Submitted:
30 December 2024
Posted:
03 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
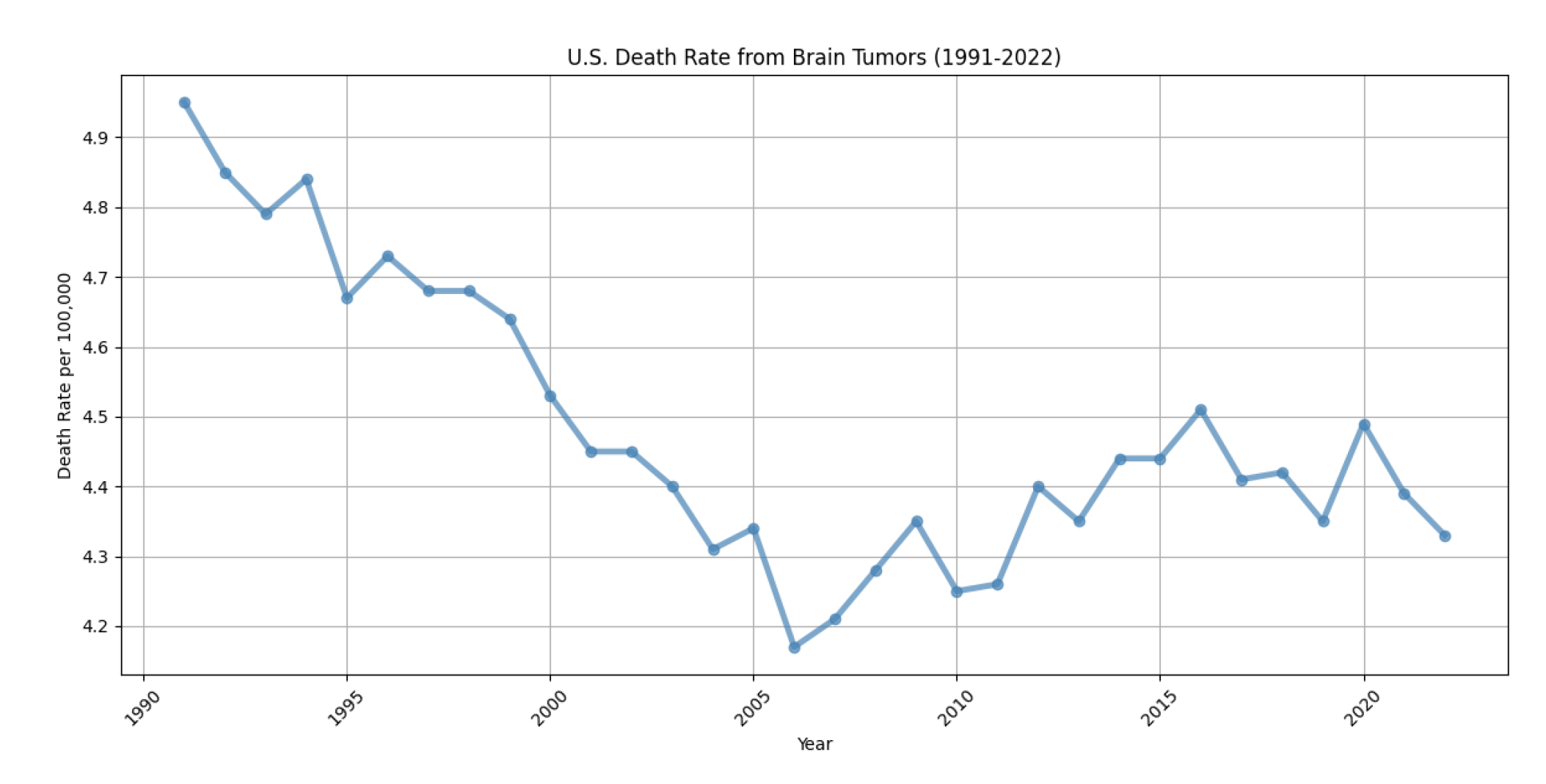
2. MRI Technology: A Critical Tool for Brain Tumor Segmentation
2.1. Evolution of MRI Extraction Technologies from 2012 to Present
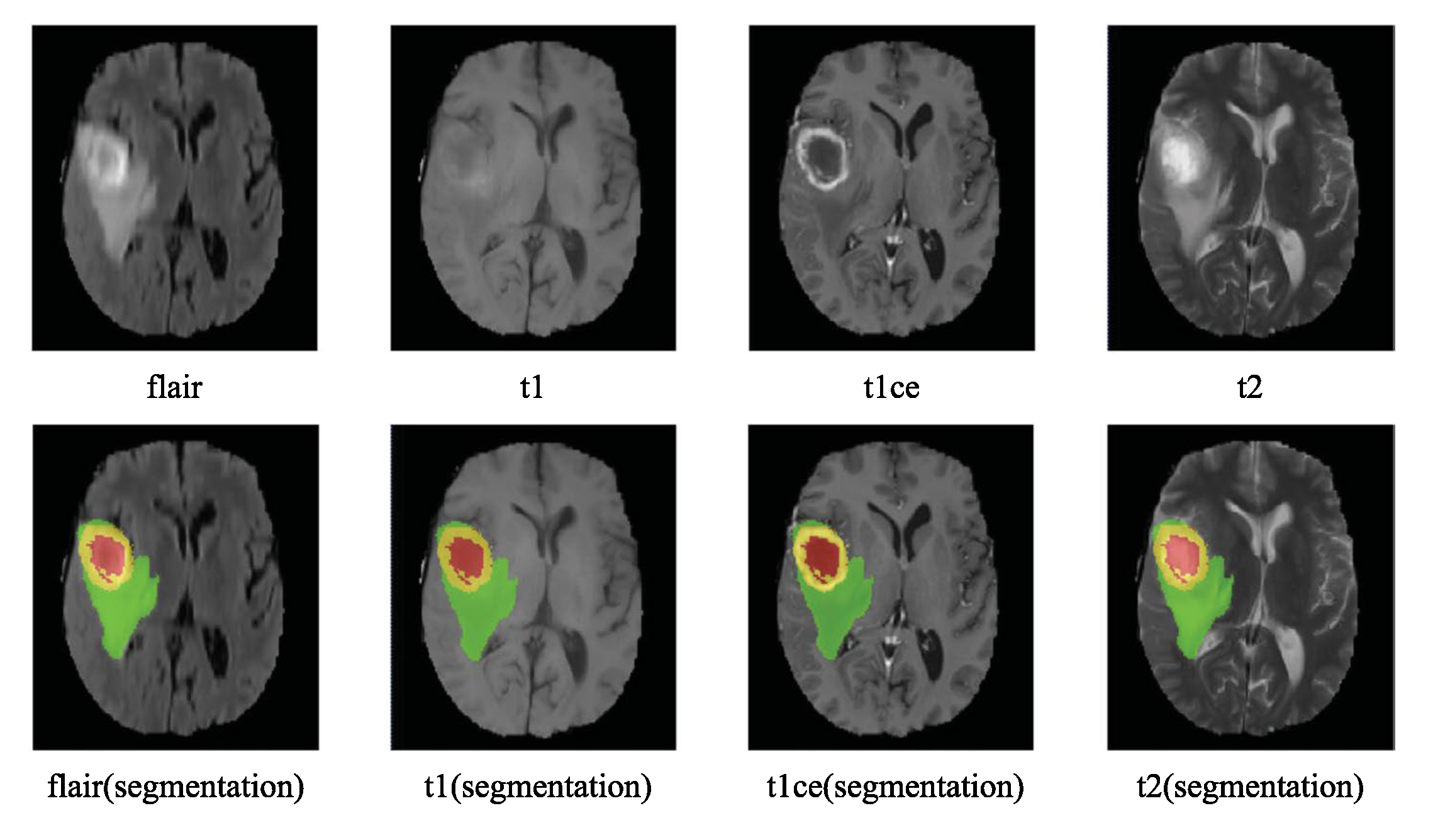
3. Overview of BraTS Challenges
4. Evolution of BraTS Datasets (2012-2024)
4.1. Dataset Review: BraTS Challenge 2012
- label 1 — ED : edema;
- label 2 — TC : tumor core.
- label 1 — NCR : necrotic tumor;
- label 2 — ED : peritumoral edema;
- label 3 — NET : non-enhancing tumor;
- label 4 — ET: enhancing tumor;
- label 0 : everything else.
- Whole tumor region (WT): Includes all four tumor structures, corresponding to labels 1+2+3+4.
- Tumor core region (TC): Contains all tumor structures except for "edema", corresponding to labels 1+3+4.
- Enhancing tumor region (ET): Includes only the "enhancing core" structures that are unique to high-grade cases, corresponding to label 4.
- "edema" was primarily segmented from T2-weighted images. FLAIR sequences were used to verify the extent of the edema and to differentiate it from ventricles and other fluid-filled structures. Initial segmentation in T2 and FLAIR included the core structures, which were then reclassified in subsequent steps.
- The gross tumor core, encompassing both enhancing and non-enhancing structures, was initially segmented by assessing hyper-intensities on T1c images (for high-grade tumors) along with inhomogeneous components of the hyper-intense lesion evident in T1 and the hypo-intense areas seen in T1.
- The “enhancing core” of the tumor was subsequently segmented by thresholding T1c intensities within the resulting gross tumor core. This segmentation included the Gadolinium-enhancing tumor rim while excluding the necrotic center and blood vessels. The intensity threshold for segmentation was determined visually for each case.
- The "necrotic (or fluid-filled) core" was identified as irregular, low-intensity necrotic areas within the enhancing rim on T1c images. This label was also applied to the occasional hemorrhages observed in the BRATS dataset.
- The "non-enhancing (solid) core" was characterized as the part of the gross tumor core remaining after the exclusion of the "Enhancing core" and the "necrotic (or fluid-filled) core."
4.2. Dataset Review: BraTS Challenge 2013
- label 1 — NCR : necrotic tumor;
- label 2 — ED : peritumoral edema;
- label 3 — NET : non-enhancing tumor;
- label 4 — ET: enhancing tumor;
- label 0 : everything else.
4.3. Dataset Review: BraTS Challenge 2014-2015-2016
- Multimodal Brain Tumor Image Segmentation — Segmentation of gliomas in pre-operative scans;
- Disease progression assessment.
- label 1 — NCR : necrotic tumor;
- label 2 — ED : peritumoral edema;
- label 3 — NET : non-enhancing tumor;
- label 4 — ET: enhancing tumor;
- label 0 : everything else.
4.4. Dataset Review: BraTS Challenge 2017
- Multimodal Brain Tumor Image Segmentation — Segmentation of gliomas in pre-operative scans;
- Prediction of patient overall survival (OS) from pre-operative scans.
- label 1 — NCR/NET : necrotic and non-enhancing tumor;
- label 2 — ED : the peritumoral edema;
- label 4 — ET : the GD-enhancing tumor;
- label 0 : everything else.
4.5. Dataset Review: BraTS Challenge 2018
- Multimodal Brain Tumor Image Segmentation — Segmentation of gliomas in pre-operative scans;
- Prediction of patient overall survival (OS) from pre-operative scans.
- label 1 — NCR/NET : necrotic and non-enhancing tumor;
- label 2 — ED : the peritumoral edema;
- label 4 — ET : the GD-enhancing tumor;
- label 0 : everything else.
4.6. Dataset Review: BraTS Challenge 2019
- Multimodal Brain Tumor Image Segmentation — Segmentation of gliomas in pre-operative scans;
- Prediction of patient overall survival (OS) from pre-operative scans;
- Quantification of Uncertainty in Segmentation.
- label 1 — NCR/NET : necrotic and non-enhancing tumor;
- label 2 — ED : the peritumoral edema;
- label 4 — ET : the GD-enhancing tumor;
- label 0 : everything else.
4.7. Dataset Review: BraTS Challenge 2020
- Multimodal Brain Tumor Image Segmentation — Segmentation of gliomas in pre-operative scans;
- Prediction of patient overall survival (OS) from pre-operative scans;
- Quantification of Uncertainty in Segmentation.
- label 1 — NCR/NET : necrotic and non-enhancing tumor;
- label 2 — ED : peritumoral edema;
- label 4 — ET : GD-enhancing tumor;
- label 0 : everything else.
4.8. Dataset Review: BraTS Challenge 2021
- Multimodal Brain Tumor Image Segmentation — Segmentation of gliomas in pre-operative scans;
- Radiogenomic Classification — The evaluation of classification methods to predict the MGMT promoter methylation status at pre-operative baseline scans.
- label 1 — NCR : necrotic tumor;
- label 2 — ED : peritumoral edema;
- label 4 — ET : GD-enhancing tumor;
- label 0 : everything else.
4.9. Dataset Review: BraTS Challenge 2022
4.10. Dataset Review: BraTS Challenge 2023
- Segmentation - Adult Glioma: RSNA-ASNR-MICCAI BraTS Continuous Evaluation Challenge;
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma;
- Segmentation - Meningioma: ASNR-MICCAI BraTS Intracranial Meningioma Challenge;
- Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge;
- Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn);
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques.
- label 1 — NCR : necrotic tumor core;
- label 2 — ED : peritumoral edematous/invaded tissue;
- label 3 — ET : GD-enhancing tumor;
- label 0 : everything else.
- label 1 — NETC : non-ehancing tumor core;
- label 2 — SNFH : surrounding non-enhancing FLAIR hyperintensity;
- label 3 — ET : enhancing tumor;
- label/subregion 0 : everything else.
- label 1 — NC : non-enhancing component (a combination of nonenhancing tumor, cystic component, and necrosis);
- label 2 — ED : peritumoral edematous area;
- label 3 — ET : Enhancing tumor;
- label/subregion 0 : everything else.
- Segmentation - Adult Glioma: RSNA-ASNR-MICCAI BraTS Continuous Evaluation Challenge: The Brain Tumor Segmentation (BraTS) Continuous Challenge aims to identify the state-of-the-art segmentation algorithms for patients with brain diffuse gliomas and their sub-regions. The training and validation datasets, identical to those used for the RSNA-ASNR-MICCAI BraTS 2021 Challenge, encompass a total of 5,880 MRI scans from 1,470 patients with brain diffuse gliomas. While the training and validation data remained consistent with those used in BraTS’21, the testing dataset for this year’s challenge was significantly expanded to include a larger number of routine clinically-acquired mpMRI scans;
-
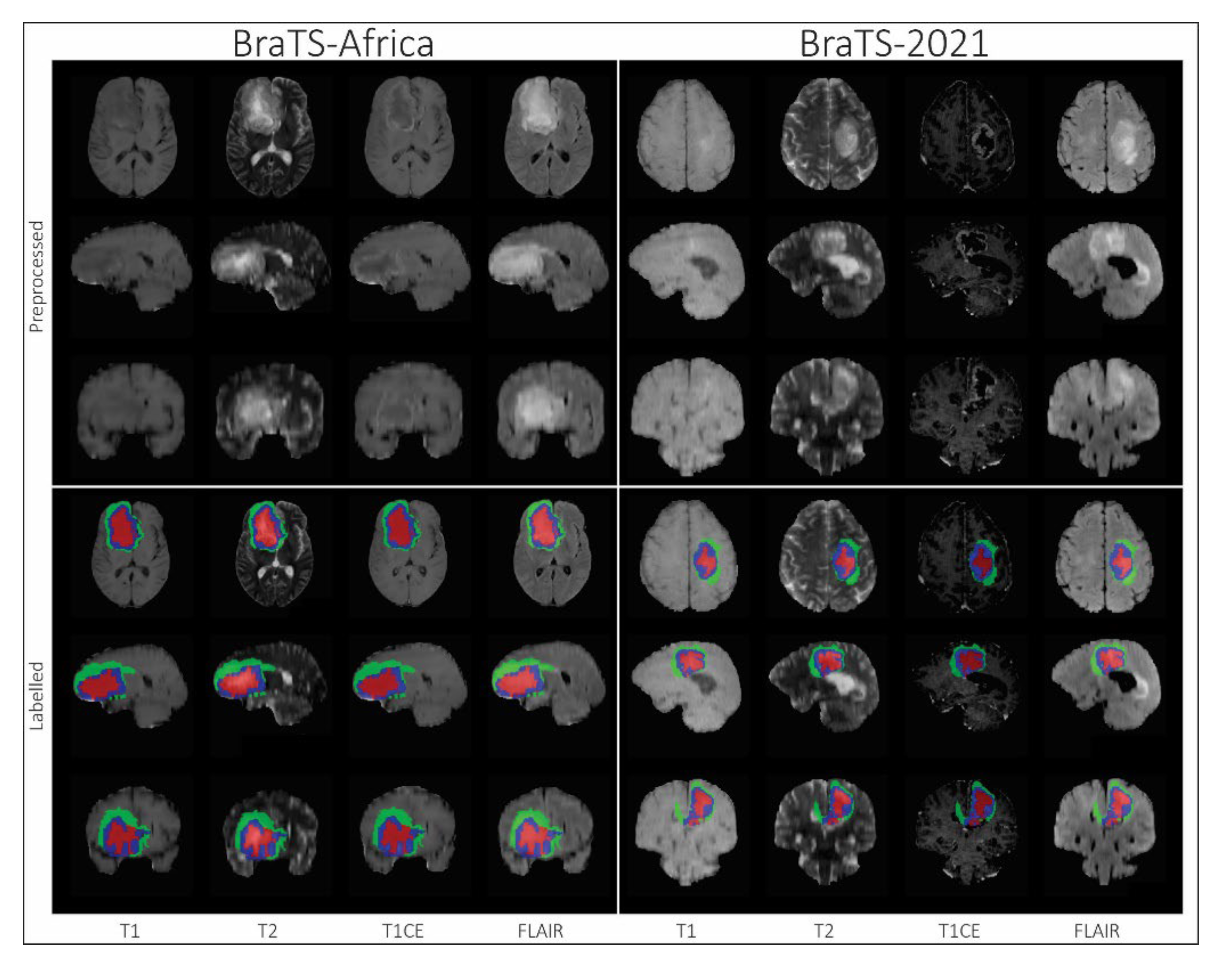
Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: The BraTS-Africa Challenge focuses on addressing the disparity in glioma treatment outcomes between high-income regions and Sub-Saharan Africa (SSA), where survival rates have not improved significantly due to factors such as the use of lower-quality MRI technology (see Figure ii), late-stage disease presentation, and unique glioma characteristics. This initiative is part of a broader effort to adapt and evaluate computer-aided diagnostic (CAD) tools for glioma detection in resource-limited settings, where they have the potential to make a significant impact on healthcare.The MICCAI-CAMERA-Lacuna Fund BraTS-Africa 2023 Challenge has assembled the largest publicly available retrospective cohort of pre-operative glioma MRI scans from adult Africans, including both low-grade glioma (LGG) and glioblastoma/high-grade glioma (GBM/HGG). These scans, obtained as part of routine clinical care from multiple institutions, encompass all four MRI modalities: T1, T1Gd, T2, T2-FLAIR. The BraTS-Africa challenge involves developing machine learning algorithms to automatically segment intracranial gliomas into three distinct classes using a new 3-label system. The sub-regions for evaluation are "enhancing tumor" (ET), "non-enhancing tumor core" (NETC), and "surrounding non-enhancing FLAIR hyperintensity" (SNFH), which are crucial for enhancing diagnostic accuracy and treatment planning in these underserved populations [10,25];
-
Segmentation - Meningioma: ASNR-MICCAI BraTS Intracranial Meningioma Challenge: Meningioma is the most prevalent intracranial brain tumor in adults, often causing significant health issues. While about 80% of these tumors are benign WHO grade 1 meningiomas, effectively managed through observation or therapy, the more aggressive grades 2 and 3 meningiomas pose greater risks, frequently recurring despite comprehensive treatment. Currently, there is no effective noninvasive technique for determining the grade of meningioma, its aggressiveness, or for predicting outcomes.Automated brain MRI tumor segmentation has evolved into a clinically useful tool that provides precise measurements of tumor volume, aiding in surgical and radiotherapy planning and monitoring treatment responses. Yet, most segmentation research has primarily focused on gliomas. Meningiomas present unique challenges for segmentation due to their extra-axial location and the likelihood of involving the skull base. Moreover, since meningiomas are often identified through imaging alone, accurate MRI analysis becomes crucial for effective treatment planning.The aim of the BraTS 2023 Meningioma Challenge was to develop an automated multi-compartment brain MRI segmentation algorithm specifically for meningiomas. This tool was intended not only to assist in accurate surgical and radiotherapy planning but also to pave the way for future research into meningioma classification, aggressiveness evaluation, and recurrence prediction based solely on MRI scans. For this challenge, all meningioma MRI scans were taken pre-operatively and pre-treatment. The objective was to automate the segmentation of these tumors using a three-label system: enhancing tumor (ET), non-enhancing tumor core (NETC), and surrounding non-enhancing FLAIR hyperintensity (SNFH);
-
Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: Brain metastases are the most prevalent type of CNS malignancy in adults and pose significant challenges in clinical assessments. This complexity is mainly due to the common occurrence of multiple, often small, metastases within a single patient. Additionally, the extensive time required to meticulously analyze multiple lesions across consecutive scans complicates detailed evaluations. Consequently, the development of automated segmentation tools for brain metastases is vital for enhancing patient care. Precisely detecting small metastatic lesions, especially those under 5 mm, is crucial for improving patient prognosis, as overlooking even a single lesion could result in treatment delays and repeated medical procedures. Moreover, the total volume of brain metastases is a critical indicator of patient outcomes, yet it remains unmeasured in routine clinical settings due to the lack of effective volumetric segmentation tools.The solution lies in crafting innovative segmentation algorithms capable of identifying and accurately quantifying the volume of all lesions. While some algorithms perform well with larger metastases, they often fail to detect or accurately segment smaller metastases. The inclusion criteria for the BraTS 2023 Brain Metastases challenge were MRI scans that showed untreated brain metastases with all 4 MRI modalities. Exclusion criteria included scans with prior treatment effects, missing required MRI sequences, or poor-quality images due to motion or other significant artifacts. Cases with post-treatment changes were deferred to BraTS-METS 2024.The dataset encompasses a collection of treatment-naive brain metastases mpMRI scans from various institutions, captured under standard clinical protocols. A total of 1303 studies were annotated, with 402 studies comprising 3076 lesions made publicly available for the competition. Additionally, 31 studies with 139 lesions were set aside for validation, and 59 studies with 218 lesions were designated for testing. Notably, the Stanford University dataset, despite being publicly accessible, was excluded from the primary dataset due to the absence of T2 sequences but was available for optional additional training [26];
-
Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: The ASNR-MICCAI BraTS Pediatrics Tumor 2023 Challenge marked the inaugural focus on pediatric brain tumors within the BraTS series, targeting a significant area of concern as pediatric central nervous system tumors are the leading cause of cancer-related mortality in children. The five-year survival rate for high-grade gliomas in children is less than 20%. Furthermore, due to their rarity, the diagnosis is often delayed and treatment relies on historical methods, with clinical trials requiring collaboration across multiple institutions.The 2023 Pediatrics Tumor Challenge, titled CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs, leveraged a comprehensive international dataset gathered from consortia dedicated to pediatric neuro-oncology. It provided the largest annotated public retrospective cohort of high-grade pediatric glioma cases.The dataset for BraTS-PEDs 2023 included multi-institutional cohort of standard clinical MRI scans, with inherent variations in imaging protocols and equipment across different institutions contributing to the diversity in image quality. The inclusion criteria for the challenge were pediatric patients with histologically confirmed high-grade gliomas, ensuring the availability of all four mpMRI sequences on treatment-naive scans. Exclusion criteria included images of poor quality or containing artifacts that hinder reliable tumor segmentation and infants younger than one month.Data for a total of 228 pediatric patients with high-grade gliomas was sourced from CBTN, Boston Children’s Hospital, and Yale University. This cohort was divided into subsets for training (99 cases), validation (45 cases), and testing (84 cases), with ground truth annotations of the tumor sub-regions validated by expert neuroradiologists specialized in pediatric neuro-oncology [27].
-
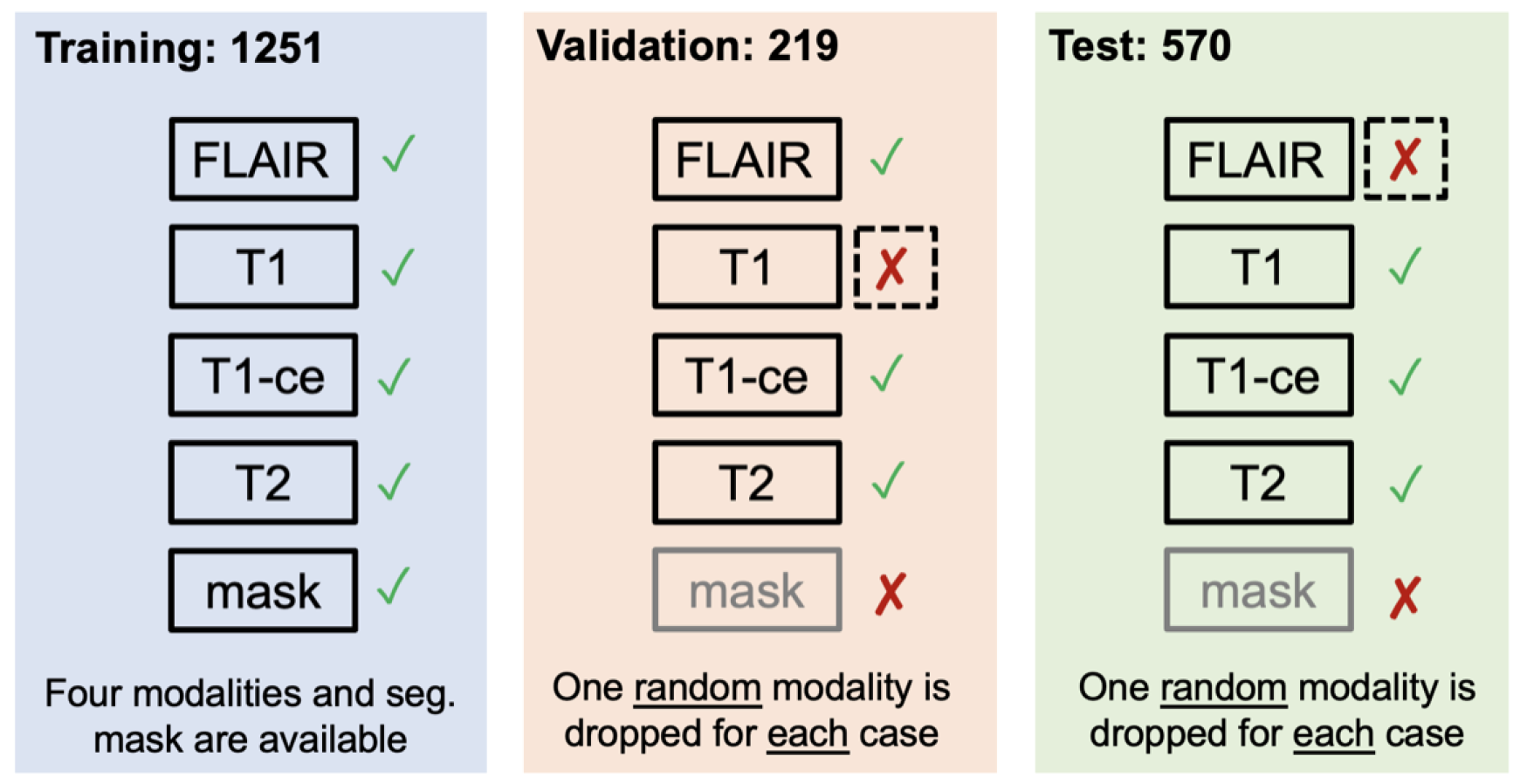
Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): Most segmentation algorithms depend on the availability of four standard MRI modalities: T1-weighted images with and without contrast, T2-weighted images, and FLAIR images. However, in clinical settings, certain sequences might be missing due to factors like time constraints or patient movement, which can introduce artifacts. Therefore, developing methods to effectively substitute missing modalities and maintain segmentation accuracy is crucial for these algorithms to be widely used in clinical routines. To address this, BraTS 2023 launched the Brain MR Image Synthesis (BraSyn) Challenge, aimed at evaluating methods that can realistically synthesize absent MRI modalities given multiple available images.The BraSyn-2023 dataset utilized the RSNA-ASNR-MICCAI BraTS 2021 dataset, comprising 1251 training datasets, 219 validation datasets, and 570 testing datasets. The challenge required participants to handle scenarios where one of the four modalities was randomly absent (’dropout’) in the test set provided for each subject, leaving only three modalities available. Participants’ algorithms were expected to generate plausible images for the missing modality. The dataset details are shown in Figure vi. The synthesized images were evaluated on three criteria: their overall quality, the accuracy of segmentation within the generated images, and the effectiveness of a subsequent tumor segmentation algorithm when applied to the completed image set [28];
-
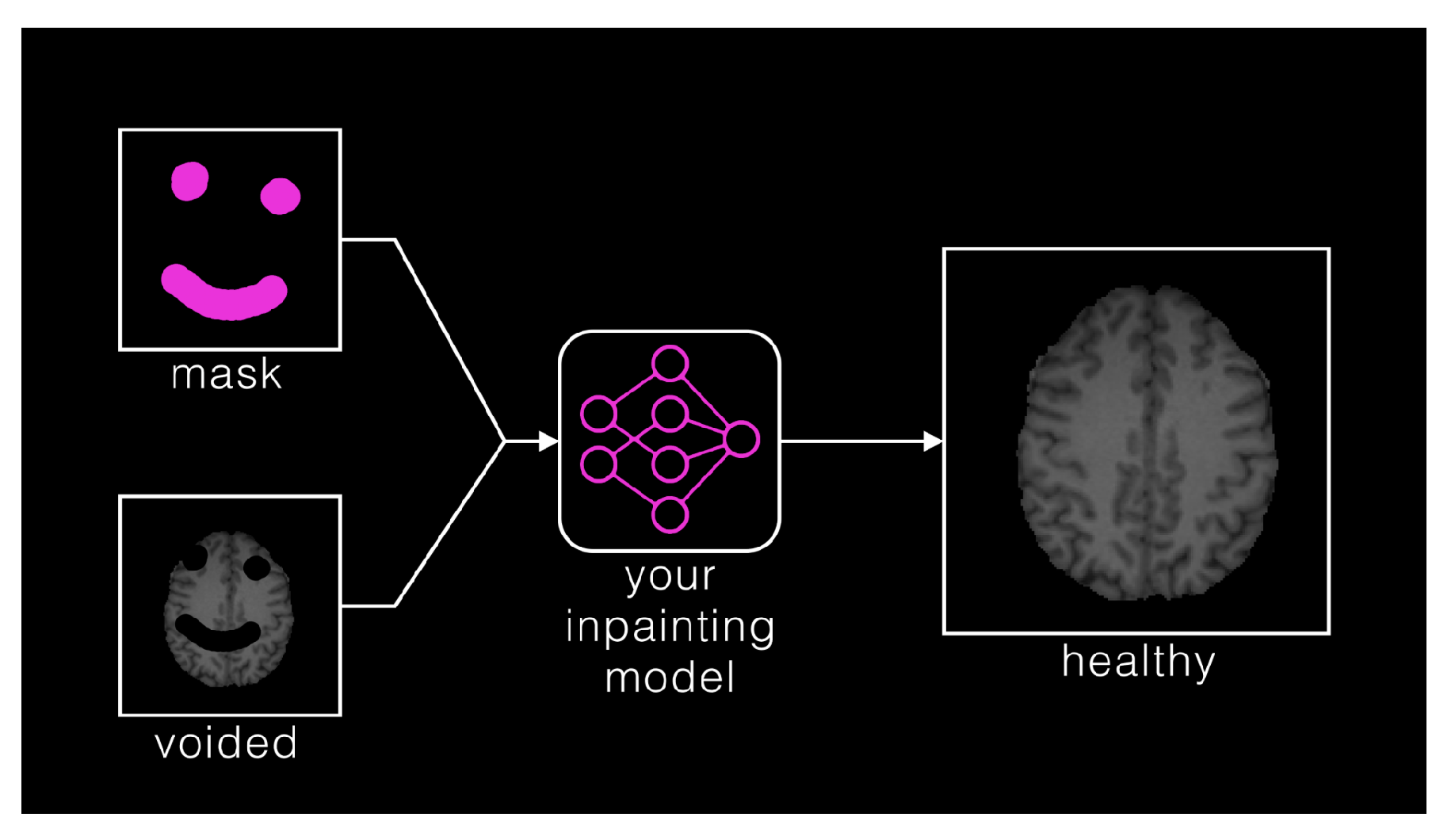
Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: In the BraTS 2023 inpainting challenge, participants were required to develop algorithmic solutions for synthesizing 3D healthy brain tissue in regions affected by glioma. This challenge arose from the need for improved tools to assist clinicians in decision-making and care provision, as most existing brain MR image analysis algorithms are optimized for healthy brains and may underperform on pathological images. These algorithms include, but are not limited to, brain anatomy parcellation, tissue segmentation, and brain extraction.The inpainting challenge addressed this issue by assigning the task of filling in pathological brain areas with synthesized healthy tissue, utilizing provided images and inpainting masks (See Figure vii). Since no actual healthy tissue data existed for tumor regions, surrogate inpainting masks created from the healthy portions of the brain were crafted using a specific protocol and provided to participants to train these algorithms. During training, participants were provided with T1 images featuring voided regions along with a corresponding void mask, the original T1 scans, and two additional masks: one for the tumor and one for the healthy area. This challenge exclusively utilized T1 scans for two main reasons: to reduce the computational demands on participants and to develop algorithms that could be generalized to other brain pathologies, as T1 scans are commonly included in MRI protocols for various conditions [29];In addition to enabling the application of standard brain image segmentation algorithms to tumor-affected brain, local inpainting also allows for the synthetic removal of tumor areas from images. This could deepen the understanding of the interplay between different brain regions and abnormal brain tissue, and is crucial for tasks such as brain tumor modeling. Moreover, inpainting helps manage local artifacts like B-field inhomogeneities, which sometimes impair the quality of brain tumor images;
-
Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: The concept of Data Centric machine learning focuses on enhancing model performance through the use of high-quality, meaningful training data. While data augmentation is recognized for boosting the robustness of machine learning models, the specific types of augmentations that benefit biomedical imaging remain uncertain. Participants were asked to develop methods to augment training data, aiming to enhance the robustness of a baseline model on newly introduced data.Software as a Medical Device (SaMD), as defined by the International Medical Device Regulators Forum (IMDRF), refers to software designed for medical use that operates independently of any hardware device. The challenge directly addressed concerns about algorithmic bias—where model predictions could be unfairly skewed against certain groups—and sought to improve algorithm robustness. Participants were tasked with creating computational methods that augmented a dataset of medical images, such that retraining a baseline AI/ML model on this augmented dataset led to improved accuracy and robustness on independent test data not previously exposed to the model. This approach would ensure that the AI/ML models used in SaMD are both effective and reliable, particularly in imaging-focused applications.The challenge used the RSNA-ASNR-MICCAI BraTS 2021 dataset to allow participants to develop and test their augmentation techniques. The goal was to improve a segmentation model with enhanced training data.
- Segmentation - Adult Glioma: RSNA-ASNR-MICCAI BraTS Continuous Evaluation Challenge : Same Pre-processing data protocol as BraTS 2021;
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: The pre-processing pipeline used for the BraTS-Africa 2023 challenge data was identical to that applied in the BraTS 2017-2022 challenges [25]. This pre-processing pipeline is publicly available through the Cancer Imaging Phenomics Toolkit (CaPTk) and the Federated Tumor Segmentation (FeTS) tool;
- Segmentation - Meningioma: ASNR-MICCAI BraTS Intracranial Meningioma Challenge: The pre-processing of the mpMRI data includes several steps: converting the images from DICOM to NIfTI format, aligning each image series to the SRI24 atlas space with uniform resampling to a 1 mm³ isotropic resolution, and performing automated skull-stripping using a deep convolutional neural network method. It’s important to note that when dealing with meningioma, which can extend through the skull and skull-base foramina, skull-stripping may inadvertently exclude any extra-cranial portions of the tumors;
- Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: A standardized pre-processing protocol was applied to all data. This included converting DICOM files to NIfTI format, aligning images to a common anatomical template (SRI24), resampling images to a consistent isotropic resolution of 1 mm³, and conducting skull-stripping. The pre-processing pipeline is publicly available through the Cancer Imaging Phenomics Toolkit (CaPTk) and the Federated Tumor Segmentation (FeTS) tool. The skull-stripping method employed utilizes an advanced deep learning technique that adapts to the brain’s shape while being robust across different MRI sequences;
- Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: The mpMRI scans were pre-processed using a standardized protocol, including conversion of the DICOM files to the NIfTI file format, co-registration to the same anatomical template (SRI24), and resampling to an isotropic resolution of 1 mm³. The pre-processing pipeline is publicly available through the Cancer Imaging Phenomics Toolkit (CaPTk) and Federated Tumor Segmentation (FeTS) tool. Skull-stripping was carried out using a previously developed pediatric-specific method, effectively de-facing the images to eliminate the possibility of facial reconstruction or identification of the patients [27];
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): A standardized pre-processing protocol was applied to all data. This included converting DICOM files to NIfTI format, aligning images to a common anatomical template (SRI24), resampling images to a consistent isotropic resolution of 1 mm³, and conducting skull-stripping. The pre-processing pipeline is publicly available through the Cancer Imaging Phenomics Toolkit (CaPTk) and the Federated Tumor Segmentation (FeTS) tool. The skull-stripping method employed utilizes an advanced deep learning technique that adapts to the brain’s shape while being robust across different MRI sequences;
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: The challenge employs scans from the multi-modal BraTS Adult Glioma 2023 challenge (task 1) and therefore shares the same pre-processing pipeline as BraTS 2021;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: The dataset is based on the RSNA-ASNR-MICCAI BraTS 2021 dataset and therefore shares the same pre-processing pipeline as BraTS 2021.
- Segmentation - Adult Glioma Dataset: The BraTS Adult Glioma training and validation data were identical to the RSNA-ASNR-MICCAI BraTS 2021 dataset, and thus used the same annotation protocol. The only change, implemented starting in 2023, was a new naming convention where the ET label value was updated from ’4’ to ’3’. The annotations include the GD-enhancing tumor (ET — label 3), the peritumoral edematous/invaded tissue (ED — label 2), and the necrotic tumor core (NCR — label 1).
-
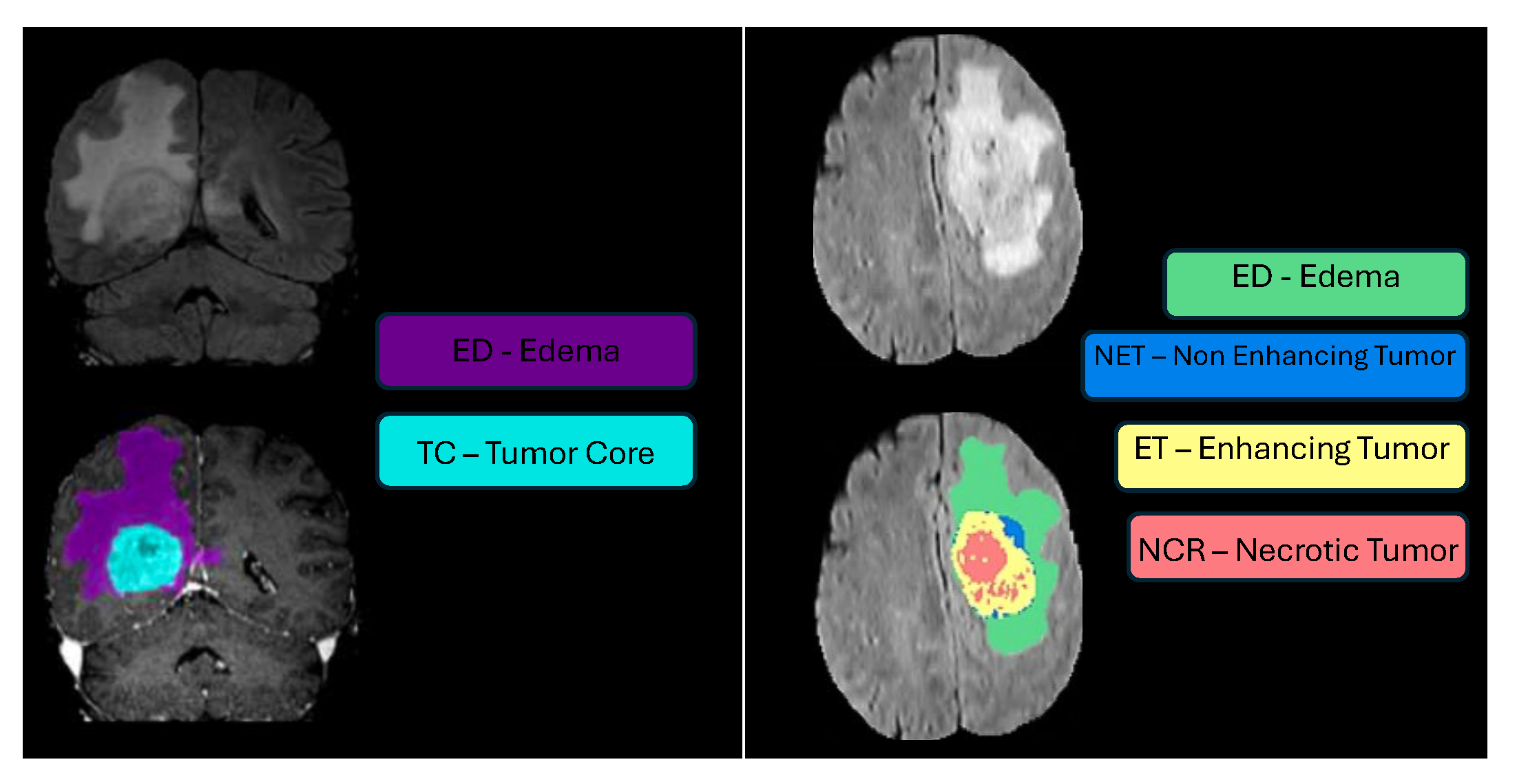
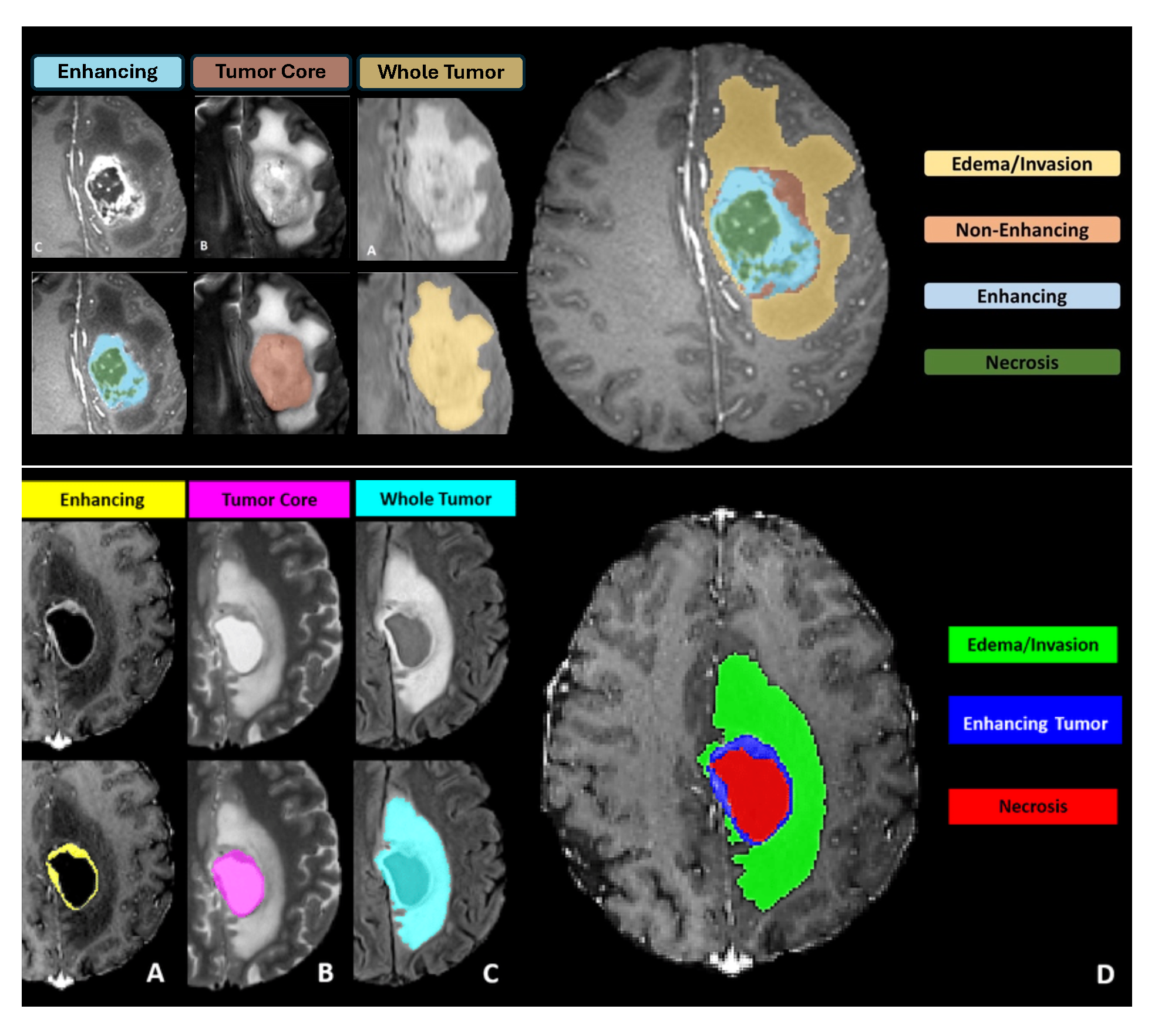
Segmentation - BraTS-Africa Dataset: All imaging data were reviewed and manually annotated by board-certified radiologists specializing in neuro-oncology, following the BraTS pre-processing and annotation protocols [10]. The new BraTS segmentation labeling introduced in 2023, and also used for the BraTS-Africa 2023 challenge, considers the following tumor sub-regions and can be visualised in Figure ii:
- Enhancing tumor (ET): Represents all tumor portions with a noticeable increase in T1 signal on post-contrast images compared to pre-contrast images, excluding adjacent blood vessels, intrinsic T1 hyperintensity, or abnormal signal in non-tumor tissues;
- Non-enhancing tumor core (NETC): Includes all non-enhancing tumor core areas, such as necrosis, cystic changes, calcification, and other non-enhancing components. Intrinsic T1 hyperintensity (e.g., intratumoral hemorrhage or fat) is also included;
- Surrounding non-enhancing FLAIR hyperintensity (SNFH): Covers the full extent of FLAIR signal abnormalities surrounding the tumor that are unrelated to the tumor core. For meningiomas, this corresponds to "vasogenic edema," excluding non-tumor-related FLAIR abnormalities like prior infarcts or microvascular ischemic changes.
-
Segmentation - Meningioma: ASNR-MICCAI BraTS Intracranial Meningioma Challenge: Prior to manual segmentation, an automated pre-segmentation model using a deep convolutional neural network (nnU-Net) was used to produce initial multi-region segmentations. This model was initially trained on a dataset of 73 manually labeled studies from a single institution, all involving meningiomas that had undergone surgical resection. While effective, this initial training set introduced a bias that could reduce model performance for non-surgical cases. To mitigate this, the model was iteratively retrained during the challenge preparation phase using additional manually corrected cases from various sites, including non-surgical meningiomas. This iterative process aimed to enhance the model’s generalizability across diverse MRI appearances and reduce pre-segmentation bias.Manual corrections of the pre-segmented labels were performed by a diverse group of annotators, ranging from medical students to experienced neuroradiologists with over 10 years of expertise. Corrections were carried out using ITK-SNAP, an open-source tool for segmenting 3D and 4D biomedical images. Annotators were provided with detailed instructions, written descriptions of tumor sub-compartment compositions, and examples of common pre-segmentation errors to ensure consistency and reduce variability in corrections. After annotators completed their corrections, each case was reviewed by a trained neuroradiologist ("approver"). Cases identified as incomplete or inaccurate were returned for additional refinement by a different annotator.For BraTS 2023 Meningioma, the task was to automatically segment intracranial meningiomas using a 3-label system: enhancing tumor (ET), non-enhancing tumor core (NETC), and surrounding non-enhancing FLAIR hyperintensity (SNFH). For additional details on the pre-segmentation and annotation procedures, refer to the task manuscript: [30];
-
Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: To ensure consistency in tumor labeling, a comprehensive annotation pipeline was established. This pipeline, designed to generate accurate ground truth (GT) labels, comprises five main stages: pre-segmentation, annotation refinement, technical quality control (QC), initial approval, and final approval. The pre-segmentation phase involved generating segmentations using three nnU-Net models trained on different datasets:1.) A model trained on the UCSF-BMSR MRI dataset to produce ET labels, fused with NETC and SNFH predictions from a model trained on the BraTS 2021 glioma dataset.2.) A model trained on the AURORA multicenter dataset to generate SNFH and tumor core (ET + NETC) labels.3.) A model trained on the Heidelberg dataset to segment SNFH and tumor core labels.The label fusion methods varied by label type: SNFH (label 2) was fused using the STAPLE algorithm to address systematic errors; ET (label 3) was combined using minority voting to improve the detection of small metastases; NETC (label 1) was derived solely from the UCSF-BMSR-trained model, overlapping ET and SNFH labels. Annotators were provided with pre-segmentations, fused segmentations, and subtraction images to refine annotations. Over 150 annotators, including students and neuroradiology experts, participated under coordinator supervision, receiving training through group reviews and lectures. Incomplete cases were returned for re-annotation. Additionally, quality control (QC) ensured proper alignment with the SRI24 atlas and validated the presence of all required segmentation masks. Each refined case underwent secondary review by another neuroradiologist, with discrepancies resolved directly by the reviewers. A final dataset review by an experienced neuroradiologist ensured uniformity and consistency. For more details, refer to the task manuscript [26];
-
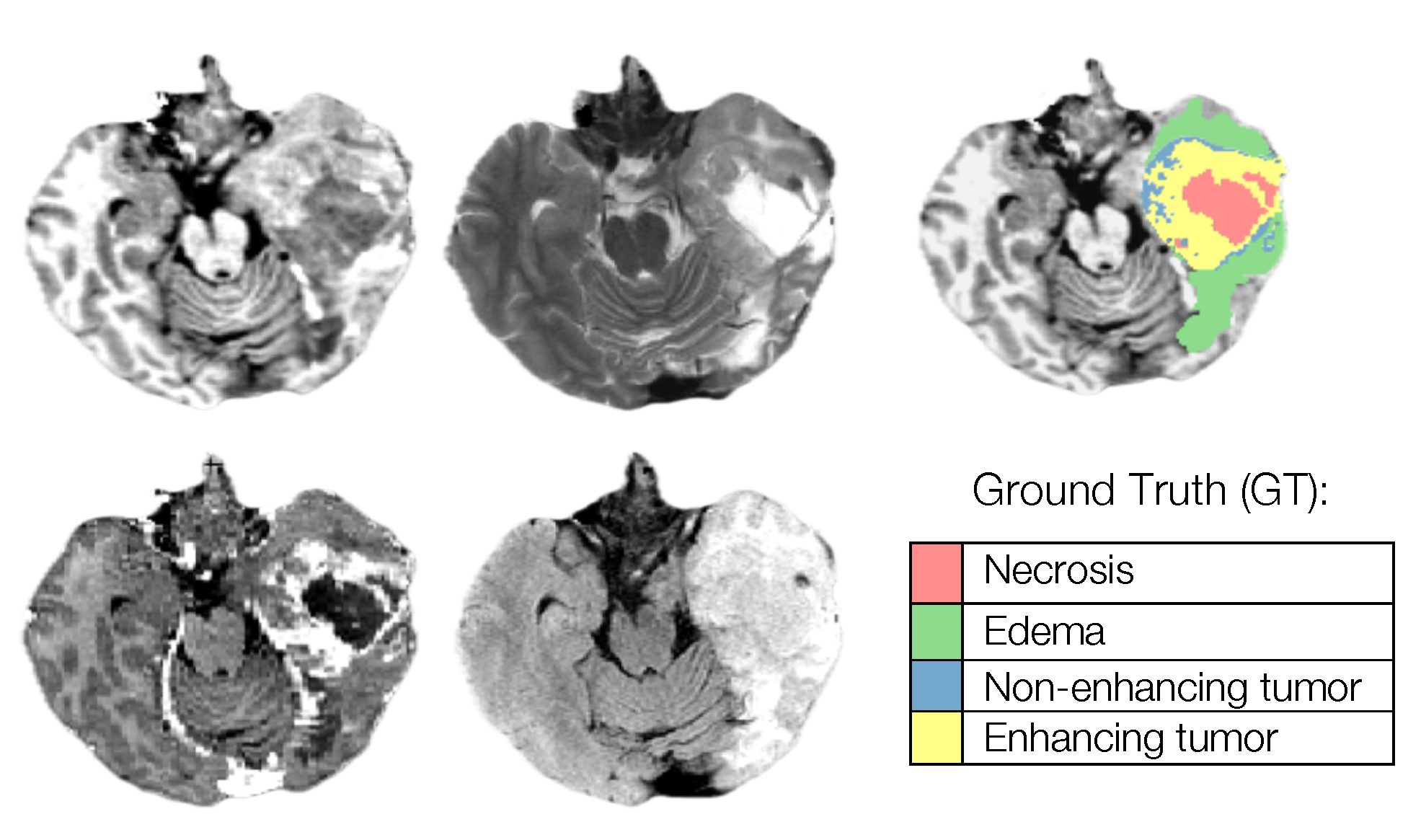
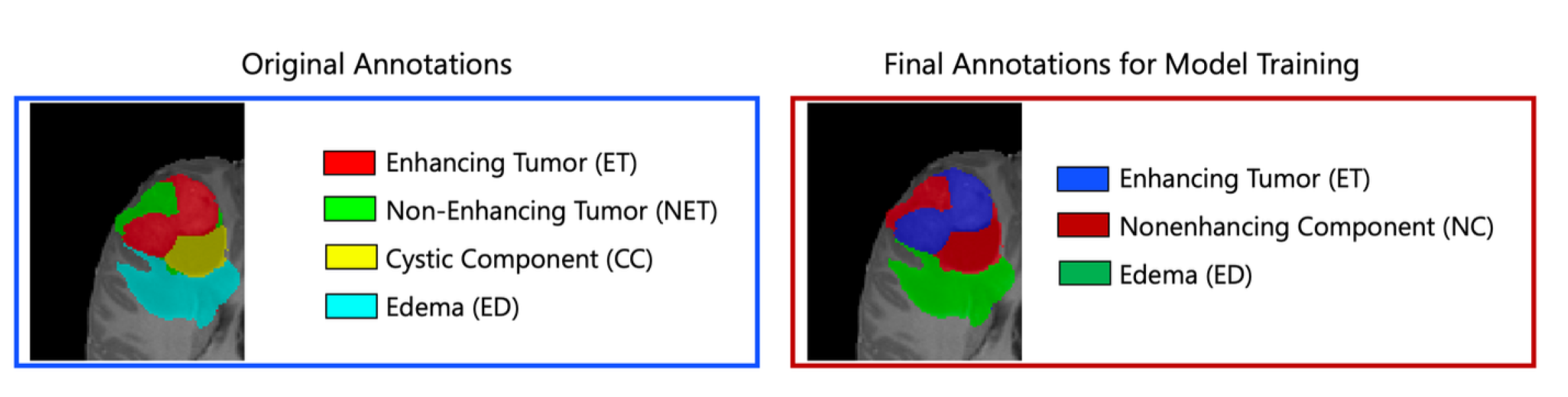
Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: Following pre-processing, the images were segmented into tumor subregions using two pediatric-specific automated deep learning models. These models produced preliminary segmentations of four tumor subregions, as recommended by the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group for assessing treatment response in high-grade gliomas and DIPGs. The subregions included:- Enhancing tumor (ET): Bright areas on T1 post-contrast images compared to T1 pre-contrast, with mild enhancement verified by comparing normal brain signal intensity.- Cystic component (CC):Very bright on T2 and dark on T1CE, resembling cerebrospinal fluid (CSF) and located within the tumor, either centrally or peripherally.- Non-enhancing tumor (NET): Any abnormal signal within the tumor not classified as enhancing or cystic.- Peritumoral edema (ED): Bright, finger-like areas on FLAIR scans, preserving underlying brain structure and surrounding the tumor.The automated segmentations served as the basis for manual refinement by volunteer neuroradiology experts using ITK-SNAP software. Annotators were provided with all four mpMRI sequences and the fused segmentation volume to guide their corrections. Refined segmentations were then reviewed by three board-certified neuroradiologists. Cases were either approved or returned for additional adjustments in an iterative process until the segmentations were deemed acceptable.For consistency with other BraTS 2023 challenges and to facilitate integration with adult glioma datasets, participants were provided with three final segmentation labels: enhancing tumor (ET); peritumoral edematous area (ED) and non-enhancing component (NC), which is a combination of non-enhancing tumor, cystic component, and necrosis. The comparison between the initial and final labels is shown in Figure v;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): The BraSyn-2023 dataset is based on the RSNA-ASNR-MICCAI BraTS 2021 dataset and therefore shares the same annotation protocol as BraTS 2021. The annotated tumor subregions include the Gd-enhancing tumor (ET - labeled as 4), peritumoral edematous/infiltrated tissue (ED - labeled as 2), and necrotic tumor core (NCR - labeled as 1) [28];
-
Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: For the BraTS 2023 challenge, T1 sequence images were used to generate two types of masks for participants. The first mask type delineates tumor-affected areas, while the second identifies likely healthy brain regions. Healthy brain masks were created by sampling existing tumor shapes and placing them in regions distant from tumors, enabling realistic training and evaluation of inpainting methods.The procedure for generating inpainting masks involves several steps. A pool of 1429 tumor masks was extracted from 1251 brains in the BraTS 2023 Adult Glioma dataset, selecting disconnected tumor compartments with at least 800 voxels. Masks were chosen based on tumor size, with small masks assigned to large tumors and vice versa, ensuring variability. Masks underwent transformations, including mirroring and random rotations, and were placed at semi-random positions distant from the tumor. Validity checks ensured masks met specific criteria, such as maintaining a minimum distance of 5 voxels from the tumor and limited overlap with the background. Once valid, both healthy and tumor masks were provided to participants. Participants were encouraged to enhance the process and create additional masks for training. To ensure healthy masks were accurate, medical experts manually reviewed and selected the best candidates from algorithm-generated options. Larger masks were preferred where possible to counteract a statistical bias toward smaller ones. For more details, refer to the task manuscript [29];
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: The dataset is based on the RSNA-ASNR-MICCAI BraTS 2021 dataset and therefore shares the same annotation protocol.
- Segmentation - Adult Glioma: RSNA-ASNR-MICCAI BraTS Continuous Evaluation Challenge: Same as BraTS 2021;
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: Contributions to the BraTS-Africa data came from a total of four medical professionals across several prestigious institutions in Nigeria;
- Segmentation - Meningioma: ASNR-MICCAI BraTS Intracranial Meningioma Challenge: The dataset for the ASNR-MICCAI BraTS Intracranial Meningioma Challenge comprises contributions from six institutions, all located in the United States (USA);
- Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: The BraTS-METS dataset included mpMRI scans from diverse institutions, representing the variability in imaging protocols and equipment reflective of global clinical practices. Eleven source institutions have contributed to the Brain Metastases Dataset, with nine located in the United States, one in Germany, and one in Egypt;
- Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge : Eight source institutions contributed to the CBTN-CONNECT-DIPGr-ASNR-MICCAI BraTS-PEDs Dataset, all located in the United States (USA);
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): The BraSyn-2023 dataset is based on the RSNA-ASNR-MICCAI BraTS 2021 dataset. Therefore, data Contributors are the same as BraTS 2021;
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: BraTS Local Inpatining challenge exclusively employs T1 scans from the multi-modal BraTS 2023 glioma segmentation challenge. Since that challenge is based on the RSNA-ASNR-MICCAI BraTS 2021 dataset, data contributors are the same as BraTS 2021;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: The dataset is based on the RSNA-ASNR-MICCAI BraTS 2021 dataset. Therefore, data Contributors are the same as BraTS 2021.
4.11. Dataset Review: BraTS Challenge 2024
- Segmentation - Adult Glioma Post Treatment: BraTS Adult Glioma Post Treatment Challenge;
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma;
- Segmentation - Meningioma Radiotherapy: BraTS Meningioma Radiotherapy Challenge;
- Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge;
- Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge;
- Segmentation - Generalizability: ASNR-MICCAI BraTS Generalizability Across Brain Tumors;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn);
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques;
- Pathology.
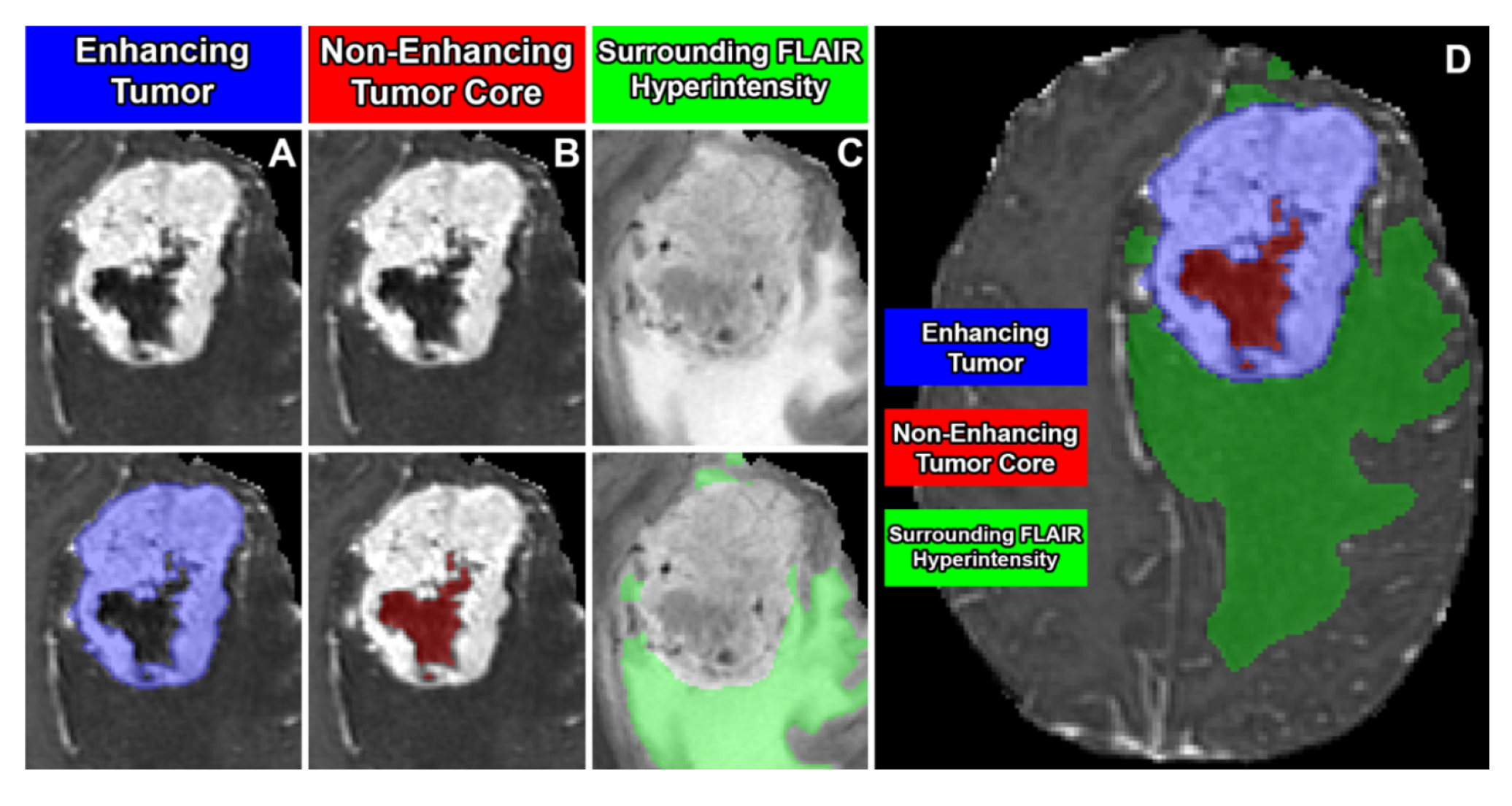
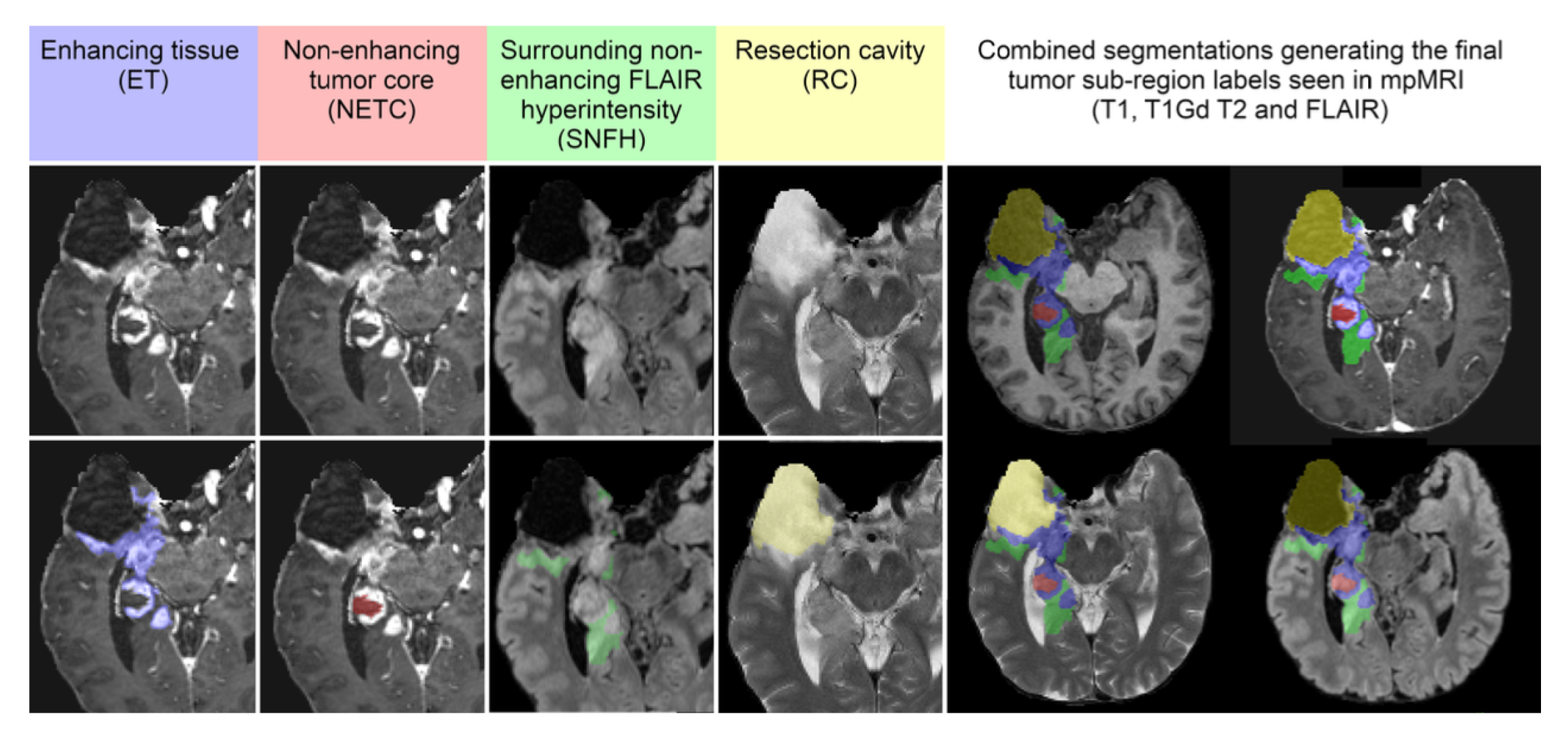
- label 1 — NETC : non-ehancing tumor core;
- label 2 — SNFH : surrounding non-enhancing FLAIR hyperintensity;
- label 3 — ET : enhancing tumor;
- label 4 — RC : resection cavity
- label 0 : everything else.
- label 1 — GTV : Gross Tumor Volume ;
- label 1 — NCR : necrotic tumor core;
- label 2 — ED : peritumoral edematous/invaded tissue;
- label 3 — ET : GD-enhancing tumor;
- label 0 : everything else.
- label 1 — NETC : non-ehancing tumor core;
- label 2 — SNFH : surrounding non-enhancing FLAIR hyperintensity;
- label 3 — ET : enhancing tumor;
- label 0 : everything else.
- label 1 — ET : enhancing tumor;
- label 2 — NET : nonenhancing tumor;
- label 3 — CC : cystic component;
- label 4 — ED : peritumoral edema;
- label 0 : everything else.
- label 1 — CT : presence of cellular tumor;
- label 2 — PN : pseudopalisading necrosis;
- label 3 — MP : areas abundant in microvascular proliferation;
- label 4 — NC : geographic necrosis;
- label 5 — IC : infiltration into the cortex;
- label 6 — WM : penetration into white matter;
- label 7 — LI : leptomeningeal infiltration;
- label 8 — DM : regions dense with macrophages;
- label 9 — PL : presence of lymphocytes;
- label 0 : everything else.
-
Segmentation - Adult Glioma Post Treatment: BraTS Adult Glioma Post Treatment Challenge: Gliomas are the most common malignant primary brain tumors in adults, with diffuse gliomas being particularly prevalent. Treatment typically involves a combination of surgery, radiation, and systemic therapies tailored to the tumor’s characteristics and the patient’s condition. MRI remains the gold standard for monitoring diffuse gliomas post-treatment, offering critical insights into tumor size, location, and morphological changes, which are essential for guiding treatment adjustments and predicting clinical outcomes.The 2024 BraTS challenge introduced a focus on post-treatment gliomas for the first time. Task 1 aimed to develop an automated multi-compartment brain tumor segmentation algorithm for high- and low-grade diffuse gliomas in post-treatment MRI, including a new subregion: the resection cavity (RC). Data and algorithms generated from this challenge have the potential to aid in objectively assessing residual tumor volume, improving treatment planning and outcome prediction [11].
-
Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: The BraTS-Africa Challenge, first launched in 2023, introduced labeled datasets specifically for adult populations in Sub-Saharan Africa. Building on its success, the second BraTS-Africa Challenge (BraTS-Africa 2024) was organized. The MICCAI-CAMERA-Lacuna Fund BraTS-Africa 2024 Challenge offered the largest publicly available annotated retrospective dataset of pre-operative glioma cases in adult Africans, including both low-grade glioma (LGG) and glioblastoma/high-grade glioma (GBM/HGG). Participants were tasked with developing segmentation methods using the clinically acquired BraTS-Africa training data, supplemented with training and validation datasets from the BraTS 2023 Adult Glioma Challenge.The evaluation focuses on segmenting glioma into three sub-regions: "enhancing tumor" (ET), "non-enhancing tumor core" (NETC), and "surrounding non-enhancing FLAIR hyperintensity" (SNFH), in line with the updated BraTS 2023 segmentation labeling;
-
Segmentation - Meningioma Radiotherapy: BraTS Meningioma Radiotherapy Challenge: Meningioma is the most common primary intracranial tumor, with about 80% being benign and effectively managed with positive outcomes. However, higher-grade meningiomas (WHO grades 2 and 3) pose greater health risks and have higher recurrence rates. They are primarily treated with radiation therapy, which may be used as the main treatment, as a postoperative adjunct, or for recurring cases. Accurate segmentation of the gross tumor volume (GTV) is critical for radiotherapy planning but remains complex and time-intensive, with no reliable automated solutions currently available.The BraTS 2024 Meningioma Radiotherapy Segmentation Challenge aimed to bridge existing gaps by developing algorithms capable of automatically segmenting gross tumor volumes (GTVs) in cranial and facial meningiomas from radiotherapy planning MRI scans.This challenge enhanced clinical relevance over previous BraTS iterations by focusing on a single tumor region and utilizing exclusively 3D post-contrast T1-weighted scans in native acquisition space, which closely resembles typical radiotherapy planning scenarios;
-
Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: Monitoring brain metastases is a time-consuming process, particularly when managing multiple metastases using manual methods. In addition, volumetric estimates of lesions and surrounding edema are increasingly recognized as critical for more accurate clinical decision-making and improved treatment outcome predictions.This challenge focused on developing versatile autosegmentation algorithms capable of precisely delineating brain metastases of varying sizes. These algorithms aim to streamline monitoring and management in both pre- and post-treatment settings, offering the potential to significantly enhance patient care and treatment planning;
-
Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: Building on the first BraTS-PEDs Challenge in 2023, the 2024 BraTS-PEDs Challenge expanded to include a larger and more diverse dataset from additional institutions, enhancing its scope and clinical relevance.The 2024 challenge provided the largest publicly available annotated cohort of high-grade pediatric gliomas, including astrocytoma and diffuse midline glioma (DMG)/diffuse intrinsic pontine glioma (DIPG).All mpMRI scans were manually annotated and validated by expert neuroradiologists specializing in pediatric neuro-oncology [11].This year, the challenge introduced significant updates to the processing pipeline and tumor subregion evaluation. The evaluated regions now include: i) “enhancing tumor” (ET); ii) “non-enhancing tumor” (NET); iii) “cystic component” (CC); iv) “edema” (ED); v) “tumor core” (TC), which combines ET, NET, and CC; and vi) the “whole tumor” (WT) [31].
-
Segmentation - Generalizability: ASNR-MICCAI BraTS Generalizability Across Brain Tumors: The overall BraTS 2024 challenge maintains its focus on creating a standardized benchmark environment while broadening its scope to address diverse brain tumor populations. While segmentation is a widely studied medical image processing task, the BraTS Generalizability Across Tumors (BraTS-GoAT) Challenge, emphasizes algorithmic generalizability across various tumor types and patient populations. The goal was to develop a segmentation algorithm that can adapt to different brain tumor types with limited training data.Candidate algorithms should demonstrate the ability to generalize across: Lesion types (Variability in lesion count, size, and brain location); Institutions (Differences in MRI scanners and acquisition protocols) and Demographics (Patient age, sex, and other factors). Although the segmentation mask labels remained consistent ("1" for NCR (necrosis), "2" for ED (edema/invaded tissue), "3" for ET (enhancing tumor), and "0" for everything else), the prevalence of each label varied within and across tumor types, reflecting the diverse imaging characteristics of the lesions.The challenge used preoperative MRI data from BraTS 2023 tasks (1 through 5), focusing on assessing the ability of algorithms to generalize beyond individual datasets and across multiple clinical applications;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn) Continuing from the success of the BraSyn 2023 Challenge, the 2024 iteration maintained the goal of evaluating image synthesis methods that create realistic image contrasts from available MRI modalities to aid automated brain tumor segmentation. The BraSyn-2024 dataset, identical in task to BraSyn-2023, was derived from the RSNA-ASNR-MICCAI BraTS 2021 dataset;
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: : The ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting Challenge continued for BraTS 2024 with the same design, task, evaluation metrics, and ranking scheme as the previous year. This iteration introduced an additional test set of 277 patients from the BraTS Meningioma Challenge, enabling an assessment of how well algorithms generalize to other pathologies and imaging data acquired from different MRI scanners [29].
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: BraTS 2024 Challenge on Relevant Augmentation Techniques shared the same objectives and dataset as BraTS 2023 Challenge on Relevant Augmentation Techniques;
-
Pathology: Glioblastoma, the most common primary brain tumor in adults, has a poor prognosis, with median survival ranging from 12-18 months with treatment and only 4 months without. Its infiltrative nature and heterogeneous molecular and micro-environmental profiles make treatment challenging. Accurate diagnosis and assessment of tumor heterogeneity are critical for selecting effective therapies and potentially improving patient outcomes. The BraTS-Path Challenge leverages whole slide histopathology images (WSI) to enhance the understanding of glioblastoma by detecting various morpho-pathological features in digitized tissue sections. It draws on the gold-standard histopathology-based approach, which traditionally focuses on identifying features such as cellular tumor regions, necrosis, microvascular proliferation, cortical infiltration, and immune cell presence. By providing a robust dataset, the challenge aims to develop deep-learning models capable of automatically classifying these distinct tumor sub-regions with varied histological profiles. These models are designed to mimic and extend the gold-standard process, supporting more consistent diagnosis and grading, thus enhancing both research and clinical applications [32].The dataset included a retrospective, multi-institutional cohort of de novo diffuse gliomas. Expert neuropathologists annotated and segmented histological regions into patches for classification based on specific characteristics. The dataset consists of: 195,000 training data from 130 digitized tissue section; 25,000 validation data from 18 digitized tissue sections and 60,000 testing data from 40 digitized tissue sections. Nine histologic areas of interest are considered as classes.
- Segmentation - Adult Glioma Post Treatment: BraTS Adult Glioma Post Treatment Challenge: The pre-processing pipeline for the 2024 BraTS post-treatment glioma challenge closely follows the approach used in the BraTS 2017-2023 challenges. This includes converting data from DICOM to NIfTI format, co-registering to the same anatomical template, resampling images to a consistent isotropic resolution of 1 mm³, and skull-stripping. Additionally, some contributing institutions employed similar preprocessing pipelines in-house or utilized the FeTS 2.0 platform. For further details, refer to the challenge manuscript [11,33];
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: Same as BraTS-Africa 2023;
-
Segmentation - Meningioma Radiotherapy: The BraTS-MEN-RT challenge exclusively includes radiotherapy planning brain MRI scans, either preoperative or postoperative, with tumors that are radiographically or pathologically consistent with meningioma. These scans consist of a single series (T1-weighted imaging) in native acquisition space, reflecting typical radiotherapy planning scenarios. This approach replaces the multi-sequence, co-registered MRI scans used in each of the BraTS 2023 automated segmentation challenges.All images underwent standardized preprocessing, including conversion from DICOM and DICOM-RT to NIfTI format using dcmrtstruct2nii, followed by automated defacing with the AFNI toolbox. Cases where the AFNI defacing algorithm removed the meningioma entirely from the field of view (e.g., anterior intraorbital meningiomas) were excluded from the dataset [33];
- Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: Since no manuscript is currently available, detailed information about the preprocessing pipeline is not accessible. However, the preprocessing protocol is expected to follow the standard BraTS preprocessing protocol;
-
Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: All mpMRI scans were processed through a three-step pipeline. First, pre-processing was carried out using the "BraTS Pipeline", a standardized approach that is publicly available through the Cancer Imaging Phenomics Toolkit (CaPTk) and Federated Tumor Segmentation (FeTS) tool. Next, a pediatric-specific automated defacing method was applied to ensure patient anonymity. Finally, tumor subregion segmentation was completed using a pediatric autosegmentation method.It is important to note that the BraTS-PEDs 2024 dataset does not include skull stripping;
- Segmentation - Generalizability: ASNR-MICCAI BraTS Generalizability Across Brain Tumors: Same pre-processing pipeline as for tasks 1,2,3,4 and 5 of BraTS 2023;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): Same pre-proceessing pipeline as BraSyn-2023;
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: Same pre-proceessing pipeline as the one used in the Synthesis Local Inpainting 2023 challenge;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: Same as BraTS 2021;
-
Pathology: The preprocessing pipeline for H&E-stained FFPE tissue sections of glioblastoma multiforme (GBM) was specifically designed to facilitate detailed histological analysis:1) Patch Extraction and Standardization: Tissue sections annotated by expert neuropathologists are segmented into standardized 512 x 512 pixel patches. This size minimizes boundary noise and ensures comprehensive coverage of annotated regions.2) Quality Control: Only high-quality sections are included, excluding those with artifacts such as tissue folding, pen markings, or glass slippage.3) Patch Classification: Each patch is categorized based on its histologic features, which are used throughout training, validation, and testing to evaluate the challenges and accuracy of feature detection.This structured pipeline ensures high-quality data for histological studies, with each patch treated as an independent unit for comprehensive analysis [32].
-
Segmentation - Adult Glioma Post Treatment: BraTS Adult Glioma Post Treatment Challenge: The BraTS 2024 post-treatment adult glioma challenge built upon the BraTS 2021-2023 framework, introducing modifications specific to the post-treatment context. All imaging datasets were manually annotated by one to four raters using a standardized, clinically approved protocol developed by expert neuroradiologists and radiation oncologists. Annotators followed detailed guidelines, including examples of challenging cases, to ensure consistency. For a full description of the protocol, refer to the challenge manuscript: [33].Preprocessed MRI volumes were segmented using five nnU-Net-based pre-segmentation methods. These outputs were fused using the STAPLE algorithm to generate consensus segmentations. Additionally, digital subtraction images (T1-Gd minus T1) were provided to radiologists to improve annotation accuracy.Annotations included the following tumor subregions: the enhancing tissue (ET — label 3); the surrounding non-enhancing FLAIR hyperintensity (SNFH — label 2); the non-enhancing tumor core (NETC — label 1) and the resection cavity (RC - label 4) which was newly added in this challenge. Annotations were iteratively refined and reviewed by board-certified neuroradiologists. This process continued until segmentations met the required quality standard for public release. Test data annotations underwent review by two sets of annotators and approvers to ensure inter-rater reliability [11,33]. The tumor sub-regions considered for the challenge are shown in Figure i;
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: Same annotation protocol as BraTS-Africa Challenge 2023;
-
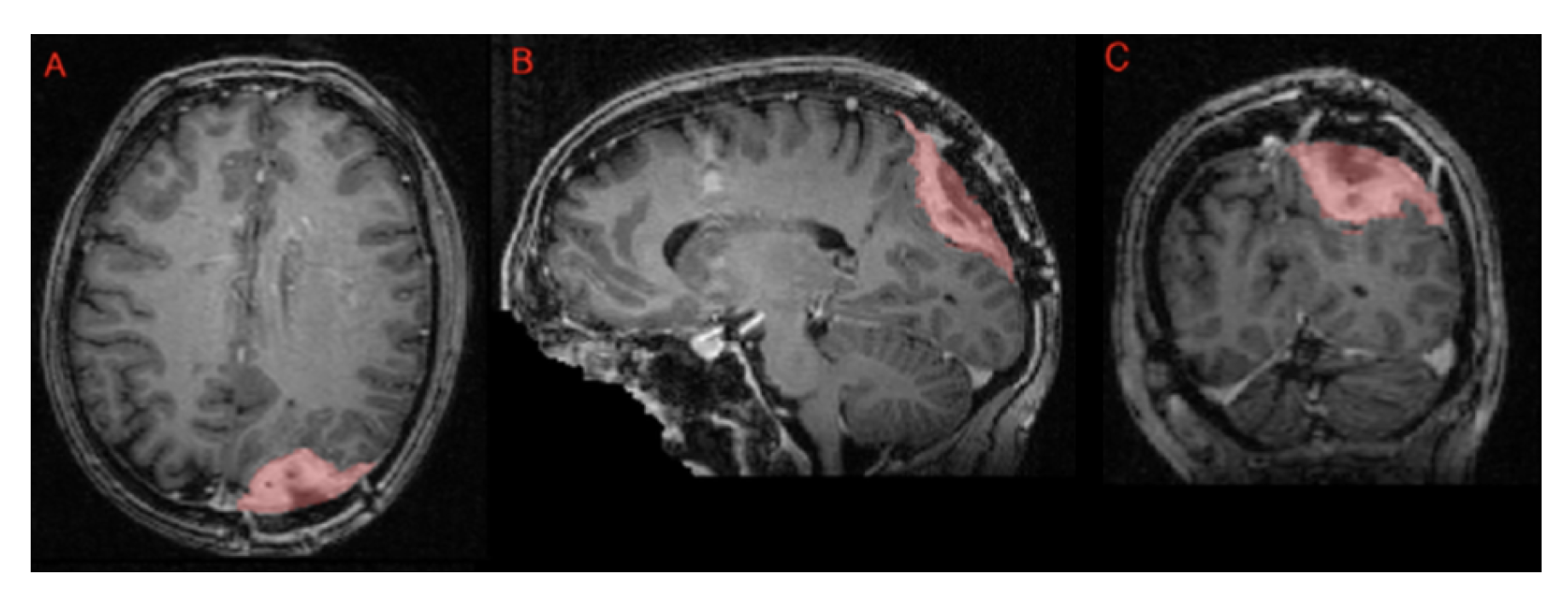
Segmentation - Meningioma Radiotherapy: BraTS Meningioma Radiotherapy Challenge: For the BraTS-MEN-RT 2024 challenge, a single target volume label was used, with definitions varying based on the radiotherapy planning context:- Preoperative setting: The target volume includes the portion of the tumor visible on T1c brain MRI.- Postoperative setting: The target volume includes the resection bed and any residual enhancing tumor (ET) visible on T1c brain MRI. Figure iii shows an example of postoperative meningioma data with its target volume label.For cases without gross tumor volume (GTV) labels provided by the treating institution (about 10% of the dataset), an automated pre-segmentation algorithm using nnU-Net was applied. All cases, whether pre-segmented or labeled by the institution, underwent manual review and correction by a senior radiation oncology resident. Subsequently, a fellowship-trained neuroradiologist ("approver") performed a final review to ensure data quality. Manual reviews and corrections were conducted using ITKSnap [34]. Figure vii shows an example of a T1-weighted meningioma radiotherapy planning MRI, with its single-label ’target volume’ representing the gross tumor volume (GTV);
-
Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: The BraTS Brain Metastasis Challenge for 2024 focused on developing an autosegmentation algorithm capable of accurately delineating brain metastases of varying sizes for both treatment-naive and post-treatment cases. The dataset includes retrospective treatment-naive brain metastasis MRI scans, adhering to the BraTS 2023 annotation protocol. The segmentation system employs three labels: Non-Enhancing Tumor Core (NETC), Surrounding Non-Enhancing FLAIR Hyperintensity (SNFH), and Enhancing Tumor (ET). The initial segmentation labels were generated using the STAPLE fusion of multiple brain metastases segmentation algorithms, followed by manual refinement by neuroradiology experts. These annotations were subsequently reviewed and approved by board-certified neuroradiologists to ensure consistency and accuracy.The post-treatment brain metastasis MRI scans, sourced from an external dataset, are expected to follow a five-label system that includes the necrotic core of tumor (NCR), FLAIR hyperintensity (SNFH), enhancing tumor (ET), hemorrhage (HM), and resection cavity (RC). While the detailed annotation protocol for the post-treatment cases has not yet been released, additional information will be provided in the forthcoming challenge manuscript, with updates available on the official challenge website (Synapse.org);
-
Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: Following pre-processing and defacing, a pediatric automated segmentation method was employed to preliminarily segment tumors into four main subregions: enhancing tumor (ET; label 1), non-enhancing tumor (NET; label 2), cystic component (CC; label 3), and peritumoral edema (ED; label 4).The automated segmentation outputs were manually revised by volunteer neuroradiology experts with varying levels of experience, following established annotation guidelines. Refinements were conducted using ITK-SNAP software. Afterward, the refined segmentations were reviewed by three board-certified neuroradiologists. Cases deemed incomplete or inaccurate were returned to the annotators for further adjustments. This iterative review process continued until the segmentations were approved by the neuroradiologists [31]. Note that for the BraTS-PEDs 2024 challenge, the tumor sub-regions considered for evaluation returned to the original four, as initially planned for the BraTS-PEDs 2023 challenge but not implemented that year (see left image in Figure v);
- Segmentation - Generalizability: ASNR-MICCAI BraTS Generalizability Across Brain Tumors: In BraTS-GoAT, preoperative MRI data from the BraTS 2023 challenge is utilized, specifically from tasks 1, 2, 3, 4, and 5. The sub-regions considered for evaluation are the "enhancing tumor" (ET), the "tumor core" (TC), and the "whole tumor" (WT). The provided segmentation labels have values of: "1" for NCR (necrosis), "2" for ED (edema/invaded tissue), "3" for ET (enhancing tumor) and "0" for everything else. Details regarding the annotation protocol have not yet been disclosed;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): Same as BraSyn 2023 Challenge;
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: Same as BraTS Local Inpainting 2023 Challenge;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques;
-
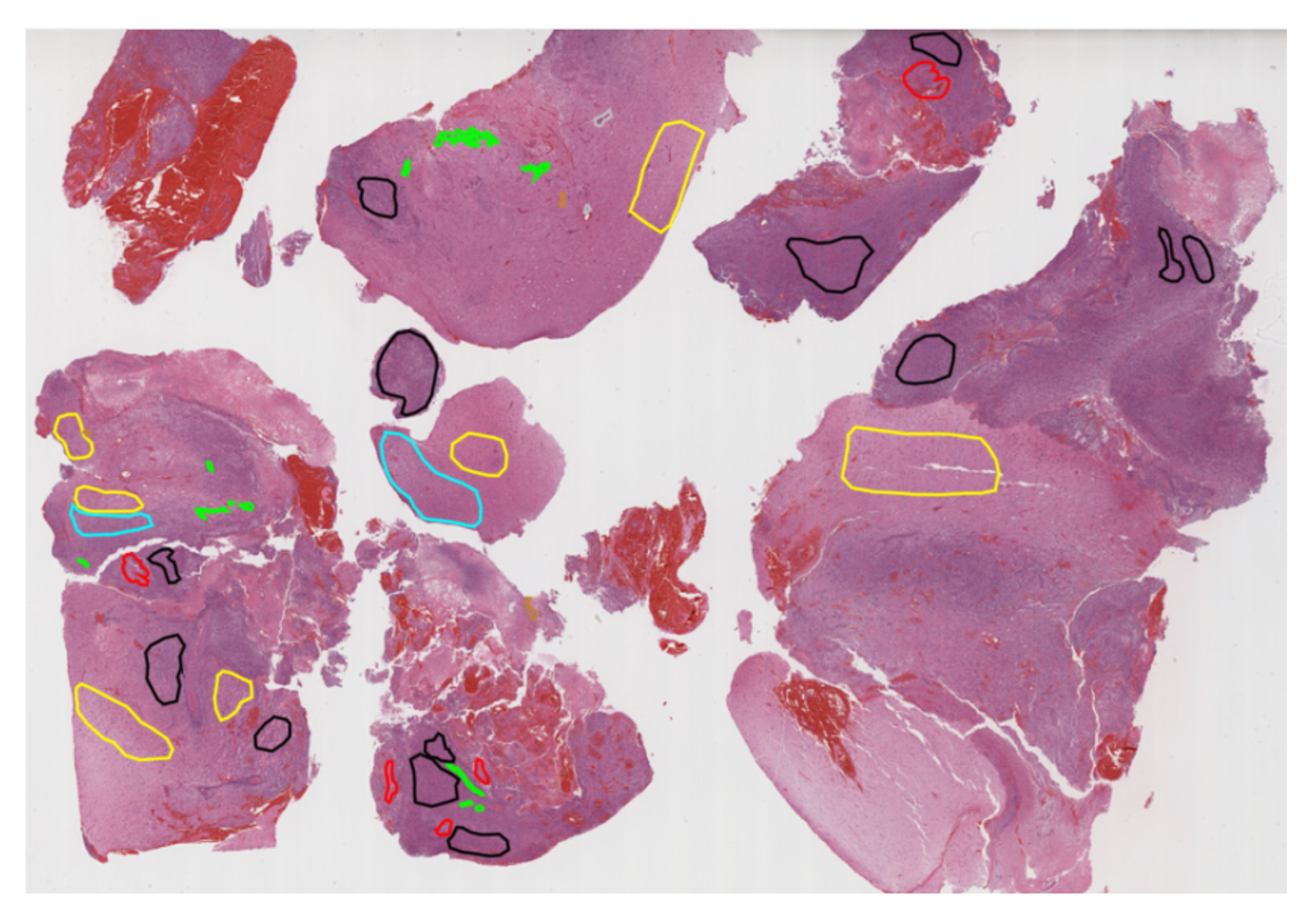
Pathology: The annotation process adhered to a clinically-approved protocol, defined by expert neuropathologists, which provided detailed instructions on segmenting each histologic feature. For further details, refer to the challenge manuscript: [32]. Each case was assigned to an annotator-approver pair. To assess inter-rater variability, three cases were annotated by all annotators. Annotators varied in experience and rank, while approvers were highly experienced, board-certified neuropathologists with over 10 years of expertise.Once annotations were completed, they were reviewed by the approvers, who evaluated their quality alongside the corresponding tissue sections. If the initial annotations on a tissue section produced fewer than approximately 1,500 patches, that section was returned to the annotators for further refinement. This iterative process continued until the approvers approved the annotations. The segmented regions were then divided into patches and classified based on specific histologic features. This established a classification task focused on accurately identifying patches with distinct morphological characteristics.Histologic areas of interest for classification included:1) presence of cellular tumor (CT);2) pseudopalisading necrosis (PN);3) areas abundant in microvascular proliferation (MP);4) geographic necrosis (NC);5) infiltration into the cortex (IC);6) penetration into white matter (WM);7) leptomeningeal infiltration (LI);8) regions dense with macrophages (DM);9)presence of lymphocytes (PL);An example of the data and classes is illustrated in Figure x.
- Segmentation - Adult Glioma Post Treatment: BraTS Adult Glioma Post Treatment Challenge: BraTS mpMRI scans come from multiple contributing institutions using various scanners and clinical protocols. Data from seven institutions contributed to the dataset, with locations across the United States and one in Germany. The total number of cases provided by these institutions approximately sums to 2,200;
- Segmentation - BraTS-Africa: BraTS Challenge on Sub-Sahara-Africa Adult Glioma: The BraTS-Africa 2024 dataset saw an expansion in the number of contributors compared to the 2023 edition, reflecting a substantial enlargement of the dataset. This increase includes new collaborations from additional healthcare institutions across several African countries, including Tanzania and Ghana;
- Segmentation - Meningioma Radiotherapy: BraTS Meningioma Radiotherapy Challenge: 750 Radiotherapy planning T1c brain MRI scans were contributed from 7 academic medical centers across the United States and United Kingdom;
- Segmentation - Brain Metastases: ASNR-MICCAI BraTS Brain Metastasis Challenge: Contributions to the dataset came from ten institutions located across the United States, Germany, and Egypt;
- Segmentation - Pediatric Tumors: ASNR-MICCAI BraTS Pediatrics Tumor Challenge: The image acquisition protocols and MRI equipment vary between institutions, leading to differences in image quality within the provided cohort. The dataset from the CBTN-CONNECT-DIPGr-ASNR-MICCAI BraTS-PEDs initiative includes contributions from a total of eight institutions, all located in the United States;
- Segmentation - Generalizability: ASNR-MICCAI BraTS Generalizability Across Brain Tumors: The data contributors include those involved in challenges 1, 2, 3, 4, and 5 from the 2023 edition. This collaborative effort provides a comprehensive and diverse dataset, supporting the development of models with improved generalizability across various brain tumor types;
- Synthesis (Global) - Missing MRI: ASNR-MICCAI BraTS MRI Synthesis Challenge (BraSyn): Same as BraTS 2021;
- Synthesis (Local) - Inpainting: ASNR-MICCAI BraTS Local Synthesis of Tissue via Inpainting: Same as BraTS 2021;
- Evaluating Augmentations for BraTS: BraTS Challenge on Relevant Augmentation Techniques: Same as BraTS 2021;
- Pathology: Contributions to the dataset came from ten institutions across the United States and one in Italy.
5. BraTS Challenges Results over the Years: SOTA Development
6. Datasets Growing Complexity over the Years
7. Datasets Challenges and Limitations
8. Dataset Usage and Impact on Research: Potential Clinical Practice
9. Future Directions and Dataset Needs
References
- SEER. Brain and Other Nervous System Cancer — Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/brain.html (accessed on December 2024).
- Bakas, S.; Reyes, M.; Jakab, A.; Bauer, S.; Rempfler, M.; Crimi, A.; Shinohara, R.T.; Berger, C.; Ha, S.M.; Rozycki, M.; et al. Identifying the Best Machine Learning Algorithms for Brain Tumor Segmentation, Progression Assessment, and Overall Survival Prediction in the BRATS Challenge. arXiv:1811.02629v3 [cs.CV] 23 Apr 2019.
- Baid, U.; Ghodasara, S.; Mohan, S.; Bilello, M.; Calabrese, E.; Colak, E.; Farahani, K.; Kalpathy-Cramer, J.; Kitamura, F. C.; Pati, S.; et al.The RSNA-ASNR-MICCAI BraTS 2021 Benchmark on Brain Tumor Segmentation and Radiogenomic Classification. arXiv:2107.02314v2 [cs.CV] 12 Sep 2021.
- Liu, J.; Li, M.; Wang, J.; Wu, F.; Liu, T.; Pan, Y.; A survey of MRI-based brain tumor segmentation methods. Tsinghua Science and Technology, vol. 19, no. 6, pp. 578-595, Dec. 2014. [CrossRef]
- Karim, S.; Tong, G.; Yu, Y.; Laghari, A.A.; Khan, A.A.; Ibrar, M. and Mehmood, F.; Developments in Brain Tumor Segmentation Using MRI: Deep Learning Insights and Future Perspectives. IEEE Access, vol. 12 (2024): 26875-26895. [CrossRef]
- Ghosh, Akhilbaran; Chakraborty, Subhadeep. Efficient Brain Tumor Segmentation with Mamba. In Proceedings of the 2024 7th International Conference of Computer and Informatics Engineering (IC2IE), 12-13 September 2024, Bali, Indonesia. IEEE, 2024. [CrossRef]
- Kose, K. Physical and technical aspects of human magnetic resonance imaging: present status and 50 years historical review. Advances in Physics: X, 2021, 6:1, 1885310. [CrossRef]
- Kabasawa, H. MR Imaging in the 21st Century: Technical Innovation over the First Two Decades. Magnetic Resonance in Medical Sciences, 2021. [CrossRef]
- Hussain, S.; Anwar, S. M. and Majid, M.; MRI-based brain tumor segmentation using FPGA-accelerated neural network. September 2021. [CrossRef]
- BraTS 2023 Challenges. Available online: https://www.synapse.org/Synapse:syn51156910/wiki/. (accessed on September 2024).
- BraTS 2024 Challenges. Available online: https://www.synapse.org/Synapse:syn53708249/wiki/626323. (accessed on September 2024).
- BraTS 2012 Challenge on Multimodal Brain Tumor Segmentation. Available online: http://www2.imm.dtu.dk/projects/BRATS2012/index.html (accessed on September 2024).
- Menze, B. H.; Jakab, A.; Bauer, S.; Kalpathy-Cramer, J.; Farahani, K.; Kirby, J.; Burren, Y.; Porz, N.; Slotboom, J.; Wiest, R.; et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans Med Imaging. 2015;34(10):1993-2024. [CrossRef]
- Narmatha, C.; Eljack, S. M.; Tuka, A. A. R. M.; Manimurugan, S. and Mustafa, M.; A hybrid fuzzy brain-storm optimization algorithm for the classification of brain tumor MRI images Journal of Ambient Intelligence and Humanized Computing, August 2020. [CrossRef]
- Fernandez, X. T. and Warfield, S. K.; Automatic Brain Tumor Segmentation based on a Coupled Global-Local Intensity Bayesian Model. Proc MICCAI-BRATS 2012.
- Ghaffari, M.; Sowmya, A. and Oliver, R.; Automated Brain Tumor Segmentation Using Multimodal Brain Scans: A Survey Based on Models Submitted to the BraTS 2012-2018 Challenges.IEEE reviews in biomedical engineering vol. 13 (2020): 156-168. [CrossRef]
- BraTS 2017 Challenge on Multimodal Brain Tumor Segmentation. Available online: https://www.med.upenn.edu/sbia/brats2017/registration.html (accessed on September 2024).
- Bakas, S.; Akbari, H.; Sotiras, A.; et al. Advancing The Cancer Genome Atlas glioma MRI collections with expert segmentation labels and radiomic features. Sci Data. 2017;4:170117. Published 2017 Sep 5. [CrossRef]
- BraTS 2018 Challenge on Multimodal Brain Tumor Segmentation. Available online: https://www.med.upenn.edu/sbia/brats2018/registration.html (accessed on September 2024).
- BraTS 2019 Challenge on Multimodal Brain Tumor Segmentation. Available online: https://www.med.upenn.edu/cbica/brats2019/registration.html (accessed on September 2024).
- BraTS 2020 Challenge on Multimodal Brain Tumor Segmentation. Available online: https://www.med.upenn.edu/cbica/brats2020/ (accessed on September 2024).
- RSNA-ASNR-MICCAI BraTS Challenge 2021. Available online: https://www.synapse.org/Synapse:syn25829067/wiki/610863 (accessed on September 2024).
- Bakas, S.; Farahani, K.; Linguraru, M.G.; et al. The Brain Tumor Segmentation Challenge (2022 Continuous Updates & Generalizability Assessment): Structured description of the challenge design. 2022.
- Synapse. Brain Tumor Segmentation Challenge (BraTS) Continuous Evaluation. Available online: https://www.synapse.org/Synapse:syn27046444/wiki/616571 (accessed on September 2024).
- Adewole, M.; Rudie, J. D.; Gbadamosi, A.; Toyobo, O.; Raymond, C.; Zhang, D.; Omidiji, O.; Akinola, R.; Suwaid, M. A.; Emegoakor, A.; et al.The Brain Tumor Segmentation (BraTS) Challenge 2023: Glioma Segmentation in Sub-Saharan Africa Patient Population (BraTS-Africa). arXiv:2305.19369v1 [eess.IV] Submitted on 30 May 2023.
- Moawad, A. W.; Janas, A.; Baid, U.; Ramakrishnan, D.; Saluja, R.; Ashraf, N.; Jekel, L.; Amiruddin, R.; Adewole, M.; Albrecht, J.; et al.The Brain Tumor Segmentation (BraTS-METS) Challenge 2023: Brain Metastasis Segmentation on Pre-treatment MRI. arXiv:2306.00838v2 [q-bio.OT] 17 Jun 2024.
- Kazerooni, A. F.; Khalili, N.; Liu, X.; Haldar, D.; Jiang, Z.; Anwar, S. M.; Albrecht, J.; Adewole, M.; Anazodo, U.; Anderson, H.; et al.The Brain Tumor Segmentation (BraTS) Challenge 2023: Focus on Pediatrics (CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs). arXiv:2305.17033v7 [eess.IV] 23 May 2024.ù.
- Pati, S., Baid, U., Edwards, B., Sheller, M. J., Foley, P., Anthony Reina, G., Thakur, S., Sako, C., Bilello, M., Davatzikos, C., Martin, J., Shah, P., Menze, B., & Bakas, S.The federated tumor segmentation (FeTS) tool: an open-source solution to further solid tumor research.Physics in medicine and biology vol. 67,20 10.1088/1361-6560/ac9449. 12 Oct. 2022. [CrossRef]
- Kofler, F.; Meissen, F.; Steinbauer, F.; Graf, R.; Ehrlich, S. K.; Reinke, A.; Oswald, E.; Waldmannstetter, D.; Hoelzl, F.; Horvath, I.; Turgut, O.; et al.The Brain Tumor Segmentation (BraTS) Challenge: Local Synthesis of Healthy Brain Tissue via Inpainting. arXiv:2305.08992v3 [eess.IV] 22 Sep 2024.
- LaBella D.; Adewole, M.; Alonso-Basanta, M.; Altes, T.; Anwar, S. M.; Baid, U.; Bergquist, T.; Bhalerao, R.; Chen, S.; Chung, V.; et al.The ASNR-MICCAI Brain Tumor Segmentation (BraTS) Challenge 2023: Intracranial Meningioma. arXiv:2305.07642v1 [cs.CV] 12 May 2023.
- Kazerooni, A. F.; Khalili, N.; Liu, X.; Gandhi, D.; Jiang, Z.; Anwar, S. M.; Albrecht, J.; Adewole, M.; Anazodo, U.; Anderson, H.; Baid, U.; et al. The Brain Tumor Segmentation in Pediatrics (BraTS-PEDs) Challenge: Focus on Pediatrics (CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs). arXiv:2404.15009v4 [cs.CV] 11 Jul 2024.
- Bakas, S.; Thakur, S. P.; Faghani, S.; Moassefi, M.; Baid, U.; Chung, V.; Pati, S.; Innani, S.; Baheti, B.; Albrecht, J.; Karargyris, A.; Kassem, H.; et al.;BraTS-Path Challenge: Assessing Heterogeneous Histopathologic Brain Tumor Sub-regions. arXiv:2405.10871v1 [cs.CV] 17 May 2024.
- de Verdier, M. C.; Saluja, R.; Gagnon, L.; LaBella, D.; Baid, U.; Tahon, N. H.; Foltyn-Dumitru, M.; Zhang, J.; Alafif, M.; Baig, S.; Chang, K.; D’Anna, G.; et al. The 2024 Brain Tumor Segmentation (BraTS) Challenge: Glioma Segmentation on Post-treatment MRI. arXiv:2405.18368v1 [cs.CV] 28 May 2024.
- LaBella, D.; Schumacher, K.; Mix, M.; Leu, K.; McBurney-Lin, S.; Nedelec, P.; Villanueva-Meyer, J.; Shapey, J.; Vercauteren, T.; Chia, K.; Al-Salihi, O.; Leu, J.; Halasz, L.; et al. Brain Tumor Segmentation (BraTS) Challenge 2024: Meningioma Radiotherapy Planning Automated Segmentation. arXiv:2405.18383v2 [cs.CV] 15 Aug 2024.
- Jiang, Z.; Ding, C.; Liu, M.; Tao, D. Two-Stage Cascaded U-Net: 1st Place Solution to BraTS Challenge 2019 Segmentation Task. Lecture Notes in Computer Science. 2020. [CrossRef]
- Isensee, F.; Jäger, P.F.; Full, P.M.; Vollmuth, P.; Maier-Hein, K.H. nnU-Net for Brain Tumor Segmentation. arXiv:2011.00848v1 [eess.IV] November 2, 2020.
- Luu, H.M.; Park, S.H. Extending nn-UNet for brain tumor segmentation. Proceedings of the RSNA-ASNR-MICCAI BraTS 2021 Benchmark. Published December 9, 2021. arXiv:2112.04653.
- Zeineldin, R.A.; Karar, M.E.; Burgert, O.; Mathis-Ullrich, F. Multimodal CNN Networks for Brain Tumor Segmentation in MRI: A BraTS 2022 Challenge Solution. arXiv preprint arXiv:2212.09310, 2022.
- Ferreira, A.; Solak, N.; Li, J.; Dammann, P.; Kleesiek, J.; Alves, V.; Egger, J. How we won BraTS 2023 Adult Glioma challenge? Just faking it! Enhanced Synthetic Data Augmentation and Model Ensemble for brain tumour segmentation. arXiv:2402.17317v2 [eess.IV] 17 Jul 2024.
- LaBella, D.; Baid, U.; Khanna, O.; McBurney-Lin, S.; McLean, R.; Nedelec, P.; Rashid, A.; Tahon, N. H.; Altes, T.; Bhalerao, R.; Dhemesh, Y.; Godfrey, D.; Hilal, F.; Floyd, S.; Janas, A.; Kazerooni, A. F.; Kirkpatrick, J.; Kent, C.; Kofler, F.; Leu, K.; Maleki, N.; Menze, B.; Pajot, M.; Reitman, Z. J.; Rudie, J. D.; Saluja, R.; Velichko, Y.; Wang, C.; Warman, P.; et al. Analysis of the BraTS 2023 Intracranial Meningioma Segmentation Challenge. arXiv:2405.09787v1 [eess.IV] 16 May 2024.
- Capellán-Martín, D.; Jiang, Z.; Parida, A.; Liu, X.; Lam, V.; Nisar, H.; Tapp, A.; Elsharkawi, S.; Ledesma-Carbayo, M.J.; Anwar, S.M.; Linguraru, M.G. Model Ensemble for Brain Tumor Segmentation in Magnetic Resonance Imaging. arXiv:2409.08232v1 [eess.IV] 12 Sep 2024.
- Baltruschat, I. M.; Janbakhshi, P.; Lenga, M. BraSyn 2023 challenge: Missing MRI synthesis and the effect of different learning objectives. arXiv:2403.07800v2 [eess.IV] 18 Mar 2024.
- Ferreira, André; Jesus, Tiago; Puladi, Behrus; Kleesiek, Jens; Alves, Victor; Egger, Jan. Improved Multi-Task Brain Tumour Segmentation with Synthetic Data Augmentation. arXiv:2411.04632v1 [cs.CV] 7 Nov 2024. Available from: https://arxiv.org/abs/2411.04632.
- Parida, Abhijeet; Capellán-Martín, Daniel; Jiang, Zhifan; Tapp, Austin; Liu, Xinyang; Anwar, Syed Muhammad; Ledesma-Carbayo, María J.; Linguraru, Marius George. Adult Glioma Segmentation in Sub-Saharan Africa using Transfer Learning on Stratified Finetuning Data. arXiv:2412.04111v1 [eess.IV] 5 Dec 2024.
- Cho, Jihoon; Park, Seunghyuck; Park, Jinah. Two-Stage Approach for Brain MR Image Synthesis: 2D Image Synthesis and 3D Refinement. arXiv:2410.10269v2 [eess.IV] 2 Dec 2024. Available from: https://arxiv.org/abs/2410.10269.
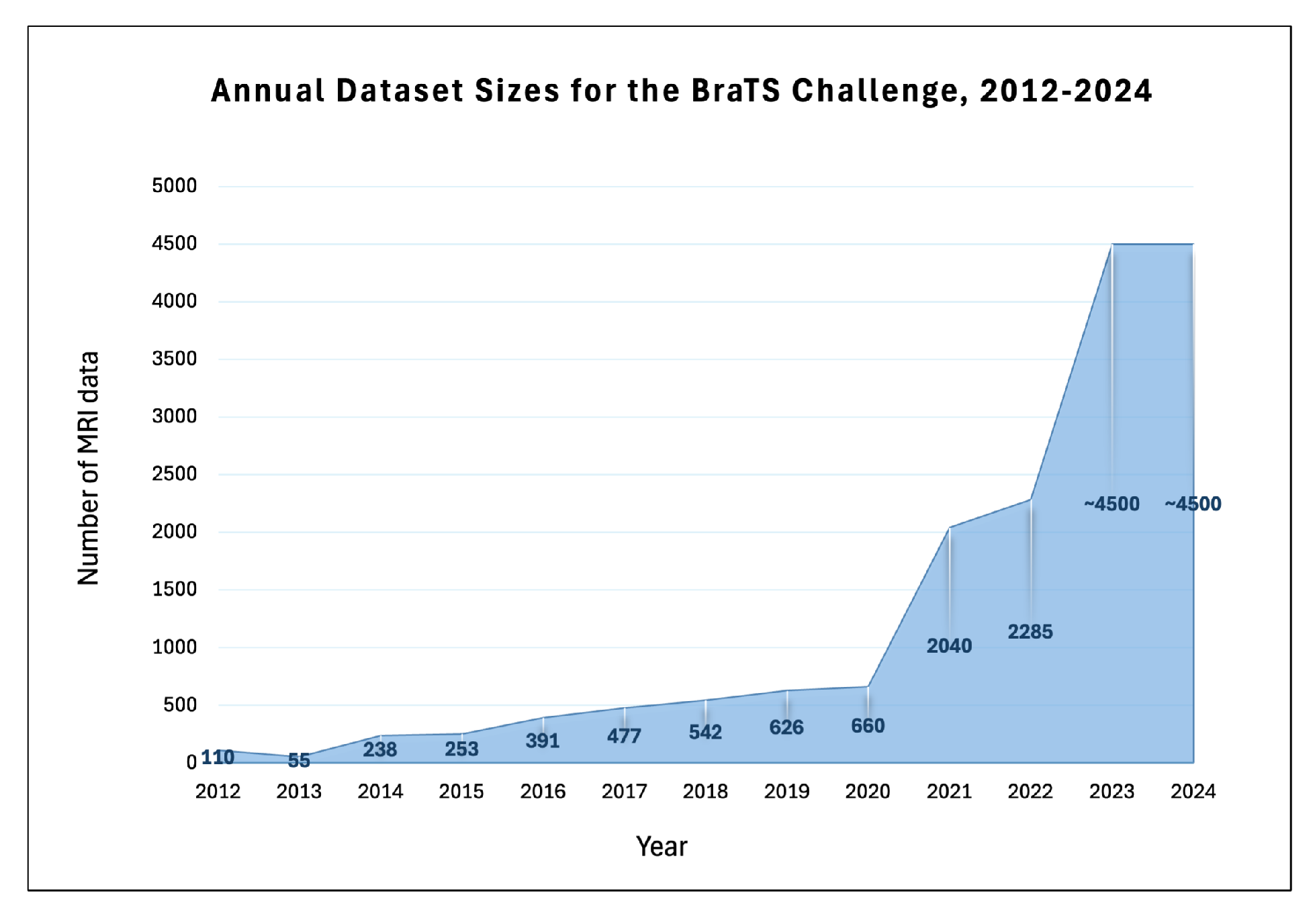
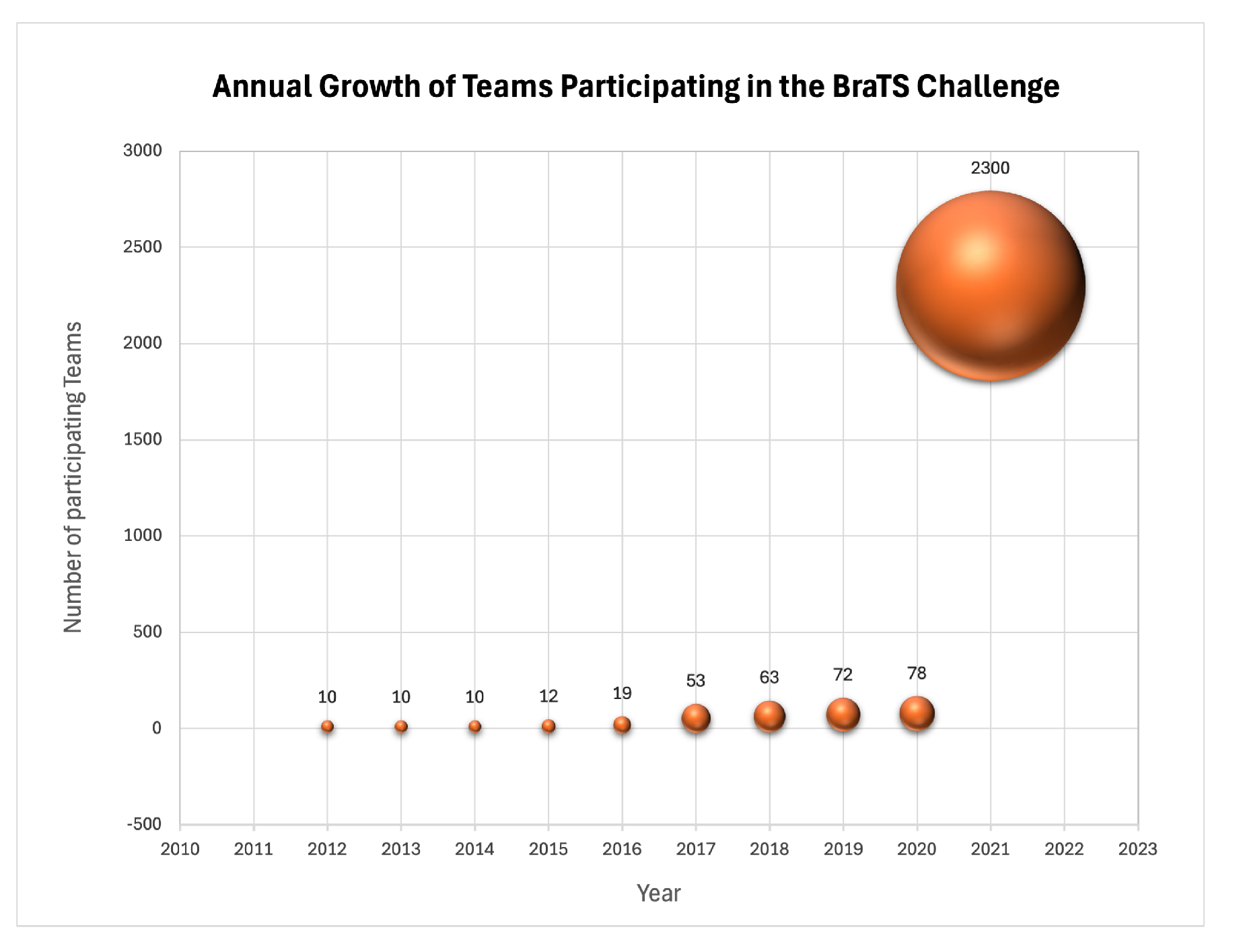
| Year | Total | Training | Validation | Testing | Tasks | Timepoint |
| Data | Data | Data | Data | |||
| 2012 | 45 real | 30 | NA | 15 | Brain Tumor Segmentation | Pre-operative |
| 65 synthetic | 50 | NA | 15 | |||
| ]1*2013 | 55 | 30 | NA | 25 | Brain Tumor Segmentation | Pre-operative |
| 2014 | 238 | 200 | NA | 38 | Brain Tumor Segmentation | Longitudinal |
| Disease Progression | ||||||
| 2015 | 253 | 200 | NA | 53 | Brain Tumor Segmentation | Longitudinal |
| Disease Progression | ||||||
| 2016 | 391 | 200 | NA | 191 | Brain Tumor Segmentation | Longitudinal |
| Disease Progression | ||||||
| 2017 | 477 | 285 | 46 | 146 | Brain Tumor Segmentation | Pre-operative |
| Overall Survival Prediction | ||||||
| 2018 | 542 | 285 | 66 | 191 | Brain Tumor Segmentation | Pre-operative |
| Overall Survival Prediction | ||||||
| 2019 | 626 | 335 | 125 | 166 | Brain Tumor Segmentation | Pre-operarive |
| Overall Survival Prediction | ||||||
| 2020 | 660 | 369 | 125 | 166 | Brain Tumor Segmentation | Pre-operative |
| Overall Survival Prediction | ||||||
| 2021 | 2040 | 1251 | 219 | 570 | Brain Tumor Segmentation | Pre-operative |
| MGMT classification | ||||||
| ]1*2022 | 2285 | 1251 | 219 | 815 | Brain Tumor Segmentation | Pre-operative |
| 2023 | 2040 | 1251 | 219 | 570 | Segmentation: Adult Glioma | Pre-operative |
| 105 | 60 | 15 | 30 | Segmentation: BraTS-Africa | Pre-operative | |
| 1424 | 1000 | 141 | 283 | Segmentation: Meningioma | Pre-operative | |
| 966 | 402 | 31 | 59 | Segmentation: Brain Metastases | Pre-operative | |
| 228 | 99 | 45 | 84 | Segmentation: Pediatric Tumors | Pre-operative | |
| 2040 | 1251 | 219 | 570 | Synthesis (Global): Inpainting | Pre-operative | |
| 2038 | 1251 | 219 | 568 | Synthesis (Local): Inpainting | Pre-operative | |
| 2040 | 1251 | 219 | 570 | Evaluating Augmentations for BraTS | Pre-operative | |
| 2024 | 2200 | 1540 | 220 | 440 | Segmentation: Adult Glioma Post Treatment | Post-operative |
| 60 | 35 | Segmentation: BraTS-Africa | Pre-operative | |||
| 750 | 500 | 70 | 180 | Segmentation: Meningioma Radiotherapy | Pre & Post -operative | |
| 652 | 88 | Segmentation: Brain Metastases | Pre & Post -operative | |||
| 464 | 261 | 91 | 112 | Segmentation: Pediatric Tumors | Pre-operative | |
| 2489 | 451 | Segmentation: Generalizability | Pre-operative | |||
| 2099 | 1251 | 219 | 629 | Synthesis (Global): Missing MRI | Pre-operative | |
| 2315 | 1251 | 219 | 845 | Synthesis (Local): Inpainting | Pre-operative | |
| 2040 | 1251 | 219 | 570 | Evaluating Augmentations for BraTS | Pre-operative | |
| 280,000 | 195,000 | 25,000 | 60,000 | Pathology | Pre-operative |
| Year | Total Data | Training Data | Validation Data | Test Data |
| BraTS 2014 | 238 | 200 data coming from both BraTS ’12-’13 and TCIA, including longitudinal data | NA | 38 unseen data from BraTS ’12-’12 test set and TCIA |
| BraTS 2015 | 253 | 200 data: Identical to the BraTS 2014 training set | NA | 53 unseen data from BraTS ’12-’12 test set and TCIA |
| BraTS 2016 | 391 | 200 data: Identical to the BraTS 2014 training set | NA | 191 unseen data from BraTS ’12-’12 test set and TCIA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




