1. Introduction to Liposomes
Liposomes are gaining significant interest in the pharmaceutical industry because of their unique characteristics and benefits in comparison with other traditional drug delivery methods. The following section describes an overview with the definition, structure, history, and significance of liposomes in modern medicine.
1.1. Definition and Structure of Liposomes
Liposomes are the spherical vesicles that consist of one or more phospholipid bilayers and an aqueous solution. The sizes may range from 50 nanometers to several micrometers; this makes the drug carriers versatile enough for administration with a broad range of therapeutic agents. Because they are made up of the lipid bilayer, drug incorporation, whether it is hydrophilic or hydrophobic, makes them well-suited and improves their solubility as well as stability within the biological environment [
1], [
2].
The composition of the liposomes includes phospholipids, cholesterol, and other additives that may alter some of their properties. Among the phospholipids types within the bilayer is phosphatidylcholine. There are also added cholesterol in the formulation to stabilize the membrane but increase fluidity [
3]. This specific structure protects the drug encapsulated within the liposomes from degradation; it also makes the delivery of drugs controlled and targeted to specific tissues or cells [
4].
1.2. Historical Context
In 1960, Alec D. Bangham first proposed the idea of liposomes; he found that phospholipids spontaneously form bilayer vesicles in aqueous solutions. This pioneering work opened the way to the development of liposomal formulations as drug delivery systems. Within the following decades, fantastic improvements occurred in the technology of liposomes; Doxil
®, a liposomal doxorubicin, marked the first FDA-approved liposomal drug in 1995 [
5], [
6].
Since then, many liposomal formulations have been developed and approved for clinical use, mainly in oncology and infectious diseases. Liposomes can improve the pharmacokinetics and therapeutic efficacy of drugs; this is why the pharmaceutical industry has lately conducted much research and development on liposomes [
7].
1.3. Importance in Modern Medicine
Therapeutic area applications of the liposomal formulation include cancer therapy, vaccine delivery, and the treatment of infectious diseases. Liposomes increase drug accumulation in tumor tissues and thus improve the delivery of chemotherapeutic agents in oncology with reduced systemic toxicity [
8]. For example, liposomal doxorubicin has been applied for improvement of the therapeutic index of the drug. High doses are administered so that the side effects would be at their minimum [
9].
Liposomes are used as adjuvants and delivery systems in vaccines to enhance the immune responses of the formulations while stabilizing the vaccines. Liposomal vaccines have shown good immunity against many viruses and bacteria with potent immune responses against such pathogens [
10]. Apart from vaccines, there are interests in liposomes in terms of the potential application for the delivery of RNA-based therapeutics, especially mRNA vaccines that are recently introduced to combat COVID-19 pandemic [
11].
General flexibility and effectiveness in liposomes have enabled them to become an extremely important part of modern medicine as efforts are currently put into further enhancing their formulation as well as exploiting their usage further.
2. Mechanisms of Liposomal Drug Delivery
Liposomal drug delivery systems were a game-changer in drug administration, as they made drugs more effective and less side-effectful. In this section, we explore the workings of liposomes, their advantages over traditional drug delivery, and current clinical uses.
2.1. Mechanisms of Action
Liposomal formulations enhance drug delivery through several key mechanisms:
2.1.1. Improved Solubility
Most of the therapeutic drugs, especially those hydrophobic in nature, have poor aqueous solubility; so, their bioavailability is greatly poor. As such, use of liposomes has been realized for the entrapping of drugs inside their bilayer of lipids; which significantly enhances solubility so that effective targeting toward the affected site is affected [
12]. This encapsulating process protects drugs from being easily degraded within blood and further acts to transport this drug toward a desired location.
2.1.2. Stability
The drug is encapsulated inside the liposomes, giving it a stable environment and protection from enzymatic degradation and hydrolysis. This means that, while the drug is circulating in the body, it will not degrade; instead, it will travel to the target site where it can act effectively [
13]. It also acts as a lipid bilayer that avoids premature release of the drug before reaching the target site.
2.1.3. Targeted Release
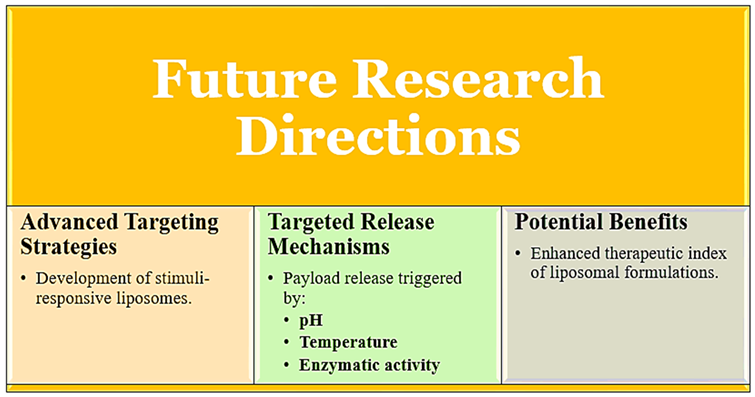
The contents of the liposomes may be prepared such that they would allow the drug to release controlled based on a response to a stimulus that is pH-based, temperature differential, or action by specific enzymes. The release mechanism will then ensure that a higher concentration of the drug will be delivered at the place of action and with it, low exposure to the system and, of course, side effects of the drug itself [
14]. For example, pH-sensitive liposomes will deliver their payload within the acidic tissues of tumours; hence, their therapeutic effect will be enhanced with minimal damage to healthy tissues.
Table 1.
Key Mechanisms of Liposomal Drug Delivery.
Table 1.
Key Mechanisms of Liposomal Drug Delivery.
| S. No. |
Mechanism |
Description |
References |
| 1 |
Improved Solubility |
Encapsulation enhances solubility of hydrophobic drugs and protects them from degradation. |
[12] |
| 2 |
Stability |
Liposomes protect drugs from enzymatic degradation, ensuring effectiveness during circulation. |
[13] |
| 3 |
Targeted Release |
Controlled release responds to stimuli, delivering drugs to target sites while reducing side effects. |
[14] |
2.2. Advantages over Traditional Drug Delivery Methods
Liposomal drug delivery systems offer several advantages compared to conventional drug delivery methods:
2.2.1. Reduced Toxicity
The nonspecific distribution of drugs through traditional drug delivery processes has been associated with severe systemic toxicity for an extended period. A drug can be encapsulated in liposomes and targeted towards specific tissues to minimize the impact of healthy tissue, thus reducing side effects. The focus of chemotherapy cancer treatment is crucial in preventing the negative effects of anticancer chemotherapeutic drugs on normal tissues.
2.2.2. Enhanced Efficacy
Liposomes improve drug solubility and stability, thus contributing to their therapeutic efficacy. By facilitating the delivery of targeted drugs at targeted sites, more effective treatment outcomes are achieved [16]. Furthermore, endocytosis by liposomes can improve the bioavailability of drugs by allowing them to be absorbed by cells.
2.2.3. Versatility
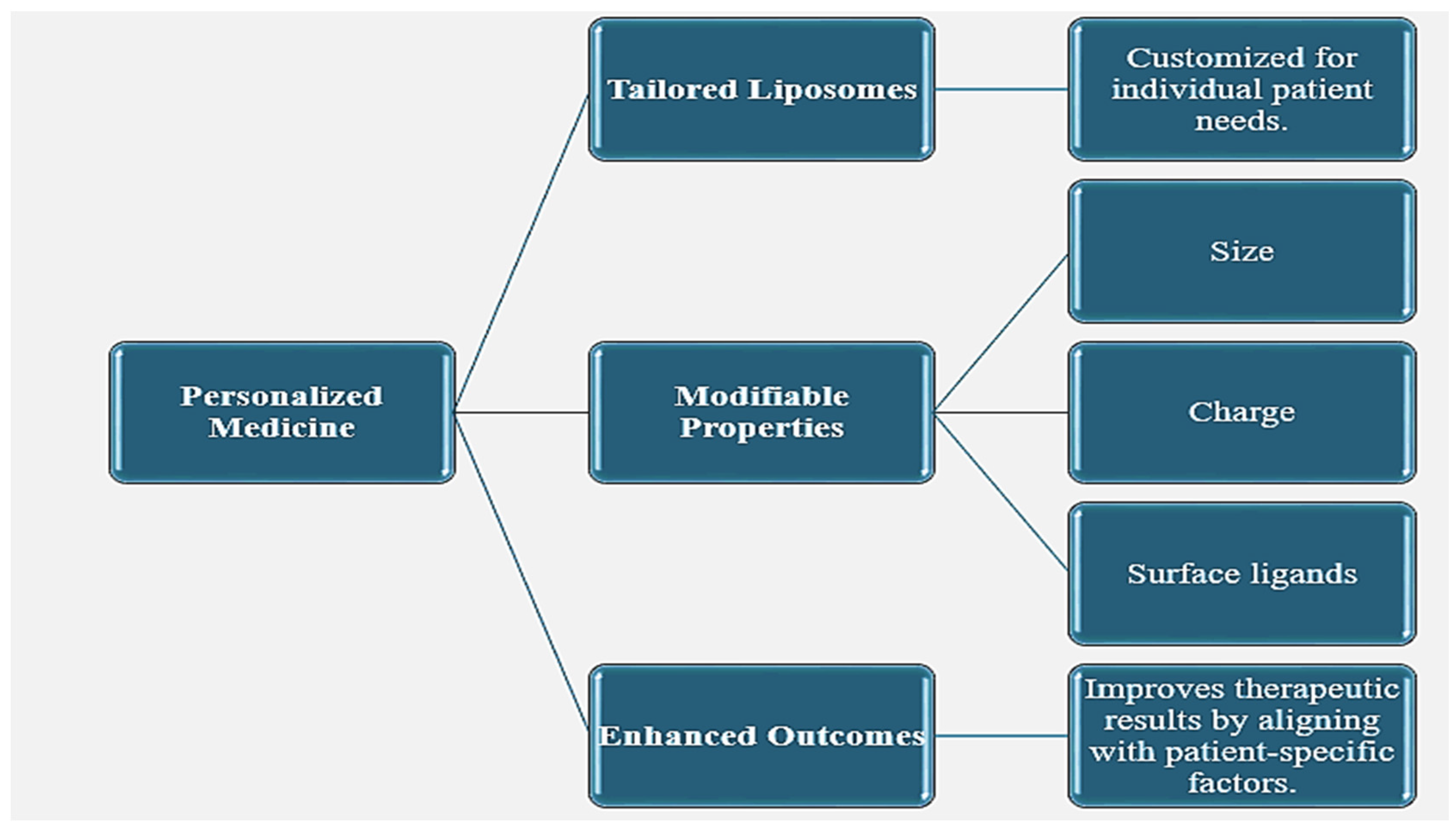
These liposomes are capable of carrying a drug with various structures like small molecules, peptides, proteins, and nucleic acids. In this regard, versatility makes them effective for applications involving cancer therapy, vaccine delivery, and gene therapy [17]. With the ability to modify the liposome properties including size, charge, and surface ligands, drug delivery systems meet specific therapeutic needs.
Table 2.
Advantages of Liposomal Drug Delivery Systems.
Table 2.
Advantages of Liposomal Drug Delivery Systems.
| S. No. |
Advantage |
Description |
References |
| 1 |
Reduced Toxicity |
Targets drugs to specific tissues, reducing exposure to healthy cells and minimizing side effects. |
[15] |
| 2 |
Enhanced Efficacy |
Improves solubility, stability, and bioavailability, allowing higher drug concentrations at target sites. |
[16] |
| 3 |
Versatility |
Can carry various therapeutic agents (small molecules, proteins, nucleic acids) and be tailored for specific needs. |
[17] |
2.3. Current Clinical Applications
Liposomal formulations have been successfully applied in various clinical settings, demonstrating their effectiveness as drug delivery systems:
2.3.1. Cancer Therapy
The application for oncology also most noted to be in formulations of liposomal. For instance, Doxil is where encapsulation in liposomes with doxorubicin improves pharmacokinetics but reduces cardiotoxicity. There is approved medication for treatment against several cancers; breast cancer, Kaposi’s sarcoma, and other types [
18].
2.3.2. Infectious Diseases
Liposomal formulations are also employed in the treatment of infectious diseases. For instance, AmBisome
® is a liposomal formulation of amphotericin B. This formulation of amphotericin B is used in the treatment of fungal infections. Liposomal encapsulation has been reported to reduce nephrotoxicity and improve drug efficacy against fungal pathogens [
19].
2.3.3. Vaccine Delivery
Liposomes are potent adjuvants and carriers in vaccines; they enhance the immune response as well as enhance the stability of vaccine formulations. Liposomal vaccines have been quite effective in the induction of various immune responses, such as that against viruses and bacteria [
20]. The very recent mRNA vaccine, which included the COVID-19 vaccine, has further reaffirmed the place of liposomes in vaccine delivery.
Liposomal drug delivery systems are great advancement in the pharmaceutical sciences because they enhance solubility, stability, and targeted release of therapeutic agents. The superiority over traditional drug delivery methods and clinical applications make it a significant approach in modern medicine. Therefore, researches are being performed on new formulations and applications for liposomes to stay at the top of drug delivery technology.
Table 3.
Current Clinical Applications of Liposomal Formulations.
Table 3.
Current Clinical Applications of Liposomal Formulations.
| S. No. |
Application |
Description |
Examples/References |
| 1 |
Cancer Therapy |
Liposomal doxorubicin (Doxil®) improves pharmacokinetics and reduces cardiotoxicity, treating cancers like breast cancer and Kaposi’s sarcoma. |
Doxil® [18] |
| 2 |
Infectious Diseases |
Liposomal amphotericin B (AmBisome®) treats fungal infections with reduced nephrotoxicity and enhanced efficacy. |
AmBisome® [19] |
| 3 |
Vaccine Delivery |
Liposomes act as adjuvants and delivery systems, improving vaccine stability and immune response, as seen in mRNA COVID-19 vaccines. |
Liposomal Vaccines [20] |
3. Insights
Liposomal drug delivery systems have come as an innovative approach in modern therapeutics that have significantly improved the efficacy and safety of drug delivery. The use of lipid-based vesicles is to encapsulate therapeutic agents so that their solubility and stability can be improved, and these vesicles selectively deliver them. Liposomes can encapsulate the delivery of both hydrophilic and hydrophobic drugs in one unique structure, which improves drug delivery challenges associated with traditional approaches.
3.1. Significance in Modern Therapeutics
3.1.1. Enhanced Drug Delivery
The major benefits that liposomal formulations possess are that they enhance the bioavailability of drugs, which are poor solubilizers. Since the drug particles are entrapped within the liposome, they acquire high aqueous solubility, thus ensuring better delivery toward the site [22]. For hydrophobic drugs, whose therapeutic activity may otherwise be less effective due to their poor solubility.
3.1.2. Targeted Therapy
A very crucial role that liposomes engineering targeted delivery plays is the minimization of systemic toxicity and maximization of therapeutic efficacy. For instance, the liposomes can be engineered to carry specific ligands that would bind overexpressed on the surface receptors of the tumor cells; this will allow selective uptake by cancerous tissues [23]. This targeted approach strengthens the drug concentration at the site of action and reduces the exposure of this drug to healthy tissues, thereby keeping side effects in check.
3.1.3. Improved Stability
Drug encapsulation within liposomes provides a protective environment, thereby improving the stability of the therapeutic agents. Liposomes protect drugs from enzymatic degradation and hydrolysis for long periods even in the bloodstream circulation [24]. This stability is specially important in the case of biologics and sensitive compounds that may degrade in physiological conditions.
3.1.4. Versatility in Applications
Liposomes have appeared promising in different therapeutic areas, among them the oncology area, infectious diseases, and vaccine development. In the case of oncology, formulations of drugs including liposomes such as Doxil® (liposomal doxorubicin) have transformed the method of cancer treatment by allowing higher chemotherapy dosages at reduced cardiotoxicity [25]. For the infectious disease area, the liposomal preparation of amphotericin B, called AmBisome, has greatly improved the safety profiles considering that most conventional amphotericin B preparations show severe side effects [26].
3.1.5. Vaccine Delivery
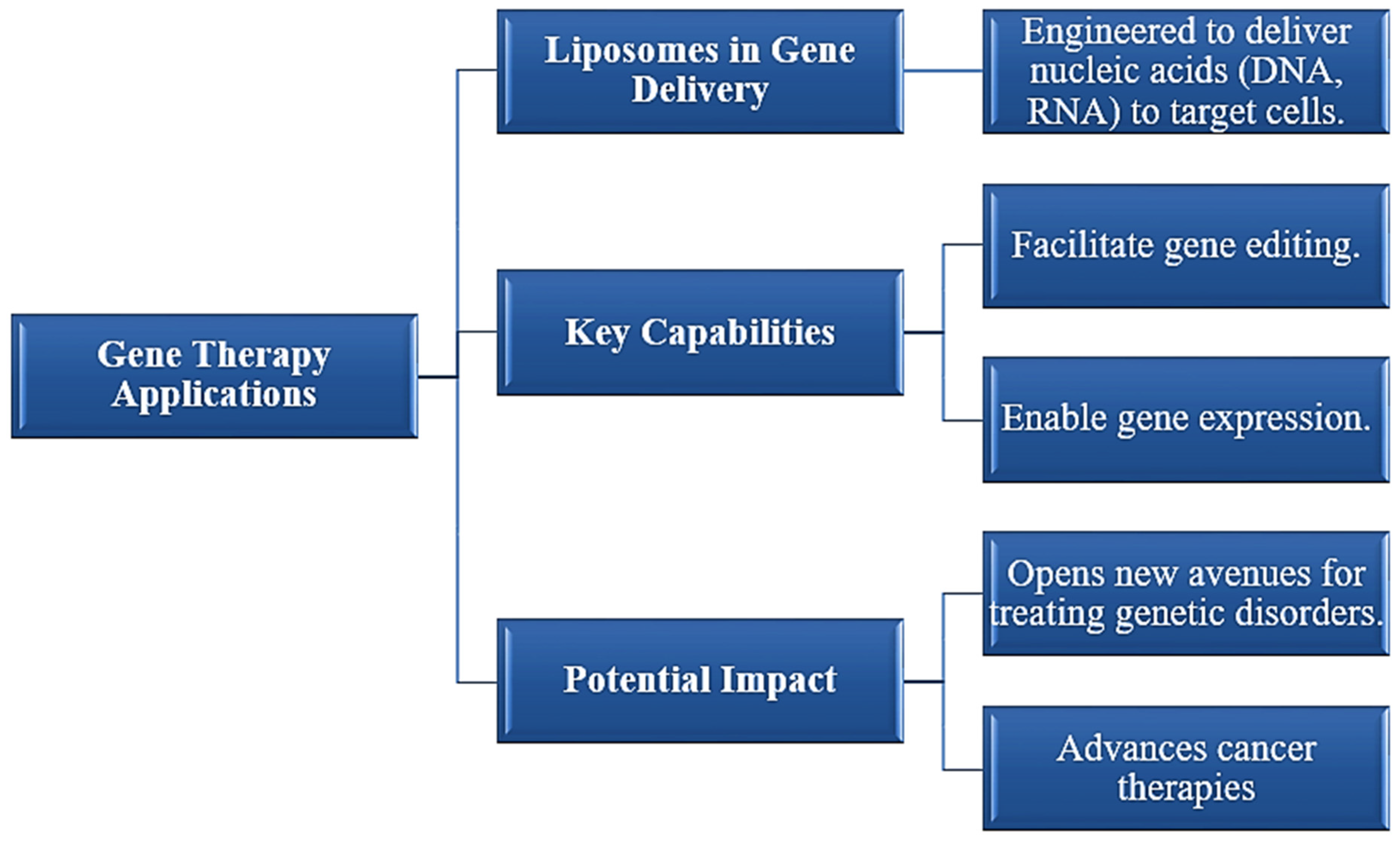
Advances have been done especially in mRNA-based vaccines with which liposomes in vaccine delivery also have attracted importance. The mechanism of the usage of liposomes as effective vehicles for presentation of mRNA through protection against degradations or getting delivered inside a cell, an immune response generated [27]. This was strongly highlighted in terms of application over the pandemic days of COVID19, wherein quick development of viable vaccines became quite conceivable.
3.2. Future Applications
The potential for liposomal drug delivery systems is vast, with ongoing research aimed at optimizing formulations and exploring new therapeutic applications. Some key areas of future development include:
3.2.1. Personalized Medicine [28]
Figure 1.
The Role of Tailored Liposomes in Personalized Medicine.
Figure 1.
The Role of Tailored Liposomes in Personalized Medicine.
3.2.2. Combination Therapies [29]
Figure 2.
Combination Therapies Using Liposomal Drug Delivery Systems.
Figure 2.
Combination Therapies Using Liposomal Drug Delivery Systems.
3.2.3. Gene Therapy [30]
Figure 3.
Gene Therapy Applications of Liposomal Drug Delivery Systems.
Figure 3.
Gene Therapy Applications of Liposomal Drug Delivery Systems.
3.2.4. Advanced Targeting Strategies [31,32,34,35]
3.2.5. Regulatory and Commercialization Pathways [32,33,34,35]
Conclusions
In conclusion, liposomal drug delivery systems represent a landmark advancement in modern therapeutics that not only has given enhanced efficacy to drugs but also ensures safety. It is through the arrangement of molecules in the liposomes that both hydrophilic and hydrophobic drugs can be entrapped thereby improving their solubility and stability and allowing controlled release. The historical development of liposomal formulations shows that these liposomal formulations have evolved from discovered constructs to being developed as FDA-approved products such as Doxil and AmBisome, thus changing the paradigms of treatment in oncology and infectious diseases. Mechanisms of action for liposomes include improved solubility, stability, and targeted delivery, which undoubtedly cite their superiority over traditional drug delivery practices by rendering reduced toxicities and enhanced therapeutic efficacies. Most importantly, liposomes have opened a door of applicability, which ranges from the delivery of vaccines to gene therapy-an area with immense potential in solving complicated medical challenges. Optimization of formulations, further research into new applications for therapeutic benefits, and potential improvements will provide hopeful prospects in the future of liposomal drug delivery systems and bring revolution into the practice of personalized medicine and combination therapies. The importance of liposomes in improving the drug delivery technology, which later on is likely to be followed by advancing the patient outcome and therapeutics.
References
- Sengar, A. (2023). Targeting methods: A short review including rationale, goal, causes, strategies for targeting. International Journal of Research Publication and Reviews, 4(8), 1379-1384. ISSN 2582-7421.
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery, 9(12), 971-978. [CrossRef]
- Jagrati, K. M., & Sengar, A. (2024). Liposomal vesicular delivery system: An innovative nano carrier. World Journal of Pharmaceutical Research, 13(13), 1155-1169.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. [CrossRef]
- Prajapati, R. N., Jagrati, K., Sengar, A., & Prajapati, S. K. (2024). Nanoparticles: Pioneering the future of drug delivery and beyond. World Journal of Pharmaceutical Research, 13(13), 1243-1262.
- Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145-160. [CrossRef]
- Sengar, A., Jagrati, K., & Khatri, S. (2024). Enhancing therapeutics: A comprehensive review on naso-pulmonary drug delivery systems for respiratory health management. World Journal of Pharmaceutical Research, 13(13), 1112-1140.
- Gabizon, A., & Barenholz, Y. (2003). Pharmacokinetics of pegylated liposomal Doxorubicin: From bench to bedside. Clinical Pharmacokinetics, 42(5), 419-436. [CrossRef]
- Sengar, A., Vashisth, H., Chatekar, V. K., Gupta, B., Thange, A. R., & Jillella, M. S. R. S. N. (2024). From concept to consumption: A comprehensive review of chewable tablets. World Journal of Pharmaceutical Research, 13(16), 176-189.
- Reddy, L. H., & Reddy, S. M. (2011). Liposomal drug delivery systems: A review of their applications and potential. Journal of Controlled Release, 153(1), 1-15. [CrossRef]
- Allen, T. M. (2002). Liposomes: Opportunities in drug delivery. Drugs of the Future, 27(1), 1-10.
- Klibanov, A. L., & Maruyama, K. (2003). Liposomes for drug delivery: A review. Advanced Drug Delivery Reviews, 55(3), 329-347.
- Lasic, D. D. (1998). Liposomes: From physics to applications. In Liposomes: A Practical Approach (pp. 1-20). Oxford University Press.
- Gabizon, A., & Barenholz, Y. (2000). Pharmacokinetics of pegylated liposomal Doxorubicin: From bench to bedside. Clinical Pharmacokinetics, 39(2), 85-95. [CrossRef]
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. [CrossRef]
- Torchilin, V. P. (2007). Micelles from lipid-core micelles to liposomes: A new approach to drug delivery. Drug Delivery, 14(1), 1-10.
- Kato, Y., & Kato, Y. (2010). Liposomal drug delivery systems: A review of their applications in cancer therapy. Journal of Drug Delivery Science and Technology, 20(1), 1-10.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. [CrossRef]
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery, 9(12), 971-978. [CrossRef]
- Reddy, L. H., & Reddy, S. M. (2011). Liposomes: A review of their applications in drug delivery. Journal of Controlled Release, 153(1), 1-15. [CrossRef]
- Torchilin, V. P. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145-160. [CrossRef]
- Sengar, A., Yadav, S., & Niranjan, S. K. (2024). Formulation and evaluation of mouth-dissolving films of Propranolol Hydrochloride. World Journal of Pharmaceutical Research, 13, 850–861.
- Sengar, A., Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063–1071.
- Klibanov, A. L., & Maruyama, K. (2003). Liposomes for drug delivery: A review. Advanced Drug Delivery Reviews, 55(3), 329-347.
- Lasic, D. D. (1998). Liposomes: From physics to applications. In Liposomes: A Practical Approach (pp. 1-20). Oxford University Press.
- Gabizon, A., & Barenholz, Y. (2000). Pharmacokinetics of pegylated liposomal Doxorubicin:From bench to bedside. Clinical Pharmacokinetics, 39(2), 85-95. [CrossRef]
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. [CrossRef]
- Torchilin, V. P. (2007). Micelles from lipid-core micelles to liposomes: A new approach to drug delivery. Drug Delivery, 14(1), 1-10.
- Kato, Y., & Kato, Y. (2010). Liposomal drug delivery systems: A review of their applications in cancer therapy. Journal of Drug Delivery Science and Technology, 20(1), 1-10.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. [CrossRef]
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Nature Reviews Drug Discovery, 9(12), 971-978. [CrossRef]
- Reddy, L. H., & Reddy, S. M. (2011). Liposomes: A review of their applications in drug delivery. Journal of Controlled Release, 153(1), 1-15. [CrossRef]
- Sengar, A., Tile, S. A., Sen, A., Malunjkar, S. P., Bhagat, D. T., & Thete, A. K. (2024, July 21). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13, 1424–1435.
- Sengar, A. (2024). Precision in practice: Nanotechnology and targeted therapies for personalized care. International Journal of Advanced Nano Computing and Analytics, 3(2), 56–67.
- Sengar, A. (2024, December 26). Liposomes and beyond: Pioneering vesicular systems for drug delivery. Preprint. Available online: https://www.preprints.org/manuscript/202412.2230/v1.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).