Trial registration number: NCT05743881
Background
Respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) are common respiratory pathogens of the
Pneumoviridae family that have similar seasonality and overlapping clinical and epidemiologic characteristics, representing significant public health threats to young children [
1,
2,
3,
4]. In pediatric populations, RSV is the most common etiological pathogen associated with lower respiratory tract illness (LRTI), followed by hMPV [
5]. Each year, RSV- and hMPV-associated LRTI are estimated to lead to a respective 3.6 million and 643,000 hospitalizations and 101,400 and 16,100 deaths in young children [
1,
2]. Furthermore, LRTI during infancy and early childhood is associated with an increased risk of chronic respiratory disorders, such as asthma, later in life [
6,
7,
8].
There are currently no active immunizations approved for RSV or hMPV in infants or children [
9,
10,
11]. Maternal immunization with the RSVpreF vaccine (Abrysvo; Pfizer, Inc., New York, NY) is recommended for pregnant women in the United States and elsewhere to prevent severe RSV-associated LRTI in their infants younger than 6 months [
12,
13,
14]. Monoclonal antibodies, nirsevimab and palivizumab, are also available as prophylaxis for RSV-LRTI; in the United States, nirsevimab is recommended for infants younger than 8 months born during or entering their first RSV season, and high-risk children aged 8 to 19 months entering their second RSV season [
15]. For hMPV, no maternal vaccine or infant prophylaxis are available, although studies have suggested that passively acquired maternal hMPV antibodies may provide transient protection to infants [
16,
17,
18].
Most children are seropositive for RSV by 2 years of age, and for hMPV by 5 years of age, reflecting the ubiquity of these childhood infections [
18,
19]. Stabilization of the RSV F glycoprotein in the prefusion (preF) conformation has advanced the development of RSV vaccines, given that this conformation is the primary target of potent RSV neutralizing antibodies (nAb)s and is highly conserved across the 2 RSV subtypes (A and B) [
20,
21,
22]. An mRNA-based vaccine encoding membrane-anchored RSV preF (mRNA-1345; mRESVIA; Moderna, Inc., Cambridge, MA) is licensed for the prevention of RSV-lower respiratory tract disease (LRTD) in adults aged ≥60 years [
23], confirming the efficacy of this vaccine strategy. In the pivotal phase 3 study in adults at least 60 years of age, mRNA-1345 demonstrated 78.7% and 80.9% vaccine efficacy (VE) against RSV-LRTD with ≥2 and ≥3 signs or symptoms, respectively, through a median follow-up of 3.7 months, with a favorable benefit:risk profile [
23,
24]. Efficacy was accompanied by an increase in nAb responses in mRNA-1345 recipients, increasing by 8.4-fold (RSV-A) and 5.1-fold (RSV-B) at Day 29 relatively to baseline [
25]. The vaccine also induces durable responses, with VE evident through a median of 18.8 months of extended follow-up [
26]; nAb responses also remained elevated relative to baseline to 12 months in a phase 1 trial in adults [
27]. Additional studies of adults in a phase 1 study of mRNA-1345 demonstrated that vaccination induces RSV-specific T-cell responses (predominantly type 1 T helper (Th1) biased) that remained detectable through 3 months of follow-up [
28].
All adults in the mRNA-1345 phase 3 efficacy trial with available data were seropositive for RSV at baseline [
25]. mRNA COVID-19 vaccines have also proven efficacious in protecting individuals seronegative for SARS-CoV-2, from infants to adults. Two doses of mRNA-1273 (Spikevax; Moderna, Inc., Cambridge, MA) or BNT162b2 (Comirnaty; Pfizer, Inc., New York, NY) were shown to be efficacious against COVID-19 and licensed in individuals as young as 6 months [
29,
30,
31,
32,
33].
Clinical development of infant RSV vaccines has proceeded in a careful and stepwise fashion (from adults to seropositive children, and then to seronegative infants) following the occurrence of enhanced respiratory disease (ERD) in a field trial of a formalin-inactivated RSV (FI-RSV) vaccine in the 1960s [
34]. In this trial, excess hospitalizations from RSV were observed in previously RSV-naïve infants after receipt of the FI-RSV vaccine. This ERD phenomenon was only observed in RSV-seronegative infants [
35]. The protective role of nAbs in infant RSV is clear, and the failure of the FI-RSV vaccine to induce robust nAb responses likely played a role in the ERD phenomenon [
35,
36]. Other potential mechanisms of the observed vaccine-associated ERD include potential imbalance in type 2 T helper (Th2) responses, infiltration of neutrophils and eosinophils into airways, a failure to elicit cytotoxic CD8+ T cells, low avidity antibodies, immune complex deposition, and complement activation [
37,
38]. In addition to the stepwise age de-escalation approach to clinical evaluation, thorough investigation of candidate RSV vaccines in well-characterized preclinical models is warranted [
39].
After demonstrating safety and immunogenicity in adults, pediatric development of mRNA-1345 began with a phase 1 trial in RSV-seropositive children aged 1 to 4 years. mRNA-1345 was found to be well-tolerated, boosted RSV nAbs, and elicited preF-biased bAbs [
40]. The safety and immunogenicity of the hMPV component of mRNA-1365, an investigational combination RSV/hMPV mRNA-based vaccine, was also previously evaluated within a mRNA-based combination hMPV/PIV3 vaccine in phase 1 studies among healthy adults 18 to 49 years [
41] and baseline-seropositive children aged 1 to 4 years [
42]. Together with nonclinical studies in rodent models, which demonstrated that RSV mRNA vaccines induced robust nAb and preF-biased binding (bAb) responses, elicited a Th1-biased cellular response, and provided dose-dependent protection from RSV challenge without enhanced lung pathology [
43], the clinical studies in seropositive children aged 1 to 4 years allowed the progression of mRNA-1345 and mRNA-1365 to clinical studies in RSV-naïve children under 2 years, per established guidelines [
44,
45,
46].
This report summarizes data from a multipart phase 1 trial designed to evaluate the safety and immunogenicity of mRNA-1345 and mRNA-1365 in RSV naïve and experienced children, conducted with oversight from an independent Data Safety Monitoring Board (DSMB). The study initiated with Part A, in which children aged 8 to 23 months were randomized to receive mRNA vaccines or placebo and were observed for a full RSV season. Following DSMB review of the Part A safety and immunogenicity findings, the study age de-escalated to children aged 5 to 7 months (Part B). Findings on mRNA-1345 and mRNA-1365 in children aged 5 to 23 months from Parts A and B of the study are reported herein, with a focus on safety, RSV surveillance, and RSV-specific immune responses. This report summarizes findings for the study through a data cutoff of October 15, 2024.
Methods
Trial Design and Participants
This is a multi-part phase 1 trial in participants aged 5 to 23 months (NCT05743881), with enrollment planned in Australia, Canada, Panama, Poland, South Africa, Spain, the United Kingdom, and the United States. Part A and B are randomized, observer-blind, placebo-controlled, age de-escalation and dose-escalation studies evaluating mRNA-1345 and mRNA-1365 (Figure S1); Part C is an open-label extension evaluating mRNA-1345 administered to participants with, or without, prior exposure to nirsevimab and will be reported separately. The study was designed to age de-escalate and dose-escalate in a stepwise approach gated by DSMB reviews. The trial employed active surveillance for RSV and hMPV disease (including weekly prompts for symptom surveillance during the RSV/hMPV season and visits for investigator assessment) and had conservative pause rules, including for ≥2 cases of severe RSV/hMPV-LRTI (see Supplement for additional details). Additionally, the DSMB reviewed unblinded data on at least a monthly basis after commencement of Part B, with a specific focus on cases of RSV/hMPV-LRTI.
Recruitment in both Parts A and B of the study was timed to fall before the local RSV season to allow for post-vaccination case monitoring. Part A enrolled healthy children aged 8 to 23 months; inclusion and exclusion criteria are shown in the Supplement. Participants across 2 cohorts were randomized in equal numbers via interactive response technology to receive a 3-dose series (administered on Day 1, Day 57 [Month 2], and Day 113 [Month 4]) of mRNA-1345 30 μg (cohort 1), mRNA-1365 30 μg (cohort 2), or saline placebo; 45 participants were planned for each cohort (30:15 [mRNA-1345:placebo] in cohort 1; 30:15 [mRNA-1365:placebo] in cohort 2). After vaccination, the plan was to follow participants through 2 years.
Age de-escalation to Part B of the study was dependent on DSMB recommendations following their review of immunogenicity data and safety data through a complete RSV season from Part A. In Part B, healthy participants aged 5 to 7 months across 4 cohorts were to be enrolled to evaluate escalating dose levels of mRNA-1345 and mRNA-1365 versus placebo. Participants were initially randomized to receive dose levels of 15 μg (cohorts 3 and 4), with dose-escalation to the first 30-µg injections (cohorts 5 and 6) commencing after DSMB review of safety data through 7 days after first 15-µg injections in cohorts 3 and 4. Inclusion and exclusion criteria are shown in the Supplement. Participants were randomly assigned using interactive response technology in equal numbers to receive a 3-dose series (Day 1, Day 57, and Day 113) of mRNA-1345, mRNA-1365, or saline placebo. To enrich for RSV-naïve participants, enrollment occurred before high local RSV activity. Participant follow up was planned through 2 years after vaccination.
The protocol and other relevant documents were approved by the central institutional review board (IRB), and the trial was conducted in accordance with the protocol, applicable laws and regulatory requirements, the International Council for Harmonization Good Clinical Practice guidelines, and the ethical principles derived from the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines. Written informed consent for participation in the study was provided for all participants by the participants’ parent(s)/legally authorized representative(s) before the conduct of study procedures.
Trial Vaccines
mRNA-1345 is a lipid nanoparticle (LNP) formulation comprising a single mRNA sequence encoding the RSV F glycoprotein stabilized in the preF conformation [
24]. mRNA-1365 is an investigational combination vaccine with an LNP formulation composed of 2 distinct mRNA sequences, 1 that encodes the membrane-anchored RSV F glycoprotein stabilized in the preF conformation, and 1 that encodes the membrane-anchored native hMPV F glycoprotein. Vaccine dose levels that were evaluated were 15 µg or 30 µg; for mRNA-1365, these doses contained half each of the RSV and hMPV encoding components. The placebo consisted of 0.9% sodium chloride (normal saline). Vaccine or placebo was administered as an intramuscular injection in the arm or leg at scheduled time points.
Study Objectives
The primary objective of this phase 1 study was to evaluate the safety and reactogenicity of mRNA-1365 and mRNA-1345, administered as a 3-injection series in healthy children aged 5 to 23 months. Secondary objectives were to evaluate the occurrence of clinical RSV or hMPV infections in study participants, and the antibody- and cellular-mediated immune responses after vaccination.
Safety and Reactogenicity Assessments
Safety assessments included solicited local and systemic adverse reactions (ARs) within 7 days after injection; unsolicited adverse events (AEs) within 28 days after injection; and serious AEs (SAEs), AEs of special interest (AESIs), medically attended AEs (MAAEs), and AEs leading to study discontinuation throughout the study. The AESIs for this study were defined as thrombocytopenia; new onset or worsening of Guillain-Barre Syndrome, acute disseminated encephalomyelitis, Bell’s palsy, or seizures; anaphylaxis; myocarditis/pericarditis; and disease caused by RSV or hMPV. The occurrence of solicited ARs were recorded daily by the participants’ parent(s)/legally authorized representative(s) via an eDiary and graded on a 1 to 4 scale based on the grading scale for pediatric participants enrolled in preventative vaccine clinical trials (Pediatric Internal Standard; Table S1).
Study staff contacted the participants’ parent(s)/legally authorized representative(s) on a regular basis to monitor for symptomatic RSV or hMPV infections (weekly during the RSV/hMPV seasons; monthly outside the seasons) and requested the immediate reporting of new/worsening symptoms or difficulty breathing or wheezing (Supplement), with in-person visits and nasal swabs taken within 5 days of respiratory symptom onset. Protocol- defined endpoints were the number and percentage of participants with respiratory tract illness (RTI), LRTI, severe LRTI, very severe LRTI, and hospitalizations associated with RSV or hMPV; these were recorded throughout the study and were classified based on World Health Organization (WHO) standardized case definitions for RSV (Table S2). In a post hoc analysis, the number of participants meeting any of the latter 3 categories (severe LRTI, very severe LRTI, and hospitalizations) were combined into a “severe/hospitalized RSV disease” category in order to optimally identify participants with clinically significant RSV-LRTI.
Immunogenicity Assessments
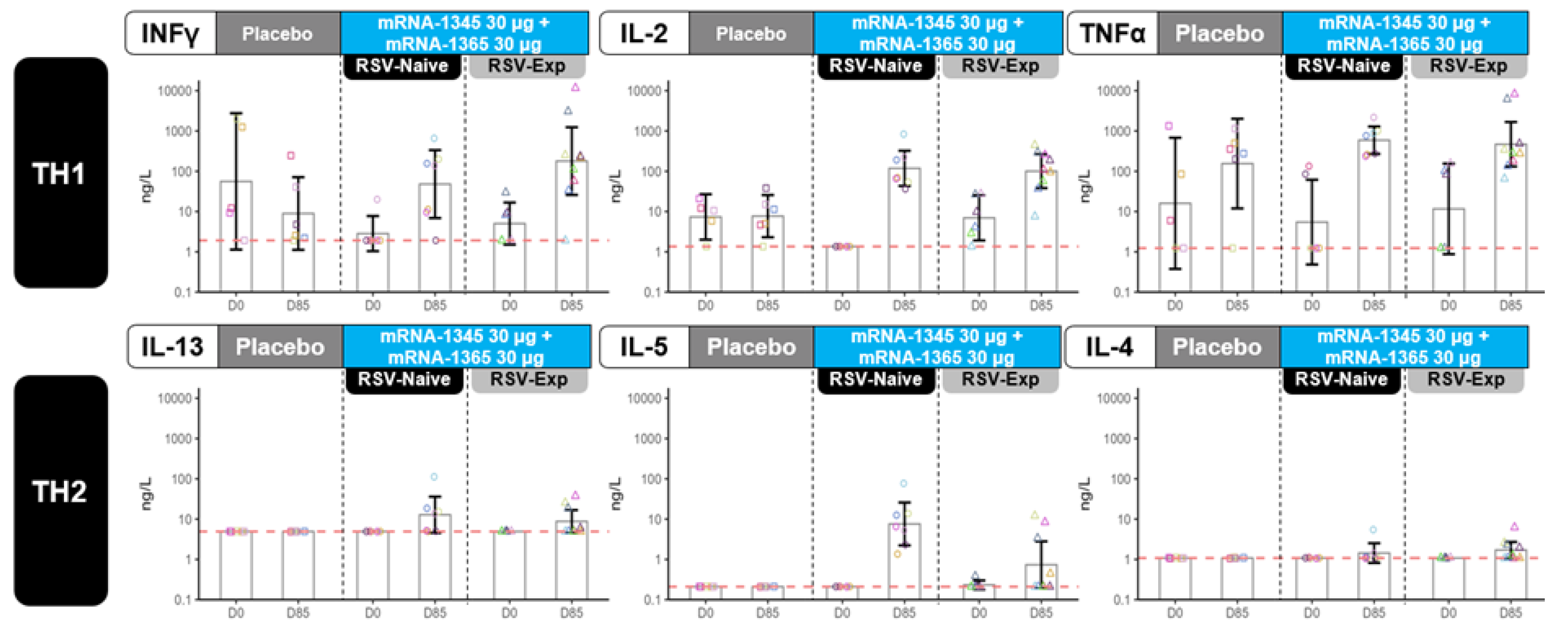
Blood samples for antibody-mediated immunogenicity were scheduled on Days 1, 29, 85, and 141 in Part A and on Days 1, 85, 141, and 365 in Part B. Protocol-defined endpoints included serum nAb titers against RSV (subtypes A and B) and hMPV (subtypes A and B), and bAb IgG concentrations against RSV and hMPV preF and postfusion (postF) conformations (Supplement). This article reports RSV nAb and bAb immune responses up to Day 141 (Part A) and up to Day 85 (Part B; cohorts 3 and 4 only). Blood samples for cell-mediated immunogenicity (CMI) were collected at Days 1 and 85 from a subset of participants ≥12 months of age at enrollment in Part A; because of blood volume constraints, no cell-mediated immunogenicity assessments were planned for participants <12 months). Assessments were performed for RSV- and hMPV-specific cellular responses (Supplement).
Statistical Analysis
No formal hypotheses were tested; the number of participants enrolled was considered sufficient to provide a descriptive summary of the safety and immunogenicity of mRNA-1345 and mRNA-1365. In Part A, with 30 participants receiving each vaccine (mRNA-1345, mRNA-1365, or placebo), the study had 99% probability to observe at least 1 participant with a specific solicited AR, assuming the solicited AR rate was 15% in the mRNA-1345 and mRNA-1365 groups. In Part B, if the underlying RSV severe LRTI rate was 1% per year in placebo recipients and 20% per year in mRNA vaccine recipients, approximately 40 placebo recipients and 80 mRNA vaccine recipients (pooled mRNA-1345 and mRNA-1365) would provide ≥80% power to detect the RSV severe LRTI rate difference between the placebo group and the pooled mRNA vaccine groups, at a 2-sided 5% alpha level.
In each study part, safety analyses were descriptive and were presented by vaccine group using the safety population and solicited safety population (see Supplement). Statistical details on immunogenicity are provided in the Supplement. In Part A, immunogenicity was analyzed in the per-protocol population, consisting of all randomly assigned participants who received study injection; complied with the vaccination schedule, the timing of blood collection for immunogenicity (including a baseline and at least 1 postvaccination assessment); and had no major protocol deviations that impacted the immune response. In Part B, immunogenicity was analyzed in the full analysis population, consisting of all randomized participants who received vaccination. Since RSV serostatus was unknown at the time of enrollment, a post- hoc analysis was performed to define baseline RSV-experienced versus RSV-naïve participants. RSV exposure status was defined based on postF bAb concentrations at baseline using a threshold of 1800 arbitrary units (AU)/mL for participants aged 5 to 7 months at enrollment (Part B), and 200 AU/mL for participants aged 8 to 23 months (Part A). These values were determined based on the assessment of baseline antibody concentrations in this study and other RSV clinical trials conducted by the sponsor in pediatric and adult populations, with the higher threshold in younger participants reflecting the persistence of transplacentally acquired maternal antibodies.
Results
Part A
Participants
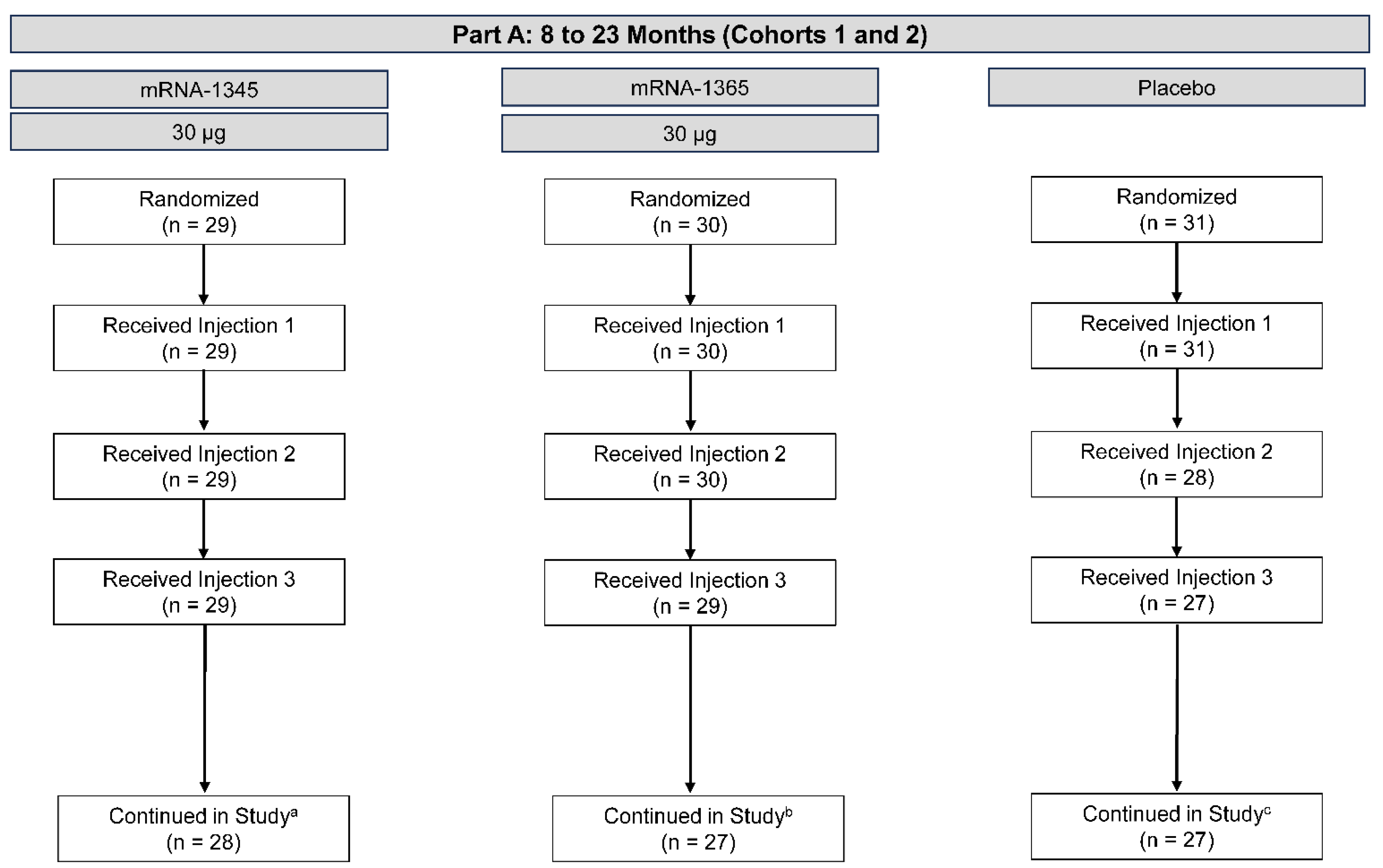
A total of 90 participants aged 8 to 23 months in cohorts 1 and 2 were enrolled between February and June 2023, 25 of whom were from the United States and 65 from Panama. The median age was 16.5 months, 54.4% of participants were male, 25.6% were White, and 85.6% were Hispanic or Latino. These participants were randomized in equal numbers to receive a 3-injection series of mRNA-1345 (30 μg), mRNA-1365 (30 μg), or placebo; 85 (94.4%) received all 3 injections (mRNA-1345, n = 29; mRNA-1365, n = 29; placebo, n = 27) (Figure 1). Demographics and other baseline characteristics were well-matched between groups (Table S3). As of the data cutoff date (October 15, 2024), the median (range) study duration from the first injection was 516 (3-596) days. Three participants discontinued study intervention due to withdrawal of consent (mRNA-1365, n = 1; placebo, n = 2), while 1 placebo participant was withdrawn due to a physician decision related to poor compliance with study activities. One participant discontinuation of the study vaccine was reported due to an AE; this was in a 15-month-old placebo recipient with a history suggestive of seizures. The physician withdrew this participant because of an SAE of grade 2 seizure on Day 26 after the first injection, which resolved the same day and was not considered related to the study injection. At baseline, 36 of the 85 participants (42.4%) with available results had RSV postF bAb GMCs <200 AU/mL and were therefore considered RSV-naïve.
Figure 1.
Participant disposition – Part A: 8 to 23 months (cohorts 1 and 2). Disposition of participants as of the October 15, 2024, data cutoff date. aNo participants in the mRNA-1345 30-µg group discontinued the study intervention. One participant discontinued the study due to physician decision. bOne participant in the mRNA-1365 30-µg group discontinued the study intervention due to withdrawal of consent by parent/guardian. Three participants discontinued the study due to lost to follow-up (n=1) or withdrawal of consent by parent/guardian (n=2). cFour participants in the placebo group discontinued study intervention due to withdrawal of consent by parent/guardian (n=2), physician decision (n=1), or adverse event (n=1). Four participants discontinued the study due to withdrawal of consent by parent/guardian (n=2) or physician decision (n=2). .
Figure 1.
Participant disposition – Part A: 8 to 23 months (cohorts 1 and 2). Disposition of participants as of the October 15, 2024, data cutoff date. aNo participants in the mRNA-1345 30-µg group discontinued the study intervention. One participant discontinued the study due to physician decision. bOne participant in the mRNA-1365 30-µg group discontinued the study intervention due to withdrawal of consent by parent/guardian. Three participants discontinued the study due to lost to follow-up (n=1) or withdrawal of consent by parent/guardian (n=2). cFour participants in the placebo group discontinued study intervention due to withdrawal of consent by parent/guardian (n=2), physician decision (n=1), or adverse event (n=1). Four participants discontinued the study due to withdrawal of consent by parent/guardian (n=2) or physician decision (n=2). .
Safety
Solicited local ARs within 7 days after vaccination were experienced by 24.1%, 23.3%, and 9.7% of participants after first injection of mRNA-1345, mRNA-1365, and placebo, respectively. Solicited systemic ARs were experienced by 31.0%, 30.0%, and 29.0% of participants, respectively. There was no trend towards increased reactogenicity with subsequent dosing. Most solicited ARs within 7 days after any injection were grade 1 or 2 in severity, and the most common local and systemic solicited ARs were injection site pain and irritability/crying (Figure S2). No grade 3 or 4 solicited local ARs were reported; one grade 4 solicited systemic AR of fever was reported on Days 1 to 3 after the first injection of mRNA-1365; the report coincided with an AE of influenza infection in the participant.
Up to October 15, 2024, 7 participants had each experienced 1 SAE: 4 in the mRNA-1345 group, 1 in the mRNA-1365 group, and 2 in the placebo group. One of these SAEs was a hospitalization for RSV pneumonia, and is included in the RSV case surveillance section (see below). One participant in the mRNA-1345 group had an SAE of hMPV pneumonia. The remaining 5 SAEs (gastroenteritis, n=1; bronchiolitis with no pathogen identified, n=1; human rhinovirus bronchiolitis, n=1; cervical adenopathy, n=1; and seizure disorder, n=1 [described above]) were considered unrelated to injection. In addition to the seizure disorder and RSV/hMPV respiratory infections, there were 2 AESIs (mRNA-1345, n=1; placebo, n=1), both of which were febrile convulsions and were not related to study injections. No deaths were reported.
RSV Case Surveillance
Per the protocol, participants in Part A were followed through a full RSV season before age de-escalating to Part B. Through March 2024 (end of the first RSV season), no cases of severe/hospitalized RSV had occurred in participants in Part A. Respiratory surveillance is ongoing, and as of October 15, 2024, a total of 38 cases of symptomatic RSV infections were identified (Table 1). Among baseline RSV-experienced participants (n=49), symptomatic RSV infections were reported in 5 of 13 (38.5%), 7 of 19 (36.8%), and 6 of 17 participants (35.3%) in the mRNA-1345, mRNA-1365, and placebo groups, respectively. Among baseline RSV-naïve participants (n=36), symptomatic RSV infections were reported in 6 of 14 (42.9%), 6 of 9 (66.7%), and 8 of 13 participants (61.5%) in the mRNA-1345, mRNA-1365, and placebo groups, respectively. No symptomatic RSV infections occurred in the 5 participants with unknown RSV-naïve/-experienced status. As of the data cutoff, 1 baseline RSV-naïve participant was hospitalized with respiratory symptoms. This 24-month-old participant received mRNA-1365 and had a positive test for RSV/rhinovirus/enterovirus co-infection during their second season of RSV surveillance (in August 2024, after the prespecified DSMB decision to de-escalate to Part B). The participant received supplemental oxygen and was discharged from the hospital within 2 days.
Table 1.
Summary of symptomatic RSV infections and severe/hospitalized RSV by RSV-naïve and RSV-experienced participants – Part A: 8 to 23 months (cohorts 1 and 2; safety population)a,b.
Table 1.
Summary of symptomatic RSV infections and severe/hospitalized RSV by RSV-naïve and RSV-experienced participants – Part A: 8 to 23 months (cohorts 1 and 2; safety population)a,b.
| |
mRNA vaccines |
|
| |
mRNA-1345
30 µg
(n=29) |
mRNA-1365
30 µg
(n=30) |
Either
vaccine
(n=59) |
Placebo
(n=31) |
| RSV-naïve (n=36) |
| Participants, n |
14 |
9 |
23 |
13 |
| Symptomatic RSV (all severity), n (%) |
6 (42.9) |
6 (66.7) |
12 (52.2) |
8 (61.5) |
| Severe/hospitalized RSV, n (%) |
0 |
1 (11.1)c,d
|
1 (4.3) |
0 |
| RSV-experienced (n=49) |
| Participants, n |
13 |
19 |
32 |
17 |
| Symptomatic RSV (all severity), n (%) |
5 (38.5) |
7 (36.8) |
12 (37.5) |
6 (35.3) |
| Severe/hospitalized RSV, n (%) |
0 |
0 |
0 |
0 |
Immunogenicity
Antibody Responses
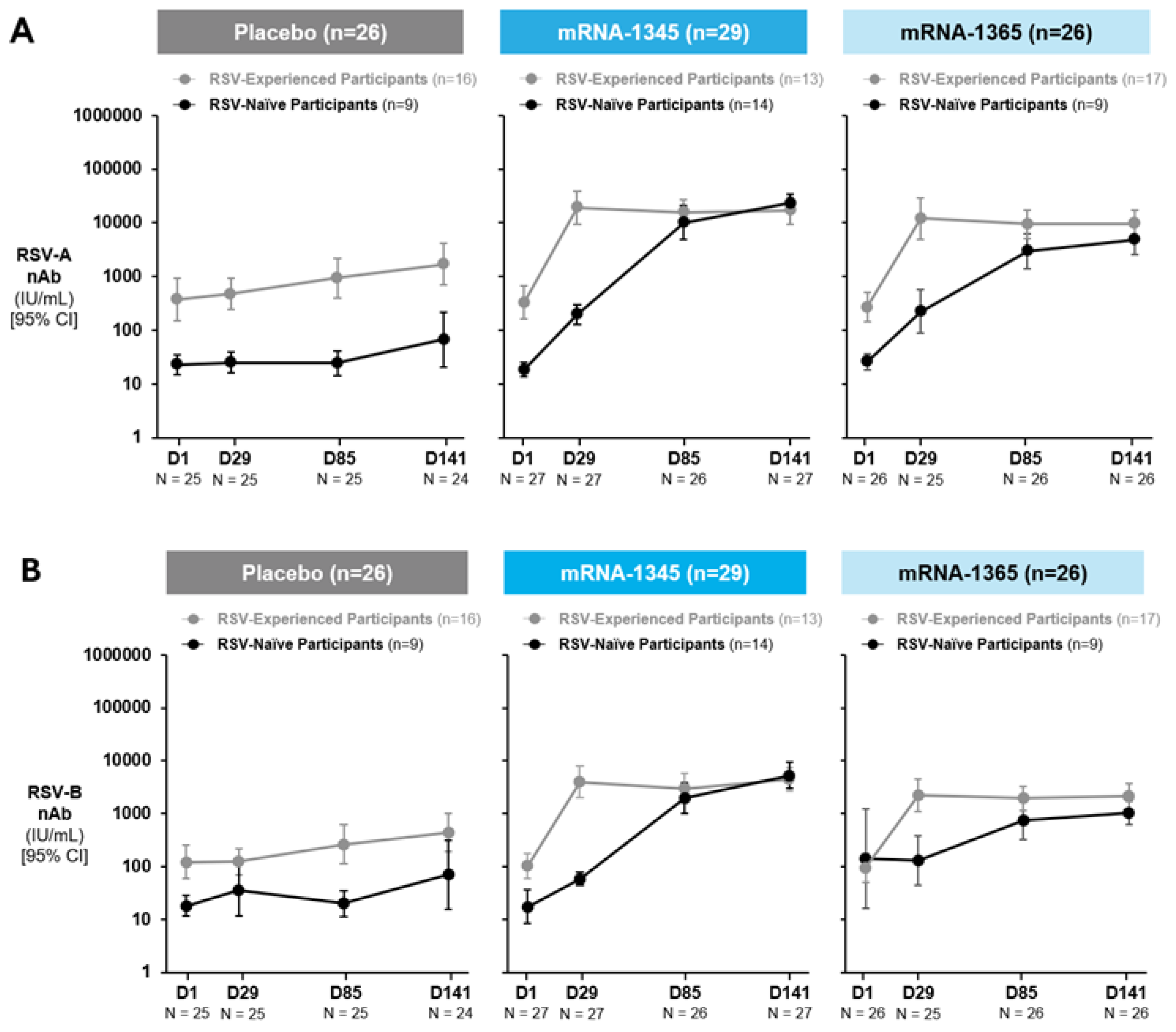
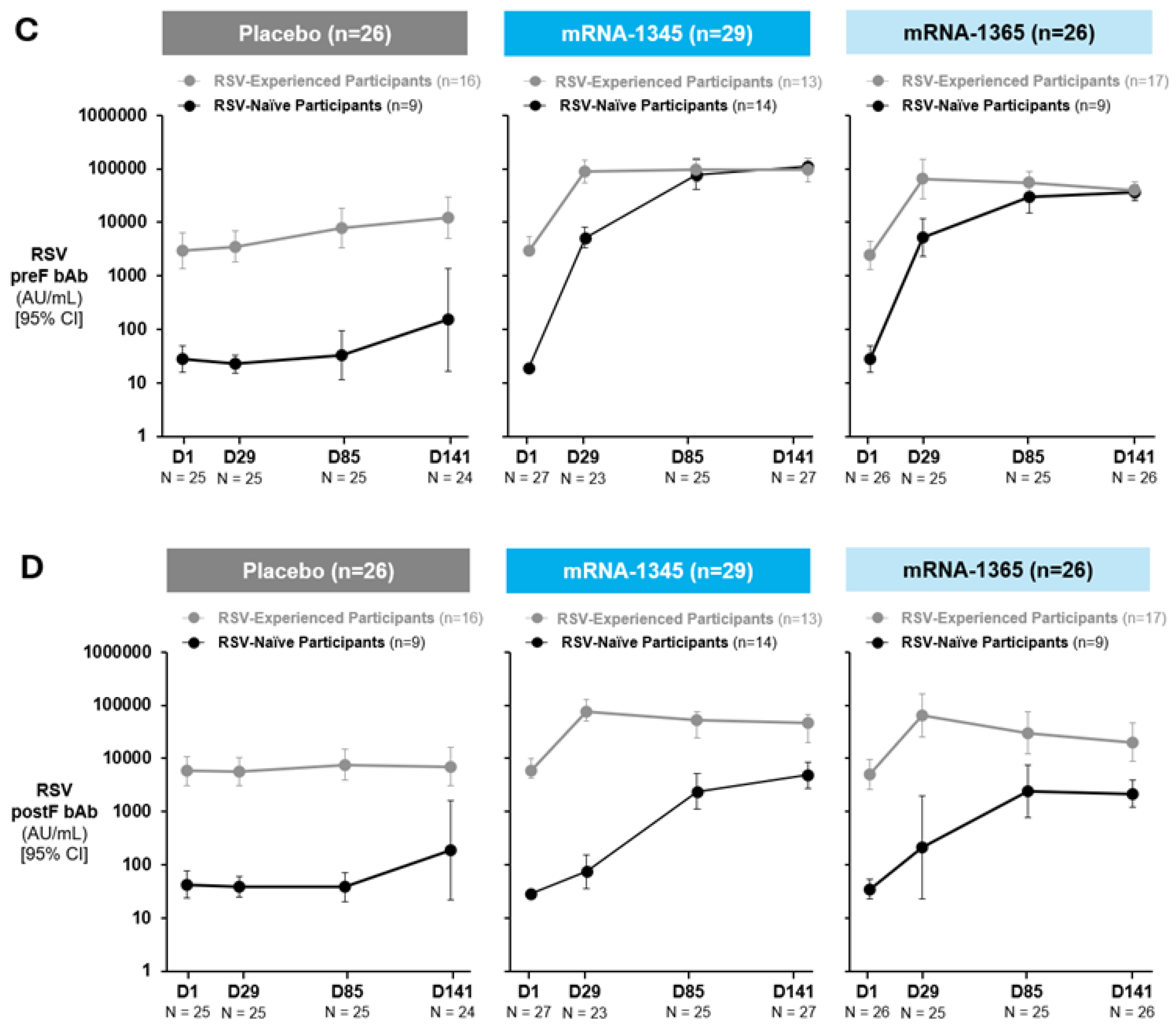
Serum samples collected at baseline and at 1 month post-injection 1 (Day 29), 1 month post-injection 2 (Day 85), and 1 month post-injection 3 (Day 141) were tested for RSV-specific antibodies across study groups (Figure 2A-B; Table S4). Among baseline RSV-experienced participants, a single injection of mRNA-1345 induced a Day 29 nAb geometric mean fold rise (GMFR) from baseline (95% CI) of 58.4 (29.7-114.8) and 38.8 (17.5-86.2) for RSV-A and -B, respectively. Similarly, a single injection of mRNA-1365 in RSV-experienced participants induced a Day 29 nAb GMFR (95% CI) from baseline of 44.3 (20.4-96.3) and 23.4 (7.8-70.2) for RSV-A and -B, respectively. Subsequent injections did not meaningfully further increase nAb titers, and nAb responses remained elevated through Day 141. A similar pattern was observed for bAb responses (Figure 2C-D; Table S5).
Figure 2.
Neutralizing antibody titers against RSV-A (A), RSV-B (B), and binding antibody concentrations against RSV preF (C) and postF (D) – Part A: 8 to 23 months (cohorts 1 and 2; per-protocol population). Study injections occurred at Day 1, Day 57, and Day 113. RSV-experienced is defined as baseline RSV postF bAbs ≥200 AU/mL. RSV-naïve was defined as baseline RSV postF bAbs <200 AU/mL. 95% CI was calculated based on the t-distribution of the log-transformed values or the difference in the log-transformed values for GM titer/concentration, then back-transformed to the original scale for presentation. Numbers indicated below each time point are those with non-missing antibody data. Data cutoff date for nAb data: October 7, 2024. Data cutoff date for bAb data: October 18, 2024. Abbreviations: bAb, binding antibody; nAb, neutralizing antibody; postF, postfusion F; preF, prefusion F; RSV, respiratory syncytial virus.
Figure 2.
Neutralizing antibody titers against RSV-A (A), RSV-B (B), and binding antibody concentrations against RSV preF (C) and postF (D) – Part A: 8 to 23 months (cohorts 1 and 2; per-protocol population). Study injections occurred at Day 1, Day 57, and Day 113. RSV-experienced is defined as baseline RSV postF bAbs ≥200 AU/mL. RSV-naïve was defined as baseline RSV postF bAbs <200 AU/mL. 95% CI was calculated based on the t-distribution of the log-transformed values or the difference in the log-transformed values for GM titer/concentration, then back-transformed to the original scale for presentation. Numbers indicated below each time point are those with non-missing antibody data. Data cutoff date for nAb data: October 7, 2024. Data cutoff date for bAb data: October 18, 2024. Abbreviations: bAb, binding antibody; nAb, neutralizing antibody; postF, postfusion F; preF, prefusion F; RSV, respiratory syncytial virus.
Among participants considered RSV-naïve at baseline, vaccination with mRNA-1345 or mRNA-1365 induced nAb responses after a single injection (Day 29), which continued to increase with subsequent injections. Among recipients of mRNA-1345, the Day 141 nAb GMFR (95% CI) from baseline was 1240.3 (655.7-2346.3) and 302.5 (98.1-932.4) for RSV-A and -B, respectively; geometric mean titers (GMTs) at Day 141 were comparable to maximal nAb GMTs for RSV-experienced participants. Similarly, for RSV-naïve participants receiving mRNA-1365, the Day 141 nAb GMFR (95% CI) from baseline was 184.5 (73.2-465.2) and 7.3 (1.1-47.3) for RSV-A and -B, respectively. A similar pattern was observed for bAb responses in this group (Figure 2C-D; Table S5). In both baseline RSV-experienced and RSV-naïve populations, a preF biased response was observed, with a higher preF/postF ratio in RSV-naïve participants than RSV-experienced through Day 141.
Part B
Participants
The study advanced to Part B following DSMB review of safety data (including results of surveillance for respiratory infections through 1 full RSV season) and immunogenicity (including Day 141 antibody results) in March 2024.
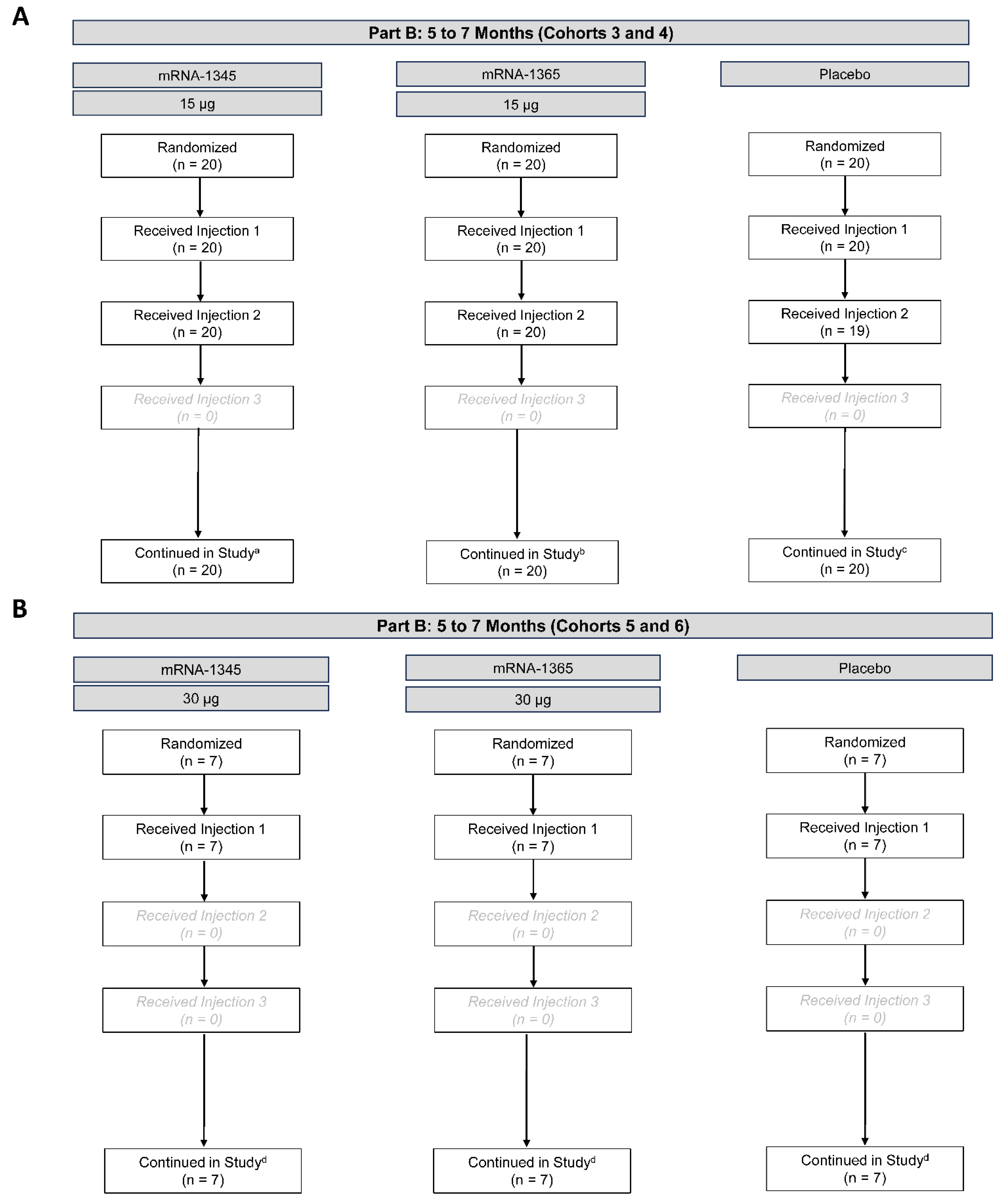
Part B began in May 2024, with enrollment of cohorts 3 and 4. A total of 60 participants were randomized to receive mRNA-1345 15 µg (n=20), mRNA-1365 15 µg (n=20), or placebo (n=20) (Figure 4). Following review of Day 7 safety data from all participants, dose escalation to 30 µg in cohorts 5 and 6 commenced in June 2024, with 21 participants randomized to mRNA-1345 30 µg (n=7), mRNA-1365 30 µg (n=7), or placebo (n=7; Figure 4). On July 17, 2024, a protocol-defined study pause was triggered by identification of 2 events meeting criteria for severe RSV-LRTI in cohorts 3 and 4. At that time, 59 of 60 participants in cohorts 3 and 4 had received 2 of the 3 planned injections (1/20 placebo participants had only received 1 injection) (Figure 4). Participants in cohorts 5 and 6 had received only the first of 3 planned injections across all groups. As defined in the clinical protocol, unblinded data were reviewed by the DSMB, which advised to maintain the pause on further dosing and enrollment to this study but to maintain continued study monitoring.
Figure 4.
Participant disposition – Part B: 5 to 7 months (cohorts 3-4 [A], 5-6 [B]). Disposition of participants as of the October 15, 2024, data cutoff date. Dosing and enrollment to Part B were incomplete due to study pause. aNo participants discontinued the study. 13 participants in the mRNA-1345 15-µg group discontinued the study intervention due to other reason. bNo participants discontinued the study. 13 participants in the mRNA-1365 15-µg group discontinued study intervention due to other reason. cNo participants discontinued the study. 14 participants in the placebo group discontinued study intervention due to other reason (n=13) or adverse event (n=1). dNo participants discontinued the study. 7 participants in each group discontinued study intervention due to other reason.
Figure 4.
Participant disposition – Part B: 5 to 7 months (cohorts 3-4 [A], 5-6 [B]). Disposition of participants as of the October 15, 2024, data cutoff date. Dosing and enrollment to Part B were incomplete due to study pause. aNo participants discontinued the study. 13 participants in the mRNA-1345 15-µg group discontinued the study intervention due to other reason. bNo participants discontinued the study. 13 participants in the mRNA-1365 15-µg group discontinued study intervention due to other reason. cNo participants discontinued the study. 14 participants in the placebo group discontinued study intervention due to other reason (n=13) or adverse event (n=1). dNo participants discontinued the study. 7 participants in each group discontinued study intervention due to other reason.
At the data cutoff date (October 15, 2024), the median (range) duration of follow-up for cohorts 3 and 4 was 160 (155-167) days from the first injection. At enrollment, the median (range) age of participants in cohorts 3 and 4 was 6 (5-7) months, and 48.3% of participants were male (Table S6). All 60 participants in cohorts 3 and 4 were recruited in Panama, and 100% were of Hispanic or Latino ethnicity. Fifty-three of 60 participants (88.3%) were considered RSV-naïve (postF bAb <1800 AU/mL). In cohorts 5 and 6, the median (range) duration of follow-up was 113-119 (96-126) days from the first injection; the median (range) age of participants was 6 (5-7) months, and 33.3% of participants were male. Most (20/21) participants were recruited in Panama, with a single participant recruited in the United Kingdom; 95.2% of cohort 5 and 6 participants were of Hispanic or Latino ethnicity. Immunogenicity data are not available for participants in cohorts 5 and 6; therefore, baseline RSV status cannot yet be determined.
Safety
Within cohorts 3 and 4, solicited local ARs within 7 days after vaccination were experienced by 0%, 20.0%, and 10.0% of participants after the first injection of mRNA-1345 15 µg, mRNA-1365 15 µg, and placebo, respectively; solicited systemic ARs were experienced by 20.0%, 30.0%, and 25.0% of participants, respectively (Figure S3). There was no consistent trend towards increased reactogenicity with subsequent dosing. Most solicited ARs within 7 days after the first and second injections were grade 1 or 2 in severity, and the most common reactions were fever and irritability/crying (Figure S3). No unsolicited AEs reported up to 28 days after any injection were considered related to study injection. No deaths were reported. Reactogenicity data for cohorts 5 and 6 will be presented in a future report.
As of October 15, 2024, 9 SAEs have occurred in 8 participants in cohorts 3 and 4. Of these, 5 were considered related to RSV-LRTI and are described in the following RSV case surveillance section. One mRNA-1365 group participant had an hMPV-LRTI, while the remaining 3 SAEs consisted of episodes of parainfluenza 3 bronchiolitis (placebo, n=1), a sensory processing disorder (placebo, n=1) and gastroenteritis (mRNA-1365 15 µg, n=1), which were considered unrelated to injection. There were no AESIs in cohorts 3 and 4, other than the RSV/hMPV respiratory infections and the sensory processing disorder. There were no SAEs or AESIs in cohorts 5 and 6.
RSV Case Surveillance
In cohorts 3 and 4, from baseline up to the data cutoff (October 15, 2024), symptomatic RSV infections among baseline RSV-experienced participants (n=7) were detected among 0 of 2, 1 of 3 (33%), and 0 of 2 mRNA-1345 15 µg, mRNA-1365 15 µg, and placebo recipients, respectively (Table 2). No severe/hospitalized RSV cases occurred among baseline RSV-experienced participants. Among baseline RSV-naïve participants (n=53), a total of 8 of 18 (44%), 8 of 17 (47%), and 12 of 18 symptomatic RSV infections (67%) were detected among mRNA-1345 15 µg, mRNA-1365 15 µg, and placebo recipients, respectively. Severe/hospitalized RSV cases among baseline RSV-naïve participants occurred in 6 participants in total: 2 of 18 (11%) in mRNA-1345 15-µg recipients, 3 of 17 (18%) in mRNA-1365 15-µg recipients, and 1 of 18 (6%) in placebo recipients. One of the severe/hospitalized cases in mRNA-1365 15 µg recipients had a co-infection with SARS-CoV-2, while the single case in the placebo recipient had a co-infection with hMPV.
Table 2.
Summary of symptomatic RSV infections and severe/hospitalized RSV by RSV-naïve and RSV-experienced participants – Part B (5 to 7 months, cohorts 3 and 4; safety population)a,b.
Table 2.
Summary of symptomatic RSV infections and severe/hospitalized RSV by RSV-naïve and RSV-experienced participants – Part B (5 to 7 months, cohorts 3 and 4; safety population)a,b.
| |
mRNA vaccines |
|
| |
mRNA-1345
15 µg
(n=20) |
mRNA-1365
15 µg
(n=20) |
Either
vaccine
(n=40) |
Placebo
(n=20) |
| RSV-naïve (n=53) |
| Participants, n |
18 |
17 |
35 |
18 |
| Symptomatic RSV (all severity), n (%) |
8 (44.4) |
8 (47.1) |
16 (45.7) |
12 (66.7) |
| Severe/hospitalized RSV, n (%) |
2 (11.1) |
3 (17.6)c
|
5 (14.3)c
|
1 (5.6)d
|
| RSV-experienced (n=7) |
| Participants, n |
2 |
3 |
5 |
2 |
| Symptomatic RSV (all severity), n (%) |
0 |
1 (33.3) |
1 (20.0) |
0 |
| Severe/hospitalized RSV, n (%) |
0 |
0 |
0 |
0 |
Among the 5 vaccine recipients in cohorts 3 and 4 with severe/hospitalized RSV, onset occurred after injection 1 in 1 participant (Day 23 post-injection) and after injection 2 in 4 participants (Days 3-26 post-injection); onset for the 1 placebo recipient was Day 37 post-injection 2 (Figure S4). Of these 6 participants, 5 were hospitalized (including the placebo recipient); 4 of these 5 participants were discharged from the hospital within 5 days. An mRNA-1365 15 µg recipient with RSV required mechanical ventilation for 7 days and was discharged after 16 days. The respiratory illnesses in all children have resolved; the infant who required mechanical ventilation has ongoing systemic hypertension. The sixth vaccine recipient (who received mRNA-1365 15 µg) was treated in the emergency room for tachypnea and subcostal retractions, received oxygen therapy, and recovered without the need for admission to the hospital.
In cohorts 5 and 6, symptomatic RSV infections were detected among 4 of 7 mRNA-1345 30-µg recipients (57.1%), 1 of 7 mRNA-1365 30-µg recipients (14.3%), and 4 of 7 placebo recipients (57.1%). No cases of severe/hospitalized RSV occurred in these participants.
Immunogenicity
Serum samples were collected at baseline and 1 month post-injection 2 (Day 85) for analyses of RSV-specific antibody responses; results are summarized here for cohorts 3 and 4 (recipients received the 15-µg dose). As a majority (88.3%) of cohort 3 and 4 participants were considered RSV-naive, analyses are not presented by baseline RSV status. Participants were instead grouped for analysis based on whether or not they had experienced an RSV infection between baseline and Day 85 (Table 3). This approach allowed the descriptive comparison of immunogenicity after RSV infection alone (placebo group), after vaccination in the absence of infection (mRNA-1345 or mRNA-1365), and after RSV infection and vaccination (mRNA-1345 or mRNA-1365).
RSV infection in placebo participants (n=6) between baseline a n indicates the number of participants with non-missing results at the corresponding visit; for GMFR, n indicates the number of participants with non-missing baseline and Day 85 results.
Table 3.
Neutralizing antibody and binding antibody responses analyzed according to presence or absence of symptomatic RSV infection between baseline and Day 85 – Part B: 5 to 7 months (cohorts 3 and 4; full analysis population).
Table 3.
Neutralizing antibody and binding antibody responses analyzed according to presence or absence of symptomatic RSV infection between baseline and Day 85 – Part B: 5 to 7 months (cohorts 3 and 4; full analysis population).
| |
Neutralizing antibodies |
Binding antibodies |
| |
RSV-A
GMT (IU/mL) |
RSV-B
GMT (IU/mL) |
RSV preF IgG GMC (AU/mL) |
RSV postF IgG GMC (AU/mL) |
| Symptomatic RSV infection between baseline and Day 85 |
| mRNA-1345 15 µg |
|
|
|
|
| Baseline (n=4) |
47.2 |
53.9 |
276.8 |
523.7 |
| Day 85 (n=4) |
15135.3 |
8959.3 |
128076.7 |
8425.7 |
|
GMFRa (95% CI) (n=4) |
320.9 (9.1-11290.2) |
166.3
(4.8-5750.7) |
462.7
(20-10695.8) |
16.1
(0.2-1337.7) |
| mRNA-1365 15 µg |
|
|
|
|
| Baseline (n=8) |
79.5 |
70.5 |
292.6 |
283.0 |
| Day 85 (n=6) |
4436.8 |
3851.6 |
49322.8 |
4474.0 |
|
GMFRa (95% CI) (n=6) |
46.4
(2.6-830.8) |
51.1
(3.8-686.0) |
157.2
(7.8-3163.8) |
13.1
(0.9-192) |
| Placebo |
|
|
|
|
| Baseline (n=8) |
66.7 |
92.1 |
261.5 |
228.8 |
| Day 85 (n=6) |
2007.0 |
557.0 |
8061.2 |
11289.8 |
|
GMFRa (95% CI) (n=6) |
32.1
(15.2-67.8) |
6.3
(2.3-17.4) |
34.7
(16.0-75.4) |
47.2
(20.0-111.2) |
| No symptomatic RSV infection between baseline and Day 85 |
| mRNA-1345 15 µg |
|
|
|
|
| Baseline (n=16) |
104.5 |
80.3 |
500.2 |
429.8 |
| Day 85 (n=16) |
3762.3 |
2102.0 |
31376.6 |
1796.2 |
|
GMFRa (95% CI) (n=16) |
36.0
(19.5-66.3) |
26.2
(15.4-44.5) |
62.7
(35.9-109.5) |
4.2
(2.2-7.9) |
| mRNA-1365 15 µg |
|
|
|
|
| Baseline (n=12) |
91.6 |
62.3 |
129.7 |
144.0 |
| Day 85 (n=11) |
2043.7 |
712.3b
|
23076.1 |
1655.3 |
|
GMFRa (95% CI) (n=11) |
20.5
(4.3-97.8) |
9.5
(2.4-37.4)b
|
148.3
(27.3-806.9) |
9.9
(2.6-38.6) |
| Placebo |
|
|
|
|
| Baseline (n=12) |
170.9 |
123.4 |
546.5 |
794.9 |
| Day 85 (n=11) |
181.6 |
116.7 |
501.0 |
581.0 |
|
GMFRa (95% CI) (n=11) |
1 (0.3-2.9) |
0.9 (0.4-2.4) |
0.9 (0.1-5,5) |
0.7 (0.1-3.6) |
nd Day 85 resulted in a Day 85 nAb GMFR (95% CI) from baseline of 32.1 (15.2-67.8) and 6.3 (2.3-17.4) against RSV-A and -B, respectively. Vaccination alone (in the absence of RSV infection) in mRNA-1345 recipients (n=16) induced Day 85 RSV-A and -B nAb GMFRs (95% CIs), respectively, of 36 (19.5-66.3) and 26.2 (15.4-44.5); in recipients of mRNA-1365, RSV-A and -B Day 85 GMFRs were 20.5 (4.3-97.8) (n=11) and 9.5 (2.4-37.4) (n=10), respectively. Vaccination therefore induced nAb responses similar in magnitude to natural infection.
Participants with RSV infection after at least 1 vaccine injection developed potent nAb responses: mRNA-1345 recipients (n=4) had a Day 85 nAb GMFR (95% CI) of 320.9 (9.1-11290.2) and 166.3 (4.8-5750.7), and mRNA-1365 recipients (n=6) had a Day 85 nAb of 46.4 (2.6-830.8) and 51.1 (3.8-686.0) for RSV-A and -B, respectively.
Similar patterns were observed for measure of preF and postF bAb responses, with a consistent strong preF bias in the vaccine groups (Table 3).
Discussion
As part of the RSV vaccine clinical development program, the phase 1 study summarized here was designed as an age de-escalation study of mRNA-based RSV (mRNA-1345) and investigational RSV/hMPV combination (mRNA-1365) vaccines. Infants and children aged 8 to 23 months were first enrolled (Part A), followed by enrollment of infants aged 5 to 7 months (Part B). Advancement to the younger age group occurred after DSMB review of safety and immunogenicity data from the older age group. The study was designed with pause rules and ongoing oversight of all ongoing safety monitoring by the blinded study team and the unblinded independent DSMB. The DSMB reviewed unblinded data at least monthly during Part B, with a specific focus on cases of RSV/hMPV-LRTI. Analysis of Part A results (in children aged 8 to 23 months) indicated that both vaccines were well-tolerated and induced RSV-specific antibody responses. Surveillance through the end of the 2023-2024 RSV season revealed a substantial number of RSV infections; all were mild and no cases of severe/hospitalized RSV-LRTI occurred, including among participants who were RSV-naive at baseline. The DSMB recommended commencement of Part B for age de-escalation according to established guidelines [
44,
45,
46]. In July 2024, a protocol-defined study pause was initiated due to the detection of 2 cases of severe RSV-LRTI in vaccine recipients in Part B. No further dosing and enrollment have occurred since the study pause, and surveillance for RSV infections continues. Despite a trend for fewer symptomatic RSV infections in Part B in vaccine recipients versus placebo recipients, as of October 15, 2024, 5 of 40 recipients of mRNA-1345 15 µg or mRNA-1365 15 µg (12.5%) have had severe/hospitalized RSV-LRTI, compared with 1 of 20 placebo recipients (5.0%).
Despite recent advances in RSV prevention in infancy, there remains a substantial burden of disease in pediatric populations beyond infancy [
2,
47,
48], which highlights the significant unmet medical need for a pediatric RSV vaccine to provide active immunity. Given the documented efficacy of mRNA vaccines against SARS-CoV-2 in children [
29,
30,
31], and the efficacy of the mRNA-1345 RSV vaccine against RSV-LRTD in older adults [
24], as well as a reassuring nonclinical program [
43], a clinical program in children was undertaken, with careful guidelines for age de-escalation to predominantly RSV-naïve participants aged 5 to 7 months.
Historically, vaccine-associated ERD was observed in RSV-naïve children after administration of an investigational FI-RSV vaccine. Both mRNA-1345 and mRNA-1365 vaccines employ the mRNA-based platform, a technology which presents the preF antigen differently from formalin-inactivated vaccines. Based on the 2016 WHO Consultation on RSV Vaccine Development and the 2017 Food and Drug Administration Vaccines and Related Biological Products Advisory Committee meeting, gene-based vaccines and live-attenuated RSV vaccines would be expected to present a lower risk of RSV–vaccine–induced ERD [
49,
50]. As mRNA-based vaccines that enable intracellular translation of viral antigens, mRNA-1345 and mRNA-1365 also fall into the lower-risk category of vaccines for RSV-vaccine induced ERD. Indeed, administration of RSV preF-encoding vaccines (mRNA-1345 and mRNA-1365) to RSV-naïve infants and children aged 8 to 23 months in this study induced nAbs against both RSV-A and -B responses and a Th1-biased cell-mediated immune response. Further, vaccination of infants aged 5 to 7 months (almost 90% of whom were RSV-naïve) induced nAb responses comparable to those measured after RSV infection alone in placebo recipients. RSV infection following priming with these RSV preF mRNA vaccines induced highly elevated levels of nAbs. Taken together, these findings confirm that mRNA vaccines encoding the preF RSV glycoprotein induce robust nAb responses in RSV-naïve infants. This stands in contrast to studies of infant recipients of the FI-RSV vaccine administered during field trial in the 1960s, in which nAb responses were minimally induced in vaccinated infants [
36].
Notably, ERD was not observed after mRNA-based COVID-19 vaccines in animal models [
51], and COVID-19 vaccine development included monitoring for any signals of potentiation of respiratory disease in both adults and children. Studies of mRNA COVID-19 vaccines in adults demonstrated a Th1 bias of CD4+ responses [
52,
53], and there has been no evidence of ERD after mRNA COVID-19 vaccination, including in seronegative children as young as 6 months [
29,
30,
31].
The theoretical risk of RSV-ERD associated with mRNA-1345 was initially and rigorously assessed in nonclinical rodent studies that demonstrated this risk was low. Further, in Part A of the current trial, no concern for RSV-ERD was identified in children aged 8 to 23 months through an entire RSV season, including in those who were RSV-naïve. Thus, despite meticulous preclinical and clinical investigation in adherence to guidelines, the detection of this increase in severe/hospitalized RSV-LRTI in Part B of this trial highlights the uncertainty regarding the mechanism of RSV-ERD. Despite the induction of a strongly preF- biased bAb response with potent neutralizing activity in RSV-naïve infants aged 5 to 7 months and evidence of a Th1-biased cellular immune response (in children aged 12 to 23 months), the trial observed cases of severe/hospitalized RSV illness that occurred after vaccination in infants aged 5 to 7 months who were RSV naive at baseline. These findings suggest that a different mechanism to that observed with FI-RSV vaccine may be responsible. Additionally, this study may suggest the need to adapt the existing guidelines for RSV pediatric vaccine development. Ongoing immunogenicity assessments and surveillance for respiratory infections are being conducted to better understand responses over time, as well as the distribution of RSV disease among study groups.
Strengths of this phase 1 trial include the randomized, observer-blind, placebo-controlled study design. In addition, the trial employed active RSV and hMPV disease surveillance methods during an intense RSV season and had pre-defined pause rules, including for ≥2 cases of severe RSV-LRTI or severe hMPV-LRTI. The study’s extensive interactions with the DSMB helped drive decision-making, such as age de-escalation and assessment after the enrollment and dosing pause. Additionally, frequent engagement with study investigators allowed for timely assessments that were needed for rapid study adaptations, as well as clear communication of the study status to IRBs, global regulatory authorities, and the families of the children in the study. The study’s small sample size, and the overall weighting to vaccine versus placebo dosing, are limitations. Additionally, at the time of this reporting, surveillance for a full RSV season in Part B has not yet been completed. These factors limit the conclusions that can be drawn from this study. Nevertheless, the suggestion of a higher number of hospitalized/severe RSV disease cases in vaccine versus placebo recipients requires ongoing scrutiny, including planned surveillance through additional complete RSV seasons and further immunologic evaluations.
Overall, vaccination with mRNA-1345 (RSV vaccine) or mRNA-1365 (RSV/hMPV vaccine) increased RSV-A and RSV-B specific nAbs in children aged 8 to 23 months (Part A) who were RSV-naïve and RSV-experienced, as well as induced de novo nAb responses in infants aged 5 to <8 months (Part B) who were RSV-naïve. Vaccination induced a preF-biased bAb response in both study parts, and induced RSV F-specific Th1-biased cellular responses in older children. Study dosing and enrollment have been paused while immunogenicity assessments and surveillance for respiratory infections are ongoing to better understand the increase in severe/hospitalized RSV-LRTI observed among vaccinated, RSV-naïve, young children.
Data Availability
Access to participant-level data presented in the article and supporting clinical documents by qualified external researchers who provide methodologically sound scientific proposals may be available upon reasonable request for products or indications that have been approved by regulators in the relevant markets and subject to review from 24 months after study completion. Such requests can be made to Moderna, Inc., 325 Binney Street, Cambridge, MA 02142; or data_sharing@modernatx.com. A materials transfer and/or data access agreement with the sponsor will be required for accessing shared data. All other relevant data are presented in the paper. Further details about the study are available online at ClinicalTrials.gov NCT05743881.
Author Contributions
MS, SKS, and CAS contributed to the study concept/design; MS and JM collected the data. All authors analyzed/interpreted the data. All authors contributed to the writing and reviewing of the manuscript, as well as approved the final draft. Potential Conflicts of Interest; MDS and JM are employees of Moderna Biotech Distributor UK Ltd., and may hold stock/stock options in the company. JD, CR, NP, SKS, and CAS are employees of Moderna, Inc., and may hold stock/stock options in the company.
Funding
This work was funded by Moderna, Inc.
Acknowledgments
Medical writing and editorial assistance were provided by Jessica Nepomuceno, PhD, Renee Gordon, PhD, Kate Russin, PhD, and Ashlea Inan, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP 2022) guidelines, funded by Moderna, Inc., and under the direction of the authors. The authors also acknowledge the work of 1365 P101 study team, including Sabine Schnyder Ghamloush, Jospeh Whitten, Barbara Jones, Louie Morsy, Vinicius Righi, Sinead Rudden, Riya Joshi, Jenni Mou, Wen Zhou, Huiling Chen, Brandon Sucher, Weijie Zhang, Lingyi Zheng, Honghong Zhou, Imy Chiu, and Laila El Asmar, as well as Dr Rituparna Das. The authors further wish to acknowledge the participants and their families, and the work of the data safety monitoring board and the study investigators.
References
- Li, Y. , et al., Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet, 2022. 399(10340): p. 2047-2064.
- Wang, X. , et al., Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health, 2021. 9(1): p. e33-e43.
- Prevention, C.f.D.C.a. Clinical Overview of RSV. 2024 [cited 2024 ]; Available from: https://www.cdc.gov/rsv/hcp/clinical-overview/index.html. 25 November.
- Centers for Disease Control and Prevention. Human Metapneumovirus: About Human Metapneumovirus, /: 2024 [cited 2024 November 25]; Available from: https, 11 April 2024.
- Ren, L. , et al., Viral infections of the lower respiratory tract. Curr Infect Dis Rep, 2012. 14(3): p. 284-91.
- Deshmukh, H. , et al., Impact of Viral Lower Respiratory Tract Infection (LRTI) in Early Childhood (0-2 Years) on Lung Growth and Development and Lifelong Trajectories of Pulmonary Health: A National Institutes of Health (NIH) Workshop Summary. Pediatr Pulmonol, 2024: p. e27357.
- Tregoning, J.S. and J. Schwarze, Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev, 2010. 23(1): p. 74-98.
- Douros, K. and M.L. Everard, Time to Say Goodbye to Bronchiolitis, Viral Wheeze, Reactive Airways Disease, Wheeze Bronchitis and All That. Front Pediatr, 2020. 8: p. 218.
- Centers for Disease Control and Prevention. Healthcare Providers: RSV Prevention Information, /: 2023 [cited 2024 July 18]; Available from: https, 28 September 2023.
- Centers for Disease Control and Prevention. Human Metapneumovirus (HMPV) About, /: [cited 2024 July 18]; Available from: https, 11 April 2024.
- National Foundation for Infectious Diseases. Respiratory Syncytial Virus (RSV), /: 2024 July 2]; Available from: https, 24 June 2024.
- Kampmann, B., D. Radley, and I. Munjal, Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. Reply. N Engl J Med, 2023. 389(11): p. 1053-1055.
- Centers for Disease Control and Prevention. Healthcare Providers: RSV Vaccination for Pregnant People, /: 2023 [cited 2024 June 17]; Available from: https, 29 September 2023.
- UK Health Security Agency. Complete routine immunisation schedule from 1 September 2024, U: 5]; Available from, 1 September 1294.
- Jones, J.M. , et al., Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep, 2023. 72(34): p. 920-925.
- Wolf, D.G. , et al., High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis, 2003. 188(12): p. 1865-7.
- van den Hoogen, B.G. , et al., A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med, 2001. 7(6): p. 719-24.
- Lu, G. , et al., Large-scale seroprevalence analysis of human metapneumovirus and human respiratory syncytial virus infections in Beijing, China. Virol J, 2011. 8: p. 62.
- Andeweg, S.P. , et al., Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci Rep, 2021. 11(1): p. 8953.
- Crank, M.C. , et al., A proof of concept for structure-based vaccine design targeting RSV in humans. Science, 2019. 365(6452): p. 505-509.
- McLellan, J.S. , et al., Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science, 2013. 342(6158): p. 592-8.
- Graham, B.S., M. S.A. Gilman, and J.S. McLellan, Structure-Based Vaccine Antigen Design. Annu Rev Med, 2019. 70: p. 91-104.
- Package Insert - MRESVIA. 2024 [cited 2024 ]; Available from: https://www.fda.gov/media/179005/download?attachment. 5 December.
- Wilson, E. , et al., Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N Engl J Med, 2023. 389(24): p. 2233-2244.
- Goswami, J. , et al., Humoral Immunogenicity of mRNA-1345 RSV Vaccine in Older Adults. J Infect Dis, 2024.
- Das, R. Update on Moderna’s RSV Vaccine, mRESVIA (mRNA-1345), in Adults ≥60 Years of Age. 2024 [cited 2024 ]; Available from: https://www.cdc.gov/acip/downloads/slides-2024-06-26-28/04-RSV-Adult-Das-508.pdf. 5 December.
- Shaw, C.A. , et al., Safety and Immunogenicity of an mRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase I Clinical Trial in Healthy Older Adults. J Infect Dis, 2024.
- Paris, R. , et al., T-Cell Responses Following Vaccination With an mRNA Respiratory Syncytial Virus Vaccine, mRNA-1345, in Older Adults, in International Federation of Ageing (IFA). 2023: Bangkok, Thailand.
- Anderson, E.J. , et al., Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med, 2022. 387(18): p. 1673-1687.
- Creech, C.B. , et al., Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age. N Engl J Med, 2022. 386(21): p. 2011-2023.
- Ali, K. , et al., Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N Engl J Med, 2021. 385(24): p. 2241-2251.
- Munoz, F.M. , et al., Evaluation of BNT162b2 Covid-19 Vaccine in Children Younger than 5 Years of Age. N Engl J Med, 2023. 388(7): p. 621-634.
- Polack, F.P. , et al., Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med, 2020. 383(27): p. 2603-2615.
- Noor, A. and L.R. Krilov, A Historical Perspective on Respiratory Syncytial Virus Prevention: A Journey Spanning Over Half a Century From the Setback of an Inactive Vaccine Candidate to the Success of Passive Immunization Strategy. J Pediatric Infect Dis Soc, 2024. 13(Supplement_2): p. S103-s109.
- Mejias, A. and O. Ramilo, RSV Prevention Within Reach for Older Infants and Toddlers: The Role of Active Immunization. J Pediatric Infect Dis Soc, 2024. 13(Supplement_2): p. S125-s130.
- Murphy, B.R. and E.E. Walsh, Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol, 1988. 26(8): p. 1595-7.
- Stephens, L.M. , et al., Prefusion F-Based Polyanhydride Nanovaccine Induces Both Humoral and Cell-Mediated Immunity Resulting in Long-Lasting Protection against Respiratory Syncytial Virus. J Immunol, 2021. 206(9): p. 2122-2134.
- Acosta, P.L., M. T. Caballero, and F.P. Polack, Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol, 2016. 23(3): p. 189-195.
- Giersing, B.K. , et al., Report from the World Health Organization's Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 7-9th Sep 2015. Vaccine, 2016. 34(26): p. 2865-2869.
- Snape, M.D. , et al., Phase 1 Safety and Immunogenicity Results of Two Investigational mRNA Vaccines, mRNA-1345, a Respiratory Syncytial Virus Vaccine, and mRNA-1653, a Human Metapneumovirus and Parainfluenza Virus Type 3 Combination Vaccine in Seropositive Young Children. Open Forum Infect Dis, 2023. 10: p. ofad500.2266.
- August, A. , et al. Safety and immunogenicity of an mRNA-based human metapneumovirus and parainfluenza virus type 3 combined vaccine in healthy adults, 2022. [Google Scholar]
- Schnyder Ghamloush, S. , et al., Safety and Immunogenicity of an mRNA-Based hMPV/PIV3 Combination Vaccine in Seropositive Children. Pediatrics, 2024. 153(6).
- Shaw, C.A. , et al., Design and preclinical assessment of mRNA-1345 prefusion F glycoprotein-encoding mRNA vaccine 1 for respiratory syncytial virus. Submitted., 2024.
- World Health Organization. Guidelines on the quality, safety and efficacy of respiratory syncytial virus vaccines, /: 2024 ]; Available from: https, 29 April 2024.
- United States Department of Health and Human Services. Respiratory Syncytial Virus Infection: Developing Antiviral Drugs for Prophylaxis and Treatment Guidance for Industry, /: 2024 ]; Available from: https, 5 December 2024.
- European Medicines Agency. Guideline on the clinical evaluation of medicinal products indicated for the prophylaxis or treatment of respiratory syncytial virus (RSV) disease, /: 2024 ]; Available from: https, 5 December 2024.
- Centers for Disease Control and Prevention. RSV Immunization Guidance for Infants and Young Children, /: 2024 ]; Available from: https, 2 December 2024.
- Li, Z. , et al., Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: a systematic review and meta-analysis. Frontiers in Immunology, 2022. 13.
- Giersing, B.K. , et al., Meeting report: WHO consultation on Respiratory Syncytial Virus (RSV) vaccine development, Geneva, 25-. Vaccine, 2019. 37(50): p. 7355-7362. 26 April.
- Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document: Development of Vaccines for Prevention of RSV Disease in RSV-Naive Infants, /: 2024 ]; Available from: https://public4.pagefreezer.com/content/FDA/01-02-2023T10:30/https, 3 December 2024.
- DiPiazza, A.T. , et al., COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity, 2021. 54(8): p. 1869-1882 e6.
- Jackson, L.A. , et al., An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med, 2020. 383(20): p. 1920-1931.
- Mateus, J. , et al., Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science, 2021. 374(6566): p. eabj9853.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).