Submitted:
13 January 2025
Posted:
14 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Senescence Overview
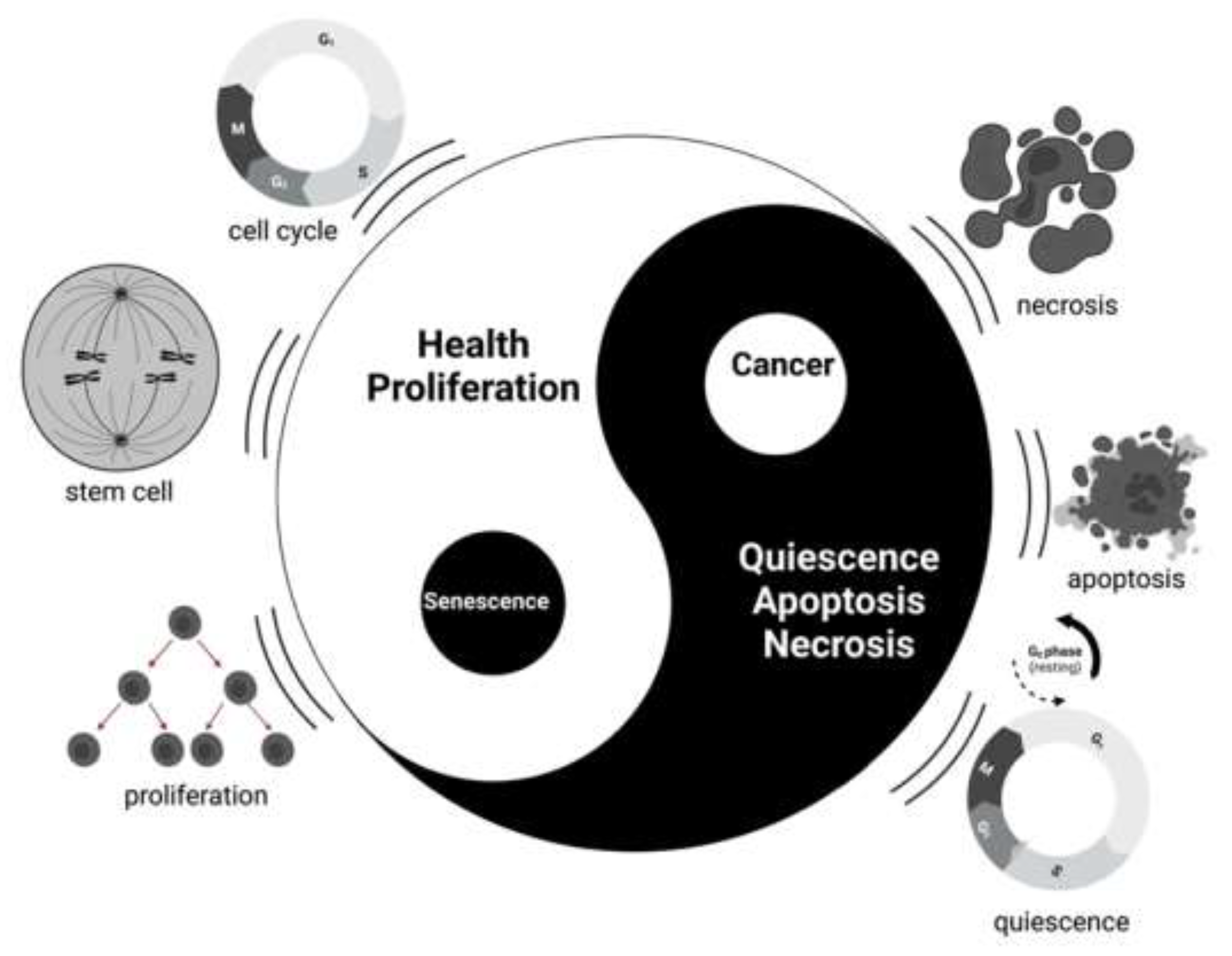
2.1. History of Senescence
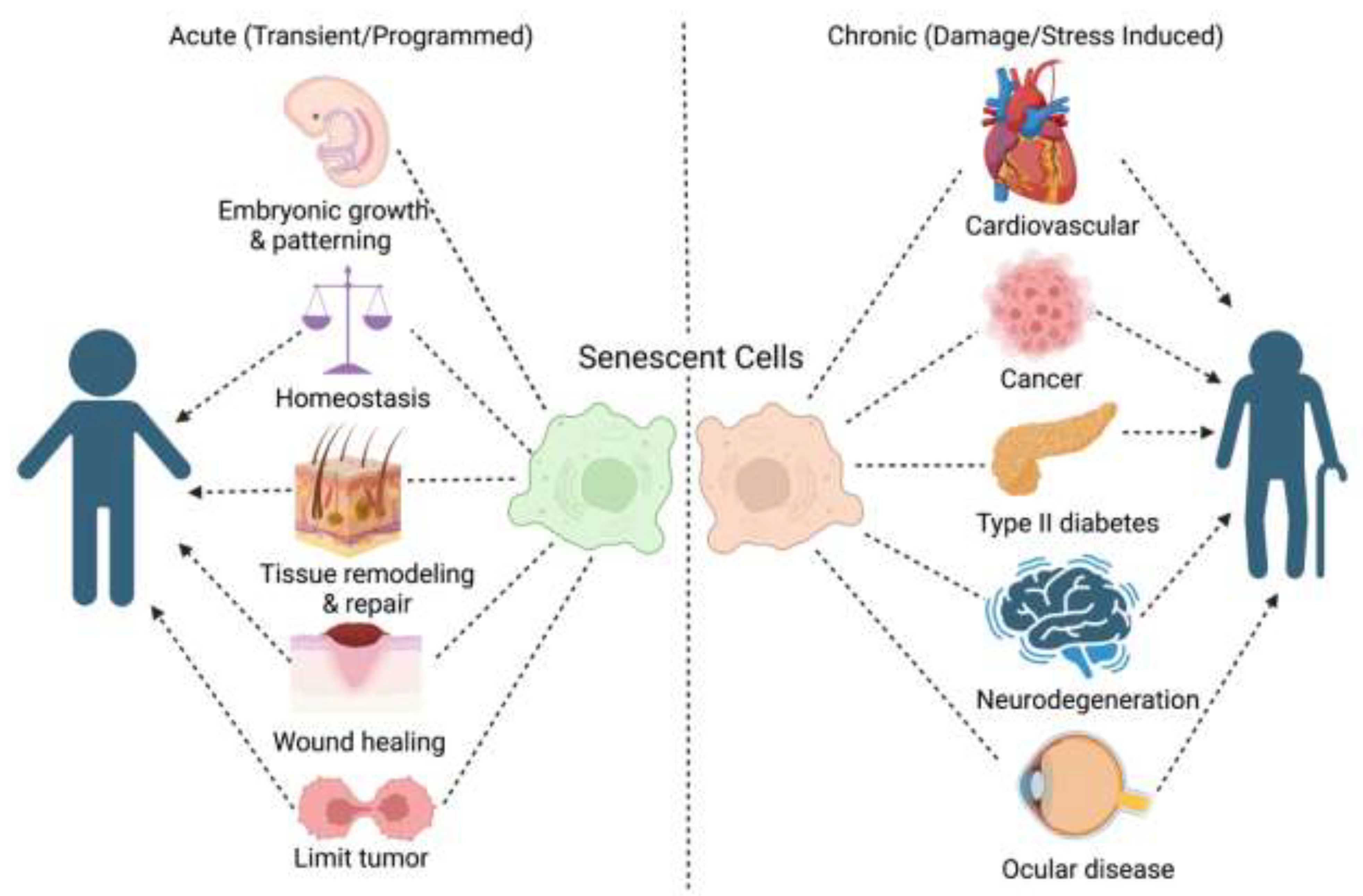
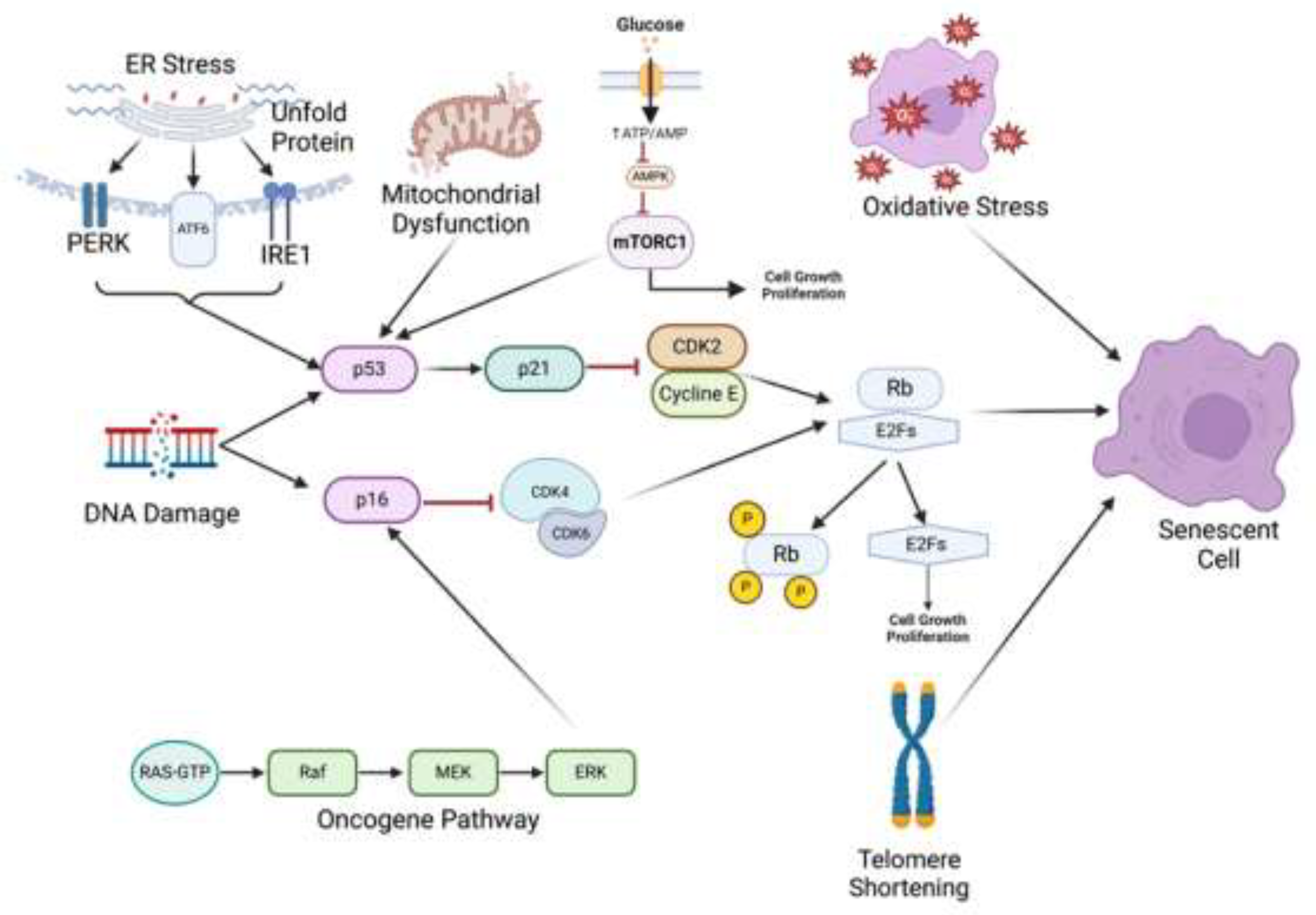
2.2. Causes of Cellular Senescence
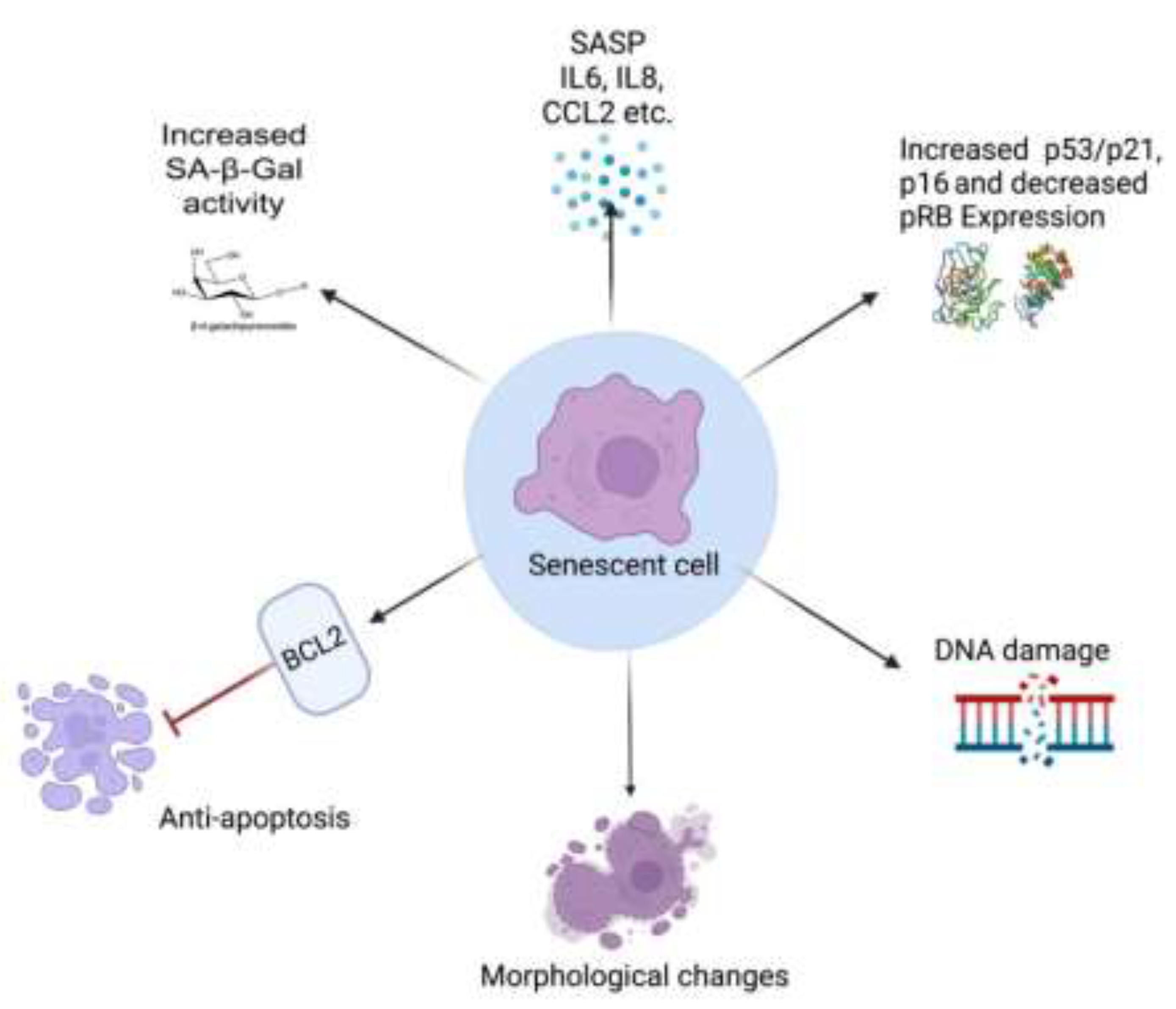
2.3. Markers for Senescence
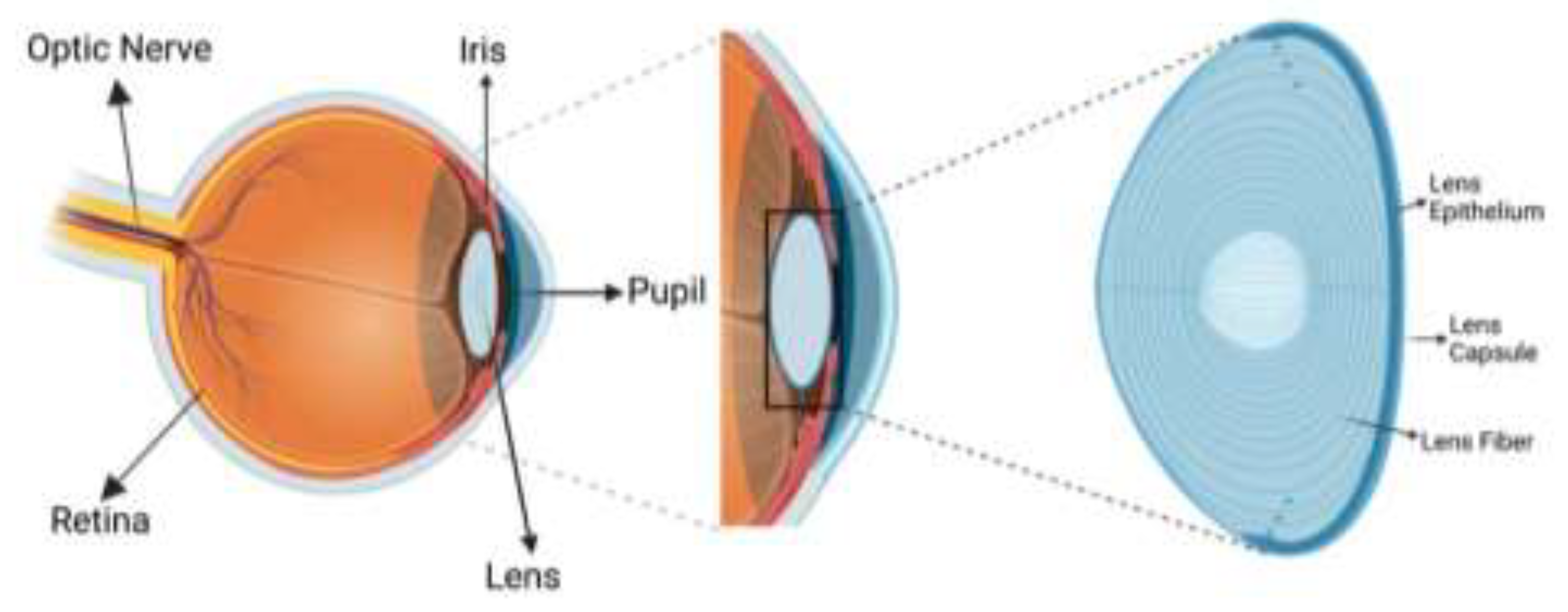
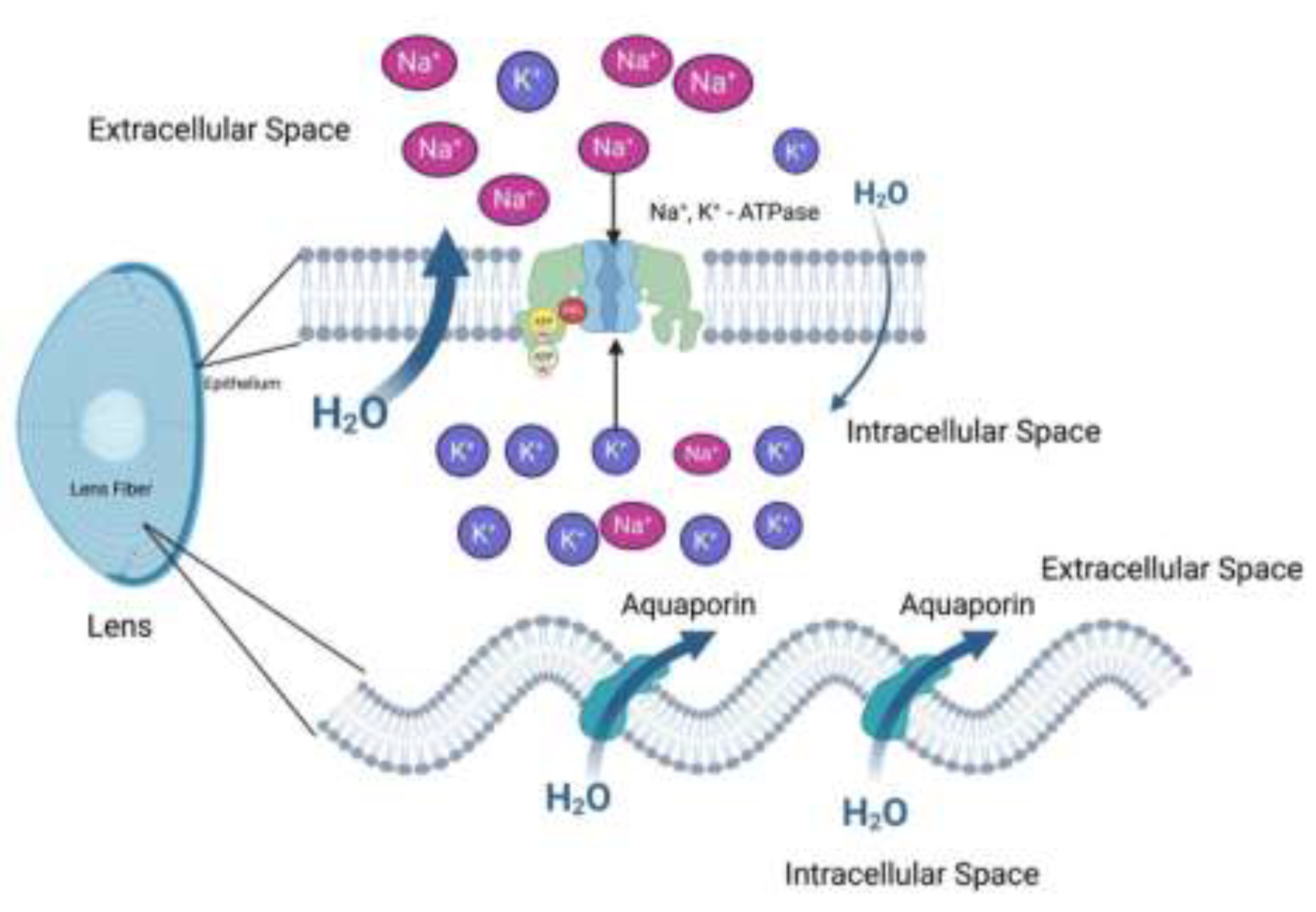
3. Eye Anatomy and Physiology
4. LECs and Senescence
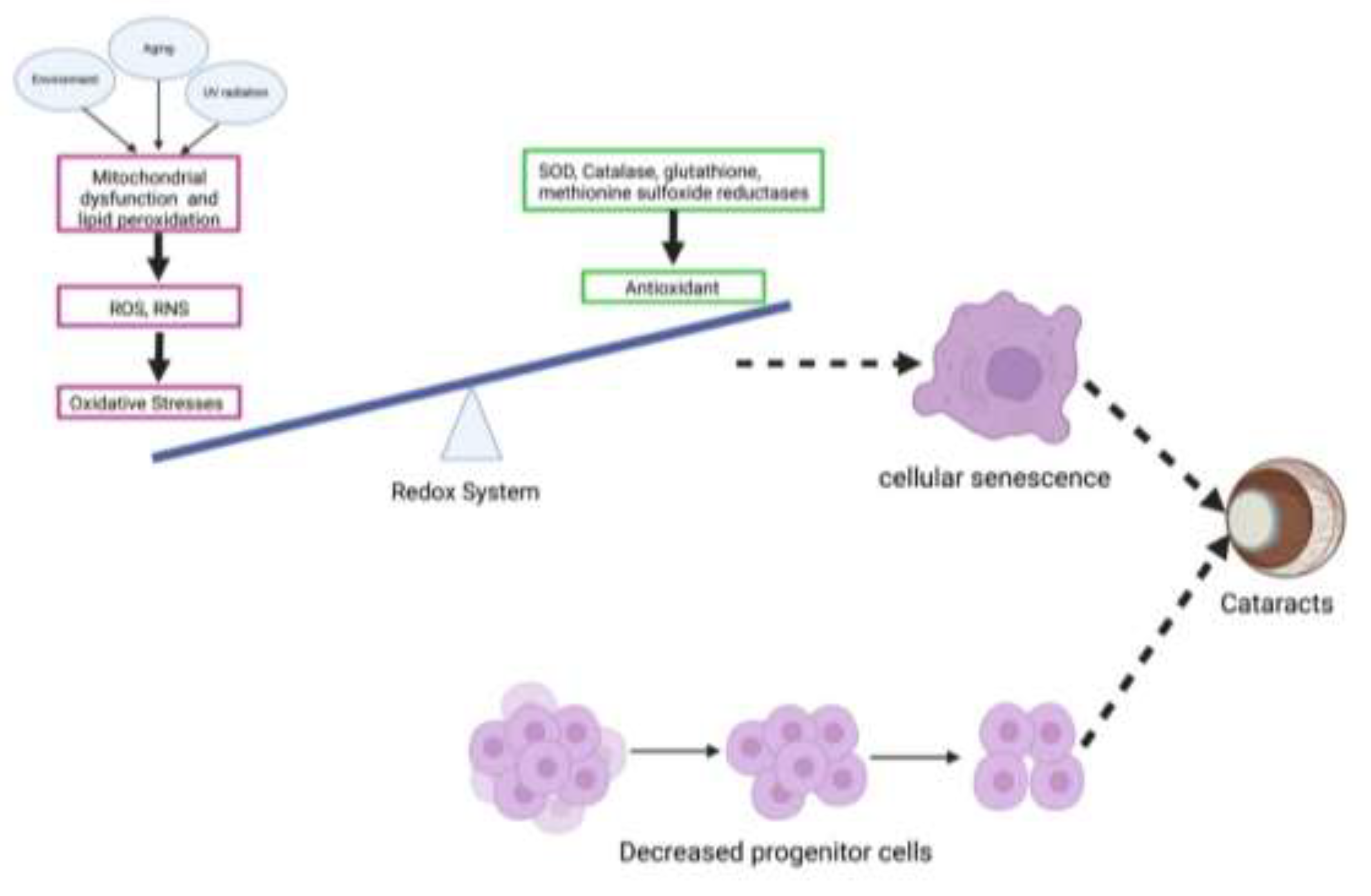
4.1. LECs Senescence and Cataract
4.2. Causes for LECs Senescence
4.3. To Apoptosis or Not to Apoptosis?
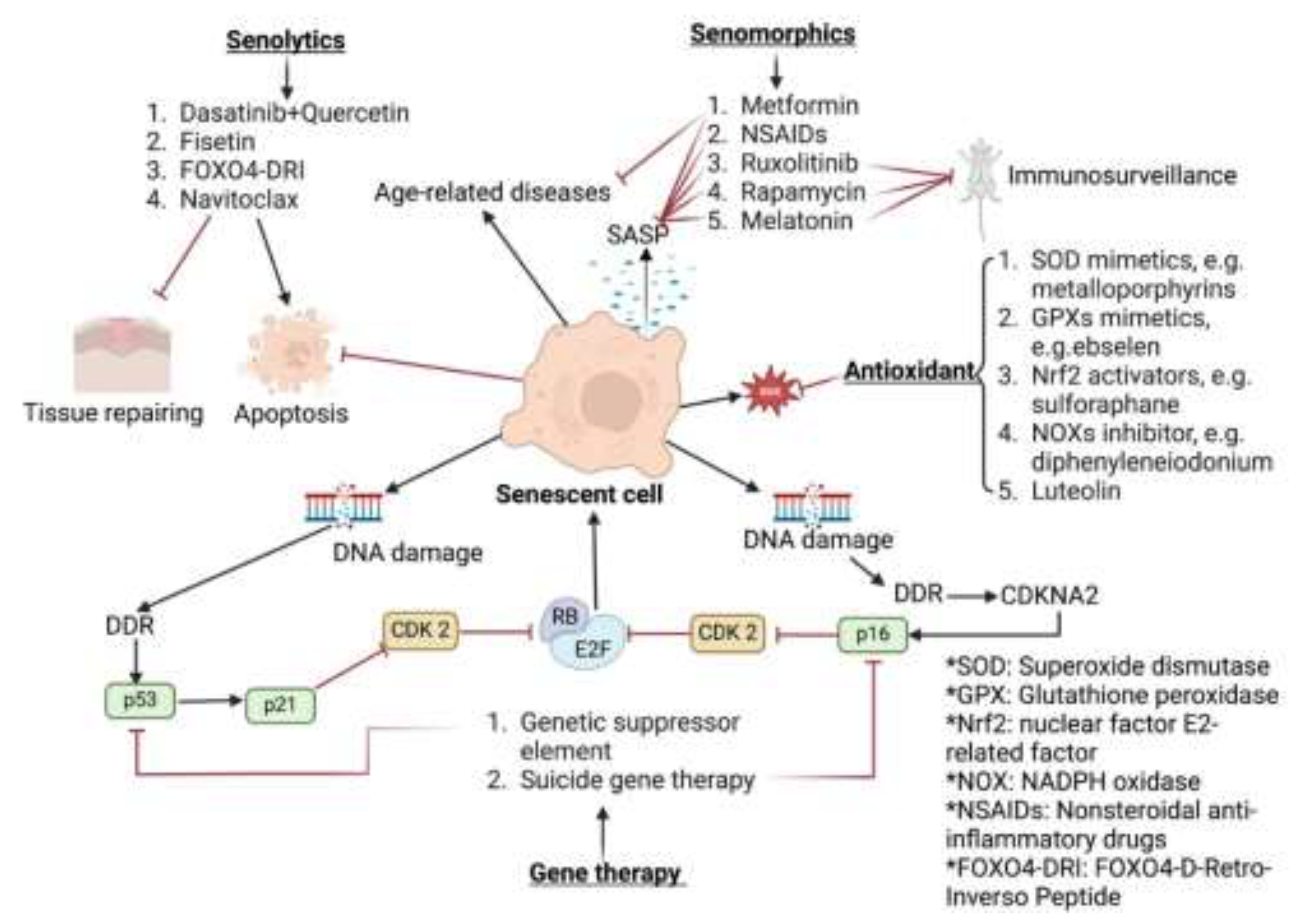
5. Senotherapeutics
6. Senotherapeutics for the Lens
7. Conclusion and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK – AMP-activated protein kinase |
| AP-1 – Activator protein 1 |
| ARE – Antioxidant response element |
| ATF6 – Activating transcription factor 6 |
| Bcl-2 – B-cell lymphoma 2 |
| CDK – Cyclin-dependent kinase |
| CDKs – Cyclin-dependent kinases |
| DDR – DNA damage response |
| DRI – D-retro-inverso |
| ETC – Electron transport chain |
| FTH1 – Ferritin heavy chain 1 |
| FOXO4 – Forkhead box O4 |
| GSH – Glutathione |
| GPx – Glutathione peroxidase |
| Grx – Glutaredoxin |
| IL-6 – Interleukin-6 |
| IL-8 – Interleukin-8 |
| LEC – Lens epithelial cell |
| LECs – Lens epithelial cells |
| MMPs – Matrix metalloproteinases |
| mTOR – Mechanistic target of rapamycin |
| MiDAS – Mitochondrial dysfunction-associated senescence |
| NF-κB – Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 – Nuclear factor erythroid 2-related factor 2 |
| OFZ – Organelle-free zone |
| OIS – Oncogene-induced senescence |
| OXPHOS – Oxidative phosphorylation |
| p16 – Cyclin-dependent kinase inhibitor 2A (CDKN2A) |
| p21 – Cyclin-dependent kinase inhibitor 1A (CDKN1A) |
| p53 – Tumor protein p53 |
| PERK – Protein kinase R-like endoplasmic reticulum kinase |
| RB – Retinoblastoma protein |
| RNS – Reactive nitrogen species |
| ROS – Reactive oxygen species |
| SASP – Senescence-associated secretory phenotype |
| SA-β-gal – Senescence-associated beta-galactosidase |
| SOD – Superoxide dismutase |
| TAF – Telomere-associated DNA damage foci |
| TUNEL – Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| UPR – Unfolded protein response |
| UV – Ultraviolet |
| VEGF – Vascular endothelial growth factor |
| WT – Wild-type |
References
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 2021, 22, 75-95. [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15-22. [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010, 5, 99-118. [CrossRef]
- Shmulevich, R.; Krizhanovsky, V. Cell Senescence, DNA Damage, and Metabolism. Antioxid Redox Signal 2021, 34, 324-334. [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol 2018, 217, 65-77. [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013, 75, 685-705. [CrossRef]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 2014, 15, 482-496. [CrossRef]
- Saito, Y.; Yamamoto, S.; Chikenji, T.S. Role of cellular senescence in inflammation and regeneration. Inflamm Regen 2024, 44, 28. [CrossRef]
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat Rev Cardiol 2022, 19, 250-264. [CrossRef]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular Senescence in Cardiovascular and Metabolic Diseases. Front Cardiovasc Med 2018, 5, 18. [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer - role and therapeutic opportunities. Nat Rev Clin Oncol 2022, 19, 619-636. [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer 2022, 22, 340-355. [CrossRef]
- Wyld, L.; Bellantuono, I.; Tchkonia, T.; Morgan, J.; Turner, O.; Foss, F.; George, J.; Danson, S.; Kirkland, J.L. Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers (Basel) 2020, 12. [CrossRef]
- Iwasaki, K.; Abarca, C.; Aguayo-Mazzucato, C. Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications. Diabetes Metab J 2023, 47, 441-453. [CrossRef]
- Murakami, T.; Inagaki, N.; Kondoh, H. Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic beta Cells. Front Endocrinol (Lausanne) 2022, 13, 869414. [CrossRef]
- Narasimhan, A.; Flores, R.R.; Robbins, P.D.; Niedernhofer, L.J. Role of Cellular Senescence in Type II Diabetes. Endocrinology 2021, 162. [CrossRef]
- Palmer, A.K.; Tchkonia, T.; LeBrasseur, N.K.; Chini, E.N.; Xu, M.; Kirkland, J.L. Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity. Diabetes 2015, 64, 2289-2298. [CrossRef]
- Lee, H.J.; Yoon, Y.S.; Lee, S.J. Molecular Mechanisms of Cellular Senescence in Neurodegenerative Diseases. J Mol Biol 2023, 435, 168114. [CrossRef]
- Sahu, M.R.; Rani, L.; Subba, R.; Mondal, A.C. Cellular senescence in the aging brain: A promising target for neurodegenerative diseases. Mech Ageing Dev 2022, 204, 111675. [CrossRef]
- Wang, Y.; Kuca, K.; You, L.; Nepovimova, E.; Heger, Z.; Valko, M.; Adam, V.; Wu, Q.; Jomova, K. The role of cellular senescence in neurodegenerative diseases. Arch Toxicol 2024, 98, 2393-2408. [CrossRef]
- Soleimani, M.; Cheraqpour, K.; Koganti, R.; Djalilian, A.R. Cellular senescence and ophthalmic diseases: narrative review. Graefes Arch Clin Exp Ophthalmol 2023, 261, 3067-3082. [CrossRef]
- Sreekumar, P.G.; Hinton, D.R.; Kannan, R. The Emerging Role of Senescence in Ocular Disease. Oxid Med Cell Longev 2020, 2020, 2583601. [CrossRef]
- Wu, R.; Wang, J.J.; Mitchell, P.; Lamoureux, E.L.; Zheng, Y.; Rochtchina, E.; Tan, A.G.; Wong, T.Y. Smoking, socioeconomic factors, and age-related cataract: The Singapore Malay Eye study. Arch Ophthalmol 2010, 128, 1029-1035. [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp Cell Res 1961, 25, 585-621. [CrossRef]
- Liao, Z.; Yeo, H.L.; Wong, S.W.; Zhao, Y. Cellular Senescence: Mechanisms and Therapeutic Potential. Biomedicines 2021, 9. [CrossRef]
- Rodriguez-Brenes, I.A.; Wodarz, D.; Komarova, N.L. Quantifying replicative senescence as a tumor suppressor pathway and a target for cancer therapy. Sci Rep 2015, 5, 17660. [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: three decades of progress. Nat Rev Genet 2019, 20, 299-309. [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S.; Thanasoula, M.; Sarandi, E.; Nikolouzakis, K.; Fragkiadaki, P.; Tsoukalas, D.; Spandidos, D.A.; Tsatsakis, A. Telomerase and telomeres in aging theory and chronographic aging theory (Review). Mol Med Rep 2020, 22, 1679-1694. [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol 2022, 24, 135-147. [CrossRef]
- Saretzki, G. Role of Telomeres and Telomerase in Cancer and Aging. Int J Mol Sci 2023, 24. [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov 2016, 6, 584-593. [CrossRef]
- Shin, J.S.; Hong, A.; Solomon, M.J.; Lee, C.S. The role of telomeres and telomerase in the pathology of human cancer and aging. Pathology 2006, 38, 103-113. [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 2017, 58, 235-263. [CrossRef]
- Rossiello, F.; Herbig, U.; Longhese, M.P.; Fumagalli, M.; d’Adda di Fagagna, F. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr Opin Genet Dev 2014, 26, 89-95. [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol 2021, 9, 645593. [CrossRef]
- Dulic, V.; Beney, G.E.; Frebourg, G.; Drullinger, L.F.; Stein, G.H. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol Cell Biol 2000, 20, 6741-6754. [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev 2017, 2017, 8416763. [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 2019, 18, 295-317. [CrossRef]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.H. Involvement of Nrf2 in Ocular Diseases. Oxid Med Cell Longev 2017, 2017, 1703810. [CrossRef]
- Faraonio, R. Oxidative Stress and Cell Senescence Process. Antioxidants (Basel) 2022, 11. [CrossRef]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol 2017, 11, 482-501. [CrossRef]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front Aging Neurosci 2022, 14, 827900. [CrossRef]
- Riordan, R.; Rong, W.; Yu, Z.; Ross, G.; Valerio, J.; Dimas-Munoz, J.; Heredia, V.; Magnusson, K.; Galvan, V.; Perez, V.I. Effect of Nrf2 loss on senescence and cognition of tau-based P301S mice. Geroscience 2023, 45, 1451-1469. [CrossRef]
- Volonte, D.; Liu, Z.; Musille, P.M.; Stoppani, E.; Wakabayashi, N.; Di, Y.P.; Lisanti, M.P.; Kensler, T.W.; Galbiati, F. Inhibition of nuclear factor-erythroid 2-related factor (Nrf2) by caveolin-1 promotes stress-induced premature senescence. Mol Biol Cell 2013, 24, 1852-1862. [CrossRef]
- von Zglinicki, T. Oxidative stress and cell senescence as drivers of ageing: Chicken and egg. Ageing Res Rev 2024, 102, 102558. [CrossRef]
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A., et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 2016, 35, 724-742. [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest 2022, 132. [CrossRef]
- Martini, H.; Passos, J.F. Cellular senescence: all roads lead to mitochondria. FEBS J 2023, 290, 1186-1202. [CrossRef]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol 2013, 169 Suppl 2, 1-8. [CrossRef]
- Murphy, M.P. How understanding the control of energy metabolism can help investigation of mitochondrial dysfunction, regulation and pharmacology. Biochim Biophys Acta 2001, 1504, 1-11. [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov 2018, 17, 865-886. [CrossRef]
- Gambino, V.; De Michele, G.; Venezia, O.; Migliaccio, P.; Dall’Olio, V.; Bernard, L.; Minardi, S.P.; Della Fazia, M.A.; Bartoli, D.; Servillo, G., et al. Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell 2013, 12, 435-445. [CrossRef]
- Liu, D.; Xu, Y. p53, oxidative stress, and aging. Antioxid Redox Signal 2011, 15, 1669-1678. [CrossRef]
- Kim, Y.; Jang, Y.; Kim, M.S.; Kang, C. Metabolic remodeling in cancer and senescence and its therapeutic implications. Trends Endocrinol Metab 2024, 35, 732-744. [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab 2021, 3, 1290-1301. [CrossRef]
- Zhang, H.; Zhou, H.; Shen, X.; Lin, X.; Zhang, Y.; Sun, Y.; Zhou, Y.; Zhang, L.; Zhang, D. The role of cellular senescence in metabolic diseases and the potential for senotherapeutic interventions. Front Cell Dev Biol 2023, 11, 1276707. [CrossRef]
- Kwon, S.M.; Hong, S.M.; Lee, Y.K.; Min, S.; Yoon, G. Metabolic features and regulation in cell senescence. BMB Rep 2019, 52, 5-12. [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 2020, 21, 183-203. [CrossRef]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat Aging 2023, 3, 642-660. [CrossRef]
- Fontana, L.; Mitchell, S.E.; Wang, B.; Tosti, V.; van Vliet, T.; Veronese, N.; Bertozzi, B.; Early, D.S.; Maissan, P.; Speakman, J.R., et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell 2018, 17, e12746. [CrossRef]
- Fontana, L.; Nehme, J.; Demaria, M. Caloric restriction and cellular senescence. Mech Ageing Dev 2018, 176, 19-23. [CrossRef]
- Aversa, Z.; White, T.A.; Heeren, A.A.; Hulshizer, C.A.; Saul, D.; Zhang, X.; Molina, A.J.A.; Redman, L.M.; Martin, C.K.; Racette, S.B., et al. Calorie restriction reduces biomarkers of cellular senescence in humans. Aging Cell 2024, 23, e14038. [CrossRef]
- Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 2012, 13, 89-102. [CrossRef]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol 2015, 308, C415-425. [CrossRef]
- Ziegler, D.V.; Martin, N.; Bernard, D. Cellular senescence links mitochondria-ER contacts and aging. Commun Biol 2021, 4, 1323. [CrossRef]
- Lee, J.H.; Lee, J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic beta-Cell Dysfunction and Senescence in Type 2 Diabetes. Int J Mol Sci 2022, 23. [CrossRef]
- Koloko Ngassie, M.L.; Drake, L.Y.; Roos, B.B.; Koenig-Kappes, A.; Pabelick, C.M.; Gosens, R.; Brandsma, C.A.; Burgess, J.K.; Prakash, Y.S. Endoplasmic reticulum stress-induced senescence in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2024, 327, L126-L139. [CrossRef]
- Takahashi, A. The discovery of oncogene-induced senescence. Nat Rev Mol Cell Biol 2024, 10.1038/s41580-024-00791-3. [CrossRef]
- Toropov, A.L.; Deryabin, P.I.; Shatrova, A.N.; Borodkina, A.V. Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells. Int J Mol Sci 2023, 24. [CrossRef]
- Liu, X.L.; Ding, J.; Meng, L.H. Oncogene-induced senescence: a double edged sword in cancer. Acta Pharmacol Sin 2018, 39, 1553-1558. [CrossRef]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838-857. [CrossRef]
- Gonzalez-Gualda, E.; Baker, A.G.; Fruk, L.; Munoz-Espin, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J 2021, 288, 56-80. [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995, 92, 9363-9367. [CrossRef]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated beta-Galactosidase Detection in Pathology. Diagnostics (Basel) 2022, 12. [CrossRef]
- Evangelou, K.; Lougiakis, N.; Rizou, S.V.; Kotsinas, A.; Kletsas, D.; Munoz-Espin, D.; Kastrinakis, N.G.; Pouli, N.; Marakos, P.; Townsend, P., et al. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 2017, 16, 192-197. [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016, 8, 3-11. [CrossRef]
- Siddiqui, M.S.; Francois, M.; Fenech, M.F.; Leifert, W.R. Persistent gammaH2AX: A promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res 2015, 766, 1-19. [CrossRef]
- Oda, T.; Gotoh, N.; Kasamatsu, T.; Handa, H.; Saitoh, T.; Sasaki, N. DNA damage-induced cellular senescence is regulated by 53BP1 accumulation in the nuclear foci and phase separation. Cell Prolif 2023, 56, e13398. [CrossRef]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front Genet 2018, 9, 247. [CrossRef]
- Hewitt, G.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 2012, 3, 708. [CrossRef]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 2014, 15, 1139-1153. [CrossRef]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J Mol Biol 2019, 431, 2629-2643. [CrossRef]
- Kohli, J.; Ge, C.; Fitsiou, E.; Doepner, M.; Brandenburg, S.M.; Faller, W.J.; Ridky, T.W.; Demaria, M. Targeting anti-apoptotic pathways eliminates senescent melanocytes and leads to nevi regression. Nat Commun 2022, 13, 7923. [CrossRef]
- Rehman, I.; Hazhirkarzar, B.; Patel, B.C. Anatomy, Head and Neck, Eye. In StatPearls, Treasure Island (FL), 2024.
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog Mol Biol Transl Sci 2015, 134, 119-127. [CrossRef]
- Harding, J.J.; Rixon, K.C.; Marriott, F.H. Men have heavier lenses than women of the same age. Exp Eye Res 1977, 25, 651. [CrossRef]
- Augusteyn, R.C. Growth of the human eye lens. Mol Vis 2007, 13, 252-257.
- Augusteyn, R.C. On the growth and internal structure of the human lens. Exp Eye Res 2010, 90, 643-654. [CrossRef]
- Delamere, N.A.; Tamiya, S. Expression, regulation and function of Na,K-ATPase in the lens. Prog Retin Eye Res 2004, 23, 593-615. [CrossRef]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal 2009, 11, 339-353. [CrossRef]
- Lou, M.F. Redox regulation in the lens. Prog Retin Eye Res 2003, 22, 657-682. [CrossRef]
- Lou, M.F. Glutathione and Glutaredoxin in Redox Regulation and Cell Signaling of the Lens. Antioxidants (Basel) 2022, 11. [CrossRef]
- Ang, M.J.; Afshari, N.A. Cataract and systemic disease: A review. Clin Exp Ophthalmol 2021, 49, 118-127. [CrossRef]
- Lam, D.; Rao, S.K.; Ratra, V.; Liu, Y.; Mitchell, P.; King, J.; Tassignon, M.J.; Jonas, J.; Pang, C.P.; Chang, D.F. Cataract. Nat Rev Dis Primers 2015, 1, 15014. [CrossRef]
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye (Lond) 2020, 34, 1357-1370. [CrossRef]
- Mencucci, R.; Stefanini, S.; Favuzza, E.; Cennamo, M.; De Vitto, C.; Mossello, E. Beyond vision:Cataract and health status in old age, a narrative review. Front Med (Lausanne) 2023, 10, 1110383. [CrossRef]
- Vasavada, A.R.; Mamidipudi, P.R.; Sharma, P.S. Morphology of and visual performance with posterior subcapsular cataract. J Cataract Refract Surg 2004, 30, 2097-2104. [CrossRef]
- Brown, N.A. The morphology of cataract and visual performance. Eye (Lond) 1993, 7 ( Pt 1), 63-67. [CrossRef]
- Shah, M.A.; Shah, S.M.; Shah, S.B.; Patel, C.G.; Patel, U.A. Morphology of traumatic cataract: does it play a role in final visual outcome? BMJ Open 2011, 1, e000060. [CrossRef]
- Fu, Q.; Qin, Z.; Yu, J.; Yu, Y.; Tang, Q.; Lyu, D.; Zhang, L.; Chen, Z.; Yao, K. Effects of senescent lens epithelial cells on the severity of age-related cortical cataract in humans: A case-control study. Medicine (Baltimore) 2016, 95, e3869. [CrossRef]
- Yan, Y.; Yu, H.; Sun, L.; Liu, H.; Wang, C.; Wei, X.; Song, F.; Li, H.; Ge, H.; Qian, H., et al. Laminin alpha4 overexpression in the anterior lens capsule may contribute to the senescence of human lens epithelial cells in age-related cataract. Aging (Albany NY) 2019, 11, 2699-2723. [CrossRef]
- Zhang, Z.F.; Zhang, J.; Hui, Y.N.; Zheng, M.H.; Liu, X.P.; Kador, P.F.; Wang, Y.S.; Yao, L.B.; Zhou, J. Up-regulation of NDRG2 in senescent lens epithelial cells contributes to age-related cataract in human. PLoS One 2011, 6, e26102. [CrossRef]
- Andley, U.P. The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol 2008, 40, 317-323. [CrossRef]
- Parreno, J.; Emin, G.; Vu, M.P.; Clark, J.T.; Aryal, S.; Patel, S.D.; Cheng, C. Methodologies to unlock the molecular expression and cellular structure of ocular lens epithelial cells. Front Cell Dev Biol 2022, 10, 983178. [CrossRef]
- Wang, Y.; Tseng, Y.; Chen, K.; Wang, X.; Mao, Z.; Li, X. Reduction in Lens Epithelial Cell Senescence Burden through Dasatinib Plus Quercetin or Rapamycin Alleviates D-Galactose-Induced Cataract Progression. J Funct Biomater 2022, 14. [CrossRef]
- Teng, H.; Hong, Y.; Cao, J.; Li, H.; Tian, F.; Sun, J.; Wen, K.; Han, G.; Whelchel, A.; Zhang, X., et al. Senescence marker protein30 protects lens epithelial cells against oxidative damage by restoring mitochondrial function. Bioengineered 2022, 13, 12955-12971. [CrossRef]
- Kubota, M.; Shui, Y.B.; Liu, M.; Bai, F.; Huang, A.J.; Ma, N.; Beebe, D.C.; Siegfried, C.J. Mitochondrial oxygen metabolism in primary human lens epithelial cells: Association with age, diabetes and glaucoma. Free Radic Biol Med 2016, 97, 513-519. [CrossRef]
- Marchetti, M.A.; Pizarro, G.O.; Sagher, D.; Deamicis, C.; Brot, N.; Hejtmancik, J.F.; Weissbach, H.; Kantorow, M. Methionine sulfoxide reductases B1, B2, and B3 are present in the human lens and confer oxidative stress resistance to lens cells. Invest Ophthalmol Vis Sci 2005, 46, 2107-2112. [CrossRef]
- Zhang, J.; Yu, Y.; Dang, T.; Lal, K.; Wu, H. The impact of glutaredoxin 1 and glutaredoxin 2 double knockout on lens epithelial cell function. Exp Eye Res 2023, 233, 109521. [CrossRef]
- Cvekl, A.; Camerino, M.J. Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology. Cells 2022, 11. [CrossRef]
- Li, W.C.; Kuszak, J.R.; Dunn, K.; Wang, R.R.; Ma, W.; Wang, G.M.; Spector, A.; Leib, M.; Cotliar, A.M.; Weiss, M., et al. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol 1995, 130, 169-181. [CrossRef]
- Harocopos, G.J.; Alvares, K.M.; Kolker, A.E.; Beebe, D.C. Human age-related cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci 1998, 39, 2696-2706.
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000-1011. [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: many therapeutic avenues. Genes Dev 2020, 34, 1565-1576. [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J 2023, 290, 1362-1383. [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: the path to the clinic. Nat Med 2022, 28, 1556-1568. [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M., et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015, 14, 644-658. [CrossRef]
- Elsallabi, O.; Patruno, A.; Pesce, M.; Cataldi, A.; Carradori, S.; Gallorini, M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules 2022, 27. [CrossRef]
- Tavenier, J.; Nehlin, J.O.; Houlind, M.B.; Rasmussen, L.J.; Tchkonia, T.; Kirkland, J.L.; Andersen, O.; Rasmussen, L.J.H. Fisetin as a senotherapeutic agent: Evidence and perspectives for age-related diseases. Mech Ageing Dev 2024, 222, 111995. [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L., et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18-28. [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B., et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428-435. [CrossRef]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A., et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132-147 e116. [CrossRef]
- Zhang, C.; Xie, Y.; Chen, H.; Lv, L.; Yao, J.; Zhang, M.; Xia, K.; Feng, X.; Li, Y.; Liang, X., et al. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging (Albany NY) 2020, 12, 1272-1284. [CrossRef]
- Li, Y.; Zhang, C.; Cheng, H.; Lv, L.; Zhu, X.; Ma, M.; Xu, Z.; He, J.; Xie, Y.; Yang, X., et al. FOXO4-DRI improves spermatogenesis in aged mice through reducing senescence-associated secretory phenotype secretion from Leydig cells. Exp Gerontol 2024, 195, 112522. [CrossRef]
- Kowald, A.; Kirkwood, T.B.L. Senolytics and the compression of late-life mortality. Exp Gerontol 2021, 155, 111588. [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab 2020, 32, 15-30. [CrossRef]
- Zajda, A.; Huttunen, K.M.; Sikora, J.; Podsiedlik, M.; Markowicz-Piasecka, M. Is metformin a geroprotector? A peek into the current clinical and experimental data. Mech Ageing Dev 2020, 191, 111350. [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577-1585. [CrossRef]
- Fujita, Y.; Inagaki, N. Metformin: clinical topics and new mechanisms of action. Diabetol Int 2017, 8, 4-6. [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front Endocrinol (Lausanne) 2019, 10, 294. [CrossRef]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne) 2021, 12, 718942. [CrossRef]
- Yang, Y.; Lu, X.; Liu, N.; Ma, S.; Zhang, H.; Zhang, Z.; Yang, K.; Jiang, M.; Zheng, Z.; Qiao, Y., et al. Metformin decelerates aging clock in male monkeys. Cell 2024, 187, 6358-6378 e6329. [CrossRef]
- Griveau, A.; Wiel, C.; Ziegler, D.V.; Bergo, M.O.; Bernard, D. The JAK1/2 inhibitor ruxolitinib delays premature aging phenotypes. Aging Cell 2020, 19, e13122. [CrossRef]
- Wang, R.; Yu, Z.; Sunchu, B.; Shoaf, J.; Dang, I.; Zhao, S.; Caples, K.; Bradley, L.; Beaver, L.M.; Ho, E., et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 2017, 16, 564-574. [CrossRef]
- Luis, C.; Maduro, A.T.; Pereira, P.; Mendes, J.J.; Soares, R.; Ramalho, R. Nutritional senolytics and senomorphics: Implications to immune cells metabolism and aging - from theory to practice. Front Nutr 2022, 9, 958563. [CrossRef]
- Martin Gimenez, V.M.; de Las Heras, N.; Lahera, V.; Tresguerres, J.A.F.; Reiter, R.J.; Manucha, W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front Aging Neurosci 2022, 14, 888292. [CrossRef]
- Cruciani, S.; Garroni, G.; Pala, R.; Barcessat, A.R.P.; Facchin, F.; Ventura, C.; Fozza, C.; Maioli, M. Melatonin finely tunes proliferation and senescence in hematopoietic stem cells. Eur J Cell Biol 2022, 101, 151251. [CrossRef]
- Tan, Y.Z.; Xu, X.Y.; Dai, J.M.; Yin, Y.; He, X.T.; Zhang, Y.L.; Zhu, T.X.; An, Y.; Tian, B.M.; Chen, F.M. Melatonin induces the rejuvenation of long-term ex vivo expanded periodontal ligament stem cells by modulating the autophagic process. Stem Cell Res Ther 2021, 12, 254. [CrossRef]
- Batinic-Haberle, I.; Reboucas, J.S.; Spasojevic, I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 2010, 13, 877-918. [CrossRef]
- Pacula, A.J.; Kaczor, K.B.; Wojtowicz, A.; Antosiewicz, J.; Janecka, A.; Dlugosz, A.; Janecki, T.; Scianowski, J. New glutathione peroxidase mimetics-Insights into antioxidant and cytotoxic activity. Bioorg Med Chem 2017, 25, 126-131. [CrossRef]
- Hariton, F.; Xue, M.; Rabbani, N.; Fowler, M.; Thornalley, P.J. Sulforaphane Delays Fibroblast Senescence by Curbing Cellular Glucose Uptake, Increased Glycolysis, and Oxidative Damage. Oxid Med Cell Longev 2018, 2018, 5642148. [CrossRef]
- Liao, K.M.; Chen, C.J.; Luo, W.J.; Hsu, C.W.; Yu, S.L.; Yang, P.C.; Su, K.Y. Senomorphic effect of diphenyleneiodonium through AMPK/MFF/DRP1 mediated mitochondrial fission. Biomed Pharmacother 2023, 162, 114616. [CrossRef]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid Redox Signal 2015, 23, 406-427. [CrossRef]
- Kohli, J.; Campisi, J.; Demaria, M. A novel suicide gene therapy for the treatment of p16(Ink4a)-overexpressing tumors. Oncotarget 2018, 9, 7274-7281. [CrossRef]
- Demaria, M. Gene therapy for p16-overexpressing cells. Aging (Albany NY) 2018, 10, 518-519. [CrossRef]
- Yao, K.; Zhang, L.; Zhang, Y.; Ye, P.; Zhu, N. The flavonoid, fisetin, inhibits UV radiation-induced oxidative stress and the activation of NF-kappaB and MAPK signaling in human lens epithelial cells. Mol Vis 2008, 14, 1865-1871.
- Sreelakshmi, V.; Sasikala, V.; Abraham, A. Luteolin Supplementation Prevents Selenite-Induced Cataractogenesis in Sprague Dawley Rat Pups. Chem Biodivers 2015, 12, 1881-1890. [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Obligatory Role of AMPK Activation and Antioxidant Defense Pathway in the Regulatory Effects of Metformin on Cellular Protection and Prevention of Lens Opacity. Cells 2022, 11. [CrossRef]
- Chen, M.; Fu, Y.; Wang, X.; Wu, R.; Su, D.; Zhou, N.; Qi, Y. Metformin protects lens epithelial cells against senescence in a naturally aged mouse model. Cell Death Discov 2022, 8, 8. [CrossRef]
- Mi, Y.; Wei, C.; Sun, L.; Liu, H.; Zhang, J.; Luo, J.; Yu, X.; He, J.; Ge, H.; Liu, P. Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed Pharmacother 2023, 157, 114048. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




