Submitted:
16 January 2025
Posted:
17 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Immunopathogenetic Background
3. Organ-Specific Immunopathology
3.1. The Respiratory Tract
3.2. The Gastrointestinal Tract
3.3. Central and Peripheral Nervous System
3.4. Bones and Joints
3.5. The Skin
3.6. Endocrine Glands
3.7. The Cardiovascular System
3.8. Challenges and Future Perspectives
Author Contributions
Funding
Scientific Support
Availability of Data and Material/Data Transparency
Conflicts of Interest/Competing Interest
References
- Bonilla, FA, Barlan, I, Chapel, H, Costa-Carvalho, BT, Cunningham-Rundles, C, de la Morena, MT, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract 2016,4,38-59. [CrossRef]
- Seidel, M, Kindle, G, Gathmann, B, Quinti, I, Buckland, M, van Monfrans, J, et al. The European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract 2019, 7, 1763–1770. [Google Scholar] [CrossRef]
- Patel, SY, Carbone, J, Jolles, S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol 2019, 10, 33. [Google Scholar] [CrossRef]
- Pandit, C, Hsu, P, van Asperen, P, Mehr, S. Respiratory manifestations and management in children with common variable immunodeficiency. Paediatr Respir Rev 2016, 19, 56–61. [Google Scholar] [CrossRef]
- Esmaeilzadeh, E, Jokar-Derisi, A, Hassani, AH, Yazdani, R, Delavari, S, Abolhassani, H, et al. Assessment of the first manifestations of common variable immunodeficiency in a large cohort of patients. BMC Immunol 2023, 24, 9. [Google Scholar] [CrossRef]
- Zainaldain, H, Rizvi, FS, Rafiemanesh, H, Alizadeh, M, Jamee, M, Mohammadi, S, et al. Infectious complications reporting in common variable immunodeficiency: a systematic review and meta-analysis. Oman Med J 2020, 35, e157. [Google Scholar] [CrossRef]
- Patuzzo, G, Barbieri, A, Tinazzi, E, Vener,i E, Argentino, G, Moretta, F, et al. Autoimmunity and infection in common variable immunodeficiency (CVID). Autoimmun Rev 2016, 15, 877–882. [Google Scholar] [CrossRef]
- Pashangzadeh, S, Delavari, S, Moeini Shad, T, Salami, F, Rasouli, SE, Yazdani, R, et al. Noninfectious complications in B-lymphopenic common variable immunodeficiency. J Investig Allergol Clin Immunol 2024, 34, 233–245. [Google Scholar] [CrossRef]
- Szczawińska- Popłonyk, A, Tąpolska-Jóźwiak, K, Schwartzmann, E, Popłonyk, N. Immune dysregulation in pediatric common variable immunodeficiency: implications for the diagnostic approach. Front Pediatr 2022, 10, 855200. [Google Scholar] [CrossRef] [PubMed]
- Szczawińska-Popłonyk, A, Schwartzmann, E, Bukowska-Olech, E, Biernat, M, Gattner, S, Korobacz, T, et al. The pediatric common variable immunodeficiency – from genetics to therapy: a review. Eur J Pediatr 2022, 181, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, G, Peroni, DG, Consolini, R. Beyond infections: new warning signs for inborn errors of immunity in children. Front Pediatr 2022, 10, 855445. [Google Scholar] [CrossRef]
- Ho, H, Cunningham-Rundles, C. Non-infectious complications of common variable immunodeficiency: updated clinical spectrum, sequelae, and insights to pathogenesis. Front Immunol 2020, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Peng, XP, Caballero-Oteyza, A, Grimbacher, B. Common variable immunodeficiency: more pathways than roads to Rome. Annu Rev Pathol Mech Dis 2023, 18, 283–310. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, FS, Tavakol, M, Aghamahdi, F, Sadri, H, Chavoshzadeh, Z, Jamee, M, et al. Immunological evaluation of pediatric patients with polyautoimmunity. Endocr Metab Immune Disord Drug Targets 2024, 24, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, MJ, Sullivan, KE, Fuleihan, R, Bingham, CO. Phenotypic characterization of patients with rheumatologic manifestations of common variable immunodeficiency. Semin Arthritis Rheum 2018, 48, 318–326. [Google Scholar] [CrossRef]
- Carrabba, M, Salvi, M, Basselli, LA, Serafino, S, Zarantonello, M, Trombetta, E, et al. Long-term follow-up in common variable immunodeficiency: the pediatric-onset and adult-onset landscape. Front Pediatr 2023, 11, 1125994. [Google Scholar] [CrossRef]
- Ramirez, NJ, Posadas-Cantera, S, Caballero-Oteyza, A, Camacho-Ordonez, N, Grimbacher, B. There is no gene for CVID – novel monogenetic causes for primary antibody deficiency. Curr Opin Immunol 2021, 72, 176–185. [Google Scholar] [CrossRef]
- Chawla, S, Barman, P, Tyagi, R, Jindal, AK, Sharma, S, Rawat, A, et al. Autoimmune cytopenias in common variable immunodeficiency are a diagnostic and therapeutic conundrum: an update. Front Immunol 2022, 13, 869466. [CrossRef]
- Cunningham-Rundless, C, Casanova, JL, Boisson, B. Genetics and clinical phenotypes in common variable immunodeficiency. Front Genet 2024, 14, 1272912. [Google Scholar] [CrossRef]
- Ameratunga, R, Woon, ST, Bryant, VL, Steele, R, Slade, C, Leung, EY, et al. Clinical implications of digenic inheritance and epistasis in primary immunodeficiency disorders. Front Immunol 2018, 8, 1965. [Google Scholar] [CrossRef]
- Ameratunga, R, Koopmans, W, Woon, ST, Leung, E, Lehnert, K, Slade, CA, et al. Epistatic interactions between mutations in TACI (TNFRSF13B) and TCF3 result in a severe primary immunodeficiency disorder and systemic lupus erythematosus. Clin Transl Immunol 2017, 6, 159. [Google Scholar] [CrossRef]
- Dieli-Crimi, R, Martinez-Gallo, M, Franco-Jarava, C, Antolin, M, Blasco, L, Paramonov, I, et al. Th1-skewed profile and excessive production of proinflammatory cytokines in an NFKB1-deficient patient with CVID and severe gastrointestinal manifestations. Clin Immunol 2018, 195, 49–58. [Google Scholar] [CrossRef]
- Massaad, MJ, Zhou, J, Tsuchimoto, D, Chou, J, Jabara, H, Janssen, E, et al. Deficiency of base excision repair enzyme NEIL3 drives increased predisposition to autoimmunity. J Clin Invest 2016, 126, 4219–4236. [Google Scholar] [CrossRef] [PubMed]
- Sic, H, Speletas, M, Cornacchione, V, Seidel, M, Beibel, M, Linghu, B, et al. An activating Janus Kinase 3 mutation is associated with Cytotoxic T Lymphocyte Antigen-4-dependent immune dysregulation syndrome. Front Immunol 2017, 8, 1824. [Google Scholar] [CrossRef] [PubMed]
- Ho, H, Cunningham-Rundles, C. Seeking relevant biomarkers in common variable immunodeficiency. Front Immunol 2022, 13, 857050. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, G, Cappelli, S, Consolini, R. Autoimmunity in primary immunodeficiency disorders: an updated review on pathogenic and clinical implications. J Clin Med 2021, 10, 4729. [Google Scholar] [CrossRef]
- Amirifar, P, Mozdarani, H, Yazdani, R, Kiaei, F, Moeini Shad, T, Shahkaram,i S, et al. Effect of class switch recombination defect on the phenotype of ataxia-telangiectasia patients. Immunol Invest 2021, 50, 201–215. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A, Tąpolska-Jóźwiak, K, Schwartzmann, E, Pietrucha, B. Infections and immune dysregulation in ataxia-telangiectasia children with hyper-IgM and non-hyper-IgM phenotypes: a single center experience. Front Pediatr 2022, 10, 972952. [Google Scholar] [CrossRef]
- Barbosa, RR, Silva, SP, Silva, SL, Tendeiro, R, Melo, AC, Pedro, E, et al. Monocyte activation is a feature of common variable immunodeficiency irrespective of plasma lipopolysaccharide levels. Clin Exp Immunol 2012, 169, 263–272. [Google Scholar] [CrossRef]
- Abyazi, ML, Bell, KA, Gyimesi, G, Baker, TS, Byun, M, Ko, HM, et al. Convergence of cytokine dysregulation and antibody deficiency in common variable immunodeficiency with inflammatory complications. J Allergy Clin Immunol 2022, 149, 315–326. [Google Scholar] [CrossRef]
- Macpherson, ME, Halvorsen, B, Ynestad, A, Ueland, T, Mollnes, T, Berge, RK, et al. Impaired HDL function amplifies systemic inflammation in common variable immunodeficiency. Sci Rep 2019, 9, 9427. [Google Scholar] [CrossRef]
- Poto, R, Pecoraro, A, Ferrara, AL, Punziano, A, Lagnese, G, Messuri, C, et al. Cytokine dysregulation despite immunoglobulin replacement therapy in common variable immunodeficiency (CVID). Front Immunol 2023, 14, 1257398. [Google Scholar] [CrossRef]
- Maglione, PJ, Cols, M, Cunningham-Rundles, C. Dysregulation of innate lymphoid cells in common variable immunodeficiency. Curr Allergy Asthma Rep 2018, 17, 77. [Google Scholar] [CrossRef]
- Lopez-Herrera, G, Segura-Mendez, NH, O’Farril-Romanillos, P, Nunez-Nunez, ME, Zarate-Hernandez, MC, Mogica-Martinez, D, et al. Low percentages of regulatory T cells in common variable immunodeficiency (CVID) patients with autoimmune diseases and its association with increased numbers of CD4+CD45RO+ T and CD21low B Cells. Allergol Immunopathol 2019, 47, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Matson, EM, Abyazi, ML, Bell, KA, Hayes, KM, Maglione, PJ. B cell dysregulation in common variable immunodeficiency interstitial lung disease. Front Immunol 2021, 11, 622114. [Google Scholar] [CrossRef] [PubMed]
- Richardson, CT, Slack, MA, Dhillon, G, Marcus, CZ, Barnard,, J, Palanichamy A, et al. Failure of B cell tolerance in CVID. Front Immunol 2019, 10, 288. [Google Scholar] [CrossRef]
- Azizi, G, Rezaei, N, Kiaee, F, Tavakolinia, N, Yazdani, R, Mirshafiey, A, et al. T cell abnormalities in common variable immunodeficiency. J Investig Allergol Clin Immunol 2016, 26, 233–243. [Google Scholar] [CrossRef]
- Ogulur, I, Kiykim, A, Karakoc-Aydiner, E, Ozen, A, Baris, S. Lymphocyte abnormalities in pediatric-onset common variable immunodeficiency. Int Arch Allergy Immunol 2020, 181, 228–237. [Google Scholar] [CrossRef]
- Rossi, S, Baronio, M, Gazzurelli, L, Tessarin, G, Baresi, G, Chiarini, M, et al. Lymphocyte alterations in patients with common variable immunodeficiency (CVID) and autoimmune manifestations. Clin Immunol, 2022; 241, 109077. [Google Scholar] [CrossRef]
- Kennedy-Batalla, R, Acevedo, D, Luo, Y, Esteve-Sole, A, Vlagea, A, Correa-Rocha, R, et al. Treg in inborn errors of immunity: gaps, knowns and future perspectives. Front Immunol 2024, 14, 1278759. [Google Scholar] [CrossRef]
- Rutkowska-Zapała, M, Grabowska-Gurgul, A, Lenart, M, Szaflarska, A, Kluczewska, A, Mach-Tomalska, M, et al. Gene signature of regulatory T cells isolated from children with selective IgA deficiency and common variable immunodeficiency. Cells 2024, 13, 417. [Google Scholar] [CrossRef]
- Azizi, G, Hafezi, N, Mohammadi, H, Yazdani, R, Alinia, T, Tavakol, M, et al. Abnormality of regulatory T cells in common variable immunodeficiency. Cell Immunol 2017, 315, 11–17. [Google Scholar] [CrossRef]
- Van de Ven, AAJM, de Jong, PA, Hoytema, van Konijnenburg, DP, Kessels, OAM, Boes, M, Sanders, EAM, et al. Airway and interstitial lung disease are distinct entities in paediatric common variable immunodeficiency. Clin Exp Immunol 2011, 165, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Correa-Jimenez, O, Restrepo-Gualteros, S, Nino, G, Cunningham-Rundles, C, Sullivan, KE, Fuleihan, RL, et al. Respiratory comorbidities associated with bronchiectasis in patients with common variable immunodeficiency in the USIDNET registry. J Clin Immunol 2023, 43, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C, Hsu, P, van Asperen, P, Mehr, S. Respiratory manifestations and management in children with common variable immunodeficiency. Pediatr Respir Rev 2016, 19, 56–61. [Google Scholar] [CrossRef]
- Esenboga, S, Oguz, B, Cagdas, D, Karaatmaca, B, Emiralioglu, N, Yalcin, E, et al. Respiratory findings in pediatric patients with primary immunodeficiency. Pediatr Pulmonol 2021, 56, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, N, Jamee, M, Bakhtyari, M, Rafiemanesh, H, Zainaldain, H, Tavakol, M, et al. Bronchiectasis in common variable immunodeficiency: a systematic review and meta-analysis. Pediatr Pulmonol 2020, 55, 292–299. [Google Scholar] [CrossRef]
- Kellner, ES, Fuleihan, R, Cuningham-Rundles, C, Wechsler, JB, USIDNET Consortium. Cellular defects in CVID patients with chronic lung disease in the USIDNET registry. J Clin Immunol 2019, 39, 569–576. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A, Jończyk-Potoczna, K, Mikoś, M, Ossowska, L, Langfort, R. Granulomatous lymphocytic interstitial lung disease in a spectrum of pediatric primary immunodeficiencies. Pediatr Dev Pathol 2021, 24, 504–512. [Google Scholar] [CrossRef]
- Sood, AK, Funkhouser, W, Handly, B, Weston, B, Wu, EY. Granulomatous-lymphocytic interstitial lung disease in 22q11.2 deletion syndrome: case report and literature review. Curr Allergy Asthma Rep 2018, 18, 14. [Google Scholar] [CrossRef]
- Marczak, H, Heropolitańska-Pliszka, E, Langfort, R, Roik, D, Grzela, K. Nijmegen Breakage Syndrome complicated with primary pulmonary granulomas. Pediatrics 2018, 142, e20180122. [Google Scholar] [CrossRef]
- Sacco, KA, Gazzin, A, Notarangelo, LD, Delmonte, OM. Granulomatous inflammation in inborn errors of immunity. Front Pediatr 2023, 11, 1110115. [Google Scholar] [CrossRef]
- Van Stigt, AC, von der Thusen, JH, Mustafa, DAM, van den Bosch, TPP, Lila, KA, Vadgama, D, et al. Granulomas in common variable immunodeficiency display different histopathological features compared to other granulomatous diseases. J Clin Immunol 2024, 45, 22. [Google Scholar] [CrossRef]
- Lopes, JP, Ho, H, Cunningham-Rundles, C. Interstitial lung disease in commona variable immunodeficiency. Front Immunol 2021, 12, 605945. [Google Scholar] [CrossRef] [PubMed]
- Lui, VG, Ghosh, T, Rymaszewski, A, Chen, S, Baxter, RM, Kong, DS., et al. Dysregulated lymphocyte antigen receptor signaling in common variable immunodeficiency with granulomatous lymphocytic interstitial lung disease. J Clin Immunol 2023, 43, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Shamriz, O, Shadur, B, NaserEddin, A, Zaidman, I, Simanovsky,, N, Elpeleg O, et al. Respiratory manifestations in LPS-responsive beige-like anchor (LRBA) protein-deficient patients. Eur J Pediatr 2018, 177, 1163–1172. [Google Scholar] [CrossRef]
- Hartono, S, Ippoliti, MR, Mastroianni, M, Torres, R, Rider, NL. Gastrointestinal disorders associated with primary immunodeficiency diseases. Clin Rev Allergy Immunol 2019, 57, 145–165. [Google Scholar] [CrossRef]
- Song, J, Lleo, A, Yang, GX, Zhang, W, Bowlus, CL, Gershwin, E, et al. Common variable immunodeficiency and liver involvement. Clin Rev Allergy Immunol 2018, 55, 340–351. [Google Scholar] [CrossRef]
- Franzblau, LE, Fuleihan, RL, Cunningham-Rundles, C, Wysocki, CA. CVID-associated intestinal disorders in the USIDNET registry: an analysis of disease manifestations, functional status, comorbidities, and treatment. J Clin Immunol 2024, 44, 32. [Google Scholar] [CrossRef]
- Andersen, IM, Jorgensen, SF. Gut inflammation in CVID: causes and consequences. Expert Rev Clin Immunol 2022, 18, 3145. [Google Scholar] [CrossRef]
- Marsden, JWN, Lacle, MM, Severs, M, Leavis, HL. Paucity of gastrointestinal plasma cells in common variable immunodeficiency. Curr Opin Allergy Clin Immunol 2024, 24, 464–471. [Google Scholar] [CrossRef]
- Van Schevick, CM, Lowe, DM, Burns, SO, Workman, S, Symes, A, Guzman, D, et al. Bowel histology of CVID patients reveals distinct patterns of mucosal inflammation. J Clin Immunol 2022, 42, 46–59. [Google Scholar] [CrossRef]
- Pikkarainen, S, Martelius, T, Ristimaki, A, Siitonen, S, Seppanen, MRJ, Farkkila, M. A high prevalence of gastrointestinal manifestations in common variable immunodeficiency. Am J Gastroenterol 2019, 114, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Leone, P, Vacca, A, Damacco, F, Racanelli, V. Common variable immunodeficiency and gastric malignancies. Int J Mol Sci 2018,19,451. [CrossRef]
- Jorgensen, SF, Reims, HM, Frydenlund, D, Holm, K, Paulsen, V, Michelsen, AE, et al. A cross-sectional study of the prevalence of gastrointestinal symptoms and pathology in patients with common variable immunodeficiency. Am J Gastroenterol 2016, 111, 1467–1475. [Google Scholar] [CrossRef]
- Lougaris, V, Ravelli, A, Villanacci, V, Salemme, M, Soresina, A, Fuoti, A, et al. Gastrointestinal pathologic abnormalities in pediatric-onset and adult-onset common variable immunodeficiency. Dig Dis Sci 2015, 60, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, A, Crescenzi, L, Varicchi, G, Marone, G, Spadaro G. Heterogeneity of liver disease in common variable immunodeficiency disorders. Front Immunol 2020, 11, 338. [Google Scholar] [CrossRef]
- Baumert, LS, Shih, A, Chung, RT. Management of liver disease and portal hypertension in common variable immunodeficiency (CVID). JHEP Rep 2023, 5, 100882. [Google Scholar] [CrossRef]
- Souza Lima, FM, Toledo-Barros, M, Ferreira Alves, VA, Seixas Duarte, MI, Takakura, C, Bernardes-Silva, CF, et al. Liver disease accompanied by enteropathy in common variable immunodeficiency: common pathophysiological mechanisms. Front Immunol 2022, 13, 933463. [Google Scholar] [CrossRef]
- Murakawa, Y, Miyagawa-Hayashino, A, Ogura, Y, Egawa, H, Okamoto, S, Soejima, Y, et al. Liver transplantation for severe hepatitis in patients with common variable immunodeficiency. Pediatr Transplant 2012, 16, 210–216. [Google Scholar] [CrossRef]
- Song, J, Lleo, A, Yang, GX, Zhang, W, Bowlus, CL, Gershwin, E, et al. Common variable immunodeficiency and liver involvement. Clin Rev Allergy Immunol 2018, 55, 340–351. [Google Scholar] [CrossRef]
- Yildrim, M, Ayvaz, DC, Konuskan, B, Gocmen, R, Tezcan, I, Topcu, M, et al. Neurologic involvement in primary immunodeficiency disorders. J Child Neurol 2018, 33, 320–328. [Google Scholar] [CrossRef]
- Kose, H, Karali, Z, Bodur, M, Cekic, S, Kilic, SS. Neurological involvement in patients with primary immunodeficiency. Allergol Immunopathol 2024, 52, 85–92. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A, Schwartzmann, E, Chmara, Z, Głukowska, A, Krysa, T, Majchrzycki, M, et al. 22q11.2 deletion syndrome: a comprehensive review of molecular genetics in the context of multidisciplinary clinical approach. Int J Mol Sci 2023, 24, 8317. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A, Olejniczak, K, Tąpolska-Jóźwiak, K, Boruczkowski, M, Jończyk-Potoczna, K, Małdyk, J, et al. Cutaneous and systemic granulomatosis in ataxia-telangiectasia: a clinico-pathological study. Adv Dermatol Allergol 2020, 37, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, M, Nguyen, J, Fuleihan, R, Grundling, K, Cunningham-Rundles, C, Otani, IM. Neurologic conditions and symptoms reported among common variable immunodeficiency patients in the USIDNET. J Clin Immunol 2020, 40, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Abati, E, Faravelli, I, Magri, F, Govoni, A, Velardo, D, Gagliari, D, et al. Central nervous system involvement in common variable immunodeficieny: a case of unilateral optic neuritis in a 26-year-old Italian patient. Front Neurol 2018, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, BI, Smith, TL, Delic, A, Esquibe,l L, Galli, J, Millsap, L, et al. Neurologic manifestations of common variable immunodeficiency. Neurol Neuroimmunol Neuroinflamm 2023, 10, 200088. [Google Scholar] [CrossRef]
- Nguyen, JT, Green, A, Wilson, MR, DeRisi, JL, Gundling, K. Neurologic complications of common variable immunodeficiency. J Clin Immunol 2016, 36, 793–800. [Google Scholar] [CrossRef]
- Van de Ven, A, Mader, I, Wolff, D, Goldacker, S, Fuhrer, H, Rauer, S, et al. Structural non-infectious manifestations of the central nervous system in common variable immunodeficiency disorders. J Allergy Clin Immunol Pract 2020, 8, 1047–1062. [Google Scholar] [CrossRef]
- Najem, CE, Springer, J, Prayson, R, Culver, DA, Fernandez, J, Tavee, J, et al. Intra-cranial granulomatous disease in common variable immunodeficiency: case series and review of the literature. Semin Arthritis Rheum 2018, 47, 890–896. [Google Scholar] [CrossRef]
- Ozdemir, O, Okan, MS, Kilic, SA. Chronic inflammatory demyelinating polyneuropathy in common variable immunodeficiency. Pediatr Neurol 2012, 46, 260–262. [Google Scholar] [CrossRef]
- Mangodt, TC, Van den Driessche, K, Norga, KK, Moes, N, De Bruyne, M, Haerynck, F, et al. Central nervous system manifestations of LRBA deficiency: case report of two siblings and literature review. BMC Pediatr 2023, 23, 353. [Google Scholar] [CrossRef]
- Chinello, M, Mauro, M, Cantalupo, G, Talenti, G, Mariotto, S, Balter, R, et al. Acute cervical longitudinally extensive transverse myelitis in a child with Lipopolysaccharide-Responsive-Beige-Like-Anchor-Protein (LRBA) deficiency: a new complication of a rare disease. Front Pediatr 2020, 16. [Google Scholar] [CrossRef]
- Azizi, G, Kiaee, F, Hedayat, E, Yazdani, R, Dolatshahi, E, Alinia, T, et al. Rheumatologic complications in a cohort of 227 patients with common variable immunodeficiency. Scand J Immunol 2018, 87, 12663. [Google Scholar] [CrossRef] [PubMed]
- Levy, E, Stolzenberg, M, Bruneau, J, Breton, S, Neven, B, Sauvion, S, et al. LRBA deficiency with autoimmunity and early onset chronic erosive polyarthritis. Clin Immunol 2016, 168, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Semo Oz, R, Tesher, MS. Arthritis in children with LRBA deficiency – case report and literature review. Pediatr Rheumatol 2019, 17, 82. [Google Scholar] [CrossRef]
- Fabozzi, F, De Vito, R, Gaspari, S, Leone, F, Delvecchio, M, Agolini, E, et al. Case report: A new pathogenic variant of LRBA deficiency with a complex phenotype and Rosai-Dorfman disease. Front Immunol 2022, 13, 944810. [Google Scholar] [CrossRef]
- Pott, NM, Atschekzei, F, Pott, CC, Ernst, D, Witte, T, Sogkas, G. Primary antibody deficiency-associated arthritis shares features with spondyloarthritis and enteropathic arthritis. RMD Open 2022, 8, 002664. [Google Scholar] [CrossRef]
- Mehrabadi, AZ, Aghamohammadi, N, Abolhassani, H, Aghamohammadi, A, Rezaei, N, Yazdani, R. Comprehensive assessment of skin disorders in patients with common variable immunodeficiency (CVID). J Clin Immunol 2022, 42, 653–664. [Google Scholar] [CrossRef]
- De Wit, J, Dalm, VASH, Totte, JEE, Kamphuis, LSJ, Vermont, CL, van Osnabrugge, FY, et al. Atopic manifestations are underestimated clinical features in various primary immunodeficiency disease phenotypes. J Investig Allergol Clin Immunol 2023, 33, 200–208. [Google Scholar] [CrossRef]
- Zheng, C, Shi, Y, Zou, Y. T cell co-stimulatory and co-inhibitory pathways in atopic dermatitis. Front Immunol 2023, 14, 1081999. [Google Scholar] [CrossRef]
- De Bruyn Carlier, T, Saiema Badloe, FM, Ring, J, Gutermuth, J, Kortekaas Krohn, I. Autoreactive T cells and their role in atopic dermatitis. J Autoimmun 2021, 120, 102634. [Google Scholar] [CrossRef]
- Sharifinejad, N, Azizi, G, Rasouli, SE, Chavoshzadeh, Z, Mahdavian,i SA, Tavakol, M, et al. Autoimmune versus non-autoimmune cutaneous features in monogenic patients with inborn errors of immunity. Biology 2023, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Rivalta, B, Zama, D, Pancaldi, G, Facchini, G, Cantarini, ME, Miniaci, A, et al. Evans syndrome in childhood: long term follow-up and the evolution in primary immunodeficiency or rheumatological disease. Front Pediatr 2019, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Al-Herz, W, Zainal, M, Nanda, A. A prospective survey of skin manifestations in children with inborn errors of immunity from a national registry over 17 years. Front Immunol 2021, 12, 751469. [Google Scholar] [CrossRef] [PubMed]
- Sillevis Smitt, JH, Kuijpers, TW. Cutaneous manifestations of primary immunodeficiency. Curr Opin Pediatr 2013, 25, 492–497. [Google Scholar] [CrossRef]
- Boudart, J, Fink, W, Theunis, A, Dangoisse, C, Ferster, A, Andre, J. Granulomatous skin disease in an immunocompromised child. Ann Dermatol Venereol 2011, 138, 38–41. [Google Scholar] [CrossRef]
- Savino, W, Mendes-da-Cruz, DA, Lepletier, A, Dardenne, M. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol 2016, 12, 77–89. [Google Scholar] [CrossRef]
- Jara, L, Munoz-Durango, N, Llanos, C, Fardella, C, Gonzalez, PA, Bueno, SM, et al. Modulating the function of the immune system by thyroid hormones and thyrothropin. Immunol Letters 2017, 84, 76–83. [Google Scholar] [CrossRef]
- Jaeger, M, Sloot, YJE, Horst, RT, Chu, X, Koenen, HJPM, Koeken, VACM, et al. Thyrotropin and thyroxin support immune homeostasis in humans. Immunology 2021, 163, 155–168. [Google Scholar] [CrossRef]
- Takasawa, K, Kanegane, H, Kashimada, K, Morio, T. Endocrinopathies in Inborn Errors of Immunity. Front Immunol 2021, 12, 786241. [Google Scholar] [CrossRef]
- Coopmans,, EC, Chunharojrith P, Neggers, SJCMM, van der Ent, MW, Swagemakers, SMA, Hollink, IH, et al. Endocrine disorders are prominent clinical features in patients with primary antibody deficiencies. Front Immunol 2019, 10, 2079. [Google Scholar] [CrossRef]
- Fathi, N, Nirouei, M, Salimian Rizi, Z, Fekrvand, S, Abolhassani, H, Salami, F, et al. Clinical, immunological, and genetic features in patients with NFKB1 and NFKB2 mutations: a systematic review. J Clin Immunol 2024, 44, 160. [Google Scholar] [CrossRef] [PubMed]
- Shi, C, Wang, F, Tong, A, Zhang, X, Song, H, Liu, Z, et al. NFKB2 mutation in common variable immunodeficiency and isolated adrenocorticotropic hormone deficiency. Medicine 2016, 95, e5081. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G, Genzano, CB, Fierabracci, A, Fousteri, G. Type 1 diabetes and inborn errors of immunity: complete starngers or 2 sides of the same coin? J Allergy Clin Immunol 2023, 151, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, B, Pacillo, L, Milardi, G, Jofra, T, Di Cesare, S, Gerosa, J. Natural history of type 1 diabetes on an immunodysregulatory background with genetic alteration in B-cell activating factor receptor: a case report. Front Immunol 2022, 13, 952715. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A, Bernat-Sitarz, K, Schwartzmann, E, Piechota, M, Badura-Stronka, M. Clinical and immunological assessment of APDS2 with features of the SHORT syndrome related to a novel mutation in PIK3R1 and reduced penetrance. Allergol Immunopathol 2022, 50, 1–9. [Google Scholar] [CrossRef]
- Ramirez, L, Tamayo, R, Hanadys, A. APDS and SHORT syndrome in a teenager with PIK3R1 pathogenic variant. J Clin Immunol 2020, 40, 1020–1025. [Google Scholar] [CrossRef]
- Goudouris, ES, Silva Segundo, GR, Poli, C. Repercussions of inborn errors of immunity on growth. J Pediatr 2019, 95, 49–58. [Google Scholar] [CrossRef]
- Ramzi, N, Yazdani, S, Talakoob, H, Jamee, M, Karim, H, Azizi, G. Acute pericarditis: a peculiar manifestation of common variable immunodeficiency. Allergol Immunopathol 2021, 49, 115–119. [Google Scholar] [CrossRef]
- Laufs H, Nigrovic PA, Schneider LC, Oettgen H, Del NP, Moskowitz IPG, et al. Giant cell myocarditis in a 12-year-old girl with common variable immunodeficiency. Mayo Clin Proc 2002,77,92-96. [CrossRef]
- Franzon, TA, Kovalszki, A, Rabah, R, Nicklas, JM. Case report of heart transplantation for giant cell myocarditis in a patient with common variable immunodeficiency. Eur Heart J Case Rep 2021, 5, ytab447. [Google Scholar] [CrossRef]
- Sener, S, Basaran, O, Batu, ED, Atalay, E, Esenboga, S, Cagdas, D, et al. Childhood-onset Takayasu arteritis and immunodeficiency: case-based review. Clin Rheumatol 2022, 41, 2883–2892. [Google Scholar] [CrossRef]
- Skeik, N, Rumery, KK, Udayakumar, P, Crandall, BM, Warrington, KJ, Sullivan, TM. Concurrent Takayasu arteritis with common variable immunodeficiency and moyamoya disease. Ann Vasc Surg 2013, 27, 240. [Google Scholar] [CrossRef]
- Bahal, M, Kumar, G, Mane, S, Chavan, S, Gupta, A. Early-onset Takayasu arteritis in childhood: a case report. Cureus 2024, 16, e53885. [Google Scholar] [CrossRef]
- Napiórkowska-Baran, K, Schmidt, O, Szymczak, B, Lubański, J, Doligalska, A, Bartuzi, Z. Molecular linkage between immune system disorders and atherosclerosis. Curr Issues Mol Biol 2023, 45, 8780–8815. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, SF, Macpherson, ME, Skarpengland, T, Berge, RK, Fevang, B, Halvorsen, B, et al. Disturbed lipid profile in common variable immunodeficiency – a pathogenic loop of inflammation and metabolic disturbances. Front Immunol 2023, 14, 1199727. [Google Scholar] [CrossRef]
- Skarpengland, T, Mackpherson, ME, Hov, JR, Kong, XY, Bohov, P, Halvorsen, B, et al. Altered plasma fatty acids associate with gut microbial composition in common variable immunodeficiency. J Clin Immunol 2022, 42, 146–157. [Google Scholar] [CrossRef]
- Yang, L, Chen, S, Zhao, Q, Sun, Y, Nie, H. The critical role of Bach2 in shaping the balance between CD4+ T cell subsets in immune-mediated diseases. Mediators Inflamm 2019, 2019, 2609737. [Google Scholar] [CrossRef]
- Lougaris, V, Baronio, M, Gazzurelli, L, Lorenzini, T, Fuoti M, Moratto, D, et al. A de novo monoallelic CTLA-4 deletion causing pediatric onset CVID with recurrent autoimmune cytopenias and severe enteropathy. Clin Immunol 2018, 197, 186–188. [Google Scholar] [CrossRef]
- Schwab, C, Gabrysch, A, Olbrich, P, Patino, V, Warnatz, K, Wolff, D, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4 insufficient subjects. J Allergy Clin Immunol 2018, 142, 1932–1946. [Google Scholar] [CrossRef]
- Liu, A, Liu, Q, Leng, S, Zhang, X, Feng, Q, Peng, J, et al. Identification of novel NFKB1 and ICOS frameshift variants in patients with CVID. Clin Exp Immunol 2023, 211, 68–77. [Google Scholar] [CrossRef]
- Keller, MD, Pandey, R, Li D, Glessner, J, Tian, L, Henrickson, SE, et al. Mutation in IRF2BP2 is responsible for a familial form of common variable immunodeficiency disorder. J Allergy Clin Immunol 2016, 138, 544–550. [Google Scholar] [CrossRef]
- Habibi, S, Zaki-Dizaji, M, Rafiemanesh, H, Lo, B, Jamee, M, Gamez-Diaz, L, et al. Clinical, immunologic, and molecular spectrum of patients with LPS-Responsive Beige-Like Anchor Protein deficiency: a systematic review. J Allergy Clin Immunol Pract 2019, 7, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Tugcu, GD, Polat, SE, Metin, A, Orhan, D, Cinel, G. Interstitial lung disease in an adolescent girl with Lipoplysaccharide-Responsive Beige-Like Anchor deficiency. Pediatr Allergy Immunol Pulmonol 2022, 35, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Dia,z L, August, D, Stepensky, P, Revel-Vilk, S, Seidel, MG, Noriko, M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol 2016, 137, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Tuijnenburg, P, Lango Allen, H, Burns, SO, Greene, D, Jansen, MH, Staples, E, et al. Loss-of-function nuclear factor κB subunit 1 (NFKB1) variants are the most common monogenic cause of common variable immunodeficiency in Europeans. J Allergy Clin Immunol 2018, 142, 1285–1296. [Google Scholar] [CrossRef]
- Klemann, C, Camacho-Ordonez, N, Yang, L, Eskandarian, Z, Rojas-Restrepo, JL, Frede, N, et al. Clinical and immunological phenotype of patients with primary immunodeficiency due to damaging mutations in NFKB2. Front Immunol 2019, 10, 297. [Google Scholar] [CrossRef]
- Trindade, BC, Chen, GY. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol Rev 2020, 297, 139–161. [Google Scholar] [CrossRef]
- Zhou, Q, Chen, D, Yu, J, Zheng, B, Zhou, W, Jia, Z, et al. A novel gain-of-function STAT3 variant in infantile-onset diabetes associated with multiorgan autoimmunity. Mol Genet Genomic Med 2024, 12, e2407. [Google Scholar] [CrossRef]
- Zhang, Y, Li. J, Zhang, Y, Zhang, X, Tao, J. Effect of TACI signaling on humoral immunity and autoimmune disease. J Immunol Res 2015, 247426. [Google Scholar] [CrossRef]
- Kołtan, S, Ziętkiewicz, M, Grześk, E, Becht, R, Berdej-Szczot, E, Cienkusz, M, et al. CoVID-19 in unvaccinated patients with inborn errors of immunity-Polish experience. Front Immunol 2022, 13, 953700. [Google Scholar] [CrossRef]
- Meyts, I, Bucciol, G, Quinti, I, Neven, B, Fischer, A, Seoane, E, et al. Coronavirus disease 19 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol 2021, 147, 520–531. [Google Scholar] [CrossRef]
- Al-Hakim, A, Kacar, M, Savic, S. The scope and impact of viral infections in common variable immunodeficiency (CVID) and CVID-like disorders: a literature review. J Clin Med 2024, 13, 1717. [Google Scholar] [CrossRef]
- Szczawińska-Popłonyk, A, Ciesielska, W, Konarczak, M, Opanowski,, J, Orska A, Wróblewska, J, et al. Immunogenetic landscape in pediatric common variable immunodeficiency. Int J Mol Sci 2024, 25, 9999. [Google Scholar] [CrossRef]
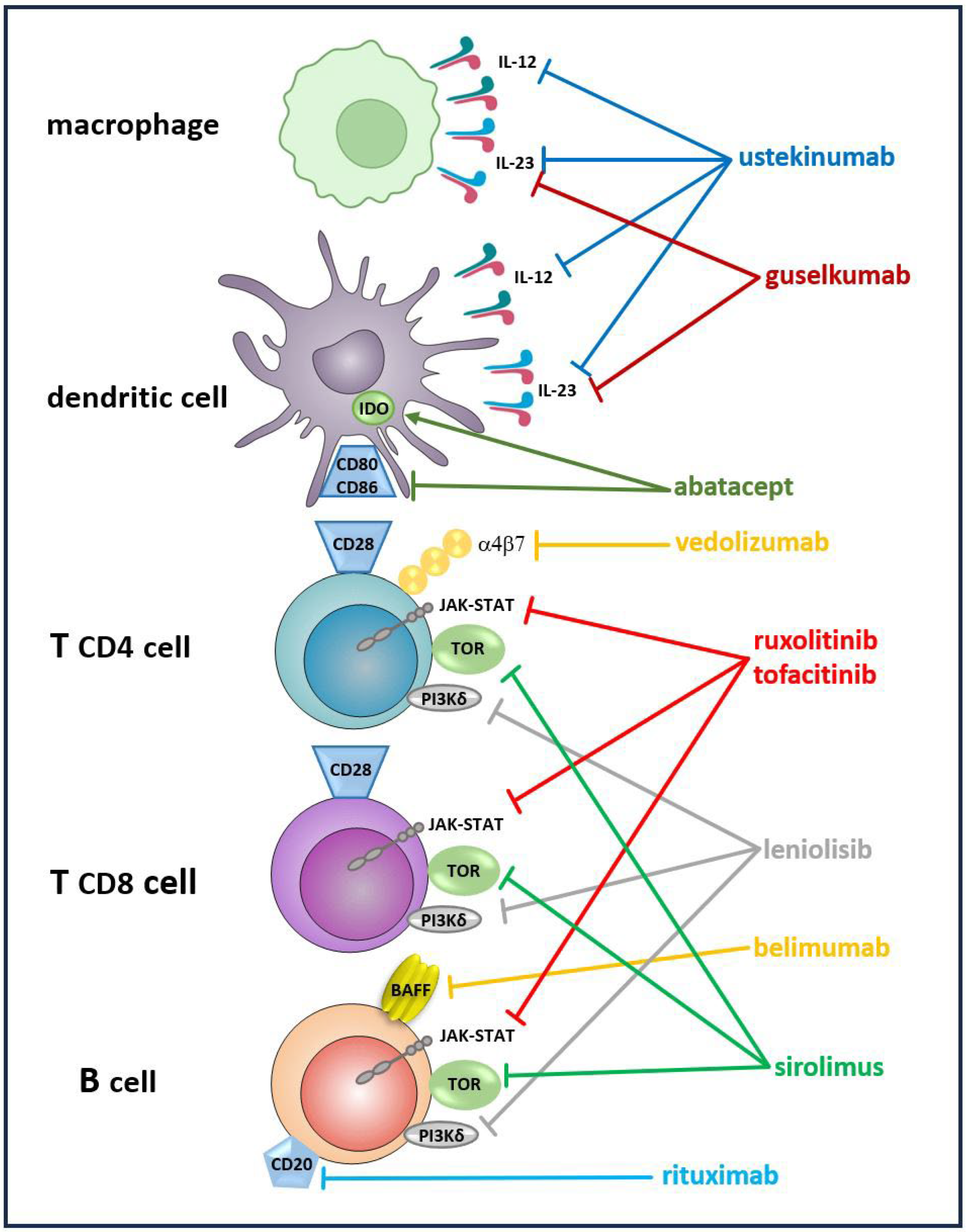
- Fevang, B. Treatment of inflammatory complications in common variable immunodeficiency (CVID): current concepts and future perspectives. Expert Rev Clin Immunol 2023, 19, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Szaflarska, A, Lenart, M, Rutkowska-Zapała, M, Siedlar, M. Clinical and experimental treatment of primary humoral immunodeficiencies. Clin Exp Immunol 2024, 216, 120–131. [Google Scholar] [CrossRef]
- De Gottardi, A, Sempoux, C, Berzigotti, A. Porto-sinusoidal vascular disorder. J Hepatol 2022, 77, 1124–1135. [Google Scholar] [CrossRef]
- Tessarin, G, Baronio, M, Lougaris, V. Monogenic forms of common variable immunodeficiency and implications on target therapeutic approaches. Curr Opin Allergy Clin Immunol 2023, 23, 461–466. [Google Scholar] [CrossRef]
- Van Stigt, A, Gualtiero, G, Cinetto, F, Dalm, VASH, Ijspert, H, Muscianisi, F. The biological basis for current treatment strategies for granulomatous disease in common variable immunodeficiency. Curr Opin Allergy Clin Immunol 2024, 24, 479–487. [Google Scholar] [CrossRef]
- Chanchal, DK, Chaudhary, JS, Kumar, P, Agnihotri, N, Porwal, P. CRISPR-based therapies: revolutionizing drug development and precision medicine. Curr Gene Ther 2024, 24, 193–207. [Google Scholar] [CrossRef]
- Verma, A, Sharma, T, Awasthi, A. CRISPR and gene editing: a game-changer in drug development. Curr Pharm Des 2024, 30, 1133–1135. [Google Scholar] [CrossRef]
| Immune dysregulation | |||
|---|---|---|---|
| Genetic variants | Immunophenotype | Organ-specific manifestation | Ref. No |
|
BACH 2 BTB Domain and CNC Homolog 2 |
Impaired B cell differentiation Defective immunoglobulin class switch recombination and somatic hypermutation Excessive Th2 cell differentiation Impaired T reg cell development |
Autoimmunity: vitiligo, endocrinopathies, IDDM, IBD | [120] |
|
CTLA-4 Cytotoxic T Lymphocyte Antigen 4 |
Low naïve T cells and recent thymic emigrants Low Treg cells |
Autoimmunity: AIHA, ITP, enteropathy, IBD, endocrinopathies, arthritis, psoriasis Inflammatory granulomatosis: GLILDAsthma Lymphoproliferation: lymphadenopathy, splenomegaly |
[121,122] |
|
ICOS Inducible T-Cell Costimulator |
Low B cell counts, in particular switched-memory B cell and plasmablast deficiency Increased numbers of immature CD21low B cells |
Autoimmunity: enteropathy, arthritis, IDDM | [123] |
|
IRF2BP2 Interferon Regulatory Factor 2-Binding Protein 2 |
Decreased B cell maturation, deficiency of switched memory B cells | Autoimmunity: IDDM, colitis, psoriasis | [124] |
|
LRBA Lipopolysaccharide (LPS)-Responsive Beige-like Anchor Protein |
Dysregulation of T cell activation and expansion Low B cell counts, in particular switched-memory B cell and plasmablast deficiency Increased numbers of immature CD21low B cells |
Autoimmunity: JIA, enteropathy, celiac disease, vitiligo, alopecia, AIHA, ITP, endocrinopathies, autoimmune hepatitis, IDDM Inflammatory: interstitial lung disease, bronchiectasis Inflammatory granulomatosis, GLILDAsthma Lymphoproliferation: lymphadenopathy, splenomegaly |
[125,126,127] |
|
NFKB1 Nuclear Factor Kappa B1 |
B cell lymphopenia, reduced non-switched and switched memory B cells CD21low B cell expansion |
Autoimmunity: AIHA, ITP, vitiligo, alopecia, Hashimoto thyroiditis Lymphoproliferation: lymphadenopathy, splenomegaly Inflammatory granulomatosis: GLILD, granulomatous liver disease |
[128] |
|
NFKB2 Nuclear Factor Kappa B2 |
Abnormalities in the T cell compartment: reduced numbers of follicular T helper cells, low Th17 cells, and Treg cells Impaired B cell differentiation: decreased switched memory and non-switched memory B cells, increased naïve B cells |
Autoimmunity: arthritis, alopecia, vitiligo, ITP, growth hormone deficiency, ACTH deficiency, enteropathy Inflammatory: bronchiectasis Lymphoproliferation: splenomegaly, lymphadenopathy |
[129] |
|
NOD2 Nucleotide-binding oligomerization domain containing 2 |
Impaired response to danger signals from pathogen-associated molecular patterns (PAMP) Deregulated inflammatory IL-6 homeostasis Disturbances in the actin cytoskeleton Disturbed B and T cell activation |
Autoimmunity: IBD, IDDM, arthritis, multiple sclerosis, autoimmune encephalomyelitis Inflammatory granulomatosis Asthma |
[130] |
|
STAT3 Signal Transducer and Activator of Transcription 3 |
Disturbed regulation of TH17/Treg cell equilibrium B cell lymphopenia |
Lymphoproliferation: lymphadenopathy, splenomegaly Autoimmunity: IDDM, growth hormone deficiency, Hashimoto thyroiditis, ITP, AIHA, enteropathy, arthritis Inflammatory: GLILD |
[131] |
|
TACI Transmembrane Activator and CAML Interactor |
Impaired central and peripheral B cell tolerance BAFF driven B cell activation Treg cell dysfunction |
Lymphoproliferation: splenomegaly, lymphadenopathy, tonsillar hypertrophy Autoimmunity: IDDM, JIA, ITP, AIHA, celiac disease, IBD Inflammatory granulomatosis |
[132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




