1. Introduction
The enzyme, choline acetyltransferase (ChAT) (EC 2.3.1.6) is the main acetylcholine (ACh) biosynthesizing enzyme. It defines the neuronal and non-neuronal cholinergic cells, i.e., cells that synthesize ACh and use it as an auto- and/or paracrine signaling molecule. ChAT is therefore widely distributed across various tissues, including non-neuronal systems in diverse cell types, such as immune cells, astroglia cells and sperm, though its canonical functional significance lies within the central nervous system (CNS) [
1,
2,
3]. In the CNS, ChAT activity is crucial for maintaining cholinergic neurotransmission, influencing processes like memory consolidation, attention and other cognitive functions. Acetylcholinesterase (AChE), is similarly expressed in a wide range of tissues, including neurons, red blood cells, and lymphocytes [
3]. These enzymes are crucial for cholinergic neurotransmission, with AChE breaking down acetylcholine in synaptic clefts and ChAT catalyzing the synthesis of acetylcholine [
4]. Proper functioning of these enzymes is essential for maintaining cholinergic balance, and their dysregulation is linked to neurodegenerative diseases, including the major dementia disorders, like Alzheimer's disease (AD), in which the major therapeutic options are still cholinesterase inhibitors (ChEIs). These drugs are used to counter the cholinergic deficit in AD by preventing degradation of ACh by AchE [
5]. Nonetheless, ChEIs show limited clinical efficacy perhaps because the ACh biosynthesis is not optimal in the cholinergic neurons which calls for a new strategy, where the catalytic function of ChAT is boosted to counteract the observed reduction in the neuronal expression of ChAT [
6,
7]. Intriguingly, beta-amyloid peptide 42 (Aβ
42) is the first documented ChAT activity booster, which are termed as ChAT-Potentiating-Ligands (CPLs) [
6,
7]. In addition, ChAT has been targeted for development of specific cholinergic biomarker for instance for development of positron emission tomography (PET) imaging probe to map early cholinergic changes in various cholinergic-related neurodegenerative diseases, such as neuromotor disorders like amyotrophic sclerosis (ALS) and dementias like AD [
8,
9]. All of these require early identification to optimize the chance of finding a viable therapeutic strategy [
8,
9]. As PET imaging probes, ChAT ligands have significant potential for the early identification of Alzheimer's disease (AD) and related dementias, including Down syndrome and Lewy body diseases [
8,
10].
The distribution of ChAT has been measured within the neuron and it appears to be 80-90% as cytosolic protein a portion of which can be associated to ionically bond to the membrane and 10-20% of the enzyme appears to be non-ionically bound to the plasma membrane [
2]. A recent report indicates that ChAT may also be in the sperm membrane extracellularly [
1]. In our work with ChAT, we have encountered with some unexplained in vitro changes in the activity of recombinant human ChAT protein in various buffer systems containing different detergent or different concentrations of the included detergents like Triton X-100 (TX100) and Tween 20 (TW20).
Detergents are indispensable component of many buffers system used in biochemical and biomedical research, due to their remarkable ability to solubilize membrane proteins while preserving their activity in solution [
11,
12,
13]. The use of detergents spans a wide range of applications, from facilitating the extraction of membrane-bound proteins, like certain enzymes or receptors, to maintaining the structural stability and functions of the proteins for various purposes [
12,
14,
15]. Two of the most common detergent in life science research are the non-ionic detergents like TX100 and TW20 [
16,
17,
18,
19]. This is largely due to their mild nature, which minimizes denaturation and preserves the native structure and function of proteins. Nonetheless, some studies indicates that TX100 can alter the function of certain enzymes [
18,
20,
21,
22]. Some studies indicate that the functional alteration of the enzymes may be related to micellar properties of the detergent [
18,
20,
21,
23]. Others suggest the changes in the activity may be caused by an increase in the solubility of the enzymes[
24,
25].
Triton X-100 has on average a molecular weight of ~625 g/mol and a critical micelle concentration (CMC) of 0.2-0.3 mM with an aggregation number typically ranging between 75-165 molecules. Commonly, TX100 is added to buffers at 0.05% (v/v) concentration, while its concentrations range from 0.1% to 0.6% in buffers for solubilizing membrane proteins while preserving structure. Tween 20 has a higher molecular weight of ~1228 g/mol than TX100 but its CMC (0.06-0.07 mM) is ~4 times lower than the CMC of TX100, with an aggregation number of 60 [
19,
26].
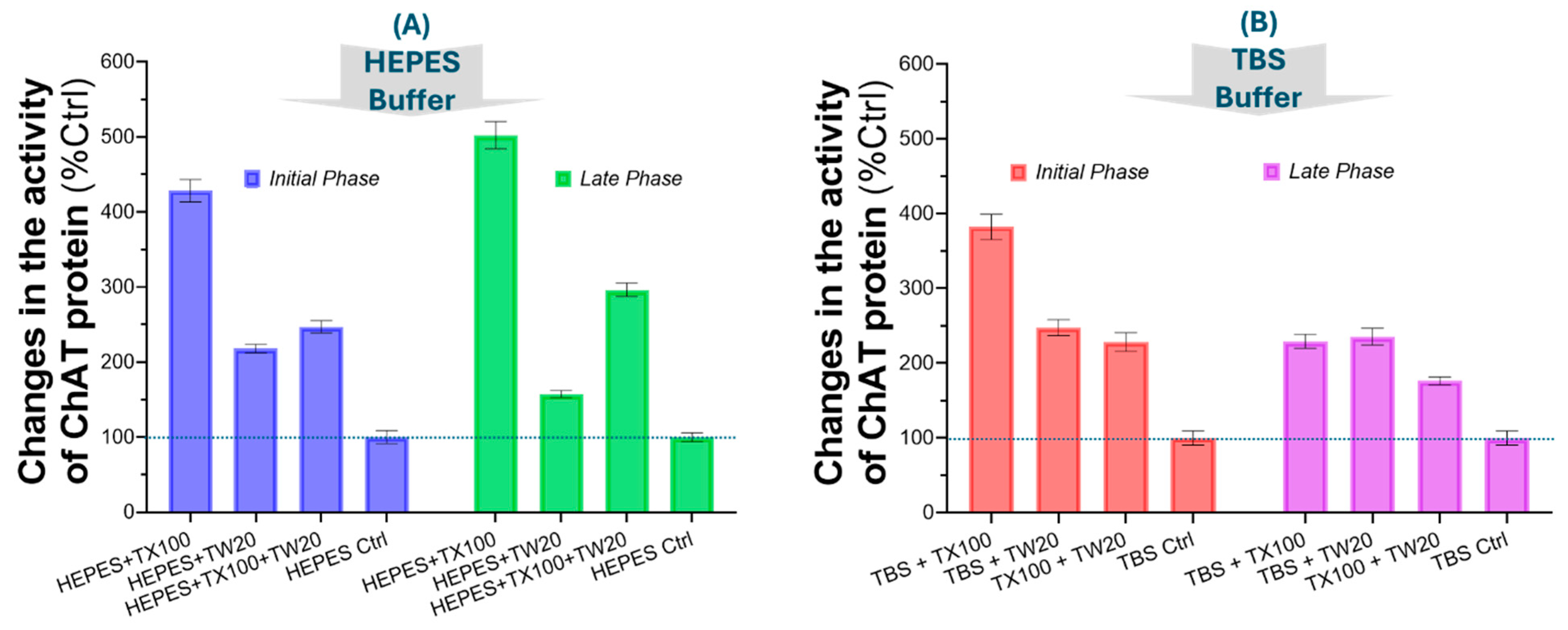
In this study we investigated the in vitro changes in ChAT activity observed in the most common buffers with and without TX100 and TW20. We show here that increases in ChAT catalytic efficiency occurs mainly at post CMC concentration of the detergent regardless of the buffer system. We also show the boosted ChAT catalytic function is unlikely to be related to an improved solubility of ChAT protein stability of its tertiary structure. Rather, the results suggest that a membrane-like microenvironment formation accounts for the altered ChAT activity because ChAT becomes catalytic ultra-fast depending on the post CMC concentrations of TX100 or TW20. This report delivers crucial insights on the function of an enzyme which is implicated in neurodegenerative diseases, as well as opens new window into a cholinergic enhancing strategy delivery of ultrafast-ChAT via functionally enhance nanoparticles [
27].
3. Discussion
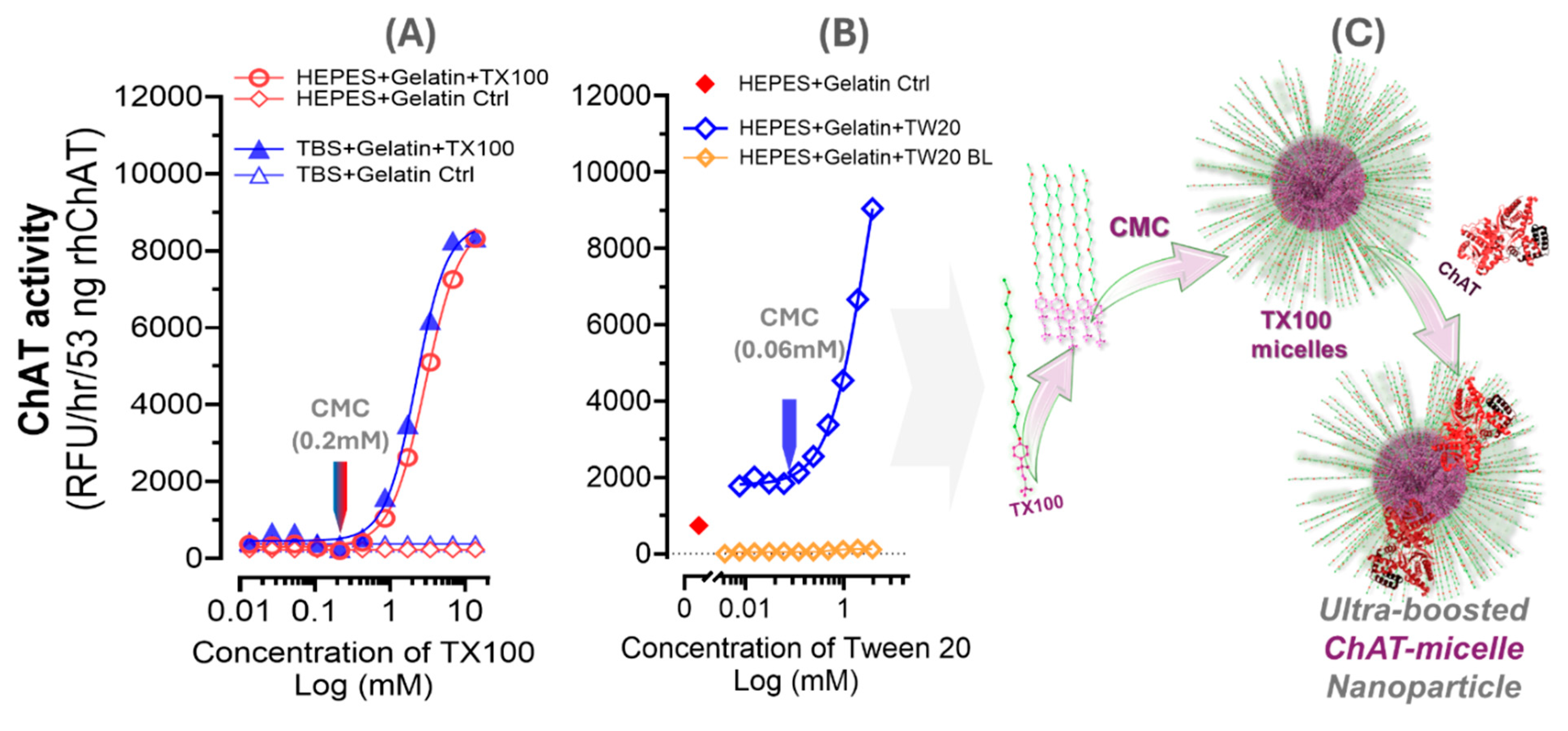
We show for the first time that ChAT becomes hyper-boosted in the presence of non-ionic detergents like TX100 and Tween-20. The boost in the catalytic rate ChAT by TX100 reached a plateau at ~10 folds over the basal levels in buffer supplemented with 1mg/mL gelatin as a protein structure stabilizer. We further showed that the observed boost was related to the critical micelle concentration (CMC), since TW20 boosted, like TX100, the catalytic rate of ChAT and in both cases, it happened when their concentrations in the buffers passed their specific CMC, highlighting the enzyme’s specific sensitivity to micelle-induced environmental changes.
To explain these observations, we hypothesized the boosting effect of TX100 and TW20 on the catalytic rate of ChAT is mediated through formation of membrane-like micellar structures which occurs as soon as the concentration of the surfactants possess their CMCs. Reports have shown that TX-100 micelles follow a multilayer organization leaving large part of hydrophilic tail in the outer region of the micelle, with a strong interaction with surrounding water molecules [
19,
28,
29]. Our results thereby indicate that ChAT become embedded into these micellar structures. These provide a microenvironment, resembling the native cellular environment, in which ChAT naturally possess a high specific activity. This is in line with several reports, indicating that ChAT exist as both soluble cytoplasmic variant and as a membrane-bound form. The difference between membrane-bound and membrane-free ChAT forms has been suggested to arise from post-translational modifications [
30,
31]. For instance, three putative membrane-associated forms of ChAT have been identified across animal models, indicating translocation to the plasma membrane, unlike the cytosolic form. ChAT seems also to exhibit certain peculiarity in a membrane-like environment, for instance being less subjected to inhibition by inhibitors of the enzyme[
32,
33]. It is also shown in several animal species that ChAT protein is compartmentalized within synaptosomes, particularly at presynaptic terminal [
33,
34]. Thereby ChAT protein seems to have affinity to membrane-like micro-environment, like presynaptic membranes, myelin fragments, and synaptosomes where it exhibits the highest specific activity[
34].
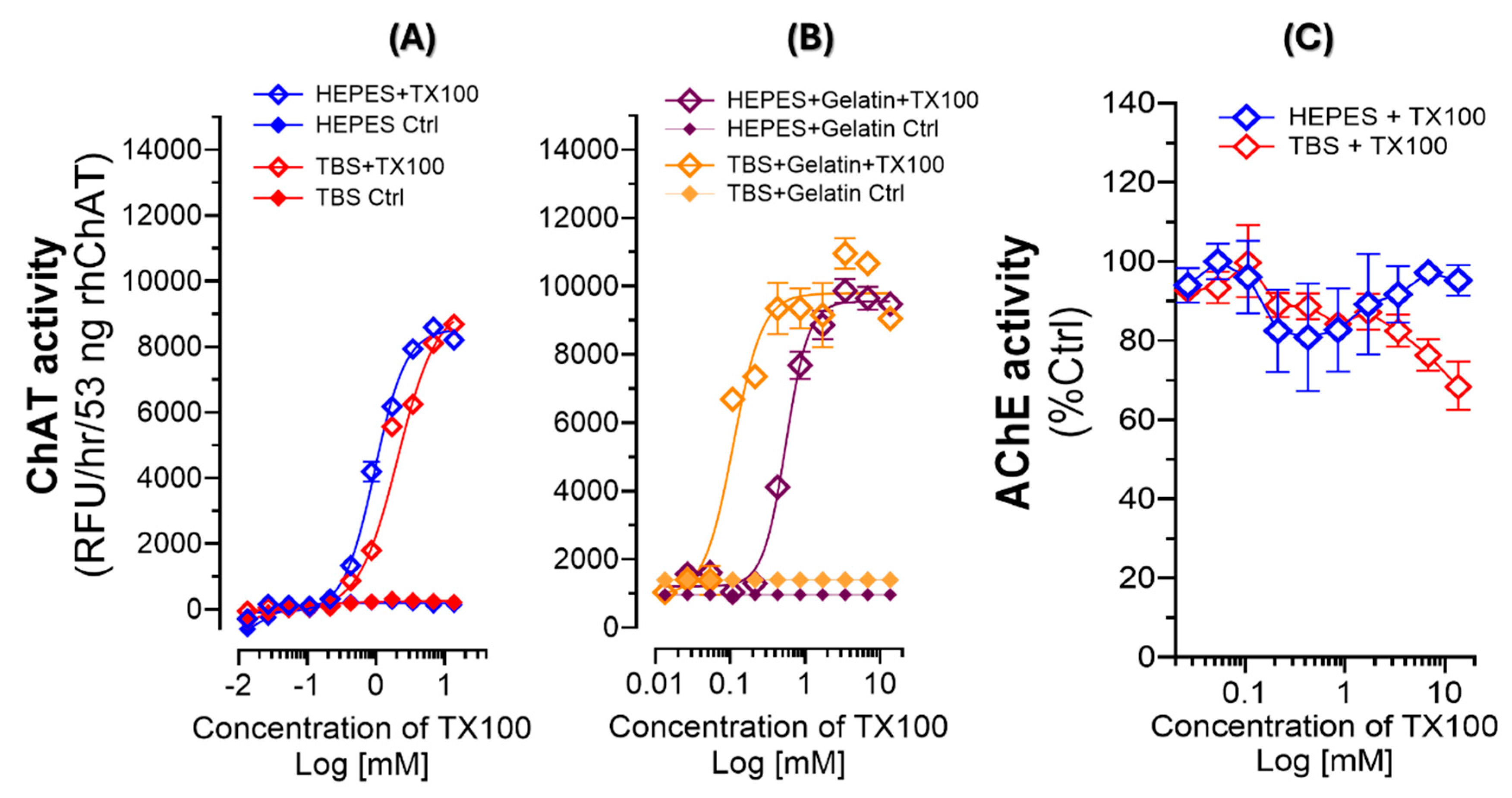
We also performed an identical experiment on AChE, another enzyme related to acetylcholine metabolism. We found no changes in the catalytic rate of AChE in the presence of various concentrations of TX100, including CMC, reinforcing the idea that the boosting effect was exclusive to ChAT, when compared to AChE. Nonetheless, there are reports about other enzymes that their activity are affected by TX100[
16,
17,
18,
20,
21,
23,
35]. For instance, phospholipase-D, another CNS-related membrane-bound enzymes, shows enhanced activity by TX100 which reaches a maximum level at TX100 concentrations of 0.1–0.2% (w/v) [
36]. Noteworthy, the CMC of TX100 in percentage term is 0.0125% (or 0.21mM).
Other enzymes, on the other hand, can be inhibited by TX-100. For instance, Cytochrome c oxidase, an enzyme involved in the mitochondria respiratory chain of the Krebs cycle, is inhibited by TX100, with an inhibition constant (Ki) of 0.3mM. Nonetheless, in this case the effect seems to be mediated by suppression of intraprotein electron transfer by a blocking interaction of TX100 at the catalytic mouth of the enzyme [
37].
ChAT can gain an enhanced activity in other ways as well. For instance, reports show that amyloid-β (Aβ) peptides, in particular Aβ
42, boosts the activity of human ChAT by ~25% through a direct interaction with ChAT protein[
6,
7]. This has resulted in the generation of a new class of small molecules, coined as ChAT-Potentiating-Ligands (CPLs) [
6,
7]. The activity of ChAT may also be boosted by phosphorylation at specific residues, such as serine 440 and threonine 456 [
31]. The phosphorylation of ChAT seems in addition to alter its binding to plasma membrane and interaction with other cellular proteins[
31]. Noteworthy, all the aforenoted mode of activation of ChAT is modest in their magnitude compared to the 10-fold ultra-boosting of ChAT protein by both TX100 and TW20.
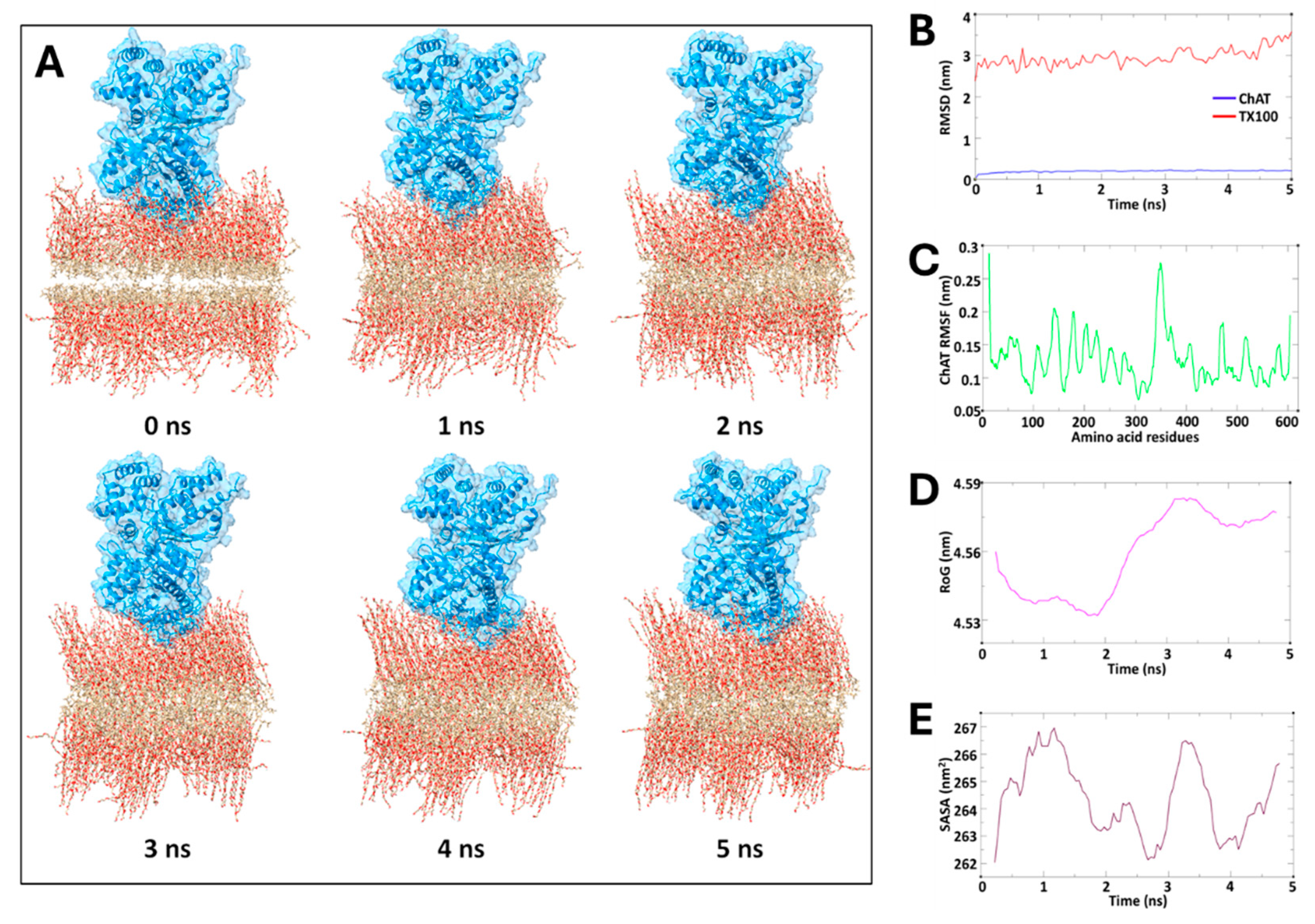
To test our hypothesis, we also performed an in-silico molecular dynamic simulation, with the assumption that TX100 can form a membrane-like bilayer construct, which previous reports have indicated[
28]. The simulated spatial configuration together with the quantified MD metrics supported our hypothesis that micelle formation provide a favorable microstructure that mimics the cellular membrane conditions, potentially enhancing compartmentation of ChAT. Thereby, formation of facilitated ChAT-micelle nanoparticles through encapsulation of ChAT by TX100 or TW20 is the most plausible explanation for the results of the current report. This might represent a form of micellar polymer encapsulation of enzymes [
29].
The micelle-embedded hyper-boosted ChAT nanoparticles may have therapeutic properties. Reports indicate that acetylcholine, in addition to its canonical function as a neurotransmitter, possess a strong anti-inflammatory function, which can effectively regulate immune responses through activation of α7 nicotinic receptor[
38,
39]. Report has also shown that ChAT exist as soluble form in both human plasma and cerebrospinal fluids[
3]. Evidence indicates that the function of extracellular ChAT is to keep an extracellular acetylcholine equilibrium for regulation of the function of immune cells in circulation and astroglial cells in the brain[
3]. It is also reported that the extracellular acetylcholine equilibrium in the brain of patients with Alzheimer´s disease is dysfunctional due to an interaction between Aβ peptides and cholinesterase, which leads to formation of complexes, called BAβACs, in which the cholinesterases are hyperactive [
3,
40]. This result in a shift in the acetylcholine equilibrium resulting in overactivation of astroglial cells and neuroinflammation [
3,
40]. It has been shown that cholinesterase inhibitors can partially restore the balance which also correlate with the improved cognition in the treated patients[
41,
42]. We hypothesize that the micelle-embedded hyper-boosted ChAT nanoparticles should be able to restore or even elevate the extracellular acetylcholine equilibrium in favor of an anti-inflammatory milieu, through an effective suppression of astrogliosis.
This study has several limitations. Firstly, we investigate the effect of only the two most common laboratory detergents. Future investigation with other non-ionic surfactants like Brij, as well as various ionic and zwitterionic may provide insights for deducing the mechanism for the boosting of ChAT catalytic function. Similarly, new studies are warranted to expand the scope of this study to include other natural fatty acids and lipid bilayer constructs as alternatives. Overall, studies are warranted to elucidate whether the observed effects are unique to non-ionic detergents or extend to broader micelle-forming agents. While the in-silico molecular dynamics (MD) simulation supported our hypothesis from the in vitro findings, they should be considered preliminary. Thereby deeper and longer MD simulation than 5 ns is required for investigating the interactions between detergents constructs and ChAT. Additionally, the focus on recombinant human ChAT leaves open the question of whether similar effects would be observed with ChAT from rodent models or from cellular extracts, which may differ in their structural properties and responses. Lastly, while gelatin served effectively as a stabilizer in our experimental setup, it remains unclear whether the result would stand if a naturally occurring carrier protein, like serum albumin, had been used instead of gelatine. Noteworthy, we could not use bovine serum albumin (BSA) since it is incompatible with our ChAT assay detection systems[
6].
In summary, our findings reveal a unique micelle-induced hyperactivation of ChAT, distinct from the behavior of other enzymes like AChE, with potentially far-reaching implications for enzyme-based therapeutics and drug delivery strategies. Future studies should aim to clarify the exact mechanisms by which micelle environments modulate this key cholinergic enzyme and explore the potential of micelle-forming compounds as both therapeutic enhancers and delivery agents.
4. Materials and Methods
4.1. Chemicals
The following material was used HEPES (Cat #H700), Trizma base (Cat #T1503), EDTA (Cat #324503), NaCl (Cat # S9888), gelatin bovine skin (Cat #G9391), Triton X-100 or 1,1,3,3-Tetramethylbutyl)phenyl-polyethylene glycol [Cat #93443, aggregation number 100-155, average micellar molecular weight (Mw) of 80000, or an average Mw of 625], Tween 20 or Polyethylene glycol sorbitan monolaurate [Cat #P1379; Mw ~1228, and CMC of 0.06mM], Choline chloride (Cat #C7527), Acetyl-CoA (Cat #A2056), acetylthiocholine iodide (ATC; Cat #A5751) and DTNB [or 5,5′-dithiobis (2-nitrobenzoic acid); Cat #322123], were all purchased from Merck (St. Louis, MO, USA).
4.2. Production and Purification of Recombinant Human ChAT Protein
Recombinant human ChAT (rhChAT) protein was produced and purified by the Protein Science Facility (PSF) at Karolinska Institute/SciLifeLab (
http://ki.se/psf), as described before [
43]. Briefly, the purity of protein was determined using sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) stained with Coomassie blue dye. The total protein concentration was measured using DC protein Assay (BioRad). The storage buffer for the protein was 20 mM HEPES buffer, pH 7.5, containing 300 mM NaCl, 0.5 mM TCEP. The protein was diluted in the storage buffer to a concentration of 212 μg/mL. The diluted enzyme solution was then aliquoted (10μL/tube), frozen on dry ice, and stored at -20°C.
4.3. Serial Solutions of TX100 and TW20 in the HEPES and TBS Buffer Systems
HEPES buffer (20mM; pH 7.4, containing 1.0 mM EDTA and 150 mM NaCl). TBS buffer (10 mM; pH 7.4, containing 1.0 mM EDTA and 150 mM NaCl). HEPES- or TBS-Gelatin buffers was prepared by adding 1.0 mg/mL (w/v) of gelatin to these buffers.
A set of TX100 solutions were prepared with 2-fold serial dilution from a 3.2% solution in the HEPES or TBS buffer, ranging from 3.2% to 0.0031%. These solutions were used to achieve four-fold lower final concentrations in the wells that ranged from 0.8% to 0.0008%. These TX100 solution series were prepared, using an average molecular weight of 625g/mol, so that a final molar concentration range of 13.7mM to 0.0134 mM were reached in the wells (final volume of 80μL).
Similarly, a set of TW20 solutions were prepared with 2-fold serial dilution from a 1.6% (v/v) solution in the HEPES or TBS buffers, ranging from 1.6% to 0.0016%. These solutions were used to achieve four-fold lower final concentrations in the wells that ranged from 0.4% to 0.0004%. Using an average molecular weight of 1227g/mol, these account for a final molar concentration of TW20 in the wells, that ranged from 3.84 mM to 0.0038 mM in the wells (final volume of 80μL).
Noteworthy, upon addition of 20μL of the TX100 or TW20 solutions series into the wells of the microtiter plate, the plate was placed in the plate reader and subjected to 30s orbital shaking at intensity level 5. Following the dispensing of all components, the plate was placed on a shaker and subjected to a second round of orbital shaking for 15 minutes at 600 rpm to ensure thorough mixing of the added Triton-X100 or Tween 20 solutions with the choline and the enzyme solutions added at previous steps. Finally, the plate was placed in the plate reader and 20μL of cocktail-A was added to each well, the changes in fluorescence intensity was monitored as noted before.
The controls for HEPES- or TBS-detergent solutions were simply the buffers lacing the detergents (i.e., TSB or HEPES buffers). The enzyme activity in the controls wells was used as reference values to compute percentage changes in the ChAT activity by TX100 or TW20. Blanks were wells that contained all components but the enzyme protein (the buffer was used instead). In the single-detergent concentration assessments, the samples were applied in fourteen replicates, while in the dose-response assessments, the samples were applied in at least at quadruplicate. The controls were in all cases over 20 replicates. The final concentration of rhChAT protein was 53ng/mL, which equals to 4.24ng of rhChAT protein per well.
4.4. In Vitro Fluorometric ChAT Activity Assay
In all the analyses, an in-house developed continuous high throughput fluorometric method was used together with the rhChAT) protein[
43]. The assay was conducted in 384-well Greiner flat black plates (Greiner Item-No. 781209). The protocol involved the sequential addition of 20μL/well of 600μM choline chloride solution (final concentration, Cf, 150 µM), 20 μL/well of the detergent solutions (TX100 and/or TW20) at certain concentrations, and then 20 μL/well of a 0.212µg/ml of a rhChAT protein solution (Cf = 0.053μg/mL). The plate was then incubated at room temperature on an orbital shaker at 100rpm for 30 minutes.
Finally, 20µL/well of a cocktail-A [dilution buffer containing 53 μM Acetyl-CoA (Cf, 13.3 µM) and 60 μM CPM (Cf, 15 µM)] was added to each well. The changes in fluorescence intensity was monitored for 30 minutes at 2-3 minutes intervals, using a microplate spectrophotometer reader (Infinite M1000, Tecan). The excitation and emission wavelengths were 390 nm and 479 nm, respectively.
4.5. In Vitro Colorimetric AChE Activity Assay
An in-house high throughput assay for the enzymatic activity of AChE was designed using a modified version of Ellman's colorimetric assay, as described [
44]. Purified recombinant human AChE (rhAChE) protein (Sigma, Cat no. C1682) was used. Briefly, 20 μL/well of a 1:768 diluted solution purified rhAChE protein (Cf = 3.5 ng/mL) was added to the wells of a 384 well plate (a flatbottom transparent plate). Then 20 μL/wells of the serial solutions set of TX100 in HEPES or in TBS was added to the assigned wells (all in quadruplicates), followed by adding 20μL/well of a 1.6 mM freshly prepared solution of DTNB (Cf = 0,4 mM) to all wells. The plate was incubated at 600rpm on an orbital shaker at RT for ~15 min. On each 384-wells plate, several enzyme wells without TX100, but the buffer vehicle were also included to serve as reference enzyme control wells. Negative controls (or blanks) were wells without enzyme. Lastly, 20 μL of a 2.0 mM ATC (Cf = 0.5 mM) was added to each well, and the changes in absorbance were continuously monitored at 412 nm wavelength for 10 min with 1 min interval, using a microplate spectrophotometer reader (Infinite M1000, Tecan). The rate of the enzyme activity was determined from the linear part of the kinetic reaction curves as ΔOD/time. The total volume in all wells was 80μL. The percentage inhibition for each compound was calculated based on the enzyme control value as a reference (100% activity).
4.6. Molecular Dynamics (MD) Simulations Study on ChAT with TX100
Molecular dynamics (MD) simulations were conducted to investigate the stability and interaction dynamics between ChAT and a bilayered micelle membrane composed of Triton X-100 (TX100) molecules. Using the GROMACS 2020 software package, the CHARMM36M force field was employed to model atomic interactions within this protein-membrane system [
45]. The initial structure of the protein was obtained from the Protein Data Bank (PDB ID: 2FY3) and assessed for missing residues with simultaneous removal of redundant solvents, ions or molecules. To focus on the interaction behavior between the protein and the membrane, the protein was positioned just above the upper leaflet of the TX100 bilayered-micelle using CHARMM-GUI, an approach designed to assess both the stability of the system and the interactions occurring at the protein-membrane interface [
46].
The bilayer micelle was generated following CHARMM-GUI protocols, specifically tailored to heterogeneous bilayer systems, and the system size in the XY plane was determined based on the protein’s cross-sectional area along the Z-axis, ensuring complete coverage of the micelle around the protein. The TIP3P water model was used for solvation, providing an accurate model for water behavior in a biological environment. A 0.15 M NaCl concentration was introduced to neutralize the system using a Monte Carlo ion placement method, ensuring electrostatic stability. Periodic boundary conditions were applied to simulate an infinite system, and the complete protein-membrane complex was enclosed within a cubic simulation box.
To prepare the system for simulation, an initial energy minimization was carried out using the steepest descent algorithm for 500,000 steps, addressing steric clashes and unfavorable interactions. Hydrogen bonds were constrained using the LINCS algorithm to stabilize bond lengths during both the equilibration and production phases [
47]. Two equilibration steps followed the energy minimization viz. a 1-nanosecond NVT phase, conducted under the canonical ensemble, and a 1-nanosecond NPT phase, conducted under the isothermal-isobaric ensemble. Temperature control was maintained at 310.15 K using the velocity-rescaling method, while the pressure was regulated at 1 bar using the Parrinello-Rahman barostat to replicate physiological conditions.
The production MD run was subsequently performed for 5 nanoseconds under constant temperature and pressure, with the Nose-Hoover thermostat and Parrinello-Rahman barostat employed for temperature and pressure control. Non-bonded interactions were managed using the Verlet force-switch function, with cutoffs set at 1.0 nm and 1.2 nm for Lennard-Jones interactions, while the Particle Mesh Ewald (PME) method was applied to account for long-range electrostatic interactions. Further, analysis of the simulation trajectories included calculations of root-mean-square deviation (RMSD) and root-mean-square fluctuation (RMSF) to evaluate the stability and flexibility of the system. Additionally, structural insights were obtained by analyzing solvent-accessible surface area (SASA) and radius of gyration (RoG) using GROMACS modules gmx_sasa and gmx_gyrate, respectively. Visualization of trajectories was performed using VMD software and the clear graphical representations of simulation results were drawn using QtGrace tool [
48,
49].