1. Introduction
Macrophages play essential roles in immune responses and inflammation, and their metabolic pathways, including metabolism of lipids and glucose, are central to their function. Lipid metabolism in macrophages is critical for achieving polarization, inflammatory responses, and overall tissue homeostasis. Although the immune response is initially enhanced by macrophage clearance of lipoproteins, excessive lipid uptake impairs the immunological response, causes buildup of atherosclerotic plaques, and prevents resolution of inflammation, thereby facilitating the development of lesions into more complex, advanced plaques involving other immune cells [
1]. Lipids are taken up by macrophages via surface scavenger receptors, such as Cluster of Differentiation 36 (CD36), and are degraded in lysosomes into free cholesterol and fatty acids 2. This process is essential in controlling lipid metabolism and avoiding lipid buildup, and consequently alleviating conditions causing atherosclerosis [
2]. The involvement of macrophages in tissue healing and the immune response is reflected by their ability to use different activation and polarization states: the pro-inflammatory macrophage 1 (M1) phenotype and the anti-inflammatory macrophage 2 (M2) phenotype; macrophages therefore adjust to various environmental cues, and subsequently influence the recovery and inflammatory processes [
3]. Macrophages account for approximately 40% of adipose tissue cells in obese mice but only 10% in lean mice. This substantial difference reflects the roles of macrophages in obesity-associated inflammation and metabolic dysfunction [
4]. Glucose Transporter 1 (GLUT1) is essential for glucose metabolism in macrophages, which are high energy-demand cells in the innate immune system [
5]. Glucose is a primary energy source in macrophages, and glucose metabolic pathways depend on macrophage activation and polarization states. Different macrophage states regulate glucose metabolism; for example, M1 macrophages increase glycolysis and decrease oxidative phosphorylation, whereas M2 macrophages increase oxidation, including fatty acid oxidation and oxidative phosphorylation, and enhance tissue repair and the resolution of inflammation. Beyond lipids and glucose, amino acids are additional energy source. Macrophages use amino acids for protein synthesis and energy production. Amino acid metabolism in macrophages also plays an important role in regulating immune responses, inflammation, and tissue homeostasis. Disruption of glucose, lipid, and amino acid metabolism in macrophages can lead to pathological conditions such as atherosclerosis, obesity, diabetes, cancer, and chronic inflammatory diseases. Understanding how macrophages use glucose, lipids, and amino acids might provide insights into developing therapies for a variety of diseases, including atherosclerosis and chronic inflammatory conditions.
Macrophages express several circadian clock genes in a rhythmic manner, and consequently control the immune response and cell metabolism [
6]. Circadian genes maintain autonomous oscillatory patterns and regulate the circadian control of macrophage responses. Moreover, several immune activation markers and clock genes are expressed rhythmically in the absence of immune stimuli [
7]. Chronic health issues have been observed in individuals with circadian disturbances [
8]. Circadian rhythm genes involved in the physiological functioning of macrophages are normally transcribed under the influence of the transcription factor Brain and Muscle ARNT-like protein-1 (BMAL1) [
9], thus regulating lipid and glucose metabolism, and directing the immune response.
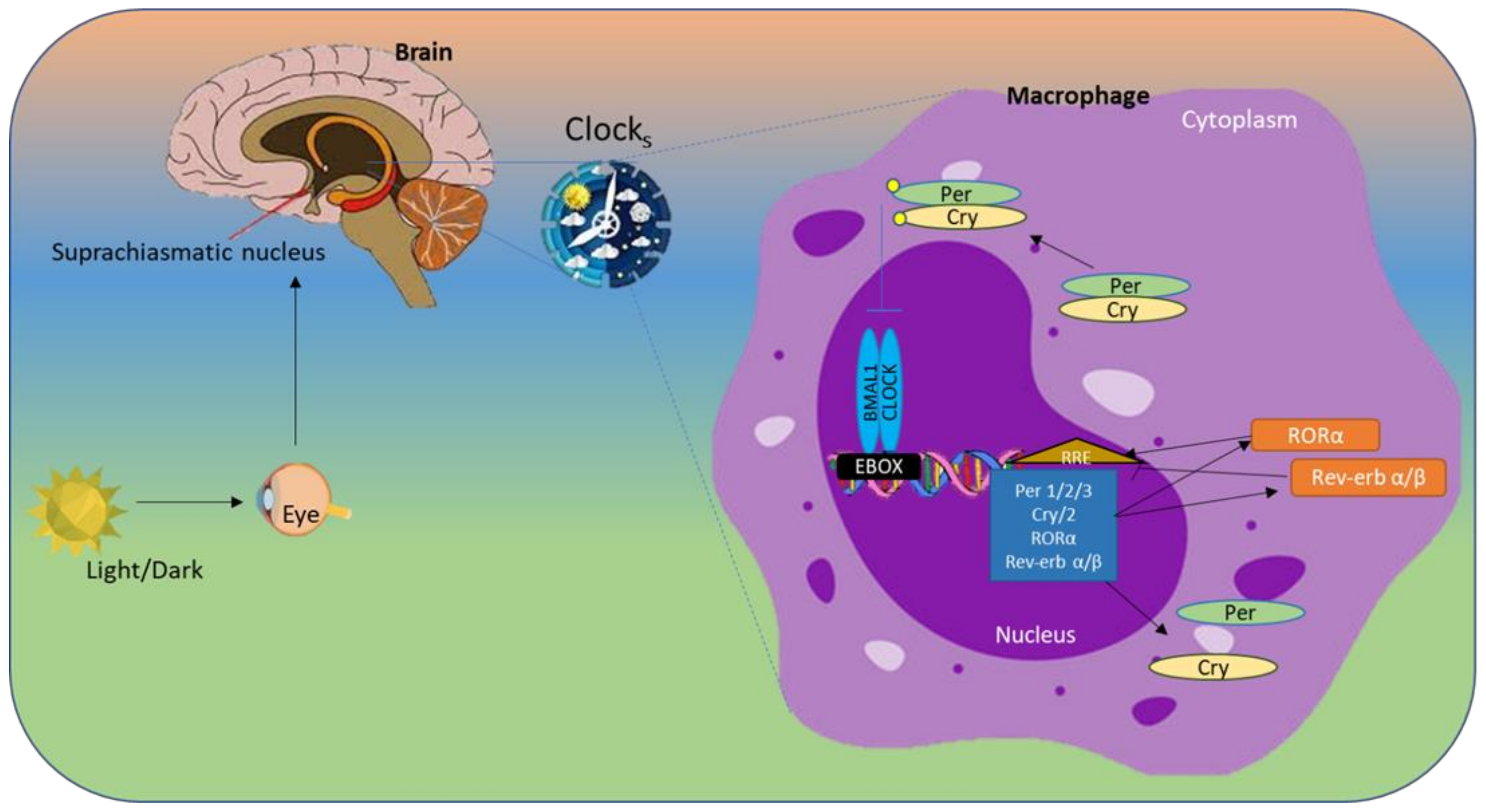
Circadian rhythms are regulated by the Bmal1 and Circadian Locomotor Output Cycles Kaput (Clock) transcription factors, which bind and subsequently stimulate transcription of the Period (Per1, 2 and 3) and Cryptochrome (Cry1, 2) genes, which are responsible for the fundamental circadian clock mechanism through inhibition of the Clock/Bmal1 heterodimer, thereby establishing a self-regulating negative feedback loop [
10]. RAR-related orphan receptor alpha (Rorα) and reverse erythroblastosis virus α (Rev-Erbα) compete for interaction with common DNA binding sites in the ROR elements of Bmal1. Rorα regulates the Clock operation by enhancing Bmal1 transcription, whereas Rev-Erbα/β inhibits it, thus regulating the timing of lipid and glucose metabolism throughout the day; therefore, these proteins are essential for maintaining the natural circadian rhythm. The circadian clock is a crucial regulator of various physiological processes, including sleep and blood pressure. This internal 24-hour "biological clock" helps coordinate the timing of various bodily functions in synchrony with the external environment, primarily light and darkness (schematic diagram of clock gene activity in
Figure 1).
This review explores the multifaceted roles of circadian clock genes in regulating macrophage metabolism, with a particular focus on how glucose and lipid pathways affect macrophage function. The literature analysis highlights the importance of glucose and lipids as key energy sources, components of cell membranes, and signaling molecules that influence macrophage activity. Additionally, this review examines the roles of lipids in modifying proteins, which in turn regulate macrophage behavior, and how they serve as ligands for essential transcription factors. In addition, this review examines how normal glucose, lipid, and amino acid metabolism supports macrophage function, whereas abnormal lipid metabolism contributes to metabolic diseases. Finally, new treatments are discussed that complement circadian cycles and enhance the effectiveness of therapies for inflammation-associated conditions, thus highlighting the importance of using cutting-edge technology to study macrophage metabolism.
2. Roles of Circadian Clock Genes in Regulating Macrophage Function and Metabolism
Molecular clocks are present in nearly all cells and play crucial roles in regulating various biological processes. The "master" clock is found in the hypothalamus, specifically the suprachiasmatic nucleus (SCN). The SCN interprets light signals from outside and creates rhythmic signals. The hypothalamic-pituitary-adrenal axis and autonomic nervous system send signals that synchronize peripheral clocks in various tissues of the body [
11]. The molecular clock is sustained by an interdependent network of transcription-translation feedback loops, under regulation by heterodimerized BMAL1 and CLOCK proteins. BMAL1 is the principal regulator of the clock, and most rhythmic activities are suppressed in its absence [
12]. BMAL1/CLOCK heterodimers bind E-box motifs of DNA and activate the transcription of genes involved in the circadian mechanism. Genes such as Period (PER) and Cryptochrome (CRY) translocate into the nucleus, inhibit the BMAL1 complex, and repress their own transcription [
13]. Finally, the regulatory scheme involves RAR-related orphan receptor alpha (RORα), which stimulates BMAL1, and REV-ERBα, which represses the transcription of BMAL1.
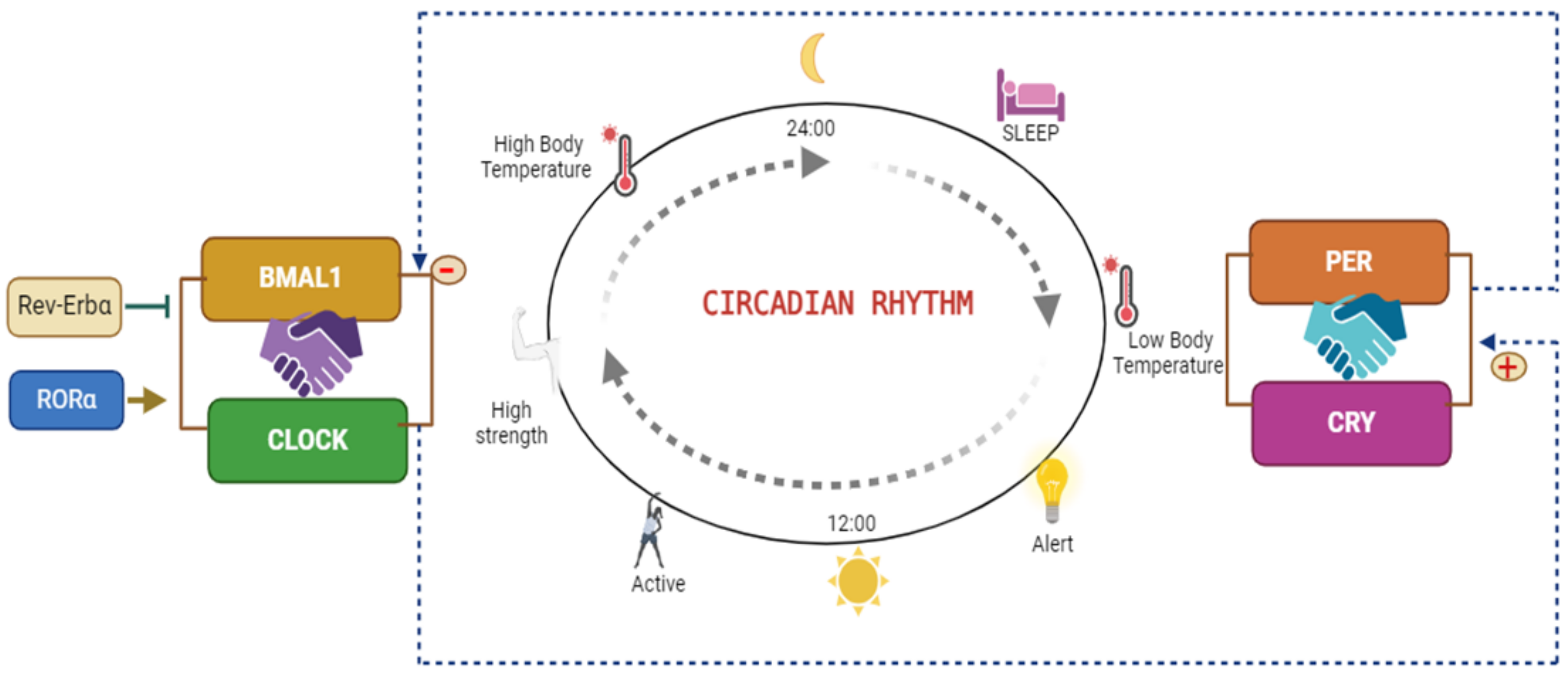
Figure 2.
The circadian rhythm is regulated primarily by the master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Signals from the SCN synchronize peripheral clocks throughout the body. The CLOCK and BMAL1 proteins form a heterodimer that binds E-box elements in specific target genes. After synthesis, PER1–3 and CRY1-2 proteins accumulate in the cytoplasm, form heterodimers, and translocate to the nucleus. Kinases such as Casein Kinase 1 (CK1) phosphorylate newly generated PER and CRY proteins; this modification is crucial for the stability and control of PER and CRY. These PER and CRY heterodimers subsequently inhibit transcription by interfering with CLOCK/BMAL1-driven activation of E-box genes [
14,
15,
16]. Additionally, Rev-Erbα binds the ROR response element (RORE) in the BMAL1 promoter and suppresses its expression, whereas RORα exerts an opposite, activating effect.
Figure 2.
The circadian rhythm is regulated primarily by the master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Signals from the SCN synchronize peripheral clocks throughout the body. The CLOCK and BMAL1 proteins form a heterodimer that binds E-box elements in specific target genes. After synthesis, PER1–3 and CRY1-2 proteins accumulate in the cytoplasm, form heterodimers, and translocate to the nucleus. Kinases such as Casein Kinase 1 (CK1) phosphorylate newly generated PER and CRY proteins; this modification is crucial for the stability and control of PER and CRY. These PER and CRY heterodimers subsequently inhibit transcription by interfering with CLOCK/BMAL1-driven activation of E-box genes [
14,
15,
16]. Additionally, Rev-Erbα binds the ROR response element (RORE) in the BMAL1 promoter and suppresses its expression, whereas RORα exerts an opposite, activating effect.
2.1. BMAL1 (Brain and Muscle ARNT-Like Protein-1)
BMAL1 is a fundamental circadian clock gene that regulates some circadian rhythm-associated genes, thereby influencing various physiological processes, including immune responses. Although BMAL1 is expressed in macrophages in a rhythmic manner, its roles in regulating cellular function in macrophages remain unclear. Previous studies have linked the molecular clock to oxidative damage regulation in various tissues. Complete Bmal1 deletion induces an advanced aging phenotype via reactive oxygen species (ROS)-induced tissue atrophy [
17]. Bmal1 regulates oxidative stress pathways in macrophages and consequently limits the production of the proinflammatory cytokine IL-1β. Specifically, in macrophages, nuclear factor erythroid 2-related factor 2 (Nrf2) activity shows a cycle according to the time of day and is directly mediated by Bmal1 in the presence of lipopolysaccharide (LPS) induction [
18,
19]. Furthermore, decreased Nrf2 activity leads to a loss of redox homeostasis and aberrant production of IL-1β, thereby explaining why macrophages that lack Bmal1 are highly pro-inflammatory in response to LPS [
20]. Disruption of BMAL1 leads to altered immune responses, and increases susceptibility to infections and chronic inflammatory diseases such as arthrosclerosis [
21]. Together, these findings demonstrate that Bmal1 regulates the epigenetic status of enhancers and consequently controls macrophage inflammatory responses.
In macrophages, BMAL1 regulates the rhythmic expression of genes involved in inflammation and metabolism: it modulates cytokine production and enhances anti-inflammatory responses during specific times of day. BMAL1 also influences macrophage polarization by promoting M2 polarization for tissue repair and resolution of inflammation [
22]. Improvements in macrophage function have been reported after the deletion of Bmal1; for example, loss of Bmal1 in macrophages protects against pneumococcal pneumonia [
23]. Bmal1 deficiency in macrophages has been suggested to contribute to increased phagocytosis [
23]. Another study has indicated that Bmal1 regulates Rev-Erb-directed enhancer RNA (eRNA) transcription and affects histone lysine acetylation-responsive enhancers [
22]. Additionally, this protein affects phagocytosis and ROS production, and contributes to pathogen clearance [
24]. Overall, BMAL1 is a crucial link between circadian rhythms and macrophage function and is involved in maintaining immune homeostasis.
2.2. CLOCK (Circadian Locomotor Output Cycles Kaput) Gene
With its partner, Bmal1, the Clock forms a transcriptional activation complex that promotes the rhythmic activation of several downstream genes associated with immunological responses and metabolism [
25,
26]. Clock-driven circadian rhythm maintains the timing of inflammatory responses, phagocytosis, and cytokine synthesis in macrophages in synchrony with the body's overall metabolic state [
27]. Clock has been demonstrated to affect the synthesis of cytokines that trigger inflammation, including Toll-like receptor 9 (TLR-9), Tumor necrosis factor (TNF)-α and IL-6, which are important components of immune responses [
28,
29]. Long-term inflammation and related diseases, such as metabolic syndrome and cardiovascular disorders, are exacerbated by dysregulated macrophages inflammatory responses caused by genetic or environmental disruption of the Clock gene (e.g., through irregular light cycles or diet). Furthermore, Clock is crucial for macrophage metabolism, particularly lipids and glucose, which directly affect macrophage phenotype and activity [
30]. Disruption in normal Clock gene activity leads to impaired lipid processing by macrophages; consequently, these cells switch from the anti-inflammatory M2 phenotype to the more pro-inflammatory M1 phenotype [
31]. This switch is important in the development of insulin resistance and atherosclerosis, as well as immunological modulation [
32,
33].
Macrophage function is closely controlled in a time-dependent manner by Clock’s interaction with Bmal1; subsequently, transcription of Per and Cry is activated in the nucleus, and the Per and Cry proteins interact, thus creating a feedback loop that prevents Clock-Bmal1 activity [
26,
34]. This cyclical feedback ensures mechanism that macrophages display time-specific inflammatory and metabolic responses synchronized with the general circadian cycles [
35]. Changes in circadian rhythms affect immunological homeostasis, lipid metabolism, and macrophage-mediated tissue repair, thereby increasing inflammation. Together with Bmal1, Clock plays important roles in obesity and diabetes [
32]. Several studies have found that polymorphisms in Clock or Bmal1 are associated with obesity and type 2 diabetes [
36,
37,
38]. A global knockout of Clock or Bmal1 is associated with impaired glucose tolerance and decreased insulin secretion [
38,
39]. These phenotypes suggest that dysfunction in macrophage Clock might play important roles in the development of metabolic diseases.
2.3. CRY (Cryptochrome)
Cry genes, specifically Cry1 and Cry2, are integral components of the circadian clock. Cry proteins are part of a complex that inhibits the activity of transcription factors such as CLOCK and BMAL1, and play crucial roles in the negative feedback loop that maintains circadian rhythms [
40]. This inhibition prevents the expression of target genes, thus contributing to the oscillations in circadian rhythms [
41]. Cry genes are also expressed in peripheral tissues, including macrophages, where they regulate immune responses and other physiological functions [
12]. These genes are involved in regulating metabolic processes, and influencing how the body processes nutrients and energy throughout the day.
Cry proteins’ light sensitivity allows them to integrate environmental cues into the circadian system. In the presence of light, they undergo conformational changes affecting their stability and function [
38,
42]. The Cry gene suppresses the inflammatory response in macrophages via negative regulation of the cyclic adenosine monophosphate-protein kinase A- nuclear factor kappa-light-chain-enhancer of activated B cells (cAMP-PKA-NF-κB) pathway [
43]. Loss of the Cry gene results in Nuclear factor-κB (NF-κB) activation and constitutive upregulation of IL-6 and TNFα. The Per/Cry complex is another mechanism of clock-related suppression of inflammatory mediators in macrophages, thus establishing a likely biochemical relationship between the arrhythmic clock mechanism and an enhanced inflammatory response [
44]. Transcriptomic analysis has demonstrated that pancreatic islet Cry2 is downregulated in people with type 2 diabetes with respect to healthy controls [
12,
45]. Overexpression of Cry1 in diabetic db/db mice lowers blood glucose and increases insulin sensitivity [
46,
47]. Furthermore, Cry1/2 knockout mice are hyperglycemic [
42]. Barclay et al. have shown elevated obesity, insulin secretion, and lipid storage in diet-induced Cry1 knockout mice [
48]. Like Bmal1 and Clock, Cry1 and Cry2 shows a circadian rhythm in gene expression in macrophages, thus influencing inflammatory immune responses, and controlling pathogen recognition and cytokine secretion [
6].
2.4. Period Gene
The Per gene, or Period gene (Per1, Per2, and Per3), is a key component of the circadian clock in various organisms, including mammals [
49]. The PER gene is integral to the molecular clock, by helping maintain a 24-hour cycle of gene expression [
50]. In macrophages, PER genes are critical regulators of the circadian rhythms influencing immune responses [
6].
Per1 deficient mice have significantly elevated numbers of Kupffer cells in the liver and show pro-inflammatory cytokine enhancement after LPS treatment [
51]; therefore, Per1 has multifaceted physiological activities, including decreasing macrophages’ recruitment to the liver and interaction with peroxisome proliferator-activated receptor-gamma (PPARγ), and consequently preventing an excessive innate immune response to liver injury.
The circadian rhythm frequently regulates gene expression, essential for cell differentiation. Its functions in the reprogramming of differentiated cells remain largely unclear. PER1, a master circadian regulator, has been found to facilitate virus-mediated reprogramming of mouse embryonic fibroblasts, thereby leading to the production of induced pluripotent stem cells and induced neurons [
50]. Circadian rhythms modulated by Per genes affect macrophages' responses to pathogens. For example, the expression of pro-inflammatory cytokines may peak at specific times, thereby optimizing the immune response to pathogen exposure [
52].
Healthy immune responses and metabolic balance are supported by proper Per gene regulation, whereas chronic inflammation and metabolic diseases, including obesity and insulin resistance, are associated with Per dysregulation. PER genes are therefore crucial for preserving the circadian regulation of macrophage activity [
53]; this function may have implications in the treatment of disorders associated with inflammation.
2.5. Rev-Erbα (Reverse Erythroblastosis Virus α)
Rev-Erbα, an orphan nuclear receptor, is a transcriptional repressor that suppresses BMAL1 and other targets. The biochemical mechanisms of Rev-Erbα, a crucial mediator between the circadian rhythm system and inflammation response, remain unknown. Rev-Erbα regulates macrophage inflammation by directly influencing CCL2 expression. Rev-Erbα directly suppresses C-C motif chemokine ligand 2 (CCL2) expression via a binding motif in the CCL2 promoter. Additionally, Rev-Erbα inhibits CCL2-activated signals, including p38 and Extracellular signal-regulated kinase (ERK), whereas this signaling is restored by the addition of exogenous CCL2 [
54]. Rev-Erbα might be a crucial link between inflammation and the circadian degradation elicited by aging or obesity. In states of chronic systemic inflammation, such as obesity and aging, the gene expression of Rev-Erbα is diminished in peritoneal macrophages, thus altering inflammatory responses 54. The rhythmic circadian repressor Rev-Erbα has been found to be essential to the mechanism coupling the pulmonary clock to innate immunity, through its regulation of the homeostasis of pulmonary inflammation [
55]. Moreover, degradation of Rev-Erbα protein results in an inflammatory response in alveolar macrophages; therefore, the stability of Rev-Erbα protein influences couple the master clock to innate immunity [
55], The macrophage clockwork has been found to enable temporal gating of systemic responses to endotoxins, and Rev-Erbα has been identified as the crucial link between the clock and immune activity. One study has shown that Rev-Erbα agonists decrease the inflammatory response in macrophages [
56]. Moreover, SR9009, despite being an agonist of Rev-Erbα, decreases macrophage M1 polarization and the rate of abortion elicited by LPS in mice. In addition, LPS has been found to repress the expression of Rev-Erbα in macrophages [
57]. Therefore, Rev-Erbα might serve as a therapeutic target for inflammatory diseases in humans [
56]. Rev-Erbα regulates the circadian clock in macrophages, thereby synchronizing metabolic and immunological processes with daily cycles. This protein regulates gene expression in metabolism, lipids, and inflammation. Proper Rev-Erbα activity decreases chronic inflammation and metabolic syndrome, whereas Rev-Erb dysregulation is associated with illnesses such as obesity and diabetes. Targeting Rev-Erbα therefore has promise in potentially curing inflammation-driven metabolic disorders.
Dysfunction in Rev-Erbα/β has been found to significantly influence the progression of metabolic diseases and affect energy equilibrium [
58,
59]. Sato et al. have shown that Rev-erbα deficiency increases the inflammatory response in obesity-associated macrophages by inhibiting Ccl2 expression [
54]. Rev-Erbα regulates macrophage inflammation by directly influencing Ccl2 expression. In states of chronic systemic inflammation, such as obesity and aging, diminished expression of the Rev-Erbα gene in peritoneal macrophages leads to altered inflammatory responses [
54]. Moreover, degradation of the Rev-Erbα protein elicits an inflammatory response in alveolar macrophages; therefore, significant changes in the stability of Rev-Erbα protein couple the master clock to innate immunity [
55]. Moreover, agonists to Rev-Erbα decrease the inflammatory response in macrophages [
56].
3. Metabolism of Micronutrients in Macrophages
3.1. Lipid Metabolism and Macrophage Function
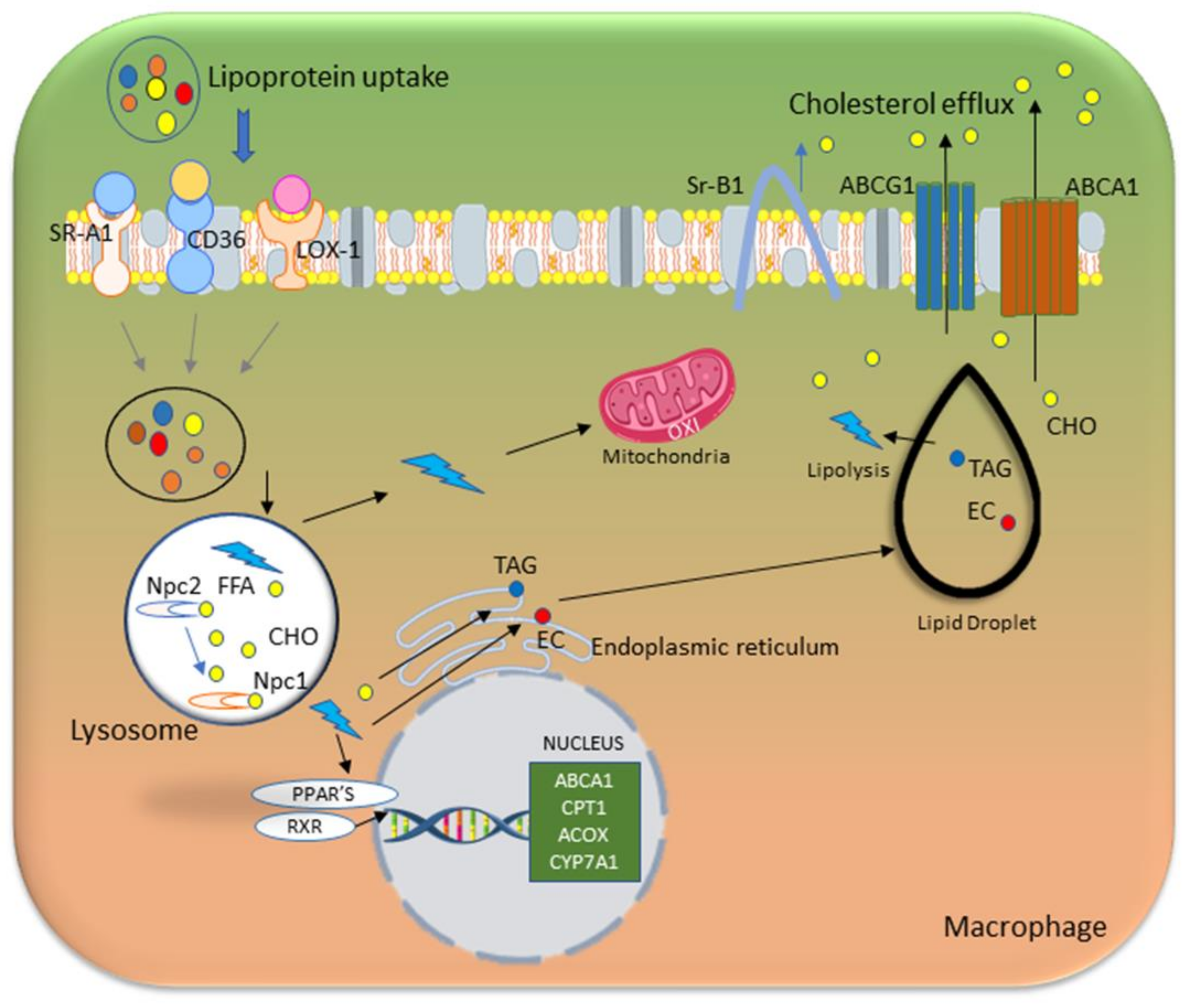
Lipid metabolism is an integral part of macrophage function and activation. Macrophages perform uptake, storage, and breakdown of lipids, thereby affecting polarization into either a pro-inflammatory state (M1) or anti-inflammatory state (M2). M1 macrophages use glycolysis as their central metabolic pathway through HIF-1α and NF-κB, following inflammatory activation, thus enabling initiation of microbicidal activity even in the context of hypoxic inflammatory tissue [
60,
61]. During tissue repair and remodeling, macrophages exhibit a switch to oxidative metabolism of glucose and fatty acids; develop an anti-inflammatory M2 phenotype; and rely predominantly on mitochondrial fatty acid oxidation (FAO) to meet their energy requirements [
62,
63].
Lipases in macrophages convert triglycerides within lipid droplets into free fatty acids (FFAs). Over time, cells continuously receive energy from FAO of these FFAs in both mitochondria and peroxisomes [
64]. Greater lipid intake and FAO enable M2 macrophages to perform anti-inflammatory and reparative functions. This metabolism supports tissue repair and regeneration through the release of anti-inflammatory cytokines and growth factors that promote the healing and remodeling of injured tissues 2. Lipid accumulation in M1 macrophages drives the production of pro-inflammatory cytokines and ROS, and consequently contributes to inflammation and tissue damage [
65]. In M1 macrophages, glycolysis is upregulated, thus resulting in rapid ATP generation and supplying the tricarboxylic acid (TCA) cycle with citrate, which is then converted to acetyl-CoA. This process is facilitated by the enzyme ATP-citrate lyase (ACLY), whose levels rise quickly after macrophage activation. Inhibiting or silencing ACLY decreases key inflammatory products such as nitric oxide and ROS, thus demonstrating ACLY’s roles in supporting the inflammatory response [
66]. Increased use of lipids stimulates FAO, which in turn supports M2 activation through acetyl-CoA production and histone acetylation [
67]. Adipose-derived stem cells transplantation enhances liver function and lipid metabolism [
68], and stimulates the transition of macrophages from the M1 to the M2 phenotype by enhancing their capacity for lipid uptake and digestion.
The activation of macrophages and their participation in lipid metabolism are significantly influenced by transcription factors. The expression of pro-inflammatory genes in response to stimuli is driven by transcription factors including JAK-signal transducer and activator of transcription 3 (STAT3), and NF-κB, which are important regulators of macrophage activation [
69,
70]. PPARγ and nuclear liver X receptor (LXR) are two factors that control lipid absorption, storage, and cholesterol efflux in lipid metabolism; activation of the PPARγ-LXRα-ABC metabolic pathway boosts cholesterol efflux, increases high-density lipoprotein (HDL) cholesterol transfer, and decreases atherosclerosis [
71,
72,
73].
Dysfunction in macrophage lipid metabolism has been implicated in several diseases, including atherosclerosis, which is characterized by the accumulation of lipid-laden macrophages or foam cells in arterial plaques [
74]. A schematic diagram of lipid metabolism in macrophages is shown in
Figure 3.
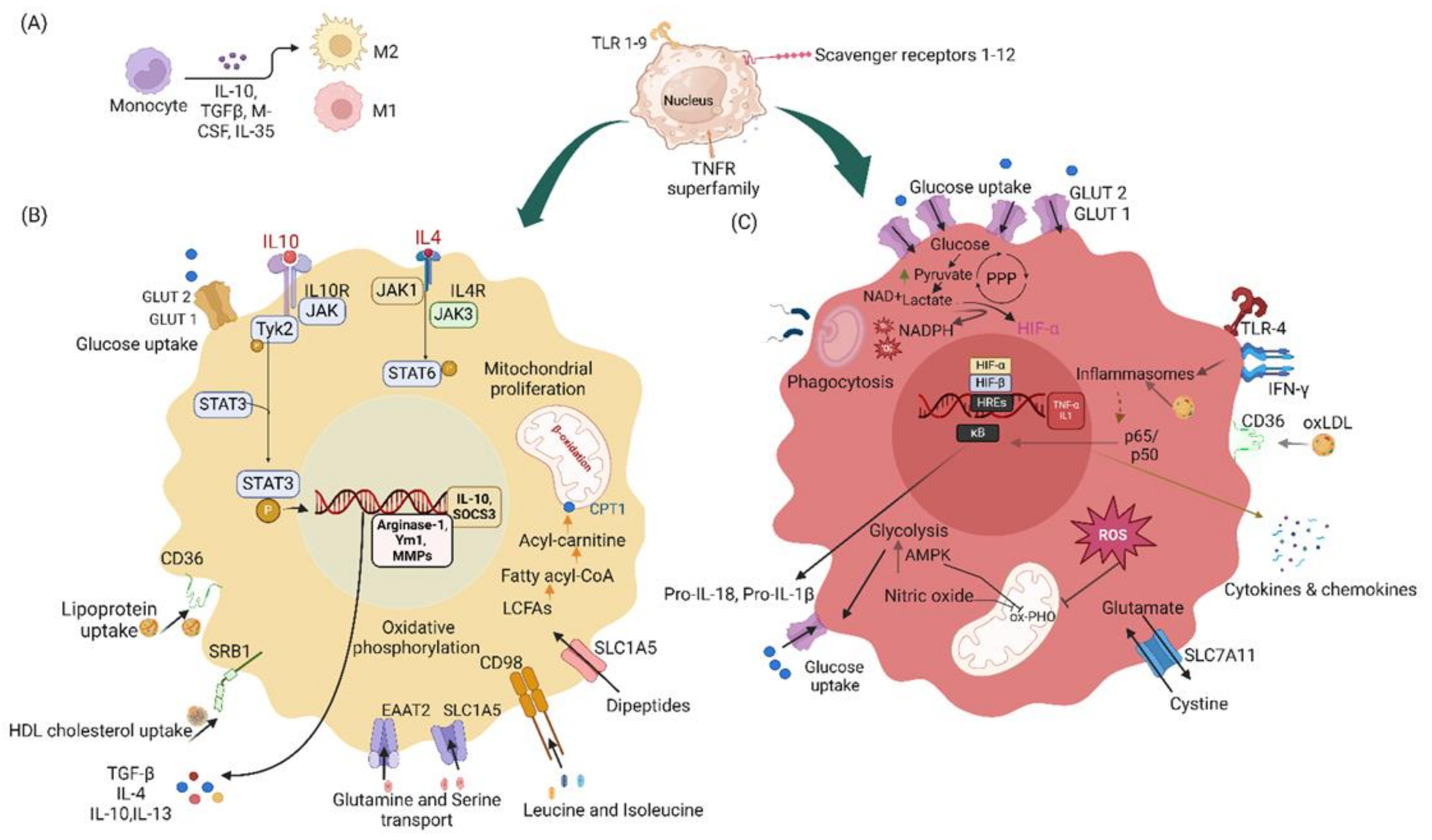
3.2. Glucose Metabolism in Macrophages: A Key to Immune Activation and Inflammation
Macrophages undergo substantial metabolic changes during activation, particularly during inflammatory reactions, including a shift toward greater absorption of glucose and catabolism to meet metabolic needs [
79,
80,
81,
82,
83,
84,
85,
86]. The need for rapid energy generation and the synthesis of macromolecules necessary for immunological activities are the main reasons for this metabolic reprogramming. Glycolysis is markedly upregulated in activated macrophages. Even when oxygen is present, activated macrophages favor glycolysis, whereas resting macrophages use primarily oxidative phosphorylation to produce ATP [
87,
88,
89]. This process, known as the Warburg effect, provides the glycolytic intermediates necessary for most biosynthetic pathways and enables rapid ATP synthesis. This rapid provision of ATP supply helps macrophages perform several essential tasks, such as phagocytosis, pro-inflammatory cytokine release, and ROS generation, which are essential for the defense against antigens [
90]. Glycolysis also helps direct carbon flow into the oxidative pentose phosphate pathway, which creates nicotinamide adenine dinucleotide phosphate (NADPH), which in turn is used to generate ROS via NADPH oxidases. NADPH is necessary for maintaining cellular redox equilibrium, because it helps regenerate glutathione, a critical antioxidant that protects cells against oxidative damage. The pentose phosphate pathway, a precursor to nucleotide synthesis, aids in the rapid multiplication of immune cells during the inflammatory response.
The phagocytic activity of M1 macrophages is also dependent on ROS generation. Inhibition of aerobic glycolysis by activating pyruvate kinase M2 (PKM2) or inhibition of pyruvate dehydrogenase kinase 1 (PDK1) decreases LPS-induced macrophage polarization toward the M1 phenotype [
91]. Glycolysis is essential for both the M1 and M2 polarization states of macrophages [
92]. M2 macrophages must undergo glycolysis to produce cytokines in response to LPS stimulation [
93]. Pro-inflammatory macrophages engage primarily in glycolysis but can also use oxidative phosphorylation and the TCA cycle, particularly in M2 macrophages or after resolution of inflammation [
94]. Fatty acids and other substrates may be preferentially used under certain situations, thereby increasing ATP synthesis and mitochondrial respiration. As macrophages shift among various functional modes, metabolic flexibility is essential to these cells’ ability to adjust to their microenvironment. An illustration of glucose and lipid metabolic pathways in M1 and M2 macrophages is shown in
Figure 4.
The importance of glucose metabolite signaling in macrophage activation and activity has also been demonstrated in many studies. Succinate and fumarate, two intermediates in the metabolism of glucose, are signaling molecules that control inflammatory reactions [
98]. For instance, elevated succinate levels boost immune responses by increasing the synthesis of pro-inflammatory cytokines [
99], whereas other metabolites have anti-inflammatory properties, thus underscoring the complex equilibrium of macrophage metabolic pathways [
84].
3.3. Amino Acid Metabolism in Macrophages
The function and activation status of macrophages are largely determined by amino acid metabolism [
100], which also affects how these cells contribute to tissue healing and immune responses [
101]. The metabolization of arginine, an important amino acid in macrophages, varies according to the anti-inflammatory (M2) or pro-inflammatory (M1) state 102. The enzyme inducible nitric oxide synthase (iNOS) in M1 macrophages transforms arginine into nitric oxide, a substance essential for resolving infections and triggering inflammatory reactions. M2 macrophages, in contrast, produce arginase-1, which changes arginine to urea and ornithine [
102]. Ornithine supports the M2 anti-inflammatory properties of macrophages by aiding in collagen synthesis and tissue healing [
103]. Ornithine decarboxylase (OD) has elicited a strong effect on M1 macrophage activation. Several findings have established that OD in macrophages modulates bacterial persistence inside the host, given that OD deletion results in microbial survival effects during gastric infection. Microbial pathogenesis in mice has been shown to be significantly affected by OD in macrophages [
104].
Under glutamine starvation in macrophages, M2-polarized macrophages increase the expression of Glutamine-synthetase (GS). Beyond causing macrophage M2 polarization and inducing tumor metastasis, GS expression (which increases under starvation) might also encourage the release of glutamine for other cells to use [
105]. When activated, macrophages increase their glutamine absorption, thereby enabling rapid cell proliferation and the generation of inflammatory mediators [
106]. Furthermore, the mechanistic target of rapamycin (mTOR) pathway, which is essential in controlling immunological responses, metabolism, and cell proliferation, is activated by amino acids such as leucine. By interfering with macrophage differentiation, proliferation, and pro-inflammatory cytokine production, mTOR signaling has been shown to modulate the immune response [
107].
The complexity of macrophage metabolism is demonstrated through the interaction of these amino acids with different metabolic pathways. This ability of macrophages to adapt metabolic changes enables them to react efficiently to various signals, and consequently affects their shift toward the pro-inflammatory (M1) or anti-inflammatory (M2) state [
108,
109]. Maintaining the equilibrium of these pathways is essential in immune homeostasis, tissue repair, and the overall response to infection and injury [
73,
110,
111]. Gaining insight into these metabolic processes involving amino acid metabolism in macrophages may clarify their functions in various diseases, such as chronic inflammatory conditions, autoimmune diseases, and cancer.
4. Circadian Clock-Associated Macrophages in the Metabolism of Lipids, Glucose, and Amino Acids, and the Inflammatory Response
Several studies have identified that circadian clock genes regulate macrophage lipid metabolism [
22,
29,
112,
113]. We have demonstrated that Clock mutant mice show elevated oxLDL uptake, diminished cholesterol efflux in macrophages, and aggravated atherosclerosis [
112]. Huo et al. have demonstrated that myeloid Bmal1 deficient mice show upregulated monocyte recruitment and atherosclerosis development [
21]. Blacher et al. have shown that the clock control gene Kruppel-like factor 4 (KLF4) plays important roles in controlling macrophage phagocytes in deficient Klf4 expression in in vivo and in vitro [
114]. microRNA-21 (Mir21) controls the circadian regulation of apoptosis in atherosclerosis [
115]. Deficiency in Mir21 in macrophages decreases atherosclerosis, and Mir21-deficient mice show enhanced Bmal1 gene expression in macrophages; therefore, miR21 might be associated with the circadian clock gene Bmal1 in the development of atherosclerosis. Together, these findings indicate that the macrophage circadian clock targets the lipid metabolism in macrophages, thus controlling macrophage function and immune responses, and protecting against atherosclerosis and metabolic syndrome.
Furthermore, recent studies have indicated that the circadian rhythm has a major effect on macrophage glucose metabolism [
116]. Circadian rhythms control several metabolic functions, such as mitochondrial activity and glycolysis, which in turn affect macrophage activity and the onset of immunological responses [
117,
118]. Timmons et al. have shown that macrophage Bmal1 controls the uptake of glucose in macrophages, and regulates PKM2, thereby controlling IL-1beta mRNA levels via STAT3 in macrophages [
9]. Changes in macrophage activity elicited by disruptions in circadian timing can exacerbate metabolic diseases and chronic inflammation [
116,
119,
120]. Gaining insight into the links between macrophage metabolism of glucose and circadian rhythms may pave the way to therapeutic interventions in several illnesses involving dysregulated immune responses.
Studies have suggested that the circadian clock also affects various amino acid metabolism-associated macrophage functions, including regulation of the synthesis, degradation, and transport of amino acids, such as arginine, glutamine, cysteine, and methionine, in macrophages.
5. New Treatments that Complement Circadian Cycles and Enhance Therapeutic Effectiveness for Conditions Associated with Inflammation
New therapies are being developed to work in harmony with circadian cycles, by timing treatments to align with the body’s natural rhythms, thereby enhancing their effectiveness in managing inflammation-associated conditions. These options include chronotherapy, whose goal is using an understanding of how biological cycles affect treatment reactions to improve treatment effectiveness, optimize health benefits, and avoid potential adverse effects [
23,
121]. Chronotherapy with PPARγ agonists has been reported by using rosiglitazone to regulate triglyceride buildup inside macrophages, thus altering circadian gene cycles. These modifications affect the rhythmic release of TNF-α and regulate the phenotypic switch in vascular smooth muscle cells [
122]. Chrononutrition is another important concept for managing metabolic conditions; its emphasis on food consumption in accordance with the circadian rhythms supports nutritional metabolic processes and regulates nutritional intake on the basis of circadian cycles [
123]. Studies have shown potential for improving macrophage metabolic activities by matching glycolysis and oxidative phosphorylation according to energy demands [
124]. Moreover, dietary amino acids have been reported to modulate intestinal macrophages by enhancing phagocytic activity, and encouraging macrophage replenishment and IL-10 release [
125]. Pharmacological targeting of circadian components such as REV-ERB and ROR enables direct control of clock-regulated metabolic genes, thereby influencing inflammatory pathways and macrophage polarization states [
126]. Furthermore, regulation of light exposure and sleep cycles promotes circadian synchronization, and therefore might decrease chronic inflammation and improve macrophage functional profiles in immune-associated illnesses, including metabolic dysregulation [
127]. Integrating these strategies into established therapies might potentially increase macrophage activity and inflammatory responses, thereby achieving better results in the treatment of metabolic diseases, including rheumatoid arthritis, inflammatory bowel disease, and metabolic syndrome.
6. Cutting-Edge Research and Emerging Areas in Circadian Biology
Cutting-edge research in circadian biology and immunometabolism has revealed new details regarding the complex interplay between the clock genes and metabolic activities within immune cells, notably macrophages, by studying how circadian rhythm alterations affect macrophage polarization and consequently chronic inflammatory disorders and metabolic dysfunction. Technological developments have enabled substantial advancements in research on circadian rhythms and macrophage metabolism, by enabling more accurate and thorough analysis [
128]. High-throughput sequencing tools, such as RNA-seq, proteomics, and transcriptomics, have allowed researchers to characterize circadian gene expression patterns in macrophages in different time periods, as well as macrophage phenotypes [
129].
RNA-seq is a high-throughput sequencing method that allows the transcriptome in a biological sample to be examined. RNA-seq detects and measures non-coding RNAs (such as miRNAs, lncRNAs, and snoRNAs), which have important regulatory functions despite not being translated into proteins [
130]. RNA-seq can be used to accurately characterize the diurnal fluctuations in gene expression exhibited by macrophages [
131], and has enabled the identification of genes with rhythmic expression patterns by capturing gene expression changes over time. This temporal accuracy is essential for understanding the circadian regulation of macrophage activity. By identifying and quantifying these different pathways and genes in macrophage, RNA-seq can help clarify how macrophages modulate their functions in response to circadian cues [
128].
Mass spectrometry enables proteomic characterization of macrophages, by providing information about macrophage heterogeneity, and offers insights into metabolic pathways and changes in response to stimuli. This method aids in the identification of important metabolites that influence energy generation, inflammatory responses, and macrophage polarization (e.g., M1 vs. M2 states). Mass spectrometry-based metabolomics has advanced knowledge of macrophage activity in both health and illness by mapping metabolic patterns [
132]. However, this technique has several limitations; for example, the purity and integrity of proteins is not maintained, and high molecular weight proteins and hydrophobicity also interfere with the efficiency of this technique [
133].
A variety of metabolites and proteins can be rapidly profiled from cell or tissue samples with matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS), which can facilitate research on macrophage metabolism. In addition to detecting multidimensional macrophage activity, MALDI-TOF MS can distinguish between unstimulated and stimulated macrophages [
134]. This method can identify particular metabolic modifications associated with macrophage polarization (e.g., M1 or M2 states), including changes in lipids and amino acids metabolism [
135]. MALDI-TOF's ability to recognize these metabolic fingerprints can aid in clarifying the macrophage metabolic changes associated with immunological responses, inflammation, and energy consumption. Furthermore, modern imaging technologies, such as live-cell imaging and bioluminescent reporters, have enabled real-time monitoring of circadian rhythms in living animals, thus offering insights into the functional implications of macrophage metabolic reprogramming. Future research is expected to focus on creating and enhancing analytical instruments that are more accurate and highly sensitive for the detection of sub-proteomes; these methods may elucidate the complex heterogeneity of macrophages.
7. Conclusions and Perspectives
The study of circadian genes in macrophages provides crucial insights into how these immune cells control their metabolic activities, such as lipid, glucose, and amino acid metabolism, during daily cycles. Circadian genes govern macrophage behaviors by fine-tuning metabolic pathways to match environmental signals, thereby modulating inflammatory and tissue-remodeling responses. Macrophage metabolism can be altered by modifying circadian genes, thus restoring balance in lipid and glucose management and amino acid processing; this balance is particularly important in disorders involving metabolic dysregulation. Controlling critical pathways involving Bmal1, Clock Per, Cry, Rev-Erbα, and other circadian clock genes enables specific metabolic modifications in macrophages. This capability might potentially lower chronic inflammation and metabolic stress, and open new routes for treating metabolic dysfunction, in conditions such as cancer, diabetes, and atherosclerosis, in which macrophage function and metabolism are radically altered. Circadian modulation might also aid in optimizing macrophages’ dynamic shifts between pro-inflammatory and anti-inflammatory phenotypes, thereby improving disease outcomes and offering a paradigm for the use of new treatments for immune-associated disorders.
Funding
This work was supported in part by National Institutes of Health grants R56 HL137912 and R01HL169313 to XP.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709-21. [CrossRef] [PubMed] [PubMed Central]
- Huang SC, Everts B, Ivanova Y, O'Sullivan D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY, O'Neill CM, Yan C, Du H, Abumrad NA, Urban JF, Jr., Artyomov MN, Pearce EL, Pearce EJ. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846-55. Epub 20140803. [CrossRef] [PubMed] [PubMed Central]
- Orliaguet L, Ejlalmanesh T, Alzaid F. Metabolic and Molecular Mechanisms of Macrophage Polarisation and Adipose Tissue Insulin Resistance. Int J Mol Sci. 2020;21(16). Epub 20200810. [CrossRef] [PubMed] [PubMed Central]
- Stansbury CM, Dotson GA, Pugh H, Rehemtulla A, Rajapakse I, Muir LA. A lipid-associated macrophage lineage rewires the spatial landscape of adipose tissue in early obesity. JCI Insight. 2023;8(19). Epub 20231009. [CrossRef] [PubMed] [PubMed Central]
- Obaid M, Udden SMN, Alluri P, Mandal SS. LncRNA HOTAIR regulates glucose transporter Glut1 expression and glucose uptake in macrophages during inflammation. Sci Rep. 2021;11(1):232. Epub 20210108. [CrossRef] [PubMed] [PubMed Central]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106(50):21407-12. Epub 2009/12/04. [CrossRef] [PubMed] [PubMed Central]
- Guzman-Ruiz MA, Guerrero-Vargas NN, Lagunes-Cruz A, Gonzalez-Gonzalez S, Garcia-Aviles JE, Hurtado-Alvarado G, Mendez-Hernandez R, Chavarria-Krauser A, Morin JP, Arriaga-Avila V, Buijs RM, Guevara-Guzman R. Circadian modulation of microglial physiological processes and immune responses. Glia. 2023;71(2):155-67. Epub 20220816. [CrossRef] [PubMed] [PubMed Central]
- Mason IC, Qian J, Adler GK, Scheer F. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020;63(3):462-72. Epub 20200108. [CrossRef] [PubMed] [PubMed Central]
- Timmons GA, Carroll RG, O'Siorain JR, Cervantes-Silva MP, Fagan LE, Cox SL, Palsson-McDermott E, Finlay DK, Vincent EE, Jones N, Curtis AM. The Circadian Clock Protein BMAL1 Acts as a Metabolic Sensor In Macrophages to Control the Production of Pro IL-1beta. Front Immunol. 2021;12:700431. Epub 20211109. [CrossRef] [PubMed] [PubMed Central]
- Cox KH, Takahashi JS. Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol. 2019;63(4):R93-R102. Epub 2019/09/27. [CrossRef] [PubMed] [PubMed Central]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517-49. [CrossRef] [PubMed]
- Fagiani F, Di Marino D, Romagnoli A, Travelli C, Voltan D, Di Cesare Mannelli L, Racchi M, Govoni S, Lanni C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct Target Ther. 2022;7(1):41. Epub 20220208. [CrossRef] [PubMed] [PubMed Central]
- Li S, Zhang L. Circadian Control of Global Transcription. Biomed Res Int. 2015;2015:187809. Epub 20151123. [CrossRef] [PubMed] [PubMed Central]
- Takahashi, J.S. Molecular components of the circadian clock in mammals. Diabetes Obes Metab. 2015;17 Suppl 1:6-11. Epub 2015/09/04. [CrossRef] [PubMed] [PubMed Central]
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320(5878):949-53. Epub 2008/05/20. [CrossRef] [PubMed] [PubMed Central]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134(5):728-42. Epub 2008/09/09. [CrossRef] [PubMed] [PubMed Central]
- Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany NY). 2009;1(12):979-87. Epub 20091230. [CrossRef] [PubMed] [PubMed Central]
- Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, Yamamoto M, Chan J, van der Horst GT, Fukada Y, Meng QJ. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548-60. Epub 2014/03/19. [CrossRef] [PubMed] [PubMed Central]
- Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, Palsson-McDermott EM, Angiari S, Ryan DG, Corcoran SE, Timmons G, Geiger SS, Fitzpatrick DJ, O'Connell D, Xavier RJ, Hokamp K, O'Neill LAJ, Curtis AM. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A. 2018;115(36):E8460-E8. Epub 2018/08/22. [CrossRef] [PubMed] [PubMed Central]
- Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, Xue C, Geiger SS, Hokamp K, Reilly MP, Coogan AN, Vigorito E, FitzGerald GA, O'Neill LA. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A. 2015;112(23):7231-6. Epub 20150520. [CrossRef] [PubMed] [PubMed Central]
- Huo M, Huang Y, Qu D, Zhang H, Wong WT, Chawla A, Huang Y, Tian XY. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017;31(3):1097-106. Epub 2016/12/09. [CrossRef] [PubMed] [PubMed Central]
- Oishi Y, Hayashi S, Isagawa T, Oshima M, Iwama A, Shimba S, Okamura H, Manabe I. Bmal1 regulates inflammatory responses in macrophages by modulating enhancer RNA transcription. Sci Rep. 2017;7(1):7086. Epub 2017/08/03. [CrossRef] [PubMed] [PubMed Central]
- Kitchen GB, Cunningham PS, Poolman TM, Iqbal M, Maidstone R, Baxter M, Bagnall J, Begley N, Saer B, Hussell T, Matthews LC, Dockrell DH, Durrington HJ, Gibbs JE, Blaikley JF, Loudon AS, Ray DW. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc Natl Acad Sci U S A. 2020;117(3):1543-51. Epub 20200103. [CrossRef] [PubMed] [PubMed Central]
- Perez S, Rius-Perez S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants (Basel). 2022;11(7). Epub 20220719. [CrossRef] [PubMed] [PubMed Central]
- Zhang W, Xiong Y, Tao R, Panayi AC, Mi B, Liu G. Emerging Insight Into the Role of Circadian Clock Gene BMAL1 in Cellular Senescence. Front Endocrinol (Lausanne). 2022;13:915139. Epub 20220606. [CrossRef] [PubMed] [PubMed Central]
- Shirato K, Sato S. Macrophage Meets the Circadian Clock: Implication of the Circadian Clock in the Role of Macrophages in Acute Lower Respiratory Tract Infection. Front Cell Infect Microbiol. 2022;12:826738. Epub 20220223. [CrossRef] [PubMed] [PubMed Central]
- Knudsen-Clark AM, Cazarin J, Altman BJ. Do macrophages follow the beat of circadian rhythm in TIME (Tumor Immune Microenvironment)? F1000Res. 2023;12:101. Epub 20230127. [CrossRef] [PubMed] [PubMed Central]
- Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36(2):251-61. [CrossRef] [PubMed] [PubMed Central]
- Vieira E, Mirizio GG, Barin GR, de Andrade RV, Nimer NFS, La Sala L. Clock Genes, Inflammation and the Immune System-Implications for Diabetes, Obesity and Neurodegenerative Diseases. Int J Mol Sci. 2020;21(24). Epub 20201221. [CrossRef] [PubMed] [PubMed Central]
- Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, Rao L, Duo Y. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8(1):207. Epub 20230522. [CrossRef] [PubMed] [PubMed Central]
- Liu Z, Gan L, Zhang T, Ren Q, Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2018;64(1). Epub 20171204. [CrossRef] [PubMed] [PubMed Central]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043-5. Epub 2005/04/23. [CrossRef] [PubMed] [PubMed Central]
- Oosterman JE, Wopereis S, Kalsbeek A. The Circadian Clock, Shift Work, and Tissue-Specific Insulin Resistance. Endocrinology. 2020;161(12). [CrossRef] [PubMed]
- Haspel JA, Anafi R, Brown MK, Cermakian N, Depner C, Desplats P, Gelman AE, Haack M, Jelic S, Kim BS, Laposky AD, Lee YC, Mongodin E, Prather AA, Prendergast BJ, Reardon C, Shaw AC, Sengupta S, Szentirmai E, Thakkar M, Walker WE, Solt LA. Perfect timing: circadian rhythms, sleep, and immunity - an NIH workshop summary. JCI Insight. 2020;5(1). Epub 20200116. [CrossRef] [PubMed] [PubMed Central]
- Alexander RK, Liou YH, Knudsen NH, Starost KA, Xu C, Hyde AL, Liu S, Jacobi D, Liao NS, Lee CH. Bmal1 integrates mitochondrial metabolism and macrophage activation. Elife. 2020;9. Epub 20200512. [CrossRef] [PubMed] [PubMed Central]
- Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond). 2008;32(4):658-62. Epub 20071211. [CrossRef] [PubMed]
- Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104(36):14412-7. Epub 2007/08/31. [CrossRef] [PubMed] [PubMed Central]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627-31. Epub 2010/06/22. [CrossRef] [PubMed] [PubMed Central]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. Epub 2004/11/04. [CrossRef] [PubMed] [PubMed Central]
- Richards J, Gumz ML. Mechanism of the circadian clock in physiology. Am J Physiol Regul Integr Comp Physiol. 2013;304(12):R1053-64. Epub 20130410. [CrossRef] [PubMed] [PubMed Central]
- Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337(6094):599-602. Epub 20120705. [CrossRef] [PubMed]
- Lin C, Todo T. The cryptochromes. Genome Biol. 2005;6(5):220. Epub 20050429. [CrossRef] [PubMed] [PubMed Central]
- Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(31):12662-7. Epub 20120709. [CrossRef] [PubMed] [PubMed Central]
- Timmons GA, O'Siorain JR, Kennedy OD, Curtis AM, Early JO. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front Immunol. 2020;11:1743. Epub 20200804. [CrossRef] [PubMed] [PubMed Central]
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152-6. Epub 20100919. [CrossRef] [PubMed] [PubMed Central]
- Jang H, Lee GY, Selby CP, Lee G, Jeon YG, Lee JH, Cheng KK, Titchenell P, Birnbaum MJ, Xu A, Sancar A, Kim JB. SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding. Nat Commun. 2016;7:12180. Epub 20160714. [CrossRef] [PubMed] [PubMed Central]
- Tanida M, Yamatodani A, Niijima A, Shen J, Todo T, Nagai K. Autonomic and cardiovascular responses to scent stimulation are altered in cry KO mice. Neurosci Lett. 2007;413(2):177-82. Epub 20061218. [CrossRef] [PubMed]
- Barclay JL, Shostak A, Leliavski A, Tsang AH, Johren O, Muller-Fielitz H, Landgraf D, Naujokat N, van der Horst GT, Oster H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab. 2013;304(10):E1053-63. Epub 20130326. [CrossRef] [PubMed]
- Chen K, Wang Y, Li D, Wu R, Wang J, Wei W, Zhu W, Xie W, Feng D, He Y. Biological clock regulation by the PER gene family: a new perspective on tumor development. Front Cell Dev Biol. 2024;12:1332506. Epub 20240515. [CrossRef] [PubMed] [PubMed Central]
- Katoku-Kikyo N, Lim S, Yuan C, Koroth J, Nakagawa Y, Bradley EW, Kikyo N. The circadian regulator PER1 promotes cell reprogramming by inhibiting inflammatory signaling from macrophages. PLoS Biol. 2023;21(12):e3002419. Epub 20231204. [CrossRef] [PubMed] [PubMed Central]
- Wang T, Wang Z, Yang P, Xia L, Zhou M, Wang S, Du J, Zhang J. PER1 prevents excessive innate immune response during endotoxin-induced liver injury through regulation of macrophage recruitment in mice. Cell Death Dis. 2016;7(4):e2176. Epub 20160407. [CrossRef] [PubMed] [PubMed Central]
- Cicchese JM, Evans S, Hult C, Joslyn LR, Wessler T, Millar JA, Marino S, Cilfone NA, Mattila JT, Linderman JJ, Kirschner DE. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev. 2018;285(1):147-67. [CrossRef] [PubMed] [PubMed Central]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112(12):1796-808. [CrossRef] [PubMed] [PubMed Central]
- Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192(1):407-17. Epub 20131204. [CrossRef] [PubMed]
- Pariollaud M, Gibbs JE, Hopwood TW, Brown S, Begley N, Vonslow R, Poolman T, Guo B, Saer B, Jones DH, Tellam JP, Bresciani S, Tomkinson NC, Wojno-Picon J, Cooper AW, Daniels DA, Trump RP, Grant D, Zuercher W, Willson TM, MacDonald AS, Bolognese B, Podolin PL, Sanchez Y, Loudon AS, Ray DW. Circadian clock component REV-ERBalpha controls homeostatic regulation of pulmonary inflammation. J Clin Investig. 2018;128(6):2281-96. Epub 20180430. [CrossRef] [PubMed] [PubMed Central]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(2):582-7. Epub 2011/12/21. [CrossRef] [PubMed] [PubMed Central]
- Cui L, Xu F, Wang S, Li X, Lin H, Ding Y, Du M. Pharmacological activation of rev-erbalpha suppresses LPS-induced macrophage M1 polarization and prevents pregnancy loss. BMC Immunol. 2021;22(1):57. Epub 20210816. [CrossRef] [PubMed] [PubMed Central]
- Hong H, Cheung YM, Cao X, Wu Y, Li C, Tian XY. REV-ERBalpha agonist SR9009 suppresses IL-1beta production in macrophages through BMAL1-dependent inhibition of inflammasome. Biochem Pharmacol. 2021;192:114701. Epub 20210726. [CrossRef] [PubMed]
- Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Med Chem. 2011;3(5):623-38. [CrossRef] [PubMed] [PubMed Central]
- Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103(48):18154-9. Epub 20061117. [CrossRef] [PubMed] [PubMed Central]
- Oishi Y, Spann NJ, Link VM, Muse ED, Strid T, Edillor C, Kolar MJ, Matsuzaka T, Hayakawa S, Tao J, Kaikkonen MU, Carlin AF, Lam MT, Manabe I, Shimano H, Saghatelian A, Glass CK. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell Metab. 2017;25(2):412-27. Epub 20161229. [CrossRef] [PubMed] [PubMed Central]
- Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T, Castegna A. The Metabolic Signature of Macrophage Responses. Front Immunol. 2019;10:1462. Epub 20190703. [CrossRef] [PubMed] [PubMed Central]
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176-85. Epub 20121129. [CrossRef] [PubMed]
- Li J, Guo T, Li Y, Wang Q, Du Y, Li R, Lin J, Fu J, Chen X, Luo S. Adipose stem cells regulate lipid metabolism by upregulating mitochondrial fatty acid beta-oxidation in macrophages to improve the retention rate of transplanted fat. Stem Cell Res Ther. 2024;15(1):328. Epub 20240927. [CrossRef] [PubMed] [PubMed Central]
- Yang B, Hang S, Xu S, Gao Y, Yu W, Zang G, Zhang L, Wang Z. Macrophage polarisation and inflammatory mechanisms in atherosclerosis: Implications for prevention and treatment. Heliyon. 2024;10(11):e32073. Epub 20240529. [CrossRef] [PubMed] [PubMed Central]
- Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440(1):105-11. Epub 20130917. [CrossRef] [PubMed]
- Cameron AM, Lawless SJ, Pearce EJ. Metabolism and acetylation in innate immune cell function and fate. Semin Immunol. 2016;28(5):408-16. Epub 20161027. [CrossRef] [PubMed] [PubMed Central]
- Liao N, Pan F, Wang Y, Zheng Y, Xu B, Chen W, Gao Y, Cai Z, Liu X, Liu J. Adipose tissue-derived stem cells promote the reversion of non-alcoholic fatty liver disease: An in vivo study. Int J Mol Med. 2016;37(5):1389-96. Epub 20160315. [CrossRef] [PubMed]
- Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF-kappaB signaling in inflammation and cancer. MedComm (2020). 2021;2(4):618-53. Epub 20211216. [CrossRef] [PubMed] [PubMed Central]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. Epub 20091007. [CrossRef] [PubMed] [PubMed Central]
- Marechal L, Laviolette M, Rodrigue-Way A, Sow B, Brochu M, Caron V, Tremblay A. The CD36-PPARgamma Pathway in Metabolic Disorders. Int J Mol Sci. 2018;19(5). Epub 20180521. [CrossRef] [PubMed] [PubMed Central]
- Zuo S, Wang Y, Bao H, Zhang Z, Yang N, Jia M, Zhang Q, Jian A, Ji R, Zhang L, Lu Y, Huang Y, Shen P. Lipid synthesis, triggered by PPARgamma T166 dephosphorylation, sustains reparative function of macrophages during tissue repair. Nat Commun. 2024;15(1):7269. Epub 20240823. [CrossRef] [PubMed] [PubMed Central]
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450-62. [CrossRef] [PubMed] [PubMed Central]
- Geeraerts X, Bolli E, Fendt SM, Van Ginderachter JA. Macrophage Metabolism As Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front Immunol. 2017;8:289. Epub 20170315. [CrossRef] [PubMed] [PubMed Central]
- Brown AJ, Mander EL, Gelissen IC, Kritharides L, Dean RT, Jessup W. Cholesterol and oxysterol metabolism and subcellular distribution in macrophage foam cells. Accumulation of oxidized esters in lysosomes. J Lipid Res. 2000;41(2):226-37. [PubMed]
- Feingold KR. Introduction to Lipids and Lipoproteins. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext. South Dartmouth (MA)2000.
- Tabas I, Boykow GC, Tall AR. Foam cell-forming J774 macrophages have markedly elevated acyl coenzyme A:cholesterol acyl transferase activity compared with mouse peritoneal macrophages in the presence of low density lipoprotein (LDL) despite similar LDL receptor activity. J Clin Investig. 1987;79(2):418-26. [CrossRef] [PubMed] [PubMed Central]
- Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARgamma in the control of lipid metabolism. J Lipid Res. 2002;43(2):177-86. Epub 2002/02/28. [PubMed]
- Krejcova G, Danielova A, Nedbalova P, Kazek M, Strych L, Chawla G, Tennessen JM, Lieskovska J, Jindra M, Dolezal T, Bajgar A. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. Elife. 2019;8. Epub 20191014. [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605-14. Epub 20100524. [CrossRef] [PubMed]
- Blagih J, Jones RG. Polarizing macrophages through reprogramming of glucose metabolism. Cell Metab. 2012;15(6):793-5. [CrossRef] [PubMed]
- O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553-65. Epub 20160711. [CrossRef] [PubMed] [PubMed Central]
- Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Dabritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O'Neill LA. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167(2):457-70 e13. Epub 20160922. [CrossRef] [PubMed] [PubMed Central]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O'Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496(7444):238-42. Epub 20130324. [CrossRef] [PubMed] [PubMed Central]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, Amir S, Lubec G, Park J, Esterbauer H, Bilban M, Brizuela L, Pospisilik JA, Otterbein LE, Wagner O. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813-26. [CrossRef] [PubMed] [PubMed Central]
- Watanabe R, Hilhorst M, Zhang H, Zeisbrich M, Berry GJ, Wallis BB, Harrison DG, Giacomini JC, Goronzy JJ, Weyand CM. Glucose metabolism controls disease-specific signatures of macrophage effector functions. JCI Insight. 2018;3(20). Epub 20181018. [CrossRef] [PubMed] [PubMed Central]
- Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016;45(4):817-30. [CrossRef] [PubMed] [PubMed Central]
- Rosa Neto JC, Calder PC, Curi R, Newsholme P, Sethi JK, Silveira LS. The Immunometabolic Roles of Various Fatty Acids in Macrophages and Lymphocytes. Int J Mol Sci. 2021;22(16). Epub 20210806. [CrossRef] [PubMed] [PubMed Central]
- Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect Immun. 1996;64(1):108-12. [CrossRef] [PubMed] [PubMed Central]
- Van den Bossche J, Baardman J, de Winther MP. Metabolic Characterization of Polarized M1 and M2 Bone Marrow-derived Macrophages Using Real-time Extracellular Flux Analysis. J Vis Exp. 2015(105). Epub 20151128. [CrossRef] [PubMed] [PubMed Central]
- Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O'Neill LA. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21(1):65-80. [CrossRef] [PubMed] [PubMed Central]
- Suzuki H, Hisamatsu T, Chiba S, Mori K, Kitazume MT, Shimamura K, Nakamoto N, Matsuoka K, Ebinuma H, Naganuma M, Kanai T. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol Lett. 2016;176:18-27. Epub 20160518. [CrossRef] [PubMed]
- Chiba S, Hisamatsu T, Suzuki H, Mori K, Kitazume MT, Shimamura K, Mizuno S, Nakamoto N, Matsuoka K, Naganuma M, Kanai T. Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol Lett. 2017;183:17-23. Epub 20170124. [CrossRef] [PubMed]
- Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, Di Conza G, Cheng WC, Chou CH, Vavakova M, Muret C, Debackere K, Mazzone M, Huang HD, Fendt SM, Ivanisevic J, Ho PC. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18(9):985-94. Epub 20170717. [CrossRef] [PubMed]
- Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267-88. [CrossRef] [PubMed] [PubMed Central]
- Yu T, Gan S, Zhu Q, Dai D, Li N, Wang H, Chen X, Hou D, Wang Y, Pan Q, Xu J, Zhang X, Liu J, Pei S, Peng C, Wu P, Romano S, Mao C, Huang M, Zhu X, Shen K, Qin J, Xiao Y. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun. 2019;10(1):4353. Epub 20190925. [CrossRef] [PubMed] [PubMed Central]
- O'Neill, L.A. A broken krebs cycle in macrophages. Immunity. 2015;42(3):393-4. [CrossRef] [PubMed]
- Benmoussa K, Garaude J, Acin-Perez R. How Mitochondrial Metabolism Contributes to Macrophage Phenotype and Functions. J Mol Biol. 2018;430(21):3906-21. Epub 20180710. [CrossRef] [PubMed]
- Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, Muller A, Tigani B, Kneuer R, Patel S, Valeaux S, Gommermann N, Rubic-Schneider T, Junt T, Carballido JM. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213(9):1655-62. Epub 20160801. [CrossRef] [PubMed] [PubMed Central]
- Kelly B, Pearce EL. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020;32(2):154-75. Epub 20200709. [CrossRef] [PubMed]
- Yurdagul, A., Jr. Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, Kolluru GK, Rymond CC, Gerlach BD, Zheng Z, Kuriakose G, Kevil CG, Koomen JM, Cleveland JL, Muoio DM, Tabas I. Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell Metab. 2020;31(3):518-33 e10. Epub 20200130. [CrossRef] [PubMed] [PubMed Central]
- Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171(3):1232-9. [CrossRef] [PubMed]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342):1072-6. Epub 20170511. [CrossRef] [PubMed] [PubMed Central]
- Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, Steeves MA, Cleveland JL, Schneider C, Piazuelo MB, Gobert AP, Wilson KT. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A. 2017;114(5):E751-E60. Epub 20170117. [CrossRef] [PubMed] [PubMed Central]
- Palmieri EM, Menga A, Martin-Perez R, Quinto A, Riera-Domingo C, De Tullio G, Hooper DC, Lamers WH, Ghesquiere B, McVicar DW, Guarini A, Mazzone M, Castegna A. Pharmacologic or Genetic Targeting of Glutamine Synthetase Skews Macrophages toward an M1-like Phenotype and Inhibits Tumor Metastasis. Cell Rep. 2017;20(7):1654-66. [CrossRef] [PubMed] [PubMed Central]
- He L, Weber KJ, Schilling JD. Glutamine Modulates Macrophage Lipotoxicity. Nutrients. 2016;8(4):215. Epub 20160412. [CrossRef] [PubMed] [PubMed Central]
- Yan H, Liu Y, Li X, Yu B, He J, Mao X, Yu J, Huang Z, Luo Y, Luo J, Wu A, Chen D. Leucine alleviates cytokine storm syndrome by regulating macrophage polarization via the mTORC1/LXRalpha signaling pathway. Elife. 2024;12. Epub 20240305. [CrossRef] [PubMed] [PubMed Central]
- Yu W, Wang Z, Zhang K, Chi Z, Xu T, Jiang D, Chen S, Li W, Yang X, Zhang X, Wu Y, Wang D. One-Carbon Metabolism Supports S-Adenosylmethionine and Histone Methylation to Drive Inflammatory Macrophages. Mol Cell. 2019;75(6):1147-60 e5. Epub 20190813. [CrossRef] [PubMed]
- Rodriguez AE, Ducker GS, Billingham LK, Martinez CA, Mainolfi N, Suri V, Friedman A, Manfredi MG, Weinberg SE, Rabinowitz JD, Chandel NS. Serine Metabolism Supports Macrophage IL-1beta Production. Cell Metab. 2019;29(4):1003-11 e4. Epub 20190214. [CrossRef] [PubMed] [PubMed Central]
- Juban G, Chazaud B. Metabolic regulation of macrophages during tissue repair: insights from skeletal muscle regeneration. FEBS Lett. 2017;591(19):3007-21. Epub 20170617. [CrossRef] [PubMed]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678-89. [CrossRef] [PubMed]
- Pan X, Jiang XC, Hussain MM. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation. 2013;128(16):1758-69. Epub 2013/09/10. [CrossRef] [PubMed] [PubMed Central]
- Yang G, Zhang J, Jiang T, Monslow J, Tang SY, Todd L, Pure E, Chen L, FitzGerald GA. Bmal1 Deletion in Myeloid Cells Attenuates Atherosclerotic Lesion Development and Restrains Abdominal Aortic Aneurysm Formation in Hyperlipidemic Mice. Arterioscler Thromb Vasc Biol. 2020;40(6):1523-32. Epub 2020/04/24. [CrossRef] [PubMed] [PubMed Central]
- Blacher E, Tsai C, Litichevskiy L, Shipony Z, Iweka CA, Schneider KM, Chuluun B, Heller HC, Menon V, Thaiss CA, Andreasson KI. Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol. 2022;23(2):229-36. Epub 20211223. [CrossRef] [PubMed] [PubMed Central]
- Schober A, Blay RM, Saboor Maleki S, Zahedi F, Winklmaier AE, Kakar MY, Baatsch IM, Zhu M, Geissler C, Fusco AE, Eberlein A, Li N, Megens RTA, Banafsche R, Kumbrink J, Weber C, Nazari-Jahantigh M. MicroRNA-21 Controls Circadian Regulation of Apoptosis in Atherosclerotic Lesions. Circulation. 2021;144(13):1059-73. Epub 20210708. [CrossRef] [PubMed]
- Gachon F, Loizides-Mangold U, Petrenko V, Dibner C. Glucose Homeostasis: Regulation by Peripheral Circadian Clocks in Rodents and Humans. Endocrinology. 2017;158(5):1074-84. [CrossRef] [PubMed]
- Krieger, D.T. Rhythms of ACTH and corticosteroid secretion in health and disease, and their experimental modification. J Steroid Biochem. 1975;6(5):785-91. [CrossRef] [PubMed]
- Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J. Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017;25(1):86-92. Epub 20161020. [CrossRef] [PubMed] [PubMed Central]
- Zlacka J, Zeman M. Glycolysis under Circadian Control. Int J Mol Sci. 2021;22(24). Epub 20211220. [CrossRef] [PubMed] [PubMed Central]
- Petrenko V, Philippe J, Dibner C. Time zones of pancreatic islet metabolism. Diabetes Obes Metab. 2018;20 Suppl 2:116-26. [CrossRef] [PubMed]
- Amiama-Roig A, Verdugo-Sivianes EM, Carnero A, Blanco JR. Chronotherapy: Circadian Rhythms and Their Influence in Cancer Therapy. Cancers (Basel). 2022;14(20). Epub 20221017. [CrossRef] [PubMed] [PubMed Central]
- Tian Y, Luan X, Yang K. Chronotherapy involving rosiglitazone regulates the phenotypic switch of vascular smooth muscle cells by shifting the phase of TNF-alpha rhythm through triglyceride accumulation in macrophages. Heliyon. 2024;10(10):e30708. Epub 20240516. [CrossRef] [PubMed] [PubMed Central]
- Arola-Arnal A, Cruz-Carrion A, Torres-Fuentes C, Avila-Roman J, Aragones G, Mulero M, Bravo FI, Muguerza B, Arola L, Suarez M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients. 2019;11(11). Epub 20191030. [CrossRef] [PubMed] [PubMed Central]
- Katsi V, Papakonstantinou IP, Soulaidopoulos S, Katsiki N, Tsioufis K. Chrononutrition in Cardiometabolic Health. J Clin Med. 2022;11(2). Epub 20220107. [CrossRef] [PubMed] [PubMed Central]
- Caprara G, Allavena P, Erreni M. Intestinal Macrophages at the Crossroad between Diet, Inflammation, and Cancer. Int J Mol Sci. 2020;21(14). Epub 20200708. [CrossRef] [PubMed] [PubMed Central]
- Zhang Y, Fang B, Emmett MJ, Damle M, Sun Z, Feng D, Armour SM, Remsberg JR, Jager J, Soccio RE, Steger DJ, Lazar MA. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbalpha couple metabolism to the clock. Science. 2015;348(6242):1488-92. Epub 20150604. [CrossRef] [PubMed] [PubMed Central]
- Potter GD, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr Rev. 2016;37(6):584-608. Epub 20161020. [CrossRef] [PubMed] [PubMed Central]
- Eligini S, Gianazza E, Mallia A, Ghilardi S, Banfi C. Macrophage Phenotyping in Atherosclerosis by Proteomics. Int J Mol Sci. 2023;24(3). Epub 20230130. [CrossRef] [PubMed] [PubMed Central]
- Chai JT, Ruparelia N, Goel A, Kyriakou T, Biasiolli L, Edgar L, Handa A, Farrall M, Watkins H, Choudhury RP. Differential Gene Expression in Macrophages From Human Atherosclerotic Plaques Shows Convergence on Pathways Implicated by Genome-Wide Association Study Risk Variants. Arterioscler Thromb Vasc Biol. 2018;38(11):2718-30. [CrossRef] [PubMed] [PubMed Central]
- Sun YM, Chen YQ. Principles and innovative technologies for decrypting noncoding RNAs: from discovery and functional prediction to clinical application. J Hematol Oncol. 2020;13(1):109. Epub 20200810. [CrossRef] [PubMed] [PubMed Central]
- Henriques F, Bedard AH, Guilherme A, Kelly M, Chi J, Zhang P, Lifshitz LM, Bellve K, Rowland LA, Yenilmez B, Kumar S, Wang Y, Luban J, Weinstein LS, Lin JD, Cohen P, Czech MP. Single-Cell RNA Profiling Reveals Adipocyte to Macrophage Signaling Sufficient to Enhance Thermogenesis. Cell Rep. 2020;32(5):107998. [CrossRef] [PubMed] [PubMed Central]
- Chen HJ, Sevin DC, Griffith GR, Vappiani J, Booty LM, van Roomen C, Kuiper J, Dunnen JD, de Jonge WJ, Prinjha RK, Mander PK, Grandi P, Wyspianska BS, de Winther MPJ. Integrated metabolic-transcriptomic network identifies immunometabolic modulations in human macrophages. Cell Rep. 2024;43(9):114741. Epub 20240913. [CrossRef] [PubMed]
- Banfi C, Baetta R, Gianazza E, Tremoli E. Technological advances and proteomic applications in drug discovery and target deconvolution: identification of the pleiotropic effects of statins. Drug Discov Today. 2017;22(6):848-69. Epub 20170308. [CrossRef] [PubMed]
- Ouedraogo R, Textoris J, Daumas A, Capo C, Mege JL. Whole-cell MALDI-TOF mass spectrometry: a tool for immune cell analysis and characterization. Methods Mol Biol. 2013;1061:197-209. [CrossRef] [PubMed]
- Ouedraogo R, Daumas A, Capo C, Mege JL, Textoris J. Whole-cell MALDI-TOF mass spectrometry is an accurate and rapid method to analyze different modes of macrophage activation. J Vis Exp. 2013(82):50926. Epub 20131226. [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).