Submitted:
05 January 2025
Posted:
06 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
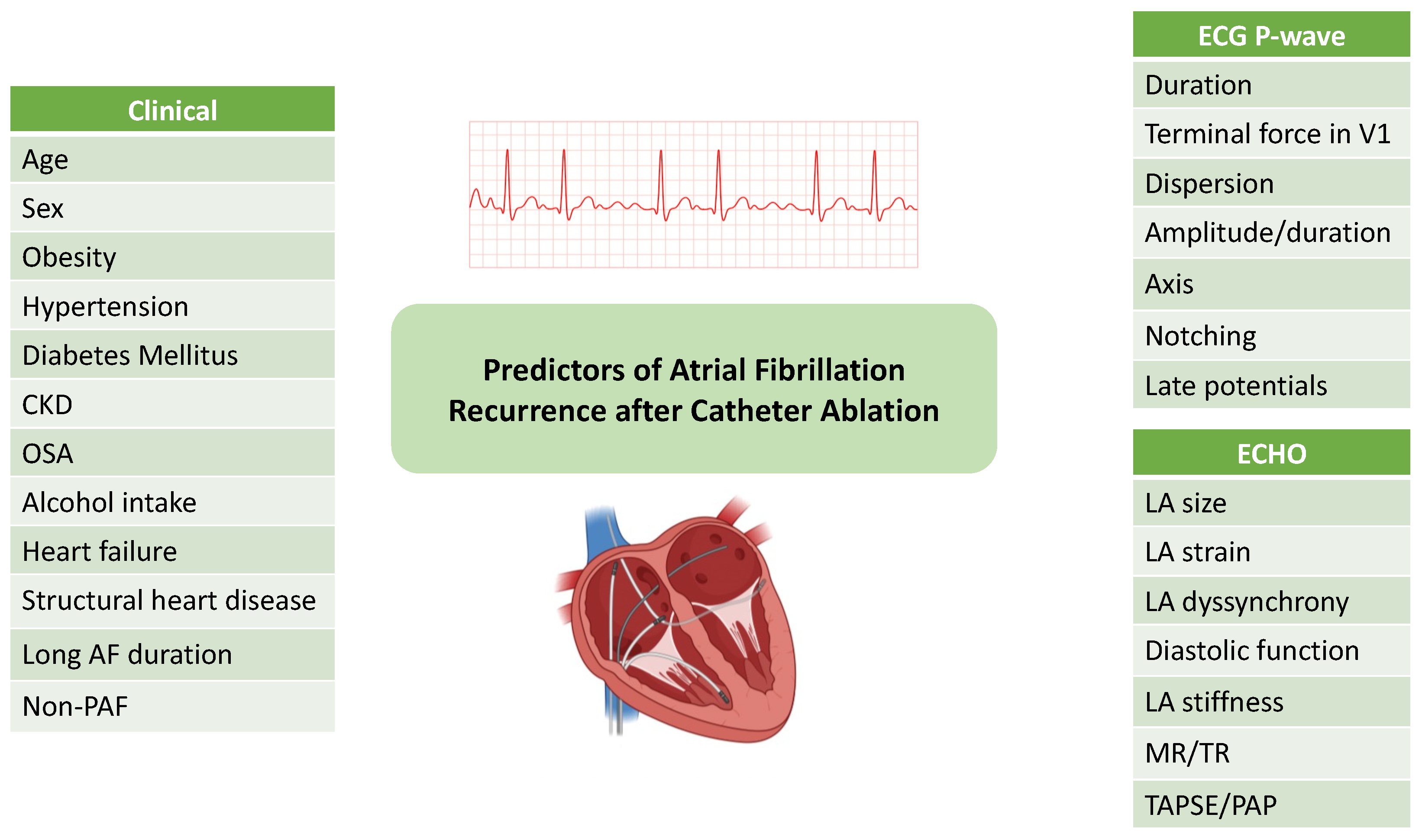
2. AF Recurrence After Catheter Ablation
3. Predictors of AF Recurrence After Catheter Ablation
3.1. Clinical Predictors
3.2. Electrocardiographic Predictors
3.3. Echocardiographic Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Gelder IC, Rienstra M, Bunting K V, et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the task force for the management of atrial fibrillation of the European Society of Ca. Eur Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen J, Ruwald MH, Pallisgaard J, et al. Lower Recurrence Rates of Atrial Fibrillation and MACE Events After Early Compared to Late Ablation: A Danish Nationwide Register Study. J Am Heart Assoc. 2024, 13, e032722. [Google Scholar] [CrossRef] [PubMed]
- Poole JE, Bahnson TD, Monahan KH, et al. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol. 2020, 75, 3105–3118. [Google Scholar] [CrossRef] [PubMed]
- Tzeis S, Gerstenfeld EP, Kalman J, et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. EP Eur. 2024, 26, euae043. [Google Scholar] [CrossRef]
- Lévy S, Steinbeck G, Santini L, et al. Management of atrial fibrillation: two decades of progress — a scientific statement from the European Cardiac Arrhythmia Society. J Interv Card Electrophysiol. 2022, 65, 287–326. [Google Scholar] [CrossRef]
- Pallisgaard JL, Gislason GH, Hansen J, et al. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J. 2018, 39, 442–449. [Google Scholar] [CrossRef]
- Chew DS, Black-Maier E, Loring Z, et al. Diagnosis-to-Ablation Time and Recurrence of Atrial Fibrillation Following Catheter Ablation: A Systematic Review and Meta-Analysis of Observational Studies. Circ Arrhythm Electrophysiol. 2020, 13, e008128. [Google Scholar] [CrossRef]
- El-Harasis MA, Quintana JA, Martinez-Parachini JR, et al. Recurrence After Atrial Fibrillation Ablation and Investigational Biomarkers of Cardiac Remodeling. J Am Heart Assoc. 2024, 13, e031029. [Google Scholar] [CrossRef]
- Musat DL, Milstein NS, Bhatt A, et al. Incidence and Predictors of Very Late Recurrence of Atrial Fibrillation Following Cryoballoon Pulmonary Vein Isolation. Circ Arrhythmia Electrophysiol. 2020, 13, e008646. [Google Scholar] [CrossRef]
- Schwab AC, Anic A, Farkowski MM, et al. Rhythm monitoring, success definition, recurrence, and anticoagulation after atrial fibrillation ablation: results from an EHRA survey. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2023, 25, 676–681. [CrossRef]
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Hear Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Erhard N, Metzner A, Fink T. Late arrhythmia recurrence after atrial fibrillation ablation: incidence, mechanisms and clinical implications. Herzschrittmachertherapie + Elektrophysiologie. 2022, 33, 71–76. [Google Scholar] [CrossRef]
- Wei Y, Bao Y, Lin C, et al. Early recurrence after cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: mechanism and implication in long-term outcome. BMC Cardiovasc Disord. 2022, 22, 400. [Google Scholar] [CrossRef]
- Noujaim C, Lim C, Mekhael M, et al. Identifying the prognostic significance of early arrhythmia recurrence during the blanking period and the optimal blanking period duration: insights from the DECAAF II study. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2023, 25(6). [CrossRef]
- Davtyan K V, Topchyan AH, Brutyan HA, et al. The predictive role of early recurrences of atrial arrhythmias after pulmonary vein cryoballoon ablation. Is blanking period an outdated concept? Insights from 12-month continuous cardiac monitoring. BMC Cardiovasc Disord. 2021, 21, 483. [Google Scholar] [CrossRef]
- Popa MA, Kottmaier M, Risse E, et al. Early arrhythmia recurrence after catheter ablation for persistent atrial fibrillation: is it predictive for late recurrence? Clin Res Cardiol. 2022, 111, 85–95. [CrossRef]
- Liang JJ, Elafros MA, Chik WW, et al. Early recurrence of atrial arrhythmias following pulmonary vein antral isolation: Timing and frequency of early recurrences predicts long-term ablation success. Hear Rhythm. 2015, 12, 2461–2468. [Google Scholar] [CrossRef]
- Steinberg C, Champagne J, Deyell MW, et al. Prevalence and outcome of early recurrence of atrial tachyarrhythmias in the Cryoballoon vs Irrigated Radiofrequency Catheter Ablation (CIRCA-DOSE) study. Hear Rhythm. 2021, 18, 1463–1470. [Google Scholar] [CrossRef]
- Iqbal SUR, Kueffer T, Knecht S, et al. Impact of shortening the blanking period to 8 weeks after PVI: Insights from COMPARE-CRYO using continuous rhythm monitoring. Hear Rhythm. December, 20 December. [CrossRef]
- Mainigi SK, Sauer WH, Cooper JM, et al. Incidence and predictors of very late recurrence of atrial fibrillation after ablation. J Cardiovasc Electrophysiol. 2007, 18, 69–74. [Google Scholar] [CrossRef]
- Themistoclakis S, Schweikert RA, Saliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Hear Rhythm. 2008, 5, 679–685. [Google Scholar] [CrossRef]
- Mo P, Fan C, Chen J, et al. Atrial Fibrillation Types and Chronic Kidney Disease are Independent Predictors of Atrial Fibrillation Recurrence After Radiofrequency Ablation. Ther Clin Risk Manag. 2024, 20, 817–828. [Google Scholar] [CrossRef]
- Farghaly AAA, Ali H, Lupo P, et al. Early versus Late Radiofrequency Catheter Ablation in Atrial Fibrillation: Timing Matters. J Clin Med. [CrossRef]
- De Greef Y, Bogaerts K, Sofianos D, Buysschaert I. Impact of Diagnosis-to-Ablation Time on AF Recurrence: Pronounced the First 3 Years, Irrelevant Thereafter. JACC Clin Electrophysiol. 2023, 9, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Tabaja C, Younis A, Santangeli P, et al. Catheter ablation of atrial fibrillation in elderly and very elderly patients: safety, outcomes, and quality of life. J Interv Card Electrophysiol. 2024, 67, 1083–1092. [Google Scholar] [CrossRef]
- Kozhuharov N, Karim N, Creta A, et al. Long-term outcomes of catheter ablation for atrial fibrillation in octogenarians. J Interv Card Electrophysiol an Int J Arrhythm pacing. August, 20 August. [CrossRef]
- Li H, Wang Z, Cheng Z, et al. Sex differences involved in persistent atrial fibrillation recurrence after radiofrequency ablation. BMC Cardiovasc Disord. 2022, 22, 549. [Google Scholar] [CrossRef]
- Bukari A, Nayak H, Aziz Z, Deshmukh A, Tung R, Ozcan C. Impact of race and gender on clinical outcomes of catheter ablation in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2017, 40, 1073–1079. [Google Scholar] [CrossRef]
- Li M, Liu T, Luo D, Li G. Systematic review and meta-analysis of chronic kidney disease as predictor of atrial fibrillation recurrence following catheter ablation. Cardiol J. 2014, 21, 89–95. [Google Scholar] [CrossRef]
- Lee W-C, Wu P-J, Fang C-Y, Chen H-C, Chen M-C. Impact of chronic kidney disease on atrial fibrillation recurrence following radiofrequency and cryoballoon ablation: A meta-analysis. Int J Clin Pract. 2021, 75, e14173. [Google Scholar] [CrossRef]
- Yamashita S, Tokuda M, Matsuo S, et al. Comparison of atrial arrhythmia recurrence after persistent atrial fibrillation ablation between patients with or without tachycardia-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2019, 30, 2310–2318. [Google Scholar] [CrossRef]
- Tan NY, Mohsin Y, Hodge DO, et al. Catheter Ablation for Atrial Arrhythmias in Patients With Cardiac Amyloidosis. J Cardiovasc Electrophysiol. 2016, 27, 1167–1173. [Google Scholar] [CrossRef]
- Barbhaiya CR, Kumar S, Baldinger SH, et al. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Hear Rhythm. 2016, 13, 383–390. [Google Scholar] [CrossRef]
- Hiraya D, Sato A, Hoshi T, et al. Impact of coronary artery disease and revascularization on recurrence of atrial fibrillation after catheter ablation: Importance of ischemia in managing atrial fibrillation. J Cardiovasc Electrophysiol. 2019, 30, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Lador A, Maccioni S, Khanna R, Zhang D. Influence of time to ablation on outcomes among patients with atrial fibrillation with pre-existing heart failure. Hear Rhythm O2 2024, 5, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Ando M, Yanagisawa S, Suzuki H, et al. Early Cryoablation After First Diagnosis of Atrial Fibrillation Reduces Arrhythmia Recurrence in Heart Failure Patients. JACC Asia. 2024, 4, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Seewöster T, Kosich F, Sommer P, Bertagnolli L, Hindricks G, Kornej J. Prediction of low-voltage areas using modified APPLE score. EP Eur. 2021, 23, 575–580. [Google Scholar] [CrossRef]
- Xin Y, Hang F, Wu Y. Predictors of Low Voltage Zone and Sex Differences in Low Voltage Zone Distribution in Patients with Atrial Fibrillation. Rev Cardiovasc Med. 2023, 24, 324. [Google Scholar] [CrossRef]
- Ribo T, Jianzeng D, Xiaohui L, et al. [Impact of CHA2DS2 VASc score on substrate for persistent atrial fibrillation and outcome post catheter ablation of atrial fibrillation]. Zhonghua Xin Xue Guan Bing Za Zhi 2015, 43, 695–699. [Google Scholar]
- Kornej J, Hindricks G, Kosiuk J, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014, 7, 281–287. [Google Scholar] [CrossRef]
- Kornej J, Schumacher K, Dinov B, et al. Prediction of electro-anatomical substrate and arrhythmia recurrences using APPLE, DR-FLASH and MB-LATER scores in patients with atrial fibrillation undergoing catheter ablation. Sci Rep. 2018, 8, 12686. [Google Scholar] [CrossRef]
- Sano M, Heeger C-H, Sciacca V, et al. Evaluation of predictive scores for late and very late recurrence after cryoballoon-based ablation of atrial fibrillation. J Interv Card Electrophysiol an Int J Arrhythm pacing. 2021, 61, 321–332. [Google Scholar] [CrossRef]
- Han J, Li G, Zhang D, Wang X, Guo X. Predicting Late Recurrence of Atrial Fibrillation After Radiofrequency Ablation in Patients With Atrial Fibrillation: Comparison of C2HEST and HATCH Scores. Front Cardiovasc Med. 2022, 9, 907817. [Google Scholar] [CrossRef]
- Kosiuk J, Dinov B, Kornej J, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Hear Rhythm. 2015, 12, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Sato T, Sotomi Y, Hikoso S, et al. DR-FLASH Score Is Useful for Identifying Patients With Persistent Atrial Fibrillation Who Require Extensive Catheter Ablation Procedures. J Am Heart Assoc. 2022, 11, e024916. [Google Scholar] [CrossRef] [PubMed]
- Bisbal F, Alarcón F, Ferrero-de-Loma-Osorio A, et al. Left atrial geometry and outcome of atrial fibrillation ablation: results from the multicentre LAGO-AF study. Eur Hear J - Cardiovasc Imaging. 2018, 19, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Kosich F, Schumacher K, Potpara T, Lip GY, Hindricks G, Kornej J. Clinical scores used for the prediction of negative events in patients undergoing catheter ablation for atrial fibrillation. Clin Cardiol. 2019, 42, 320–329. [Google Scholar] [CrossRef]
- Kornej J, Hindricks G, Shoemaker MB, et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015, 104, 871–876. [Google Scholar] [CrossRef]
- Mujović N, Marinković M, Marković N, Shantsila A, Lip GYH, Potpara TS. Prediction of very late arrhythmia recurrence after radiofrequency catheter ablation of atrial fibrillation: The MB-LATER clinical score. Sci Rep. 2017, 7, 40828. [Google Scholar] [CrossRef]
- Akkaya E, Berkowitsch A, Greiss H, et al. PLAAF score as a novel predictor of long-term outcome after second-generation cryoballoon pulmonary vein isolation. EP Eur. 2018, 20, f436–f443. [Google Scholar] [CrossRef]
- Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: Promising results from the Turkish Cryoablation Registry. Int J Cardiol. 2013, 169, 201–206. [Google Scholar] [CrossRef]
- Mesquita J, Ferreira AM, Cavaco D, et al. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure – ATLAS score. EP Eur. 2018, 20, f428–f435. [Google Scholar] [CrossRef]
- Winkle RA, Jarman JWE, Mead RH, et al. Predicting atrial fibrillation ablation outcome: The CAAP-AF score. Hear Rhythm. 2016, 13, 2119–2125. [Google Scholar] [CrossRef]
- Sanhoury M, Moltrasio M, Tundo F, et al. Predictors of arrhythmia recurrence after balloon cryoablation of atrial fibrillation: the value of CAAP-AF risk scoring system. J Interv Card Electrophysiol. 2017, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Peigh G, Kaplan RM, Bavishi A, et al. A novel risk model for very late return of atrial fibrillation beyond 1 year after cryoballoon ablation: the SCALE-CryoAF score. J Interv Card Electrophysiol an Int J Arrhythm pacing. 2020, 58, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Luo Y, Tang Y, Huang W, Xiong S, Long Y, Liu H. Age, creatinine, and ejection fraction (ACEF) score as predictive values for late non-valvular atrial fibrillation recurrence after radiofrequency ablation. Clin Exp Hypertens. 2023, 45, 2207784. [Google Scholar] [CrossRef]
- Chen LY, Ribeiro ALP, Platonov PG, et al. P Wave Parameters and Indices: A Critical Appraisal of Clinical Utility, Challenges, and Future Research—A Consensus Document Endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardio. Circ Arrhythmia Electrophysiol. 2022, 15, e010435. [Google Scholar] [CrossRef]
- Intzes S, Zagoridis K, Symeonidou M, et al. P-wave duration and atrial fibrillation recurrence after catheter ablation: a systematic review and meta-analysis. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2023, 25, 450–459. [Google Scholar] [CrossRef]
- Bayés de Luna A, Escobar-Robledo LA, Aristizabal D, et al. Atypical advanced interatrial blocks: Definition and electrocardiographic recognition. J Electrocardiol. 2018, 51, 1091–1093. [Google Scholar] [CrossRef]
- Pranata R, Yonas E, Vania R. Prolonged P-wave duration in sinus rhythm pre-ablation is associated with atrial fibrillation recurrence after pulmonary vein isolation-A systematic review and meta-analysis. Ann noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiology, Inc. 2019, 24, e12653. [Google Scholar] [CrossRef]
- Koutalas E, Kallergis E, Nedios S, Kochiadakis G, Kanoupakis E. P-wave duration as a marker of atrial remodeling in patients referred to ablation for atrial fibrillation: A new stratification tool emerging? Hell J Cardiol. 2023, 73, 53–60. [Google Scholar] [CrossRef]
- MORRIS JJJ, ESTES EHJ, WHALEN RE, THOMPSON HKJ, MCINTOSH HD. P-WAVE ANALYSIS IN VALVULAR HEART DISEASE. Circulation 1964, 29, 242–252. [Google Scholar] [CrossRef]
- Tiffany Win T, Ambale Venkatesh B, Volpe GJ, et al. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Hear Rhythm. 2015, 12, 155–162. [Google Scholar] [CrossRef]
- Qiu Y, Sun J, Wang Y, et al. Association between P-wave terminal force in lead V(1) and extent of left atrial low-voltage substrate in older patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol an Int J Arrhythm pacing. 2024, 67, 1153–1160. [Google Scholar] [CrossRef]
- Huang Z, Zheng Z, Wu B, et al. Predictive value of P wave terminal force in lead V1 for atrial fibrillation: A meta-analysis. Ann noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiology, Inc. 2020, 25, e12739. [Google Scholar] [CrossRef] [PubMed]
- Sudo Y, Morimoto T, Tsushima R, et al. P-wave terminal force in lead V1 and outcomes in patients with persistent atrial fibrillation undergoing catheter ablation. Am Heart J. 2023, 260, 141–150. [Google Scholar] [CrossRef]
- Wang Z, Wang B, Yang Y, Yang X, Che Y, Xia Y. P-wave terminal force in lead V1 is associated with recurrence after catheter ablation in patients with paroxysmal atrial fibrillation and normal left atrial size. Front Cardiovasc Med. 2024, 11, 1467585. [Google Scholar] [CrossRef]
- Wakabayashi Y, Uesako H, Kobayashi M, Ichikawa T, Koyama T, Abe H. P-wave terminal force is related to left pulmonary vein reconnection in patients with atrial fibrillation recurrence after pulmonary vein isolation. Heart Vessels. September, 20 September. [CrossRef]
- Dilaveris P, Batchvarov V, Gialafos J, Malik M. Comparison of different methods for manual P wave duration measurement in 12-lead electrocardiograms. Pacing Clin Electrophysiol. 1999, 22, 1532–1538. [Google Scholar] [CrossRef]
- Chávez-González E, Donoiu I. Utility of P-Wave Dispersion in the Prediction of Atrial Fibrillation. Curr Heal Sci J. 2017, 43, 5–11. [Google Scholar] [CrossRef]
- Pérez-Riera AR, de Abreu LC, Barbosa-Barros R, Grindler J, Fernandes-Cardoso A, Baranchuk A. P-wave dispersion: an update. Indian Pacing Electrophysiol J. 2016, 16, 126–133. [Google Scholar] [CrossRef]
- Perez M V, Dewey FE, Marcus R, et al. Electrocardiographic predictors of atrial fibrillation. Am Heart J. 2009, 158, 622–628. [Google Scholar] [CrossRef]
- Liu P, Lv T, Yang Y, Gao Q, Zhang P. Use of P wave indices to evaluate efficacy of catheter ablation and atrial fibrillation recurrence: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2022, 65, 827–840. [Google Scholar] [CrossRef]
- Kizilirmak F, Demir GG, Gokdeniz T, et al. Changes in Electrocardiographic P Wave Parameters after Cryoballoon Ablation and Their Association with Atrial Fibrillation Recurrence. Ann noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiology, Inc. 2016, 21, 580–587. [Google Scholar] [CrossRef]
- Doğduş M, Turan OE, Başkurt AA, et al. An Effective Novel Index for Predicting the Recurrence of Atrial Fibrillation Ablation: P Wave Duration-to-Amplitude Ratio. Turk Kardiyol Dern Ars. 2022, 50, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang ZR, Ragot D, Massin SZ, et al. P-Wave Duration/Amplitude Ratio Quantifies Atrial Low-Voltage Area and Predicts Atrial Arrhythmia Recurrence After Pulmonary Vein Isolation. Can J Cardiol. 2023, 39, 1421–1431. [Google Scholar] [CrossRef]
- Maheshwari A, Norby FL, Soliman EZ, et al. Refining Prediction of Atrial Fibrillation Risk in the General Population With Analysis of P-Wave Axis (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2017, 120, 1980–1984. [Google Scholar] [CrossRef]
- Dhaliwal KK, Upadhya B, Soliman EZ, et al. Association of P-Wave Axis With Incident Atrial Fibrillation in Diabetes Mellitus (from the ACCORD Trial). Am J Cardiol. 2020, 128, 191–195. [Google Scholar] [CrossRef]
- Salah A, Zhou S, Liu Q, Yan H. P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arq Bras Cardiol. 2013, 101, 519–527. [Google Scholar] [CrossRef]
- Wakatsuki D, Asano T, Mase H, Kurata M, Onuki T, Suzuki H. The characteristic of an abnormal p-wave axis in patients with atrial fibrillation. J Electrocardiol. 2022, 73, 1–7. [Google Scholar] [CrossRef]
- Okuyama T, Kabutoya T, Kario K. Notched P-wave on digital electrocardiogram predicts the recurrence of atrial fibrillation in patients who have undergone catheter ablation. J Arrhythmia. 2024, 40, 472–478. [Google Scholar] [CrossRef]
- Yanagisawa S, Inden Y, Okamoto H, et al. Electrocardiogram characteristics of P wave associated with successful pulmonary vein isolation in patients with paroxysmal atrial fibrillation: Significance of changes in P-wave duration and notched P wave. Ann noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiology, Inc. 2020, 25, e12712. [Google Scholar] [CrossRef]
- Ehrlich JR, Zhang GQ, Israel CW, Hohnloser SH. [P-wave signal averaging-ECG: normal values and reproducibility]. Z Kardiol. 2001, 90, 170–176. [Google Scholar] [CrossRef]
- Yugo D, Kuo M-J, Hu Y-F, et al. Dynamic changes in signal-averaged P wave after catheter ablation of atrial fibrillation. J Chinese Med Assoc. /: https, 2022.
- Aytemir K, Aksoyek S, Yildirir A, Ozer N, Oto A. Prediction of atrial fibrillation recurrence after cardioversion by P wave signal-averaged electrocardiography11This study was presented as an oral presentation at NASPE’s 19th Annual Scientific Sessions, San Diego, May 6–9, 1998. Int J Cardiol. 1999, 70, 15–21. [Google Scholar] [CrossRef]
- Okumura Y, Watanabe I, Ohkubo K, et al. Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal-averaged P-wave duration. Pacing Clin Electrophysiol. 2007, 30, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Tang R-B, Lv W-H, Long D-Y, et al. Catheter ablation of atrial fibrillation in patients with left bundle branch block. Pacing Clin Electrophysiol. 2024, 47, 518–524. [Google Scholar] [CrossRef]
- Yano M, Egami Y, Ukita K, et al. Impact of Baseline Right Bundle Branch Block on Outcomes After Pulmonary Vein Isolation in Patients With Atrial Fibrillation. Am J Cardiol. 2021, 144, 60–66. [Google Scholar] [CrossRef]
- Wang L, Yang G, Cui C, et al. The feasibility of atrial Fibrillatory wave amplitude in predicting ablation outcomes in persistent atrial fibrillation. J Electrocardiol. 2024, 86, 153766. [Google Scholar] [CrossRef]
- Hunuk B, de Asmundis C, Mugnai G, et al. Early repolarization pattern as a predictor of atrial fibrillation recurrence following radiofrequency pulmonary vein isolation. Ann noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiology, Inc. 2019, 24, e12627. [Google Scholar] [CrossRef]
- Wen S-N, Zhu H-J, Sun P-Y, et al. Depolarization and repolarization parameters on ECG predict recurrence after atrial fibrillation ablation in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2019, 30, 2405–2413. [Google Scholar] [CrossRef]
- Wazni OM, Tsao H-M, Chen S-A, et al. Cardiovascular imaging in the management of atrial fibrillation. J Am Coll Cardiol. 2006, 48, 2077–2084. [Google Scholar] [CrossRef]
- Zhao Z, Zhang F, Ma R, et al. Development and Validation of a Risk Nomogram Model for Predicting Recurrence in Patients with Atrial Fibrillation After Radiofrequency Catheter Ablation. Clin Interv Aging. 2022, 17, 1405–1421. [Google Scholar] [CrossRef]
- Lee H-L, Hwang Y-T, Chang P-C, Wen M-S, Chou C-C. A three-year longitudinal study of the relation between left atrial diameter remodeling and atrial fibrillation ablation outcome. J Geriatr Cardiol. 2018, 15, 486–491. [Google Scholar] [CrossRef]
- Taylan G, Gök M, Kurtul A, et al. Integrating the Left Atrium Diameter to Improve the Predictive Ability of the Age, Creatinine, and Ejection Fraction Score for Atrial Fibrillation Recurrence After Cryoballoon Ablation. Anatol J Cardiol. 2023, 27, 567–572. [Google Scholar] [CrossRef]
- Zhuang J, Wang Y, Tang K, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. EP Eur. 2012, 14, 638–645. [Google Scholar] [CrossRef]
- Njoku A, Kannabhiran M, Arora R, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2018, 20, 33–42. [Google Scholar] [CrossRef]
- Kranert M, Shchetynska-Marinova T, Liebe V, et al. Recurrence of Atrial Fibrillation in Dependence of Left Atrial Volume Index. In Vivo 2020, 34, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Sanna G, Moccia E, Canonico ME, et al. Left atrial remodeling in heart failure: the role of sphericity index (the SPHERICAT-HF study). Int J Cardiovasc Imaging. [CrossRef]
- Shi J, Xu S, Chen L, et al. Impact of Left Atrial Sphericity Index on the Outcome of Catheter Ablation for Atrial Fibrillation. J Cardiovasc Transl Res. 2021, 14, 912–920. [Google Scholar] [CrossRef]
- Olsen FJ, Christensen LM, Krieger DW, et al. Relationship between left atrial strain, diastolic dysfunction and subclinical atrial fibrillation in patients with cryptogenic stroke: the SURPRISE echo substudy. Int J Cardiovasc Imaging. 2020, 36, 79–89. [Google Scholar] [CrossRef]
- Morris DA, Parwani A, Huemer M, et al. Clinical Significance of the Assessment of the Systolic and Diastolic Myocardial Function of the Left Atrium in Patients With Paroxysmal Atrial Fibrillation and Low CHADS2 Index Treated With Catheter Ablation Therapy. Am J Cardiol. 2013, 111, 1002–1011. [Google Scholar] [CrossRef]
- Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Hear J - Cardiovasc Imaging. 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Li Y, Li Y, Sun L, et al. Left atrial strain for predicting recurrence in patients with non-valvular atrial fibrillation after catheter ablation: a single-center two-dimensional speckle tracking retrospective study. BMC Cardiovasc Disord. 2022, 22, 468. [Google Scholar] [CrossRef]
- Ma X-X, Boldt L-H, Zhang Y-L, et al. Clinical Relevance of Left Atrial Strain to Predict Recurrence of Atrial Fibrillation after Catheter Ablation: A Meta-Analysis. Echocardiography. 2016, 33, 724–733. [Google Scholar] [CrossRef]
- Khan HR, Yakupoglu HY, Kralj-Hans I, et al. Left Atrial Function Predicts Atrial Arrhythmia Recurrence Following Ablation of Long-Standing Persistent Atrial Fibrillation. Circ Cardiovasc Imaging. 2023, 16, e015352. [Google Scholar] [CrossRef]
- Knappe D, Vogler J, Weimann J, et al. Left Atrial Reservoir Strain and Recurrence of Atrial Fibrillation Following De-Novo Pulmonary Vein Isolation - Results of the ASTRA-AF Pilot Study. Circ J. June, 20 June. [CrossRef]
- Chang S, Zhang X, Ge C, et al. Automatic Echocardiographic Assessment of Left Atrial Function for Prediction of Low-Voltage Areas in Non-Valvular Atrial Fibrillation. Int J Gen Med. 2024, 17, 4493–4506. [Google Scholar] [CrossRef] [PubMed]
- Müller P, Weijs B, Bemelmans NMAA, et al. Echocardiography-derived total atrial conduction time (PA-TDI duration): risk stratification and guidance in atrial fibrillation management. Clin Res Cardiol. 2021, 110, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- den Uijl DW, Gawrysiak M, Tops LF, et al. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. EP Eur. 2011, 13, 1533–1540. [Google Scholar] [CrossRef]
- Evranos B, Aytemir K, Oto A, et al. Predictors of atrial fibrillation recurrence after atrial fibrillation ablation with cryoballoon. Cardiol J. 2013, 20, 294–303. [Google Scholar] [CrossRef]
- Ejima K, Kato K, Arai K, et al. Impact of Atrial Remodeling on the Outcome of Radiofrequency Catheter Ablation of Paroxysmal Atrial Fibrillation. Circ J. 2014, 78, 872–877. [Google Scholar] [CrossRef]
- Fukushima K, Fukushima N, Ejima K, et al. Left Atrial Appendage Flow Velocity and Time from P-Wave Onset to Tissue Doppler–Derived A’ Predict Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation. Echocardiography 2015, 32, 1101–1108. [Google Scholar] [CrossRef]
- Shang Z, Su D, Cong T, et al. Assessment of left atrial mechanical function and synchrony in paroxysmal atrial fibrillation with two-dimensional speckle tracking echocardiography. Echocardiography. 2017, 34, 176–183. [Google Scholar] [CrossRef]
- Sarvari SI, Haugaa KH, Stokke TM, et al. Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. Eur Hear J - Cardiovasc Imaging. 2016, 17, 660–667. [Google Scholar] [CrossRef]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Masuda M, Fujita M, Iida O, et al. An E/e′ ratio on echocardiography predicts the existence of left atrial low-voltage areas and poor outcomes after catheter ablation for atrial fibrillation. EP Eur. 2018, 20, e60–e68. [Google Scholar] [CrossRef]
- Gong K-Z, Yan Q-D, Huang R-D, et al. The impact of echocardiographic parameter ratio of E/E’ on the late recurrence paroxysmal atrial fibrillation in patients accepted radiofrequency catheter ablation: A retrospective clinical study. Medicine 2020, 99, e19897. [Google Scholar] [CrossRef]
- Wada R, Shinohara M, Fujino T, et al. Significance of mitral L-waves in predicting late recurrences of atrial fibrillation after radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2023, 46, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kosiuk J, Breithardt O-A, Bode K, et al. The predictive value of echocardiographic parameters associated with left ventricular diastolic dysfunction on short- and long-term outcomes of catheter ablation of atrial fibrillation. EP Eur. 2014, 16, 1168–1174. [Google Scholar] [CrossRef]
- Li A, Zhang M, Ning B. Predictive value of the left atrioventricular coupling index for recurrence after radiofrequency ablation of paroxysmal atrial fibrillation. J Cardiothorac Surg. 2024, 19, 552. [Google Scholar] [CrossRef]
- Khurram IM, Maqbool F, Berger RD, et al. Association Between Left Atrial Stiffness Index and Atrial Fibrillation Recurrence in Patients Undergoing Left Atrial Ablation. Circ Arrhythm Electrophysiol. [CrossRef]
- Kishima H, Mine T, Fukuhara E, Ashida K, Ishihara M. The association between left atrial stiffness and low-voltage areas of left atrium in patients with atrial fibrillation. Heart Vessels. 2019, 34, 1830–1838. [Google Scholar] [CrossRef]
- Qiao Y, Wu L, Hou B, et al. Functional mitral regurgitation: predictor for atrial substrate remodeling and poor ablation outcome in paroxysmal atrial fibrillation. Medicine (Baltimore). /: https, 2016.
- Sunaga A, Matsuoka Y, Nakatani D, et al. Extensive ablation for persistent atrial fibrillation patients with mitral regurgitation: Insights from the EARNEST-PVI prospective randomized trial. Int J Cardiol. 2024, 410, 132231. [Google Scholar] [CrossRef]
- Zhao Y, Zhao L, Huang Q, et al. Nomogram to predict recurrence risk factors in patients with non-valvular paroxysmal atrial fibrillation after catheter radiofrequency ablation. Echocardiography. 2024, 41, e15779. [Google Scholar] [CrossRef]
- Nakamura K, Takagi T, Kogame N, et al. Impact of atrial mitral and tricuspid regurgitation on atrial fibrillation recurrence after ablation. J Electrocardiol. 2021, 66, 114–121. [Google Scholar] [CrossRef]
- Choi YY, Choi J-I, Jeong JH, et al. Impact of pulmonary artery pressure on recurrence after catheter ablation in patients with atrial fibrillation. Front Cardiovasc Med. 2023, 10, 1187774. [Google Scholar] [CrossRef]
- Yano M, Egami Y, Ukita K, et al. Clinical impact of right ventricular-pulmonary artery uncoupling on predicting the clinical outcomes after catheter ablation in persistent atrial fibrillation patients. Int J Cardiol Hear Vasc. 2022, 39, 100991. [Google Scholar] [CrossRef]
- Moon J, Jin Hong Y, Shim J, et al. Right Atrial Anatomical Remodeling Affects Early Outcomes of Nonvalvular Atrial Fibrillation After Radiofrequency Ablation. Circ J. 2012, 76, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Akutsu Y, Kaneko K, Kodama Y, et al. Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation: a pilot study. Circ Cardiovasc Imaging. 2011, 4, 524–531. [Google Scholar] [CrossRef]
- Han J-M, Xie Q, Song X-Y, Ma Y-L. Right atrial volume index and right atrial volume predict atrial fibrillation recurrence: A meta-analysis. PLoS One 2024, 19, e0315590. [Google Scholar] [CrossRef]
- Kim M, Uhm J-S, Park J-W, et al. The Effects of Radiofrequency Catheter Ablation for Atrial Fibrillation on Right Ventricular Function. Korean Circ J. 2024, 54, 203–217. [Google Scholar] [CrossRef]
- Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef]
- Mohanty S, Torlapati PG, Casella M, et al. Redefining the blanking period after pulsed-field ablation in patients with atrial fibrillation. Hear Rhythm. August, 20 August. [CrossRef]
- Liu C-M, Chen W-S, Chang S-L, et al. Use of artificial intelligence and I-Score for prediction of recurrence before catheter ablation of atrial fibrillation. Int J Cardiol. 2024, 402, 131851. [Google Scholar] [CrossRef]
- Jiang J, Deng H, Liao H, et al. An Artificial Intelligence-Enabled ECG Algorithm for Predicting the Risk of Recurrence in Patients with Paroxysmal Atrial Fibrillation after Catheter Ablation. J Clin Med. [CrossRef]
- Sun S, Wang L, Lin J, Sun Y, Ma C. An effective prediction model based on XGBoost for the 12-month recurrence of AF patients after RFA. BMC Cardiovasc Disord. 2023, 23, 561. [Google Scholar] [CrossRef]
- Fan X, Li Y, He Q, et al. Predictive Value of Machine Learning for Recurrence of Atrial Fibrillation after Catheter Ablation: A Systematic Review and Meta-Analysis. Rev Cardiovasc Med. 2023, 24, 315. [Google Scholar] [CrossRef]
- Truong ET, Lyu Y, Ihdayhid AR, Lan NSR, Dwivedi G. Beyond Clinical Factors: Harnessing Artificial Intelligence and Multimodal Cardiac Imaging to Predict Atrial Fibrillation Recurrence Post-Catheter Ablation. J Cardiovasc Dev Dis. [CrossRef]
| Score | Parameters included | ||||||
|---|---|---|---|---|---|---|---|
| CHADS2 | CHF | Age≥75 | DM | TIA/Stroke | |||
| CHA2DS2-VASc [41] | CHF | Age≥75 | DM | TIA/Stroke | Vascular disease | Age≥65 | Female |
| R2CHADS2 [41] | CHF | Age≥75 | DM | TIA/Stroke | Renal dysfunction | ||
| APPLE [49] | Age > 65 | Persistent AF | eGFR< 60 | LAD≥43mm | LVEF < 50% | ||
| MB-LATER [50] | Male | BBB on ECG | LAD>47mm | ERAF | Persistent AF | ||
| C2HEST [44] | CAD/COPD | HTN | Age >75 | HF | Thyroid disease | ||
| HATCH [44] | HTN | Age≥75 | TIA/Stroke | COPD | HF | ||
| DR-FLASH [45] | DM | CKD | Persistent AF | LAD>45mm | Age>65 | Female | HTN |
| PLAAF [51] | Persistent AF | LA area | Abnormal PV anatomy | AF history | Female | ||
| BASE-AF2 [52] | BMI>28 | LAD>40mm | Smoking | ERAF | AF duration>6 years | Non-PAF | |
| ATLAS [53] | Age > 60 | Female | Non-PAF | Smoking | LAVi | ||
| CAAP-AF [54,55] | CAD | LAD>40 | Age>50 | Persistent AF | AADs failure | Female | |
| SCALE-CryoAF [56] | SHD | CAD | LAD>43mm | LBBB | ERAF | Non-PAF | |
| LAGO [47] | SHD | AF type | CHA2DS2-VASc ≤ 1 | LAD>40mm | LA sphericity | ||
| ACEF [57] | Age | Creatinine | LVEF | ||||
| Score | Ablation strategy | Type of recurrence |
|---|---|---|
| CHADS2 | RFA | ERAF, LRAF |
| CHA2DS2-VASc | RFA | ERAF, LRAF |
| R2CHADS2 | RFA | ERAF, LRAF |
| APPLE | RFA | LRAF, VLRAF |
| MB-LATER | RFA, CBA | LRAF, VLRAF |
| C2HEST | RFA | LRAF |
| HATCH | RFA | LRAF |
| DR-FLASH | RFA | Substrate |
| PLAAF | CBA | LRAF, VLRAF |
| BASE-AF2 | CBA | LRAF, VLRAF |
| ATLAS | RFA | Any |
| CAAP-AF | RFA, CBA | LRAF |
| SCALE-CryoAF | CBA | VLRAF |
| LAGO | RFA | Any |
| ACEF | RFA | LRAF |
| P-wave parameter | Abnormal values |
|---|---|
| P-wave duration (PWD) [60] | >120 ms |
| PTFV1 [66] | >0.04 mm*s |
| P-wave dispersion [71,73] | >40 ms or >80ms |
| PWD/PWA [76] | >830ms/mV |
| P-wave axis [73] | <0 or > 75 ̊ |
| P-wave notch [82,83] | peak-to-peak distance in lead II of more than 20ms |
| Echocardiographic parameter | Recurrence | No Recurrence |
|---|---|---|
| LA diameter (LAD) [97] | Variable, >40mm is enlarged | |
| LA volume (LAV)/LA volume index (LAVi) [98] | Variable, most agree >153ml/>34ml/m2 favours recurrence | |
| LA sphericity index [101] | >0.678 | ≤0.678 |
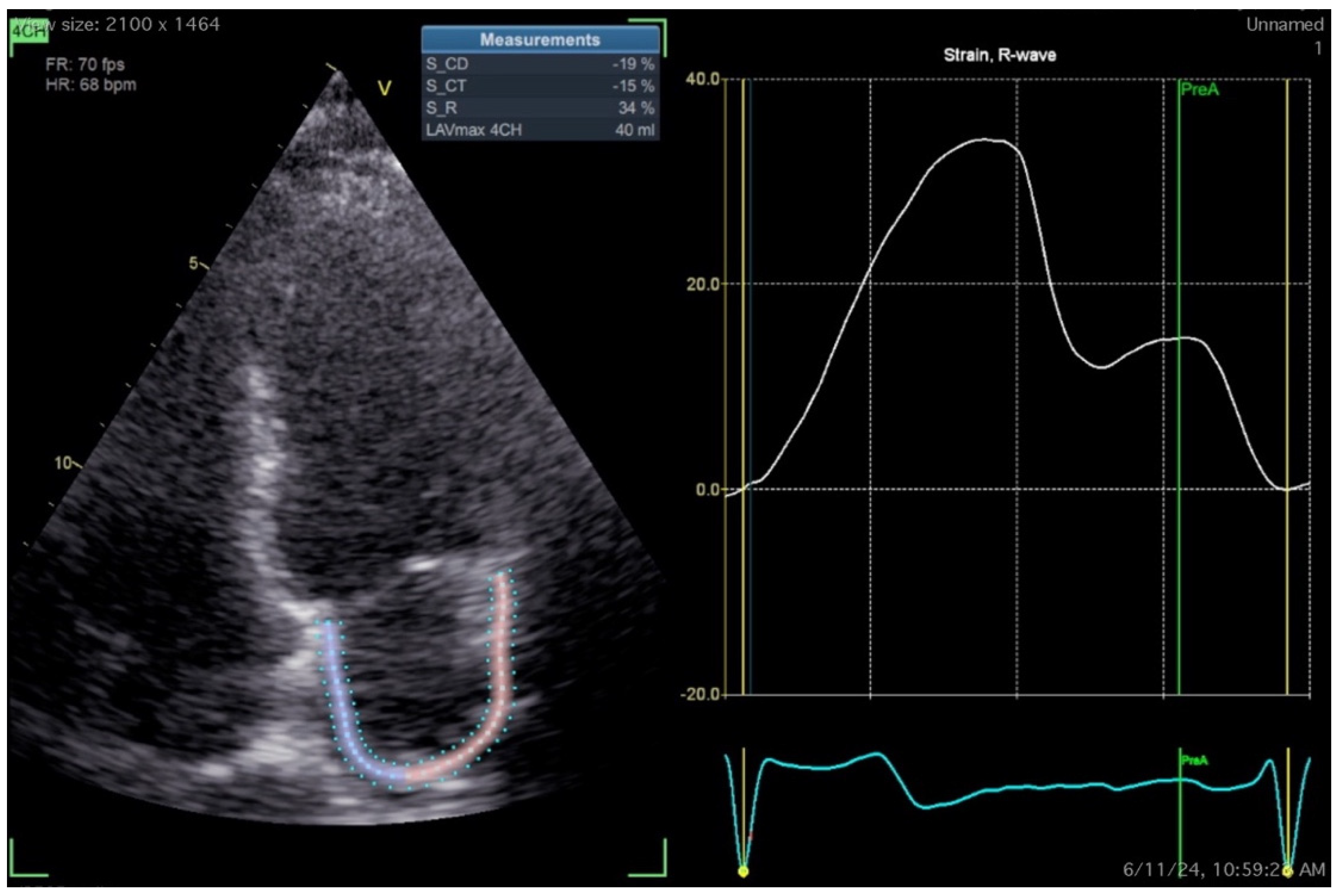
| LA reservoir strain (LASr) [135] | 39% (95% CI, 38%–41%) | |
| LA conduit strain (LAScd)* [135] | 23% (95% CI, 21%–25%) | |
| LA contraction strain (LASct)* [135] | 17% (95% CI, 16%–19%) | |
| Total atrial conduction time (PA-TDI)* [110] | 146.7±20.4 ms | 130.1 ±23.0 ms |
| LA mechanical dispersion (LA-MD) [116] | 38 ± 14 ms | 30 ± 12 ms |
| E/A [121] | 1.8 ± 0.9 | 1.5 ± 0.9 |
| DT [121] | 214 ± 67 ms | 243 ± 68 ms |
| E/E’ [118] | >14 | ≤14 |
| L-wave [120] | Presence | - |
| Left atrioventricular coupling index (LACI) [122] | 44.0 (43.0–45.0)% | 49.5 (47.0–53.0) % |
| LA stiffness index (LASi) [123,124] | 0.83±0.46 or 1.64 ± 1.70 | 0.40±0.22 or 0.61 ± 0.46 |
| Mitral/tricuspid regurgitation (MR/ TR) [128] | More than mild | - |
| Pulmonary artery pressure (PAP) [129] | ≥35 mmHg | < 35mmHg |
| TAPSE/PAP [130] | ≤ 0.57 | >0.57 |
| Right atrial volume (RAV) [132] | ≥87 ml | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





