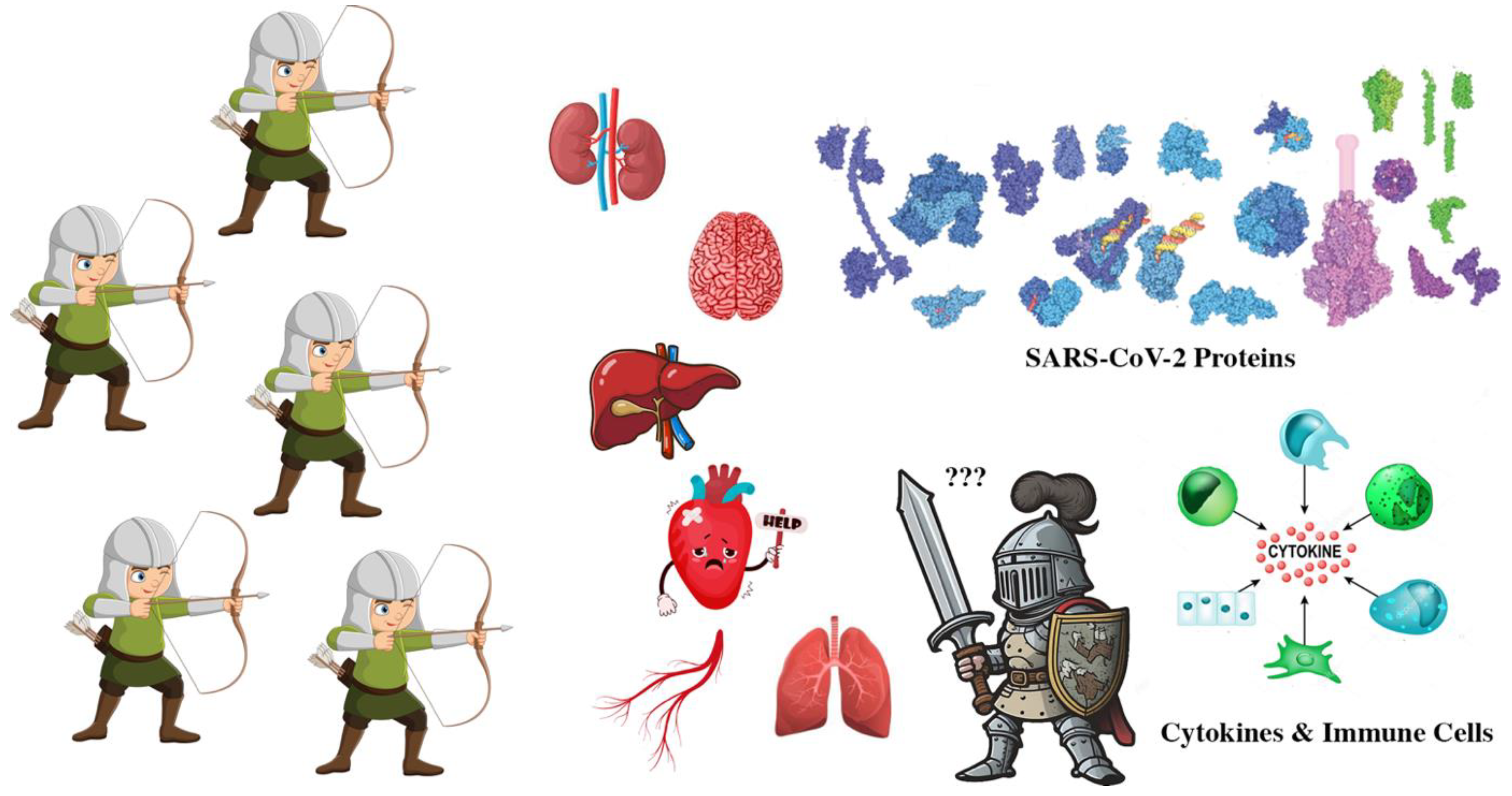
Introduction
Though there were almost 5,000 FDA-tracked trials, no FDA or EU approved COVID-19 drug was discovered during the pandemic! However, those discovered before the pandemic proved very effective even though monoclonal antibodies were disabled by variants.
COVID-19 attacks All major organs several different ways.
|
Direct Viral Damage |
Hyper-
Inflammation
|
Blood Clots |
Lack of Oxygen |
Other |
| Heart |
X |
X |
X |
X |
Stress |
| Lungs |
X |
X |
X |
X |
Scaring |
| Kidneys |
X |
X |
X |
X |
Microvascular damage |
| Liver |
X |
X |
X |
X |
COVID-19 drug injury |
| Pancreas |
X |
X |
X |
X |
Increased triglycerides
Reduced blood flow
|
| Neurological |
X |
X |
X |
X |
Metabolic dysfunction |
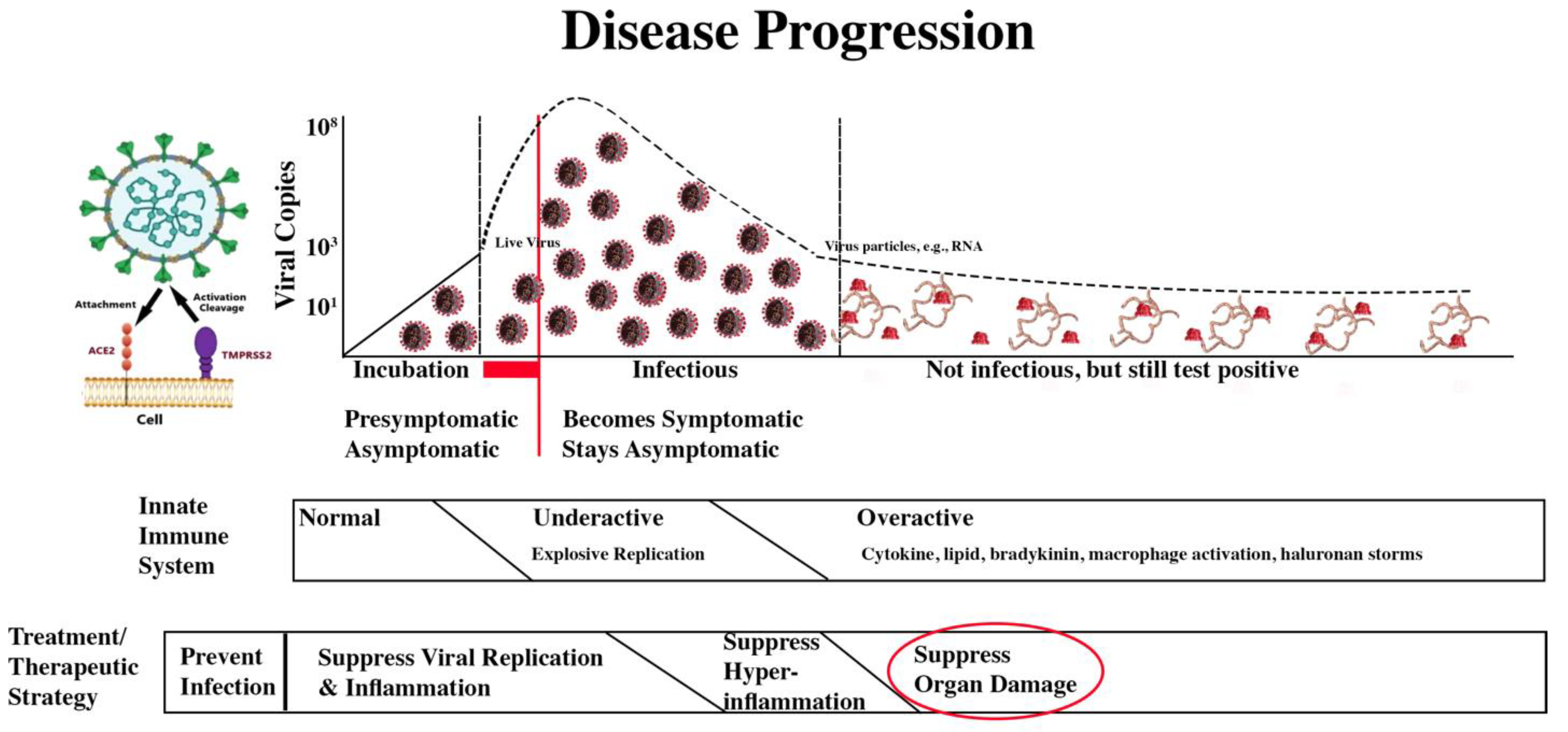
The answer to the very simple question of what my outcome will be if I get COVID-19 and follow a treatment regime is impossible to answer. The combination of variants, vaccine history, comorbidities, infection history, and therapeutics is greater than the number of stars in the Milky Way galaxy!
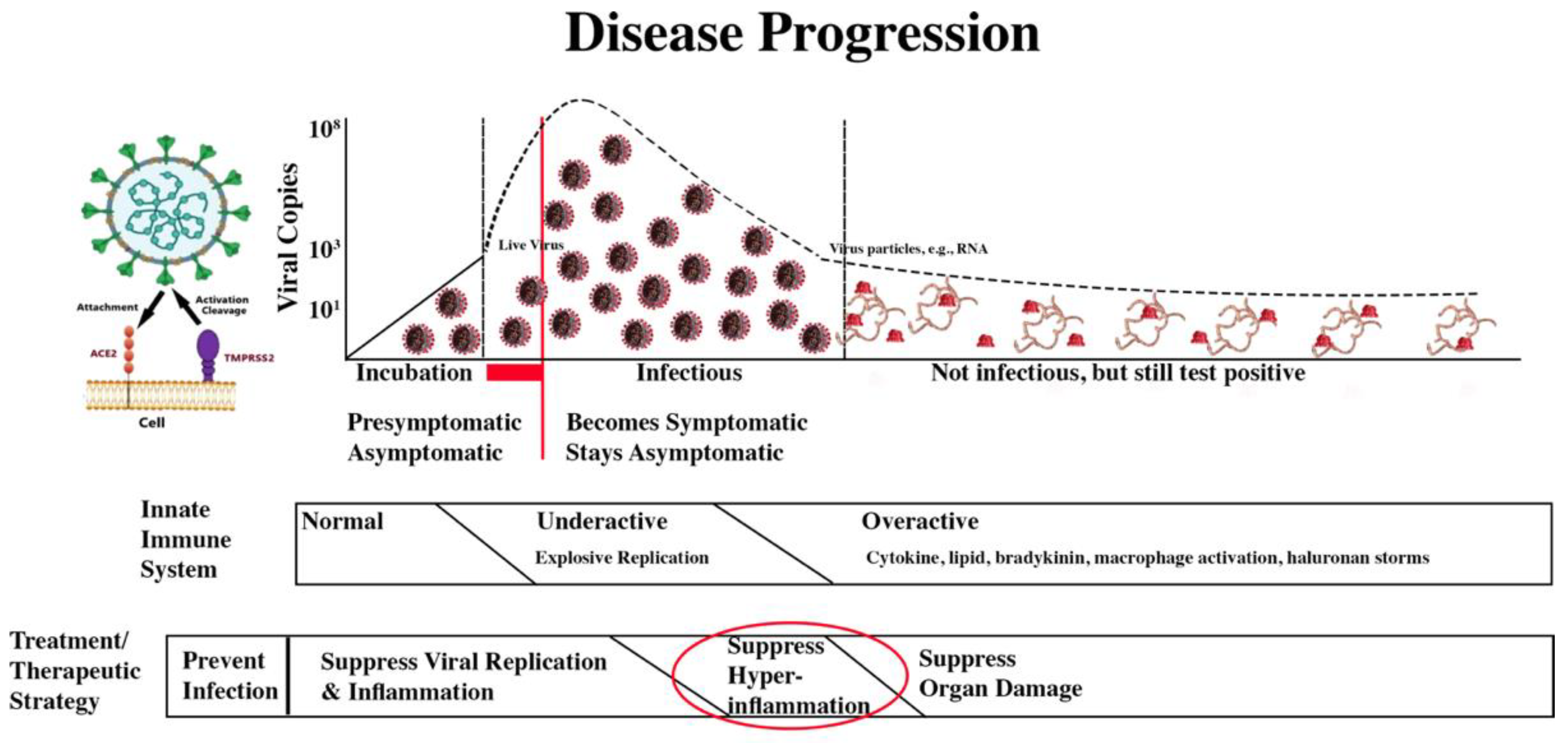
With that discouraging introduction, however, the therapeutic path should be taken is clear. After vaccination, reduce viral replication through the rapid use of antivirals, with PAXLOVD now being the best choice for those who can take it. If the virus has explosively replicated, therapeutic steps to reduce severe COVID-19’s impacts are also relatively clear.
Treatment/Therapeutic Strategy
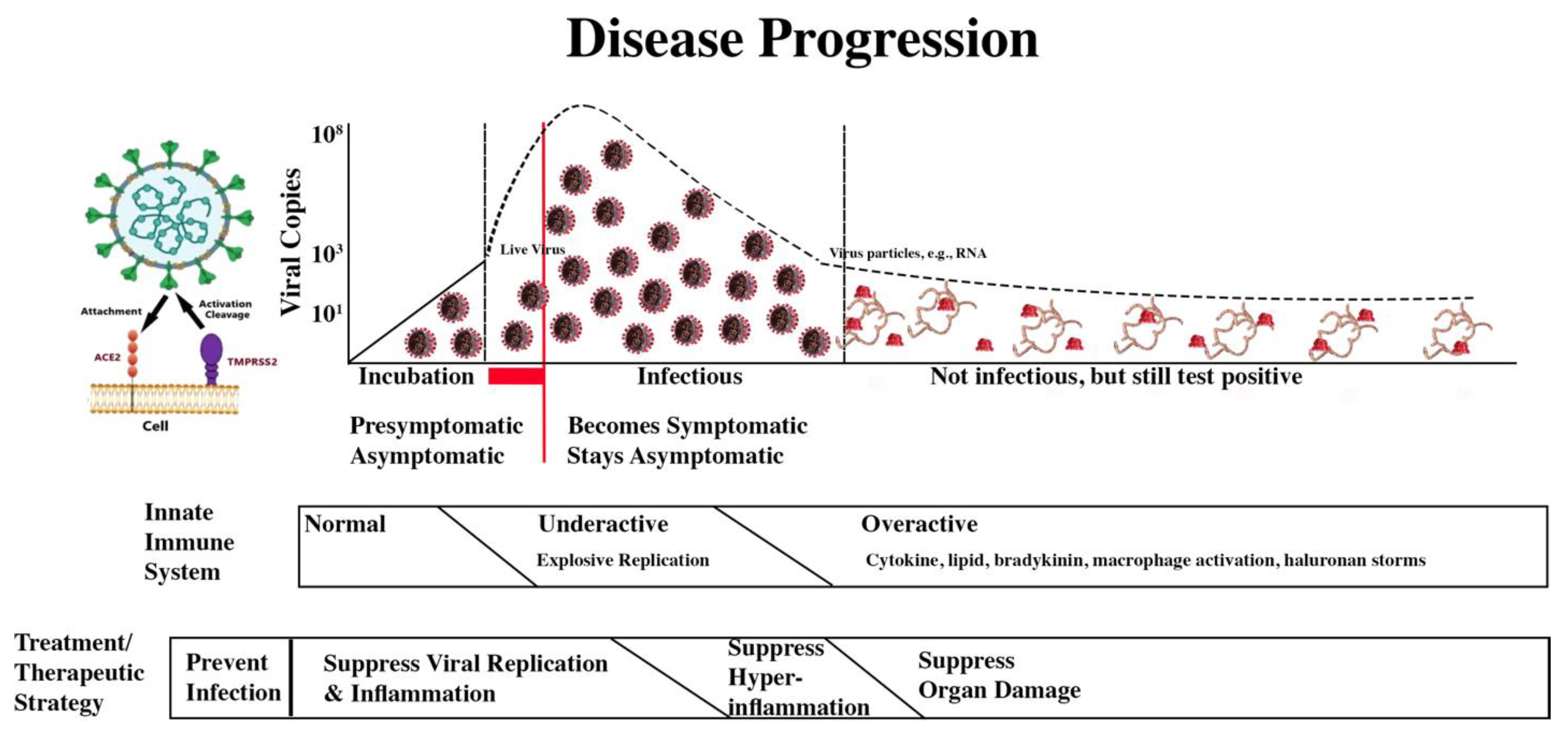
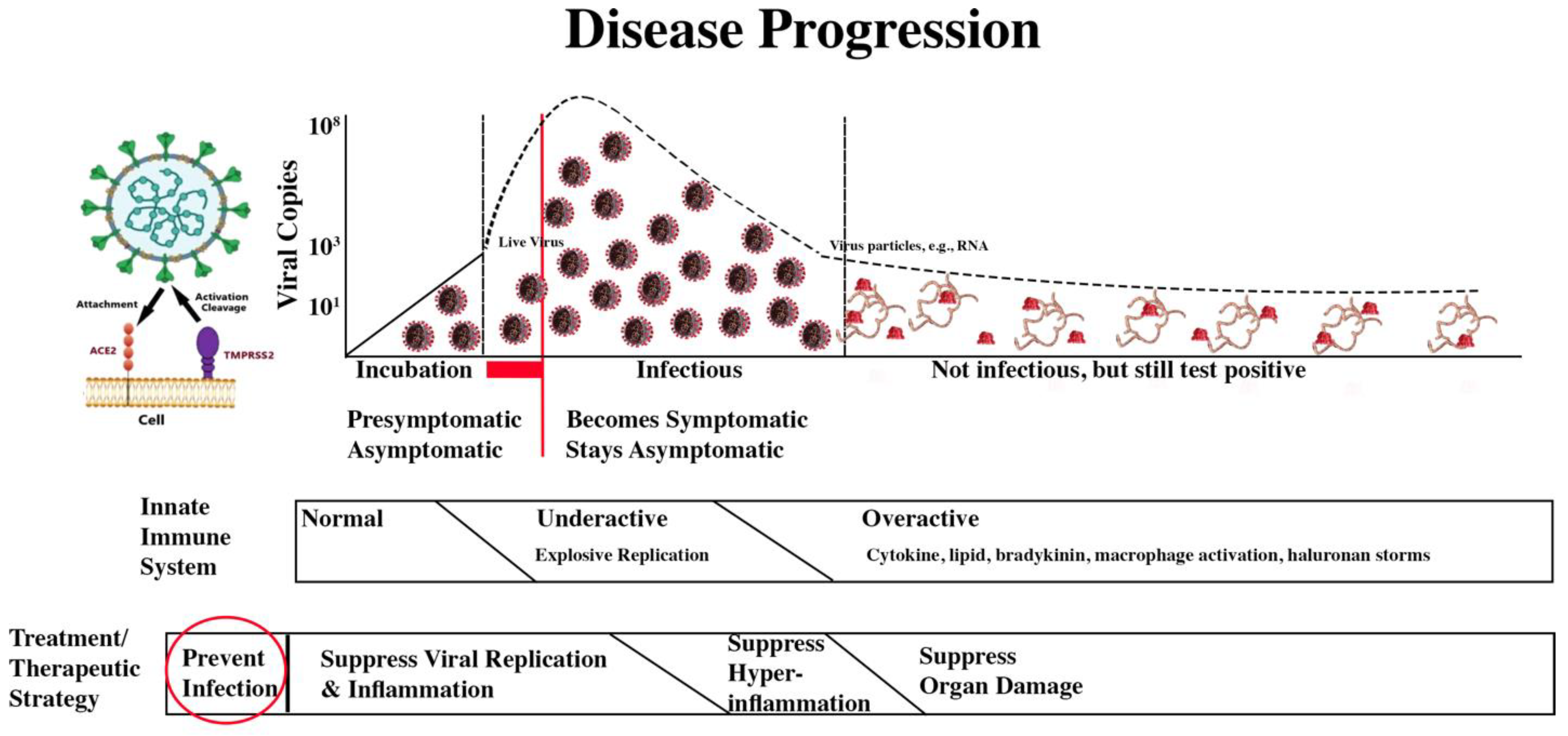
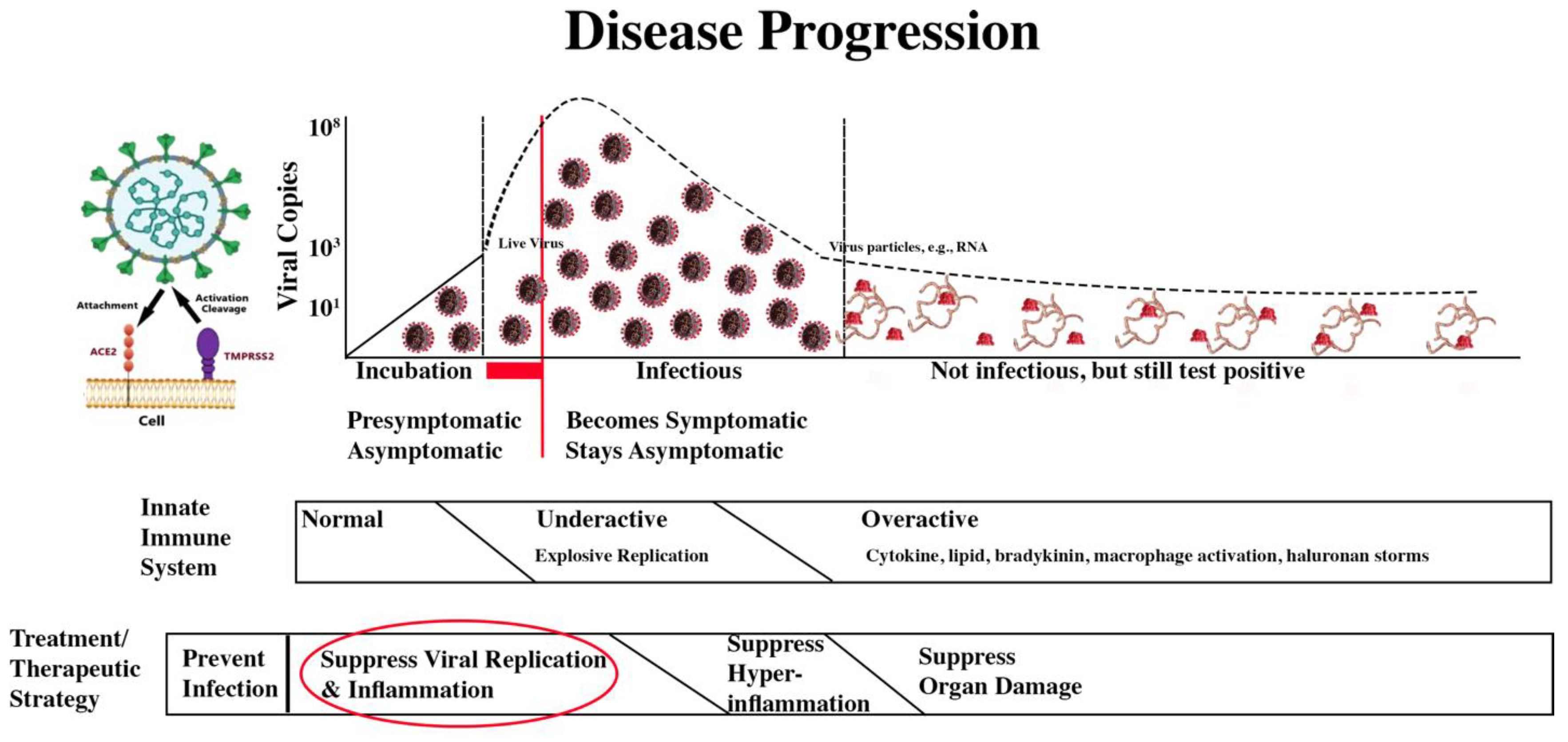
The treatment/therapeutic strategy is summarized in the second bar below the disease progression image.
COVID-19 Progression and Treatment/Therapeutic Strategy
The four treatment/therapeutic phases are:
| |
Damage Being Addressed |
| Treatment/Therapeutic Phase |
Direct Viral Damage |
Hyper-
Inflammation
|
Blood Clots |
Lack of Oxygen |
| Prevent infection. |
X |
|
|
|
| Suppress viral replication and inflammation. |
X |
X |
X |
|
| Suppress hyperinflammation. |
|
X |
X |
X |
| Suppress organ damage. |
|
|
X |
X |
It is discouraging to note:
Many diseases, especially those that are rare, genetic, or complex lack definitive cures.
No respiratory disease has ever been cured.
National Institute of Health Guidelines
The NIH produced 72 versions of very comprehensive treatment guidelines. They grew from 90 pages long to 278 pages after 12 months, and finally to 478 pages. The final update, Last NIH COVID Treatment Guidelines, was on February 29, 2024.
Therapeutic Flood
With the pandemic, pharmaceutical companies and academic researchers pursued new therapeutics with World War II-like urgency and focus. Several websites tracked global therapeutic trials:
No longer operational, The Global Coronavirus COVID-19 Clinical Trial Tracker listed 2,533 trials on December 31, 2020.
No longer being updated, the World Health Organization Treatment Tacker listed 4,634 trials in August 2023.
The FDA Clinical Trial Tracker is still operational.
A May 2021, Nature paper [
1] reported that there were multiple trials of the same drug, most too small to draw definitive conclusions. As will be seen, therapeutics discussed here had many papers whose results were often contradictory.
Trials of the Same Drug Drug Trial Size
For perspective, the Pfizer mRNA vaccine phase 3 trial had 43,448 participants, and its first PAXLOVID phase 3 trial had 2,246 adult participants.
A July 2024, Heliyon paper [
2] summarized COVID clinical trials through April 23, 2023.
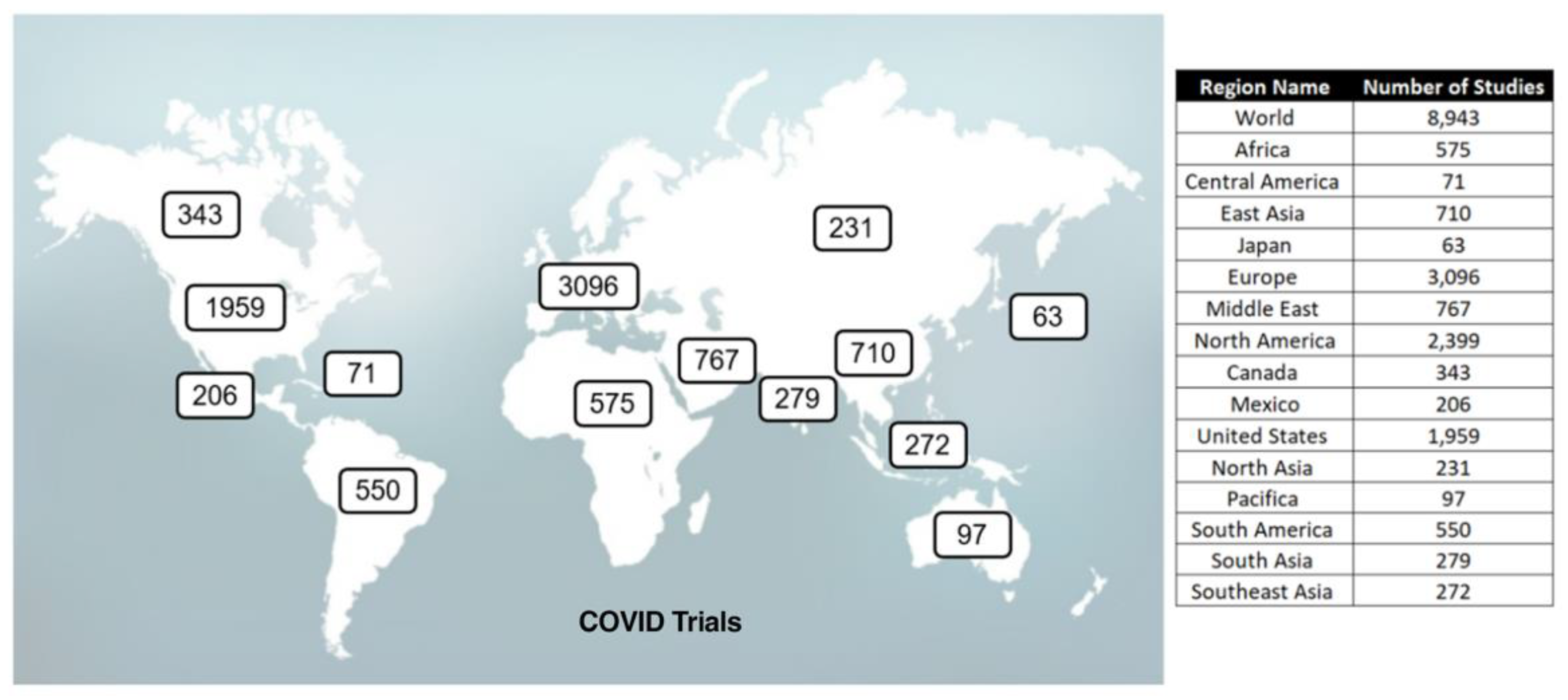
Global Clinical Trials
The following summarizes the FDA Clinical Trials and
The Mouse That Roared therapeutic paper statistics as of November 1, 2024. It excludes 1,686 COVID-19 vaccine trials.
| FDA COVID-19 Therapeutic Clinical Trials |
Google Scholar |
Mouse papers |
| Total |
Completed |
Terminated |
Results |
| 4,855 |
2,222 |
427 |
528 |
~3,000,0001
|
3,065 |
Google Scholar reported 5,550,000 papers for COVID. COVID scholar reported 143,711, the NIH 374,733, and PubMed 53,165.
For perspective, there were 1,107 FDA clinical trials for triple negative breast cancer, 720 for the flu, 193 for RSV, and 18 for Addison’s disease.
For the high-impact therapeutics, I used Google Scholar to find the paper with the most citations and/or impact. Even there, there is a sample bias as the prestigious journals’ papers and older papers often get the most citations. For example, of the 20 therapeutics with referenced papers in this article, the highest cited papers on 8 of them was the New England Journal of Medicine. Further, many of the early, highly cited papers suggested a therapeutic, e.g., probiotics, should be investigated or were the first to trial it often with only a few people.
A therapeutic goes through three trial phases and a Regulatory Review, Phase 1 - safety with a small group, Phase 2 - effectiveness with a larger group, Phase 3 - effectiveness and safety with a very large group.
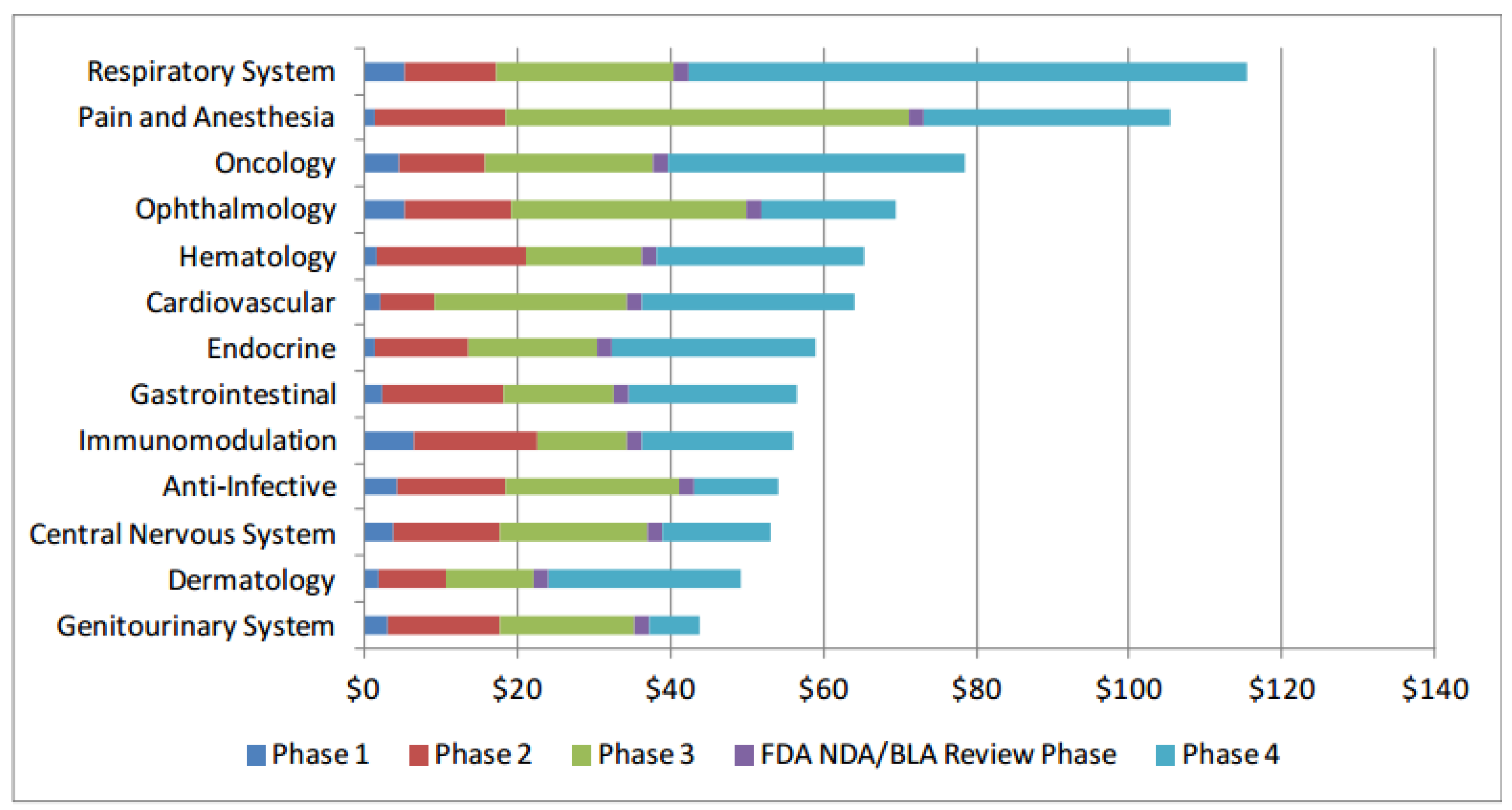
Clinical trial costs and failure rates are significant barriers to drug development. Costs were reported in a July 2014, Office of the Assistant Secretary for Planning and Evaluation paper [
3].
2012 Drug Trial Costs
Further, the paper reported 12% odds of making it all the way through regulatory approval.
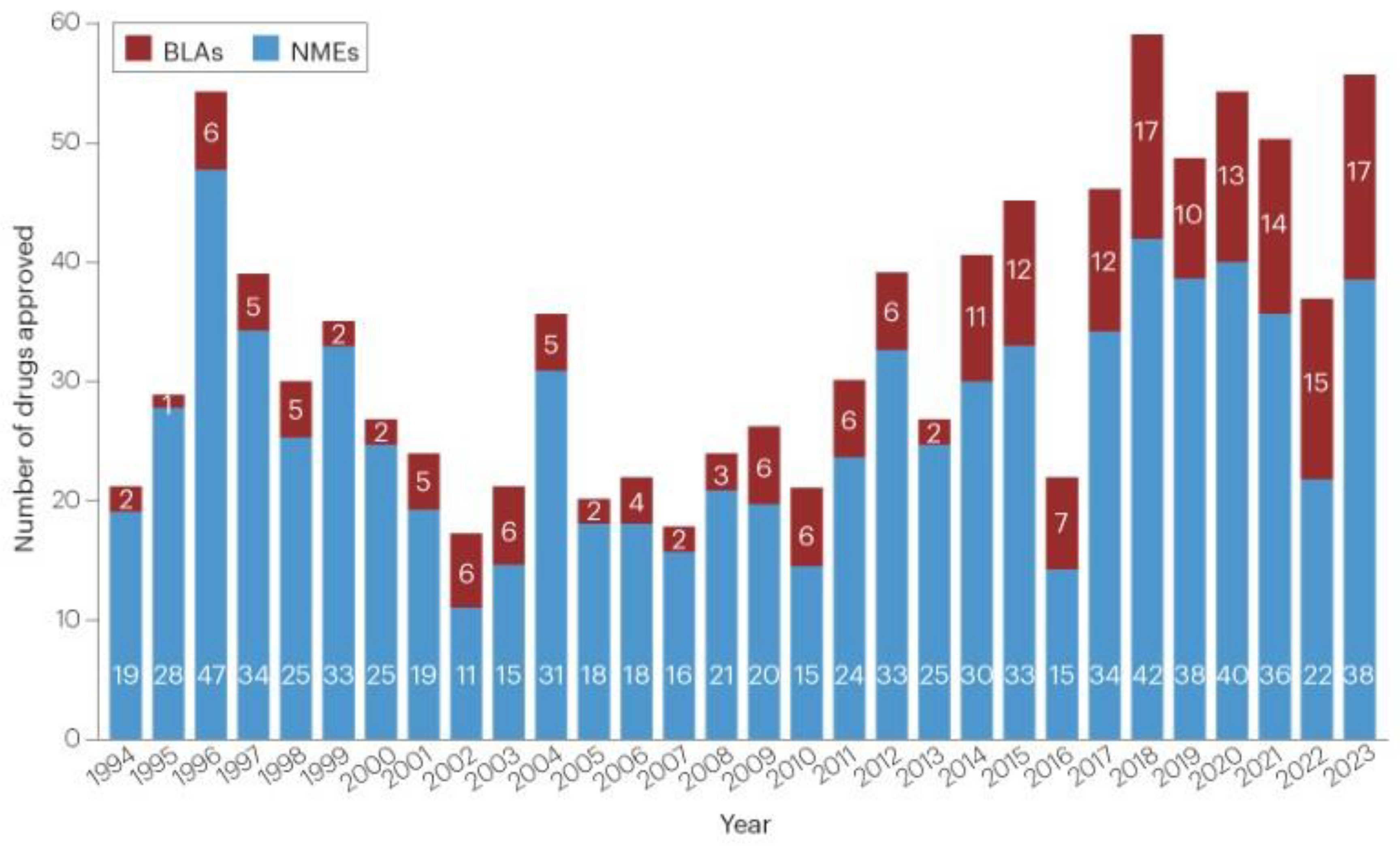
Cost, success rates, and therapeutic differential advantage are factors behind how few therapeutics are approved by the FDA each year as shown in the next image from a January 2024, Nature paper [
4].
Yearly FDA Drug Approval
(New molecular entities (NMEs) and biologics license applications (BLAs). Vaccines and gene therapies are not included.)
As an example of a drug that has not made it to market because a “good enough drug” is available might be masitinib. A July 2021 Science paper [
5], which has been cited 208 times, reported masitinib (MPRO) reduced the SARS-CoV-2 viral load by more than 99% and reduced inflammatory cytokine levels in mice. Since early 2023, it has been attempting to recruit people for a clinical trial.
Testing and Targets
There are many potential therapeutic targets including the viral proteins, cytokines, immune system cells, and organs.
Therapeutic Targets
Viral proteins are juicy therapeutic targets. Antiviral, anti-inflammatory and specialized therapeutics were used as weapons against them.
| |
Antiviral |
Anti-inflammatory |
Specialized |
| Structural Proteins |
| S |
X |
X |
|
| N |
X |
X |
Heart, amyloidosis |
| M |
X |
X |
|
| E |
X |
X |
Kidney, lung |
| Nonstructural Proteins |
| Nsp1 |
X |
X |
|
| Nsp2 |
|
|
|
| Nsp3 (PLpro) |
X |
|
|
| Nsp5 (Mpro) |
X |
X |
|
| Nsp6 |
|
X |
mRNA transport |
| Nsp7 |
X |
|
|
| Nsp8 |
X |
|
|
| Nsp9 |
X |
X |
|
| Nsp10 |
X |
X |
|
| Nsp11 |
|
|
|
| Nsp12 (RdRp) |
X |
|
|
| Nsp13 |
X |
|
|
| Nsp14(Exon) |
X |
X |
|
| Nsp15 |
X |
X |
|
| Nsp16 |
X |
X |
|
| Accessory Proteins |
| ORF3a |
X |
X |
Mitochondrial function |
| ORF3b |
|
X |
|
| ORF3c |
|
|
|
| ORF6 |
|
X |
|
| ORF7a |
X |
|
|
| ORF7b |
X |
|
|
| ORF 8 |
|
X |
Cholesterol formation |
| ORF9b |
|
|
|
| ORF9c |
|
|
Mitochondrial functionHeart cellsCholesterol formation |
| ORF10 |
|
X |
|
Squeakinstein
Therapeutic testing first involves animals. While COVID-19 has been reported in wild mice, their ACE2 receptors aren’t good matches for SARS-CoV-2. In 2000, mice were genetically engineered to have human-like ACE2 receptors. Recent tricks improved this genetic engineering and even made mice for Long COVID studies. The resulting transgenic mouse would have made Mary Shelly proud - a Squeakinstein. All kinds of therapeutics were tried on them.
Squeakinstein – A Transgenic, COVID-19 Infected K18-hACE2 Mouse
One study had mice do three months of swim training to test exercise immune system impact.
Mouse That Roared Papers
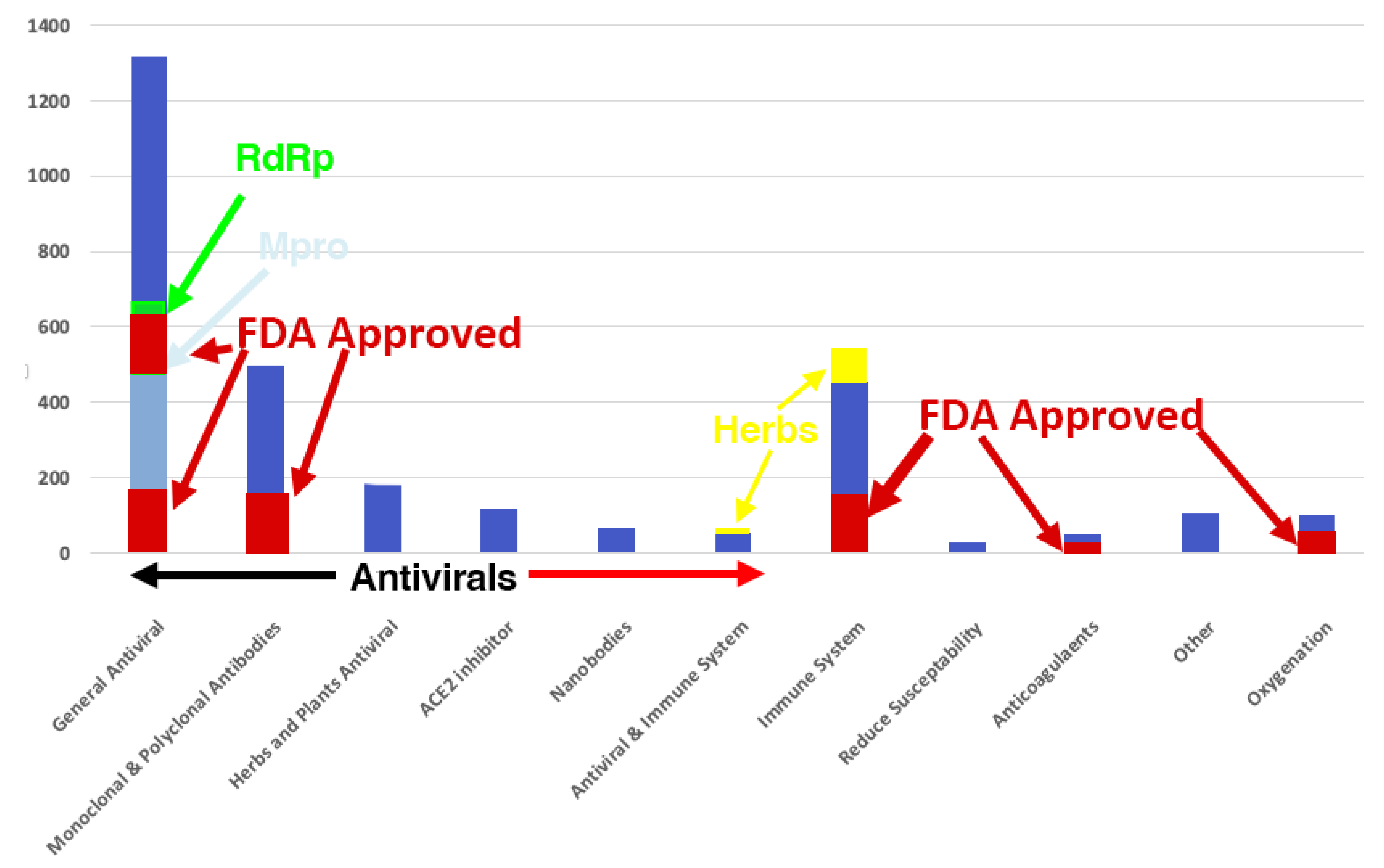
The following chart shows the distribution of all The Mouse That Roared Therapeutics and indicates how many were FDA approved. Notice 75% of the papers were on antivirals.
The Mouse That Roared COVID-19 Therapeutic Papers
Prevent Infection
We are here:
Prevent Infection
These are FDA Clinical Trials and
The Mouse That Roared prevent infection papers’ statistics.
| |
FDA Clinical Trials |
Google
Scholar
|
Mouse Papers |
| Total |
Completed |
Results |
| Prevention1
|
326 |
145 |
53 |
|
|
| Nasal sprays |
53 |
35 |
4 |
23,200 |
78 |
| BCG Vaccination |
9 |
5 |
0 |
31,000 |
26 |
| Vitamin D |
89 |
52 |
4 |
1,370,000 |
49 |
| Probiotics |
22 |
12 |
2 |
51,800 |
7 |
| Pemgarda |
1 |
0 |
0 |
38 |
1 |
Nasal Sprays
Since muscularly administered COVID-19 vaccines don’t provide good upper respiratory protection, nasal sprays and gargles are appealing therapeutics. In fact, an engineered lama nanobodies nasal spray protected Squeakinsteins or fully cured them if infected.
0.5 - 1% Povidone Iodine Nasal Spray or Gargle
Several pre-pandemic studies reported iodine nasal spray or gargle killed
all viruses in the nose or mouth. A September 2020, Ear Nose and Throat paper [
6] with 102 citations reported all evaluated concentrations of nasal antiseptics and oral rinse antiseptics completely inactivated SARS-CoV-2 after 60 seconds. A September 2022, Ear Nose & Throat paper [
7] reported povidone iodine nasal spray resulted in an 8.57 reduction of COVID-19 hospitalization or death rates. The NIH Guidelines are silent on nasal sprays.
Bacille Calmette-Guérin Vaccination
The 80-year-old Bacille Calmette-Guérin (BCG) tuberculous vaccination is one of the world’s most widely used vaccines. An April 2020 Lancet paper [
8], which was cited 389 times, reported it was believed BCG stimulated “trained immunity” to respiratory infections.
Early in the pandemic, African countries with extensive BCG vaccination had 65% of the case rates of neighboring countries with less extensive BCG vaccination. However, a September 2022, PLOS ONE paper [
9] reported that the differences in COVID-19 burden associated with BCG vaccination rates generally diminished in magnitude and usually lost statistical significance as the pandemic progressed. Though a few subsequent papers reported some minor protective effect, most reported no effect. The NIH Guidelines are silent on it.
Vitamin D
Researchers assessed vitamins A, C, D or K’s preventative effects. Most of the action was in vitamin D, which had wide ranging assessments on its effectiveness. The NIH Guidelines said there was insufficient evidence to recommend for or against vitamin C or D.
Probiotics
A November 2020, medRxiv preprint [
10] reported that in an analysis of 327,720 UK participants, some dietary supplements lowered SARS-CoV-2 infection risk. Probiotics provided a 14% reduced infection chance (95%CI: [8%,19%]). No
The Mouse That Roared paper reported negative results. The NIH Guidelines are silent on probiotics.
Pemgarda
The company who makes Pemgarda reported phase three trial results:
Through month 6, 84% relative infection risk reduction.
In months 7-12, 64% risk reduction of symptomatic COVID-19 in an immunocompetent adult population.
However, a November 2024, bioRxiv preprint [
11] reported variants KP.3.1.1 and XEC significantly impacted its neutralization rates. Subsequent New England Journal of Medicine papers reported the same impacts.
Suppress Viral Replication and Inflammation
We are here:
Suppress Viral Replication and Inflammation
The biggest take away from this review is that it is better to keep the barbarians at the gate than to battle them in the courtyard.
Keep the Barbarians at the Gate
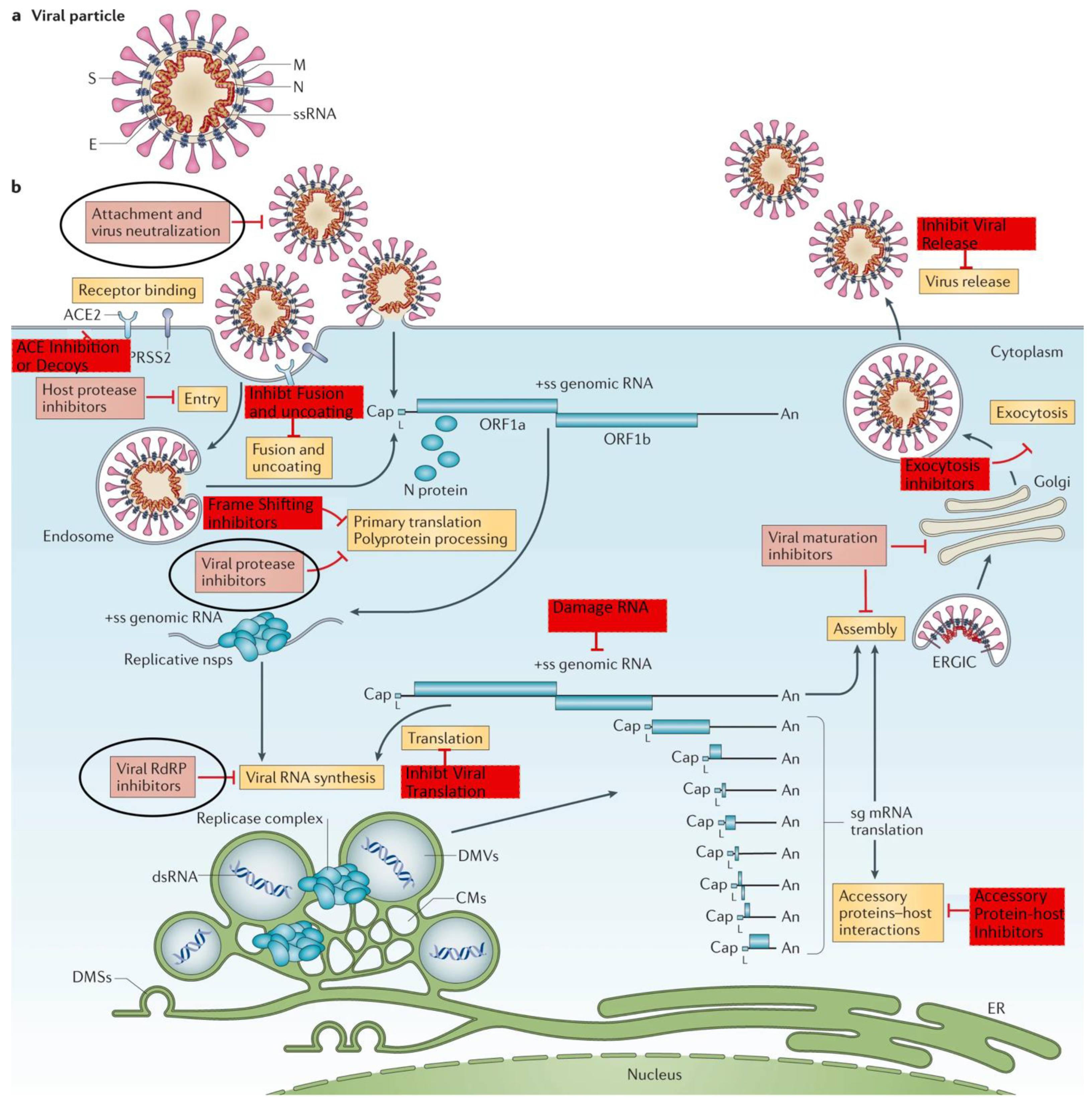
The following image is an update of one from an October 2020, Nature paper [
12] which was cited 1,878 times. The red and pick boxes show what parts of the viral life cycle can be impacted by antivirals. The pink boxes were as of October 2020. The red boxes represent viral impact subsequently discovered. The circled boxes correspond to FDA approved antivirals.
Viral Lifecycle and Antiviral Targets
Following are the number of the FDA Clinical Trials and The Mouse That Roared antiviral papers’ statistics. Included are therapeutics that will receive special attention. As will become clear, almost all antivirals targeted the spike protein or two nonstructural proteins – nsp5 (Mpro also called 3CLpro) or nsp12 (RdRp). Even though disabling other structural, e.g. the Nucleocapsid, or nonstructural, e.g., nsp1, proteins would have disabled the virus, there were relatively few papers and no approved therapeutics that addressed them.
These three targets were discovered early in 2020!
A February 2020, Nature paper [
13] investigated drugs that had worked on other viruses, e.g. SARS-CoV-1, MERS and Ebola. It reported Remdesivir was likely to be effective.
A March 2020, Science paper [
14] reported the development of an MPRO inhibitor.
A February 2020, Nature paper [
15] reported the spike protein was an ideal target for vaccines and therapeutics.
| |
FDA Clinical Trials |
Google
Scholar
|
Mouse Papers |
| Total |
Completed |
Results |
| Antiviral |
736 |
304 |
102 |
3,200,000 |
2331 |
| Spike Protein Monoclonal Antibody1 |
99 |
39 |
18 |
24,400 |
497 |
| Casirivimab/imdevimab2
|
25 |
8 |
11 |
6,830 |
38 |
| Sotrovimab2
|
24 |
9 |
10 |
5,720 |
53 |
| Tixagevimab/cilgavimab2
|
18 |
12 |
6 |
4,230 |
39 |
| MPRO |
|
|
|
18,900 |
502 |
| PAXLOVID |
45 |
14 |
7 |
9,780 |
176 |
| RdRp3 |
23,300 |
193 |
| Molnupiravir3
|
20 |
7 |
5 |
11,000 |
107 |
| Remdesivir4
|
108 |
51 |
26 |
41,200 |
73 |
| Innate Immunity |
|
|
|
|
|
| Interferon |
68 |
36 |
0 |
491,000 |
37 |
| Other Types |
|
|
|
|
|
| Renin-Angiotensin System Inhibitors |
9 |
2 |
0 |
38,000 |
4 |
| ACE2 |
31 |
10 |
2 |
63,700 |
102 |
| Simvastatin |
4 |
1 |
0 |
16,900 |
8 |
| Promising But Generally Ineffectual |
|
|
|
| Convalescent Plasma |
175 |
87 |
30 |
42,300 |
48 |
| Favipiravir |
65 |
36 |
9 |
23,100 |
26 |
There were 71 nanobody The Mouse That Roared papers and none with an FDA match.
Many papers discussed multiple monoclonal antibodies.
Many papers discussed RdRp therapeutics with other therapeutics, e.g., PAXLOVID and monoclonal antibodies.
About 20 The Mouse That Roared Molnupiravir papers were co-studies with PAXLOVID.
Monoclonal Antibodies and Nanobodies
Monoclonal antibodies and nanobodies bind to various targets, e.g., cancer cells or a viral protein.
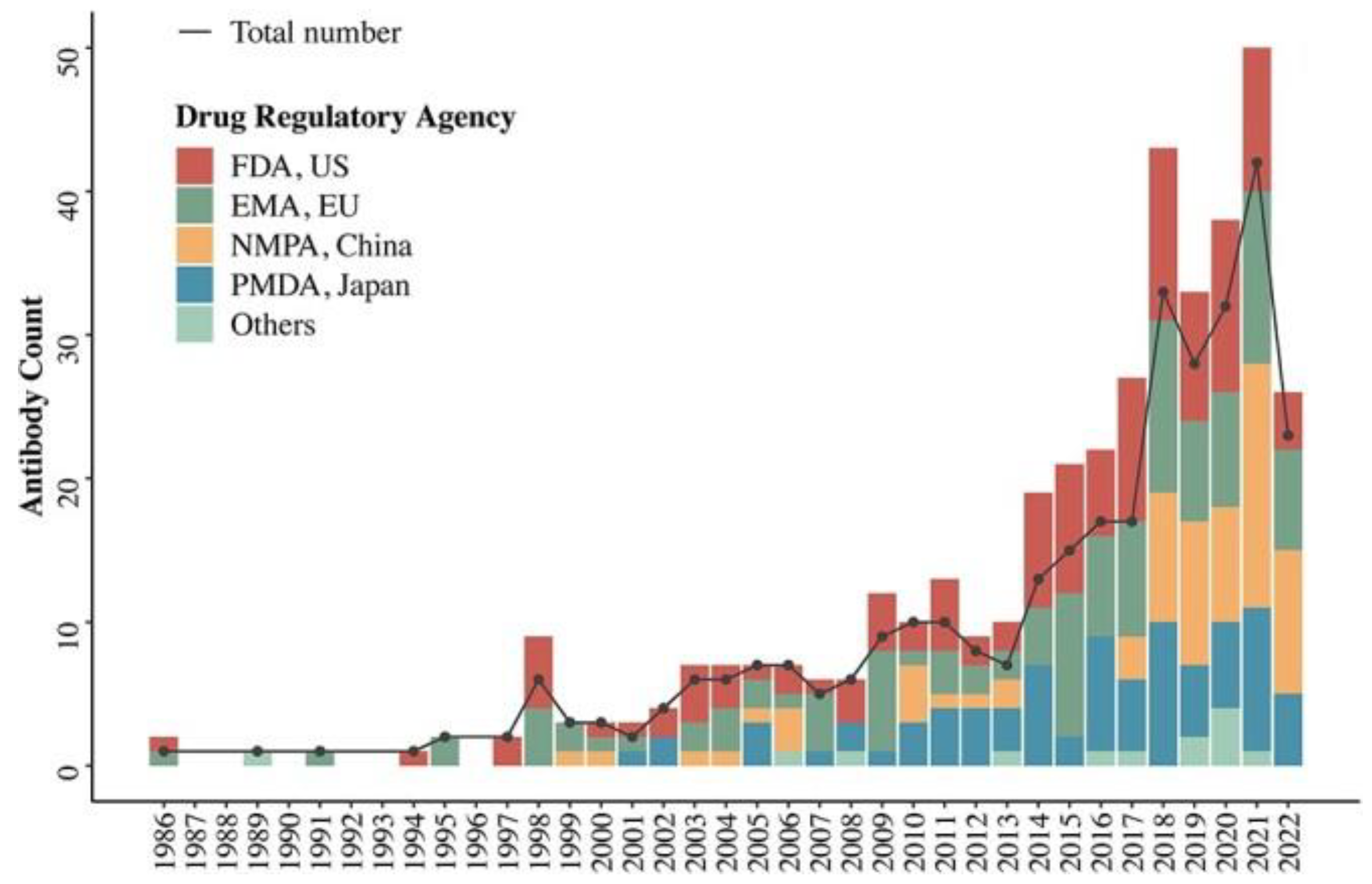
The FDA approved its first monoclonal antibody in 1986. It helped prevent kidney transplant rejection. According to a June 2022, Antibody Therapeutics paper [
16], 162 had been approved around the world by June 30, 2022.
Biosimilar, diagnostic, and veterinary antibodies are not included.
Monoclonal antibodies address transplants, cancers, sepsis, multiple sclerosis, arthritis, blood clotting, stroke, bacterial infection, autoimmune diseases, RSV, degenerative myopia, diabetes, macular degeneration, and many obscure diseases. The flu has no monoclonal antibody drugs because of its rapid mutation rate. In March 2024, a Cell Immunity paper [
17] reported a promising new approach. Time will tell.
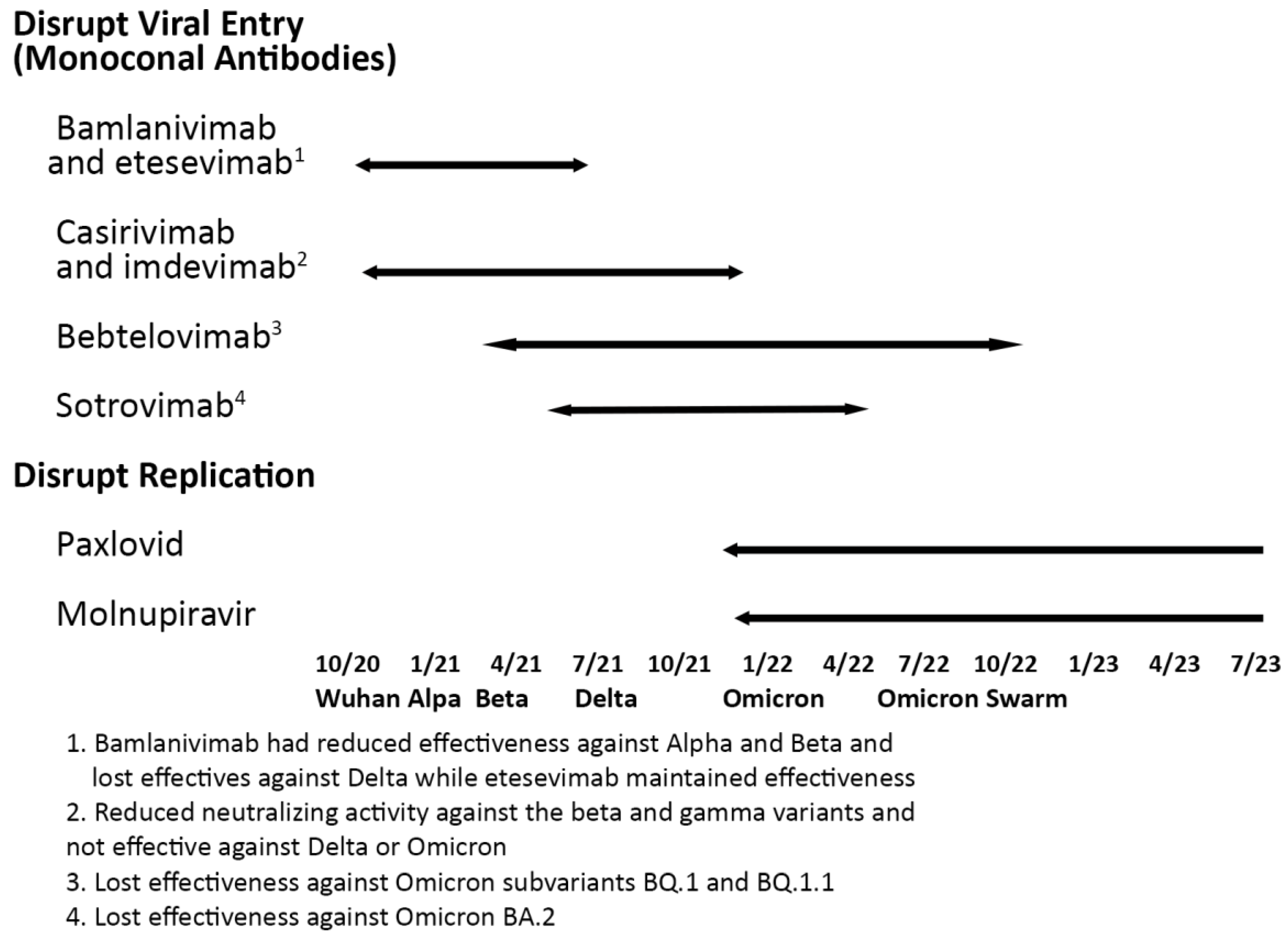
Monoclonal antibodies were highly effective until Variants of Concern emerged.
Interestingly, a November 2024, Cell paper [
18] reported several
non-neutralizing antibodies provided protection against SARS-CoV-2 in different animal models.
On March 22, 2024, the FDA approved Pemgarda (pemivibart) as a preventative monoclonal antibody for immunocompromised people. It was effective against the JN.1 variant but as will be discussed later lost some of its effectiveness against KP3.1.1 and XEC.
The FDA-approved monoclonal antibodies were quite effective if taken early and could be taken by pregnant women. Once taken, the FDA recommended delaying vaccination by 90 days as they could interfere with the COVID-19 vaccines. Here is data on their effectiveness.
Casirivimab with imdevimab – a February 2022, Lancet paper [
19] which was cited 223 times reported in seronegative patients, 396 (24%) of 1633 patients allocated to casirivimab and imdevimab versus 452 (30%) of 1520 patients allocated to usual care died within 28 days (rate ratio [RR] 0.79, 95% CI 0.69–0.91;).
Bamlanivimab plus etesevimab – A July 2021, New England Journal of Medicine paper [
20] which was cited 701 times reported that by day 29, 11 of 518 patients (2.1%) in the bamlanivimab–etesevimab group had a COVID-19–related hospitalization or death from any cause, as compared with 36 of 517 patients (7.0%) in the placebo group (absolute risk difference, −4.8 percentage points; 95% confidence interval [CI], −7.4 to −2.3; relative risk difference, 70%; P<0.001). No deaths occurred in the bamlanivimab–etesevimab group; 10 deaths occurred in the placebo group. Beta and Gamma wiped it out.
Sotrovimab
- An October 2021, New England Journal of Medicine paper [
21] which was cited 1,136 times reported that it had hospitalization or death 85%relative risk reduction, 97.24% confidence interval, 44 to 96. Though it had some effectiveness in Omicron BA.1 and BA.2, later Omicron subvariants wiped it out.
Evusheld (tixagevimab and cilgavimab) – An April 2022, New England Journal of Medicine paper [
22] which was cited 700 times reported that follow-up at a median of 6 months showed a relative infection risk reduction of 82.8% (95% CI, 65.8 to 91.4). Though it had some effectiveness in Omicron BA.1 and BA.2, later Omicron subvariants wiped it out. It was extensively used for infection protection.
The NIH Guidelines noted these monoclonal antibodies are no longer effective.
Llama, hamster, or ferret monoclonal antibodies and nanobodies were developed for:
The neuropilin-1 binding domain
The furin/TMPRSS2 cleavage site
Several structural and nonstructural proteins
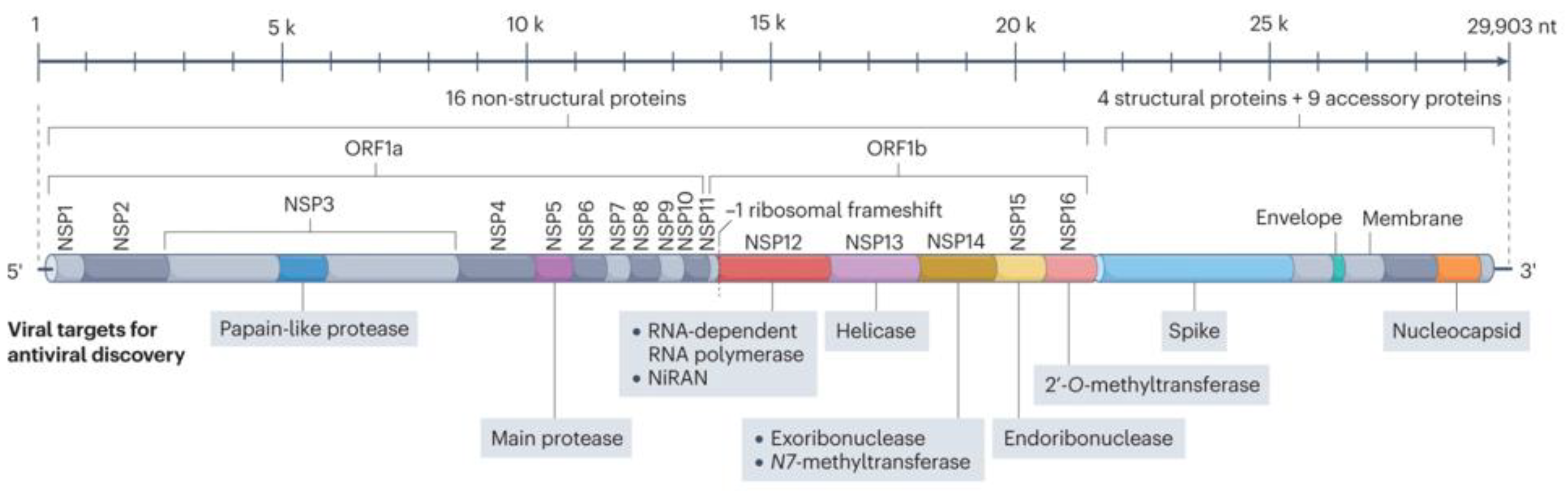
Several viral proteins and protein complexes are essential for the viral replication. Here is an image of the SARS-CoV-2 proteins.
SARS-CoV-2 Viral Proteins
The virus replicates by hijacking our replication machinery and material. It produces two long polyproteins called ORF1a and ORF1b which are cleaved into individual nonstructural proteins. Two viral enzymes do this cleaving after the replication process is jumpstarted by our less efficient cleaving enzymes. MPRO (nsp5), the main protease, cleaves the ORF1a polyprotein. Disabling MPRO, disables viral replication. Likewise, disabling Plpro (nsp3), the papain-like protease, disabled viral replication. There are no approved nsp3 drugs.
PAXLOVID
PAXLOVID, nirmatrelvir plus ritonavir, is the most effective FDA-approved COVID-19 antiviral. Nirmatrelvir was tested against MERS in 2012. It disables MPRO by cleaving it. Though used for treating HIV/AIDS, Ritonavir is seldom employed for its own antiviral activity but instead boosts other protease inhibitors. The FDA granted early use authorization on December 21, 2021, and full approval on May 23, 2023. No MPRO mutation has disabled it.
China developed a PAXLOVID look-alike called Azvudine and developed another MPRO drug called leritrelvir which they claim is as effective as PAXLOVID.
Some people can’t take PAXLOVID because of ritonavir drug interactions. FDA PAXLOVID Drug Interactions and University of Liverpool Drug Interactions websites assess these interactions. The assessments include when not to take various COVID-19 drugs, take them with careful monitoring, or take them only after pausing their medications, e.g., simvastatin in the case of PAXLOVID. PAXLOVID is safe for pregnant women.
There have been many studies reporting slightly different results. A June 2022, New England Journal of Medicine [
23] which was cited 2023 times on the PAXLOVID phase three, adult trial reported:
89.1% hospitalization risk reduction. No one died while 1% in the placebo group died.
3.1% of the people taking PAXLOVID reported diarrhea compared to 1.6% in the placebo group.
5.6% of the people taking PAXLOVID reported a very bitter, metallic taste later called PAXLOVID mouth. Sucking on sweets or taking saccharin helped reduce the bitter taste. Only .03% of the patients reported a very bitter taste in the placebo group.
A July 2023, Lancet paper [
24] reported that PAXLOVID’s effectiveness in preventing hospital admission or death differed greatly by when it was taken.
within 30 days of a positive test for SARS-CoV-2, 53.6% (95% CI 6.6-77.0),
within 5 days of symptom onset, 79.6% (95% CI 33.9-93.8)
on first day of symptom onset, 89.6% (95% CI 50.2-97.8).
The NIH recommends “treatment should be initiated as soon as possible and within 5 days of symptom onset.”
Viral rebound happens in about 2%-5% of those who took PAXLOVID. Viral rebound is not understood, and studies reported wildly differing results. The CDC [
25] reported that with a virologic response through day 5, viral rebound occurred in 6.4%-8.4% of PAXLOVID recipients and 5.9%-6.5% of placebo recipients during the 2021 pre-Omicron and 2022 Omicron periods.
Because of cost, rebound fear, and side effects, PAXLOVID is significantly underutilized. A November 2022, CDC report [
26] reported that among 699,848 adults aged ≥18 years eligible for PAXLOVID during April–August 2022, 28.4% received a PAXLOVID prescription within 5 days of COVID-19 diagnosis. Further, usage dropped precipitously in lower socioeconomic groups even when the government was paying for it.
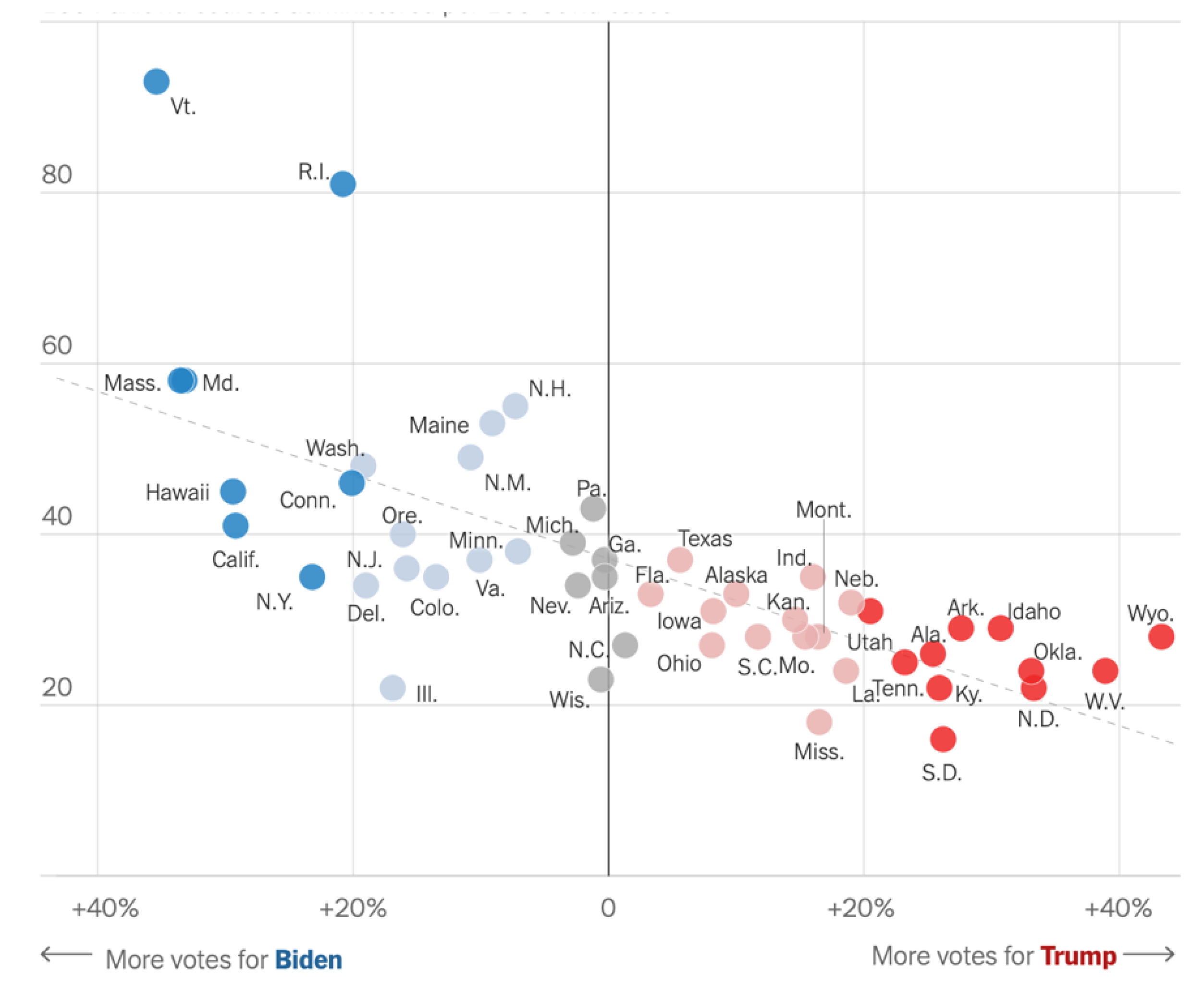
In October 2022, the NY Times [27] reported use by political party.
100 PAXLOVID Courses per 100 COVID-19 Cases
Unlike many viruses including the flu, SARS-CoV-2 replication has error correction which improves its replication rates. Two approved therapeutics reduced its error correction effectiveness by interfering with the nsp12 nonstructural protein called RdRp.
Remdesivir is an intravenous, hospital-administered therapeutic. It was effective against Hepatitis C and RSV in 2009, Ebola in 2014 and Marburg in 2015. The FDA approved it for COVID-19 use on Oct 22, 2020.
Lagevrio (Molnupiravir) is a tablet therapeutic. It was used against influenza, Ebola, chikungunya, and various coronaviruses including MERS. The FDA approved it for COVID-19 use on December 23, 2021.
Remdesivir
A May 2020, New England Journal of Medicine [
28] paper which was citied 6,317 times reported:
| |
Remdesivir |
Placebo |
| Recovery time - days |
10 |
15 |
| Mortality - 29th day |
11.4% |
15.2% |
| Serious adverse events |
24.6% |
31.6% |
A March 2024, Therapeutic Advances in Infectious Diseases paper [
29] reported remdesivir was associated with an absolute risk reduction of 6.4% and a relative risk reduction of 66% for all-cause hospitalization or death. It was often taken with PAXLOID. RdRp mutations have not disabled it.
Remdesivir helps keep patients alive, often for a long time, while they are COVID-19 positive. Extended infections are a rich breeding ground for viral mutations. Five The Mouse That Roared papers reported substantial viral mutations occurred in remdesivir-treated patients.
The NIH Guidelines recommend using remdesivir within 7 days of symptom onset in a hospital setting often with other drugs. They recommend using PAXLOVID over it.
Lagevrio (Molnupiravir)
A December 2021, New England Journal of Medicine [
30] paper which was cited 1,852 times reported its effectiveness was:
| |
Molnupiravir |
Placebo |
| Hospitalization or death - 29th day |
6.9% |
9.7% |
| Serious adverse events |
30.4% |
31.6% |
A July 2022, medR
xiv [
31] preprint reported a retrospective study of 92 million patients with PAXLOVID (n =11,270) or Molnupiravir (n =2,374) taken within 5 days of COVID-19 symptoms.
| |
PAXLOVID |
Molnupiravir |
| |
7 Days |
30 Days |
7 Days |
30 Days |
| Symptoms |
2.31% |
5.87% |
3.75% |
8.21% |
| Hospitalization |
0.44% |
0.77% |
0.84% |
1.39% |
| Rebound1 |
3.53% |
5.40% |
5.86% |
8.59% |
All joint studies reported greater PAXLOVID effectiveness. However, Molnupiravir can be taken by more people.
The NIH Guidelines recommended Molnupiravir after PAXLOVID and remdesivir.
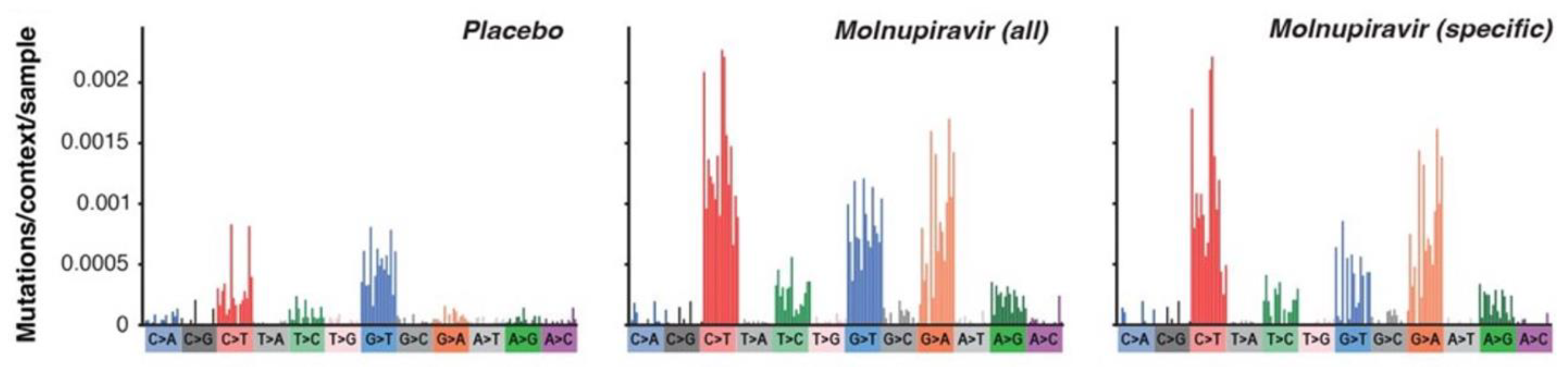
A January 2023, medR
xiv [
30] preprint reported Molnupiravir induced SARS-CoV-2 mutations.
Molnupiravir Created Mutation Rates
Molnupiravir potentially could change to our DNA. However, the FDA reported that the genotoxicity data after treatment implied Molnupiravir has a low risk for damaging our DNA. Nonetheless, the FDA recommended:
Against pregnant patients use unless therapy is indicated and there are no options.
The use of effective contraception during and following Molnupiravir treatment.
Only people ages 18 or greater use it.
ACE2 Inhibitors
Recall the primary way SARS-CoV-2 enters our cells is after the spike protein attaches to a cell’s ACE2 receptor, breaks into two pieces with the larger piece containing the viral RNA entering the cell. Monoclonal antibodies interfere with such entry by attaching to the spike protein. Several promising ACE2-based approaches were reported in The Mouse That Roared papers:
Alter the ACE2 receptor’s biochemistry so the spike couldn’t bind to it.
Reduce the number of cell ACE2 receptors.
Provide particles that bind to the ACE2 receptor so the spike could not.
Provide ACE2 decoys - nanoparticles, soluble, or inhaled - for the spike binding.
Though papers reported extraordinary success rates across variants, little progress has been made in commercializing ACE2 receptors. The NIH Guidelines are silent on them.
Simvastatin
Many people take statins to control cholesterol levels. Of many statins tested, only simvastatin had some (small) impact on COVID-19 outcomes in some studies. An October 2023, New England Journal of Medicine paper [
32] which had been cited 27 times reported that although recruitment was stopped because cases had decreased, among critically ill patients with COVID-19, simvastatin did not meet the prespecified criteria for superiority relative to control.
Promising But Generally Ineffectual
Convalescent Plasma
{ XE "Therapeutics:Plasma" }Blood plasma is the yellowish liquid part of blood that holds the blood cells in suspension and carries cells and proteins throughout the body. The first Nobel Prize in Physiology and Medicine was awarded in 1901 to Emil von Behring for injecting antibodies from animals’ plasma who had recovered from diphtheria into diphtheria patients.
It is believed that { XE "1918 Spanish Flu:Plasma" } blood plasma halved the 1918 Spanish Flu’s mortality and was particularly effective when patients received it early in an infection. It was used against measles in the 1930’s, the Korean hemorrhagic fever in the 1950’s, and is one of Ebola’s best treatments.
Arturo Casadevall, a Johns Hopkins Bloomberg Distinguished Professor of Molecular Microbiology and Immunology and its department chairman, ran a vigorous campaign in 2020 to get the world to investigate COVID-19 plasma therapy.
The trial results were conflicted. An August 2023, Nature [
34] paper analyzed 19 trials and reported estimated mortality relative to placebo was 0.94 (95% CI 0.81-1.08, p = 0.33).
The NIH Guidelines said there is insufficient evidence to recommend either for or against the use of high-titer convalescent plasma for hospitalized or nonhospitalized immunocompromised patients.
Favipiravir
Though it has uncertain effectiveness and is not available in the US, Japan uses favipiravir for the flu. With COVID-19, it might inhibit RdRp and disrupt the envelope and ORF7a proteins.
An October 2020, Engineering paper [
35] which was cited 1,602 times reported that for the 35 favipiravir (FPV) patients and the 45 control patients, FPV had shorter viral clearance median time - 4 days, interquartile range 2.5–9 days versus 11 days interquartile range 8–13 days. FPV also showed 91.43% versus 62.22% improvement in chest CT’s.
World trials concluded favipiravir did not improve clinical outcomes though appeared to offer some relief in mild to moderate cases.
The NIH Guidelines mentioned it only in one trial with ivermectin.
Herbs
Here are the FDA Clinical Trials and
The Mouse That Roared herb therapeutic papers’ statistics.
| |
FDA Clinical Trials |
Google Scholar |
Mouse Papers |
| Total |
Completed |
Results |
| Herbs |
15 |
6 |
0 |
57,000 |
263 |
| Traditional Chinese Medicine1
|
21 |
7 |
0 |
447,000 |
53 |
Viral Lifecycle and Antiviral Targets
One paper reported Cipra cut down viral replication 98%. Here is a breakdown of the herbs into various categories.
| |
|
Herbs excluding Chinese Medicine |
Chinese Medicine |
| |
All |
Anti-Viral |
Treat – mainly Inflammation |
Anti-viral and treat |
Anti-viral |
Treat - mainly Inflammation |
Anti-viral and treat |
| Total |
314 |
179 |
58 |
21 |
24 |
28 |
4 |
| Mpro |
45 |
36 |
|
1 |
8 |
|
0 |
| RdRp |
12 |
9 |
|
0 |
3 |
|
0 |
The February 2023, Chinese COVID-19 Guidelines for Treating COVID-19 guidelines are 26 pages long. 10 pages address Traditional Chinese Medicine. They included “Western Therapeutics” like PAXLOVID.
The NIH Guidelines only mention herbs as potential medical contraindications.
Anti-Inflammatory Cytokines
The most discussed anti-inflammatory cytokines were interferons which "interfere" with viral replication by protecting cells from infection. Though there are no approved interferon drugs,
The Mouse That Roared papers
addressed many other interferons, e.g., IFN-1, β, β1a, γ, λ2 and α16. An April 2023, International Journal of Molecular Sciences [
36] paper reported interferon IFN-α2b reduced lung injuries by 50% and reduced the odds of severe COVID-19 by a factor of 5.
The NIH Guidelines:
Recommended against interferon α or β, for nonhospitalized patients with mild to moderate COVID-19 except in a clinical trial.
Recommended against the use of interferon α, except in a clinical trial or interferon β for hospitalised patients.
Recommended neither for nor against the use of interferon λ.
Other Antivirals
In addition to the antivirals already discussed, about 550 additional ones were discussed in The Mouse That Roared. The remaining 550 papers addressed every pink and red box in the above figure titled Viral Lifecycle and Antiviral Targets. The therapeutics used many different tricks, e.g., used amino acids, peptides, aptamers, short-RNA, micro-RNA, ultra-violet light in lungs, plasma activated water, to disrupt a biochemical process either by just changing molecules’ shapes or damaging them, e.g., through proteases or kinases. Many therapeutics addressed disrupting the N-protein’s glycans. There were extensive drug and compound searches, e.g., 350,000, 33,590, 22,000, 18,000, 6,710, 6,218, 2100, 1,900, 1796, and 850. They were administered by chewing gum, nasal spray, mouth wash, table and needle.
The drug compounds and sources included - H2 Sulfur compounds, NaCl, ZnO, CeO2, hydrophobic C60, copper, gold, silver, ClO2, CuFe204, boron, NO, Se, vitamin B-a and B-12 supplements, TMPRSS2 inhibitor, phosphatidic acid, nucleic acid, antiandrogen, human breast milk, pluripotent stem cell, earthworms, chewing gum, shark immune cells, mesenchymal stem cells, fungi, bacteria such as e-coli, marine sponges, scorpion venom, interferons, NK cells, sea cucumbers, mink lung epithelial cell, CRISPR to find or modify, cow milk, monoclonal antibodies, coffee, bee venom, electric fields, mouth wash, purified IgG, anti-protozoal, induce vomiting, spider hemocytes, salamandra, sweet potato roots, oriental wasp, human defensins, bile acids, antibiotics, hen egg yolks, bovine herpesvirus, male contraceptive, cardiac imaging, and inhaled heparin.
Many drugs that had effectiveness against other diseases showed some effectiveness against COVID-19. The diseases included gas, leprosy, irritable bowel syndrome, tapeworms, anti-epileptic, gout, vaginal infection, hepatitis, HIV/AIDS, parasites, sleeping sickness, Prozac, anticonvulsants, laxatives, hyponatremia, over active bladder, circadian rhythm, cystinosis, influenza, anti-fungal, anti-biotic, macular degeneration, asthma, malaria, RSV, cardiac imaging, ring stage parasites, antipsychotic, diuretic, kidney damage, HPV, bipolar, epilepsy, schizophrenia, anxiety, migraine, tranquilizers, cholesterol, hepatitis C, pulmonary fibrosis, gall stones, liver disease, type I Gaucher, Fabry disease, anemia and hypovolemia, herpes, pancreatitis, reflux esophagitis, weight loss, hypertension, ectopic pregnancies, abnormal hemoglobin, allergy, hay fever, common cold, rheumatoid arthritis, rapid heartbeats, shortness of breath, pale skin, cold hands and feet, dark urine, jaundice, mitochondrial antioxidant, lung transplantation, antiandrogen, lactate-lowering, viral hemorrhagic fevers, throat and tonsils infections, ulcerative colitis, gallstones, diabetic kidney disease, lupus, and methemoglobinemia.
Cancer drugs against many different types of diseases were: leprosy, irritable bowel syndrome, tapeworms, anti-epileptic, gout drug, vaginal infection, hepatitis, HIV/AIDS, antiparasitic; sleeping sickness, anticonvulsants, laxatives, hyponatremia, over active bladder, circadian rhythm, cystinosis, influenza, anti-fungal, anti-biotic, macular degeneration, asthma, malaria, RSV, ring stage parasites, antipsychotic, diuretic, kidney damage, HPV, depression, bipolar, epilepsy, schizophrenia, anxiety, migraine, tranquilizers, cholesterol, hepatitis C, hydrophobic C60, pulmonary fibrosis, gall stones, liver disease, type I Gaucher, Fabry disease, anemia and hypovolemia, herpes, pancreatitis, reflux esophagitis, weight loss, hypertension, ectopic pregnancies, abnormal hemoglobin, allergy, hay fever, common cold, rheumatoid arthritis, rapid heartbeats, shortness of breath, pale skin, cold hands and feet, dark urine, jaundice, mitochondrial antioxidant, lung transplantation, lactate-lowering, viral hemorrhagic fevers, ulcerative colitis, gallstones, diabetic kidney disease, lupus, methemoglobinemia.
Suppress Hyperinflammation
We are here:
Suppress Hyperinflammation
Sadly, the barbarians have broached the gate and are winning the fight. Either there will be:
More likely in severe cases, Long COVID can follow either outcome.
Inflammation
Inflammation is a natural response to injury to eliminate its cause, to remove damaged cells, and to start healing. Inflammation:
Recognizes the harmful stimuli like injury or pathogens, e.g. SARS-Co-2.
Releases signaling molecules like histamines and cytokines.
Recruits immune cells: White blood cells, particularly neutrophils and macrophages, to neutralize pathogens and remove debris.
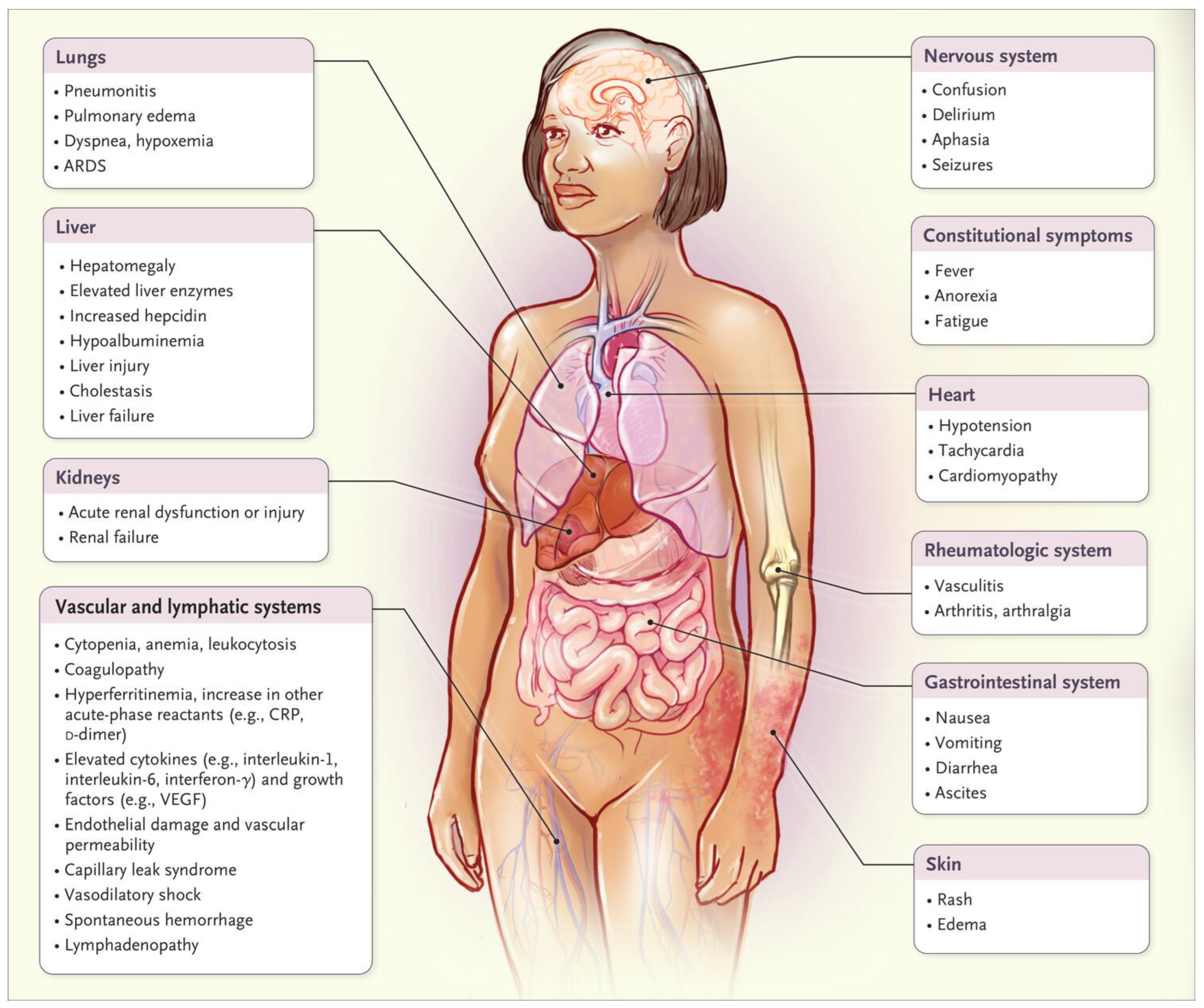
Hyperinflammation is excessive inflammation. COVID hyperinflammation damages many parts of the body as shown by this image from a December 2020, New England Journal of Medicine paper [
37] which has been cited 2,889 times.
Cytokine Storm Damage
Causes
Hyperinflammation has two causes.
When SARS-CoV-2 binds to the ACE2 receptor, it dysregulates the renin-angiotensin-aldosterone system (RAAS) which has important roles in inflammation and blood pressure control. Through a complex process, this results in an overproduction of cytokines which are small proteins that act as signaling molecules in the immune system. This over production is called the cytokine storm. There are hundreds of cytokines, of which most proinflammatory cytokines are tumor necrosis factor-alpha (TNF-α):Interleukin-1 (IL-1), (IL-6), IL-17, (IL-12), (IL-23) and interferon IFN-γ.
Cytokines work together to amplify inflammation and coordinate immune responses, but when overproduced, they can lead to excessive inflammation and tissue damage, as seen in autoimmune diseases, cancer, infections and physical damage.
- 2.
In addition, the innate immune system’s workhorses, the effector cells, are damaged and release cytokines. This damage also promotes viral replication as it takes 4-6 days for our adaptive immune system to kick in.
If hospitalized, the fight for one’s life is now very serious. Hyper inflammation is very hard to treat because treating inflammation risks impairing the body's defense mechanisms, the biology is complex, it has several causes, it is self-sustaining, there is individual variability, and the treatments have side effects.
Many of the hyperinflammation drugs also treat COVID-19 comorbidities such as cancer, obesity, diabetes, or the gut’s microbiology. Thus, there is a confusing interplay of the drugs’ targets and the reasons for their outcomes.
The FDA Clinical Trial and
The Mouse That Roared anti-inflammatory therapeutics statistics are:
| |
FDA Clinical Trials |
Google Scholar |
Mouse Papers1 |
| |
Total |
Completed |
Results |
| Anti-Inflammatory |
241 |
97 |
22 |
214,000 |
457 |
| Tocilizumab |
75 |
31 |
7 |
38,600 |
43 |
| Anakinra |
25 |
13 |
3 |
14,600 |
16 |
| Baricitinib |
27 |
8 |
3 |
13,400 |
24 |
| Corticosteroid |
79 |
34 |
3 |
127,000 |
65 |
| Dexamethasone |
93 |
40 |
7 |
131,000 |
56 |
| Metformin |
9 |
2 |
1 |
42,600 |
24 |
The two therapeutic approaches to hyperinflammation are:
Address the cause, i.e., protect the renin-angiotensin and innate immune systems.
Address the result, i.e., reduce inflammation.
Attack Hyperinflammation Cause
Renin-Angiotensin System Inhibitors
A dysregulated renin-angiotensin system is the major cytokine storm villains. ACE Inhibitors, angiotensin receptor blockers, and direct renin inhibitors drugs were trialed. None reported strong results.
The NIH Guidelines recommended that people who were already using drugs for blood pressure control continue to do so. No recommendations were made to start them.
Innate Immune System Protection
One can protect the innate immune system by inhibiting pro-inflammatory cytokines or protecting the effector cells or the complement system.
Inhibit Pro-Inflammatory Cytokines
Interleukins, the cytokines most discussed for their pro-inflammatory impacts, modulate growth, differentiation, and activation during inflammatory and immune responses. They stimulate immune cell recruitment and activation, increase their vascular permeability, and induce fever, all of which are critical for fighting off invading pathogens. They can be anti-inflammatory, e.g., interleukin IL-1, IL-4, IL-6, IL-10, IL-11, and IL-13.
Though it can be anti-inflammatory, IL-6 came up often as a particularly proinflammatory interleukin. A February 2023, PLOS ONE paper [
38] reported that inhibiting IL-6 with Jusvinza reduced mortality to 10% in the treated group vs 60% in the untreated group. Many Chinese herbs addressed IL-6.
The Mouse That Roared papers addressed many other interleukins, e.g., interleukin-1Ra, 1β, 2C, 7, 17i, 18, 23, 23i, and 33.
The NIH Guidelines addressed IL-6 suppression in hospitalized COVID-19 patients and recommended tocilizumab in combination with dexamethasone.
Protect Effector Cells
A few of The Mouse That Roared papers discussed therapeutics which helped reduce the inflammatory impacts to innate effector cells, e.g., macrophages with hydroxyl-polyamidoamine dendrimer-N-acetyl cysteine conjugate, natural killer cells with 7DW8-5 glycolipid, tumor necrosis factor with nanochelating technology, neutrophils with sivelestat, and very importantly NETS (neutrophil extracellular traps) with thiocyanate and DNA proteases.
A June 2024, Blood Advances reported infusing cytotoxic t-cells led to viral elimination in all patients at 4 days (≥88%) and 14 days (>99%). [
39].
Boost the Complement System
The complement system enhances (complements) the ability of antibodies and cells that ingest pathogens, promotes inflammation, and attacks the pathogen's cell membrane. It is dysregulated by COVID-19. A June 2020, Nature paper [
40] reported the Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib helped normalize it.
The NIH Guidelines were silent on the complement system.
Attack The Result of Hyperinflammation
We shall first discuss approved drugs and a few with high promise that reduced hyperinflammation. We will then characterize the others that were discussed in The Mouse That Roared.
Tocilizumab
Tocilizumab is a monoclonal antibody immunosuppressive directed against interleukin-6. Approved for use in 2008 in Japan, it is used to treat rheumatoid arthritis. It targets pro-inflammatory cytokines like TNF-α, IL-6, IL-1, and IL-17, as well as immune cell surface receptors. The FDA granted it COVID-19 Emergency Use Authorization on December 21, 2022.
A May 2021, JAMA paper [
41] reported that 8 trials had different outcomes. New England Journal of Medicine papers reported tocilizumab:
improved outcomes including survival [
42] - 36% vs. 26%.
had no effect on severity or mortality. [
43]
The highest cited paper was early in the pandemic and reported the results of only 15 patients.
Anakinra
Used to treat rheumatoid arthritis, anakinra is a recombinant and slightly modified version of a human interleukin 1 (IL-1) receptor antagonist. It targets pro-inflammatory cytokines like TNF-α, IL-6, IL-1, and IL-17, as well as immune cell surface receptors. It was used for Familial Mediterranean Fever in 1998. The FDA granted it COVID-19 Emergency Use Authorization on November 8, 2022, one year after Europe.
An October 2021, Lancet [
44] paper reported a six-trial study. Anakinra showed a survival benefit when given
without dexamethasone (OR 0.23 [95% CI 0.12-0.43]), but not with it (OR 0.72 [95% CI 0.37-1.41]). Other papers did not report as impressive outcomes.
Baricitinib
Baricitinib was approved for use for rheumatoid arthritis in 2017. (Got the pattern?) It blocks Janus kinase (JAK) enzymes, which are critical in the signaling pathways of many pro-inflammatory cytokines, such as IL-6 and IFN-γ. The FDA granted it COVID-19 Emergency Use Authorization on May 10, 2022.
A December 2020, New England Journal of Medicine paper [
45] reported patients receiving baricitinib had a median 7 days to recovery of (95% confidence interval [CI], 6 to 8), as compared with 8 days (95% CI, 7 to 9) for controls, and a 30% higher odds of improvement in clinical status at day 15 (odds ratio, 1.3; 95% CI, 1.0 to 1.6). Patients receiving high-flow oxygen or noninvasive ventilation at enrollment had a time to recovery of 10 days with combination treatment and 18 days for controls (rate ratio for recovery, 1.51; 95% CI, 1.10 to 2.08). The 28-day mortality was 5.1% in the combination group and 7.8% in the control group.
The highest cited paper was early in the pandemic and had only 13 patients in the baricitinib group.
The NIH Guidelines noted that the use of immunomodulators such as dexamethasone, Janus kinase inhibitors (e.g., baricitinib, tofacitinib), interleukin-6 inhibitors (e.g., tocilizumab, sarilumab), tumor necrosis factor inhibitors (e.g., infliximab), or abatacept, which also treats rheumatoid arthritis, to treat COVID-19 may also increase the risk of infectious complications. However, when these therapies are used appropriately, the benefits outweigh the risks.
Glucocorticoids
Glucocorticoids are steroids which generate diverse and strong immune system suppressive effects by downregulating proinflammatory cytokines such as interleukins and blocking inflammatory signaling pathways.
Dexamethasone, the go-to
glucocorticoid, was often mixed with antivirals. An October 2020, New England Journal of Medicine paper [
46] which was cited 9,664 times reported that in the dexamethasone group, the incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and among those receiving oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94) but not among those who weren’t receiving support (17.8% vs. 14.0%; rate ratio, 1.19; 95% CI, 0.92 to 1.55).
Other papers reported mortality reductions of 42%, 33%, 32.2%, and 20% (twice). It had some risks, particularly changed in bacterial compositions and increased hyperglycemia in diabetic patients.
Regarding dexamethasone, the NIH Guidelines noted:
Spirulina
Spirulina is a bacteria which significantly reduced IL-6, TNF-α, IL-10, and IP-10. An April 2024, Frontiers in Immunology paper [
47] reported mortality hazard ratios were non-ICU HR, 0.13; 95% CI, 0.02 to 0.97; ICU, HR, 0.16; 95% CI, 0.05 to 0.48.
Other Anti-inflammatory Therapeutics
What follows is a characterization of the 275 The Mouse That Roared ant-inflammatory papers that haven’t already been discussed. They addressed:
Cytokines - IL-6 (main target), IL-2, TNF-αi, I-17 IL-23, interleukin 7, IFNγ, TNFα, IL-1β, IL-18, TGF-β1, IFN-α and β , ANG2, NLRP3.
Effector Cells – macrophages, cytotoxic T cells, neutrophils, and mast cells
Kinases, an enzyme that catalyzes the transfer of a phosphate group from ATP to a specified molecule - Janus and Casein kinase 2, TANK-binding kinase 1, spleen tyrosine kinase.
Miscellaneous others, e.g. transcription factors, a gene that encodes a protein that detects products of damaged cells and triggers an immune response, GSDMD inhibitors exosomes, adenine dinucleotide metabolism, NETS.
The drugs had many ingredients: cluster of differentiation 24, N-Protein antibodies, monoclonal antibodies for selected cytokines, interferon, genes, gene expression, CRISPR modified T cells, stem cell transplants, hydrogen, immunoglobulins, molecules that cause cell death, bacteria, blood purification, plasma exchange, algae, synthetic angiotensin (1-7) (TXA-127) or an angiotensin II type1 receptor–biased ligand, low dose radiation, low-intensity pulsed ultrasound, pharmacologic stress agent used in cardiac perfusion imaging studies, drugs to maintain general anesthesia, hyperimmune plasma from sheep, mesenchymal stromal sells from many sources, injectable porous silicon particles, silver, selenium, lithium, sodium nitrate, hydrogen rich water, cytotoxic T lymphocytes, cellular human amniotic fluid, degalactosylated bovine glycoproteins, activated protein C, vitamin E, peptide angiotensin-(1-7) [Ang-(1-7)], blood filter, antioxidant enzymes.
They addressed many different diseases.
Autoimmune Diseases: rheumatoid arthritis, ankylosing spondylitis, psoriasis (including plaque psoriasis), Crohn's disease, inflammatory bowel disease, ulcerative colitis, ankylosing spondylitis, alopecia areata, multiple sclerosis, chronic immune thrombocytopenia, Behçet's disease, eczema, atopic dermatitis, seborrheic dermatitis, gout, plaque psoriasis, and keratoconjunctivitis sicca associated with Sjögren's syndrome.
Many human diseases, particularly immunosuppressed diseases like inflammatory bowel syndrome are associated with inflammation. From a 2013 national estimate provided in a December 2016, JAMA paper [
46], US adults’ immunosuppression prevalence from health conditions and medication use was 2.7%. Selected autoimmune drugs are useful with COVID because they attack cytokines, autoantibodies, and interfere with cell signaling pathways that drive inflammation
Cancer: bone marrow, small cell lung cancer, leukemias (chronic myeloid leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia), multiple myeloma, prostate cancer, castration-resistant prostate cancer, high-risk primary or secondary myelofibrosis, testicular, and ovarian cancer, glioblastoma multiforme and myelodysplastic syndromes.
Selected cancer drugs are useful with COVID because they attack cytokines, have strong anti-inflammatory effects, and shrink tumors would release pro-inflammatory mediators.
Other Lung Diseases - chronic obstructive pulmonary disease (COPD), chronic bronchopulmonary disorders, idiopathic pulmonary fibrosis, acute lung injury, bleomycin lung injury, ARDS (acute respiratory distress syndrome), and TB (tuberculosis).
Others - abnormal blood lipid levels, acute pancreatitis, alcohol use disorder, Alzheimer’s, ALS, angina pectoris, chronic hepatitis, chronic kidney disease, diabetes, diabetic kidney disease, disseminated intravascular coagulation and pancreatitis, epilepsy, gastroesophageal reflux disease, graft/host diseases, heart problems, hemophagocytic lymphohistiocytosis, high blood pressure, hyperlipidemia, hypertriglyceridemia, lactobezoar, malaria, migraines, nausea, nephrotic syndrome, neuropathic pain, osteoporosis in postmenopausal women, peptic ulcer disease, prion diseases, depression, psychosis and anxiety, schizophrenia, sepsis, solid organ transplant, superficial mycoses, tapeworm, viscid or excessive mucus, Zollinger-Ellison syndrome
Suppress Organ Damage
We are here.
Suppress Organ Damage
One is very, very ill, and there is no silver bullet. These are the FDA clinical trial and
The Mouse that Roared papers that addressed two key areas of organ treatment.
| |
FDA Clinical Trials |
Google Scholar |
Mouse papers |
| |
Lungs |
| Oxygen |
25 |
1,570,000 |
71 |
| ECMO |
3 |
40,800 |
52 |
| Vilobelimab |
1 |
446 |
6 |
| |
Cardiovascular |
| Anti-coagulants1 |
|
54 |
| Heparin |
12 |
133,000 |
32 |
Lungs
As previously noted, lungs can be damaged by direct viral damage, inflammation, blood clots, and/or lack of oxygen. Oxygenation will be assessed here. Yet again, a key message is to act quickly. Following are the increasingly aggressive oxygen treatments.
Conventional oxygen.
High-Flow Nasal Cannula, the nasal delivery of an adjustable mixture of heated and humidified air and oxygen at rates that exceed spontaneous inspiratory flow.
Mechanical ventilation.
Extracorporeal Membrane Oxygenation.
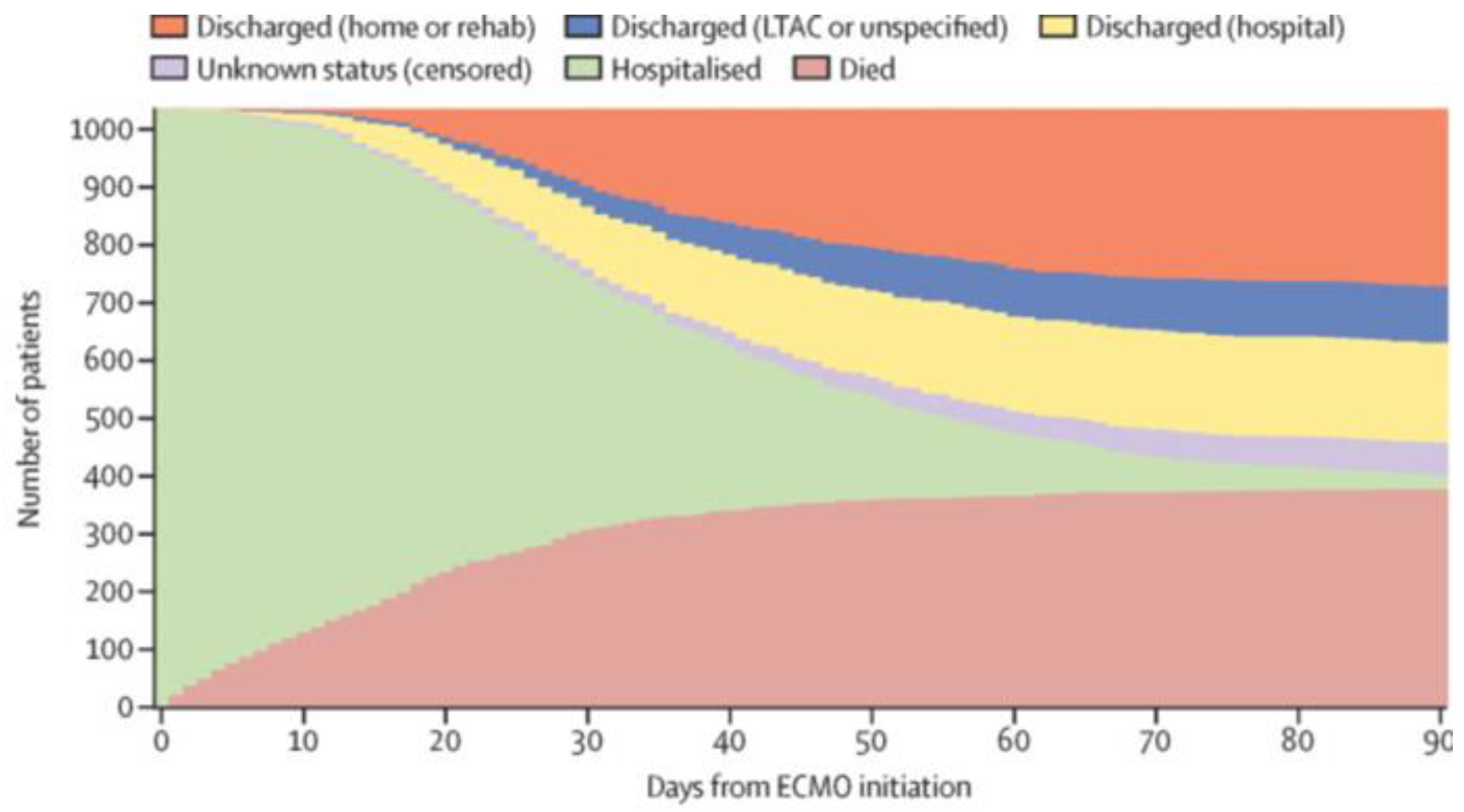
ECMO is a mechanically supported ventilation system that is used on average 15 days with an interquartile range of 8-17 days for COVID-19 patients. It is the predominate form of mechanical ventilation with one meta study reporting 98.6% use. A May 2024, BMC Journal paper [
47] reported 54% of the patients experienced hemorrhagic complications. Even with it, lung transplantation may be proposed. These are the ECMO outcome statistics from a September 2020, Lancet paper [
49].
ECMO Outcome Rates
An August 2023, Journal of Clinical Medicine paper [
51] reported selected study ARDS patient mortality with and without ECMO.
| Characteristic |
Follow-Up |
ECMO |
Conventional |
| ARDS-H1N1 |
6 months |
37% |
53% |
| ARDS |
60 days |
35% |
46% |
| ARDS |
60 days |
34% |
47% |
| ARDS |
90 days |
36% |
48% |
| COVID ARDS |
60 days |
26% |
33% |
| COVID ARDS |
60 days |
35% |
47% |
The NIH Guidelines recommend starting with high-flow nasal cannula oxygen followed by noninvasive ventilation or intubation and mechanical ventilation. Though ECMO was referenced on 51 pages in the NIH guidelines regarding the use of other therapeutics with it, there were no explicit discussion of it.
The monoclonal antibody Vilobelimab was trialed for septic shock in 2012. A December 2022, Lancet paper [
52] which was cited 88 times reported all-cause mortality rate at 28 days was 32% (95% CI 25–39) in the vilobelimab group and 42% (35–49) in the placebo group (hazard ratio 0.73, 95% CI 0.50–1.06; p=0.094). The FDA granted early use authorization on April 4, 2023.
The NIH Guidelines stated that though vilobelimab received a COVID-19 FDA EUA, there was insufficient evidence to recommend either for or against its use.
The Mouse That Roared papers reported that other oxygenation treatments were attempted without much success - hyperbaric chamber along with a cytokine therapeutic (erythropoietin), repurposed drugs imatinib (cancer), methylthioninium chloride and nebulized recombinant tissue plasminogen activator (stroke).
Cardiovascular
As previously noted, the cardiovascular system can be damaged by direct viral infection, hyper-inflammation, blood clots, and/or lack of oxygen. Dealing with blood clots will now be assessed. Well-known anti-coagulants, e.g., heparin, xarelto and aspirin, address them. However, anti-coagulants must be used very carefully as they can cause bleeding.
In August 2021, the New England Journal of Medicine [
53] paper which has been cited 1,111 times reported available evidence did not support therapeutic-dose heparin fuse or thrombosis prevention in critically ill patients. While there was some evidence that it was effective for people with severe comorbidities there must be proactive bleeding monitoring as bleeding accounted for 3-6% of COVID-19 deaths.
A March 2024, Frontiers in Cardiovascular Medicine [
54] reported the relative risks and benefits of different heparin doses.
| |
Therapeutic Dose Compared to
Standard Dose
|
Intermediate Dose Compared to Therapeutic Dose |
| VTE1 Risk |
OR=1.09, 95% CI: 0.58-2.02 |
OR=0.85, 95% CI: 0.52-1.38 |
| All-Cause Mortality |
OR=1.12, 95% CI: 0.75-1.67 |
OR=1.34, 95% CI: 0.83-2.17 |
| Bleeding Risk |
OR=2.59, 95% CI: 1.87-3.57 |
OR=2.42, 95% CI: 1.58-3.70 |
Venous thromboembolism (VTE) is when a blood clot forms in a vein. VTE includes deep vein thrombosis (DVT) and pulmonary embolism (PE). DVT occurs when a blood clot forms in a deep vein, usually in the lower leg, thigh, or pelvis.
The NIH Guidelines on heparin recommended its use only in hospital settings.
Another approach to clotting is to dissolve neutrophil extracellular traps (NETS), networks of extracellular fibers, primarily composed of neutrophils DNA. Neutrophils are the immune system's first line of defense and kill invading pathogens by engulfing them and secreting anti-microbials. In 2004, a novel third function was identified: formation of NETs. NETs allow neutrophils to kill extracellular pathogens. Early in the pandemic, it was discovered that NETs can propagate inflammation and microvascular thrombosis particularly in ARDS patients’ lungs. Several drugs reduced NET formation including colchicine, IL1β blockers and anakinra.
The NIH Guidelines were silent on NETS.
Other anti-clotting approaches discussed in The Mouse that Roared papers included blood vessel dilation, antiplatelet, plasma exchange, caplacizumab, and repurposed drugs for treating tapeworm (niclosamide), leprosy (clofazimine), multiple myeloma (bortezomib), and hypertension (bosentan).
Dumb and Dumber
There are just some things that one can’t make up, including a few popular therapeutics.
| |
FDA Clinical Trials |
Google Scholar |
Mouse Papers |
| Trump Recommended |
Total |
Completed |
Results |
| Azithromycin |
115 |
42 |
15 |
55,200 |
19 |
| Hydroxychloroquine |
237 |
85 |
29 |
64,200 |
32 |
| Other |
|
|
|
|
|
|
| Ivermectin |
91 |
34 |
14 |
25,700 |
40 |
| |
|
|
|
|
|
|
Regarding ivermectin which treats infections caused by roundworms, threadworms, and other parasites, the FDA said, “You are not a horse, you are not a cow. Seriously, y’all. Stop it.”
What’s Next
Present antivirals, if used early, when combined with vaccination are quite effective. While variants have disabled all monoclonal antibodies, the MRPO and RdRp drugs, have been mutation resistant. That might not continue to be the case. Thus, working on mutation resistant antivirals would be wise.
I think the remaining big challenge is hyperinflammation. All approved drugs address the result, not the cause. Three attacks might provide good results.
Determine how to keep the RAAS system from being dysregulated.
Protect the innate immune system from nonstructural proteins attack.
Attack highly inflammatory cytokines, e.g. IL-6.
Summary and Conclusions
Even though there were 4855 FDA COVID-19 clinical trials through October 2024, none of the FDA or EU approved therapeutics was discovered during the pandemic. Fortunately, the impressive arsenal of therapeutics which were discovered before the pandemic are quite effective
if they are used early.
| Prevention |
| mRNA vaccines |
They are the most important drug to avoid severe COVID. |
| Pemgarda |
A monoclonal for immunocompromised people. Originally 84% risk reduction through six months. KP.3.1.1 and XEC impacted effectiveness. |
| Suppress Viral Replication and Inflammation - Antivirals |
| Monoclonal antibodies |
About an 85% mortality reduction early in the pandemic. All but Pemgarda were completely disabled by variants. |
| PAXLOVID |
There are many different assessments of impact. There was 89% mortality reduction in unvaccinated in a trial where 80% took PAXLOVID and 20% took Molnupiravir. |
| Remdesivir |
It is often recommended in combination with another therapeutic. It is the NIH’s choice after PAXLOVID though it must be hospital administered. |
| Molnupiravir |
This is a very good pill choice if one can’t take PAXLOVID. |
| Suppress Hyperinflammation - Anti-inflammatories |
| Dexamethasone |
None is a magic bullet. Dexamethasone is the most frequently used followed by baricitinib. Use recommendations are quite complex. Remdesivir is often co-recommended. |
| Anakinra |
| Baricitinib |
| Tocilizumab |
| Suppress Organ Damage - Lungs insufficient oxygenation |
| ECMO |
After other less aggressive oxygenation approaches have been attempted, ECMO is used. 36% mortality early start vs. 58.9% late start. For patients receiving prolonged mechanical ventilation, the one-year and five-year survival rates were 24.3% and 14.6%. |
| Vilobelimab |
It is a monoclonal antibody with a 31.7%, 28-day mortality vs. 41.6% for the placebo. |
| Suppress Organ Damage - Cardiovascular clotting |
| Heparin |
It must be used with extreme care because of bleeding risks |
| Though the NIH is silent on the following therapeutics, they are worth considering. |
|
|
|