1. Introduction
Papillary thyroid carcinoma (PTC) accounts for 80–90% of all thyroid cancers in children [
1,
2,
3]. Advances in diagnostic evaluation, particularly neck ultrasonography (USG) and fine-needle aspiration biopsy (FNAB), have significantly enhanced the detection of microcarcinomas, contributing to the rising number of reported cases over the past decade [
3,
4,
5].
The ultrasonographic features characteristic of PTC include solid hypoechoic nodules with irregular margins and shape, extrathyroidal extension, a taller-than-wide configuration, microcalcifications (punctate echogenic foci), and cervical lymphadenopathy [
1]. In pediatric patients, the primary presentation of PTC is typically a thyroid nodule. However, it may also present as cervical lymphadenopathy, with or without a palpable thyroid nodule, or as an incidental lesion identified during neck imaging or surgery performed for unrelated reasons [
1].
Autoimmune thyroiditis (AIT), the most common autoimmune disorder affecting the thyroid, is a well-established independent risk factor for PTC [
6,
7,
8]. AIT and PTC frequently exhibit overlapping clinical features, including goiter and both functional and structural thyroid abnormalities, complicating the distinction between benign and malignant conditions. This challenge is particularly pronounced in early-stage PTC or specific variants seen in pediatric patients [
1,
9]. A notable subtype, pediatric diffuse sclerosing PTC, is characterized by widespread infiltration leading to enlargement of the affected thyroid lobe or the entire gland, often accompanied by palpable cervical lymphadenopathy [
1,
9]. This variant is commonly associated with microcalcifications, necessitating fine-needle aspiration biopsy (FNAB) for definitive diagnosis [
1,
9].
Classic ultrasound (US) criteria for differentiating benign from malignant lymph nodes in children are similar to those used in adults [
1]. B-scan criteria for benign lymph nodes include small size, oval shape, the presence of a hilum, moderate or low echogenicity, and sharply defined margins. Soft tissue edema may also be observed. Doppler ultrasound findings indicative of benign lymph nodes include absent vascular flow, centrally located vessels, a single vascular pattern, and low impedance values [
10].
B-scan criteria for malignant lymph nodes include large size, rounded shape, absence of a hilum, marked hypoechogenicity, irregular, blurred, angular, or invasive margins, and structural changes such as focal cortical nodules, intranodal necrosis, reticulation, calcification, or matting [
10]. Soft tissue edema is typically absent. Doppler ultrasound findings consistent with malignancy include the presence of vascular flow, peripherally located vessels, multiple vascular pedicles, a disorganized (chaotic) vascular pattern, and high impedance values [
10]. Knowledge of these differences, coupled with improved access to high-resolution ultrasound equipment, facilitates the timely detection of suspected malignancies in at-risk patient groups.
We report the case of an 11-year-old patient with a history of AIT, where cervical lymph node ultrasound was instrumental in raising suspicion of PTC in the context of an inflamed thyroid gland.
3. Case Report
An 11-year-old female patient with a two-year history of autoimmune thyroiditis (AIT) presented to the outpatient thyroid clinic with complaints of difficulty waking in the mornings, persistent hair loss, sensations of heat, and mild weight gain over the past few months. Her family history was significant for maternal hypothyroidism and vitiligo, as well as a paternal grandmother who had undergone thyroidectomy for thyroid follicular nodular disease.
Physical examination revealed a mildly enlarged thyroid gland with a firm consistency, particularly notable in the right lobe. Initial laboratory investigations from her past medical records showed significantly elevated thyroid-stimulating hormone (TSH) levels at 19.530 μIU/mL (reference range: 0.3–4.0 μIU/mL), with normal free triiodothyronine (fT3) at 5.59 pmol/L (4.0–7.8 pmol/L) and free thyroxine (fT4) at 12.09 pmol/L (10.0–25.0 pmol/L). Thyroid autoantibodies were elevated, with thyroid peroxidase antibodies (TPOAb) at 36 IU/mL (reference <20 IU/mL) and thyroglobulin antibodies (TgAb) at 47 IU/mL (reference <30 IU/mL). She was started on levothyroxine therapy (50 μg daily).
An initial thyroid ultrasound performed during an earlier consultation demonstrated a heterogeneous, hypoechoic thyroid gland consistent with autoimmune thyroiditis. The right thyroid lobe measured 1.3 × 1.32 × 3.6 cm, the left lobe measured 1.58 × 1.26 × 3.12 cm, and the isthmus was 0.26 cm thick. No cystic or solid nodules were identified at that time.
At the follow-up visit, despite ongoing levothyroxine therapy, her TSH levels remained elevated at 13.17 μIU/mL, likely due to an insufficient dosage adjustment by the managing physician. Additionally, there was a marked increase in thyroid autoantibodies over time, with TPOAb rising from 36 IU/mL to 562.6 IU/mL and TgAb increasing from 47 IU/mL to >4000 IU/mL. The patient’s mother reported noticeable thyroid enlargement, prompting a repeat thyroid ultrasound.
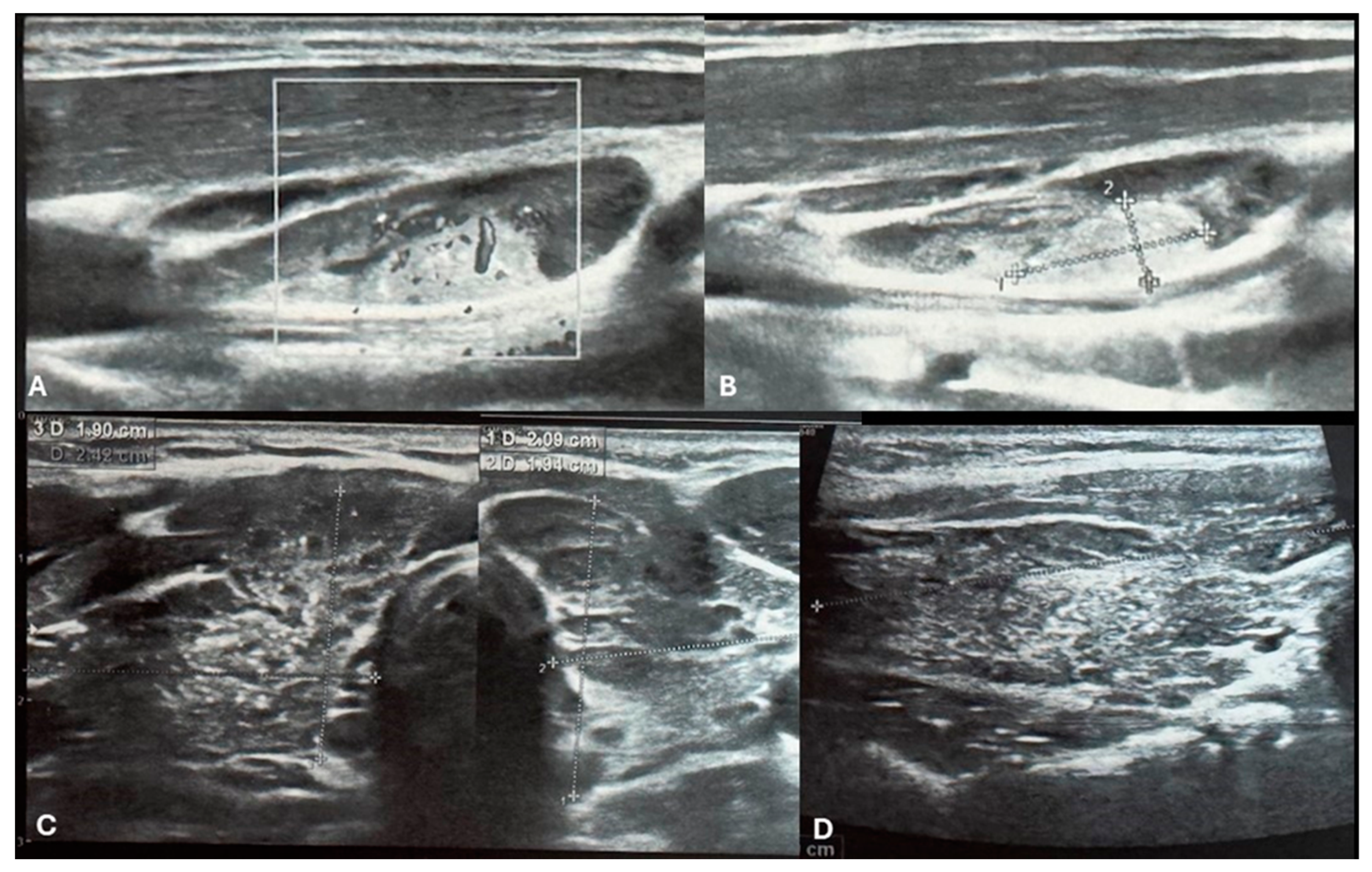
Figure 1.
Ultrasound imaging of the thyroid and neck region. [A, B] Ultrasound images depict a vascularized hyperechoic lesion measuring 1.01 x 0.45 cm located within a lymph node in the upper cervical level. This finding raises suspicion for malignancy due to pathological vascularization and its proximity to the right thyroid lobe. [C] Transversal images of right and left lobe showing remodeled thyroid gland as a result of autoimmune thyroiditis. The thyroid parenchyma demonstrates characteristic heterogeneity, consistent with the chronic inflammatory changes seen in AIT. Additionally, multiple small microcalcifications are visible throughout the right thyroid lobe [C&D].
Figure 1.
Ultrasound imaging of the thyroid and neck region. [A, B] Ultrasound images depict a vascularized hyperechoic lesion measuring 1.01 x 0.45 cm located within a lymph node in the upper cervical level. This finding raises suspicion for malignancy due to pathological vascularization and its proximity to the right thyroid lobe. [C] Transversal images of right and left lobe showing remodeled thyroid gland as a result of autoimmune thyroiditis. The thyroid parenchyma demonstrates characteristic heterogeneity, consistent with the chronic inflammatory changes seen in AIT. Additionally, multiple small microcalcifications are visible throughout the right thyroid lobe [C&D].
Repeat ultrasonography revealed multiple small microcalcifications scattered throughout the right thyroid lobe and a suspicious lymph node adjacent to the right thyroid lobe. The lymph node exhibited a hyperechogenic lesion with pathological vascularization, raising concern for malignancy [
Figure 1.A,B]. Fine-needle aspiration biopsy (FNAB) of the right thyroid lobe was performed, revealing cytological findings of dispersed groups and sheets of thyrocytes with nuclear enlargement, clearing, occasional intranuclear grooves, and focal oxyphilic metaplasia. The background contained numerous lymphocytes, scattered multinucleated giant cells, colloid, and blood. These findings were classified as Bethesda category III (atypia of undetermined significance, AUS) with concurrent features of chronic lymphocytic thyroiditis.
FNAB of the suspicious lymph node was initially deferred due to the patient’s non-cooperation during the procedure. Under sedation, repeat FNAB of the right thyroid lobe and FNAB of the suspicious right lateral cervical lymph node were performed. Cytology of the thyroid sample again yielded Bethesda category III findings [
Figure 2A]. The lymph node aspirate revealed the presence of thyrocytes, suggestive of possible metastatic involvement.
Given the suspicious cytological and ultrasonographic findings, surgical intervention was planned. One month later, the patient underwent total thyroidectomy with central neck dissection (level VI) and right-sided modified radical neck dissection (levels II–V). Intraoperative recurrent laryngeal nerve (RLN) monitoring was utilized to ensure nerve integrity and minimize the risk of injury. Intraoperative frozen section examination was performed during the procedure. Notably, enlarged lymph nodes were observed in the central and right lateral neck compartments, further corroborating the concern for malignancy.
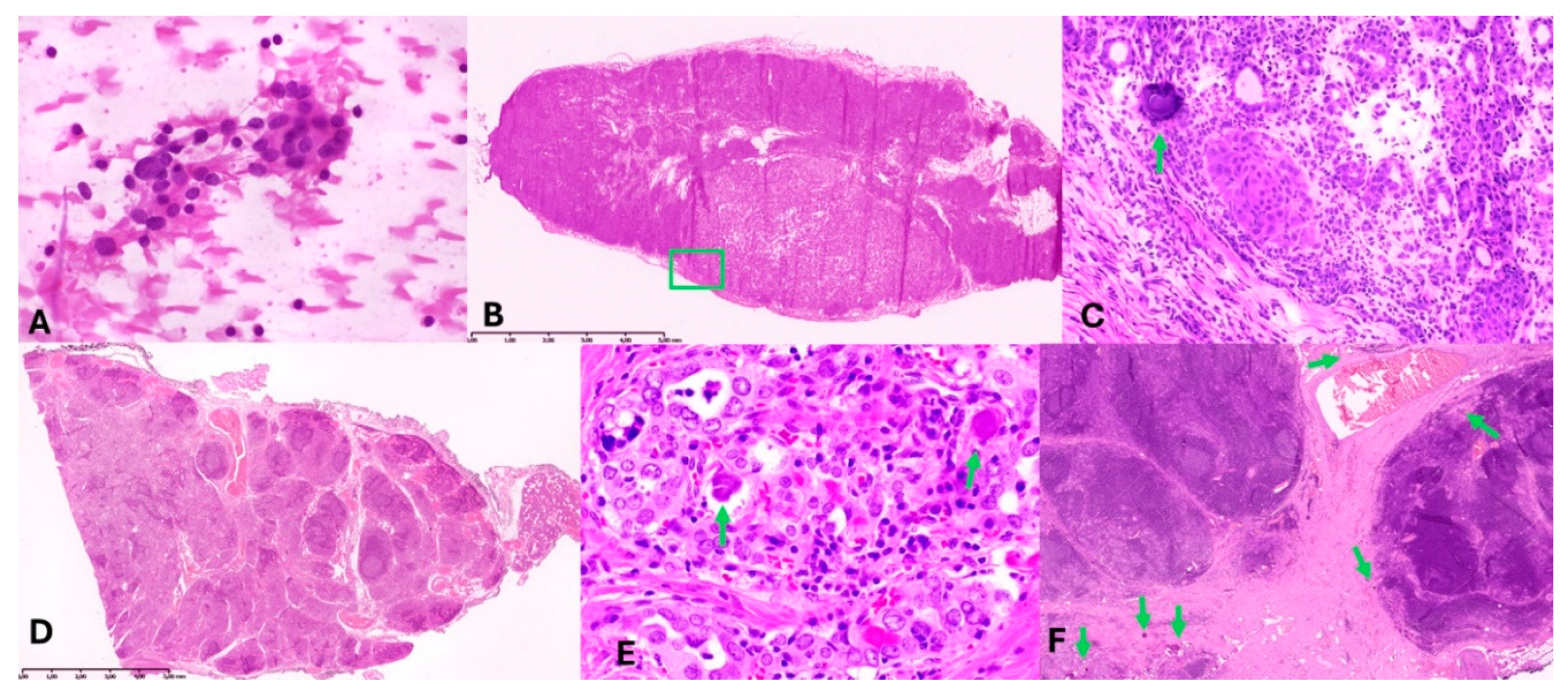
Figure 2.
Morphologic findings. A – slide presenting cytological result from FNAB of the right thyroid lobe; B – intraoperative examination revealing lymph node metastasis; C – enlarged part of B containing psammoma body [green arrow] in metastasis; D – Autoimmune thyroiditis’s-related changes seen in the left lobe of the thyroid gland; E – microfoci of carcinoma cells in the right thyroid lobe; F – fibrotic area with psammoma bodies in thyroid gland [down left], lymph node without metastasis [right], and parathyroid fat tissue [over the vessel].
Figure 2.
Morphologic findings. A – slide presenting cytological result from FNAB of the right thyroid lobe; B – intraoperative examination revealing lymph node metastasis; C – enlarged part of B containing psammoma body [green arrow] in metastasis; D – Autoimmune thyroiditis’s-related changes seen in the left lobe of the thyroid gland; E – microfoci of carcinoma cells in the right thyroid lobe; F – fibrotic area with psammoma bodies in thyroid gland [down left], lymph node without metastasis [right], and parathyroid fat tissue [over the vessel].
Intraoperative and Histopathological Findings
Intraoperative frozen sections of the right lateral lymph nodes confirmed metastatic papillary thyroid carcinoma (PTC) [
Figure 2B and C]. Metastases were identified in 2 of 28 lymph nodes from the right lateral neck [levels II–V], with the largest metastatic deposit measuring 1.1 cm. Psammoma bodies were noted in two additional lymph nodes, while one lymph node contained ectopic thyroid tissue.
Permanent histopathological analysis of the thyroid revealed extensive chronic lymphocytic thyroiditis (autoimmune thyroiditis), characterized by dense lymphocytic infiltration forming lymphoid follicles with germinal centers, prominent oxyphilic (Hürthle cell) metaplasia, and focal fibrosis. Numerous psammoma bodies were identified in the right lobe, predominantly in fibrotic areas adjacent to lymphatic vessels and lymph nodes. Minute foci (0.25–0.5 mm) of atypical follicular epithelial cells were noted, demonstrating nuclear features characteristic of papillary thyroid carcinoma (PTC), including nuclear enlargement, clearing, grooves, and occasional intranuclear inclusions [
Figure 2D–F]. These findings confirmed the diagnosis of PTC, despite the absence of a dominant tumor mass.
Immunohistochemical staining for cytokeratin 19 (CK19) confirmed the presence of PTC in atypical follicular epithelial cells and metastatic lymph nodes. Additionally, in central neck lymph nodes (level VI), psammoma bodies were identified in 3 of 27 nodes without overt metastatic carcinoma. Microscopic foci of PTC metastases were also identified intraoperatively in two lymph nodes adjacent to the left thyroid lobe.
The final pathological diagnosis was multifocal papillary thyroid carcinoma (PTC) with diffuse involvement of the right thyroid lobe, scattered minute foci of carcinoma, and metastatic involvement of central (level VI) and right lateral neck lymph nodes (levels II–V). The diffuse sclerosing variant of PTC (dsvPTC) was identified. The tumor was staged as pT3aN1bM0 according to the AJCC 8th edition staging system [
11].
Postoperative Course
The patient tolerated the surgery well, with no immediate complications. Vocal cord function was intact, and calcium levels remained within normal limits. Prophylactic calcium and vitamin D supplementation were initiated. The patient was discharged in stable condition on suppressive levothyroxine therapy (Euthyrox 150 μg daily).
Four months postoperatively, the patient underwent radioactive iodine (RAI) ablation therapy with 100 mCi of I-131 following recombinant human TSH (rhTSH) stimulation. A post-therapy whole-body scan revealed no abnormal iodine uptake, indicating the absence of residual or metastatic iodine-avid disease. Serum thyroglobulin levels under rhTSH stimulation were undetectable (<0.04 ng/mL), with negative anti-thyroglobulin antibodies, reflecting an excellent biochemical response. Suppressive levothyroxine therapy was continued, targeting a TSH level of 0.1–0.4 μIU/mL.
4. Discussion
Papillary thyroid carcinoma (PTC), originating from the follicular cells of the thyroid gland, affects both adults and children, with a notable female predominance [
12,
13,
14]. In pediatric cases, PTC often presents with distinct clinical and pathological features compared to its adult counterpart. Pediatric PTC is more likely to be multifocal and involve the entire gland. Tumors in children are generally larger at the time of diagnosis and exhibit a higher propensity for extrathyroidal extension, lymph node metastases, and recurrence [
15,
16,
17]. Tumour size and metastatic patterns differ among pediatric age groups; children under 14 years are more likely to present with larger tumors and central cervical lymph node involvement [
16]. Despite these aggressive features, pediatric PTC has an excellent prognosis, with 5-year survival rates of approximately 98% in children aged 0–14 years and 99% in adolescents aged 15–19 years [
13]. Children have a longer life expectancy than adults, making it essential to carefully consider the long-term consequences of thyroid cancer treatment in pediatric patients. Radioiodine (RAI) therapy carries a risk of complications, including transient reductions in male fertility, bone marrow suppression, dysfunction of the salivary and lacrimal glands (the most common adverse effects), and an increased risk of secondary cancers [
1]. Therefore, earlier detection of thyroid cancer offers hope for avoiding the need for RAI or other adjuvant therapies, thereby reducing the potential for long-term complications.
Currently, at our center, we utilize the 2023 Bethesda categories for thyroid cytology, which are defined as follows: I – Nondiagnostic (ND); II – Benign (B); III – Atypia of Undetermined Significance (AUS; previously included FLUS, Follicular Lesion of Undetermined Significance); IV – Follicular Neoplasm (FN; formerly included SFN, Suspicion of Follicular Neoplasm); V – Suspicious for Malignancy (SM) and VI – Malignant (M) [
18]. The risk of malignancy (ROM) in thyroid nodules is notably higher in children compared to adults, with rates of 29.6% for Bethesda category III, 42.3% for category IV, and 90.8% for category V [
1]. Among cytological diagnoses classified as Bethesda categories III and IV, thyroid cancer is postoperatively diagnosed in children at significantly higher rates than in adults. Consequently, for children with indeterminate cytological findings in these categories, surgical treatment should be strongly considered. As outlined in the 2024 Polish Guidelines on Papillary Thyroid Carcinoma (PTC) in children, the recommended surgical approach involves removal of the affected lobe (lobectomy) along with the isthmus, rather than pursuing a repeat fine-needle aspiration biopsy (FNAB) [
1]. Kujdowicz et al. demonstrated that the sensitivity of fine-needle aspiration biopsy (FNAB) for detecting non-benign neoplasms across Bethesda categories III through VI was approximately 86% in both AIT and non-AIT patients [
4]. However, for papillary thyroid carcinoma (PTC), sensitivity in Bethesda categories V and VI decreased significantly in AIT patients, from 86% in non-AIT patients to 61.5% in those with AIT [
4]. These findings highlight the need for heightened consideration of surgical intervention in pediatric patients with Bethesda III–VI cytology, particularly in the context of concurrent AIT, where diagnostic sensitivity is reduced.
Intraoperative examination can be valuable in determining the extent of neck lymph node surgery and distinguishing between parathyroid tissue and lymph nodes. However, routine intraoperative examination is not recommended for diagnosing thyroid nodules, particularly follicular nodules or small-sized tumors[
1]. Nevertheless, its use may be warranted for evaluating suspicious lymph nodes, as illustrated in this report.
The rate of malignancy among pediatric patients with autoimmune thyroiditis is reported to range from 0.67% to 3%, significantly higher than the background risk of approximately 0.02% in the general pediatric population [
14]. The coexistence of autoimmune thyroiditis (AIT) is more commonly observed in pediatric patients diagnosed with thyroid cancer, particularly papillary thyroid carcinoma (PTC), with rates reaching up to 50% of operated cases [
14,
19].
Studies highlight notable differences between PTC in children [≤15 years] and adolescents [>15 years], particularly in clinical presentation and preoperative ultrasound [USG] features [
20,
21]. PTC in younger children is generally more aggressive, with a higher likelihood of regional lymph node metastases, extrathyroidal invasion, and pulmonary metastases. These cases are also associated with higher pathological tumor-node-metastasis [pTNM] staging and a greater prevalence of microcalcifications within nodules, as seen on ultrasound [
21]. Pediatric thyroid cancer should be suspected in the presence of a solid, rapidly growing thyroid nodule with calcifications. Additionally, cervical lymph node metastases should be strongly considered in patients with a PTC diagnosis [
20].
A study comparing the USG features of pediatric PTC patients with and without AIT found that most patients displayed typical features of PTC, including solid or predominantly solid nodules, marked hypoechogenicity, irregular shape and margins, punctate echogenic foci, and lateral neck lymph node metastases. Punctate echogenic foci were significantly more frequent in PTC patients with AIT compared to those without AIT [
21].
In children, thyroid cancer typically presents as an asymptomatic neck mass, sometimes accompanied by cervical lymphadenopathy [
14]. Imaging is recommended for children at high risk of thyroid cancer, as well as in cases where palpable nodules, thyroid gland asymmetry, or cervical lymphadenopathy are detected during physical examination [
5,
20]. Typically ultrasonographic features suggestive of PTC include irregular shape and margins, solid nodules, hypoechogenicity, punctate echogenic foci (microcalcifications), and lymph node metastasis. PTC is also characterized by a wider-than-tall shape and predominantly intranodular vascularity, as opposed to peripheral vascularity [
9,
21,
22].
Microscopically, PTC commonly displays fibrovascular papillae surrounded by one or more layers of cells with oval, crowded nuclei described as clear, empty, or "Orphan Annie-eyed" nuclei [
23,
24]. A similar nuclear appearance can be observed in AIT and other autoimmune thyroiditis. Psammoma bodies, which represent calcified remnants of necrotic papillae, are typically located within the tumor stroma or lymphatic vessels at the cores of papillae [
24]. Several histologic subtypes of PTC have been identified apart from classic variant, including the "high-risk" tall cell variant, follicular variant, solid/trabecular variant as well as diffuse sclerosing variant (dsvPTC) [
14,
23]. The diffuse sclerosing variant (dsvPTC) is distinguished by widespread involvement of one or both thyroid lobes, prominent squamous metaplasia, a high density of psammoma bodies, significant interstitial fibrosis, and marked lymphocytic infiltration with germinal center formation [
23]. In pediatric PTC, histologic features differ from those in adults, including a higher prevalence of psammoma bodies and rounded, non-overlapping nuclei [
14]. The dsvPTC subtype may not form a distinct gross tumor but presents with diffuse glandular enlargement, squamous metaplasia, and psammoma bodies, with increased vascularity and microcalcifications frequently observed in pediatric cases [
21,
25,
26,
27]. In some cases, the presence of microcalcifications on thyroid imaging may be the only distinguishing feature between autoimmune thyroiditis (AIT) and papillary thyroid carcinoma (PTC), as observed in the present case and previous reports [
25,
26,
27,
28,
29,
30,
31]. The similar echostructure of dsvPTC and benign conditions, such as AIT, likely contributes to the delayed diagnosis [
30,
31].
The ultrasonographic characteristics of thyroid cancer in patients with AIT remain poorly documented [
1,
4,
9,
28]. However, cases like this highlight the critical importance of performing detailed ultrasound evaluations, including thorough examination of cervical lymph nodes, to identify potential abnormalities and improve diagnostic accuracy.
The pathogenesis linking autoimmune thyroiditis (AIT) and thyroid cancer remains poorly understood. One hypothesis suggests that AIT, as a chronic inflammatory condition, induces structural damage to the thyroid gland, creating a microenvironment conducive to carcinogenesis. Another hypothesis posits that organ-specific regulatory T-cell dysfunction in AIT patients impairs local immune surveillance, facilitating thyroid cancer development [
19]. Molecular studies have implicated genes such as p53, BCL-2, and RET in thyroid cancer pathogenesis, offering further insights into its development [
29]. AIT is often characterized by dense lymphocytic infiltration and lymphoid follicle formation, where chronic antigenic stimulation may drive neoplastic hyperplasia of thyroid follicles, ultimately leading to malignant transformation. Additionally, high iodine intake, which increases AIT prevalence, may exacerbate thyroid epithelial cell damage and immune dysregulation, further promoting carcinogenesis [
29].
In the present case, the patient exhibited persistently elevated thyroid-stimulating hormone (TSH) levels despite levothyroxine therapy, likely due to an insufficient dosage adjustment by the managing physician. This was accompanied by progressively rising levels of anti-thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-Tg) antibodies. Interestingly, Min et al. identified a significant association between elevated serum thyroglobulin antibody (TgAb) levels and central lymph node metastasis (LNM) in patients with PTC and AIT, suggesting TgAb as a potential biomarker for LNM in this population (32). Furthermore, Min et al. demonstrated in adults that a TgAb/TPOAb ratio exceeding 2 was strongly correlated with more extensive disease (32). Similarly, Xu et al. reported that larger PTC lesions and LNM were significantly associated with elevated TgAb levels, reinforcing its value as a biomarker for disease severity [
33]. Given the limited data in pediatric series, multicenter research is crucial to validate these findings and better understand their clinical implications in children [
28].