1. Introduction
A new stage for obtaining experimental results related to the functional performance of friction pairs (contact pressure, sliding speed, lubrication requirements and conditions, friction and wear behavior, etc.) is constituted by tribosystems, which are based on selective transfer (the transfer of material from one element of the friction pair to another in the presence of a lubricant forming a layer - tribolayer - superficial, anti-friction, skinny, of the order of a few microns, behaving very well in friction and wear), to implement them in the design and execution of machine elements [
1,
2].
The use of such friction pairs (tribosystems) implies using those pairs of materials and lubricants that will surely reduce friction and wear and, thereby, extend the working life of machines, installations, and equipment [
3]. Initially, the main direction of action in the fight against wear and friction reduction was to increase the hardness of the friction surfaces, proving to be a great help in increasing the reliability, service life, and wear resistance of the friction pairs. However, traditional methods are not sufficient to cope with increasing demands, high speeds, and worsening lubrication conditions. Another reason is that the effective contact surfaces are small, due to the high hardness of the asperities and thus, in the contact areas, the pressures are very high, leading to intensive wear.
One way to solve this problem is to use friction pairs with selective transfer. In this case, a thin layer of copper is applied to the hard steel surface, on which a thin layer of polymer is present, as a result of operating in contact with a bronze surface which also has a thin layer of copper, thus allowing the realization of low-wear friction pairs [
4,
5]. Thus, selective transfer is a contact interaction by frictional, characterized by special molecular exchange interactions.
This occurs as a result of various physic-chemical reactions, which lead to an independent decrease in friction and wear; typical is the formation of a thin metallic film, in which a typical mechanism of movement of defects (dislocations) by diffusion occurs. The film appears in the initial phase, through the selective dissolution of the anodic components in the surface layers of the metal or alloy, upon the appearance of the selective transfer from the lubricant that determines the metallic coating. It is a protective polymer film, which originates from the products of tribodestruction and the hydrocarbons of the lubricant in the contact area through the chemisorption of these products to the anodic components of the alloys of the respective pair and favors the occurrence of selective transfer [
2,
6,
7,
8,
9]. The lubricant that achieves metallic coatings is the one that determines selective transfer in the friction pairs made of materials that allow this phenomenon to occur (an example is brass or bronze/steel lubricated with glycerin, etc.).
In the base material, there are also surface-active surfactant elements, or they are formed by the decomposition of additives. Ionic lubrication is a lubrication based on the discharge of metal ions that arise through electrochemical processes in the friction zone. As a result, a so-called servowitte (life-saving - after the principle of friction pairs from living nature) film appears [
2,
9]. Through selective transfer, wear can be reduced, and the friction coefficient (COF) can be of the order of magnitude of fluid friction. The causes that lead at wear and COFs low, are:
- lowering the real contact pressure by leveling the microscopic irregularities and forming a metallic film;
- compensating the deformations and reducing the resistance to movement in the friction areas by reducing the number of accidents in the surface layers;
- the reflux of wear particles or metal ions in the contact area and the formation of the film through the development of electro-kinetic potentials in dispersed media.
Through the presence of an electrically charged double layer, they cause an electrophoretic movement of the particles in the field of direct contact and a displacement of the ions corresponding to the direction of the field. Preventing metal oxidation, by forming a solid adsorption layer of active substances at surface, ensures plasticity and deformation stability of the protective polymer film, reduces contact pressure and creates additional sliding areas with low displacement resistance [
5].
Therefore, for the study of these physic-chemical processes that take place in the contact area of the friction pairs operating with selective transfer, polarization is used, applicable after some experiments on the mode of influence on friction and wear, by changing the lubricant input speed (here glycerin). For the experiments with the help of polarization, steel with a copper alloy (bronze) lubricated with glycerin was combined (associated) by friction, due to the special properties of glycerin [
2,
6,
9,
10]. Thus, through the polarization method, it is possible to analyze the qualitative and quantitative changes of glycerin, the establishment of the properties of the selective solution, and the deterioration of the materials of the bronze/steel friction pair in the friction process.
2. Materials and Method
Experiments were carried out with the help of polarization to combination/association by friction of a pair from copper alloy with steel in glycerin, for the research by friction under the conditions of a selective transfer of the processes of mutual action and by the input way, of the lubricant in the contact areas. With the help of this method, the quantitative and qualitative transformations of the lubricant (here glycerin) can be studied very easily, as well as the determination of the properties of the selective solution, respectively the damage of materials to friction, even after the frictional stress. This combination of materials was thus chosen because the elementary processes of the selective transfer phenomenon can be explained through the special properties of glycerin.
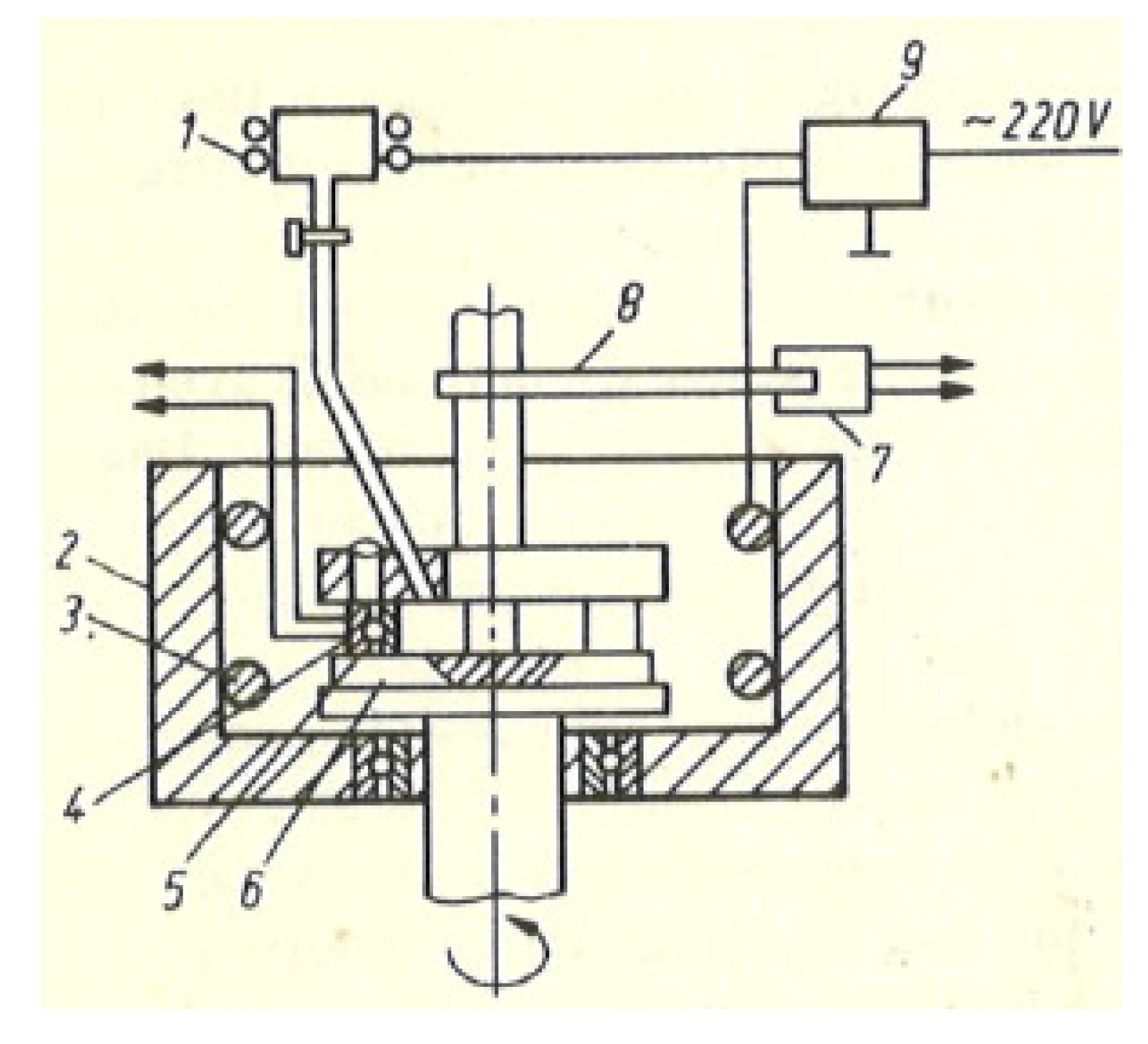
The tests were carried out on a test machine that works on the pin-disc tribometer principle with the steel disc (OLC 45 (AISI/SAE 1045)), and the pin made of a copper alloy (bronze CuSn12T (UNS-C90800)) with a sliding speed of 0.3 m/s. The measurements were performed in continuous current using a mercury drop electrode and presented in
Figure 1a, which is an electrochemical analytical tool for the qualitative and quantitative analysis of the content of different ions, atoms, and molecules in an electrolyte solution based on the calibration curve (current-voltage) obtained when the substance is electrolyzed with controlled potential. The test result is a polarography curve or polar-gram (
Figure 1b). The half-wave potential of each substance on the polar plot is the basis for qualitative analysis, while the wave height represents the limiting diffusion current for quantitative analysis.
The drain period was 1 to 3 seconds, and the recording was performed in a time of 0.7, drain period. A silver chloride electrode was used as a control electrode. The concentration of the researched material (mixture of glycerin, zinc, and copper) was determined with the help of a calibration curve (see
Figure 1b)
The content of zinc and copper in the lubricant (glycerin) was determined after the test in a closed circuit of the lubricant. This procedure makes it possible to determine the content of wear particles in the lubricant.
In this case, no losses of materials were reported, as happens with the use of liquid lubricants. The absolute measurement was determined experimentally with the help of a calibrating solution.
To examine the influence of the lubricant's entry speed in the friction contact area, an installation (
Figure 2) was used with the possibility of changing the temperature at a speed of 0.33 m/s and a pressure of 10.5 MPa, under the conditions, of continuous assurance with fresh lubricant (glycerin).
The possibility of the continuous entry of glycerin into the contact area of the friction pair was done from a lubricant tank, allowing comparative research on the evolution of the COF (implicit and wear) under the conditions of a selective transfer.
3. Results and Discussion
When it is choosing a lubricant, not only the output properties lubricant (here glycerin) should be taken into consideration, but also those of the changes that occur in the process of requesting during operation. This condition is directly related to the problem of the stability to carry out a selective transfer in time [
11,
12]. Such a bond is constituted by the aldehyde of acrylic acid called acrolein [
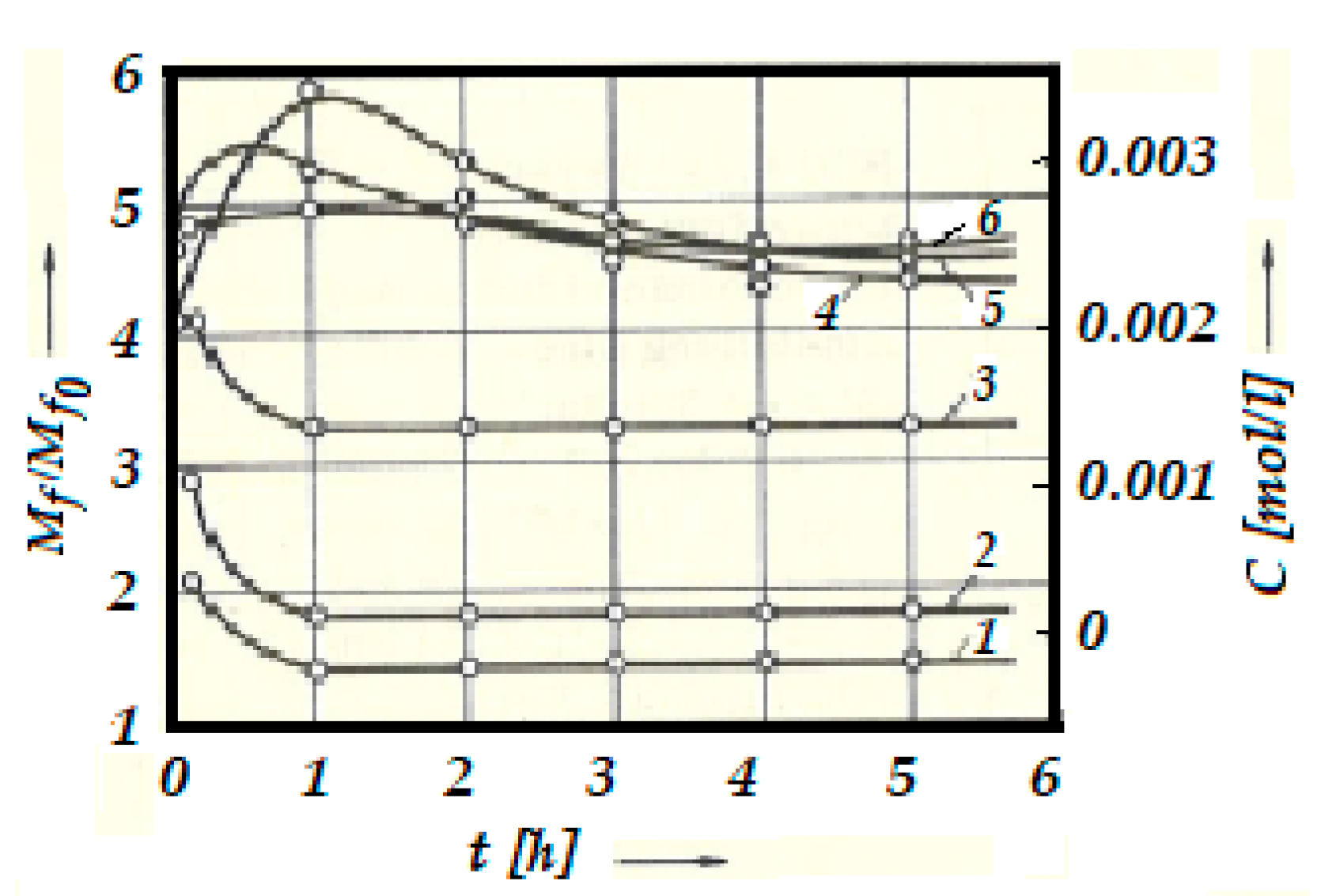
13]. The experimental results highlighted the change in the concentration of acrolein, C in glycerin for bronze/steel friction pair, these being shown in
Figure 3.
The starting points, in this functional figure (
Figure 3), of the relative moments of friction
Mf/Mfo (Mfo is the starting moment of friction, and
Mf is the moment during the friction process) correspond to the time of entry into the function under the action of different contact pressures. After about an hour, a pronounced selective transfer can be observed, which precedes the very low value of the relative moment, but also the presence of a copper layer on the friction areas. At the same moment, the concentration,
C reaches its maximum value.
The increase in concentration,
C at the beginning of the rubbing period is related to oxidation and other chemical transformations of glycerin, especially the formation of acrolein [
14]. The cause is the increase in temperature when the catalytic action of the metals is activated during the association by friction. After this period, the acrolein concentration,
C no longer depends, except to a very small extent, on the contact pressure. It even decreases with time, this being a result of acrolein’s superficiality and its ability to dissolve through oxidation and dissolution reactions.
It should also be noted as too much quantity of chemical products, which appear in the transformations of the lubricant (glycerin), deregulates the stability of the selective transfer. From here it follows that the lubricant (glycerin) as a simple product, but active for adjusting the selective transfer is still indicated for this. Stable conditions is find out only, in established ratio of the oxidation and reduction properties of the lubricant (glycerin) and they change, as the result of an aging of the lubricant (glycerin) in the friction process.
From here it emerges that the regulation of the stability of a selective transfer consists especially in maintaining an optimal ratio of the lubricant and the aging products, within well-established limits. They also ensure a minimum friction force and takes place, for example, by adjusting the speed of the fresh lubricant (glycerin) input.
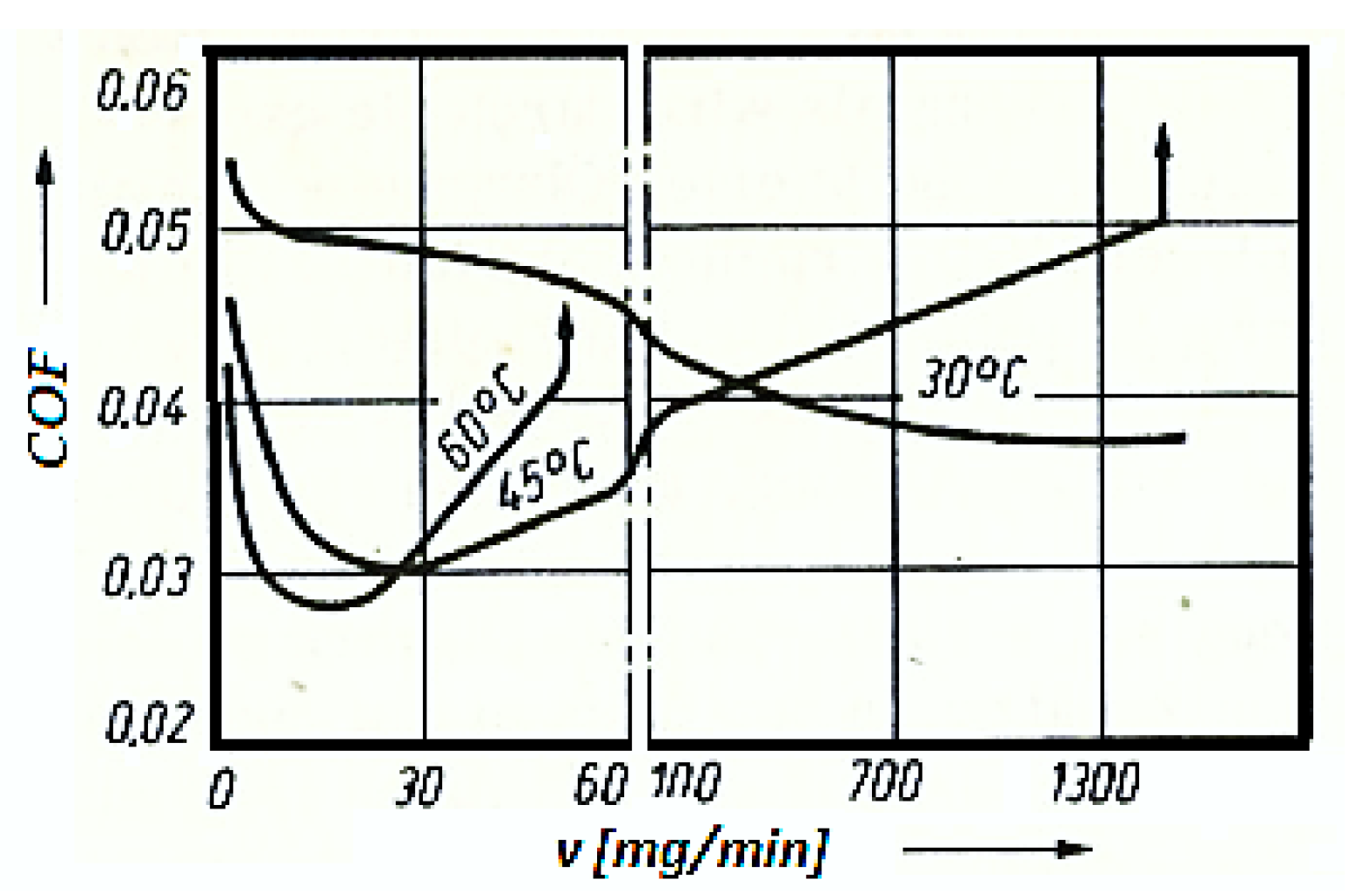
To examine such aspects, the variation of the COF with the speed of the lubricant (glycerin) entering the friction contact area was plotted (
Figure 4), for the considered pair (bronze/steel), at the temperatures of 30
0C, 45
0C, and 60
0C.
The experiment was carried out on an installation provided with the possibility of temperature changing at a speed of 0.33 m/s and the pressure of 10.5 MPa, under the conditions of a continuous fresh lubricant. From the obtained results (see
Figure 4) it is visible that for the chosen friction conditions there is an optimal speed of entry of the fresh glycerin into the contact area, which also ensures a minimum value of the COF, respectively of wear. This occurs especially at high temperatures and low lubricant (glycerin) input speeds.
At high lubricant (glycerin) input speeds, the COF has minimum values at low temperatures, after which it tends to stabilize, from where a selective transfer can no longer unfold under these conditions. At high temperatures, the range of different glycerin input speeds shrinks, at which the COF has a very small value and highlights a selective transfer, and at the same time causes the chemical transformation process into the lubricant (glycerin). Comparative research on the COF in the conditions of a selective transfer to a continuous input of the lubricant from the lubricant tank, it is observed from the beginning of the friction that, there is a very large exchange of lubricant in the tank.
After long attempts by friction, the contact conditions in the tank are still the same, which were also with the reduced glycerin change. The test time, the minimum COF, and wear depend on the amount of lubricant for the respective friction conditions. An increase in the value of the COF at a higher glycerin input speed proves that glycerin no longer has the same properties that are valid for a selective transfer. Only, at a well-determined amount of the chemical transformation products in the lubricant are conditions created for a stable and stationary selective transfer at the operating by friction.
The analysis of the experimental results showed that both in the case of the lack of the aged products as well as in those with surplus, the stability conditions for a selective transfer are damaged. Realizing lubrication with a continuous supply of lubricant (glycerin) gives the possibility of avoiding such unintended consequences and an optimal choice of the speed of flow of the lubricant (glycerin). This must be ensured after a certain time in order to obtain stable conditions for a selective transfer, where the values of the COF and wear are low [
15,
16].
This possibility is established following the experiments carried out and their results, in which the friction pair was kept in an oil tank with an unchanged amount of lubricant (glycerin). In this case, the COF values become stable, which is typical for a selective transfer, after long-term tests. It is possible, that no copper servowitte layer forms [
2,
9], but apart from this, one can observe the dark layer on the friction area as well as the traces of destruction, but also the change in the color of the lubricant (glycerin).
Thus, the stability of a selective transfer is closely related to the aging of the glycerin, and it can be achieved by introducing surplus lubricant, which then changes the oxidizing capacity of the glycerin and protects the friction areas from micro-wear and therefore, avoids excessive wear and, at the same time, it regulates also the temperature in the friction zone. A capitalization of the oxidation-reduction properties of the lubricant makes possible the operation of a friction pair under the conditions of a selective transfer for a known contact pressure and sliding speed. Both the kinematics of the wear process and the particularities of obtaining a selective transfer depend on the physic-chemical properties of the lubricant (glycerin).
Thus, at the start of operation take place a friction on the contact area of the copper alloy (bronze), influenced by the separate dislocations of the structure, to which corresponds a correlation ratio of copper and zinc concentration in glycerin, with the bronze pin, that rubs with the disk of steel. Then, the surface dispersion of the bronze follows, without changes in its composition.
When the static conditions are cancellation, another ratio appears, namely that of the concentration between copper and zinc in the lubricant.
This certifies the beginning of a selective transfer during the wear of copper alloy (bronze), when stable organo-metallic bonds are formed, for example complex bonds, in which zinc is no longer catalytic, a process that can be observed at tests with loads of 1.72 and 5.8 MPa. The results of the tests are presented in
Table 1, which contains, on ones for taking up zinc from lubricant (glycerin) at the pair between bronze and steel.
The proportion of the zinc loss is a sensitive recognition quantity for the kinematics and the wear characteristics of bronze at a load of 1.72 MPa, where the zinc loss coefficient is already 1.6, after 0.5 h (30 min) from the beginning of the frictional stress following that, under the conditions corresponding to the beginning of wear, to approaches to 1 (see
Table 1). This proves the fact that we have no, nor a wear process. At a contact load of 5.8 MPa, part of the selective wear changes in the start period through the leakage effect and at the same time, with this, also the friction of the copper alloy (bronze) in the area of separate dislocations of the structure.
In this case, the copper alloy is passed without any change in composition, in the friction zone of the steel, which hardens in the cold. When transforming the lubricant (glycerin), products appear that act, as an antioxidant substance in the processes that take place in the friction areas of the surfaces. An analysis of the change in zinc loss coefficients during frictional stress gives the possibility of obtaining an important control factor, for the wear process in the presence of an active lubricant (here glycerin). The change in lubricant (glycerin) activity is influenced by kinetic processes and it favors or disfavors the stable positioning of friction conditions.
The results of experimental research in the presence of a lubricant with a zinc and copper content are important because through a glycerin/lubricant exchange the wear products from the lubricant must be removed, and the start of a new leakage process will be done with a new lubricant (glycerin). Here it must be remembered that the formation of the real contact surface after a change of glycerin follows again, even if not with the same intensity as in the previous process or as at the beginning of the tests, i.e. with friction areas not yet polished. These results correspond very well with the results obtained by the concentration of the products of the chemical transformations from glycerin (lubricant) and in researching the particularities of obtaining the conditions for a selective transfer.
The increase in zinc loss coefficients, as well as the increase in the respective ionization speed for zinc, is probably caused, through taking over the active products of transformation from glycerin, as well as through the intensity of the electrochemical corrosion processes on the friction areas under the conditions of a selective transfer.
To the extent that the properties of the lubricant (glycerin) are established in stationary conditions, then decreases also the ionization speed, taking into account the balance, destruction, and formation of wear particles in the friction areas. This balance determines the low values of wear intensity and COF.
In the conditions, in which the copper alloy (bronze) is rubbed before the test on the steel surface, and both rubbing areas are consistent with the copper concentration, they will be selectively correlated, and the coefficient of zinc losses in the initial stage is equal to 1. It then grows during friction. This has a great influence on the selective transfer at the wear of copper alloy (bronze) for the specific friction conditions.
The probable increase in wear and simultaneously with shredding through a strong pressing caused by pressure and high temperatures can favor the appearance of layers from the copper alloy at frictional stress. In this way, a stratification of the bronze friction is produced. This process is subordinate to the lubricant and results from the possibility of the quality regulation of these friction layers by choosing an appropriate lubricant (glycerin).
By plasticization the friction areas during the friction stress, as a result of the action of the active materials on the surface, which are in the lubricant (glycerin), through the minimal hardness of the formed surfaces, the real contact area increases during friction. The real pressure and temperature in the contact areas decrease, and the friction process is reduced.
Therefore, similar research on the wear products of copper-zinc alloys as well as the transformation products of glycerin in the friction contact area gives the possibility of the dependence of the wear process on the physico-chemical transformations of the lubricant (glycerin).