Submitted:
27 November 2024
Posted:
02 December 2024
You are already at the latest version
Abstract
Keywords:
Declaration of Authorship
- An online market for medicine ” and the work presented in it are my own. I confirm that:
- This work was done wholly or mainly while in candidature for a research de- gree at this University.
- Where any part of this thesis has previously been submitted for a degree or any other qualification at this University or any other institution, this has been clearly stated.
- Where I have consulted the published work of others, this is always clearly attributed.
- Where I have quoted from the work of others, the source is always given. With the exception of such quotations, this thesis is entirely my own work.
Acknowledgements
Contents
| Declaration of Authorship | iii | ||
| Abstract | v | ||
| Acknowledgements | vii | ||
| 1 | Introduction | 1 | |
| 1.1 | Background of the research................................................................................ | 1 | |
| 1.2 | Aim of this research............................................................................................. | 2 | |
| 1.3 | Research Question................................................................................................ | 2 | |
| 1.3.1 Sub research question............................................................................. | 2 | ||
| 1.4 | Objectives.............................................................................................................. | 2 | |
| 1.5 | Scope and limitations of the research................................................................ | 3 | |
| 1.6 | Dissertation road map......................................................................................... | 3 | |
| 2 | Literature Review | 5 | |
| 2.1 | Existing distribution system............................................................................... | 5 | |
| 2.2 | Unavailability of medicines................................................................................ | 5 | |
| 2.3 | Distribution and pricing issues of pharmaceutical drugs in Ireland............. | 5 | |
| 2.3.1 Differential pricing of drugs and its effects on public........................ | 6 | ||
| 2.4 | Generic medicines, its importance and policy of Ireland government on promoting generic drugs............................................................................... | 6 | |
| 2.5 | Classification of pharmaceutical drugs............................................................. | 7 | |
| 2.6 | Effect of Common market place on the pricing of goods................................ | 8 | |
| 2.7 | Legal Scope of the Online pharmacies In European Union............................ | 9 | |
| 2.8 | Online Virtual Market as the common market place....................................... | 9 | |
| 2.9 | Existing Online retailers In Ireland.................................................................. | 10 | |
| 2.10 | Challenges and solutions.................................................................................. | 11 | |
| 2.11 | Summary............................................................................................................. | 11 | |
| 3 | Research Methodology and System Analysis | 13 | |
| 3.1 | Research philosophy.......................................................................................... | 13 | |
| 3.2 | Research Approach............................................................................................ | 13 | |
| 3.3 | Research ethics................................................................................................... | 13 | |
| 3.4 | Preliminary system planning............................................................................ | 14 | |
| 3.4.1 Data collection methodology............................................................... | 14 | ||
| 3.4.2 Questionnaire......................................................................................... | 14 | ||
| 3.4.3 Data analysis procedures...................................................................... | 14 | ||
| 3.4.4 Time horizon.......................................................................................... | 15 | ||
| 3.4.5 Twelve-week timeline.......................................................................... | 15 | ||
| 3.5 | System analysis.................................................................................................. | 15 | |
| 3.5.1 Identify problem and Observations.................................................... | 15 | ||
| 3.5.2 Feasibility study.................................................................................... | 16 | ||
| 3.6 Detailed System Study...................................................................................... | 16 | ||
| 3.7 Summary............................................................................................................. | 16 | ||
| 4 | Artefact Design | 19 | |
| 4.1 | Logical design.................................................................................................... | 19 | |
| 4.1.1 System Workflows................................................................................ | 20 | ||
| 4.2 | Physical design................................................................................................... | 21 | |
| 4.2.1 Product dashboard creation................................................................. | 21 | ||
| 4.2.2 Checkout creation................................................................................. | 22 | ||
| 4.2.3 Payment-page creation......................................................................... | 22 | ||
| 4.2.4 Modules.................................................................................................. | 23 | ||
| 4.3 | Architecture Design........................................................................................... | 25 | |
| 4.4 | Detailed Design.................................................................................................. | 26 | |
| 4.4.1 Software Development Methodology................................................. | 26 | ||
| 4.4.2 Minimum Viable Product..................................................................... | 27 | ||
| 4.4.3 Software Requirement Specification................................................... | 28 | ||
| 4.4.4 U M L Diagram...................................................................................... | 28 | ||
| 4.5 | Development technologies and tools.............................................................. | 29 | |
| 4.5.1 Technologies.......................................................................................... | 29 | ||
| 4.5.2 Development Environment(s)............................................................. | 29 | ||
| 4.5.3 Configuration Management tool......................................................... | 29 | ||
| 4.6 | Database design................................................................................................. | 29 | |
| 4.6.1 Business rules........................................................................................ | 29 | ||
| 4.7 | Summary............................................................................................................. | 30 | |
| 5 | Artefact Development | 31 | |
| 5.1 | Web Application Development........................................................................ | 31 | |
| 5.1.1 Models.................................................................................................... | 31 | ||
| 5.1.2 Controllers.............................................................................................. | 31 | ||
| Admin controller................................................................................... | 31 | ||
| Checkout controller............................................................................... | 31 | ||
| Account Controller................................................................................ | 31 | ||
| Home controller..................................................................................... | 31 | ||
| 5.1.3 Views...................................................................................................... | 32 | ||
| The landing page................................................................................... | 32 | ||
| Shopping cart page............................................................................... | 32 | ||
| Payment page........................................................................................ | 32 | ||
| Information window............................................................................. | 32 | ||
| Contact admin page.............................................................................. | 32 | ||
| Admin page........................................................................................... | 32 | ||
| Authentication page.............................................................................. | 32 | ||
| 5.2 | Mobile Application............................................................................................ | 33 | |
| 5.3 | Authorization and Authentication.................................................................. | 33 | |
| 5.4 | Application dashboard...................................................................................... | 33 | |
| 5.5 | Summary............................................................................................................. | 34 | |
| 6 | Data Analysis and Findings | 35 | |
| 6.1 | Introduction........................................................................................................ | 35 | |
| 6.2 | Findings............................................................................................................... | 35 | |
| 6.2.1 General size of the market................................................................... | 35 | ||
| 6.2.2 Awareness among the people.............................................................. | 35 | ||
| 6.2.3 Customer attitude and trends.............................................................. | 37 | ||
| Change in perspective.......................................................................... | 38 | ||
| Technology Adaptiveness Index......................................................... | 39 | ||
| Privacy Concern Index......................................................................... | 40 | ||
| 6.3 | Summary............................................................................................................. | 41 | |
| 7 | Discussions | 43 | |
| 7.1 | General perspectives.......................................................................................... | 43 | |
| 7.1.1 Medicine prices will be more transparent and market-oriented . | 43 | ||
| 7.1.2 Centralization of pharmaceutical industry........................................ | 43 | ||
| 7.1.3 Expand the pharmaceutical logistics market..................................... | 43 | ||
| 7.1.4 A complete service-oriented model. ................................................... | 44 | ||
| 7.1.5 Improved retail drugstores profitability............................................. | 44 | ||
| 7.1.6 A panel for information innovation.................................................... | 44 | ||
| 7.1.7 Health Benefits....................................................................................... | 44 | ||
| 7.2 | Social benefits..................................................................................................... | 45 | |
| 7.2.1 Better healthcare system....................................................................... | 45 | ||
| 7.2.2 A big financial relief.............................................................................. | 45 | ||
| 7.3 | System Feedback................................................................................................ | 45 | |
| 7.4 | System Benefits................................................................................................... | 46 | |
| 7.4.1 An easy window for showcasing products....................................... | 46 | ||
| 7.4.2 Review and ratings................................................................................ | 46 | ||
| 7.4.3 Price comparison................................................................................... | 46 | ||
| 7.5 | Summary............................................................................................................. | 46 | |
| 8 | Conclusion and Future scope | 47 | |
| 8.1 | Conclusion.......................................................................................................... | 47 | |
| 8.2 | Future scope........................................................................................................ | 48 | |
| A | Prototyping | 55 | |
| A.1 | Mobile Application - Prototype........................................................................ | 55 | |
| B | Questionnaire | 57 | |
| B.1 | Face to face interview Questionnaire for Pharmaceutical company Rep- resentative.................................................................................................. | 57 | |
| B.2 | Answers............................................................................................................... | 57 | |
| B.3 | Face to face interview Questionnaire for Pharmaceutical product re- tailers................................................................................................................... | 57 | |
| B.4 | General Questionnaire....................................................................................... | 58 | |
| C | Source code | 69 | |
| D | Application Screenshots | 71 | |
List of Figures
| 4.1 | EHR based Online pharmacy........................................................................... | 19 |
| 4.2 | Doctor Activity Diagram................................................................................... | 20 |
| 4.3 | Action Diagram.................................................................................................. | 20 |
| 4.4 | System overall workflow diagram................................................................... | 21 |
| 4.5 | Landing page-Activity diagram....................................................................... | 21 |
| 4.6 | Activity diagram- Checkout............................................................................. | 22 |
| 4.7 | Activity diagram- Payment.............................................................................. | 22 |
| 4.8 | Use-case diagram............................................................................................... | 23 |
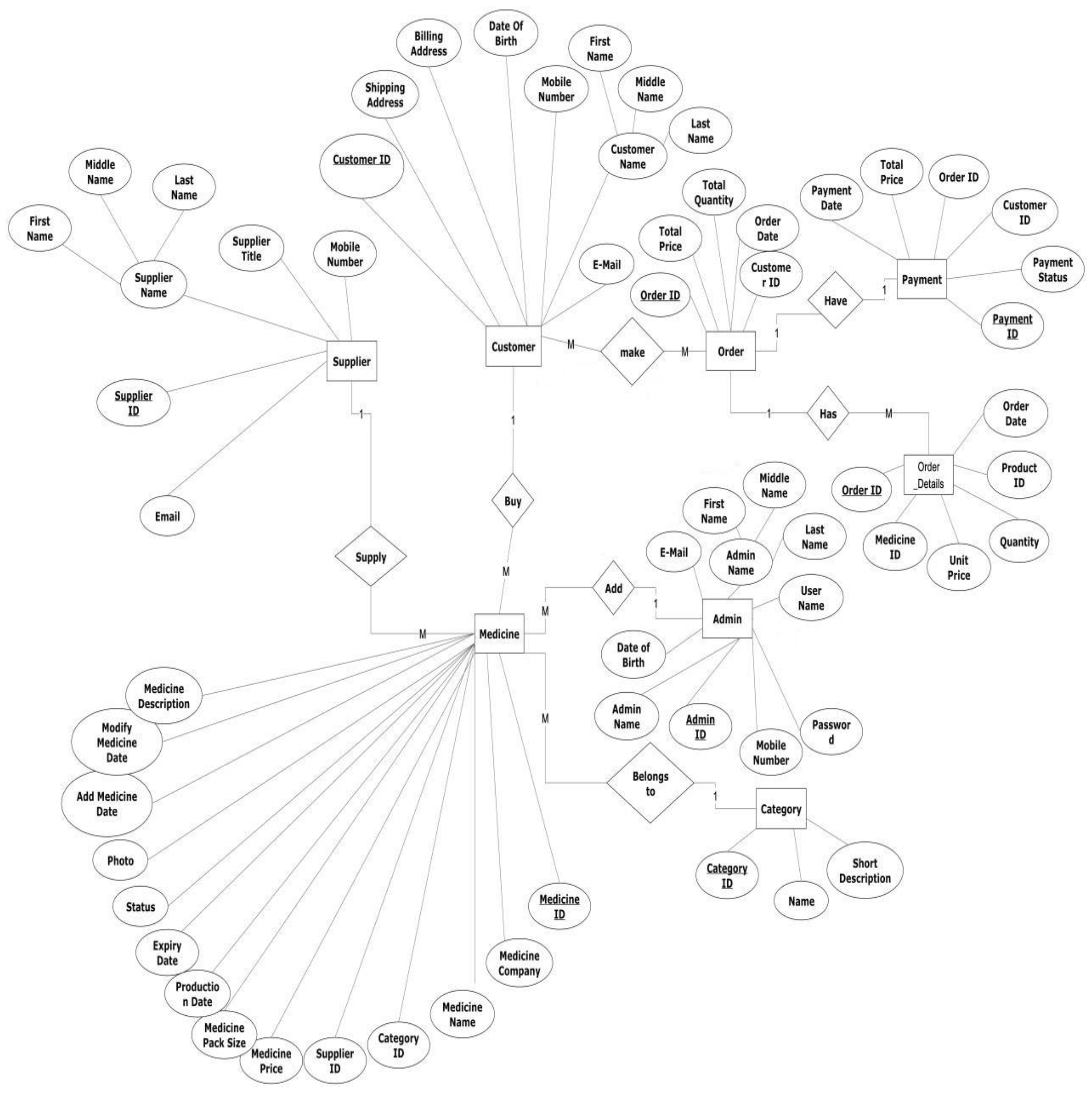
| 4.9 | Entity relationship diagram.............................................................................. | 24 |
| 4.10 | Incremental Development................................................................................. | 26 |
| 4.11 | Product prototyping.......................................................................................... | 26 |
| 4.12 | Quality management process........................................................................... | 27 |
| 4.13 | Development process........................................................................................ | 27 |
| 4.14 | UML class diagram............................................................................................ | 28 |
| 4.15 | Database-tables................................................................................................... | 30 |
| 4.16 | Database-Diagram.............................................................................................. | 30 |
| 5.1 OWIN Schematic representation......................................................................33 | ||
| 5.2 Application dashboard......................................................................................34 | ||
| 6.1 | Survey candidates requiring continuous medication.................................... | 35 |
| 6.2 | Awareness on medication identification based on chemical composition | 36 |
| 6.3 | Awareness on the existence of generic medications and its price ad- vantage................................................................................................................ | 36 |
| 6.4 | Generic medicine prescription trends............................................................. | 37 |
| 6.5 | Public awareness on EHR and e-prescription................................................ | 37 |
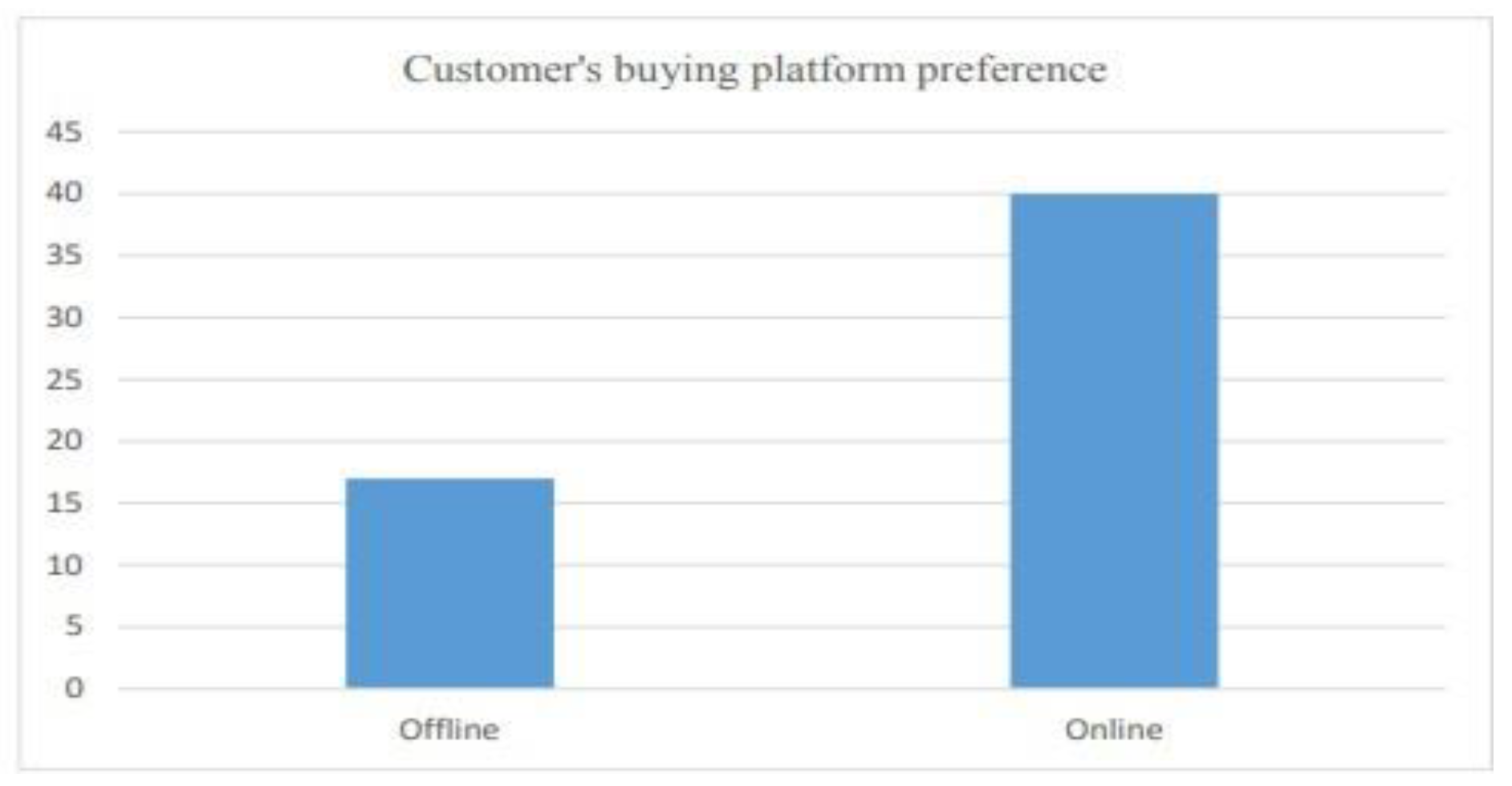
| 6.6 | Buying platform preference of customers....................................................... | 38 |
| 6.7 | Customer opinion on online medical stores................................................... | 38 |
| 6.8 | Trends in opting generic medications.............................................................. | 38 |
| 6.9 | Internet usage statistics..................................................................................... | 39 |
| 6.10 | Internet usage purpose statistics...................................................................... | 39 |
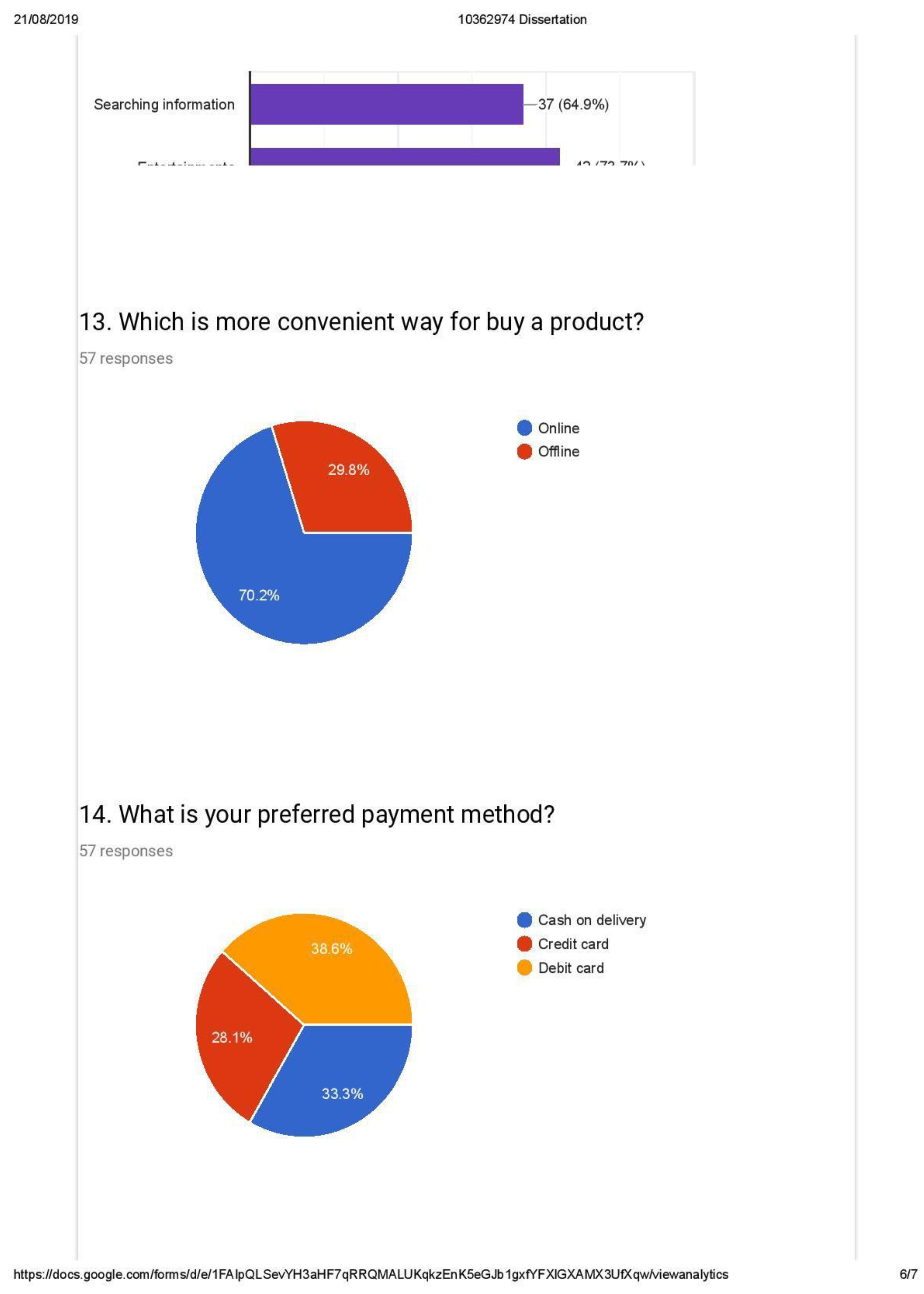
| 6.11 | Customer payment preference......................................................................... | 40 |
| 6.12 | Customer acceptance in linking EHR and prescription................................ | 40 |
| 6.13 | Customer acceptance on online marketplace having access to EHR for e-prescription...................................................................................................... | 40 |
| A.1 | Mobile Application............................................................................................ | 55 |
| A.2 | Mobile Application 2......................................................................................... | 55 |
| A.3 | Mobile Application 3......................................................................................... | 56 |
| B.1 Answer 1..............................................................................................................58 | ||
| B.2 Answer 2..............................................................................................................59 | ||
| B.3 Answer 3..............................................................................................................60 | ||
| B.4 | Answer 4 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 61 | |||
| B.5 | Survey 1 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 62 | |||
| B.6 | Survey 2 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 63 | |||
| B.7 | Survey 3 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 64 | |||
| B.8 | Survey 4 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 65 | |||
| B.9 | Survey 5 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 66 | |||
| B.10 | Survey 6 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 67 | |||
| B.11 | Survey 7 | . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 68 | |||
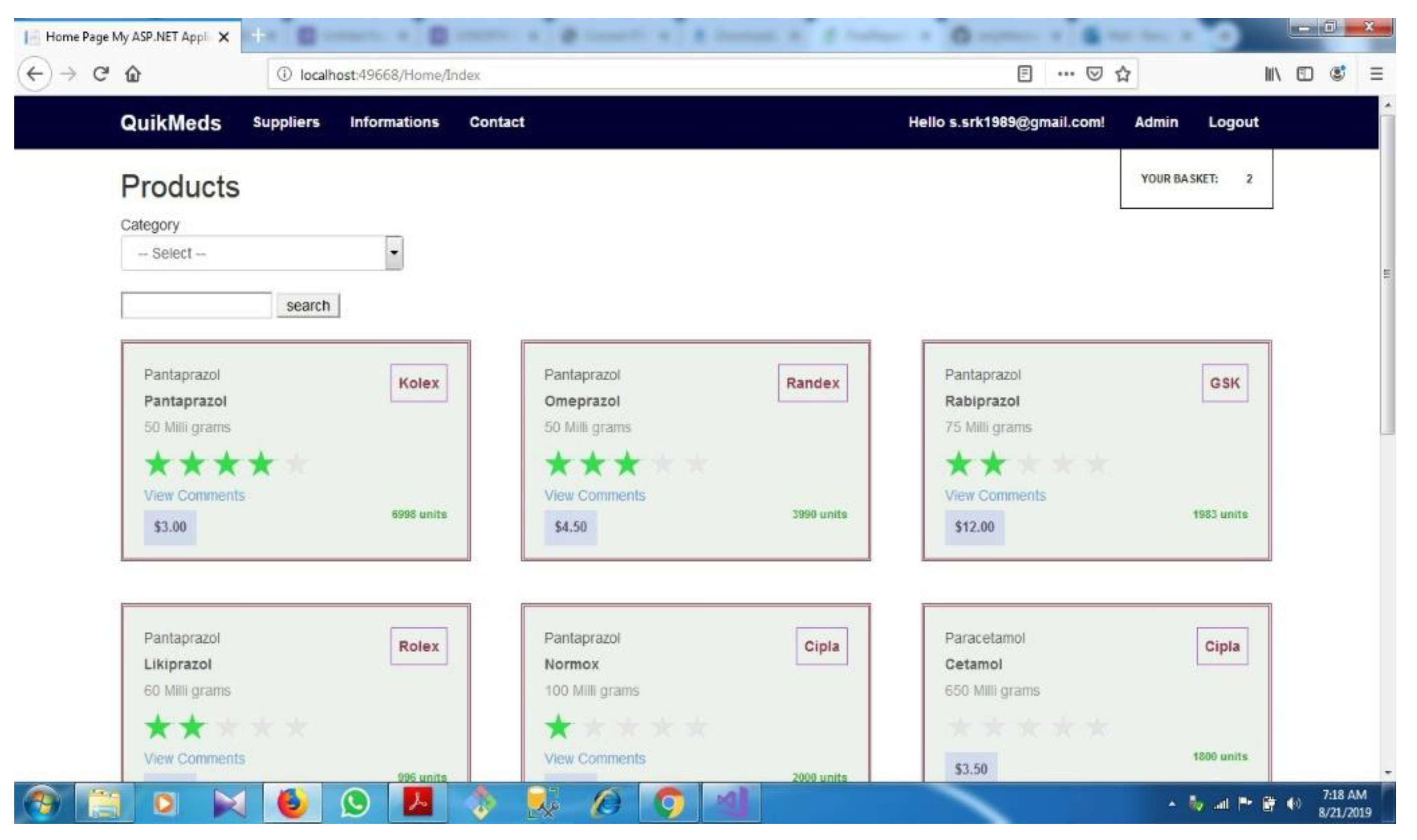
| D.1 | Application Dash Board.................................................................................... | 71 | ||||
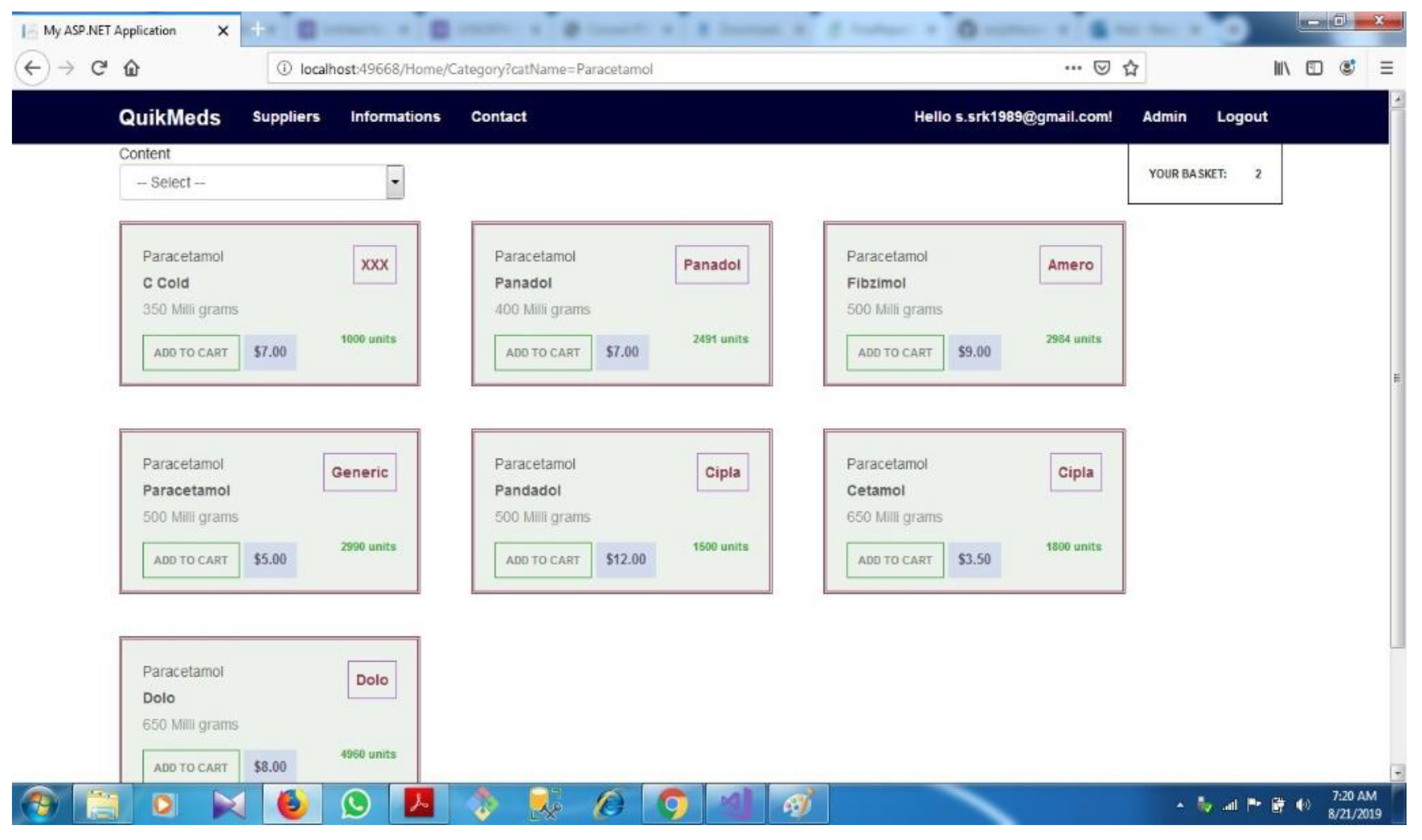
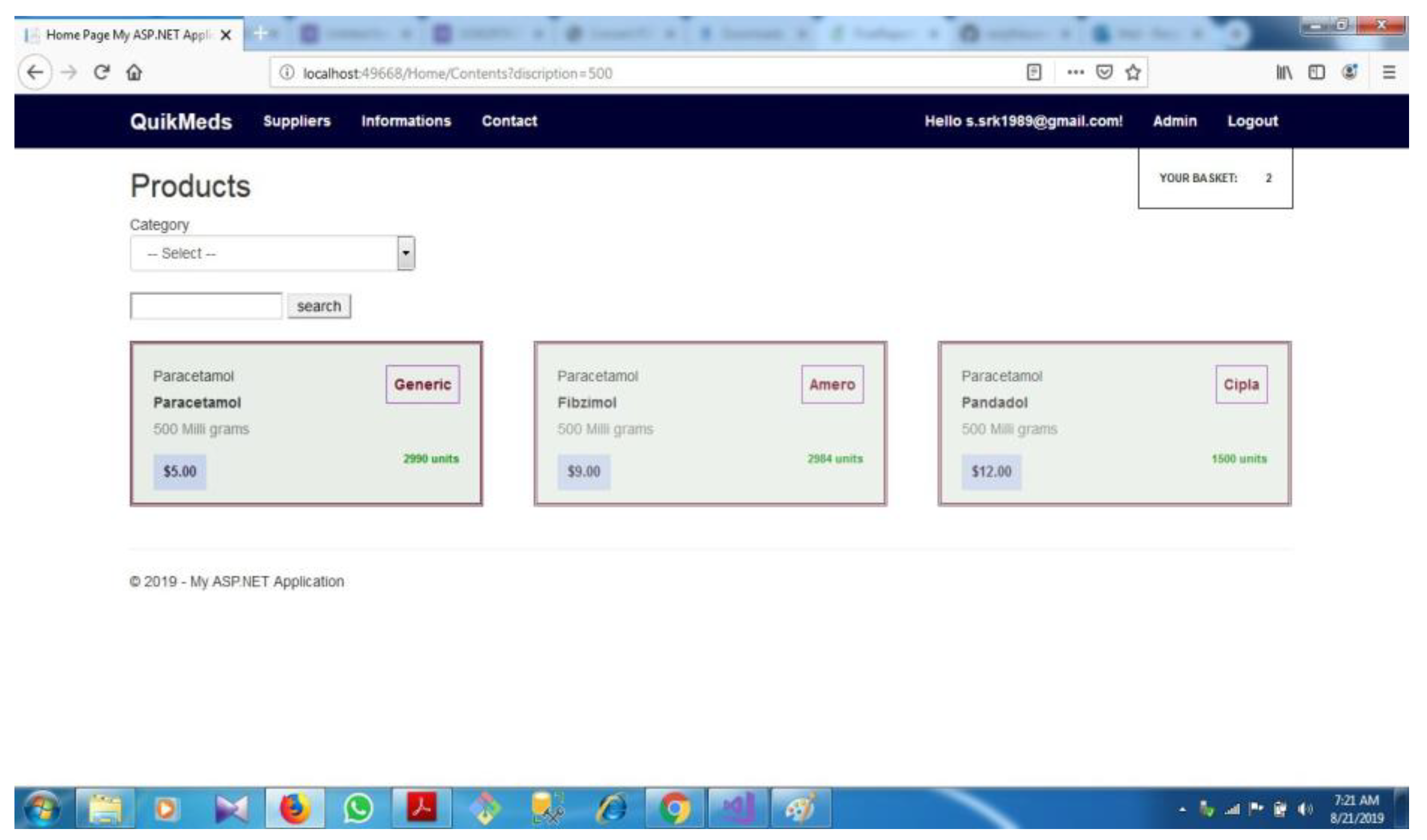
| D.2 | Category based selection................................................................................... | 71 | ||||
| D.3 | Content based selection..................................................................................... | 72 | ||||
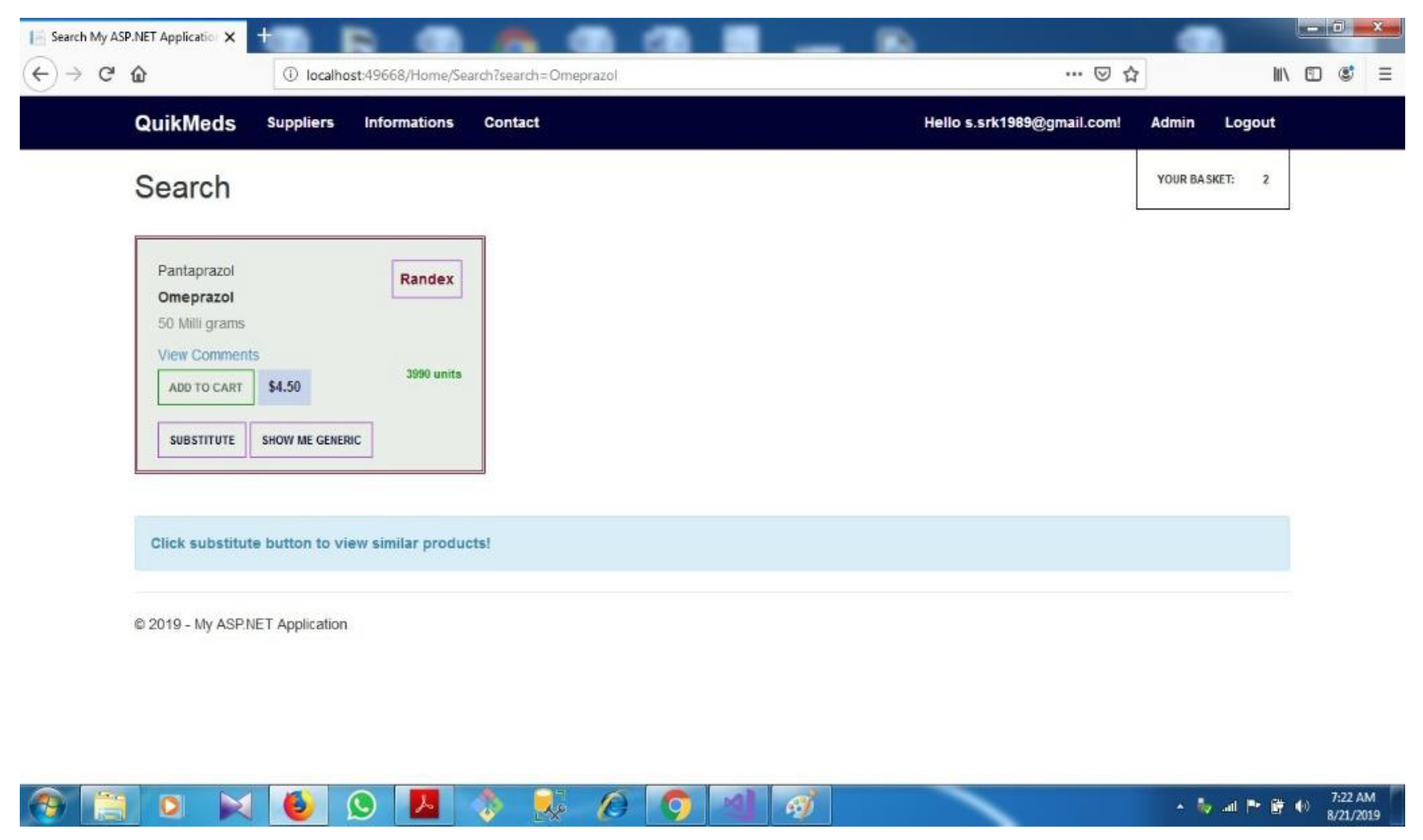
| D.4 | Search option...................................................................................................... | 72 | ||||
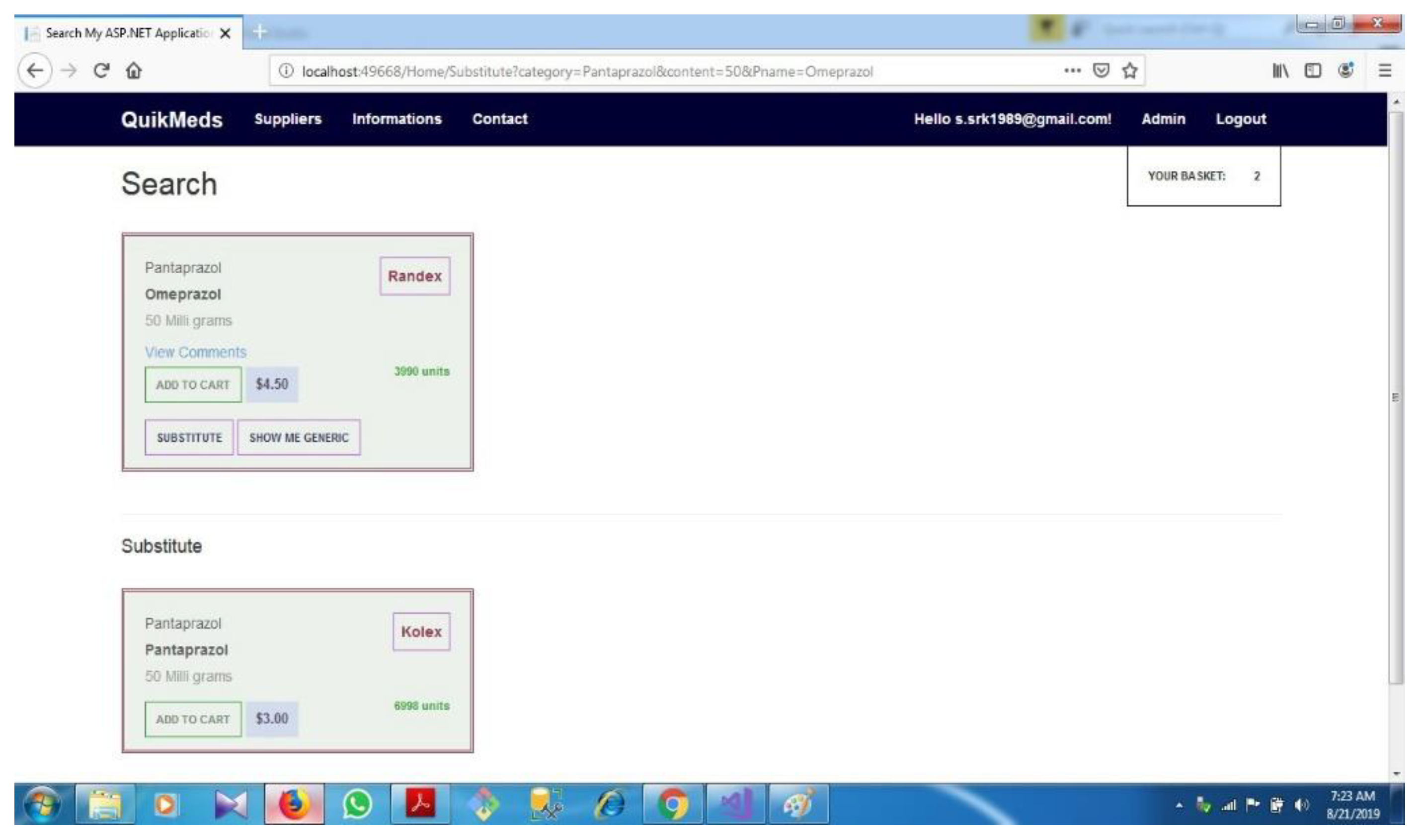
| D.5 | Finding suitable Substitute............................................................................... | 72 | ||||
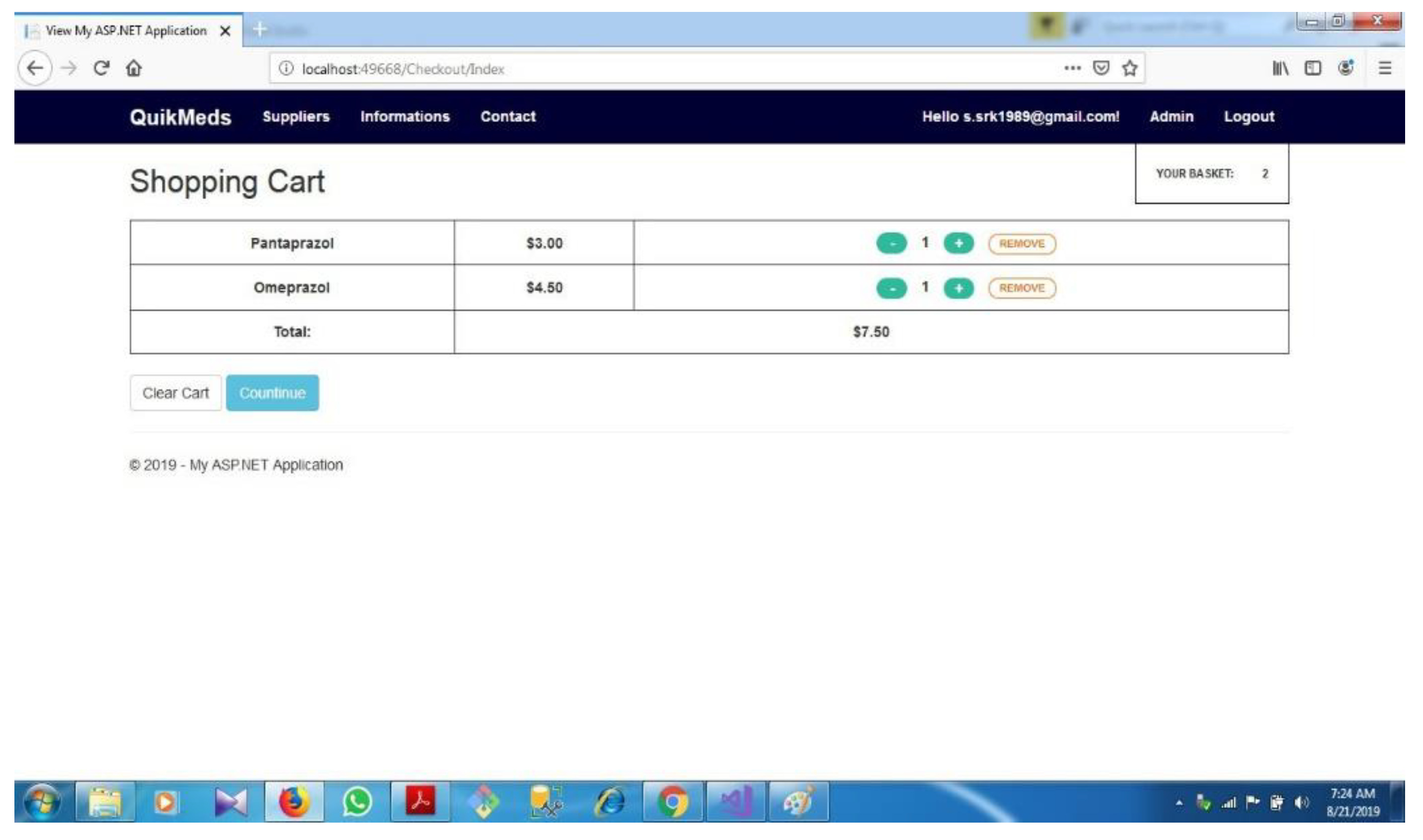
| D.6 | Shopping cart...................................................................................................... | 73 | ||||
| D.7 | Admin section.................................................................................................... | 73 | ||||
| D.8 | Admin operations.............................................................................................. | 73 | ||||
Chapter 1
1. Introduction
1.1. Background of the research
1.2. Aim of this research
1.3. Research Question
1.3.1. Sub research question
- How to implement a new system for online sales of medicines?
- What kind of services could be provided to educate the citizens towards the usage of medicines?
1.4. Objectives
- 1.
- To eliminate the differential pricing of identical drugs.
- 2.
- To promote generic medications in a user-centric way.
- 3.
- To provide support for educates clients about the products, details with medi- cation guidance and other value-added services through information portals.
1.5. Scope and limitations of the research
1.6. Dissertation road map
- Chapter 1 provides the background, aims, and objectives of the research fol- lowed by the research questions. This chapter also talks about the scope, limi- tations of the research and the major contributions of the research.
- Chapter 2 reviews the existing literature to understand key terminologies and their significance.
- Chapter 3 talks about the research plan and the methods used in the study.
- Chapter 4 Explains the system design in detail.
- Chapter 5 Explains the system in detail. This chapter describes the system features and implementation of the system along with project management.
- Chapter 6 presents the data analysis and findings generated a part of the re- search survey.
- Chapter 7 talks about the insights gathered after examining the data findings.
- Chapter 8 provides the conclusion of the research concerning the research ques- tions and discusses the future scope of the system.
Chapter 2
2. Literature Review
2.1. Existing distribution system
2.2. Unavailability of medicines
2.3. Distribution and pricing issues of pharmaceutical drugs in Ireland
2.3.1. Differential pricing of drugs and its effects on public
2.4. Generic medicines, its importance and policy of Ireland government on promoting generic drugs
2.5. Classification of pharmaceutical drugs
- By their therapeutic use, i.e. based on the conditions they are used to treat.
- By their mechanism of action or the biochemical reaction, the drug has when it is taken.
- By their body responds to the drug.
- By their chemical structure
- Level one – describes the organ system treats.
- Level Two – describes the drug’s therapeutic effect.
- Level Three – describes the mechanism.
- Level Four – general chemical properties of the drug.
- Level Five - describes the chemical composition.
2.6. Effect of Common market place on the pricing of goods
2.7. Legal Scope of the Online pharmacies In European Union
2.8. Online Virtual Market as the common market place
2.9. Existing Online retailers In Ireland
2.10. Challenges and solutions
2.11. Summary
Chapter 3
3. Research Methodology and System Analysis
3.1. Research philosophy
3.2. Research Approach
3.3. Research ethics
- Consent based on free will: All the data collected from participants online or offline is based on consent on free will, and volunteered to the research contri- bution, without any physical/psychological compulsion/harm.
- Data Integrity: No data collected from any resources including, but not limited to, the volunteer contributions are tampered with in any way and is an actual representation.
- Source Integrity: All sources from which data has been collected for reference was verified in the best way possible for its data integrity, minimizing any margin of data misrepresentation.
- Data Confidentiality and Anonymity: No data that could completely identify any individual volunteered for contributing data to the research or otherwise are collected for any purpose. No data collected shall be shared to any third party for monetary benefits or otherwise and only aggregated data are kept and published for research purposes.
3.4. Preliminary system planning
3.4.1. Data collection methodology
3.4.2. Questionnaire
3.4.3. Data analysis procedures
3.4.4. Time horizon
3.4.5. Twelve-week timeline
- Week 1: Analysis of problem in detail.
- Week 2: Find out related articles, documents, and literature to support the solution.
- Week 3: Data collection via surveys, oral talk, and observations.
- Week 4: Data analysis and findings.
- Week 5: System development.
- Week 6: System development.
- Week 7: Testing and user reviews
- Week 8: Feedback collection about the system, Report generation.
- Week 9: System alteration based on Feedback.
- Week 10: Error correction
- Week 11: Discussions with clients, Report generation.
- Week 12: Final set up of Minimum Viable Product for delivery.
3.5. System analysis
3.5.1. Identify problem and Observations
3.5.2. Feasibility study
3.6. Detailed System Study
- A page for display all medicines and its price. It should give the details of the manufacturer and supplier. And there must be a rating display for knowing the acceptability and experience of the customer.
- A page for selecting medicine based on category. This system must have an option to filter the medicines and display only the medicines which are pre- scribed by the doctor for that particular customer.
- All operations must be done after authentication and authorization.
- An option for searching a particular medicine. And there must be an option to search its substitutes.
- There must be a page for giving feedback.
3.7. Summary
Chapter 4
4. Artefact Design
4.1. Logical design
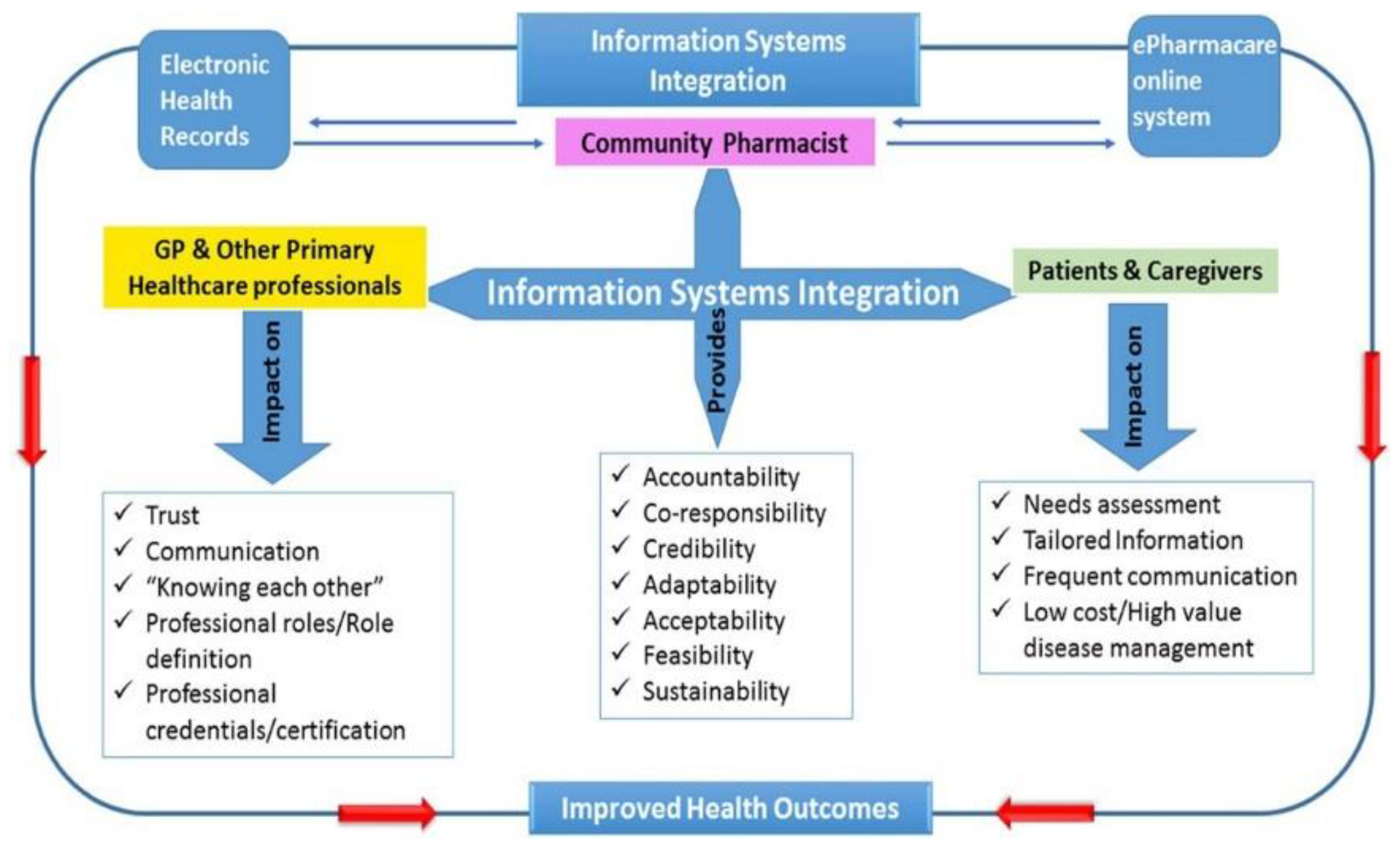
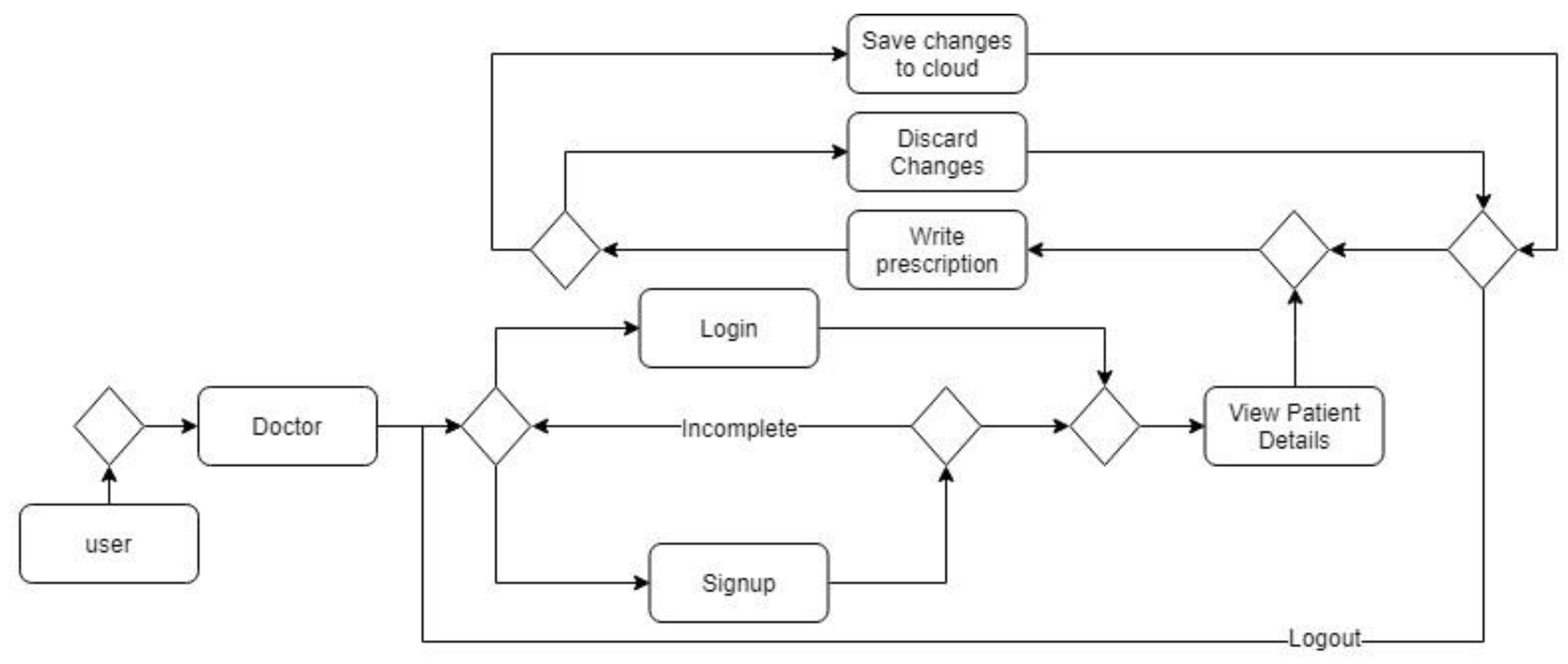
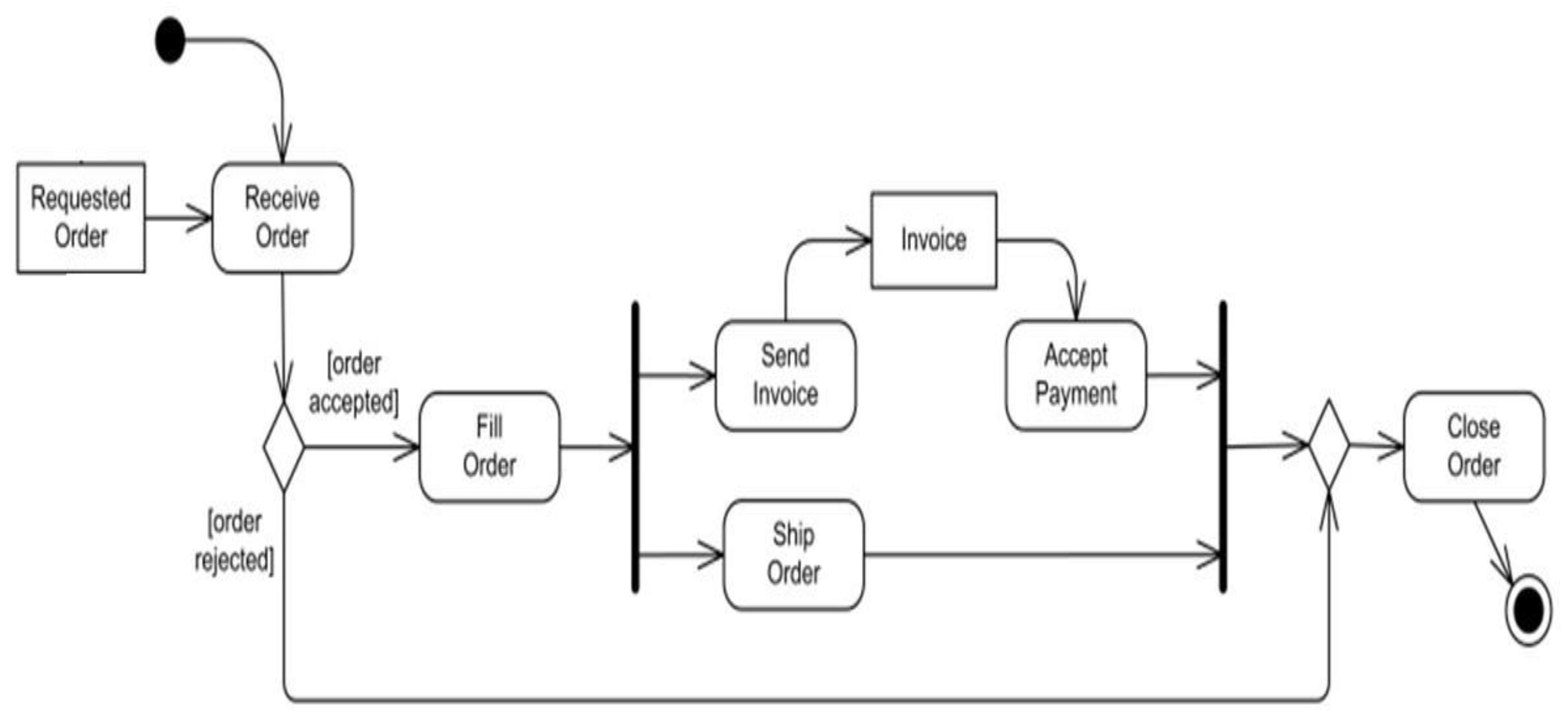
4.1.1. System Workflows
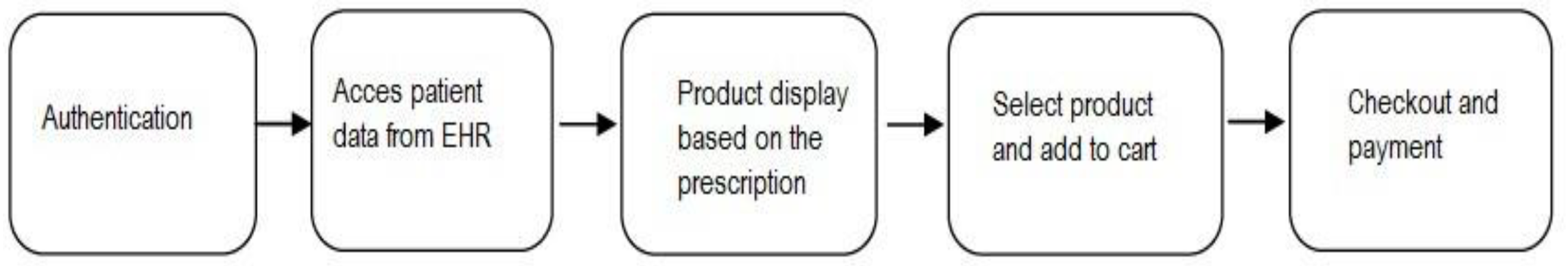
4.2. Physical design
4.2.1. Product dashboard creation
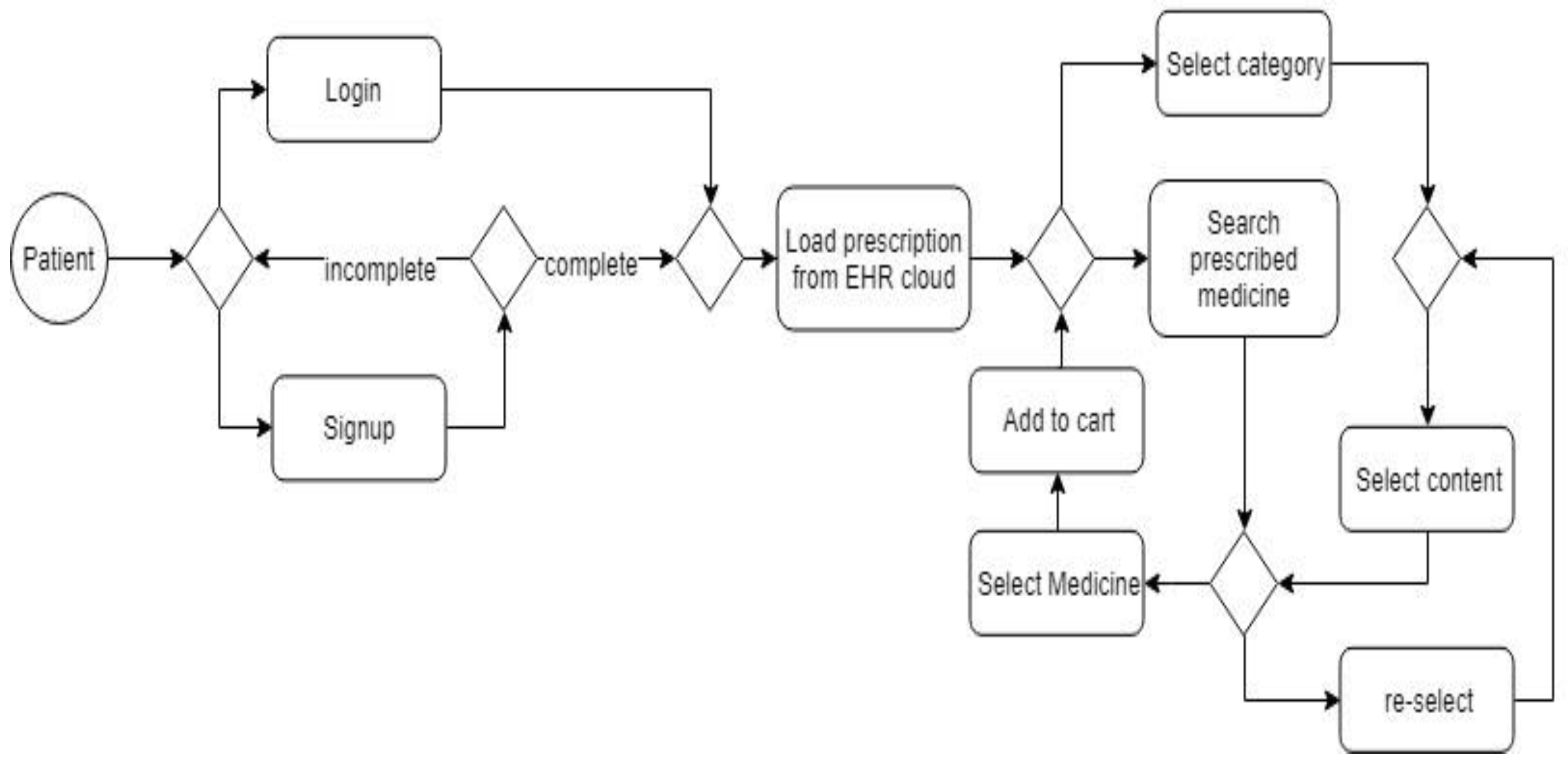
4.2.2. Checkout creation
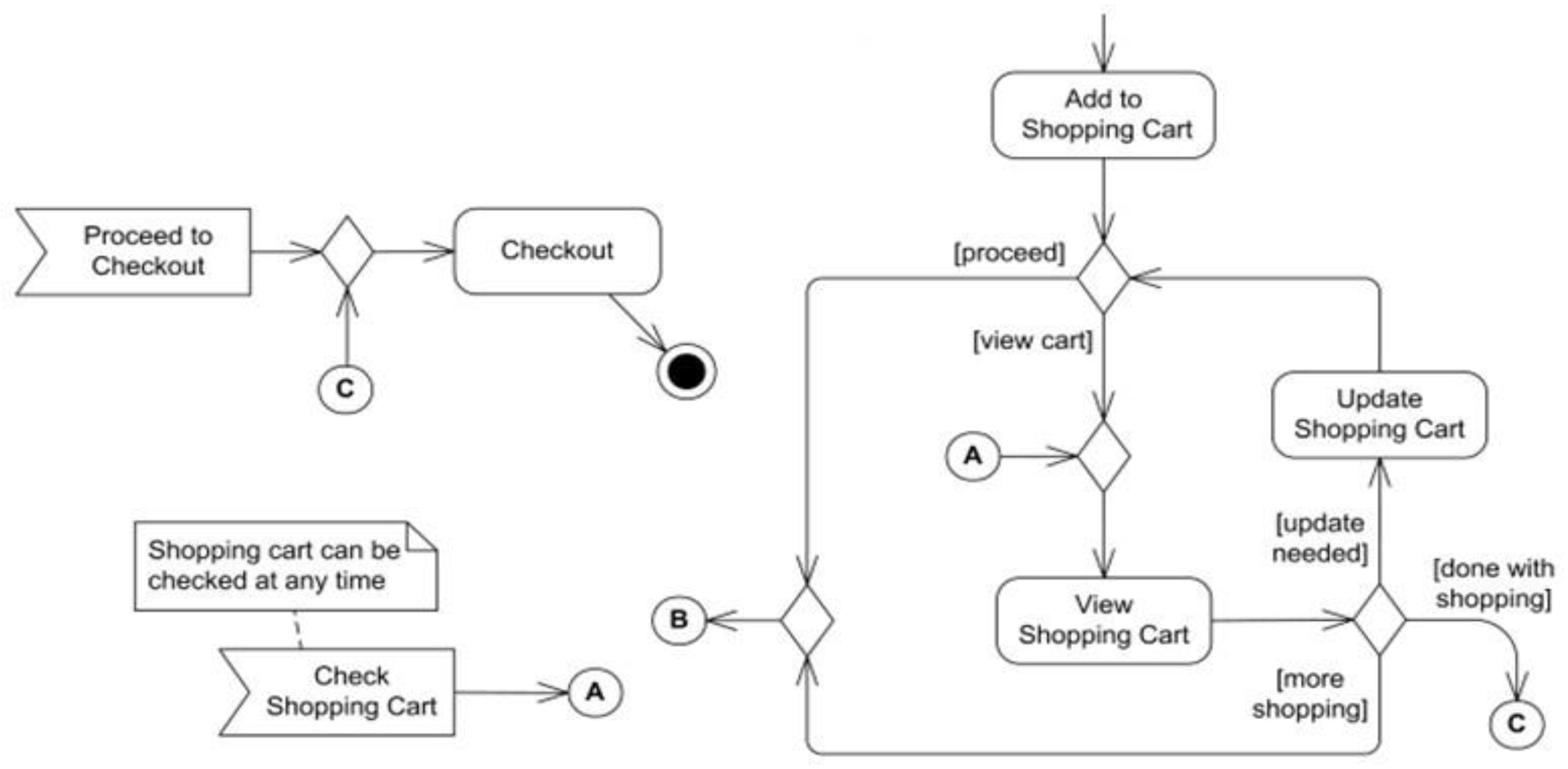
4.2.3. Payment-page creation
4.2.4. Modules
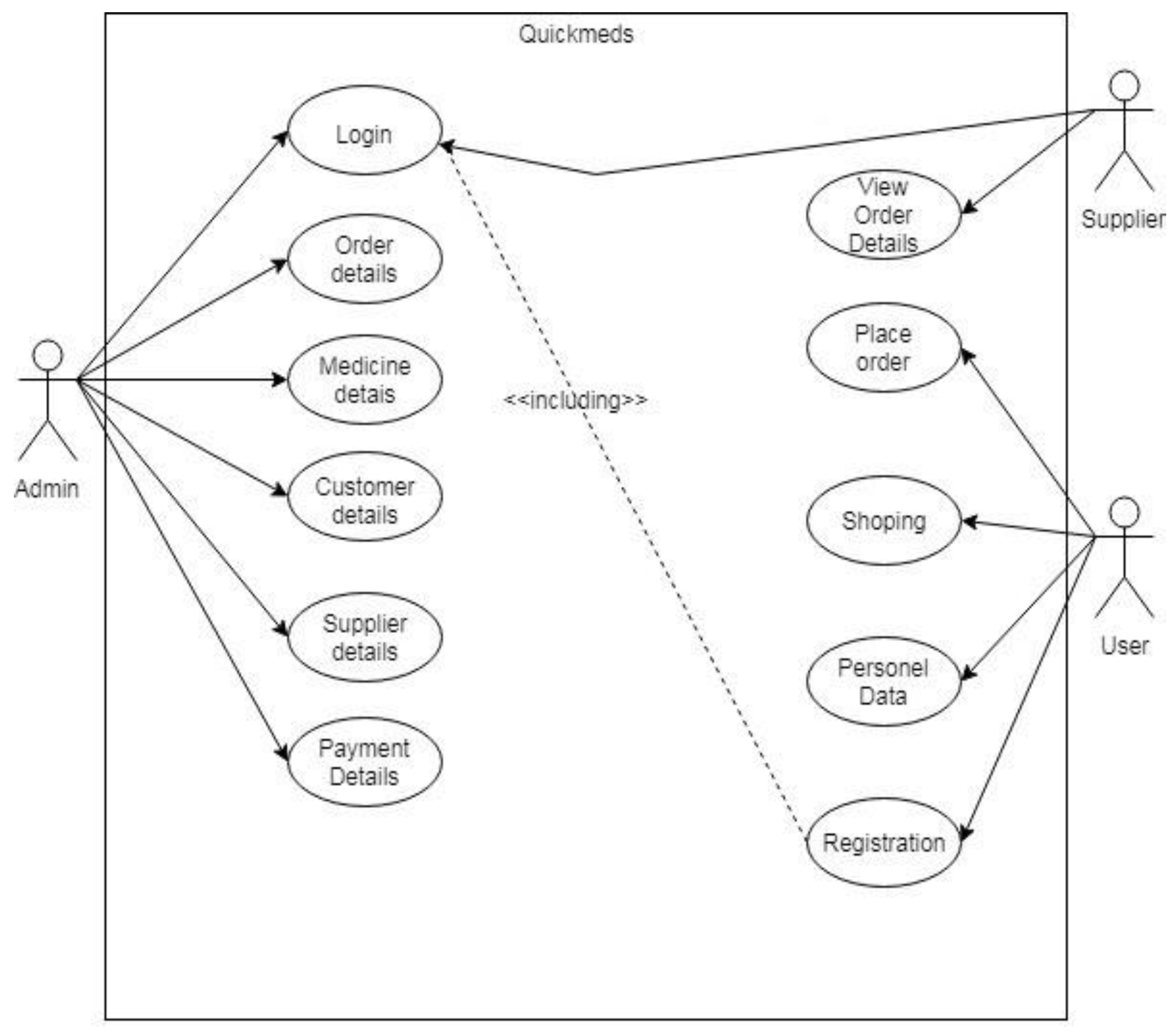
- Admin module
- User module
- Supplier module
- Add Supplier details: Admin can add different supplier details across the county by using the basic details like store name, address, email id, and phone no. Once added, the login credentials will be sent to the supplier’s email id.
- Add products: Admin is provided with the functionality to add the various products. can add medicine with details like medicine name, category, generic name and re-order level.
- Add Category: Admin can provide a list of common medications.
- Manage Stock: Admin can manage the information of medicine stock by in- cluding information like quantity, and rate of medicine.
- Fast Moving Items: Admin can view the list of medicines which are quickly sold out.
- Health Tips: Can add health information and medical updates on healthy liv- ing ideas.
- View Stock: Can check the stoke status.
- View Request: Can view client’s request for medicine...
- Make Conveyance: Once the request is placed the customer will be contacted through a mail.
- Update Profile: Can alter the profile as per the requirement.
- Quick Moving Items: Can see and arrange the order of fast-moving goods.
- Search medicine: Users have the option to view medical products as per the proscription updated on the EHR cloud.
- Update Profile: An additional option to change registration details.
- Search Supplier: Users are also provided with a facility to view supplier details and contact details.
- Health Tips: Have the option to see helpful tips and collect information about certain drugs example OTC drugs.
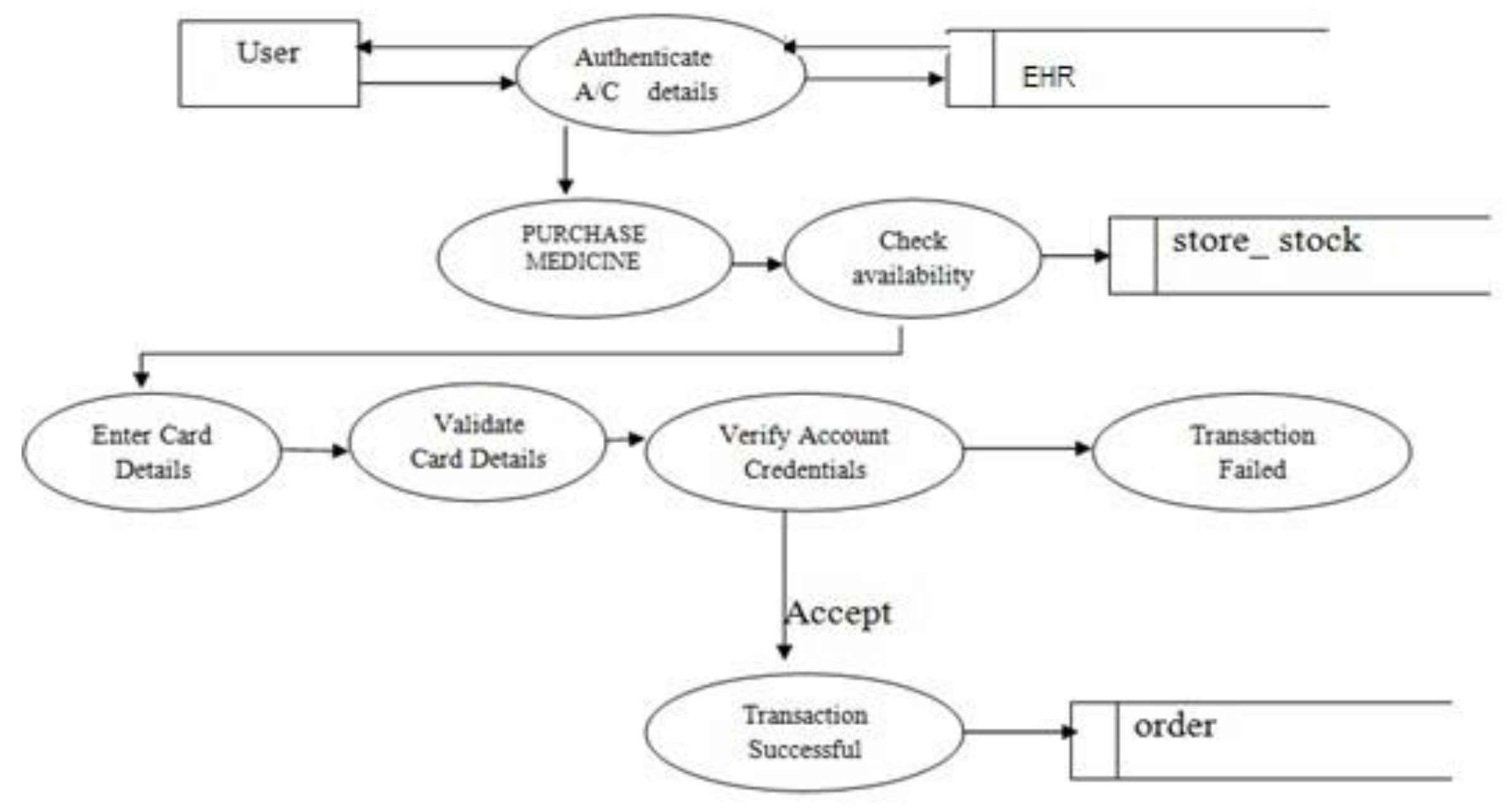
- Purchase Medicine: User can purchase medicine using a credit card or debit card.
4.3. Architecture Design
- performance:- By localizing critical operations and minimizing communica- tions performance can be increased up to a certain extent. In this context researcher mainly focused on using large components rather than fine-grain components.
- Security:- Used multilayered architecture with critical assets in the inner layers.
- Safety:- Localise safety-critical features in a small number of sub-systems.
- Availability:- Include redundant components and mechanisms for fault toler- ance.
- Maintainability:-For easy maintenance the researcher used fine-grain and re- placeable components.
4.4. Detailed Design
4.4.1. Software Development Methodology
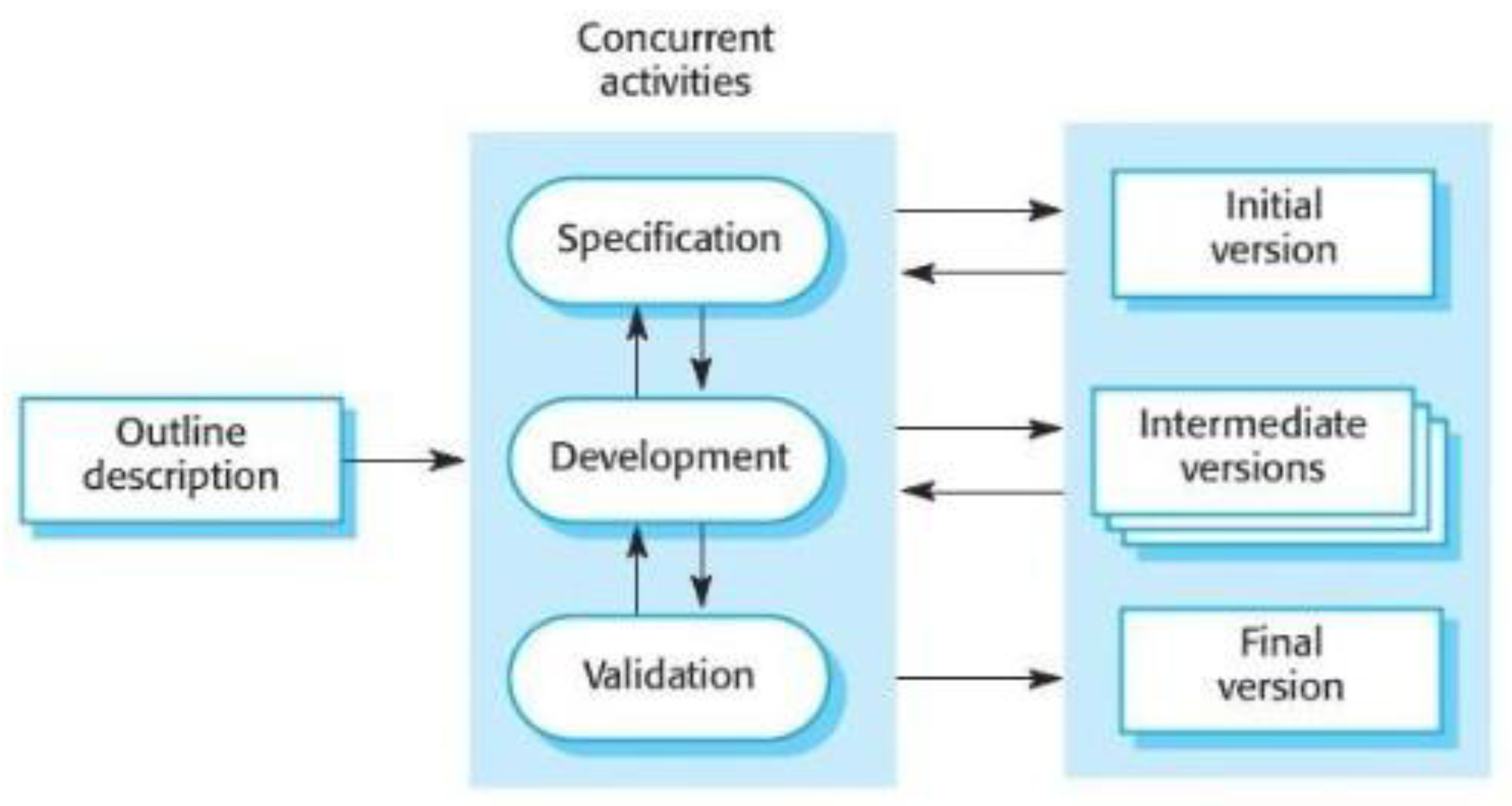
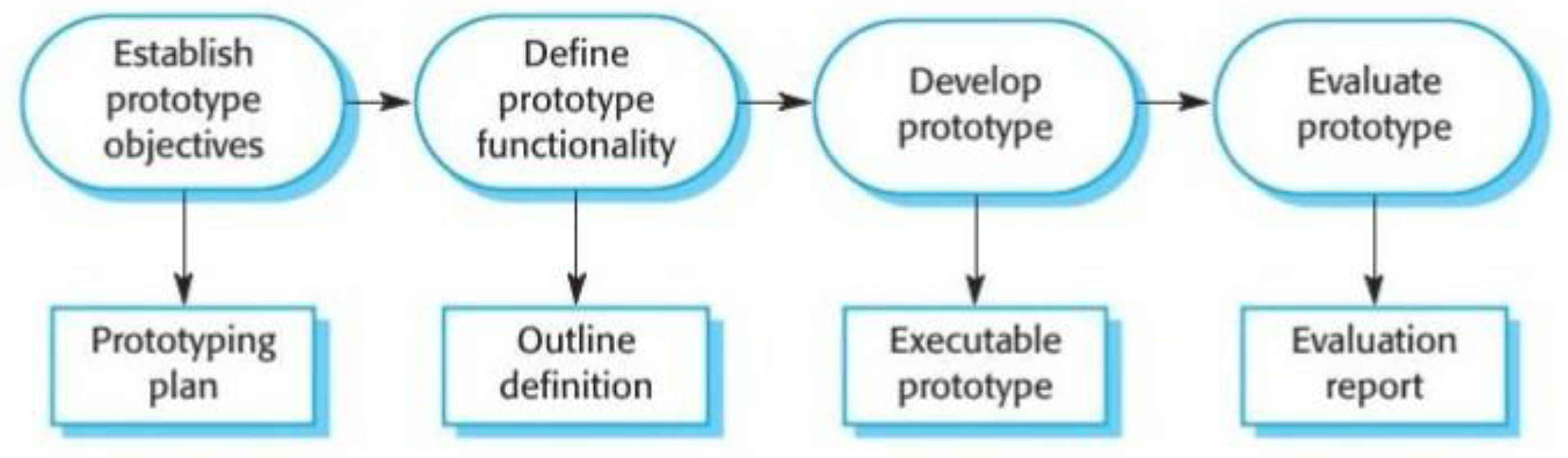
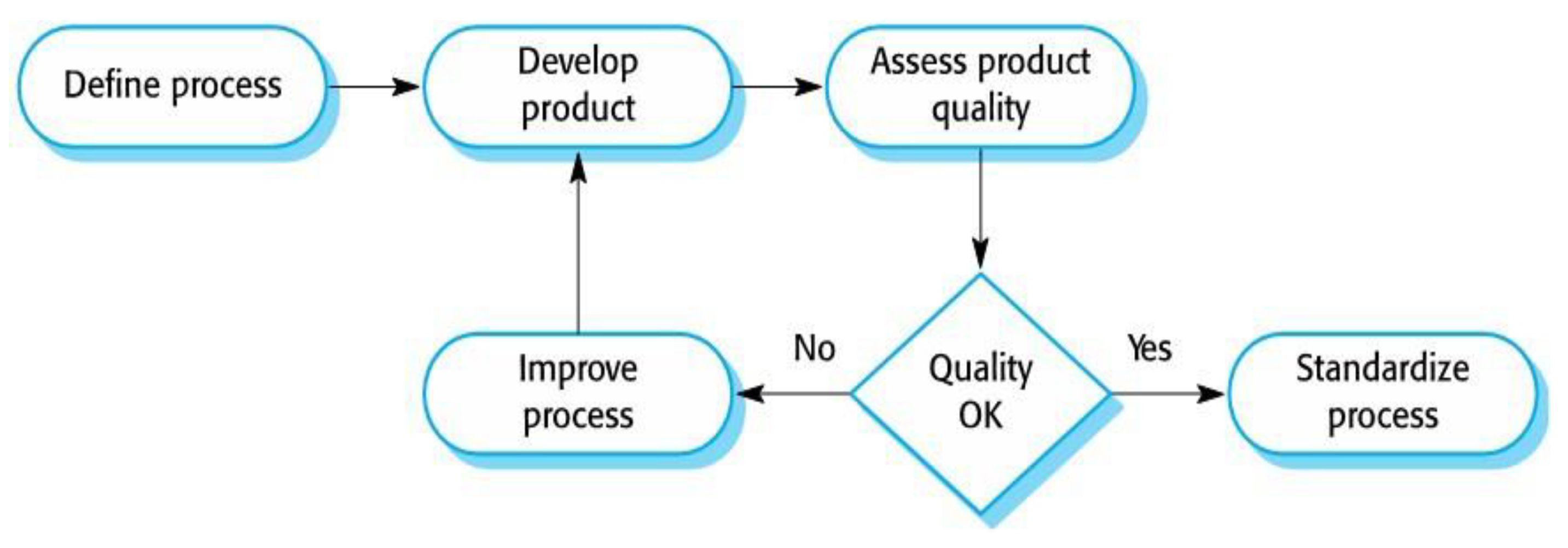
4.4.2. Minimum Viable Product
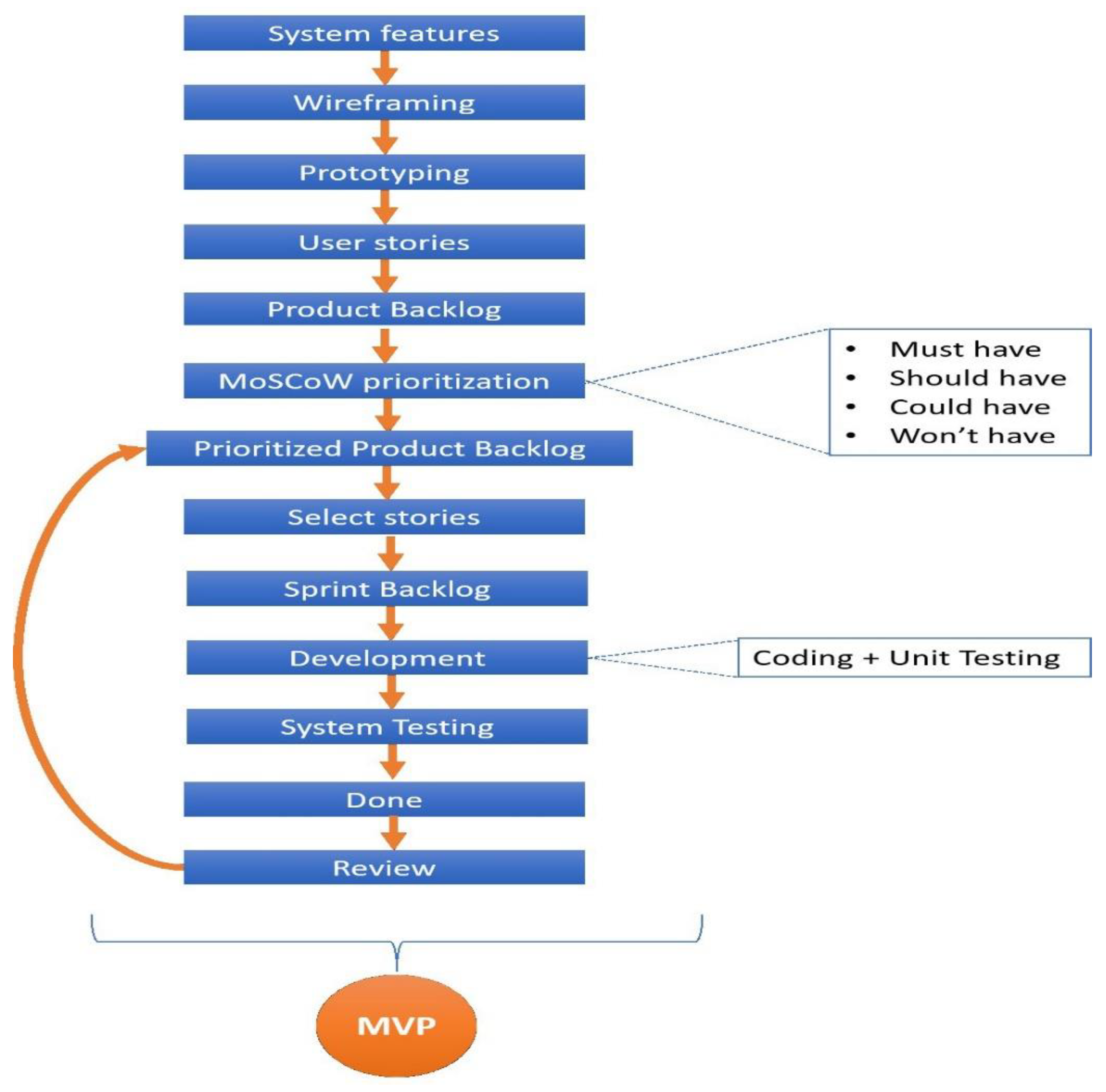
4.4.3. Software Requirement Specification
4.4.4. U M L Diagram
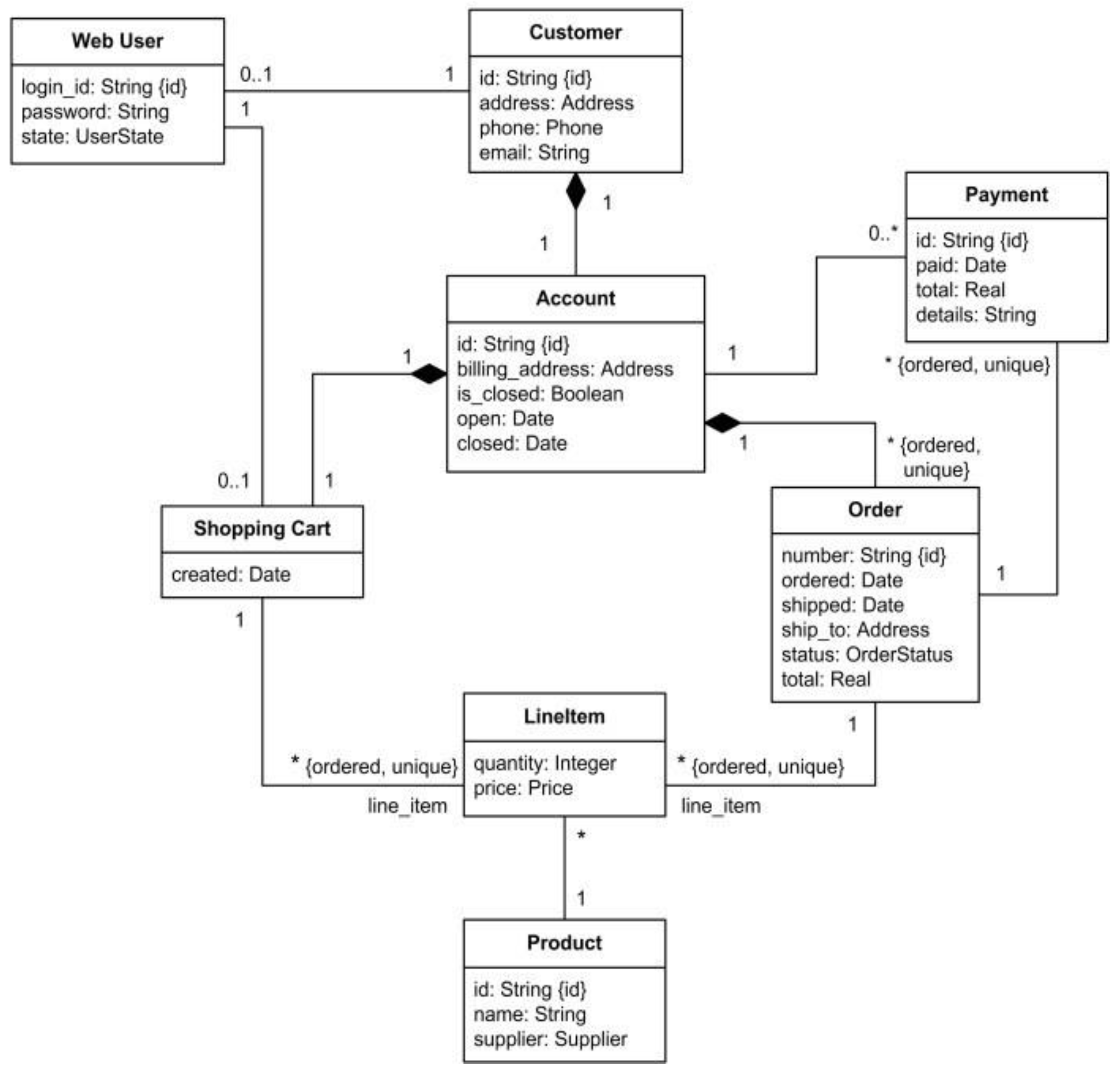
4.5. Development technologies and tools
4.5.1. Technologies
4.5.2. Development Environment(s)
4.5.3. Configuration Management tool
4.6. Database design
4.6.1. Business rules
- Medical prescription will be downloaded from EHR cloud-based on customer authentication.
- Allow customers to become a part of the buying process while assisting cus- tomers through the experience with reviews and ratings.
- Customers must be able to do site navigation with easiness. So customers can search for products by category and content.
- Customers must understand what kind of products they are going to buy, there must be an information window providing with details of all drugs.
- All operations and transaction should be protected by an authentication pro- cedure. The user should register with the organization before doing any trans- actions.
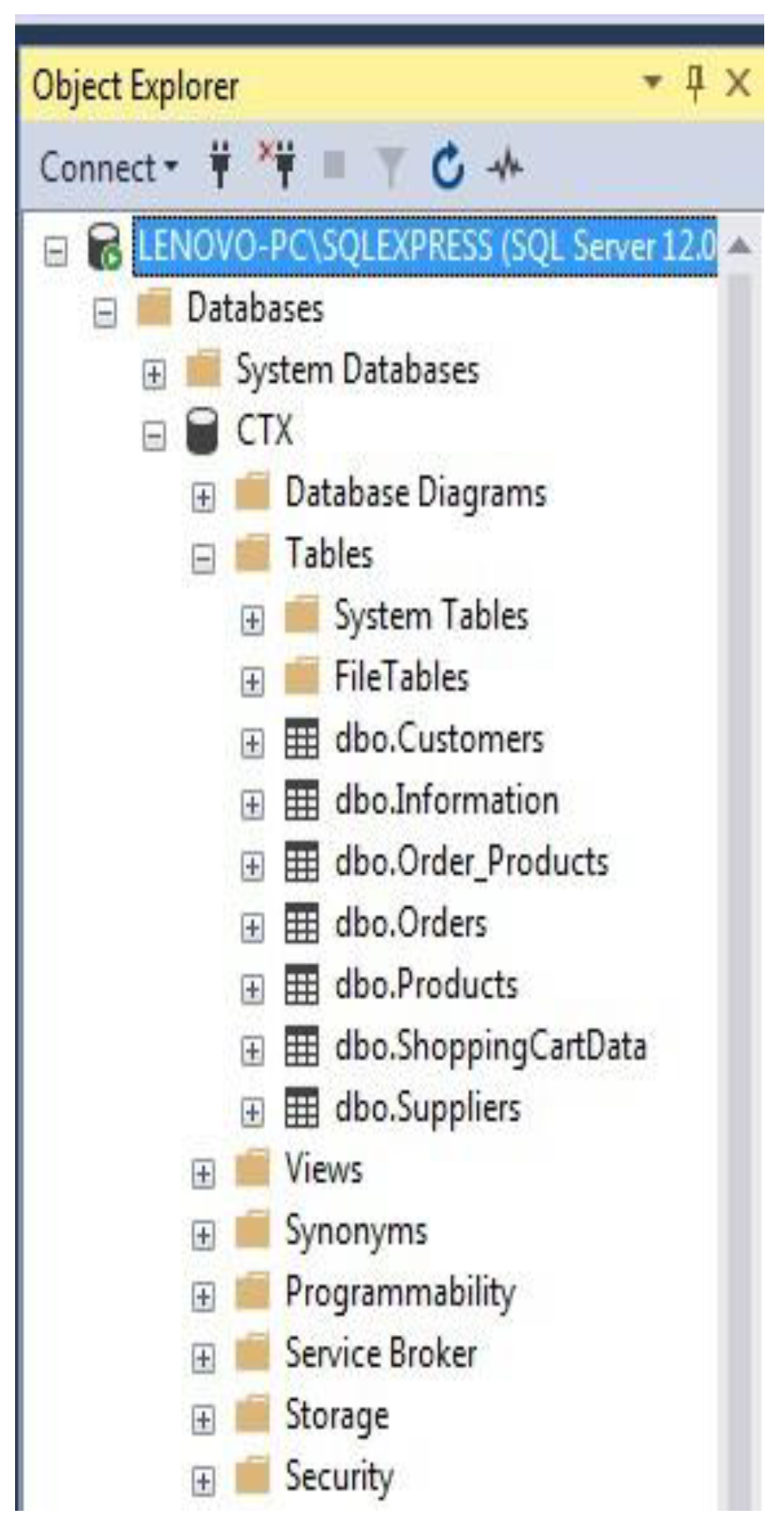
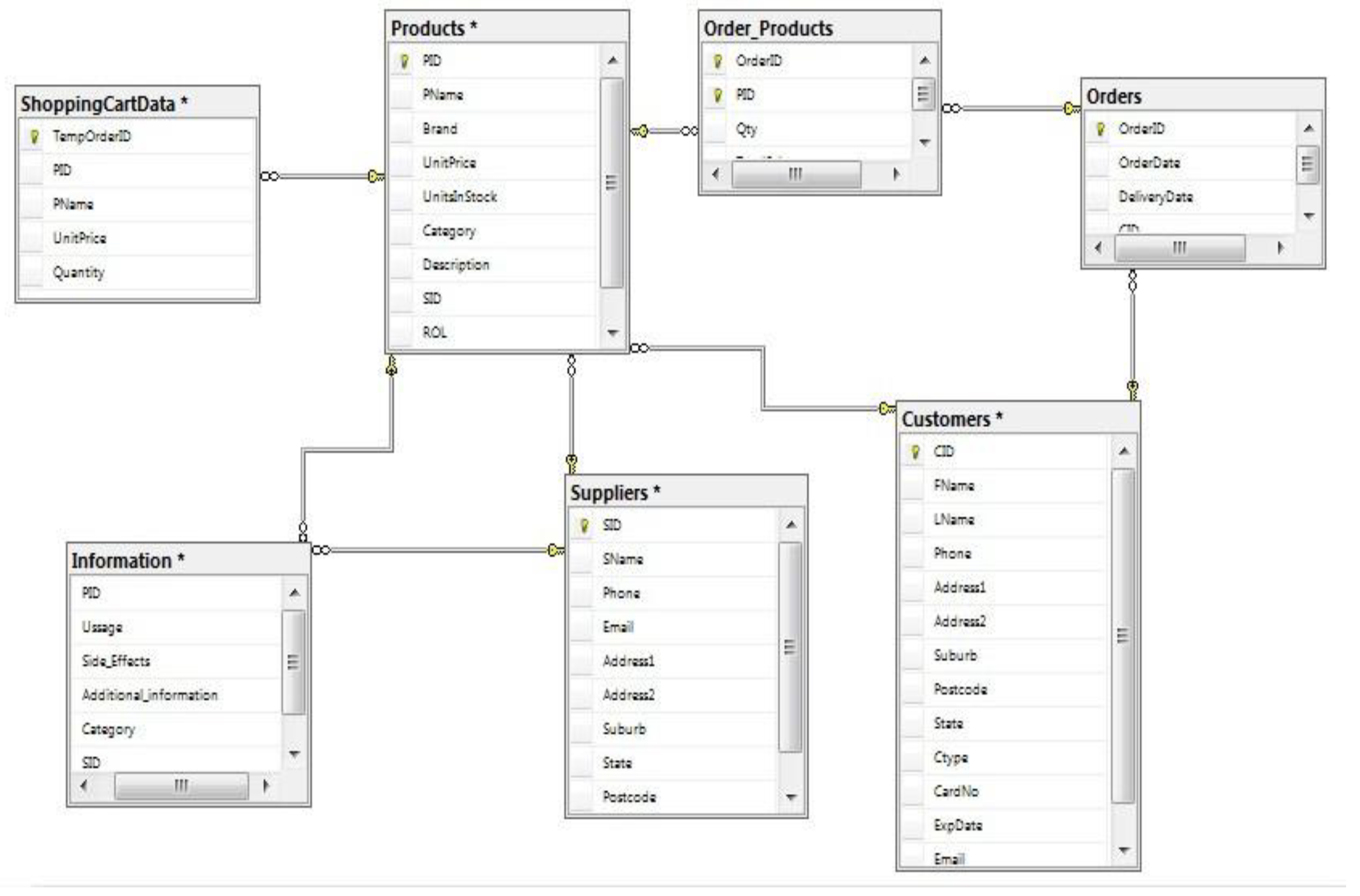
4.7. Summary
Chapter 5
5. Artefact Development
5.1. Web Application Development
5.1.1. Models
5.1.2. Controllers
5.1.3. Views
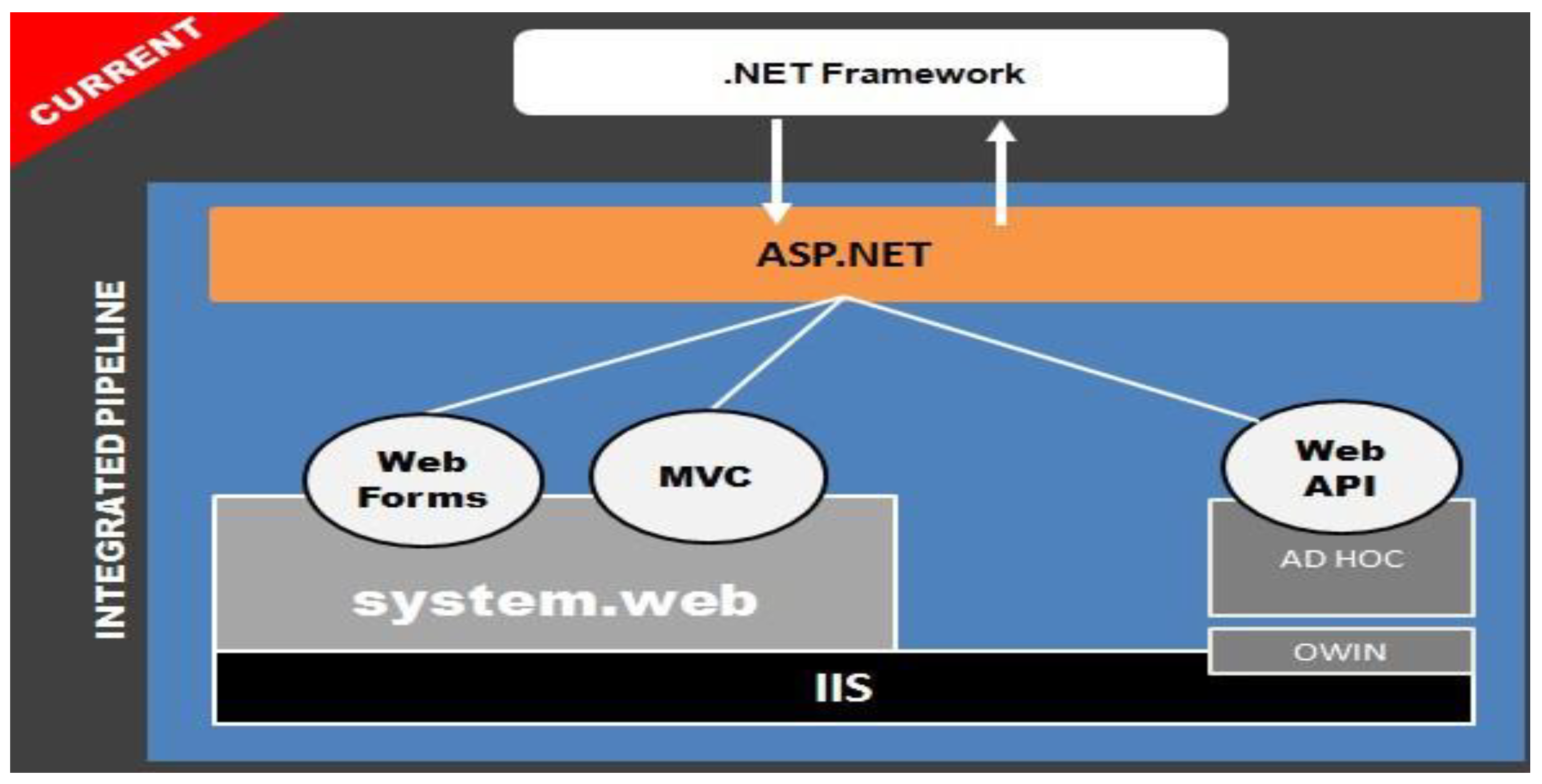
5.2. Mobile Application
5.3. Authorization and Authentication
5.4. Application dashboard
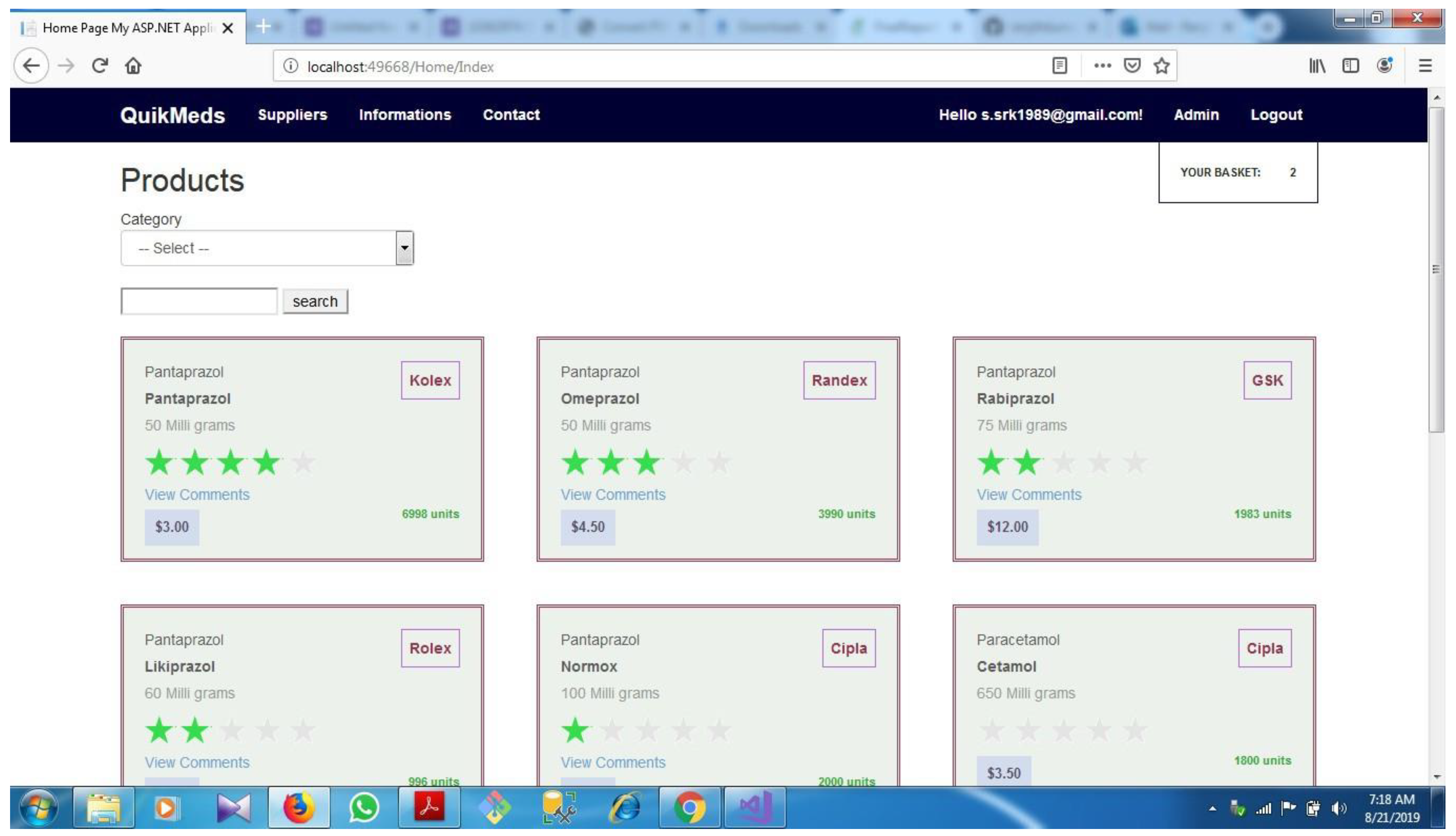
5.5. Summary
Chapter 6
6. Data Analysis and Findings
6.1. Introduction
6.2. Findings
6.2.1. General size of the market
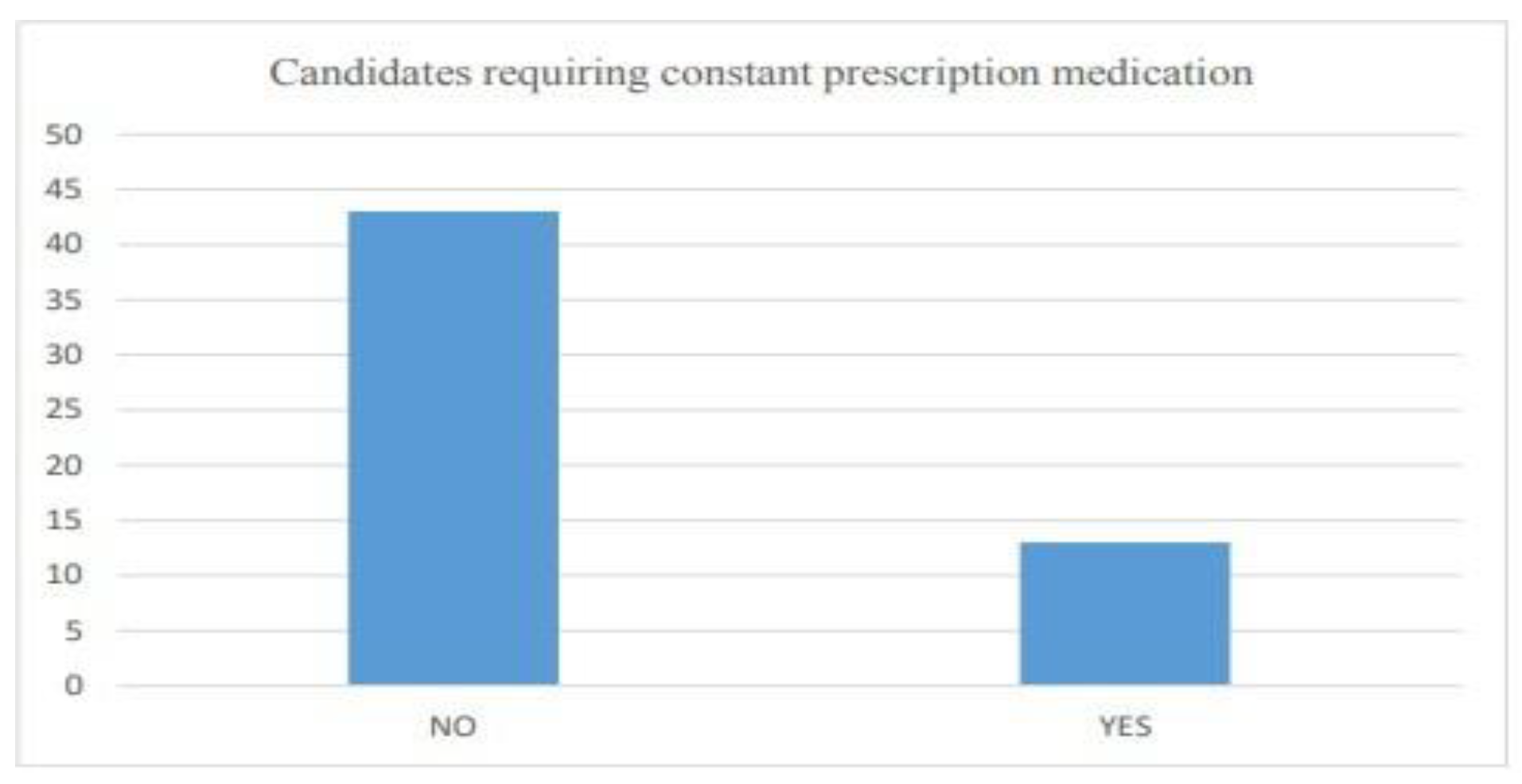
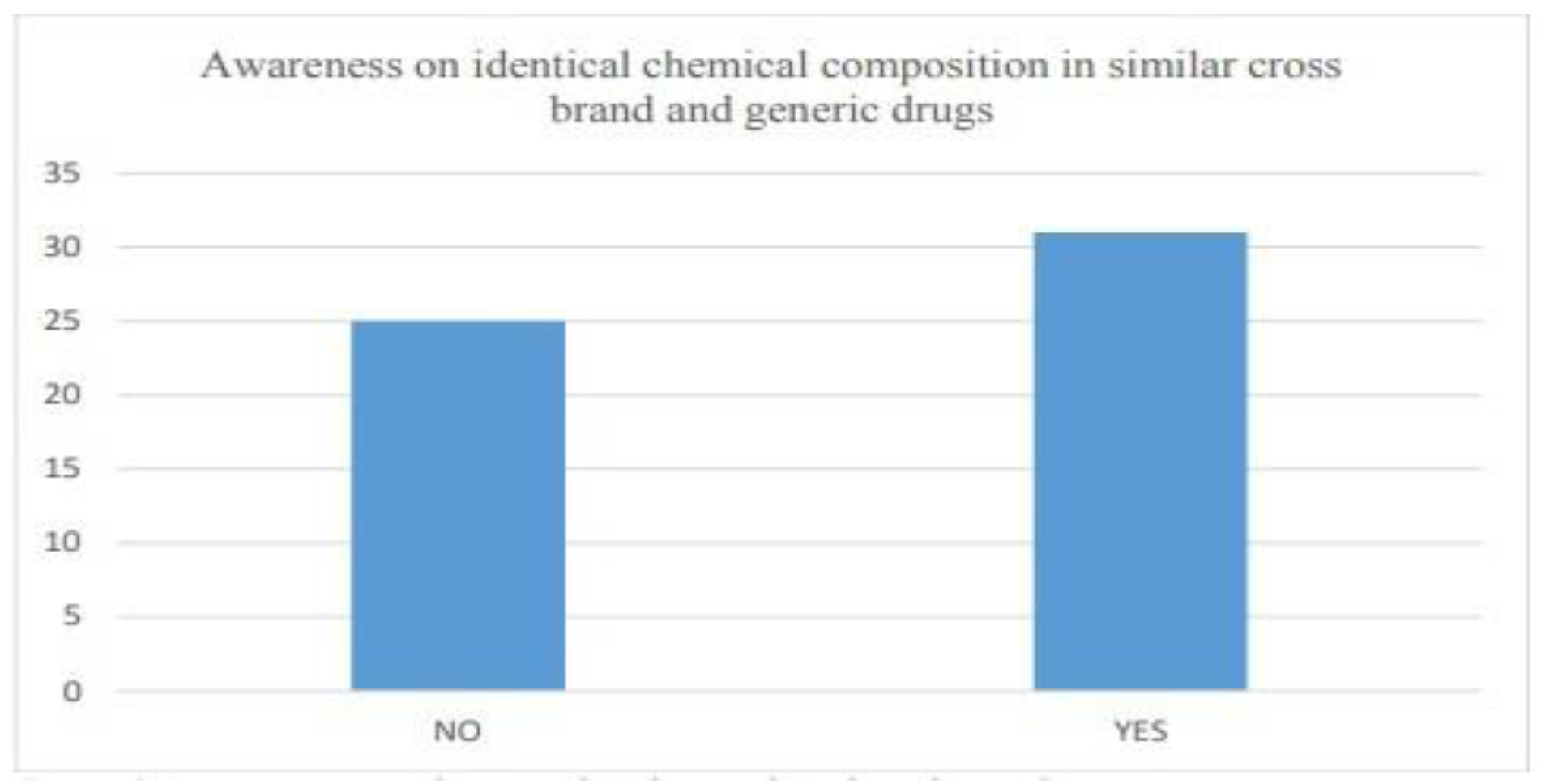
6.2.2. Awareness among the people
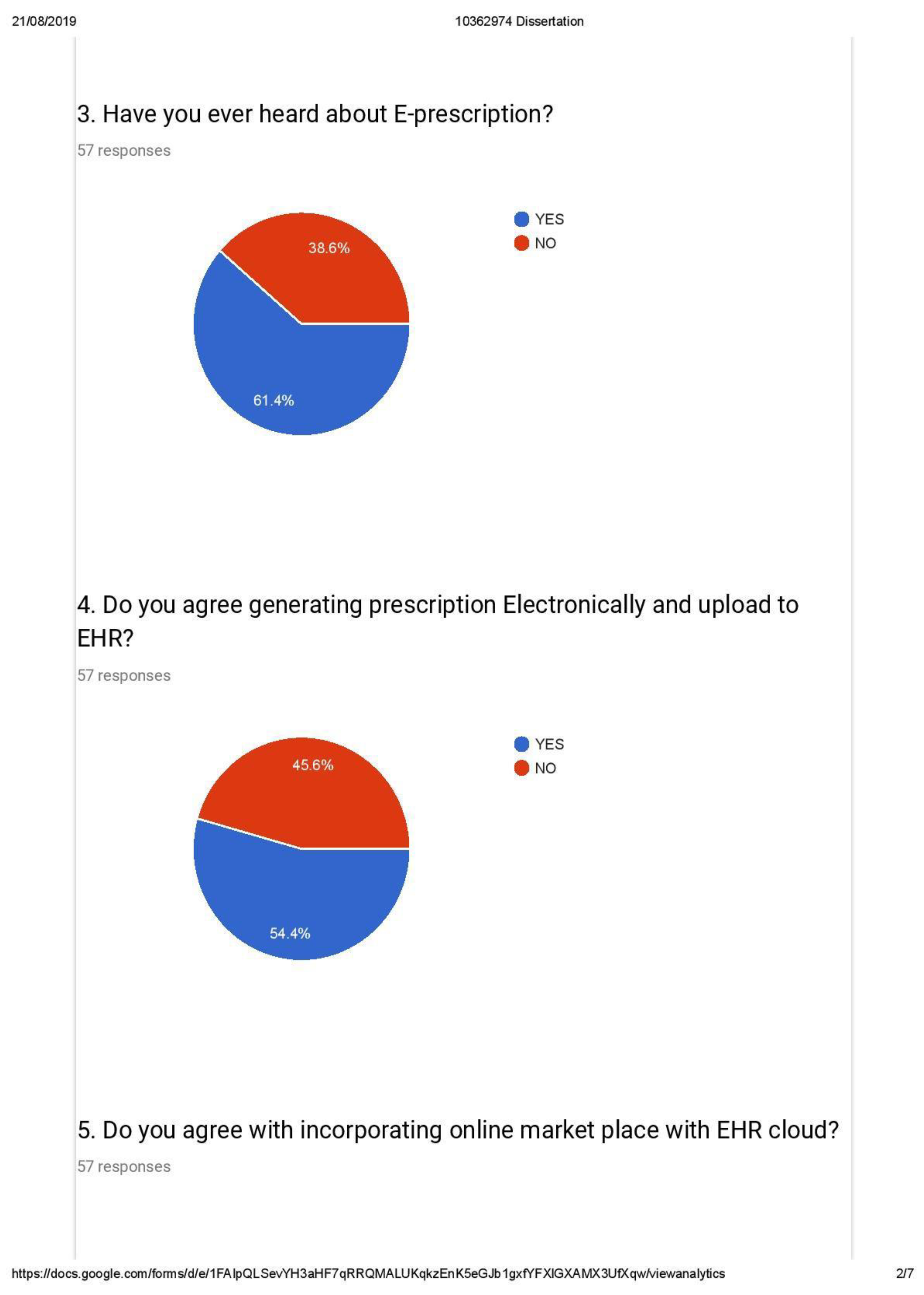
- Awareness on EHR and E-prescription
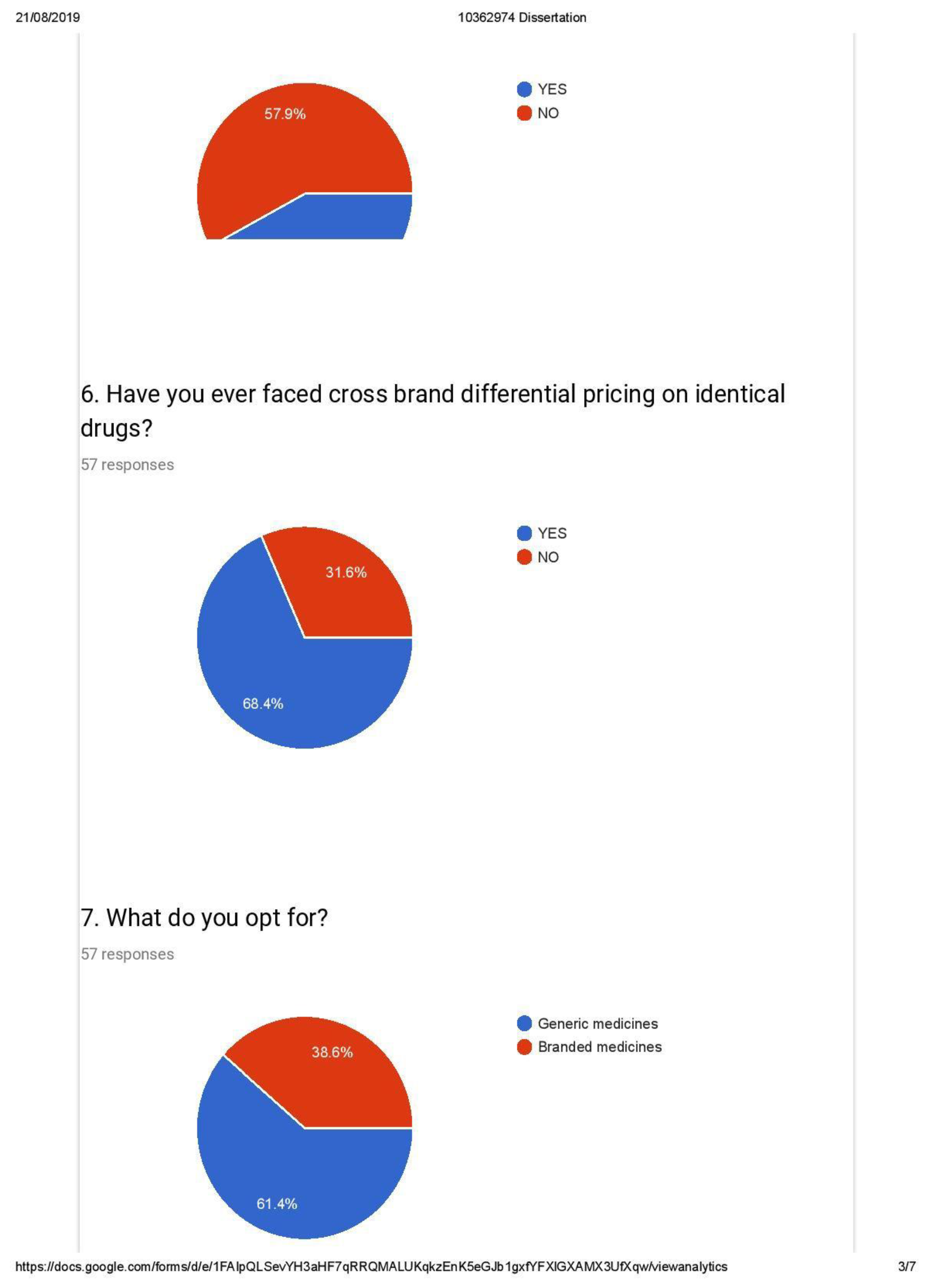
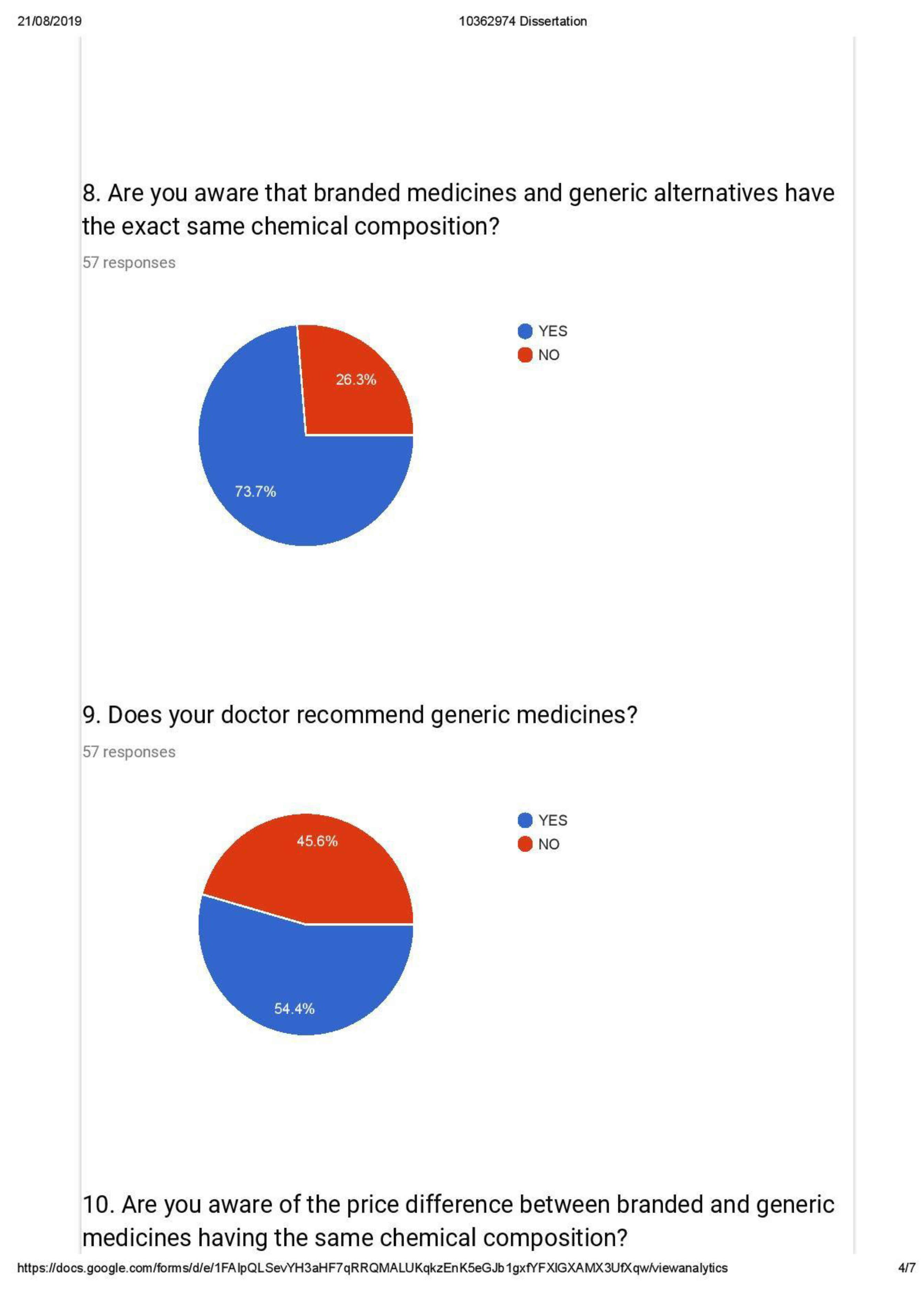
- Identification of drugs based on content and not brands
- Awareness of Generic alternatives and
- Trends in prescription by medical practitioners
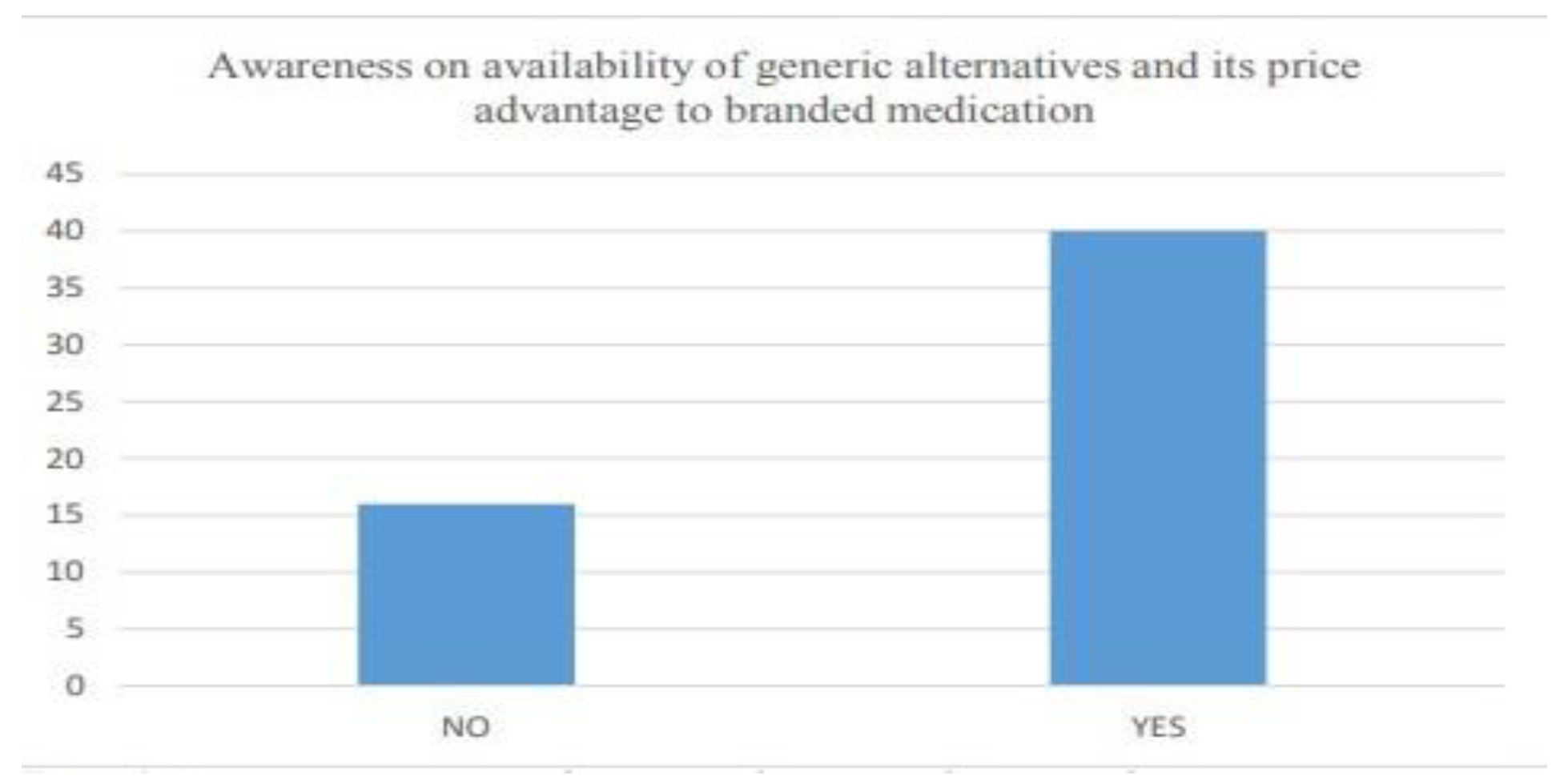
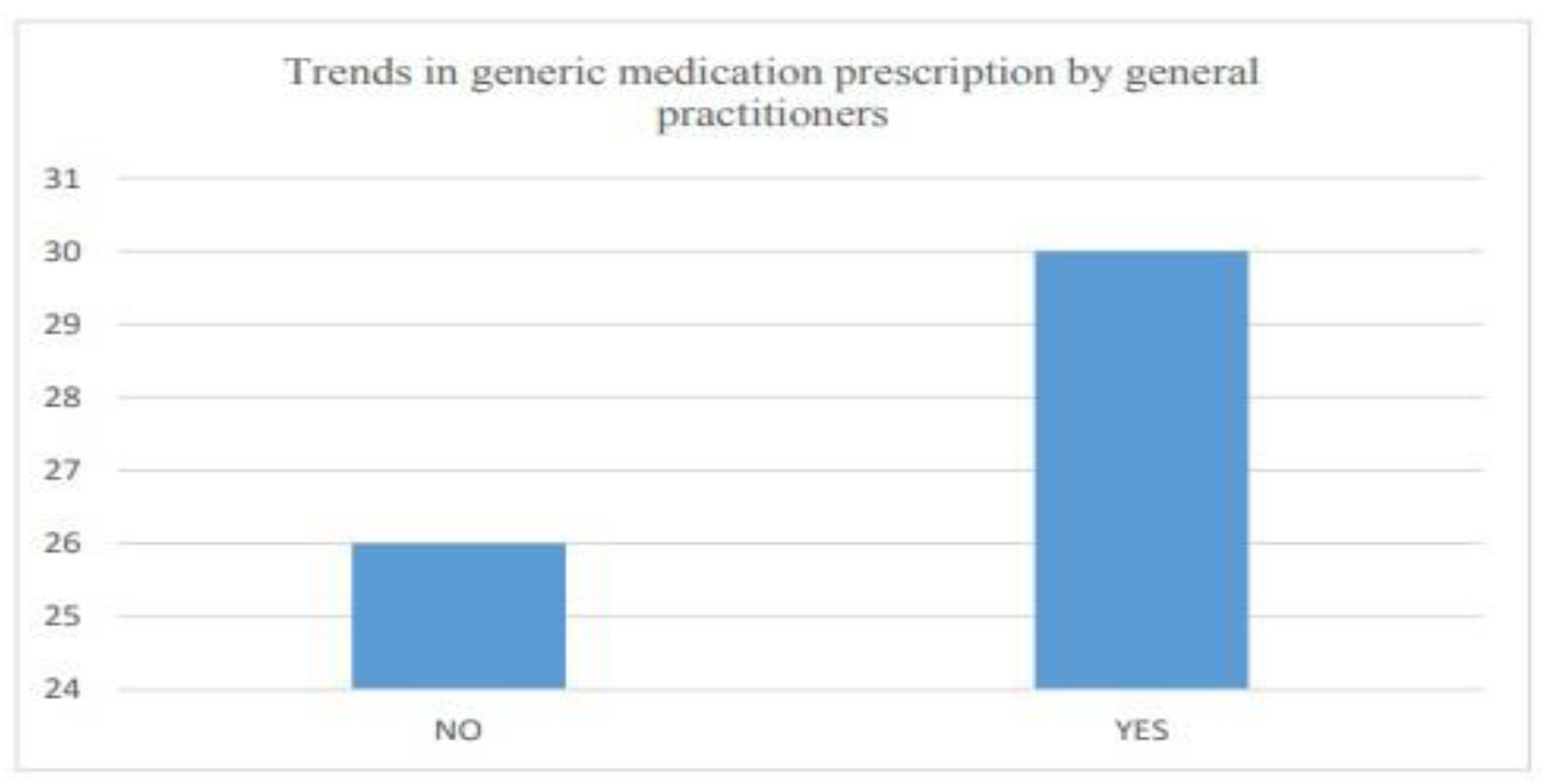
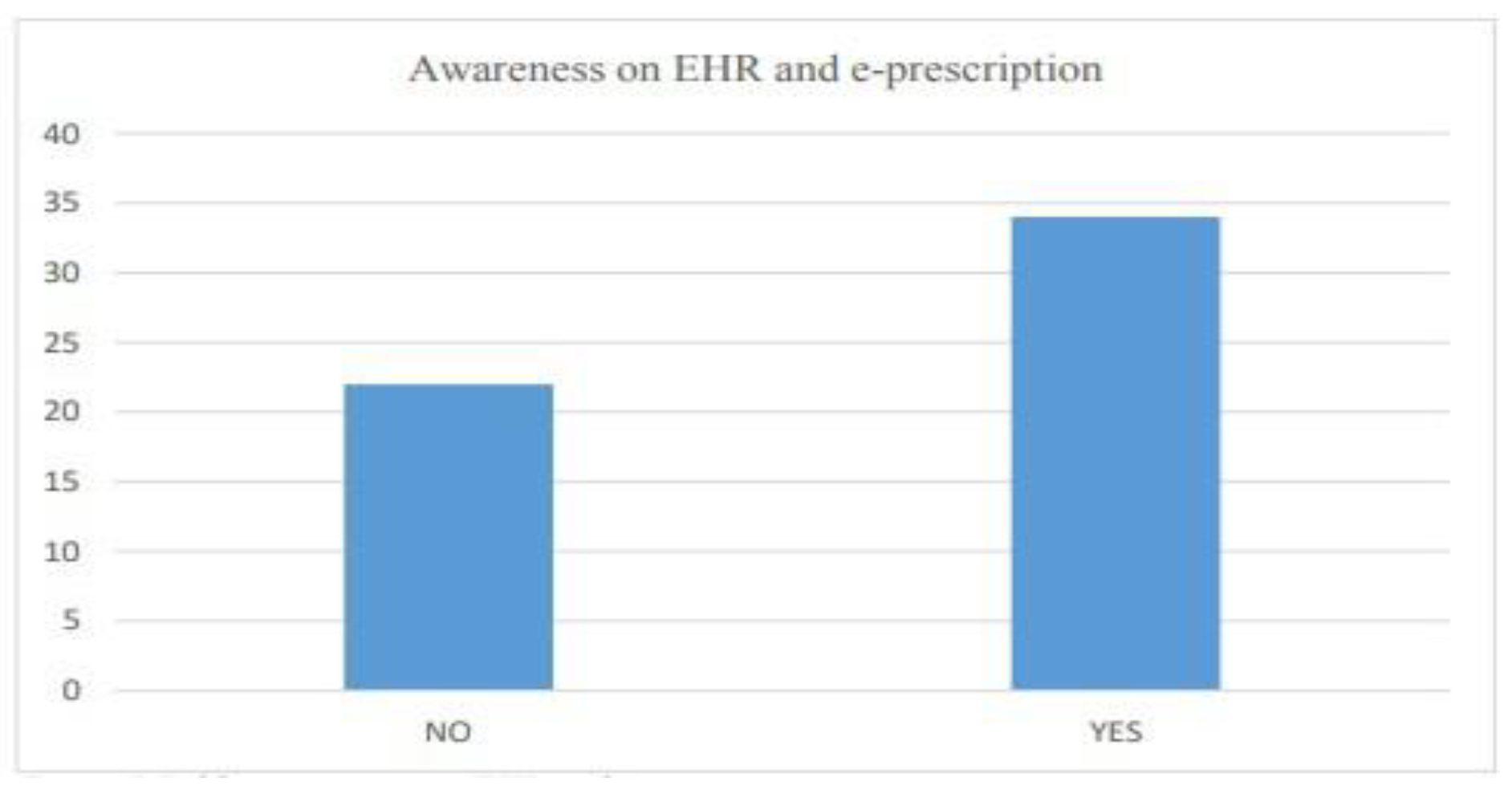
6.2.3. Customer attitude and trends
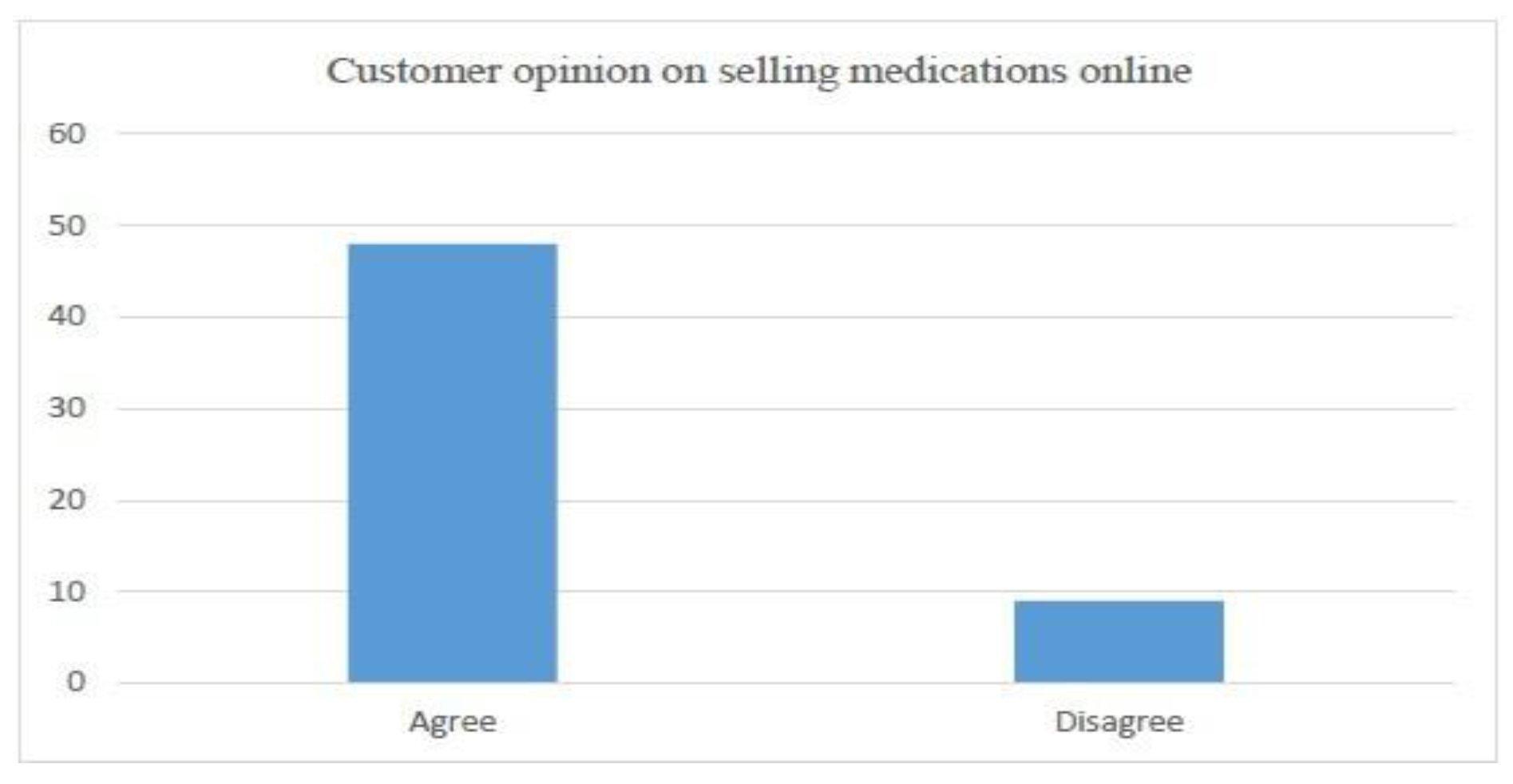
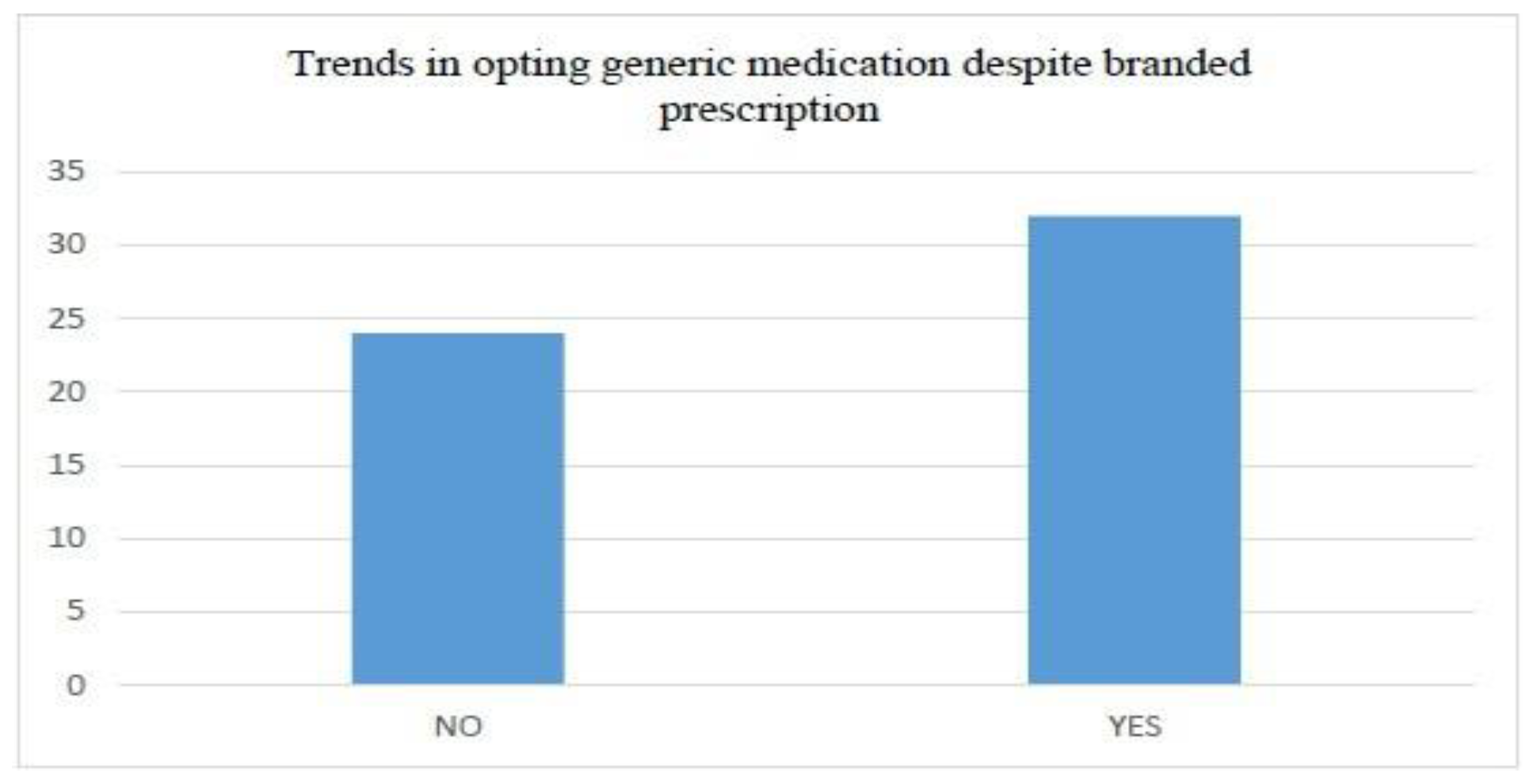
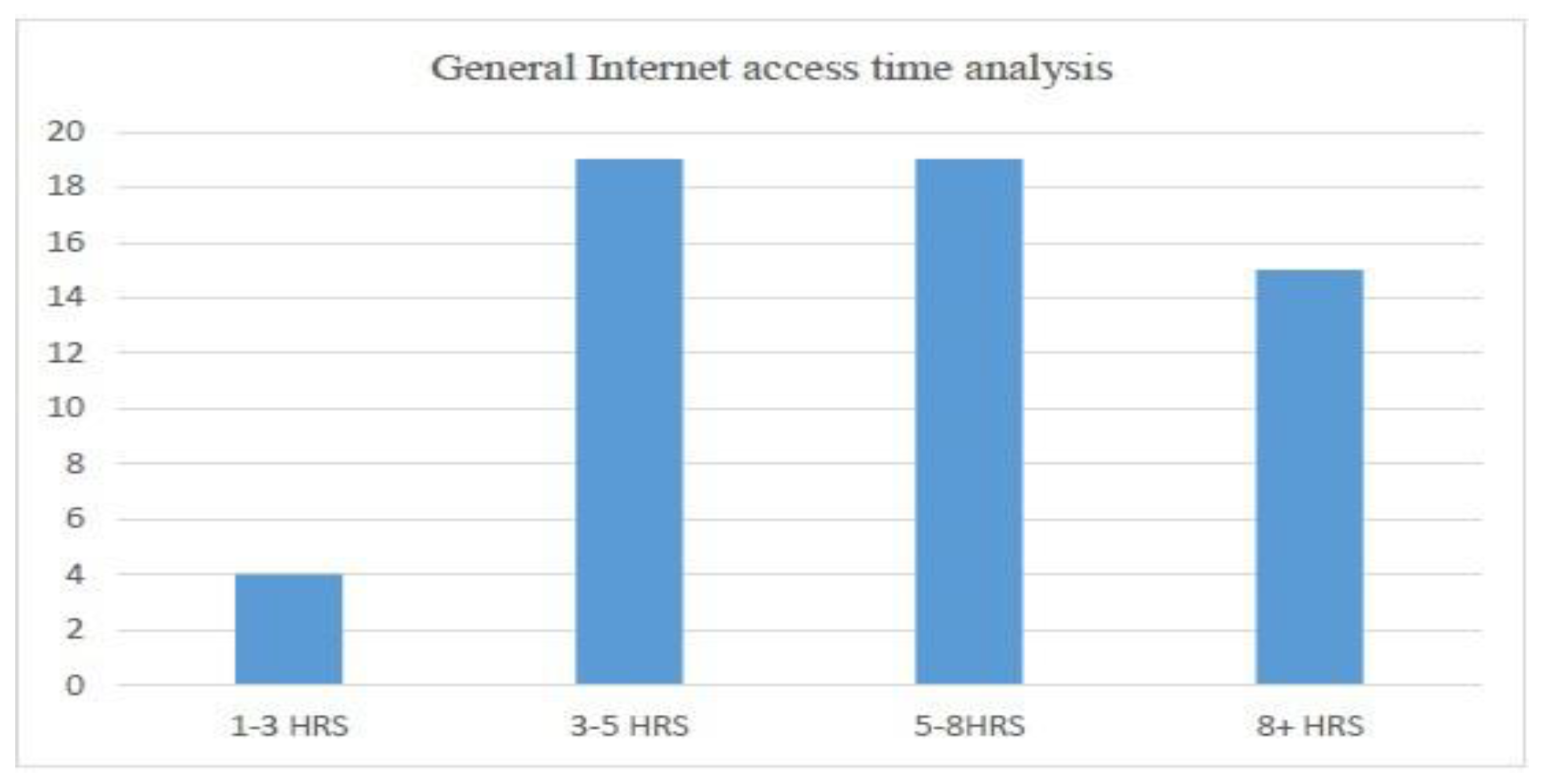
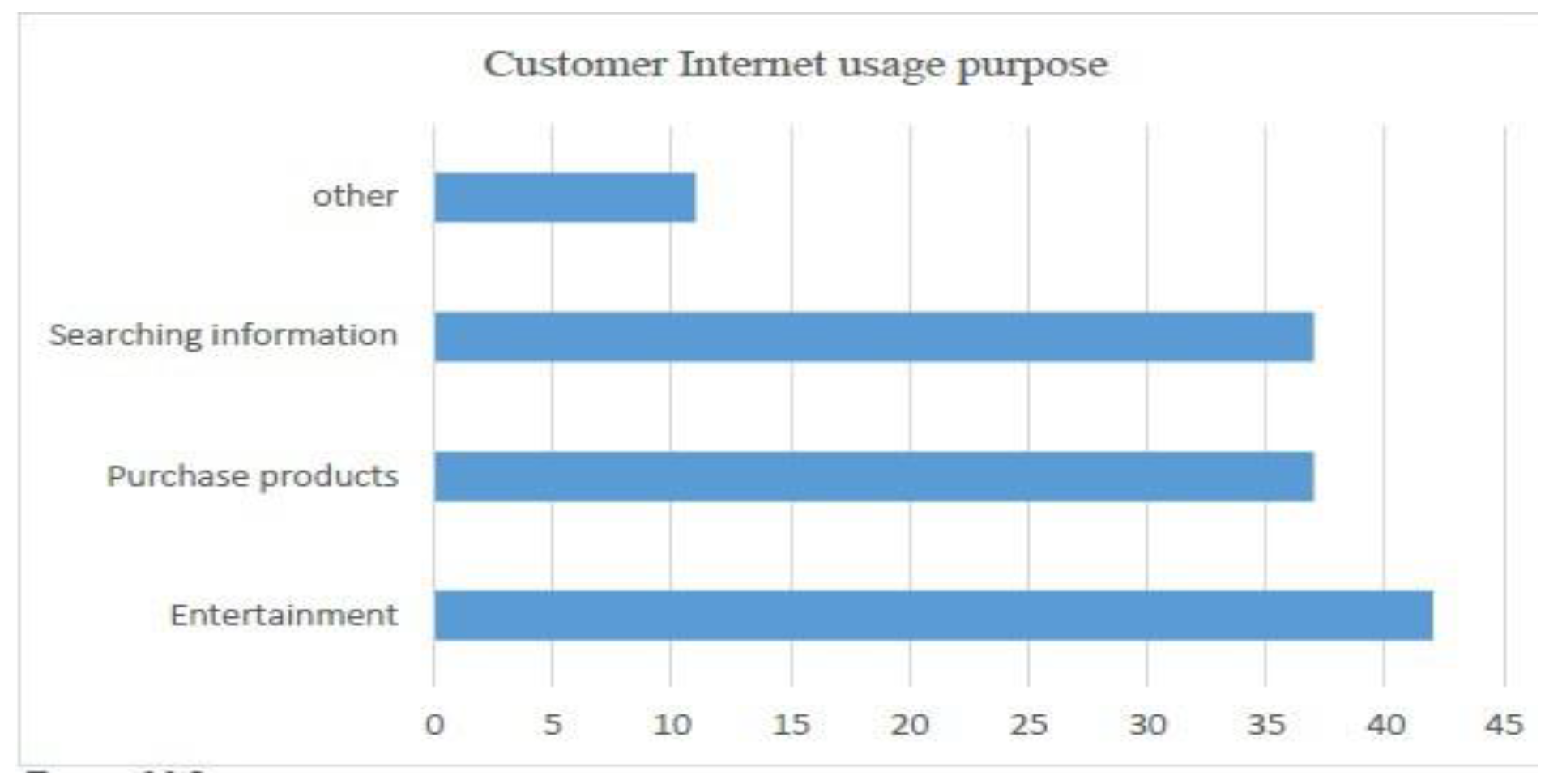
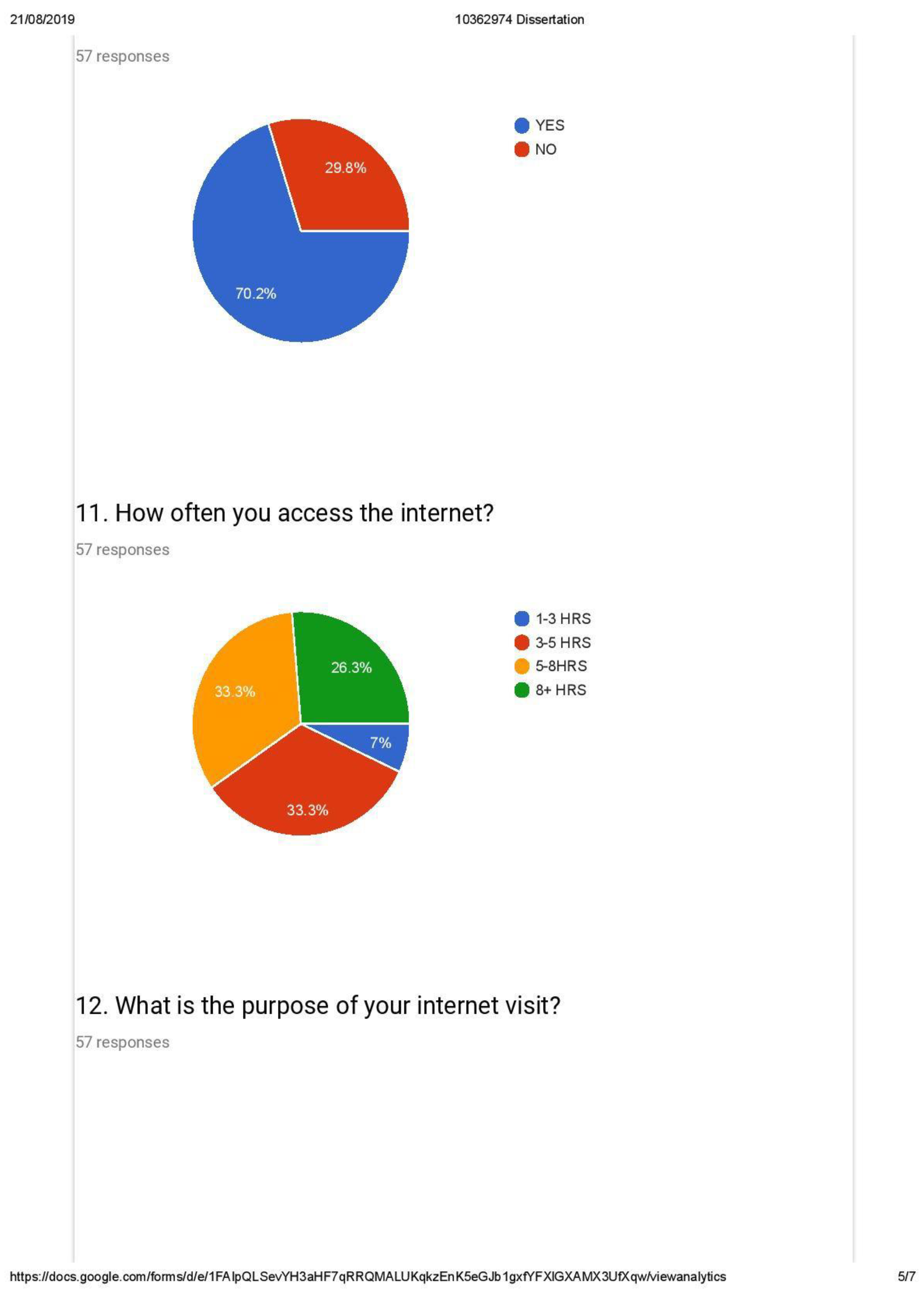
- Average Internet usage time and (weightage of 5 percent)
- Customer usage of the Internet for the purchase of goods (weightage of 40 percent)
- Customer’s buying platform preference (weightage of 15 percent)
- Preferred payment method for purchased goods (Weightage of 40 percent)
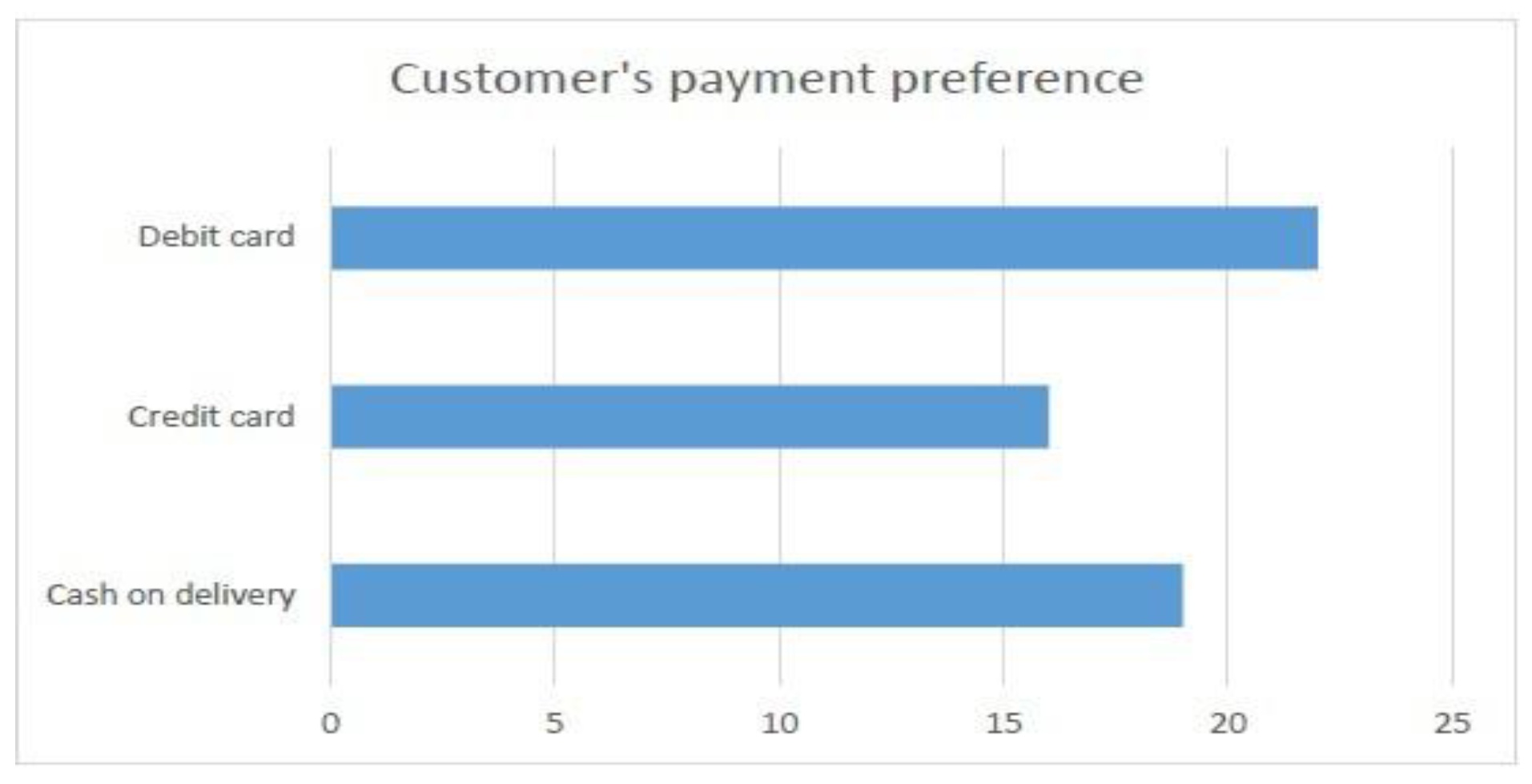
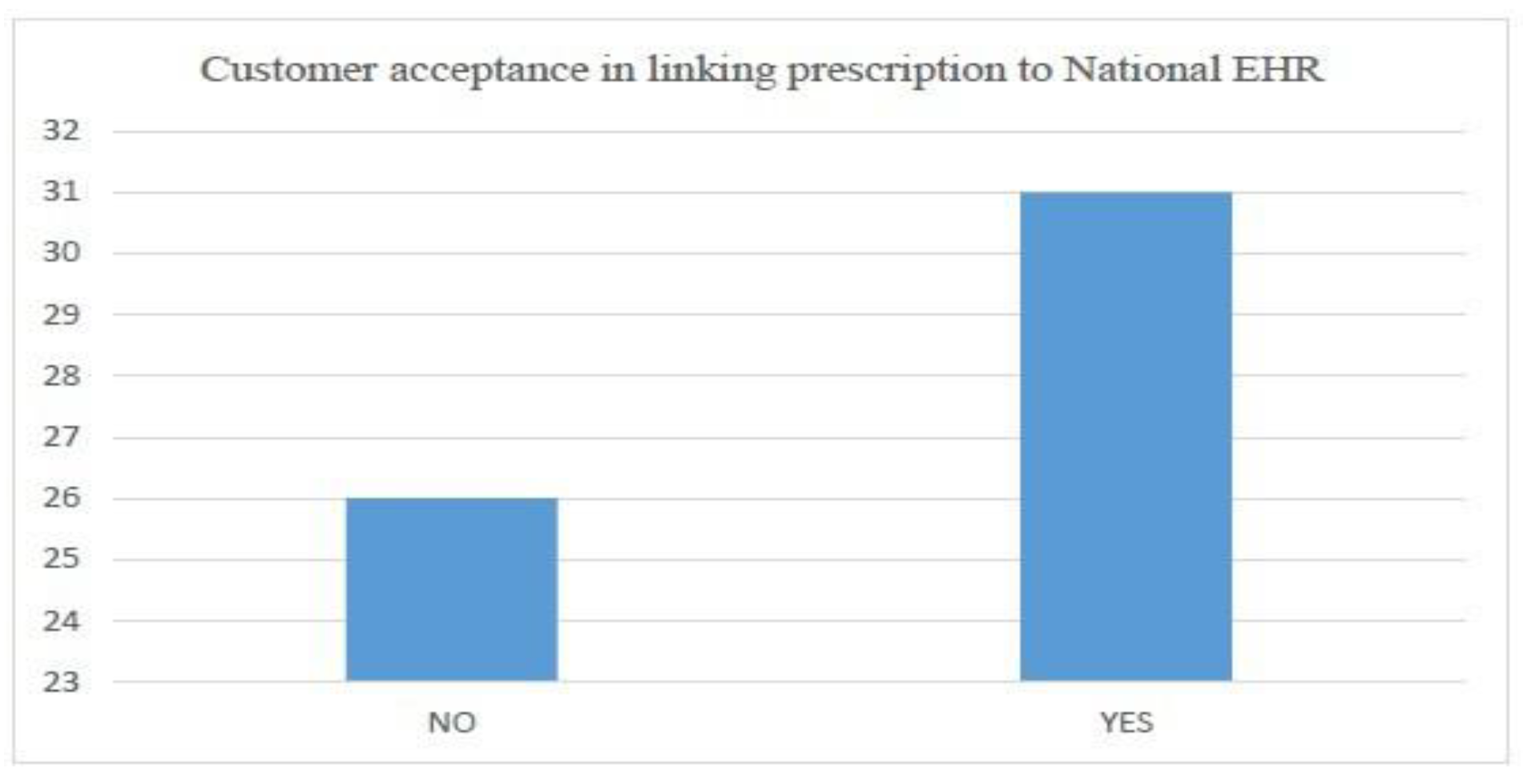
- Customer acceptance in generating e-prescription connected with national EHR (with weightage of 20 percent).
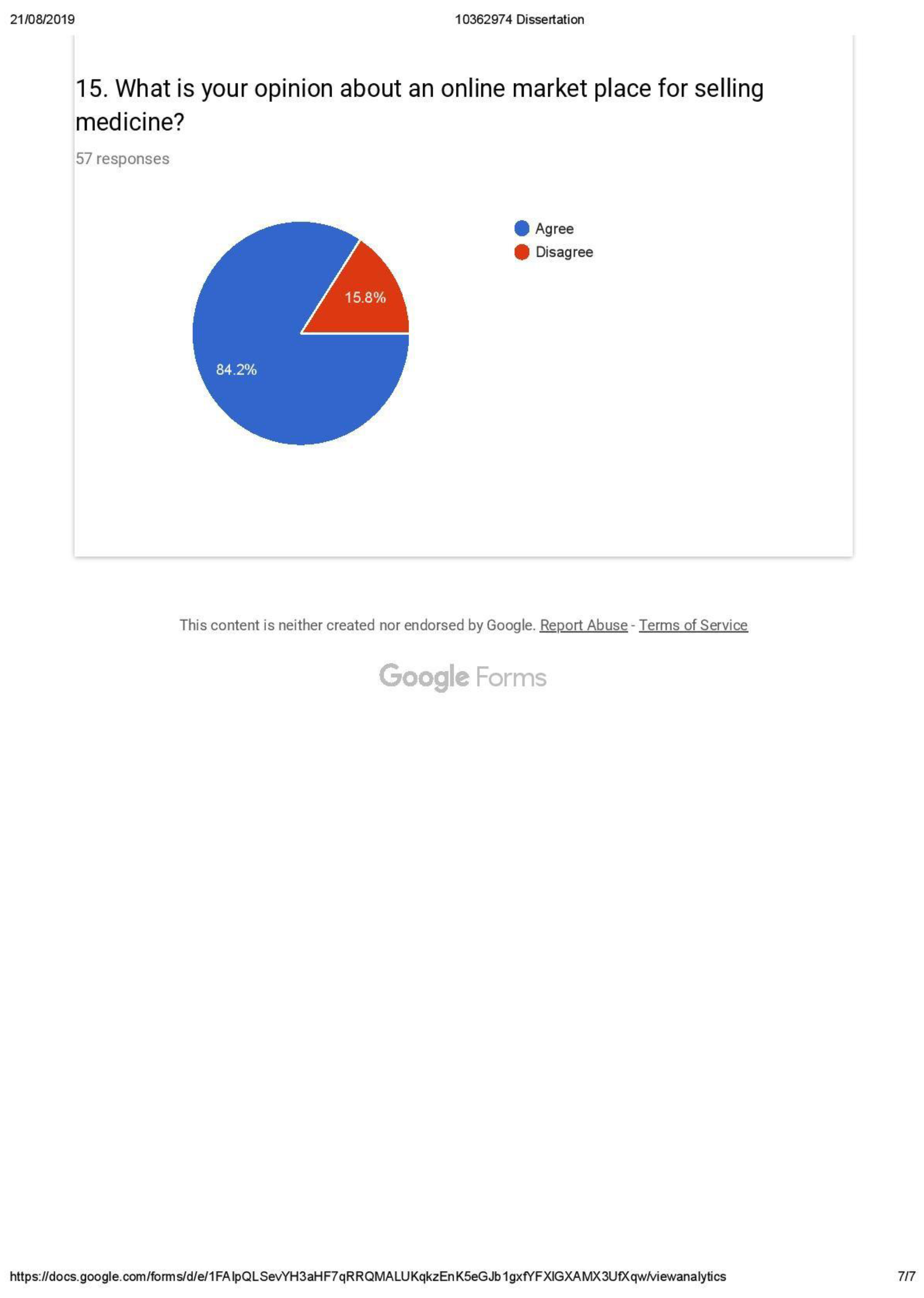
- Customer acceptance of having an online market place with access to e-prescription from national EHR (with weightage of 80 percent).
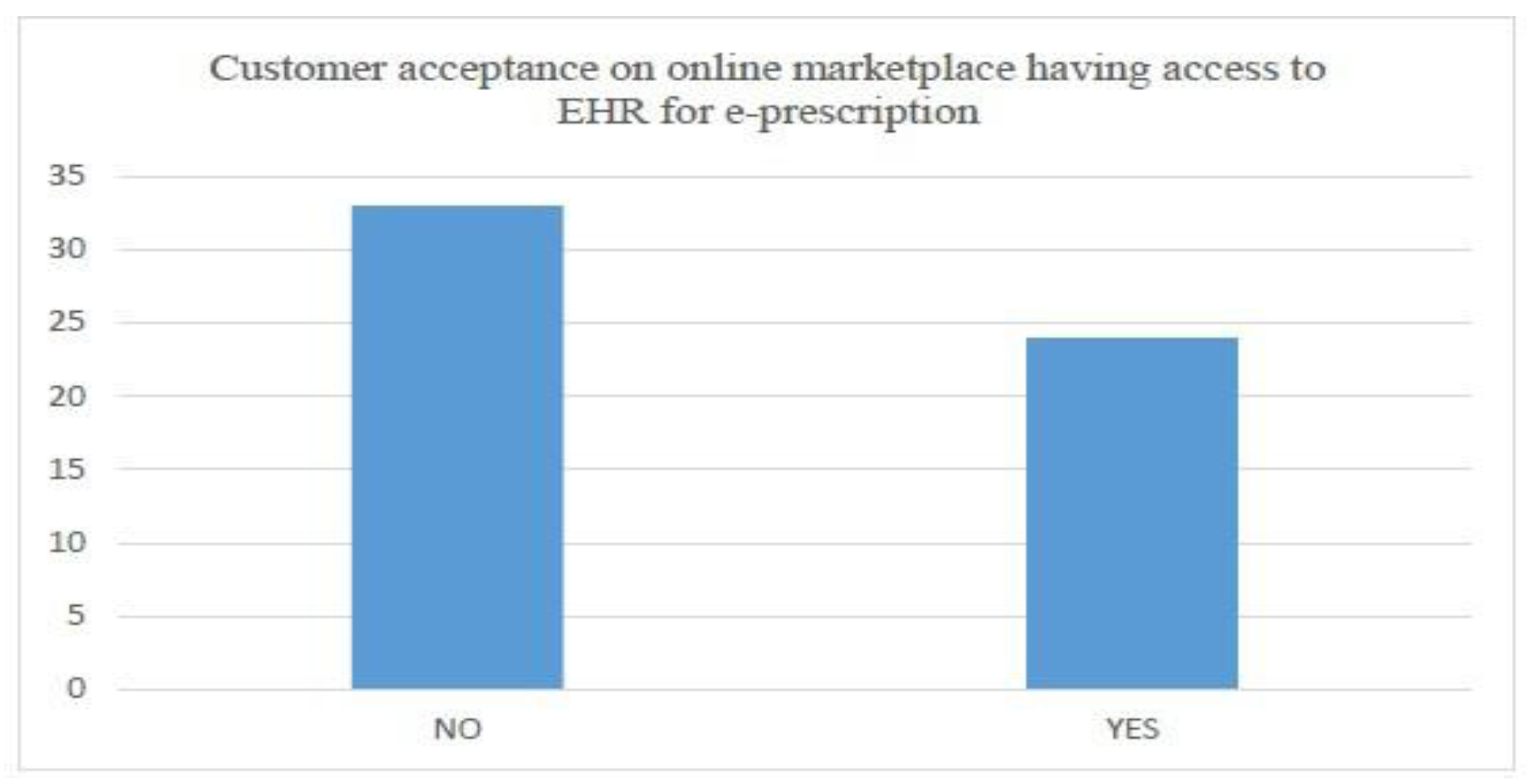
6.3. Summary
Chapter 7
7. Discussions
7.1. General perspectives
7.1.1. Medicine prices will be more transparent and market-oriented
7.1.2. Centralization of pharmaceutical industry
7.1.3. Expand the pharmaceutical logistics market
7.1.4. A complete service-oriented model.
7.1.5. Improved retail drugstores profitability
7.1.6. A panel for information innovation.
7.1.7. Health Benefits
7.2. Social benefits
7.2.1. Better healthcare system
7.2.2. A big financial relief
7.3. System Feedback
7.4. System Benefits
7.4.1. An easy window for showcasing products
7.4.2. Review and ratings
7.4.3. Price comparison
7.5. Summary
Chapter 8
8. Conclusion and Future scope
8.1. Conclusion
8.2. Future scope
Appendix A
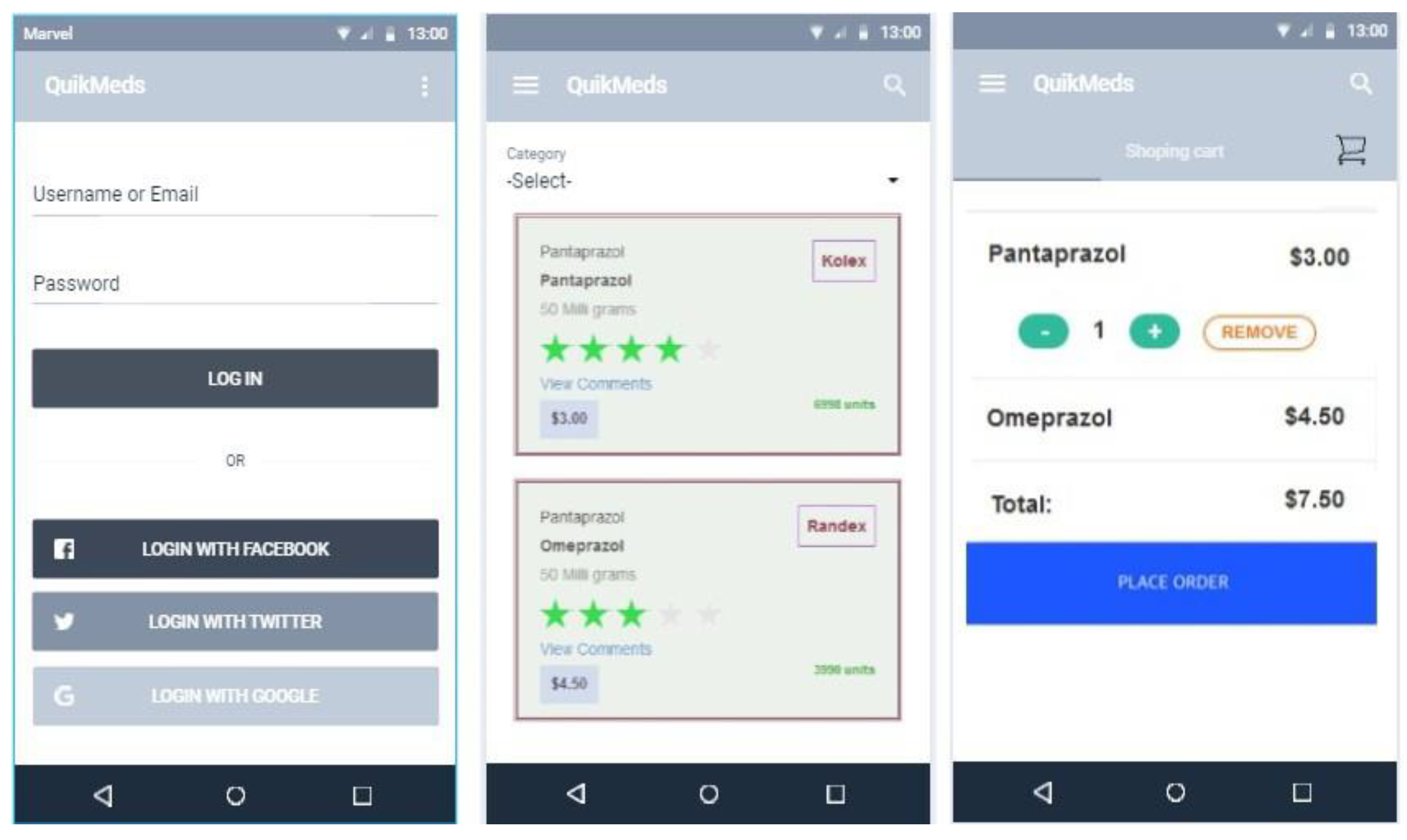
Appendix A1 Mobile Application - Prototype
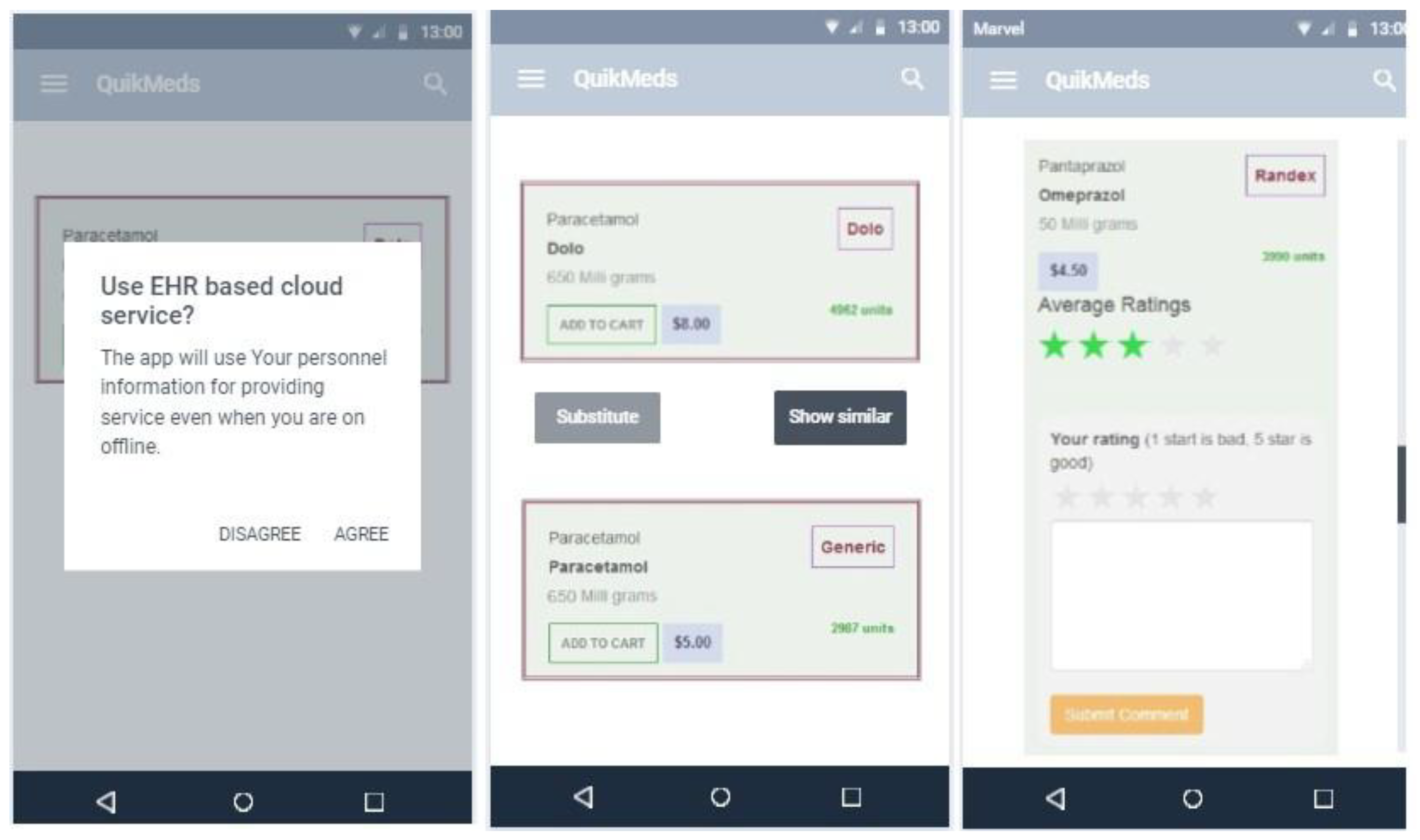
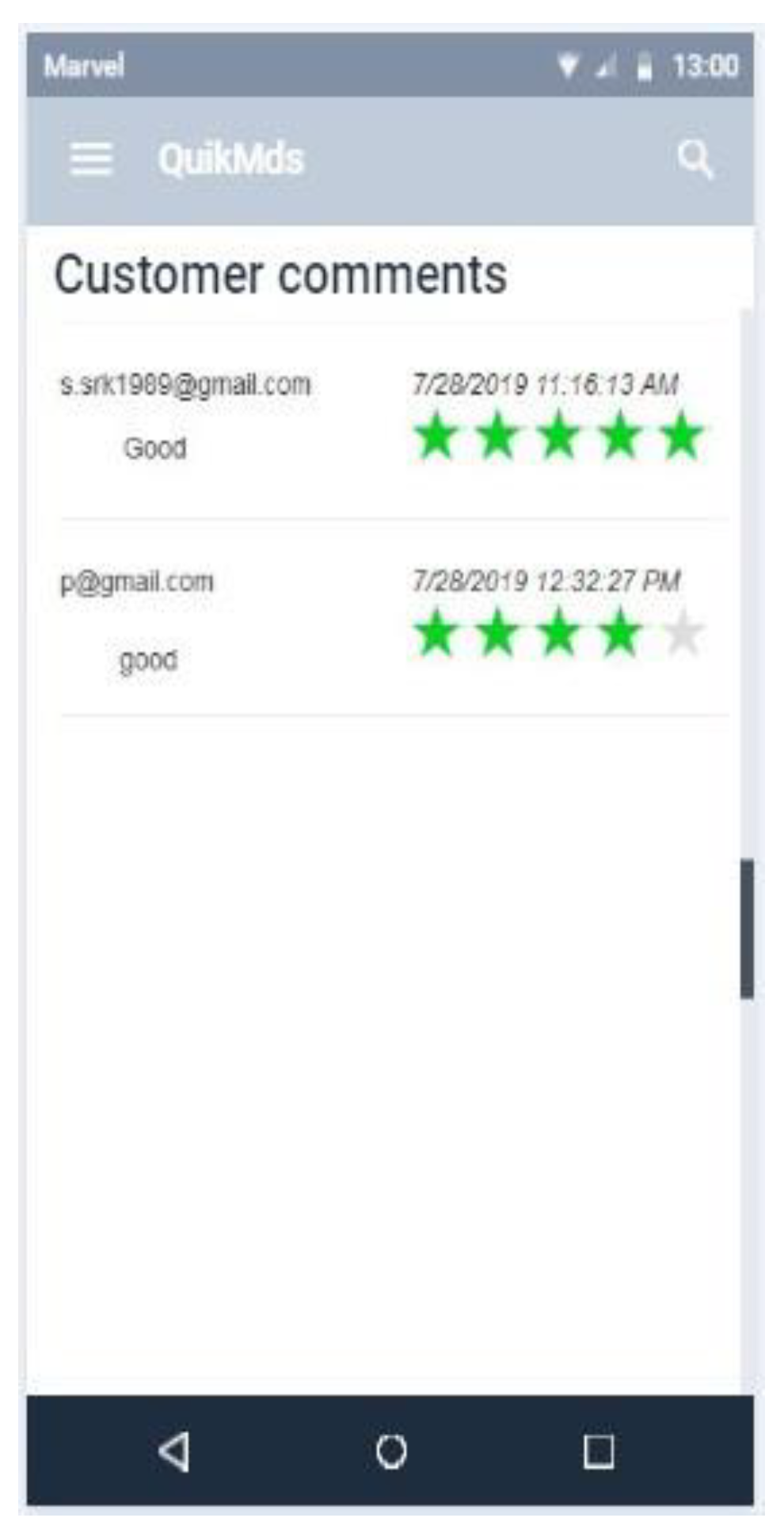
Appendix B
Appendix B1 Face to face interview Questionnaire for Pharmaceutical company Representative
- Will the availability of an online sales channel enabling the registered retailers improve your business volume and distribution ease or will it disrupt your business?
- Do you support a healthy competitive online market space?
- Why is there a huge pricing difference between generic and brand medicines?
- Does creating an online market space where branded and generic medicines are simultaneously traded affect your business volume? If you feel it would, can you state the reasons?
Appendix B2 Answers
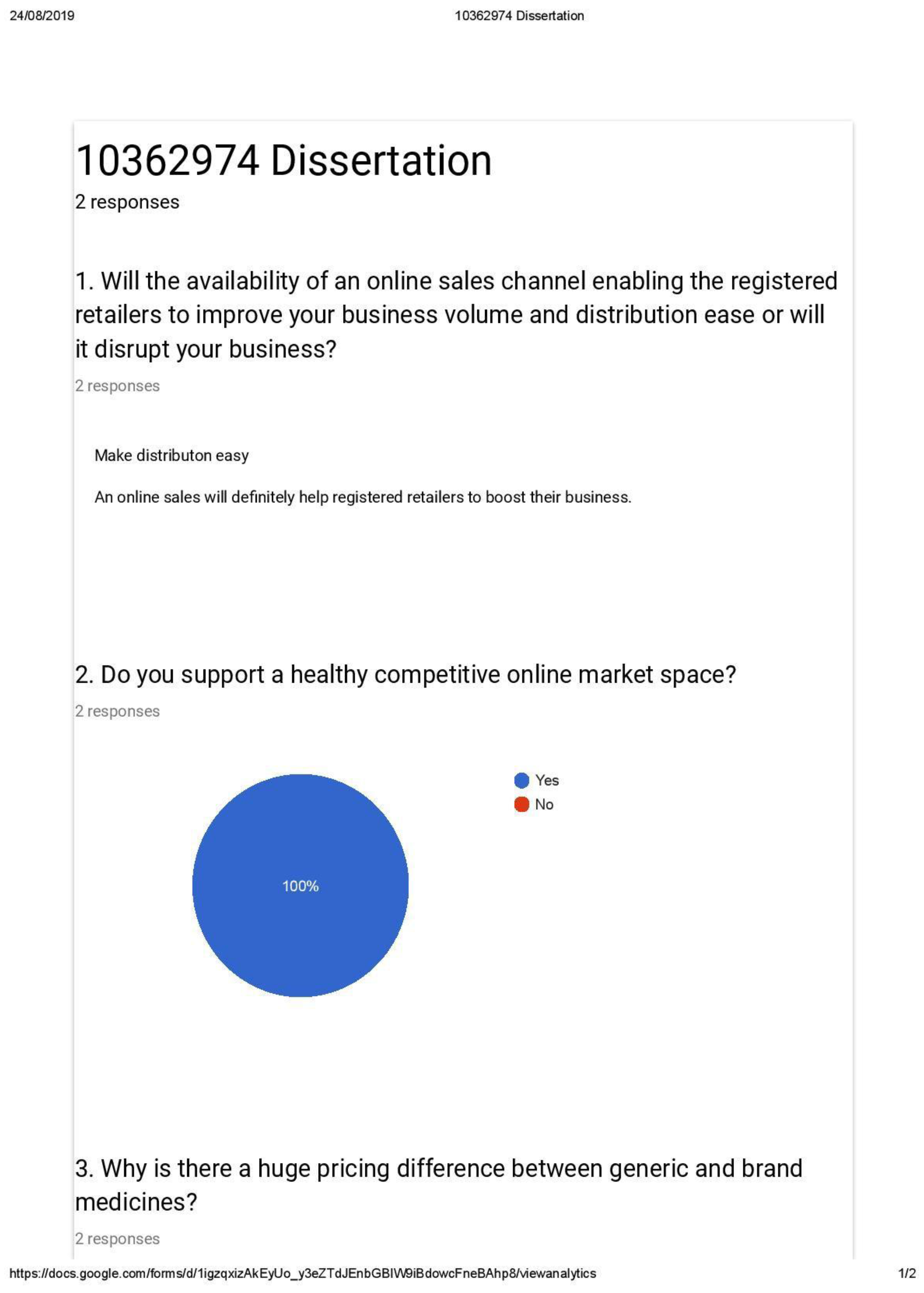
Appendix B3 Face to face interview Questionnaire for Pharmaceutical product retailers
- Will an online market, where you will be able to list your products, and receive your order be helpful to you?
- Will such a platform help to improve your volume of business?
- What is your biggest concern in such a platform?
- Will you be able to compete with other retailers in such an online platform?
- Are you ready to undercut your profit margin if you can increase your volume of sales?
- Up to what percentage of your sales are you ready to give as facilitation/operational fee towards such a platform?
- What are the facilities you are expecting in relation with the facilitation fee you have specified in the previous question?
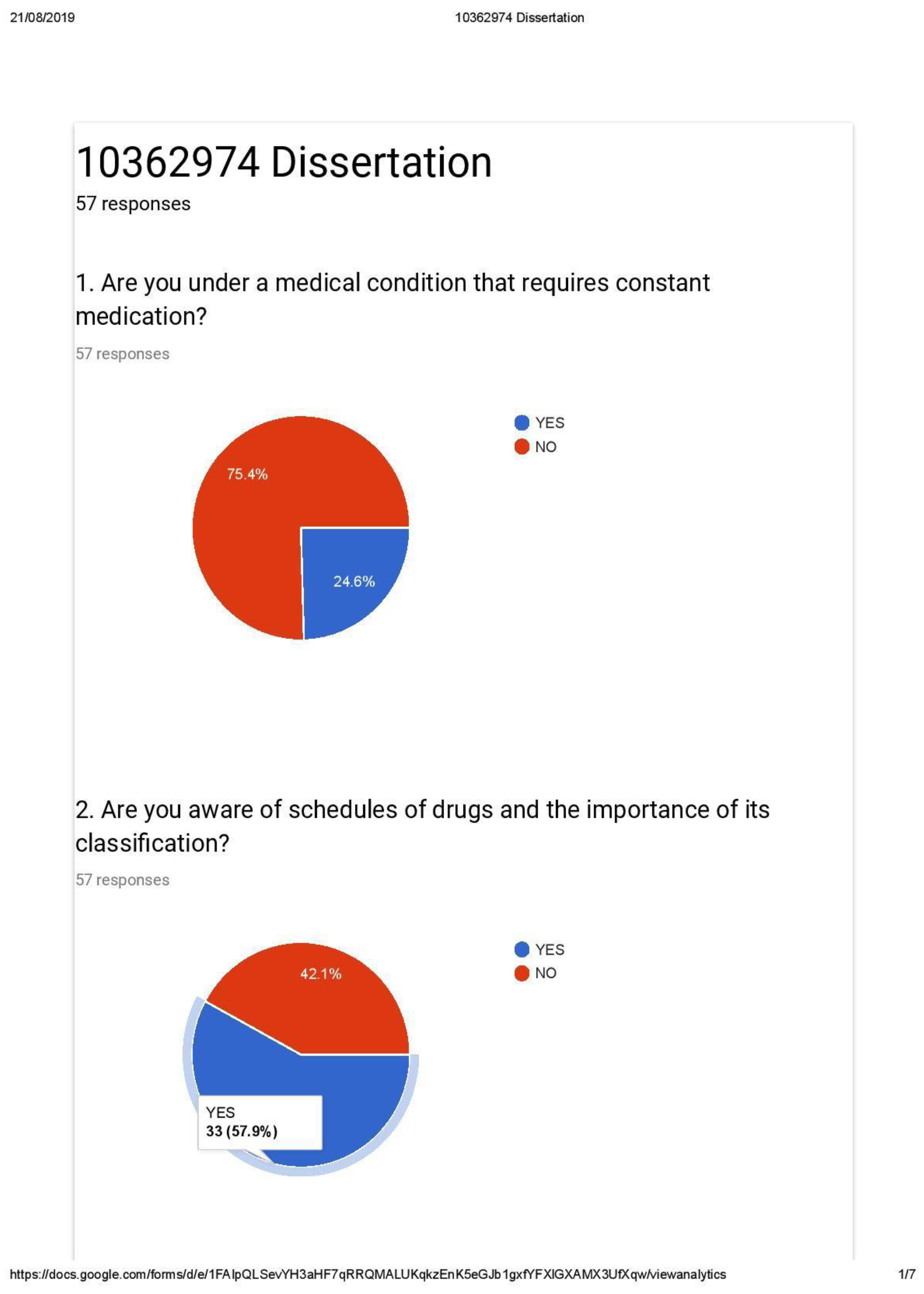
Appendix B4 General Questionnaire



Appendix C
Appendix D
References
- Agnieszka, Szewczyk (2015). Usability Of Internet Shops Quating The Example Of E- pharmacies. Vol. 72. University of Szczecin.
- Apoteket and European Commission Stokeholm (2015). Prescriptions Apoteket and Stockholm County Council Sweden-eRecept an E-Prescribing Application.
- Bershidsky, Leonid (2018). The influence of a growing online retail industry increases con- sumers’ exposure to the fluctuations of energy prices and exchange rates. URL: https:.
- //www.bloomberg.com/opinion/articles/2018-10-12/the-amazon-effect- can-drive-prices-up-too.
- Bihari, Michael (2019). Drug Classes: Making Sense of What Medication Classifications Mean. URL: https://www.verywellhealth.com/drug-classes-1123991.
- Brennan, Cianan (2017). Pharmaceutical industry paid Irish doctors 3.7 million for "undis- closed" reasons in 2015. URL: https://www.thejournal.ie/big-pharma-ireland- 3068146-Nov2016/.
- Burke, Elaine (2019). Ireland is a home for 24 of the world’s top biotech and pharma com- panies. URL: https://www.siliconrepublic.com/careers/biotech- pharma- companies-ireland.
- Buying medicines online (2019). URL: https : / / www . ema . europa . eu / en / human - regulatory/overview/public-health-threats/falsified-medicines/buying- medicines-online.
- Car, Josip and Ashly Black (2008). “Prevention Using Information Technology Sys- tems”. In: Medication Errors: Prevention Using Information Technology Systems 62.1,.
- pp. 1–20.
- Department of Health (2017). URL: http://health.gov.ie/.
- Directive (2001). Directive 2001/83/Ec Of The European Parliament. URL: https : / / www . ema . europa . eu / en / documents / regulatory - procedural - guideline / directive- 2001/83/ec- european- parliament- council- 6- november- 2001- community-code-relating-medicinal-products-human-use_en.pdf.
- —(2011). Directive 2001/62/Ec Of The European Parliament. URL: https://eur-lex. europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:174:0074:0087:EN: PDF.
- Electronic Irish Statute Book (2013). URL: http://www .irishstatutebook.ie/eli/ 2013/act/31/enacted/en/print#part1.
- European Association of Mail Service Pharmacy (2019). URL: https : / / www . eamsp . pharmacy/.
- Generic Drugs: Questions And Answers (2018). URL: https://www.fda.gov/drugs/ questions-answers/generic-drugs-questions-answers.
- Giuseppe, Colangelo and Tortie Valerio (2017). Selective distribution and online market- place restrictions under EU competition rules after Coty Prestige. 1st ed. Vol. 1. 81-109. Europiean Competition Journal.
- Gorecki, Paul (2017). PharmacoEconomics. URL: https://www.esri.ie/publications/ availability-and-pricing-new-medicines-in-ireland-reflections-and- reform.
- Home - PMC - NCBI. URL: https://www.ncbi.nlm.nih.gov/pmc/articles.
- Ian, Sommerville (2016). Software Engineering, 10th Edition. URL: https : / / www . pearson.com/us/higher-education/program/Sommerville-Software-Engineering- 10th-Edition/PGM35255.html.
- Ltd, White October (2018). FoodRisc Resource Centre. URL: http://resourcecentre. foodrisc.org/mixed-methods-research_185.html.
- Lynch, Priscilla (2014). Generic medicine: What’s in a name? URL: https://www.irishtimes. com/life-and-style/health-family/generic-medicine-what-s-in-a-name- 1.1834124.
- McDermid, Douglas (2011). “Pragmatism”. In: Pragmatism 62.1, pp. 1–20. URL: https:.
- //www.iep.utm.edu/pragmati/.
- Murphy, Kieran C and Caroline Spillane (2011). The Role of the Medical Council Pro- tecting Patients, Supporting Doctors. URL: https : / / www . medicalcouncil . ie / News - and - Publications / Publications / Annual - Reports - Statistics - /A - Statistical-Report,-June-2011.pdf/.
- Murray, Sean (2017). ’Oh, that’s just the price’: 11 difference in cost of same medication in two Dublin pharmacies. URL: https://www.thejournal.ie/pharmacy- prices- different-3544949-Aug2017/.
- Natalie, Richards and Hudson Ian (2016). UK medicines regulation: responding to cur- rent challenges. URL: https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10. 1111/bcp.13077.
- National Medicines Information Centre (2008). URL: http :// www . stjames . ie / GPs / HealthcareProfessionals/Newsletters/NMICBulletins/NMICBulletins2008/.
- Non-prescription medicines (2018). URL: http://www.aesgp.eu/self-care/regulation/ non-prescription-medicines/.
- O’conel, Ronal (2019). Pharmacy. URL: https://www.google.com/publicdata/.
- O’Regan, Eilish (2016). ’My medication costs 144 a month in Republic - but I can get six-month supply for 50 in North’. URL: https:/ / www .independent .ie/ irish- news/health/my-medication-costs-144-a-month-in-republic-but-i-can- get-sixmonth-supply-for-50-in-north-34750761.html.
- Pharmaceutical Society Of Ireland (2007). URL: https://www.thepsi.ie/tns/about- psi/legislation.aspx.
- Pharmaceutical Society Of Ireland (2008). URL: https://www.thepsi.ie/tns/about- psi/legislation.aspx.
- Porecha, Maitri (2018). Sales at Jan Aushadhi drug stores cross 150 crore in Q2. URL: https://www.thehindubusinessline.com/economy/sales-at-jan-aushadhi- drug-stores-cross-150-crore-in-q2/article25382362.ece.
- Prescription - e-Estonia. URL: https://e- estonia.com/solutions/healthcare/e- prescription/.
- PSI Internet supply list (2019). URL: https://www.thepsi.ie/Libraries/Approved_ companies / PSI _ List _ of _ approved _ companies _ for _ the _ sale _ of _ non - prescription_medicines_online.sflb.ashx.
- Ries, Eric (2018). Minimum Viable Product. URL: http://www.startuplessonslearned. com/2009/08/minimum-viable-product-guide.html.
- Tripathy, Ansuman (2018). India Emerges As Top Five Pharmaceuticals Markets Of The World. URL: http://www.businessworld.in/article/India-Emerges-As-Top- Five-Pharmaceuticals-Markets-Of-The-World/05-05-2018-148349/.
- WHO (2017). 2. Anatomical Therapeutic Chemical (ATC) Classification. URL: https:// www.who.int/medicines/regulation/medicines-safety/toolkit_atc/en/.
- Wholesale Distribution (2019). URL: http :// www . hpra . ie / homepage / medicines / regulatory-information/wholesalers-and-distributors.
- Workman, Daniel (2019). Drugs and Medicine Exports by Country. URL: http://www. worldstopexports.com/drugs-medicine-exports-country/.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




