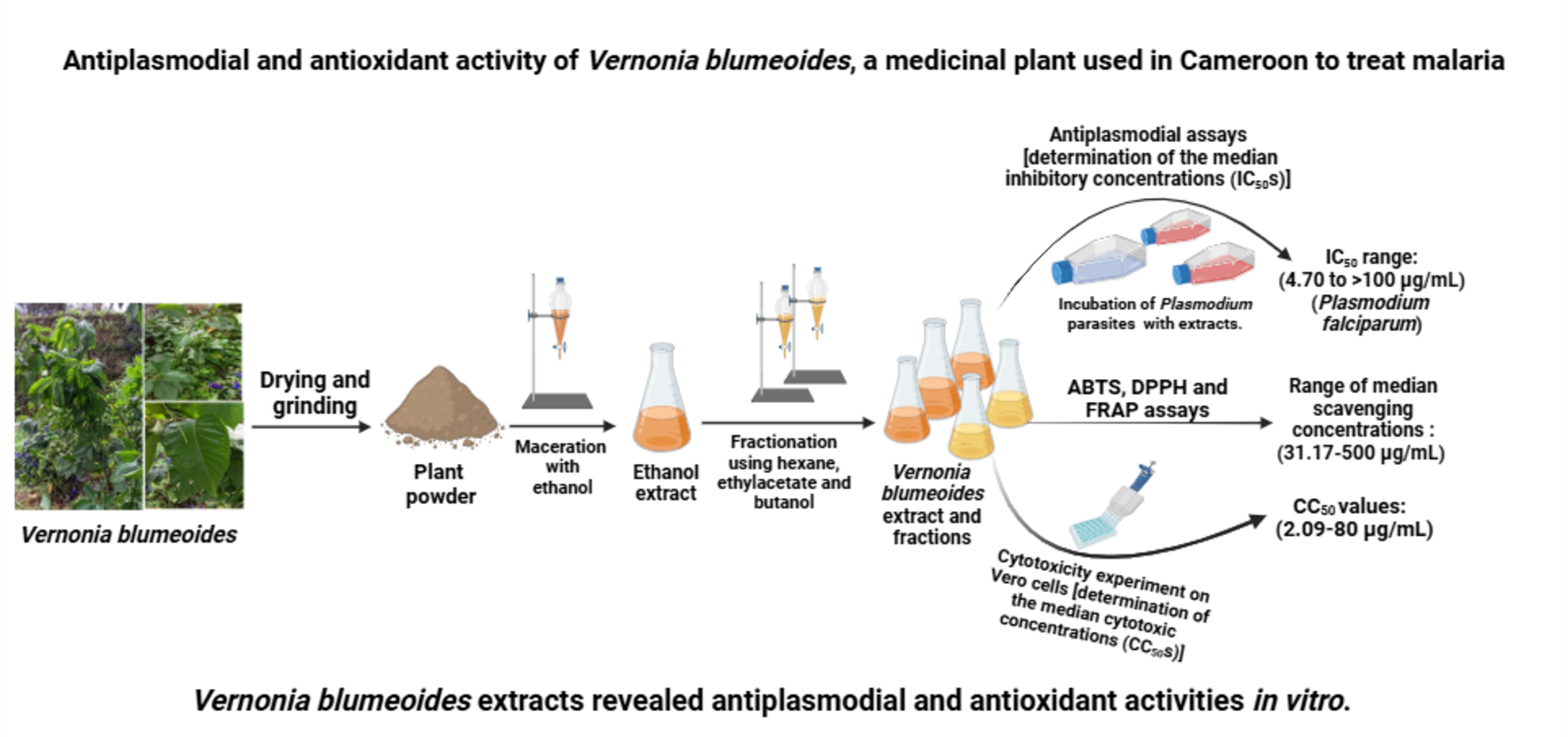
1. Introduction
Malaria is an infectious disease caused by parasites of the genus
Plasmodium and transmitted to humans through the bite of an infected female Anopheles mosquito [
1]. There are five species of
Plasmodium that are responsible for this disease. These include
Plasmodium falciparum,
P. vivax,
P. ovale,
P. malariae, and
P. knowlesi [
2,
3]. Of these microorganisms,
P. falciparum is the most severe parasite that causes the highest number of deaths [
4]. There are about 200 million cases of malaria, resulting in almost half a million deaths each year [
4]. Anopheles mosquitoes (
Anopheles gambiae,
Anopheles stephensi, etc.), the vectors of malaria, are influenced by environmental and climatic factors, which have an impact on the parasite’s transmission [
5]. The pathogenesis of malaria starts when the sporozoites enter the blood stream following a female Anopheles mosquito’s bite. Next, the parasites travel to the liver host, where they invade the hepatocytes and mature into liver-stage schizonts, which burst to release 2,000 to 40,000 uninucleate merozoites, which infect other red cells. During the mosquito blood meal, gametocytes, which can be ingested, develop in the mosquito gut to gametes, which undergo fertilization and mature in 2 to 3 weeks to sporozoites [
6,
7].
There are four major classes of antimalarial drugs that have been used for the treatment of malaria. These include arylamino alcohol compounds (quinine, quinidine, chloroquine, amodiaquine, mefloquine, halofantrine, piperaquine and lumefantrine) ; antifolates (sulfadoxine, pyrimethamine, proguanil, chlorproguanil and trimethoprim) ; 8-aminoquinoline (primaquine and tafenoquine); artemisinin derivatives (artemisinin, artesunate, artemether, β-arteether and dihydroartemisinin) and antibacterials (tetracycline, doxycycline and clindamycin), as well as their combination with other drugs [
8].
Due to the emergence of multidrug resistance, artemisinin-based combination therapies have been recommended by WHO as the first line treatment for uncomplicated malaria [
9]. The rationale behind combination therapy being the fact that two drugs used with different modes of action might target different resistance mechanisms; thus, the probability of developing resistance to both drugs at the same cell division is the product of their individual probabilities. However, recent reports highlighted
Plasmodium falciparum resistance of artemisinin-based combination therapy [
10,
11]. Therefore, there is a pressing need to search for effective treatments against malaria.
Furthermore, oxidative stress is intricately involved in the pathogenesis of malaria and includes various mechanisms, such as the overproduction of free radicals by immune cells and the generation of pro-inflammatory cytokines. The as-generated oxidative stress may participate in the development of malaria by accelerating the spread of infection, damaging host tissues and disrupting the immune response [
12]. The abrogation of reactive oxygen species (ROS) and the reduction of infection-induced oxidative stress are the key roles played by antioxidant substances to overcome parasitic infections. Antioxidant compounds also strengthen the body's defences against oxidative damage. By promoting an effective immune response against pathogens, antioxidants can therefore help mitigate cellular damage [
13,
14]. Therefore, the need for a dual therapy that defeats the pathogen and neutralizes the redox imbalance generated during infection is paramount.
Medicinal plants are among the important sources to produce pharmacophores with high selectivity [
15,
16]. Drug discovery based on natural products and secondary metabolites is considered as alternative approaches for antimalarial therapy [
17].
The isolation of secondary metabolites with antimalarial properties from different plant organs was already reported with the advent of quinine from chincona tree and artemisinin from
Artemisia annua [
17,
18,
19].
A number of medicinal plants, including the Vernonia species (Vernonia amygdalina, Vernonia brachycalyx, Vernonia cinerea, Vernonia colorata, Vernonia fastigiata, Vernonia guineensis, Vernonia lasiopus, Vernonia myriantha, and Vernonia oligocephala, among others) were reported to exhibit antiplasmodial activity [
20,
21,
22]. Despite the excessive use of the aerial parts of Vernonia blumeoides in treating malaria and diarrhea symptoms by Nigerian tribes [
23], the scientific validation of V. blumeoides (also known as Bagashi in the Hausa tribe, Africa) as an antiplasmodial plant is not yet reported. Other traditional uses of Vernonia blumeoides include treatment of various parasitic and unspecified infectious diseases [
24,
25].
Plants of the
Vernonia genus produce characteristic compounds such as sesquiterpene lactones, with several reported biological activities, such as fungistatic [
26], and cytotoxic activities [
27]. Some other compounds that have been isolated from
Vernonia include flavonoids [
28,
29,
30], steroids [
31,
32] and polysaccharides [
33,
34].
Based on the foregoing considerations, this study sought to investigate the antiplasmodial activity of V. blumeoides extracts against sensitive and resistant strains of P. falciparum. The antioxidant and cytotoxic effects of the antiplasmodial extracts are also investigated.
2. Materials and Methods
2.1. Materials
2.1.1. Plant Collection and Identification
The leaves and stems of Vernonia blumeoides (Asteraceae) were collected in January 2023 at Metep (5°06'21.3"N 12°49'46.5"E) in the Nsem district, Department of Nanga-Eboko (Centre Region, Cameroon). The identity of the plant was confirmed by Mr Nana Victor (Botanist) at the National Herbarium of Cameroon (Yaounde, Cameroon), where a specimen was deposited under voucher number 35087/HNC as compared to a plant sample from R. Letouzey N: 13276 of 22th November 1974. Then, the plant was brought to the Research Unit of Environmental and Applied Chemistry of the University of Dschang (Dschang, Cameroon), washed to remove dirt and other contaminants, then shade dried at room temperature. Then, the dried material was coarsely powdered and kept at 4°C until further use.
Figure 1.
Photography of Vernonia blumeoides collected at Metep (5°06'21.3"N 12°49'46.5"E) in the Nsem district, Department of Nanga-Eboko (Centre Region, Cameroon) (By Batana MFL).
Figure 1.
Photography of Vernonia blumeoides collected at Metep (5°06'21.3"N 12°49'46.5"E) in the Nsem district, Department of Nanga-Eboko (Centre Region, Cameroon) (By Batana MFL).
2.1.2. Plasmodium falciparum Strains
The plant extracts were tested in vitro for antiplasmodial activity on chloroquine-sensitive (3D7) and multidrug-resistant (Dd2) strains of P. falciparum, supplied by BEI-Resource (Biodefense and Emerging Infections Research Resources Repository). These strains are maintained in continuous culture at the Laboratory for Phytobiochemistry and Medicinal Plants Studies at the University of Yaounde 1.
2.1.3. Mammalian Cells
The in vitro cytotoxicity of the active extract and fractions was evaluated on murine macrophage Raw 264.7 cell line, which was gifted by the Noguchi Memorial Institute for Medical Research, University of Ghana. The cells were maintained in continuous culture at the Laboratory for Phytobiochemistry and Medicinal Plants Studies, Department of Biochemistry, University of Yaounde 1.
2.2. Methods
2.2.1. Plant Extraction
The coarsely powdered
Vernonia blumeoides leaves and stems (2.09 kg) were macerated in 14 L of ethanol for 72 hours. The mixture was stirred twice a day and the obtained macerate was filtered through a hydrophilic cotton, then evaporated using a rotary evaporator (Rotavapor® R-100) at 60°C under reduced pressure, and dried under ventilation. The process was repeated 3 times. The dry crude extract was weighed using a mechanical balance (Triple Beam Balance 2610) and stored at 4°C for further use. The extraction yield was calculated according to the following formula:
2.2.2. Phytochemical Screening
A qualitative analysis of the ethanol extract was conducted to identify different constituents, such as steroids, triterpenes, flavonoids, and phenolic compounds [
35], sugars [
36], alkaloids and tannins [
37], which could be responsible for the antiplasmodial activity.
a. Liebermann-Burchard test
In this assay, 1 mg of extract was dissolved in chloroform, followed by an addition of acetic anhydride and concentrated sulphuric acid. After stirring, the presence of triterpenes was indicated by a purplish-red colour, whereas the presence of steroids was indicated by a greenish-blue colour [
35].
b. Molisch assay
To identify the presence or absence of sugars in the test sample, 1 mg of extract was dissolved in ethanol, followed by an addition of 1% ethanol solution of α-naphthol, then a few drops of concentrated sulfuric acid. Next, the presence of sugars was indicated by the appearance of a purplish-red ring at the interface of the solution [
36].
c. Ferric chloride (FeCl3) test
To identify the presence of phenolic compounds, 1 mg of extract was dissolved in ethanol, followed by an addition of FeCl
3 solution. The occurrence of phenolic compounds resulted in the development of blue or violet complexes [
35].
d. Shinoda test
To a solution of ethanol extract of
V. blumeoides, a few drops of concentrated hydrochloric acid and magnesium sheets. The presence of flavonoids was revealed by the appearance of a purple colour [
35].
e. Dragendorff test
In this experiment, 10 mg of the ethanol extract of
V. blumeoides were added to 1% ethanolic solution of HCl, followed by the addition of the Dragendorff reagent. The generation of an orange precipitate revealed the presence of alkaloids [
37].
f. Tannins test
Briefly, 15 mg of
V. blumeoides extract was dissolved in 1 mL of ethanol, followed by an addition of a ferric chloride solution. The appearance of a dark brownish-green or blue-black colour revealed the presence of tannins in the extract [
37].
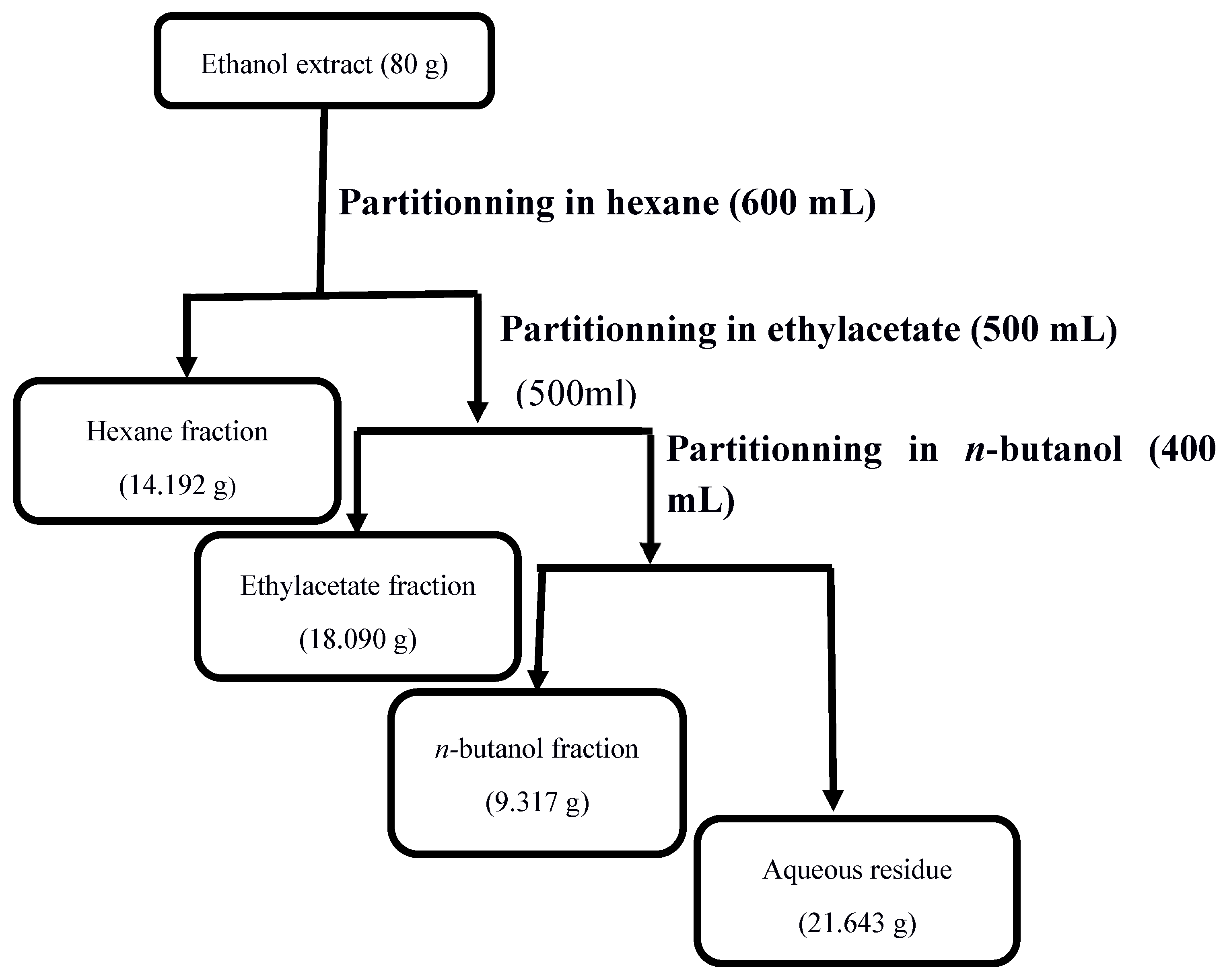
2.2.3. Fractionation of the Ethanol Extract
Eighty grams (80 g) of the ethanol extract of
Vernonia blumeoides were dissolved in 600 mL of distilled water and introduced into a 1000 mL separating funnel. Next, partitioning of constituents was performed using solvents of increasing polarity, such as hexane, ethyl acetate and n-butanol. After addition of hexane to the
V. blumeoides solution, the mixture was shaken vigorously and left to stand alone, so as to separate the organic phase from the aqueous phase. Then, the organic phase was dried using a rotary evaporator to remove the solvent in order to obtain the hexane extract. The same process was repeated for ethylacetate and n-butanol [
38]. In fact, the aqueous phase was successively resuspended in ethylacetate, then in n-butanol to afford ethylacetate and n-butanol fractions, respectively (
Figure 2).
2.2.4. Assay for the Inhibition of Plasmodium falciparum
a. Preparation of the intermediate plates
The extract and fractions from V. blumeoides were prepared by dissolving 100 mg of each sample in 1 mL of dimethyl sulfoxide (DMSO ; 100%), to reach a final concentration of 100 mg/mL. Afterwards, 2 μL of each extract’s solution were introduced into 96 well round bottom microplates (Sigma Aldrich). Next, 198 μL of incomplete RPMI 1640 medium were added into the first wells to obtain a concentration of 1 mg/mL, whereas only 160 μL of incomplete RPMI 1640 were added to the remaining wells. Then, a series of dilutions of geometric sequence 5 were performed to obtain concentrations ranging from 1 mg/mL to 0.0016 mg/mL and from 1% to 0.0016% for extracts and DMSO, respectively. Artemisinin (Sigma-Aldrich), was used as a positive control and was diluted from a stock solution (10 mM) to obtain concentrations ranging from 10 μM to 0.016 μM. The as-prepared intermediate plates were stored at -80°C for further use.
b. Maintenance of Plasmodium falciparum through in vitro culture
Chloroquine-sensitive (3D7) and multidrug-resistant (Dd2) strains of Plasmodium falciparum were cultured with human erythrocytes with reference to a previously described procedure [
39]. Cryotubes containing
P. falciparum strains were collected from the -80°C freezer and thawed to 37°C for 2 minutes. Then, the content of each cryotube was transferred into a 50 mL Falcon conical tube, followed by a drop-wise addition of 100 μL of 12% NaCl solution. After 5 minutes had elapsed, an additional 5 ml of NaCl solution (1.6%) was gradually added to the mixture. The as-prepared solution was centrifuged at 2500 rpm for 5 minutes, then the pellet was removed and washed with complete RPMI 1640 medium. Afterwards, the remaining pellet was cultured and maintained in suspension at 4% haematocrit in fresh human erythrocytes from an O+ blood group donor and complete RPMI 1640 medium.
P. falciparum cultures were grown at 37°C in a humidified atmosphere containing 5% CO
2. The culture medium was renewed daily with a fresh complete medium to ensure optimal and continuous growth of the parasites. Blood smears were prepared on cleaned slides, stained with 10% Giemsa and then observed under a light microscope (Huma Scope Classic) at 100X objective with immersion oil to examine the
P. falciparum-infected red blood cells. Forty-eight (48) hours prior to the antiplasmodial test, parasite cultures containing mainly the ring stage were synchronized.
c. Culture synchronisation
To test the antiplasmodial activity of
V. blumeoides extracts over a complete parasite cycle [
40], parasites were synchronized at ring stage by culture treatment with 5% (w/v) sorbitol to eliminate trophozoites and schizonts (advanced stages). Then, parasitic cultures containing at least 80% of the ring stage were centrifuged at 2500 rpm for 5 min, then the supernatant was removed and replaced with 5 ml of 5% (w/v) sorbitol solution [
41]. Next, the mixture was homogenized and incubated at 37°C for 10 minutes, then centrifuged again at 2500 rpm for 5 minutes, followed by three washes with incomplete RPMI 1640 medium to remove as much sorbitol as possible. After synchronisation, the parasites were re-suspended in complete RPMI 1640 medium with 4% haematocrit. After 48 hours of incubation,
V. blumeoides extracts were tested for antiplasmodial activity [
42].
d. Inhibitory effects of Vernonia blumeoides extracts vis-à-vis Plasmodium falciparum
The antiplasmodial activity of
V. blumeoides extracts was carried out in 96-well flat-bottom microplates (Sigma Aldrich) using a SYBR green I-based fluorescence assay [
43]. In brief, sorbitol-synchronized ring stage parasites (hematocrit: 4%, parasitemia: 2%, 90 µL) under normal culture conditions were incubated with
V. blumeoides extracts and artemisinin, the reference drug (10 µL), followed by an incubation at 37℃ for 72 hours. After incubation, 100 µL of SYBR Green I buffer [6 µL of 10,000 × SYBR Green I (Invitrogen) + 600 µL of red blood cells lysis buffer {Tris (25 mM; pH 7.5)} + 360 µL of EDTA (7.5 mM) + 19.2 µL of parasites lysis solution {saponin (0.012%, wt/vol)} and 28.8 µL of Triton X-100 (0.08%; vol/vol)}] was added to each well, mixed twice gently with a multichannel pipette and incubated in the dark at 37℃ for 1 hour [
44]. Fluorescence was measured using a microtiter plate reader (TECAN-Infinite M200, Tecan Austria GmbH, Grödig Flachgau, Austria) at excitation and emission wavelengths of 485 and 538 nm, respectively. The fluorescence counts were plotted against the logarithm of
V. blumeoides extracts’ concentration and the half-maximal inhibitory concentration (IC
50) was determined by analysis of dose-response curves using GraphPad Prism 5.0. Experiments were done in duplicate.
2.2.5. Antioxidant Tests
a. DPPH radical scavenging assay
The free radical scavenging assay of the most promising extracts was tested using the 1,1-diphenyl-2-picryl hydrazyl (DPPH) method [
45]. Briefly, 25 μL of each extract at different concentrations (2000 to 31.25 µg/mL) were added to 75 μL of 0.02% DPPH solution to reach final concentrations ranging from 500 to 3.90625 μg/mL. The reaction mixtures were stored in the dark at room temperature for 30 minutes. Next, the absorbance was measured against the blank (solution of DPPH in methanol only) using microplate reader TECAN Infinite M200 at 517 nm. The positive control ascorbic acid was also treated with DPPH (0.02%) solution to achieve final concentrations ranging from 25 to 0.195 μg/mL. The inhibition percentages, which were calculated from the measured optical densities, were used to express the median scavenging concentrations (SC
50s) using GraphPad Prism 8.0.1. software.
b. ABTS radical scavenging assay
The ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay is a colorimetric assay in which the ABTS radical suffers a color decrease in the presence of antioxidants.
For the ABTS assay, the radical scavenging activity of extracts was evaluated using the discoloration method [
46]. Briefly, 25 μL of extract at different concentrations (from 2000 to 31.25 µg/mL) were added to 75 μL of the ABTS
+ solution (0.175 mM) and incubated for 30 minutes in the dark at room temperature. After incubation, the absorbance of the as-prepared solutions was measured at 734 nm using the microtiter plate reader TECAN Infinite M200. ABTS reagent and ascorbic acid, which were prepared at final concentrations ranging from 25 to 0.390 μg/mL, were used as negative and positive controls, respectively. The tests were carried out in triplicate in 96 well microplates. The reduction of ABTS by
V. blumeoides extracts leads to a discoloration of the blue ABTS solution measured after 30 min of incubation at 734 nm.
c. Ferric reducing antioxidant power assay (FRAP)
The ferric iron reducing activity of
V. blumeoides extracts was evaluated using the method described by Moffatt et al. [
47]. Briefly, 25 μL of each extract at different concentrations (from 2000 to 31.25 µg/mL) were mixed to 25 μL of a solution of Fe
3+ (1.2 mg/mL). The plates were pre-incubated for 15 minutes at room temperature, followed by an addition of 50 μL of orthophenanthroline (0.2%). Then, the as-prepared solution was incubated for an additional 20 minutes at the same temperature. Afterwards, the absorbance of the solution was measured at 505 nm against the blank (25 μL of methanol + 25 μL Fe
3+ + 50 μL of ortho-phenanthroline). The positive control ascorbic acid was prepared in methanol to yield final concentrations ranging from 25 to 0.390 μg/mL.
2.2.6. Cytotoxicity assay
In this study, the cytotoxicity of extracts and fractions was evaluated against Raw 264.7 cells using a colorimetric method as described by Bowling et al. [
48].
a. In vitro culture of Raw cells
Raw cells were maintained in complete DMEM (Dulbecco’s Modified Eagle medium) (containing 1% antibiotic and 10% FBS) and stored in a 25 cm2 culture flask (T-Flask) under standard conditions at 37 °C and 5% CO2. The culture medium was renewed every 72 hours. At cell confluence (70-90%), cells were detached by treatment with 1 mL of 0.05 % trypsin-EDTA for 5 min. Afterwards, 9 mL of complete DMEM was added for trypsin inactivation, followed by a centrifugation of detached cells at 1800 rpm for 5 min. The resulting pellet was suspended in 1 mL of medium, and the cell viability was assessed using Trypan blue to standardize the cell load on the Neubauer hemocytometer.
b. Determination of median cytotoxic concentrations (CC50s)
The cytotoxicity of
V. blumeoides extracts was determined as previously described by Bowling et al. [
48]. Briefly, 100 μL of cells’ suspension containing 1×10
4 cells/well were added to each well of the plates and incubated at 37°C with 5% CO
2 for 18 hours to confirm adhesion of cells. Afterwards, the culture medium was renewed with 90 μL of fresh medium, then 10 μL of each extract of
V. blumeoides at different concentrations (final concentrations: 500, 100, 20, 4 and 0.8 μg/mL) were added to all wells except for those that served as the positive (10 μM DMSO) and negative (pure culture medium) controls. Next, the plates were incubated at 37 °C and 5% CO
2 for 48 hours, followed by an addition of 10 μL of a freshly prepared resazurin solution (0.15 mg/mL in PBS) and an additional incubation for 4 hours at 37 °C in a 5% CO
2. Subsequently, the fluorescence of the as-prepared solution was measured using a microtiter plate reader (TECAN-Infinite M200, Tecan Austria GmbH, Grödig Flachgau, Austria) at excitation and emission wavelengths of 530 and 590 nm, respectively. The test was carried out in duplicate in sterile 96-well microplates (CoStar, Washington, DC, United States of America).
From the values of optical densities, the percentage of cell viability was calculated with Microsoft excel software using the following equation:
where, At = Absorbance of test samples, Ac= Absorbance of negative control (cells without test samples).
To determine the median cytotoxic concentration (CC50), a dose-response curve (cell viability vs. concentration of samples) was plotted using Graph Pad Prism 8.0.1 software.
2.3. Statistical Analyses
Microsoft® Excel 2020 software was used to calculate the percentages of inhibition of Plasmodium falciparum (% growth inhibition). Then, the IC50 values were determined by non-linear regression analysis using Graph Pad Prism 8 software. Results were expressed as mean ± standard deviation (mean ± SD). Means were analysed by one-way analysis of variance (ANOVA), followed by comparisons of means using the Dunnett test. To minimise confounding factors, the test was performed in duplicate and inference was made by comparing the values of test samples with the positive control values. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Yield of Extraction
The yield of extraction for the ethanol extract of Vernonia blumeoides stems and leaves was calculated as 4.39%. The partitioning of V. blumeoides ethanol extract afforded hexane, ethyl acetate, and n-butanol fractions, as well as the aqueous residue, with extraction yields of 14.19, 18.09, 9.32 and 21.64%, respectively.
3.2. Half-Maximal Inhibitory Concentrations
Table 1 summarizes the half-maximal inhibitory concentrations of the ethanol extract and fractions obtained from
V. blumeoides. In fact, extract and fractions from
V. blumeoides inhibited the growth of
P. falciparum Dd2 and 3D7 with IC
50 values ranging from 5.32 to 21.48 µg/mL and from 4.50 to 180.2 µg/mL, respectively. Meanwhile, artemisinin, the standard antiplasmodial drug, displayed IC
50 value of 0.02 µg/mL. The resistance index, which is the ratio of IC
50 values obtained upon test on
P. falciparum resistant strains to the IC
50 values from the parent strains. As a result, the higher the RI, the higher the level of resistance.
V. blumeoides extracts revealed levels of resistance ranging from 1.03 to 1.13. The highest parasite resistance was observed with the hexane extract of
V. blumeoides, followed by ethanol, ethyl acetate and n-butanol extracts. However, artemisinin revealed more chances to parasite resistance as evidenced by the value of resistance index (1.45) (
Table 1).
3.3. Antioxidant Activity
Crude extracts from V. blumeoides were evaluated for antioxidant activity using DPPH, ABTS, and FRAP assays. Gallic acid was used as a reference antioxidant compound.
3.3.1. DPPH Assay
The
in vitro incubation of
V. blumeoides extracts with 1,1-diphenyl-2-picryl hydrazyl (DPPH) solution demonstrated the scavenging potential of
V. blumeoides with median scavenging concentrations (SC
50) ranging from 31.17 to 372.65 µg/mL (
Table 2). Notably, ethylacetate extract was the most active antioxidant extract, whereas the least active was found to be the water extract. Gallic acid, which was used as the positive control, showed SC
50 value of 4.6 µg/mL.
3.3.2. ABTS Test
Table 3 summarizes the concentrations of
V. blumeoides extracts required to scavenge 50% of the ABTS free radicals (SC
50). The
in vitro treatment of ABTS solution with
V. blumeoides extracts showed range of median scavenging concentrations ranging from 31.17 µg/mL to 372.65 µg/mL, vs gallic acid (SC
50 : 4.07 µg/mL). Noteworthy, the ethylacetate extract displayed the lowest scavenging concentration@31.17 µg/mL, thus revealing the highest scavenging potential among the
V. blumeoides extracts tested.
3.3.3. FRAP Assay
Table 4 summarizes the concentrations of
V. blumeoides extracts that reduced 50% (RC
50) of Fe
3+ en Fe
2+ upon FRAP assay. As much as 162.55 µg/mL of ethanol extract of
V. blumeoides were required to reduce 50% of Fe
3+ en Fe
2+, whereas the incubation of the ethylacetate fraction with Fe
3+ solution and 0.2% orthophenanthroline afforded median reducing concentration (RC
50) of 33.95 µg/mL, thus revealing the most active extract upon FRAP test. Meanwhile, gallic acid, which was used as a positive control yielded RC
50 of 2.83 µg/mL (
Table 4).
Overall, ethylacetate extract from V. blumeoides was found to be the most active extract upon DPPH and FRAP tests, followed by the n-butanol extract.
3.4. Median Cytotoxic Concentrations
Extract and fractions from
V. blumeoides were evaluated for cytotoxicity against the macrophage Raw 264.7 cells. Except ethylacetate and n-butanol fractions that showed mild cytotoxicity, none of the extracts were found to be cytotoxic to the macrophage cells as evidenced by the values of median cytotoxic concentrations that ranged from 12.03 to 80.41 µg/mL. The selectivity indices of ethanol and hexane extracts were found to be 5.90 and 9.60, as well as 5.31 and 8.48 for
PfDd2 and
Pf3D7, respectively (
Table 5).
3.5. Phytochemical Screening
Phytochemical analysis confirmed the presence of alkaloids, tannins, steroids, flavonoids, phenolic compounds, and sugars, but did not detect the presence of terpenoids. This information offers important prospects for analysing the chemical composition of classes of secondary metabolites of
V. blumeoides extracts (
Table 6).
4. Discussion
Malaria drug resistance has become a serious concern worldwide, thus rendering the so-called antimalarial treatments obsolete in many endemic regions and setting back the progress toward malaria eradication. The search for effective antimalarial therapies might overcome this issue. As recommended by WHO, the use of medicinal plant preparations is encouraged in its Traditional Medicine Strategy, especially since chemotherapy has failed due to drug resistance [
4]. One such plant includes
V. blumeoides, which has long been used by traditional healers to treat fever and malaria conditions, without scientific validation.
This study aims to evaluate the antiplasmodial activity of extracts prepared from leaves and stems of
Vernonia blumeoides. To this end, the
in vitro incubation of the ethanol extract of
V. blumeoides with sensitive and resistant strains of
P. falciparum in culture led to parasite inhibition as evidenced by the experimental IC
50 values (15.15 and 13.63 µg/mL for PfDd2 and Pf3D7, respectively). The phytochemical screening of the ethanol extract revealed the presence of alkaloids, tannins, steroids, flavonoids and phenolic compounds, among others, which chemotypes might be responsible for the observed antiplasmodial activity. In fact, growing evidence has shown the potential of alkaloids in inhibiting the growth of
P. falciparum [
49,
50]. A number of review articles have been published on the antiplasmodial activity of flavonoids [
51,
52]. Noteworthy, reports point out the antiplasmodial potential of tannins [
53], steroids [
54], and phenolic compounds [
55,
56].
Further partitioning of the ethanol extract yielded four subfractions
viz. hexane, ethylacetate, and water fractions, which exhibited antiplasmodial activity against the two
Plasmodium falciparum strains Dd2 and 3D7. Noteworthy, hexane and ethylacetate fractions (IC
50<5.32 µg/mL) were more active than the ethanol extract (13.63<IC
50<15.15 µg/mL) from which they were obtained after partitioning. This suggests that non polar and medium polar compounds extracted respectively by hexane and ethylacetate were intricately involved in antiplasmodial action of
V. blumeoides. In 2015, Aliyu et al. [
23] isolated and characterized four sesquiterpene lactones (blumeoidolide-A, blumeoidolide-B, blumeoidolide-C and blumeoidolide-D) from the aerial parts of
V. blumeoides [
23], in addition to the flavonoids chrysin and apigenin, a steroid (stigmasterol) and a pentacyclic triterpenoid (lupeol) [
23]. Other
Vernonia species were also reported to contain a number of sesquiterpene lactones [
57,
58,
59]. In the lactone functionality, the peroxy bond is key to the antimalarial action of a number of sesquiterpene lactones, including artemisinin and its derivatives. In addition, heme and iron produced by hemozoin can activate lactone-based chemotypes to produce free radicals. The activated chemotypes disrupt the physiological functions of
Plasmodium by targeting proteins, lipids and nucleic acids to cause the parasites’ death [
60]. Accumulated evidence has shown the antiplasmodial effects of cyclic esters (lactones) through inhibition of β-hematin (a synthetic counterpart of hemozoin) formation [
61,
62,
63,
64]. As
V. blumeoides is also rich in flavonoids, it is not irrational to hypothesize that these compounds might have been implicated in the antiplasmodial activity of
V. blumeoides extracts. Though the molecular mechanism of antiplasmodial action of flavonoids is not fully elucidated, it is believed that flavonoids act by inhibiting the fatty acid biosynthesis in the parasite biochemistry. They also act probably by inhibiting the influx of L-glutamine and myoinositol into infected erythrocytes during the intraerythrocytic phase of the
Plasmodium life cycle [
51,
65,
66,
67]. The phytochemical analysis of
V. blumeoides extracts revealed the presence of terpenoids and steroids. Growing evidence has shown that terpenoids and steroids exhibit antimalarial activity perhaps by inhibiting the enzymes, which are linked to the metabolic pathways (e.g. the isoprenoid biosynthesis pathway) of the apicoplast of
P. falciparum [
68,
69,
70]. Thus, it is not unreasonable to speculate that
V. blumeoides extracts might have exerted antimalarial activity by at least one of these mechanisms of action.
Infection by
P. falciparum triggers oxidative stress that induce a redox imbalance owing to the overproduction of reactive oxygen species to the detriment of the host’s endogenous antioxidant defenses [
14]. The production of free radicals is consistent with the degradation of hemoglobin by the parasite that yields a number of aminoacids for its nutrition [
71]. Modern research has shown the potential benefits of antioxidant therapy in malaria conditions, especially in cerebral malaria [
14,
72,
73]. As a matter of fact, experiments performed in small animals have shown that antioxidants prevent the development of cerebral complications [
72]. Antioxidant chemotypes also reinforce the body's defenses against oxidative stress. By promoting an effective immune response against pathogens, antioxidants can therefore overcome cellular damage [
13,
14]. Thus, the identification of dual chemotypes that inhibit the pathogen and nullifies the oxidative damage produced during infection is of outstanding importance.
V. blumeoides extracts exhibited antioxidant activity upon DPPH and FRAP assays. This antioxidant indication might contribute to the plant’s potential in inhibiting the growth of
P. falciparum, especially under
in vivo conditions. Plant species of the genus
Vernonia are well known to exhibit antioxidant properties [
74,
75]. Moreover,
V. blumeoides is rich in compounds, such as flavonoids, alkaloids, terpenoids, etc. that are well known for their antioxidant properties. The antioxidant action mechanisms of these compounds mostly include direct reactive oxygen species (ROS) scavenging, inhibition of ROS production via the chelation of trace elements or inhibition of free radical-generating enzymes (i.e., microsomal monooxygenase, glutathione S-transferase, etc.) [
76,
77,
78].
Overall, this novel contribution reveals the antiplasmodial activity of V. blumeoides extracts, which is supported by their phytochemical composition (flavonoids, alkaloids, terpenoids and steroids, etc.), and their antioxidant potential. These results substantiate the ethnopharmacological use of V. blumeoides in the treatment of certain parasitic infections, including malaria.
5. Conclusions
Vernonia blumeoides aerial parts, leaves and stems are used traditionally to overcome parasitic diseases, such as malaria. Thus, the scientific validation of V. blumeoides in the treatment of malaria conditions is of outstanding importance. In this study, the antiplasmodial activity of V. blumeoides extracts was evaluated against chloroquine sensitive and resistant strains of P. falciparum using phenotypic screenings. As a result, the ethanol extract inhibited the growth of P. falciparum as evidenced by the low IC50 value obtained. The phytochemical screening of the ethanol extract revealed the presence of flavonoids, phenolic compounds, alkaloids, terpenoids and steroids, etc. Further partitioning of the ethanol extract afforded hexane, ethylacetate, n-butanol and water residue, which were also screened for antiplasmodial activity. Hexane and ethylacetate fractions were found to be the most active antiplasmodial fractions. Furthermore, V. blumeoides extracts demonstrated antioxidant activity following DPPH and FRAP assays, thus revealing that these extracts might aid in mitigating the oxidative stress caused by malaria pathogenesis under in vivo conditions. Cytotoxicity experiments of V. blumeoides extracts vis-à-vis murine macrophage Raw cells revealed non toxicity. Vernonia blumeoides exhibited significant antiplasmodial and antioxidant properties, thus validating its traditional use for the treatment of malaria conditions. However, in-depth antiplasmodial mechanisms of action, pharmacokinetics, and in vivo experiments are required to support the safe use of Vernonia blumeoides in ethnomedicine.
Author Contributions
Conceptualization, V.N., B.P.K., R.B.T., L.A.T. and F.F.B; methodology, F.L.M.B., E.A.M.K., R.K., D.D., A.C.T. and M.J.T.N.; software, P.V.T.F., A.C.T., J.M., P.L.K., and B.P.K.; validation, V.N., P.K.L., B.P.K., R.B.T., L.A.T., and F.F.B.; formal analysis, F.L.M.B., E.A.M.K., R.K., P.K.L., A.C.T., P.V.T.F., M.J.T.N., J.M., and B.P.K. ; investigation, F.L.M.B., E.A.M.K., R.K., D.D., J.M., and M.J.T.N.; resources, V.N.; R.B.T., L.A.T. and F.F.B.; data curation, F.L.M.B., P.V.T.F., A.C.T., J.M., E.A.M.K., R.K., D.D., M.J.T.N., and B.P.K. ; writing—original draft preparation, F.L.M.B., E.A.M.K., R.K., A.C.T., D.D., P.V.T.F., J.M., M.J.T.N., and V.N.; writing—review and editing, B.P.K. P.L.K., R.B.T., L.A.T., and V.N.; visualization, P.K.L., B.P.K. and V.N.; supervision, V.N., R.B.T., and F.F.B; project administration, V.N., R.B.T., L.A.T.; B.P.K. and F.F.B; funding acquisition, V.N., R.B.T, and F.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was externally funded by the Yaounde-Bielefeld Bilateral Graduate School for Natural Products with Anti-parasite and Antibacterial Activity (YaBiNaPA) (grant number 57316173) and the Seeding Labs’ Instrumental Access.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding (s) author (s) upon reasonable request.
Acknowledgments
The authors would like to thank to the Biodefense and Emerging Infections Research Resources Repository (BEI-Resource) for providing the chloroquine-sensitive (3D7) and multidrug-resistant (Dd2) strains of P. falciparum and the Noguchi Memorial Institute for Medical Research, University of Ghana for providing the murine macrophage Raw cells 264.7. This work received support from the Yaoundé-Bielefeld Bilateral Graduate School for Natural Products with Anti-parasite and Antibacterial Activity (YaBiNaPA). The authors are also very grateful to the Alexander von Humboldt Foundation, Bonn, Germany (Research Hub 3.4-CMR-Hub) for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koram, K.A.; Molyneux, M. When is ‘malaria’ malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg 2007, 77 (Suppl 6), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Gupta, E.; Singh, P.; Soni, S.; Noor, U. Insight on Vernonia plant for its pharmacological properties: A review. Recent Adv Food Nutr Agric 2023, 14(2), 84–93. [Google Scholar] [CrossRef]
- Foster, D.; Cox-Singh, J.; Mohamad, D.S.; Krishna, S.; Chin, P.P.; Singh, B. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar J 2014, 13, 60. [Google Scholar] [CrossRef]
- The World Health Organization (WHO), 2024a. Monitoring malaria drug efficacy and resistance. https://www.who.int/activities/monitoring-malaria-drug-efficacy-and-resistance, Accessed on 09th September 2024.
- The World Health Organization (WHO), 2021. World Malaria Report 2021: Regional Data and Trends. https://www.who.int/publications-detail-redirect/9789240040496 (2021, accessed 12th May 2024).
- Briquet, S.; Marinach, C.; Silvie, O.; Vaquero, C. Preparing for transmission: Gene regulation in Plasmodium sporozoites. Front Cell Infect Microbiol 2021, 10, 618430. [Google Scholar] [CrossRef]
- Ouologuem, D.T.; Dara, A.; Kone, A.; Ouattara, A.; Djimde, A.A. Plasmodium falciparum development from gametocyte to oocyst: Insight from functional studies. Microorganisms 2023, 11(8), 1966. [Google Scholar] [CrossRef]
- Na-Bangchang, K.; Karbwang, J. Pharmacology of antimalarial drugs, current anti-malarials. In: Kremsner, P., Krishna, S. (eds) Encyclopedia of Malaria. Springer, New York, NY, 2019. [CrossRef]
- The World Health Organization (WHO), 2024b. WHO traditional medicine strategy: 2014-2023. https://www.who.int/publications/i/item/9789241506096, Accessed on 01st October 2024.
-
Ouji, M.; Augereau, J.M.; Paloque, L.; Benoit-Vical; F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite 2018, 25, 24. [CrossRef]
- Ward, K.E.; Fidock, D.A.; Bridgford, J.L. Plasmodium falciparum resistance to artemisinin-based combination therapies. Curr Opin Microbiol 2022, 69, 102193. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol 2015, 4, 180–3. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol 2002, 82(1), 47–95. [Google Scholar] [CrossRef]
- Gomes, A.R.Q.; Cunha, N.; Varela, E.L.P.; Brígido, H.P.C.; Vale, V.V.; Dolabela, M.F.; De Carvalho, E.P.; Percário, S. Oxidative stress in malaria: Potential benefits of antioxidant therapy. Int J Mol Sci 2022, 23(11), 5949. [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; Rollinger, J.M.; Schuster, D.; Breuss, J.M.; Bochkov, V.; Mihovilovic, M.D.; Kopp, B.; Bauer, R.; Dirsch, V.M.; Stuppner, H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv 2015 33(8), 1582-1614.
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic Agents. Molecules 2022, 27(2), 349. [CrossRef]
- Mohammadi, S.; Jafari, B.; Asgharian, P.; Martorell, M.; Sharifi-Rad, J. Medicinal plants used in the treatment of malaria: A key emphasis to Artemisia, Cinchona, Cryptolepis, and Tabebuia genera. Phytother Res 2020, 34(7), 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Wallaart, T.E.; Pras, N.; Quax, W.J. Isolation and identification of dihydroartemisinic acid hydroperoxide from Artemisia annua : A novel biosynthetic precursor of artemisinin. J Nat Prod 1999, 62, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, I.P.; Aguiar, A.C.; Adebayo, J.O.; Krettli, A.U. Studies on activities and chemical characterization of medicinal plants in search for new antimalarials: A ten year review on ethnopharmacology. Front Pharmacol 2021, 12, 734263. [Google Scholar] [CrossRef] [PubMed]
- Bekono, B.D.; Ntie-Kang, F.; Onguéné, P.A.; Lifongo, L.L.; Sippl, W.; Fester, K.; Owono, L.C.O. The potential of anti-malarial compounds derived from African medicinal plants: a review of pharmacological evaluations from 2013 to 2019. Malar J 2020, 19(1), 183. [Google Scholar] [CrossRef]
- Tajbakhsh, E.; Kwenti, T.E.; Kheyri, P.; Nezaratizade, S.; Lindsay, D.S.; Khamesipour, F. Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: a systematic review of literature. Malar J 2021, 20(1), 349. [Google Scholar] [CrossRef]
- Angupale, J.R.; Tusiimire, J.; Ngwuluka, N?C. A review of efficacy and safety of Ugandan anti-malarial plants with application of RITAM score. Malar J 2023, 22(1), 97. [CrossRef]
- Aliyu, A.B.; Moodley, B.; Chenia, H.; Koorbanally, N.A. Sesquiterpene lactones from the aerial parts of Vernonia blumeoides growing in Nigeria. Phytochemistry 2015, 111, 163–168. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Aliyu, A.B.; Abdullahi, H.; Solomon, T.; Toko, E.; Garba, A.; Bashir, M.; Habila, N. Lactone-rich fraction from Vernonia blumeoides: antitrypanosomal activity and alleviation of the parasite-induced anemia and organ damage. J Nat Med 2013, 67, 750–757. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; de Souza, E.B.; Fontenelle, R.O. dos S. A review on antimicrobial potential of species of the genus Vernonia (Asteraceae). J Med Plants Res 2015, 9(31), 838–850. [Google Scholar]
- Kumari, K.G.N.; Masilamani, S.; Ganesh, M.R.; Aravind, S.; Sridhar, S.R. Zaluzanin D: a fungistatic sesquiterpene from Vernonia arborea. Fitoterapia 2003, 74(5), 479–482. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Harahap, U.; Sitorus, P.; Satria, D. The anticancer activities of Vernonia amygdalina Delile. leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon 2020, 6(7), e04449. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, Z.-H.; Liu, J.-K. A New highly oxygenated flavone from Vernonia saligna. Z. Naturforsch 2003, 58 c, 347Ð350.
- Erasto, P.; Grierson, D.S.; Afolayan, A.J. Antioxidant constituents in Vernonia amygdalina leaves. Pharmaceutical Biology 2007, 45, 195–199. [Google Scholar] [CrossRef]
- Collins Njonte Wouamba, S.; Mouthé Happi, G.; Nguiam Pouofo, M.; Tchamgoue, J.; Jouda, J.B.; Longo, F.; Ndjakou Lenta, B.; Sewald, N.; Fogue Kouam, S. Antibacterial flavonoids and other compounds from the aerial parts of Vernonia guineensis Benth. (Asteraceae). Chem Biodivers 2020, 17(9), e2000296. [CrossRef]
- Liu, X.; Zhou, M.; He, S.; Xu, Q.; Du, C.; Zhu, H.; Lin, T.; Wang, G.; Tian, W.; Chen, H. Polyhydric stigmastane-type steroids derivative from Vernonia amygdalina and their anti-neuroinflammatory activity. Pharmaceuticals (Basel) 2022, 15(9), 1160. [Google Scholar] [CrossRef] [PubMed]
- Tseme Wandji, N.; Bitchagno, G.T.M.; Mawabo Kamga, I.; Tchamgoue, J.; Nkenfou, C.N.; Lenta, B.N.; Sewald, N.; Kouam; S.F. Polyoxygenated stigmastane-type steroids from Vernonia kotschyana Sch. Bip. ex Walp. and their chemophenetic significance. Molecules, 2023; 28(13), 5278. [CrossRef]
- Nergard, C.S.; Diallo, D.; Michaelsen, T.E.; Malterud, K.E.; Kiyohara, H.; Matsumoto, T.; Yamada, H.; Paulsen, B.S. Isolation, partial characterisation and immunomodulating activities of polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp. J Ethnopharmacol. 2004, 91(1), 141–152. [Google Scholar] [CrossRef] [PubMed]
- Inngjerdingen, K.T.; Meskini, S.; Austarheim, I.; Ballo, N.; Inngjerdingen, M.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Chemical and biological characterization of polysaccharides from wild and cultivated roots of Vernonia kotschyana. J Ethnopharmacol 2012, 139(2), 350–358. [Google Scholar] [CrossRef]
- Evans, W.C.; Evans, D. General methods associated with the phytochemical investigation of herbal products. In: Trease and Evans’ Pharmacognosy. 1989, Elsevier, pp. 135–147.
- Odebiyi, O.O.; Sofowora, E.A. Antimicrobial alkaloids from a Nigerian chewing stick (Fagara zanthoxyloides). Planta Med 1979, 36, 204–207. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical methods. Dordrecht: Springer Netherlands. Epub ahead of print 1984. [CrossRef]
- Boniface, P.K.; Verma, S.; Shukla, A.; Cheema, H.S.; Srivastava, S.K.; Khan, F.; Darokar, M.P.; Pal, A. Bioactivity-guided isolation of antiplasmodial constituents from Conyza sumatrensis (Retz. ) E.H. Walker. Parasitol Int 2015, 64(1), 118–123. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Continuous culture of Plasmodium falciparum: its impact on malaria research. Int J Parasitol 1997, 27, 989–1006. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P.; Falciparum of Plasmodium Synch stages in culture, Society 2012, 65 (2012) 418–420.
- Yuan, L.; Hao, M.; Wu, L.; Zhao, Z.; Rosenthal, B.M.; Li, X.; He, Y.; Sun, L.; Feng, G.; Xiang, Z.; Cui, L.; Yang, Z. Refrigeration provides a simple means to synchronize in vitro cultures of Plasmodium falciparum. Exp Parasitol 2014, 140, 18–23. [Google Scholar] [CrossRef]
- Maier, A.G.; Rug, M. In vitro culturing Plasmodium falciparum erythrocytic stages. Methods Mol Biol 2013, 923, 3–15. [Google Scholar] [PubMed]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Bagavan, A.; Rahuman, A.A.; Zahir, A.A.; Kamaraj, C.; Elango, G.; ayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S.; Rajakumar, G.; Tiwari, S.K.; Sahal, D. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Easter Ghats. Malar J 2015, 7, 14–65. [Google Scholar] [CrossRef]
- Brice Rostan, P.; Boniface, P.K.; Eutrophe Le Doux, K.; Vincent, N.; Yanick Kevin, M.D.; Paul, K.L.; Fabrice, F.B. , 2024. Extracts from Cardiospermum grandiflorum and Blighia welwitschii (Sapindaceae) reveal antibacterial activity against Shigella species. S Afr J Bot 2024, 164, 419–428. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999, 26(9-10), 1231–1237. [Google Scholar] [CrossRef]
- Moffatt, M.E.K.; Longstaffe, S.; Besant, J.; Dureski, C. Prevention of iron deficiency and psychomotor decline in high-risk infants through use of iron-fortified infant formula : A randomized clinical trial. J Pediatr 1994, 125(4), 527–534. [Google Scholar] [CrossRef]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int J Parasitol Drugs Drug Resist 2012, 2, 262–270. [Google Scholar] [CrossRef]
- Uzor, P.F. Alkaloids from plants with antimalarial activity: A review of recent studies. Evid Based Complement Alternat Med 2020, 2020, 8749083. [Google Scholar] [CrossRef]
- García Díaz, J.; Tuenter, E.; Escalona Arranz, J.C.; Llauradó Maury, G.; Cos, P.; Pieters, L. Antiplasmodial activity of alkaloids from Croton linearis leaves. Exp Parasitol. 2022, 236-237, 108254. [CrossRef]
- Rudrapal, M.; Chetia, D. Plant flavonoids as potential source of future antimalarial leads. Sys Rev Pharm 2017, 8, 1–1. [Google Scholar] [CrossRef]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and dengue. Phytother Res 2019, 33(10), 2473–2517. [Google Scholar] [CrossRef]
- Dell'agli, M.; Galli, G.V.; Bulgari, M.; Basilico, N.; Romeo, S.; Bhattacharya, D.; Taramelli, D.; Bosisio, E. Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar J 2010, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Safar, H.F.; Ali, A.H.; Zakaria, N.H.; Kamal, N.; Hassan, N.I.; Agustar, H.K.; Talip, N.; Latip, J. Steroids from Diplazium esculentum: Antiplasmodial activity and molecular docking studies to investigate their binding modes. Trop Biomed 2022, 39(4), 552–558. [Google Scholar] [PubMed]
- Azebaze, A.G.; Teinkela, J.E.; Nguemfo, E.L.; Valentin, A.; Dongmo, A.B.; Vardamides, J.C. Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia. Afr Health Sci 2015, 15(3), 835–840. [Google Scholar] [CrossRef]
- Yun, Y.F.; Hermanto, F.; Aisyah, L.S.; Saputra, T.R.; Hakim, A.R.; Ningsih, A.K.; Herlina, T.; Julaeha, E.; Zainuddin, A.; Supratman, U. The phenolic compound from Kalanchoe blossfeldiana (Crassulaceae) leaf and its antiplasmodial activity against Plasmodium falciparum 3D7. Indones J Chem 2016, 16(2), 156–161. [Google Scholar] [CrossRef]
- Chea A, Hout S, Long C, Marcourt L, Faure R, Azas N, Elias R. Antimalarial activity of sesquiterpene lactones from Vernonia cinerea. Chem Pharm Bull 2006, 54, 1437–1439.
- Chukwujekwu, J.C.; Lategan, C.A.; Smith, P.J.; Van Heerden, F.R.; Van Staden, J. Antiplasmodial and cytotoxic activity of isolated sesquiterpene lactones from the acetone leaf extract of Vernonia colorata. S Afr J Bot 2009, 75, 176–179. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, T.; Yu, S.; Liu, Z.; Wang, J.; Wang, Y. Antimalarial mechanisms and resistance status of artemisinin and its derivatives. Trop Med Infect Dis 2024, 9(9) 223. [CrossRef]
- Buskuhl, H.; Oliveira, F.L.; Blind, L.Z.; Freitas, R.A.; Barison, A.; Campos, F.R.; Corilo, Y.E.; Eberlin, M.N.; Caramori, G.F.; Biavatti, M.W. Sesquiterpene lactones from Vernonia scorpioides and their in vitro cytotoxicity. Phytochemistry 2010, 71, 1539–1544. [Google Scholar] [CrossRef]
- Benoit-Vical, F.; Lelièvre, J.; Berry, A.; Deymier, C.; Dechy-Cabaret, O.; Cazelles, J.; Loup, C.; Robert, A.; Magnaval, J.F.; Meunier, B. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob Agents Chemother 2007, 51(4), 1463–1472. [Google Scholar] [CrossRef]
- Coronado, L.M.; Nadovich, C.T.; Spadafora, C. Malarial hemozoin: from target to tool. Biochim Biophys Acta 2014, 1840(6), 2032–2041. [Google Scholar] [CrossRef]
- Odhiambo, O.C.; Wamakima, H.N.; Magoma, G.N.; Kirira, P.G.; Malala, B.J.; Kimani, F.T.; Muregi, F.W. Efficacy and safety evaluation of a novel trioxaquine in the management of cerebral malaria in a mouse model. Malar J 2017, 16(1), 268. [CrossRef]
- Olanlokun, J.O.; Adetutu, J.A.; Olorunsogo, O.O. ln vitro inhibition of beta-hematin formation and in vivo effects of Diospyros mespiliformis and Mondia whitei methanol extracts on chloroquine-susceptible Plasmodium berghei-induced malaria in mice. Interv Med Appl Sci 2019, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Kim, H.S.; Lee, D.U. In vitro antimalarial activity of flavonoids and chalcones. Bull Korean Chem Soc 2007, 28(12), 2495. ; [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malaria J 2011, 10(1), S4. [CrossRef]
- Ntie-Kang, F.; Onguéné, P.A.; Lifongo, L.L.; Luc, M.M. The potential of anti-malarial compounds derived from African medicinal plants, part II: a pharmacological evaluation of non-alkaloids and non-terpenoids. Malar J 2014, 13(1):81. [CrossRef]
- Guggisberg, A.M.; Amthor, R.E.; Odom, A.R. Isoprenoid biosynthesis in Plasmodium falciparum. Eukaryot Cell 2014, 13(11), 1348–1359. [Google Scholar] [CrossRef]
- Saggu, G.S.; Pala, Z.R.; Garg, S.; Saxena, V. New Insight into isoprenoids biosynthesis process and future prospects for drug designing in Plasmodium. Front Microbiol 2016, 7, 1421. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Rajaram, K.; Swift, R.P.; Mixon, A.; Maschek, J.A.; Prigge, S.T.; Sigala, P.A. 2022. Critical role for isoprenoids in apicoplast biogenesis by malaria parasites. eLife 2022, 11, e73208. [Google Scholar] [CrossRef]
- Percário, S.; Moreira, D.R.; Gomes, B.A.; Ferreira, M.E.; Gonçalves, A.C.; Laurindo, P.S.; Green, M.D. Oxidative stress in malaria. Int J Mol Sci 2012, 13, 16346–16372. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Comim, C.M.; Hermani, F.; Silva, B.; Barichello, T.; Portella, A.C.; Gomes, F.C.A.; Sab, I.M.; Frutuoso, V.S.; Oliveira, M.F.; Bozza P.T.; Bozza F.A.; Dal-Pizzol, F.; Zimmerman, G.A.; Quevedo, J.; Castro-Faria-Neto, H.C. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy, PLoS Pathogens 2010, 6, e1000963, 2-s2.0-77954664054. [CrossRef]
- Arrey Tarkang, P.; Nwachiban Atchan, A.P.; Kuiate, J.R.; Okalebo, F.A.; Guantai, A.N.; Agbor, G.A. Antioxidant potential of a polyherbal antimalarial as an indicator of its therapeutic value. Adv Pharmacol Sci 2013, 2013, 678458. [Google Scholar] [CrossRef]
- Sonibare, M.A.; Aremu, O.T.; Okorie, P.N. Antioxidant and antimicrobial activities of solvent fractions of Vernonia cinerea (L.) Less leaf extract. Afr Health Sci 2016, 16(2), 629-39. [CrossRef]
- Hussen, E.M., Endalew, S.A. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement Med Ther 23, 146 (2023). [CrossRef]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The influence of alkaloids on oxidative stress and related signaling pathways. Free Radic Biol Med. 2019, 134, 429–444. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J Phytomed 2023, 13(4), 354–376. [Google Scholar] [PubMed]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A comprehensive review of free radicals, oxidative stress, and antioxidants : Overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds. J Chem 2024, Article ID 5594386, 21 p. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).