Submitted:
17 November 2024
Posted:
19 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. European Regions Analysis
2.2. Statistical Analysis
- “DTP3t” represents the DTP3 vaccination coverage in year t.
- Year is the year of the calendar.
- “Pandemic “is a dummy variable, taking 1 for the Covid-19 pandemic years (2020–2023) and 0 for pre-pandemic years.
2.3. Software and Tools
3. Results
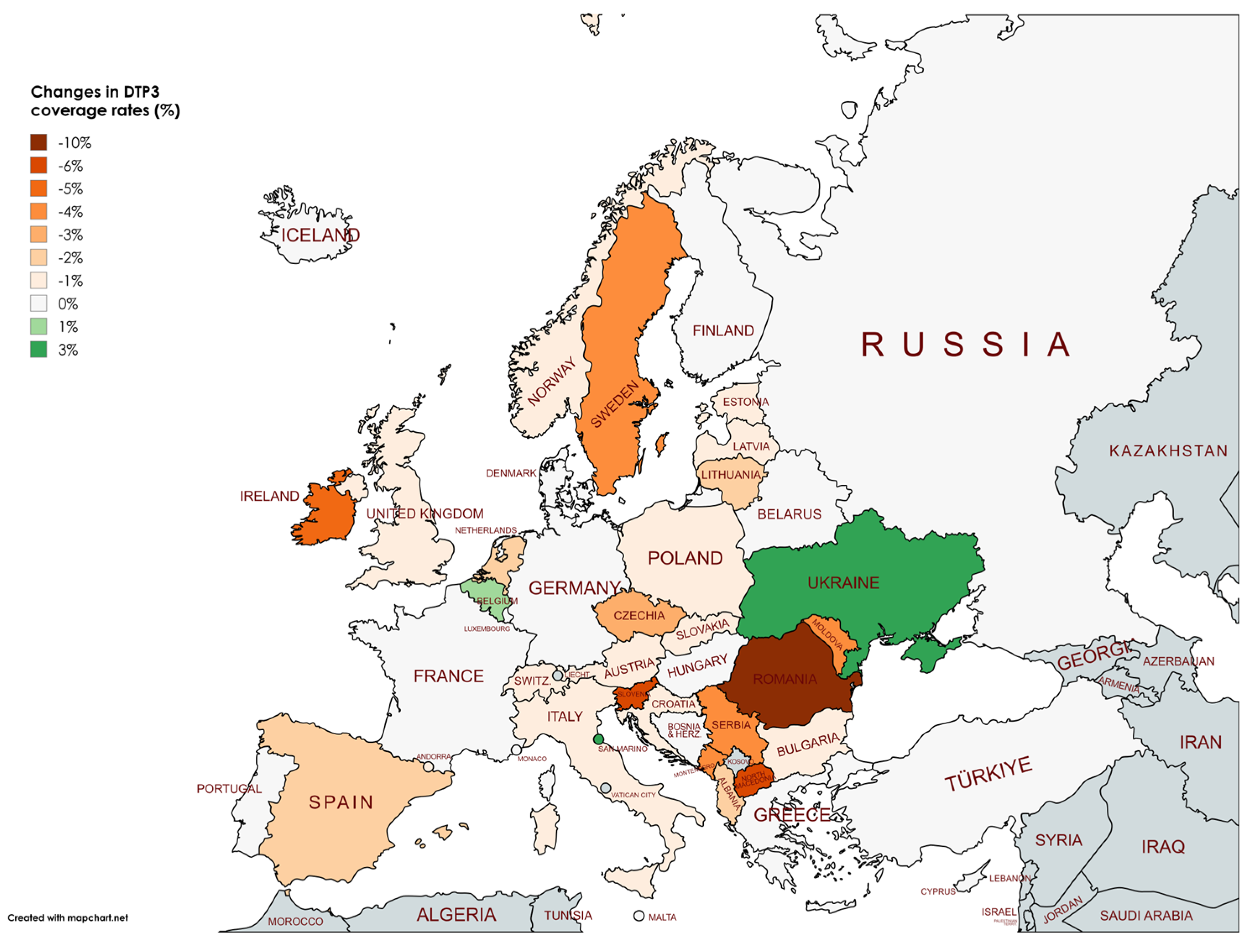
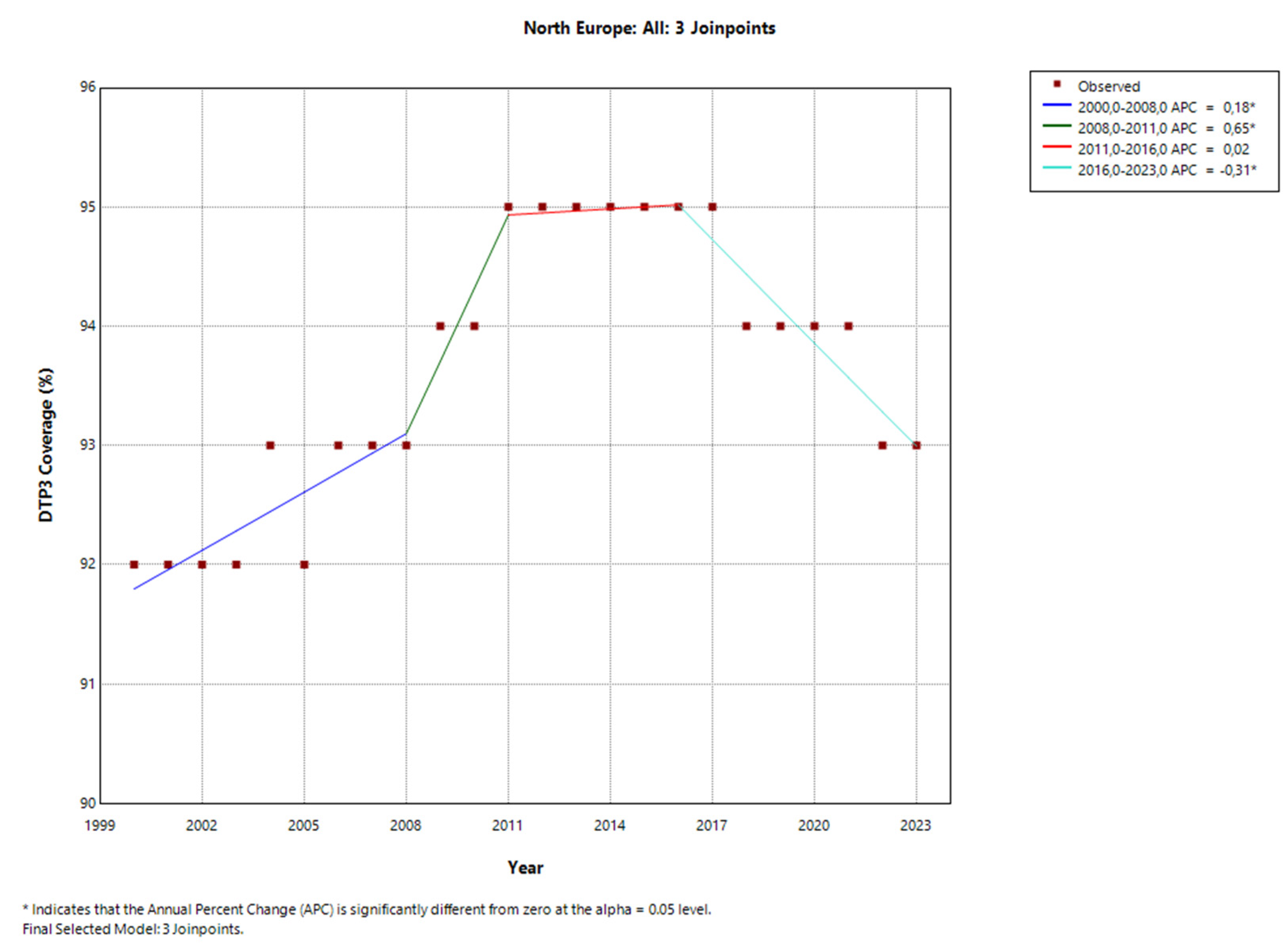
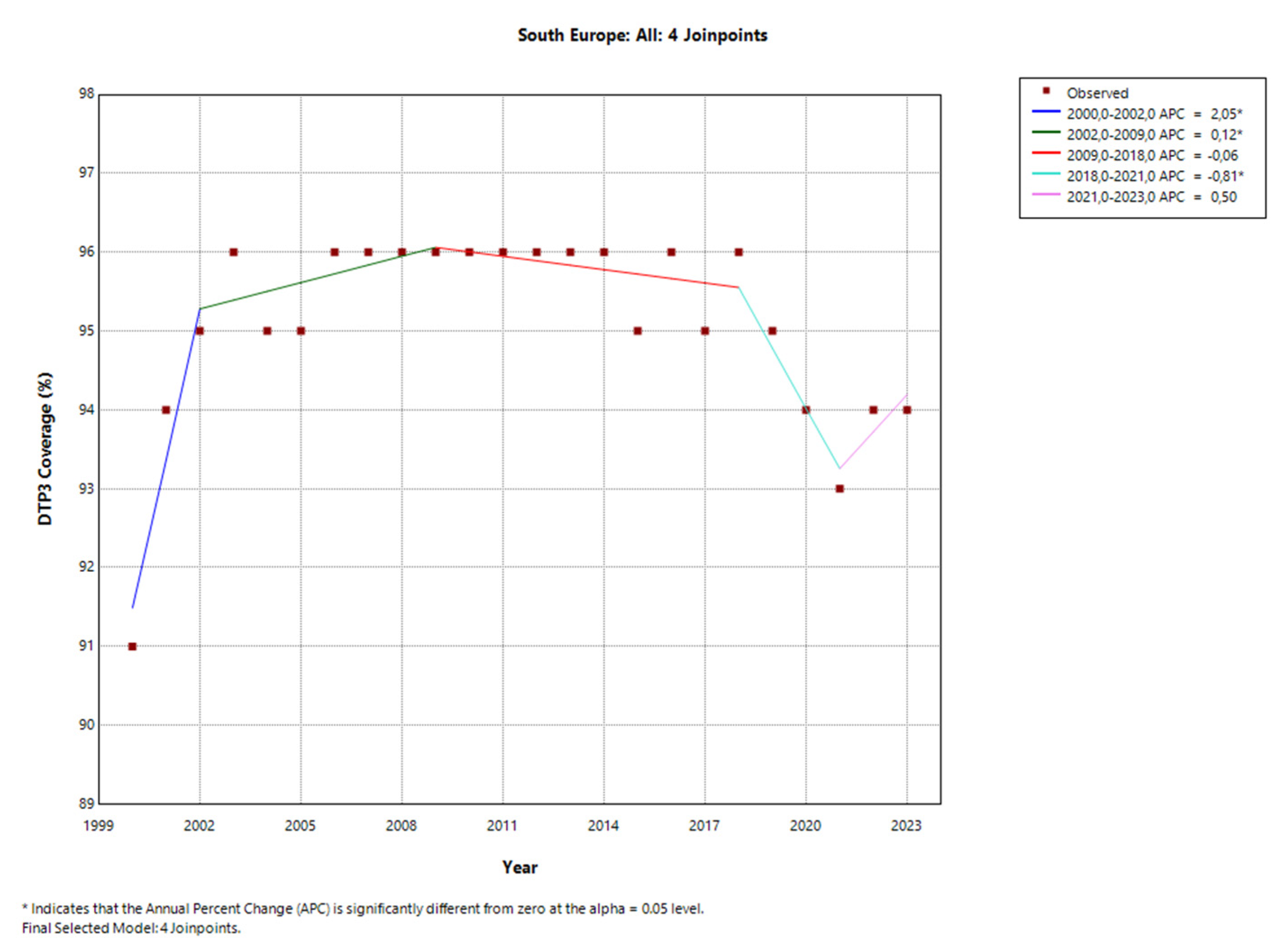
4. Discussion
4.1. Limitations
4.2. Country by Country Analysis
4.1.1. Albania
4.1.2. Austria
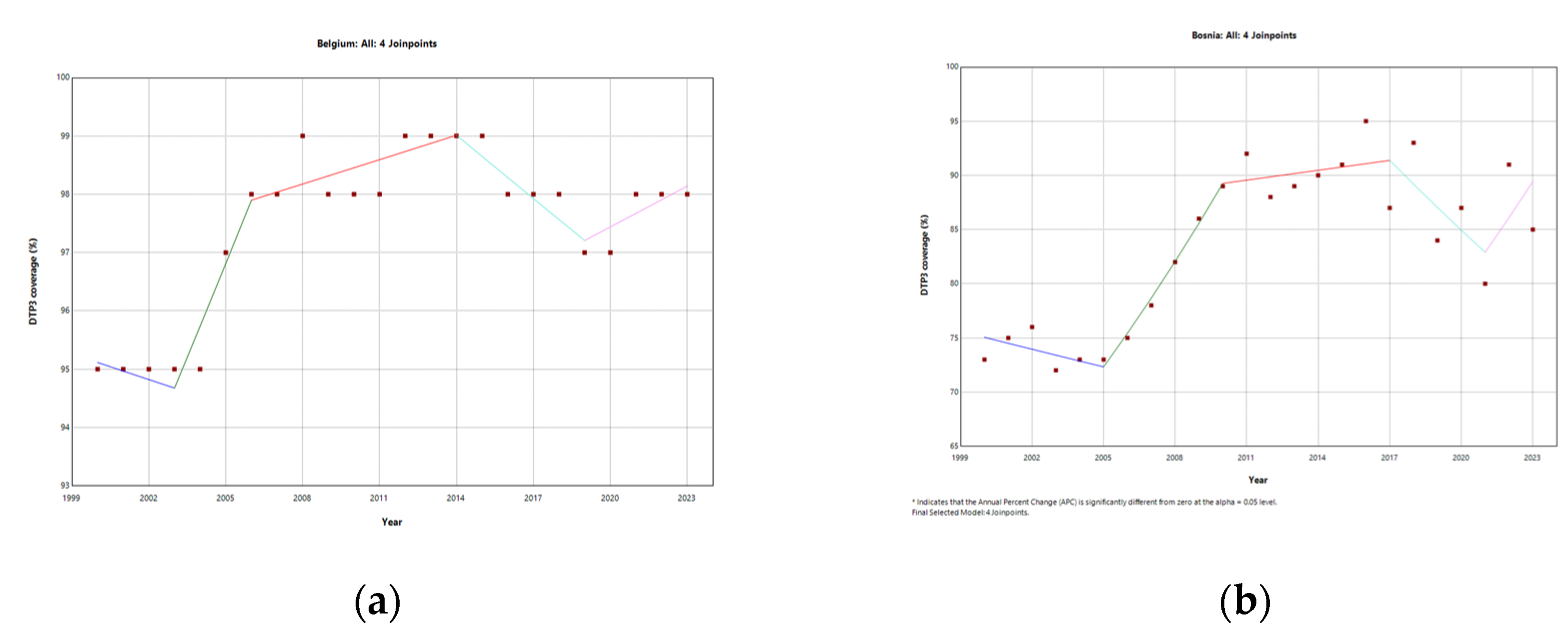
4.1.3. Belgium
4.1.4. Bosnia and Herzegovina
4.1.5. Bulgaria
4.1.6. Croatia
4.1.7. Cyprus
4.1.8. Czech Republic
4.1.9. Estonia
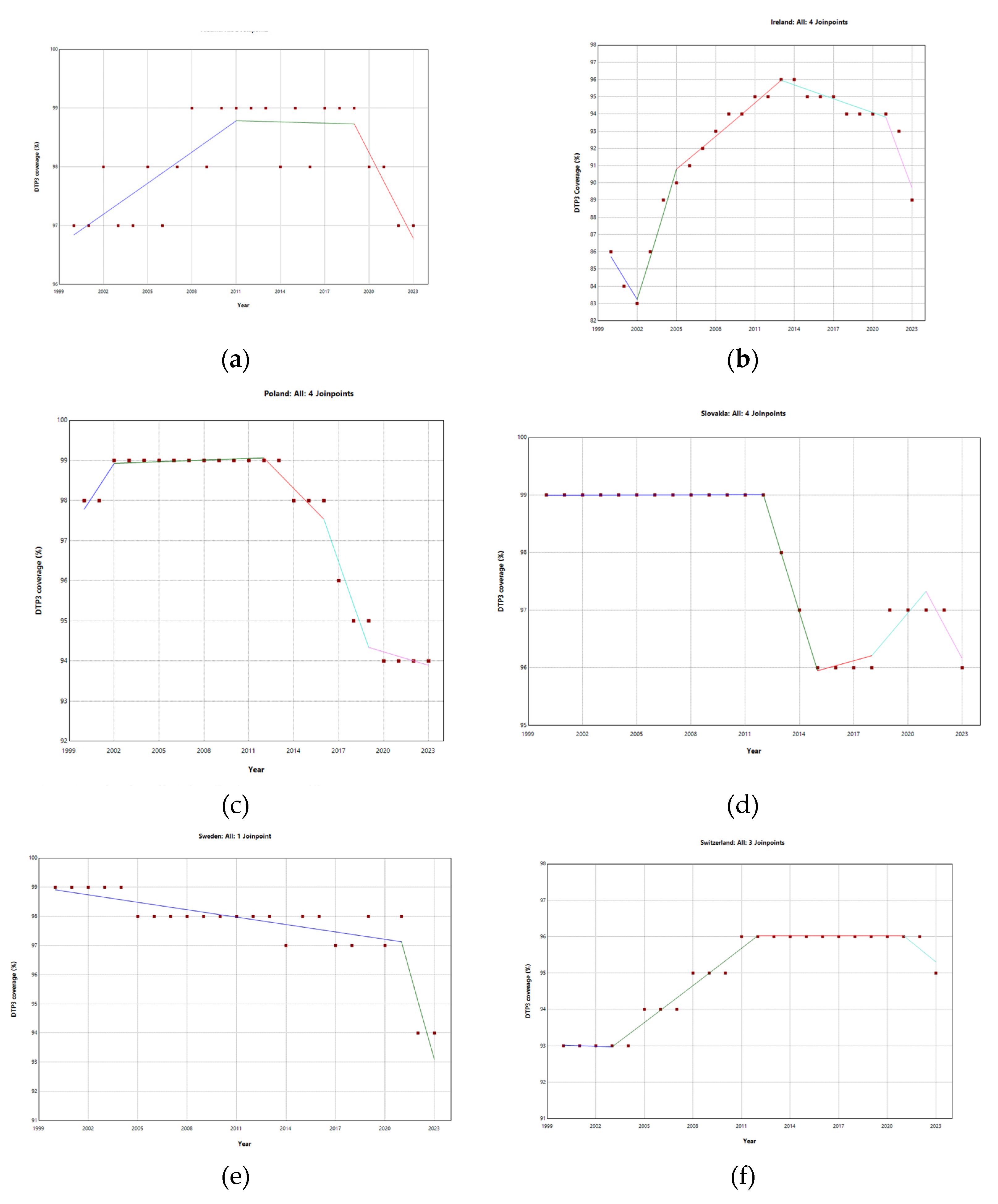
4.1.10. Ireland
4.1.11. Italy
4.1.12. Latvia
4.1.13. Lithuania
4.1.14. Moldova
4.1.15. Montenegro
4.1.16. Netherlands
4.1.17. North Macedonia
4.1.18. Norway
4.1.19. Poland
4.1.20. Romania
4.1.21. Serbia
4.1.22. Slovenia
4.1.23. Spain
4.1.24. Sweden
4.1.25. Switzerland
4.1.25. Türkiye
4.1.26. Ukraine
4.1.27. United Kingdom
4.3. Strategies for Enhancing DTP3 Vaccination Coverage in Europe Amidst and Beyond COVID-19 Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aguinaga-Ontoso, I.; Guillen-Aguinaga, S.; Guillen-Aguinaga, L.; Alas-Brun, R.; Onambele, L.; Aguinaga-Ontoso, E.; Guillen-Grima, F. COVID-19 Impact on DTP Vaccination Trends in Africa: A Joinpoint Regression Analysis. Vaccines (Basel) 2023, 11, 1103. [Google Scholar] [CrossRef]
- Aguinaga-Ontoso, I.; Guillén-Aguinaga, S.; Guillén-Aguinaga, L.; Alas-Brun, R.; Aguinaga-Ontoso, E.; Rayón-Valpuesta, E.; Guillén-Grima, F. Has COVID-19 Affected DTP3 Vaccination in the Americas? Vaccines (Basel) 2024, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Skirrow, H.; Bedford, H. Routine Vaccination during Covid-19 Pandemic Response. BMJ 2020, m2392. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Haq, F.U.; Imran, M.; Shah, A.; Bibi, N.; Khurshid, R.; Romman, M.; Gaffar, F.; Khan, M.I. Impact of the COVID-19 Lockdown on Routine Vaccination in Pakistan: A Hospital-Based Study. Hum Vaccin Immunother 2024, 17, 4934–4940. [Google Scholar] [CrossRef]
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the Impact of COVID-19 Vaccine Misinformation on Vaccination Intent in the UK and USA. Nat Hum Behav 2021, 5, 337–348. [Google Scholar] [CrossRef]
- Allington, D.; McAndrew, S.; Moxham-Hall, V.L.; Duffy, B. Media Usage Predicts Intention to Be Vaccinated against SARS-CoV-2 in the US and the UK. Vaccine 2021, 39, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Cellini, M.; Pecoraro, F.; Rigby, M.; Luzi, D. Comparative Analysis of Pre-Covid19 Child Immunization Rates across 30 European Countries and Identification of Underlying Positive Societal and System Influences. PLoS One 2022, 17, e0271290. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Naseem, R.; Salam, R.A.; Siddiqui, F.; Das, J.K. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int J Environ Res Public Health 2021, 18, 988. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Medic, S.; Cassimos, D.C.; Effraimidou, E.; Poland, G.A. Decreasing Routine Vaccination Rates in Children in the COVID-19 Era. Vaccine 2022, 40, 2525–2527. [Google Scholar] [CrossRef]
- Bramer, C.A.; Kimmins, L.M.; Swanson, R.; Kuo, J.; Vranesich, P.; Jacques-Carroll, L.A.; Shen, A.K. Decline in Child Vaccination Coverage During the COVID-19 Pandemic — Michigan Care Improvement Registry, May 2016–May 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 630–631. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Borrow, R.; Safadi, M.A.P.; van Damme, P.; Munoz, F.M. Vaccines and Routine Immunization Strategies during the COVID-19 Pandemic. Hum Vaccin Immunother 2021, 17, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.; Nathawad, R.; Arpin, E.; Johnson, S. Pandemics, Epidemics and Inequities in Routine Childhood Vaccination Coverage: A Rapid Review. BMJ Paediatr Open 2020, 4, e000842. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Samara, A.; Campbell, H.; Ladhani, S.N.; Amirthalingam, G. Recent Increase in Infant Pertussis Cases in Europe and the Critical Importance of Antenatal Immunizations: We Must Do Better…now. International Journal of Infectious Diseases 2024, 146, 107148. [Google Scholar] [CrossRef]
- World Health Organization. Half a million children in WHO European Region not fully vaccinated in their first year of life: New 2023 data reveal both gaps and gains in immunization coverage https://www.who.int/europe/news/item/15-07-2024-half-a-million-children-in-who-european-region-not-fully-vaccinated-in-their-first-year-of-life--new-2023-data-reveal-both-gaps-and-gains-in-immunization-coverage (accessed 2024 -10 -19).
- World Health Organization. European Region achieves high routine immunization coverage, but falls short of pre-pandemic levels.
- Director-General WHO. Immunization Agenda 2030 Progress towards Global Immunization Goals and Implementation of the Immunization Agenda 2030.EXECUTIVE BOARD EB154/9 154th Session 4 December 2023 Provisional Agenda Item 9 Immunization Agenda 2030 Progress towards Global Immunization Goals and Implementation of the Immunization Agenda 2030 Report by the Director-General; Geneva, 2023.
- WHO The Global Health Observatory. Immunization and vaccine-preventable communicable diseases.
- WHO. Varied impact of COVID-19 on routine immunization in the European Region https://www.who.int/europe/news/item/16-07-2021-varied-impact-of-covid-19-on-routine-immunization-in-the-european-region.
- WHO. COVID-19 pandemic leads to major backsliding on childhood vaccinations, new WHO, UNICEF data shows https://www.who.int/news/item/15-07-2021-covid-19-pandemic-leads-to-major-backsliding-on-childhood-vaccinations-new-who-unicef-data-shows (accessed 2024 -10 -20).
- Effraimidou, E.; Cassimos, D.C.; Medic, S.; Topalidou, M.; Theodoridou, M.; Maltezou, H.C. Vaccination Programs for Children Aged up to 18 Years in Europe, 2020. Journal of Child Health Care 2023, 27, 336–350. [Google Scholar] [CrossRef]
- Saidu, Y. Exploring Barriers to Immunization Coverage within a Sub-Saharan African Setting, Università degli Studi di Siena, Siena, 2023.
- Ackerson, B.K.; Sy, L.S.; Glenn, S.C.; Qian, L.; Park, C.H.; Riewerts, R.J.; Jacobsen, S.J. Pediatric Vaccination During the COVID-19 Pandemic. Pediatrics 2021, 148. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.; Truche, P.; Sousa Salgado, L.; Meireles, T.; Santana, V.; Buda, A.; Bentes, A.; Botelho, F.; Mooney, D. The Impact of COVID-19 on Routine Pediatric Vaccination Delivery in Brazil. Vaccine 2022, 40, 2292–2298. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Medic, S.; Cassimos, D.C.; Effraimidou, E.; Poland, G.A. Decreasing Routine Vaccination Rates in Children in the COVID-19 Era. Vaccine 2022, 40, 2525–2527. [Google Scholar] [CrossRef] [PubMed]
- Santoli, J.M.; Lindley, M.C.; DeSilva, M.B.; Kharbanda, E.O.; Daley, M.F.; Galloway, L.; Gee, J.; Glover, M.; Herring, B.; Kang, Y.; Lucas, P.; Noblit, C.; Tropper, J.; Vogt, T.; Weintraub, E. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration — United States, 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 591–593. [Google Scholar] [CrossRef]
- Ji, C.; Piché-Renaud, P.-P.; Apajee, J.; Stephenson, E.; Forte, M.; Friedman, J.N.; Science, M.; Zlotkin, S.; Morris, S.K.; Tu, K. Impact of the COVID-19 Pandemic on Routine Immunization Coverage in Children under 2 Years Old in Ontario, Canada: A Retrospective Cohort Study. Vaccine 2022, 40, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Cabrera, M.; Moore, M.; Lomazzi, M. Driving Paediatric Vaccine Recovery in Europe. Vaccines (Basel) 2023, 11, 184. [Google Scholar] [CrossRef]
- Polašek, O.; Wazny, K.; Adeloye, D.; Song, P.; Chan, K.Y.; Bojude, D.A.; Ali, S.; Bastien, S.; Becerra-Posada, F.; Borrescio-Higa, F.; Cheema, S.; Cipta, D.A.; Cvjetković, S.; Castro, L.D.; Ebenso, B.; Femi-Ajao, O.; Ganesan, B.; Glasnović, A.; He, L.; Heraud, J.M.; Igwesi-Chidobe, C.; Iversen, P.O.; Jadoon, B.; Karim, A.J.; Khan, J.; Biswas, R.K.; Lanza, G.; Lee, S.W.; Li, Y.; Liang, L.-L.; Lowe, M.; Islam, M.M.; Marušić, A.; Mshelia, S.; Manyara, A.M.; Htay, M.N.; Parisi, M.; Peprah, P.; Sacks, E.; Akinyemi, K.O.; Shahraki-Sanavi, F.; Sharov, K.; Rotarou, E.S.; Stankov, S.; Supriyatiningsih, W.; Chan, B.T.; Tremblay, M.; Tsimpida, D.; Vento, S.; Glasnović, J.V.; Wang, L.; Wang, X.; Ng, Z.X.; Zhang, J.; Zhang, Y.; Campbell, H.; Chopra, M.; Cousens, S.; Krstić, G.; Macdonald, C.; Mansoori, P.; Patel, S.; Sheikh, A.; Tomlinson, M.; Tsai, A.C.; Yoshida, S.; Rudan, I. Research Priorities to Reduce the Impact of COVID-19 in Low- and Middle-Income Countries. J Glob Health 2022, 12, 09003. [Google Scholar] [CrossRef]
- Jovanović, V.; Lazić, M. Vaccination Attitudes Examination (VAX) Scale: A Bifactor-ESEM Approach in a Youth Sample (15–24 Years). BMC Psychol 2023, 11, 351. [Google Scholar] [CrossRef]
- Tzanakaki, G.; Cabrnochová, H.; Delić, S.; Draganescu, A.; Hilfanova, A.; Onozó, B.; Pokorn, M.; Skoczyńska, A.; Tešović, G. Invasive Meningococcal Disease in South-Eastern European Countries: Do We Need to Revise Vaccination Strategies? Hum Vaccin Immunother 2024, 20. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga-Ontoso, I.; Guillen-Aguinaga, S.; Guillen-Aguinaga, L.; Alas-Brun, R.; Onambele, L.; Aguinaga-Ontoso, E.; Guillen-Grima, F. COVID-19 Impact on DTP Vaccination Trends in Africa: A Joinpoint Regression Analysis. Vaccines (Basel) 2023, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga-Ontoso, I.; Guillén-Aguinaga, S.; Guillén-Aguinaga, L.; Alas-Brun, R.; Aguinaga-Ontoso, E.; Rayón-Valpuesta, E.; Guillén-Grima, F. Has COVID-19 Affected DTP3 Vaccination in the Americas? Vaccines (Basel) 2024, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- United Nations. WHO: “Alarming” rise of measles cases in Europe https://unric.org/en/who-alarming-rise-of-measles-cases-in-europe/ (accessed 2024 -09 -23).
- Mboussou, F.; Kada, S.; Danovaro-Holliday, M.C.; Farham, B.; Gacic-Dobo, M.; Shearer, J.C.; Bwaka, A.; Amani, A.; Ngom, R.; Vuo-Masembe, Y.; Wiysonge, C.S.; Impouma, B. Status of Routine Immunization Coverage in the World Health Organization African Region Three Years into the COVID-19 Pandemic. Vaccines (Basel) 2024, 12, 168. [Google Scholar] [CrossRef]
- Cardoso Pinto, A.M.; Ranasinghe, L.; Dodd, P.J.; Budhathoki, S.S.; Seddon, J.A.; Whittaker, E. Disruptions to Routine Childhood Vaccinations in Low- and Middle-Income Countries during the COVID-19 Pandemic: A Systematic Review. Front Pediatr 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- GAVI. Raising Generation Immunity The 2023 Mid-Term Review Report; Geneva,Switzerland, 2023.
- O’Leary, S.T.; Opel, D.J.; Cataldi, J.R.; Hackell, J.M.; O’Leary, S.T.; Campbell, J.D.; Ardura, M.I.; Banerjee, R.; Bryant, K.A.; Caserta, M.T.; Frenck, R.; Gerber, J.S.; John, C.C.; Kourtis, A.P.; Myers, A.; Pannaraj, P.; Ratner, A.J.; Shah, S.S.; Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H.; Bernstein, H.H.; Cardemil, C.; Farizo, K.M.; Kafer, L.M.; Kim, D.; Medina, E.L.; Moore, D.; Panagiotakopoulos, L.; Romero, J.R.; Sauvé, L.; Starke, J.R.; Thompson, J.; Wharton, M.; Woods, C.R.; Frantz, J.M.; Gibbs, G.; Hackell, J.M.; Almendarez, Y.M.; Berhane, A.M.; Cantrell, P.E.; Kafer, L.M.; Latimer, T.; Warner, R.; Wiskind, R.H.; Schafer, K.; Skatrud, A.; Magnus, M.A.; Laventhal, N.T.; Geis, G.M.; Loeff, D.S.; Michelson, K.; Ott, M.; Elster, N.; Diekema, D.S.; Arora, K.; McGee, M.; Emanuel, A. Strategies for Improving Vaccine Communication and Uptake. Pediatrics 2024, 153. [Google Scholar] [CrossRef]
- Stepovic, M.; Dragojevic Simic, V.; Zivanovic Macuzic, I.; Simic, R.; Vekic, S.; Sekulic, M.; Radovanovic, S.; Maricic, M.; Sorak, M.; Suljagic, V.; Vojinovic, R.; Rancic, N. The Last 3 Decade of Vaccination Coverage in the Balkan and Eastern Europe Countries with Reference to the Impact of the COVID-19 Pandemic. Front Pharmacol 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Lindstrand, A.; Cherian, T.; Chang-Blanc, D.; Feikin, D.; O’Brien, K.L. The World of Immunization: Achievements, Challenges, and Strategic Vision for the Next Decade. J Infect Dis 2021, 224 (Supplement_4), S452–S467. [Google Scholar] [CrossRef] [PubMed]
- Data Warehouse - UNICEF DATA https://data.unicef.org/resources/data_explorer/unicef_f/?ag=UNICEF&df=IMMUNISATION&ver=1.0&dq=.IM_DTP3..&startPeriod=2000&endPeriod=2024 (accessed 2024 -07 -27).
- United Nations. Standard country or area codes for statistical use (M49) https://unstats.un.org/unsd/methodology/m49/ (accessed 2024 -07 -27).
- World Bank. Population, total | Data https://data.worldbank.org/indicator/SP.POP.TOTL (accessed 2024 -07 -28).
- UNICEF. Number of Births. Data Warehouse - UNICEF DATA https://data.unicef.org/resources/data_explorer/unicef_f/?ag=UNICEF&df=GLOBAL_DATAFLOW&ver=1.0&dq=.DM_BRTS..&startPeriod=2019&endPeriod=2023 (accessed 2024 -07 -28).
- United Nations, D. of E. and S. A. P. D. World Population Prospects 2022, Online Edition https://population.un.org/wpp (accessed 2024 -07 -28).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation Tests for Joinpoint Regression with Applications to Cancer Rates. Stat Med 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Joinpoint Regression Program Version 5.2.0. May, 2023; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute. 2023.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2023.
- Muggeo, V.M.; Atkins, D.C.; Gallop, R.J.; Dimidjian, S. Segmented Mixed Models with Random Changepoints: A Maximum Likelihood Approach with Application to Treatment for Depression Study. Stat Modelling 2014, 14, 293–313. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Interval Estimation for the Breakpoint in Segmented Regression: A Smoothed Score-based Approach. Aust N Z J Stat 2017, 59, 311–322. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Testing with a Nuisance Parameter Present Only under the Alternative: A Score-Based Approach with Application to Segmented Modelling. J Stat Comput Simul 2016, 86, 3059–3067. [Google Scholar] [CrossRef]
- Fasola, S.; Muggeo, V.M.R.; Küchenhoff, H. A Heuristic, Iterative Algorithm for Change-Point Detection in Abrupt Change Models. Comput Stat 2018, 33, 997–1015. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, 2016. [Google Scholar]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files_. R Package Version 1.4.3. 2023.
- MapChart Version 6.0.10. MapChart. November 30, 2024.
- Brewer, C.A.; Hatchard, G.W.; Harrower, M.A. COLORBREWER 2.0 Color Advice for Cartograph. 2013.
- Brewer, C.A.; Hatchard, G.W.; Harrower, M.A. ColorBrewer in Print: A Catalog of Color Schemes for Maps. Cartogr Geogr Inf Sci 2003, 30, 5–32. [Google Scholar] [CrossRef]
- Nelson, R. COVID-19 Disrupts Vaccine Delivery. Lancet Infect Dis 2020, 20, 546. [Google Scholar] [CrossRef] [PubMed]
- WHO. At least 80 million children under one at risk of diseases such as diphtheria, measles and polio as COVID-19 disrupts routine vaccination efforts, warn Gavi, WHO and UNICEF https://www.who.int/news-room/detail/22-05-2020-at-least-80-million-children-under-one-at-risk-of-diseases-such-as-diphtheria-measles-and-polio-as-covid-19-disrupts-routine-vaccination-efforts-warn-gavi-who-and-unicef (accessed 2024 -10 -23).
- Dinleyici, E.C.; Borrow, R.; Safadi, M.A.P.; van Damme, P.; Munoz, F.M. Vaccines and Routine Immunization Strategies during the COVID-19 Pandemic. Hum Vaccin Immunother 2021, 17, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Badker, R.; Miller, K.; Pardee, C.; Oppenheim, B.; Stephenson, N.; Ash, B.; Philippsen, T.; Ngoon, C.; Savage, P.; Lam, C.; Madhav, N. Challenges in Reported COVID-19 Data: Best Practices and Recommendations for Future Epidemics. BMJ Glob Health 2021, 6, e005542. [Google Scholar] [CrossRef] [PubMed]
- Rotulo, A.; Kondilis, E.; Thwe, T.; Gautam, S.; Torcu, Ö.; Vera-Montoya, M.; Marjan, S.; Gazi, Md. I.; Putri, A.S.; Hasan, R.B.; Mone, F.H.; Rodríguez-Castillo, K.; Tabassum, A.; Parcharidi, Z.; Sharma, B.; Islam, F.; Amoo, B.; Lemke, L.; Gallo, V. Mind the Gap: Data Availability, Accessibility, Transparency, and Credibility during the COVID-19 Pandemic, an International Comparative Appraisal. PLOS Global Public Health 2023, 3, e0001148. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.; Bach Habersaat, K.; Jackson, C.; Kulo, A.; Primorac, E.; Smjecanin, M.; Funk, S. Tailoring Immunization Programmes: Using Patient File Data to Explore Vaccination Uptake and Associated Factors. Hum Vaccin Immunother 2021, 17, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.; Ruta, S.M.; Cernescu, C.; Pistol, A. Suboptimal MMR Vaccination Coverages—A Constant Challenge for Measles Elimination in Romania. Vaccines (Basel) 2024, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Mayerová, D.; Abbas, K. Childhood Immunisation Timeliness and Vaccine Confidence by Health Information Source, Maternal, Socioeconomic, and Geographic Characteristics in Albania. BMC Public Health 2021, 21, 1724. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, F.; Carter, C.; Lyons, B.A.; Reifler, J. Association of Vaccine Hesitancy and Immunization Coverage Rates in the European Union. Vaccine 2021, 39, 3935–3939. [Google Scholar] [CrossRef]
- Bali, D.; Kuli-Lito, G.; Ceka, N.; Godo, A. Maternal and Child Health Care Services in Albania. J Pediatr 2016, 177, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Petro, E.; Perumal-Pillay, V.; Mantel-Teeuwisse, A.K.; van den Ham, H.A.; Suleman, F. Evaluation of Alignment of the Reimbursement Medicines List for Children in Albania with the WHO Essential Medicines List for Children. J Pharm Policy Pract 2024, 17. [Google Scholar] [CrossRef]
- Mayerová, D.; Abbas, K. Childhood Immunisation Timeliness and Vaccine Confidence by Health Information Source, Maternal, Socioeconomic, and Geographic Characteristics in Albania. BMC Public Health 2021, 21, 1724. [Google Scholar] [CrossRef] [PubMed]
- Gjini, E.; Moramarco, S.; Carestia, M.C.; Cenko, F.; Ylli, A.; Mehmeti, I.; Palombi, L.; Buonomo, E. Parents’ and Caregivers’ Role toward Childhood Vaccination in Albania: Assessment of Predictors of Vaccine Hesitancy. Ann Ig 2023, 35, 75–83. [Google Scholar] [CrossRef]
- Pojani, P.C.E.; Nelaj, E.; Ylli, A.P.M.A. Overview of the Immunization Situation in Albania. European Journal of Multidisciplinary Studies 2017, 5, 259. [Google Scholar] [CrossRef]
- Gjini, E.; Carestia, M.; Cenko, F.; Di Giovanni, D.; Mehmeti, I.; Moramarco, S.; Yulli, A.; Buonomo, E. Hesitancy toward Childhood Vaccinations: Preliminary Results from an Albanian Nursing Staff’s Investigation. Nurs Res Pract 2022, 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Measles on the Rise in the EU/EEA: Considerations for Public Health Response.; Stockholm, 2024. [CrossRef]
- Bauer, A.; Tiefengraber, D.; Wiedermann, U. Towards Understanding Vaccine Hesitancy and Vaccination Refusal in Austria. Wien Klin Wochenschr 2021, 133, 703–713. [Google Scholar] [CrossRef]
- Magyar, R.; Voitl, P.K.; Voitl, J.J.M.; Diesner-Treiber, S.C. Vaccine Hesitancy among Parents of Children in Their First Two Years of Life. Front Public Health 2024, 12. [Google Scholar] [CrossRef]
- Gobert, C.; Semaille, P.; Van der Schueren, T.; Verger, P.; Dauby, N. Prevalence and Determinants of Vaccine Hesitancy and Vaccines Recommendation Discrepancies among General Practitioners in French-Speaking Parts of Belgium. Vaccines (Basel) 2021, 9, 771. [Google Scholar] [CrossRef]
- Partouche, H.; Gilberg, S.; Renard, V.; Saint-Lary, O. Mandatory Vaccination of Infants in France: Is That the Way Forward? European Journal of General Practice 2019, 25, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Carrico, J.; Mellott, C.E.; Talbird, S.E.; Bento-Abreu, A.; Merckx, B.; Vandenhaute, J.; Benchabane, D.; Dauby, N.; Ethgen, O.; Lepage, P.; Luyten, J.; Raes, M.; Simoens, S.; Van Ranst, M.; Eiden, A.; Nyaku, M.K.; Bencina, G. Public Health Impact and Return on Investment of Belgium’s Pediatric Immunization Program. Front Public Health 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Glatman-Freedman, A.; Nichols, K. The Effect of Social Determinants on Immunization Programs. Hum Vaccin Immunother 2012, 8, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Varbanova, V.; Verelst, F.; Hens, N.; Beutels, P. Determinants of Basic Childhood Vaccination Coverage in European and OECD Countries. Hum Vaccin Immunother 2022, 18. [Google Scholar] [CrossRef] [PubMed]
- Kolesar, R.J.; Spruk, R.; Tsheten, T. Evaluating Country Performance After Transitioning From Gavi Assistance: An Applied Synthetic Control Analysis. Glob Health Sci Pract 2023, 11, e2200536. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Europe. Ukraine Crisis Strategic Response Plan for January–December 2023.; Copenhagen, 2023.
- Tull, K. Vaccine Hesitancy: Guidance and Interventions. ; 2019.
- Hadjipanayis, A.; van Esso, D.; del Torso, S.; Dornbusch, H.J.; Michailidou, K.; Minicuci, N.; Pancheva, R.; Mujkic, A.; Geitmann, K.; Syridou, G.; Altorjai, P.; Pasinato, A.; Valiulis, A.; Soler, P.; Cirstea, O.; Illy, K.; Mollema, L.; Mazur, A.; Neves, A.; Zavrsnik, J.; Lapii, F.; Efstathiou, E.; Kamphuis, M.; Grossman, Z. Vaccine Confidence among Parents: Large Scale Study in Eighteen European Countries. Vaccine 2020, 38, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Riad, A. COVID-19 Vaccine Hesitancy: A Tale of Two Pandemics, Charles University Faculty of Medicine in Pilsen, Pilsen, 2013.
- Vijest HIR. Krivotvorenje COVID potvrda postalo pravi mali biznis: Desetero palo u Zagrebu, lažno upisivali cijepljenja [Forgery of COVID certificates has become a real small business: Ten fell in Zagreb, falsely registering vaccinations] https://kaportal.net.hr/aktualno/vijesti/148105/krivotvorenje-covid-potvrda-postalo-pravi-mali-biznis-desetero-palo-u-zagrebu-lazno-upisivali-cijepljenja/ (accessed 2024 -10 -25).
- Tomljenovic, H.; Bubic, A.; Hren, D. Decision Making Processes Underlying Avoidance of Mandatory Child Vaccination in Croatia – a Qualitative Study. Current Psychology 2022, 41, 6210–6224. [Google Scholar] [CrossRef]
- Jerković, H.; Šitum, M. VACCINE HESITANCY - FROM PARENTAL DISTRUST TO COVID-19 CONSPIRACIES. Psychiatr Danub 2023, 35, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Pivac, I.; Markić, J.; Poklepović Peričić, T.; Aranza, D.; Marušić, A. Evaluating Health Claim Assessment Skills of Parents with Preschool Children: A Cross-Sectional Study Using Informed Health Choices Claim Evaluation Tool. J Glob Health 2023, 13, 04156. [Google Scholar] [CrossRef] [PubMed]
- Fakonti, G.; Kyprianidou, M.; Toumbis, G.; Giannakou, K. Attitudes and Acceptance of COVID-19 Vaccination Among Nurses and Midwives in Cyprus: A Cross-Sectional Survey. Front Public Health 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Kyprianidou, M.; Fakonti, G.; Tzira, E.; Pylli, M.; Giannakou, K. Associations between Socio-Demographic Characteristics and Maternal Attitudes towards Childhood Vaccination in Cyprus—A Cross-Sectional Survey. COVID 2023, 3, 1042–1051. [Google Scholar] [CrossRef]
- Vuolanto, P.; Almeida, A.N.; Anderson, A.; Auvinen, P.; Beja, A.; Bracke, P.; Cardano, M.; Ceuterick, M.; Correia, T.; De Vito, E.; Delaruelle, K.; Delicado, A.; Esposito, M.; Ferrara, M.; Gariglio, L.; Guerreiro, C.; Marhánková, J.H.; Hilário, A.P.; Hobson-West, P.; Iorio, J.; Järvinen, K.-M.; Koivu, A.; Kotherová, Z.; Kuusipalo, A.; Lermytte, E.; Mendonça, J.; Morais, R.; Numerato, D.; Polak, P.; Rudek, T.; Sbaragli, S.; Scavarda, A.; Silva, K.; da Silva, P.A.; Sivelä, J.; Moura, E.S.; Świątkiewicz-mośny, M.; Tipaldo, G.; Wagner, A. Trust Matters: The Addressing Vaccine Hesitancy in Europe Study. Scand J Public Health 2024, 52, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Macounová, P.; Macounová, D. Mumps and Its Occurrence in the Czech Republic and Slovakia. Epidemiol Mikrobiol Imunol 2021, 70, 253–263. [Google Scholar]
- Riad, A.; Jouzová, A.; Üstün, B.; Lagová, E.; Hruban, L.; Janků, P.; Pokorná, A.; Klugarová, J.; Koščík, M.; Klugar, M. COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. Int J Environ Res Public Health 2021, 18, 13373. [Google Scholar] [CrossRef] [PubMed]
- Merilind, E.; Salupere, R.; Västra, K.; Kalda, R. The Influence of Performance-Based Payment on Childhood Immunisation Coverage. Health Policy (New York) 2015, 119, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.O.; Moriarty, F.; Lawlor, L.; Gorman, K.M.; Beamish, J. Vaccine Hesitancy and Reported Non-Vaccination in an Irish Pediatric Outpatient Population. Eur J Pediatr 2021, 180, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; Levita, L.; Martinez, A.P.; Stocks, T.V.A.; Karatzias, T.; Hyland, P. Psychological Characteristics Associated with COVID-19 Vaccine Hesitancy and Resistance in Ireland and the United Kingdom. Nat Commun 2021, 12, 29. [Google Scholar] [CrossRef]
- Ceannt, R.; Vallieres, F.; Burns, H.; Murphy, J.; Hyland, P. Covid-19 Vaccine Hesitancy and Resistance amongst Parents of Children under 18 Years of Age in Ireland. Vaccine 2022, 40, 6196–6200. [Google Scholar] [CrossRef] [PubMed]
- Marron, L.; Ferenczi, A.; O’Brien, K.M.; Cotter, S.; Jessop, L.; Morrissey, Y.; Migone, C. Views on COVID-19 Vaccination of Young Children in Ireland, Results from a Cross-Sectional Survey of Parents. Vaccine 2022, 40, 5716–5725. [Google Scholar] [CrossRef] [PubMed]
- Marron, L.; Ferenczi, A.; O’Brien, K.M.; Cotter, S.; Jessop, L.; Morrissey, Y.; Migone, C. A National Survey of Parents’ Views on Childhood Vaccinations in Ireland. Vaccine 2023, 41, 3740–3754. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Moore, A.C.; Sahm, L.J.; Fleming, A. Parent Attitudes about Childhood Vaccines: Point Prevalence Survey of Vaccine Hesitancy in an Irish Population. Pharmacy 2021, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, L.; Cortassa, G.; Trilla, A. Effectiveness of Mandatory and Incentive-Based Routine Childhood Immunization Programs in Europe: A Systematic Review of the Literature. Vaccines (Basel) 2021, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Tan, L. (L. J.; Safadi, M.A.P.; Horn, M.; Regojo Balboa, C.; Moya, E.; Schanbaum, J.; Pimenta, P.; Lambert, E.; Soumahoro, L.; Sohn, W.-Y.; Bruce, T.; Ruiz García, Y. Pandemic’s Influence on Parents’ Attitudes and Behaviors toward Meningococcal Vaccination. Hum Vaccin Immunother 2023, 19. [Google Scholar] [CrossRef]
- Tondo, L. Italian Nurse Accused of Giving Fake Covid Jabs to Anti-Vaxxers Arrested. The Guardian 2022. [Google Scholar]
- Poeta, M.; Moracas, C.; Albano, C.; Petrarca, L.; Maglione, M.; Pierri, L.; Carta, M.; Montaldo, P.; Venturini, E.; De Luca, M.; Buonsenso, D.; Brambilla, I.; Giacomet, V.; Lo Vecchio, A.; Bruzzese, E.; Midulla, F.; Colomba, C.; Guarino, A. Pertussis Outbreak in Neonates and Young Infants across Italy, January to May 2024: Implications for Vaccination Strategies. Eurosurveillance 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi-Lanna, L.; Valente, R.; Cataldi, S.; Martire, F. Predictors of Young People’s Anti-Vaccine Attitudes in the Context of the COVID-19 Pandemic. Public Understanding of Science 2024, 33, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Kassianos, G.; Cohen, J.-M.; Civljak, R.; Davidovitch, N.; Pecurariu, O.F.; Froes, F.; Galev, A.; Ivaskeviciene, I.; Kõivumägi, K.; Kristufkova, Z.; Kuchar, E.; Kyncl, J.; Maltezou, H.C.; Marković, M.; Nitsch-Osuch, A.; Ortiz de Lejarazu, R.; Rossi, A.; Schelling, J.; van Essen, G.A.; Zavadska, D. The Influenza Landscape and Vaccination Coverage in Older Adults during the SARS-Cov-2 Pandemic: Data from Several European Countries and Israel. Expert Rev Respir Med 2024, 18, 69–84. [Google Scholar] [CrossRef]
- Lucane, Z.; Kursite, M.; Sablinskis, K.; Gailite, L.; Kurjane, N. COVID-19 Vaccination Coverage and Factors Influencing Vaccine Hesitancy among Patients with Inborn Errors of Immunity in Latvia: A Mixed-Methods Study. Vaccines (Basel) 2023, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.B.; Godøy, A.A.; Vinjerui, K.H.; Kour, P.; Kjøllesdal, M.K.R.; Indseth, T. Vaksinasjonsdekning Mot Covid-19 Etter Innvandrerbakgrunn. Tidsskrift for Den norske legeforening 2022. [Google Scholar] [CrossRef] [PubMed]
- Čeponytė, K.; Narkevičiūtė, E.; Beržanskytė, A.; Burokienė, S. Determinants of Parental Attitudes towards Children’s Vaccination in Lithuania: An Online Survey. Acta Med Litu 2024, 31, 23–34. [Google Scholar] [CrossRef]
- Svist, V.; Maciuleviciene, A.; Naudziunaite, S.; Petraitiene, S.; del Torso, S.; Grossman, Z.; Magelinskiene, G.; Valiulis, A. Vaccine-Hesitant Families Are More Susceptible to Verbal Communication Messaging. Cent Eur J Public Health 2023, 31, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, S.P.; Silver, D.; Domenti, O.; Gasoyan, H.; Paatashvili, E.; Gellin, B.G.; Gordon, J.L. Strengthening Legal Frameworks for Vaccination: The Experiences of Armenia, Georgia, and Moldova. Vaccine 2019, 37, 4840–4847. [Google Scholar] [CrossRef]
- Melovic, B.; Jaksic Stojanovic, A.; Vulic, T.B.; Dudic, B.; Benova, E. The Impact of Online Media on Parents’ Attitudes toward Vaccination of Children—Social Marketing and Public Health. Int J Environ Res Public Health 2020, 17, 5816. [Google Scholar] [CrossRef]
- Knijff, M.; van Lier, A.; Boer, M.; de Vries, M.; Hament, J.-M.; de Melker, H.E. Parental Intention, Attitudes, Beliefs, Trust and Deliberation towards Childhood Vaccination in the Netherlands in 2022: Indications of Change Compared to 2013. Vaccine 2024, 42, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, D.; van Hoek, A.J.; Veldhuijzen, I.; Hahné, S.; Wallinga, J. Social Clustering of Unvaccinated Children in Schools in the Netherlands. Epidemiol Infect 2022, 150, e200. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, M.; van Lier, A.; van der Maas, N.; Veldhuijzen, I.; Freudenburg, W.; van Sorge, N.M.; Sanders, E.A.M.; Knol, M.J.; de Melker, H.E. Short Term Impact of the COVID-19 Pandemic on Incidence of Vaccine Preventable Diseases and Participation in Routine Infant Vaccinations in the Netherlands in the Period March-September 2020. Vaccine 2021, 39, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Shumanov, G.; Todosieva, J.; Nikolovska, E.; Shumanova, C.; Panova, G. Condition and Prospects of Vaccination of the Population in the Republic of Macedonia. KNOWLEDGE-International Journal 2018, 23, 461–466. [Google Scholar]
- Mustafa, Z.; Memeti, S.; Karadzovski, Z.; Arsova Sarafinovska, Z.; Mihajloska, E.; Anchevska Netkovska, K.; Grozdanova, A. The Influence of Covid-19 Pandemic on the Vaccination of the Population with the Influenza Vaccine in the Republic of North Macedonia. MEDIS – International Journal of Medical Sciences and Research 2022, 1, 49–53. [Google Scholar] [CrossRef]
- Berild, J.; Berg, A.; Bruun, T.; Christensen, A. Barnevaksinasjonsprogrammet i Norge. Rapport for 2021. Barnevaksinasjonsprogrammet i Norge; Oslo, 2022.
- Price, D.; Bonsaksen, T.; Leung, J.; McClure-Thomas, C.; Ruffolo, M.; Lamph, G.; Kabelenga, I.; Ostertun Geirdal, A. Factors Associated with Trust in Public Authorities Among Adults in Norway, United Kingdom, United States, and Australia Two Years after the COVID-19 Outbreak. Int J Public Health 2023, 68. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Price, D.; McClure-Thomas, C.; Bonsaksen, T.; Ruffolo, M.; Kabelenga, I.; Lamph, G.; Geirdal, A.Ø. Motivation and Hesitancies in Obtaining the COVID-19 Vaccine—A Cross-Sectional Study in Norway, USA, UK, and Australia. Vaccines (Basel) 2023, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Nybru Gleditsch, R.; Skogset Ofitserova, T.; Aubrey White, R.; Karoline Råberg Kjøllesdal, M.; Dvergsdal, E.; Hansen, B.T.; Askeland Winje, B. Vaccine Coverage among Children Born to Immigrant Parents in Norway, 2000–2020. Vaccine 2024, 42, 3049–3056. [Google Scholar] [CrossRef]
- Melović, B.; Stojanović, A.J.; Vulić, T.B.; Baynazoğlu, M.E. Perceptions and Attitudes of Parents Toward Vaccination of Children in Western Balkan Countries: Trust in Function of Improving Public Health. Balkan Med J 2020. [Google Scholar] [CrossRef] [PubMed]
- Jenness, S.M.; Aavitsland, P.; White, R.A.; Winje, B.A. Measles Vaccine Coverage among Children Born to Somali Immigrants in Norway. BMC Public Health 2021, 21, 668. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccination schedule for Poland https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-country_name?DISEASECODE=&TARGETPOP_GENERAL= (accessed 2024 -10 -25).
- Finnegan, G. MMR rates fall in Poland – despite mandatory vaccination rules https://www.vaccinestoday.eu/stories/mmr-rates-fall-poland-despite-mandatory-vaccination-rules/ (accessed 2024 -10 -25).
- Ganczak, M.; Kalinowski, P.; Pasek, O.; Duda-Duma, Ł.; Sobieraj, E.; Goławski, J.; Biesiada, D.; Jansen, D.; Vervoort, J.P.M.; Edelstein, M.; Kowalska, M. Health System Barriers to Child Mandatory and Optional Vaccination among Ukrainian Migrants in Poland in the Context of MMR and HPV Vaccines—A Qualitative Study. Int J Environ Res Public Health 2022, 20, 712. [Google Scholar] [CrossRef] [PubMed]
- Iova, C.F.; Badau, D.; Daina, M.D.; Șuteu, C.L.; Daina, L.G. Knowledge, Attitudes, Intentions and Vaccine Hesitancy among Postpartum Mothers in a Region from the Northwest of Romania. Vaccines (Basel) 2023, 11, 1736. [Google Scholar] [CrossRef]
- Herdea, V.; Tarciuc, P.; Ghionaru, R.; Lupusoru, M.; Tataranu, E.; Chirila, S.; Rosu, O.; Marginean, C.O.; Leibovitz, E.; Diaconescu, S. Vaccine Hesitancy Phenomenon Evolution during Pregnancy over High-Risk Epidemiological Periods—“Repetitio Est Mater Studiorum. ” Vaccines (Basel) 2023, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.; Ruta, S.M.; Cernescu, C.; Pistol, A. Suboptimal MMR Vaccination Coverages—A Constant Challenge for Measles Elimination in Romania. Vaccines (Basel) 2024, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, M.; Loncarevic, G.; Kanazir, M.; Kisic-Tepavcevic, D.; Gazibara, T. Trend in Mandatory Immunisation Coverage: Linear and Joinpoint Regression Approach, Serbia, 2000 to 2017. Eurosurveillance 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Milošević Đorđević, J.; Mari, S.; Vdović, M.; Milošević, A. Links between Conspiracy Beliefs, Vaccine Knowledge, and Trust: Anti-Vaccine Behavior of Serbian Adults. Soc Sci Med 2021, 277, 113930. [Google Scholar] [CrossRef]
- Aritonovic Pribakovic, J.; Katanic, N.; Ilic, A.; Stojanovic Tasic, M.; Mitic, N.R.; Mirkovic, M.; Radomirovic, D.; Milentijevic, M.; Mirkovic, M.; Katanic, R. The Measles Epidemic in Northern Kosovo and Metohija, Serbia, October 2017−August 2019. The Journal of Infection in Developing Countries 2022, 16, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Burgess, C.; Lisul, B.; Pawaskar, M.; Petigara, T.; Murtagh, J.; Kanazir, M.; Loncarevic, G.; Carias, C. Impact of the COVID-19 Pandemic on Measles Vaccination Coverage and Estimated Catch-up Efforts for Serbia. Pediatric Infectious Disease Journal 2024, 43, 1011–1017. [Google Scholar] [CrossRef]
- Loncarevic, G.S.; Jovanovic, A.L.; Kanazir, M.S.; Kisic Tepavcevic, D.B.; Maric, G.D.; Pekmezovic, T.D. Are Pediatricians Responsible for Maintaining High MMR Vaccination Coverage? Nationwide Survey on Parental Knowledge and Attitudes towards MMR Vaccine in Serbia. PLoS One 2023, 18, e0281495. [Google Scholar] [CrossRef]
- Štrbac, M.; Vuković, V.; Pustahija, T.; Nikolić, N.; Rajčević, S.; Ilić, S.; Dugandžija, T.; Patić, A.; Ristić, M.; Petrović, V. Motives and Attitudes of Parents toward HPV Vaccination: Results from the Initial Period of HPV Vaccine Rollout in Serbia. PLoS One 2023, 18, e0287295. [Google Scholar] [CrossRef]
- Kregar Velikonja, N.; Dobrowolska, B.; Stanisavljević, S.; Erjavec, K.; Globevnik Velikonja, V.; Verdenik, I. Attitudes of Nursing Students towards Vaccination and Other Preventive Measures for Limitation of COVID-19 Pandemic: Cross-Sectional Study in Three European Countries. Healthcare 2021, 9, 781. [Google Scholar] [CrossRef]
- Moraga-Llop, F.A.; Fernández-Prada, M.; Grande-Tejada, A.M.; Martínez-Alcorta, L.I.; Moreno-Pérez, D.; Pérez-Martín, J.J. Recuperando Las Coberturas Vacunales Perdidas En La Pandemia de COVID-19. Vacunas 2020, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Garces-Sanchez, M.; Cilleruelo-Ortega, M.J.; Hernandez-Merino, A.; Alvarez-Garcia, F.J. The Impact of the Pandemic on Vaccination Coverage in Spain: A Challenge for Pediatrics and Public Health. Anales de Pediatría (English Edition) 2023. [Google Scholar] [CrossRef] [PubMed]
- Burgaya-Subirana, S.; Balaguer, M.; Miró Catalina, Q.; Sola, L.; Ruiz-Comellas, A. Influenza Vaccination Coverage in Children: How Has COVID-19 Influenced It? A Review of Five Seasons (2018–2023) in Central Catalonia, Spain. Vaccines (Basel) 2024, 12, 925. [Google Scholar] [CrossRef]
- Gómez-Acebo, I.; Barquín-Ruiz, A.; Llorente, S.; Alonso-Molero, J.; Llorca, J.; Cabero-Perez, M.J.; Dierssen-Sotos, T. The Impact of the COVID-19 Pandemic on Childhood Vaccination Rates and the Role of Sociodemographic Factors: A Cohort Study. Vaccine 2024, 42, 126207. [Google Scholar] [CrossRef] [PubMed]
- Falcon, M.; Rodríguez-Blázquez, C.; Romay-Barja, M.; Ayala, A.; Burgos, A.; De Tena-Dávila, M.J.; Forjaz, M.J. COVID-19 Vaccine Hesitancy in Spain and Associated Factors. Front Public Health 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Ganem, F.; Folch, C.; Colom-Cadena, A.; Bordas, A.; Alonso, L.; Soriano-Arandes, A.; Casabona, J. Determinants of COVID-19 Vaccine Hesitancy among Students and Parents in Sentinel Schools Network of Catalonia, Spain. PLoS One 2023, 18, e0282871. [Google Scholar] [CrossRef]
- Zürcher, S.J.; Signorell, A.; Léchot-Huser, A.; Aebi, C.; Huber, C.A. Childhood Vaccination Coverage and Regional Differences in Swiss Birth Cohorts 2012–2021: Are We on Track? Vaccine 2023, 41, 7226–7233. [Google Scholar] [CrossRef] [PubMed]
- Seiler, M.; Goldman, R.D.; Staubli, G.; Hoeffe, J.; Gualco, G.; Manzano, S. Parents’ Intent to Vaccinate against Influenza during the COVID-19 Pandemic Im Two Regions in Switzerland. Swiss Med Wkly 2021, 151, w20508. [Google Scholar] [CrossRef]
- Baysson, H.; Pullen, N.; De Mestral, C.; Semaani, C.; Pennacchio, F.; Zaballa, M.-E.; L’Huillier, A.G.; Lorthe, E.; Guessous, I.; Stringhini, S.; The Specchio-COVID19 study group. Parental Willingness to Have Children Vaccinated against COVID-19 in Geneva, Switzerland: A Cross-Sectional Population-Based Study. Swiss Med Wkly 2023, 153, 40049. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, N.; Suman, M.; Ersoy, M.; Örün, E. Parents’ Attitudes toward Childhood Vaccines and COVID-19 Vaccines in a Turkish Pediatric Outpatient Population. Vaccines (Basel) 2022, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, E.C.; Borrow, R.; Safadi, M.A.P.; van Damme, P.; Munoz, F.M. Vaccines and Routine Immunization Strategies during the COVID-19 Pandemic. Hum Vaccin Immunother 2021, 17, 400–407. [Google Scholar] [CrossRef]
- Kara, A.; İlbay, S.; Topaç, O.; Arabulan, E.A.; Tezer, H.; Tavukçu, N.; Şimşek, Ç. Alteration in Vaccination Rates and an Evaluation of Physicians’ Perceptions of the Possible Impact of the SARS-CoV-2 Pandemic on Childhood Vaccinations in Ankara, Turkey. Hum Vaccin Immunother 2021, 17, 3457–3462. [Google Scholar] [CrossRef]
- Akbulut, S.; Boz, G.; Ozer, A.; Sahin, T.T.; Colak, C. Evaluation of the Turkish Population’s Perspective on COVID-19 Vaccine Hesitancy and Routine Childhood Vaccine Applications: National Survey Study. Vaccines (Basel) 2023, 11, 779. [Google Scholar] [CrossRef]
- Ota, M.O.C.; Badur, S.; Romano-Mazzotti, L.; Friedland, L.R. Impact of COVID-19 Pandemic on Routine Immunization. Ann Med 2021, 53, 2286–2297. [Google Scholar] [CrossRef]
- Duran, S.; Duran, R.; Acunaş, B.; Şahin, E.M. Changes in Parents’ Attitudes towards Childhood Vaccines during COVID-19 Pandemic. Pediatrics International 2023, 65. [Google Scholar] [CrossRef]
- Romaniuk, P.; Semigina, T. Ukrainian Health Care System and Its Chances for Successful Transition from Soviet Legacies. Global Health 2018, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.; Goncharova, M.; Germanovich, M.; Koshalko, O.; Kutalek, R.; Dückers, M.; Rodyna, R. Locating Vaccine Uptake and Public Participation in Ukraine: An Exploratory Qualitative Study on Attitudes and Barriers to Early Childhood Vaccination. Glob Public Health 2023, 18. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Torchia, G.; Nardi, A. Vaccine Hesitancy among Ukrainian Refugees. Journal of preventive medicine and hygiene 2022, 63, E566–E572. [Google Scholar] [CrossRef]
- Vignier, N.; Halley des Fontaines, V.; Billette de Villemeur, A.; Cazenave-Roblot, F.; Hoen, B.; Chauvin, F.; Lepelletier, D.; Chidiac, C.; Billaud, E. Public Health Issues and Health Rendezvous for Migrants from Conflict Zones in Ukraine: A French Practice Guideline. Infect Dis Now 2022, 52, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Loboda, A. Systematic Review of Health and Disease in Ukrainian Children Highlights Poor Child Health and Challenges for Those Treating Refugees. Acta Paediatr 2022, 111, 1341–1353. [Google Scholar] [CrossRef]
- Rzymski, P.; Falfushynska, H.; Fal, A. Vaccination of Ukrainian Refugees: Need for Urgent Action. Clinical Infectious Diseases 2022, 75, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Pitchforth, E.; Edwards, N.; Alderwick, H.; McGuire, A.; Mossialos, E. United Kingdom: Health System Review. Health Syst Transit 2022, 24, 1–194. [Google Scholar]
- Vagnoni, C.; Rough, E.; Bunn, S. UK Vaccination Policy; House of Commons Library: London, 2022. [Google Scholar]
- Measles in These Days Can Cause a Lot of Anxiety. Lancet Microbe 5, e203.
- Hobson-West, P. Understanding Vaccination Resistance: Moving beyond Risk. Health Risk Soc 2003, 5, 273–283. [Google Scholar] [CrossRef]
- Petts, J.; Niemeyer, S. Health Risk Communication and Amplification: Learning from the MMR Vaccination Controversy. Health Risk Soc 2004, 6, 7–23. [Google Scholar] [CrossRef]
- Casiday, R.; Cresswell, T.; Wilson, D.; Panter-Brick, C. A Survey of UK Parental Attitudes to the MMR Vaccine and Trust in Medical Authority. Vaccine 2006, 24, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.A.; Mahgoub, H.; Shankar, A.G. Vaccine Acceptance: The UK Perspective. Hum Vaccin Immunother 2013, 9, 2658–2660. [Google Scholar] [CrossRef] [PubMed]
- Torracinta, L.; Tanner, R.; Vanderslott, S. MMR Vaccine Attitude and Uptake Research in the United Kingdom: A Critical Review. Vaccines (Basel) 2021, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Sythes, L.; Bedford, H. Motherhood and Vaccine Refusal in the United Kingdom: A New Examination of Gender, Identity and the Journey to Contemporary Non-vaccination. Child Care Health Dev 2022, 48, 979–989. [Google Scholar] [CrossRef]
- Cookson, D.; Jolley, D.; Dempsey, R.C.; Povey, R. A Social Norms Approach Intervention to Address Misperceptions of Anti-Vaccine Conspiracy Beliefs amongst UK Parents. PLoS One 2021, 16, e0258985. [Google Scholar] [CrossRef]
- Mellott, C.E.; Jaworski, R.; Carrico, J.; Talbird, S.E.; Dobrowolska, I.; Golicki, D.; Bencina, G.; Clinkscales, M.; Karamousouli, E.; Eiden, A.L.; Sabale, U. Public Health Impact and Return on Investment of the Pediatric Immunization Program in Poland. Expert Rev Vaccines 2023, 22, 1114–1125. [Google Scholar] [CrossRef]
- Autzen, B.; Dineen, K.; Vaughan, D. Vaccinating Children: Fairness and Childism. Lancet Infect Dis 2021, 21, 1354–1355. [Google Scholar] [CrossRef]
- Rigby, M.J.; Chronaki, C.E.; Deshpande, S.S.; Altorjai, P.; Brenner, M.; Blair, M.E. European Union Initiatives in Child Immunization— the Need for Child Centricity, e-Health and Holistic Delivery. Eur J Public Health 2020, 30, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Lentakis, E.; Seale, H.; Lazarus, R.; Mounier-Jack, S. Exploring the Attitudes and Practices of Adult Service Users and Providers towards Vaccination in Non-Primary Care Settings: A Mixed Methods Scoping Review. Vaccine 2024, 42, 126472. [Google Scholar] [CrossRef]
- Tesorone, M.; Ungaro, C.; Graziano, L.; Vitagliano, A.; Luminoso, I.; Corvino, M.; Papa, M.; Verdoliva, C. Vaccinations at Home: A New Strategy to Contain Vaccine Hesitancy? The Experience of ASL Napoli 1 Centro, Italy. Ann Ig 2024. [Google Scholar] [CrossRef]
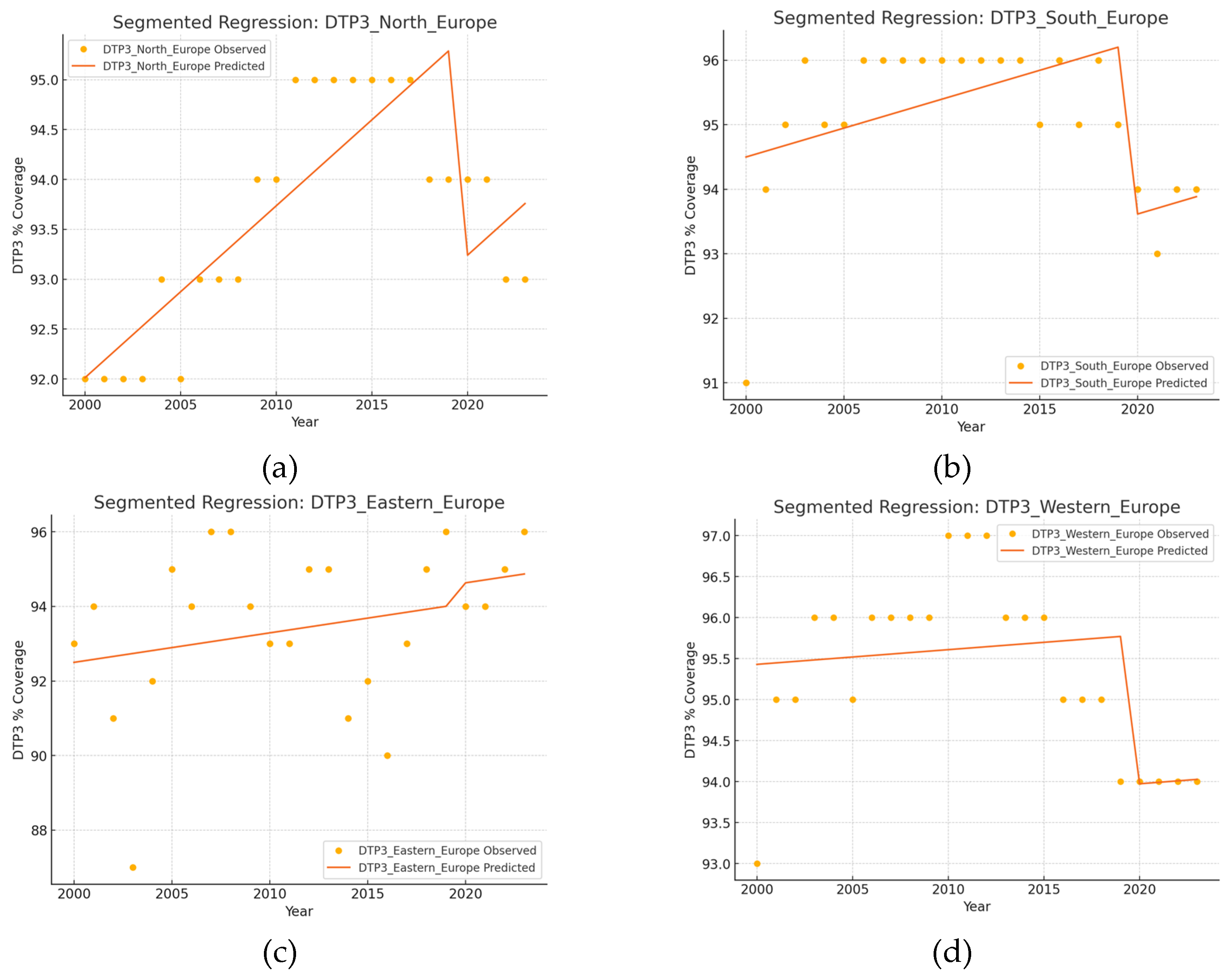
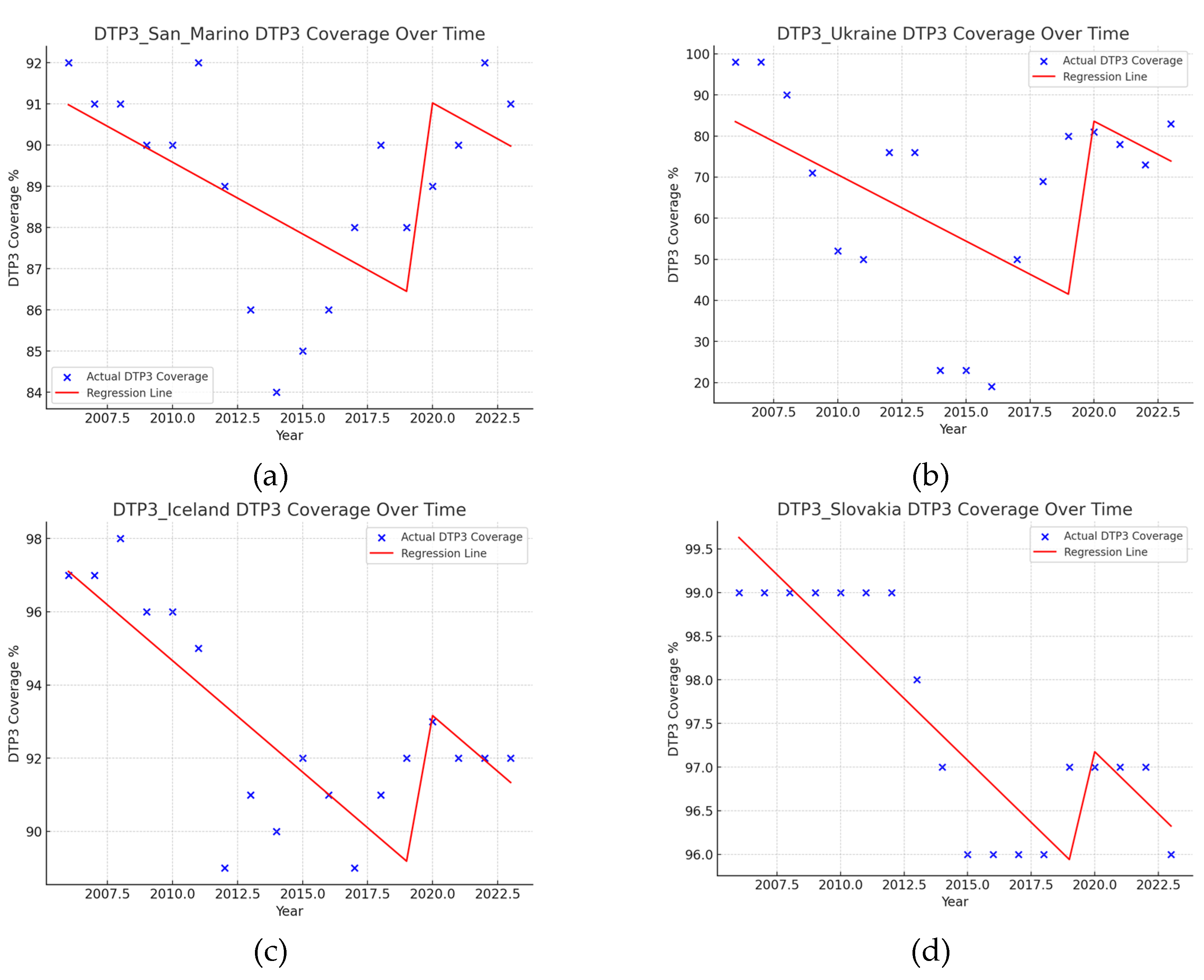
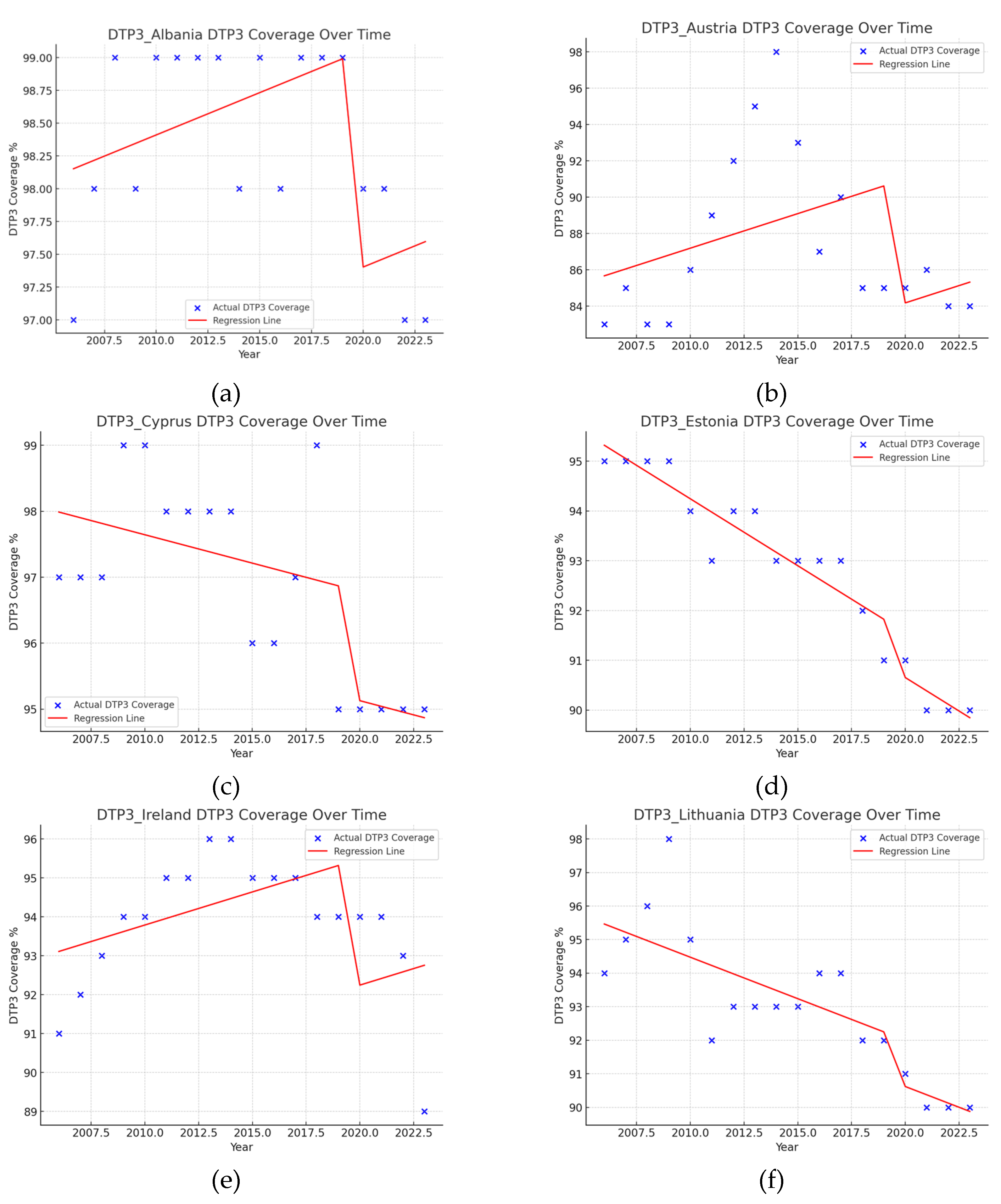
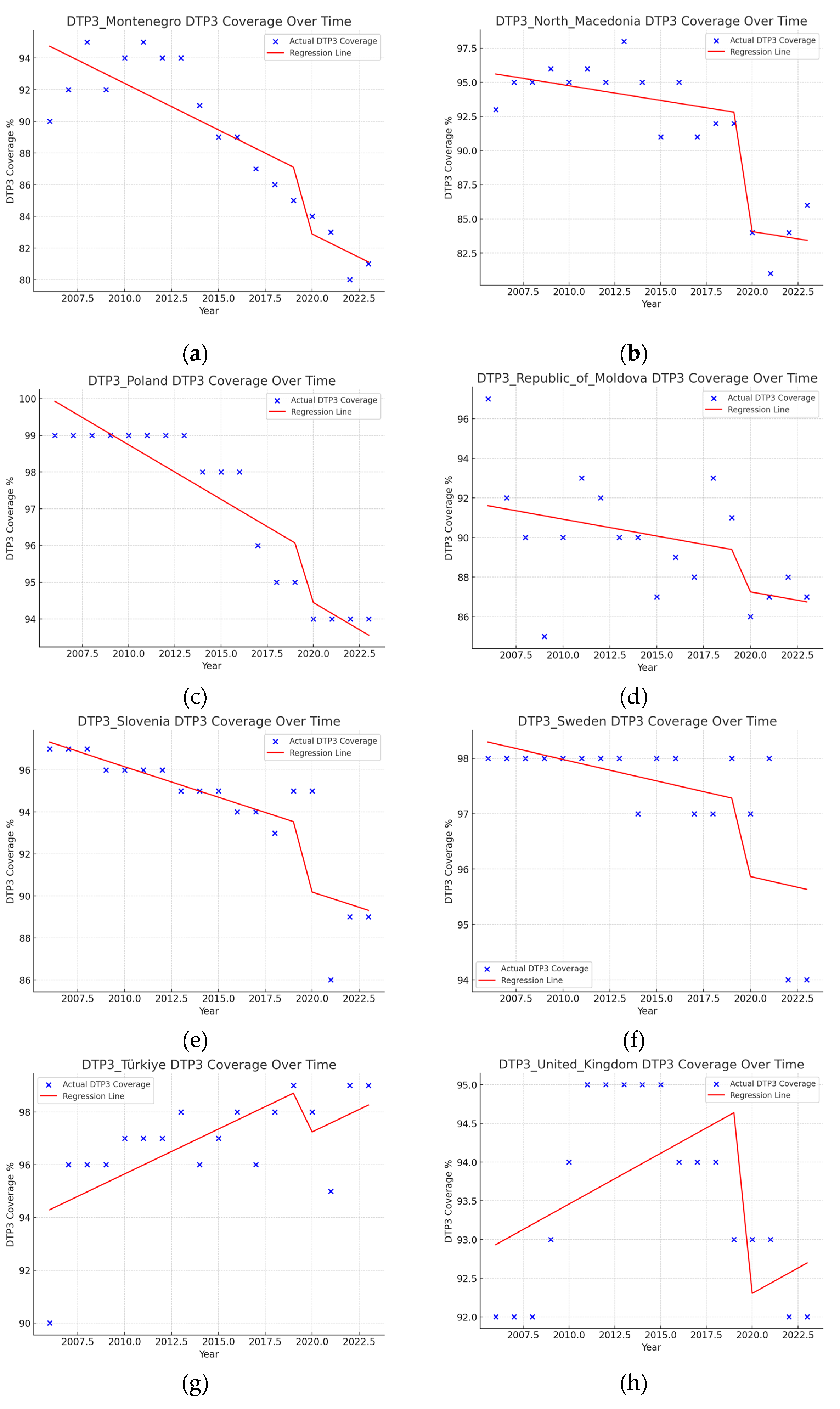
| Region | Number of Joint Points (Jointpoints) | APC Total Period | APC1 | APC2 | APC3 | APC4 | APC5 |
|---|---|---|---|---|---|---|---|
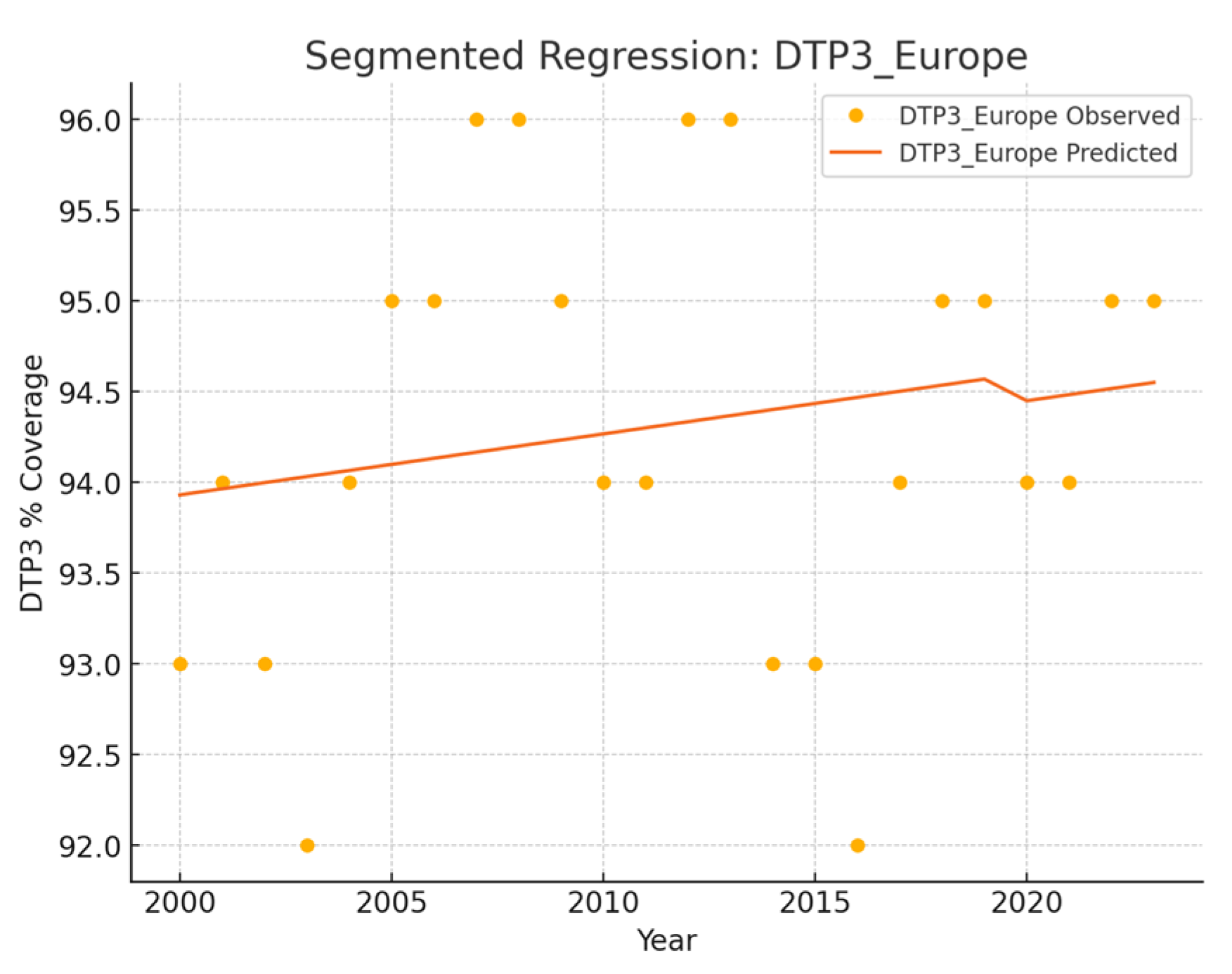
| Europe | 0 | 0.03 | - | - | - | - | - |
| North | 3 (2008, 2011, 2016) | 0,11* | 0,18* | 0,65* | 0,02 | -0,31* | - |
| South | 4 (2002, 2009, 2018, 2021) | -0.02 | 2.05* | 0.12* | -0.06 | -0.81* | 0.50 |
| East | 0 | 0.11 | - | - | - | - | - |
| West | 4 (2002, 2008, 2011, 2020) | -0,12 | 1,17* | 0,06 | 0,41 | -0,37* | 0,03 |
| Country | Number of Joint Points (Jointpoints) | APC Total Period | APC1 | APC2 | APC3 | APC4 | APC5 |
|---|---|---|---|---|---|---|---|
| Albania | 2 (2011, 2019) | 0.04 | 0.18* | -0.01 | -0.50* | ||
| Andorra | 0 | 0.06 | |||||
| Austria | 3 (2009, 2014, 2017) | 0.19 | 0.1 | 3.0* | -3.8* | -0.5 | |
| Belarus | 2 (2003, 2006) | 0.50 | -13.3* | 11.6* | -0.2 | ||
| Belgium | 4 (2003, 2006, 2014, 2019) | 0.12 | -0.15 | 1.12* | 0.14* | -0.37* | 0.24* |
| Bosnia | 4 (2005, 2010, 2017, 2021) | 0.96 | -0.74 | 4.30* | 0.34 | -2.41* | 3.87* |
| Bulgaria | 1 | -0.21* | 0.76 | -0.31* | |||
| Croatia | 1 (2008) | -0.12 | 0.41* | -0.34* | |||
| Cyprus | 1 (2011) | -0.10 | -0.14 | 0.31* | |||
| Czechia | 1 (2011) | -0.15 | 0.14 | -0.41* | |||
| Denmark | 3 (2004, 2007, 2018) | 0.13 | -0.38 | -2.88* | 0.91* | -0.06 | |
| Estonia | 1 (2007) | -0.21* | 0.33* | -0.37* | |||
| Finland | 2 (2014, 2017) | -0.46* | 0.06 | -3.11* | 0.17 | ||
| France | 4 (2003, 2006, 2013, 2016) | -0.09* | 0.04 | 0.58 | 0.02 | -1.00 | -0.00 |
| Germany | 3 (2003, 2010, 2017) | -0.21* | 2.18* | -0.05 | -0.76* | 0.02 | |
| Greece | 2 (2003, 2007) | 0.33 | 1.71* | 1.36 | -0.00 | ||
| Hungary | 0 | 0.00 | 0.27 | -2.55* | 0.18 | ||
| Iceland | 2 (2009, 2012) | -0.30* | 0.27 | -2.55* | 0.18 | ||
| Ireland | 4 (2002, 2005, 2013, 2021) | 0.40* | -1.49* | 2.95* | 0.69* | -0.28* | |
| Italy | 1 (2003) | 0.09 | 2.85* | -0.08 | |||
| Latvia | 1 (2011) | -0.01 | -0.55* | 0.46* | |||
| Lithuania | 1 (2009) | -0.21* | 0.14 | -0.40* | |||
| Luxembourg | 0 | 0.01 | |||||
| Malta | 3 (2005, 2008, 2012) | 0.60 | -0.77 | -9.17* | 9.46* | -0.06 | |
| Monaco | 0 | 0.00 | |||||
| Montenegro | 1 (2011) | -0.91* | 0.94* | -1.43* | |||
| Netherlands | 0 | 0.29 | |||||
| North Macedonia | 1 (2013) | -0.48* | 0.21 | -1.47* | |||
| Norway | 0 | 0.29 | |||||
| Poland | 2 (2013, 2020) | -0.23* | 0.06 | -0.76* | -0.03 | ||
| Portugal | 0 | 0.14 | |||||
| Republic of Moldova | 0 | -0.50 | |||||
| Romania | 0 | -0.83 | |||||
| Russian Federation | 2 (2007, 2011) | 0.01 | 0.36* | -0.32* | 0.00 | ||
| San Marino | 1 (2015) | -0.40* | -0.92* | 0.90* | |||
| Serbia | 0 | -0.02 | |||||
| Slovakia | 3 (2012, 2015, 2021) | -0.15 | 0.00 | -1.10* | 0.23* | -0.50* | |
| Slovenia | 1 (2008) | -0.16 | 0.86* | -0.57* | |||
| Spain | 1 (2012, 2016) | -0.16* | 1.24 | -0.07 | -0.61 | ||
| Sweden | 1 (2021) | -0.14* | -0.09* | -1.94* | |||
| Switzerland | 3 (2003, 2012, 2021) | 0.16* | -0.01 | 0.36* | 0.00 | -0.45* | |
| Türkiye | 2 (2003, 2006) | 0.89* | -6.17* | 9.35* | 0.18 | ||
| Ukraine | 3 (2012, 2015, 2018) | -2.63 | -3.75 | -35.32* | 56.47* | ||
| United Kingdom | 2 (2008, 2012) | 0.11* | 0.18 | 0.91* | -0.34* | ||
| * Annual Percent Change (APC) p < 0.05 |
| 2019 | 2023 | |||||
|---|---|---|---|---|---|---|
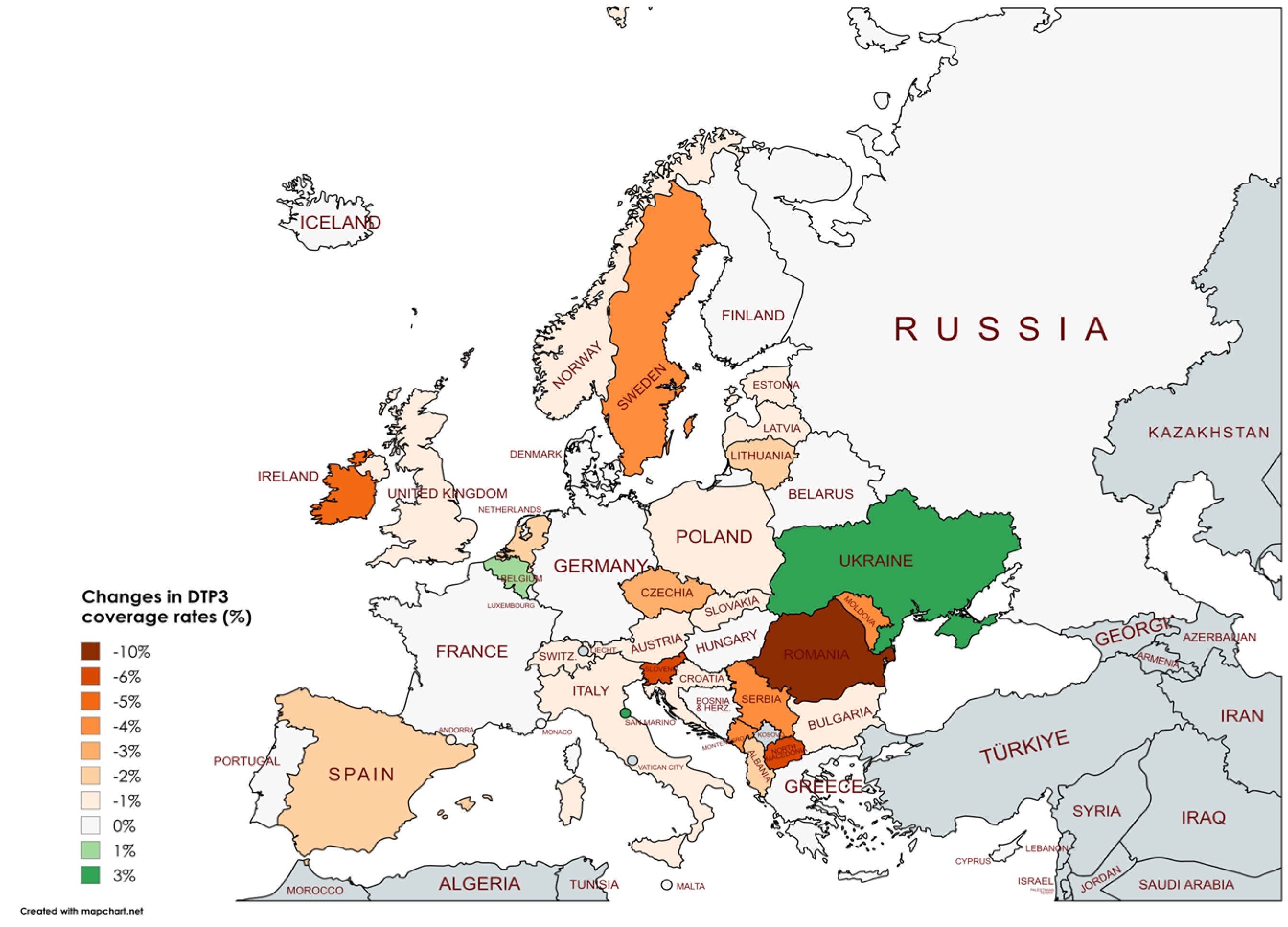
| Country | DTP3 (%) | Births (n) | DTP3 (%) | Births (n) | Change in DTP3 (%) | p † |
| Albania | 99 | 29773 | 97 | 28538 | -2 | <0.001 |
| Andorra | 99 | 590 | 98 | 569 | -1 | ns |
| Austria | 85 | 84744 | 84 | 84132 | -1 | <0.001 |
| Belarus | 98 | 93218 | 98 | 86154 | 0 | ns |
| Belgium | 97 | 116136 | 98 | 116823 | 1 | <0.001 |
| Bosnia and Herzegovina |
73 | 29754 | 73 | 26279 | 0 | ns |
| Bulgaria | 93 | 61637 | 92 | 55495 | -1 | <0.001 |
| Croatia | 94 | 36700 | 93 | 33537 | -1 | <0.001 |
| Cyprus | 95 | 13237 | 95 | 12348 | 0 | ns |
| Czechia | 97 | 109738 | 94 | 99736 | -3 | <0.001 |
| Denmark | 97 | 60455 | 97 | 65003 | 0 | ns |
| Estonia | 91 | 14158 | 90 | 13102 | -1 | 0.005 |
| Finland | 91 | 45790 | 91 | 47143 | 0 | ns |
| France | 96 | 701300 | 96 | 671363 | 0 | ns |
| Germany | 91 | 771867 | 91 | 755129 | 0 | ns |
| Greece | 99 | 81148 | 99 | 76080 | 0 | ns |
| Hungary | 99 | 92375 | 99 | 104762 | 0 | ns |
| Iceland | 92 | 4402 | 92 | 4553 | 0 | ns |
| Ireland | 94 | 60463 | 89 | 56728 | -5 | <0.001 |
| Italy | 96 | 418113 | 95 | 406243 | -1 | <0.001 |
| Latvia | 99 | 18693 | 98 | 15996 | -1 | <0.001 |
| Lithuania | 92 | 28075 | 90 | 25605 | -1 | <0.001 |
| Luxembourg | 99 | 6167 | 99 | 6724 | -2 | ns |
| Malta | 98 | 4318 | 98 | 4863 | 0 | ns |
| Monaco | 99 | 331 | 99 | 323 | 0 | ns |
| Montenegro | 85 | 7429 | 81 | 6773 | 0 | <0.001 |
| Netherlands (Kingdom of the) | 94 | 169827 | 92 | 182435 | -2 | <0.001 |
| North Macedonia | 92 | 20447 | 86 | 19769 | -6 | <0.001 |
| Norway | 97 | 54384 | 96 | 54464 | -1 | <0.001 |
| Poland | 95 | 382527 | 94 | 423741 | -1 | <0.001 |
| Portugal | 99 | 85439 | 99 | 79465 | 0 | ns |
| Republic of Moldova |
91 | 40266 | 87 | 49667 | -4 | <0.001 |
| Romania | 88 | 203903 | 78 | 214232 | -10 | <0.001 |
| Russian Federation |
97 | 1486730 | 97 | 1375609 | 0 | ns |
| San Marino | 88 | 209 | 91 | 199 | 3 | ns |
| Serbia | 97 | 69672 | 93 | 65195 | -4 | <0.001 |
| Slovakia | 97 | 57020 | 96 | 62451 | -1 | <0.001 |
| Slovenia | 95 | 19865 | 89 | 18520 | -6 | <0.001 |
| Spain | 95 | 354681 | 93 | 351908 | -2 | <0.001 |
| Sweden | 98 | 114109 | 94 | 113685 | -4 | <0.001 |
| Switzerland | 96 | 85930 | 95 | 86100 | -1 | <0.001 |
| Türkiye | 99 | 1303201 | 99 | 1214053 | 0 | ns |
| Ukraine | 80 | 353538 | 83 | 184455 | 3 | <0.001 |
| United Kingdom | 93 | 709079 | 92 | 677708 | -1 | <0.001 |
| Region | Intercept | Year | Pandemic |
|---|---|---|---|
| Europe | 26,77 | 0,0336 | -0,1530 |
| North Europe | -252,76 | 0,1724*** | -2,2187*** |
| South Europe | -84,61 | 0,0896* | -2,6746*** |
| Eastern Europe | -65,71 | 0,0791 | 0,5507 |
| Western Europe | 59,61 | 0,0179 | -1,8149* |
| Nation | Intercept | Year | Pandemic | Interaction |
| Albania | -31.2673 | 0.0645 | -1.6521** | N/A |
| Andorra | -252.7788 | 0.1742 | -0.8535 | N/A- |
| Austria | -677.9055 | 0.3806 | -6.8187 | N/A- |
| Belarus | -23.6828 | 0.0602 | -0.2919 | N/A- |
| Belgium | 145.8932 | -0.0237 | -0.3228 | N/A- |
| Croatia | 687.9301 | -0.2946*** | 0.4016 | N/A- |
| Cyprus | 270.5469 | -0.0860 | -1.6544† | N/A- |
| Czechia | 569.6045 | -0.2344*** | -0.9975 | N/A- |
| Denmark | -1253.2089 | 0.6688*** | -1.8051 | N/A- |
| Estonia | 634.5661 | -0.2688*** | -0.9021* | N/A- |
| Finland | 1455.1928 | -0.6753*** | 0.1131 | N/A- |
| France | 643.1797 | -0.2710*** | 0.5816 | N/A |
| Germany | 1058.9201 | -0.4796*** | 1.5304* | N/A- |
| Greece | 42.6651 | 0.0280† | -0.1802 | N/A- |
| Hungary | 99.0000 | 0.0000*** | 0.0000 | N/A- |
| Iceland | 1317.9547 | -0.6086*** | 4.5846** | N/A- |
| Ireland | -247.6943 | 0.1699 | -3.2433* | -1.8087** |
| Italy | 355.2488 | -0.1290* | 0.0899 | N/A |
| Latvia | -494.0307 | 0.2925† | -0.7037 | N/A- |
| Lithuania | 591.5722 | -0.2473** | -1.3813 | N/A- |
| Luxembourg | 99.0000 | 0.0000 | 0.0000 | N/A- |
| Malta | -3965.2956 | 2.0150*** | -9.8854 | N/A- |
| Monaco | 99.0000 | 0.0000 | 0.0000 | N/A- |
| Montenegro | 1272.4608 | -0.5871** | -3.6447† | N/A- |
| Netherlands (Kingdom of the) | 667.1475 | -0.2839*** | 0.1977 | N/A- |
| North Macedonia | 527.0100 | -0.2151*** | -8.5288*** | N/A- |
| Norway | -368.5200 | 0.2301*** | 0.1076 | N/A- |
| Poland | 695.2581 | -0.2968*** | -1.3290† | N/A- |
| Portugal | -222.5545 | 0.1591*** | -0.1465 | N/A |
| Republic of Moldova | 432.4086 | -0.1699 | -1.9710 | N/A- |
| Romania | 1960.9631 | -0.9290*** | 1.0756 | N/A- |
| Russian Federation | 270.4040 | -0.0860*** | 0.4885* | N/A- |
| San Marino | 789.8433 | -0.3484* | 4.9212* | N/A- |
| Serbia | 179.9877 | -0.0430 | -0.0415 | N/A- |
| Slovakia | 669.0760 | -0.2839*** | 1.5191* | N/A- |
| Slovenia | 679.7028 | -0.2903* | -3.0657* | N/A- |
| Spain | 321.4823 | -0.1118* | -2.4221** | N/A- |
| Sweden | 253.5922 | -0.0774† | -1.3389† | -1.2494*** |
| Switzerland | -190.1452 | 0.1419*** | -1.0274** | -0.4516* |
| Türkiye | -587.3172 | 0.3398** | -1.8081 | N/A |
| Ukraine | 6563.0914 | -3.2301* | 45.3210* | N/A- |
| United Kingdom | -170.2197 | 0.1312† | -2.4664** | N/A- |
| Country | Segmented | Chi-Square | JoinPoint |
| Albania | - | - | - |
| Austria | - | - | |
| Belgium | + | + | |
| Bosnia | + | ||
| Bulgaria | - | ||
| Croatia | - | ||
| Cyprus | - | ||
| Czechia | - | ||
| Estonia | - | - | |
| Ireland | - | - | - |
| Italy | - | ||
| Latvia | |||
| Lithuania | - | - | |
| Moldavia | - | - | |
| Montenegro | - | - | |
| Netherland | - | ||
| North Macedonia | - | - | |
| Norway | - | ||
| Poland | - | - | - |
| Romania | - | ||
| Serbia | - | ||
| Slovakia | - | - | |
| Slovenia | - | - | |
| Spain | - | - | |
| Sweden | - | - | - |
| Switzerland | - | - | - |
| Türkiye | - | ||
| Ukraine | + | ||
| United Kingdom | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





