1. Introduction
Strabismus is characterized by a misalignment of the visual axes of the eyes, wherein the deviated eye loses coordination with the fixing eye, leading to impaired or absent binocular vision [
1].
To correct ocular deviation, the first step involves assessing the potential for realignment using optical correction. If this approach proves ineffective or inadequate, surgical intervention may be necessary [
1]. Strabismus surgery plays a vital role in enhancing visual quality in cases of early-onset strabismus, restoring normal binocular vision in late-onset strabismus with well-preserved preexisting binocular vision, eliminating abnormal head posture associated with strabismus, and addressing aesthetic concerns. Aligned eyes not only improve physical appearance but also facilitate social interactions and enhance overall quality of life [
2].
Surgical techniques for strabismus involve realigning the eyes by modifying the extraocular muscles, either by weakening an overactive muscle (muscle recession) or strengthening an underactive muscle (muscle plication or resection).
Surgery for strabismus can be performed under topical/local anesthesia or general anesthesia, with the choice of anesthesia determined by several factors, including the patient’s age, cognitive function, and the expected duration of the procedure [
3].
Ophthalmic surgeries are generally perceived to cause minimal or no postoperative pain, as they involve less extensive tissue trauma compared to other surgical procedures [
4]. However, limited attention has been given to the factors contributing to postoperative pain and discomfort in oculoplastic surgery performed under general anesthesia. These factors are critical not only for analyzing surgical outcomes but also for improving the subjective patient experience. Understanding the determinants of postoperative pain and discomfort may enable modifications that enhance patient care and healthcare delivery systems [
5].
This observational study seeks to identify which types of strabismus surgery are associated with a higher risk of postoperative complications, considering the influence of the anesthesia type and the patient’s age.
2. Material and Methods
Patients undergoing strabismus surgery were recruited based on the following inclusion criteria: no age restrictions, inclusion of both sexes and all ethnicities, ability to provide informed consent for adult patients, or parental/legal guardian assent for patients under 18 years of age.
All patients underwent a standard preoperative evaluation for strabismus surgery. This included performing all necessary diagnostic tests to determine the most appropriate surgical technique for each case. Subsequently, a consultation was held between the surgeon and the patient—or, for patients under 18 years old, their parents or legal guardians—to discuss the surgical procedure and any potential complications. Written informed consent was obtained before proceeding further.
Anesthetic evaluation followed the consent process to confirm the patient’s suitability for surgery under general anesthesia. This assessment involved an anesthetic consultation, blood tests, and an electrocardiogram (ECG).
The study received approval from the Human Subjects Ethics Committee of the University of Milan and was performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and its later amendments.
2.1. Protocols of Anesthesia and Surgical Techniques
Standard monitors—including ECG, heart rate, blood pressure, and oxygen saturation—were applied to all patients in the operating room.
During the intraoperative period, the administration of premedication was determined based on the patient’s age and anxiety levels. Children under 12 years of age typically receive oral midazolam to minimize anxiety, alleviate pain, and reduce resistance during the anesthesia preparation phase [
6].
For most patients undergoing straightforward strabismus procedures, such as rectus muscle recessions, anesthesia was induced intravenously using fentanyl and propofol, with maintenance provided by sevoflurane, an inhalation agent. Dosages were adjusted according to the patient’s weight to ensure optimal anesthesia management [
7].
For more complex procedures, such as plications or surgeries involving the oblique muscles, stronger analgesics were administered because of the increased complexity and surgery duration. Pediatric patients received ketoprofen, while adult patients were treated with ketorolac. These analgesics were used to mitigate the risk of postoperative complications associated with longer and more invasive procedures [
8].
In some cases, such as patients with a mild cold who were otherwise fit for surgery, dexamethasone, an anti-inflammatory agent, was used. Postoperative pain management includes the administration of the antiemetic ondansetron and the analgesic paracetamol to ensure patient comfort and reduce postoperative symptoms [
9].
Exceptions to the standard anesthesia protocol were made for patients with neuromuscular disorders. In these cases, anesthesia induction and maintenance were performed using remifentanil and propofol, omitting the use of volatile inhalation agents. This adjustment was necessary because agents like sevoflurane can trigger malignant hyperthermia in patients with neuromuscular conditions. Malignant hyperthermia is a life-threatening pharmacogenetic disorder of the skeletal muscles, characterized by an exaggerated hypermetabolic response to volatile anesthetic agents and depolarizing muscle relaxants, which causes circulatory shock [
10].
Several surgical techniques, ranging from simple to complex procedures, were used, with key differences as variables in the analysis. These included longer surgical durations, the potential need for additional anesthesia, and greater postoperative discomfort for patients. The most commonly performed procedure was rectus muscle recession, both medial and lateral, which involves weakening an overactive muscle by repositioning it to a new insertion point. Conversely, strengthening an underactive muscle was achieved through plication or resection techniques [
11].
More advanced procedures were also conducted, including the anterior transposition of the inferior oblique (ATIO) for cases of fourth cranial nerve paralysis [
12], and the modified Nishida technique, which involves transposing the superior and inferior rectus muscles toward the lateral rectus to address sixth cranial nerve paralysis [
13]. For patients diagnosed with Heavy Eye Syndrome (HES), the Yokoyama technique was used, wherein the superior and lateral rectus muscles are connected to correct the condition [
14]. Also, some surgeries involved re-intervention on previously operated muscles or using mixed and/or multiple techniques during the same procedure.
2.2. Outcome Measures
Patients were reassessed approximately 3 to 4 hours after surgery to evaluate postoperative symptoms, including headache, nausea, vomiting, burning sensation, and the feeling of a foreign body.
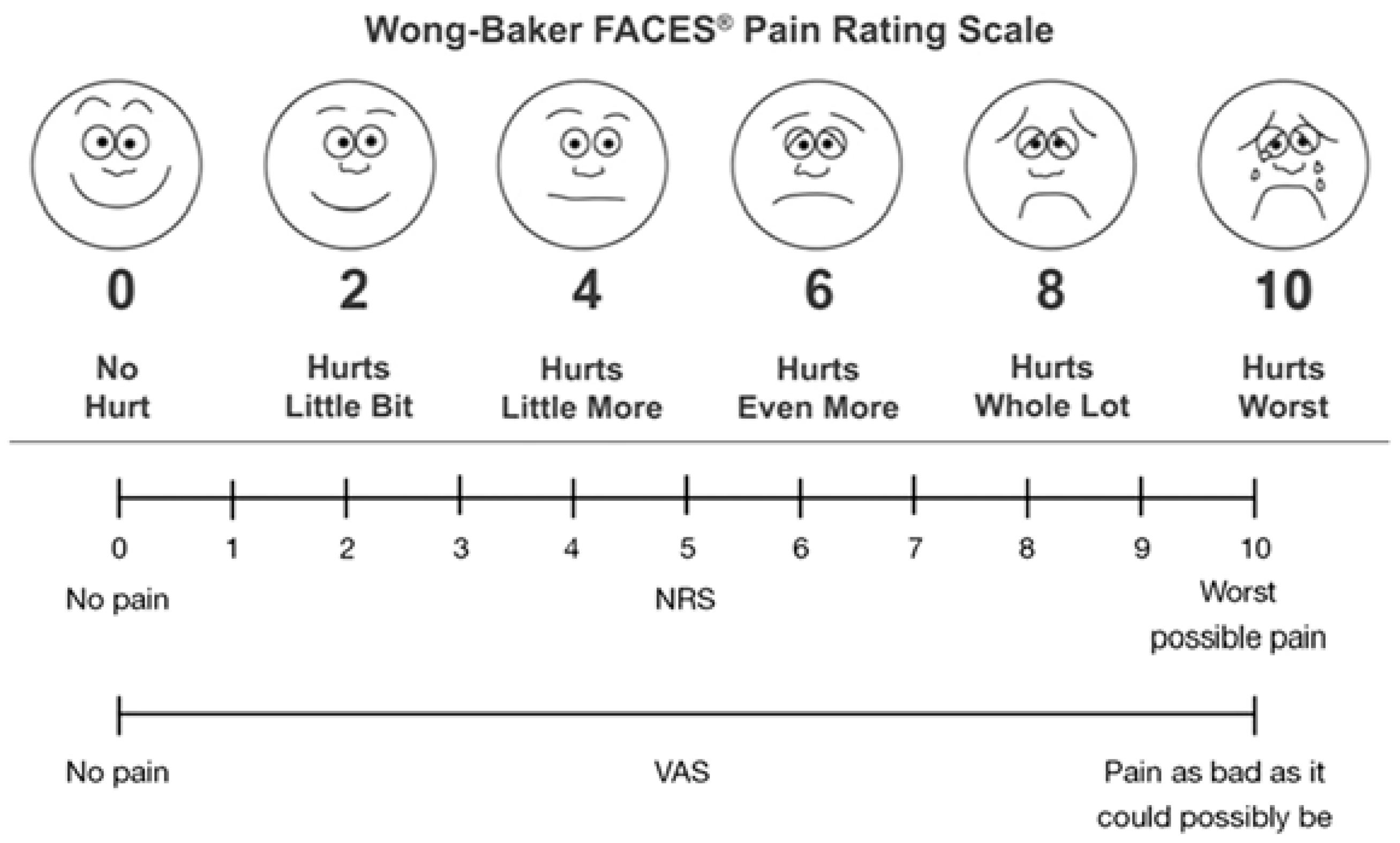
Age-appropriate scales were used to objectively measure postoperative symptoms and pain. For children under 10 years old, the Wong-Baker Faces Pain Scale (WBFPS) was used [
15]. This simple, intuitive, and effective tool allows children to visually indicate their discomfort by selecting from six faces representing different levels of pain. The WBFPS was used to assess symptoms such as burning sensations, the sensation of a foreign body, eye pain, headache, nausea, vomiting, and episodes of inconsolable crying. This scale provides a more objective and child-friendly method for evaluating the type and intensity of pain experienced postoperatively [
16].
For patients aged 10 years and older, either the Numeric Rating Scale (NRS) or the Visual Analogue Scale (VAS) was employed [
17]. These scales required patients to rate their level of pain or discomfort on a numerical scale ranging from 0 (no pain) to 10 (worst pain imaginable), facilitating a standardized and reliable assessment of postoperative symptoms.
Figure 1 illustrates the WBFPS, the NRS, and the VAS as presented to the patients.
A single operator administered one of the three pain assessment scales to every 100 patients, providing verbal explanations to ensure an understanding of how each number or face corresponded to specific symptoms. The scoring system was predetermined during the study design phase, with higher scores indicating more severe symptoms.
For the WBFPS
Score 0 = no symptoms
Score 2 = ocular discomfort, such as burning or a foreign body sensation
Score 4 = eye pain
Score 6 = eye pain and headache
Score 8 = severe pain with possible nausea or vomiting (as reported by the parent)
Score 10 = episodes of inconsolable crying
While for the VAS and the NRS
Score 0–1 = no symptoms
Score 2–3 = ocular burning or foreign body sensation
Score 4–5 = eye pain
Score 6–7 = eye pain and headache
Score 8–9 = nausea
Score 10 = vomiting
2.3. Statistics
Descriptive data were summarized as the mean and standard deviation for continuous variables with normal distribution and as the median and interquartile range (25th–75th percentiles) for non-normally distributed continuous variables. Categorical variables were reported as absolute frequencies and percentages. Factors associated with different outcomes, including symptoms and pain, were analyzed using logistic regression. The analysis was conducted in two stages: univariate models were applied initially, followed by multivariate models, to account for potential confounders.
3. Results
Between January and June 2024, 100 patients (45.0% female) were recruited from San Giuseppe Hospital in Milan, Italy. The mean age of the sample was 26.1 years (±20.81).
Table 1 summarizes the baseline characteristics, including demographic and surgical details.
Most patients (59.0%) underwent rectus recession procedures on either the right medial rectus muscle (RRMM) or the right lateral rectus muscle (RRLL). Most surgeries (77.0%) were conducted under standard anesthesia protocols. Also, for 66.0% of the patients, the surgery duration was 20 minutes or less.
Table 2 outlines the postoperative outcomes, including symptoms reported during the follow-up visit. 37 patients (37.0%) experienced at least one symptom, with headache being the most common (25.0%), followed by ocular pain (7.0%). Most patients reported no pain (median: 0; range: 0–6).
Table 3 presents an analysis of factors influencing the presence or absence of at least one postoperative symptom.
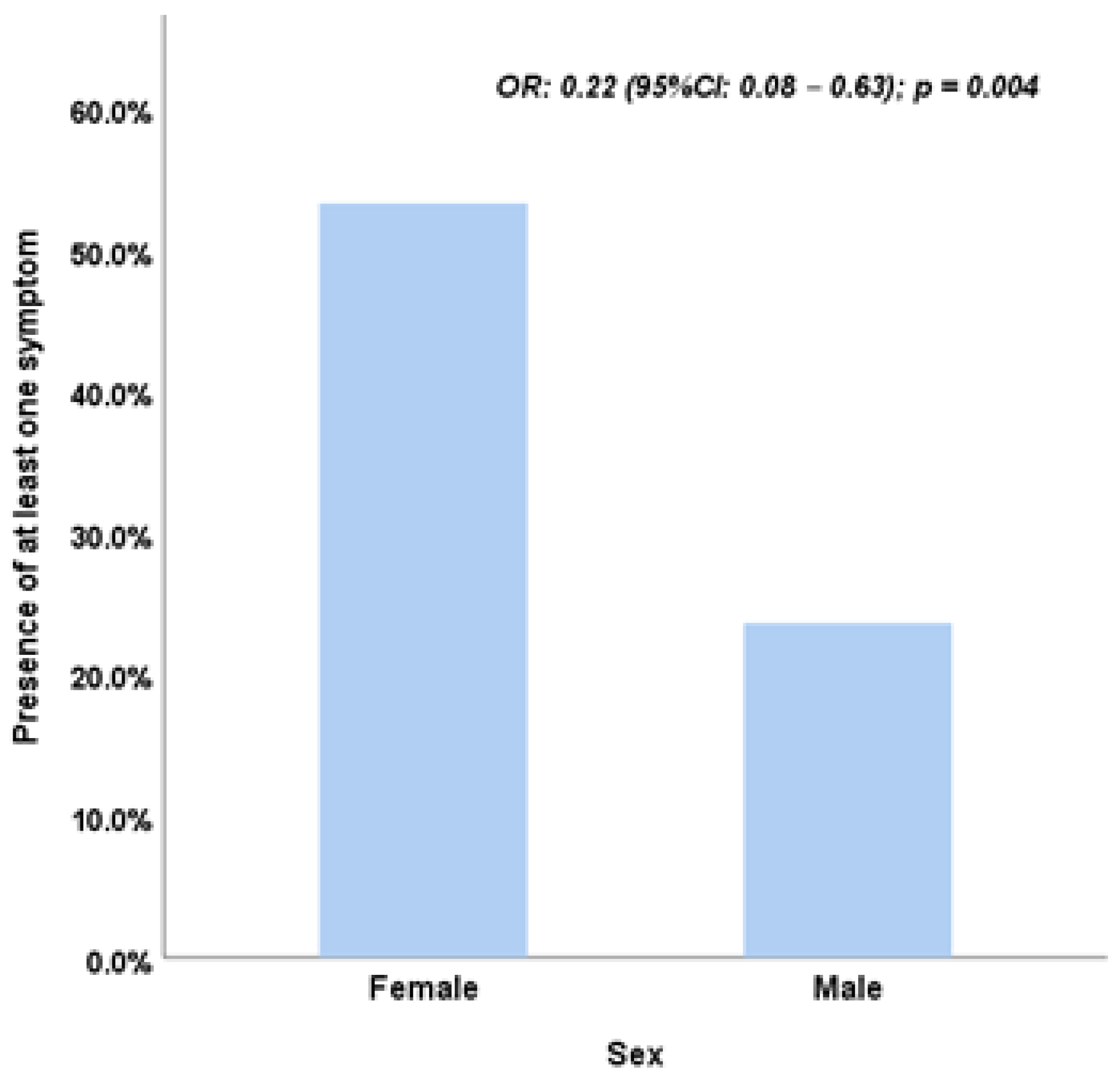
Univariate analysis identified several factors significantly associated with at least one postoperative symptom. Female sex was linked to a higher likelihood of symptoms (OR = 3.70, p = 0.003). Other significant factors included mixed procedures (OR = 5.04, p = 0.002), the use of additional anesthesia (OR = 6.10, p = 0.001), and surgery durations exceeding 20 minutes (OR = 2.78, p = 0.019). However, in the multivariate model, only sex remained statistically significant, with males being less likely than females to experience symptoms (OR = 0.22; 95% CI: 0.08–0.63; p = 0.004) (
Figure 2).
The analysis of factors associated with pain (VAS ≥ 6) is summarized in
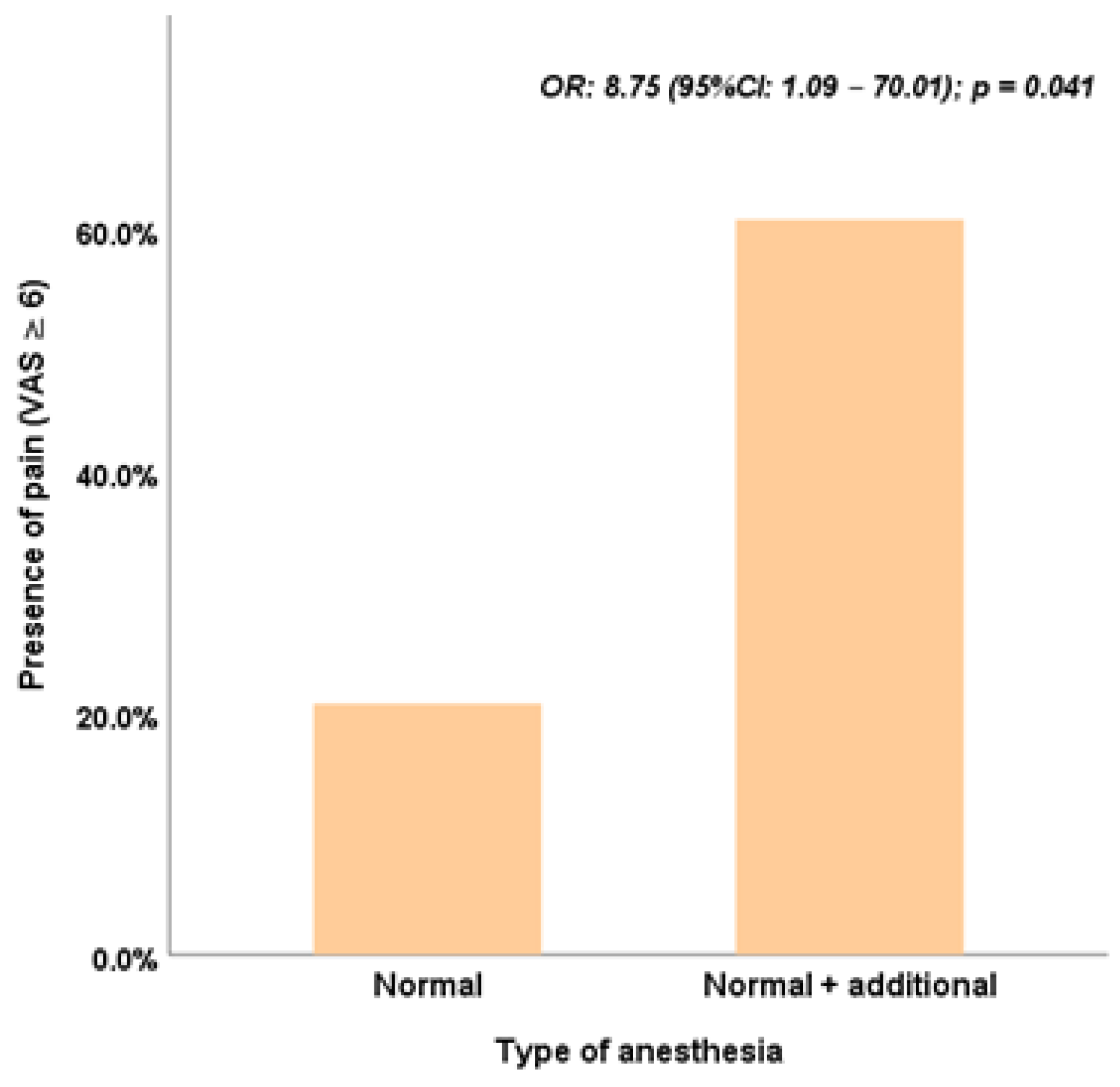
Table 4. The type of anesthesia emerged as the only significant factor in both univariate and multivariate models (univariate model: OR = 5.93, 95% CI: 2.18–16.16, p < 0.001; multivariate model: OR = 8.75, 95% CI: 1.09–70.01, p = 0.041) (
Figure 3). While mixed surgery procedures showed significance in the univariate model (OR = 3.86, 95% CI: 1.39–10.75, p = 0.010), this association was not sustained in the multivariate analysis.
Sex demonstrated a borderline association with pain in the univariate analysis, suggesting that males were less likely to report pain (OR = 0.42, 95% CI: 0.18–1.00, p = 0.05). However, this relationship was not statistically significant in the multivariate model (OR = 0.41, 95% CI: 0.15–1.14, p = 0.09).
4. Discussion
This study evaluates differences in postoperative outcomes following strabismus surgery, considering variables such as anesthesia type, surgical technique, and patient demographics. The multivariate analysis identifies sex as the sole factor significantly associated with postoperative symptoms, with males less likely to report symptoms after adjusting for confounders (OR: 0.22, 95% CI: 0.08–0.63, p = 0.004).
Table 3 shows that the univariate analysis initially highlighted significant variables, including type of surgery, anesthesia, and surgery duration. However, in the multivariate model, many of these lost significance, likely because of confounding relationships. For example, "type of surgery" was significantly associated with the need for additional anesthesia (p < 0.001) and longer surgery durations (p < 0.001), as confirmed by chi-square tests. Also, "sex" was significantly linked to "type of surgery" (p = 0.026), with males more likely to undergo procedures carrying a lower inherent risk of symptoms. These interrelationships diminish the apparent influence of other variables.
In
Table 4, the multivariate analysis underscores the significant role of anesthesia type in influencing postoperative symptoms (OR: 8.75, 95% CI: 1.09–70.01, p = 0.041), reinforcing the independent impact of anesthesia protocols on postoperative pain and discomfort.
Previous research, such as Huang et al. [
18], also identified sex and surgical type as key determinants of postoperative outcomes, particularly for symptoms like vomiting and dizziness. These findings align with our results, though factors such as anxiety, a significant determinant in their study, were not assessed in this research.
Limitations
A notable limitation of this study is the relatively small sample size, which may limit the statistical power and the generalizability of the findings. Larger-scale studies with more robust and controlled designs are warranted to validate these results and provide a more comprehensive understanding of the factors influencing postoperative outcomes following strabismus surgery.
5. Conclusions
The findings of this study indicate that sex is a significant determinant in predicting the likelihood of postoperative symptoms within hours of strabismus surgery. Males appear to have a lower risk of experiencing at least one postoperative symptom than females. To confirm and strengthen these observations, future research with a larger sample size is recommended, enabling more robust analyses and greater statistical reliability. Also, the analysis highlights that when considering pain intensity—a score greater than 6 on the Visual Analogue Scale (VAS)—the type of anesthesia emerges as the most critical factor. This association remains significant even in the multivariate model, underscoring its independent role in influencing postoperative pain outcomes.
References
- Ticho BH. Strabismus. Pediatr Clin North Am. 2003 Feb;50(1):173-88. PMID: 12713111. [CrossRef]
- Gunton KB. Impact of strabismus surgery on health-related quality of life in adults. Curr Opin Ophthalmol. 2014 Sep;25(5):406-10. PMID: 25029092. [CrossRef]
- Modi NC, Jones DH. Strabismus: background and surgical techniques. J Perioper Pract. 2008 Dec;18(12):532-5. PMID: 19192548. [CrossRef]
- Henzler D, Kramer R, Steinhorst UH, Piepenbrock S, Rossaint R, Kuhlen R. Factors independently associated with increased risk of pain development after ophthalmic surgery. Eur J Anaesthesiol. 2004 Feb;21(2):101-6. PMID: 14977340. [CrossRef]
- Fekrat S, Elsing SH, Raja SC, Campochiaro PA, de Juan E Jr, Haller JA. Eye pain after vitreoretinal surgery: a prospective study of 185 patients. Retina. 2001;21(6):627-32. PMID: 11756886. [CrossRef]
- Lethin M, Paluska MR, Petersen TR, Falcon R, Soneru C. Midazolam for Anesthetic Premedication in Children: Considerations and Alternatives. Cureus. 2023 Dec 11;15(12):e50309. PMID: 38089942; PMCID: PMC10711689. [CrossRef]
- Sridharan K, Sivaramakrishnan G. Comparison of Fentanyl, Remifentanil, Sufentanil and Alfentanil in Combination with Propofol for General Anesthesia: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr Clin Pharmacol. 2019;14(2):116-124. PMID: 30868958; PMCID: PMC7011685. [CrossRef]
- Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017 Jul 1;152(7):691-697. PMID: 28564673. [CrossRef]
- Shen YD, Chen CY, Wu CH, Cherng YG, Tam KW. Dexamethasone, ondansetron, and their combination and postoperative nausea and vomiting in children undergoing strabismus surgery: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2014 May;24(5):490-8. Epub 2014 Mar 10. PMID: 24612183. [CrossRef]
- Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015 Aug 4;10:93. PMID: 26238698; PMCID: PMC4524368. [CrossRef]
- Modi NC, Jones DH. Strabismus: background and surgical techniques. J Perioper Pract. 2008 Dec;18(12):532-5. PMID: 19192548. [CrossRef]
- González C, Cinciripini G. Anterior transposition of the inferior oblique in the treatment of unilateral superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1995 Mar-Apr;32(2):107-13. PMID: 7629664. [CrossRef]
- Murthy SR, Pappuru M. Modified Nishida's procedure for monocular elevation deficiency. J AAPOS. 2018 Aug;22(4):327-329.e1. Epub 2018 May 9. PMID: 29752994. [CrossRef]
- Wabbels B, Fricke J, Schittkowski M, Gräf M, Lorenz B, Bau V, Nentwich MM, Atili A, Eckstein A, Sturm V, Beisse C, Sterker I, Neppert B, Mauschitz MM. Yokoyama procedure for esotropia associated with high myopia: real-world data from a large-scale multicentre analysis. Acta Ophthalmol. 2021 Dec;99(8):e1340-e1347. Epub 2021 Mar 2. PMID: 33655633. [CrossRef]
- Tomlinson D, von Baeyer CL, Stinson JN, Sung L. A systematic review of face scales for children's self-reported pain intensity. Pediatrics. 2010 Nov;126(5):e1168-98. Epub 2010 Oct 4. PMID: 20921070. [CrossRef]
- Garra G, Singer AJ, Domingo A, Thode HC Jr. The Wong-Baker pain FACES scale measures pain, not fear. Pediatr Emerg Care. 2013 Jan;29(1):17-20. PMID: 23283256. [CrossRef]
- Rosas S, Paço M, Lemos C, Pinho T. Comparison between the Visual Analog Scale and the Numerical Rating Scale in the perception of esthetics and pain. Int Orthod. 2017 Dec;15(4):543-560. Epub 2017 Nov 13. PMID: 29146313. [CrossRef]
- Huang J, Lin J, Xiong Y, Wang Z, Zhu Y, Ye H, Guo W. Risk Factors Associated with Postoperative Discomfort After Ambulatory Strabismus Surgery Under General Anesthesia. J Pain Res. 2020 May 5;13:947-953. PMID: 32440200; PMCID: PMC7211303. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).