1. Introduction
Acute ischemic stroke (AIS) remains a major public health concern, affecting more than 13 million people annually and ranking as a leading cause of disability and mortality worldwide [
1]. Standard treatment protocols such as thrombolysis (TL) and mechanical thrombectomy (MT) have improved outcomes for eligible patients [
2] but often fail to account for individual variability [
3]. As a result, many patients experience suboptimal outcomes, highlighting the need for personalized approaches to AIS treatment.
To address this need, the Stroke-SCORE (Simplified Clinical Outcome Risk Evaluation), a decision-support tool designed to provide personalized treatment recommendations for AIS patients, was developed. Using readily available clinical data, the Stroke-SCORE incorporates key factors such as age, National Institutes of Health Stroke Scale (NIHSS) score at admission, and pre-morbid modified Rankin Scale (pre-mRS) score to help clinicians make more informed decisions. By predicting individual prognoses, the Stroke-SCORE aims to complement existing guidelines, bridging the gap between generalized protocols and individualized care.
2. Materials and Methods
2.1. Study Design and Dataset
This retrospective cohort study was conducted using data from the prospective Transzlációs Idegtudományi Nemzeti Laboratórium (TINL) STROKE-registry. Between February 2023 and September 2024 914 AIS patients were admitted to the Department of Neurology, University of Pécs. After 121 patients with incomplete data were excluded, a total of 793 patients were analyzed. AIS was defined as the sudden onset of a focal neurological deficit lasting more than 24 h without evidence of acute intracranial hemorrhage on imaging. The collected data included patient demographics, clinical characteristics, treatment details, and 90-day outcomes.
2.2. Outcome Assessment
The primary outcome was assessed 90 days after stroke using the modified Rankin Scale (mRS), a well-established measure of functional independence. A favorable outcome was defined as an mRS score of 0-2, whereas an mRS score >2 indicated an unfavorable outcome. Functional outcomes were evaluated during follow-up visits or via structured telephone interviews conducted by trained personnel.
2.3. Development of the Stroke-SCORE
Key predictors of unfavorable outcomes at 90 days were identified via logistic regression analysis and later confirmed via SHapley Additive exPlanations (SHAP) (
Table 1). Age, NIHSS score at admission, and pre-mRS score emerged as the strongest predictors, whereas other factors, such as the plasma-glucose level, the international normalized ratio (INR) at admission, onset-door-time, hypertension, and diabetes mellitus were found to be less influential.
Each factor was assigned weighted points on the basis of its contribution to the outcome probability: age ≥ 80 years: +1 point, NIHSS score at admission > 15: +2 points, and pre-mRS score ≥ 3: +1 point.
A Gradient Boosting Classifier was employed to develop the predictive model due to its capacity to manage complex, nonlinear relationships. The dataset was split into training (80%) and testing (20%) subsets for rigorous evaluation, and features were scaled with StandardScaler to enhance performance. All the statistical analyses were conducted in Python, ensuring replicability and robustness.
The cumulative score stratified patients into low (0–1 points, < 30% probability of unfavorable outcome), moderate (2–3 points, 30–70% probability of unfavorable outcome), and high-risk (4+ points, >70% probability of unfavorable outcome) categories. The cut-off values for age, NIHSS score, and pre-mRS score were data-driven, as supported by the statistical analysis of the initial dataset. The model was internally validated via receiver operating characteristic (ROC) analysis and isotonic regression with bootstrapping.
2.4. Implementation of the Stroke-SCORE
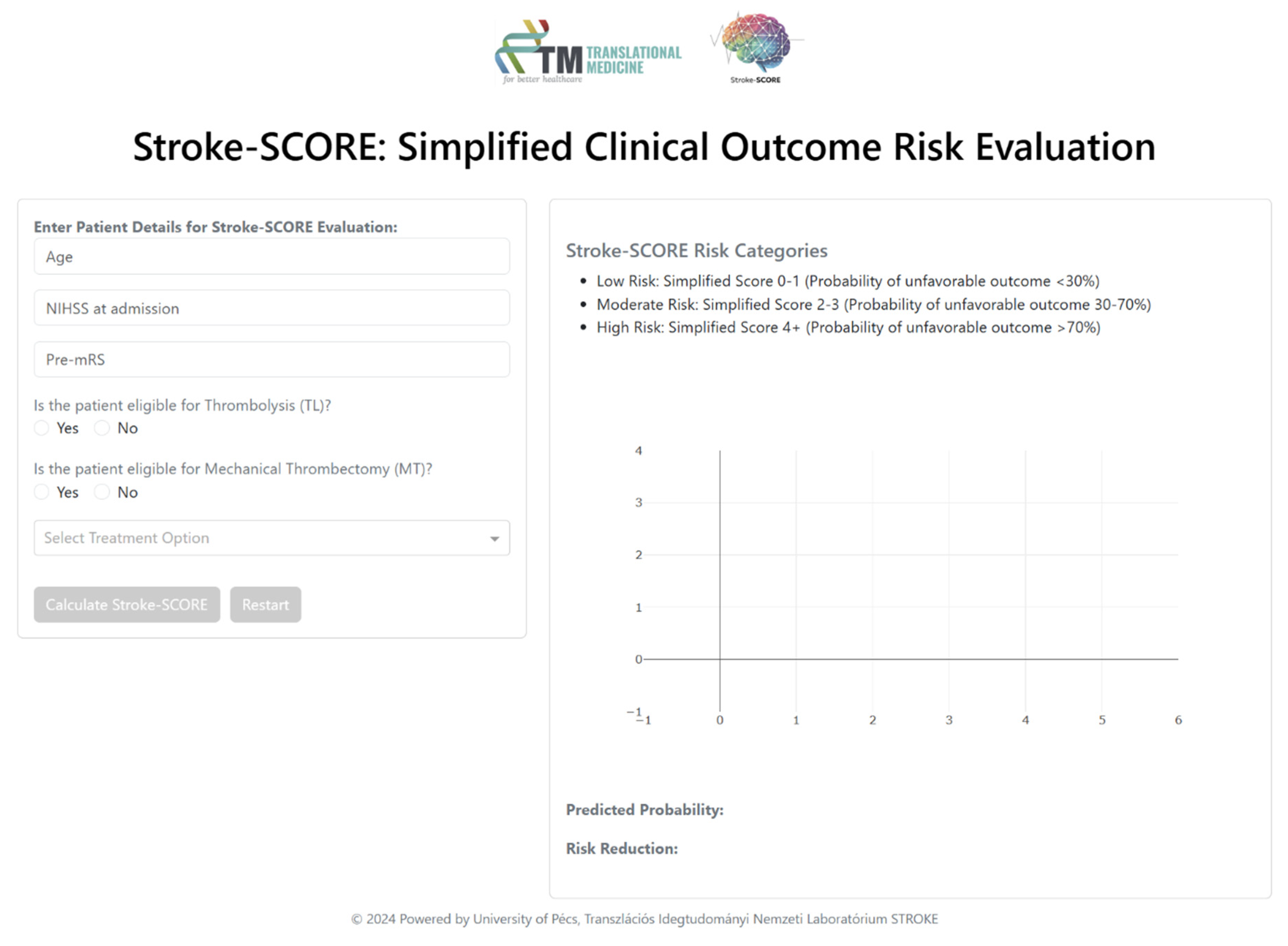
The Stroke-SCORE was integrated into an interactive decision-support tool built with Dash, enabling real-time clinical application (
Figure 1). This interface automatically classifies patients into risk categories, allowing clinicians to assess prognosis efficiently. Additionally, it provides visualizations of the predicted probability of unfavorable outcomes, demonstrating the potential impact of various treatment options. Through interactive decision-making, the tool offers individualized treatment recommendations aimed at minimizing the risk of unfavorable outcomes.
3. Results
3.1. Performance of the Stroke-SCORE
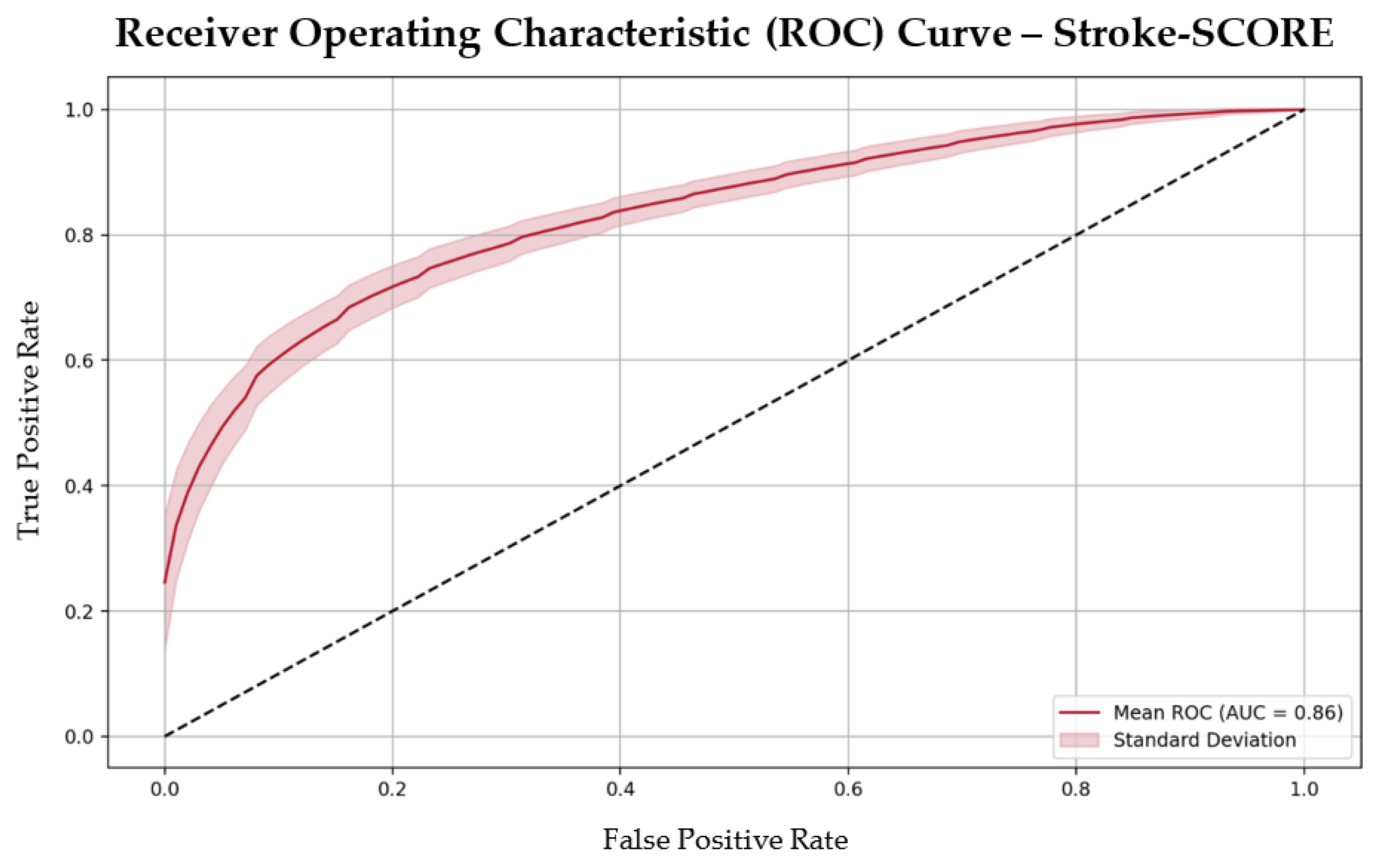
The Stroke-SCORE had a moderate positive correlation with 90-day mRS outcomes, (correlation coefficient 0.51,
p < 0.001) and demonstrated strong predictive accuracy, with an area under the curve (AUC) of 0.86 and an overall accuracy of 80%. The model’s performance metrics included a precision of 0.82, recall of 0.79, sensitivity of 0.79, and specificity of 0.81 (
Figure 2). These metrics highlight the model’s ability to effectively differentiate between patients likely to achieve favorable versus unfavorable outcomes.
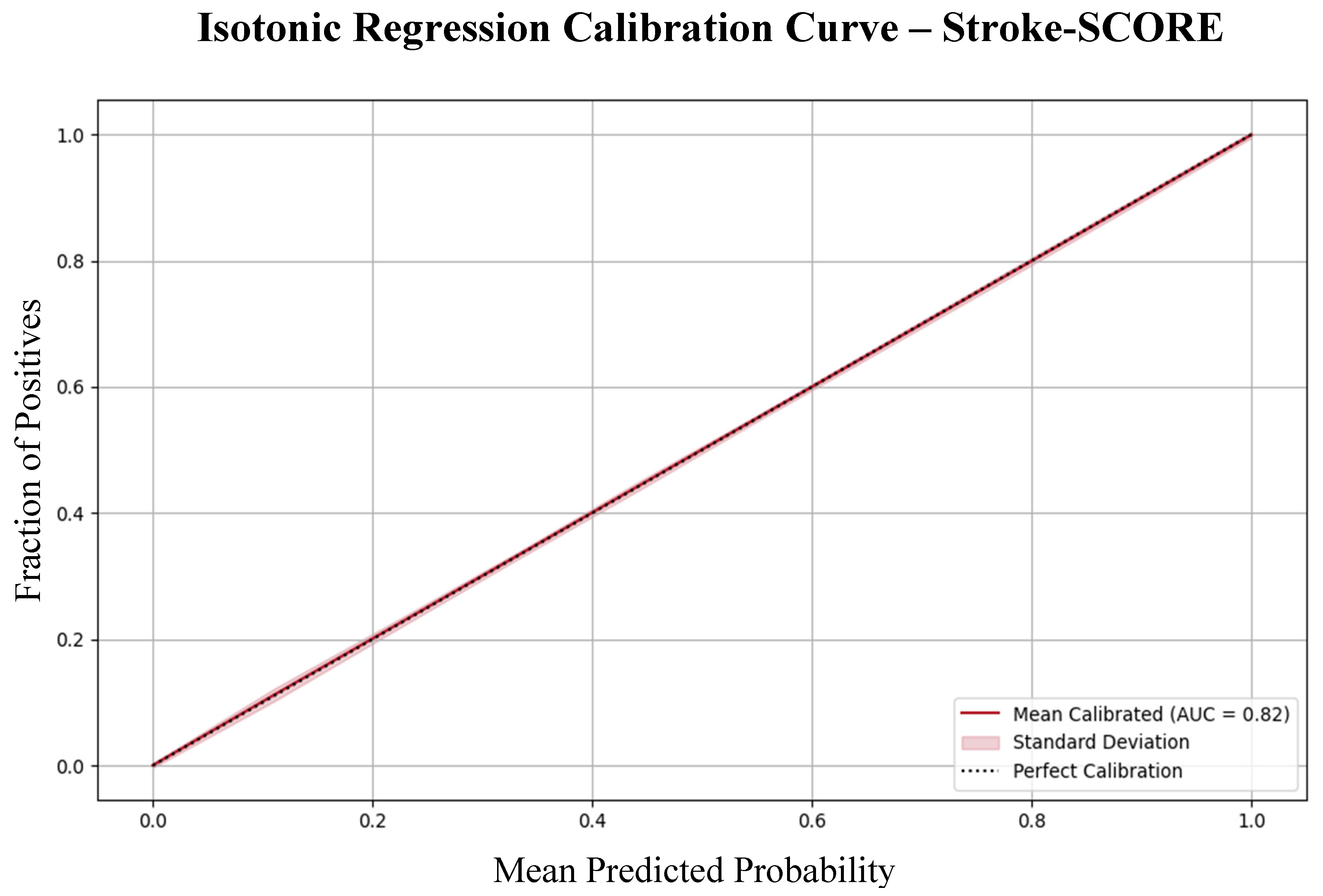
Moreover, calibration analysis confirmed that the model’s predicted probabilities aligned well with the observed outcomes, indicating that the Stroke-SCORE provides reliable risk estimates for patient prognosis (
Figure 3).
3.2. Patient Characteristics
Among the 793 patients analyzed, the median age was 72 years (interquartile range [IQR]: 63-80), with 49.3% being male. The median NIHSS score at admission was 5 (IQR: 3-10), indicating that most patients experienced strokes of moderate severity. The median pre-stroke mRS score was 0 (IQR: 0-1), suggesting a generally low level of pre-existing disability. The treatment distribution included 181 patients (22.8%) receiving TL, 150 (18.9%) undergoing MT, 63 (7.9%) receiving combination treatment (TL + MT), and 399 (50.3%) receiving standard care (SC).
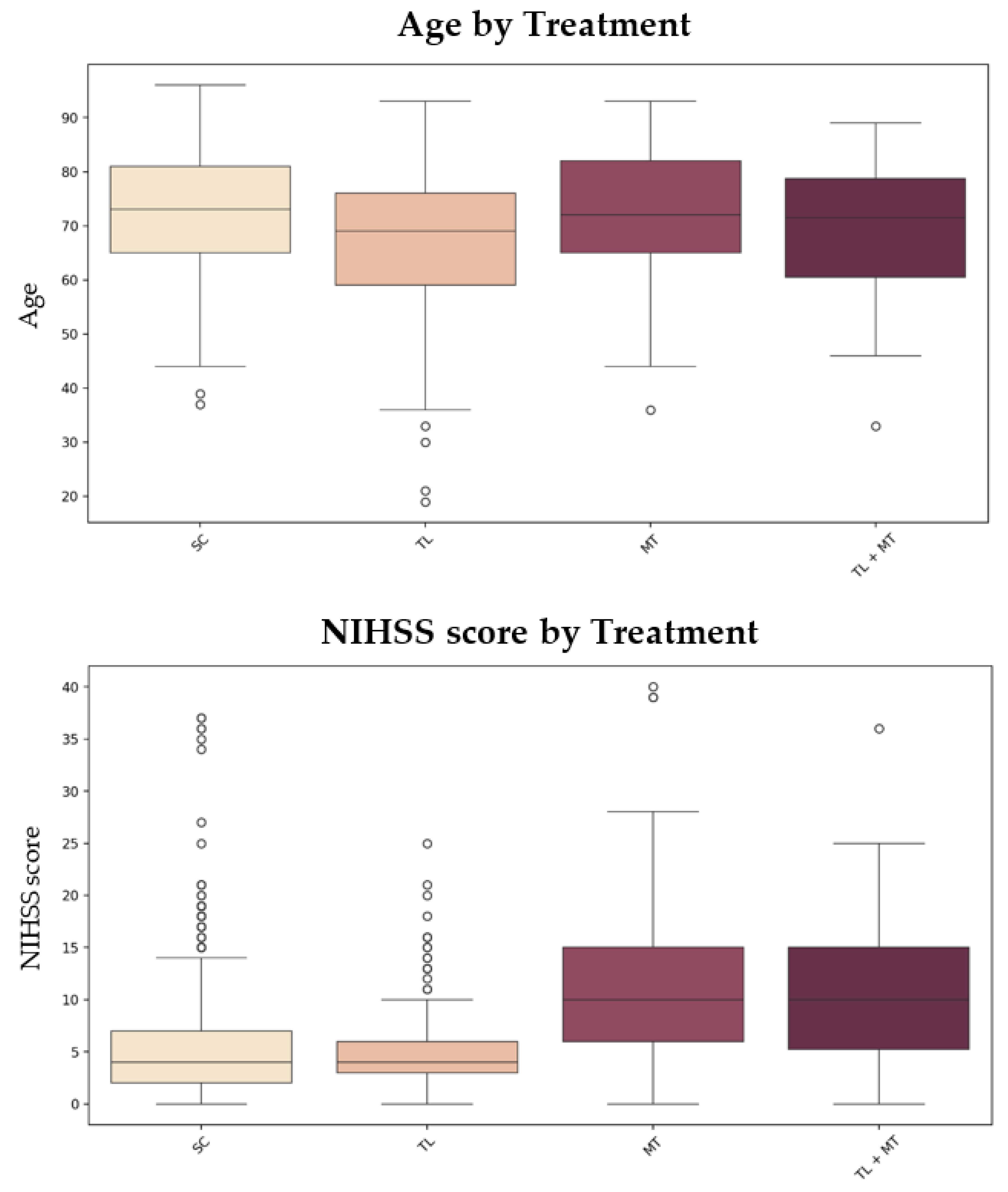
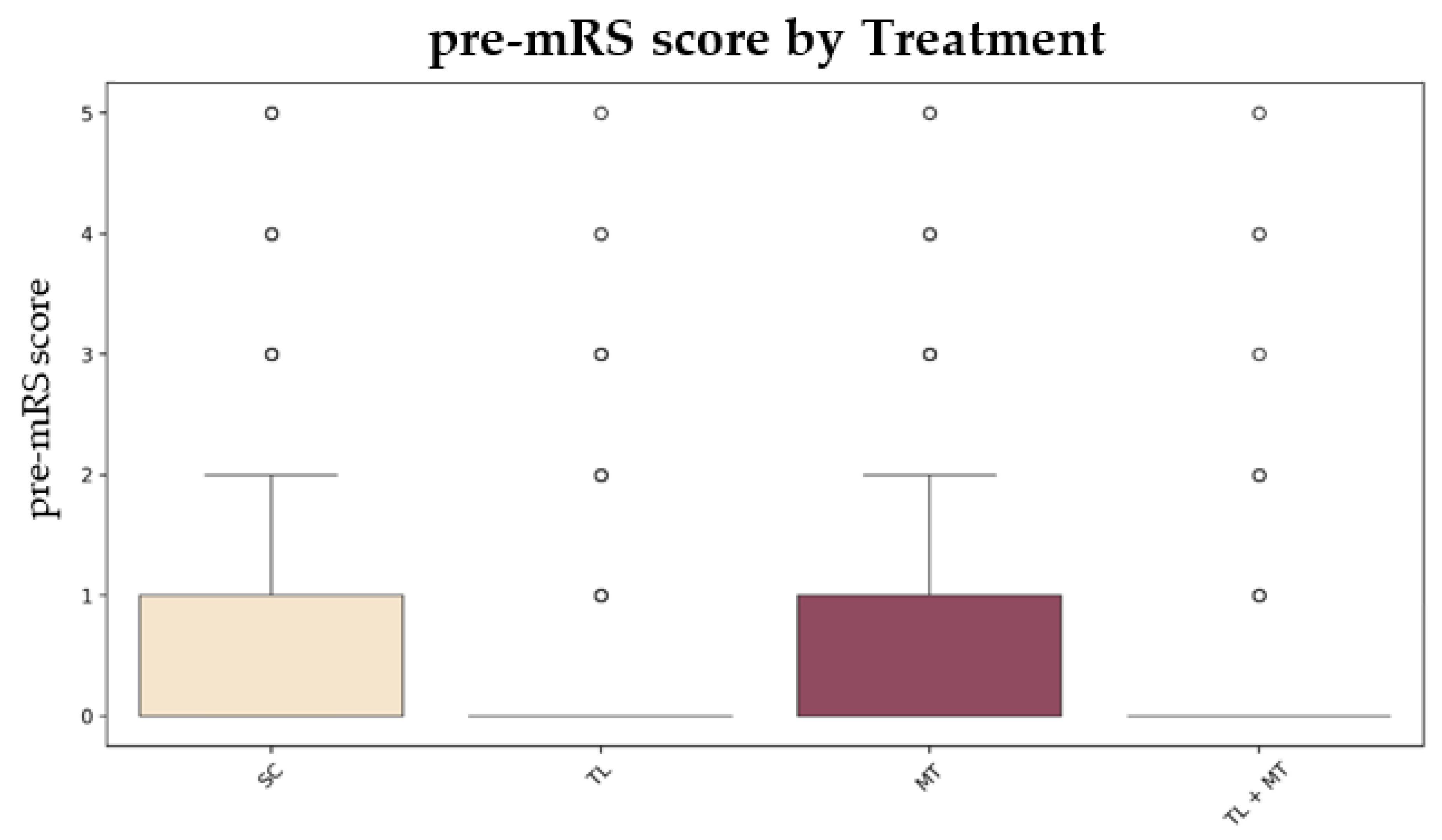
To illustrate how patient characteristics influence treatment selection,
Figure 4 presents box plots of age, NIHSS score, and pre-mRS score across treatment groups. These visualizations underscore significant differences in patient characteristics across treatment strategies (
Figure 4).
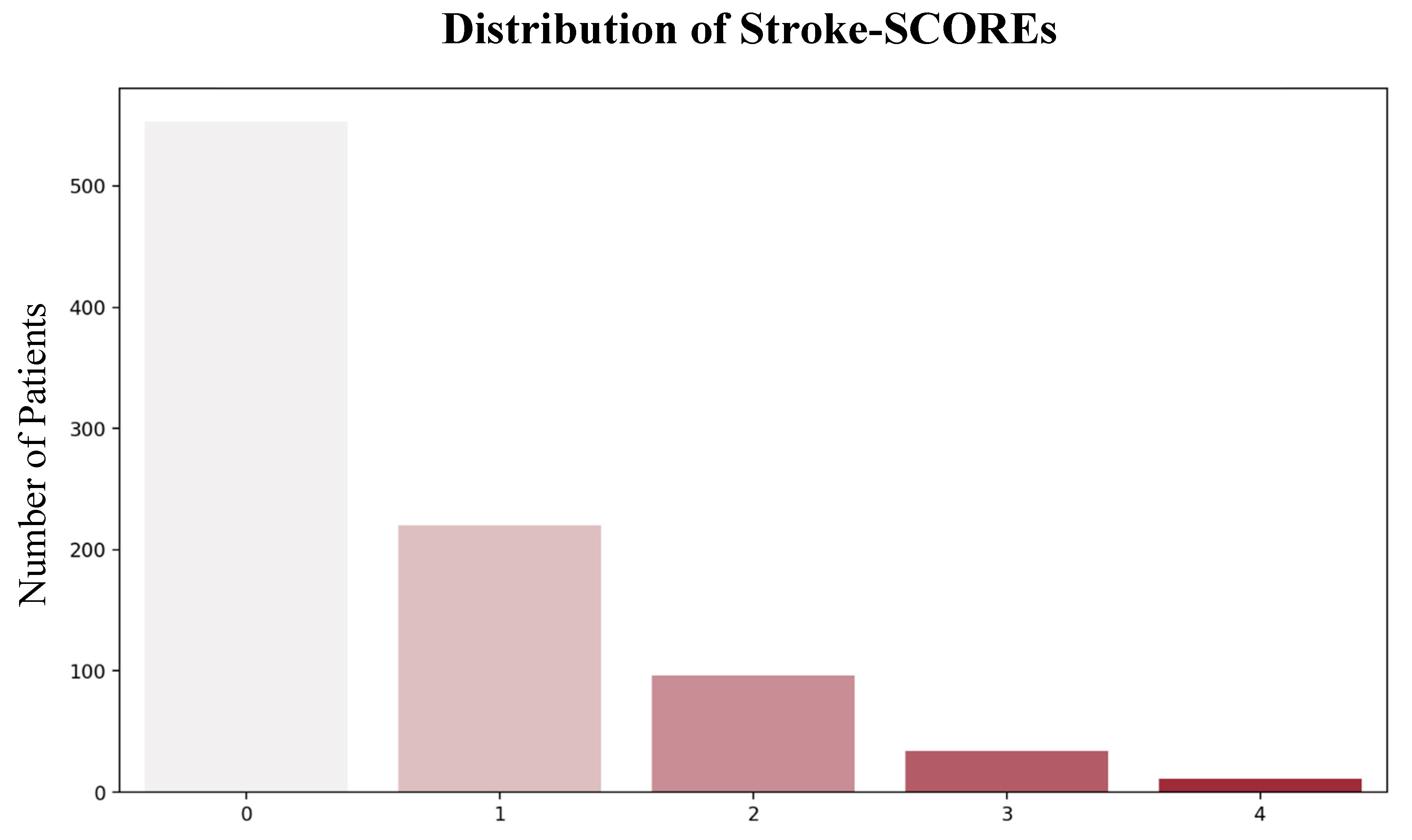
3.3. Patient Risk Stratification and Treatment Efficacy Across Different Stroke-SCOREs
The majority of patients were classified as low-risk (671; 85%), while 113 (14%) were classified as moderate risk, and 9 (1%) were classified as high-risk (
Figure 5).
Table 2 summarizes the average 90-day mRS score across Stroke-SCOREs for different treatment approaches. Logistic regression analysis indicated that low-risk patients (Stroke-SCORE of 0 or 1) experienced the greatest benefit from TL. These patients tended to be younger (average age: 67.1 years vs. 71.9 years), had lower NIHSS scores (mean 4.9 vs. 7.7) and pre-mRS scores (mean 0.38 vs. 0.75) compared to other treatment groups (
p < 0.001). Even after adjusting for these variables, TL was independently associated with a 30% reduction in unfavorable outcomes compared with other treatments.
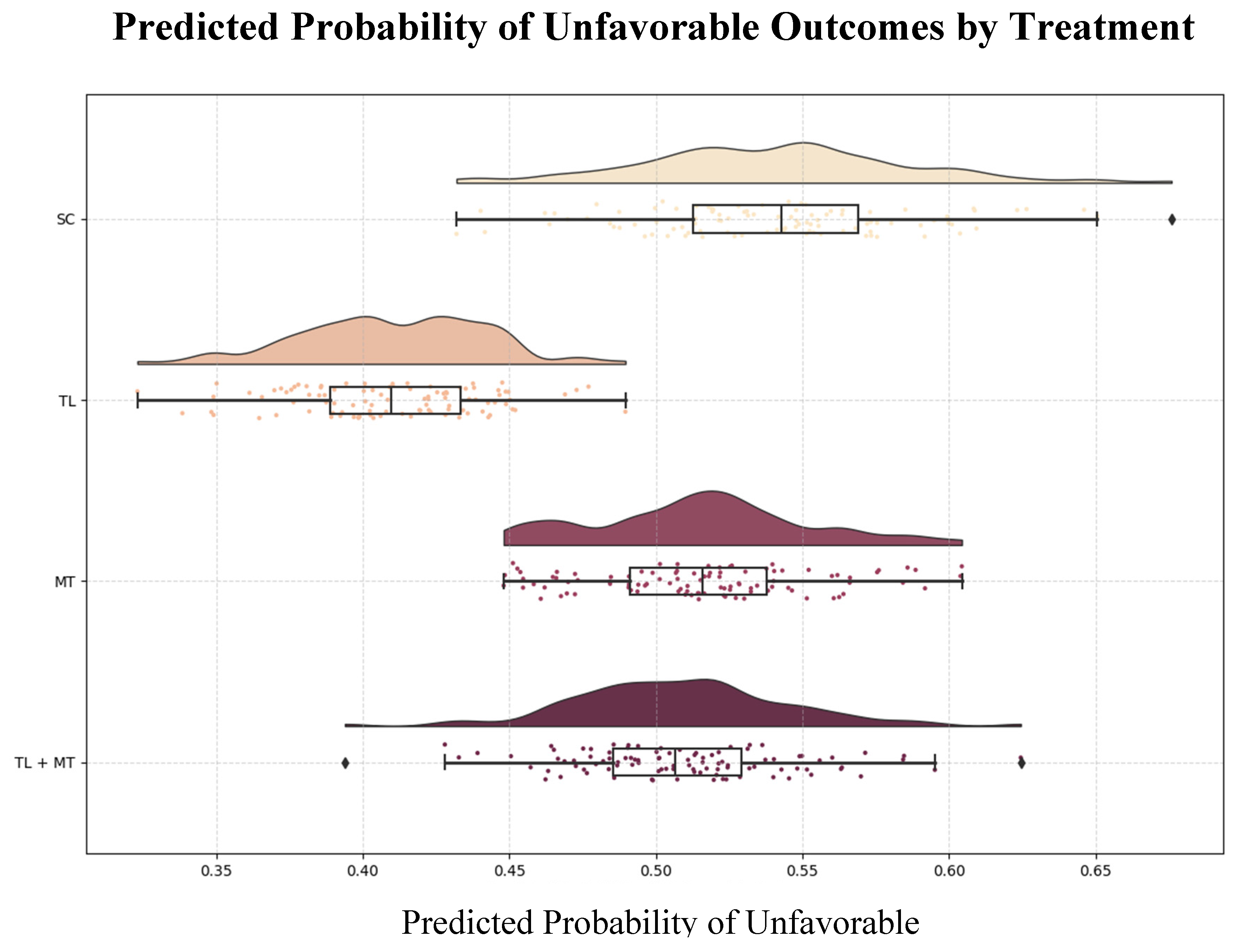
3.4. Simulated Outcomes
The model was used to create a counterfactual analysis that estimated potential 90-day outcomes if different treatments were applied universally to the cohort: TL was most effective for low-risk patients, with a predicted probability of unfavorable outcomes of 41%. MT was associated with a greater likelihood of unfavorable outcomes (54%), particularly in high-risk patients. Combination therapy yielded a predicted probability of unfavorable outcomes of 51% (
Figure 6).
4. Discussion
4.1. Navigating High-Risk Treatment Decisions with the Stroke-SCORE
To demonstrate the utility of the Stroke-SCORE model, consider a hypothetical case: an 82-year-old patient presents with a large vessel occlusion within 4.5 h of symptom onset. The patient has an NIHSS score of 18 and a pre-mRS score of 3, resulting in a total Stroke-SCORE of 4, which categorizes the patient as high-risk.
In this scenario, the Stroke-SCORE model predicts only a modest chance of a favorable outcome with TL or MT, with risk reduction estimates of just 0.14% for TL and 1.02% for MT compared with SC. These figures indicate a relatively high risk of an unfavorable outcome, even when aggressive interventions are used. This prediction underscores that despite eligibility for these treatments, the likelihood of substantial functional recovery is limited.
The Stroke-SCORE supports clinicians in navigating the complexity of making treatment decisions. This highlights that while the patient meets eligibility for both TL and MT, the expected improvement in functional outcome may not justify the risks of intervention. By providing such insights, the model facilitates a more informed discussion among the medical team, the patient, and their family, helping them weigh the potential benefits of intervention against the risks, such as procedural complications and the patient’s overall health status.
4.2. Comparison with Existing Scoring Systems
In recent years, several scoring systems have been developed to predict outcomes after AIS. Notable examples include the Acute Stroke Registry and Analysis of Lausanne (ASTRAL) score [
4], the DRAGON score (Diabetes, Rankin scale, Age, Glucose, Onset-to-treatment time, and NIHSS score) [
5], and the Totaled Health Risks in Vascular Events (THRIVE) score [
6]. These tools rely on readily available clinical features to estimate functional outcomes and are designed for use at patient admission. All three scores have undergone external validation, with AUC values ranging between 0.70 and 0.80 [
7,
8].
Treatment-Specific Limitations of Existing Models
While convenient and practical for estimating general functional outcomes, these scores have important limitations. Many do not account for specific treatment options or are tailored to a single intervention, such as TL (in the DRAGON) or MT (in the THRIVE score), restricting their application across broader AIS patient populations, particularly when choosing between multiple treatment strategies.
In the internal validation, the ASTRAL, DRAGON, and THRIVE scores achieved AUCs of 0.85, 0.84, and 0.75, respectively. In comparison, the Stroke-SCORE not only demonstrated slightly better predictive accuracy, with an AUC of 0.86 but also offered a unique advantage: incorporating treatment-specific considerations. This makes the Stroke-SCORE a more comprehensive tool, capable of guiding individualized treatment decisions rather than merely predicting outcomes on the basis of baseline characteristics.
Population-Restricted Prediction Models
Many existing prediction models are limited in their applicability to specific patient subgroups. For example, the PREDICT score developed by Hoffmann et al. is designed for younger stroke patients (under 55 years) and uses features such as the mASPECTS (manual Alberta Stroke Program Early CT Score), plasma-glucose level, and large vessel occlusion type to predict 90-day outcomes. These specialized models lack the flexibility to be applied across a diverse range of AIS patients, making them less functional in general clinical practice [
9].
Complex Models with Limited Real-Time Applicability
Other advanced models use complex metrics or specialized imaging biomarkers, making their incorporation into clinical workflows challenging, particularly in emergency settings where simplicity and speed are critical. For example, Forkert
et al.’s model employed magnetic resonance fluid-attenuated inversion recovery (MR FLAIR) imaging to assess the final infarction volume and location to predict 30-day outcomes [
10]. Despite its accuracy, this approach requires specialized imaging that may not always be feasible in real-time emergency scenarios. Similarly, the radiomics-based model developed by Yang and Guo relies on advanced imaging biomarkers that demand significant expertise and time-consuming preprocessing [
11].
In contrast, the Stroke-SCORE offers a practical, treatment-specific prediction tool that relies on just three easily obtainable clinical characteristics: age, NIHSS score, and pre-mRS. This simplicity makes the Stroke-SCORE particularly suitable for real-time decision-making at the bedside, enabling clinicians to make quick yet informed treatment decisions based on individual risk profiles.
4.3. Limitations and Future Directions
While internal validation has shown promising results, the study’s retrospective design and single-center dataset limit its generalizability, therefore our results should be interpreted as hypothesis-generating rather than definite evidence. Furthermore, assessments such as NIHSS and pre-mRS scores, which require evaluation by neurologists, introduce subjectivity that may affect prediction consistency.
To address these limitations, we will first validate the Stroke-SCORE prospectively within our patient cohort to assess its performance in real-world scenarios. Future research should focus on external, multicenter prospective validation to determine its broader applicability. Integration into real-time clinical workflows and the inclusion of additional predictors may further improve model performance.
5. Conclusions
The Stroke-SCORE is a practical, data-driven tool for personalizing AIS treatment. By utilizing three simple predictors—age, the NIHSS score, and the pre-mRS score—it balances predictive accuracy with clinical simplicity, making it suitable for real-world application.
By complementing existing treatment guidelines, the Stroke-SCORE empowers clinicians to make informed decisions that align with individual patient needs, ultimately improving outcomes and reducing unnecessary interventions.
Author Contributions
Conceptualization, J.S. and B.C.; methodology, J.S. and L.S.; validation, Z.N.K. and E.B.; formal analysis, J.S.; data curation, J.S.; writing—original draft preparation, J.S. and B.C.; writing—review and editing, Z.N.K. and E.B.; visualization, J.S.; supervision, L.S.; project administration, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Scientific and Research Ethics Committee of the Medical Research Council of the University of Pécs (RRF-2.3.1-21-2022-00011).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| AIS |
acute ischemic stroke |
| TL |
thrombolysis |
| MT |
mechanical thrombectomy |
| SCORE |
Simplified Clinical Outcome Risk Evaluation |
| NIHSS |
National Institute of Health Stroke Scale |
| pre-mRS |
pre-morbid modified Rankin Scale |
| mRS |
modified Rankin Scale |
| SC |
standard care |
| ROC |
receiver operating characteristics |
| AUC |
area under the curve |
| TINL |
Transzlációs Idegtudományi Nemzeti Laboratórium |
| SHAP |
SHapley Additive exPlanations |
| INR |
international normalized ratio |
| IQR |
interquartile range |
| ASTRAL |
Acute Stroke Registry and Analysis of Lausanne |
| DRAGON |
Diabetes, Rankin scale (pre-stroke), Age, Glucose, Onset-to-treatment time, NIHSS score |
| THRIVE |
Totaled Health Risks in Vascular Events |
| mASPECTS |
manual Alberta Stroke Program Early CT Score |
| MR FLAIR |
Magnetic resonance fluid-attenuated inversion recovery |
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50. [Google Scholar] [CrossRef]
- Chye, A.; Hackett, M.L.; Hankey, G.J.; Lundström, E.; Almeida, O.P.; Gommans, J.; Dennis, M.; Jan, S.; Mead, G.E.; Ford, A.H.; et al. Repeated Measures of Modified Rankin Scale Scores to Assess Functional Recovery From Stroke: AFFINITY Study Findings. J Am Heart Assoc 2022, 11. [Google Scholar] [CrossRef]
- Ntaios, G.; Faouzi, M.; Ferrari, J.; Lang, W.; Vemmos, K.; Michel, P. An Integer-Based Score to Predict Functional Outcome in Acute Ischemic Stroke. Neurology 2012, 78, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Strbian, D.; Meretoja, A.; Ahlhelm, F.J.; Pitkäniemi, J.; Lyrer, P.; Kaste, M.; Engelter, S.; Tatlisumak, T. Predicting Outcome of IV Thrombolysis–Treated Ischemic Stroke Patients. Neurology 2012, 78, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Cullen, S.P.; Faigeles, B.S.; Rao, V.A. Predicting Long-Term Outcome after Endovascular Stroke Treatment: The Totaled Health Risks in Vascular Events Score. American Journal of Neuroradiology 2010, 31, 1192–1196. [Google Scholar] [CrossRef]
- Cooray, C.; Mazya, M.; Bottai, M.; Dorado, L.; Skoda, O.; Toni, D.; Ford, G.A.; Wahlgren, N.; Ahmed, N. External Validation of the ASTRAL and DRAGON Scores for Prediction of Functional Outcome in Stroke. Stroke 2016, 47, 1493–1499. [Google Scholar] [CrossRef]
- Flint, A.C.; Faigeles, B.S.; Cullen, S.P.; Kamel, H.; Rao, V.A.; Gupta, R.; Smith, W.S.; Bath, P.M.; Donnan, G.A.; Lees, K.R.; et al. THRIVE Score Predicts Ischemic Stroke Outcomes and Thrombolytic Hemorrhage Risk in VISTA. Stroke 2013, 44, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, V.S.; Schönecker, S.; Amin, M.; Reidler, P.; Brauer, A.; Kopczak, A.; Wunderlich, S.; Poli, S.; Althaus, K.; Müller, S.; et al. A Novel Prediction Score Determining Individual Clinical Outcome 3 Months after Juvenile Stroke (PREDICT-Score). J Neurol 2024, 271, 6238–6246. [Google Scholar] [CrossRef] [PubMed]
- Forkert, N.D.; Verleger, T.; Cheng, B.; Thomalla, G.; Hilgetag, C.C.; Fiehler, J. Multiclass Support Vector Machine-Based Lesion Mapping Predicts Functional Outcome in Ischemic Stroke Patients. PLoS One 2015, 10, e0129569. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Ischemic Stroke Outcome Prediction with Diversity Features from Whole Brain Tissue Using Deep Learning Network. Front Neurol 2024, 15. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).