Submitted:
31 December 2024
Posted:
02 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Sample Preparation
2.4. Preparation of Standards
2.5. Total Phenolic Content Assay
2.6. DPPH Antioxidant Assay
2.7. ABTS Antioxidant Assay
2.8. FRAP Antioxidant Assay
2.9. Acetylcholinesterase Inhibition Assay
2.10. HPTLC–DPPH Fingerprint
2.11. HPLC/UV/MS Analysis
2.12. Statistical Analysis
3. Results
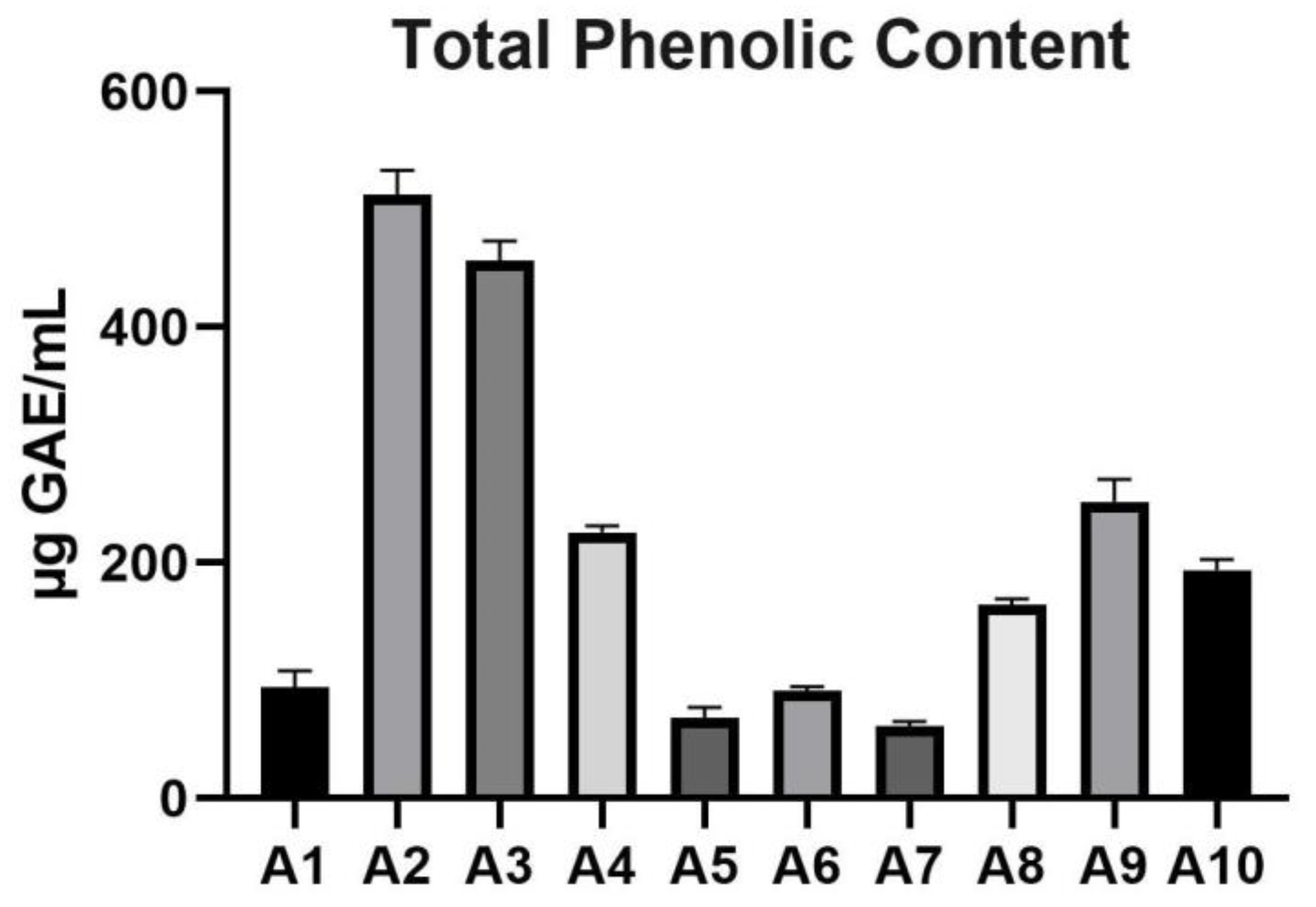
3.1. Total Phenolic Content
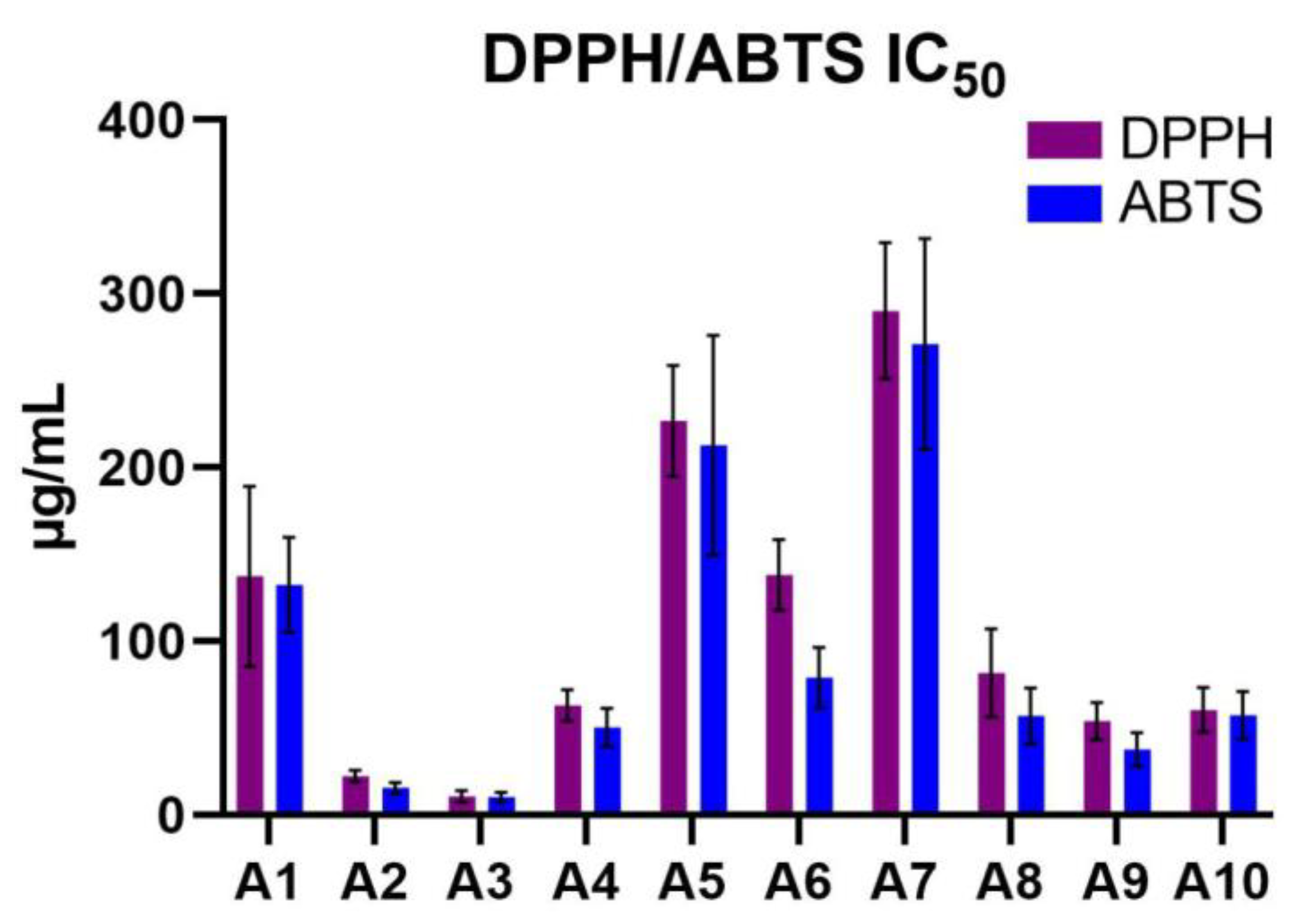
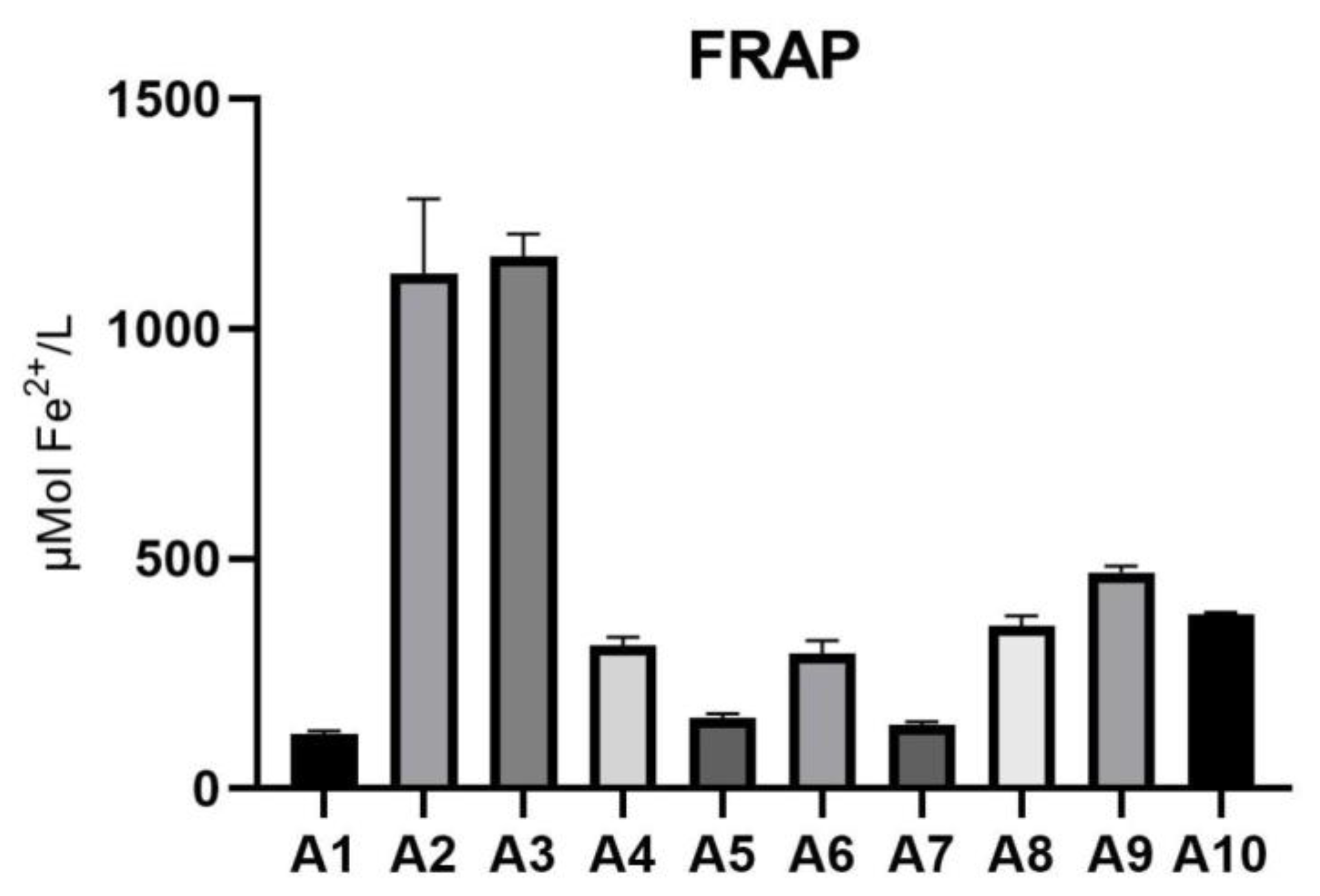
3.2. Antioxidant Activity
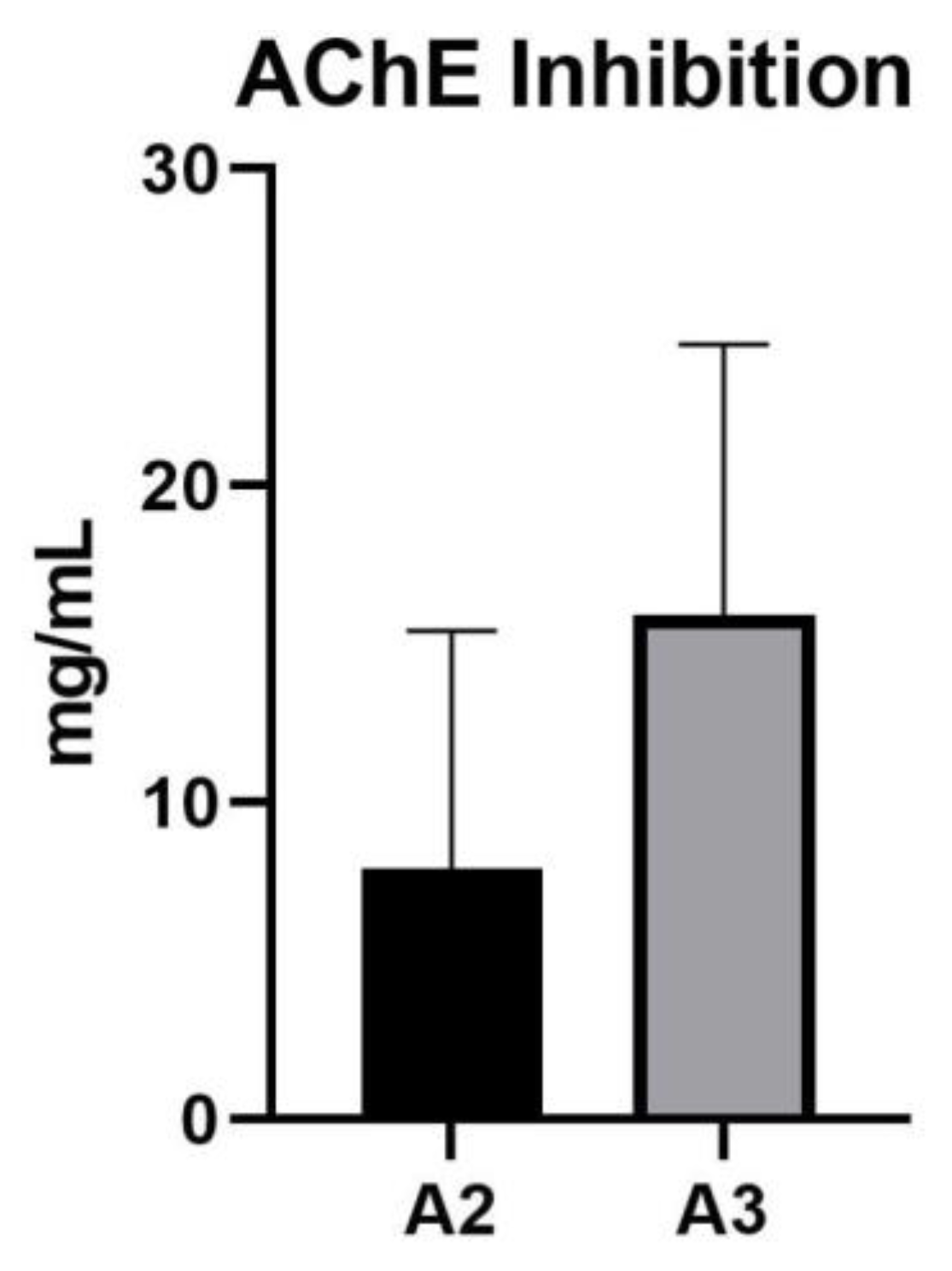
3.3. Acetylcholinesterase Inhibition Activity
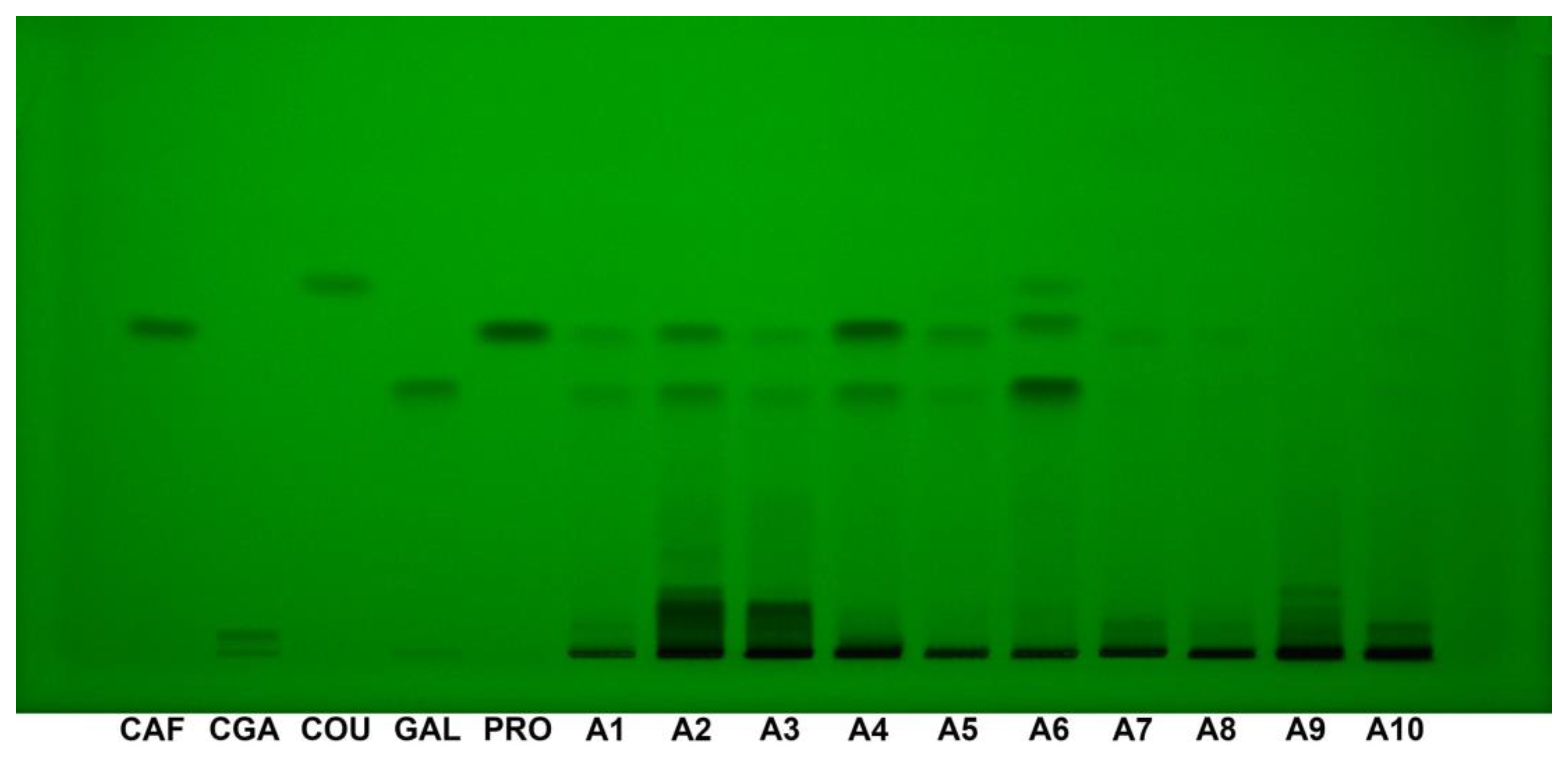
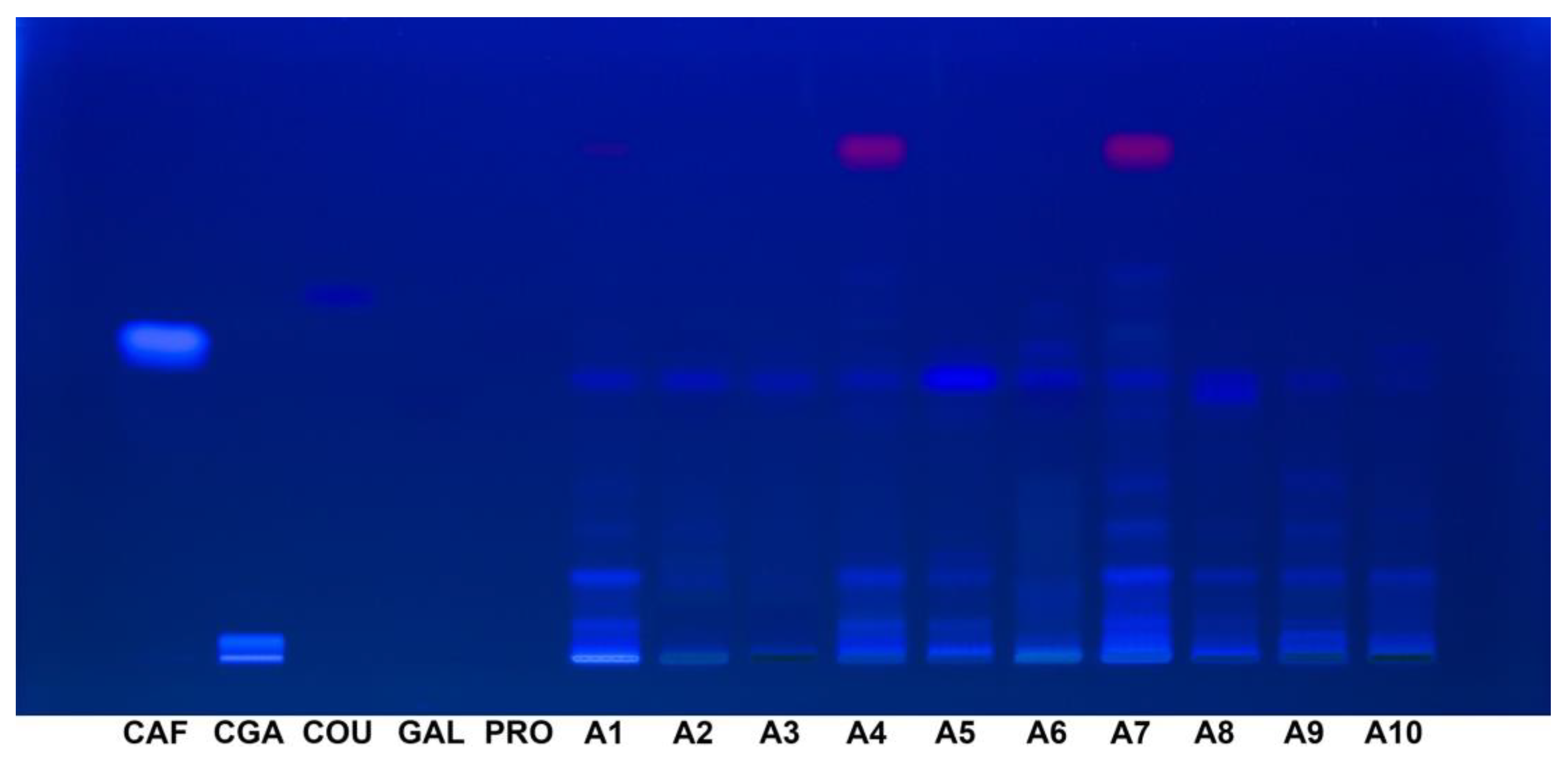
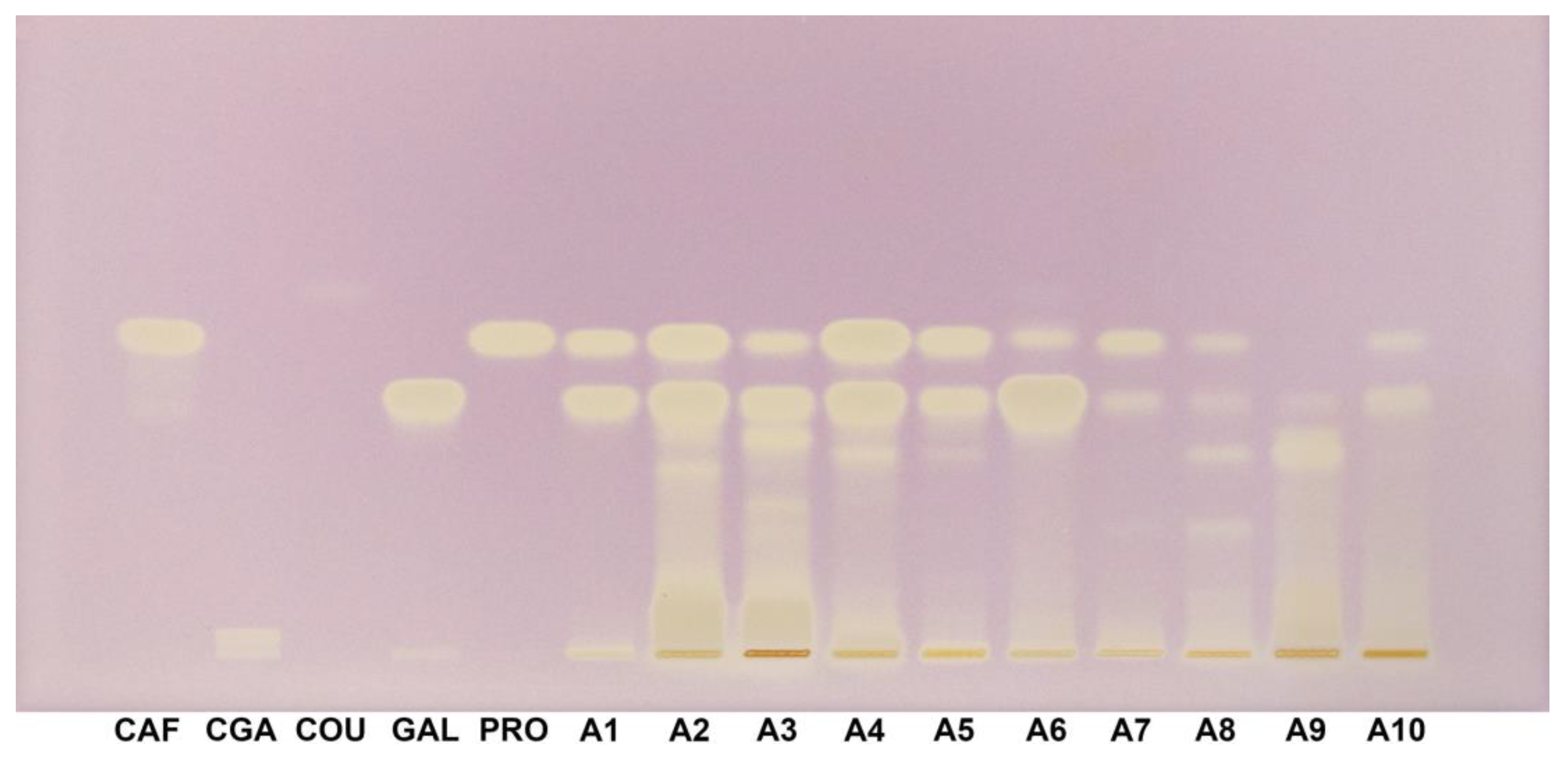
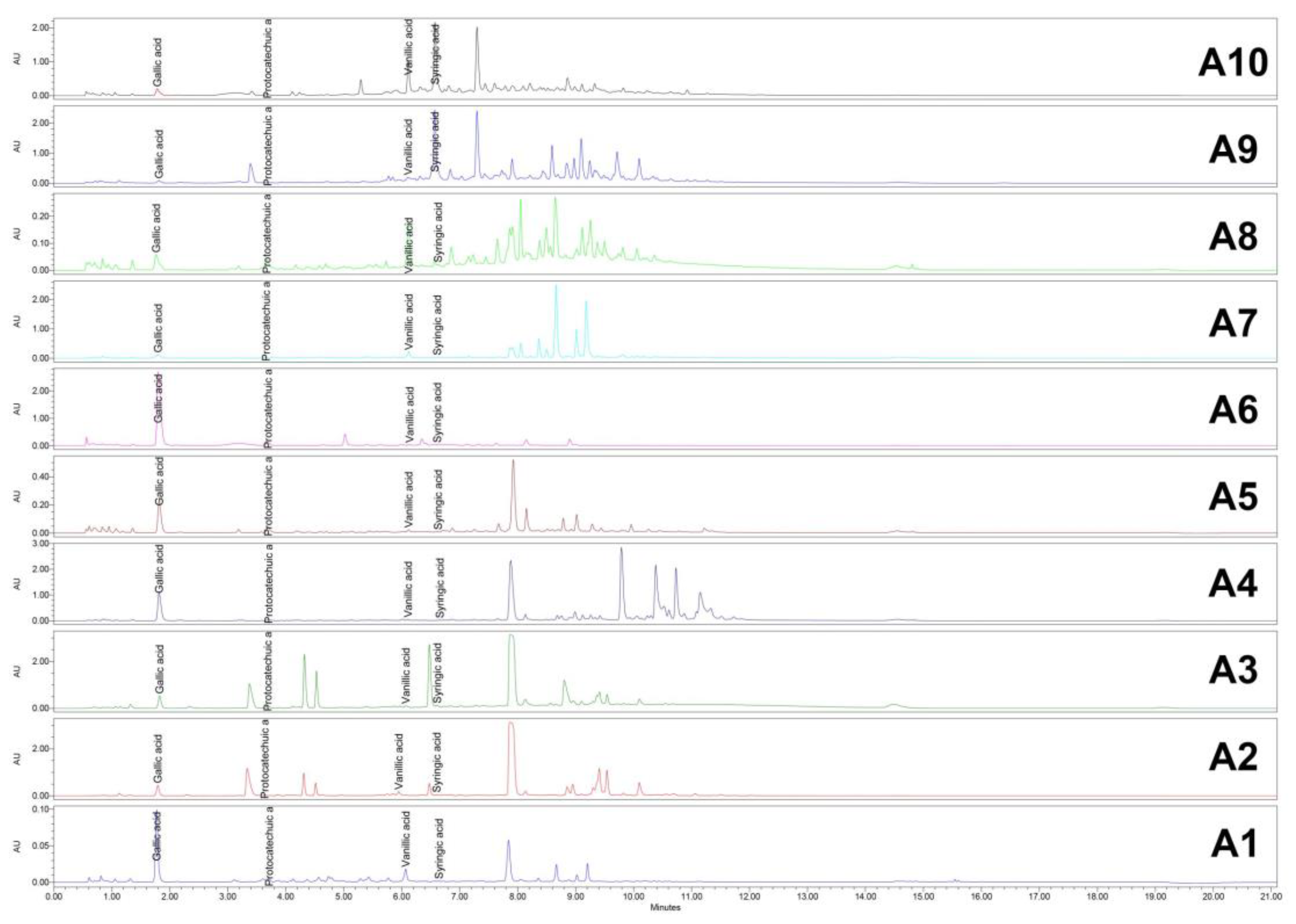
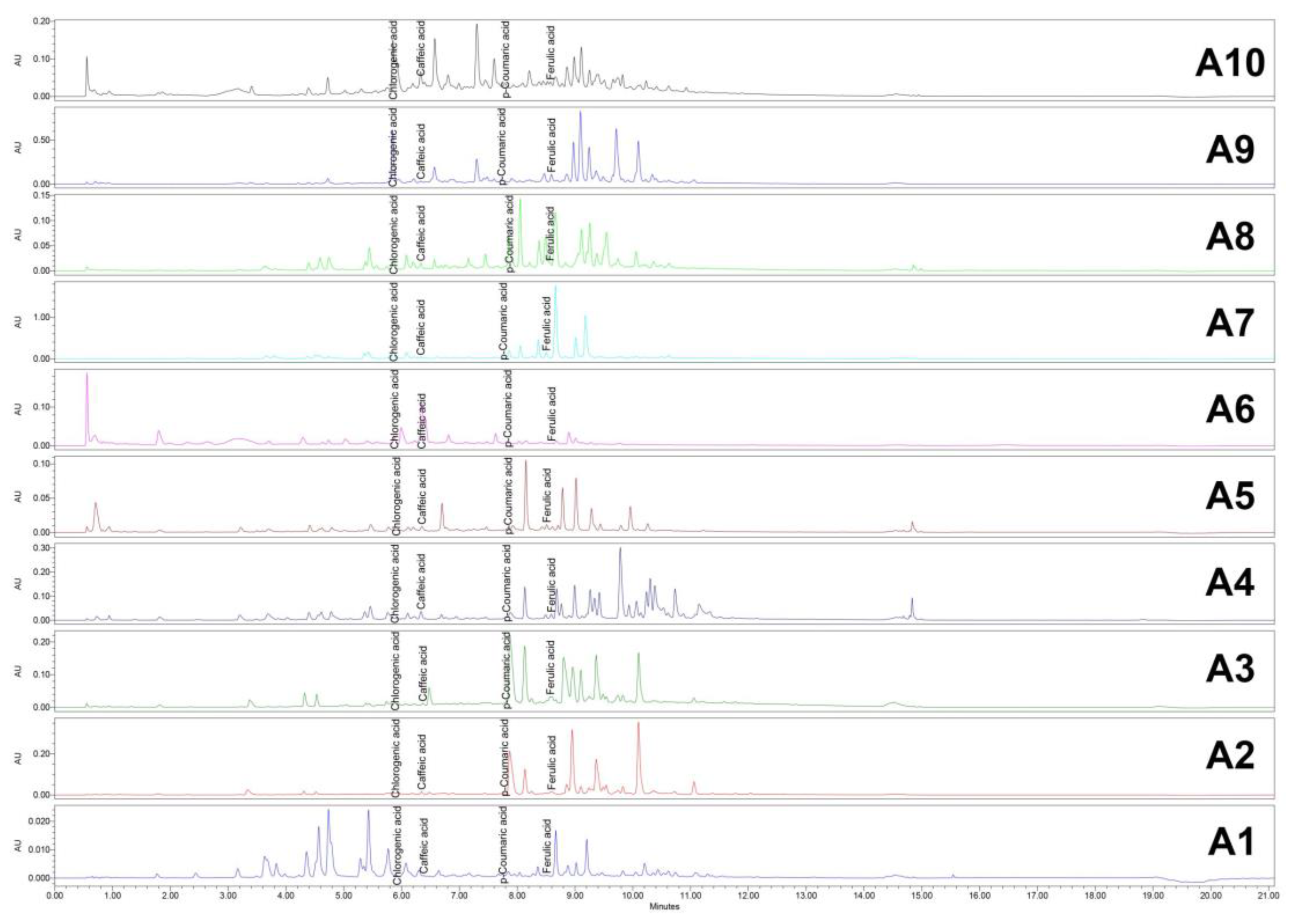
3.4. HPTLC–DPPH Fingerprint
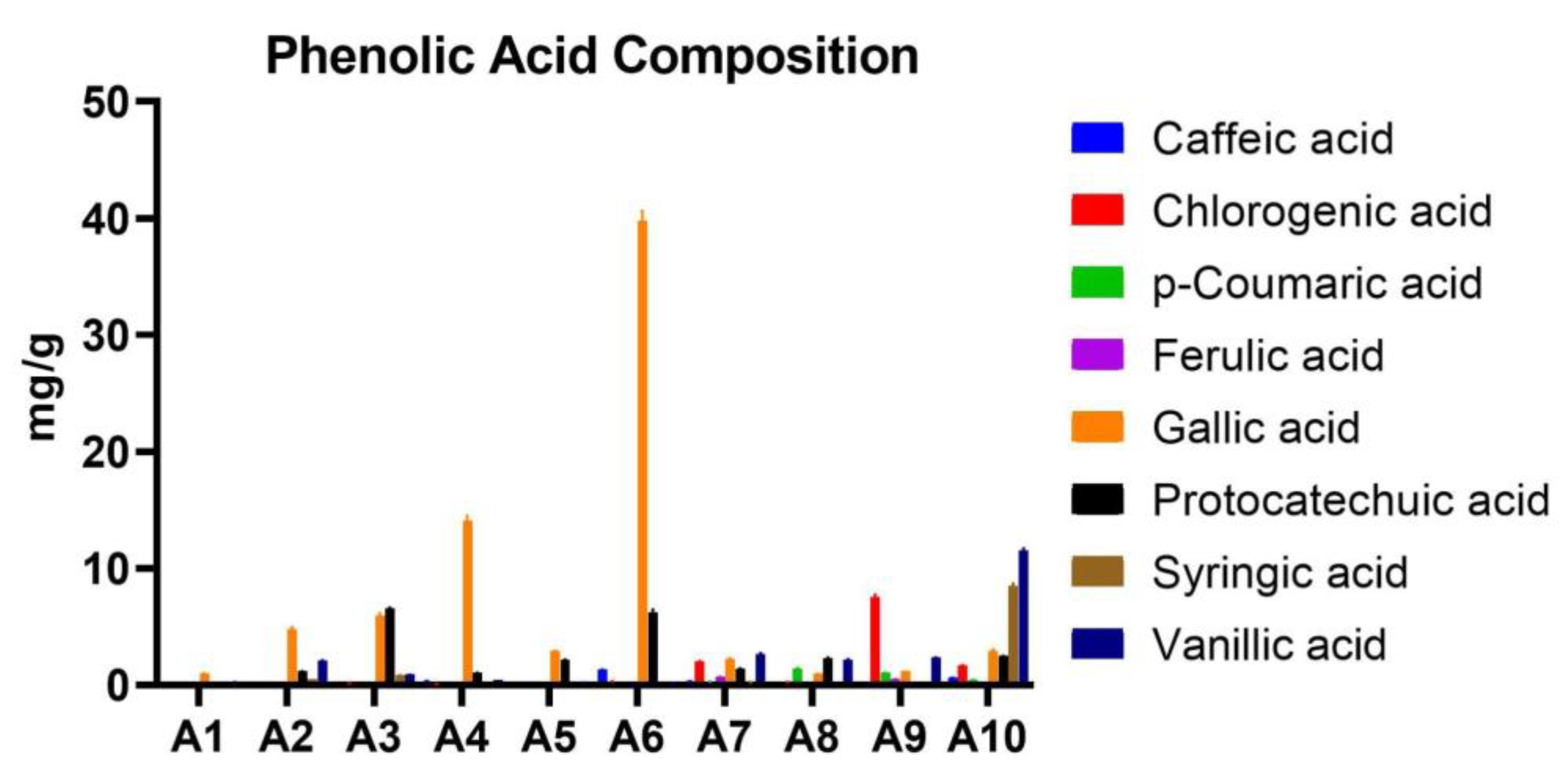
3.5. HPLC/UV/MS Analysis
4. Discussion
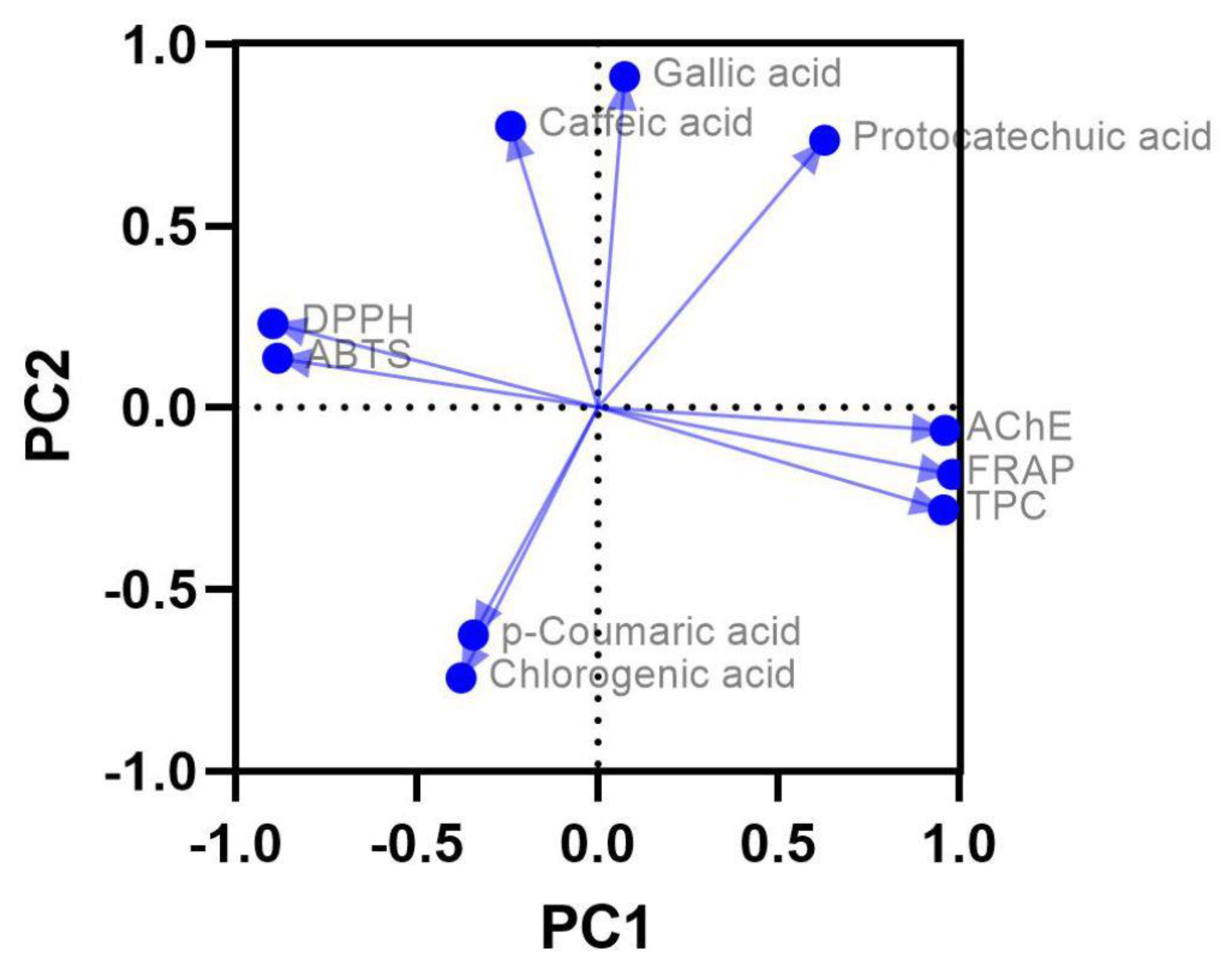
4.1. Correlation of TPC with Antioxidant and AChE Inhibitory Activities
4.2. Correlation of Phenolic Acids with TPC, Antioxidant and AChE Inhibitory Activities
4.3. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Săvulescu, T.; Nyarady, E.I. (Eds). Flora R.P.R., 1st ed.; Romanian Academy Publishing House: Bucharest, Romania, 1958; Volume VI. (in Romanian) [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds). Flora Europaea. Vol. 2: Rosaceae to Umbelliferae, 1st ed.; Cambridge University Press: Cambridge, UK, 1968; pp. 237–239. [Google Scholar]
- Ciocârlan, V. Flora ilustrată a României. Pteridophyta et Spermatophyta, 3rd ed.; Ceres Publishing House: Bucharest, Romania, 2009. (in Romanian) [Google Scholar]
- Sârbu, I.; Ştefan, N.; Oprea, A. Plante vasculare din România. Determinator ilustrat de teren., 1st ed.; Victor B Victor Publishing House: Bucharest, Romania, 2013. (in Romanian) [Google Scholar]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lin, F.; Zhang, R.; Wang, M.; Gu, R.; Long, C. Acer truncatum Bunge: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 282, 114572. [Google Scholar] [CrossRef] [PubMed]
- Şen Utsukarçi, B.; Bal, Y.; Gurdal, B.; Bardakçı Altan, H.; Yılmaz, F.N.; Döşler, S. Pharmacognostical Studies on Acer campestre L. subsp. campestre. Int. J. Second. Metab. 2020, 7, 126–138. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, R.; Liu, Y.; Liu, J.; Zhang, X.; Gong, Z.; Qu, T. Metabolomics Combined with Transcriptomics Analysis Reveals the Regulation of Flavonoids in the Leaf Color Change of Acer truncatum Bunge. Int. J. Mol. Sci. 2024, 25, 13325. [Google Scholar] [CrossRef]
- Liu, W.; Ouyang, Y.; Wan, C.P. Flavonoids of the Genus of Acer: A Mini Review. Asian J. Chem. 2013, 25, 7075–7078. [Google Scholar] [CrossRef]
- Chang, B.Y.; Jung, Y.S.; Yoon, C.-S.; Oh, J.S.; Hong, J.H.; Kim, Y.-C.; Kim, S.Y. Fraxin Prevents Chemically Induced Hepatotoxicity by Reducing Oxidative Stress. Molecules 2017, 22, 587. [Google Scholar] [CrossRef]
- García-Pérez, M.-E.; Kasangana, P.-B.; Stevanovic, T. Bioactive Molecules from Myrianthus arboreus, Acer rubrum, and Picea mariana Forest Resources. Molecules 2023, 28, 2045. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, T.R.; Meda, N.R.; Stevanovic, T. Suitability of DPPH spiking for antioxidant screening in natural products: the example of galloyl derivatives from red maple bark extract. Anal. Bioanal. Chem. 2017, 409, 5225–5237. [Google Scholar] [CrossRef]
- Dong, L.-P.; Ni, W.; Dong, J.-Y.; Li, J.-Z.; Chen, C.-X.; Liu, H.-Y. A New Neolignan Glycoside from the Leaves of Acer truncatum. Molecules 2006, 11, 1009–1014. [Google Scholar] [CrossRef]
- Aouinti, H.; Touhami, I.; Khouja, M.; Messaoud, C.; Khaldi, A. Chemical composition analysis of seed oil from the three wild Tunisian provenances of Acer monspessulanum L. J. New Sci. 2022, 90, 5106–5112. [Google Scholar] [CrossRef]
- Hovanet, M.V.; Dociu, N.; Dinu, M.; Ancuceanu, R.; Morosan, E.; Oprea, E. A Comparative Physico-chemical Analysis of Acer platanoides and Acer pseudoplatanus Seed Oils. Rev. Chim. (Bucharest) 2015, 66, 987–991. [Google Scholar]
- Le, X.; Zhang, W.; Sun, G.; Fan, J.; Zhu, M. Research on the Differences in Phenotypic Traits and Nutritional Composition of Acer truncatum Bunge Seeds from Various Regions. Foods 2023, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Huang, Y.; Dong, Y.; Zhang, W.; Xia, F.; Bai, H.; Stevanovic, Z.D.; Li, H.; Shi, L. Effective Improvement of the Oxidative Stability of Acer truncatum Bunge Seed Oil, a New Woody Oil Food Resource, by Rosemary Extract. Antioxidants 2023, 12, 889. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Ma, J.; Xin, H.; Wang, S.; Chen, Z.; Hong, X.; Zhang, B.; Li, L. Comparative Study of the Fatty Acid Composition of the Acer truncatum Bunge from Different Producing Areas. Forests 2022, 13, 1409. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Liu, T.; Ding, Z.; Liu, W.; Gu, Q.; Mu, Y.; Xu, J.; Seeram, N.P.; Huang, X.; et al. Glucitol-Core Containing Gallotannins-Enriched Red Maple (Acer rubrum) Leaves Extract Alleviated Obesity via Modulating Short-Chain Fatty Acid Production in High-Fat Diet-Fed Mice. J. Funct. Foods 2020, 70, 103970. [Google Scholar] [CrossRef]
- Kausar, F.; Farooqi, M.-A.; Farooqi, H.-M.-U.; Salih, A.-R.-C.; Khalil, A.-A.-K.; Kang, C.-w.; Mahmoud, M.H.; Batiha, G.-E.-S.; Choi, K.-h.; Mumtaz, A.-S. Phytochemical Investigation, Antimicrobial, Antioxidant and Anticancer Activities of Acer cappadocicum Gled. Life 2021, 11, 656. [Google Scholar] [CrossRef]
- Bhatta, S.; Ratti, C.; Poubelle, P.E.; Stevanovic, T. Nutrients, Antioxidant Capacity and Safety of Hot Water Extract from Sugar Maple (Acer saccharum M.) and Red Maple (Acer rubrum L.) Bark. Plant Foods Hum. Nutr. 2018, 73, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Meda, N.R.; Poubelle, P.E.; Stevanovic, T. Antioxidant Capacity, Phenolic Constituents and Toxicity of Hot Water Extract from Red Maple Buds. Chem. Biodivers. 2017, 14, e1700028. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.-H.; Park, E.; Cho, W.-K.; Kim, Y.-H.; Ma, J.Y. Therapeutic Effects of Acer palmatum Thumb. Leaf Extract (KIOM-2015E) on Benzalkonium Chloride-Induced Dry Eye in a Mouse Model. Int. J. Mol. Sci. 2022, 23, 14964. [Google Scholar] [CrossRef]
- Meda, N.R.; Stevanovic, T.; Poubelle, P.E. Anhydroglucitol-core gallotannins from red maple buds modulate viability of human blood neutrophils. Toxicol. In Vitro 2019, 60, 76–86. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Oh, T.W.; Park, E.; Yim, N.-H.; Park, K.I.; Cho, W.K.; Ma, J.Y. Anti-Inflammatory and Anti-Apoptotic Effects of Acer palmatum Thumb. Extract, KIOM-2015EW, in a Hyperosmolar-Stress-Induced In Vitro Dry Eye Model. Nutrients 2018, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Cha, B.-Y.; Woo, J.-T.; Kim, Y.-C.; Jang, J.-H. Acerogenin A from Acer nikoense Maxim Prevents Oxidative Stress-Induced Neuronal Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression in Mouse Hippocampal HT22 Cell Line. Molecules 2015, 20, 12545–12557. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Cho, E.J. The Protective Effects of Acer okamotoanum and Isoquercitrin on Obesity and Amyloidosis in a Mouse Model. Nutrients 2020, 12, 1353. [Google Scholar] [CrossRef]
- Son, S.-Y.; Choi, J.-H.; Kim, E.-B.; Yin, J.; Seonu, S.-Y.; Jin, S.-Y.; Oh, J.-Y.; Lee, M.-W. Chemopreventive Activity of Ellagitannins from Acer pseudosieboldianum (Pax) Komarov Leaves on Prostate Cancer Cells. Plants 2023, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, H.M.U.; Sammantasinghar, A.; Kausar, F.; Farooqi, M.A.; Chethikkattuveli Salih, A.R.; Hyun, K.; Lim, J.-H.; Khalil, A.A.K.; Mumtaz, A.S.; Choi, K.H. Study of the Anticancer Potential of Plant Extracts Using Liver Tumor Microphysiological System. Life 2022, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Li, L.; Seeram, N.P. Effects of Maple (Acer) Plant Part Extracts on Proliferation, Apoptosis and Cell Cycle Arrest of Human Tumorigenic and Non-Tumorigenic Colon Cells. Phytother. Res. 2012, 26, 995–1002. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Lee, S.R.; Kim, B.; Hong, J.-H.; Jang, Y.S.; Lee, D.E.; Pang, C.; Kang, K.S.; Kim, K.H. Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Signaling Pathways. Plants 2022, 11, 3387. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, Y.J.; Han, Y.B.; Lee, J.C.; Lee, S.; Park, H.-J.; Lee, H.-J.; Kim, K.H. Isoamericanoic Acid B from Acer tegmentosum as a Potential Phytoestrogen. Nutrients 2018, 10, 1915. [Google Scholar] [CrossRef]
- Ha, H.; Shim, K.-S.; Kim, T.; An, H.; Lee, C.-J.; Lee, K.J.; Ma, J.Y. Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef]
- Phong, N.V.; Min, B.S.; Yang, S.Y.; Kim, J.A. Inhibitory Effect of Coumarins and Isocoumarins Isolated from the Stems and Branches of Acer mono Maxim. against Escherichia coli β-Glucuronidase. Appl. Sci. 2022, 12, 10685. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liu, Y.-H.; Liu, D.-Z.; Xu, J.-Y.; Zhang, Q. Insulin-Mimic Components in Acer truncatum Leaves: Bio-Guided Isolation, Annual Variance Profiling and Regulating Pathway Investigated by Omics. Pharmaceuticals 2021, 14, 662. [Google Scholar] [CrossRef]
- Liang, Y.; Kong, F.; Ma, X.; Shu, Q. Inhibitory Effect of Acer truncatum Bunge Seed Coat Extract on Fatty Acid Synthase, Differentiation and Lipid Accumulation in 3T3-L1 Adipocytes. Molecules 2022, 27, 1324. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-H.; Lee, S.-J.; Kim, S.-H.; Jang, S.-H.; Won, C.; Kim, H.-D.; Kim, T.H.; Cho, J.-H. Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet. Nutrients 2020, 12, 3753. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Cho, E.J. Flavonoids from Acer okamotoanum Inhibit Adipocyte Differentiation and Promote Lipolysis in the 3T3-L1 Cells. Molecules 2020, 25, 1920. [Google Scholar] [CrossRef]
- Ma, H.; Xu, J.; DaSilva, N.A.; Wang, L.; Wei, Z.; Guo, L.; Johnson, S.L.; Lu, W.; Xu, J.; Gu, Q.; Seeram, N.P. Cosmetic applications of glucitol-core containing gallotannins from a proprietary phenolic-enriched red maple (Acer rubrum) leaves extract: inhibition of melanogenesis via down-regulation of tyrosinase and melanogenic gene expression in B16F10 melanoma cells. Arch. Dermatol. Res. 2017, 309, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Roy, T.; Boateng, S.T.; He, H.; Liu, C.; Liu, W.; Li, D.; Wu, P.; Seeram, N.P.; Chamcheu, J.C.; et al. Standardized Pomegranate (Pomella®) and Red Maple (Maplifa®) Extracts and Their Phenolics Protect Type I Collagen by the Inhibition of Matrix Metalloproteinases, Collagenase, and Collagen Cross-Linking. Molecules 2022, 27, 7919. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-J.; Ji, Y.; Jang, Y.-P.; Choung, S.-Y. Acer tataricum subsp. ginnala Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-κB, and TGFβ/Smad Signaling in UVB-Irradiated Human Dermal Fibroblasts. Molecules 2021, 26, 662. [Google Scholar] [CrossRef]
- Ma, H.; Guo, H.; Liu, C.; Wan, Y.; Seeram, N.P. Protective Effects of a Red Maple (Acer rubrum) Leaves Extract on Human Keratinocytes against H2O2-induced Oxidative Stress. FASEB J. 2018, 32, 656.33–656.33. [Google Scholar] [CrossRef]
- Liu, J.; Liang, S.; Qin, K.; Jia, B.; Ren, Z.; Yang, X.; Yang, X. Acer truncatum leaves extract modulates gut microbiota, improves antioxidant capacity, and alleviates lipopolysaccharide-induced inflammation in broilers. Poult. Sci. 2023, 102, 102951. [Google Scholar] [CrossRef]
- Chen, X.; Song, Y.; Song, W.; Han, J.; Cao, H.; Xu, X.; Li, S.; Fu, Y.; Ding, C.; Lin, F.; Shi, Y.; Li, J. Multi-omics reveal neuroprotection of Acer truncatum Bunge Seed extract on hypoxic-ischemia encephalopathy rats under high-altitude. Commun. Biol. 2023, 6, 1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, W.; Song, Y.; Cao, H.; Xu, X.; Li, S.; Fu, Y.; Xue, T.; Ding, C.; Lin, F.; Shi, Y.; Li, J. Lipidomics reveal the cognitive improvement effects of Acer truncatum Bunge seed oil on hypoxic-ischemic encephalopathy rats. Food Funct. 2023, 14, 6610–6623. [Google Scholar] [CrossRef]
- Song, W.; Zhang, K.; Xue, T.; Han, J.; Peng, F.; Ding, C.; Lin, F.; Li, J.; Sze, F.T.A.; Gan, J.; Chen, X. Cognitive improvement effect of nervonic acid and essential fatty acids on rats ingesting Acer truncatum Bunge seed oil revealed by lipidomics approach. Food Funct. 2022, 13, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, H.; Pesce, M.; Patruno, A.; Bahrami, A.; Pour, P.M.; Farzaei, M.H. A Review of Plant Extracts and Plant-Derived Natural Compounds in the Prevention/Treatment of Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2021, 22, 833. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Shen, J.; Gao, Y.; He, C.; Peng, Y.; Xiao, P. Ku-jin tea (Acer tataricum subsp. ginnala or A. tataricum subsp. theiferum), an underestimated functional beverage rich in antioxidant phenolics. J. Funct. Foods 2016, 24, 75–84. [Google Scholar] [CrossRef]
- El-Khatib, A.H.; Engel, A.M.; Weigel, S. Co-Occurrence of Hypoglycin A and Hypoglycin B in Sycamore and Box Elder Maple Proved by LC-MS/MS and LC-HR-MS. Toxins 2022, 14, 608. [Google Scholar] [CrossRef]
- Renaud, B.; Kruse, C.-J.; François, A.-C.; Grund, L.; Bunert, C.; Brisson, L.; Boemer, F.; Gault, G.; Ghislain, B.; Petitjean, T.; et al. Acer pseudoplatanus: A Potential Risk of Poisoning for Several Herbivore Species. Toxins 2022, 14, 512. [Google Scholar] [CrossRef]
- Kamiński, P.; Gruba, M.; Fekner, Z.; Tyśkiewicz, K.; Kobus, Z. The Influence of Water Extraction Parameters in Subcritical Conditions and the Shape of the Reactor on the Quality of Extracts Obtained from Norway Maple (Acer platanoides L.). Processes 2023, 11, 3395. [Google Scholar] [CrossRef]
- Zhang, M.; Choe, J.; Bu, T.; Liu, S.; Kim, S. Comparison of Antioxidant Properties and Metabolite Profiling of Acer pseudoplatanus Leaves of Different Colors. Antioxidants 2023, 12, 65. [Google Scholar] [CrossRef]
- Kim, I.; Ha, J.-H.; Jeong, Y. Optimization of Extraction Conditions for Antioxidant Activity of Acer tegmentosum Using Response Surface Methodology. Appl. Sci. 2021, 11, 1134. [Google Scholar] [CrossRef]
- Atroune, F.; Chaker, S.; Djebbar, R.; Dahmani-Megrerouche, M. Comparative evaluation of phenolics content and antioxidant activity of leaves and branches of field maple (Acer campestre) from two populations of Northeastern Algeria. An. Univ. Oradea Fasc. Biol. 2019; XXVI, 7–13. Available online: https://www.bioresearch.ro/2019-1.html (accessed on 5 December 2024).
- Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef]
- Meng, R.; Ou, K.; Chen, L.; Jiao, Y.; Jiang, F.; Gu, R. Response Surface Optimization of Extraction Conditions for the Active Components with High Acetylcholinesterase Inhibitory Activity and Identification of Key Metabolites from Acer truncatum Seed Oil Residue. Foods 2023, 12, 1751. [Google Scholar] [CrossRef] [PubMed]
| Sample | Species / vegetal product | Date / site of collection (southwest Romania flora) | Voucher specimen |
|---|---|---|---|
| A1 | A. pseudoplatanus / folium | 1 July 2024 / Craiova City, Dolj County | ACER-PPT-2024-0701 |
| A2 | A. tataricum / folium | 12 June 2024 / Craiova City, Dolj County | ACER-TAT-2024-0612 |
| A3 | A. tataricum / fructus | ||
| A4 | A. campestre / folium | 12 June 2024 / Craiova City, Dolj County | ACER-CAM-2024-0612 |
| A5 | A. campestre / fructus | ||
| A6 | A. pseudoplatanus / fructus | 1 July 2024 / Craiova City, Dolj County | ACER-PPT-2024-0701 |
| A7 | A. platanoides / folium | 7 July 2024 / Craiova City, Dolj County | ACER-PLT-2024-0707 |
| A8 | A. platanoides / fructus | ||
| A9 | A. monspessulanum / folium | 21 July 2024 / Dubova Village, Mehedinţi County | ACER-MON-2024-0721 |
| A10 | A. monspessulanum / fructus |
| Sample | TPC (μg GAE/mL) |
|---|---|
| A1 | 94.01±14.41 |
| A2 | 512.8±20.54 |
| A3 | 456.1±16.94 |
| A4 | 225.8±5.527 |
| A5 | 68.45±8.563 |
| A6 | 91.04±3.572 |
| A7 | 61.48±3.755 |
| A8 | 164.1±4.978 |
| A9 | 251.8±19.06 |
| A10 | 193.8±8.927 |
| Sample | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | FRAP (μmol Fe2+/L) |
|---|---|---|---|
| A1 | 137.4±51.9 | 132.5±27.3 | 117.8±7.659 |
| A2 | 22.61±3.28 | 15.68±3.31 | 1119±164.6 |
| A3 | 10.88±3.385 | 10.39±2.861 | 1158±48.98 |
| A4 | 63.14±8.96 | 50.45±10.89 | 310.6±18.36 |
| A5 | 226.9±32 | 212.7±63.3 | 154.4±9.225 |
| A6 | 138.2±20.4 | 79.29±17.3 | 294.5±26.92 |
| A7 | 290.2±39.2 | 271.2±60.7 | 139.8±5.361 |
| A8 | 81.78±25.27 | 57.28±15.97 | 354.7±20.75 |
| A9 | 54.25±10.59 | 37.75±9.7 | 471±12.87 |
| A10 | 60.62±12.72 | 57.58±13.61 | 378.1±5.64 |
| Compound | tR (min) |
|---|---|
| Gallic acid | 1.84 |
| Protocatechuic acid | 3.70 |
| Chlorogenic acid | 5.83 |
| Vanillic acid | 6.11 |
| Caffeic acid | 6.36 |
| Syringic acid | 6.64 |
| p-Coumaric acid | 7.80 |
| Ferulic acid | 8.54 |
| Sample | Caffeic acid (mg/g) | Chlorogenic acid (mg/g) | p-Coumaric acid (mg/g) | Ferulic acid (mg/g) | Gallic acid (mg/g) | Protocatechuic acid (mg/g) | Syringic acid (mg/g) | Vanillic acid (mg/g) |
|---|---|---|---|---|---|---|---|---|
| A1 | 0.003±0.001 | 0.005±0.001 | 0.012±0.001 | 0.001±0.001 | 0.977±0.015 | 0.169±0.002 | 0.012±0.001 | 0.24±0.01 |
| A2 | 0.122±0.004 | 0.114±0.004 | 0.024±0.001 | 0.109±0.005 | 4.75±0.205 | 1.171±0.021 | 0.459±0.007 | 2.076±0.036 |
| A3 | 0.043±0.001 | 0.14±0.004 | 0.062±0.001 | 0.19±0.004 | 5.929±0.204 | 6.528±0.101 | 0.82±0.017 | 0.863±0.021 |
| A4 | 0.35±0.009 | 0.07±0.002 | 0.158±0.002 | 0.08±0.002 | 14.05±0.473 | 1.04±0.012 | 0.081±0.002 | 0.436±0.007 |
| A5 | 0.071±0.001 | 0.011±0.001 | 0.091±0.004 | 0.029±0.001 | 2.878±0.063 | 2.145±0.029 | 0.009±0.001 | 0.21±0.005 |
| A6 | 1.323±0.019 | 0.302±0.009 | 0.227±0.002 | 0.01±0.001 | 39.796±0.809 | 6.219±0.227 | 0.154±0.003 | 0.173±0.005 |
| A7 | 0.312±0.009 | 2.016±0.035 | 0.283±0.013 | 0.665±0.027 | 2.19±0.104 | 1.371±0.062 | 0.235±0.007 | 2.613±0.122 |
| A8 | 0.105±0.001 | 0.239±0.004 | 1.401±0.016 | 0.025±0.001 | 0.94±0.024 | 2.304±0.048 | 0.091±0.003 | 2.167±0.052 |
| A9 | 0.282±0.005 | 7.488±0.237 | 1.04±0.016 | 0.471±0.019 | 1.15±0.014 | 0.001±0 | 0.001±0 | 2.317±0.041 |
| A10 | 0.622±0.006 | 1.648±0.07 | 0.412±0.015 | 0.061±0.002 | 2.926±0.119 | 2.459±0.031 | 8.49±0.206 | 11.519±0.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





