Graphical Abstract
1. Introduction
Onychomycosis, also referred to as tinea unguium, is a fungal infection affecting the toenails or fingernails, leading to discoloration, thickening, surface irregularities, and separation from the nail bed [
1,
2]. It is primarily caused by dermatophytes, yeast, and non-dermatophyte moulds, with common pathogens including
Trichophyton rubrum, Trichophyton mentagrophytes,
Epidermophyton floccosum,
Candida albicans, and
Trichophyton inerdigitale [
3]. A transungual drug delivery system targets the infection through the keratinized nail plate, offering advantages such as minimal systemic side effects, ease of removal, prolonged medication residence time, and reduced risk of drug-drug interactions, particularly in elderly patients or those on multiple medications [
4]. Sertaconazole nitrate, an imidazole antifungal with broad-spectrum activity, is effective against the causative organisms of onychomycosis by inhibiting ergosterol synthesis, a crucial component of the fungal cell wall [
5]. Despite its efficacy, traditional transungual delivery systems face challenges such as inadequate drug penetration through the nail plate, limited vascular access to the nail bed, and short residence times of topical treatments [
6,
7]. To address these issues, novel formulations of Sertaconazole nitrate were developed to enhance drug release, increase permeability, and prolong residence time, thereby improving efficacy and patient compliance.
2. Materials and Methods
Sertaconazole nitrate was obtained as a gift from Micro Labs Ltd., Banglore. Propylene glycol,PEG 400, Polymethyl methacrylate, Ethyl cellulose, Tween 80 and Tween 20 was obtained from S.D. Fine Chemicals Ltd., Mumbai. Peceol, Maisine and Labrafil M 1944CS was procured from Gattefose, Mumbai, India. Other chemicals used were of pharmaceutical and analytical grade.
2.1. Emulsion Stability Testing
After selecting the oils, surfactants, and co-surfactants, emulsions were prepared with Surfactant: Co-surfactant (S: CoS) ratios of 1:1 and 1:2, and oil was added to the S: CoS mix in a 1:4 ratio [
8]. The emulsion concentrates underwent various stability tests for optimization:
Freeze-Thaw Cycles: Emulsions underwent three freeze-thaw cycles, alternating between -5°C for 24 hours and 35°C for the next 24 hours, and were checked for precipitation, phase separation, or turbidity.
Heat-Cool Cycles: Emulsions were subjected to three heat-cool cycles by heating at 40°C for 6 hours, followed by cooling in an ice bath and maintaining at room temperature for 18 hours. Visual inspections were done for signs of instability.
Centrifugation: Emulsions were centrifuged at 1000 rpm for 15 minutes and assessed for phase separation or turbidity.
2.2. Dispersibility Test
The Dispersibility test was conducted using a USP XXII dissolution apparatus II. In this test, 1 ml of each formulation was added to 500 ml of water at 37°C ± 1°C, with gentle agitation provided by a stainless-steel paddle rotating at 50 rpm 9. The formulations were visually evaluated based on the following grading system:
Grade A: Forms a clear or bluish nano-emulsion within 1 minute.
Grade B: Forms a slightly less clear bluish-white emulsion within 1 minute.
Grade C: Forms a fine milky emulsion within 2 minutes.
Grade D: Forms a dull, greyish-white emulsion with a slightly oily appearance, taking longer than 2 minutes to emulsify.
Grade E: Shows poor emulsification with large oil globules on the surface.
2.3. Preparation of Micro Emulsion Concentrate
Self Microemulsifying concentrate were prepared by mixing required quantity of oils, Surfactant and Co-surfactants as shown in
Table 1.
2.4. Selection of Self Microemulsifying Drug Delivery System
The drug’s solubility in the selected oil ratio was evaluated by adding a fixed dose to the mixture, shaking for 24 hours, and then centrifuging for 10 minutes at 3000 rpm [
10]. The supernatant was filtered, and Sertaconazole nitrate’s UV absorbance was measured at 259.60 nm after methanol dilution.
2.5. Characterization of SMEDDS
2.5.1. Drug Content
1 ml of the emulsion was diluted to 10 ml with methanol. From this, 1 ml was further diluted to 10 ml with methanol. The drug content was then measured using UV absorbance at 259.60 nm [
11].
2.5.2. Particle size Distribution
The size distribution of globules of the formulation was measured by dynamic light scattering particle size analyzer [
12].
2.5.3. Zeta Potential
Zeta potential was measured by using Zetameter instrument
2.6. Formulation of Transungual Patches
2.6.1. Formulation of Backing Membrane
The ring bottom was wrapped with aluminum foil on which backing membrane was casted by pouring polymeric solution of ethyl cellulose and kept for drying at controlled rate using an inverted funnel as shown in
Table 2.
2.6.2. Formulation of Plain Drug Matrix
The matrix was prepared by solvent evaporation method in which homogenous dispersion of Sertaconazole nitrate with optimized ratio of Polymethylmethacrylate (PMMA) and chitosan was prepared [
13]. The composition of plain drug matrix is shown in
Table 3 and
Table 4.
The plasticizer was added to above polymeric dispersion and was poured on the backing membrane in petri dish and was kept at room temperature for controlled evaporation. The dried patches were kept in desiccator until use [
14].
2.6.3. Drug in Transcutol
The drug was dissolved in Transcutol and mixed with an optimized Chitosan ratio. A plasticizer was added, and the polymeric dispersion was poured onto a backing membrane in a petri dish. After 6 hours of slow evaporation, the dried drug-polymer matrix patches were stored in a desiccator until use.
2.6.4. Drug in Self Microemulsifying Drug Delivery System
The drug was dissolved in SMEDDS and mixed with an optimized Chitosan ratio. A plasticizer was added, and the polymeric dispersion was poured onto a backing membrane in a petri dish. The Chitosan dispersion was dried uniformly at room temperature for 6 hours to form polymer matrix patches, which were then stored in a desiccator until use. The composition of the drug-loaded transungual patches is detailed in
Table 5.
2.7. Evaluation of Transungual Patches
2.7.1. Appearance
The drug loaded transungual patches were visually examined for color, physical form or appearance.
2.7.2. Folding Endurance
The number of times the film could be folded at the same place without breaking is the folding endurance of the film.
2.7.3. Percentage Moisture Loss
The films were stored in a desiccator with activated silica at room temperature for 24 hours. They were weighed repeatedly until a constant weight was achieved [
15,
16]. The moisture content percentage was calculated based on the difference between the initial and final weights relative to the final weight.
2.7.4. Percentage of Moisture Uptake
A weighed film, stored in a desiccator at room temperature for 24 hours, was exposed to 75% relative humidity (potassium chloride solution) in a desiccator until a constant weight was achieved [
17].
2.7.5. Thickness and Weight Variation
Patch thickness was measured with a digital vernier caliper, while weight variation was assessed by cutting patches from six different locations and weighing them with a digital balance. Average weight and standard deviation were then calculated.
2.7.6. Drug Content
The patch was taken into a 100 ml volumetric flask containing methanol and sonicated for 2h. Subsequent dilutions were made and analyzed using UV spectrophotometer at the wavelength maxima (λ
max) of 259.60nm [
18].
2.7.7. In-Vitro Diffusion Study
In-vitro diffusion studies were performed using a Franz diffusion cell at 37±5°C with an egg shell membrane. The receptor compartment, filled with 25 ml Phosphate buffer pH 7.4 and SLS (50:1 ratio), held a 1x1 cm patch in the donor compartment, drug side facing the membrane. The system was stirred at 600 rpm for 7 hours [19]. At each hour, 5 ml samples were withdrawn and replaced with fresh solvent. Each experiment was conducted in triplicate, with drug analysis at 261.40 nm using a UV-VIS Spectrophotometer.
2.7.8. Kinetic Analysis
Various models were tested for explaining the kinetics of drug release. To analyze the mechanism of the drug release rate kinetics of the dosage form, the obtained data were fitted into zero-order, First order, Higuchi and Korsmeyer-Peppa’s release model [20,21,22].
Results
The emulsion concentrates were tested for emulsion stability. The formulations were subjected to Freeze thawing, Heat- cool cycle and centrifugation. As shown in
Table 6 formulation A1, A2, A3 and A4 were found to be adequately stable over a long period of time and devoid of any phase separation or turbidity. Hence, these formulations were chosen for the preparation of micro-emulsion and characterization studies.
The formulations were subjected to dispersibility test and the formulation A1 and A2 were categorized as grade D i.e., these were the emulsions with oily appearance. While A3 and A4 were grade C i.e., milky emulsion. Thus, these prototypes were used for preparation of SMEDDS.
Sertaconazole nitrate (80 mg) was added to a mixture of surfactants, co-surfactants, and oil, and stirred for 24 hours to reach equilibrium. The most stable microemulsions were selected for characterization. Based on solubility, stability, and dispersibility tests, A4 was chosen as the optimized SMEDDS system for incorporation into the transungual patch. The drug content in all transungual formulations ranged from 98.5% to 101% of Sertaconazole nitrate, as per BP standards. The globule size of the SMEDDS (A4) was 200 nm (50th percentile) and its zeta potential was -32 mV, indicating moderate stability.
The transungual patches were assessed for thickness, weight, moisture content, moisture uptake, and folding endurance. Patches F2CA, F2CB, F2CC, and A6PD exhibited optimal properties. Those with a dibutyl phthalate to plasticizer ratio of 15%:15% were notably flexible, elastic, and smooth. All patches demonstrated excellent film flexibility with folding endurance exceeding 50 folds. Moisture content ranged from 0.4% to 2%, indicating stability and resistance to becoming brittle. Hydrophilic polymers absorbed more moisture (0.1%–3.0%) compared to hydrophobic ones, which showed lower absorption. Thickness and weight variations among the patches were minimal.
The drug content of all transungual patch formulations adhered to the Sertaconazole nitrate limits of 98.5%-101% as specified by BP. Specifically, F2CA had a drug content of 100.1%, F2CB 99.69%, F2CC 99.92%, and A6PD 100.8%, demonstrating that all formulations were within the acceptable range.
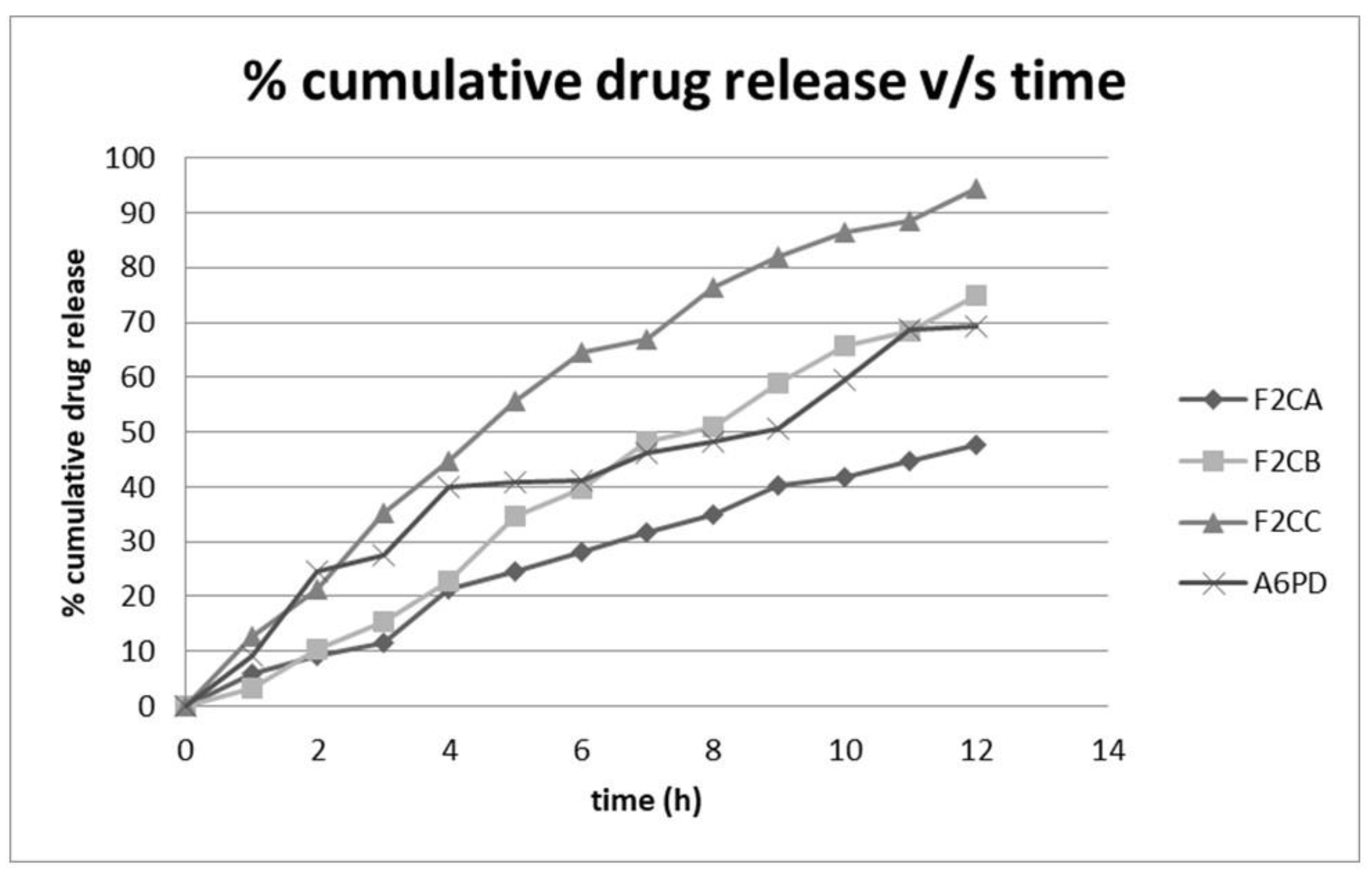
In the in vitro drug release study of Sertaconazole nitrate from transungual patches (F2CA, F2CB, F2CC, and A6PD) conducted in phosphate buffer pH 7.4 over 12 hours, the cumulative drug release varied among the formulations. F2CA and F2CB showed slower release profiles, with F2CA reaching 47.61% and F2CB 74.78% by the end of the study. In contrast, F2CC and A6PD exhibited higher release rates, with F2CC achieving 94.52% and A6PD 69.2% cumulative drug release as shown in
Figure 1.
Kinetic analysis of the in-vitro diffusion profiles of F2CA, F2CB, F2CC, and A6PD showed that all formulations best fit the first-order release model, as indicated by the higher r2 values compared to the zero-order model. The Korsmeyer-Peppas model revealed varying n values, suggesting different release mechanisms across the formulations.
3. Discussion
The screening of penetration enhancers revealed that Span 80 and PEG 200 had minimal impact on nail thickness, while thioglycolic acid increased it, possibly due to hydration and swelling, creating pores for drug diffusion. Oleic acid and transcutol decreased nail thickness, enhancing drug solubility and permeation through their amphiphilic nature. Saturation solubility studies indicated that Peceol, Labrafil M 1944, and Transcutol exhibited higher solubility for Sertaconazole nitrate. The chosen penetration enhancers were then incorporated into self-micro emulsifying drug delivery systems (SMEDDS) for improved drug solubility and permeability. The selected formulations demonstrated stability, making them suitable for further development.
Dispersibility tests categorized formulations A1 and A2 as grade D (oily appearance) and A3 and A4 as grade C (milky emulsion). Formulation A4, with the highest solubility, stability, and desirable dispersibility, was selected as the optimized SMEDDS system for incorporation into transungual patches. The evaluation of transungual patches (A1P, A2P, A3P, A4P, A5P, A6P, F1C, F2C, F3C, F4C, F5C, F6C, F2CA, F2CB, F2CC, A6PD) encompassed parameters like thickness, weight, moisture content, moisture uptake, and folding endurance. Patches F2CA, F2CB, F2CC, and A6PD demonstrated favourable properties, with A6PD showing good flexibility and stability.
Drug content analysis confirmed that all formulations complied with specified limits. In vitro drug release studies indicated varied release profiles among formulations, with A6PD demonstrating 69.2% release, while F2CB and F2CC exhibited higher releases of 74.78% and 94.52%, respectively, after 12 hours. The kinetic analysis suggested that the release mechanism followed first-order kinetics with non-Fickian diffusion.
4. Conclusion
The development and evaluation of transungual patches containing Sertaconazole nitrate for the treatment of onychomycosis proved to be a promising approach. The study successfully addressed critical challenges associated with drug penetration across the nail plate by employing the Self-Microemulsifying Drug Delivery System (SMEDDS) technique. This technique not only improved the solubility and permeability of Sertaconazole nitrate but also facilitated the formulation of patches with favourable characteristics. The comprehensive evaluation of various patch formulations, considering parameters such as thickness, weight variation, drug content, folding endurance, moisture content, moisture uptake, and in vitro drug release, provided a thorough understanding of the developed transungual patches. The utilization of kinetic models for analyzing the in vitro release data further contributed valuable insights into the mechanism of drug release from the patches.
The selected formulations demonstrated stability under accelerated conditions according to International Conference on Harmonization (ICH) guidelines, highlighting their potential for stable and effective transungual drug delivery systems. This indicates a significant advancement in the treatment of onychomycosis, offering a targeted and sustainable solution for enhanced therapeutic outcomes.
References
- Zaias, N. The nail in health and disease. 2nd edition. Appleton and Lange, Connecticut; 1990:1-255.
- Chabasse D, Baran R, De Chauvin MF. Onychomycosis 1: epidemiology and etiology. J Mycol Med. 2000; 10:177-190.
- Baran R, Hay R, Tosti A and Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998; 139:567-571.
- Baran R, Kaoukhov A. Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J Eur Acad Dermatol Venereol. 2005; 19:21-29.
- Barot B, Parejiya P, Patel H, Mehta D, Shelat P. Microemulsion-based antifungal gel delivery to nail for the treatment of onychomicosis: formulation, optimization, and efficacy studies. AAPS PharmSciTech. 2012 March; 13(1):184-192.
- Bhowmik D, Kumar K. Recent trends in dermal and transdermal drug delivery systems: current and future prospects. Pharma Innov. 2013; 2(6).
- Bseiso E, Nasr M, Sammour O. Novel nail penetration enhancer containing vesicles nPEVs for the treatment of onychomicosis. J Drug Deliv. 2: 2016; 23(8), 2016.
- Chein, YW. Transdermal drug delivery and delivery system. Novel Drug Delivery System. 2nd ed. New York: M. Dekker; 2009; 50(2): 301-380.
- Chen Y, Quan P, Liu X, Wang M. Fang L. Review on novel chemical permeation enhancers for transdermal drug delivery. Asian J Pharm Sci. 2014; 9:51-64.
- Chouhan P, Saini T. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm. 2012; 426(1-2):179-182.
- Chouhan P, Saini T. D-optimal Design and development of microemulsion based transungual drug delivery formulation of ciclopirox olamine for the treatment of onychomicosis. Indian J Pharm Sci. 2016; 78(4):498-511.
- Chouhan P, Saini TR. Hydroxypropyl-ß-cyclodextrin: A novel transungual permeation enhancer for development of topical drug delivery system for onychomycosis. J Drug Deliv. 1: 2014, 2014.
- Cohen P, Scher R. Topical and surgical treatment of onychomycosis. J Am Acad Dermatol. 1994; 31:74-77.
- Dash S, Murthy P, Nath L, Chowdhary P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Poloniae Pharmaceutica Drug Research. 2010; 67(3):217-223.
- Deepthi BV, Poornachandra Rao K, Chennapa G, Naik MK, Chandrashekara KT, Sreenivasa MY. Antifungal attributes of Lactobacillus plantarum MYS6 against fumonisin producing Fusarium proliferatum associated with poultry feeds. PLoS One. 2016 Jun 10;11(6):e0155122.
- Higuchi, T. Mechanism of sustained- action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. Journal of Pharmaceutical Sciences. 1963; 52:114-1149.
- Jha S, Dey S, Karki R. Microemulsions- potential carrier for improved drug delivery.Asian Journal of Biomedical and Pharmaceutical Sciences. 2011; 1(1):5-9.
- Kansagra H, Subrata M, Asif K. Formulation and evaluation of transdermal patch of sertaconazole nitrate. International research Journal of Pharmacy. 2012; 3(11):109-113.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).