Submitted:
13 December 2024
Posted:
16 December 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Anti-Glioblastoma Activity of Sesquiterpene Lactones
2.1. Anti-Tumor Potency of STLs
2.2. Criteria for Selection of Published Material and Structure of Analyzed Data
2.3. Direct Toxic Effect of PTLs on Glioblastoma Cells: Key Processes and Mechanisms
2.3.1. Pro-Apoptotic Effect of STLs in Glioblastoma Cells
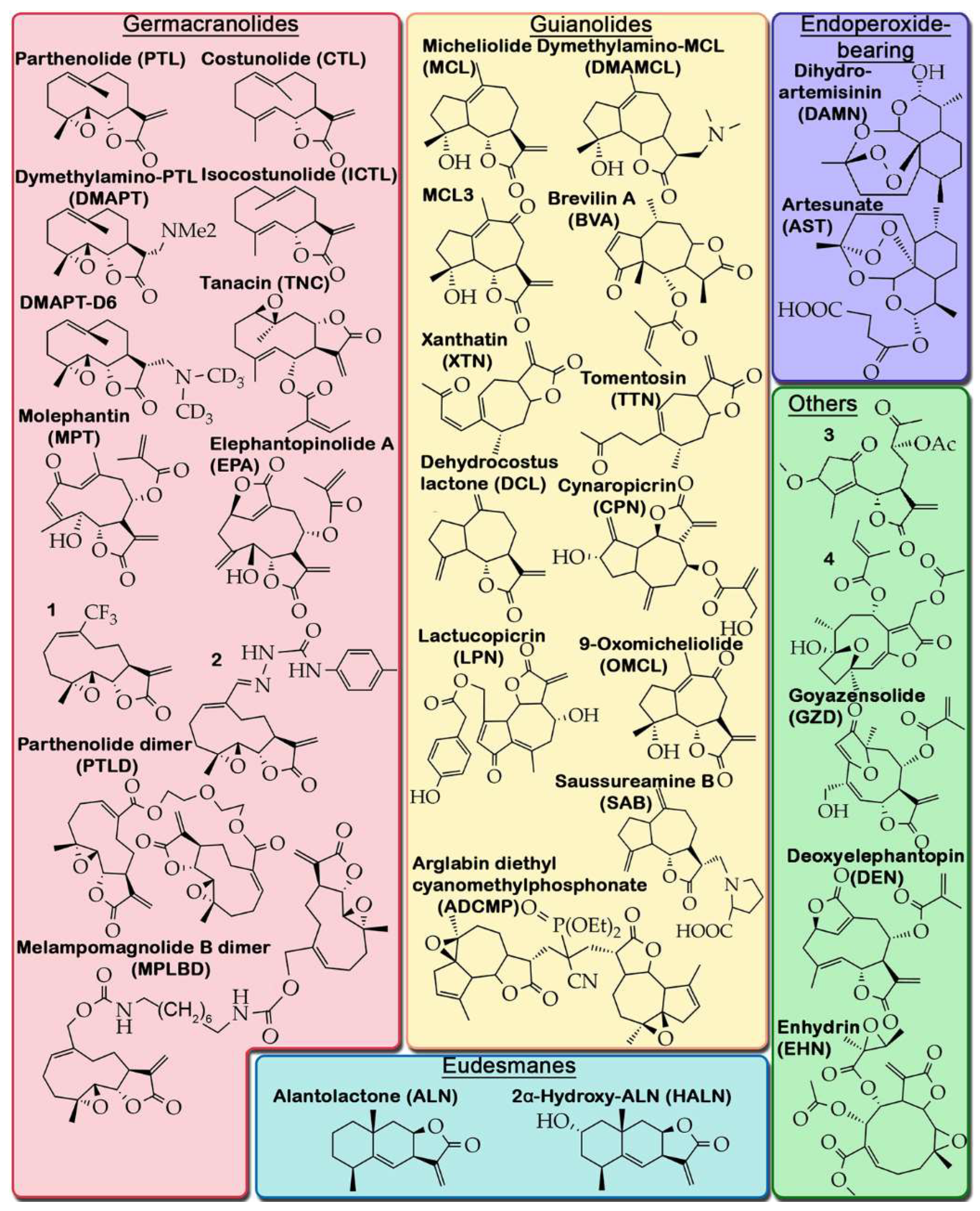
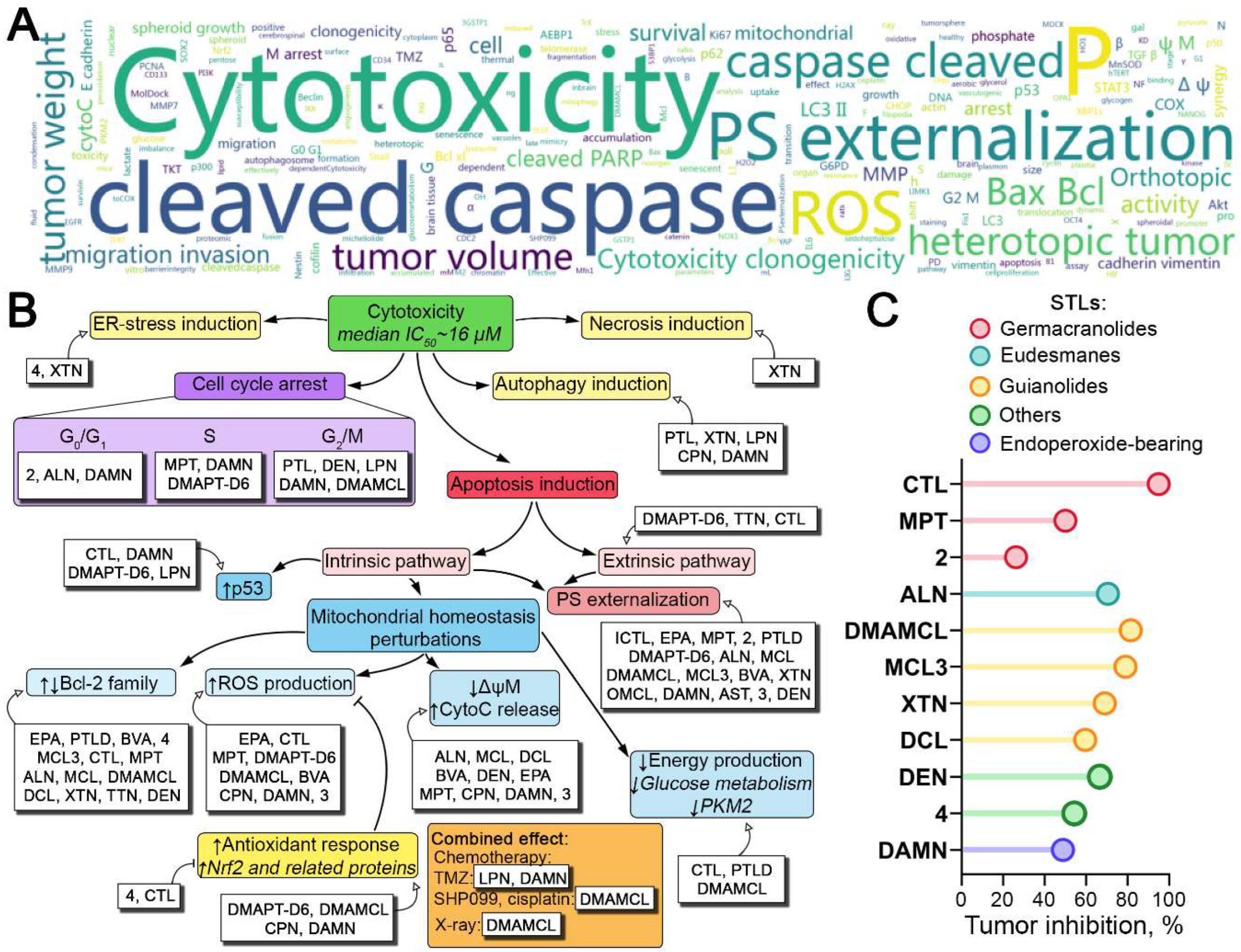
2.3.2. Pro-Oxidant Effect of STLs in Glioblastoma Cells
| Type | Compound | Cell line | Concentration | Biological effects | Effects on cell signaling | Ref. |
| Germacranolides | Parthenolide (PTL) | U373 | 8–16 µM | Cytotoxicity (IC50(24 h) ~ 17 µM); ↓survivin, G2/M arrest, ↑phosphatydilserine (PS) externalization, ↑cleaved caspase-3, ↑cytoplasm vacuoles, ↑LC3-II/LC3-I | ↓Cdk2, ↑Chk2, ↑ULK1 | [46] |
| Dimethylaminoparthenolide (DMAPT) |
9LSF | 5–25 µM 40 mg/kg, i.p.1 |
In vitro: Cytotoxicity (IC50(48 h) ~ 7 µM) In vivo (BBB permeability): Effective uptake by orthotopic 9LSF tumor in rats |
ND2 | [81] | |
| U87, GBM6, GL261 | 1–10 µM; 100 mg/kg (30 times) |
In vitro: Cytotoxicity (IC50 = 3.5–8.8 µM) In vivo (BBB permeability): accumulation in brain of healthy mice (6251 ng/g (1 h); brain-to-plasma ratio: 2.1 (1 h) and 3.0 (4 h)) In vivo (GL261; orthotopic): ↑survival |
ND | [82] | ||
| Tanacin (TNC) | U87 | 1–20 µg/mL | Cytotoxicity (IC50(48 h) = 4.5 µg/mL) | ND | [83] | |
| Isocostunolide (ICTL) | Glioma stem cell lines: GSC-3#, GSC-12#, GSC-18# |
0.1–10 µg/mL | Cytotoxicity (IC50(72 h) = 1.1–2.8 µg/mL), ↑PS externalization, ↑cleaved caspase-3, ↓spheroidal growth, ↓colony formation capacity |
ND | [45] | |
| Elephantopinolide A (EPA) | U87 | 1–50 µM | Cytotoxicity (IC50(48 h) = 4.22±0.11 µM), ●3GSTP1 (molecular docking (MolDock), thermal shift assay), ↓GSTP1, ↑PS externalization, ↑chromatin condensation, apoptosis (Acridine orange/ethidium bromide staining), ↑cleaved caspase-7, ↑Bax, ↓Bcl-xl, ↓ΔψM4, oxidative stress: ↑mitochondrial ROS, ↑H2O2, ↑·OH, ↑lipid peroxidation | ↓JNK1 (mRNA, protein), ↑p-JNK, ↑p-STAT3 | [50] | |
| Costunolide (CTL) | A173, U87 | 10–40 µM 5 mg/kg, i.p. (10 times) |
In vitro: ROS-dependent cytotoxicity (IC50(24 h) ~ 30 µM), ↑ROS, ↓telomerase activity, ↓hTERT, ↑p53, ↑caspase-3/8 activity, ↑Bax/Bcl-2, ↓glucose metabolism: ↓G6PD, ↓TKT, ↓TKT activity; ↑senescence: ↑β-gal-cell staining, ↑GS(P), ↑glycogen accumulation In vivo (U87, heterotopic): ↓tumor volume, ↓tumor weight, ↓telomerase activity, ↑ROS, ↑caspase-3/8 activity, ↓TERT, ↓G6PD, ↓TKT, ↓GS(P) |
↓Nrf2 | [51] | |
| Molephantin (MPT) | U251, U87 | 3–100 µM 10 and 30 mg/kg, i.p. (10 times) |
In vitro: Cytotoxicity (IC50(72 h) = 10.6–22.6 µM), ↓colony forming capacity, S arrest, ↓migration, ↓invasion, ↓vimentin, ↓Snail, ↓N-cadherin, ↑E-cadherin, ↑PS externalization, ↑Bax/Bcl-2, ↑ROS, ↑cleaved caspase-7/9/3, ↑cleaved PARP, ↑mitochondrial ROS, ↓ΔψM, ↑mitochondrial dynamic imbalance (↓Mfn1/2, ↓OPA1, ↑Fis1, ↑Drp1, ↑mitochondrial fragmentation), ↓late stage mitophagy, ↓autophagosome-lysosome fusion, ↓spheroid growth In vivo (U87, heterotopic): brain tissue accumulation, ↓tumor volume, ↓tumor weight, no organ toxicity, ↑Bax/Bcl-2, ↑cleaved caspase-9/7/3, ↑cleaved PARP |
↓CDK4, ↓CDK2, ↑p21, ↓p-PI3K, ↓p-Akt, ↓p-mTOR, | [52] | |
| Melampomagnolide B dimer (MPLBD) | 9L-SF | 3–10 µM | Cytotoxicity | ND | [84] | |
| 1 (смoтри 32) | C6 | ND | IC50 = 3.0±0.8 µM | ND | [85] | |
| 2 (4d) | U87, MC38 | 1–16 µM 40 mg/kg, p.o.5 (12 times) |
In vitro: Cytotoxicity (IC50(96 h) = 2.8 µM), ↑PS externalization, G0/G1 arrest In vivo (MC38, heterotopic): ↓tumor weight, no organ toxicity |
●NF-κB (MolDock) | [49] | |
| Parthenolide dimer (смoтри 5) (PTLD) | U87, U118, SF126, SHG44, U251, C6 | 1–10 µM 50 mg/kg, i.p. (6 times) |
In vitro: Cytotoxicity (IC50(72 h) = 1.66–7.93 µM), ↓clonogenicity, ↑PS externalization, ↓migration, ↓invasion, ●PKM2 (molecular docking, thermal shift assay), ↑E-cadherin, ↓vimentin, ↑Bax/Bcl-2, ↓Bcl-xl In vivo (U118, heterotopic): ↓tumor volume, ↓tumor weight, ↑Bax/Bcl-2, ↑E-cadherin, ↓vimentin, ↓STAT3 MDCK cells: ↓barrier integrity |
↓STAT3, ↓p-STAT3, ↑PDK4 | [47] | |
| DMAPT-D6 | U87, LN229 | 2.5–40 µM | ROS-dependent cytotoxicity (IC50 = 11.15–15.5 µM), ↓clonogenicity, S arrest, ↑ROS, ↑DNA damage (↑γH2AX, ↑p53, ↑53BP1, ↑LIG IV), ↑PS externalization, ↑cleaved caspase-3, ↑cleaved PARP | ↓cyclin B, ↓cyclin E, ↓CDK1, ↓CDK2, ↑p27, ↑Nrf2, ↑DR3, ↑DR5, ↑FADD, ↑TRADD | [48] | |
| Eudesmanes | Alantolactone (ALN) | U87, U251, U118 | 1–50 µM 10 and 20 mg/kg, i.p. (15 times) |
In vitro: Cytotoxicity (IC50(48 h) = 16.33–29.16 µM), ↓clonogenicity, G0/G1 arrest, ↓migration, ↓invasion, ↓MMP-2, ↓MMP-9, ↑PS externalization, ↑cleaved caspase-3/9, ↑cleaved PARP, ↑Bax/Bcl-2, ↑cytoplasmic cytochrome C (cytoC), ↓COX-2 In vivo (U87, heterotopic): ↓tumor weight, ↓tumor volume, ↓COX-2, ↓p-p65 In vivo (BBB penetration): present in cerebrospinal fluid |
↓Cyclin D1, ↓CDK4, ↓binding of NF-κB p50/p65 and p300 to COX-2 promoter, ↓nuclear translocation of p65/p50, ↓p-IκB-α, ↓p-IKKβ, ↓IKKβ kinase activity, ●IKKβ (MolDock) | [65] |
| U87, U251 | 10 µM 20 mg/kg, i.p (15 times) |
In vitro: ↑G-actin, ↓F-actin, ↑mitochondrial transition of F-actin, ↓p-cofilin, ↑mitochondrial transition of cofilin, ↓migration, ↓invasion, ↓MMP-2, ↓MMP-9, ↑PS externalization, ↑cleaved caspase-3/9, ↑cleaved PARP, ↑cytoC In vivo (U87, heterotopic): ↓p-cofilin, ↓p-LIMK1/2 |
↓p-LIMK1/2 | [66] | ||
| HCM3, U87, U251 | 10–50 µM 20 mg/kg, i.p. (7 times) |
In vitro: Cytotoxicity (IC50 = 10–30 µM), ↓spheroid growth, ↓CD133, ↓OCT4, ↓SOX2, ↓NANOG In vivo (U87, orthotopic): ↑survival, ↓tumor size, ↓p-EGFR, ↓p-YAP |
↓YAP, ↑p-YAP, ↓p-EGFR, ↑p-LATS1 | [86] | ||
| 2α-Hydroxyalantolactone (HALN) | U87, U87ΔEGFR | 0.1–100 µM | Cytotoxicity (IC50 = 15.15–49.22 µM) | ND | [87] | |
| Guaianolides | Micheliolide (MCL) | U251 | 2.5–20 µM | Cytotoxicity (IC50(48 h) = 12.5±1.6 µM), ↓filopodia formation, ↓clonogenicity, ↓migration, ↓invasion, ↓MMP-9, ↓N-cadherin, ↓vimentin, ↑PS externalization, ↑cytoC, ↑cleaved caspase-3/9, ↑Bax/Bcl-2, ↓COX-2 | ↓p-IκBα/ IκBα | [53] |
| Dimethylaminomicheliolide (DMAMCL, ACT001) | C6, U87 | 1–120 µM 25–100 mg/kg, p.o. (21 times) |
In vitro: Cytotoxicity (IC50(72 h) = 20.58–27.18 µM), ↑PS externalization, ↑Bax/Bcl-2 In vivo (C6, orthotopic): ↓tumor weight, ↑survival, no organ and brain toxicity In vivo (BBB permeability): effectively accumulated in brain tissue (19.0±9.6 µg/mL (0.5 h)) |
ND | [54] | |
| U118, U251, U87, SF126, SHG44 | 5–40 µM | Cytotoxicity (IC50(48 h) = 17.9–37.1 µM), ↓clonogenicity, ●PKM2 (micheliolide (DMAMCL metabolite), pull-down), ↑pyruvate kinase activity, ↓aerobic glycolysis and ↓pentose phosphate pathway (↓lactate, ↓glucose-6-phosphate, ↓sedoheptulose-7-phosphate, ↓glycerol-3-phosphate) | ND | [21] | ||
| GL261 | 0.2–100 µM 50 mg/kg, p.o. + 2 Gy X-ray (5 times) |
In vitro: ↑susceptibility of GL261 cells to X-ray, ↑ROS, ↑DNA damage, ↑cleaved caspase-3 In vivo (GL261, heterotopic): ↓tumor volume (+X-ray) |
ND | [55] | ||
| U87 | 10 µM 200 mg/kg, p.o. (6 times) |
In vitro: ●PAI-1 (proteomic analysis, thermal shift assay, pull-down, surface plasmon resonance (KD = 2.31 mM), MolDock), ↓migration, ↓invasion, ↓vasculogenic mimicry, ↑PS externalization, synergy with cisplatin, ↑E-cadherin, ↓vimentin, ↓Snail, ↓β-catenin In vivo (U118, heterotopic): ↓tumor size, ↓tumor weight, ↓p-PI3K, ↓p-Akt |
↓p-PI3K, ↓p-Akt | [56] | ||
| U251, TJ905 | 20–80 µM 400 mg/kg/day, p.o. |
In vitro: ↓PD-L1 In vivo (GL261, orthotopic): ↑survival, ↓PD-L1, ↓p-STAT3, ↓M2 macrophage infiltration |
↓p-STAT3, ●STAT3 (pull-down) | [15] | ||
| U118, U251, SF126, SHG44, GL261 | 0.1–100 µM 200 and 400 mg/kg/day, p.o. |
In vitro: Cytotoxicity (IC50 = 7.3–77.3 µM), G2/M arrest, ↑PS externalization. ↑ROS, ↑NOX1, ↑TrX, ↑HO1, ↓MnSOD In vivo (U118, heterotopic): ↓tumor weight, ↓tumor volume In vivo (GL261, orthotopic): ↓tumor volume, ↓CDC2, ↓cyclin B1, ↓p-p65, ↓MnSOD, ↓Ki67 |
●IKKβ (pull-down, LC-MS/MS), ↓IKKγ, ↓p- IKKβ, ↓p-IκBα, ↓p-p65, ↓p-p65 nuclear translocation, ↓β-TRCP, ↑Nrf2 |
[57] | ||
| patient-derived GSC 1123, R39 | 1–100 µM 100 mg/kg, p.o. (5 times) |
In vitro: Cytotoxicity (IC50 = 15.87–19.88 µM), ↓spheroid growth, ↓AEBP1, ↓TGF-β-induced parameters (↓AEBP1, ↓p-Akt, ↓ cell proliferation, ↓spheroid growth), synergy with SHP099 In vivo (1123, orthotopic): ↓tumor growth, ↑survival, ↓p-Akt, ↓AEBP1, ↓Ki67, ↓Nestin |
↓p-Akt | [24] | ||
| MCL3 | G442, U87, U251, Hs683 | 3–30 µM 10, 20, and 40 mg/kg, p.o. (14 times) |
In vitro: Cytotoxicity (IC50(96 h) = 6.44–18.90 µM), ↑PS externalization, ↓IL6, ↓HIF-1α, ↓MMP-2, ↓Bcl-2, ↓Mcl-1 In vivo (G442, heterotopic): ↓tumor volume, ↓tumor weight, ↓PCNA, ↓CD34 (angiogenesis), ↓IL6 |
↓p-NF-κB, ↓nuclear p-NF-κB, ↓p-STAT3, ↓nuclear p-STAT3 | [58] | |
| Dehydrocostus lactone (DCL) | U118, U251, U87 | 1–100 µM 10 and 20 mg/kg, i.p. (14 times) |
In vitro: Cytotoxicity (IC50(48 h) = 17.16–26.42 µM), ↓clonogenicity, ↓migration, ↑cytoC, ↑Bax/Bcl-2, ↓COX-2, ↓p300/p50/p65 NF-κB nuclear translocation, ↓p300/p50/p65 NF-κB binding to COX-2 promoter In vivo (U87, heterotopic): ↓tumor volume, ↓tumor weight, ↓COX-2, ↓p-p65, ↓p-IKKβ In vivo (BBB permeability): effectively accumulated in brain tissue |
↓p-IKKα/β, ↓p-IκBα, ↓p-p65, ●IKKβ (MolDock) |
[18] | |
| Brevilin A (BVA) | U87, U373, LN229 | 5–80 µM | Cytotoxicity (IC50(24 h) = 30–40 µM), ↑PS externalization, ↑ROS, ↓GSH, ↑Bak, ↓Bcl-xl, ↑cytoC, ↓ΔψM, ↑cleaved caspase-3/9, ↑cleaved PARP, ↓XIAP | ↓p-JNK, ↓p-p38 | [59] | |
| Xanthatin (XTN) | C6, U251 | 1–15 µM 10, 20 and 40 mg/kg, i.p. (ND) |
In vitro: Cytotoxicity (IC50(24 h) ~ 15 µM), ↑PS externalization, ↑TUNEL-positive cells, ↑cleaved caspase-3, ↑Bax/Bcl-2, ↑ER stress (↑GRP78, ↑XBP1s, ↑nuclear translocation of CHOP) In vivo (C6, heterotopic): ↓tumor weight, ↑necrotic areas, ↑p-IRE1, ↑ATF6, ↑p-EIF2α, ↑XBP1s, ↑ATF4, ↑CHOP, ↑cleaved caspase-3 |
↑p-IRE1α, ↑p-EIF2α, ↑ATF4 | [60] | |
| C6, U251 | 1–15 µM 10, 20 and 40 mg/kg, i.p. (14 times) |
In vitro: Cytotoxicity (IC50(12 h) ~ 15 µM), ↓PCNA, ↓clonogenicity, ↑cleaved PARP, ↑cleaved caspase-3, ↓LC3-II/LC3-I, ↓autophagosome formation, ↑p62, ↓Beclin-1, ↓BECN1, ↓ATG5, ↓ATG7, ↓ATG12 In vivo (ND, ND): ↓LC3-II/LC3-I, ↑p62, ↑Beclin-1, ↑p-Akt, ↑p-mTOR, ↓p-ULK1 |
↑p-Akt, ↑p-mTOR, ↓p-ULK1, no effect on p-ERK1/2, p-JNK, and p-p38 | [61] | ||
| Lactucopicrin (LPN) | U87 | 1–10 µM | Cytotoxicity (IC50(24 h) = 12.5±1.1 µM), ↓clonogenicity, ↓migration, autophagy induction (↓p62, ↑LC3-II, rearrangement of vimentin and α-tubulin cytoskeleton), G2/M arrest, ↑p53, ↓pro-caspase-6, ↑cleaved PARP, synergy with temozolomide (TMZ) | ↓p-Akt, ↓p-ERK1/2, ↑p21, ↓CDK2, ↓p65 NF-κB | [22] | |
| Tomentosin (TTN) | U87 | 5–100 µM | Cytotoxicity (IC50(48 h) = 28.8 µM), ↑BAX, ↑CASP3, ↑CASP8, ↑CASP9, ↑CYCS, ↑FADD, ↑TNF, ↑TNFR1, ↑TNFR2, ↑TIMP2, ↓clonogenicity | ND | [62] | |
| Cynaropicrin (CPN) | U87 | 4–10 µM | ROS-dependent cytotoxicity (IC50(48 h) = 12.8±3.3), ↓clonogenicity, ↑ROS, ↓ΔψM, ↑cytoC, ↓pro-caspase-9/3, ↑LC3-II/I, ↓p62, ↑senescence (↑β-gal-positive cells), additive effect with TMZ | ↓p-ERK, ↓p-p65 NF-κB, ↑nuclear translocation of Nrf2 | [63] | |
| Cynaropicrin (CPN) Dehydrocstus lactone (DCL) Saussureamine B (SAB) |
U251 CSCs, U251 |
ND | Cytotoxicity (U251 CSCs: IC50 = 7.9–20.4 µM; U251: IC50 = 4.0–10.9 µM) | ND | [88] | |
| Arglabin diethyl cyanomethylphosphonate (8d) (ADCMP) | T98G | ND | Cytotoxicity (IC50(48 h) = 16.9±1.3 µM), selectivity index = 3.2 | ND | [89] | |
| 9-Oxomicheliolide (OMCL) |
U87 | ND | Cytotoxicity (IC50 = 13.15 µM), ↑PS externalization | ND | [64] | |
| Endoperoxide- containing STLs |
Dihydroartemisinin (DAMN) | GL261 GL261 GSCs |
10–80 µM | Cytotoxicity (GL261: IC50(24 h) ~ 80 µM, GL261 GSCs: IC50(24 h) ~ 40 µM), ↓spheroid growth, G1 arrest, ↑cleaved caspase-3 | ↓p-Akt | [67] |
| U87 | 5–160 µM | Cytotoxicity (IC50 ~ 70 µM), ↓migration, ↓invasion, ↓ADAM17 | ↓p-EGFR, ↓p-Akt | [90] | ||
| LN-229, LN-Z308, T269 | 5–9 µM | Cytotoxicity, ↓clonogenicity, synergy with TMZ, ↑ROS, ↑CAT, ↑GPX1, ↑GPX4, ↑SOD2, ↑LC3-II, ↓Sox2, ↓Nestin | ND | [91] | ||
| U87, U251 | 50–600 µM 2, 10, and 50 mg/kg, p.o. (45 times) |
In vitro: Cytotoxicity (IC50(24 h) = 200–210 µM), ↓migration, ↓invasion, ↓MMP9, ↓MMP9 activity, ↓MMP7, ↓MMP7 activity, ↑ROS, ↑p53, ↑p-p53 In vivo (U87, U251, heterotopic): ↓tumor volume |
↓EGFR, ↓β-catenin, ↓p-β-catenin |
[76] | ||
| U87, U251 | 3.125–200 µM 100 mg/kg, i.p. (28 times) |
In vitro: Cytotoxicity (IC50(24 h) = 16.12–25.05 µM), ↓DNA synthesis, ↓clonogenicity, S and G2/M arrest, ↑PS externalization, ↑caspase-3, ↑cleaved caspase-3/9, ↑cleaved PARP, ↓ΔψM, ↓glucose uptake, ↓L-lactate, ↓glycolytic capacity, synergy with TMZ, ↓spheroid formation In vivo (U87, orthotopic): ↑median survival time, ↑caspase-3 |
↓PGC-1α, ●ERRα (MolDock, TR-FRET) | [68] | ||
| Artesunate (AST) | LN229, A172 | 15 µM | Senolytic activity: ↓proliferation of senescent cells, ↑PS externalization in senescent cells. Non-toxic for non-senescent cells. | ND | [69] | |
| Other | 3 (смoтри 3) | U251, C6 | 1–100 µM | Cytotoxicity (IC50 = 36.6–41.6 µM), ↑activated caspases, ↑sub-G0/G1, ↑PS externalization, ↑ROS, ↓ΔψM | ND | [72] |
| 4 (смoтри 6) | U251 | 5–8 µM 2 mg/kg, p.o. (14 times) |
In vitro: Cytotoxicity (IC50 = 1.7 µM), mitotic catastrophe, ↓c-Myc, ↓Bcl-2, ↓Mcl-1, ↓Bcl-xl, ↓spheroid growth, ↑spheroid cell disaggregation, ↓migration from the spheroid, ↓Hsp105, ↓vimentin, ↓TNAP2, ↓G6PD, ↓GCN1, ↓TrxR1 In vivo (U251, heterotopic): ↓tumor volume, ↓p-STAT3, ↓STAT3 |
↓STAT3 DNA-binding activity, ↓p-STAT3, ●STAT3 (NMR, MolDock) | [73] | |
| Goyazensolide (GZD) | U87, T98G | 10–100 µM | Cytotoxicity (IC50 ~ 6 µM), ↓clonogenicity, no effect on migration, ↑apoptosis, ↑cleaved caspase-3 | ND | [70] | |
| Deoxyelephantopin (DEN) | GL261 | 0.5–2 µg/mL 10 mg/kg, p.o. (14 times) |
In vitro: Cytotoxicity (IC50(24 h) ~ 2 µg/mL), ↑PS externalization, G2/M arrest, ↓VEGF, ↓TGF-β, ↑caspase-3, ↑Bax/Bcl-2, ↑cytoC In vivo (GL261, heterotopic): ↓tumor volume, ↓tumor weight, ↑survival |
↓CDK4, ↓cyclin D2, ↓p-Akt, ↓p-STAT | [71] | |
| Enhydrin (EHN) | U87, LN229 | 2–8 µM 15–25 µM, intracraneal |
In vitro: Cytotoxicity (IC50(24 h) = 1.6–2.6 µM), ↓migration, ↓invasion, ↓N-cadherin, ↓vimentin, ↑E-cadherin In vivo (ND, orthotopic): ↓tumor size, ↑survival, ↓Ki67, ↓Jun, ↓TGF-β1, ↑Smad7 |
↓Jun, ↓TGF-β1, ↓p-Smad2, ↓nuclear p-Smad2, ↓p-Smad3, ↓nuclear p-Smad3, ↑Smad7 | [92] |
2.3.3. Effect of STLs on Energy Metabolism of Glioblastoma Cells
2.3.4. Effect of STLs on Other Processes Associated with Glioblastoma Cell Proliferation
2.4. Permeability of STLs Through Blood-Brain Barrier
2.5. Anti-Glioblastoma Efficacy of STLs In Vivo
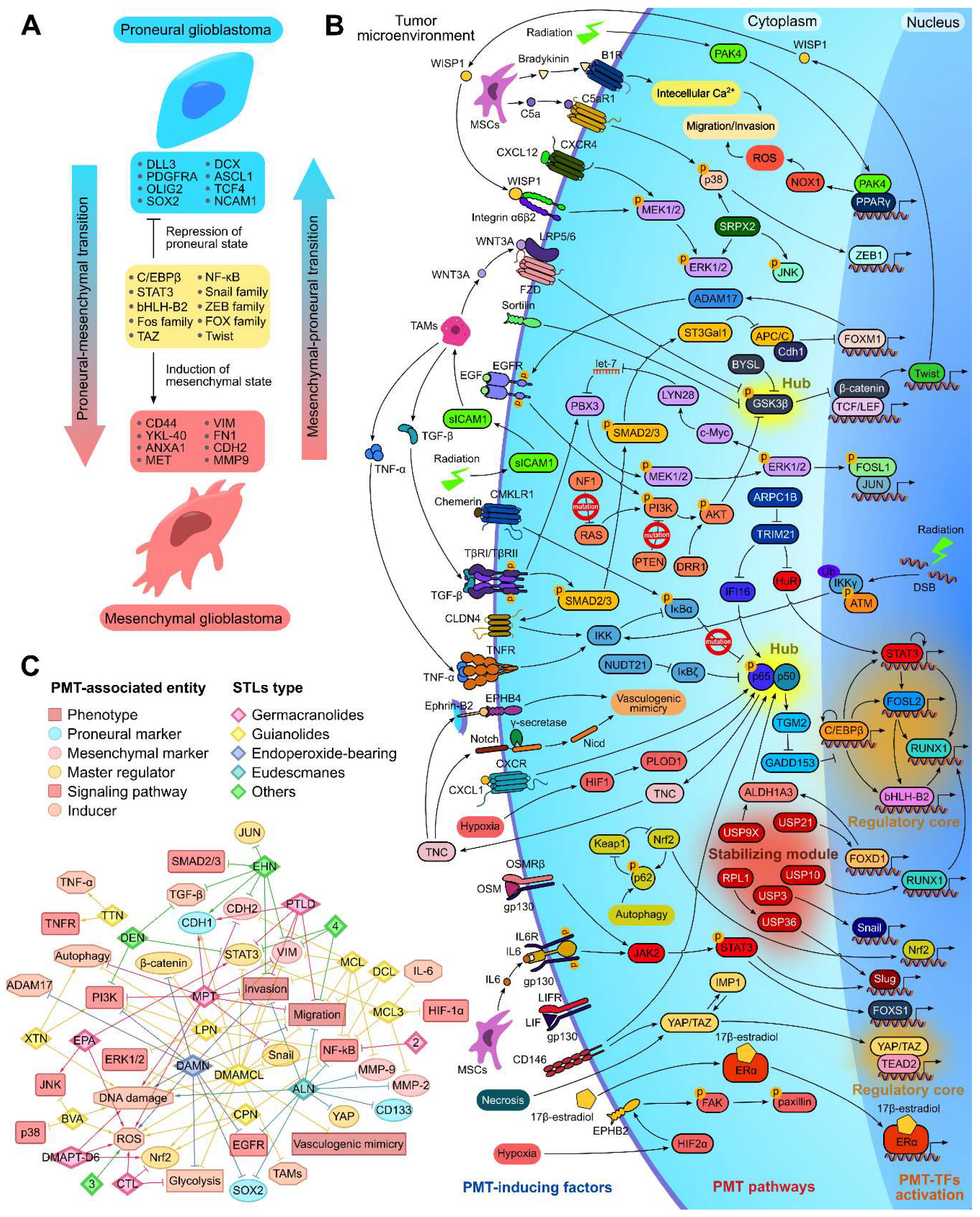
2.6. Pharmacological Potential of STLs Against Proneural-Mesenchymal Transition of Glioblastoma Cells
2.6.1. Proneural-Mesenchymal Transition as Promising Target for Glioblastoma Therapy
2.6.2. Effect of STLs on Key Regulators of PMT
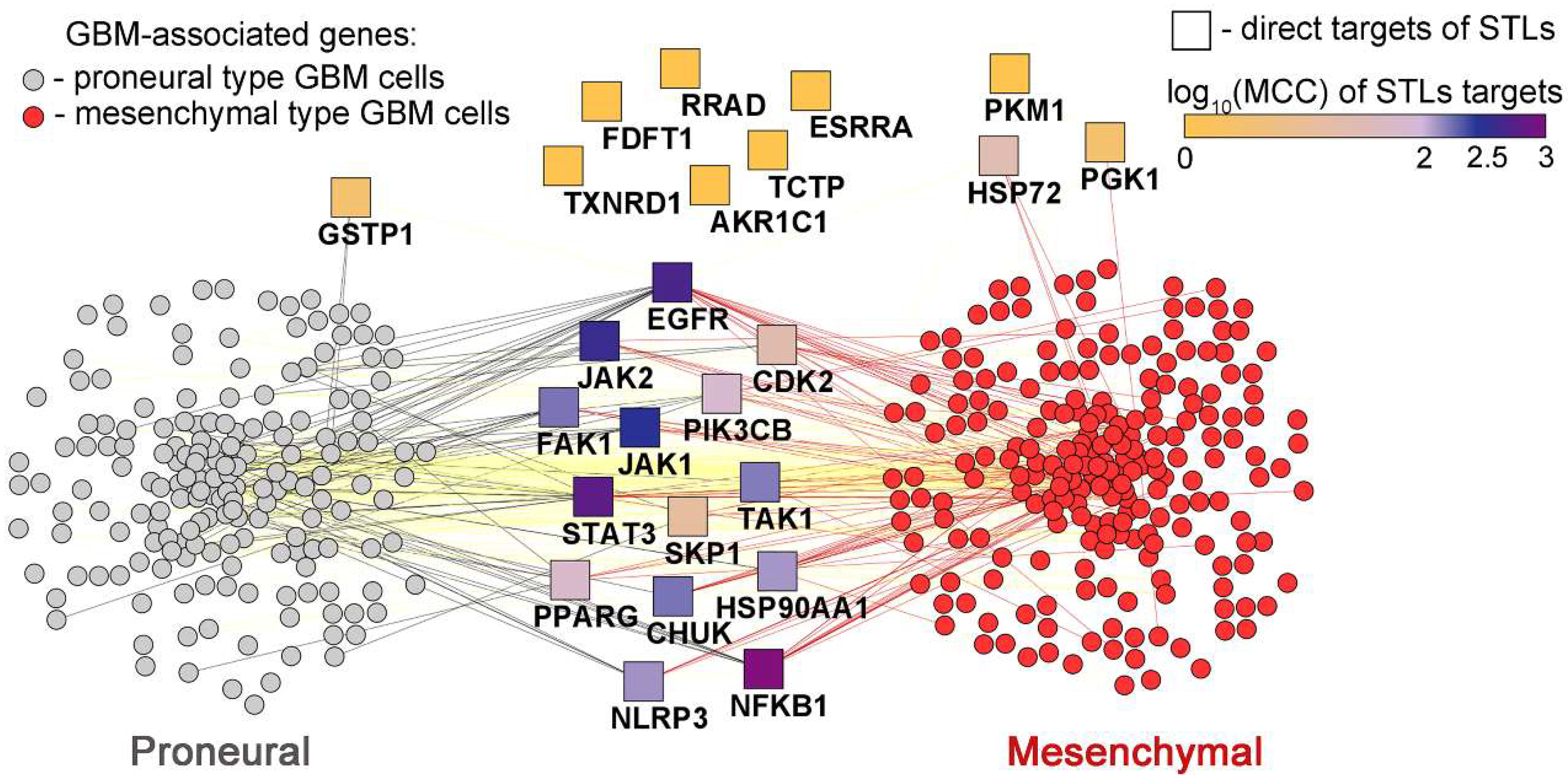
2.6.3. The Association of Protein Interactome of STLs with PMT
3. Conclusion and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawly, J.; Murcar, M.G.; Schcolnik-Cabrera, A.; Issa, M.E. Glioblastoma stem cell metabolism and immunity. Cancer Metastasis Rev. 2024, 43, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, F.; Alberghina, C.; D’Aprile, S.; Pavone, A.M.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Di Rosa, M.; Zappalà, A.; Cammarata, F.P.; et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Fedele, M.; Cerchia, L.; Pegoraro, S.; Sgarra, R.; Manfioletti, G. Proneural-Mesenchymal Transition: Phenotypic Plasticity to Acquire Multitherapy Resistance in Glioblastoma. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Chiariello, M.; Inzalaco, G.; Barone, V.; Gherardini, L. Overcoming challenges in glioblastoma treatment: targeting infiltrating cancer cells and harnessing the tumor microenvironment. Front. Cell. Neurosci. 2023, 17, 1327621. [Google Scholar] [CrossRef] [PubMed]
- Yalamarty, S.S.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers (Basel). 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Li, X.; Zhang, X. Glioblastoma: An Update in Pathology, Molecular Mechanisms and Biomarkers. Int. J. Mol. Sci. 2024, 25, 3040. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lu, X.; Liao, Y.; Ouyang, P.; Wang, H.; Zhang, X.; Huang, G.; Qi, S.; Li, Y. Crosstalk between glioblastoma and tumor microenvironment drives proneural–mesenchymal transition through ligand-receptor interactions. Genes Dis. 2024, 11, 874–889. [Google Scholar] [CrossRef] [PubMed]
- Kalya, M.; Beißbarth, T.; Kel, A.E. Master Regulators Associated with Poor Prognosis in Glioblastoma Multiforme. Biochem. (Moscow), Suppl. Ser. B Biomed. Chem. 2021, 15, 263–273. [Google Scholar] [CrossRef]
- Koehler, A.; Karve, A.; Desai, P.; Arbiser, J.; Plas, D.R.; Qi, X.; Read, R.D.; Sasaki, A.T.; Gawali, V.S.; Toukam, D.K.; et al. Reuse of Molecules for Glioblastoma Therapy. Pharmaceuticals 2021, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Kesari, S. Chapter 14 - Repurposing drugs in glioblastoma. In; Vitorino, C., Balaña, C., Cabral, C.B.T.-N.I.I.G., Eds.; Academic Press, 2023; pp. 285–317 ISBN 978-0-323-99873-4.
- Xu, F.; Yang, Y.-H.; Yang, H.; Li, W.; Hao, Y.; Zhang, S.; Zhang, Y.-Z.; Cao, W.-X.; Li, X.-X.; Du, G.-H.; et al. Progress of studies on natural products for glioblastoma therapy. J. Asian Nat. Prod. Res. 2024, 26, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, F.; Mao, J. Progress of natural sesquiterpenoids in the treatment of hepatocellular carcinoma. Front. Oncol. 2024, 14, 1445222. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Chu, C.; Qin, J.-J.; Guan, X. Research progress on antitumor mechanisms and molecular targets of Inula sesquiterpene lactones. Chin. Med. 2023, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Li, J.; Li, Q.; Wang, X.; Medikonda, R.; Zhao, T.; Li, T.; Ma, H.; Yi, L.; Liu, P.; et al. ACT001 reduces the expression of PD-L1 by inhibiting the phosphorylation of STAT3 in glioblastoma. Theranostics 2020, 10, 5943–5956. [Google Scholar] [CrossRef] [PubMed]
- Laurella, L.C.; Mirakian, N.T.; Garcia, M.N.; Grasso, D.H.; Sülsen, V.P.; Papademetrio, D.L. Sesquiterpene Lactones as Promising Candidates for Cancer Therapy: Focus on Pancreatic Cancer. Molecules 2022, 27, 3492. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Dos Santos, C.N. Sesquiterpene lactones: Promising natural compounds to fight inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Z.; Wang, C.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Ma, J.; Zhang, B.; et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-κB/COX-2 signaling pathway by targeting IKKβ. Am. J. Cancer Res. 2017, 7, 1270–1284. [Google Scholar]
- Yan, X.; Bai, M.; Yao, G.; Wang, X. Research progress on anti-glioma mechanism of natural sesquiterpene lactones. Chinese J. Clin. Pharmacol. Ther. 29, 1174–1184.
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xue, Q.; Liu, K.; Ge, W.; Liu, W.; Wang, J.; Zhang, M.; Li, Q.; Cai, D.; Shan, C.; et al. Dimethylaminomicheliolide (DMAMCL) Suppresses the Proliferation of Glioblastoma Cells via Targeting Pyruvate Kinase 2 (PKM2) and Rewiring Aerobic Glycolysis. Front. Oncol. 2019, 9, 993. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, R.; Oliva, M.A.; Staffieri, S.; Castaldo, S.; Giangaspero, F.; Arcella, A. Implication of Lactucopicrin in Autophagy, Cell Cycle Arrest and Oxidative Stress to Inhibit U87Mg Glioblastoma Cell Growth. Molecules 2020, 25, 5843. [Google Scholar] [CrossRef]
- Ma, J.-H.; Qi, J.; Liu, F.-Y.; Lin, S.-Q.; Zhang, C.-Y.; Xie, W.-D.; Zhang, H.-Y.; Li, X. Ivalin Inhibits Proliferation, Migration and Invasion by Suppressing Epithelial Mesenchymal Transition in Breast Cancer Cells. Nutr. Cancer 2018, 70, 1330–1338. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, B.; Liu, W.; Yu, B.; Shi, Q.; Luo, F.; Bai, Y.; Feng, H. Targeting of glioma stem-like cells with a parthenolide derivative ACT001 through inhibition of AEBP1/PI3K/AKT signaling. Theranostics 2021, 11, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Moujir, L.; Callies, O.; Sousa, P.M.C.; Sharopov, F.; Seca, A.M.L. Applications of sesquiterpene lactones: A review of some potential success cases. Appl. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Rajabi, S.; Hamzeloo-Moghadam, M.; Kumar, A.; Maresca, M.; Ghildiyal, P. Sesquiterpene lactones as emerging biomolecules to cease cancer by targeting apoptosis. Front. Pharmacol. 2024, 15, 1371002. [Google Scholar] [CrossRef]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, B.T., Ed.; Elsevier, 2006; Vol. 33, pp. 309–392 ISBN 1572-5995.
- Patrushev, S.S.; Rybalova, T. V.; Ivanov, I.D.; Vavilin, V.A.; Shults, E.E. Synthesis of a new class of bisheterocycles via the Heck reaction of eudesmane type methylene lactones with 8-bromoxanthines. Tetrahedron 2017, 73, 2717–2726. [Google Scholar] [CrossRef]
- Liu, X.; Bian, L.; Duan, X.; Zhuang, X.; Sui, Y.; Yang, L. Alantolactone: A sesquiterpene lactone with diverse pharmacological effects. Chem. Biol. Drug Des. 2021, 98, 1131–1145. [Google Scholar] [CrossRef]
- De Ford, C.; Ulloa, J.L.; Catalán, C.A.N.; Grau, A.; Martino, V.S.; Muschietti, L. V.; Merfort, I. The sesquiterpene lactone polymatin B from Smallanthus sonchifolius induces different cell death mechanisms in three cancer cell lines. Phytochemistry 2015, 117, 332–339. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Xiong, L.-A.; Ma, L.-F.; Fang, L.; Zhan, Z.-J. Natural product-derived ferroptosis mediators. Phytochemistry 2024, 219, 114002. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.B.; Fu, P.Y.; Ky, N.; Zhu, H.S.; Feng, X.L.; Li, J.; Srinivasan, K.G.; Hamza, M.S.; Zhao, Y. NF-κB p65 repression by the sesquiterpene lactone, Helenalin, contributes to the induction of autophagy cell death. BMC Complement. Altern. Med. 2012, 12, 1–12. [Google Scholar] [CrossRef]
- Yang, R.; Ma, S.; Zhuo, R.; Xu, L.; Jia, S.; Yang, P.; Yao, Y.; Cao, H.; Ma, L.; Pan, J.; et al. Suppression of endoplasmic reticulum stress-dependent autophagy enhances cynaropicrin-induced apoptosis via attenuation of the P62/Keap1/Nrf2 pathways in neuroblastoma. Front. Pharmacol. 2022, 13, 977622. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Nishimura, Y.; Takeda, T.; Kurita, M.; Uchiyama, T.; Suzuki, T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J. Pharmacol. Sci. 2015, 127, 397–403. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, H.; Ji, S.Y.; Kim, S.Y.; Hwangbo, H.; Park, S.-H.; Kim, G.-Y.; Park, C.; Leem, S.-H.; Hong, S.H.; et al. Induction of Apoptosis by Isoalantolactone in Human Hepatocellular Carcinoma Hep3B Cells through Activation of the ROS-Dependent JNK Signaling Pathway. Pharmaceutics 2021, 13, 1627. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, E.; Çınar Ayan, İ.; Dursun, H.G.; Vural, H. Tomentosin induces apoptosis in pancreatic cancer cells through increasing reactive oxygen species and decreasing mitochondrial membrane potential. Toxicol. Vitr. 2022, 84, 105458. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.W.; Park, E.S.; Lee, C.S. Parthenolide induces apoptosis by activating the mitochondrial and death receptor pathways and inhibits FAK-mediated cell invasion. Mol. Cell. Biochem. 2014, 385, 133–144. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Q.; zhou, H.; Huang, C.; Liu, G.; Zhao, X.; Cheng, Z.; You, X. Dihydroartemisinin inhibited vasculogenic mimicry in gastric cancer through the FGF2/FGFR1 signaling pathway. Phytomedicine 2024, 134, 155962. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Aleksandrova, Y.R.; Sharova, E. V; Smirnova, E. V; Artyushin, O.I.; Nikolaeva, N.S.; Semakov, A. V; Schagina, I.A.; Akylbekov, N.; Kurmanbayev, R.; et al. Conjugates of 3,5-Bis(arylidene)-4-piperidone and Sesquiterpene Lactones Have an Antitumor Effect via Resetting the Metabolic Phenotype of Cancer Cells. Molecules 2024, 29, 2765. [Google Scholar] [CrossRef]
- Ma, X.; Wu, K.; Xu, A.; Jiao, P.; Li, H.; Xing, L. The sesquiterpene lactone eupatolide induces apoptosis in non-small cell lung cancer cells by suppressing STAT3 signaling. Environ. Toxicol. Pharmacol. 2021, 81, 103513. [Google Scholar] [CrossRef]
- Qu, Z.; Lin, Y.; Mok, D.K.-W.; Bian, Q.; Tai, W.C.-S.; Chen, S. Brevilin A, a Natural Sesquiterpene Lactone Inhibited the Growth of Triple-Negative Breast Cancer Cells via Akt/mTOR and STAT3 Signaling Pathways. Onco. Targets. Ther. 2020, 13, 5363–5373. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, X.; Shen, C.; Yuan, J.; Lou, S.; Ma, X.; Chen, X.; Yang, B.; Zhao, H. A novel sesquiterpene lactone fraction from Eupatorium chinense L. suppresses hepatocellular carcinoma growth by triggering ferritinophagy and mitochondrial damage. Phytomedicine 2023, 112, 154671. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef]
- Trimble, C.L.; Levinson, K.; Maldonado, L.; Donovan, M.J.; Clark, K.T.; Fu, J.; Shay, M.E.; Sauter, M.E.; Sanders, S.A.; Frantz, P.S.; et al. A first-in-human proof-of-concept trial of intravaginal artesunate to treat cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol. Oncol. 2020, 157, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, S.-R.; Zhu, P.-F.; Liu, L.; Wang, B.; Liu, Y.-P.; Luo, X.-D.; Zhao, X.-D. Isocostunolide inhibited glioma stem cell by suppression proliferation and inducing caspase dependent apoptosis. Bioorg. Med. Chem. Lett. 2017, 27, 2863–2867. [Google Scholar] [CrossRef]
- Tang, T.K.; Chiu, S.C.; Lin, C.W.; Su, M.J.; Liao, M.H. Induction of survivin inhibition, G 2 /M cell cycle arrest and autophagic on cell death in human malignant glioblastoma cells. Chin. J. Physiol. 2015, 58, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xue, Q.; Liu, S.; Hu, K.; Wang, D.; Wang, T.; Li, Y.; Guo, H.; Hao, X.; Ge, W.; et al. Identification of Parthenolide Dimers as Activators of Pyruvate Kinase M2 in Xenografts of Glioblastoma Multiforme in Vivo. J. Med. Chem. 2020, 63, 1597–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Yang, D.L.; Qin, H.X.; He, L.J.; Huang, J.H.; Tang, D.Y.; Xu, Z.G.; Chen, Z.Z.; Li, Y. DMAPT-D6 induces death-receptor-mediated apoptosis to inhibit glioblastoma cell oncogenesis via induction of DNA damage through accumulation of intracellular ROS. Oncol. Rep. 2021, 45, 1261–1272. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Wang, S.; Zeng, B.; Du, G.; Zhang, C.; Li, Y. Synthesis, cytotoxicity, and in vivo antitumor activity study of parthenolide semicarbazones and thiosemicarbazones. Bioorg. Med. Chem. 2020, 28, 115557. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.-L.; Wang, X.-Y.; Bai, M.; Zhang, X.; Song, S.-J.; Yao, G.-D. Sesquiterpene lactones from Elephantopus scaber exhibit cytotoxic effects on glioma cells by targeting GSTP1. Bioorg. Chem. 2022, 129, 106183. [Google Scholar] [CrossRef]
- Ahmad, F.; Dixit, D.; Sharma, V.; Kumar, A.; Joshi, S.D.; Sarkar, C.; Sen, E. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. 2016, 7, e2213. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Pan, J.; Zhang, Z.; Chen, G.; Geng, J.; Lin, Q.; Zhang, T.; Cao, S.; Chen, C.; Lin, J.; et al. Small-molecule Molephantin induces apoptosis and mitophagy flux blockage through ROS production in glioblastoma. Cancer Lett. 2024, 592, 216927. [Google Scholar] [CrossRef]
- Feng, D.; Liu, M.; Liu, Y.; Zhao, X.; Sun, H.; Zheng, X.; Zhu, J.; Shang, F. Micheliolide suppresses the viability, migration and invasion of U251MG cells via the NF-κB signaling pathway. Oncol. Lett. 2020, 20, 67. [Google Scholar] [CrossRef]
- An, Y.; Guo, W.; Li, L.; Xu, C.; Yang, D.; Wang, S.; Lu, Y.; Zhang, Q.; Zhai, J.; Fan, H.; et al. Micheliolide derivative DMAMCL inhibits glioma cell growth in vitro and in vivo. PLoS One 2015, 10, e0116202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ni, K.; Chan, C.; Guo, N.; Luo, T.; Han, W.; Culbert, A.; Weichselbaum, R.R.; Lin, W. Dimethylaminomicheliolide Sensitizes Cancer Cells to Radiotherapy for Synergistic Combination with Immune Checkpoint Blockade. Adv. Ther. 2022, 5, 2100160. [Google Scholar] [CrossRef]
- Xi, X.; Liu, N.; Wang, Q.; Chu, Y.; Yin, Z.; Ding, Y.; Lu, Y. ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma. Cell Death Dis. 2019, 10, 757. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.; Liu, B.; Li, J.; Hao, X.; Ge, W.; Zhang, X.; Bao, S.; Gong, J.; Jiang, Z.; et al. ACT001 modulates the NF-κB/MnSOD/ROS axis by targeting IKKβ to inhibit glioblastoma cell growth. J. Mol. Med. 2020, 98, 263–277. [Google Scholar] [CrossRef]
- Du, Q.-Q.; Tang, M.; Huang, L.-L.; Zhao, R.; Yan, C.; Li, Y.; Pan, X.-D. The antitumor activity and mechanism of MCL3 in G422 glioblastoma. World J. Tradit. Chinese Med. 2020, 6, 353. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin A promotes oxidative stress and induces mitochondrial apoptosis in U87 glioblastoma cells. Onco. Targets. Ther. 2018, 11, 7031–7040. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Di, Z.-M.; Cao, Q.; Xu, W.-S.; Bi, S.-X.; Yu, J.-S.; Shen, Y.-J.; Yu, Y.-Q.; Shen, Y.-X.; Feng, L.-J. Xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating endoplasmic reticulum stress-dependent CHOP pathway. Acta Pharmacol. Sin. 2020, 41, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, T.; Huang, X.; Xu, W.; Di, Z.; Ma, Y.; Xue, M.; Bi, S.; Shen, Y.; Yu, Y.; et al. Xanthatin suppresses proliferation and tumorigenicity of glioma cells through autophagy inhibition via activation of the PI3K-Akt–mTOR pathway. Pharmacol. Res. Perspect. 2023, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ayan, Ç.İ.; Güçlü, E.; Dursun, H.G.; Vural, H. Tomentosin shows anticancer effect on U87 human glioblastoma multiforme cells. Bull. Biotechnol. 2021, 2, 23–26. [Google Scholar] [CrossRef]
- Rotondo, R.; Oliva, M.A.; Arcella, A. The Sesquiterpene Lactone Cynaropicrin Manifests Strong Cytotoxicity in Glioblastoma Cells U-87 MG by Induction of Oxidative Stress. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Zeng, B.; Cheng, Y.; Zheng, K.; Liu, S.; Shen, L.; Hu, J.; Li, Y.; Pan, X. Design, synthesis and in vivo anticancer activity of novel parthenolide and micheliolide derivatives as NF-κB and STAT3 inhibitors. Bioorg. Chem. 2021, 111, 104973. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Wang, C.; Cheng, W.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Xing, J.; et al. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKβ kinase activity and interrupting NF-κB/COX-2-mediated signaling cascades. J. Exp. Clin. Cancer Res. 2017, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zou, S.; Ren, T.; Zhao, L.-J.; Yu, L.-F.; Li, X.-Y.; Yan, X.; Zhang, L.-J. Alantolactone suppresses the metastatic phenotype and induces the apoptosis of glioblastoma cells by targeting LIMK kinase activity and activating the cofilin/G-actin signaling cascade. Int. J. Mol. Med. 2021, 47, 68. [Google Scholar] [CrossRef]
- Cao, L.; Duanmu, W.; Yin, Y.; Zhou, Z.; Ge, H.; Chen, T.; Tan, L.; Yu, A.; Hu, R.; Fei, L.; et al. Dihydroartemisinin exhibits anti-glioma stem cell activity through inhibiting p-AKT and activating caspase-3. Pharmazie 2014, 69, 752–758. [Google Scholar] [PubMed]
- Zhang, W.; Wang, Y.; Chen, L.; Chen, H.; Qi, H.; Zheng, Y.; Du, Y.; Zhang, L.; Wang, T.; Li, Q. Dihydroartemisinin suppresses glioma growth by repressing ERRα-mediated mitochondrial biogenesis. Mol. Cell. Biochem. 2024, 479, 2809–2825. [Google Scholar] [CrossRef]
- Beltzig, L.; Christmann, M.; Kaina, B. Abrogation of Cellular Senescence Induced by Temozolomide in Glioblastoma Cells: Search for Senolytics. Cells 2022, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Izumi, C.; Laure, H.J.; Barbosa, N.G.; Thomé, C.H.; Ferreira, G.A.; Sousa, J.P.B.; Lopes, N.P.; Rosa, J.C. Sequesterpene Lactones Isolated from a Brazilian Cerrado Plant (Eremanthus spp.) as Anti-Proliferative Compounds, Characterized by Functional and Proteomic Analysis, are Candidates for New Therapeutics in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 4713. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-W.; Chen, H.-H.; Sheu, J.J.-C. Deoxyelephantopin induces apoptosis and cell cycle arrest in GL261 glioblastoma cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Trifunović, S.; Isaković, A.M.; Isaković, A.; Vučković, I.; Mandić, B.; Novaković, M.; Vajs, V.; Milosavljević, S.; Trajković, V. Isolation, characterization, and in vitro cytotoxicity of new sesquiterpenoids from Achillea clavennae. Planta Med. 2014, 80, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, G.; Youn, U.J.; Yue, P.; Zhang, M.; Chen, C.-H.; Hilliard, T.S.; Paladino, D.; Li, Y.; Choi, J.; Sarkaria, J.N.; et al. Hirsutinolide Series Inhibit Stat3 Activity, Alter GCN1, MAP1B, Hsp105, G6PD, Vimentin, TrxR1, and Importin α-2 Expression, and Induce Antitumor Effects against Human Glioma. J. Med. Chem. 2015, 58, 7734–7748. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J. Cell. Physiol. 2003, 195, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dube, C.; Gibert, M.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers (Basel). 2018, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.; Wang, P.; Hu, Y.; Xue, Y.; Liu, X.; Qu, C.; Ma, J.; Liu, Y. Dihydroartemisin inhibits glioma invasiveness via a ROS to P53 to β-catenin signaling. Pharmacol. Res. 2017, 119, 72–88. [Google Scholar] [CrossRef]
- Sun, X.; Dyson, H.J.; Wright, P.E. A phosphorylation-dependent switch in the disordered p53 transactivation domain regulates DNA binding. Proc. Natl. Acad. Sci. 2021, 118, e2021456118. [Google Scholar] [CrossRef]
- Fung, T.S.; Chakrabarti, R.; Higgs, H.N. The multiple links between actin and mitochondria. Nat. Rev. Mol. Cell Biol. 2023, 24, 651–667. [Google Scholar] [CrossRef]
- Namme, J.N.; Bepari, A.K.; Takebayashi, H. Cofilin Signaling in the CNS Physiology and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 10727. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Zhao, J.; Wu, L.; Qi, Q.; Liu, Y.; Li, G.; Li, J.; Liu, H.; Wu, H. Cofilin: A Promising Protein Implicated in Cancer Metastasis and Apoptosis. Front. Cell Dev. Biol. 2021, 9, 599065. [Google Scholar] [CrossRef] [PubMed]
- Penthala, N.R.; Janganati, V.; Alpe, T.L.; Apana, S.M.; Berridge, M.S.; Crooks, P.A.; Borrelli, M.J. N-[(11)CH(3)]Dimethylaminoparthenolide (DMAPT) uptake into orthotopic 9LSF glioblastoma tumors in the rat. Bioorg. Med. Chem. Lett. 2016, 26, 5883–5886. [Google Scholar] [CrossRef] [PubMed]
- Hexum, J.K.; Becker, C.M.; Kempema, A.M.; Ohlfest, J.R.; Largaespada, D.A.; Harki, D.A. Parthenolide prodrug LC-1 slows growth of intracranial glioma. Bioorg. Med. Chem. Lett. 2015, 25, 2493–2495. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.A.; Bejcek, B.E.; Zhang, C.-R.; Nair, M.G. Sesquiterpenoid Lactones in Tanacetum huronense Inhibit Human Glioblastoma Cell Proliferation. Nat. Prod. Commun. 2016, 11, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Janganati, V.; Ponder, J.; Jordan, C.T.; Borrelli, M.J.; Penthala, N.R.; Crooks, P.A. Dimers of Melampomagnolide B Exhibit Potent Anticancer Activity against Hematological and Solid Tumor Cells. J. Med. Chem. 2015, 58, 8896–8906. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-J.; Ge, W.-Z.; Li, Q.-Y.; Lu, Y.; Gong, J.-M.; Kuang, B.-J.; Xi, X.; Wu, H.; Zhang, Q.; Chen, Y. Syntheses and Biological Evaluation of Costunolide, Parthenolide, and Their Fluorinated Analogues. J. Med. Chem. 2015, 58, 7007–7020. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, M.M.; Chen, X.; Li, Y.; Khan, M.; Ma, T. Alantolactone Suppresses YAP Signaling and Stemness Properties in Glioblastoma Cells. Pak. J. Zool. 2023, 55. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Dawood, M.; Mahmoud, N.; Elbadawi, M.; Sugimoto, Y.; Klauck, S.M.; Mohamed, N.; Efferth, T. 2α-Hydroxyalantolactone from Pulicaria undulata: activity against multidrug-resistant tumor cells and modes of action. Phytomedicine 2021, 81, 153409. [Google Scholar] [CrossRef]
- Araki, K.; Hara, M.; Hamada, S.; Matsumoto, T.; Nakamura, S. Antiproliferative Activities of Cynaropicrin and Related Compounds against Cancer Stem Cells. Chem. Pharm. Bull. (Tokyo). 2024, 72, 200–208. [Google Scholar] [CrossRef]
- Salin, A. V; Shabanov, A.A.; Khayarov, K.R.; Islamov, D.R.; Voloshina, A.D.; Amerhanova, S.K.; Lyubina, A.P. Phosphine-Catalyzed Synthesis and Cytotoxic Evaluation of Michael Adducts of the Sesquiterpene Lactone Arglabin. ChemMedChem 2024, 19, e202400045. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Wang, F.; Gao, H.; Hu, W. Dihydroartemisinin suppresses glioma proliferation and invasion via inhibition of the ADAM17 pathway. Neurol. Sci. 2015, 36, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Lemke, D.; Pledl, H.-W.; Zorn, M.; Jugold, M.; Green, E.; Blaes, J.; Löw, S.; Hertenstein, A.; Ott, M.; Sahm, F.; et al. Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. Oncotarget 2016, 7, 56713–56725. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, J.; Li, X.; Zong, S.; Zhang, G.; Guo, Z.; Jing, Z. Enhydrin suppresses the malignant phenotype of GBM via Jun/Smad7/TGF-β1 signaling pathway. Biochem. Pharmacol. 2024, 226, 116380. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Asthana, S.; Kumar, D. Role of Oxidative Stress in Metabolic Reprogramming of Brain Cancer. Cancers (Basel). 2023, 15, 4920. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ramiro, A.; Ramírez-Ortega, D.; Pérez de la Cruz, V.; Hérnandez-Pedro, N.Y.; González-Esquivel, D.F.; Sotelo, J.; Pineda, B. Role of Redox Status in Development of Glioblastoma. Front. Immunol. 2016, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiao, Y.; Sun, Q.; Peng, L.; Sun, L. A novel SLC25A1 inhibitor, parthenolide, suppresses the growth and stemness of liver cancer stem cells with metabolic vulnerability. Cell Death Discov. 2023, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, Y.; Li, T.; Gan, X.; Yu, H. The Role of PKM2 in the Regulation of Mitochondrial Function: Focus on Mitochondrial Metabolism, Oxidative Stress, Dynamic, and Apoptosis. PKM2 in Mitochondrial Function. Oxid. Med. Cell. Longev. 2022, 2022, 7702681. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Valieva, Y.; Ivanova, E.; Fayzullin, A.; Kurkov, A.; Igrunkova, A. Senescence-Associated β-Galactosidase Detection in Pathology. Diagnostics 2022, 12, 2309. [Google Scholar] [CrossRef]
- Chojak, R.; Fares, J.; Petrosyan, E.; Lesniak, M.S. Cellular senescence in glioma. J. Neurooncol. 2023, 164, 11–29. [Google Scholar] [CrossRef]
- Salam, R.; Saliou, A.; Bielle, F.; Bertrand, M.; Antoniewski, C.; Carpentier, C.; Alentorn, A.; Capelle, L.; Sanson, M.; Huillard, E.; et al. Cellular senescence in malignant cells promotes tumor progression in mouse and patient Glioblastoma. Nat. Commun. 2023, 14, 441. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Jäättelä, M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007, 14, 1576–1582. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, Z.; Xu, J.; Zhang, L.; Cui, H. Endoplasmic reticulum stress-induced cell death as a potential mechanism for targeted therapy in glioblastoma (Review). Int J Oncol 2021, 59, 60. [Google Scholar] [CrossRef]
- Khabibov, M.; Garifullin, A.; Boumber, Y.; Khaddour, K.; Fernandez, M.; Khamitov, F.; Khalikova, L.; Kuznetsova, N.; Kit, O.; Kharin, L. Signaling pathways and therapeutic approaches in glioblastoma multiforme (Review). Int. J. Oncol. 2022, 60, 69. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37. [Google Scholar] [CrossRef]
- Bors, L.A.; Erdö, F. Overcoming the blood-brain barrier. Challenges and tricks for CNS drug delivery. Sci. Pharm. 2019, 87. [Google Scholar] [CrossRef]
- Velumani, K.; John, A.; Shaik, M.R.; Hussain, S.A.; Guru, A.; Issac, P.K. Exploring sesquiterpene lactone as a dual therapeutic agent for diabetes and oxidative stress: insights into PI3K/AKT modulation. 3 Biotech 2024, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.N.A.; Choucry, M.A.; El Senousy, A.S.; Hassan, A.; El-Marasy, S.A.; El Awdan, S.A.; Omar, F.A. Ambrosin, a potent NF-κβ inhibitor, ameliorates lipopolysaccharide induced memory impairment, comparison to curcumin. PLoS One 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; Rai, V.; Awasthee, N.; Dhasmana, A.; Rajalaksmi, D.S.; Nair, M.S.; Gupta, S.C. Isodeoxyelephantopin, a Sesquiterpene Lactone Induces ROS Generation, Suppresses NF-κB Activation, Modulates LncRNA Expression and Exhibit Activities Against Breast Cancer. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; Marques, D.; Figueira, I.; Cankar, K.; Bosch, D.; Brito, M.A.; dos Santos, C.N. Costunolide and parthenolide: Novel blood-brain barrier permeable sesquiterpene lactones to improve barrier tightness. Biomed. Pharmacother. 2023, 167, 115413. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.J.; Huang, L.F.; Deng, J. Le; Wang, Y.M.; Guo, C.; Peng, X.N.; Liu, Z.; Gao, J.M. Cognitive enhancement and neuroprotective effects of OABL, a sesquiterpene lactone in 5xFAD Alzheimer’s disease mice model. Redox Biol. 2022, 50, 102229. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.; Gerstberger, R.; Roth, J.; Hübschle, T. Parthenolide attenuates LPS-induced fever, circulating cytokines and markers of brain inflammation in rats. Cytokine 2011, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, Y.L.; Xing, J.S.; Lan, X.Q.; Wang, L.T.; Zhang, B. Alantolactone plays neuroprotective roles in traumatic brain injury in rats via anti-inflammatory, anti-oxidative and anti-apoptosis pathways. Am. J. Transl. Res. 2018, 10. [Google Scholar]
- Xu, C.; Hou, P.; Li, X.; Xiao, M.; Zhang, Z.; Li, Z.; Xu, J.; Liu, G.; Tan, Y.; Fang, C. Comprehensive understanding of glioblastoma molecular phenotypes: classification, characteristics, and transition. Cancer Biol. Med. 2024, 21, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wu, H.; Tian, M.; Liu, Q.; Zhu, Y.; Zhang, H.; Zhang, X.; Shen, H. Isolinderalactone suppresses pancreatic ductal adenocarcinoma by activating p38 MAPK to promote DDIT3 expression and trigger endoplasmic reticulum stress. Int. Immunopharmacol. 2024, 143, 113497. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, B.; Zhao, X.; Tian, S.; Li, Y.; Pei, H.; Yu, S.; Liu, C.; Lin, Z.; Zuo, Z.; et al. Dehydrocostus lactone inhibits the proliferation and metastasis of hepatocellular carcinoma cells via modulating p53-p21-CDK2 signaling pathway. Arab. J. Chem. 2023, 16, 104994. [Google Scholar] [CrossRef]
- Zhu, S.M.; Park, Y.R.; Seo, S.Y.; Kim, I.H.; Lee, S.T.; Kim, S.W. Parthenolide inhibits transforming growth factor β1-induced epithelial-mesenchymal transition in colorectal cancer cells. Intest. Res. 2019, 17, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Y.; Ying, C.; Jiang, Y.; Hu, J. Hypoxia-induced PLOD1 overexpression contributes to the malignant phenotype of glioblastoma via NF-κB signaling. Oncogene 2021, 40, 1458–1475. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Song, S.; Chen, W.; Zhang, J.; Yang, H.; Chen, Y. Hypoxia-induced EPHB2 promotes invasive potential of glioblastoma. Int. J. Clin. Exp. Pathol. 2019, 12, 539–548. [Google Scholar] [PubMed]
- Yee, P.P.; Wei, Y.; Kim, S.-Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 2020, 11, 5424. [Google Scholar] [CrossRef] [PubMed]
- Kesanakurti, D.; Maddirela, D.; Banasavadi-Siddegowda, Y.K.; Lai, T.-H.; Qamri, Z.; Jacob, N.K.; Sampath, D.; Mohanam, S.; Kaur, B.; Puduvalli, V.K. A novel interaction of PAK4 with PPARγ to regulate Nox1 and radiation-induced epithelial-to-mesenchymal transition in glioma. Oncogene 2017, 36, 5309–5320. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.-C.; Kang, J.-H.; Choi, M.-Y.; Suh, Y.; Zhao, Y.; Kim, M.-J.; Chang, J.H.; Shim, J.-K.; Yoon, S.-J.; Kang, S.-G.; et al. Soluble ICAM-1 a Pivotal Communicator between Tumors and Macrophages, Promotes Mesenchymal Shift of Glioblastoma. Adv. Sci. (Weinheim, Baden-Wurttemberg, Ger. 2022, 9, e2102768. [Google Scholar] [CrossRef]
- Liu, Z.; Kuang, W.; Zhou, Q.; Zhang, Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int J Mol Med 2018, 42, 3395–3403. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Pillat, M.M.; Motaln, H.; Ulrich, H.; Lah, T.T. Kinin-B1 Receptor Stimulation Promotes Invasion and is Involved in Cell-Cell Interaction of Co-Cultured Glioblastoma and Mesenchymal Stem Cells. Sci. Rep. 2018, 8, 1299. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-J.; Kim, S.; Oh, Y.; Suh, Y.; Kaushik, N.; Lee, J.-H.; Lee, H.-J.; Kim, M.-J.; Park, M.-J.; Kim, R.-K.; et al. Crosstalk between GBM cells and mesenchymal stemlike cells promotes the invasiveness of GBM through the C5a/p38/ZEB1 axis. Neuro. Oncol. 2020, 22, 1452–1462. [Google Scholar] [CrossRef]
- Yan, T.; Tan, Y.; Deng, G.; Sun, Z.; Liu, B.; Wang, Y.; Yuan, F.; Sun, Q.; Hu, P.; Gao, L.; et al. TGF-β induces GBM mesenchymal transition through upregulation of CLDN4 and nuclear translocation to activate TNF-α/NF-κB signal pathway. Cell Death Dis. 2022, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shen, S.; Liu, T.; Ren, X.; Zhu, C.; Liang, Q.; Cui, X.; Chen, L.; Cheng, P.; Cheng, W.; et al. Chemerin enhances mesenchymal features of glioblastoma by establishing autocrine and paracrine networks in a CMKLR1-dependent manner. Oncogene 2022, 41, 3024–3036. [Google Scholar] [CrossRef]
- Alafate, W.; Li, X.; Zuo, J.; Zhang, H.; Xiang, J.; Wu, W.; Xie, W.; Bai, X.; Wang, M.; Wang, J. Elevation of CXCL1 indicates poor prognosis and radioresistance by inducing mesenchymal transition in glioblastoma. CNS Neurosci. Ther. 2020, 26, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Yamini, B. NF-κB, Mesenchymal Differentiation and Glioblastoma. Cells 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Liu, X.; Wang, Z.; Zhang, C.; Wu, F.; Jiang, H.; Zhang, W.; Bao, Z.; Wang, Y.; et al. ALDH1A3 induces mesenchymal differentiation and serves as a predictor for survival in glioblastoma. Cell Death Dis. 2018, 9, 1190. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Voshart, D.; Paridaen, J.T.M.L.; Oosterhof, N.; Liang, D.; Thiruvalluvan, A.; Zuhorn, I.S.; den Dunnen, W.F.A.; Zhang, G.; Lin, H.; et al. CD146 increases stemness and aggressiveness in glioblastoma and activates YAP signaling. Cell. Mol. Life Sci. 2022, 79, 398. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Oh, Y.T.; Kim, J.-Y.; Kim, S.S.; Choi, E.; Kim, T.H.; Hong, J.H.; Chang, N.; Cho, H.J.; Sa, J.K.; et al. Transglutaminase 2 Inhibition Reverses Mesenchymal Transdifferentiation of Glioma Stem Cells by Regulating C/EBPβ Signaling. Cancer Res. 2017, 77, 4973–4984. [Google Scholar] [CrossRef]
- Angel, I.; Pilo Kerman, O.; Rousso-Noori, L.; Friedmann-Morvinski, D. Tenascin C promotes cancer cell plasticity in mesenchymal glioblastoma. Oncogene 2020, 39, 6990–7004. [Google Scholar] [CrossRef]
- Yang, W.; Wu, P.-F.; Ma, J.-X.; Liao, M.-J.; Wang, X.-H.; Xu, L.-S.; Xu, M.-H.; Yi, L. Sortilin promotes glioblastoma invasion and mesenchymal transition through GSK-3β/β-catenin/twist pathway. Cell Death Dis. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Han, X.; Xu, X.; Zhou, Z.; Chen, X.; Tang, Y.; Cheng, J.; Moazzam, N.F.; Liu, F.; Xu, J.; et al. FoxM1 drives ADAM17/EGFR activation loop to promote mesenchymal transition in glioblastoma. Cell Death Dis. 2018, 9, 469. [Google Scholar] [CrossRef]
- Sha, Z.; Zhou, J.; Wu, Y.; Zhang, T.; Li, C.; Meng, Q.; Musunuru, P.P.; You, F.; Wu, Y.; Yu, R.; et al. BYSL Promotes Glioblastoma Cell Migration, Invasion, and Mesenchymal Transition Through the GSK-3β/β-Catenin Signaling Pathway. Front. Oncol. 2020, 10, 565225. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Chu, C.; Zhou, W.; Huang, Z.; Zhai, K.; Fang, X.; Huang, Q.; Zhang, A.; Wang, X.; Yu, X.; et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat. Commun. 2020, 11, 3015. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Zhang, Q.; Yu, H.; Zhao, Y.; Shen, L. Identification of WISP1 as a novel oncogene in glioblastoma. Int. J. Oncol. 2017, 51, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vega, A.M.; Del Moral-Morales, A.; Zamora-Sánchez, C.J.; Piña-Medina, A.G.; González-Arenas, A.; Camacho-Arroyo, I. Estradiol Induces Epithelial to Mesenchymal Transition of Human Glioblastoma Cells. Cells 2020, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; Lee, S.; Harmanci, A.S.; Patel, R.; Latha, K.; Yang, Y.; Marisetty, A.; Lee, H.-K.; Heimberger, A.B.; Fuller, G.N.; et al. CXCR4 expression is associated with proneural-to-mesenchymal transition in glioblastoma. Int. J. cancer 2023, 152, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Xiang, W.; Zhang, Q.; Wang, H.; Zhou, Y.; Tian, H.; Abdelmaksou, A.; Xue, J.; Sun, M.; Yi, D.; et al. CD90low glioma-associated mesenchymal stromal/stem cells promote temozolomide resistance by activating FOXS1-mediated epithelial-mesenchymal transition in glioma cells. Stem Cell Res. Ther. 2021, 12, 394. [Google Scholar] [CrossRef]
- Lv, S.-Q.; Fu, Z.; Yang, L.; Li, Q.-R.; Zhu, J.; Gai, Q.-J.; Mao, M.; He, J.; Qin, Y.; Yao, X.-X.; et al. Comprehensive omics analyses profile genesets related with tumor heterogeneity of multifocal glioblastomas and reveal LIF/CCL2 as biomarkers for mesenchymal subtype. Theranostics 2022, 12, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Natesh, K.; Bhosale, D.; Desai, A.; Chandrika, G.; Pujari, R.; Jagtap, J.; Chugh, A.; Ranade, D.; Shastry, P. Oncostatin-M differentially regulates mesenchymal and proneural signature genes in gliomas via STAT3 signaling. Neoplasia 2015, 17, 225–237. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Salazar, K.L.; Balasubramaniyan, V.; Wani, K.; Heathcock, L.; Hollingsworth, F.; James, J.D.; Gumin, J.; Diefes, K.L.; Kim, S.H.; et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011, 25, 2594–2609. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.S.; Lim, W.K.; Alvarez, M.J.; Bollo, R.J.; Zhao, X.; Snyder, E.Y.; Sulman, E.P.; Anne, S.L.; Doetsch, F.; Colman, H.; et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature 2010, 463, 318–325. [Google Scholar] [CrossRef]
- Fan, L.; Chen, Z.; Wu, X.; Cai, X.; Feng, S.; Lu, J.; Wang, H.; Liu, N. Ubiquitin-Specific Protease 3 Promotes Glioblastoma Cell Invasion and Epithelial–Mesenchymal Transition via Stabilizing Snail. Mol. Cancer Res. 2019, 17, 1975–1984. [Google Scholar] [CrossRef]
- Chesnelong, C.; Hao, X.; Cseh, O.; Wang, A.Y.; Luchman, H.A.; Weiss, S. SLUG Directs the Precursor State of Human Brain Tumor Stem Cells. Cancers (Basel). 2019, 11, 1635. [Google Scholar] [CrossRef] [PubMed]
- Pölönen, P.; Jawahar Deen, A.; Leinonen, H.M.; Jyrkkänen, H.-K.; Kuosmanen, S.; Mononen, M.; Jain, A.; Tuomainen, T.; Pasonen-Seppänen, S.; Hartikainen, J.M.; et al. Nrf2 and SQSTM1/p62 jointly contribute to mesenchymal transition and invasion in glioblastoma. Oncogene 2019, 38, 7473–7490. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, J.; Waghmare, I.; Sartini, S.; Coviello, V.; Zhang, Z.; Kim, S.-H.; Mohyeldin, A.; Pavlyukov, M.S.; Minata, M.; et al. FOXD1-ALDH1A3 Signaling Is a Determinant for the Self-Renewal and Tumorigenicity of Mesenchymal Glioma Stem Cells. Cancer Res. 2016, 76, 7219–7230. [Google Scholar] [CrossRef]
- Chong, Y.K.; Sandanaraj, E.; Koh, L.W.H.; Thangaveloo, M.; Tan, M.S.Y.; Koh, G.R.H.; Toh, T.B.; Lim, G.G.Y.; Holbrook, J.D.; Kon, O.L.; et al. ST3GAL1-Associated Transcriptomic Program in Glioblastoma Tumor Growth, Invasion, and Prognosis. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.-W.; Wang, S.; Fan, L.; Feng, S.; Cai, X.; Peng, C.; Wu, X.; Lu, J.; Chen, D.; et al. USP9X deubiquitinates ALDH1A3 and maintains mesenchymal identity in glioblastoma stem cells. J. Clin. Invest. 2019, 129, 2043–2055. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, Z.; Yang, Y.; Jiang, L.; Song, S.; Qi, X.; Chen, Y.; Yang, H.; Liu, J.; Chu, L. USP10 deubiquitinates RUNX1 and promotes proneural-to-mesenchymal transition in glioblastoma. Cell Death Dis. 2023, 14, 207. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Z.; Tang, Q.; Wang, Z.; Lu, J.; You, Y.; Wang, H. USP21 promotes self-renewal and tumorigenicity of mesenchymal glioblastoma stem cells by deubiquitinating and stabilizing FOXD1. Cell Death Dis. 2022, 13, 712. [Google Scholar] [CrossRef]
- Qiu, W.; Cai, X.; Xu, K.; Song, S.; Xiao, Z.; Hou, Y.; Qi, X.; Liu, F.; Chen, Y.; Yang, H.; et al. PRL1 Promotes Glioblastoma Invasion and Tumorigenesis via Activating USP36-Mediated Snail2 Deubiquitination. Front. Oncol. 2021, 11, 795633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-W.; Cheng, H.-Y.; Kuo, C.-L.; Way, T.-D.; Lien, J.-C.; Chueh, F.-S.; Lin, Y.-L.; Chung, J.-G. Tetrandrine inhibits human brain glioblastoma multiforme GBM 8401 cancer cell migration and invasion in vitro. Environ. Toxicol. 2019, 34, 364–374. [Google Scholar] [CrossRef]
- Zou, M.; Duan, Y.; Wang, P.; Gao, R.; Chen, X.; Ou, Y.; Liang, M.; Wang, Z.; Yuan, Y.; Wang, L.; et al. DYT-40, a novel synthetic 2-styryl-5-nitroimidazole derivative, blocks malignant glioblastoma growth and invasion by inhibiting AEG-1 and NF-κB signaling pathways. Sci. Rep. 2016, 6, 27331. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, X.; Wu, L.; Zhou, D.; Song, Y.; Zhang, L.; Wu, Q.; He, Q.; Wang, G.; Liu, X.; et al. Quercetin Suppresses Human Glioblastoma Migration and Invasion via GSK3β/β-catenin/ZEB1 Signaling Pathway. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, K.; Li, C.; Zhao, Y.; Li, H.; Liu, X.; Long, Y.; Yao, J. Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma cells. Oncol. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Liu, H.-W.; Su, Y.-K.; Bamodu, O.A.; Hueng, D.-Y.; Lee, W.-H.; Huang, C.-C.; Deng, L.; Hsiao, M.; Chien, M.-H.; Yeh, C.-T.; et al. The Disruption of the β-Catenin/TCF-1/STAT3 Signaling Axis by 4-Acetylantroquinonol B Inhibits the Tumorigenesis and Cancer Stem-Cell-Like Properties of Glioblastoma Cells, In Vitro and In Vivo. Cancers (Basel). 2018, 10, 491. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, T.; Xu, Y.; Wu, C.; Chen, J.; Ren, Y.; Kong, L.; Sun, S.; Guo, W.; Wang, Y.; et al. A novel STAT3 inhibitor, HJC0152, exerts potent antitumor activity in glioblastoma. Am. J. Cancer Res. 2019, 9, 699–713. [Google Scholar]
- Jia, M.; Wang, Y.; Guo, Y.; Yu, P.; Sun, Y.; Song, Y.; Zhao, L. Nitidine chloride suppresses epithelial-mesenchymal transition and stem cell-like properties in glioblastoma by regulating JAK2/STAT3 signaling. Cancer Med. 2021, 10, 3113–3128. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Xu, R.; Ji, J.; Xu, Y.; Han, M.; Wei, Y.; Huang, B.; Chen, A.; Zhang, Q.; et al. YM155 decreases radiation-induced invasion and reverses epithelial–mesenchymal transition by targeting STAT3 in glioblastoma. J. Transl. Med. 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Lin, C.; Wu, S.; Wang, F.; Wang, H.; Wang, Y.; Peng, Y.; Hutchinson, M.R.; Li, H.; et al. Artemisinin inhibits TLR4 signaling by targeting co-receptor MD2 in microglial BV-2 cells and prevents lipopolysaccharide-induced blood–brain barrier leakage in mice. J. Neurochem. 2021, 157. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, G.; Luo, L.; Lin, T.; Chu, X.; Liu, K.; Lai, T.; Liao, Y.; Lin, X.; Chen, J. Artemisitene induces apoptosis of breast cancer cells by targeting FDFT1 and inhibits the growth of breast cancer patient-derived organoids. Phytomedicine 2024, 135, 156155. [Google Scholar] [CrossRef] [PubMed]
- Omotuyi, I.O.; Nash, O.; Metibemu, S.D.; Iwegbulam, G.C.; Olatunji, O.M.; Agbebi, E.; Falade, C.O. Dihydroartemisinin binds human PI3K-β affinity pocket and forces flat conformation in P-loop MET783: A molecular dynamics study. Comput. Toxicol. 2023, 27. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Liu, J.; Zhang, C.; Jian, C.; Wang, L.; Zhang, Y.; Shi, C. Natural Product Alantolactone Targeting AKR1C1 Suppresses Cell Proliferation and Metastasis in Non-Small-Cell Lung Cancer. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Li, J.-K.; Jiang, X.-L.; Zhang, Z.; Chen, W.-Q.; Peng, J.-J.; Liu, B.; Zhu, P.-L.; Yung, K.-K.-L. Isoalantolactone exerts anti-melanoma effects via inhibiting PI3K/AKT/mTOR and STAT3 signaling in cell and mouse models. Phytother. Res. 2024, 38, 2800–2817. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Lin, W.; Zhou, J.; Liu, Y.; Ma, L.; Li, M.; Hu, Y.; Yu, C.; Zhang, Y.; et al. Discovery of Novel Sesquiterpene Lactone Derivatives as Potent PKM2 Activators for the Treatment of Ulcerative Colitis. J. Med. Chem. 2023, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, W.; Niu, R.; Cong, L.; Jiang, M.; Bai, G. Costunolide covalently targets and inhibits CaMKII phosphorylation to reduce ischemia-associated brain damage. Phytomedicine 2023, 115. [Google Scholar] [CrossRef]
- Liu, Y.C.; Feng, N.; Li, W.W.; Tu, P.F.; Chen, J.P.; Han, J.Y.; Zeng, K.W. Costunolide Plays an Anti-Neuroinflammation Role in Lipopolysaccharide-Induced BV2 Microglial Activation by Targeting Cyclin-Dependent Kinase 2. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, W.; Chen, R.; Vladimir, K.; Dong, X.; Zia, K.; Sun, X.; Dai, X.; Bao, M.; Shen, X.; Liang, G. Costunolide specifically binds and inhibits thioredoxin reductase 1 to induce apoptosis in colon cancer. Cancer Lett. 2018, 412. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.L.; Wang, X.Y.; Bai, M.; Zhang, X.; Song, S.J.; Yao, G.D. Sesquiterpene lactones from Elephantopus scaber exhibit cytotoxic effects on glioma cells by targeting GSTP1. Bioorg. Chem. 2022, 129. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Hu, Q.; Liu, L.; Yuan, Y.; Zhang, C.; Tang, J.; Shen, X. Eupalinolide B attenuates lipopolysaccharide-induced acute lung injury through inhibition of NF-κB and MAPKs signaling by targeting TAK1 protein. Int. Immunopharmacol. 2022, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, L.; Yang, Q.; Duan, A.; Ju, X.; Cai, B.; Chen, L.; An, T.; Li, Y. Parthenolide inhibits ubiquitin-specific peptidase 7 (USP7), Wnt signaling, and colorectal cancer cell growth. J. Biol. Chem. 2020, 295. [Google Scholar] [CrossRef]
- Berdan, C.A.; Ho, R.; Lehtola, H.S.; To, M.; Hu, X.; Huffman, T.R.; Petri, Y.; Altobelli, C.R.; Demeulenaere, S.G.; Olzmann, J.A.; et al. Parthenolide Covalently Targets and Inhibits Focal Adhesion Kinase in Breast Cancer Cells. Cell Chem. Biol. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; McGowan, A.; Dinatale, G.J.; Chiramanewong, T.; Cai, T.; Connor, R.E. Hsp72 Is an Intracellular Target of the α,β-Unsaturated Sesquiterpene Lactone, Parthenolide. ACS Omega 2017, 2. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Lindenmeyer, M.T.; Merfort, I. Role of cysteine residues of p65/NF-κB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ju, X.; Yang, Q.; Zhu, Y.; Fan, D.; Su, G.; Kong, L.; Li, Y. USP47 maintains the stemness of colorectal cancer cells and is inhibited by parthenolide. Biochem. Biophys. Res. Commun. 2021, 562. [Google Scholar] [CrossRef] [PubMed]
- El Gaafary, M.; Morad, S.A.F.; Schmiech, M.; Syrovets, T.; Simmet, T. Arglabin, an EGFR receptor tyrosine kinase inhibitor, suppresses proliferation and induces apoptosis in prostate cancer cells. Biomed. Pharmacother. 2022, 156. [Google Scholar] [CrossRef] [PubMed]
- La, C.; Li, M.; Wang, Z.; Liu, T.; Zeng, Q.; Sun, P.; Ren, Z.; Ye, C.; Liu, Q.; Wang, Y. Isolation and anti-neuroinflammation activity of sesquiterpenoids from Artemisia argyi: computational simulation and experimental verification. BMC Complement. Med. Ther. 2024, 24, 264. [Google Scholar] [CrossRef] [PubMed]
- Pyun, H.; Kang, U.; Seo, E.K.; Lee, K. Dehydrocostus lactone, a sesquiterpene from Saussurea lappa Clarke, suppresses allergic airway inflammation by binding to dimerized translationally controlled tumor protein. Phytomedicine 2018, 43. [Google Scholar] [CrossRef]
- Wang, M.R.; Huang, L.F.; Guo, C.; Yang, J.; Dong, S.; Tang, J.J.; Gao, J.M. Identification of NLRP3 as a covalent target of 1,6-O,O-diacetylbritannilactone against neuroinflammation by quantitative thiol reactivity profiling (QTRP). Bioorg. Chem. 2022, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Kuang, S.; Wang, Y.; Sun, X.X.; Gu, Y.; Hu, L.H.; Yu, Q. Bigelovin inhibits STAT3 signaling by inactivating JAK2 and induces apoptosis in human cancer cells. Acta Pharmacol. Sin. 2015, 36. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Maryam, A.; Saleem, M.Z.; Shakir, H.A.; Qazi, J.I.; Li, Y.; Ma, T. Brevilin A induces ROS-dependent apoptosis and suppresses STAT3 activation by direct binding in human lung cancer cells. J. Cancer 2020, 11. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Jiang, Y.; Yuan, Y.; Yang, L.; Hu, Q.; Tang, J.; Meng, X.; Xie, C.; Shen, X. Brevilin A Ameliorates Acute Lung Injury and Inflammation Through Inhibition of NF-κB Signaling via Targeting IKKα/β. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhu, J.; Teng, L.; Chen, C.; Bi, L.; Chen, W. Britannin inhibits hepatocellular carcinoma development and metastasis through the GSK-3β/β-catenin signaling pathway. Phytomedicine 2024, 135, 156126. [Google Scholar] [CrossRef] [PubMed]
- Büchele, B.; Zugmaier, W.; Lunov, O.; Syrovets, T.; Merfort, I.; Simmet, T. Surface plasmon resonance analysis of nuclear factor-κB protein interactions with the sesquiterpene lactone helenalin. Anal. Biochem. 2010, 401. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Q.; Wen, T.; Luo, L.; Liu, L.; Wang, L.; Shen, X. Arteannuin B, a sesquiterpene lactone from Artemisia annua, attenuates inflammatory response by inhibiting the ubiquitin-conjugating enzyme UBE2D3-mediated NF-κB activation. Phytomedicine 2024, 124. [Google Scholar] [CrossRef]
- Tang, P.; Zhao, S.; Wang, X.; Wang, S.; Wang, Y.; Kong, L.; Luo, J. Chloranthalactone B covalently binds to the NACHT domain of NLRP3 to attenuate NLRP3-driven inflammation. Biochem. Pharmacol. 2024, 226, 116360. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Yan, Q.L.; Bai, M.; Liu, Q.; Song, S.J.; Yao, G.D. Deoxyelephantopin, a germacrane-type sesquiterpene lactone from Elephantopus scaber, induces mitochondrial apoptosis of hepatocarcinoma cells by targeting Hsp90α in vitro and in vivo. Phyther. Res. 2023, 37. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, R.; Serba, C.; Abegg, D.; Hoch, D.G.; Adibekian, A.; Winssinger, N. Divergent synthesis and identification of the cellular targets of deoxyelephantopins. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Hong, L.; Chen, J.; Wu, F.; Wu, F.; Shen, X.; Zheng, P.; Shao, R.; Lu, K.; Liu, Z.; Chen, D.; et al. Isodeoxyelephantopin Inactivates Thioredoxin Reductase 1 and Activates ROS-Mediated JNK Signaling Pathway to Exacerbate Cisplatin Effectiveness in Human Colon Cancer Cells. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Voli, A.; Mozzicafreddo, M.; Pollastro, F.; Tosco, A.; Monti, M.C. Targeting phosphoglycerate kinases by tatridin A, a natural sesquiterpenoid endowed with anti-cancer activity, using a proteomic platform. Front. Mol. Biosci. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Patel, M.; Ruzevick, J.; Jackson, C.; Lim, M. STAT3 Activation in Glioblastoma: Biochemical and Therapeutic Implications. Cancers (Basel). 2014, 6, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Takaesu, G.; Ninomiya-Tsuji, J.; Kishida, S.; Li, X.; Stark, G.R.; Matsumoto, K. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol. Cell. Biol. 2001, 21, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
| Type | Compound | Protein | Сonstant, μM | Method | Ref |
| Endorepoxide-bearing | Artemisinin | MD2 | KD1 = 2.6 | Fluorescence titrations, thermal shift assay | [169] |
| Artemisitene | FDFT1 | KD = 165 | Thermal shift assay, SPR2 | [170] | |
| DAMN | ERRα | - | TR-FRET | [68] | |
| PI3K-β | - | Computational approaches | [171] | ||
| Eudesmanes | Alantolactone | AKR1C1 | KD = 11.8 | SPR, enzyme activity assay | [172] |
| Isoalantolactone | STAT3 | KD = 100 | Thermal shift assay, SPR | [173] | |
| Germacranolides |
Costunolide derivative D5 | PKM2 | KD = 0.018 | Thermal shift assay, SPR | [174] |
| Costunolide |
CaMKII | KD = 21.57 | DARTS3, thermal shift assay | [175] | |
| CDK2 | KD = 32.02 | DARTS, thermal shift assay, SPR | [176] | ||
| TrxR1 | - | SPR, enzyme activity assay | [177] | ||
| Elephantopinolide A Cis-scabertopin Elephantopinolide F |
GSTP1 | - | Thermal shift assay | [178] | |
| Eupalinolide B | TAK1 | - | Thermal shift assay | [179] | |
| Parthenolide | USP7 | - | Thermal shift assay, SPR, enzyme activity assay | [180] | |
| FAK1 | - | Proteomics, enzyme activity assay | [181] | ||
| HSP72 | - | LC-MS/MS | [182] | ||
| NFκB | - | EMSA | [183] | ||
| USP47 | IC504 = 24.97 | Thermal shift assay, enzyme activity assay | [184] | ||
| Parthenolide dimer | PKM2 | - | Thermal shift assay | [47] | |
| Guianolides and pseudoguaianolides | Arglabin | EGFR | - | Phospho-RTK array | [185] |
| Argyinolide S | JAK1 | - | DARTS | [186] | |
| Dehydrocostus lactone | TCTP | KD = 5.33 | SPR | [187] | |
| DMAMCL | PAI-1 | KD = 2310 | Thermal shift assay, SPR, pull-down | [56] | |
| STAT3 | - | Pull-down | [15] | ||
| IKKβ | - | Pull-down, LC-MS/MS | [57] | ||
| Micheliolide | PKM2 | - | Pull-down assay | [21] | |
| 1,6-O,O-diacetylbritannilactone | NLRP3 | - | Thermal shift assay | [188] | |
| Bigelovin | JAK2 | IC50 = 44.24 | Enzyme activity assay | [189] | |
| Brevilin A | STAT3 | KD = 0.01 | SPR | [190] | |
| IKKα/β | - | Thermal shift assay, LC-MS/MS | [191] | ||
| Britannin | GSK-3β | KD = 30.1 | Enzyme activity assay, SPR | [192] | |
| Helenalin | NF-κB | KD = 4.8 | SPR | [193] | |
| Others |
4 | STAT3 | - | Nuclear magnetic resonance | [73] |
| Arteannuin B | UBE2D3 | KD = 1.2 | Thermal shift assay, DARTS, LC-MS/MS | [194] | |
| Chloranthalactone B | NLRP3 | KD = 10.3 | Pull-down, DARTS, thermal shift assay | [195] | |
| Deoxyelephantopin | Hsp90α | - | DARTS | [196] | |
| PPARγ | - | Enzyme activity assay | [197] | ||
| Isodeoxyelephantopin | TrxR1 | - | Enzyme activity assay | [198] | |
| Tatridin A | PGK1 | IC50 = 3.76 | DARTS, enzyme activity assay | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





