Submitted:
10 January 2025
Posted:
11 January 2025
You are already at the latest version
Abstract
In these times and with the pace of life we have developed, many people need help falling asleep due to poor sleep hygiene, among other reasons. Thus, in mild cases, it is recommended to use natural therapies, such as phytotherapy, avoiding in the first instance the use of drugs. Melatonin is considered a versatile molecule widely used today. It is included as a main ingredient in dietary supplements that are, in some cases, accompanied by medicinal plants as botanical mix, generating beneficial products for sleep disorders among other conditions. The dietary phytotherapeutic supplements evaluated in this work contain various concentrations of melatonin and other products, resulting in different effects on sleep therapy. The aim of this work is to reveal the quantitative differences that exist between the melatonin contents labeled in the products and those analyzed. The degradation rate of this hormone at three years in the phytotherapeutic supplements is also studied, in order to re-evaluate the expiration dates of these products. As conclusion, the mixture between synthetic melatonin and different botanical mix is very common in the supplements studied here and aimed at improving sleep. However, the most natural thing would be to be able to use only plants with sufficient phytomelatonin content to eliminate the inclusion of chemically synthesized melatonin in the preparations. We propose the use of a particular raw plant material with excellent characteristics for this purpose.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Melatonin/Phytomelatonin
2.3. Analysis of Melatonin/Phytomelatonin by HPLC with Fluorimetric Detection (LC-FLUO)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Melatonin/Phytomelatonin Contents in the Phytotherapeutic Supplements
3.2. Study on the Stability of Melatonin in the Phytotherapeutic Supplements
4. Conclusions
- In 11 of the supplements, the total melatonin value is below the manufacturer's specifications, between 5-68%. It would be advisable to re-examine the products on future occasions to continue checking the veracity of the stipulated contents, but more measures should also be implemented to regulate the amount of actual melatonin they should contain.
- Only in supplements with extra melatonin content (+31.1% and +104%) can we assume that their botanical components truly provide phytomelatonin, which is added to the synthetic melatonin added in all other products.
- Among the proposals to be highlighted, we recommend the performance of residue analyses of the chemical synthesis of melatonin to demonstrate the synthetic origin of this added molecule. We also recommend encouraging the use of natural preparations rich in phytomelatonin that do not contain by-products of the synthetic molecule.
- The stability of the preparations is increased under the following storage conditions: i) pills in their packaging (blister pack, airtight bottle), ii) protected from light, and iii) protected from the action of air (oxidation and changes in color).
- If we consider that the differences in some of the contents measured in 2021 with respect to the manufacturer's specifications are due to early degradation of melatonin molecule, we propose to reevaluate the established caducity periods by carrying out future studies and analyses.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, a Pineal Factor That Lightens Melanocytes. J Am Chem Soc 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem Mol Biol Int 1995, 35, 627–634. [Google Scholar] [PubMed]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol Rhythm Res 1995, 26, 406–409. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J Pineal Res 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. The Melatonin Rhythm: Both a Clock and a Calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef]
- Blume, C.; Angerer, M.; Raml, M.; del Giudice, R.; Santhi, N.; Pichler, G.; Kunz, A.B.; Scarpatetti, M.; Trinka, E.; Schabus, M. Healthier Rhythm, Healthier Brain? Integrity of Circadian Melatonin and Temperature Rhythms Relates to the Clinical State of Brain-Injured Patients. Eur J Neurol 2019, 26, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.A.; Manchester, L.C. The Universal Nature, Unequal Distribution and Antioxidant Functions of Melatonin and Its Derivatives. Mini-Rev Med Chem 2013, 13, 373–384. [Google Scholar]
- Reiter, R.J.; Sharma, R.; Tan, D.-X.; Chuffa, L.G. de A.; da Silva, D.G.H.; Slominski, A.T.; Steinbrink, K.; Kleszczynski, K. Dual Sources of Melatonin and Evidence for Different Primary Functions. Front. Endocrinol. 2024, 15. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Hardeland, R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P. Melatonin and Healthy Aging. In Vitamins and Hormones Hormones and Aging; Litwack, G., Ed.; Academic Press, 2021; Volume 115, pp. 67–88. ISBN 0083-6729. [Google Scholar]
- Cardinali, D.P. Melatonin as a Chronobiotic/Cytoprotective Agent in Bone. Doses Involved. Journal of Pineal Research 2024, n/a, e12931. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Mania, M.; Abbate, F.; Navarra, M.; Guerrera, M.C.; Laura, R.; Vega, J.A.; Levanti, M.; German�, A. Melatonin Treatment Suppresses Appetite Genes and Improves Adipose Tissue Plasticity in Diet-Induced Obese Zebrafish. Endocrine 2018. [Google Scholar] [CrossRef] [PubMed]
- Dahlitz, M.; Alvarez, B.; Vignau, J.; English, J.; Arendt, J.; Parkes, J. Delayed Sleep Phase Syndrome Response to Melatonin. Lancet 1991, 337, 1121–1124. [Google Scholar] [CrossRef]
- Takahashi, T.; Sasaki, M.; Itoh, H.; Ozone, M.; Yamadera, W.; Hayshida, K.I.; Ushijima, S.; Matsunaga, N.; Obuchi, K.; Sano, H. Effect of 3 Mg Melatonin on Jet Lag Syndrome in an 8-h Eastward Flight. Psych Clin Neurosci 2000, 54, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.; Poeggeler, B.; Menendez-Pelaez, A.; Chen, L.; Saarela, S. Melatonin as a Free-Radical Scavenger. Implications for Aging and Age-Related Diseases. Annual New York Academic Science 1994, 714, 1–12. [Google Scholar] [CrossRef]
- Reiter, R.J.; Melchiorri, D.; Sewerynek, E.; Poeggeler, B.; Barlow-Walden, L.; Chuang, J.; Ortiz, G.G.; Acuña-Castroviejo, D. A Review of the Evidence Supporting Melatonin’s Role as an Antioxidant. J Pineal Res 1995, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bantounou, M.; Plascevic, J.; Galley, H.F. Melatonin and Related Compounds: Antioxidant and Anti-Inflammatory Actions. Antioxidants 2022, 11, 532. [Google Scholar] [CrossRef]
- Carrascal, L.; Nu�ez-Abades, P.; Ayala, A.; Cano, M. Role of Melatonin in the Inflammatory Process and Its Therapeutic Potential. Current Pharmaceutical Design 2018, 24, 1563–1588. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, H.S.; Kwon, M.J.; Jang, J.E.; Kim, T.N.; Lee, S.H.; Kim, M.K.; Park, J.H. Melatonin Protects INS-1 Pancreatic B-Cells from Apoptosis and Senescence Induced by Glucotoxicity and Glucolipotoxicity. Islets 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, M.A.; Suárez, S.; Puente-Moncada, N.; Anítua, J.M.; et al. Mechanisms Involved in the Pro-Apoptotic Effect of Melatonin in Cancer Cells. Int J Mol Sci 2013, 14, 6597–6613. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A Review of the Multiple Actions of Melatonin on the Immune System. Endocrine 2012, 27, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kvetnoy, I.; Ivanov, D.; Mironova, E.; Evsyukova, I.; Nasyrov, R.; Kvetnaia, T.; Polyakova, V. Melatonin as the Cornerstone of Neuroimmunoendocrinology. International Journal of Molecular Sciences 2022, 23, 1835. [Google Scholar] [CrossRef]
- Cardinali, D.; Brown, G.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Malabadi, R.; Kolkar, K.; Meti, N.; Chalannavar, R. Melatonin: One Molecule One-Medicine for Many Diseases, Coronavirus (SARS-CoV-2) Disease (Covid- 19); Function in Plants. International Journal of Research and Scientific Innovation 2021, 08, 155–181. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a Potential Adjuvant Treatment. Life Sciences 2020, 250, 117583. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ogugofor, M.O.; Nkwoemeka, N.E.; Nweze, E.J.; Okoye, C.O. Phytomelatonin: A Potential Phytotherapeutic Intervention on COVID-19-Exposed Individuals. Microbes and Infection 2022, 24, 104886. [Google Scholar] [CrossRef] [PubMed]
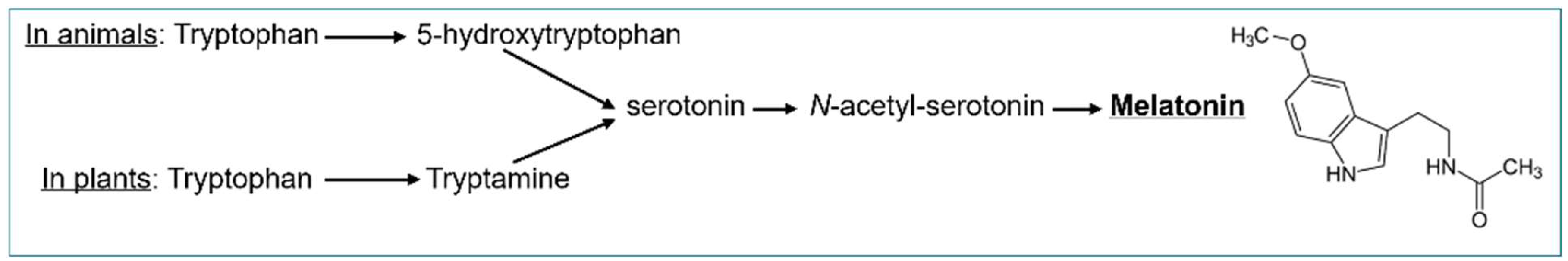
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin, Natural Melatonin from Plants as a Novel Dietary Supplement: Sources, Activities and World Market. Journal of Functional Foods 2018, 48, 37–42. [Google Scholar] [CrossRef]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. The Plant Journal 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
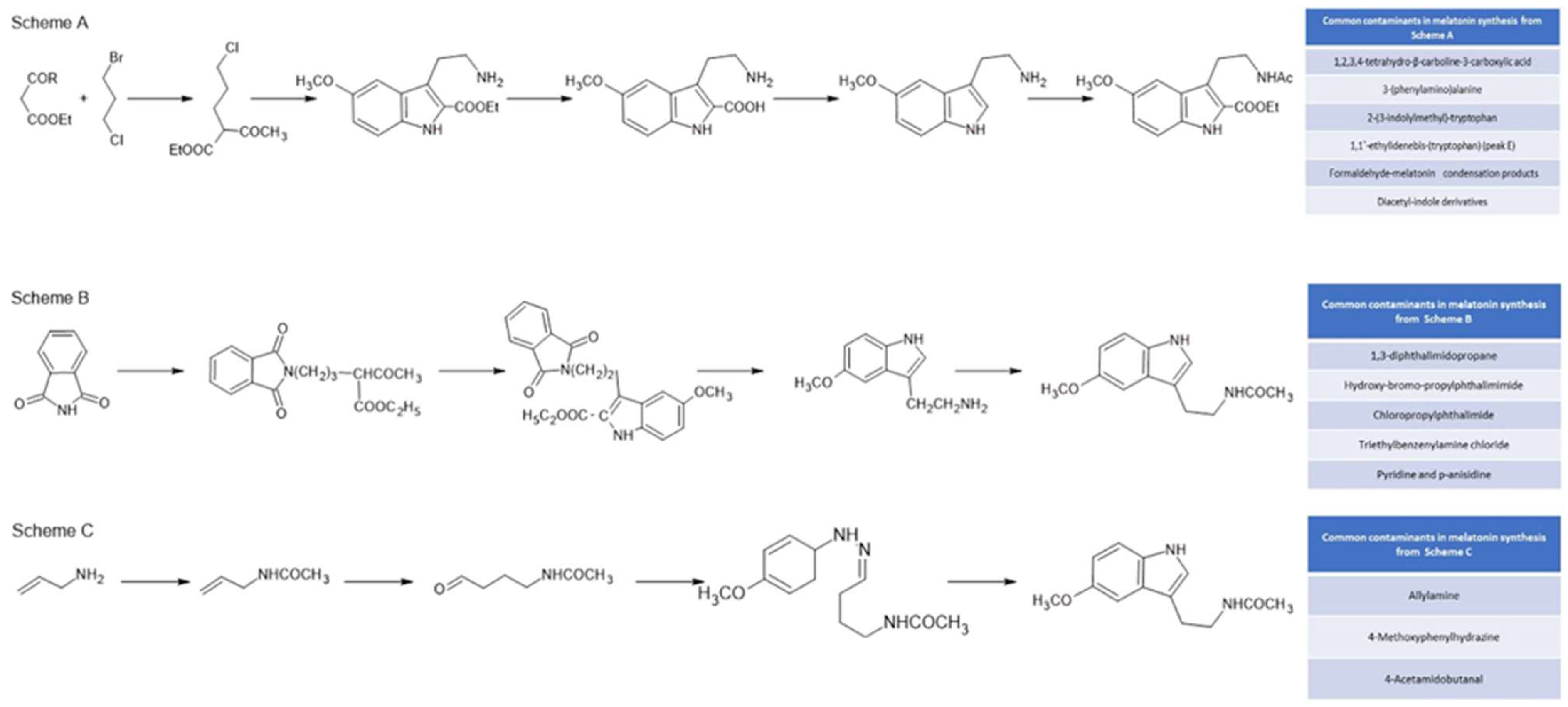
- Hugel, H.M.; Kennaway, D.J. Synthesis and Chemistry of Melatonin and of Related Compounds. A Review. Organic Preparations and Procedures Int 1995, 27, 1–31. [Google Scholar] [CrossRef]
- He, L.; Li, J.L.; Zhang, J.J.; Su, P.; Zheng, S.L. Microwave Assisted Synthesis of Melatonin. Synthetic Communications 2003, 33, 741–747. [Google Scholar] [CrossRef]
- Arnao, M.B.; Giraldo-Acosta, M.; Castejón-Castillejo, A.; Losada-Lorán, M.; Sánchez-Herrerías, P.; El Mihyaoui, A.; Cano, A.; Hernández-Ruiz, J. Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin. Metabolites 2023, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Naylor, S.; Johnson, K.L.; Williamson, B.L.; Klarskov, K.; Gleich, G.J. Structural Characterization of Contaminants in Commercial Preparations of Melatonin by On-Line HPLC-Electrospray Ionization-Tandem Mass Spectrometry. Adv Exp Med Biol 1999, 467, 769–777. [Google Scholar] [PubMed]
- Williamson, B.L.; Tomlinson, A.J.; Naylor, S.; Gleich, G.J. Contaminats in Commercial Preparations of Melatonin. Mayo Clinic Proceed. 1997, 72, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.L.; Tomlinson, A.J.; Mishra, P.K.; Gleich, G.J.; Naylor, S. Structural Characterization of Contaminants Found in Commercial Preparations of Melatonin: Similarities to Case-Related Compounds from L-Tryptophan Associated with Eosinophilia-Myalgia Syndrome. Chem Res Toxicol 1998, 11, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Mayeno, A.H. The Eosinophilia-Myalgia Syndrome: Lessons from Germany. Mayo Clinic Proceed. 1994, 69, 702–704. [Google Scholar]
- OECD-Organisation for Economic Co-operation & Development Initial Assessment Report on Phthalimide. Screening Information DataSet (SIDS) 2006, SIAM 20, Paris, France, ID-85-41-6.
- Verspui, G.; Elbertse, G.; Sheldon, F.A.; Hacking, M.A.P.J.; Sheldon, R.A. Selective Hydroformylation of N-Allylacetamide in an Inverted Aqueous Two-Phase Catalytic System, Enabling a Short Synthesis of Melatonin. Chem. Commun. 2000, 1363–1364. [Google Scholar] [CrossRef]
- Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Common Methods of Extraction and Determination of Phytomelatonin in Plants. In ROS Signaling in Plants; Corpas, F.J., Palma, J.M., Eds.; Methods in Molecular Biology; Springer US: New York, NY, 2024; Volume 2798, pp. 161–181. ISBN 978-1-07-163825-5. [Google Scholar]
- Losada-Lorán, M.; Cano, A.; Hernández-Rui̇z, J.; Arnao, M.B. Phytomelatonin Content in Valeriana Officinalis L. and Some Related Phytotherapeutic Supplements. International Journal of Plant Based Pharmaceuticals 2022, 2, 176–181. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Assessment of Different Sample Processing Procedures Applied to the Determination of Melatonin in Plants. Phytochem Anal 2009, 20, 14–18. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Distribution of Melatonin in Different Zones of Lupin and Barley Plants at Different Ages in the Presence and Absence of Light. J Agr Food Chem 2008, 56, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High Levels of Melatonin in the Seeds of Edible Plants. Possible Function in Germ Tissue Protection. Life Sci 2000, 67, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv Bot 2014, e815769. [Google Scholar] [CrossRef]
- Marioni, F.; Bertoli, A.; Pistelli, L. A Straightforward Procedure to Biosynthesis Melatonin Using Freshly Chopped Achillea Millefolium L. as Reagent. Phytochem Lett 2008, 1, 107–110. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Szwajgier, D.; Gawel-Beben, K.; Strzepek-Gomolka, M.; Glowniak, K.; Meissner, H.O. Is Phytomelatonin Complex Better Than Synthetic Melatonin? The Assessment of the Antiradical and Anti-Inflammatory Properties. Molecules 2021, 26, 6087. [Google Scholar] [CrossRef] [PubMed]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality Analysis of Commercial Chlorella Products Used as Dietary Supplement in Human Nutrition. Journal of Applied Phycology 2010, 22, 265–276. [Google Scholar] [CrossRef]
- Roy-Lachapelle, A.; Solliec, M.; Bouchard, M.F.; Sauvé, S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins 2017, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Current State of the Natural Melatonin: The Phytomelatonin Market. Melatonin Research 2024, 7, 242–248. [Google Scholar] [CrossRef]
| # |
Product Trade Name (Lab, Location) |
Melatonin content (in brochure) | Content of plant material |
| 1 |
Meladispert Melatonin Herbatonin 100% Vegetal (Vemedia Pharma Lab, Netherlands) |
1.90 mg |
Oryza sativa Medicago sativa Chlorella vulgaris Chlorella pyrenoidosa |
| 2 |
Kneipp Sueño Complet (Hartmann Lab, Germany) |
1.85 mg |
120 mg Valeriana officinalis 80 mg Melissa officinalis 50 mg Passiflora incarnata |
| 3 |
Farline Melatonin Forte (Farline Lab, Madrid, Spain) |
1.85 mg |
100 mg Eschscholzia californica 100 mg Passiflora incarnata 50 mg Valeriana officinalis |
| 4 |
Aquilea Sueño (Uriach Lab, Barcelona, Spain) |
1.95 mg |
100 mg Eschscholzia californica 100 mg Passiflora incarnata 50 mg Valeriana officinalis |
| 5 |
PrismaNatural Melato+ (Best Medical Lab, Sevilla, Spain) |
1.80 mg |
117 mg Eschscholzia californica 117 mg Passiflora incarnata 36 mg Melissa officinalis 18 mg Tilia platyphyllos 12 mg Valeriana officinalis |
| 6 |
Melatonin Zentrum (Ynsadiet Lab, Madrid, Spain) |
1.80 mg |
117 mg Eschscholzia californica 117 mg Passiflora incarnata 36 mg Melissa officinalis 18 mg Tilia platyphyllos 12 mg Valeriana officinalis |
| 7 |
Dulces Sueños Deliplus (Korott Lab, Alicante, Spain) |
1.00 mg |
200 mg Passiflora incarnata 100 mg Humulus lupulus |
| 8 |
Arkosueño Forte (Arkopharma Lab, France) |
1.90 mg |
160 mg Eschscholzia californica 150 mg Valeriana officinalis 100 mg Passiflora incarnata |
| 9 |
Buenas Noches Total (Eladiet Lab, Barcelona, Spain) |
1.85 mg |
100 mg Valeriana officinalis 75 mg Pasiflora incarnata 25 mg Eschscholzia californica |
| 10 |
ActiveComplex Melatonin (PharmaNord Lab, Denmark) |
1.00 mg | - |
| 11 |
Somniplant (Lavigor 7000 Lab, Bizkaia, Spain) |
1.99 mg |
200 mg Passiflora incarnata 100 mg Eschscholzia californica 50 mg Crataegus monogyna |
| 12 |
Sedasor Sueño (PharmaSor Lab, Soria, Spain) |
mg |
150 mg Valeriana officinalis 150 mg Eschscholzia californica 88 mg Passiflora incarnata |
| 13 |
Dormesan Forte (A. Vogel Lab, Switzerland) |
- |
2090 mg Passiflora incarnata* 1331 mg Melissa officinalis* 1050 mg Avena sativa* 306 mg Valeriana officinalis* 251 mg Humulus lupulus* *Fresh plant extracts |
| # | Product |
Advertised melatonin (mg/tablet) |
Measured melatonin (mg/tablet) | Difference(mg) | Difference (%) |
| 1 | Meladispert Melatonin | 1.90 mg | 1.47 ± 0.15 | -0.43 | -22.6 |
| 2 | Kneipp Sueño Complet | 1.85 mg | 1.73 ± 0.14 | -0.12 | -6.5 |
| 3 | Farline Melatonin Forte | 1.85 mg | 1.17 ± 0.17 | -0.68 | -36.8 |
| 4 | Aquilea Sueño | 1.95 mg | 1.14 ± 0.05 | -0.81 | -41.5 |
| 5 | PrismaNatural Melato+ | 1.80 mg | 2.38 ± 0.14 | +0.58 | +31.1 |
| 6 | Melatonin Zentrum | 1.80 mg | 1.56 ± 0.02 | -0,24 | -13.3 |
| 7 | Dulces Sueños Deliplus | 1.00 mg | 0.95 ± 0.03 | -0,05 | -5.0 |
| 8 | Arkosueño Forte | 1.90 mg | 1.47 ± 0.01 | -0.43 | -22.6 |
| 9 | Buenas Noches Total | 1.85 mg | 1.16 ± 0.12 | -0.69 | -37.3 |
| 10 | ActiveComplex Melatonin | 1.00 mg | 0.58 ± 0.05 | -0.42 | -42.0 |
| 11 | Somniplant | 1.99 mg | 1.16 ± 0.11 | -0.83 | -41.7 |
| 12 | Sedasor Sueño | 1.80 mg | 0.58 ± 0.07 | -1.22 | -67.8 |
| 13 | Dormesan Forte | - | 1.04 µg ± 0.35 | +1.04 µg | - |
| # | Product |
2021 Measured melatonin (mg/tablet) |
2024 Measured melatonin (mg/tablet) |
Difference(mg) |
Difference (%) |
| 1 | Meladispert Melatonin | 1.47 ± 0.15 | 0.89 ± 0.16 | -0.58 | -39.5 |
| 2 | Kneipp Sueño Complet | 1.73 ± 0.14 | 1.08 ± 0.08 | -0.65 | -37.6 |
| 3 | Farline Melatonin Forte | 1.17 ± 0.17 | 0.96 ± 0.08 | -0.21 | -17.9 |
| 4 | Aquilea Sueño | 1.14 ± 0.05 | 0.71 ± 0.09 | -0.43 | -37.7 |
| 5 | PrismaNatural Melato+ | 2.38 ± 0.14 | 1.26 ± 0.05 | -1.12 | -47.1 |
| 7 | Dulces Sueños Deliplus | 0.95 ± 0.03 | 0.72 ± 0.02 | -0.23 | -24.2 |
| 8 | Arkosueño Forte | 1.47 ± 0.01 | 0.81 ± 0.02 | -0.66 | -44.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





