggest that seizure freedom is one of the main determinants of variance within the expression data.
3.3. Differential Expression Analysis
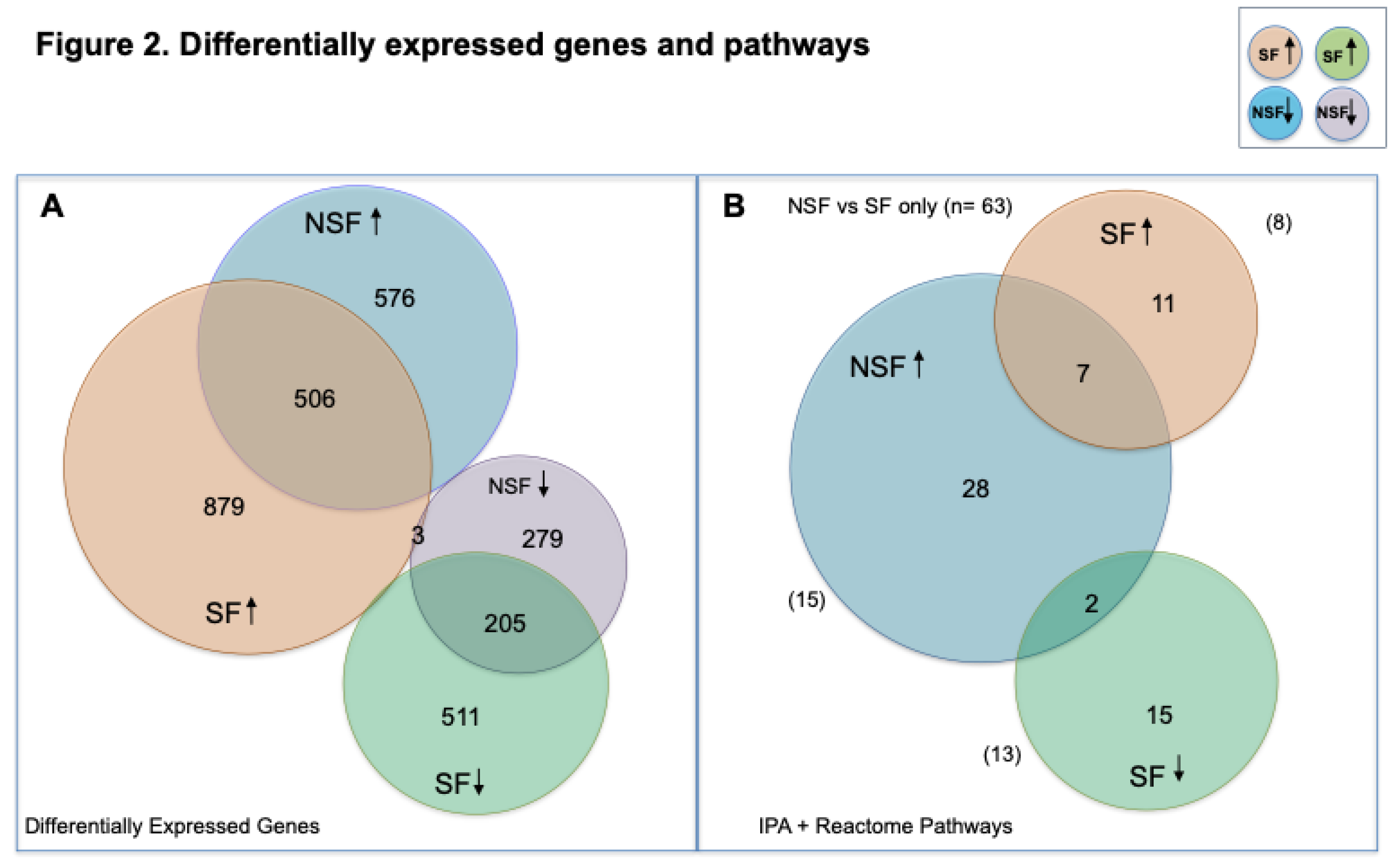
In comparing gene expression differences between NSF and SF, 1548 genes were significantly differentially expressed (p-value ≤0.05, FDR ≤0.05), 952 with elevated transcript abundance in NSF (≥2-fold, range 2.0- 457) and 632 with lower transcript abundance (≥2-fold, range 2.0- 98.5). To infer the number of genes that were upregulated and downregulated, we performed comparisons of transcript abundance in NSF
versus controls and SF
versus controls. A total of 1082 transcripts were upregulated and 403 downregulated in NSF (FDR-adjusted p-value ≤0.05) (
Figure 2A). The SF
versus controls analysis yielded a larger number of differentially expressed genes (DEGs): 1406 upregulated and 531 downregulated. There were 506 upregulated and 205 downregulated DEGs shared between NSF and SF, respectively.
3.4. Canonical Pathways Altered in NSF and SF Cohorts
We investigated results of pathway enrichment procedures, limiting reporting to significant results shared among comparisons of NSF vs SF (FDR≤ 0.05) and NSF or SF versus controls (FDR ≤0.01) and z-scores with absolute values ≥2.0 (
Figure 3). For the NSF cohort, pathway enrichment procedures identified 37 canonical pathways, all of which were predicted to be activated (
Figure 2B). A total of 35 significantly altered canonical pathways were identified in the SF cohort, 17 of which were predicted to be deactivated and 18 to be activated. Shared canonical pathways between NSF and SF included 2 predicted to be activated in NSF and deactivated in SF (Adrenergic Receptor Signaling and Neurexins and Neuroligins) and 7 predicted to be activated in both (S100 Family Signaling, Interleukin 17A (IL-17A) Signaling in Fibroblasts, Macrophage Alternative Activation Signaling, Class A/1 Rhodopsin-like receptors, Interleukin-10 signaling, Cell surface interactions at the vascular wall and Inducible nitric oxide synthase (iNOS) Signaling) (
Figures 2B and 3).
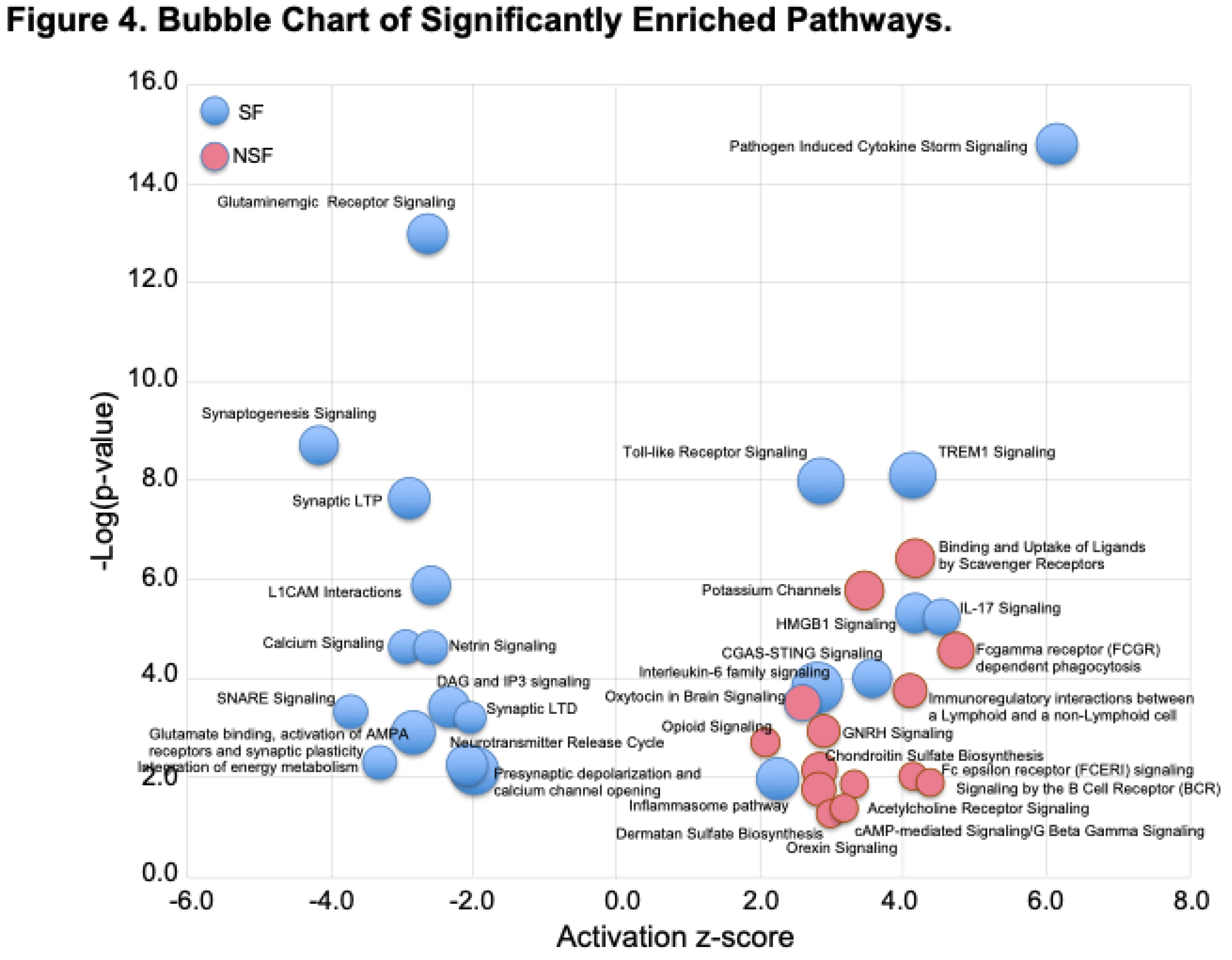
The top five pathways predicted to be solely activated in NSF include Binding and Uptake of Ligands by Scavenger Receptors, G-Protein Coupled Receptor Signaling, Potassium Channels, cAMP response element-binding protein (CREB) Signaling in Neurons, and G alpha (q) signaling events. For SF, the top unique activated pathways include Pathogen Induced Cytokine Storm Signaling, Atherosclerosis Signaling, Triggering receptor expressed on myeloid cells-1 (TREM1) Signaling, Toll-like Receptor Signaling, and High mobility group box 1 (HMGB1) Signaling (
Figure 4). Atherosclerosis is the main cause of ischemic stroke and cardiovascular disease and is considered to be an inflammatory disease—providing a pathway link with epileptogenesis [
14,
23]. The top five deactivated pathways for SF were Glutaminergic Receptor Signaling, Synaptogenesis Signaling, Synaptic Long-Term Potentiation, Dopamine–dopamine and cAMP-regulated phosphoprotein (DARPP32) Feedback in cAMP Signaling, and Netrin Signaling.
3.5. Hierarchical Pathway Categories
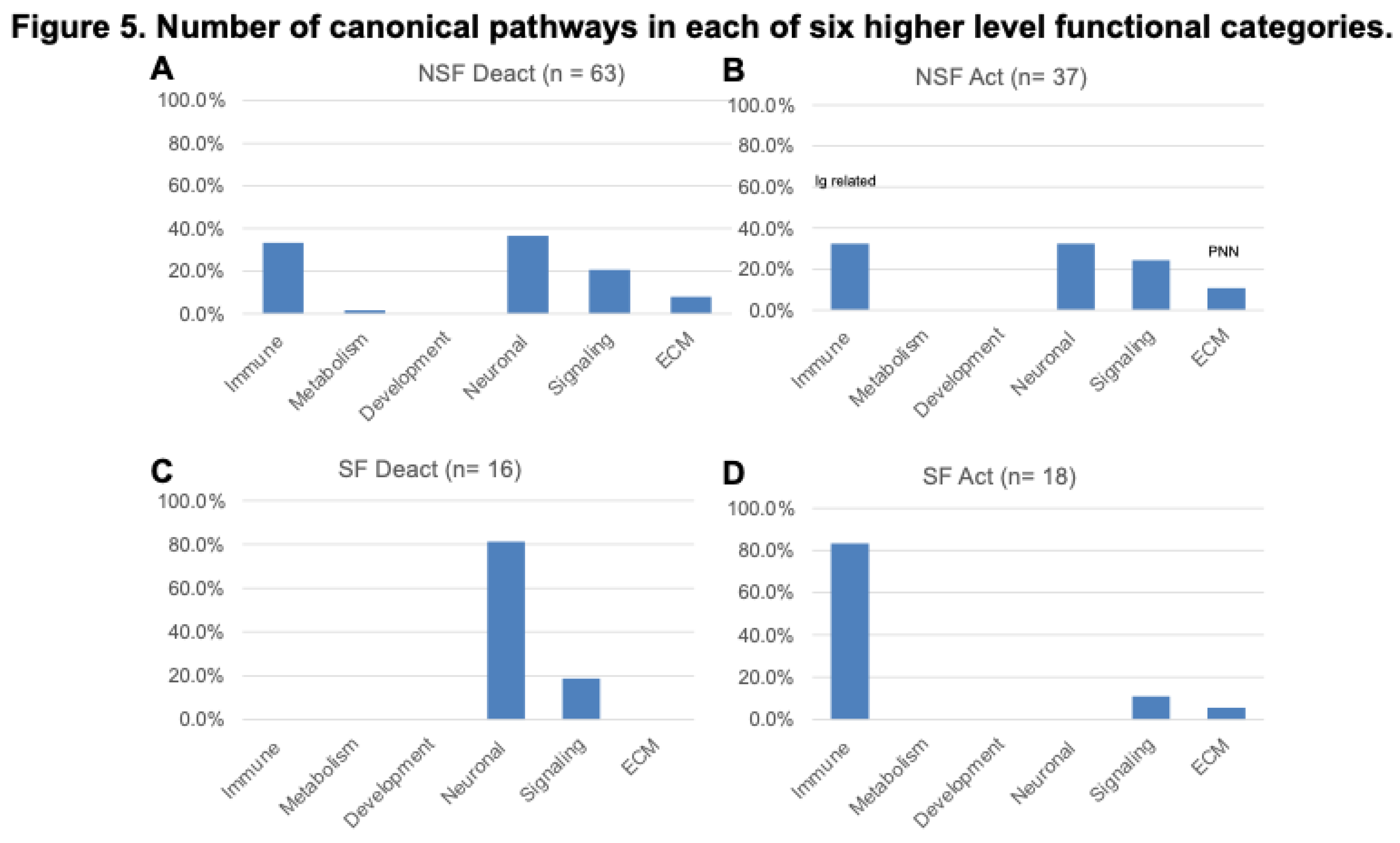
Figure 4 shows a bubble chart representing a subset of the 63 pathways (n= 36 “selected” pathways) that most strongly distinguish NSF and SF in terms of enrichment p-value and activation score. The SF cohort is characterized by a nearly equal representation of activated and deactivated pathways, many of which function in immune and neuronal systems, while NSF shows a pattern dominated by activation of pathways associated with adaptive immune, neuronal and ECM functionality. These higher categories can be visualized in
Figure 5A, where we classify all 63 significantly enriched pathways into one of six higher level functional categories: Immune, Metabolism, Development, Neuronal, Signaling, and Extracellular Matrix (ECM). Nearly equal percentages of NSF activated pathways fall under Immune and Neuronal categories, followed by Signaling and ECM (
Figure 5B). In contrast, the majority of SF deactivated pathways are in the neuronal category (
Figure 5C) and nearly all of the SF activated pathways are Immune related (
Figure 5D).
3.6. Predicted Upstream Regulators
To identify potential drivers of the differential expression patterns observed within each dataset we used the upstream regulator function in IPA. In general, the top predicted activated molecules had higher z-scores for the SF compared with the NSF cohort (top five mean= 8.6 versus 6.7, respectively). Lipopolysaccharide (LPS) was the top predicted activator for both NSF and SF (z-score= 7.8 and 10.7, respectively). However, Cyclic-AMP response element binding protein 1 (CREB1) was the next top predicted upstream activator for the NSF cohort (z-score= 6.9) while the immune related molecules, Interleukin-1 beta (IL1B) and Tumor necrosis factor (TNF), were the next top predicted activators for SF cohort (z-score= 8.8 and 8.2, respectively).
3.7. Pathway Effects
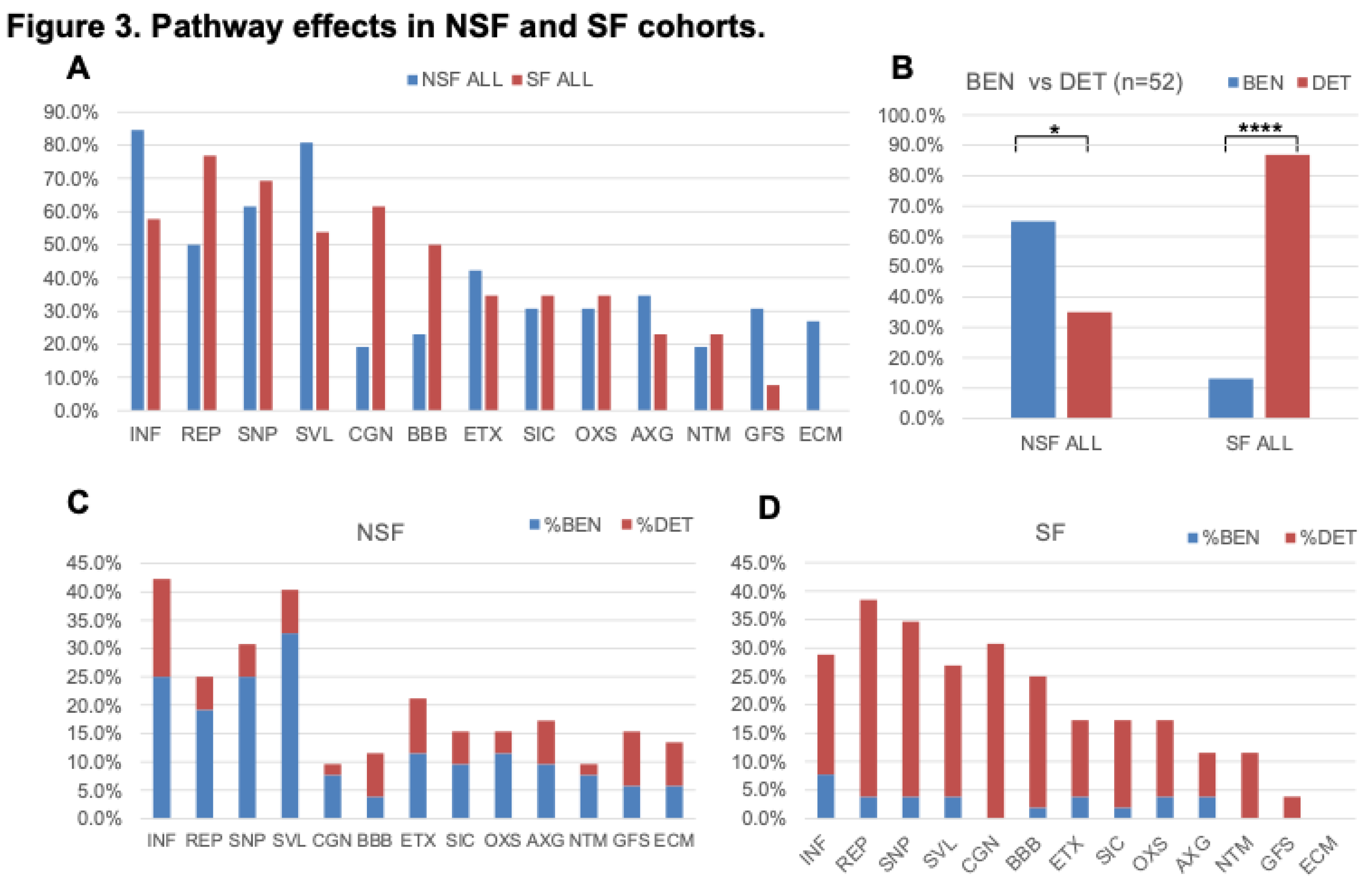
Literature searches querying cellular processes associated with the 52 canonical pathways that were unique to NSF or SF cohorts in
Figure 3 resulted in many that are commonly reported in brain injury or other neurological disorders. A total of 13 such effects was compiled with tallies of the number of times each effect was involved for all 52 pathways. Many of these pathway effects are considered hallmarks of neurological disease or known to play prominent roles in disease pathogenesis or response to brain insults [
24,
25]. The most common pathway effect was ‘neuroinflammation’ (INF), which was involved in 37 of the 52 (71.2%) pathways. This was followed by ‘cell survival/apoptosis’ (SVL, 67.3%), ‘synaptic plasticity’ (SNP, 65.4%), ‘repair/recovery’ (REP, 63.5%), ‘cognition’ (CGN, 40.4%), ‘excitotoxicity’ (ETX, 38.5%), ‘blood-brain barrier’ (BBB, 36.5%), ‘secondary injury cascade’ (SIC, 32.7%), ‘oxidative stress’ (OXS, 32.7%), ‘axon guidance/neurite outgrowth’ (AXG, 28.8%), ‘neurotransmission’ (NTM, 21.2%), ‘glial/fibrotic scar’ (GFS, 19.2%), and ‘extracellular matrix’ (ECM, 13.5%) (
Figure 3).
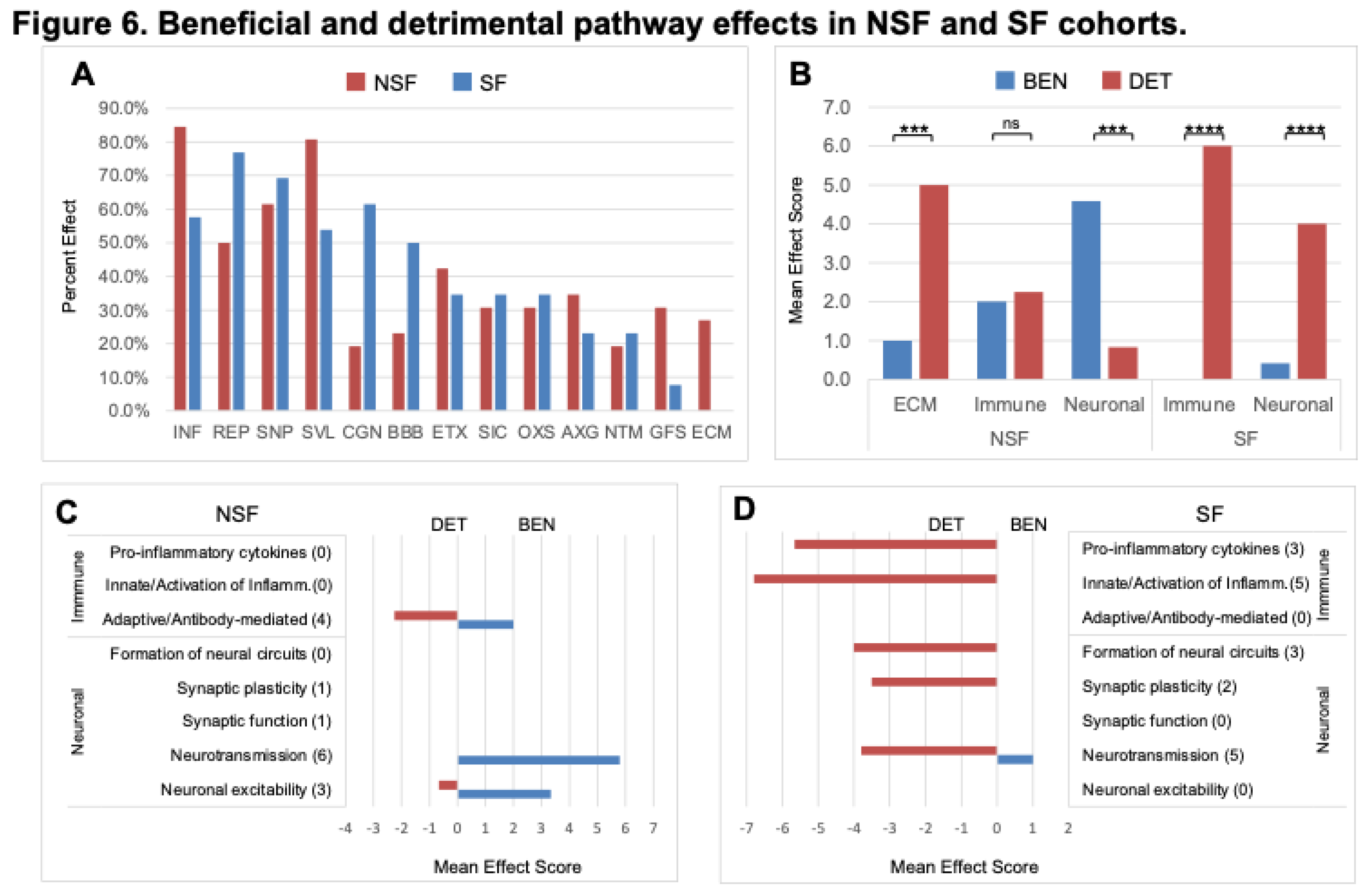
NSF
versus SF effect frequencies differ; five pathway effects are higher in NSF compared with SF: INF (84.6% vs 57.7%), SVL (80.8% vs 53.8%), AXG (34.6% vs 23.1%), GFS (30.8% vs 7.7%), and ECM (26.9% vs 0.0%), respectively, while three are more frequent in SF: REP (76.9% vs 50.0%), CGN (61.5% vs 19.2%), and BBB (50.0% vs 23.1%), respectively (
Figure 6A). The mean number of effects associated with a pathway is 2.7 ± 2.5. For the combined cohort, the mean number of beneficial effects (2.1 ± 2.4) is lower than that for detrimental effects (3.2 ± 2.5) (t-test p-value= 0.010) (
Figure 3). This trend is more extreme for the SF cohort, which has a much lower mean number of beneficial (0.9 ± 1.9)
versus detrimental effects (4.3 ± 2.0) (t-test p-value= <1 x 10
-5). The NSF cohort shows the opposite trend with a larger mean number of beneficial effects (3.5 ± 2.2) compared with detrimental effects (1.9 ± 2.3) (t-test p-value= 6.3 x 10
-3) (
Figure 3). This shift toward increased detrimental effects in the SF cohort is clearest for SVL, CGN, OXS, and NTM (
Figure 3).
3.8. Divergent Immune and Neuronal System-Related Pathway Effects
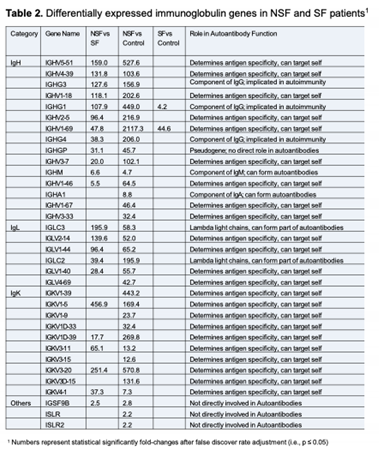
As reported above, NSF and SF cohorts differ in the activation status of immune- and neuronal-related pathways (
Figure 5). A striking difference between NSF and SF hippocampal tissue is the over-expression of nearly three dozen immunoglobulin genes in NSF. Of the 40 immunoglobulin DEGs in both NSF and SF cohorts, 34 were found to be over-expressed in NSF (Table 2), with only two expressed at lower levels. The genes uniquely upregulated in NSF include several that can form autoantibodies. The top hit when performing an overrepresentation test for these 34 DEGs in the Reactome knowledgebase was ‘complement cascade’. Our analysis reveals distinct effects of deactivation/activation of these pathways whereby NSF has a mixed beneficial to detrimental profile for immune-related pathways and a predominantly beneficial profile for neuronal-related pathways. In contrast, SF has a detrimental profile for both immune and neuronal related pathway alterations (
Figure 6B). Further subclassification of immune and neuronal functions indicates SF pathways characterized by detrimental activation of pro-inflammatory cytokines and inflammatory immune cells, with no evidence of adaptive immune/antibody-mediated processes as seen in NSF (
Figure 6D). NSF has several activated pathways that function in neurotransmission and neuronal excitability with chiefly beneficial effects (
Figure 5C), while SF has several deactivated pathways that function in formation of neural circuits (axon guidance, neurite outgrowth), synaptic plasticity, and neurotransmission—all with mainly detrimental effects in the context of injured brain tissue (
Figure 6D).
4. Discussion
While clinical features have shown utility in predicting post-operative outcome [
11,
26,
27], the process of evaluating which TLE patients are the best surgical candidates based solely on clinical/imaging data is complex [
28]. For example, models to predict surgical outcomes have <75% discrimination [
16,
27]. The incorporation of molecular data to improve clinical decision-making has shown promise [
17,
29,
30,
31] including recent genome-wide approaches. Hershberger et al. [
16] found that upregulation of genes related to immune response and inflammation was associated with higher risk of seizure recurrence and that altered expression of genes involved in synaptic transmission and neuronal plasticity was associated with lower risk of seizure recurrence. Similarly, Louis et al. [
17] found that upregulation of genes related to neuroinflammation, glial cell activation, and oxidative stress was associated with seizure recurrence and downregulation of genes involved in Gamma-aminobutyric acid (GABA)ergic signaling and synaptic plasticity was associated with seizure freedom. Focusing more on risks of late seizure recurrence, Jehi et al. [
14] found associations related to neuronal plasticity, synaptic transmission, and immune response. These findings underscore the known complexity of epilepsy pathophysiology and molecular mechanisms that may contribute to post-surgical seizure outcomes. The main unanswered question is how localized alterations of these pathways (i.e., in the resected tissue itself) predict post-surgical outcome (i.e., more widespread and/or persistent effects).
Our transcriptome analyses of resected hippocampal tissue identified several cellular signaling pathways that distinguish NSF from SF patients. In the following sections we attempt to infer how these alterations predict or influence postoperative seizure freedom
versus seizure recurrence. We do not favor an explanation based solely on pre-surgical clinical factors because evaluations of our patients were performed at a single neurosurgical center and NSF and SF cohorts did not vary significantly in clinical features that have been shown to be predictors of surgical outcome [
9,
11,
12,
24] (Table 1).
4.1. Shared Pathways Indicative of Common Processes in Epileptogenesis.
We used a two-prong strategy to identify molecular and cellular signaling processes that may explain postsurgical seizure outcome. By comparing genome-wide changes in transcript abundance in NSF
versus SF we filtered out many common alterations that underly the epileptogenic process
per se. Similar rates of ASM usage, epilepsy duration, and seizure frequencies help to homogenize the impact of these variables on the genetic makeup of the tissue [
14]. The drawback of using internal cohort comparisons is that it cannot determine whether genes are upregulated or downregulated relative to baseline expression levels expected under physiological conditions. Therefore, we also compared each TLE cohort with a set of autopsy controls without epilepsy [
20]. The results reported in
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6 represent only those genes and pathways that were statistically significant in both analyses.
4.2. Pathways Distinguishing NSF from SF
Deactivated and activated pathways in hippocampal tissue from the NSF cohort primarily fell into four higher level categories: ECM, Immune, Neuronal and Signaling systems (
Figure 5A,B). This pattern contrasts with that of the SF cohort, which primarily exhibitied deactivation of Neuronal pathways and activation of Immune pathways (
Figure 5C,D). Morover, the ratio of beneficial to detrimental pathway effects differed significantly for the higher level categories shared between the NSF and SF cohorts (
Figure 6B). Neuronal system pathway effects were generally inferred to be beneficial in the NSF and detrimental in the SF cohort (
Figure 3). While immune-related pathways were of mixed effect in NSF, they were exclusively associated with detrimental effects in the SF cohort (
Figure 6B). In the next section we discuss pathways and pathway effects, that uniquely characterize the NSF and SF cohorts, to identify potential factors underlying seizure freedom or recurrence.
4.3. Increased Expression of Immunoglobulins in NSF
A striking difference between NSF and SF hippocampal tissue is the over-expression of nearly three dozen immunoglobulin genes in NSF. Of the 40 immunoglobulin DEGs in both NSF and SF cohorts, 34 were found to be over-expressed in NSF (Table 2), with only two expressed at lower levels. The genes uniquely upregulated in NSF include several that can form autoantibodies. The top hit when performing an overrepresentation test for these 34 DEGs in the Reactome knowledgebase was ‘complement cascade’. The complement system is a critical part of the immune response that enhances the ability of antibodies and phagocytic cells to clear damaged cells and promote inflammation. Antibodies that participate in autoimmunity can interact with the complement cascade in several significant ways, contributing to both physiological immune function and the pathology of autoimmune diseases. In fact, our results bear some resemblance to a recent study that found increased inflammatory response characterized by the activation of the complement system in drug-resistant TLE patients who experienced seizure recurrence after hippocampectomy [
25].
How might increased immunoglobulin expression, particularly in the context of autoimmunity and persistent inflammation, contribute to the risk of post-surgery seizure recurrence in TLE and other neurological disorders? Autoantibodies targeting neuronal proteins (e.g., anti-N-methyl-D-aspartic acid (NMDA) receptor; anti-Gamma-aminobutyric acid (GABA) receptor) can have widespread effects on neural circuit function and plasticity, contributing to the development and persistence of seizures even after surgical resection of the epileptic focus [
32]. In spinal cord injury, B cells producing pathogenic antibodies have been shown to impair recovery [
33] and spinal cord injury can trigger systemic autoimmunity, characterized by chronic B lymphocyte activation and autoantibody synthesis [
34,
35]. The high-affinity IgG receptor, Fc gamma receptor I (FcγRI), has also been implicated in modulating neuropathic pain after peripheral nerve injury [
36], suggesting a role for immunoglobulins in the pathogenesis of neurological disorders.
The risk of developing autoimmune epilepsy after TLE surgery may be elevated in patients with increased expression of immunoglobulins in the resected hippocampal tissue [
37,
38]. Such increased expression may suggest the presence of pre-existing autoimmune activity, even if it has not yet manifested as clinical autoimmune epilepsy [
39]. Thus, it is plausible that autoimmune epilepsy could potentially be triggered by the resection of hippocampal tissue in TLE patients after surgery and affect other brain regions [
40]. In other cases, the exposure of neuronal antigens during surgery may lead to the production of autoantibodies that cross-react with other proteins in the brain due to molecular mimicry [
41].
4.4. The Role of Increased Activation of Chondroitin and Dermatan Synthesis in NSF
Chondroitin sulfate proteoglycans (CSPGs) are key components of the extracellular matrix (ECM) in the central nervous system (CNS). Increased activation of chondroitin and dermatan synthesis, particularly after CNS injury or in neurological disorders like epilepsy, can lead to the formation of a dense, inhibitory CSPG-rich matrix [
42,
43]. This inhibitory matrix can limit synaptic plasticity, axonal growth, and regeneration, thus hindering repair processes and functional recovery [
44,
45,
46]. Interestingly, enzymatic digestion of CSPGs using chondroitinase ABC (ChABC) has shown promise in promoting functional recovery and reducing pathology in spinal cord injury models [
44,
47]. In the context of temporal lobe epilepsy, increased chondroitin 6-sulfation has been implicated in the formation of aberrant neural circuits and the persistence of seizures [
48]. The upregulation of CSPGs can also contribute to the formation of a glial scar, which acts as a physical and chemical barrier to regeneration [
47,
49].
4.5. What Factors Explain the Association of Increased Pro-Inflammatory Markers and Post-Surgery Seizure Freedom?
In contrast to our NSF patients, patients in the SF cohort had increased levels of neuroinflammatory markers involved in innate immunity, production of pro-inflammatory cytokines, regulation of inflammation, and recruitment of immune cells to the site of injury. The activation of these pathways is consistent with findings in other TLE studies, especially in patients with hippocampal sclerosis (HS) [
50,
51]. Interestingly, cells of adaptive immunity such as T and B cells or natural killer (NK) cells were not detected in human epileptic tissue [
51], which differentiated brain inflammation in TLE from inflammation in Rasmussen's encephalitis, where cells of adaptive immunity are strongly represented in the lesional tissue [
52]. The association of activated neuroinflammatory processes in patients with better post-surgical outcomes appears to be contrary to the aforementioned transcriptome studies [
14,
16,
17].
Figure 6 shows significant presence of detrimental inflammatory pathways in SF compared with NSF. Current surgical practices appear to be resecting detrimental inflammatory hippocampal pathophysiology in SF patients and “ineffectively” resecting detrimental ECM hippocampal pathophysiology in NSF patients. Continued research is needed to establish whether the increased expression of neuroinflammatory markers and signaling processes in resected tissue could be an indicator of a more localized pathology that is amenable to surgical treatment (as opposed to increased CSPGs and immunoglobulin levels, which may have a more extensive and complex pathology that is less likely to be resolved by surgery alone).
4.6. Implications for Pre- and Post-Surgery Surveillance
Future work is needed to determine whether these results have implications for pre- and post-surgery surveillance such as 1) identifying patients with higher likelihood of post-surgery seizure freedom and 2) monitoring patients after surgery and treatment when increased expression of CSPGs and immunoglobulins are identified in resected tissue. Incorporating circulating inflammatory cytokine (along with CSPG and immunoglobulin expression data, if possible) into pre-surgical evaluation protocols could help guide patient counseling, surgical decision-making, and post-operative management strategies. Indeed, gene expression profiles are known to differ in pre-surgical peripheral blood samples taken from NSF and SF patients, and several candidate biomarkers have been identified [
53]. For example, BGN codes for biglycan, which is a structural component of ECM that also acts as a danger signal that stimulates multifunctional proinflammatory signaling linking the innate to the adaptive immune response [
54]. Other genes with potential prognostic value include several with ECM and/or inflammation-related roles (e.g., Bridging Integrator 3 (BIN3); Matrix Metallopeptidase 8 (MMP8); Interferon Alpha Inducible Protein 27 (IFI27); Interleukin 22 Receptor Subunit Alpha 1 (IL22RA1); Radical S-Adenosyl Methionine Domain Containing 2 (RSAD2); Platelet Factor 4 Variant 1 (PF4V1)) as well as with roles in synaptic plasticity and repair (e.g., Proteolipid Protein 1 (PLP1); Glial Fibrillary Acidic Protein (GFAP); Nectin Cell Adhesion Molecule 2 (PVRL2); Cytoplasmic Polyadenylation Element Binding Protein 4 (CPEB4); and MAM Domain Containing Glycosylphosphatidylinositol Anchor 1 (MDGA1)) [
53].
Validating these results on a biological level may also inform on patients at risk for seizure recurrence and other neurological deficits after surgery. In addition to standard ASMs, some patients may benefit from immunomodulatory therapies, such as intravenous immunoglobulin (IVIG) or plasmapheresis, which have been used to treat autoimmune epilepsy and other neurological disorders associated with pathogenic autoantibodies [
40,
55]. It may also be of benefit to consider continued monitoring of circulating cytokines with a neuroinflammatory profile during long-term follow-up as an additional tool that may be used as an indication of favorable outcome after temporal lobe surgery [
50].
5. Conclusions and Limitations
In summary, this study revealed a distinct set of immunological and neurological processes in hippocampal tissue resected from patients who became seizure free or who experienced recurrent seizures following temporal lobectomy and amygdalohippocampectomy. In both the NSF and SF cohorts, there is ample evidence of alterations involving the regulation of neuroinflammation. However, the immune response in the NSF cohort is mainly geared to the clearance of cellular debris and potentially harmful molecules through phagocytosis and antibody-dependent cellular cytotoxicity, while the response in the SF cohort is aimed at the production of pro-inflammatory cytokines and chemokines, which can exacerbate neuroinflammation and secondary damage in pathologic brain tissue. There is a similar division in the neuronal response, with NSF pathways primarily involved in the modulation of neuronal excitability and neurotransmission, activation of which may have a neuromodulatory effect. On the other hand, many neuronal pathways were deactivated in the SF cohort, which may have the effect of impairing synaptic function, reducing neurotransmitter release, and disrupting the formation of neural circuits. Finally, this study identified pathways that involve chondroitin and dermatan synthesis that were activated in the NSF cohort, which may have a more widespread inhibitory effect on neuronal plasticity.
An important open question for future work is whether the increased activation of these pathways and the concomitant upregulation of immunoglobulin expression can work in concert to inhibit repair processes and contribute to the persistence of seizures after surgery. Targeting both the inhibitory CSPG matrix and the autoimmune/inflammatory components may be necessary to effectively promote repair and prevent the recurrence of seizures and other neurological deficits [
56]. While uncovering many pathological processes that may be prognostic for surgery outcome in patients with TLE, we point out two limitations of this study, including a relatively small cohort size and the lack of suitable control tissue. While we prioritized the NSF versus SF comparisons, we also relied on post-mortem hippocampal tissue to predict whether pathways were activated or deactivated relative to baseline at physiological conditions. It is also important to note that future studies are needed to validate these findings at the biological level and to test the potential role of autoimmunity in the post-surgical non-seizure free condition. Finally, the discovery of biomarkers in samples that are easily accessed from patients in presurgical examinations (e.g., peripheral blood, including leukocytes) will greatly facilitate translation of this research.
6. Patents
Dr. Weinand is author of US Non-Provisional Patent Application 18/754,715 (UA23-142, ARIZ 23.12 NP), “Methods for the prognosis and treatment of temporal lobe epilepsy”, submitted June 26, 2024.
Author Contributions
Conceptualization: MEW; Methodology: MFH. Validation: MFH; Formal analysis: MFH; Investigation: MEW; Resources: MEW; Writing - original draft: MFH.; Writing - review & editing: MFH, MEW; Visualization: MFH; Supervision: MEW; Project administration: MFH, MEW; Funding acquisition: MEW.
Funding
This research was funded by the United States National Institutes of Health (NIH) RO1MH065151 (Yuri Persidsky, Temple University) (HCS 04–42), NIH subcontract from Temple University to University of Arizona by which Dr. Weinand, Co-Principal Investigator, received support for purchase of laboratory materials and gene expression analysis.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Arizona (Protocol Number 1401194084, The University of Arizona, Date of Initial Approval: 02-04-2014, Updated Approval: 05-05-2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from all patients to report their results in the public domain consistent with publication of this paper.
Data Availability Statement
Acknowledgments
We thank Branden Lau in the Arizona Genetics Core at the University of Arizona for bioinformatic analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DeBarros Lourence, F. H.; Marques, L. H. N.; DeAraujo Filho, G. M. Electroencephalogram alterations associated with psychiatric disorders in temporal lobe epilepsy with mesial sclerosis: A systematic review. Epilepsy Behav. 2020, 108. [Google Scholar] [CrossRef] [PubMed]
- Cascino, G. D. Temporal lobe epilepsy: more than hippocampal pathology. Epilepsy Curr. 2005, 5, 187–9. [Google Scholar] [CrossRef] [PubMed]
- Panina, Y. S.; Timechko, E. E.; Usoltseva, A. A.; Yakovleva, K. D.; Kantimirova, E. A.; Dmitrenko, D. V. Biomarkers of Drug Resistance in Temporal Lobe Epilepsy in Adults. Metabolites 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A. T.; Brodie, M. J.; Allen Hauser, W.; Mathern, G.; Moshe, S. L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–77. [Google Scholar] [CrossRef]
- Sanchez, J.; Centanaro, M.; Solis, J.; Delgado, F.; Yepez, L. Factors predicting the outcome following medical treatment of mesial temporal epilepsy with hippocampal sclerosis. Seizure 2014, 23, 448–53. [Google Scholar] [CrossRef]
- Gallek, M. J.; Skoch, J.; Ansay, T.; Benbahani, M.; Mount, D.; Manziello, A.; Witte, M.; Bernas, M.; Labiner, D. M.; Weinand, M. E. Cortical gene expression: prognostic value for seizure outcome following temporal lobectomy and amygdalohippocampectomy. Neurogenetics 2016, 17, 211–218. [Google Scholar] [CrossRef]
- Asadi-Pooya, A. A.; Rostami, C. History of surgery for temporal lobe epilepsy. Epilepsy Behav. 2017, 70, 57–60. [Google Scholar] [CrossRef]
- Donos, C.; Breier, J.; Friedman, E.; Rollo, P.; Johnson, J.; Moss, L.; Thompson, S.; Thomas, M.; Hope, O.; Slater, J.; et al. Laser ablation for mesial temporal lobe epilepsy: Surgical and cognitive outcomes with and without mesial temporal sclerosis. Epilepsia 2018, 59, 1421–1432. [Google Scholar] [CrossRef]
- Bjellvi, J.; Olsson, I.; Malmgren, K.; Wilbe Ramsay, K. Epilepsy duration and seizure outcome in epilepsy surgery: A systematic review and meta-analysis. Neurology 2019, 93, e159–e166. [Google Scholar] [CrossRef]
- Hemb, M.; Palmini, A.; Paglioli, E.; Paglioli, E. B.; Costa da Costa, J.; Azambuja, N.; Portuguez, M.; Viuniski, V.; Booij, L.; Nunes, M. L. An 18-year follow-up of seizure outcome after surgery for temporal lobe epilepsy and hippocampal sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 800–5. [Google Scholar] [CrossRef]
- Ivanovic, J.; Larsson, P. G.; Ostby, Y.; Hald, J.; Krossnes, B. K.; Fjeld, J. G.; Pripp, A. H.; Alfstad, K. A.; Egge, A.; Stanisic, M. Seizure outcomes of temporal lobe epilepsy surgery in patients with normal MRI and without specific histopathology. Acta Neurochir. (Wien) 2017, 159, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Tomlinson, G.; Snead, C.; Sander, B.; Widjaja, E. Systematic review and network meta-analysis of resective surgery for mesial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, E.; Jain, P.; Demoe, L.; Guttmann, A.; Tomlinson, G.; Sander, B. Seizure outcome of pediatric epilepsy surgery: Systematic review and meta-analyses. Neurology 2020, 94, 311–321. [Google Scholar] [CrossRef]
- Jehi, L.; Yehia, L.; Peterson, C.; Niazi, F.; Busch, R.; Prayson, R.; Ying, Z.; Bingaman, W.; Najm, I.; Eng, C. Preliminary report: Late seizure recurrence years after epilepsy surgery may be associated with alterations in brain tissue transcriptome. Epilepsia Open 2018, 3, 299–304. [Google Scholar] [CrossRef]
- Hammer, M. F.; Sprissler, R.; Bina, R. W.; Lau, B.; Johnstone, L.; Walter, C. M.; Labiner, D. M.; Weinand, M. E. Altered expression of signaling pathways regulating neuronal excitability in hippocampal tissue of temporal lobe epilepsy patients with low and high seizure frequency. Epilepsy Res. 2019, 155. [Google Scholar] [CrossRef]
- Hershberger, C. E.; Louis, S.; Busch, R. M.; Vegh, D.; Najm, I.; Bazeley, P.; Eng, C.; Jehi, L.; Rotroff, D. M. Molecular subtypes of epilepsy associated with post-surgical seizure recurrence. Brain Commun. 2023, 5. [Google Scholar] [CrossRef]
- Louis, S.; Busch, R. M.; Lal, D.; Hockings, J.; Hogue, O.; Morita-Sherman, M.; Vegh, D.; Najm, I.; Ghosh, C.; Bazeley, P.; et al. Genetic and molecular features of seizure-freedom following surgical resections for focal epilepsy: A pilot study. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Fiala, M.; Avagyan, H.; Merino, J. J.; Bernas, M.; Valdivia, J.; Espinosa-Jeffrey, A.; Witte, M.; Weinand, M. Chemotactic and mitogenic stimuli of neuronal apoptosis in patients with medically intractable temporal lobe epilepsy. Pathophysiology 2013, 20, 59–69. [Google Scholar] [CrossRef]
- Sprissler, R.; Hammer, M.; Labiner, D.; Joshi, N.; Alan, A.; Weinand, M. Leukocyte differential gene expression prognostic value for high versus low seizure frequency in temporal lobe epilepsy. BMC Neurol. 2024, 24. [Google Scholar] [CrossRef]
- Hwang, T.; Park, C.; Leung, A.; Gao, Y.; Hyde, T.; Kleinman, J.; Rajpurohit, A.; Tao, R.; Shin, J.; Weinberger, D. Dynamic regulation of RNA editing in human brain development and disease. Nature Neuroscience 2016, 19, 1093–1099. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–9. [Google Scholar] [CrossRef] [PubMed]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Schetinger, M. R.; Morsch, V. M.; Bonan, C. D.; Wyse, A. T. NTPDase and 5'-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors 2007, 31, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Josephson, C. B.; Dykeman, J.; Fiest, K. M.; Liu, X.; Sadler, R. M.; Jette, N.; Wiebe, S. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology 2013, 80, 1669–76. [Google Scholar] [CrossRef]
- Grote, A.; Heiland, D. H.; Taube, J.; Helmstaedter, C.; Ravi, V. M.; Will, P.; Hattingen, E.; Schure, J. R.; Witt, J. A.; Reimers, A. , et al. 'Hippocampal innate inflammatory gliosis only' in pharmacoresistant temporal lobe epilepsy. Brain 2023, 146, 549–560. [Google Scholar] [CrossRef]
- Athreya, A.; Fasano, R. E.; Drane, D. L.; Millis, S. R.; Willie, J. T.; Gross, R. E.; Karakis, I. Withdrawal of antiepileptic drugs after stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Epilepsy Res. 2021, 176. [Google Scholar] [CrossRef]
- Jehi, L.; Yardi, R.; Chagin, K.; Tassi, L.; Russo, G. L.; Worrell, G.; Hu, W.; Cendes, F.; Morita, M.; Bartolomei, F.; et al. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 2015, 14, 283–90. [Google Scholar] [CrossRef]
- Anyanwu, C.; Motamedi, G. K. Diagnosis and Surgical Treatment of Drug-Resistant Epilepsy. Brain Sci. 2018, 8, 49. [Google Scholar] [CrossRef]
- Pernhorst, K.; Herms, S.; Hoffmann, P.; Cichon, S.; Schulz, H.; Sander, T.; Schoch, S.; Becker, A. J.; Grote, A. TLR4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure 2013, 22, 675–8. [Google Scholar] [CrossRef]
- Strauss, K. I.; Elisevich, K. V. Brain region and epilepsy-associated differences in inflammatory mediator levels in medically refractory mesial temporal lobe epilepsy. J. Neuroinflammation 2016, 13. [Google Scholar] [CrossRef]
- Teocchi, M. A.; Ferreira, A. E.; Da Luz De Oliveira, E. P.; Tedeschi, H.; D'Souza-Li, L. Hippocampal gene expression dysregulation of Klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J. Neuroinflammation 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Bien, C. G.; Vincent, A.; Barnett, M. H.; Becker, A. J.; Blumcke, I.; Graus, F.; Jellinger, K. A.; Reuss, D. E.; Ribalta, T.; Schlegel, J.; et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 2012, 135, 1622–38. [Google Scholar] [CrossRef]
- Ankeny, D. P.; Guan, Z.; Popovich, P. G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Invest. 2009, 119, 2990–9. [Google Scholar] [CrossRef]
- Ankeny, D. P.; Lucin, K. M.; Sanders, V. M.; McGaughy, V. M.; Popovich, P. G. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006, 99, 1073–87. [Google Scholar] [CrossRef]
- Popovich, P. G.; Stokes, B. T.; Whitacre, C. C. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J. Neurosci. Res. [CrossRef]
- Liang, Y.; Zhang, Z.; Juan, Z.; Zhang, R.; Zhang, C. The high-affinity IgG receptor FcgammaRI modulates peripheral nerve injury-induced neuropathic pain in rats. Mol. Brain 2019, 12. [Google Scholar] [CrossRef]
- Flammer, J.; Neziraj, T.; Ruegg, S.; Probstel, A. K. Immune Mechanisms in Epileptogenesis: Update on Diagnosis and Treatment of Autoimmune Epilepsy Syndromes. Drugs 2023, 83, 135–158. [Google Scholar] [CrossRef]
- Ryding, M.; Mikkelsen, A. W.; Nissen, M. S.; Nilsson, A. C.; Blaabjerg, M. Pathophysiological Effects of Autoantibodies in Autoimmune Encephalitides. Cells 2023, 13, 15. [Google Scholar] [CrossRef]
- Chen, B.; Lopez Chiriboga, A. S.; Sirven, J. I.; Feyissa, A. M. Autoimmune Encephalitis-Related Seizures and Epilepsy: Diagnostic and Therapeutic Approaches. Mayo Clin. Proc. 2021, 96, 2029–2039. [Google Scholar] [CrossRef]
- Geis, C.; Planaguma, J.; Carreno, M.; Graus, F.; Dalmau, J. Autoimmune seizures and epilepsy. J. Clin. Invest. 2019, 129, 926–940. [Google Scholar] [CrossRef]
- Husari, K. S.; Dubey, D. Autoimmune Epilepsy. Neurotherapeutics 2019, 16, 685–702. [Google Scholar] [CrossRef]
- Dyck, S. M.; Karimi-Abdolrezaee, S. Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Exp. Neurol. 2015, 269, 169–87. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J. W.; Kwok, J. C. F. Proteoglycan Sulphation in the Function of the Mature Central Nervous System. Front. Integr. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E. J.; Moon, L. D.; Popat, R. J.; King, V. R.; Bennett, G. S.; Patel, P. N.; Fawcett, J. W.; McMahon, S. B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–40. [Google Scholar] [CrossRef] [PubMed]
- Burnside, E. R.; Bradbury, E. J. Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol. Appl. Neurobiol. 2014, 40, 26–59. [Google Scholar] [CrossRef]
- Sorg, B. A.; Berretta, S.; Blacktop, J. M.; Fawcett, J. W.; Kitagawa, H.; Kwok, J. C.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef]
- Bartus, K.; James, N. D.; Didangelos, A.; Bosch, K. D.; Verhaagen, J.; Yanez-Munoz, R. J.; Rogers, J. H.; Schneider, B. L.; Muir, E. M.; Bradbury, E. J. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J. Neurosci. 2014, 34, 4822–36. [Google Scholar] [CrossRef]
- Yutsudo, N.; Kitagawa, H. Involvement of chondroitin 6-sulfation in temporal lobe epilepsy. Exp. Neurol. 2015, 274, 126–33. [Google Scholar] [CrossRef]
- Bradbury, E. J.; Burnside, E. R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Quirico-Santos, T.; Meira, I. D.; Gomes, A. C.; Pereira, V. C.; Pinto, M.; Monteiro, M.; Souza, J. M.; Alves-Leon, S. V. Resection of the epileptogenic lesion abolishes seizures and reduces inflammatory cytokines of patients with temporal lobe epilepsy. J. Neuroimmunol. 2013, 254, 125–30. [Google Scholar] [CrossRef]
- Ravizza, T.; Gagliardi, B.; Noe, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–60. [Google Scholar] [CrossRef]
- Vezzani, A.; Granata, T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 2005, 46, 1724–43. [Google Scholar] [CrossRef] [PubMed]
- Sprissler, R.; Bina, R.; Kasoff, W.; Witte, M. H.; Bernas, M.; Walter, C.; Labiner, D. M.; Lau, B.; Hammer, M. F.; Weinand, M. E. Leukocyte expression profiles reveal gene sets with prognostic value for seizure-free outcome following stereotactic laser amygdalohippocampotomy. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Nastase, M. V.; Young, M. F.; Schaefer, L. Biglycan: a multivalent proteoglycan providing structure and signals. J. Histochem. Cytochem. 2012, 60, 963–75. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Britton, J. W.; McKeon, A.; Shin, C.; Lennon, V. A.; Quek, A. M.; So, E.; Worrell, G. A.; Cascino, G. D.; Klein, C. J. , et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology 2014, 82, 1578–86. [Google Scholar] [CrossRef]
- Shafqat, A.; Albalkhi, I.; Magableh, H. M.; Saleh, T.; Alkattan, K.; Yaqinuddin, A. Tackling the glial scar in spinal cord regeneration: new discoveries and future directions. Front. Cell. Neurosci. 2023, 17. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).