Submitted:
04 December 2024
Posted:
04 December 2024
You are already at the latest version
Abstract
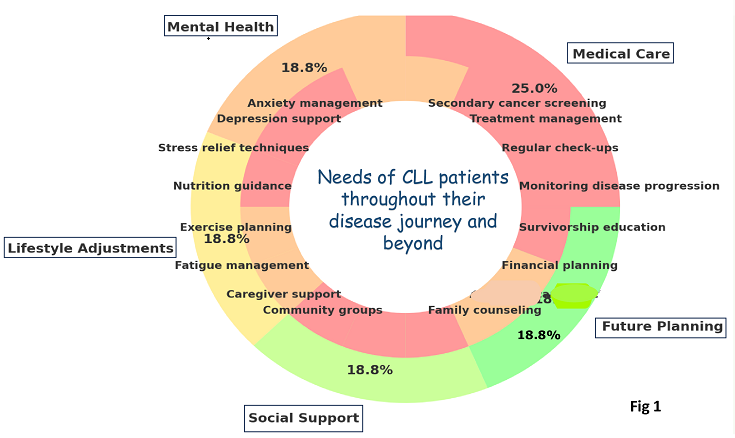
Keywords:
Introduction
The Changing Treatment Landscape of CLL: A Success Story
Challenges Faced by Long-Term CLL Survivors
The Management of CLL-Related Immune Dysfunction
Cardiovascular Complications of BTKis: Risks and Management
Second Primary Malignancies: Prevalence and Implications for Screening and Long-Term Care
Bone Health in CLL: Addressing Fracture Risk and the Potential of BTK Inhibitors
Managing Frailty in CLL
Enhancing Quality of Life for CLL Patients
Conclusions
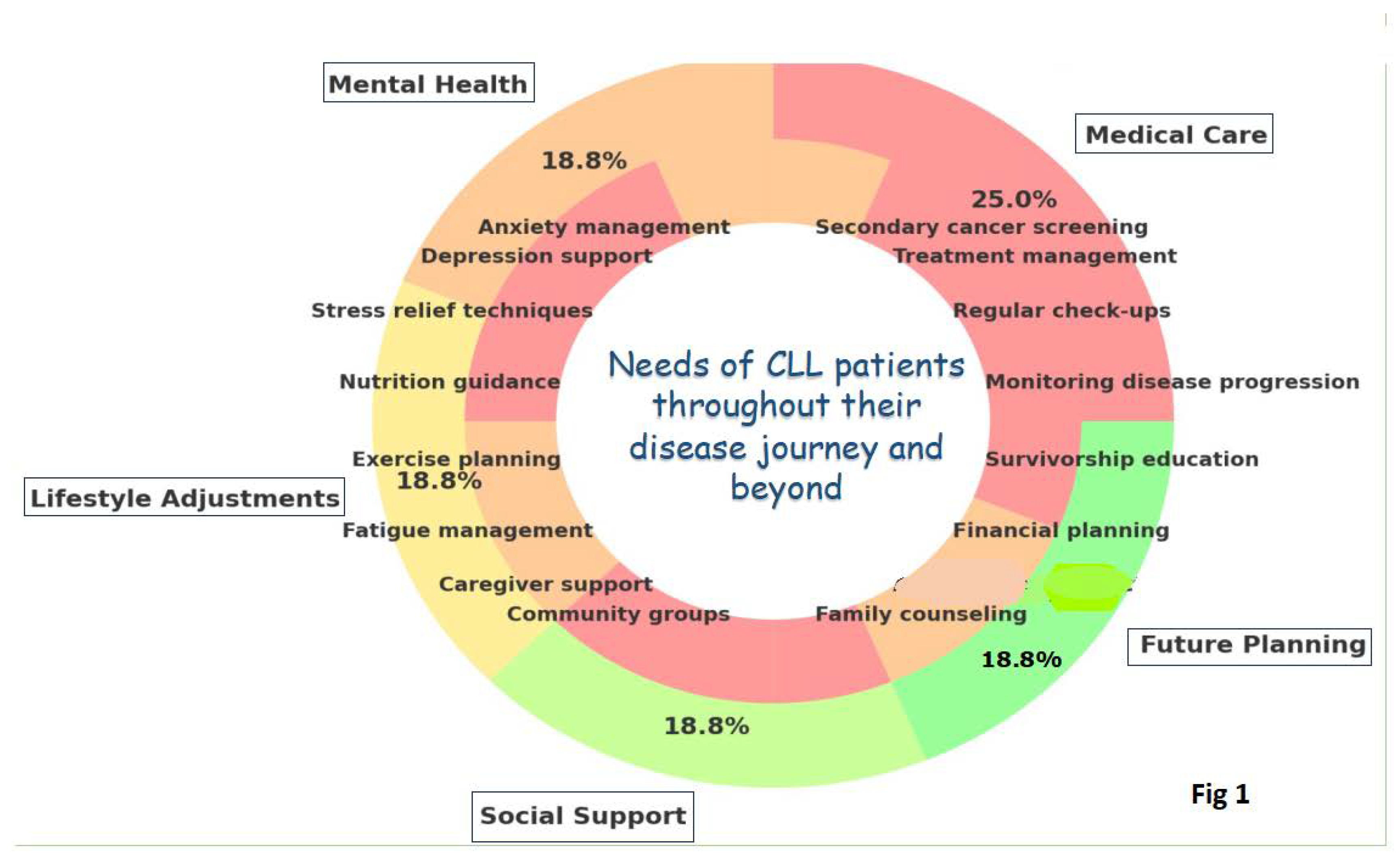
Funding
Data Availability Statement
Conflicts of Interest
References
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute; 2023. https://seer.cancer.gov/statistics-network/explorer/. Data source(s): SEER Incidence Data, November 2022 Submission (1975-2020), SEER 22 registries.
- Jain N, Wierda WG, O'Brien S Chronic lymphocytic leukaemia. Lancet. 2024, 404, 694–706. [CrossRef] [PubMed]
- Hemminki K, Hemminki J, Försti A, et al: Survival trends in hematological malignancies in the Nordic countries through 50 years. Blood Cancer J 2022, 12, 150. [CrossRef]
- van der Straten L, Maas C, Levin MD, et al: Long-term trends in the loss in expectation of life after a diagnosis of chronic lymphocytic leukemia: A population-based study in the Netherlands, 1989-2018. Blood Cancer J 2022, 12, 72. [CrossRef]
- Kajuter H, Wellmann I, Khil L, et al: Survival of patients with chronic lymphocytic leukemia before and after the introduction of chemoimmunotherapy in Germany. Blood Cancer J 2021, 11, 174. [CrossRef] [PubMed]
- Wang Y, Achenbach SJ, Rabe KG, et al: Cause of death in patients with newly diagnosed chronic lymphocytic leukemia (CLL) stratified by the CLL-International Prognostic Index. Blood Cancer J 2021, 11, 140. [CrossRef] [PubMed]
- Fedele PL, Opat S. Chronic Lymphocytic Leukemia: Time to Care for the Survivors. J Clin Oncol. 2024, 42, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007, 370, 230–239. [Google Scholar] [CrossRef]
- Eichhorst BF Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006, 107, 885–891. [Google Scholar]
- Flinn IW, Neuberg DS. , Grever MR et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol 2007, 25, 793–798. [Google Scholar] [CrossRef]
- Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016, 127, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012, 30, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Goede V, Fischer K, Busch R, et al Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014, 370, 1101–1110. [CrossRef] [PubMed]
- Hallek M, Fischer K, Fingerle-Rowson G, et al Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [CrossRef]
- Thompson PA, Bazinet A, Wierda WG, et al Sustained remissions in CLL after frontline FCR treatment with very-long-term follow-up. Blood 2023, 142, 1784–1788. [CrossRef]
- Davids, MS. Functional cure reported in CLL. Blood 2023, 142, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Scarfò L, Chatzikonstantinou T, Rigolin GM, et al COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 2020, 34, 2354–2363. [CrossRef]
- Molica S, Tam C, Polliack A. Current perspectives regarding SARS-CoV-2 vaccination in chronic lymphocytic leukemia. Hematol Oncol 2022, 40, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt TD, Wang XV, Hanson CA, et al Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood 2022, 140, 112–120. [CrossRef]
- Eichhorst B, Niemann CU, Kater AP, et al First-Line Venetoclax Combinations in Chronic Lymphocytic Leukemia. N Engl J Med 2023, 388, 1739–1754. [CrossRef] [PubMed]
- Eichhorst B, Ghia P, Niemann CU, et al ESMO Clinical Practice Guideline interim update on new targeted therapies in the first line and at relapse of chronic lymphocytic leukaemia. Ann Oncol 2024, 35, 762–768. [CrossRef]
- https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Version 1.2025 — October 1, 2024 Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients withchronic lymphocytic leukemia. Blood Adv 2022, 6, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in firstline treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019, 20, 43–56. [Google Scholar] [CrossRef]
- Moreno C, Greil R, Demirkan F, et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: final analysis of the randomized,phase III iLLUMINATE trial. Haematologica 2022, 107, 2108–2020. [Google Scholar] [CrossRef] [PubMed]
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versu chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018, 379, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Woyach JA, Ruppert AS, Heerema NA, et al. Long-term results of Alliance A041202 show continued advantage of ibrutinib-based regimens compared with bendamustine plus rituximab (BR) chemoimmunotherapy. Blood 2024, 143, 1616–1627. [Google Scholar]
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 2019, 381, 432–443. [Google Scholar] [CrossRef]
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib ± obinutuzumab vs obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: 6-year follow-up of Elevate-TN. Blood 2023, 142 (Suppl. 1). [Google Scholar]
- Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and smalllymphocytic lymphoma (SEQUOIA): a randomised, controlled,phase 3 trial. Lancet Oncol 2022, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and Obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Munir T, Cairns DA, Bloor A, et al. Chronic lymphocytic leukemia therapy guided by measurable residual disease. N Engl J Med 2024, 390, 326–337. [Google Scholar] [CrossRef]
- Al-Sawaf O, Zhang C, Jin HY, et al. Transcriptomic profiles and 5-year results from the randomized CLL14 study of venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab in chronic lymphocytic leukemia. Nat Commun 2023, 14, 2147–2156. [Google Scholar] [CrossRef]
- Burger J, Barr P, Robak T, et al Final Analysis of the RESONATE-2 Study: Up to 10 Years of Follow-Up of First-Line Ibrutinib Treatment in Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Clin Lymphoma Myeloma Leukemia Volume 2024, 24 (Suppl. 1), S1–S678.
- Ghia P, Owen C, Allan JN, et al. First-line ibrutinib treatment in patients with chronic lymphocytic leukemia is associated with overall survival rates similar to those of an age-matched general population: A pooled post hoc analysis. Hemasphere. 2024, 8, e74. [Google Scholar] [CrossRef] [PubMed]
- Molica S, Shanafelt T, Allsup D, et al. Impact of targeted agents on survival of CLL patients age > 65 relative to age and sex matched population. Am J Hematol. 2023, 99, 480–483. [Google Scholar]
- Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015, 126, 573–581. [Google Scholar] [CrossRef]
- Moreira J, Rabe KG, Cerhan JR, et al Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia 2013, 27, 136–141. [CrossRef]
- Galitzia A, Maccaferri M, Mauro FR, et al Chronic Lymphocytic Leukemia: Management of Adverse Events in the Era of Targeted Agents. Cancers (Basel). 2024, 16, 1996. [CrossRef]
- Vassilopoulos S, Shehadeh F, Kalligeros M, et al Targeted therapies in CLL/SLL and the cumulative incidence of infection: A systematic review and meta-analysis. Front Pharmacol 2022, 13, 989830. [CrossRef]
- Chong EA, Kumashie KG, Chong ER, et al Immunologic Predictors of Vaccine Responsiveness in Patients With Lymphoma and Chronic Lymphocytic Leukemia. J Infect Dis 2024, 230, 15–27. [CrossRef] [PubMed]
- Visentin A, Chatzikonstantinou T, Scarfò L, et al The evolving landscape of COVID-19 and post-COVID condition in patients with chronic lymphocytic leukemia: A study by ERIC, the European research initiative on CLL. Am J Hematol 2023, 98, 1856–1868. [CrossRef]
- Mikulska M, Cesaro S, de Lavallade H, et al: Vaccination of patients with haematological malignancies who did not have transplantations: Guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019, 19, e188–e199. [CrossRef]
- Tomasulo E, Paul S, Mu R, et al Interruption of BTK inhibitor improves response to SARS-CoV-2 booster vaccination in patients with CLL. Leuk Lymphoma. 2023, 64, 2306–2315. [CrossRef] [PubMed]
- Solman IG, Blum LK, Hoh HY, et al Ibrutinib restores immune cell numbers and function in first-line and relapsed/refractory chronic lymphocytic leukemia. Leuk Res 2020, 97, 106432. [CrossRef]
- Moreno C, Solman IG, Tam CS, et al Immune restoration with ibrutinib plus venetoclax in first-line chronic lymphocytic leukemia: the phase 2 CAPTIVATE study. Blood Adv 2023, 7, 5294–5303. [CrossRef] [PubMed]
- Parikh SA, Leis JF, Chaffee KG, et al Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer 2015, 121, 2883–2891. [CrossRef]
- Khan S, Allsup D, Molica S. An updated perspective on immunoglobulin replacement in chronic lymphocytic leukaemia in the era of targeted therapies. Front Oncol 2023, 13, 1135812. [Google Scholar] [CrossRef]
- Chai KL, Wong J, Weinkove R, et al Interventions to reduce infections in patients with hematological malignancies: a systematic review and meta-analysis. Blood Adv 2023, 7, 20–31. [CrossRef]
- Larsson K, Mattsson M, Ebrahim F, et al: High prevalence and incidence of cardiovascular disease in chronic lymphocytic leukaemia: A nationwide population-based study. Br J Haematol 2020, 190, e245–e248.
- Larsson K, Soderling J, Hoglund M, et al: Cardiovascular disease in patients with chronic lymphocytic leukemia: A Swedish nationwide register study with matched comparators. Am J Hematol 2022, 97, E255–E257.
- Dickerson T, Wiczer T, Waller A, et al Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [CrossRef]
- Fernandez Turizo MJ, Kim E, Zhang C, et al Pre-existing cardiovascular disease is associated with an increased risk of cardiovascular events during Bruton tyrosine kinase inhibitor therapy. Oncologist 2024, oyae229. [CrossRef] [PubMed]
- Brown JR, Moslehi J, O'Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017, 102, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Diamond A, Bensken WP, Vu L, et al Ibrutinib Is Associated With Increased Cardiovascular Events and Major Bleeding in Older CLL Patients. JACC CardioOncol. 2023, 5, 233–243. [CrossRef] [PubMed]
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020, 136, 2038–2050. [Google Scholar] [CrossRef]
- Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Brown JR, Eichhorst B, Lamanna N, et al Sustained Benefit of Zanubrutinib vs Ibrutinib in Patients With R/R CLL/SLL: Final Comparative Analysis of ALPINE. Blood, 2024.
- Lampson BL, Yu L, Glynn RJ, et al Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017, 129, 2581–2584. [CrossRef]
- Sharman JP, Ghia P, Miranda P, et al Analysis of ventricular arrhythmias and sudden death from prospective, randomized clinical trials of acalabrutinib. Br J Haematol. 2024, 205, 529–533. [CrossRef]
- Sanam Habib A, Shaaban A, Kola-Kehinde O, et al Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2023, 5, 570–590.
- Abdel-Qadir, H. , Sabrie N., Leong D., et al. Cardiovascular risk associated with ibrutinib use in chronic lymphocytic leukemia: a population-based cohort study. J Clin Oncol. 2021, 39, 3453–3462. [Google Scholar] [CrossRef]
- Zheng Y, Guo X, Chen C, et al Cardiovascular Toxicities of Ibrutinib: A Pharmacovigilance Study Based on the United States Food and Drug Administration Adverse Event Reporting System Database. Pharmaceuticals (Basel) 2023, 16, 98. [CrossRef]
- Brown, J.R. , Byrd J.C., Ghia P., et al. Cardiovascular adverse events in patients with chronic lymphocytic leukemia receiving acalabrutinib monotherapy: pooled analysis of 762 patients. Haematologica. 2022, 107, 1335–1346. [Google Scholar] [CrossRef]
- Lyon AR, López-Fernández T, Couch LS, et al: 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022, 43, 4229–4361.
- Awan FT, Addison D, Alfraih F, et al International consensus statement on the management of cardiovascular risk of Bruton's tyrosine kinase inhibitors in CLL. Blood Adv 2022, 6, 5516–5525. [CrossRef] [PubMed]
- Kumar V, Ailawadhi S, Bojanini L, et al Trends in the risk of second primary malignancies among survivors of chronic lymphocytic leukemia. Blood Cancer J 2019, 9, 75. [CrossRef] [PubMed]
- van der Straten L, Levin MD, Dinnessen MAW, et al Risk of second primary malignancies in patients with chronic lymphocytic leukemia: a population-based study in the Netherlands, 1989-2019. Blood Cancer J. 2023, 13, 15. [CrossRef] [PubMed]
- Chatzikonstantinou T, Scarfò L, Karakatsoulis G, et al Other malignancies in the history of CLL: an international multicenter study conducted by ERIC, the European Research Initiative on CLL, in HARMONY. EClinicalMedicine 2023, 65, 102307. [CrossRef] [PubMed]
- Cramer P, Isfort S, Bahlo J, et al Outcome of advanced chronic lymphocytic leukemia following different first-line and relapse therapies: a meta-analysis of five prospective trials by the German CLL Study Group (GCLLSG). Haematologica 2015, 100, 1451–1459. [CrossRef]
- Bond DA, Huang Y, Fisher JL, et al Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia 2020, 34, 3197–3205. [CrossRef] [PubMed]
- Falchi L, Vitale C, Keating MJ, et al: Incidence and prognostic impact of other cancers in a population of long-term survivors of chronic lymphocytic leukemia. Ann Oncol 2016, 27, 1100–1106. [CrossRef]
- Olszewski AJ, Gutman R, Eaton CB: Increased risk of axial fractures in patients with untreated chronic lymphocytic leukemia: A population-based analysis. Haematologica 2016, 101, e488–e491. [CrossRef]
- Ferrajoli A, Keating MJ, Manshouri T, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood 2002, 100, 1215–1219. [Google Scholar] [CrossRef]
- Lai R, O'Brien S, Maushouri T, et al. Prognostic value of plasma interleukin-6 levels in patients with chronic lymphocytic leukemia. Cancer. 2002, 95, 1071–1075. [Google Scholar] [CrossRef]
- Yan XJ, Dozmorov I, Li W, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood. 2011, 118, 5201–5210. [Google Scholar] [CrossRef]
- Schmiedel BJ, Scheible CA, Nuebling T, et al: RANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemia. Cancer Res 2013, 73, 683–694. [CrossRef] [PubMed]
- Shanafelt TD, Drake MT, Maurer MJ, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011, 117, 1492–1498. [Google Scholar] [CrossRef]
- Molica S, Digiesi G, Antenucci A, et al Vitamin D insufficiency predicts time to first treatment (TFT) in early chronic lymphocytic leukemia (CLL). Leuk Res 2012, 36, 443–447. [CrossRef] [PubMed]
- Ariza, Y. , Murata M., Ueda Y., Yoshizawa T. Bruton’s tyrosine kinase (Btk) inhibitor tirabrutinib suppresses osteoclastic bone resorption. Bone Rep. 2019, 10, 100201. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, N.K. , Kim Y.G., Kim H.J., Kim H.J., Lee J.H., Choi S.Y., Kwon T.G., Lee H.J., Kim J.Y., Lee Y. A novel Bruton’s tyrosine kinase inhibitor, acalabrutinib, suppresses osteoclast differentiation and Porphyromonas gingivalis lipopolysaccharide-induced alveolar bone resorption. J. Periodontol. 2019, 90, 546–554. [Google Scholar]
- Giannoni P, Marini C, Cutrona G, et al Unraveling the Bone Tissue Microenvironment in Chronic Lymphocytic Leukemia. Cancers (Basel) 2023, 15, 5058. [CrossRef] [PubMed]
- Molica S, Brugiatelli M, Morabito F, et al Treatment of elderly patients with chronic lymphocytic leukemia: an unmet cinical need. Expert Rev Hematol. 2013, 6, 441–449. [CrossRef] [PubMed]
- Molica, S. Defining treatment success in chronic lymphocytic leukemia: exploring surrogate markers, comorbidities, and patient-centered endpoints. Expert Rev Hematol. 2024, 17, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Goede, V. Frailty is also a target for targeted drugs in CLL. Blood 2023, 142, 1107–1108. [Google Scholar] [CrossRef] [PubMed]
- van der Straten L, Stege CAM, Kersting S, et al. Fixed-duration venetoclax plus obinutuzumab improves quality of life and geriatric impairments in FCR-unfit patients with CLL. Blood 2023, 142, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Martino EA, Mauro FR, Reda G, et al Ibrutinib as first line therapy in chronic lymphocytic leukemia patients over 80 years old: A retrospective real-life multicenter Italian cohort. Hematol Oncol 2024, 42, e3249. [CrossRef] [PubMed]
- Simon F, Ligtvoet R, Nösslinger T, et al Safety of acalabrutinib treatment in very old (≥80 y) and/or frail patients with chronic lymphocytic leukemia - interim safety analysis of the ongoing phase II CLL-Frail trial. Hematological Oncology 2023, 41, 468–469. [CrossRef]
- González-Gascón-Y-Marín I, Ballesteros-Andrés M, Martínez-Flores S, et al The Five "Ws" of Frailty Assessment and Chronic Lymphocytic Leukemia: Who, What, Where, Why, and When. Cancers (Basel) 2023, 15, 4391. [CrossRef] [PubMed]
- Crowder SL, Hoogland AI, Small BJ, et al Associations among frailty and quality of life in older patients with cancer treated with chemotherapy. J Geriatr Oncol 2022, 13, 1149–1155. [CrossRef]
- Johnson PC, Woyach JA, Ulrich A, et al Geriatric assessment measures are predictive of outcomes in chronic lymphocytic leukemia. J Geriatr Oncol. 2023, 14, 101538. [CrossRef] [PubMed]
- Soumerai JD, Barrientos JC, Ahn IE, et al Consensus Recommendations from the 2024 Lymphoma Research Foundation Workshop on Treatment Selection and Sequencing in CLL or SLL. Blood Adv 2024.
- S. Molica, D. Allsup, A. Polliack, D. Giannarelli. The net clinical benefit of targeted agents in the upfront treatment of elderly/unfit chronic lymphocytic leukemia patients: Results of network meta-analysis. Eur. J. Haematol. 2023, 110, 774–777. [Google Scholar] [CrossRef]
- Shanafelt TD, Bowen D, Venkat C, et al Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 2007, 139, 255–264. [CrossRef] [PubMed]
- Holzner B, Kemmler G, Kopp M, et al: Quality of life of patients with chronic lymphocytic leukemia: Results of a longitudinal investigation over 1 yr. Eur J Haematol 2004, 72, 381–389. [CrossRef] [PubMed]
- Waweru C, Kaur S, Sharma S, et al: Health-related quality of life and economic burden of chronic lymphocytic leukemia in the era of novel targeted agents. Curr Med Res Opin 2020, 36, 1481–1495. [CrossRef] [PubMed]
- Russell K, Moghaddam N, Tickle A: Examining anxiety and depression in haematology cancer patients in ongoing treatment and under watchful waiting: A systematic review and meta-analysis. Eur J Cancer Care 2022, 31, e13678.
- Fifer S, Godsell J, Opat S, et al Understanding the experience, treatment preferences and goals of people living with chronic lymphocytic leukemia (CLL) in Australia. BMC Cancer 2024, 24, 831.
- Deering KL, Sundaram M, Harshaw Q,et al. Health-related quality of life and treatment satisfaction in Chronic Lymphocytic Leukemia (CLL) patients on ibrutinib compared to other CLL treatments in a real-world US cross sectional study. PLoS One 2022, 17, e0270291. [Google Scholar]
- Tam CS, Lamanna N, O'Brien SM, et al Health-related quality of life outcomes associated with zanubrutinib versus ibrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: results from the ALPINE Trial. Curr Med Res Opin. 2023, 39, 1497–1503.
- Molica S, Bombaci F, Cuneo A, et al. LIVING WITH CHRONIC LYMPHOCYTIC LEUKEMIA (CLL): A QUANTITATIVE CROSS-SECTIONAL STUDY OF ITALIAN PATIENTS' EXPERIENCES ON BEHALF OF AIL (ASSOCIAZIONE ITALIANA CONTRO LE LEUCEMIE-LINFOMI E MIELOMI). EHA, 2020; EP1743.
- Molica S, Shanafelt TD, Allsup D, Giannarelli D. Impact of Targeted Agents on Survival of Chronic Lymphocytic Leukemia Patients Fit for Fludarabine, Cyclophosphamide, and Rituximab (FCR) Relative to Age- and Sex-Matched Population. Cancers (Basel) 2024, 16, 1085. [Google Scholar] [CrossRef] [PubMed]
- Wang Y, Achenbach SJ, Rabe KG, et al Cause of death in patients with newly diagnosed chronic lymphocytic leukemia (CLL) stratified by the CLL-International Prognostic Index. Blood Cancer J 2021, 11, 140. [CrossRef]
- Villavicencio A, Solans M, Zacarías-Pons L, et al Comorbidities at Diagnosis, Survival, and Cause of Death in Patients with Chronic Lymphocytic Leukemia: A Population-Based Study. Int J Environ Res Public Health 2021, 18, 701. [CrossRef] [PubMed]
- Shanafelt TD, Kay NE. Comprehensive management of the CLL patient: a holistic approach. Hematology Am Soc Hematol Educ Program. 2007, 324–331.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




