Submitted:
22 December 2024
Posted:
23 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Among the Nanotechnology Applications
2.1. Application of Nanomaterials to Natural and Synthetic Bioactive Molecules
2.1.1. NPs-Mediated Controlled Release of APs
2.1.2. Main NPs Developed to Nano Formulate Natural and Synthetic APs
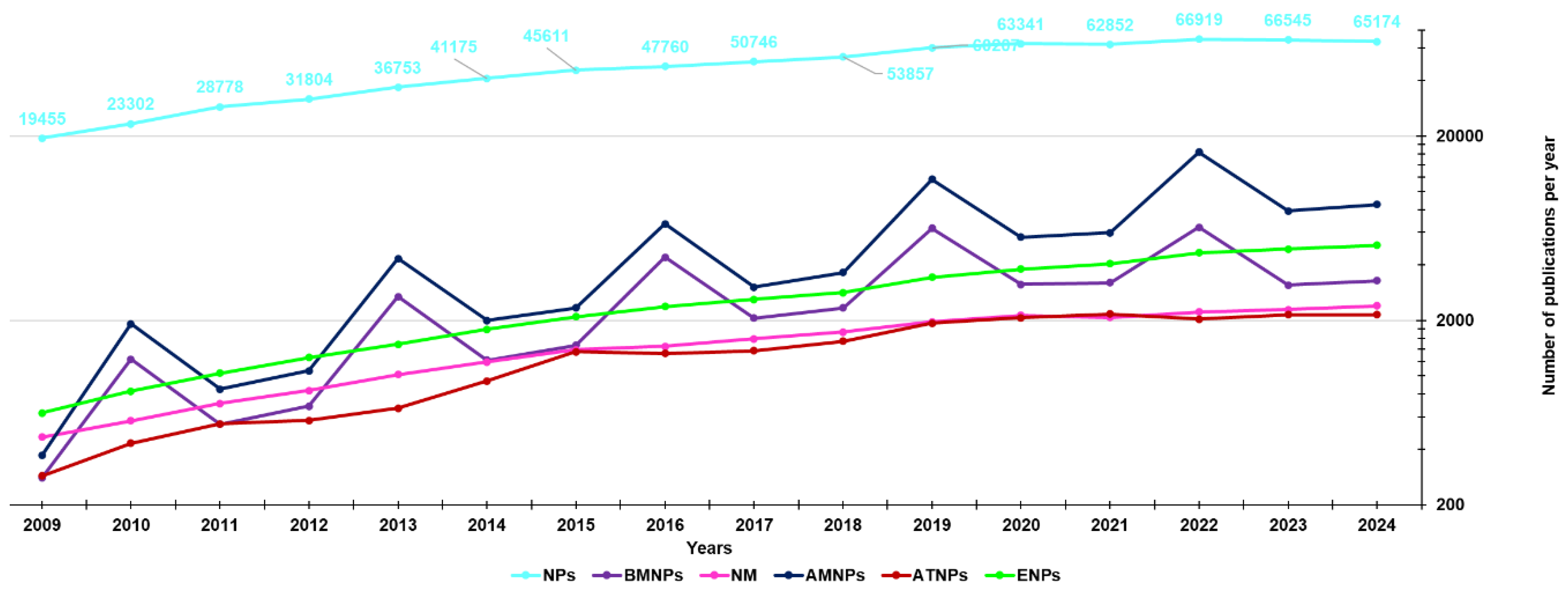
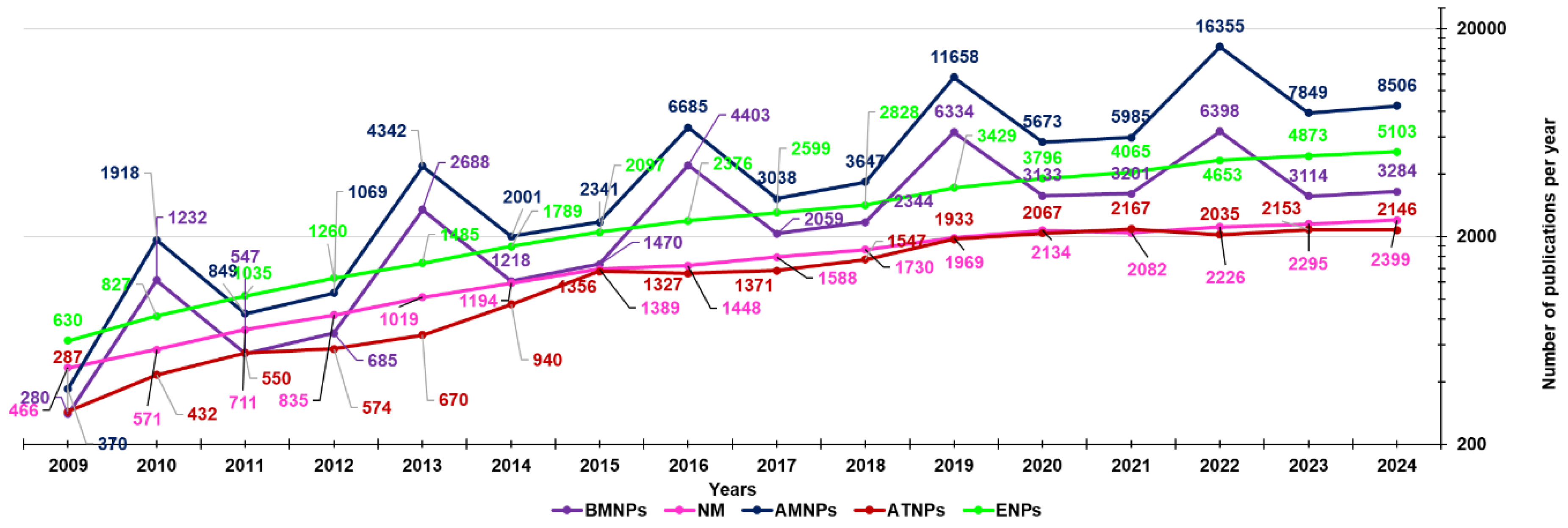
3. Our Dealing with Nanotechnology Applications: Last 15 Years Studies
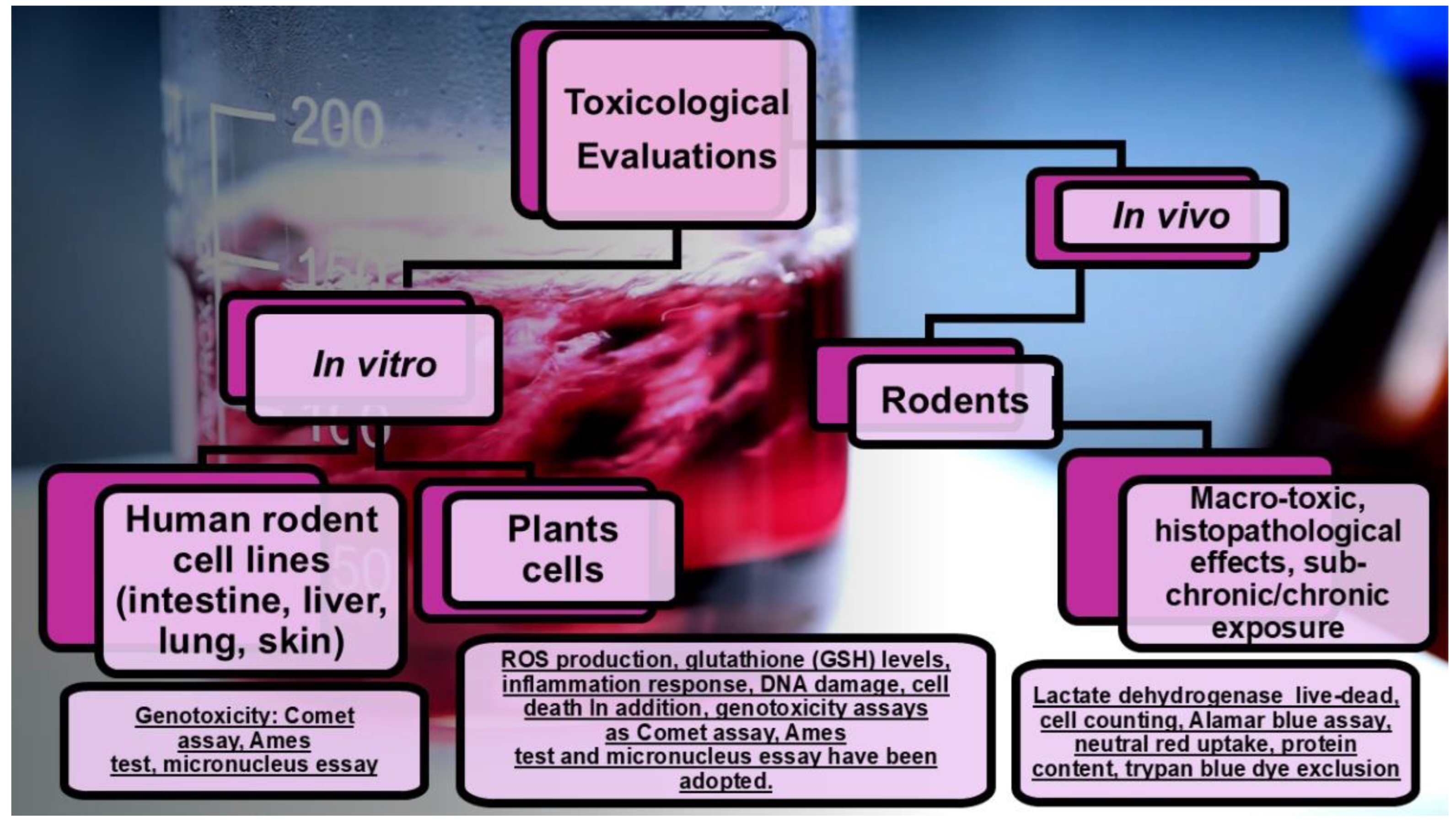
4. Nanotoxicology
The Possible Migration of NPs From FP to Food and Toxicity of Ingesting Them
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
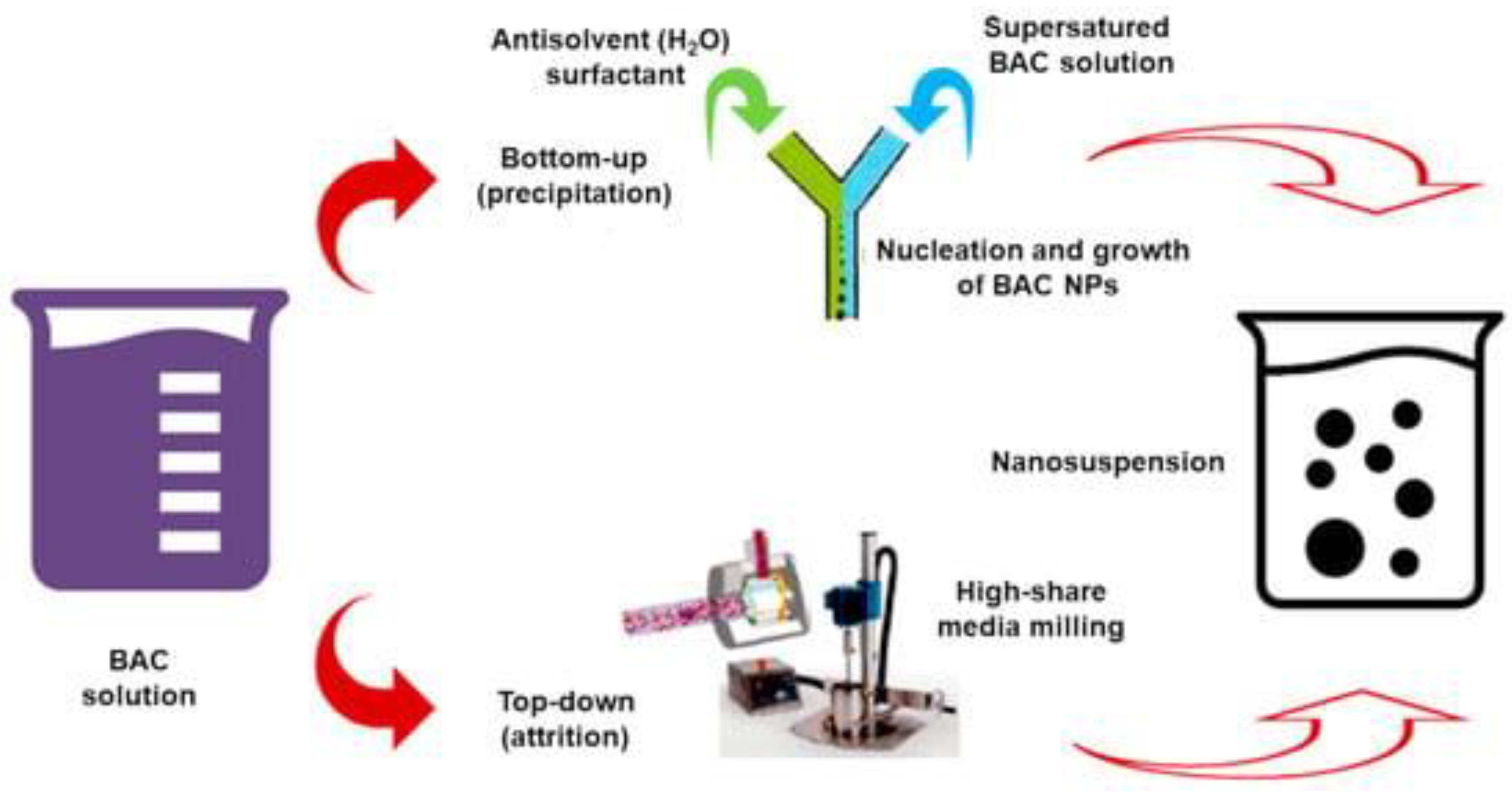

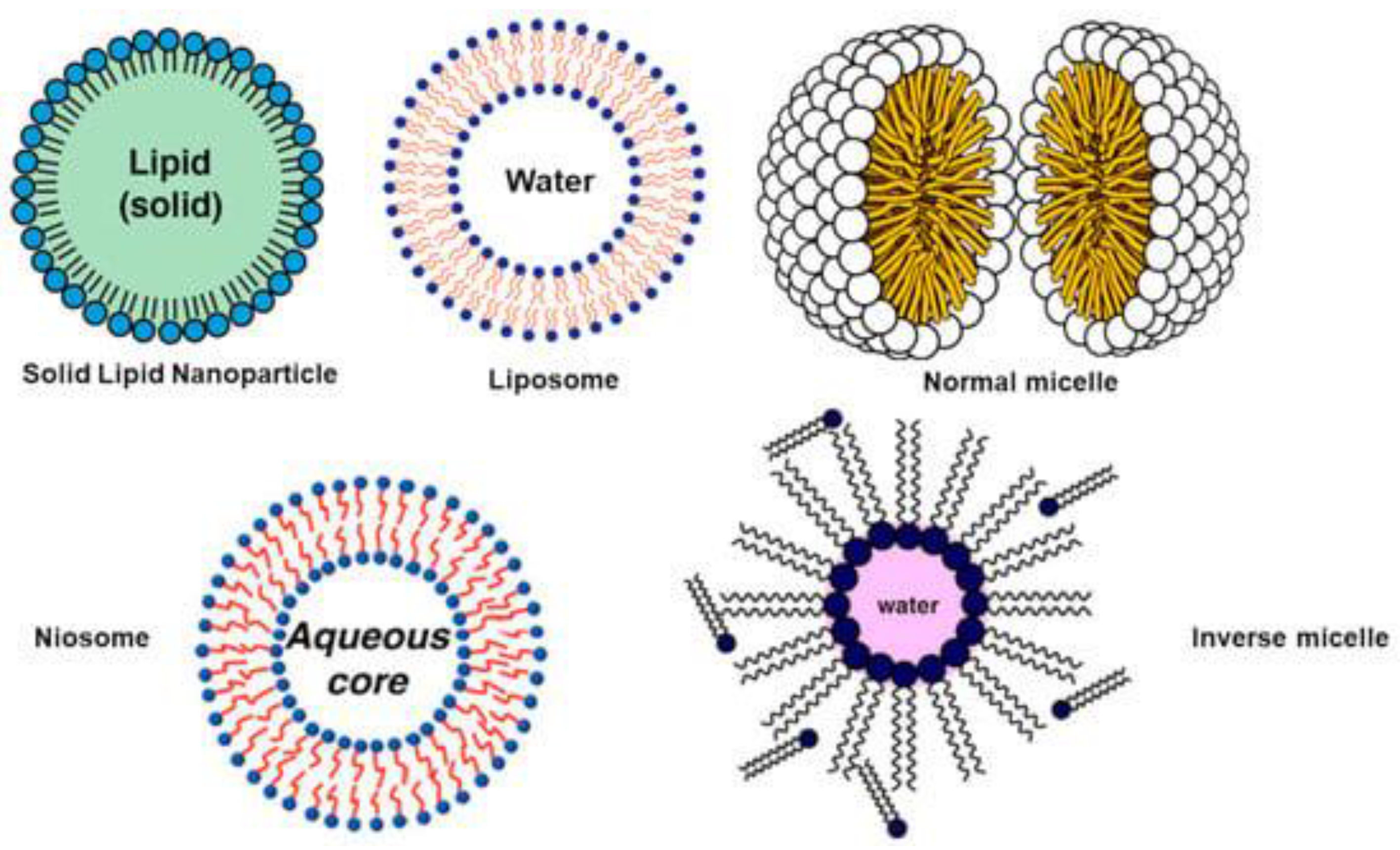
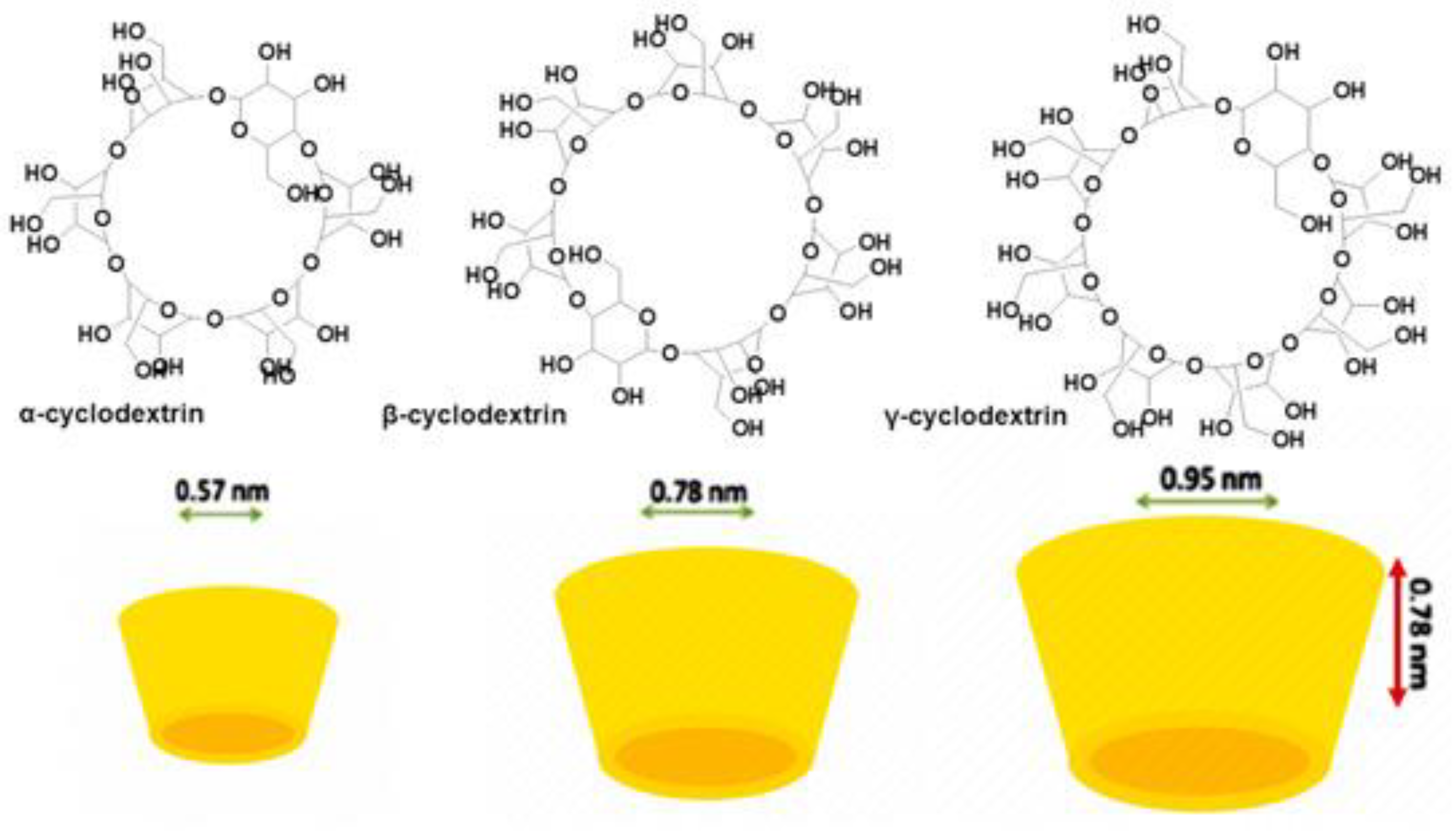
References
- Pocci, M.; Alfei, S.; Lucchesini, F.; Bertini, V.; Idini, B. Nanostructured Styrenic Co-Polymers Containing Glucopyranosyl Residues and Their Functionalization. Tetrahedron 2009, 65, 5684–5692. [Google Scholar] [CrossRef]
- Pocci, M.; Alfei, S.; Lucchesini, F.; Castellaro, S.; Bertini, V. Synthesis and NMR Investigation of Styrene Glycopolymers Containing <scp>d</Scp> -Galactose Units Functionalized with 4-(4-Hydroxybutoxy)Benzylamine Residues. Polym. Chem. 2013, 4, 740–751. [Google Scholar] [CrossRef]
- Pocci, M.; Alfei, S.; Lucchesini, F.; Castellaro, S.; Bertini, V. Synthesis, Glycosylation and NMR Characterization of Linear Peracetylated <scp>d</Scp> -Galactose Glycopolymers. RSC Adv 2015, 5, 23835–23846. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S. Synthesis and Characterization of Polyester-Based Dendrimers Containing Peripheral Arginine or Mixed Amino Acids as Potential Vectors for Gene and Drug Delivery. Macromol Res 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR Characterization of Dendrimers Based on 2, 2-Bis-(Hydroxymethyl)-Propanoic Acid (Bis-HMPA) Containing Peripheral Amino Acid Residues for Gene Transfection. Organic Communications 2017, 10, 144–177. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and Characterization of Versatile Amphiphilic Dendrimers Peripherally Decorated with Positively Charged Amino Acids. Polym Int 2018, 67, 1572–1584. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and Characterization of Fourth Generation Polyester-based Dendrimers with Cationic Amino Acids-modified Crown as Promising Water Soluble Biomedical Devices. Polym Adv Technol 2018, 29, 2735–2749. [Google Scholar] [CrossRef]
- Alfei, S.; Taptue, G.B.; Catena, S.; Bisio, A. Synthesis of Water-Soluble, Polyester-Based Dendrimer Prodrugs for Exploiting Therapeutic Properties of Two Triterpenoid Acids. Chinese Journal of Polymer Science 2018, 36, 999–1010. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Ponassi, M.; Rosano, C.; Zoppi, V.; Spallarossa, A. Hydrophilic and Amphiphilic Water-Soluble Dendrimer Prodrugs Suitable for Parenteral Administration of a Non-Soluble Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitor Thiocarbamate Derivative. European Journal of Pharmaceutical Sciences 2018, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Signorello, M.G.; Schito, A.; Catena, S.; Turrini, F. Reshaped as Polyester-Based Nanoparticles, Gallic Acid Inhibits Platelet Aggregation, Reactive Oxygen Species Production and Multi-Resistant Gram-Positive Bacteria with an Efficiency Never Obtained. Nanoscale Adv 2019, 1, 4148–4157. [Google Scholar] [CrossRef]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of Ellagic Acid Micro and Nano Formulations with Amazingly Increased Water Solubility by Its Entrapment in Pectin or Non-PAMAM Dendrimers Suitable for Clinical Applications. New Journal of Chemistry 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. ChemistrySelect 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Development of a Fast, Low-Cost, Conservative and Ecological Method for Quantifying Gallic Acid in Polymeric Formulations by FTIR Spectroscopy in Solution. ChemistrySelect 2020, 5, 4381–4388. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and Biocompatible Spherical Dendrimer Nanoparticles with a Gallic Acid Shell and a Double-Acting Strong Antioxidant Activity as Potential Device to Fight Diseases from “Oxidative Stress. ” Drug Deliv Transl Res 2020, 10, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Alfei, S. Antibacterial Activity of Non-Cytotoxic, Amino Acid-Modified Polycationic Dendrimers against Pseudomonas Aeruginosa and Other Non-Fermenting Gram-Negative Bacteria. Polymers (Basel) 2020, 12, 1818. [Google Scholar] [CrossRef]
- Alfei, S.; Piatti, G.; Caviglia, D.; Schito, A. Synthesis, Characterization, and Bactericidal Activity of a 4-Ammoniumbuthylstyrene-Based Random Copolymer. Polymers (Basel) 2021, 13, 1140. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Valenti, G.; Domenicotti, C. Synthesis of Polystyrene-Based Cationic Nanomaterials with Pro-Oxidant Cytotoxic Activity on Etoposide-Resistant Neuroblastoma Cells. Nanomaterials 2021, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Schito, G.C.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers (Basel) 2021, 13, 521. [Google Scholar] [CrossRef]
- Alfei, S.; Brullo, C.; Caviglia, D.; Piatti, G.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Schito, A.M. Pyrazole-Based Water-Soluble Dendrimer Nanoparticles as a Potential New Agent against Staphylococci. Biomedicines 2021, 10, 17. [Google Scholar] [CrossRef]
- Alfei, S.; Brullo, C.; Caviglia, D.; Zuccari, G. Preparation and Physicochemical Characterization of Water-Soluble Pyrazole-Based Nanoparticles by Dendrimer Encapsulation of an Insoluble Bioactive Pyrazole Derivative. Nanomaterials 2021, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Increased Water-Solubility and Maintained Antioxidant Power of Resveratrol by Its Encapsulation in Vitamin E TPGS Micelles: A Potential Nutritional Supplement for Chronic Liver Disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef]
- Schito, A.M.; Caviglia, D.; Piatti, G.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Schito, G.C.; Alfei, S. Efficacy of Ursolic Acid-Enriched Water-Soluble and Not Cytotoxic Nanoparticles against Enterococci. Pharmaceutics 2021, 13, 1976. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M.; Zuccari, G. Considerable Improvement of Ursolic Acid Water Solubility by Its Encapsulation in Dendrimer Nanoparticles: Design, Synthesis and Physicochemical Characterization. Nanomaterials 2021, 11, 2196. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Alfei, S. Broad-Spectrum Bactericidal Activity of a Synthetic Random Copolymer Based on 2-Methoxy-6-(4-Vinylbenzyloxy)-Benzylammonium Hydrochloride. Int J Mol Sci 2021, 22, 5021. [Google Scholar] [CrossRef]
- Alfei, S.; Caviglia, D.; Piatti, G.; Zuccari, G.; Schito, A.M. Bactericidal Activity of a Self-Biodegradable Lysine-Containing Dendrimer against Clinical Isolates of Acinetobacter Genus. Int J Mol Sci 2021, 22, 7274. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Bactericidal Activity of Non-Cytotoxic Cationic Nanoparticles against Clinically and Environmentally Relevant Pseudomonas Spp. Isolates. Pharmaceutics 2021, 13, 1411. [Google Scholar] [CrossRef]
- Alfei, S.; Caviglia, D.; Piatti, G.; Zuccari, G.; Schito, A.M. Synthesis, Characterization and Broad-Spectrum Bactericidal Effects of Ammonium Methyl and Ammonium Ethyl Styrene-Based Nanoparticles. Nanomaterials 2022, 12, 2743. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Zuccari, G.; Caviglia, D.; Brullo, C. Synthesis and Characterization of Pyrazole-Enriched Cationic Nanoparticles as New Promising Antibacterial Agent by Mutual Cooperation. Nanomaterials 2022, 12, 1215. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Spallarossa, A.; Lusardi, M.; Zuccari, G. Successful Dendrimer and Liposome-Based Strategies to Solubilize an Antiproliferative Pyrazole Otherwise Not Clinically Applicable. Nanomaterials 2022, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Caviglia, D.; Zorzoli, A.; Marimpietri, D.; Spallarossa, A.; Lusardi, M.; Zuccari, G.; Schito, A.M. Potent and Broad-Spectrum Bactericidal Activity of a Nanotechnologically Manipulated Novel Pyrazole. Biomedicines 2022, 10, 907. [Google Scholar] [CrossRef]
- Alfei, S.; Zorzoli, A.; Marimpietri, D.; Schito, A.M.; Russo, E. Mutual Jellification of Two Bactericidal Cationic Polymers: Synthesis and Physicochemical Characterization of a New Two-Component Hydrogel. Pharmaceutics 2022, 14, 2444. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Caviglia, D.; Brullo, C.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Enhanced Antibacterial Activity of a Cationic Macromolecule by Its Complexation with a Weakly Active Pyrazole Derivative. Biomedicines 2022, 10, 1607. [Google Scholar] [CrossRef]
- Alfei, S.; Zorzoli, A.; Marimpietri, D.; Zuccari, G.; Russo, E.; Caviglia, D.; Schito, A.M. A Self-Forming Hydrogel from a Bactericidal Copolymer: Synthesis, Characterization, Biological Evaluations and Perspective Applications. Int J Mol Sci 2022, 23, 15092. [Google Scholar] [CrossRef]
- Zuccari, G.; Russo, E.; Villa, C.; Zorzoli, A.; Marimpietri, D.; Marchitto, L.; Alfei, S. Preparation and Characterization of Amorphous Solid Dispersions for the Solubilization of Fenretinide. Pharmaceuticals 2023, 16, 388. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Brullo, C.; Alfei, S. Pyrazole-Enriched Cationic Nanoparticles Induced Early- and Late-Stage Apoptosis in Neuroblastoma Cells at Sub-Micromolar Concentrations. Pharmaceuticals 2023, 16, 393. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.E.; Marengo, B.; Milanese, M.; Zuccari, G.; Brullo, C.; Domenicotti, C.; Alfei, S. Imidazo-Pyrazole-Loaded Palmitic Acid and Polystyrene-Based Nanoparticles: Synthesis, Characterization and Antiproliferative Activity on Chemo-Resistant Human Neuroblastoma Cells. Int J Mol Sci 2023, 24, 15027. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Milanese, M.; Brullo, C.; Valenti, G.E.; Domenicotti, C.; Russo, E.; Marengo, B. Antiproliferative Imidazo-Pyrazole-Based Hydrogel: A Promising Approach for the Development of New Treatments for PLX-Resistant Melanoma. Pharmaceutics 2023, 15, 2425. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Giannoni, P.; Signorello, M.G.; Torazza, C.; Zuccari, G.; Athanassopoulos, C.M.; Domenicotti, C.; Marengo, B. The Remarkable and Selective In Vitro Cytotoxicity of Synthesized Bola-Amphiphilic Nanovesicles on Etoposide-Sensitive and -Resistant Neuroblastoma Cells. Nanomaterials 2024, 14, 1505. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Zuccari, G.; Bacchetti, F.; Torazza, C.; Milanese, M.; Siciliano, C.; Athanassopoulos, C.M.; Piatti, G.; Schito, A.M. Synthesized Bis-Triphenyl Phosphonium-Based Nano Vesicles Have Potent and Selective Antibacterial Effects on Several Clinically Relevant Superbugs. Nanomaterials 2024, 14, 1351. [Google Scholar] [CrossRef]
- Alfei, S.; Giordani, P.; Zuccari, G. Synthesis and Physicochemical Characterization of Gelatine-Based Biodegradable Aerogel-like Composites as Possible Scaffolds for Regenerative Medicine. Int J Mol Sci 2024, 25, 5009. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Zuccari, G.; Athanassopoulos, C.M.; Domenicotti, C.; Marengo, B. Strongly ROS-Correlated, Time-Dependent, and Selective Antiproliferative Effects of Synthesized Nano Vesicles on BRAF Mutant Melanoma Cells and Their Hyaluronic Acid-Based Hydrogel Formulation. Int J Mol Sci 2024, 25, 10071. [Google Scholar] [CrossRef] [PubMed]
- Orienti, I.; Zuccari, G.; Carosio, R.; G. Montaldo, P. Improvement of Aqueous Solubility of Fenretinide and Other Hydrophobic Anti-Tumor Drugs by Complexation with Amphiphilic Dextrins. Drug Deliv 2009, 16, 389–398. [Google Scholar] [CrossRef]
- Zuccari, G.; Bergamante, V.; Carosio, R.; Gotti, R.; Montaldo, P.G.; Orienti, I. Micellar Complexes of All-Trans Retinoic Acid with Polyvinylalcohol-Nicotinoyl Esters as New Parenteral Formulations in Neuroblastoma. Drug Deliv 2009, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Carosio, R.; Pistoia, V.; Orienti, I.; Formelli, F.; Cavadini, E.; Mangraviti, S.; Montaldo, P.G.; Ognio, E.; Emionite, L.; Zuccari, G. Enhanced Anti-Neuroblastoma Activity of a Fenretinide Complexed Form after Intravenous Administration. Journal of Pharmacy and Pharmacology 2012, 64, 228–236. [Google Scholar] [CrossRef]
- Orienti, I.; Zuccari, G.; Falconi, M.; Teti, G.; Illingworth, N.A.; Veal, G.J. Novel Micelles Based on Amphiphilic Branched PEG as Carriers for Fenretinide. Nanomedicine 2012, 8, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, D.; Pastorino, F.; Zuccari, G.; Caffa, I.; Loi, M.; Marimpietri, D.; Brignole, C.; Perri, P.; Cilli, M.; Nico, B.; et al. Enhanced Anti-Tumor and Anti-Angiogenic Efficacy of a Novel Liposomal Fenretinide on Human Neuroblastoma. Journal of Controlled Release 2013, 170, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Milelli, A.; Pastorino, F.; Loi, M.; Petretto, A.; Parise, A.; Marchetti, C.; Minarini, A.; Cilli, M.; Emionite, L.; et al. Tumor Vascular Targeted Liposomal-Bortezomib Minimizes Side Effects and Increases Therapeutic Activity in Human Neuroblastoma. Journal of Controlled Release 2015, 211, 44–52. [Google Scholar] [CrossRef]
- Parise, A.; Milelli, A.; Tumiatti, V.; Minarini, A.; Neviani, P.; Zuccari, G. Preparation, Characterization and in Vitro Evaluation of Sterically Stabilized Liposome Containing a Naphthalenediimide Derivative as Anticancer Agent. Drug Deliv 2015, 22, 590–597. [Google Scholar] [CrossRef]
- Alfei, S. Nanotechnology Applications to Improve Solubility of Bioactive Constituents of Foods for Health-Promoting Purposes. In; 2020; pp. 189–257.
- Alfei, S. Cationic Materials for Gene Therapy: A Look Back to the Birth and Development of 2,2-Bis-(Hydroxymethyl)Propanoic Acid-Based Dendrimer Scaffolds. Int J Mol Sci 2023, 24, 16006. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Alfei, S. Development of Phytochemical Delivery Systems by Nano-Suspension and Nano-Emulsion Techniques. Int J Mol Sci 2023, 24, 9824. [Google Scholar] [CrossRef]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Zuccari, G. Attempts to Improve Lipophilic Drugs’ Solubility and Bioavailability: A Focus on Fenretinide. Pharmaceutics 2024, 16, 579. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Caviglia, D. Prevention and Eradication of Biofilm by Dendrimers: A Possibility Still Little Explored. Pharmaceutics 2022, 14, 2016. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers (Basel) 2020, 12, 1195. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C. Nanotubes: Carbon-Based Fibers and Bacterial Nano-Conduits Both Arousing a Global Interest and Conflicting Opinions. Fibers 2022, 10, 75. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology Application in Food Packaging: A Plethora of Opportunities versus Pending Risks Assessment and Public Concerns. Food Research International 2020, 137, 109664. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers (Basel) 2021, 13, 2262. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-Based Commercial Formulations: Current Developments and Future Prospects. J Pharm Investig 2023, 53, 19–33. [Google Scholar] [CrossRef]
- Jagtiani, E. Advancements in Nanotechnology for Food Science and Industry. Food Front 2022, 3, 56–82. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-Physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J Am Chem Soc 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Deshpande, D.; Korde, A.; Amiji, M. A Review of Multifunctional Nanoemulsion Systems to Overcome Oral and CNS Drug Delivery Barriers. Mol Membr Biol 2010, 27, 260–273. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the Limits for Ellagic Acid Bioavailability: A Crossover Pharmacokinetic Study in Healthy Volunteers after Consumption of Pomegranate Extracts. J Funct Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Ellagic Acid (EA): A Green Multi-Target Weapon Reducing Oxidative Stress and Inflammation Thus Preventing and Ameliorating Alzheimer Disease (AD) Condition. Preprints 2024, 2024111130. [Google Scholar]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin Delivery Using Nanotechnology. Drug Discov Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Applied Sciences 2020, 10, 3353. [Google Scholar] [CrossRef]
- Avachat, A.M.; Patel, V.G. Self Nanoemulsifying Drug Delivery System of Stabilized Ellagic Acid–Phospholipid Complex with Improved Dissolution and Permeability. Saudi Pharmaceutical Journal 2015, 23, 276–289. [Google Scholar] [CrossRef]
- Madrigal-Carballo, S.; Lim, S.; Rodriguez, G.; Vila, A.O.; Krueger, C.G.; Gunasekaran, S.; Reed, J.D. Biopolymer Coating of Soybean Lecithin Liposomes via Layer-by-Layer Self-Assembly as Novel Delivery System for Ellagic Acid. J Funct Foods 2010, 2, 99–106. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Rahmati-yamchi, M.; Shanehbandi, D.; Nazari Soltan Ahmad, S.; Hasani, A. Enhanced Anti-Cancer Capability of Ellagic Acid Using Solid Lipid Nanoparticles (SLNs). Int J Cancer Manag 2018, 11. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.S.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion Complex of Ellagic Acid with β-Cyclodextrin: Characterization and in Vitro Anti-Inflammatory Evaluation. J Mol Struct 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Mady, F.M.; Ibrahim, S.R.-M. Cyclodextrin-Based Nanosponge for Improvement of Solubility and Oral Bioavailability of Ellagic Acid. Pak J Pharm Sci 2018, 31, 2069–2076. [Google Scholar]
- Sha, X.; Guo, J.; Chen, Y.; Fang, X. Effect of Phospholipid Composition on Pharmacokinetics and Biodistribution of Epirubicin Liposomes. J Liposome Res 2012, 22, 80–88. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Liu, B.; Feng, S.-S. A Strategy for Precision Engineering of Nanoparticles of Biodegradable Copolymers for Quantitative Control of Targeted Drug Delivery. Biomaterials 2010, 31, 9145–9155. [Google Scholar] [CrossRef] [PubMed]
- de Paz, E.; Martín, Á.; Estrella, A.; Rodríguez-Rojo, S.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J. Formulation of β-Carotene by Precipitation from Pressurized Ethyl Acetate-on-Water Emulsions for Application as Natural Colorant. Food Hydrocoll 2012, 26, 17–27. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Huang, Q. Quercetin Nanosuspensions Produced by High-Pressure Homogenization. J Agric Food Chem 2014, 62, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Vargas, V.; Saldarriaga, S.; Sánchez, F.S.; Cuellar, L.N.; Paladines, G.M. Effects of the Spray-Drying Process Using Maltodextrin on Bioactive Compounds and Antioxidant Activity of the Pulp of the Tropical Fruit Açai (Euterpe Oleracea Mart.). Heliyon 2024, 10, e33544. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E. α-Tocopherol Nanosuspensions Produced Using a Supercritical Assisted Process. J Food Eng 2015, 149, 131–136. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J Nutr 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Koutelidakis, A.E.; Argyri, K.; Sevastou, Z.; Lamprinaki, D.; Panagopoulou, E.; Paximada, E.; Sali, A.; Papalazarou, V.; Mallouchos, A.; Evageliou, V.; et al. Bioactivity of Epigallocatechin Gallate Nanoemulsions Evaluated in Mice Model. J Med Food 2017, 20, 923–931. [Google Scholar] [CrossRef]
- Martínez-Ballesta, Mc.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Baccarin, T.; Lemos-Senna, E. Potential Application of Nanoemulsions for Skin Delivery of Pomegranate Peel Polyphenols. AAPS PharmSciTech 2017, 18, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, H.; Kim, J.E.; Song, K. Bin; Lee, Y.S.; Chung, D.S.; Min, S.C. Plum Coatings of Lemongrass Oil-incorporating Carnauba Wax-based Nanoemulsion. J Food Sci 2013, 78. [Google Scholar] [CrossRef]
- Salazar, J.; Müller, R.H.; Möschwitzer, J.P. Combinative Particle Size Reduction Technologies for the Production of Drug Nanocrystals. J Pharm (Cairo) 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Jonaitis, T.S.; Card, J.W. A Brief Review of the Occurrence, Use, and Safety of Food-Related Nanomaterials. J Food Sci 2011, 76. [Google Scholar] [CrossRef]
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.-M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of Curcumin-Loaded Lipid Nanoemulsions with Cell-Penetrating Peptides Increases Their Cellular Uptake and Enhances the Anti-Inflammatory Effects in Endothelial Cells. Journal of Pharmacy and Pharmacology 2016, 68, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Cao, Y.; Huang, Q. Edible Nanoencapsulation Vehicles for Oral Delivery of Phytochemicals: A Perspective Paper. J Agric Food Chem 2017, 65, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Food-Grade Nanoparticles for Encapsulation, Protection and Delivery of Curcumin: Comparison of Lipid, Protein, and Phospholipid Nanoparticles under Simulated Gastrointestinal Conditions. RSC Adv 2016, 6, 3126–3136. [Google Scholar] [CrossRef]
- Osorno, L.; Brandley, A.; Maldonado, D.; Yiantsos, A.; Mosley, R.; Byrne, M. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef]
- Thatipamula, R.; Palem, C.; Gannu, R.; Mudragada, S.; Yamsani, M. Formulation and in Vitro Characterization of Domperidone Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. Daru 2011, 19, 23–32. [Google Scholar] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Moacă, E.-A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef]
- Miladi, K.; Ibraheem, D.; Iqbal, M.; Sfar, S.; Fessi, H.; Elaissari, A. Particles from Preformed Polymers as Carriers for Drug Delivery. EXCLI J 2014, 13, 28–57. [Google Scholar] [PubMed]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-Based Nanoparticles: From Preparation to Encapsulation of Active Molecules. Int J Pharm 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, Y.; Yang, F.; Wan, K.; Fan, D.; Wang, D. Novel Oral Administrated Ellagic Acid Nanoparticles for Enhancing Oral Bioavailability and Anti-Inflammatory Efficacy. J Drug Deliv Sci Technol 2018, 46, 215–222. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Zheng, J.; Liu, F.; Qiu, C.-Y.; Lin, W.-F.; Tang, C.-H. The Influence of Ionic Strength on the Characteristics of Heat-Induced Soy Protein Aggregate Nanoparticles and the Freeze–Thaw Stability of the Resultant Pickering Emulsions. Food Funct 2017, 8, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Fragr J 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M. del M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Riaz, M.; Ko, S.; Park, S. Novel Technologies to Enhance Solubility of Food-Derived Bioactive Compounds: A Review. J Funct Foods 2017, 39, 63–73. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols – a Review. Trends Food Sci Technol 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Wahyudiono; Fukuzato, R. ; Higashiura, T.; Youn, Y.-S.; Lee, Y.-W.; Goto, M. Nanoparticle Formation of Lycopene/β-Cyclodextrin Inclusion Complex Using Supercritical Antisolvent Precipitation. J Supercrit Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- de Lima Petito, N.; da Silva Dias, D.; Costa, V.G.; Falcão, D.Q.; de Lima Araujo, K.G. Increasing Solubility of Red Bell Pepper Carotenoids by Complexation with 2-Hydroxypropyl-β-Cyclodextrin. Food Chem 2016, 208, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int J Mol Sci 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Salatin, S.; Jelvehgari, M. Natural Polysaccharide Based Nanoparticles for Drug/Gene Delivery. Pharmaceutical Sciences 2017, 23, 84–94. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-Based Delivery Systems for Protein Therapeutics and Antigens. Adv Drug Deliv Rev 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Duceppe, N.; Tabrizian, M. Advances in Using Chitosan-Based Nanoparticles for in Vitro and in Vivo Drug and Gene Delivery. Expert Opin Drug Deliv 2010, 7, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Ojea-Jiménez, I.; Tort, O.; Lorenzo, J.; Puntes, V.F. Engineered Nonviral Nanocarriers for Intracellular Gene Delivery Applications. Biomedical Materials 2012, 7, 054106. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a Rheology Modifier: Origin, Structure, Commercial Production and Rheology. Carbohydr Polym 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Kim, S.-K.; Ahn, C.-B.; Je, J.-Y. Preparation, Characterization, and Antioxidant Properties of Gallic Acid-Grafted-Chitosans. Carbohydr Polym 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Ramana, L.N.; Sethuraman, S.; Krishnan, U.M. Ellagic Acid Encapsulated Chitosan Nanoparticles as Anti-Hemorrhagic Agent. Carbohydr Polym 2014, 111, 215–221. [Google Scholar] [CrossRef]
- Rocha, D.S.; Casagrande, L.; Model, J.F.A.; dos Santos, J.T.; Hoefel, A.L.; Kucharski, L.C. Effect of Yerba Mate (Ilex Paraguariensis) Extract on the Metabolism of Diabetic Rats. Biomedicine & Pharmacotherapy 2018, 105, 370–376. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Roggeri, A.; Olivero, G.; Cisani, F.; Bonfiglio, T.; Summa, M.; Grilli, M.; Caviglioli, G.; Alfei, S.; et al. Neuroinflammation in Aged Brain: Impact of the Oral Administration of Ellagic Acid Microdispersion. Int J Mol Sci 2020, 21, 3631. [Google Scholar] [CrossRef] [PubMed]
- Al Shaal, L.; M. R.H.; S.R. Smart Crystal Combination Technology – Scale up from Lab to Pilot Scale and Long Term Stability. Die Pharmazie - An International Journal of Pharmaceutical Sciences 2010, 65, 877–884. [Google Scholar]
- McClements, D.J.; Li, Y. Review of in Vitro Digestion Models for Rapid Screening of Emulsion-Based Systems. Food Funct 2010, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Oms-Oliu, G.; MartÃn-Belloso, O. Nanoemulsion-Based Delivery Systems to Improve Functionality of Lipophilic Components. Front Nutr 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Ho, M.J.; Choi, Y.W.; Kang, M.J. A Polyvinylpyrrolidone-Based Supersaturable Self-Emulsifying Drug Delivery System for Enhanced Dissolution of Cyclosporine A. Polymers (Basel) 2017, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; McClements, D.J. Formation of Nanoemulsions Stabilized by Model Food-Grade Emulsifiers Using High-Pressure Homogenization: Factors Affecting Particle Size. Food Hydrocoll 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Chow, P.Y.; Gue, S.Z.; Leow, S.K.; Goh, L.B. Solid Self-Microemulsifying System (S-SMECS) for Enhanced Bioavailability and Pigmentation of Highly Lipophilic Bioactive Carotenoid. Powder Technol 2015, 274, 199–204. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arabian Journal of Chemistry 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv Pharm Bull 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int J Mol Sci 2013, 14, 4242–4282. [Google Scholar] [CrossRef]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid Lipid Based Nanocarriers: An Overview / Nanonosači Na Bazi Čvrstih Lipida: Pregled. Acta Pharmaceutica 2012, 62, 433–472. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in Liposomal Drug Delivery to Cancer: An Overview. J Drug Deliv Sci Technol 2020, 56, 101549. [Google Scholar] [CrossRef]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and Pitfalls in the Development of Liposomal Delivery Systems for Cancer Therapy. Semin Cancer Biol 2021, 69, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H. Applications of Nanotechnology in Agriculture. In; 2019; pp. 115–142.
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical Properties and Skin Permeation of Span 60/Tween 60 Niosomes of Ellagic Acid. Int J Pharm 2012, 423, 303–311. [Google Scholar] [CrossRef]
- Kurl, S.; Kumar, A.; Reena; Mittal, N. ; Singh, D.; Bassi, P.; Kaur, G. Challenges, Opportunities, and Future Prospects of Polysaccharide-Based Nanoparticles for Colon Targeting: A Comprehensive Review. Carbohydrate Polymer Technologies and Applications 2023, 6, 100361. [Google Scholar] [CrossRef]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Songkro, S.; Reanmongkol, W. Topical Anti-inflammatory Potential of Standardized Pomegranate Rind Extract and Ellagic Acid in Contact Dermatitis. Phytotherapy Research 2014, 28, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Kaewnopparat, N.; Songkro, S.; Panichayupakaranant, P.; Reanmongkol, W. Physicochemical Properties, in Vitro Release and Skin Permeation Studies of a Topical Formulation of Standardized Pomegranate Rind Extract. Pak J Pharm Sci 2015, 28, 29–36. [Google Scholar] [PubMed]
- Abd-Rabou, A.A.; Ahmed, H.H. CS-PEG Decorated PLGA Nano-Prototype for Delivery of Bioactive Compounds: A Novel Approach for Induction of Apoptosis in HepG2 Cell Line. Adv Med Sci 2017, 62, 357–367. [Google Scholar] [CrossRef] [PubMed]
- LI, L.; LI, W.; JUNG, S.-W.; LEE, Y.-W.; KIM, Y.-H. Protective Effects of Decursin and Decursinol Angelate against Amyloid β-Protein-Induced Oxidative Stress in the PC12 Cell Line: The Role of Nrf2 and Antioxidant Enzymes. Biosci Biotechnol Biochem 2011, 75, 434–442. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Ortega-Regules, A.E.; Lozada-Ramírez, J.D.; Pérez-Pérez, M.C.I.; Vernon-Carter, E.J.; Welti-Chanes, J. Color and Chemical Stability of Spray-Dried Blueberry Extract Using Mesquite Gum as Wall Material. Journal of Food Composition and Analysis 2011, 24, 889–894. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins Encapsulated by PLGA@PEG Nanoparticles Potentially Improved Its Free Radical Scavenging Capabilities via P38/JNK Pathway against Aβ1–42-Induced Oxidative Stress. J Nanobiotechnology 2017, 15, 12. [Google Scholar] [CrossRef]
- Mady, F.; Shaker, M. Enhanced Anticancer Activity and Oral Bioavailability of Ellagic Acid through Encapsulation in Biodegradable Polymeric Nanoparticles. Int J Nanomedicine 2017, Volume 12, 7405–7417. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Z.; Kessler, M.R.; Brehm-Stecher, B.; Larock, R.C. Antibacterial Soybean-Oil-Based Cationic Polyurethane Coatings Prepared from Different Amino Polyols. ChemSusChem 2012, 5, 2221–2227. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics 2023, 15, 889. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging Nanoformulation Strategies for Phytocompounds and Applications from Drug Delivery to Phototherapy to Imaging. Bioact Mater 2022, 14, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common Delivery Systems for Enhancing in Vivo Bioavailability and Biological Efficacy of Nutraceuticals. J Funct Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Tsai, M.-J.; Wu, P.-C.; Tsai, Y.-H.; Wu, Y.-H.; Fang, J.-Y. Elastic Liposomes as Carriers for Oral Delivery and the Brain Distribution of (+)-Catechin. J Drug Target 2011, 19, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an Oral Carrier System in Rats: Bioavailability and Antioxidant Properties of Liposome-Encapsulated Curcumin. J Agric Food Chem 2009, 57, 9141–9146. [Google Scholar] [CrossRef] [PubMed]
- ELSAMALIGY, M.; AFIFI, N.; MAHMOUD, E. Evaluation of Hybrid Liposomes-Encapsulated Silymarin Regarding Physical Stability and in Vivo Performance. Int J Pharm 2006, 319, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Rizvi, M.M.A.; Kar, S.K. Oral Delivery of Curcumin Bound to Chitosan Nanoparticles Cured Plasmodium Yoelii Infected Mice. Biotechnol Adv 2012, 30, 310–320. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, J.; Chen, H.; Xiao, Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B. Comparative Pharmacokinetics and Bioavailability Studies of Quercetin, Kaempferol and Isorhamnetin after Oral Administration of Ginkgo Biloba Extracts, Ginkgo Biloba Extract Phospholipid Complexes and Ginkgo Biloba Extract Solid Dispersions in Rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin–Phospholipid Complex: Preparation, Therapeutic Evaluation and Pharmacokinetic Study in Rats. Int J Pharm 2007, 330, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Liu, S.; Chen, X.; Wu, M.; Wang, H.; Yin, H.; He, D.; Xiong, H.; Zhang, J. Design and Evaluation of a Novel Evodiamine-Phospholipid Complex for Improved Oral Bioavailability. AAPS PharmSciTech 2012, 13, 534–547. [Google Scholar] [CrossRef] [PubMed]
- YANYU, X.; YUNMEI, S.; ZHIPENG, C.; QINENG, P. The Preparation of Silybin–Phospholipid Complex and the Study on Its Pharmacokinetics in Rats. Int J Pharm 2006, 307, 77–82. [Google Scholar] [CrossRef]
- Hüsch, J.; Gerbeth, K.; Fricker, G.; Setzer, C.; Zirkel, J.; Rebmann, H.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Effect of Phospholipid-Based Formulations of Boswellia Serrata Extract on the Solubility, Permeability, and Absorption of the Individual Boswellic Acid Constituents Present. J Nat Prod 2012, 75, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- FILBURN, C.R.; KETTENACKER, R.; GRIFFIN, D.W. Bioavailability of a Silybin–Phosphatidylcholine Complex in Dogs. J Vet Pharmacol Ther 2007, 30, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hong, J.; Shen, G.; Wu, R.T.; Wang, Y.; Huang, M.; Newmark, H.L.; Huang, Q.; Khor, T.O.; Heimbach, T.; et al. Pharmacokinetics of Dietary Cancer Chemopreventive Compound Dibenzoylmethane in Rats and the Impact of Nanoemulsion and Genetic Knockout of Nrf2 on Its Disposition. Biopharm Drug Dispos 2011, 32, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, J.; Chikamori, H.; Sato, H.; Uchida, S.; Debari, K.; Onoue, S.; Yamada, S. Physicochemical and Pharmacological Characterization of α-Tocopherol-Loaded Nano-Emulsion System. Int J Pharm 2010, 396, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gui, Sh.Y. ; G. Sh.Y.; G.Sh.Y.; W.L.; W.L.; P.D.Y.; P.D.Y.; P.D.Y.; L.Q.Y.; L.Q.Y.; Y.B.P.; S.J.Zh. Preparation and Evaluation of a Microemulsion for Oral Delivery of Berberine. 2008, 63, 516–519. [Google Scholar]
- Yu, A.; Wang, H.; Wang, J.; Cao, F.; Gao, Y.; Cui, J.; Zhai, G. Formulation Optimization and Bioavailability After Oral and Nasal Administration in Rabbits of Puerarin-Loaded Microemulsion. J Pharm Sci 2011, 100, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, J.; Lu, Y.; Liang, B.; Yang, C. Body Distribution of Camptothecin Solid Lipid Nanoparticles after Oral Administration. Pharm Res 1999, 16, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of Gastrointestinal Absorption of Quercetin by Solid Lipid Nanoparticles. Journal of Controlled Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- MEI, Z.; LI, X.; WU, Q.; HU, S.; YANG, X. The Research on the Anti-Inflammatory Activity and Hepatotoxicity of Triptolide-Loaded Solid Lipid Nanoparticle. Pharmacol Res 2005, 51, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Improving the Oral Bioavailability of Curcumin Using Novel Organogel-Based Nanoemulsions. J Agric Food Chem 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- S. Chopraa, K.K.S.A.R.K.K. In-Situ Nano-Emulsification Technique for Enhancing Oral Bioavailability of Curcumin and Thereby Evaluating Its Anticancer Efficacy on Human Lung Adeno-Carcinoma Epithelial Cell Line. J Pharm Res 2011, 4, 4087–4093. [Google Scholar]
- Tang, J.; Sun, J.; He, Z.-G. Self-Emulsifying Drug Delivery Systems: Strategy for Improving Oral Delivery of Poorly Soluble Drugs. Curr Drug ther 2007, 2, 85–93. [Google Scholar] [CrossRef]
- Shao, B.; Cui, C.; Ji, H.; Tang, J.; Wang, Z.; Liu, H.; Qin, M.; Li, X.; Wu, L. Enhanced Oral Bioavailability of Piperine by Self-Emulsifying Drug Delivery Systems: In Vitro, in Vivo and in Situ Intestinal Permeability Studies. Drug Deliv 2015, 22, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tian, R.; Hu, W.; Jia, Y.; Jiang, H.; Zhang, J.; Zhang, L. Preparation and Evaluation of Self-Microemulsifying Drug Delivery System of Baicalein. Fitoterapia 2012, 83, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Cui, F.; Li, Q.; Han, X.; Yu, Y.; Yang, M. A Novel Formulation Design about Water-Insoluble Oily Drug: Preparation of Zedoary Turmeric Oil Microspheres with Self-Emulsifying Ability and Evaluation in Rabbits. Int J Pharm 2005, 288, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Feng, N.; Xu, J. Preparation and Evaluation of Self-Microemulsifying Drug Delivery System of Oridonin. Int J Pharm 2008, 355, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.-J.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Peracetylation as a Means of Enhancing in Vitro Bioactivity and Bioavailability of Epigallocatechin-3-Gallate. Drug Metabolism and Disposition 2006, 34, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, X.; Wang, Q.; Cheng, S.; Zhang, S.; Zhang, M. Pharmacokinetics, Tissue Distribution and Excretion Study of Resveratrol and Its Prodrug 3,5,4′-Tri-O-Acetylresveratrol in Rats. Phytomedicine 2013, 20, 558–563. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Ferry, D.R.; Anderson, D.; Hussain, S.A.; Young, A.M.; Cook, J.E.; Hodgkin, E.; Seymour, L.W.; Kerr, D.J. Pre-Clinical and Clinical Study of QC12, a Water-Soluble, pro-Drug of Quercetin. Annals of Oncology 2001, 12, 245–248. [Google Scholar] [CrossRef]
- Belluti, F.; Fontana, G.; Bo, L.D.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, Synthesis and Anticancer Activities of Stilbene-Coumarin Hybrid Compounds: Identification of Novel Proapoptotic Agents. Bioorg Med Chem 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan Nanoparticles Enhance the Plasma Exposure of (−)-Epigallocatechin Gallate in Mice through an Enhancement in Intestinal Stability. European Journal of Pharmaceutical Sciences 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y. ; Sun; Wang; Sui; She; Zhai; Wang Effect of Particle Size on Solubility, Dissolution Rate, and Oral Bioavailability: Evaluation Using Coenzyme Q10 as Naked Nanocrystals. Int J Nanomedicine 2012, 5733. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.T.; Chen, N.; Ko, K.M.; Ng, K.M. Product Design: A Nanomized Nutraceutical with Enhanced Bioactivity and Bioavailability. Ind Eng Chem Res 2012, 51, 7320–7326. [Google Scholar] [CrossRef]
- Ghosh, D.; Choudhury, S.T.; Ghosh, S.; Mandal, A.K.; Sarkar, S.; Ghosh, A.; Saha, K. Das; Das, N. Nanocapsulated Curcumin: Oral Chemopreventive Formulation against Diethylnitrosamine Induced Hepatocellular Carcinoma in Rat. Chem Biol Interact 2012, 195, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing Anti-Inflammation Activity of Curcumin through O/W Nanoemulsions. Food Chem 2008, 108, 419–424. [Google Scholar] [CrossRef] [PubMed]
- MAITI, K., M. K., G.A., A.H.N., P.S.B., & M.P.K. ENHANCED THERAPEUTIC BENEFIT OF QUERCETIN–PHOSPHOLIPID COMPLEX IN CARBON TETRACHLORIDE–INDUCED ACUTE LIVER INJURY IN RATS: A COMPARATIVE STUDY. Https://Sid.Ir/Paper/297090/En. IRANIAN JOURNAL OF PHARMACOLOGY AND THERAPEUTICS (IJPT) 2005, 4, 84–90. [Google Scholar]
- Date, A.A.; Nagarsenker, M.S.; Patere, S.; Dhawan, V.; Gude, R.P.; Hassan, P.A.; Aswal, V.; Steiniger, F.; Thamm, J.; Fahr, A. Lecithin-Based Novel Cationic Nanocarriers (Leciplex) II: Improving Therapeutic Efficacy of Quercetin on Oral Administration. Mol Pharm 2011, 8, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Mandal, A.K.; Sarkar, S.; Panda, S.; Das, N. Nanoencapsulation of Quercetin Enhances Its Dietary Efficacy in Combating Arsenic-Induced Oxidative Damage in Liver and Brain of Rats. Life Sci 2009, 84, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Guazelli, C.F.S.; Fattori, V.; Colombo, B.B.; Georgetti, S.R.; Vicentini, F.T.M.C.; Casagrande, R.; Baracat, M.M.; Verri, W.A. Quercetin-Loaded Microcapsules Ameliorate Experimental Colitis in Mice by Anti-Inflammatory and Antioxidant Mechanisms. J Nat Prod 2013, 76, 200–208. [Google Scholar] [CrossRef] [PubMed]
- ELSAMALIGY, M.; AFIFI, N.; MAHMOUD, E. Evaluation of Hybrid Liposomes-Encapsulated Silymarin Regarding Physical Stability and in Vivo Performance. Int J Pharm 2006, 319, 121–129. [Google Scholar] [CrossRef]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Nanoparticles Formulation of Cuscuta Chinensis Prevents Acetaminophen-Induced Hepatotoxicity in Rats. Food and Chemical Toxicology 2008, 46, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-L.; Wu, T.-H.; Lin, L.-T.; Cham, T.-M.; Lin, C.-C. Naringenin-Loaded Nanoparticles Improve the Physicochemical Properties and the Hepatoprotective Effects of Naringenin in Orally-Administered Rats with CCl4-Induced Acute Liver Failure. Pharm Res 2009, 26, 893–902. [Google Scholar] [CrossRef]
- Pathan, R.A.; Bhandari, U.; Javed, S.; Nag, T.C. Anti-Apoptotic Potential of Gymnemic Acid Phospholipid Complex Pretreatment in Wistar Rats with Experimental Cardiomyopathy. Indian J Exp Biol 2012, 50, 117–127. [Google Scholar] [PubMed]
- Wang, Y.; Ma, Y.; Zheng, Y.; Song, J.; Yang, X.; Bi, C.; Zhang, D.; Zhang, Q. In Vitro and in Vivo Anticancer Activity of a Novel Puerarin Nanosuspension against Colon Cancer, with High Efficacy and Low Toxicity. Int J Pharm 2013, 441, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomé-Carneiro, J.; Yáñez-Gascón, M.J.; Alcántara, D.; Selma, M. V.; Beltrán, D.; García-Conesa, M.T.; Urbán, C.; Lucas, R.; Tomás-Barberán, F.; et al. Preventive Oral Treatment with Resveratrol Pro-Prodrugs Drastically Reduce Colon Inflammation in Rodents. J Med Chem 2010, 53, 7365–7376. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Sang, S.; Cheng, K.-H.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Peracetylated (−)-Epigallocatechin-3-Gallate (AcEGCG) Potently Prevents Skin Carcinogenesis by Suppressing the PKD1-Dependent Signaling Pathway in CD34 + Skin Stem Cells and Skin Tumors. Carcinogenesis 2013, 34, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Moradi, M. Improvement of Food Packaging Based on Functional Nanomaterial. In Nanotechnology: Applications in Energy, Drug and Food; Springer International Publishing: Cham, 2019; pp. 309–344. [Google Scholar]
- Pal, S.; Sarantsev, A. A Note on Transportation Cost Inequalities for Diffusions with Reflections. Electronic Communications in Probability 2019, 24. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J Agric Food Res 2022, 7, 100270. [Google Scholar] [CrossRef]
- Kuswandi, B. Environmental Friendly Food Nano-Packaging. Environ Chem Lett 2017, 15, 205–221. [Google Scholar] [CrossRef]
- Pocci, M.; Alfei, S.; Lucchesini, F.; Castellaro, S.; Bertini, V. Synthesis, Glycosylation and NMR Characterization of Linear Peracetylated d-Galactose Glycopolymers. RSC Adv 2015, 5. [Google Scholar] [CrossRef]
- Alfei, S.; Pintaudi, F.; Zuccari, G. Synthesis and Characterization of Amine and Aldehyde-Containing Copolymers for Enzymatic Crosslinking of Gelatine. Int J Mol Sci 2024, 25, 2897. [Google Scholar] [CrossRef]
- Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Alfei, S. Development of Mixed Micelles for Enhancing Fenretinide Apparent Solubility and Anticancer Activity Against Neuroblastoma Cells. Curr Drug Deliv 2024, 22. [Google Scholar] [CrossRef] [PubMed]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent Advances and Challenges on Applications of Nanotechnology in Food Packaging. A Literature Review. Food and Chemical Toxicology 2019, 134, 110814. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J Food Sci 2015, 80. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Nanotechnologies in the Food Industry – Recent Developments, Risks and Regulation. Trends Food Sci Technol 2012, 24, 30–46. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials 2018, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Xiao, H.; Bhattacharya, K.; Bitounis, D.; Demokritou, P.; McClements, D.J. Development of a Standardized Food Model for Studying the Impact of Food Matrix Effects on the Gastrointestinal Fate and Toxicity of Ingested Nanomaterials. NanoImpact 2019, 13, 13–25. [Google Scholar] [CrossRef]
- Schmidt, B.; Katiyar, V.; Plackett, D.; Larsen, E.H.; Gerds, N.; Koch, C.B.; Petersen, J.H. Migration of Nanosized Layered Double Hydroxide Platelets from Polylactide Nanocomposite Films. Food Additives & Contaminants: Part A 2011, 28, 956–966. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. Migration of Nanoparticles from Plastic Packaging Materials Containing Carbon Black into Foodstuffs. Food Additives & Contaminants: Part A 2014, 31, 1769–1782. [Google Scholar] [CrossRef]
- Gallocchio, F.; Cibin, V.; Biancotto, G.; Roccato, A.; Muzzolon, O.; Carmen, L.; Simone, B.; Manodori, L.; Fabrizi, A.; Patuzzi, I.; et al. Testing Nano-Silver Food Packaging to Evaluate Silver Migration and Food Spoilage Bacteria on Chicken Meat. Food Additives & Contaminants: Part A 2016, 33, 1063–1071. [Google Scholar] [CrossRef]
- Tiimob, B.J.; Mwinyelle, G.; Abdela, W.; Samuel, T.; Jeelani, S.; Rangari, V.K. Nanoengineered Eggshell–Silver Tailored Copolyester Polymer Blend Film with Antimicrobial Properties. J Agric Food Chem 2017, 65, 1967–1976. [Google Scholar] [CrossRef]
- Su, Q.-Z.; Lin, Q.-B.; Chen, C.-F.; Wu, L.-B.; Wang, Z.-W. Effect of Organic Additives on Silver Release from Nanosilver–Polyethylene Composite Films to Acidic Food Simulant. Food Chem 2017, 228, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Yu, Y.; Li, N.; Wang, L. Application and Safety Assessment for Nano-Composite Materials in Food Packaging. Chinese Science Bulletin 2011, 56, 1216–1225. [Google Scholar] [CrossRef]
- Maisanaba, S.; Pichardo, S.; Puerto, M.; Gutiérrez-Praena, D.; Cameán, A.M.; Jos, A. Toxicological Evaluation of Clay Minerals and Derived Nanocomposites: A Review. Environ Res 2015, 138, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Evaluation and Simulation of Silver and Copper Nanoparticle Migration from Polyethylene Nanocomposites to Food and an Associated Exposure Assessment. J Agric Food Chem 2014, 62, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Becaro, A.A.; Siqueira, M.C.; Puti, F.C.; de Moura, M.R.; Correa, D.S.; Marconcini, J.M.; Mattoso, L.H.C.; Ferreira, M.D. Cytotoxic and Genotoxic Effects of Silver Nanoparticle/Carboxymethyl Cellulose on Allium Cepa. Environ Monit Assess 2017, 189, 352. [Google Scholar] [CrossRef] [PubMed]
- Demir, E. A Review on Nanotoxicity and Nanogenotoxicity of Different Shapes of Nanomaterials. Journal of Applied Toxicology 2021, 41, 118–147. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, M.; Gatti, A.M.; Savarino, G.; Quattroni, P.; Martinelli, L.; Monari, E.; Boraschi, D. Innate Defence Functions of Macrophages Can Be Biased by Nano-Sized Ceramic and Metallic Particles. Eur Cytokine Netw 2004, 15, 339–346. [Google Scholar] [PubMed]
- Huang, L.-H.; Sun, X.-Y.; Ouyang, J.-M. Shape-Dependent Toxicity and Mineralization of Hydroxyapatite Nanoparticles in A7R5 Aortic Smooth Muscle Cells. Sci Rep 2019, 9, 18979. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in Vitro and in Vivo Toxicity of Gold Nanoparticles. Chinese Chemical Letters 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Perez, J.E.; Contreras, M.F.; Vilanova, E.; Felix, L.P.; Margineanu, M.B.; Luongo, G.; Porter, A.E.; Dunlop, I.E.; Ravasi, T.; Kosel, J. Cytotoxicity and Intracellular Dissolution of Nickel Nanowires. Nanotoxicology 2016, 10, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D.G. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Res Lett 2009, 4, 1409. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Unger, R.E.; Kirkpatrick, C.J.; Gatti, A.M.; Monari, E. Effects of Nano-Scaled Particles on Endothelial Cell Function in Vitro: Studies on Viability, Proliferation and Inflammation. J Mater Sci Mater Med 2004, 15, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-Y.; Zhu, B.-S.; Wang, X.-F.; Lu, Q.-H. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells. Chem Res Toxicol 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem Res Toxicol 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Haynes, C.L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J Am Chem Soc 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Martucci, N.J.; Liu, Y.; Yoo, E.; Tako, E.; Mahler, G.J. Silicon Dioxide Nanoparticle Exposure Affects Small Intestine Function in an in Vitro Model. Nanotoxicology 2018, 12, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Retracted Article: ZnO Nanoparticles Affect Intestinal Function in an in Vitro Model. Food Funct 2018, 9, 1475–1491. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.L.; Leong, D.T. Reducing ZnO Nanoparticles Toxicity through Silica Coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, L.D.; Reed, R.B.; Loguinov, A. V.; Antczak, P.; Tagmount, A.; Aloni, S.; Nowinski, D.T.; Luong, P.; Tran, C.; Karunaratne, N.; et al. Silver Nanowire Exposure Results in Internalization and Toxicity to Daphnia Magna. ACS Nano 2013, 7, 10681–10694. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, K.; Roy, B.; Chandrasekaran, N.; Krishnan, S.P.; Mukherjee, A. Silver Nanorods Induced Oxidative Stress and Chromosomal Aberrations in the Allium Cepa Model. IET Nanobiotechnol 2020, 14, 161–166. [Google Scholar] [CrossRef]
- Rajeshwari, A.; Roy, B.; Chandrasekaran, N.; Mukherjee, A. Cytogenetic Evaluation of Gold Nanorods Using Allium Cepa Test. Plant Physiology and Biochemistry 2016, 109, 209–219. [Google Scholar] [CrossRef]
- Alaraby, M.; Hernández, A.; Marcos, R. Systematic in Vivo Study of NiO Nanowires and Nanospheres: Biodegradation, Uptake and Biological Impacts. Nanotoxicology 2018, 12, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Demir, E. An in Vivo Study of Nanorod, Nanosphere, and Nanowire Forms of Titanium Dioxide Using Drosophila Melanogaster : Toxicity, Cellular Uptake, Oxidative Stress, and DNA Damage. J Toxicol Environ Health A 2020, 83, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Shim, H.-W.; Lee, G.-H.; Kim, J.-H.; Kim, D.-W. Comparison of Toxicity between the Different-Type TiO2 Nanowires in Vivo and in Vitro. Arch Toxicol 2013, 87, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Marcos, R. Antigenotoxic Potential of Boron Nitride Nanotubes. Nanotoxicology 2018, 12, 868–884. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Ohnishi, M.; Sasaki, T.; Matsumoto, M. Carcinogenicity of Multi-Walled Carbon Nanotubes: Challenging Issue on Hazard Assessment. J Occup Health 2018, 60, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, K.; Schneider, M.; Hammarin, G.; Häcker, U.; Prinz, C.N. Ingestion of Gallium Phosphide Nanowires Has No Adverse Effect on Drosophila Tissue Function. Nanotechnology 2013, 24, 285101. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Adolfsson, K.; Ekvall, M.T.; Borgström, M.T.; Linse, S.; Hansson, L.-A.; Cedervall, T.; Prinz, C.N. Translocation of 40 Nm Diameter Nanowires through the Intestinal Epithelium of Daphnia Magna. Nanotoxicology 2016, 10, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, M.; Li, Q.; Zhang, H.; Alvarez, P.J.J.; Liu, H.; Chen, W. Genotoxicity and Cytotoxicity of Cadmium Sulfide Nanomaterials to Mice: Comparison Between Nanorods and Nanodots. Environ Eng Sci 2014, 31, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Anzai, Y.; Piccoli, C.W.; Outwater, E.K.; Stanford, W.; Bluemke, D.A.; Nurenberg, P.; Saini, S.; Maravilla, K.R.; Feldman, D.E.; Schmiedl, U.P.; et al. Evaluation of Neck and Body Metastases to Nodes with Ferumoxtran 10–Enhanced MR Imaging: Phase III Safety and Efficacy Study. Radiology 2003, 228, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, G.; Kauser, H.; Athar, M. Iron Augments Stage-I and Stage-II Tumor Promotion in Murine Skin. Cancer Lett 2002, 183, 113–122. [Google Scholar] [CrossRef]
- Sadeghiani, N.; Barbosa, L.S.; Silva, L.P.; Azevedo, R.B.; Morais, P.C.; Lacava, Z.G.M. Genotoxicity and Inflammatory Investigation in Mice Treated with Magnetite Nanoparticles Surface Coated with Polyaspartic Acid. J Magn Magn Mater 2005, 289, 466–468. [Google Scholar] [CrossRef]
- Almansour, M.; Alarifi, S.; Jarrar, B. In Vivo Investigation on the Chronic Hepatotoxicity Induced by Intraperitoneal Administration of 10-Nm Silicon Dioxide Nanoparticles. Int J Nanomedicine 2018, Volume 13, 2685–2696. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Douglas, T.; Witwer, B.; Zhang, S.-C.; Strable, E.; Lewis, B.K.; Zywicke, H.; Miller, B.; van Gelderen, P.; Moskowitz, B.M.; et al. Magnetodendrimers Allow Endosomal Magnetic Labeling and in Vivo Tracking of Stem Cells. Nat Biotechnol 2001, 19, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Veranth, J.M.; Kaser, E.G.; Veranth, M.M.; Koch, M.; Yost, G.S. Cytokine Responses of Human Lung Cells (BEAS-2B) Treated with Micron-Sized and Nanoparticles of Metal Oxides Compared to Soil Dusts. Part Fibre Toxicol 2007, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell Uptake and in Vitro Toxicity of Magnetic Nanoparticles Suitable for Drug Delivery. Mol Pharm 2009, 6, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B.; Lai, T.C.; Huang, J.H.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Hwu, Y.K. Biocompatibility of Fe 3 O 4 Nanoparticles Evaluated by in Vitro Cytotoxicity Assays Using Normal, Glia and Breast Cancer Cells. Nanotechnology 2010, 21, 075102. [Google Scholar] [CrossRef] [PubMed]
- Faux, S.P.; Francis, J.E.; Smith, A.G.; Chipman, J.K. Induction of 8-Hydroxydeoxyguanosine in Ah -Responsive Mouse Liver by Iron and Aroclor 1254. Carcinogenesis 1992, 13, 247–250. [Google Scholar] [CrossRef]
- Ansar, S.; Abudawood, M.; Hamed, S.S.; Aleem, M.M. Exposure to Zinc Oxide Nanoparticles Induces Neurotoxicity and Proinflammatory Response: Amelioration by Hesperidin. Biol Trace Elem Res 2017, 175, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Senapati, V.A.; Gupta, G.S.; Pandey, A.K.; Shanker, R.; Dhawan, A.; Kumar, A. Zinc Oxide Nanoparticle Induced Age Dependent Immunotoxicity in BALB/c Mice. Toxicol Res (Camb) 2017, 6, 342–352. [Google Scholar] [CrossRef]
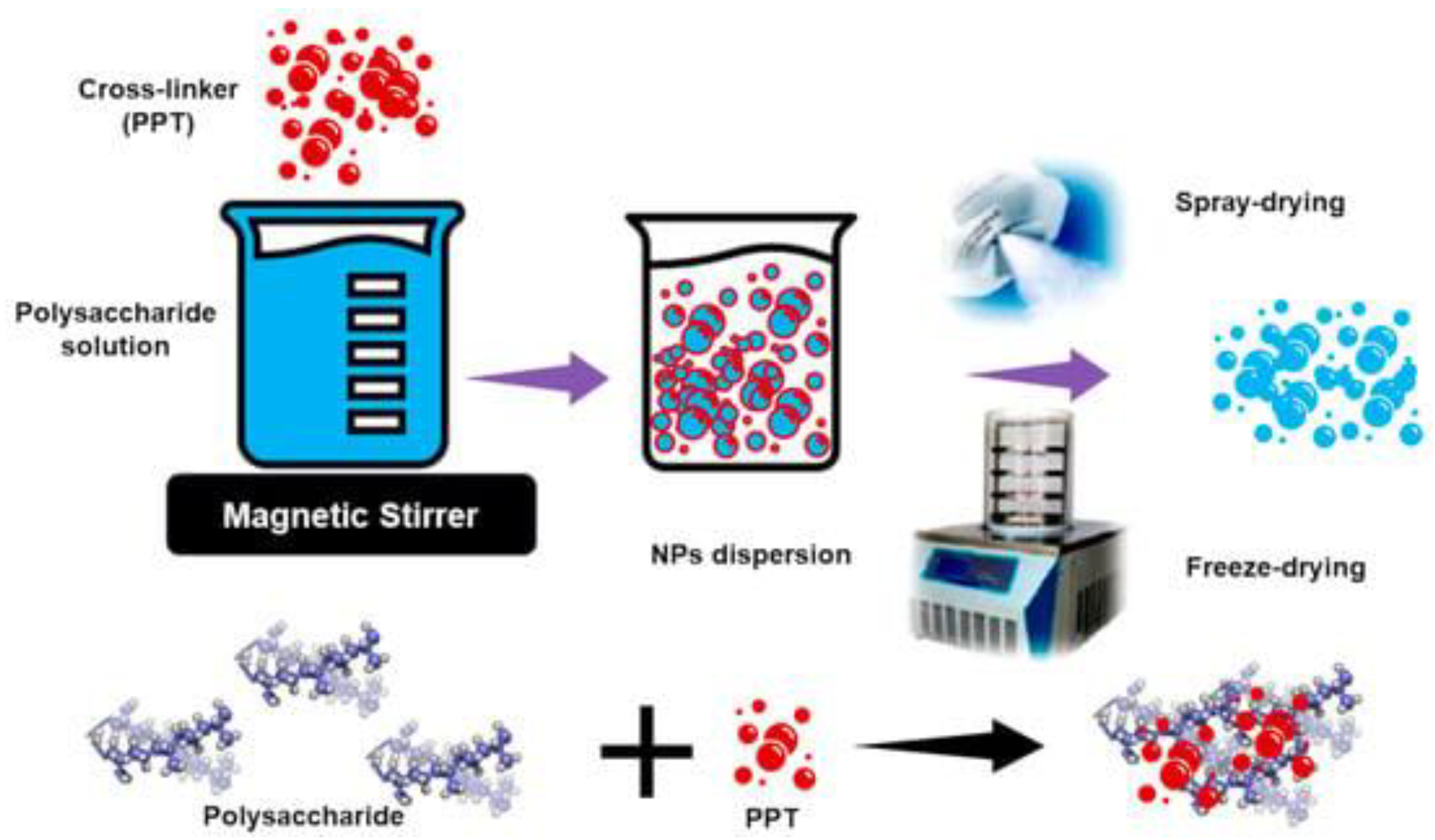
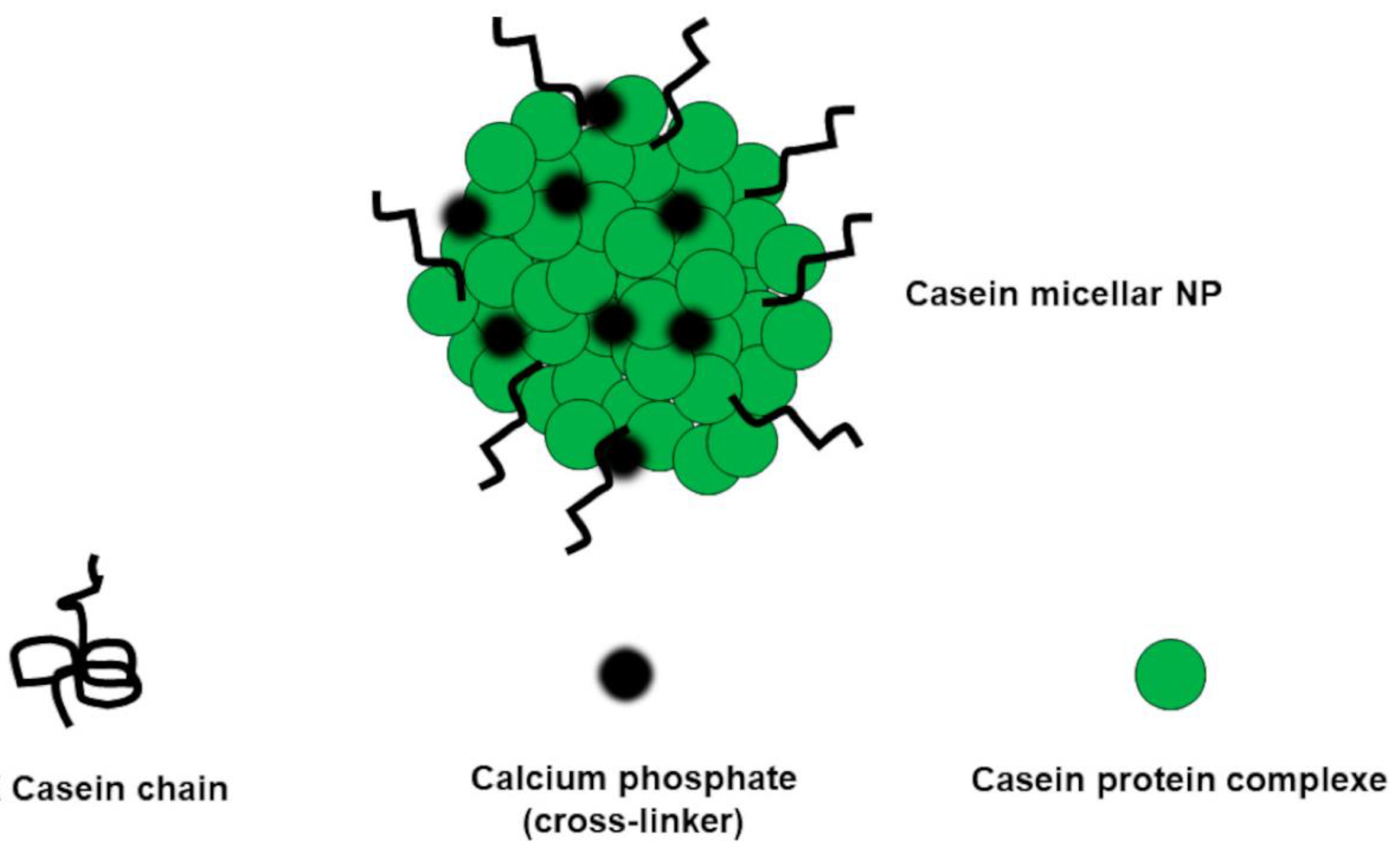
| Method | Description | General Indications/Uses | Properties | Main APs | Refs. | |||
|---|---|---|---|---|---|---|---|---|
| NSs | Colloidal dispersion of NPs (10-900 nm) in water Surfactants, co-surfactants, polymers * |
To improve solubility/bioavailability of both HAPs/LAPs | ↑Dispersibility, ↑solubility Sustained, controlled, targeted delivery ↑Stability ↑Therapeutic effects in cells and tissues |
[52] | ||||
| β-carotene | [78] | |||||||
| Quercetin | [79] | |||||||
| Acai fruits | [80] | |||||||
| α-Tocopherol | [81] | |||||||
| NEs | Kinetically stable liquid-in-liquid dispersions Droplet sizes 100-500 nm |
↓Particles size of HAPs/LAPs, H/L food additives ** Orally administrable drugs Protected drug delivery Suitable for food, cosmetics, pharmaceuticals Suitable for material synthesis |
↑Solubility/bioavailability Sustained, controlled, targeted delivery Extended half-life Obtained either by low energy techniques or by high energy techniques |
[82] | ||||
| Turmeric | [52] | |||||||
| Curcumin | [83] | |||||||
| di-Benzoyl-methane | [52] | |||||||
| Tannins Stilbene Flavonoids |
[84] | |||||||
| ECGC | [85] | |||||||
| Lipids Carotenoids |
[52] | |||||||
| Pomegranate extracts | [52,86] | |||||||
| LEO | [87] | |||||||
| SEDDSs | SMEDDSs 100–200 nm |
Anhydrous nano-dispersions achieved by drying A an oil phase, surfactants, co-surfactants/co-solvents, and LAPs Powders will spontaneously arrange in colloidal NEs when merged with water or with GIT fluids by small agitation or by the digestive motility of the stomach and intestine |
For orally delivering LAPs, food-grade chemicals, additives, drugs For low therapeutic dose APs |
↑Oral bioavailability improvement Possibility of an easy scale-up ↑DL% Allow delivering peptides and lipids without the risk of lipid digestion |
[88,89] | |||
| SNEDDSs < 50 nm |
EGCG | [90] | ||||||
| SDDSs | ↑ Soluble bioactive NPs with the AP physically entrapped or covalently linked (20–1000 nm) Nano carriers can be made of PEG, PUR, PCL, PLGA, PVA, P2VP, PLA, PPO, Pluronics®, PGA, PAE, PLL, mPEG, PasP, PLH, PEI, PVP, PLLeu, DOCA, HPMC, PHB, PEO, PBLG, PS, PIHCA, PAH, biocompatible polyester-based dendrimers |
For delivering HAPs/LAPs, food-grade chemicals additives, drugs For low therapeutic dose APs |
↑Solubility, bioavailability, dispersion and stability in GIT ↑APs systemic spread, transportation through the endothelial cell layer ↑Release at the target site Controlled microbiota metabolism ↑ APs’ bio-efficacy ↑Cellular uptake Favourable drug release profile protracted in time |
[91,92,93] | ||||
| Paclitaxel B Doxorubicin B mPEG-PLGA-Paclitaxel B PEGylated factor VII C Estradiol C PEGylated antibody fragment C Erythropoiesis stimulating agent C PEGylated IFNbeta-1a C |
[94] | |||||||
| DexamethasoneDocetaxel Rifampin Genistein + paclitaxel + quercetinHydrochlorothiazide Cisplatin Curcumin Diminazen aceturate Paclitaxel Folic acid siRNA + paclitaxel Docetaxel +siRNA-Bcl-2 Doxorubicin Lidocaine Cripofloxacin HCl DexamethasoneInsulin FITC-Dextran LevonorgestrelDNA | ||||||||
| OSNPs | LNPs | SLNPs | An external lipid monolayer with a solid-lipid core Spherical morphology (10–1000 nm) Surfactants/emulsifiers to stabilize Ideal fat/aqueous medium ratio 0.1/30.0 (w/w) |
For delivering LAPs | Biocompatible | Domperidone | [95] | |
| LPs | Artificial vesicles achieved by mixing phospholipids + cholesterol Lipid bilayer enclosing an aqueous core |
Immunological adjuvants and drug carriers | ↑ EE% of APs with different polarities Preserve APs from enzymes activity and degrading agents Biodegradable, biologically inactive Non-antigenic, non-pyrogenic, no intrinsic toxicity, instability in plasma D |
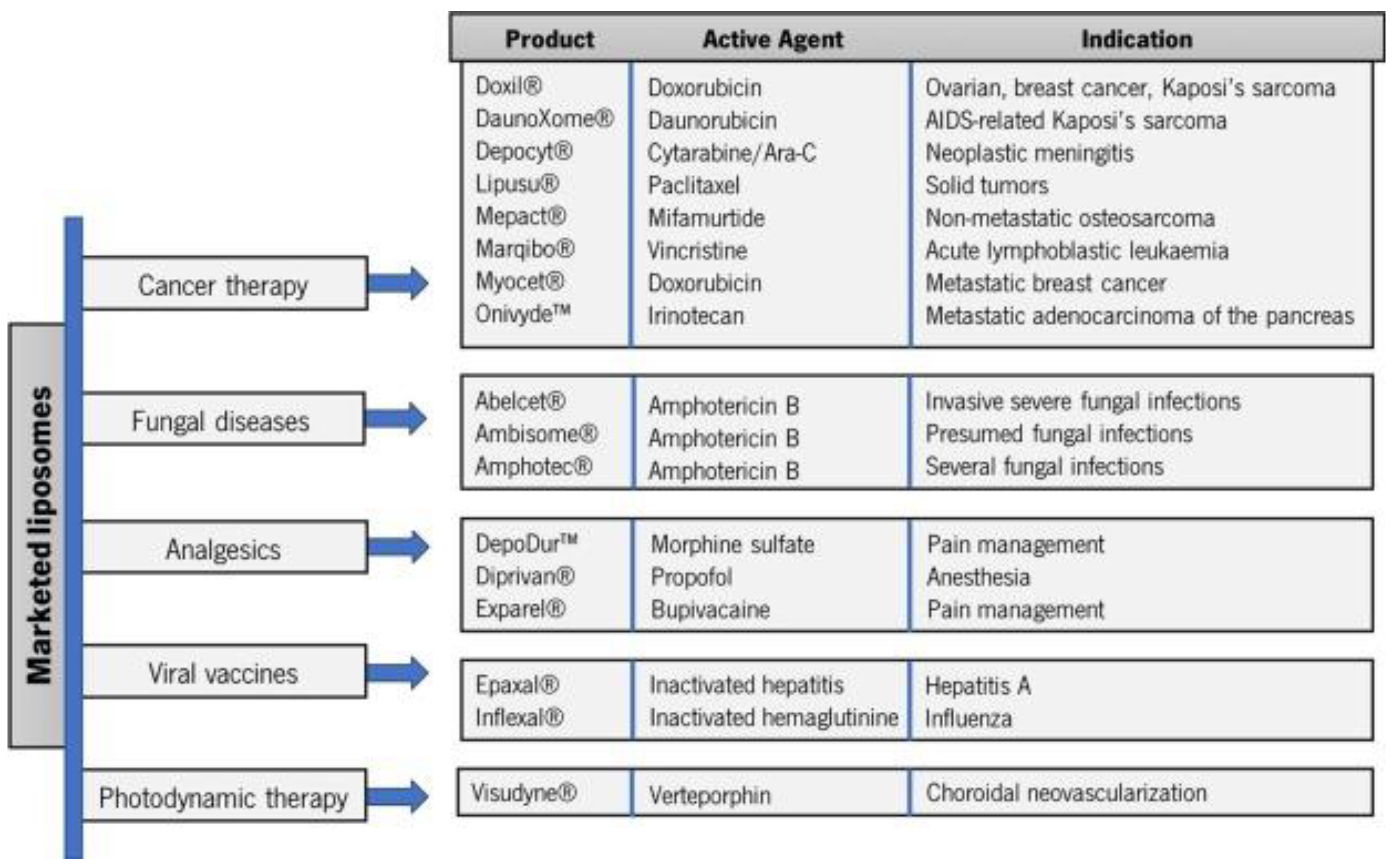
Irinotecan Amphotericin B Verteporfin Morphine sulphate Bupivacaine Inactivated hepatitis A Inactivated hemagglutinin of influenza A and B |
[52] [96] |
|||
| n-MIC | Very slim, spherical lipid particles (10–400 nm) | n-MICs form in aqueous medium n-MIC can solubilize LAPs |
↑ Bioavailability ↑Systemic residence time Protect APs from early inactivation ↑DL% and good stability |
Paclitaxel Doxorubicin Curcumin Dextran/Doxorubicin Doxorubicin/SN-38 Podophyllotoxin LCA Doxorubicin/siPD-L1 β-Lapachone/camptothecin Doxorubicin/CD147 miR-34a mimic/volasertib (BI6727) siRNA siRNA/ Doxorubicin Docetaxel Sorafenib Camptothecin Paclitaxel/siRNA Dexamethasone JQ1 Estradiol Adriamycin Doxorubicin/Fe3O4 NPs |
[97] | |||
| i-MIC | i-MICs form in oil medium i-MIC solubilize HAPs |
|||||||
| NIOs | Uncharged or charged lipid-based lamellar nanostructures Merge non-ionic E, cationic F or anionic G surfactants + cholesterol Vesicles osmotically active/stable |
For ↑oral bioavailability of APs with limited absorption | ↓Toxicity for cells *** Act as reservoir systems Provide controlled and sustained delivery |
Tamoxifen Docetaxel Metformin Celecoxib Gemcitabine Ascorbic acid Geranium oil Curcumin Cisplatin, Epirubicin Folic acid Letrozole Cyclophosphamide, Farnesol Gingerol Doxorubicin Hyaluronic acid Morusin Melittin Paclitaxel 2,5-Diketopiperazine Carnosine Trastuzumab Mcl-1 Nioplex Nintedanib Artemisin Silibinin Sunitinib 5-Fluorouracil Oxaliplatin Saccharomyces Cerevisiae Lycopene Hippadine γ-Oryzanol Amygdalin Ozonated olive oil |
[98] | |||
| Pro NPs | Made of both animal I and vegetable proteins L through proteins precipitation and crosslinking agents § De-solvating agents M |
For carrying several molecules | Simple manufacturing Compatible with the ↑-pressure Emulsification processes ↑ Freeze–thaw stability Suitable to being transformed Biocompatibility ↑ Stability ↑ Permeation ability in vitro Sustained delivering of APs ↓Toxicity for cells # ↑Shelf-life of APs ↑Resistance of APs to acidic gastric pH |
EGCG, GA Probiotics |
[15] [99,100,101,102] |
|||
| ONPs | CDs | Cyclic oligosaccharides consisting of six (α-CD), seven (β-CD), eight (γ-CD), or more glucopyranose units linked by α-(1, 4) bonds | For preparing FFs, FSs, IFT, APs, by the monomolecular inclusion complex technique To delivery different LAPs |
↑Hydrophilicity and water solubility of LAPs ↑Chemical stability ↓Early degradation and metabolism Can modify unpleasant tastes and flavours Realize a controlled release of LAPs |
Linoleic acid Resveratrol Carotenoids Lycopene (Lyc) Hesperidin Olive leaf extracts 1 Quercetin Myricetin Kaempferol 3-Hydroxyflavone Morin Rutin Curcumin Ferulic acid Ellagic acid Amino acids Hydrolysed soy pro 2 |
[52] [103] [104,105] [62] [106,107,108] |
||
| PNPs | Prepared from natural hydrophilic polysaccharides Comprise polyelectrolytes (cationic, anionic, neutral saccharides) and non-polyelectrolytes |
To deliver different APs To develop APs-load FFs and additives |
↑Solubility ↑Controlled and target release ↑Stability, ↑food shelf-life ↑Cellular uptake |
Olive leaf extract Gallic acid Caffeic acid Yerba mate H Caffeine Theobromine Saponins Polyphenols Probiotics Flavors Anthocyanins Procyanidins Ellagic acid Gliadin |
[52] [109,110,111,112,113,114,115,116,117,118,119,120] |
|||
| NPs | Nuts | Bioactivities | (nm) | Animal | Refs. |
|---|---|---|---|---|---|
| Phospholipid-based delivery systems | |||||
| Liposome | (+)-Catechin | Antioxidant, neuro-protective | 35–70 | Wistar Albino rats | [148] |
| Liposome | Curcumin | Anti-HIV, anti-tumour, antioxidant Anti-inflammatory |
263 | Sprague-Dawley rats | [149] |
| HL | Silymarin | Hepatoprotective | 660 | Albino rats | [150] |
| PLS | Silymarin | 196 | Beagle dogs | [151] | |
| PPC | Ginkgo biloba * | ↓Platelet aggregation Radical scavenger, antioxidant, protection of CNS |
N/A | Sprague-Dawley rats | [152] |
| PPC | Curcumin | Antioxidant, anti-inflammatory Anti-carcinogenic, anti-bacteria ↓Cholesterol, antitumor, antispasmodic Wound healing, anti-coagulant, hepatoprotective |
Wistar Albino rats | [153] | |
| PPC | Evodiamine | Anti-tumour, anti-inflammatory, anti-obesity Anti-nociceptive, thermoregulatory |
Sprague-Dawley rats | [154] | |
| PPC | Silybin | Hepatoprotective | Rats | [155] | |
| PPC | Boswellic acid | Anti-inflammatory, hepatoprotective ↓5-Lipoxygenase |
Rats | [156] | |
| PPC | Silybin | Hepatoprotective, antioxidant | Dogs | [157] | |
| Emulsion-based delivery systems | |||||
| NEs | DBM | Anti-cancer activities, anti-proliferation | 70 | Sprague-Dawley rats | [158] |
| NEs | α-Tocopherol | Antioxidant, neuroprotective | 85 | Wistar rats | [159] |
| MEs | Berberine | Anti-bacteria, anti-tumour, anti-diabetes ↑Cerebral ischemia |
24 | Sprague-Dawley rats | [160] |
| MEs | Puerarin | Cardiovascular diseases, antioxidant, anti-diabetes | 40 | Kunming mice | [161] |
| SLNPs | Camptothecin | Anti-cancer | 197 | C57BL/6J mice | [162] |
| SLNPs | Quercetin | Antioxidant, ↓blood lipid, anti-cancer ↓Platelet aggregation, anti-anaemia Anti-inflammation, anti-anaphylaxis Dilate coronary arteries |
155 | Wistar rats | [163] |
| SLNPs | Triptolide | Immune-suppressive activity Anti-fertility, anti-neoplastic activity |
116 | Wistar rats | [164] |
| OG NEs | Curcumin | Anti-cancer, anti-inflammatory, antioxidant | 218 | CD-1 mice | [165] |
| SEDDSs | Curcumin | Anti-inflammatory, antioxidant, anti-cancer | 85 | Wistar rats | [166] |
| SEDDSs | Ginkgo biloba * | ↓Platelet aggregation, radical scavenging Antioxidant, protection of CNS |
∼100 | Dogs | [167] |
| SEDDSs | Wurenchun | Antihepatotoxic, hepatoprotective | 240 | Sprague-Dawley rats | [168] |
| SEDDSs | Baicalein | Anti-inflammatory, anti-cancer, antioxidant Anti-virus, anti-allergic |
27-54 | Sprague-Dawley rats | [169] |
| SEDDSs | ZTO | Hepatoprotective, ↓tumour, anti-bacteria ↑White blood cell, anti-thrombosis |
182 | Rabbits | [170] |
| SEDDSs | Oridonin | Anti-tumour, anti-bacteria, antioxidant Anti-inflammatory |
24 | SD rats | [171] |
| Chemical modifications | |||||
| PAc | EGCG | Antioxidant, anti-viral, anti-inflammatory Cardio-protective, neuro-protective Anti-cancer |
N/A | CF-1 mice | [172] |
| 3,5,4′-TAR** | Resveratrol | Anti-cardiovascular disease, antioxidant Anti-inflammatory, anti-tumour |
Rats | [173] | |
| QC-12** | Quercetin | Antioxidant, ↓blood lipid, anti-anaemia ↓Platelet aggregation, anti-cancer Anti-inflammation, anti-anaphylaxis Dilate coronary arteries |
Human | [174] | |
| HAA | Tricin | Antioxidant, anti-viral, anti-inflammatory Antihistamine, anti-cancer |
SD rats | [175] | |
| Other delivery methods | |||||
| Ch NPs | EGCG | Antioxidant, anti-viral, anti-inflammatory Cardio-protective, neuro-protective, anti-cancer |
440 | Swiss outbred mice | [176] |
| Ch NPs | Curcumin | Antioxidant, anti-inflammatory, anti-proliferative, anti-angiogenic | 178 | Swiss mice | [151] |
| Naked NCs | Coenzyme Q10 | Co-factor of the mitochondrial electron transport chain, antioxidant, cardio-protective neuro-protective |
400, 700 | Beagle dogs | [177] |
| NCs | Schisandrin B | Hepatoprotective, neuroprotective | 45, 168 | Sprague-Dawley rats |
[178] |
| Nuts | Technologies | Efficacy evaluated | Models | Refs. |
|---|---|---|---|---|
| Curcumin | PPC | Antioxidant, hepatoprotective | CCl4-I liver OD in mice | [153] |
| Nanoencapsulation | Chemo preventive | DENA-I liver carcer in rat | [179] | |
| NE | Anti-inflammation | TPA-I acute mouse ear edema | [180] | |
| Quercetin | PPC | Antioxidant, hepatoprotective | CCl4-I liver OD in rats | [181] |
| Cationic NPs | Anti-tumorigenic | B16F10 melanoma cells subcutaneously injected into C57BL/6 mice | [182] | |
| Nanoencapsulation | Antioxidant, protective against liver and brain damage | As-I liver/brain OD in rats | [183] | |
| Microcapsules | Anti-inflammatory, antioxidant | AA-I acute colitis in mice | [184] | |
| Silymarin | Liposomes | Hepatoprotective | CCl4-I liver damage in rats | [185] |
| Triptolide | Solid lipid NPs | Anti-inflammatory | Carrageenan-I rat paw edema | [164] |
| CU | NPs | Hepatoprotective | AcAPh-I hepatotoxicity in rats | [186] |
| Naringenin | NPs | Hepatoprotective | CCl4-I acute rat liver failure | [187] |
| α-TPh | NE | Anti-diabetes, antioxidant | STZ-I diabetes | [159] |
| GA | PPC | Anti-apoptotic, cardioprotective | DOX-I cardiac toxicity in rats | [188] |
| Puerarin | Nanodispersion | Anti-colorectal cancer | HT-29 human colon carcinoma cell subcutaneously injected to BALB/c nude mice | [189] |
| Resveratrol | Pro-drug | Anti-inflammation | 1% DSS-I in drinking water for 8 days mice colon inflammation | [190] |
| EGCG | Peracetylation | Anti-inflammation, anti-tumorigenesis | DSS-I mice colitis/tumour | [191] |
| Advanced FPs type | Purposes and Description | Key nanomaterials currently used |
|---|---|---|
| Physically improved packaging | Packaging materials incorporated with NPs to improve physical properties, such as temperature and moisture stability, mechanical strength, gas barrier, durability and flexibility | Metal oxides NPs, nano clays Carbon nanotubes, metallic NPs |
| Active packaging | NPs endowed with antimicrobial or other functionalities (e.g. antioxidant, UV absorbents) and the ability to release them into-packaging. Food packaged into AP results improved in term of taste, freshness and shelf life | Ag NPs, Au NPs, metal oxides NPs, antimicrobial and/or antioxidant NPs functionalized NPs |
| Smart packaging | Packaging materials incorporated with nano-sensors to monitor and report on the condition of the food (e.g. oxygen indicators, freshness indicators and pathogen) | ↑ Variability of nano-sensors |
| NPs Type | Size (nm), ζP (mV), PDI EE%,MW * |
Bioactivity | Characteristics | APs | Refs. |
|---|---|---|---|---|---|
| Sty-CPs (C8) |
589 nm | Adaptable to biochemical interaction studies with CAOs | Soluble, positive Schiff's fuchsin-sulphite reagent Spherical morphology |
Functionalized Glucopyranose | 2009 [1] |
| 4-HPR-A-DEX NPs | 150-350 nm 0.112-0.176 (PDI) |
↑Cytotoxicity to HTLA-230 | CPXs with 4-HPR were prepared by kneading method TGA suggested a ↑↑ thermal stability, ↑ DL%, ↑EE% Sustained drug release, possible parenteral administration ↑ Cytotoxicity to HTLA-230 than free 4-HPR ↑ Drug bioavailability, biodegradable |
Entrapped 4-HPR | 2009 [45] |
| ATRA-NIC-PVA | < 400 nm 0.202-0.450 (PDI) |
Cytotoxic to NB cells (LAN-5 cells) |
↑ATRA solubilization and release ↑↑ATRA aqueous solubility, drug fractional release < 8% ↑↑ Growth inhibition effect than free ATRA Suitable for parenteral injection, drug targeting Long-term storage |
Entrapped ATRA | 2009 [46] |
| 4-HPR-OL-DEX NPs | ~ 310 nm | In vitro (HTLA-230, LAN-5 and IMR32 NB cells) and in vivo antitumor activity | Tested both in vitro and in vivo, ↑↑ Cytotoxicity in vitro ↑↑Fraction of sub-G1 cells, no haemolytic activity Suitable for injections, ↑↑AUC, ↓ clearance ↑↑Lifespan and long-term survival of treated mice ↑↑Aqueous solubility, ↑↑ bioavailability |
Entrapped 4-HPR | 2012 [47] |
| 4-HPR-C14-PEG NPs |
50-137 nm 0.165-0.221 (PDI) |
In vitro (SH-SY5Y and NGP NB cells) antitumor activity | ↑↑Aqueous solubility, suitable for injection Stable aggregates, drug-targeting to solid tumors No release of free 4-HPR in an aqueous environment asso ↑↑Intracellular concentrations and activity than 4-HPR ↓↓4-HPR early metabolism, protracted release |
Complexed 4-HPR | 2012 [48] |
| StyGlyco-CCPs (R1) |
~ 300 nm | Suitable for interaction with CAOs | Cross linked resins, spherical morphology | Functionalized D-Glucose | 2013 [2] |
| 4-HPR-NGR-NLs | 142 nm -19.2 mV 0.073 (PDI) 69% (EE) |
In vivo ↑↑ the life of NB mice by apoptotic/anti-angiogenic effects ↓↓of tumour progression ↓↓of intra-tumoral vessels ↓↓ of VEGF expression ↓↓ of metalloproteinases MMP2/MMP9 |
By reverse phase evaporation method ↑↑ Structural integrity of NL in organic fluids Target the tumour endothelial cell marker |
Complexed 4-HPR | 2013 [49] |
| StyGlyco-LCPs (P1G2) |
N.R. | Substrates/inhibitors of CAOs | Soluble | Benlyl amine D-Glactose | 2015 [196] |
| SL[LM-BTZ] | 179 nm -33.9 mV 0.070 (PDI) |
In vivo ↑↑ the life of NB mice | Lyophilization with cryoprotectants Targeted drug delivery systems, ↑↑ therapeutic index ↑↑ Efficacy, ↑↑ EE%, suitable for intravenous injection ↓↓of BTZ systemic adverse effects |
Complexed BTZ | 2015 [50] |
| NGR-SL[LM-BTZ]) | 173 nm -30.2 mV 0.093 (PDI) |
||||
| AN169-PEG-NLs | 143.9-153.8 nm 0.052-0.077 (PDI) |
In vitro antitumor activity in human cancer cell lines (HTLA-230, Mel 3.0, OVCAR-3, SV620) | By thin-film hydration method, slow drug release Antitumour activity as free AN169 (72h) Lyophilization with cryoprotectants Long-term stability, ↑↑EE%, for intravenous injection |
Entrapped AN169 | 2015 [51] |
| PolyE-Ds | 4.4-5.4 nm 31.2-51.8 mV |
Carriers for gene and drug delivery | ↑ Water-soluble, excellent β Well-defined sizes, shapes, ↑controlled architecture Polycationic, biodegradable, 13593-25661 * |
Linked amino acids | 2017 [4] |
| b-HMPA-Ds. | N.R. | For gene transfection with p-DNA and si-RNA | ↑ Water-soluble, excellent β Well-defined sizes, shapes, ↑Controlled architecture, polycationic, biodegradable Not cytotoxic, 9834-60725* |
2017 [5] |
|
| PolyE-ADs | 3.3-3.6 nm 5.4-5.9 mV |
Drug delivery, gene transfection | Well-tailored polymeric structure, excellent β Symmetric tree-like shape, ↑functional groups Inner cavities, hydrolysable, amphiphilic by a C-18 chain Polycationic, biodegradable, not cytotoxic, 2932-6762 * |
2018 [6] |
|
| 4G PolyE-HDs | 4.5-4.6 nm 32.8-33.4 mV |
Water-soluble biomedical devices. | Well-tailored polymeric structure; excellent β Symmetric tree-like shape, ↑functional groups Inner cavities, hydrolysable, hydrophilic; ↑ solubility Polycationic, biodegradable, not cytotoxic, 2932-6762 * |
2018 [7] |
|
| 4G-5G PolyE-DPX | 4.4-36.3 nm 15.5-51.8 mV |
Several beneficial effects of UOA | Well-tailored polymeric structure; excellent β Symmetric tree-like shape, inner cavities, hydrolysable ↑Water solubility, polycationic, biodegradable Not cytotoxic, 1360-3130 * |
Entrapped UOA | 2018 [8] |
| PolyE-A/H-DPX | 18.9-47.7 nm 2.4-15.3 mV |
Non-nucleoside HIV-1 reverse transcriptase inhibitor Suitable for parenteral administration |
↑ Water-soluble, excellent β, well-defined sizes, shapes, ↑Controlled architecture, polycationic, biodegradable, Not cytotoxic, sustained release, 5851-24203 * |
Entrapped (1) | 2018 [9] |
| 5G PE-PD-D/GA (GAD) |
348.6 nm | Platelet aggregation inhibition ** ROS production inhibition ** Antibacterial (Gram-positive) ** |
↑ Water-soluble, excellent β, well-defined sizes, Spherical morphology, ↑controlled architecture 192 OH groups, biodegradable, not cytotoxic, 17010 * |
Linked GA | 2019 [10] |
| PolyE-A/H-DPX | 60–70 nm | Beneficial effects of EA For clinical applications RSA |
300-1000-fold ↑water solubility, non-PAMAM dendrimers Biodegradable, not cytotoxic, excellent β, 14287-25604 * Sustained release, ↑ DL% |
Entrapped EA | 2019 [11] |
| 5G PE-PD-D/GA (GAD) |
348.6 nm | Long-Term Preservation of EOs | Nano-spherical dendrimer, preservative power ↑than GA No pro-oxidant action, spherical morphology (SEM) ↑Compatible with lipids and oily matrices RSA 4-fold ↑than GA (DPPH), biodegradable by esterase hydrolytic actions, release GA, not cytotoxic, 17010 * |
Linked GA | 2019 [12] |
| 5G PE-PD-D/GA (GAD) |
348.6 nm | To treat diseases by OS | Nano-spherical dendrimer, no pro-oxidant action Spherical morphology (SEM), RSA 4-fold ↑than GA, biodegradable (lipase), release GA, not cytotoxic, 17010 * |
Linked GA | 2020 [16] |
| 5G-PE-PD-D-OH | 44.5 nm -21.2 mV 0.208 (PDI) |
ROS-dependent per se cytotoxicity against NB cells sensitive to ETO | Per se activity, nano-spheric, ↑ solubility, 7275 * | Empty dendrimer | 2020 [13] |
| 5G PE-PD-DPX (CPX 5) |
70 nm -45 mV |
Prevention and treatment of NB cells | ↑ Cytotoxicity and Pro-Oxidant Effects than ETO Protection of ETO, synergistic action with ETO Sustained release of ETO, ↑ DL%, ↑EE Nano-spherical morphology, ↑ solubility |
Entrapped ETO | |
| 5G-PE-PD-D-OH | 44.5 nm -21.2 mV 0.208 (PDI) |
ROS-dependent per se cytotoxicity against NB cells both sensitive and resistant to ETO | ↑ Cytotoxicity and Pro-Oxidant Effects than ETO Nano-spherical morphology, 7275 *, 64 peripherals OH Water soluble |
Empty dendrimer | 2020 [15] |
| 5G PE-PD-D/GA (GAD) |
348.6 nm | Nano-formulation nullify the pro-oxidant activity of GA To treat diseases by OS To prevent DNA oxidative damage and tumors onset |
Nano-spherical dendrimer, no pro-oxidant action Spherical morphology (SEM), RSA 4-fold ↑than GA Biodegradable (lipase), release GA by hydrolysis, Water-soluble, not cytotoxic, 17010 * |
Linked GA | |
| 5G PE-PD-DPX (GALD) |
349.9 nm -29.2 mV 0.708 (PDI) |
Nano-spherical hygroscopic dendrimer, ↑water soluble no pro-oxidant action, ↑ DL%, ↑EE, Spherical morphology (SEM), biodegradable (lipase) Sustained and quantitative release of GA Not cytotoxic, 28610 * |
Entrapped GA | ||
| PolyE-Ds | N.R. | Bactericidal (Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia) |
Non-Cytotoxic, amino acid-modified polycationic Ds More potent than colistin against P. aeruginosa (5GK) Not lithic behaviour, membrane disruptor Broad spectrum of action |
Linked K, H, KH | 2020 [17] |
| 4-AMSTY-CP (P5) | 334 nm +57.6 mV 1.012 (PDI) |
Bactericidal (Enterococcus, Staphylococcus, Pseudomonas, Klebsiella, Escherichia coli, A. baumannii, S. maltophilia | Random copolymer, ↑water soluble Rapid (0.5h) and broad spectrum non-lytic bactericidal activity, stability in solution, excellent buffer capacity Activity by membrane disruption, 5,100 (Mn) |
No AP NH3+ groups |
2021 [18] |
| 4-AMSTY-CP (P5) | 334 nm +57.6 mV 1.012 (PDI) |
ROS-dependent cytotoxic activity on ETO-resistant NB | Random copolymer, ↑water soluble Stability in solution, excellent buffer capacity Membrane disruptor, cause ↑ROS generation, 5,100 (Mn) |
No AP NH3+ groups |
2021 [19] |
| Sty-CP (P7) | 220 nm +49.8 mV 0.809 (PDI) |
Random copolymer, ↑water soluble Stability in solution, excellent buffer capacity Membrane disruptor, cause ↑ROS generation, 13,719 (Mn) |
|||
| 4G-5G-PolyE-Ds | 16.1-24.9 nm +24.8-34.0 mV |
↑Antibacterial effects (MIC = 0.5–8.7 µM vs. Enterococci, Staphylococci) | Activity depended on the density and on the type of cationic amino acid-conjugated dendrimers and not on the presence and the release of UOA, ↑water soluble Stability in solution, excellent buffer capacity Protracted release of UOA, membrane disruptor 14,600-29,300 * |
Linked K, R, KR Entrapped UOA |
2021 [20] |
| 4G-BBB4-PolyE-Ds | 112.1 nm +28.9 mV 0.289 (PDI) |
Antibacterial vs. Staphylococci To treat skin infections |
↑↑↑Water solubility than BBB4, good DL and EE SI = 1.4-5.5, protracted release of BBB4 Membrane disruptor ↓Cytotoxicity on HaCat than BBB4, 21176 * |
Linked K Entrapped BBB4 |
2021 [21] |
| N.D. | ↑↑↑Water solubility than BBB4, good DL and EE Sustained/protracted release of BBB4, 21176 * |
Linked K Entrapped BBB4 |
2021 [22] |
||
| RES-TPGS | 9.6-12.7 nm § -1.6—4.8 mV § 0.13-0.26 (PDI)§ |
Antioxidant Anti-inflammatory Protective action in the liver |
Micellar NPs, ↑↑↑water solubility than RES Good DL% and EE% Sustained/protracted release of RES ↓Cytotoxicity on HaCat than RES, 21176 |
Entrapped RES | 2021 [23] |
| UA-4G PolyE-D (UA-G4K NPs) |
577.5 vs. 333.4 nm 4GK -42.6 vs +66.1 mV 4GK 0.235 vs 0.286 4GK (PDI) |
N.D. | Biodegradable, not cytotoxic (Hela cells), ↑DL%, spheric Protracted release profile governed by diffusion Water solubility 1868-fold ↑ than UA Clinical applicability, 30,069 * |
Linked K Entrapped UA |
2021 [26] |
| Antibacterial vs. enterococci (MICs =0.5-4.3 µM) Bactericidal vs. E. faecium |
↓Cytotoxicity on HaCat (IC50 96.4 µM), SIs = 22-193 Valuable as a novel oral-administrable therapeutic option to-treat enterococcal infections. |
2021 [24] |
|||
| ATRA-TPGS NPs | 14.1-21.0 nm -7.2—-13.0 mV 0.19-0.32 (PDI, water) |
To prepare topical gel Treatment for skin diseases Treatment for melanoma |
↓ATRA cutaneous side effects, ↑stability than ATRA ↑ATRA solubilization, good EE% 22 ± 4 µ cm-2 permeation after 24 h ↑Cytotoxic effects on melanoma cells |
Entrapped ATRA | 2021 [25] |
| Sty-CP (P7) | 220 nm +49.8 mV 0.809 (PDI) |
Antibacterial activity vs. enterococci, staphylococci Acinetobacters, Pseudomonas Klebsielle, Escherichia coli Stenotrophomonas maltophylia Rapid bactericidal effects on S. aureus, K. pneumoniae, P. aeruginosa |
Random copolymer, ↑water soluble ↑Stability in solution, excellent buffer capacity Membrane disruptor, ↓tendency to develop resistance ↓Toxicity, long-term activity, 13,719 (Mn) Lowest MICs = 0.6–1.2 µM |
No AP NH3+ groups |
2021 [27] |
| 5G-PolySty-D (5G-PDK) |
203.0 nm +19.2 mV 0.282 (PDI) |
Rapid bactericidal affects vs. A. baumannii, A. pittii A. ursingii |
Membrane disrupters, MICs = 3.2-12.7 µM Electrostatic interactions with bacterial surface Self-biodegradable, 20,145.3 * |
64 Linked K | 2021 [28] |
| Antibacterial and bactericidal vs. Pseudomonadaceae | MICs depending on pigment production P. aeruginosa = 1.6-> 6.4 µM P. putida producing pyoverdine = 3.2-6.4 µM P. putida producing non-pigmented colonies = 0.2-1.6 µM ↓Cytotoxicity on HaCat, ↑↑↑ SIs (13-404) |
2021 [29] |
|||
| 4-AMSTY CP (CP1) |
833.4 nm +27.3 mV 0.2235 (PDI) 157,306 * |
Potent broad-spectrum antibacterial effects Kill pathogens rapidly |
Cationic macromolecules acting as membrane disruptors CP1 MICs = 0.1–0.8 µM, OP2 MICs = 0.35–2.8 µM Promising ingredients for the development of novel antibacterial dosage forms for topical applications (hydrogel) Spherical morphology |
No AP NH3+ groups |
2022 [30] |
| 4-AESTY OP (OP2) |
163.4 nm +31.1 mV 0.301 (PDI) 44,514 * |
||||
| CB1H-P7 NPs | 142.9 nm +36.7 mV 0.626 (PDI) |
N.D. | Spherical morphology, positive surface charge ↑↑↑ DL%, ↑↑↑ EE%, protracted release profile, 26,623.9 * |
CB1H | 2022 [31] |
| Antibacterial vs. G+/G- Bactericidal vs. S. aureus E. coli, P. aeruginosa. |
MICs ↓↓ of pristine CB1H and matrix P7 NPs displayed MICs = 0.6-4.8 µM on 34 out of 36 isolates ↓Cytotoxicity on HaCat, SIs up to 2.4 |
2022 [35] |
|||
| New curative option vs. NB (IMR 32 and SHSY 5Y cells) |
Membrane disruptors, IC50 = 0.43-0.54 µM vs. IMR 32 and SHSY 5Y cells Early-stage (66-85%) and late-stage apoptosis (52-65%) Effects of CB1H and P7 ↑ by 54-57 and 2.5-4-times (IMR32) ↑ By 53-61 and 1.3-2 times against SHSY 5Y 1-12-fold more potent than fenretinide ↓Cytotoxicity on HaCat, SIs = 2.8-3.3 |
2023 [38] |
|||
| CR232-SUVs | 173.4 nm +17.8 mV 0.118 (PDI) |
N.D. | Biocompatible, DL%, EE% ↑ with ↑ lipids/CR232 ratio Prolonged release profile ruled by zero-order kinetics 1764-fold more soluble than the untreated CR232 |
Linked K Entrapped CR232 |
2022 [32] |
| 5GK PoliE-D NPs (CR232-G5K NPs) | 529.7 nm +37.2 mV 0.472 (PDI) |
N.D. | 2311-fold more water-soluble than pristine CR232 No use of harmful organic solvents/additives, spheric Biodegradable, ↑↑↑ DL%, ↑↑↑EE%, ↓Cytotoxicity on Hela Quantitative release profile (Weibull kinetics), 44,153.1 * |
||
| Antibacterial vs G+ and G-Rapid bactericidal activity | MICs = 0.36–2.89 µM vs. all of the considered G+ and G- MICs = 0.72 µM vs. colistin-resistant P. aerginosa and K. pneumoniae carbapenemases (KPCs)-producing ↓Cytotoxicity on HaCat, SIs up to 8 |
2022 [33] |
|||
| 4-HPR-P5 | 249 nm +41.3 mV 0.210 (PDI) |
Antiproliferative activity IC50 = 1.25 µM (IMR32), 1.93 µM (SH-SY5Y) |
Molecularly dispersed 4-HPR using P5 as solubilizing agent by antisolvent co-precipitation method ↑↑↑Clinical outcomes of 4-HPR 4-HPR apparent solubility 1134-fold ↑, faster dissolution Suitable for intravenous administration, ↑↑↑ DL% Extended release over time, excellent β |
Dispersed 4-HPR | 2023 [37] |
| P5PA-4I NPs | 541 nm +8.39 mV 0.194(PDI) |
New promising treatment for chemo-resistant NB Cytotoxic to ETO-sensitive (HTLA-230) and to ETO-resistant (HTLA-ER) cells |
↑↑↑ Activity of 4I, ↑↑↑ DL%, ↑↑↑ EE% ↑↑↑ hydrophilic–lipophilic balance (HLB) Excellent buffer capacity, ↑↑↑ residence time inside cells Chemically stable in an aqueous medium > 40 days Assumed low hemolytic toxicity ROS-dependent cytotoxic effects ↑↑↑ than 4I ↑↑↑Efficacy than ETO in HTLA-ER cells |
Loaded 4I | 2023 [39] |
| TPP-BA-NVs | 49.3 nm +18.2 mV 0.529 (PDI) 852.7 * |
Cytotoxic to MDR HR-NB IC50 = 0.2 µM (HTLA-230) IC50 = 1.1 µM (HTLA-ER) |
Tested on HTLA-230 human stage-IV NB cells and HTLA-ER NB cells resistant to ETO, DOX, etc. IC50 = 538-fold ↓ than ETO (HTLA-ER) Limited cytotoxic effects against mammalian cell lines (Cos-7, IC50 = 4.9 µM, HepG2, IC50 = 9.6 µM, MRC-5, IC50 = 2.8 µM, RBCs, IC50 = 14.9 µM, SIs = 2.5–74.6 |
No additional AP Linked BPPB |
2024 [41] |
| ↑↑↑ Antibacterial effects on 50 G+ and G- MDR and ESKAPE pathogens | Characterization of BPPB by ATR-FTIR, NMR, UV, FIA-MS (ESI), elemental analysis, potentiometric titrations. Spherical vesicles, MICs = 0.250-32 µg/mL, SIs > 10 | 2024 [42] |
|||
| New treatments for CMM by MeOV and MeTRAV IC50 = 49 nM on MeOV (72 h) |
Cytoplasmic membrane disruptors trigging OS ROS-correlated apoptotic effects ↓Cytotoxicity to non-tumoral cells and RBCs SIs up to 299 on MeOV (72 h) |
2024 [44] |
|||
| Biodegradable HA-based hydrogel formulation HA-BPPB-HA possesses ↑↑↑ swelling capability ↑↑↑ Porosity, viscous elastic rheological behavior | |||||
| CP5 (P5)/DMAA 11b,c/DMAA CP MA/DMAA CP |
334, 2590, 373, 112 nm +58, +6.5, +25, +18 mV 1.012, 0.281, 0.326, 0.590PDI |
Possibility to develop M21 as a new scaffold for TE | Amine or aldehyde containing CPs were developed CPs by 5 (P5), 11b, and 11c excellent substrates for LO CPs by 5, 11b, and 11c and MA crosslinked Gel B M21 by P5/DMMA have 71% crosslinking M21 is biocompatible |
NH3+ groups CHO groups |
2024 [43,197] |
| 4-HPR-TPGS- DSPE-PEG | 11.4-15.7 nm -4- -14 mV 0.12-0.46 (PDI) |
Cytotoxic to MDR HR-NB | Micelles prepared using the solvent casting technique Good DL%, ↑↑↑ EE %, stable colloidal dispersions Apparent solubility 363-fold ↑ than 4-HPR Slow-release behaviour of about 28% (24 h) ↑ Cytotoxicity than 4-HPR on SK-N-BE-2C NB cells |
Entrapped 4-HPR | 2024 [198] |
| Food/simulant | NPs/Nanocomposite | Additive/Fortifier | Migration | Ref. |
|---|---|---|---|---|
| Vegetables | Starch/clay | None | In conformity with European directive | [61] |
| Fatty food simulant * | PLA/laurate | LHD-C12 | Below legal migration limits | [204] |
| Food simulant | CNT/LDPE/PS | None | No migration | [205] |
| Chicken meatballs | AgNPs ** | None | Slow migration | [206] |
| Chicken breast | AgNPs/co-PEFs | None | No transfer | [207] |
| Distilled water | ||||
| Acidic food simulants *** | AgNPs/PE | Irganox 1076 Irgafos 168 Chimassorb 944 Tinuvin 622 UV-531, UV-P |
Transfer promoted by organic additives | [208] |
| NPs | Polymer | FCMs | Migration result |
|---|---|---|---|
| ZnONPs | LDPE | Films | 0.009–3.416 mg/L |
| Ti2ONPs | PET | Films | 1.88–3.32 ng/kg |
| AgNPs | LDPE | Baby products | 1.05–2.25 ng/L |
| Nanoclay | LDPE-EVA | Films | N.D. |
| AgNPs | LDPE | Commercial cutting board | 0.24–0.60 µg/g |
| Ti2ONPs | PLA | Films | 2.19–3.5 µg/kg |
| Ti2ONPs/AgNPs | PLA | Films | 2.36 µg/kg |
| Ti2ONPs/AgNPs | Films | 0.593–0.8 µg/kg | |
| graphene | LDPE | Films | 1.02–1.29 MG/kg |
| Ti2ONPs | LDPE | Films | 0.61 mg/kg |
| ZnONPs | LDPE | Films | 14.17 mg/kg |
| ZnONPs | LDPE | Plaques | 0.05–2 mg/kg |
| AgNPs | PP | Tow plastic containers | 62–18887 ng/dm2 |
| PC | Baby feeding bottle | ||
| Food pox | |||
| AgNPs | PE, HDPE | Food storage boxex Commercial storage boxex |
< 0.04–0.31 µg/g |
| Commercial container Commercial bags |
0.5–46 µg/L | ||
| ZnONPs | PE, HDPE | Commercial containers, bags, dishes, cups | 0.54–46 µg/L |
| AgNPs | PE | Commercial containers | 3.17–5.66 µg/L |
| PE with a 10 µm AgNPs coating | Commercial cing films | 0.01–28.92 µg/L | |
| PP, LDPE, PS | baby bottles cutting boards food storage bags food storage containers |
6.60–35.8 μg/g | |
| AgNPs | PP, LDPE | Commercial food containers | <0.0001–0.1 ng/g |
| SiNPs | LDPE | Films | N.D. |
| Cloisit 20 A | PET | Bottle | 0.18–9.5 mg/kg |
| Carbon black | LDPE, PS | Injection moulded plaques | N.D. |
| TiN | LDPE | Films | 0.09–0.24 μg/kg |
| AgNPs | PE | Films | 0.003–0.005 mg/dm2 |
| LDPE | 0.30–1.43 mg/kg | ||
| CuNPs | PE | Films | 0.024–0.049 mg/dm2 |
| Ti2ONPs | PE | Films | 0.5–12.1 µg/kg |
| AgNPs | PP, PE | Commercial plastic container | 4.75–9.5 ng/cm2 |
| AgNPs | Plasticized PVC | Commercial plastic bags | 0.5 ng/cm2 |
| Film | 0.01–0.37 mg/dm2 | ||
| AgNPs | LDPE | Commercial bags, containers | 3.1x10-3-3.74 ng/cm2 |
| PP | Commercial bags, containers | 50.3 x 10-3-31.46 ng/cm2 | |
| PE | Commercial bags | 1–4 µg/dm−2 | |
| AgNPs | PE | Commercial food contact film | 0.22–5.6% |
| ZnONPs | LDPE | Film | 0.11–0.68 μg/L |
| Cloisite | PLA | Film | N.D. |
| NPs | Size/shape/concentration | Effects on cells | Refs. |
|---|---|---|---|
| Ag NPs | 2-8 nm | Genotoxic and cytotoxic effects on root meristematic cells of Allium cepa (A. cepa) ↓ Mitotic index ↑ Chromosomal aberration number |
[212] |
| Ag NWs | 20,000 × 65 nm 0.39-25 μg/mL |
Less toxic than nanoplates Toxicity was not only caused by Ag+ release No established LC50 value |
[213] |
| 100 nm 4 μg/cm2 |
Toxicity to human monocyte-derived macrophage THP-1 cells ↓Cell proliferation, ↑ increase of membrane instability |
||
| 40 nm 5-30 μg/mL |
Toxicity to RBCs Cell deformability, aggregation, haemolysis in a dose-dependent manner |
||
| Ag NSs | 30 nm 0.05-5 μg/cm2 |
Cytotoxic and genotoxic to fish OLHNI2 cells Chromosomal aberrations |
|
| 10, 20, 40 nm 0.39-25 μg/mL |
Cytotoxicity and superoxide generation in a fish gill cell line ↓Toxic than nanoplates None of these contributions established an AgNW LD or LC50 value |
||
| SPM Fe NPs | > 100 µg/mL | Impaired DNA, nucleus, mitochondria in different cell lines Causes ↑ ROS, inflammation |
[214] |
| Fe NWs | 50 nm 10,000 NWs per cell |
Toxicity to HeLa cells No significant effect MTT assay Up to 10,000 NWs per cell (72 h) ↑ cell viability of about 80%. |
[213] |
| Zr NPs | 5-30 nm | ↑ Viral receptor expressions, causes inflammation | [215] |
| Ce NRs | >200 nm | Progressive pro-inflammatory effect and cytotoxicity in THP-1 cells | [213] |
| HAP | Crystals, H-rod H-needle, H-sphere H-plate |
Decreased cell viability and consequent necrosis in rat aortic smooth muscle cells | [216] |
| AuNPs | 5 nm | Toxicity, ↑cytokine production in mouse fibroblasts | [217] |
| Au NRs | 54 nm 30-100 μM |
Toxicity to human prostate cancer cell line DU145, cervix carcinoma cell line HeLa, male C57/BL6 mice No genotoxicity, induction of autophagy Destabilization of lysosomes, alterations of actin cytoskeleton Impairments in cell migration |
[213] |
| 65 nm 0.5 mM |
No toxicity in HeLa cells > 90% viability after 24 h |
||
| Ni NWs | 33 nm diameter 5 μg/mL |
↓ Viability in human colorectal carcinoma HCT 116 cells | [218] |
| 200 nm 106 NPs/mL |
Toxicity to rat marrow stromal cells (MSC), MC3T3-E1 osteoblast cells, UMR-106 osteosarcoma cells Binding to cytoplasm metalloproteins Trigger lysosome reorganization around the nucleus Cell viability was more than 95% up to 5 days after internalization |
[213] | |
| 200 nm 35,000 NPs/mL |
Cytotoxicity to L929 mouse fibroblast cells No cytotoxicity. |
||
| 33 nm 5 μg/mL |
Cytotoxicity to HCT 116 cells Viability of HCT 116 cells ↓↓ at 24, 48, and 72 h exposure |
||
| Al2O3 NWs | 200-400 nm 50-200 mg/mL |
Viability of L929 and RAW264 cells was not ↓↓, no ↑ LDH release Not cytotoxic, no nuclei damage |
[213] |
| Zn NPs | 4–20 nm | Low viability, ROS production, cytotoxicity in human immune cells | [219] |
| Ti NPs | 70 nm 50 μg/mL |
Inflammation ↑ IL-8 in human microvascular endothelial cells |
[220] |
| 3/600 μg/mL | Shrinking of cells, lower metabolic activity, releasing of LDH ROS production in mouse fibroblastL929 |
[221] | |
| 70 nm | ↑ Viral receptor expressions, causes inflammation | [215] | |
| Ti NWs | 10 μg/mL | RAW264.7, H9C2, Chang human liver cells HACAT, MH-S, HEK-293, TM3, BEAS-2B cells Toxicity depended on the surface area of TiO2NWs |
[213] |
| <10 nm 12.5-350 μg/mL |
Toxicity to Caco-2/HT29 intestinal cells Non-cytotoxic damage was detected (24 h) Viability was above 80% Different interactions and cellular responses related to differently shaped TiO2NPs |
||
| Ti NRs: | <100 nm 12.5-350 μg/mL |
||
| Ti NSs | 25 nm 12.5-350 μg/mL |
||
| GO NPs | Up to 25 μg/mL | Effected antigen inhibition ↓ Intracellular levels of immune proteasome |
[222] |
| Co NPs | 50-200 nm | Pro-inflammatory effect on naïve macrophages ↓ Anti-inflammatory IL-1Ra ↑ Inflammatory TNF-a |
[215] |
| Co NWs | 12.5-175 μg/mL | Apparent cytotoxicity to 3T3 and 4T1 cells after 9 h (50 μg/ml) | [213] |
| MSP Si NPs | 100 nm | Membrane deformities and haemolysis in RBC | [223] |
| 15 nm | Strongly biased naïve macrophages towards inflammation ↑ Inflammatory cytokines IL-1b, TNF-a. ↑ Inflammatory phenotype of LPS-polarised M1 macrophages |
[215] | |
| Si NWs | 100 nm 6.25-100 μg/mL |
Pre-osteoblast subclones (MC3T3-E1) cells Induce apoptosis due to OS in MC3T3-E1 cells (48 h) Cell viability remains ↑ after 24 h |
[213] |
| 50/150 mg/mL | HeLa, HepG2, HEK293T, human normal liver-7702 cells Cytotoxicity ↑dependent on cell lines, concentration, incubation time |
||
| Cloisite® Na+ | 0–125 mg/mL | Cytotoxicity and mutagenicity in the HUVEC No cytotoxic or mutagenic effect |
[210] |
| Cloisite®130B | 0-250 mg/mL | Cytotoxicity and mutagenicity in the HUVEC toxic effects |
| NPs | Concentration(s) | Organism | Cytotoxicity/genotoxicity/Assays | Findings | Refs. |
|---|---|---|---|---|---|
| Ag NWs 100 nm | 4 μg/cm2 | D. magna | Toxicity varied as a function of AgNW dimension, coating and solution chemistry | ↑Toxic ↓Toxic than Au+ |
[227] |
| Ag NRs | 5-15 μM | Allium cepa | Mitotic index, chromosomal aberrations ROS assays |
↑ROS and chromosomal damage (15 μM) | [228] |
| Au NRs TAB-capped 46.4 nm PEG-capped 48.1nm |
0.1-10 μg/mL | A. cepa | OS, lipid peroxidation assay | ↑Mitotic index and OS | [229] |
| Ni NWs 20 nm Ni NSs < 50 nm |
0.016-10 mM | D. melanogaster | Insignificant toxic effects | No toxic or mutagenic impacts | [230] |
| Ti NRs <100 nm Ti NWs <10 nm Ti NSs <25 nm |
0.01-10 mM | D. melanogaster | Viability, internalization, intracellular ROS production, genotoxicity (comet assay) | ROS and DNA damage (10 mM) Dose–effect in hemocytes |
[231] |
| Ti NWs 14-95 m2/g | 10 μg/mL | 5-week-old ICR mice | Toxicity depended on the surface area of TiO2NWs | ↑Th2-type inflammatory cytokines ↑ Interleukin (IL)-1, ↑Tumor necrosis factor-alpha (TNFα) ↑ IL-6 |
[232] |
| BNNTs 4.56 nm | 0.01-10 mM | D. melanogaster | Non-significant toxic effects. | BNNTs ↓ genotoxic effects of K2CrO7 ↓Intracellular levels of ROS | [233] |
| CNTs | Mice/rats | Injected into the animal peritoneal cavity | Peritoneal mesothelioma | [234] | |
| GaP NWs 80 nm | * 10 NWs nL−1 * ** 50 μL 6 × 107NWs mL−1 |
D. melanogaster | Not significantly affected life span or somatic mutation rate | Not taken up into Drosophila tissues No measurable immune response No changes in genome-wide gene expression |
[235] |
| GaP NWs 40 nm | 6.2 × 1010 NWs/L | D. magna | No mortality was observed | Penetration of biological barriers governed by the NW diameter. | [236] |
| CdS NRs 30–50 nm | 1,000-10,000 mg/kg | Kunming mice (17-22 g/mouse) | Apparent toxic effects | OS and DNA damage | [237] |
| USPIO | N.R. | Humans | Phase III clinical trial | Urticaria, diarrhea, nausea | [238] |
| SPION | 1.0 mg Fe/mouse per day for 15 days | Mice | Trigger skin cancer | ↑ Stage-I, stage-II skin tumor | [239] |
| PAMNPs 8.5 nm | 0.6-1.6 × 1010 Ps/mL | Swiss mice | Time and dose-dependent toxicity | ↑Micronucleus frequency | [240] |
| SiO2 NPs 10 nm | *** 2 mg/kg | Rats | Alterations in morphometry, biochemistry, hematology, liver tissues and the expression of drug-metabolizing enzyme genes | ↑Alkaline phosphatase, LDH, low-density lipids, procalcitonin, aspartate aminotransferase, alanine aminotransferase ↑K, P, Fe concentrations |
[241] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





