Submitted:
30 December 2024
Posted:
31 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
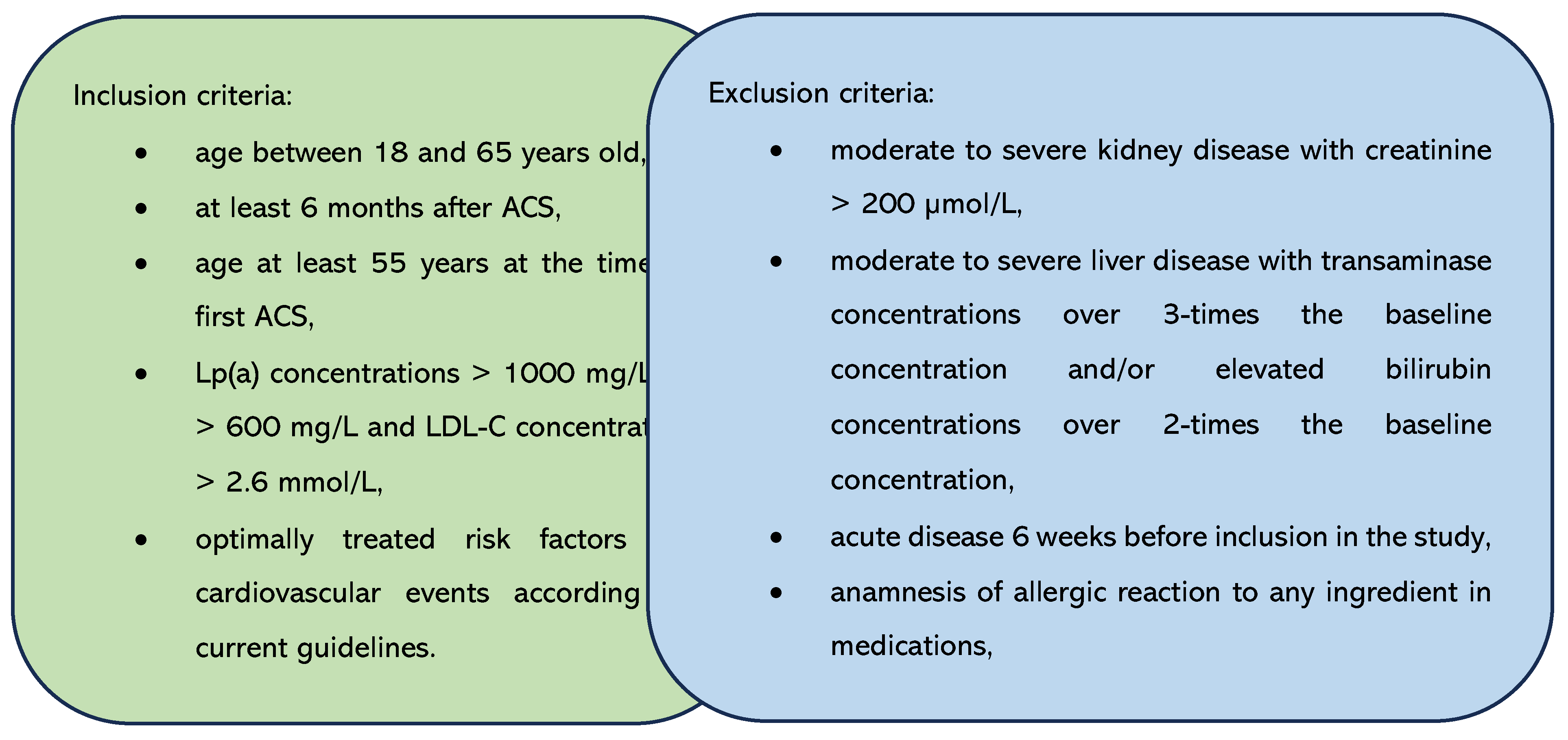
2.1. Patients and Controls
2.2. Clinical Examination
2.3. Laboratory Analyses
2.4. Gene Expression Measurement
2.5. Statistical Analysis
3. Results
3.1. Subjects’ Characteristics
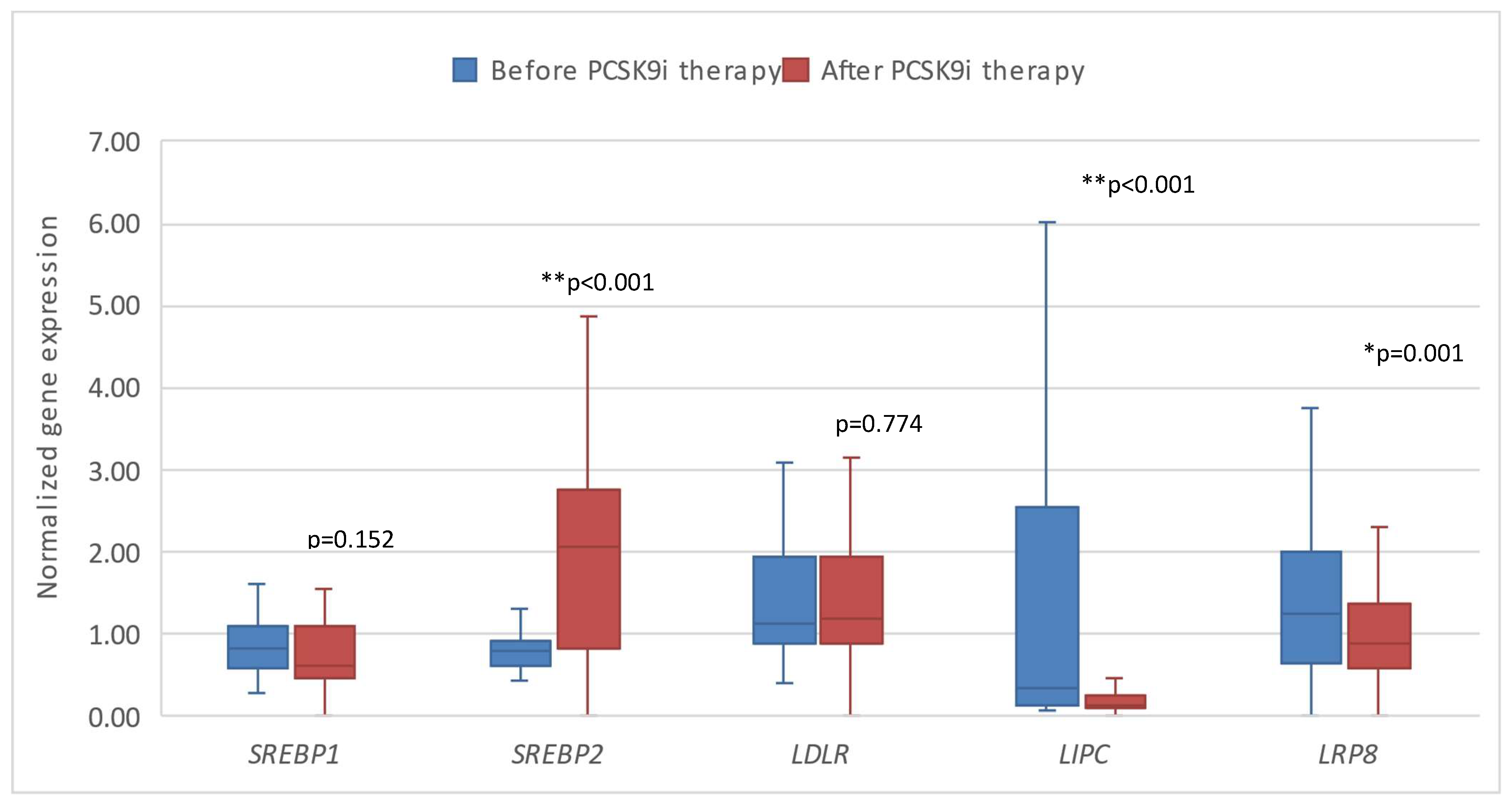
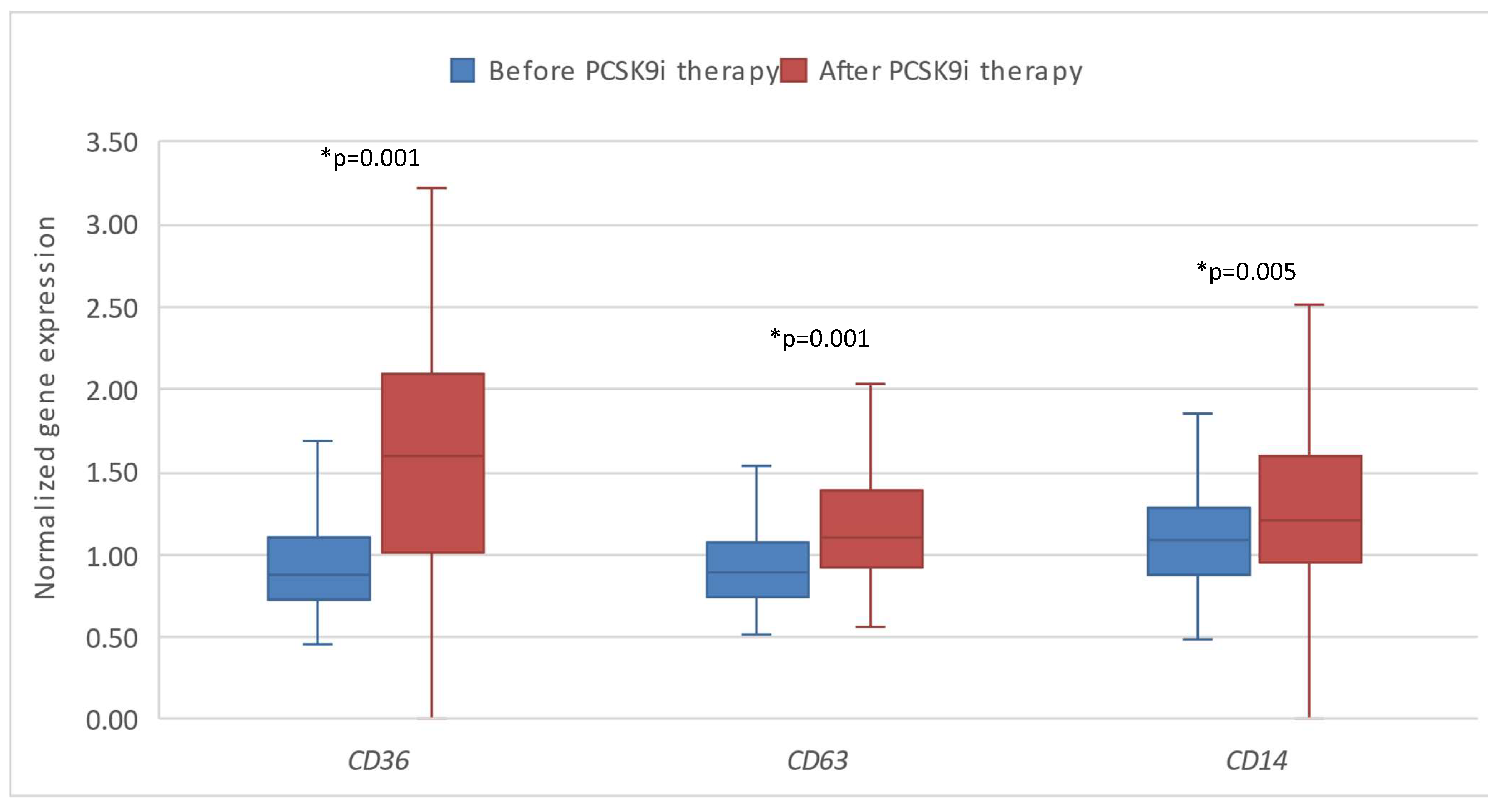
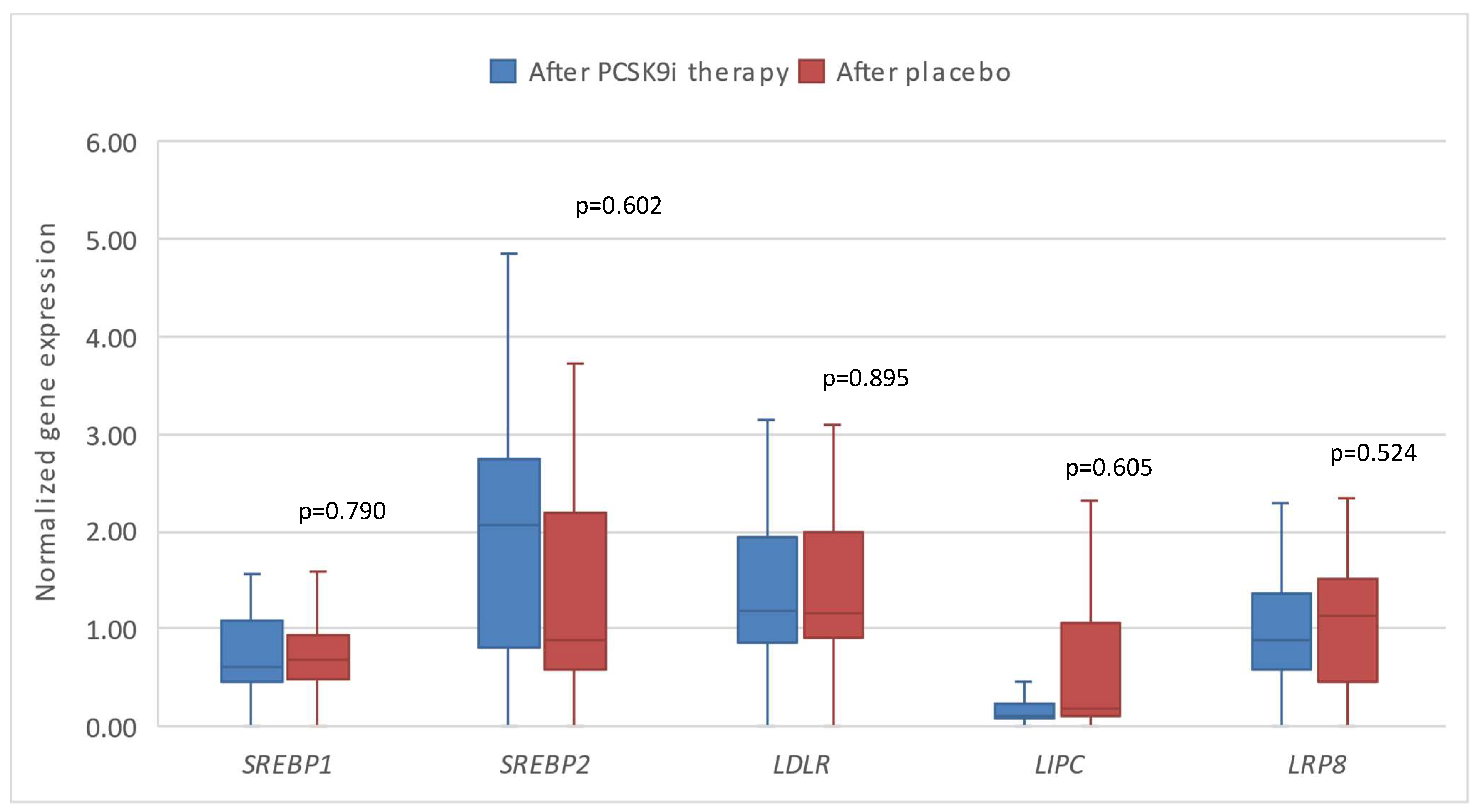
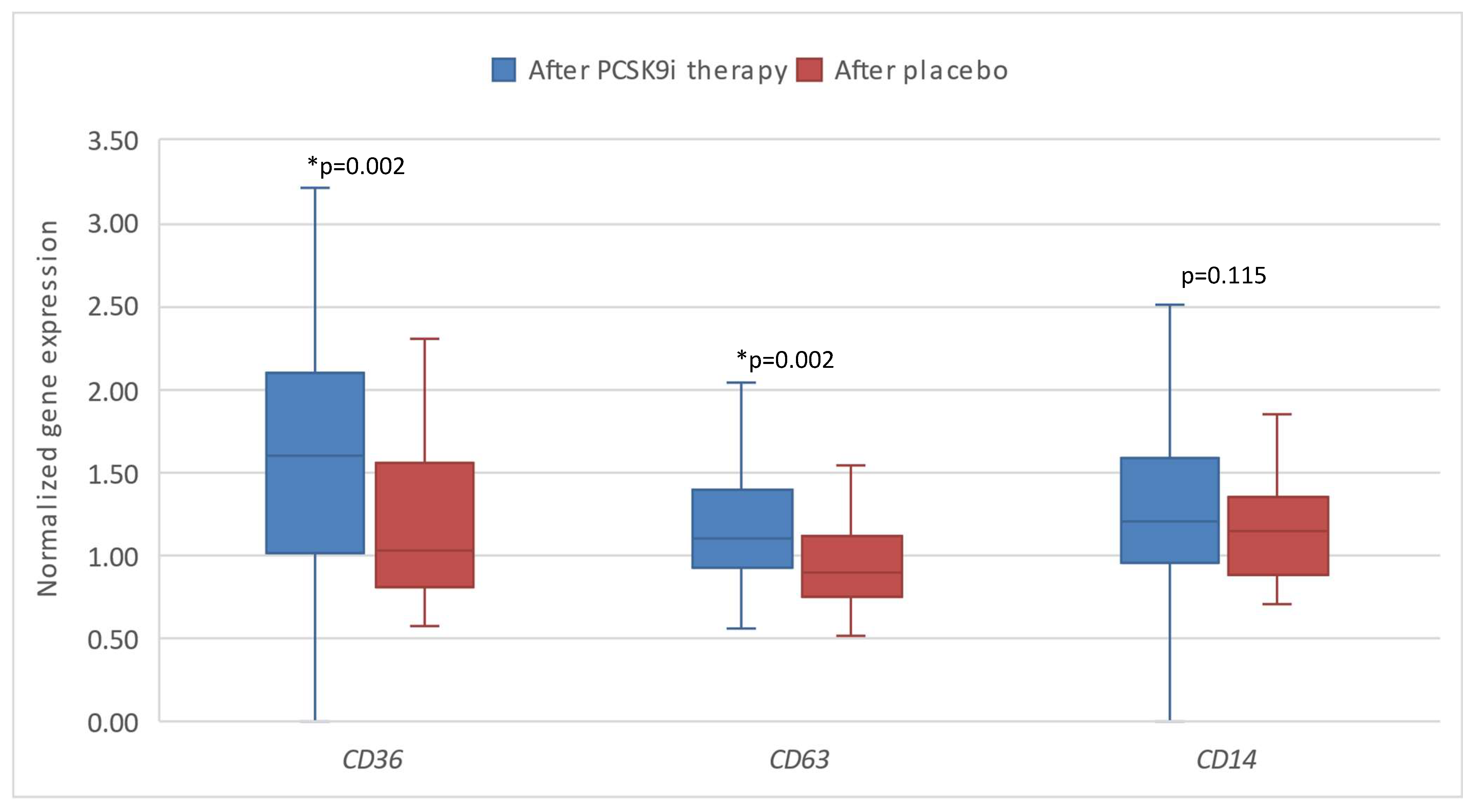
3.2. The Results of Expression of the Tested Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. New England Journal of Medicine 2017, 376, 1713–1722. [CrossRef]
- Roth, E.M.; Davidson, M.H. PCSK9 Inhibitors: Mechanism of Action, Efficacy, and Safety. Rev Cardiovasc Med 2018, 19, 31–46. [CrossRef]
- Paciullo, F.; Momi, S.; Gresele, P. PCSK9 in Haemostasis and Thrombosis: Possible Pleiotropic Effects of PCSK9 Inhibitors in Cardiovascular Prevention. Thromb Haemost 2019, 119, 359–367. [CrossRef]
- Doi, T.; Hori, M.; Harada-Shiba, M.; Kataoka, Y.; Onozuka, D.; Nishimura, K.; Nishikawa, R.; Tsuda, K.; Ogura, M.; Son, C.; et al. Patients With LDLR and PCSK9 Gene Variants Experienced Higher Incidence of Cardiovascular Outcomes in Heterozygous Familial Hypercholesterolemia. J Am Heart Assoc 2021, 10. [CrossRef]
- Peng, C.; Lei, P.; Li, X.; Xie, H.; Yang, X.; Zhang, T.; Cao, Z.; Zhang, J. Down-Regulated of SREBP-1 in Circulating Leukocyte Is a Risk Factor for Atherosclerosis: A Case Control Study. Lipids Health Dis 2019, 18, 177. [CrossRef]
- Sobati, S.; Shakouri, A.; Edalati, M.; Mohammadnejad, D.; Parvan, R.; Masoumi, J.; Abdolalizadeh, J. PCSK9: A Key Target for the Treatment of Cardiovascular Disease (CVD). Adv Pharm Bull 2020, 10, 502–511. [CrossRef]
- Dijk, W.; Di Filippo, M.; Kooijman, S.; van Eenige, R.; Rimbert, A.; Caillaud, A.; Thedrez, A.; Arnaud, L.; Pronk, A.; Garçon, D.; et al. Identification of a Gain-of-Function LIPC Variant as a Novel Cause of Familial Combined Hypocholesterolemia. Circulation 2022, 146, 724–739. [CrossRef]
- Ramesh, S.; Morrell, C.N.; Tarango, C.; Thomas, G.D.; Yuhanna, I.S.; Girardi, G.; Herz, J.; Urbanus, R.T.; de Groot, P.G.; Thorpe, P.E.; et al. Antiphospholipid Antibodies Promote Leukocyte–Endothelial Cell Adhesion and Thrombosis in Mice by Antagonizing ENOS via Β2GPI and ApoER2. Journal of Clinical Investigation 2011, 121, 120–131. [CrossRef]
- Yang, X. V.; Banerjee, Y.; Fernández, J.A.; Deguchi, H.; Xu, X.; Mosnier, L.O.; Urbanus, Rolf.T.; de Groot, P.G.; White-Adams, T.C.; McCarty, O.J.T.; et al. Activated Protein C Ligation of ApoER2 (LRP8) Causes Dab1-Dependent Signaling in U937 Cells. Proceedings of the National Academy of Sciences 2009, 106, 274–279. [CrossRef]
- Yang, M.; Li, W.; Harberg, C.; Chen, W.; Yue, H.; Ferreira, R.B.; Wynia-Smith, S.L.; Carroll, K.S.; Zielonka, J.; Flaumenhaft, R.; et al. Cysteine Sulfenylation by CD36 Signaling Promotes Arterial Thrombosis in Dyslipidemia. Blood Adv 2020, 4, 4494–4507. [CrossRef]
- Hamamoto, K.; Ohga, S.; Nomura, S.; Yasunaga, K. Cellular Distribution of CD63 Antigen in Platelets and in Three Megakaryocytic Cell Lines. Histochem J 1994, 26, 367–375. [CrossRef]
- Wu, Z.; Zhang, Z.; Lei, Z.; Lei, P. CD14: Biology and Role in the Pathogenesis of Disease. Cytokine Growth Factor Rev 2019, 48, 24–31. [CrossRef]
- Sharygin, D.; Koniaris, L.G.; Wells, C.; Zimmers, T.A.; Hamidi, T. Role of <scp>CD14</Scp> in Human Disease. Immunology 2023, 169, 260–270. [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem 1972, 18, 499–502. [CrossRef]
- Hrovat, K.; Rehberger Likozar, A.; Zupan, J.; Šebeštjen, M. Gene Expression Profiling of Markers of Inflammation, Angiogenesis, Coagulation and Fibrinolysis in Patients with Coronary Artery Disease with Very High Lipoprotein(a) Levels Treated with PCSK9 Inhibitors. J Cardiovasc Dev Dis 2022, 9, 211. [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin Chem 2009, 55, 611–622. [CrossRef]
- SREBP2: Https://Www.Origene.Com/Catalog/Gene-Expression/Qpcr-Primer-Pairs/Hp207891-Srebp2-Srebf2-Human-Qpcr-Primer-Pair-Nm-004599.
- ApoER2 (LRP8): Https://Www.Origene.Com/Catalog/Gene-Expression/Qpcr-Primer-Pairs/Hp231155-Apoer2-Lrp8-Human-Qpcr-Primer-Pair-Nm-004631.
- CD36: Https://Www.Origene.Com/Catalog/Gene-Expression/Qpcr-Primer-Pairs/Hp200058-Cd36-Human-Qpcr-Primer-Pair-Nm-000072.
- CD63: Https://Www.Origene.Com/Catalog/Gene-Expression/Qpcr-Primer-Pairs/Hp227481-Cd63-Human-Qpcr-Primer-Pair-Nm-001780.
- CD14: Https://Www.Origene.Com/Catalog/Gene-Expression/Qpcr-Primer-Pairs/Hp200558-Cd14-Human-Qpcr-Primer-Pair-Nm-000591.
- Varghese, J.F.; Patel, R.; Yadav, U.C.S. Sterol Regulatory Element Binding Protein (SREBP) -1 Mediates Oxidized Low-Density Lipoprotein (OxLDL) Induced Macrophage Foam Cell Formation through NLRP3 Inflammasome Activation. Cell Signal 2019, 53, 316–326. [CrossRef]
- Horton, J.D.; Shimomura, I. Sterol Regulatory Element-Binding Proteins. Curr Opin Lipidol 1999, 10, 143–150. [CrossRef]
- Roglans, N.; Verd, J.C.; Peris, C.; Alegret, M.; Vázquez, M.; Adzet, T.; Diaz, C.; Hernández, G.; Laguna, J.C.; Sánchez, R.M. High Doses of Atorvastatin and Simvastatin Induce Key Enzymes Involved in VLDL Production. Lipids 2002, 37, 445–454. [CrossRef]
- Retterstøl, K.; Svendsen, M.; Narverud, I.; Holven, K.B. Effect of Low Carbohydrate High Fat Diet on LDL Cholesterol and Gene Expression in Normal-Weight, Young Adults: A Randomized Controlled Study. Atherosclerosis 2018, 279, 52–61. [CrossRef]
- Zhang, L.; McCabe, T.; Condra, J.H.; Ni, Y.G.; Peterson, L.B.; Wang, W.; Strack, A.M.; Wang, F.; Pandit, S.; Hammond, H.; et al. An Anti-PCSK9 Antibody Reduces LDL-Cholesterol On Top Of A Statin And Suppresses Hepatocyte SREBP-Regulated Genes. Int J Biol Sci 2012, 8, 310–327. [CrossRef]
- Young, S.G.; Fong, L.G. Lowering Plasma Cholesterol by Raising LDL Receptors — Revisited. New England Journal of Medicine 2012, 366, 1154–1155. [CrossRef]
- Santamarina-Fojo, S.; González-Navarro, H.; Freeman, L.; Wagner, E.; Nong, Z. Hepatic Lipase, Lipoprotein Metabolism, and Atherogenesis. Arterioscler Thromb Vasc Biol 2004, 24, 1750–1754. [CrossRef]
- Brunzell, J.D.; Zambon, A.; Deeb, S.S. The Effect of Hepatic Lipase on Coronary Artery Disease in Humans Is Influenced by the Underlying Lipoprotein Phenotype. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2012, 1821, 365–372. [CrossRef]
- Han, H.; Dai, D.; Wang, W.; Zhu, J.; Zhu, Z.; Lu, L.; Zhang, R. Impact of Serum Levels of Lipoprotein Lipase, Hepatic Lipase, and Endothelial Lipase on the Progression of Coronary Artery Disease. Journal of Interventional Medicine 2019, 2, 16–20. [CrossRef]
- Yu, X.; Lu, J.; Li, J.; Guan, W.; Deng, S.; Deng, Q.; Ye, H.; Han, W.; Yu, Y.; Zhang, R. Serum Triglyceride Lipase Concentrations Are Independent Risk Factors for Coronary Artery Disease and In-Stent Restenosis. J Atheroscler Thromb 2019, 26, 762–774. [CrossRef]
- Shen, G.-Q.; Li, L.; Girelli, D.; Seidelmann, S.B.; Rao, S.; Fan, C.; Park, J.E.; Xi, Q.; Li, J.; Hu, Y.; et al. An LRP8 Variant Is Associated with Familial and Premature Coronary Artery Disease and Myocardial Infarction. The American Journal of Human Genetics 2007, 81, 780–791. [CrossRef]
- Ackers, I.; Szymanski, C.; Duckett, K.J.; Consitt, L.A.; Silver, M.J.; Malgor, R. Blocking Wnt5a Signaling Decreases CD36 Expression and Foam Cell Formation in Atherosclerosis. Cardiovascular Pathology 2018, 34, 1–8. [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The Role of CD36 in Cardiovascular Disease. Cardiovasc Res 2022, 118, 115–129. [CrossRef]
- Li, N. Platelets as an Inter-player between Hyperlipidaemia and Atherosclerosis. J Intern Med 2024, 296, 39–52. [CrossRef]
- Schrör, K.; Verheugt, F.W.A.; Trenk, D. Drug–Drug Interaction between Antiplatelet Therapy and Lipid-Lowering Agents (Statins and PCSK9 Inhibitors). Thromb Haemost 2023, 123, 166–176. [CrossRef]
- Ackers, I.; Szymanski, C.; Duckett, K.J.; Consitt, L.A.; Silver, M.J.; Malgor, R. Blocking Wnt5a Signaling Decreases CD36 Expression and Foam Cell Formation in Atherosclerosis. Cardiovasc Pathol 2018, 34, 1–8. [CrossRef]
- Demers, A.; Samami, S.; Lauzier, B.; Des Rosiers, C.; Ngo Sock, E.T.; Ong, H.; Mayer, G. PCSK9 Induces CD36 Degradation and Affects Long-Chain Fatty Acid Uptake and Triglyceride Metabolism in Adipocytes and in Mouse Liver. Arterioscler Thromb Vasc Biol 2015, 35, 2517–2525. [CrossRef]
- MURAKAMI, T.; KOMIYAMA, Y.; MASUDA, M.; KIDO, H.; NOMURA, S.; FUKUHARA, S.; KARAKAWA, M.; IWASAKA, T.; TAKAHASHI, H. Flow Cytometric Analysis of Platelet Activation Markers CD62P and CD63 in Patients with Coronary Artery Disease. Eur J Clin Invest 1996, 26, 996–1003. [CrossRef]
- Cha, J.-K.; Jeong, M.-H.; Jang, J.-Y.; Bae, H.-R.; Lim, Y.-J.; Kim, J.S.; Kim, S.-H.; Kim, J.W. Serial Measurement of Surface Expressions of CD63, P-Selectin and CD40 Ligand on Platelets in Atherosclerotic Ischemic Stroke. Cerebrovascular Diseases 2003, 16, 376–382. [CrossRef]
- Du, P.; Guo, R.; Gao, K.; Yang, S.; Yao, B.; Cui, H.; Zhao, M.; Jia, S. Identification of Differentially Expressed Genes and the Role of PDK4 in CD14+ Monocytes of Coronary Artery Disease. Biosci Rep 2021, 41. [CrossRef]
- Krychtiuk, K.A.; Lenz, M.; Hohensinner, P.; Distelmaier, K.; Schrutka, L.; Kastl, S.P.; Huber, K.; Dostal, E.; Oravec, S.; Hengstenberg, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Are Associated with Monocyte Subsets in Patients with Stable Coronary Artery Disease. J Clin Lipidol 2021, 15, 512–521. [CrossRef]
- Filatova, A.Y.; Afanasieva, O.I.; Arefieva, T.I.; Potekhina, A. V; Tyurina, A. V; Klesareva, E.A.; Razova, O.A.; Ezhov, M. V; Pokrovsky, S.N. The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis. J Pers Med 2023, 13. [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur Heart J 2020, 41, 111–188. [CrossRef]
- Corrigendum to: 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur Heart J 2020, 41, 4255–4255. [CrossRef]
- Krychtiuk, K.A.; Kastl, S.P.; Hofbauer, S.L.; Wonnerth, A.; Goliasch, G.; Ozsvar-Kozma, M.; Katsaros, K.M.; Maurer, G.; Huber, K.; Dostal, E.; et al. Monocyte Subset Distribution in Patients with Stable Atherosclerosis and Elevated Levels of Lipoprotein(a). J Clin Lipidol 2015, 9, 533–541. [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [CrossRef]
| Patients (n=96) |
Controls (n=25) |
p Value | |
|---|---|---|---|
| Gender (M/F) | 91/5 | 23/2 | 0.641 |
| Age (years) | 50.46 ± 8.74 | 48.92 ± 7.14 | 0.076 |
| BMI (kg/m2) | 28.57 ± 3.79 | 25.40 ± 3.32 | *0.045 |
| Total cholesterol [mmol/L] | 4.25 ± 0.88 | 5.80 ± 0.67 | *0.034 |
| LDL-C [mmol/L] | 2.33 ± 0.77 | 3.56 ± 0.63 | *0.023 |
| HDL-C [mmol/L] | 1.17 ± 0.27 | 1.52 ± 0.42 | *0.034 |
| TG [mmol/L] | 1.47 (1.04-2.10) | 1.31 (0.99 – 2.01) | 0.096 |
| Lp(a) [mg/L] | 1431.00 (1203.00-1658.00) | 11.00 (4.00 – 18.50) | **<0.001 |
| Patients (n=96) |
Controls (n=25) |
p Value |
|
|---|---|---|---|
| SREBP1 | 0.82 (0.60-1.11) | 0.80 (0.49-1.72) | 0.976 |
| SREBP2 | 0.77 (0.63-0.89) | 2.33 (1.90-2.87) | **<0.001 |
| LDLR | 1.18 (0.89-2.24) | 1.32 (1.04-2.33) | **<0.001 |
| LIPC | 0.35 (0.14-2.60) | 0.08 (0.06-0.11) | **<0.001 |
| LRP8 | 1.56 (0.94-2.73) | 0.75 (0.52 – 1.20) | **<0.001 |
| CD36 | 0.86 (0.72-1.02) | 1.69 (1.34 – 2.14) | **<0.001 |
| CD63 | 0.87 (0.76-1.02) | 1.23 (1.03 – 1.48) | **<0.001 |
| CD14 | 1.07 (0.85-1.25) | 1.31 (1.13 – 1.74) | **<0.001 |
| ΔSREBP1 | ΔSREBP2 | ΔLDLR | ΔLIPC | ΔLRP8 | ΔCD36 | ΔCD63 | ΔCD14 | |
|---|---|---|---|---|---|---|---|---|
| ΔTC | ρ=0.252 p=0.045* |
ρ=-0.077 p=0.544 |
ρ=-0.023 p=0.859 |
ρ=0.163 p=0.198 |
ρ=-0.062 p=0.641 |
ρ=0.072 p=0.576 |
ρ=0.218 p=0.086 |
ρ=-0.065 p=0.610 |
| ΔLDL-C | ρ=0.082 p=0.521 |
ρ=-0.031 p=0.806 |
ρ=-0.136 p=0.282 |
ρ=0.091 p=0.477 |
ρ=-0.015 p=0.910 |
ρ=0.144 p=0.263 |
ρ=0.230 p=0.069 |
ρ=0.050 p=0.694 |
| ΔHDL-C | ρ=-0.094 p=0.461 |
ρ=-0.075 p=0.554 |
ρ=-0.043 p=0.734 |
ρ=-0.172 p=0.173 |
ρ=-0.123 p=0.354 |
ρ=-0.055 p=0.672 |
ρ=-0.294 p=0.019* |
ρ=-0.231 p=0.066 |
| ΔTG | ρ=0.108 p=0.397 |
ρ=-0.043 p=0.735 |
ρ=0.089 p=0.483 |
ρ=-0.044 p=0.728 |
ρ=-0.156 p=0.237 |
ρ=-0.081 p=0.531 |
ρ=0.179 p=0.160 |
ρ=-0.021 p=0.871 |
| ΔLp(a) | ρ=-0.013 p=0.921 |
ρ=-0.096 p=0.457 |
ρ=-0.002 p=0.985 |
ρ=0.098 p=0.450 |
ρ=0.050 p=0.715 |
ρ=0.105 p=0.427 |
ρ=0.187 p=0.149 |
ρ=0.053 p=0.684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





