1. Introduction
In recent years, the convergence of Artificial Intelligence (AI) and precision medicine has sparked a revolution in healthcare, promising to transform the way diseases are diagnosed, treated, and managed. Precision medicine, also known as personalized or stratified medicine, aims to tailor medical interventions to individual patient characteristics, including genetic makeup, environmental factors, and lifestyle choices. AI, on the other hand, encompasses a diverse set of computational techniques that enable machines to mimic human intelligence, learn from data, and make predictions or decisions. The integration of AI and precision medicine represents a paradigm shift in healthcare delivery, offering unprecedented opportunities to improve patient outcomes, enhance clinical decision-making, and advance biomedical research. By leveraging AI-driven analytics, predictive modeling, and decision support systems, healthcare providers can unlock valuable insights from vast amounts of healthcare data, including genomic, clinical, imaging, and wearable device data. [
1]
AI-driven precision medicine holds promise across various healthcare domains, including disease diagnosis, risk stratification, treatment selection, and patient management. For example, AI algorithms can analyze genomic data to identify genetic variants associated with disease risk, treatment response, and drug metabolism, guiding personalized treatment approaches tailored to individual patients [
2]. Additionally, AI-driven diagnostic tools, such as deep learning algorithms for medical imaging analysis, can assist clinicians in detecting diseases at earlier stages with greater accuracy, facilitating timely interventions and improved patient outcomes [
3]. Moreover, AI-powered predictive analytics models can forecast disease trajectories, predict treatment responses, and identify individuals at risk of developing specific diseases, enabling proactive interventions and preventive strategies to mitigate health risks and improve population health [
4]. By leveraging real-time data streams from electronic health records, wearable devices, and other sources, AI-driven systems can continuously monitor patient health status, detect early warning signs of disease exacerbation, and provide personalized recommendations for disease management and lifestyle modifications.
The transformative potential of AI and precision medicine extends beyond clinical practice to encompass biomedical research, drug discovery, public health, and healthcare policy. AI-driven approaches accelerate the pace of biomedical research by analyzing large-scale datasets, uncovering disease biomarkers, and identifying novel therapeutic targets [
5]. Additionally, AI technologies enable the development of innovative healthcare solutions, such as AI-driven drug discovery platforms, remote patient monitoring systems, and predictive analytics models for disease surveillance and outbreak prediction. The integration of AI and precision medicine is paving the way for future treatment by revolutionizing healthcare delivery, biomedical research, and public health initiatives. By harnessing the power of AI-driven analytics and personalized treatment strategies, healthcare providers can optimize patient care, improve health outcomes, and advance the practice of medicine in the 21st century.
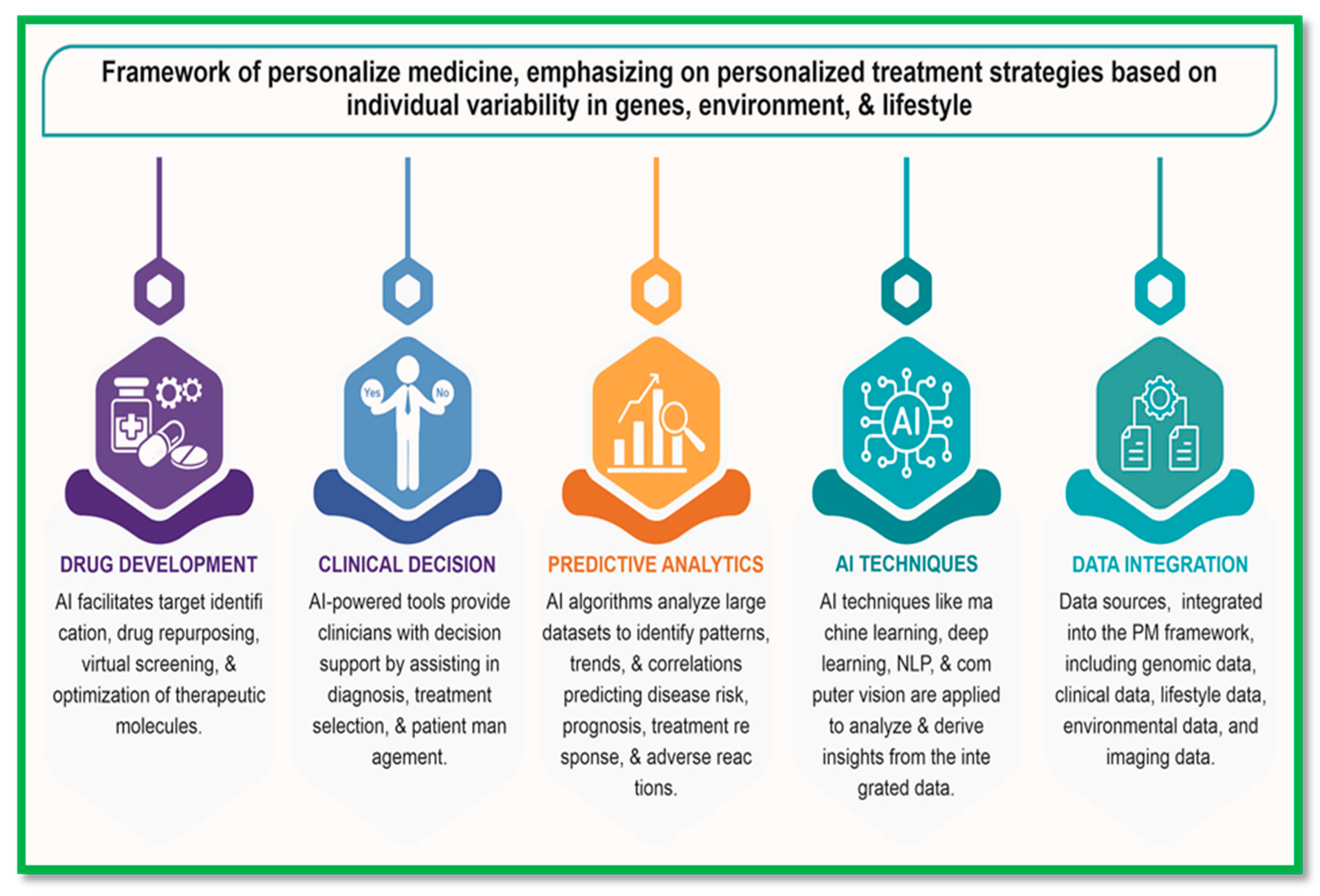
Precision Medicine:
Precision medicine, also known as personalized or stratified medicine, is a revolutionary approach to healthcare that emphasizes tailoring medical interventions to individual patient characteristics, including genetic makeup, environmental factors, and lifestyle choices. This paradigm shift from a one-size-fits-all approach to a more personalized and targeted approach holds immense promise for improving patient outcomes, optimizing treatment efficacy, and advancing biomedical research. At the core of precision medicine is the concept of leveraging comprehensive patient data, including genomic, clinical, imaging, and lifestyle information, to inform medical decision-making and treatment strategies. Genomic sequencing technologies, such as next-generation sequencing (NGS), enable the identification of genetic variants associated with disease susceptibility, treatment response, and drug metabolism. For instance, studies have demonstrated the utility of genomic profiling in guiding targeted therapies for cancer patients based on specific genetic mutations [
9,
10].
In addition to genomic data, clinical and phenotypic information play a crucial role in precision medicine. Electronic health records (EHRs) and clinical databases provide valuable insights into patient demographics, medical history, comorbidities, and treatment outcomes. Integrating these data sources with advanced analytics and machine learning algorithms enables the development of predictive models for disease risk assessment, prognosis prediction, and treatment optimization [
11,
12]. Furthermore, precision medicine extends beyond individual patient care to encompass population health management and public health initiatives. By analyzing large-scale datasets and identifying patterns of disease prevalence, risk factors, and disparities, precision medicine approaches can inform targeted interventions, preventive strategies, and healthcare policies aimed at improving population health outcomes [
13,
14,
15]. Precision medicine represents a transformative approach to healthcare that harnesses the power of comprehensive patient data, advanced analytics, and personalized treatment strategies to optimize patient care, advance biomedical research, and improve population health. By integrating genomic, clinical, and lifestyle information, precision medicine enables healthcare providers to deliver more effective, targeted and personalized interventions tailored to individual patient needs and characteristics.
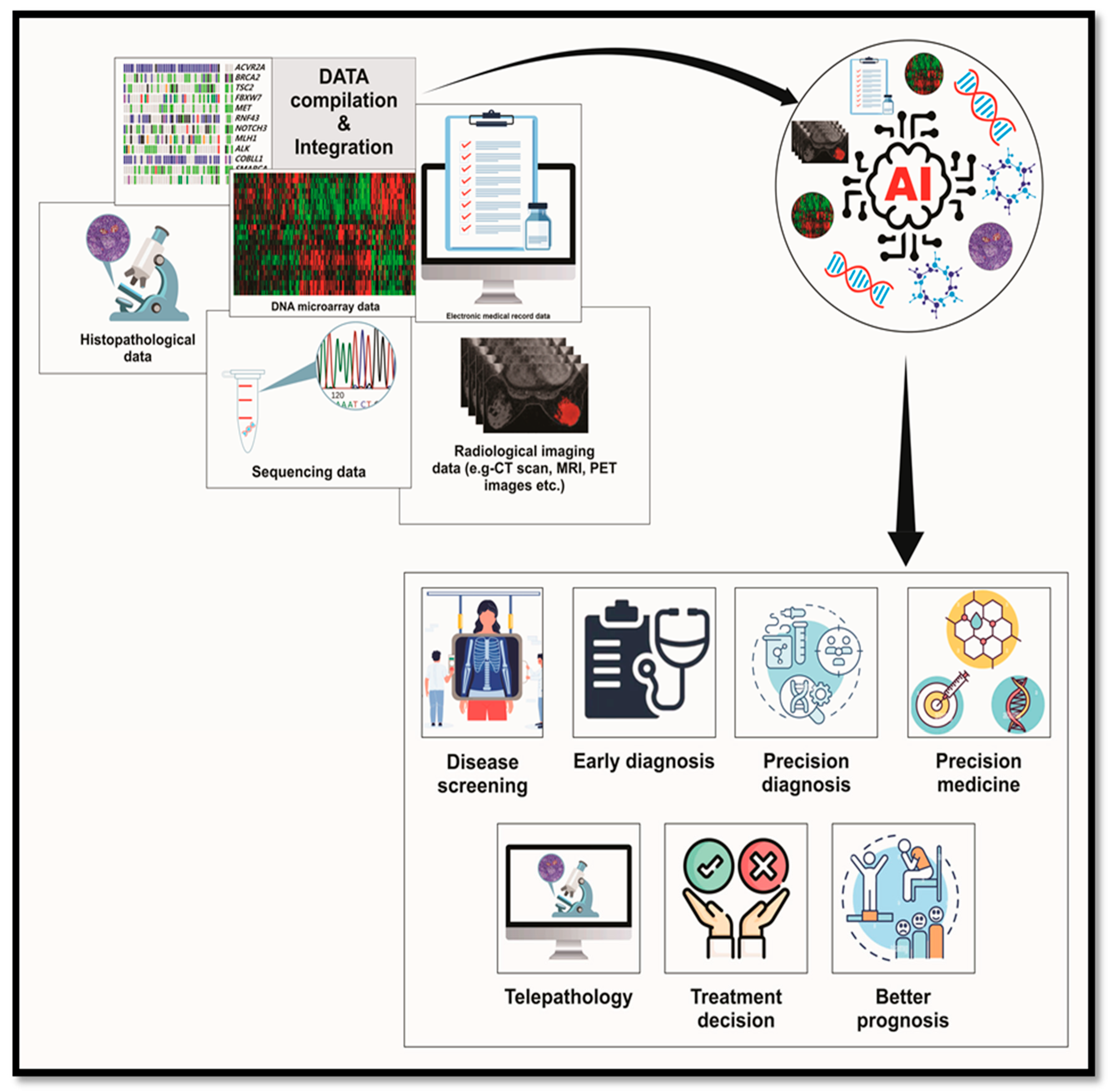
Table 1.
summarizing the role of AI in precision medicine and its potential for future treatments:.
Table 1.
summarizing the role of AI in precision medicine and its potential for future treatments:.
| Aspect |
Role of AI in Precision Medicine |
Future Potential |
| Data Integration |
Combines genomic, proteomic, and clinical data to create comprehensive patient profiles. |
Enables personalized treatment plans by integrating multi-omics and real-time patient data. |
| Disease Screening |
Identifies patterns in large datasets to detect diseases early through imaging and biomarkers. |
Expands capabilities for non-invasive, population-wide screening programs. |
| Precision Diagnosis |
Uses machine learning to analyse imaging, pathology, and molecular data for accurate, personalized diagnoses. |
Develops models that predict disease progression and subtypes for tailored interventions. |
| Treatment Decision-Making |
Assists clinicians by predicting treatment responses and recommending optimal therapies based on patient-specific data. |
Advances in AI could lead to fully automated, adaptive treatment systems in clinical practice. |
| Drug Development |
Accelerates drug discovery by identifying molecular targets and predicting compound efficacy using AI simulations. |
Revolutionizes pharmaceutical development with faster, cost-effective trials and AI-driven designs. |
| Telemedicine |
Supports remote consultations with AI-driven diagnostic tools and real-time data analysis. |
Enhances access to personalized care, especially in resource-limited regions. |
| Prognosis Prediction |
Predicts disease outcomes and survival rates using AI-based risk models. |
Develops dynamic prognosis tools adaptable to evolving patient health data. |
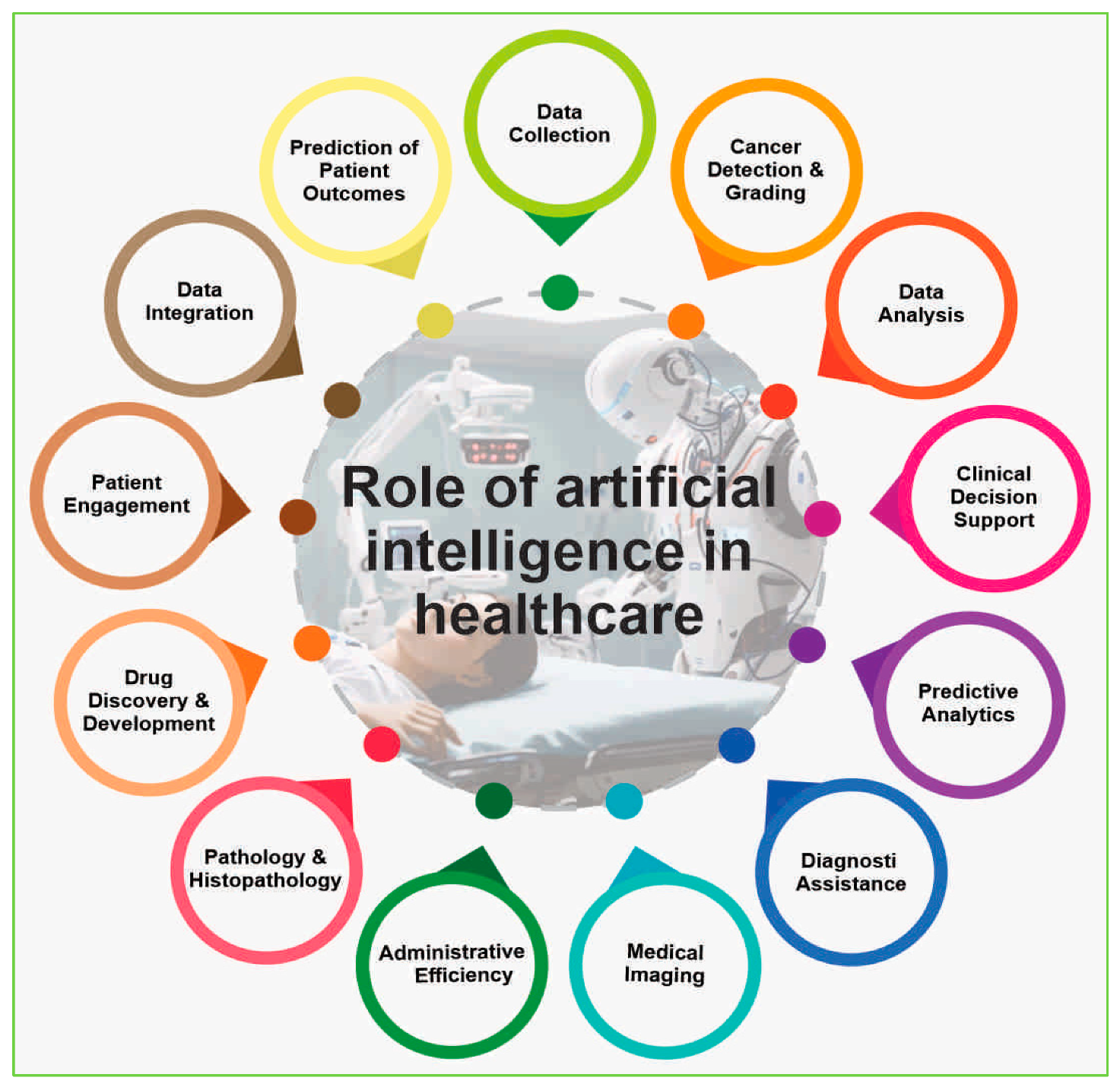
Role of AI in Healthcare
The role of artificial intelligence (AI) in healthcare is multifaceted, encompassing a range of applications that aim to enhance diagnostics, treatment planning, and overall patient care. Here is an overview of the key roles AI plays in healthcare (
Figure 2).
1-In Diagnostic Assistance: Artificial Intelligence (AI) has emerged as a powerful tool in diagnostic assistance, particularly in the field of pathology and histopathology, where it offers significant advancements in diagnostic accuracy, efficiency, and patient outcomes. By leveraging deep learning algorithms and automated image recognition, AI enhances the interpretation of medical images and microscopic tissue samples, aiding healthcare professionals in faster and more accurate diagnoses. In diagnostic imaging, AI algorithms analyze medical images such as X-rays, MRIs, and CT scans to identify patterns and abnormalities, assisting radiologists in interpreting complex imaging data [
6]. Similarly, AI algorithms aid pathologists in analyzing microscopic images of tissue samples, automating image recognition and pattern detection to identify anomalies like cancer cells or tissue abnormalities with high precision [
16].
One of the significant contributions of AI in pathology is the early detection of cancer, as AI algorithms accurately identify cancerous cells in pathology slides, enabling timely intervention and treatment planning [
17]. Additionally, AI assists in grading tumors, providing valuable information for treatment decisions and patient management. Furthermore, AI models analyze histopathological data to predict patient outcomes, including survival rates and disease recurrence, aiding clinicians in developing personalized treatment plans [
18]. By processing pathology slides much faster than human counterparts, AI reduces turnaround times for diagnoses, particularly crucial in critical cases requiring prompt action. AI also helps standardize diagnostic practices by minimizing inter-observer variability and providing consistent, objective analyses, contributing to improved diagnostic accuracy and reliability [
19]. Moreover, AI facilitates telepathology by enabling remote analysis of pathology slides, allowing for consultations, collaborations, and second opinions across geographical distances [
20]. Furthermore, AI integrates histopathological data with other patient information, such as genomic and clinical data, providing a comprehensive understanding of disease mechanisms and facilitating personalized medicine [
21]. This integration enables clinicians to tailor treatment plans to individual patient characteristics, optimizing therapeutic efficacy and patient outcomes. In pathology and histopathology AI represents a paradigm shift in diagnostic assistance, offering the potential to significantly improve diagnostic accuracy, efficiency, and patient outcomes. As technology continues to advance, the collaboration between AI and healthcare professionals is likely to further enhance the field of pathology, contributing to more precise and personalized healthcare.
Pathology and Histopathology: In the realm of pathology and histopathology, the integration of Artificial Intelligence (AI) has ushered in a new era characterized by enhanced diagnostic capabilities and streamlined processes. AI algorithms, particularly those employed in automated image analysis, have revolutionized the field by facilitating rapid and precise interpretation of microscopic images. These systems serve as invaluable aids to pathologists, enabling them to identify and characterize tissue anomalies with unprecedented accuracy and efficiency [
22]. In the context of cancer pathology, AI plays a pivotal role in early tumor detection and grading, providing critical insights that inform treatment decisions and prognoses. By accelerating diagnostic workflows, AI significantly reduces turnaround times, thereby facilitating prompt interventions and improving patient outcomes [
23,
24]. Moreover, AI ensures standardization and quality assurance in pathology practices by mitigating inter-observer variability, thereby offering consistent and objective evaluations across diverse cohorts of pathologists. This aspect is particularly crucial in maintaining diagnostic accuracy and reliability [
25,
26]. The integration of AI extends beyond mere diagnostic assistance, encompassing predictive analytics for patient outcomes. By leveraging vast datasets, AI-powered models support clinicians in devising personalized treatment strategies tailored to individual patient profiles, thereby optimizing therapeutic efficacy and clinical outcomes [
27,
28]. Telepathology stands as another domain enriched by the advent of AI, facilitating remote slide analysis and enabling collaborative consultations among pathologists irrespective of geographical barriers. This innovation not only enhances diagnostic efficiency but also fosters knowledge exchange and interdisciplinary collaborations [
29]. The transformative synergy between AI and pathology not only augments diagnostic accuracy but also opens avenues for comprehensive data integration. By linking histopathological findings with genomic and clinical data, AI facilitates a deeper understanding of disease mechanisms and fosters the advancement of precision medicine paradigms. The integration of AI is poised to revolutionize pathology practices, ultimately enhancing patient care and outcomes in the years to come.
Cancer Detection and Grading: AI has ushered in a new era in cancer detection and grading, fundamentally transforming pathology practices and significantly enhancing diagnostic accuracy and efficiency. By leveraging advanced algorithms and machine learning techniques, AI revolutionizes the analysis of pathology slides, enabling rapid and precise identification of cancerous cells and anomalies with a level of precision that often surpasses human capabilities (25, 22). Early detection is paramount in effective cancer treatment, and AI plays a pivotal role in enhancing this aspect by swiftly identifying cancerous cells and abnormalities in pathology slides. The ability of AI algorithms to detect subtle morphological changes indicative of malignancy enables pathologists to diagnose cancer at its nascent stages, facilitating timely interventions and improving patient outcomes (23, 17). Furthermore, AI contributes to tumor grading, a critical aspect of cancer diagnosis and treatment planning. By objectively evaluating the microscopic features of cancer cells, AI provides standardized and reproducible assessments of tumor grade, helping clinicians stratify patients based on the aggressiveness of the disease and tailor treatment strategies accordingly (28, 25). The integration of AI in cancer pathology holds immense promise in reducing subjectivity and variability inherent in traditional diagnostic methods. By providing consistent and objective evaluations, AI algorithms enhance the reliability and validity of tumor grading, thereby improving the accuracy of prognostic assessments and treatment decisions (16, 29). Moreover, AI accelerates diagnostic timelines by expediting the analysis of pathology slides, significantly reducing turnaround times and enabling prompt initiation of treatment interventions. This rapidity is particularly crucial in the context of cancer diagnosis, where timely intervention can substantially impact patient outcomes and survival rates [
24,
27]. The integration of AI in cancer detection and grading represents a paradigm shift in pathology practices, offering unparalleled opportunities to improve diagnostic accuracy, efficiency, and patient outcomes. By harnessing the power of advanced algorithms and machine learning techniques, AI enables early detection of cancer, objective evaluation of tumor grade, and personalized treatment planning, ultimately contributing to the ongoing fight against cancer.
Prediction of Patient Outcomes: AI has emerged as a transformative force in healthcare, particularly in predicting patient outcomes through the utilization of advanced analytics and machine learning algorithms. By analyzing vast and diverse sets of patient data, encompassing clinical records, genetic information, imaging results, and more, AI models excel at discerning subtle patterns and correlations that may elude human observation [
30,
31]. This predictive capability spans across various medical conditions, enabling clinicians to anticipate disease progression, treatment responses, and overall prognosis with remarkable accuracy. In healthcare settings, AI-driven predictive analytics play a crucial role in risk stratification, identifying patients at higher risk for complications or adverse events [
32]. Armed with this predictive insight, healthcare providers can implement personalized interventions and allocate resources more effectively, ultimately improving patient outcomes. For chronic conditions such as diabetes or cardiovascular diseases, AI holds the promise of forecasting the likelihood of future complications, thus facilitating preventive measures and timely management strategies [
33]. By leveraging AI-powered predictive models, healthcare teams can proactively intervene to mitigate risks and optimize patient care. In the context of cancer, AI algorithms analyze a plethora of data including tumor characteristics, treatment responses, and patient profiles to predict survival rates, recurrence risks, and treatment outcomes [
34,
35]. This predictive insight not only aids oncologists in developing tailored treatment plans but also enables them to optimize therapeutic strategies based on individual patient needs, thereby improving the overall quality of cancer care. The integration of AI in predicting patient outcomes not only enhances clinical decision-making but also contributes to the ongoing paradigm shift towards personalized medicine. By tailoring treatments to individual patient profiles and predicting outcomes with precision, AI empowers healthcare providers to deliver more effective and patient-centric care.
Speeding up Diagnostics: AI is at the forefront of revolutionizing diagnostic processes in healthcare by significantly enhancing speed and efficiency, particularly in image-based diagnostics such as radiology and pathology. AI applications play a pivotal role in expediting diagnostic workflows by swiftly analyzing and interpreting medical images, including X-rays, MRIs, and pathology slides, with unparalleled speed and accuracy [
36,
37]. In radiology, AI facilitates the rapid detection and interpretation of abnormalities, substantially reducing the time required for diagnostic imaging procedures. By automating image analysis tasks, AI algorithms enable radiologists to efficiently review and interpret a large volume of images, thereby accelerating the diagnostic process and enabling timely interventions and treatment planning [
6,
17]. Similarly, in pathology, AI algorithms revolutionize the analysis of microscopic images, significantly decreasing the time pathologists spend reviewing and interpreting slides. By automating tedious tasks and providing rapid insights into tissue characteristics, AI expedites the diagnostic process, enabling pathologists to make timely and accurate diagnoses [
38,
39]. The speed and efficiency with which AI processes and interprets complex medical data not only accelerate diagnosis but also facilitate quicker decision-making by healthcare professionals. This rapid turnaround time is particularly beneficial in emergency situations or when dealing with rapidly progressing diseases where prompt action is essential. Furthermore, the integration of AI technologies holds the promise of further reducing diagnostic turnaround times and enhancing overall healthcare efficiency as these systems continue to advance. By leveraging AI-driven solutions, healthcare institutions can streamline diagnostic processes, improve patient throughput, and ultimately deliver more timely and effective care to patients. AI-driven technologies are revolutionizing diagnostic workflows in healthcare, significantly enhancing speed and efficiency in image-based diagnostics such as radiology and pathology. By expediting the interpretation of medical images and automating tedious tasks, AI accelerates diagnosis, facilitates quicker decision-making, and ultimately improves patient outcomes.
Quality Assurance and Standardization: AI serves as a cornerstone in maintaining quality assurance and standardization within healthcare, particularly in diagnostic fields such as pathology, radiology, and medical imaging. AI applications contribute significantly to reducing inter-observer variability and enhancing diagnostic accuracy by automating image analysis and pattern recognition tasks [
40,
41]. Through automated processes, AI algorithms provide consistent and objective evaluations, mitigating the inherent subjectivity associated with human interpretation. This standardization introduced by AI not only improves the reliability of diagnostic results but also ensures that patients receive consistent and high-quality care, irrespective of the interpreting physician. By minimizing variability, AI-driven quality assurance processes help identify and rectify errors or discrepancies in diagnostic practices, ultimately enhancing patient care and safety [
42]. Furthermore, AI-driven quality assurance contributes to the ongoing refinement of AI models through continuous learning cycles. By identifying areas for improvement and incorporating feedback, AI systems evolve to deliver even higher levels of diagnostic accuracy and reliability over time [
43]. The standardization introduced by AI not only streamlines diagnostic practices within healthcare but also holds the potential to facilitate large-scale epidemiological studies. By ensuring that the data collected and analyzed is uniform and reliable, AI contributes to the robustness of research outcomes and enables more informed decision-making in public health initiatives [
44,
45]. AI continues to evolve and mature in maintaining high standards of quality assurance in healthcare. By promoting more reliable and reproducible diagnostic practices across diverse medical settings, AI empowers healthcare professionals to deliver optimal care and improve patient outcomes.
Robotics in Surgery:
The integration of AI in robotics has ushered in a new era in the field of surgery, transforming traditional procedures and enhancing precision, efficiency, and patient outcomes. AI-driven robotic surgical systems have become indispensable tools, assisting and augmenting the capabilities of surgeons across various surgical disciplines, from minimally invasive surgeries to complex interventions [
46]. AI algorithms embedded within robotic surgical platforms enable real-time data analysis, empowering surgeons to make immediate decisions based on patient-specific information. This contributes to enhanced surgical precision, reduced error rates, and improved postoperative recovery [
47,
48]. By providing surgeons with critical insights and actionable data during procedures, AI-driven robotics elevate the quality of surgical care and patient safety. One of the key advantages of AI in robotic surgery is its ability to aid surgeons in navigating intricate anatomical structures with unparalleled accuracy. In procedures requiring precise manipulation and dexterity, AI-guided robotic systems offer unparalleled control and stability, minimizing the risk of inadvertent tissue damage and optimizing surgical outcomes [
49,
50]. Furthermore, AI supports predictive analytics during surgery, leveraging real-time data streams to anticipate potential complications and recommend optimal courses of action. By continuously monitoring physiological parameters and surgical variables, AI-driven robotic systems enable proactive intervention, mitigating risks and ensuring a smoother intraoperative experience [
51,
52].
A prime example of AI-enabled robotic surgery is the da Vinci Surgical System, a widely adopted platform that leverages AI to assist surgeons in performing complex procedures with robotic precision. The system's intuitive interface and AI-guided instrumentation facilitate intricate maneuvers, enabling surgeons to execute tasks with unparalleled accuracy and control [
53,
54]. By combining the expertise of surgeons with the analytical capabilities of AI-driven robotic systems, the da Vinci Surgical System has revolutionized surgical practice, making procedures less invasive, more precise, and ultimately safer for patients. As AI technology continues to advance, the integration of robotics and AI in surgery holds immense promise for the future. By refining surgical techniques, expanding the scope of minimally invasive procedures, and enhancing patient care, AI-driven robotic systems are poised to reshape the landscape of surgery in the years to come [
55]. The integration of AI in robotics has revolutionized the field of surgery, empowering surgeons with advanced tools and capabilities to deliver optimal patient care. Through real-time data analysis, precise navigation, and predictive analytics, AI-driven robotic systems enhance surgical precision, efficiency, and safety, ultimately improving patient outcomes and advancing the practice of surgery.
The integration of Artificial Intelligence (AI) and precision medicine:
The integration of AI and precision medicine represents a transformative approach to healthcare that holds immense promise for revolutionizing diagnosis, treatment, and patient care. Precision medicine, also known as personalized medicine, focuses on tailoring medical interventions to individual characteristics, such as genetic makeup, lifestyle, and environmental factors. AI, on the other hand, refers to the simulation of human intelligence processes by machines, particularly through algorithms that analyze vast amounts of data to derive insights and make predictions. Combining these two fields offers unprecedented opportunities to enhance healthcare delivery and outcomes. [
56] At the heart of precision medicine lies the concept of biomarkers, which are biological indicators that can be used to identify disease risk, progression, and response to treatment. [
57] AI algorithms excel at analysing complex datasets, including genomic, proteomic, and clinical data, to identify relevant biomarkers and patterns that may not be apparent to human observers. By leveraging AI, healthcare providers can develop more accurate and reliable biomarker-based tests for diagnosing diseases, stratifying patients based on their likelihood of responding to specific treatments, and predicting disease outcomes. [
58]
One area where the integration of AI and precision medicine has shown significant promise is in oncology. [
59] Cancer is a highly heterogeneous disease, with variations in tumour biology and patient characteristics influencing treatment response and prognosis. AI-powered algorithms can analyse multi-omic data, such as genomics, transcriptomics, and imaging data, to identify molecular signatures associated with different cancer subtypes and predict patient responses to various therapies. This enables oncologists to tailor treatment plans to individual patients, optimizing therapeutic efficacy while minimizing side effects. [
60] Moreover, AI-driven precision oncology platforms can facilitate the discovery of novel therapeutic targets and the development of targeted therapies and immunotherapies. By mining large-scale genomic databases and integrating diverse datasets, AI algorithms can identify genetic alterations and pathways driving cancer progression, guiding the development of precision medicines that specifically target these molecular aberrations. This approach has led to the emergence of breakthrough cancer treatments, such as PARP inhibitors for BRCA-mutated breast and ovarian cancers, and immune checkpoint inhibitors for various malignancies. [
61] Beyond oncology, the integration of AI and precision medicine is also transforming other areas of healthcare, including cardiology, neurology, and infectious diseases. In cardiology, AI algorithms can analyze electrocardiogram (ECG) data to detect subtle abnormalities indicative of cardiac conditions, such as arrhythmias and ischemia, enabling early intervention and risk stratification. [
62,
63] Similarly, in neurology, AI-powered imaging analysis tools can assist in the early diagnosis of neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease, by detecting structural and functional brain changes that precede clinical symptoms. [
64]
In infectious diseases, AI-driven models can analyze epidemiological data, genomic sequences of pathogens, and clinical data from infected individuals to track disease transmission, predict outbreaks, and optimize treatment strategies. [
65] During the COVID-19 pandemic, AI algorithms played a crucial role in accelerating the development of diagnostics, therapeutics, and vaccines, facilitating rapid responses to the evolving public health crisis. [
66] Despite the tremendous potential of AI-driven precision medicine, several challenges remain to be addressed. These include ensuring data privacy and security, overcoming barriers to data interoperability and integration, addressing algorithm bias and interpretability issues, and navigating regulatory and ethical considerations. Additionally, there is a need for robust validation and clinical translation of AI-driven models to ensure their reliability and effectiveness in real-world healthcare settings. The integration of AI and precision medicine represents a paradigm shift in healthcare, offering unprecedented opportunities to improve patient outcomes, enhance diagnostic accuracy, and accelerate the development of personalized therapies. By harnessing the power of AI to analyze vast amounts of data and derive actionable insights, healthcare providers can deliver more precise, effective, and personalized care to patients, ushering in a new era of precision medicine. However, realizing the full potential of AI-driven precision medicine will require collaboration across disciplines, investment in infrastructure and technology, and ongoing efforts to address the technical, regulatory, and ethical challenges inherent in this transformative approach to healthcare.
Benefits of AI in Precision Medicine:
Personalized Treatment: AI holds immense potential in revolutionizing personalized treatment approaches across various medical specialties. By leveraging AI algorithms to analyze vast amounts of patient data, including genomic, clinical, and lifestyle information, healthcare providers can tailor treatments to individual patients with unprecedented precision and effectiveness. [
67] One of the primary benefits of AI in personalized treatment is the ability to predict treatment responses and outcomes based on patient-specific characteristics. AI algorithms can identify biomarkers and patterns within patient data that correlate with treatment efficacy, allowing clinicians to select the most appropriate therapy for each patient. For example, in oncology, AI-driven models can analyze tumor genomic profiles to predict which patients are likely to respond to specific cancer therapies, such as targeted therapies or immunotherapies. [
68] This personalized approach maximizes treatment efficacy while minimizing adverse effects, leading to improved patient outcomes and quality of life. Moreover, AI facilitates the discovery of novel therapeutic targets and the development of targeted therapies tailored to individual patient profiles. By mining large-scale genomic and proteomic datasets, AI algorithms can identify genetic alterations and molecular pathways driving disease progression, enabling the development of precision medicines that specifically target these molecular aberrations. [
69,
70] This approach has led to the emergence of breakthrough treatments for various diseases, including cancer, cardiovascular disorders, and rare genetic conditions. Furthermore, AI-driven personalized treatment approaches enable clinicians to optimize treatment regimens and dosages based on real-time patient data and feedback. By continuously analyzing patient responses to treatment and adjusting therapy parameters accordingly, AI algorithms can ensure that patients receive the most effective and personalized care throughout their treatment journey. [
71] Overall, the integration of AI in personalized treatment holds the promise of revolutionizing healthcare delivery by providing tailored and effective therapies that address the unique needs of each patient. By harnessing the power of AI to analyze patient data and derive actionable insights, healthcare providers can optimize treatment outcomes, minimize adverse effects, and improve patient satisfaction and quality of life. As AI technology continues to advance, the potential for personalized treatment approaches to transform healthcare delivery and outcomes will only continue to grow.
Early Disease Detection: AI plays a pivotal role in early disease detection by leveraging advanced algorithms to analyze vast amounts of data and identify subtle patterns indicative of disease onset. One of the primary benefits of AI in early disease detection is its ability to sift through complex datasets, including genomic, proteomic, imaging, and clinical data, to uncover biomarkers and signatures associated with various diseases at their earliest stages. [
72] In medical imaging, AI algorithms can analyze radiological images, such as X-rays, MRIs, and CT scans, to detect abnormalities and lesions that may indicate the presence of disease. For example, AI-powered mammography and lung cancer screening tools can assist radiologists in identifying suspicious findings, such as tumors or nodules, at early stages when treatment is most effective. [
73] Moreover, AI-driven diagnostic tools can analyze biochemical markers and physiological data to detect changes indicative of disease progression or risk. For instance, AI algorithms can analyze blood glucose levels, heart rate variability, and other physiological parameters to predict the onset of conditions such as diabetes, cardiovascular diseases, and neurodegenerative disorders. [
74,
75] By enabling early detection and intervention, AI-powered systems have the potential to significantly improve patient outcomes, reduce healthcare costs, and alleviate the burden on healthcare systems. As AI technology continues to advance, the scope and accuracy of early disease detection tools will further enhance, ushering in a new era of preventive and personalized medicine.
Improved Diagnosis: AI enhances diagnostic accuracy by analyzing vast datasets to identify subtle patterns indicative of disease. AI-driven algorithms, trained on diverse datasets, excel in medical imaging analysis, pathology interpretation, and diagnostic decision-making. By integrating AI into clinical practice, healthcare professionals benefit from augmented diagnostic capabilities, leading to earlier and more accurate disease detection and characterization. [
76] Through continuous learning and refinement, AI contributes to improved patient outcomes and optimized healthcare delivery. Enhanced Drug Discovery: AI accelerates the drug discovery process by analyzing large datasets to identify potential drug targets, predict drug interactions, and optimize drug formulations. This leads to the development of more effective and targeted therapies. [
77]
Precision Oncology: AI-driven precision oncology represents a groundbreaking approach in cancer care, leveraging advanced algorithms to analyze genomic, proteomic, and clinical data to tailor treatment strategies to individual patients. By integrating AI into oncology practice, healthcare providers can identify molecular signatures associated with different cancer subtypes, predict patient responses to specific therapies, and optimize treatment regimens. AI-driven models analyze complex genomic data to uncover genetic alterations and pathways driving cancer progression, guiding the development of targeted therapies and immunotherapies. [
78] Additionally, AI-powered imaging analysis tools assist in tumor detection, characterization, and response assessment, facilitating personalized treatment planning. The implementation of AI-driven precision oncology has led to significant advancements in cancer treatment, with improved outcomes and reduced toxicity for patients. By selecting the most effective therapies based on molecular profiles and predicting treatment responses, AI enhances treatment efficacy while minimizing adverse effects. Furthermore, AI-driven approaches enable the discovery of novel therapeutic targets and the development of innovative treatment modalities, leading to personalized and more effective cancer care. [
79,
80] As AI technology continues to evolve, the potential for AI-driven precision oncology to transform cancer diagnosis, treatment, and outcomes remains unparalleled, ushering in a new era of precision medicine in oncology.
Public Health Surveillance: Public health surveillance involves monitoring, analyzing, and interpreting health-related data to detect and control the spread of diseases, assess health trends, and inform public health interventions. AI is revolutionizing public health surveillance by enhancing the speed, accuracy, and efficiency of data analysis and interpretation. AI-powered algorithms can analyze diverse datasets, including epidemiological data, genomic sequences of pathogens, clinical records, and social media posts, to identify patterns and trends indicative of disease outbreaks and public health threats. For example, AI can analyze real-time data streams from sources such as electronic health records, hospital admissions, and symptom reporting systems to detect early warning signs of infectious disease outbreaks. Moreover, AI facilitates predictive modeling and risk assessment, enabling public health authorities to anticipate disease spread, allocate resources effectively, and implement targeted interventions to mitigate health risks. [
81] AI-driven predictive analytics can forecast disease trajectories, identify high-risk populations, and optimize vaccination campaigns and resource allocation. Additionally, AI enhances disease surveillance through automated data processing and anomaly detection, enabling rapid identification of unusual patterns or clusters that may signal emerging threats or outbreaks. [
82] AI-powered surveillance systems can continuously monitor diverse data sources, flagging anomalies in real-time and enabling prompt investigation and response. [
83] Overall, the integration of AI into public health surveillance holds immense promise for enhancing disease detection, response, and prevention efforts. By leveraging AI-driven analytics and predictive modeling, public health authorities can improve situational awareness, allocate resources more effectively, and implement timely interventions to protect population health and prevent the spread of infectious diseases. [
84] As AI technology continues to advance, the potential for AI-driven public health surveillance to revolutionize disease control and prevention efforts globally is vast.
Challenges of AI in Precision Medicine:
Data Quality and Availability: One of the primary challenges faced by Artificial Intelligence (AI) in healthcare is the quality and availability of data. AI relies on high-quality, diverse datasets for training and validation, yet healthcare data often falls short in meeting these requirements. Healthcare data is frequently fragmented across different systems, institutions, and formats, making it challenging to access and integrate into AI models. Moreover, healthcare data may be incomplete, containing gaps or missing values that can compromise the performance of AI algorithms. [
85] Additionally, data in healthcare settings may be subject to various biases, including demographic disparities, clinical variability, and sampling biases, which can skew the results of AI-driven analyses and predictions. [
86] Addressing these challenges requires concerted efforts to improve data quality, promote data sharing and interoperability, and mitigate biases in healthcare data to enable the development and deployment of robust and reliable AI-driven models in healthcare.
Data Privacy and Security: Data privacy and security present significant challenges for AI in healthcare, protecting patient privacy and ensuring the confidentiality of sensitive health information are paramount concerns in healthcare settings. AI algorithms require access to large volumes of patient data for training and analysis, raising concerns about data breaches and unauthorized access. Compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR) imposes strict requirements on data handling, storage, and sharing, further complicating AI development and deployment. Additionally, the inherent complexity of AI algorithms and their reliance on sensitive patient data increase the risk of potential vulnerabilities and cyberattacks, necessitating robust cybersecurity measures and ethical guidelines to safeguard patient privacy and prevent data breaches in AI-driven healthcare applications. Algorithm Bias and Interpretability, AI models may exhibit biases inherent in the training data, leading to disparities in healthcare outcomes. Additionally, the complexity of AI algorithms makes their decisions difficult to interpret and explain, raising concerns about trust and accountability. [
87,
88]
Regulatory and Ethical Considerations: Regulatory and ethical considerations pose significant challenges for AI in healthcare. The rapid advancement of AI technology has outpaced the development of regulatory frameworks, leading to uncertainty and inconsistency in oversight. Ethical concerns arise regarding transparency, accountability, and fairness in AI-driven decision-making, particularly in sensitive areas such as diagnosis, treatment, and patient care. [
89,
90] Balancing innovation with patient safety and ethical principles requires clear guidelines and standards for AI development, deployment, and governance. [
91] Collaboration among stakeholders, including policymakers, regulators, healthcare professionals, and technology developers, is essential to address these challenges and ensure the responsible and ethical use of AI in healthcare.
Integration with Clinical Workflow: Integrating AI-driven tools into clinical workflows presents logistical challenges, including interoperability with electronic health records (EHRs), user interface design, and training healthcare professionals. Seamless integration with existing systems and workflows is essential to ensure the efficient adoption and utilization of AI tools in clinical practice. User-friendly interfaces and intuitive design are crucial for facilitating acceptance and usability among healthcare providers. Furthermore, comprehensive training and ongoing support are necessary to empower healthcare professionals with the skills and confidence to effectively leverage AI-driven tools in patient care, optimizing their impact on clinical decision-making and patient outcomes. [
92]
Cost and Accessibility: AI-driven precision medicine necessitates substantial investment in technology, infrastructure, and training, posing challenges in terms of cost and accessibility. The adoption of AI technologies often requires substantial financial resources to procure advanced hardware, software, and data infrastructure, along with ongoing maintenance and support costs. [
93] Additionally, healthcare organizations must allocate resources for staff training and education to ensure proficiency in using AI-driven tools effectively. However, the cost of implementing AI-driven precision medicine extends beyond initial investments to encompass ongoing operational expenses, including data management, algorithm refinement, and compliance with regulatory requirements. [
94] These financial considerations may present barriers to adoption, particularly for healthcare institutions with limited budgets or competing priorities. Moreover, ensuring equitable access to AI-driven healthcare solutions is essential to address disparities in healthcare delivery. Underserved communities, including rural areas and low-income populations, may face challenges in accessing AI technologies due to financial constraints, limited infrastructure, and lack of technical expertise. Efforts to promote inclusivity and accessibility in AI-driven healthcare solutions are essential to bridge the digital divide and ensure that all patients benefit from the potential of precision medicine to improve health outcomes and reduce disparities. [
95,
96] Collaboration among stakeholders, including government agencies, healthcare organizations, technology providers, and community leaders, is critical to address these challenges and promote the widespread adoption of AI-driven precision medicine while ensuring affordability and accessibility for all. Addressing these challenges requires collaboration among healthcare stakeholders, including clinicians, researchers, policymakers, technology developers, and patients. By working together to overcome these obstacles, we can harness the full potential of AI and precision medicine to improve patient outcomes, enhance healthcare delivery, and advance the practice of medicine.
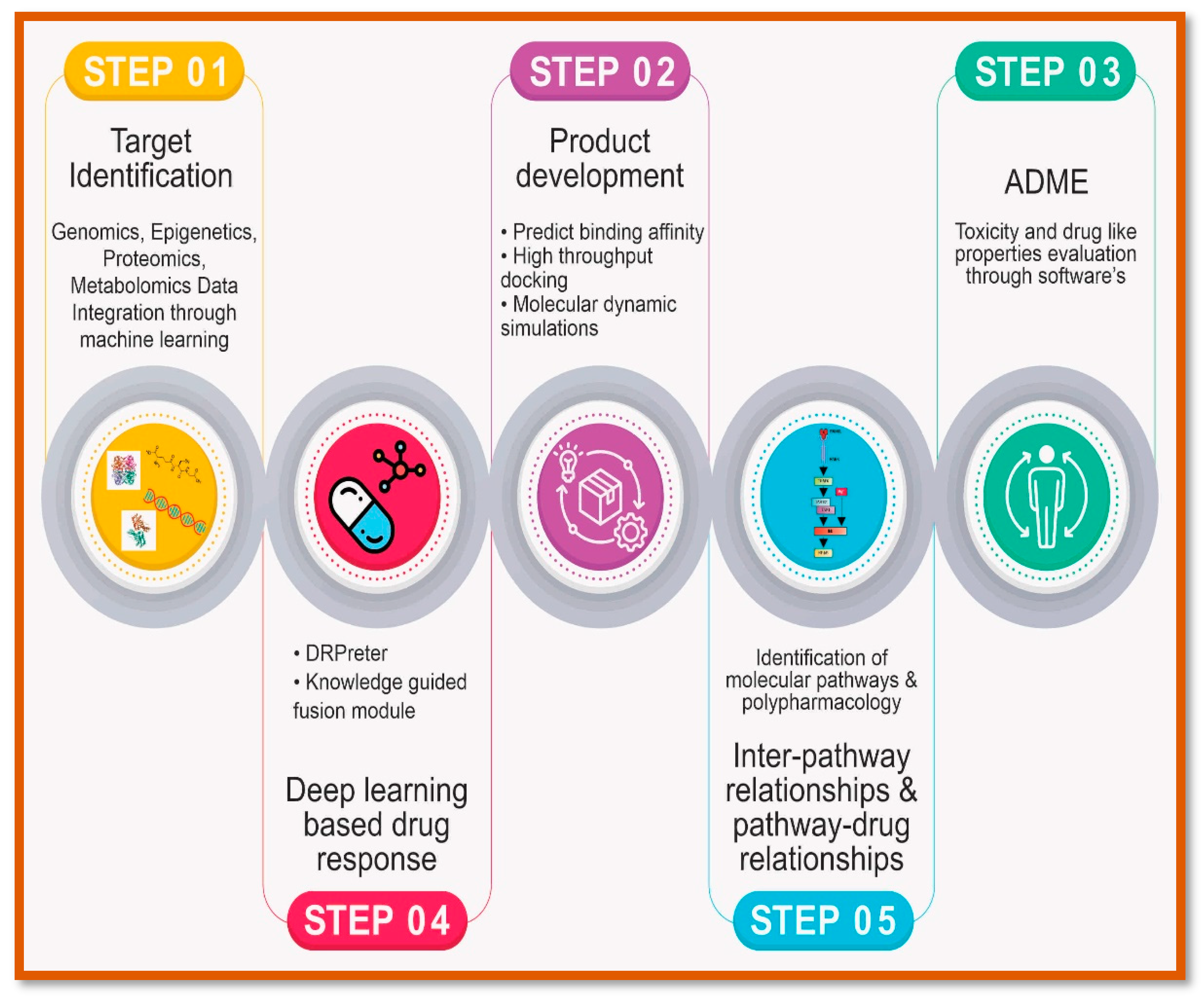
Future Prospects of AI and precision medicine:
The future prospects of AI and precision medicine hold immense promise for transforming healthcare delivery, diagnosis, treatment, and patient care. Ongoing research and technological advancements are driving innovation in AI-driven precision medicine, leading to potential breakthroughs that will continue to shape the future of healthcare (
Figure 4). One area of ongoing research is the development of AI-driven predictive analytics models for disease prevention and early intervention. These models leverage machine learning algorithms to analyze diverse datasets, including genomic, clinical, and environmental data, to identify individuals at risk of developing various diseases. By predicting disease onset or progression before symptoms manifest, AI-driven predictive analytics enable proactive interventions and personalized preventive strategies, ultimately improving health outcomes and reducing healthcare costs [
101]. Technological advancements in AI algorithms, such as deep learning and natural language processing, are enhancing the accuracy and reliability of AI-driven diagnostics and treatment recommendations. Deep learning algorithms can analyze complex medical imaging data, such as MRI and CT scans, with unprecedented precision, assisting radiologists and pathologists in diagnosing diseases at earlier stages with greater accuracy (Esteva et al., 2019). Furthermore, natural language processing algorithms can extract valuable insights from unstructured clinical notes and literature, facilitating evidence-based decision-making and advancing medical knowledge [
102].
Another area of research focuses on the integration of AI with other emerging technologies, such as genomics, proteomics, and wearable devices, to enable personalized and proactive healthcare. AI-driven genomic analysis tools can interpret vast amounts of genomic data to identify genetic variants associated with disease risk, treatment response, and drug metabolism, guiding precision medicine approaches tailored to individual patients [
103]. Additionally, wearable devices equipped with AI algorithms can continuously monitor physiological parameters, detect early signs of disease or deterioration, and provide real-time feedback to patients and healthcare providers, empowering individuals to take proactive control of their health [
104]. The combination of AI and precision medicine holds the potential to revolutionize healthcare delivery by shifting towards a more patient-centered, preventive, and proactive approach. By leveraging AI-driven insights and personalized treatment strategies, healthcare providers can optimize resource allocation, improve clinical outcomes, and enhance patient satisfaction and engagement. Furthermore, AI-driven precision medicine has the potential to address healthcare disparities by delivering tailored interventions to underserved populations and reducing inequities in access to quality care [
105]. The future of AI and precision medicine is characterized by ongoing research, technological advancements, and potential breakthroughs that will continue to shape the landscape of healthcare (
Figure 4).
Ethical Considerations of AI in Precision Medicine:
The integration of Artificial Intelligence (AI) in precision medicine raises various ethical considerations that must be carefully addressed to ensure responsible and equitable deployment of these technologies. Key ethical issues include data privacy, consent, transparency, fairness, and accountability. AI-driven precision medicine relies on vast amounts of sensitive health data, including genomic, clinical, and lifestyle information. Protecting patient privacy and confidentiality is paramount to maintain trust and ensure compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) and the General Data Protection Regulation (GDPR). Healthcare organizations must implement robust data encryption, anonymization techniques, and access controls to safeguard patient data from unauthorized access or misuse [
106].
Informed consent is essential when utilizing patient data for AI-driven precision medicine research or clinical applications. Patients should be fully informed about how their data will be used, the potential risks and benefits, and their rights regarding data sharing and privacy. Obtaining explicit consent from patients ensures respect for individual autonomy and fosters trust between patients, healthcare providers, and researchers [
107]. AI algorithms used in precision medicine should be transparent and explainable to facilitate understanding and trust among stakeholders. Clinicians and patients should have access to information about how AI models make predictions or recommendations, including the data inputs, decision-making processes, and potential biases. Transparent AI systems enable clinicians to validate predictions, understand limitations, and make informed decisions about patient care [
108].
AI-driven precision medicine must address issues of bias and fairness to ensure equitable healthcare delivery. Biases in training data or algorithms can lead to disparities in diagnosis, treatment, and outcomes among different demographic groups. Developers must carefully evaluate AI models for bias, mitigate biases through algorithmic adjustments or data pre-processing techniques, and actively promote fairness and inclusivity in AI-driven healthcare solutions [
109]. Clear accountability mechanisms are essential to address liability and responsibility in AI-driven precision medicine. Healthcare organizations, developers, and regulators must establish guidelines and standards for the responsible development, deployment, and monitoring of AI systems. Transparent reporting of AI performance metrics, regular audits, and mechanisms for addressing errors or adverse events ensure accountability and mitigate potential harms to patients [
110]. Therefore, addressing the ethical implications of AI in precision medicine requires a comprehensive approach that prioritizes data privacy, informed consent, transparency, fairness, and accountability. By adhering to ethical principles and guidelines, we can ensure the responsible and equitable deployment of AI technologies in healthcare, ultimately benefiting patients and advancing medical research and practice.
Conclusion:
In conclusion, the present review highlights the transformative potential of AI in conjunction with precision medicine and its implications for the future of healthcare. AI-driven precision medicine represents a paradigm shift in healthcare delivery, offering personalized and proactive approaches to disease prevention, diagnosis, treatment, and patient care. AI holds promise in revolutionizing various aspects of healthcare, including disease detection, diagnosis, treatment selection, and patient management. AI algorithms, such as machine learning and deep learning, analyse vast amounts of healthcare data, including genomic, clinical, imaging, and wearable device data, to generate actionable insights and support clinical decision-making. By leveraging AI-driven analytics and predictive modelling, healthcare providers can identify individuals at risk of developing diseases, predict treatment responses, and tailor interventions to individual patient needs, ultimately improving health outcomes and quality of life. AI-driven precision medicine promotes data integration, interoperability, and collaboration across healthcare systems, enabling seamless exchange of information and coordinated care delivery. Moreover, AI algorithms enhance diagnostic accuracy, reduce diagnostic errors, and optimize treatment strategies, leading to more effective and efficient healthcare delivery. The transformative potential of AI in precision medicine extends beyond clinical practice to encompass research, drug discovery, public health, and healthcare policy. AI-driven approaches accelerate biomedical research by analyzing complex datasets, identifying disease biomarkers, and uncovering novel therapeutic targets. Additionally, AI technologies enable the development of innovative healthcare solutions, such as AI-driven drug discovery platforms, remote patient monitoring systems, and predictive analytics models for disease surveillance and outbreak prediction.
Looking ahead, the integration of AI and precision medicine is poised to shape the future of healthcare by driving innovation, improving patient outcomes, and advancing population health. However, realizing the full potential of AI in healthcare requires addressing various challenges, including data privacy, consent, transparency, fairness, and accountability. By prioritizing ethical considerations and responsible deployment of AI technologies, stakeholders can harness the transformative power of AI to revolutionize healthcare delivery and create a more equitable and sustainable healthcare system for all. Therefore, it represents a ground-breaking approach to healthcare that has the potential to revolutionize clinical practice, biomedical research, and public health. By leveraging AI-driven insights and personalized treatment strategies, healthcare providers can optimize patient care, improve health outcomes, and advance the practice of medicine in the 21st century.