1. Introduction
Ventilator-associated pneumonia (VAP) is the leading nosocomial infection in intensive care units (ICUs) [
1]. VAP is associated with more days of invasive mechanical ventilation, increased antibiotic consumption and higher hospital costs [
2]. However, the association of VAP with increased mortality remains controversial [
3]. This complication affects 8-28% of patients undergoing mechanical ventilation [
1], with large variations depending on country, type of ICU and criteria used to identify VAP [
4,
5].
In older series, the most common pathogens recovered from bronchoscopy specimens were
Pseudomonas aeruginosa (24.4%),
Staphylococcus aureus (20.4%) and E
nterobacterales (14.1%) [
1]. Several studies have shown that initial treatment of VAP with inadequate antimicrobial agents is associated with a worse prognosis [
6,
7]. It is therefore particularly important to understand the epidemiology and resistance trends of the bacteria involved.
The last decade has seen significant changes both in the respiratory management of mechanically ventilated patients, especially those affected by VAP [
8,
9], and in the epidemiology of multidrug-resistant (MDR) bacteria in Europe [
10,
11], especially after the SARS-CoV-2 pandemic, where a significant increase in resistance among Gram-negative bacilli was observed [
12,
13]. Both conditions may affect the outcome of critically ill patients on mechanical ventilation.
The National Healthcare Safety Network of the Centers for Disease Control and Prevention (CDC) has reported large declines in the incidence of VAP in medical and surgical ICUs over the past 15 years [
14]. However, these results were not confirmed by an analysis using a stable definition of VAP conducted by the Medicare Patient Safety Monitoring System between 2005 and 2013 [
15]. However, other authors [
16] in countries where there is no financial penalty associated with infectious complications have shown a reduction in the incidence of VAP over the years.
We hypothesize that changes in the management of ventilated patients and the evolving epidemiology of multidrug-resistant bacteria, particularly in the post-SARS-CoV-2 era, have influenced ICU mortality in patients with VAP. To address this, our study aims to investigate variations in crude ICU mortality among patients with VAP over the past decade. Furthermore, we seek to identify associated risk factors for mortality, evaluate changes in the microbiological etiology of VAP, and assess the incidence and impact of inappropriate empirical antibiotic treatment (IEAT). These findings will provide critical insights to inform future strategies for improving patient outcomes in the ICU
2. Materials and Methods
2.1. Study Design and Participants
A retrospective, observational, single-center study was conducted over a 10-year period (2014-2024), including critically ill patients (≥18 years of age) consecutively admitted to a 30-bed ICU. The analysis included all critically ill patients who developed ventilator-associated pneumonia (VAP) with confirmed microbiology. Only bacterial VAP was considered; patients with fungal etiologies were excluded. Furthermore, only the first episode of microbiologically confirmed VAP per patient was included in this analysis. During the 10-year study period, the population was divided into three periods. The first period was from 2014 to 2018 (pre-COVID-19 period). The second period, from 2019 to 2021, coincided with the SARS-CoV-2 pandemic (COVID-19 period), and the third period, from 2022 to 2024 (post-COVID-19 period). (
Supplementary Figure S1).
2.2. Definitions
VAP was defined as a respiratory infection occurring in mechanically ventilated patients according to the guidelines of the European Respiratory Society (ERS), the European Society of Intensive Care Medicine (ESICM), the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the Asociación Latinoamericana del Tórax (ALAT)[
17]. VAP was defined as pneumonia occurring more than 48 h after endotracheal intubation with fever without other apparent cause, with new or increased sputum production, positive endotracheal aspirate (ETA) culture (> 10
6 CFU/mL) or bronchoalveolar lavage (BAL) culture (> 10
4 CFU/mL) with at least one respiratory pathogen known to cause pneumonia, and radiographic evidence of nosocomial pneumonia.
Empirical antimicrobials were selected based on specialist clinical judgment and internal ICU protocols, which could subsequently be modified by the ASP (Antimicrobial Stewardship Program) team, based on clinical response in the days following VAP diagnosis or final microbiology results.
During the entire study period, the same bundles of VAP prevention measures recommended by the Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC) within the Pneumonia zero programme were applied. Our ICU does not use digestive decontamination in ventilated patients. From 2019, a rapid microbiological diagnostic protocol based on the Biofire© Filmarray Pneumonia Panel Plus© was implemented in all patients with suspected multi-drug resistant (MDR) microorganisms, as previously published [
18]. From 2014 to 2019, the treatment protocol in our ICU included the administration of an antipseudomonal agent: carbapenem (meropenem) or piperacillin/tazobactam with or without another agent with activity against resistant cocci (linezolid or tigecycline). In patients with no apparent risk for
Pseudomonas aeruginosa, Ertapenem was the empirical treatment option accepted by our ICU protocol.
As of 2019, due to the high resistance observed for carbapenem and piperacillin/tazobactam (>40%) for Pseudomonas aeruginosa, empirical treatment was changed to aztreonam or ceftolozane/tazobactam or ceftazidime/avibactam according to the sensitivity reported by the microbiology service.
Empirical antibiotic therapy was considered appropriate (AEAT) if the bacteria detected in vitro were susceptible to at least one antibiotic administered. Empirical antibiotic therapy was considered inappropriate (IEAT) if the isolate did not show sensitivity. Multidrug resistance (MDR) was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, and extensively drug resistant (XDR) was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e., bacterial isolates remain susceptible to only one or two categories) [
19].
2.3. Objectives and Follow-Up
2.3.1. Primary Objective
The primary objective was to assess variations in crude ICU mortality among patients with VAP over a 10-year observation period and to identify associated risk factors.
2.3.2. Secondary Objectives
To evaluate potential variations in the etiology of VAP.
To assess the incidence of IEAT, the associated microorganisms, and their impact on mortality.
2.3.3. Primary Outcome
The primary outcome was all-cause mortality in the ICU. Patients discharged alive from the ICU were considered survivors, while mortality was defined as any death occurring during the ICU admission.
2.3.4. Follow-Up
All complications and outcomes were monitored throughout the ICU admission to provide a comprehensive understanding of patient trajectories and outcomes
2.4. Statistical Analysis
Prior to the study, we did not perform any statistical calculation of sample size. Instead, we selected the sample size to be equal to the number of patients admitted to the ICUs of the participants with microbiologically confirmed VAP during the study period. To provide an overview of the baseline characteristics, the continuous variables were expressed as a median (Q1-Q3 = IQR), while the categorical variables were expressed as the number of cases (percentage). For demographic and clinical characteristics, differences between groups were assessed using the following tests: chi-squared test and Fisher’s exact test or “t” test for categorical variables and Mann-Whitney U test for continuous variables.
Binary logistic regression was used to identify which variables were independently associated with ICU mortality. All variables with a statistical significance (p < 0.05) in the bivariate comparison between groups were included in the GLM (generalized linear model). In addition, period 2 coinciding with the COVID-19 pandemic and the implementation of a protocol for rapid microbiological diagnosis using molecular techniques were incorporated into the final model, as they are factors of clinical interest that may be related to the evolution of patients despite not reaching statistical significance in the comparative table.
To validate the regression model, the population was randomly divided into a training group (70% of the population) and a validation group (30% of the population) stratifying by ICU-mortality. The results are presented as odds ratios (OR) with 95% confidence intervals (95% CI). Values below 0.05 were considered significant.
The model’s performance was evaluated based on its accuracy, precision, sensitivity, specificity, and area under the ROC curve (AUC). Furthermore, the Hosmer–Lemeshow goodness of fit was determined, and the presence of collinearity between the explanatory variables was examined using variance inflation factors (VIFs) [ ]. To check whether the linear functional form of the regression model is correct, the Ramsey regression equation specification error test (RESET) was applied. It checks for overlooked variables, incorrect functional forms or omitted non-linearities by adding terms constructed as powers or products of the fitted values and assessing their significance.[ ] Furthermore, we conducted k-fold cross-validation (K = 10), which entailed partitioning the original data into two distinct sets: a primary development set (Train) and a secondary validation set (Test). The training set is then divided into k subsets. During the training process, each k subset is used as the model test set, while the remaining data is used as the training set. Once the required number of iterations has been completed, the accuracy and error rates are calculated for each of the models produced. The final accuracy and error rates are then obtained by averaging the k-trained models.
The statistical analyses were performed using R software (R-4.4.2 for Windows).
3. Results
3.1. General
A total of 227 patients met the eligibility criteria. The initial empiric treatment was administered to 90 patients (39.6%) who received meropenem (MRP), 79 (34.8%) received piperacillin/tazobactam (PTZ), 51 (22.5%) received ertapenem (ETP), and only 7 patients received alternative treatments (aztreonam = 2; cloxacillin = 2; ceftriaxone = 1; fluoroquinolone = 2). For the purposes of this analysis, only the three majority groups were considered, resulting in a final sample size of 220 patients with VAP (
Supplementary Figure S1).
Over the course of the 10-year study, the population was divided into three periods. The first period included 47 patients, with an incidence density (ID) of 2.8 episodes of VAP per 1,000 ventilator days (MV). The second period, coincided with the SARS-CoV-2 pandemic and included 96 patients with an incidence density of 4.9 episodes per 1,000 ventilator days. The third period, included 77 patients with an incidence density of 3.4 episodes per 1,000 days of mechanical ventilation. Please refer to supplementary
Figure S2 for a visual representation of the annual incidence in each period.
The first period cohort exhibited elevated levels of organ dysfunction (SOFA) and inflammation (CRP), while displaying a reduced incidence of medical admissions. In contrast, there was a higher prevalence of hypertension and immunosuppression in the second and third periods. Please refer to
Table 1 for a comprehensive overview of the patient
s’ characteristics across all three periods.
3.2. Etiology of VAP over the Years
The most frequently isolated microorganisms were gram-negative bacilli (GNB).
Pseudomonas aeruginosa and
Klebsiella spp. were the most frequently isolated GNB across all periods (see
Table 2). There was an upward trend in the isolation of these microorganisms in periods 2 and 3, although this was not statistically significant. Similarly, there was no significant change observed in the isolation of Gram-positive cocci. Methicillin-resistant
Staphylococcus aureus and
Acinetobacter spp. isolates were anecdotal in all 3 periods.
Stenotrophomonas maltophilia was more frequent in period 1 than in the other periods, but without significant differences.
A total of 69 microorganisms were isolated from the 47 patients in period 1. Of these, 22 patients (47%) had 2 microorganisms isolated and 6 patients (12.7%) had 3 microorganisms isolated. In period 2, a total of 166 microorganisms were isolated in 96 patients. Among them, 39 patients (56.5%) had two microorganisms isolated, while 16 patients (16.6%) had three microorganisms isolated. Finally, in period 3, a total of 130 microorganisms were isolated in 77 patients. Among them, 28 (36.3%) had two microorganisms isolated, while 13(16.8%) has three microorganisms isolated.
3.3. Empiric Antibiotic Treatment
Of the 220 patients, 75% (n=165) received empiric antibiotics as monotherapy. In the remaining 25% (n=55), the second antimicrobial was linezolid (n=50, 91%) or tigecycline (n=5, 9%).
The overall incidence of inappropriate empirical antibiotic treatment (IEAT) was 4.5% (10/220), with no differences between the study periods: 4.3% (n=2), 3.1% (n=3) and 6.5% (n=5) for periods 1, 2 and 3 respectively. Furthermore, there was no discernible difference in the incidence of IEAT according to the antimicrobial agent used, whether ertapenem (n=2; 3.9%), meropenem (n=3; 3.3%) or piperacillin/tazobactam (n=5; 6.3%). Stenotrophomonas maltophilia and Pseudomonas aeruginosa MDR were the microorganisms most commonly associated with IEAT (supplementary
Table S1).
Of the 210 patients with appropriate empirical antibiotic treatment, 150 (71.4%) had their treatment adjusted. In 138 (92%) de-escalation was realized and in 12 (8%) the number of antibiotics was reduced. This adjustment did not differ between periods 1 (77.8%) and 2 (66.7%, p=0.18), but was significantly lower in period 3 (56.9%, p<0.05).
3.4. ICU Mortality Rate
Crude ICU mortality in the general population was 33.6% (n=74). While patients with inadequate empirical antimicrobial treatment (IEAT) demonstrated a higher mortality rate of 40% (n=4) compared to those who received adequate treatment (33.3%, n=70), this difference was not statistically significant (p=0.73). In patients with appropriate empirical antibiotics, crude ICU mortality decreased significantly over the years from 42.2% (19/95) in period 1, 37.6% (35/93) in period 2 to 22.2% (16/72) in period 3 (p<0.001). In particular, a significant decrease was observed in period 3 compared to period 1 (p=0.02) and period 2 (p=0.03).
Although patients receiving meropenem as adequate empirical treatment had lower mortality (29.9%, 26/87) than those receiving piperacillin/tazobactam (37.8%, 28/74, p=0.28) or ertapenem (32.7%, 16/49, p=0.30), these differences did not reach significance.
Patients who did not survive were older, had a higher level of organ dysfunction as measured by the SOFA score, a higher frequency of chronic heart disease, a lower SO2/FiO2 ratio and a higher frequency of hypotension and reduced diuresis volume (
Table 3).
3.4.1. Lineal Regression Analysis (GLM)
To study the impact of variables on mortality a multivariate regression model (GLM) was developed. The population was randomly divided into a training subset with 70% of the population (n=154) and a test subset with the remaining 30% (n=66).
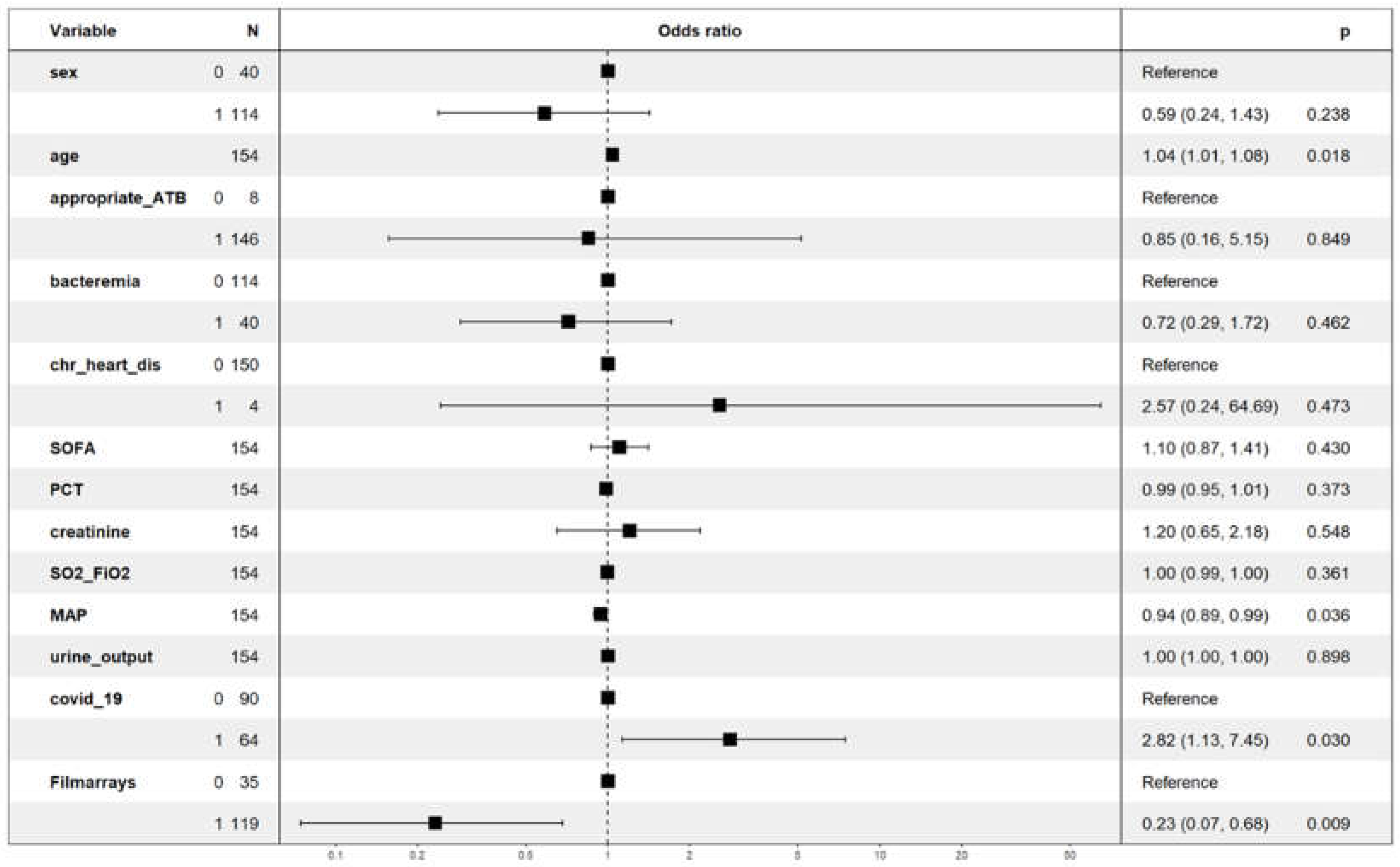
The dependent variable was ICU mortality, while the covariates included were age, sex, chronic heart disease, bacteraemia, inappropriate antibiotic treatment, period 3, PCT, SOFA score, mean arterial pressure, SO2/FiO2, serum creatinine, COVID-19 period and filmarrays protocol. All covariates were considered on the day of diagnosis of VAP.
Only age with an OR=1.04 (95% CI=1.01-1.08) for each year and COVID-19 period with an OR=2.8 (95% CI=1.1-7.4) were independently associated with an increased risk of mortality. In contrast, mean arterial pressure with OR=0.94 (95% CI=0.69-0.99) and the use of rapid microbiological diagnosis with Biofire
® Filmarray Pneumonia with OR=0.23 (95% CI=0.07-0.68) were associated with a lower risk of death. Period 3 had high collinearity with a VIF greater than 15 and had to be removed from the model.
Figure 1.
Internal validation of the model showed an accuracy of 74% with a sensitivity of 63% and a high specificity of 80% with a positive predictive value of 55% and a negative predictive value of 84%. The Hosmer-Lemeshow test was p=0.37 showing no difference between predicted and observed and the RESET linearity test was p=0.12 showing linearity in the model. Finally, the area under the ROC curve was 0.76 (0.64-0.88) (
Supplementary Figure S3). Cross-validation (K=10) did not improve model performance while maintaining an accuracy of 74% (supplementary
Table S2).
4. Discussion
Our main finding was that the crude ICU mortality of VAP decreased significantly over the years, with a significant reduction in period 3 (2022-24). This reduction was observed despite no significant change in etiology and no difference in empirical antibiotic treatment between the controlled periods. Conditions associated with crude ICU mortality were patient age and period 2, which coincided with the COVID-19 pandemic. On the other hand, mean arterial pressure and especially the implementation of a rapid diagnostic protocol based on molecular techniques were associated with a lower mortality risk.
Although crude ICU mortality associated with VAP has been reported to be as high as 50% [
20], there remains considerable controversy about the extent to which VAP contributes to death in ICU patients. In contrast, VAP has been consistently associated with prolonged mechanical ventilation and ICU stay. In our study, crude mortality decreased significantly over the years from 44.7% to 22.1%. Similarly, de Miguel-Díez J et al. [
16] demonstrated, in an elegant study of a large administrative database including more than 9000 patients, that crude mortality decreased significantly over the study period, from 35.74% in 2010 to 32.81% in 2014. Although mortality decreased by only 2.9%, this difference is significant due to the large number of patients included, so the clinical impact of this decrease appears to be very small. On the other hand, this mortality behavior was not observed by Fihmana V et al. [
21], who did not find a decrease in mortality when studying patients with VAP during the period 2007-2011, with incidences of 37%, 37%, 27%, 31% and 36% for the 5 years studied.
Our study was not designed to assess the attributable mortality of VAP, so several factors that may influence the outcome should be discussed. The first of these is empirical antimicrobial treatment. Our results show that there is a low percentage of IEAT in all periods, which may be due to empirical treatment with broad-spectrum agents, possibly more than indicated. This may explain the high rate of de-escalation observed in all periods. Although early reports observed a strong association between IEAT and mortality [
6,
22,
23,
24], more recent studies have questioned this association [
25]. In our study, IEAT was not found to be a factor associated with mortality in multivariate analysis, in contrast to other reports [
6,
22,
23,
24]. The low incidence of IEAT observed may be related to the lack of significance of the 6% increase in mortality observed in patients with IEAT.
In addition, the type of empirical treatment should be considered, with meropenem, piperacillin/tazobactam and ertapenem being the most commonly used drugs. Although unusual, our protocol includes the option of ertapenem if
Pseudomonas aeruginosa is not suspected or proven. Ertapenem is a carbapenem with antimicrobial activity against most Gram-positive and Gram-negative aerobic and anaerobic bacterial infections, including ESBL-producing pathogens. It is a valid option when it is not desired to pressure on the development of
Pseudomonas spp. resistance. In addition, ertapenem has demonstrated efficacy in the clinical and microbiological resolution of VAP [
26]. However, alterations in the Cmax and AUC
0-last of ertapenem have been reported in critically ill patients related to critical pathology and hypo-albuminemia [
27]. Bosselli E et al.[
28] observed satisfactory results with unbound ertapenem concentrations in both serum and ELF (approximately 40% of the serum value) exceeding the MIC
90 values for most pathogens found in early-onset VAP for 50-100% of the time.
In our study, we did not observe a higher incidence of IEAT in ertapenem patients, nor differences in crude mortality in this subgroup of patients compared to the meropenem or piperacillin/tazobactam. Since PK/PD disturbances related to volume of distribution and hypo-albuminemia can be partly addressed with increased dosing frequency, our protocol envisages the administration of 1 g every 12 hours in patients with VAP.
Another point of interest is the variation in etiology. The microorganisms associated with VAP vary according to many factors, such as the duration of mechanical ventilation, the length of hospital and intensive care unit stays prior to VAP, the timing and cumulative antimicrobial exposure, the local ecology, the occurrence of potential epidemic phenomena and, most importantly, the local antimicrobial policy that exerts pressure on the development of resistance.
Gram-negative organisms commonly implicated in VAP are
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and
Acinetobacter spp.;
Staphylococcus aureus is the primary Gram-positive organism [
29,
30]. However, there is a large variability related to local conditions. Our ICU is free of
Acinetobacter baumannii and methicillin-resistant
S. aureus, and the ASP team applies a strict antimicrobial policy. Various authors [
31,
32] have reported considerable variation in the etiology of VAP, with an increasing number of MDR microorganisms being isolated over the years. However, this epidemiological behaviour was not observed in our study, as also reported by de Miguel-Díez J [
16] et al.
It is important to note that, as suggested by Barbier F et al. the 63% of complications related to mechanical ventilation-associated infections, were neither VAP nor due to a documented infection in the ICU [
33], indicating that efforts should focus on the diagnostic strategy, using carbapenems only in patients with true infection and withholding carbapenems when the likelihood of infection is low. In this context, it is important to highlight the impact on mortality seen with the implementation of a rapid microbiological diagnostic protocol. Although our study cannot establish a causal relationship between mortality and the use of the rapid diagnostic protocol, multivariate analysis showed a strong and independent association between the two conditions. Our group [
18] already reported the impact that the implementation of a rapid microbiological diagnostic protocol had on the reduction of meropenem and piperacillin/tazobactam resistance as well as on the decrease of antimicrobial consumption measured in defined daily doses (DDD) in the whole ICU. The present study’s findings confirm the favorable impact of implementing protocols that include molecular microbiological diagnosis for the optimization of antimicrobial therapy.
Our study has the strength of being a 10-year analysis of the evolution of NAV using automatically generated real-world data. However, it has several limitations that must be taken into account.
First, it is a longitudinal but single-center study, so the results cannot be extrapolated to other centers.
Secondly, we did not design a study to assess attributable mortality with VAP, as our aim was to assess crude mortality over the 10 years. Different methods have been used to calculate attributable mortality in VAP, with estimates ranging from 0 to 60%.[
34]. Quantifying the impact of this condition on patient outcomes is also complicated by the time-dependent nature of the disease, which may include time-dependent bias, and by the fact that mortality and ICU discharge act as competing endpoints[
34]. Given the difficulties and conflicting results of the studies on attributable mortality, our aim was to assess which factors affect crude mortality as a ’hard’ outcome variable, rather than using complex statistical methods.
Finally, we do not differentiate between early and late VAP. It is generally recognized that early-onset VAP (within the first 5-7 days of mechanical ventilation) in previously healthy patients not receiving antibiotics usually involves isolation of microorganisms from the oropharynx without resistance mechanisms. In contrast, late-onset VAP (>5-7 days of ventilation) and VAP in patients with risk factors for multidrug-resistant (MDR) pathogens are more likely to be due to pathogens with different resistance mechanisms [
20]. However, MDR pathogens can be isolated in early-onset VAP, mainly in the presence of certain risk factors, such as exposure to antimicrobials in the previous 90 days, and some authors have found comparable rates of MDR pathogens in patients with early- versus late-onset VAP [
35,
36,
37].
5. Conclusions
Our study shows a reduction in crude VAP mortality over the years, with no change in VAP incidence, etiology or inadequate empirical antibiotic treatment, and irrespective of the choice of initial antimicrobial agent. The favorable effect on mortality observed with the implementation of a rapid microbiological diagnostic protocol suggests that this measure, should be carefully considered by ASP teams for implementation. However, our results should be interpreted with caution and confirmed by studies in other units.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Flow Chart of included patients according to period study; Figure S2: Incidence density of ventilator-associated pneumonia /1000 ventilator days according to controlled periods; Figure S3: Area under the ROC curve for the prediction of the multivariate mortality model; Table S1: Micro-organisms isolated and empirical treatment in the 10 patients with inappropriate empirical antibiotic treatment (IEAT).; Table S2: Cross-validation results (K=10).
Author Contributions
Conceptualization, Alejandro Rodríguez, Julen Berrueta, Carolina Páez, Ronny Huertas, Marco Marotta, Laura Claverias, Josep Gómez, Sandra Trefler, Frederic Gómez- Bertomeu, María Dolores Guerrero-Torres, Sergio Pardo-Granell, Ester Picó-Plana, Alicia Selles-Sánchez, Francisco Javier Candel, Ignacio Martín-Loeches and María Bodí; Data curation, Alejandro Rodríguez, Julen Berrueta, Josep Gómez, Sandra Trefler and Alicia Selles-Sánchez; Formal analysis, Alejandro Rodríguez, Julen Berrueta, Laura Claverias, Ignacio Martín-Loeches and María Bodí; Funding acquisition, Alejandro Rodríguez; Investigation, Alejandro Rodríguez, Julen Berrueta, Carolina Páez, Ronny Huertas, Marco Marotta, Sergio Pardo-Granell, Ester Picó-Plana and Alicia Selles-Sánchez; Methodology, Julen Berrueta, Carolina Páez, Marco Marotta, Laura Claverias, Josep Gómez, Sandra Trefler, Frederic Gómez- Bertomeu, María Dolores Guerrero-Torres, Sergio Pardo-Granell, Ester Picó-Plana, Francisco Javier Candel, Ignacio Martín-Loeches and María Bodí; Project administration, Alejandro Rodríguez; Resources, Ronny Huertas and Laura Claverias; Software, Julen Berrueta and Josep Gómez; Supervision, Alejandro Rodríguez; Validation, Alejandro Rodríguez, Julen Berrueta, Ronny Huertas, Josep Gómez, María Dolores Guerrero-Torres, Sergio Pardo-Granell, Ester Picó-Plana, Alicia Selles-Sánchez, Ignacio Martín-Loeches and María Bodí; Writing – original draft, Alejandro Rodríguez, Julen Berrueta, Carolina Páez, Ronny Huertas, Marco Marotta, Sandra Trefler, Frederic Gómez- Bertomeu, María Dolores Guerrero-Torres, Francisco Javier Candel, Ignacio Martín-Loeches and María Bodí; Writing – review & editing, Alejandro Rodríguez, Julen Berrueta, Frederic Gómez- Bertomeu, Francisco Javier Candel, Ignacio Martín-Loeches and María Bodí.
Funding
This study was supported with protected research time (AR and MB) by a grant from the Ricardo Barri Casanovas Foundation (FRBC01/2024). The sponsors were not involved in the study design, data collection, data analysis, data interpretation or report writing.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and the European Community Directive on Clinical Trials (2001/20/EC) of the European Parliament on Good Clinical Practice guidelines.
Informed Consent Statement
Ethical review and approval have been waived because this is an observational, retrospective (10years) study with the automated collection of anonymized data to assess the quality of care as part of the routine evaluation of the implementation of the ASP group measures (antimicrobial optimization program).
Data Availability Statement
The data supporting the conclusions of this study are available from the Joan XXIII de Tarragona Hospital (Spain), but restrictions are placed on the free availability of these data by the health authorities of Catalonia, so they are not publicly available. However, the data can be obtained from the corresponding author (AR) upon reasonable request and with the permission of the Technical Secretary and the person responsible for data management at Joan XXIII de Tarragona Hospital (Spain).
Acknowledgments
We express our gratitude to the dedicated staff members of the Pharmacy, Intensive Care, Infectious Disease, Microbiology, and Nursing Departments at Hospital Universitari Joan XXIII. This project would not have been possible without their collaborative efforts.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chastre, J.; Fagon, J.Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867–903. [CrossRef]
- Bekaert, M.; Timsit, J.F.; Vansteelandt, S.; Depuydt, P.; Vesin, A.; Garrouste-Orgeas, M.; et al. Attributable mortality of ventilator-associated pneumonia:a reappraisal using causal analysis. Am J Respir Crit Care Med 2011;184:1133–9. [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis ofindividual patient data from randomised prevention studies. Lancet Infect Dis 2013;13:665–71. [CrossRef]
- American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired,ventilator-associated, and healthcare-associated pneumonia. Am Respir Crit Care Med 2005; 171:388–416.
- Reignier, J.; Mercier, E.; Le Gouge, A.; Boulain, T.; Desachy, A.; Bellec, F.; Clavel, M.; Frat, J.P.; et al. Efect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013; 309: 249–256. [CrossRef]
- Kuti, E.L.; Patel, A.A.; Coleman, C.I. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood streaminfection: a meta-analysis. J Crit Care 2008;23:91–100. [CrossRef]
- Ohnuma, T.; Chihara, S.; Costin, B.; Treggiari, M.M.; Bartz, R.R.; Raghunathan, K.; Krishnamoorthy, V. Association of Appropriate Empirical Antimicrobial Therapy With In-Hospital Mortality in Patients With Bloodstream Infections in the US. JAMA Netw Open. 2023 3;6(1):e2249353. [CrossRef]
- Esteban, A.; Frutos-Vivar, F.; Muriel, A.; Ferguson, N.D.; Peñuelas, O.; Abraira, V.; Raymondos, K.; Rios, F.; et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188(2):220-30. [CrossRef]
- Rubulotta,F.; Blanch Torra,Ll.; Naidoo,K.D.; Aboumarie,H.S; Mathivha,L.R.; Asiri,A.Y.;Sarlabous Uranga,L.; Soussi,S. Mechanical Ventilation, Past, Present, and Future. Anesth Analg 2024 ;138(2):308-325. [CrossRef]
- Foglia, F.; Della Rocca, M.T.; Melardo, C.; Nastri, B.M.; Manfredini, M.; Montella, F.; De Filippis, A.; Finamore, E.; Galdiero, M. Bloodstream infections and antibiotic resistance patterns: a six-year surveillance study from southern Italy. Pathog Glob Health. 2023 ;117(4):381-391. [CrossRef]
- de Kraker, M.E.; Jarlier, V.; Monen, J.C.; Heuer, O.E.; van de Sande, N.; Grundmann, H. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 2013;19:860–8. [CrossRef]
- Denny, S.; Rawson, T.M.; Hart, P.; Satta, G.; Abdulaal, A.; Hughes, S.; Gilchrist, M.; Mughal, N.; Moore, L.S.P. Bacteraemia variation during the COVID-19 pandemic; a multi-centre UK secondary care ecological analysis. BMC Infect Dis. 2021;21(1):556. [CrossRef]
- Rodríguez, A.; Moreno, G.; Bodi, M.; Martín-Loeches, I. Antibiotics in development for multiresistant gram-negative bacilli. Med Intensiva (Engl Ed). 2022 ;46(11):630-640. [CrossRef]
- Dudeck, M.A.; Edwards, J.R.; Allen-Bridson, K.; Gross, C.; Malpiedi, P.J.; Peterson, K.D.; Pollock, D.A.; Weiner, L.M.; Sievert, D.M. National healthcare safety network report, data summary for 2013, device-associated module. Am J Infect Control 2015;43:206–221. [CrossRef]
- Metersky, M.L.; Wang, Y.; Klompas, M.; Eckenrode, S.; Bakullari, A.; Eldridge, N. Trend in ventilator-associated pneumonia rates between 2005 and 2013. JAMA 2016; 316:2427–2429. [CrossRef]
- de Miguel-Díez, Javier.; López-de-Andrés, Ana.; Hernández-Barrera, Valentín.; Jiménez-Trujillo, Isabel.; Méndez-Bailón, Manuel.; Miguel-Yanes, José. M. de; del Rio-López, Benito. Decreasing incidence and mortality among hospitalized patients suffering a ventilator-associated pneumonia Analysis of the Spanish national hospital discharge database from 2010 to 2014. Medicine 2017;96(30):pe7625. [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S. ; Fernandez-Vandellos,P.; Hanberger,H.; Kollef,M.; 8, Li Bassi,G.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J 2017 Sep 10;50(3). [CrossRef]
- Rodríguez, A.; Gómez, F.; Sarvisé, C.; Gutiérrez, C.; Giralt, M.G.; Guerrero-Torres, M.D.; Pardo-Granell, S.; Picó-Plana, E.; et al. Clinical and Microbiological Impact of Implementing a Decision Support Algorithm through Microbiologic Rapid Diagnosis in Critically Ill Patients: An Epidemiological Retrospective Pre-/Post-Intervention Study. Biomedicines. 2023;11(12):3330. [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M. ; Luyt, C-E. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med 2020; 46:888-906. [CrossRef]
- Fihman, V.; Messika, J.; Hajage, D.; Tournier, V.; Gaudry, S.; Magdoud, F.; Barnaud, G.; Billard-Pomares, T.; et al. Five-year trends for ventilator-associated pneumonia: Correlation between microbiological findings and antimicrobial drug consumption. International Journal of Antimicrobial Agents 2015; 46(5): 518-525. [CrossRef]
- Strich, J.R.; Heil, E.L.; Masur, H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. Journal of Infectious Diseases 2020;222(S2): S119–31. [CrossRef]
- Zilberberg, M.D.; Brian, H. Nathanson, B.H.; Laura A. Puzniak, L.A.; Dillon, R.J.; Shorr, A. F. The risk of inappropriate empiric treatment and its outcomes based on pathogens in non-ventilated (nvHABP), ventilated (vHABP) hospital-acquired and ventilator-associated (VABP) bacterial pneumonia in the US, 2012–2019. BMC Infectious Diseases (2022) 22:775. [CrossRef]
- Magnotti, L.J.; Schroeppel, T.J.; Fabian, T.C.; Clement, L.P.; Swanson, J.M.; Fischer, P.E.; Bee, T.K.; Maish, G.O.; Minard, G.; et al. . Reduction in Inadequate Empiric Antibiotic Therapy for Ventilator-Associated Pneumonia: Impact of a Unit-Specific Treatment Pathway. The American Surgeon 2008;74(6):516-523. [CrossRef]
- Ticac, M.; Grubic Kezele, T.; Bubonja Šonje, M. Impact of Appropriate Empirical Antibiotic Treatment on the Clinical Response of Septic Patients in Intensive Care Unit: A Single-Center Observational Study. Antibiotics 2024, 13, 569. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Fasce, R.; Molinari, M.P.; Rosso, R.; Di Biagio, A.; Mussap, M.; Bobbio Pallavicini, F.; Viscoli, C. Efficacy of ertapenem in the treatment of early ventilator-associated pneumonia caused by extended-spectrum b-lactamase-producing organisms in an intensive care unit. J Antimicrob Chemother 2007;60(2):433-5. [CrossRef]
- Burkhardt, O.; Kumar, V.; Katterwe, D.; Majcher-Peszynska, J.; Drewelow, B.; Derendorf, H. ; Welte,T. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. Journal of Antimicrobial Chemotherapy (2007) 59, 277–284. [CrossRef]
- Boselli, E.; Breilh, D. ; Saux, M-C.; Gordien, J-B.; Allaouchiche, B. Pharmacokinetics and lung concentrations of ertapenem in patients with ventilator-associated pneumonia. Intensive Care Med 2006; 32:2059–2062. [CrossRef]
- Di Pasquale, M.; Ferrer, M.; Esperatti, M.; Crisafulli, E.; Giunta, V.; Li Bassi, G.; Rinaudo, M.; Blasi, F.; et al. Assessment of severity of ICU-acquired pneumonia and association with etiology. Crit Care Med 2014; 42:303–312. [CrossRef]
- Esperatti, M.; Ferrer, M.; Theessen, A.; Liapikou, A.; Valencia, M.; Saucedo, L.M.; Zavala, E.; Welte, T.; Torres, A. Nosocomial pneumonia in the intensive care unit acquired during mechanical ventilation or not. Am J Respir Crit Care Med 2010; 182:1533–1539. [CrossRef]
- Rao, S.V.; Thilakchand, K.R.; Boloor, R.; Suresh, S.; George, T.; Pais, M.L.J.; Jakribettu, R.P.; Baliga, M.S. Antimicrobial resistance pattern in aerobic bacteria isolated from endotracheal aspirate in ventilator-associated pneumonia: Ten years observation from a tertiary care hospital. J Anaesthesiol Clin Pharmacol 2024;40(2):324-329. [CrossRef]
- Yu, Z.; Li, X.; Lv, C.; Tian,Y.; Suo, J.; Yan, Z.; Bai, Y.; Liu, B.; et al. Epidemiological characteristics of ventilator-associated pneumonia in neurosurgery: A 10-year surveillance study in a Chinese tertiary hospital. Infectious Medicine 2024; 3(3)100128. [CrossRef]
- Barbier, F.; Bailly, S.; Schwebel, C.; Papazian, L.; Azoulay, E.; Kallel, H.; Siami, S.; Argaud, L.; et al. Infection-related ventilator-associate complications in ICU patients colonised with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Intensive Care Med 2018; 44:616–626. [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R. H-H.; Bergmans, D.C.J.J.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 2013; 13: 665–71. [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; et al. Management of adults with hospital acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis 2016; 63:e61–e111. [CrossRef]
- Khan, R.; Al-Dorzi, H.M.; Tamim, H.M.; Rishu, A.H.; Balkhy, H.; El-Saed, A.; Arab, Y.M. The impact of onset time on the isolated pathogens and outcomes in ventilator associated pneumonia. J Infect Public Health 2016; 9:161–171. [CrossRef]
- Martin-Loeches, I.; Deja, M.; Koulenti, D.; Dimopoulos, G.; Marsh, B.; Torres, A.; Niederman, M.S.; Rello, J.; et al. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med 2013; 39:672–681. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).